-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaReplication Fork Reversal after Replication–Transcription Collision
Replication fork arrest is a recognized source of genetic instability, and transcription is one of the most prominent causes of replication impediment. We analyze here the requirement for recombination proteins in Escherichia coli when replication–transcription head-on collisions are induced at a specific site by the inversion of a highly expressed ribosomal operon (rrn). RecBC is the only recombination protein required for cell viability under these conditions of increased replication-transcription collisions. In its absence, fork breakage occurs at the site of collision, and the resulting linear DNA is not repaired and is slowly degraded by the RecJ exonuclease. Lethal fork breakage is also observed in cells that lack RecA and RecD, i.e. when both homologous recombination and the potent exonuclease V activity of the RecBCD complex are inactivated, with a slow degradation of the resulting linear DNA by the combined action of the RecBC helicase and the RecJ exonuclease. The sizes of the major linear fragments indicate that DNA degradation is slowed down by the encounter with another rrn operon. The amount of linear DNA decreases nearly two-fold when the Holliday junction resolvase RuvABC is inactivated in recB, as well as in recA recD mutants, indicating that part of the linear DNA is formed by resolution of a Holliday junction. Our results suggest that replication fork reversal occurs after replication–transcription head-on collision, and we propose that it promotes the action of the accessory replicative helicases that dislodge the obstacle.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002622
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002622Summary
Replication fork arrest is a recognized source of genetic instability, and transcription is one of the most prominent causes of replication impediment. We analyze here the requirement for recombination proteins in Escherichia coli when replication–transcription head-on collisions are induced at a specific site by the inversion of a highly expressed ribosomal operon (rrn). RecBC is the only recombination protein required for cell viability under these conditions of increased replication-transcription collisions. In its absence, fork breakage occurs at the site of collision, and the resulting linear DNA is not repaired and is slowly degraded by the RecJ exonuclease. Lethal fork breakage is also observed in cells that lack RecA and RecD, i.e. when both homologous recombination and the potent exonuclease V activity of the RecBCD complex are inactivated, with a slow degradation of the resulting linear DNA by the combined action of the RecBC helicase and the RecJ exonuclease. The sizes of the major linear fragments indicate that DNA degradation is slowed down by the encounter with another rrn operon. The amount of linear DNA decreases nearly two-fold when the Holliday junction resolvase RuvABC is inactivated in recB, as well as in recA recD mutants, indicating that part of the linear DNA is formed by resolution of a Holliday junction. Our results suggest that replication fork reversal occurs after replication–transcription head-on collision, and we propose that it promotes the action of the accessory replicative helicases that dislodge the obstacle.
Introduction
Replication arrest is a recognized source of genetic instability in all organisms. Proteins that protect, process, and restart arrested replication forks have been identified, and in eukaryotes their action is coordinated with the induction of a check-point response to prevent cell cycle progression until replication resumes [1], [2], [3], [4]. Model organisms have been used to amplify specific causes of replication arrest in a controlled way, revealing the existence of dedicated pathways of replication resumption. In bacteria, it has been shown that in spite of the existence of several well-characterized replication-restart machineries capable of reloading a replisome at a replication fork, which depend on the nature of the obstruction, most often arrested replication forks do not simply restart [5]. They are first targeted by various enzymes including accessory replicative helicases and recombination proteins [6], [7], [8]. It thus appears that different causes of replication arrest trigger different responses, and that arrested replication forks are channeled to various pathways depending on the original cause of arrest.
One of the first replication impediments recognized as important is the one created by transcription [9], [10], [11], [12], [13]. Enzymes that facilitate replication across highly transcribed regions have been identified in yeast [14]. In bacteria, replication, transcription and translation occur concomitantly, and replication progresses more than 10 times faster than transcription. Consequently, replication-transcription collisions are predicted to occur quite frequently. Head-on collisions between replication and transcription are more dramatic than co-directional collisions [9], [15], [16], nevertheless, under standard growth conditions replication arrests in highly transcribed regions are frequent enough to turn them into detectable hotspots of replication restart [17].
In Escherichia coli, as in other organisms, recombination proteins have been shown to facilitate replication progression under various conditions of replication impairment [6], [18]. In several replication mutants, recombination proteins play a specific role by participating in a reaction named replication fork reversal (RFR) [5], [6], [19], [20], [21]. During RFR, the newly synthesized strands are unwound from the daughter duplexes and base pair to form a Holliday junction (HJ) adjacent to a DNA double-stranded (dsDNA) end. This dsDNA end, made by the annealing of the leading and lagging strand ends, is used to reset a functional replication fork either by RecBCD - RecA - RuvABC - catalyzed homologous recombination (Figure 1A, pathway C), or by RecBCD - catalyzed DNA degradation (Figure 1A, pathway D). RecBC is essential for the viability of replication mutants that undergo RFR, because in its absence the dsDNA end is not efficiently processed, and resolution by RuvABC of the HJ produced by fork reversal results in the formation of a lethal, one-ended, chromosome break (Figure 1A pathway E). Hallmarks of the replication fork reversal reaction are (i) the requirement for viability for RecBC, but not RecA or RuvAB (in contrast with bona fide DNA double strand-breaks which requires all these enzymes for repair [22], [23], [24]), and (ii) the observation of RuvABC-dependent fork breakage in the absence of RecBC, while in its presence, fork breakage does not occur [19], [25].
Fig. 1. Replication fork reversal model and schematic representation of the I-SceI fragment carrying the inverted region in InvA and InvBE. 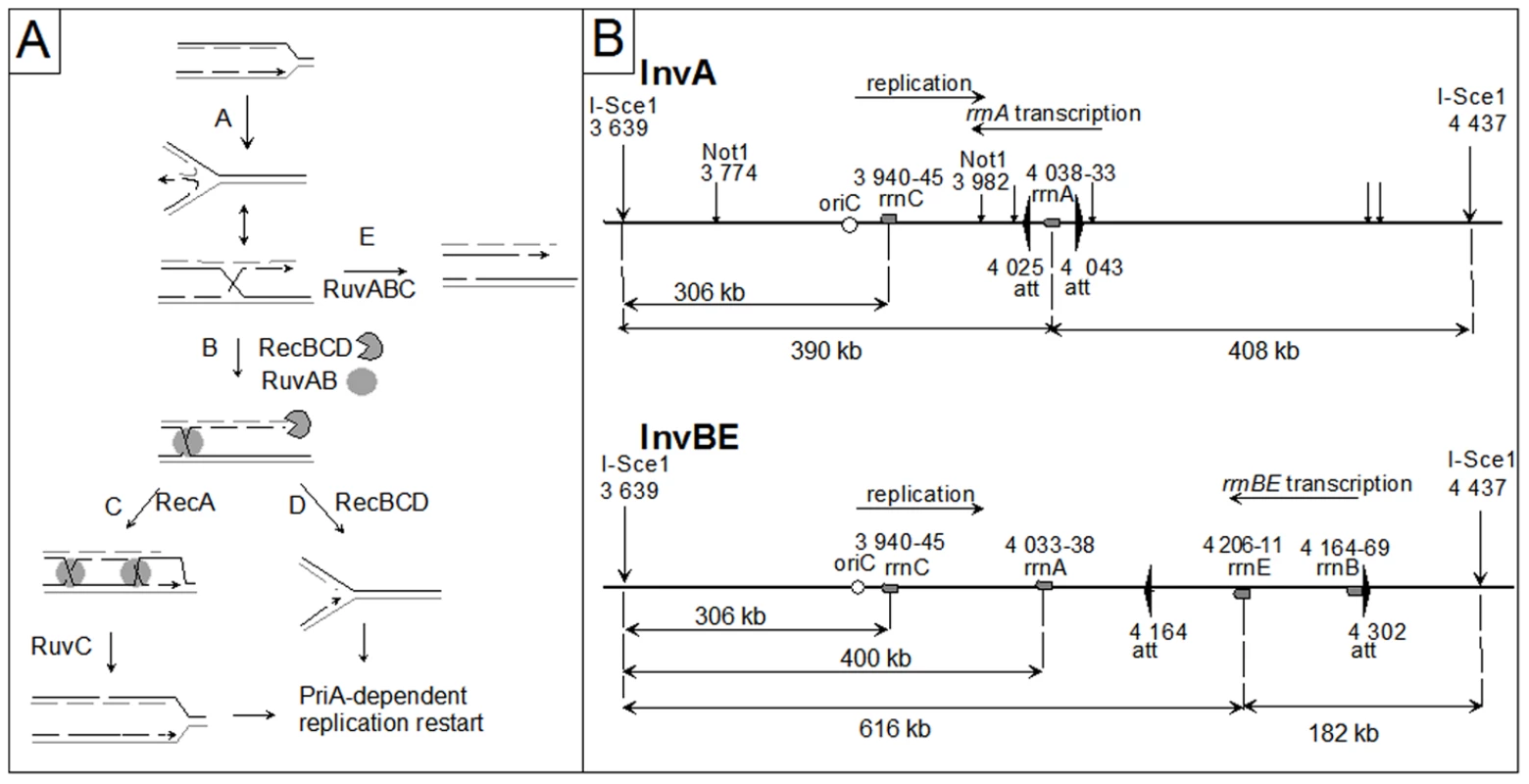
A Replication fork reversal model. In the first step (A), the replication fork is arrested, and the leading and lagging strand ends of the newly synthesized strands anneal. The reversed fork forms a four-arm structure (Holliday junction, HJ; two alternative representations of this structure are shown, open X and parallel stacked X). RecBC is essential for resetting of the fork, either by RecA-dependent homologous recombination (B–C) or by DNA degradation (B–D). Either pathway creates a substrate for replication restart proteins (PriA and its partners). In the absence of RecBCD (E), resolution of the HJ causes chromosome linearization. Continuous lines: parental chromosome. Dashed lines: newly-synthesized strands. Circle: RuvAB. Incised circle: RecBCD. B Schematic representation of the I-SceI fragment carrying the inverted region in InvA and InvBE. Positions of inversion ends (att) are indicated by wide flat arrowheads. Positions of the cleavage sites (I-SceI, NotI) are indicated by vertical arrows. Grey boxes represent the rrn genes and the open circle shows the position of oriC. The direction of replication and the direction of transcription of the inverted rrn are indicated. The distances between the two I-SceI sites and between the I-SceI sites and the 3′ end of the rrn operons are shown. The drawing is not to scale. A related reaction was later proposed to promote replication restart after blockage by a RNA polymerase that is itself arrested by a UV lesion [26]. Based on measures of cell survival after UV irradiation in various mutants, it was concluded that replication forks arrested by RNA polymerase in UV irradiated cells were reversed by RecG, an enzyme that binds multiple branched structures in vivo and in vitro [27]. In contrast with replication mutants, reversed forks in UV-irradiated cells were proposed to be targeted primarily by HJ-binding enzymes, and not by RecBCD: either RecG would convert them back to fork structures and thereby promote restart, or RuvABC would resolve them causing fork breakage. However, this model was later challenged, when direct measures of DNA synthesis showed that the inactivation of recG does not prevent replication restart in UV irradiated cells [28] and even promotes it [29], [30]. Furthermore, quantification of UV-induced chromosome fragmentation in recBC mutants showed that it completely depends on RuvABC but is hardly influenced by the RecG status of the cell [31].
Interestingly, RNA polymerase is now recognized as the main replication obstacle in the rep mutant, where RFR was first described [8], [32]. In E. coli, three accessory replicative helicases act at forks blocked by replication-transcription collisions: Rep, UvrD and DinG [8], [33]. Rep is the most critical of the three helicases since it is the only one required for normal replication. In rep mutants chromosome replication is twice slower than in wild-type cells [34], probably owing to frequent replication arrest since rep mutants undergo RFR and need replication restart proteins for growth [19]. Furthermore, Rep is driven to arrested replication forks by a direct interaction with the replicative helicase DnaB [35]. UvrD can substitute for Rep in its absence, since the uvrD single mutant has no replication defect whereas cells that lack both Rep and UvrD have a very low viability [32], [36]. DinG acts as a second back-up; its inactivation is not deleterious in rep or uvrD single mutants but prevents the residual growth of rep uvrD double mutants, as well as the growth of rep uvrD mutants in which viability is restored by a suppressor mutation that reduces replication arrest or limits its deleterious consequences (recF, rpoB, rpoC mutations, [8], [32]).
In order to better characterize the action of enzymes recruited to replication-transcription collision sites, we constructed strains in which the inversion of a specific ribosomal operon (rrn) creates a strong, locally defined replication obstacle under rapid growth conditions, owing to the high level of rrn operon expression (Inv strains, Figure 1B; [8]). This particular experimental setup allowed us to directly demonstrate that in vivo Rep, UvrD and DinG helicases indeed act at sites of replication-transcription collisions. Furthermore, in Inv strains the presence of any combination of two of these three accessory replicative helicases is required for growth in rich medium, i.e. under high collision conditions, suggesting that two of these helicases act together [8]. In contrast, the transcription-coupled repair factor Mfd does not seem to play a role in the viability of Inv mutants (Table S1, Ref. in Text S1), although this helicase dislodges transcription complexes blocked in vivo by a DNA lesion and in vitro by various obstacles, including replication forks [37], [38], [39].
In the present work, we used strains carrying one or two inverted rrn operons (Inv mutants) to address the question of the role of recombination proteins following replication-transcription collisions. First, we tested the effects of several recombination mutations and combination of mutations on the viability of Inv mutants. We previously reported that Inv strains are not affected by the absence of RecF or RecA, essential for the repair of single-stranded DNA gaps [8]; we identify here the main DSB repair complex RecBC as the only recombination function essential for viability under conditions of high replication-transcription collisions. Second, we looked for the occurrence of DSBs in the region of replication-transcription collisions by direct analysis of chromosomes of different mutants; we actually observed chromosome breakage under conditions of hyper-collisions. Third, we characterized these chromosome breaks and show that only the origin-proximal DNA double-strand end of the transcription-replication collision site can be detected, which is indicative of fork breakage. We also show that linear DNA ends that result from fork breakage are slowly degraded even in the absence of Exo V, the major dsDNA exonuclease; we show that this DNA degradation is mainly RecJ-dependent and is slowed down upon the encounter of other rrn loci on the chromosome. Finally, we addressed the question of the enzyme(s) involved in the fork breakage reaction by inactivating candidate genes. Only ruvAB inactivation was found to affect the level of fork breakage, indicating a role for the RuvAB complex. Altogether, these results lead us to propose a model for the processing of replication forks arrested by the encounter of an oppositely-oriented highly-transcribed locus.
Results
Inv mutants require RecBC for growth on rich medium
In order to test the role of recombination proteins upon replication-transcription collisions we used the InvA and InvBE strains, which carry a 18 kb inversion encompassing rrnA, and a 138 kb inversion containing rrnB and rrnE, respectively (Figure 1B). rrn inversions increase the level of replication-transcription collisions particularly in rich medium (Luria Broth, LB), i.e. under conditions of high rrn expression and high replication fork density [8]. Recombinational repair of DNA double-stranded breaks (DSBs) requires (i) RecBC, the pre-synaptic protein that loads RecA at dsDNA ends, (ii) RecA, which promotes strand invasion and homology search, and (iii) RuvAB and RuvC (or RecG) which resolve HJs formed by strand exchange [22]. Inverted rrn genes do not render homologous recombination essential, as RecA is not required for the growth of Inv strains on LB (Figure 2; [8]). Similarly, we observed that the inactivation of RuvABC and/or RecG, which catalyze the final step of homologous recombination as two redundant pathways of HJ resolution, did not affect the growth of Inv mutants on LB (Inv ruv, Inv recG and Inv ruv recG mutants, Figure 2; the 5-fold reduction of viability of the ruv recG combination of mutations in minimal medium was similarly observed in non-inverted strains (data not shown), and the slightly higher reduction of viability in LB might result from an increased need for the resolution of homologous recombination intermediates). However, a recB null mutation, which by inactivating the RecBCD complex prevents both the recombinational repair and the degradation of dsDNA ends, strongly decreased the plating efficiency of Inv mutants on LB medium (Figure 2). This observation suggests that dsDNA ends, which need to be acted upon by RecBCD, are formed during replication of the inverted chromosome region. The recD mutation, which leaves intact the homologous recombination activity of the RecBC complex but inactivates the efficient dsDNA degradation function of RecBCD, exonuclease V, did not prevent growth of Inv mutants on LB medium, suggesting that in the absence of exo V the recombination function of RecBC (helicase and RecA loading activities) is sufficient for viability.
Fig. 2. Inv recB strains are LB–sensitive. 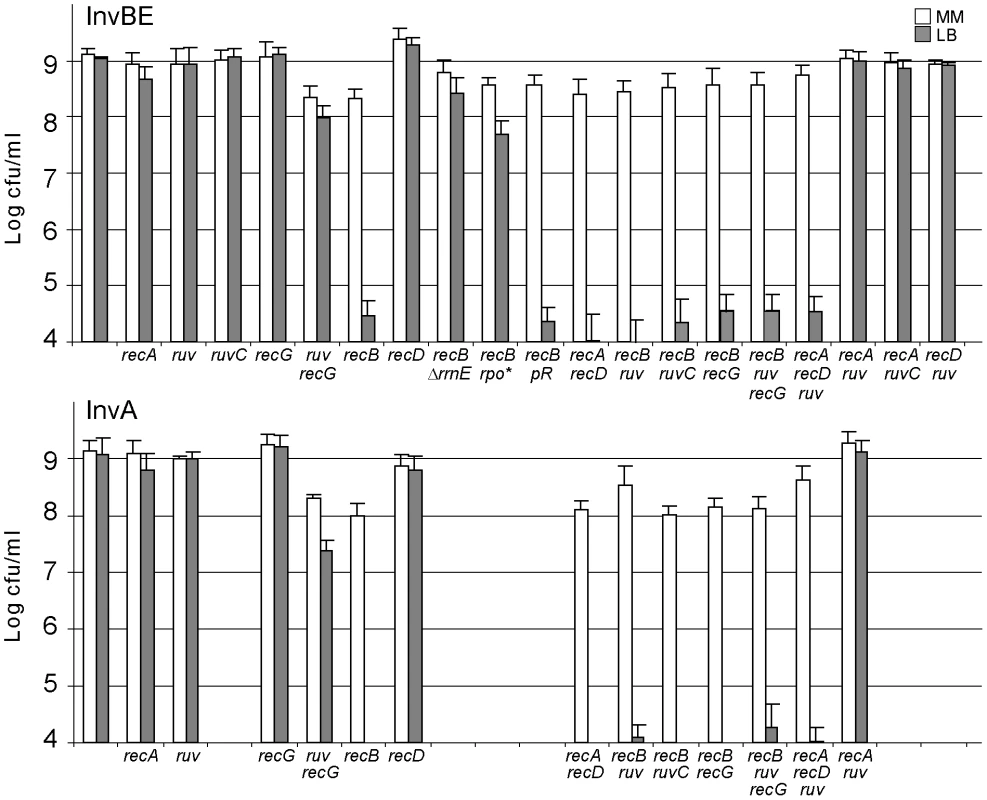
Appropriate dilutions of overnight cultures grown at 37°C in MM (OD 0.8 to 1.5) were plated on MM and LB plates, which were incubated at 37°C. White boxes: colony forming units (cfu)/ml on MM plates after 48 h incubation; grey boxes: cfu/ml on LB plates after 16–24 h incubation. Bars indicate standard deviations. Top: InvBE strains; bottom: InvA strains. ruv stands for ruvAB inactivation. rpo* stands for the rpoCΔ215–220 mutation, pR stands for pEM001, the plasmid encoding RNaseH. Colonies were small in 48 h on MM for Inv recB ruv and Inv recB ruv recG mutants, but a similar growth delay was observed for non-inverted strains. A small percentage of small colonies was observed after two to four days of incubation on LB with the InvBE recB ruvAB (or recG) and InvBE recB ruvAB recG mutants, however the number of these colonies was highly variable and no colony was ever observed with the InvA recB ruvAB (and/or recG) mutants, indicating that these mutants still require RecBC for growth on rich medium. Although InvBE carries a large inversion, the LB sensitivity of Inv recB mutants is due to the inversion of the only highly expressed of the two inverted operons, rrnE, since the precise deletion of this operon restored 100% colony formation on LB compared to MM (InvBE ΔrrnE recB mutant, Figure 2). It should be noted that the inverted region in the InvBE ΔrrnE recB mutant still carries an inverted rrn locus, rrnB. However, rrnB is expressed here at the same level in LB and in MM (minimal medium), owing to a deletion of the promoter enhancer region [8]. The full viability of the InvBE ΔrrnE recB mutant shows that RecBC is only needed when the inverted rrn operon is highly expressed. In addition, a RNA polymerase mutation that decreases rrn operon expression in LB [8], strongly increased the viability of the InvBE recB mutant (InvBE rpoCΔ215–220 recB mutant, Figure 2). Finally, rrn genes are prone to the formation of R-loops [40]; however, the requirement for RecBC is not linked to R-loop formation since over-expression of RNaseH, which destroys R-loops, did not restore viability (InvBE recB pR; Figure 2). Altogether, these results suggest that the requirement for RecBC is caused by collisions between replication forks and RNA polymerases transcribing the inverted rrn operons.
The LB sensitivity of Inv recB mutants, together with the lack of LB sensitivity of Inv recA, and Inv ruv recG mutants, suggest that a dsDNA end is formed upon replication collisions with the inverted rrn without actual DNA breakage. These results can be accounted for by the RFR model (Figure 1A). This model predicts that inactivation of both recombination (by a recA mutation) and RecBCD-catalyzed degradation (by a recD mutation) should be lethal, because it inactivates both pathways of fork resetting. This prediction was tested and, as expected, the combination of a recA and a recD mutation prevented growth of Inv mutants (InvA recA recD and InvBE recA recD, Figure 2).
Inactivation of the function catalyzing the first step of fork reversal should restore the viability of Inv recB mutants on LB, by precluding the formation of dsDNA ends. RFR has been shown to be catalyzed by various enzymatic activities. Depending on the cause of replication arrest, RecA, RuvAB, and RecG have been implicated in the formation of reversed forks in vivo or in vitro [6]. The observation that the Inv recA mutant is killed by a recD mutation indicates that a dsDNA end is still formed in the absence of RecA and that RecA is not the enzyme responsible for fork reversal. As shown in Figure 2, in the absence of either RuvAB or RecG, Inv recB and Inv recA recD mutants remain lethal on LB, indicating that fork reversal still occurs (Inv recB ruvAB, Inv recD recA ruvAB, and Inv recB recG, Figure 2). Inv recB mutants also remained LB sensitive in the absence of both RuvAB and RecG (Inv recB ruvAB recG, Figure 2), excluding a redundant function for these two enzymes. Control Inv recA ruv and Inv recD ruv mutants remained resistant to LB. Therefore, either RFR is not catalyzed in Inv mutants by the RecA, RuvAB or RecG enzymes, or in their absence a redundant, yet unknown enzyme can still catalyze the reaction. High levels of positive super-coiling can promote RFR in vitro [41], and could conceivably accumulate at sites of replication-transcription collisions, if the activity of gyrase (the enzyme that removes positive super-coils) was limiting in vivo. However, increasing (by inserting a gyrase-specific hotspot sequence at the collision site), or decreasing (by a gyrBts mutation) gyrase activity did not affect the viability of any Inv recombination mutant (our unpublished results), which renders unlikely a spontaneous reaction driven by positive super-coiling.
Fork cleavage at inverted rrnA in the InvA recBC mutant
According to the RFR model, the dsDNA ends that are formed at blocked forks are converted into one-ended double-strand breaks (DSBs) by the action RuvABC in the absence of RecBC (Figure 1A, pathway E). To ensure that the RecBC requirement results from fork breakage at the inverted rrn genes, we performed a molecular analysis of the region of replication-transcription collision. I-SceI sites were introduced into the chromosome on both sides of the inverted regions (Figure 1B). Chromosome breakage at rrnA should cleave the ∼800 kb I-SceI fragment into two fragments of about 400 kb, whereas fork breakage is expected to produce only the origin-proximal one of these two fragments (Figure 1B; Figure 1A, pathway E). We analyzed the I-SceI cleavage products of the InvA recB chromosome by pulse field gel electrophoresis (PFGE) and Southern blotting, prior to and after a shift to LB, using a probe that hybridizes with the origin-proximal part of the I-SceI fragment carrying the inversion (Figure 3A, 3B). The intact I-SceI fragment observed in MM disappeared after a shift to LB, while a ∼400 kb fragment appeared in 1 hr and seemed to be converted to DNA fragments of smaller size with time, with the accumulation of a fragment of ∼300 kb in three hours (Figure 3A, 3B). The length of the DNA fragment observed after 1 hr of incubation in LB (∼400 kb) corresponds to the distance between the I-Sce1 site and the inverted rrnA operon (Figure 1B). However, its conversion to smaller fragments at later times suggests that this DNA fragment is degraded in vivo. In E. coli, one of the main exonucleases besides RecBCD is the RecJ exonuclease, which degrades 5′-ended single-stranded DNA produced by the action on dsDNA ends of RecQ or another helicase [42]. We tested whether the I-SceI - rrnA fragment of ∼400 kb was being degraded by RecJ and observed that indeed inactivation of RecJ prevented most of the conversion of this fragment to smaller ones (Figure 3A, 3B). This result shows that RecJ degrades dsDNA ends made by breakage at rrnA in the InvA recB mutant. No production of linear DNA in vivo could be detected with control strains (non-inverted recB mutants, InvA RecBCD+ strains, Figure S1).
Fig. 3. Chromosome breakage in InvA recB and InvA recA recD mutants. 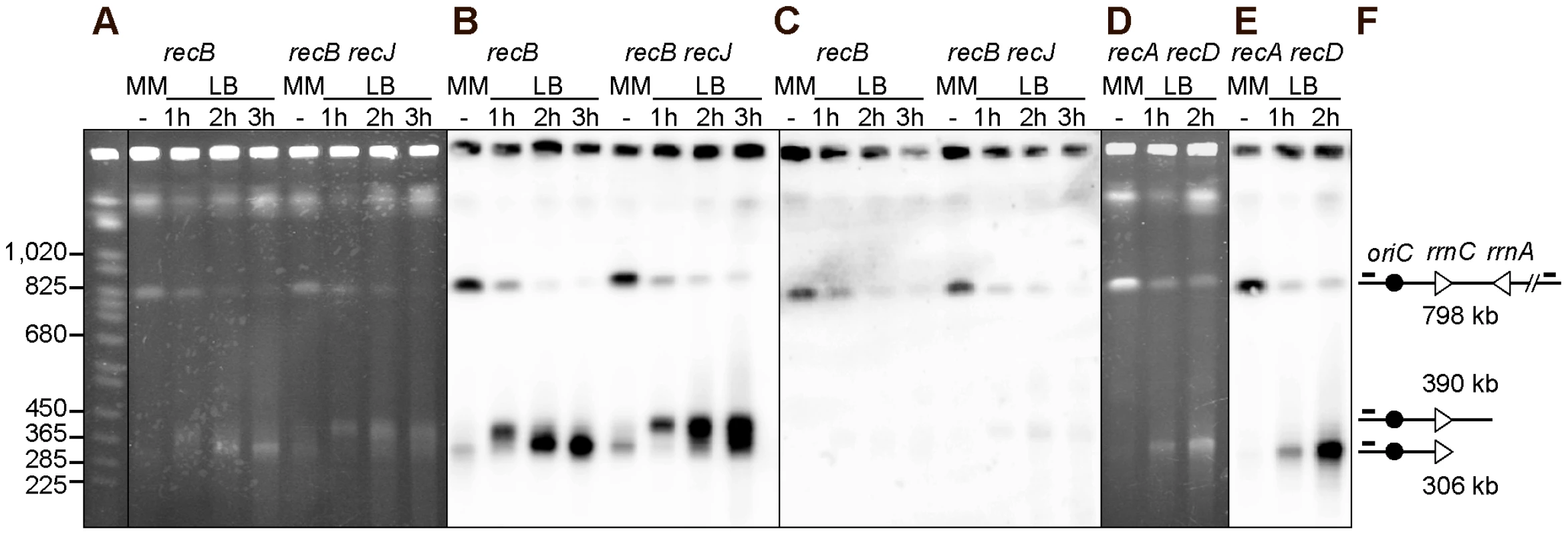
Chromosomes of InvA recombination mutants, grown in minimal medium (MM) or for 1, 2 or 3 hours in LB, were cleaved with the I-SceI enzyme and fragments were separated by PFGE. A. Ethidium bromide stained gel with I-SceI cut chromosomes from InvA recB and InvA recB recJ mutants. First lane Saccharomyces cerevisiae chromosome ladder, relevant sizes are indicated on the left. B. Southern blot of the gel shown in A using the origin-proximal probe in the 800 kb I-SceI fragment, both the intact fragment and fragments of smaller sizes hybridize with the probe. C. Southern blot of the gel shown in A using the origin-distal probe in the 800 kb I-SceI fragment, only the intact I-SceI fragment hybridizes with the probe. D. Ethidium bromide stained gel with I-SceI cut chromosomes from the InvA recA recD mutant. E. Southern blot of the gel shown in D using the origin-proximal probe in the 800 kb I-SceI fragment, both the intact fragment and fragments of smaller sizes hybridize with the probe. F. Schematic representation of the different DNA fragments. The triangles represent the two rrn operons as indicated, the black circle represent oriC and the little bars above the lines represent the position of the origin-proximal (left) and origin distal (right) probes. In order to determine whether the origin-distal part of the I-SceI fragment is also produced by cleavage at rrnA, the same membranes were hybridized to a probe within this region. The only fragment detected was in the intact I-SceI fragment (Figure 3C), which, as seen with the origin-proximal probe, decreased in intensity with time. As previously observed for other fork-breakage reactions [43], the absence of detectable origin-distal fragment is consistent with the conclusion that the origin-proximal fragments result from fork breakage (one-ended break) and not from a bona fide DSB (two-ended break) at rrnA.
Our attempts to quantify the bands produced by fork cleavage at rrnA provided highly variable results, owing partly to the nearly full disappearance of the intact I-SceI fragment, an unexpected observation, and partly to the fact that a large fraction of the probed DNA remained trapped in the wells. Non-migrating DNA may result from partial cleavage and/or from the presence of structures blocking DNA migration (forks, HJ, replication bubbles), thus contributing to the disappearance of the intact I-SceI fragment upon growth in LB. In order to determine whether the non-migrating DNA is trapped in wells by unresolved recombination intermediates, we analyzed chromosome breakage in the InvA recA recD mutant. A large amount of DNA fragments smaller than the intact I-Sce1 fragment was detected in the InvA recA recD mutant (Figure 3D, 3E), in contrast with in the non-inverted recA recD control strain (Figure S1). The level of non-migrating DNA remained very high and the intact I-SceI fragment disappeared when cells were propagated in LB, still preventing quantification (Figure 3D, 3E). This observation suggests that non-migrating DNA might be trapped in wells owing to the presence of replication intermediate structures. Interestingly, growth of the InvA recA recD mutant in LB triggered the appearance of a DNA band that hybridized specifically with the origin proximal probe, and was not the ∼400 kb fragment expected from breakage at rrnA but the smaller ∼300 kb one, observed in the recBC mutant in the presence of the RecJ exonuclease (Figure 3D, 3E). Since in a recA recD context, RecBC and RecJ are active, this ∼300 kb fragment, which is not observed in a recB recJ context, is likely to result from the degradation by the combined action of the RecBC helicase and the RecJ exonuclease of a dsDNA end produced by fork cleavage at rrnA.
Fork cleavage at the inverted rrnE locus in the InvBE recBC mutant
To determine whether fork breakage at the inverted rrnA locus is specific for this ribosomal operon, we analyzed the formation of linear DNA in the inverted region of the InvBE recB mutant by I-Sce1 enzyme. When the InvBE recB recJ mutant was propagated in LB medium, a fragment of about 616 kb that hybridizes with the origin-proximal probe was observed, as expected from fork breakage at rrnE (Figure 4A, 4B). In the InvBE recB mutant, this ∼616 kb fragment was transformed into a smear, owing to RecJ-mediated DNA degradation (Figure 4A, 4B). The corresponding origin-distal fragment of about 180 kb was not observed using an origin-distal probe, supporting the conclusion that the origin proximal fragments result from fork breakage and not from a bona fide DSB (Figure 4C). In the InvBE recA recD mutant, the origin-proximal probe revealed a smear of fragments smaller than 616 kb and two main fragments of ∼400 kb and ∼300 kb (Figure 4D, 4E), which presumably result from degradation of the ∼616 kb fragment by RecBC and RecJ as they are not observed in a InvBE recB recJ mutant. As in InvA mutants, the disappearance of the intact I-SceI band and the high level of non-migrating I-SceI fragments prevented the quantification of linear DNA species. No production of linear DNA in vivo could be detected with control strains (non-inverted recB or recA recD mutants, InvBE RecBCD+ strains, Figure S1).
Fig. 4. Chromosome breakage in InvBE recB and InvA recA recD mutants. 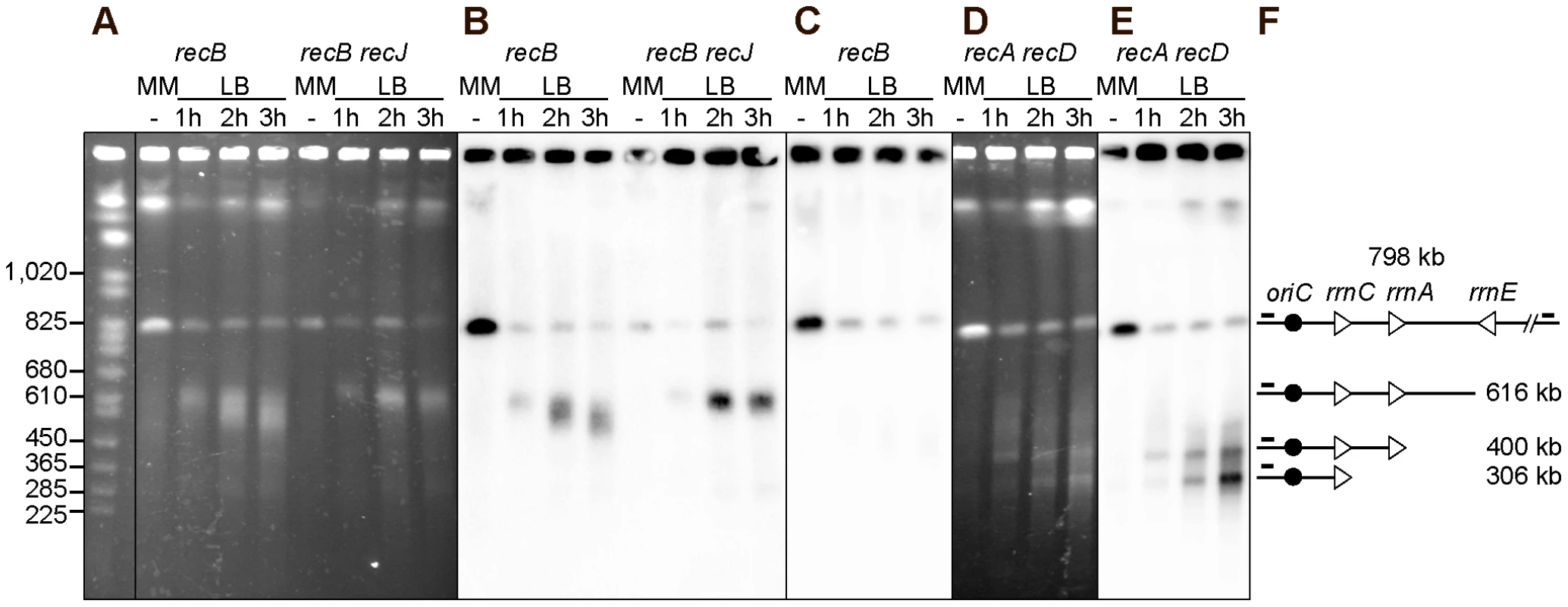
Chromosomes of the indicated InvBE mutants, grown in minimal medium (MM) or for 1, 2 or 3 hours in LB, were cleaved with the I-SceI enzyme and fragments were separated by PFGE. A. Ethidium bromide stained gel with I-SceI cut chromosomes from InvBE recB and InvBE recB recJ mutants. First lane Saccharomyces cerevisiae chromosome ladder, relevant sizes are indicated on the left. B. Southern blot of the gel shown in A using the origin-proximal probe in the 800 kb I-SceI fragment, both the intact fragment and fragments of smaller sizes hybridize with the probe. C. Southern blot of a gel made with the InvBE recB I-SceI cut chromosomes, using the origin-distal probe in the 800 kb I-SceI fragment, only the intact I-SceI fragment hybridizes with the probe. D. Ethidium bromide stained gel with I-SceI cut chromosomes from the InvBE recA recD mutant. E. Southern blot of the gel shown in D using the origin-proximal probe in the 800 kb I-SceI fragment, both the intact fragment and fragments of smaller sizes hybridize with the probe. F. Schematic representation of the different DNA fragments. The triangles represent the three rrn operons as indicated, the black circle represent oriC and the little bars above the lines represent the position of the origin-proximal (left) and origin-distal (right) probes. rrn genes are a barrier to DNA degradation
We noted that the ∼300 kb and the ∼400 kb fragments observed in the InvBE recA recD mutant have a size corresponding to the distance between the I-SceI site and the normally oriented rrnC and rrnA operons, respectively (Figure 1B). We hypothesized that the product of fork breakage at the inverted rrnE locus might be degraded until the first non-inverted rrn is encountered by exonucleases. This DNA degradation is not observed in a recB recJ mutant, is slow in a recB mutant where it is mainly catalyzed by RecJ (it does not reach rrnA, 231 kb away, in two hours), and is more efficient in a recA recD mutant, where the helicase RecBC and RecJ exonuclease are both active (Figure 4). To test the hypothesis that rrn genes could be a barrier to DNA degradation, membranes such as the one shown in Figure 4E were hybridized with probes located about 5 kb apart, immediately upstream or downstream of rrnC and rrnA (Figure 5, only the MM and LB 2 hours lanes are shown, the kinetics of appearance of bands is shown Figure 4E). The ∼300 kb fragment hybridized with the rrnC promoter region but not with the rrnC terminator region, indicating that it ends within rrnC (Figure 5, probes 2 and 3). Similarly, the ∼400 kb fragment hybridized with the rrnA promoter region but not with the rrnA terminator region, indicating that it ends within rrnA (Figure 5, probes 4 and 5). These results indicate that linear DNA ending in rrn sequences accumulates after DNA breakage, suggesting that degradation of linear DNA is slowed down by the encounter with rrn sequences. In the recA recD context, DNA degradation reaches rrnA, 231 kb away from the inverted rrnE in 1 hr, and rrnC, 325 kb away, in 2 hours (Figure 4D, 4E).
Fig. 5. rrn operons are a barrier to DNA degradation. 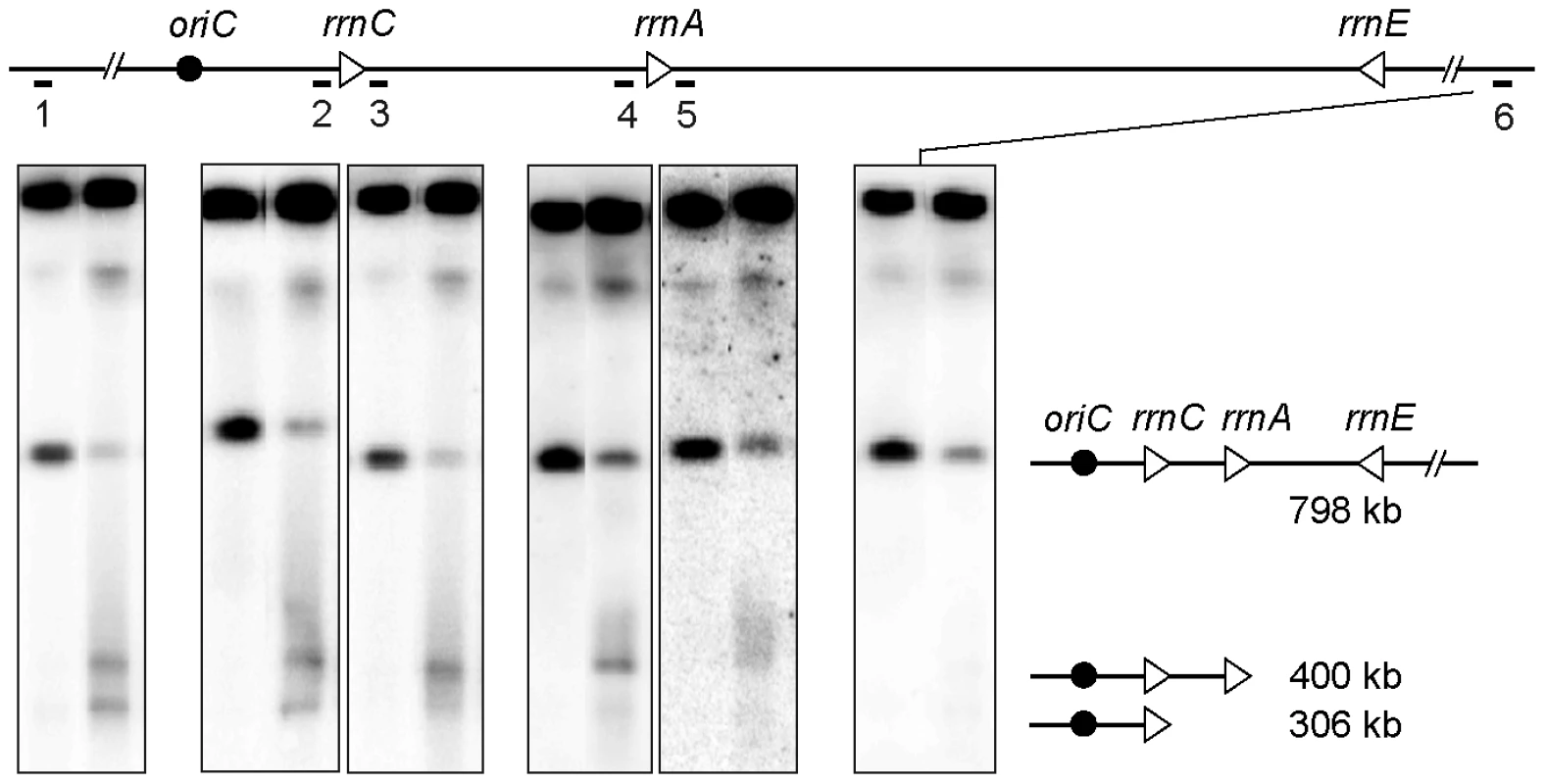
Top; schematic representation of the I-SceI fragment carrying the inverted rrnE operon. Triangles represent rrn operons as indicated, the black circle represents oriC, the black line represents the DNA I-SceI fragment and the numbered bars under this line show the positions of the different probes. Bottom; chromosomes of InvBE recA recD cells grown in MM or in LB for 2 hours were cleaved with I-SceI and fragments were separated by PFGE, for each panel: left lane, cells grown in MM, right lane, cells grown in LB for 2 hours. Southern blots were hybridized with the different probes indicated above each panel. From left to right: probe 1 - origin-proximal probe, probe 2 - rrnC promoter probe, probe 3 - rrnC terminator probe, probe 4 - rrnA promoter probe, probe 5 - rrnA terminator probe, probe 6 - origin-distal probe. A schematic representation of the fragments of different length is shown on the right. For each probe all hybridizing fragments are necessarily larger than the distance between the origin-proximal I-SceI site and the probe, so that the smear stops at the position of the probe. In the InvA genome, NotI restriction produces a 208 kb fragment carrying rrnC (NotI kb 3774 to 3982, Figure 1B), and degradation of the origin distal part of this DNA fragment until rrnC is expected to produce a 171 kb DNA fragment (NotI kb 3774 to rrnC, Figure 1B). Actually, when the InvA recB mutant was grown for 2 hrs in LB, an additional ∼170 kb fragment was observed after NotI restriction, compared to the restriction profile of chromosomes extracted from the same cells grown in MM (Figure 6A). This DNA fragment hybridized only with a probe adjacent to the rrnC promoter and not with a probe adjacent to the rrnC terminator sequence (Figure 6B, 6C). This result indicates that the fragment ends within rrnC, which suggests that DNA degradation is slowed down in rrnC in InvA recB cells. This NotI fragment corresponds to the ∼300 kb fragment observed after cleavage by I-SceI (Figure 1B), and indicates that DNA 93 kb in length, the distance between rrnC and the inverted rrnA, is degraded in 2 hours by RecJ in a recB context, and in 1 hour by RecJ and RecBC in a recA recD context (Figure 3). No 171 kb DNA fragments were observed with control strains (Figure S1).
Fig. 6. Fork breakage is partially RuvABC-dependent in InvA mutants. 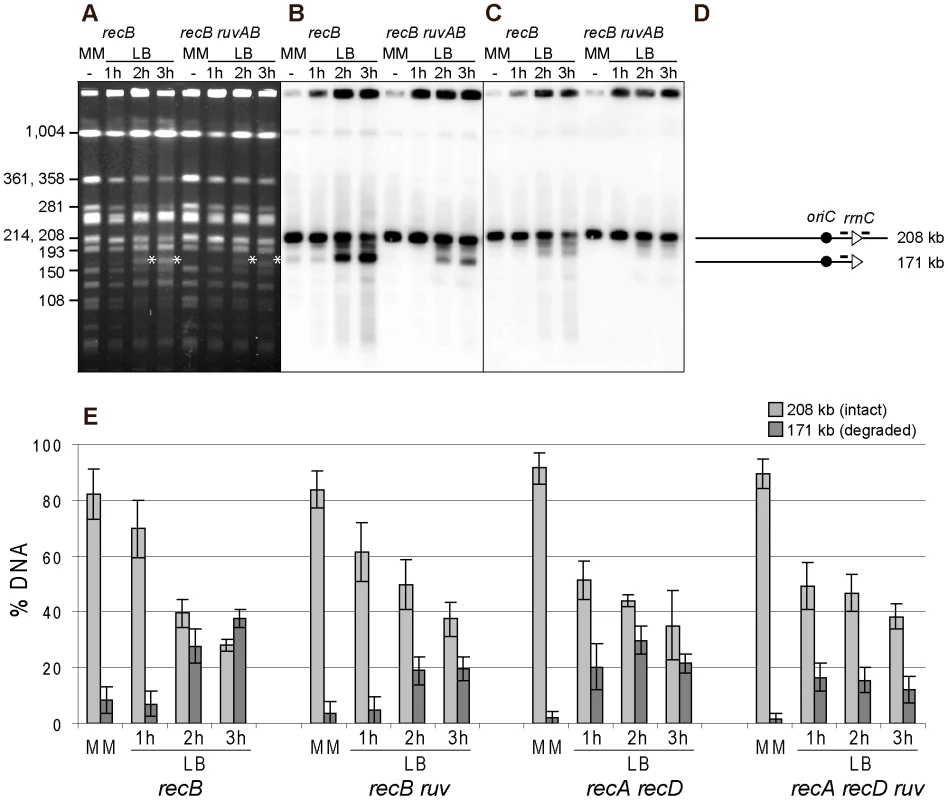
A. Chromosomes of the indicated InvA mutants, grown in minimal medium (MM) or for 1, 2 or 3 hours in LB, were restricted with the NotI enzyme and fragments were separated by PFGE. A. Ethidium bromide stained gel with NotI restricted chromosomes from InvA recB and InvA recB ruvAB mutants. Fragment sizes are indicated on the left. The star indicates the position of migration of the 171 kb DNA fragment formed by fork breakage and DNA degradation. B. Southern blot of the gel shown in A using the rrnC promoter probe, both the intact 208 kb NotI fragment and fragments of smaller sizes hybridize with the probe (the minor hybridization with the 193 kb Not1 restriction fragment may result from co-migration of broken DNA with this fragment). C. Southern blot made with the gel shown in A, using the rrnC terminator probe, only the intact 208 kb NotI fragment hybridizes with the probe. D. Schematic representation of the different DNA fragments. The triangles represent the two rrn operons as indicated, the black circle represents oriC and the bars above the lines show the positions of the probes. E. For each mutant, Southern hybridizations of 3 to 6 gels were quantified, and the percentage of hybridized DNA that remains in wells, that migrates at the 208 kb position and that migrates at the 171 kb position were calculated. For clarity, only the percentages of migrating DNA are shown here (see Table S2 for complete results); light grey, 208 kb fragment (intact), dark grey, 171 kb fragment (resulting from fork breakage and DNA degradation up to rrnC). Vertical bars indicate standard deviations. DNA breakage at an inverted rrn locus is RecA-independent and partly RuvABC-dependent
The above experiments indicate that following replication arrest at an inverted rrnA locus, the products of fork breakage are converted by DNA degradation, in the absence of the exonuclease V activity of RecBCD, to smaller chromosome fragments, most of which have a specific length and can be analyzed by NotI restriction enzyme digestion of chromosomes. As the dramatic loss of the intact band was not observed after NotI cleavage, in contrast to I-SceI cleaved chromosomes, we used Southern blots of NotI fragments to quantify the linear DNA resulting from breakage at the inverted rrnA locus, at different times after a shift to LB and in different recombination mutants. The intensities of bands corresponding to the 208 kb NotI fragment (i) trapped in wells, (ii) intact (208 kb), or (iii) degraded to rrnC after fork breakage at InvA (171 kb), were quantified by Southern hybridization. The proportion of DNA present in each of these three bands was calculated and averages from 3 to 5 experiments are shown in Figure 6E and Table S2. In the InvA recB mutant, the proportion of the 171 kb DNA fragment produced by fork breakage and DNA degradation up to rrnC increased from 9% in MM to 27%±5%, and 37%±3%, after two and three hours in LB, respectively. In the InvA recA recD mutant, this proportion increased to 20%±8% after 1 hour in LB and 30%±5% after two hours, and then decreased, possibly because DNA degradation progresses beyond rrnC. The 171 kb DNA band appeared earlier on gels in a recA recD context than in a recB context, probably because DNA degradation is more rapid in the former mutant, owing to the combined action of RecBC and RecJ. The observation of a similar efficiency of fork breakage in InvA recB and in InvA recA recD cells confirms that fork breakage is independent of RecA. As expected, the 171 kb fragment was not observed in control strains: recombination mutants without chromosome inversion, a recombination proficient InvA mutant, and an InvA mutant in which only recA or only recD is inactivated (data not shown; Table S2).
It should be noted that, given the absence of the origin-distal dsDNA end at the replication-transcription collision site (Figure 3C, Figure 4C), the origin-proximal dsDNA end is produced by either a reaction where only one of the two replicated chromosome arms at the fork is broken, or by over-replication of blocked replication forks. Either of these reactions will produce an intact copy of the chromosome in addition to the linear DNA fragment interrupted at the position of replication-transcription collision (as in Figure 1A, step E). Therefore, the percentage of 171 kb fragments can be at most 50% of the migrating DNA, assuming 100% breakage. The percentage of chromosomes that have been replicated without DNA damage can be calculated by deducing from the measured proportion of intact 208 kb fragment that of broken DNA (171 kb fragment). These calculations show that most of the DNA is broken upon collision of replication forks with the inverted rrn, in a recB and in a recA recD context (Table S2).
The RFR model predicts that fork cleavage may be RuvABC dependent (Figure 1A, step E). Since RuvABC does not have any known exonuclease activity, and is not suspected to affect the activity of E. coli exonucleases, we used this assay to compare fork breakage in RuvABC+ and ruvABC mutant cells. The proportion of the 171 kb DNA fragment was nearly two-fold lower in InvA recB ruvABC compared to InvA recB, and in InvA recA recD ruvABC compared to InvA recA recD (Figure 6; Table S2). These observations suggest that two types of reactions are responsible for the generation of a broken chromosome arm at replication-transcription collision sites, a RuvABC-dependent (RFR) and a RuvABC-independent reaction. As expected from the LB sensitivity of Inv recA recD mutants, RFR is RecA-independent since the proportion of RuvABC-dependent breakage is similar in recB and recA recD contexts. Similarly, as expected from the LB sensitivity of Inv recB recG mutants, fork breakage was RecG-independent in an InvA recB context (Table S2).
Discussion
In this work we analyze the action of recombination proteins at replication forks arrested by a collision with a highly-transcribed, oppositely-oriented rrn operon. We find that RecBC is crucial for replication across this region and that in its absence fork breakage, which is partly RuvABC-dependent, occurs. The RecBC requirement is caused by collisions of replication with RNA polymerases within the inverted ribosomal operon, and does not involve R-loop formation. Furthermore, in the course of this work we observed that DNA degradation of linear DNA is also arrested by an oppositely-oriented rrn locus.
RFR occurs at forks arrested by a highly expressed, oppositely oriented rrn operon
Several lines of evidence argue for the occurrence of RFR upon collision of replication with inverted rrn operons. First, Inv recB and Inv recA recD mutants are sensitive to growth in rich medium, whereas Inv recA and Inv recD mutants are not. Second, the inverted rrn genes are sites of chromosome breakage, half of which is dependent of RuvABC. We conclude that half of the linear DNA observed in Inv recB cells results from the resolution of reversed forks by RuvABC while the other half results from one or more other processes (Figure 7). We hypothesize that the linear DNA observed in recB ruvAB mutants may result from re-initiation at the chromosome origin oriC. Running of these new replication forks into blocked forks is expected to form linear DNA by copying the newly-synthesized strands of the first forks to the end (Figure 7). Such a run-off reaction has previously been observed at extra Ter sites inserted in the chromosome, and in over-initiation mutants [44], [45]. Strains carrying extra Ter sites are only viable if the dsDNA end is repaired by homologous recombination, suggesting that Inv cells that suffer re-replication should also require homologous recombination for replication restart and viability. Re-replication in Inv mutants may not frequent enough to render RecA essential for growth, probably owing to a significant proportion of blocked forks processed by RFR (Figure 7). Replication re-initiation at oriC might explain the high level of trapped I-SceI fragments in LB, a RecA-independent phenomenon that was less important with the smaller NotI oriC-carrying fragment.
Fig. 7. Model for the restart of forks arrested by a highly expressed, oppositely oriented rrn operon. 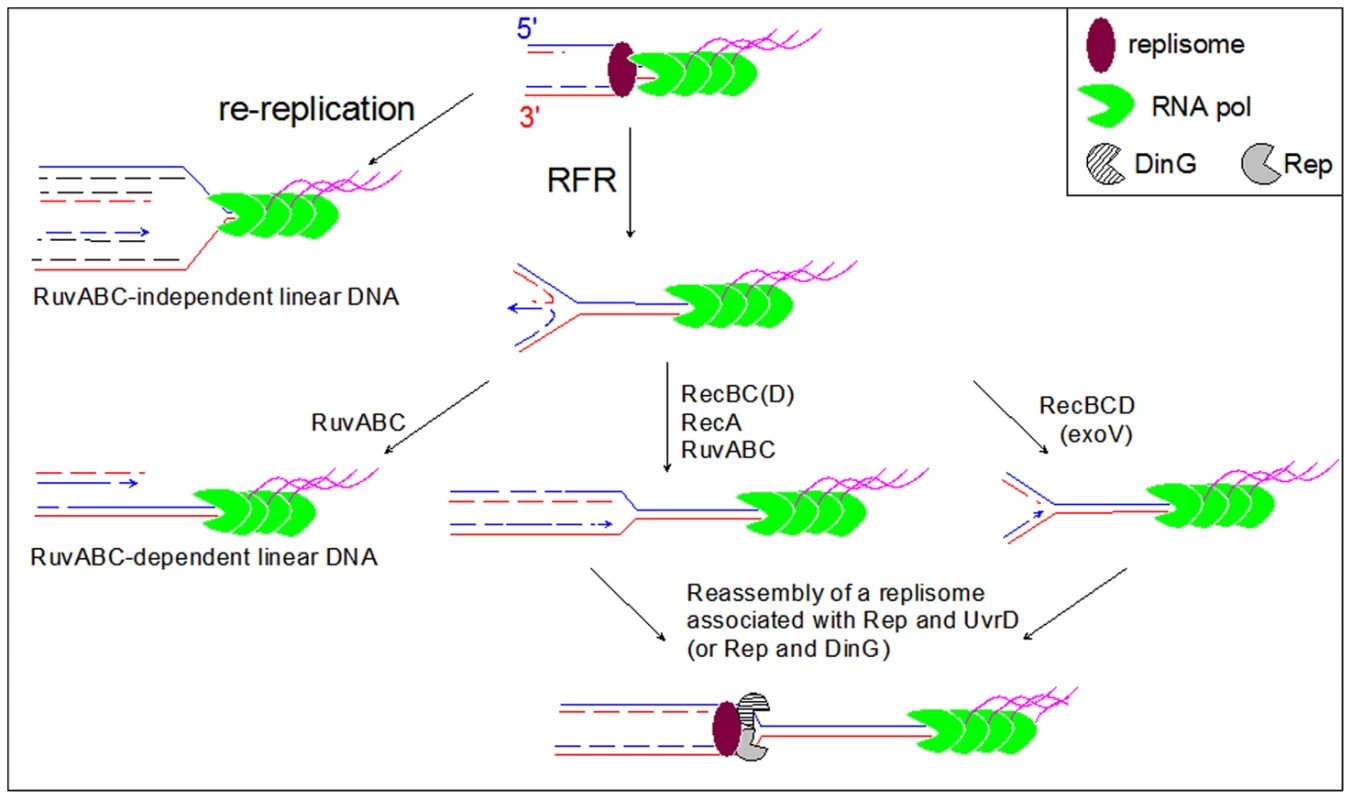
A blocked fork is either reversed (RFR) or re-replicated by a following round of replication initiated at the replication origin (re-replication). The product of RuvABC-catalyzed resolution of the HJ formed by fork reversal, and the product of re-replication are similar origin-proximal dsDNA ends (left part of the model). These DNA ends are repaired in Rec+ cells by homologous recombination catalyzed by RecBCD, RecA and RuvABC (not shown), but remain unrepaired in recBC and recA recD mutants, where they are detected by electrophoresis of Not1- or I-Sce1-treated DNA (Figure 3, Figure 4, Figure 5, Figure 6). In Rec+ cells the dsDNA end formed by fork reversal can be directly acted upon by RecBCD (see Figure 1) and processed by either homologous recombination (RecBC(D)-RecA-RuvABC pathway) or by DNA degradation (RecBCD (exo V) pathway). Reversed forks resetting by either pathway produces a replication fork that has moved backward, further from the obstacle than the original blocked fork. We propose that the reloading of new replisome at such forks favors the binding of a second accessory helicase (DinG or UvrD), required with Rep for replication across the inverted rrn operon. DNA degradation is slowed down by the encounter of rrn operons
We observed that the dsDNA fragments detected in PFGE are subjected to DNA degradation by RecJ, and this reaction is more efficient in the presence of the RecBC helicase. Degradation of dsDNA ends and homologous recombination catalyzed by the combined action of RecBC and various exonucleases including RecJ, have been reported previously in a recD mutant [46], [47]. The reaction observed here is slow, on average around 3 kb per minute in a recA recD context where both RecJ and RecBC are active, and around 1 kb per minute in a recB mutant where it depends primarily on RecJ. RecJ is a 5′ to 3′ ssDNA exonuclease, but it can also digest dsDNA in vitro, a reaction that is stimulated in the presence of the RecQ helicase [48]. RecBCD digests dsDNA at a rate close to 1 kb per second [49], which allows it to rescue reversed forks prior to the resolution of the HJ (Figure 1A step D). In contrast, the genetic properties of strains that undergo RFR indicate that RecJ and RecBC do not to rescue reversed forks by DNA degradation. Actually, RecJ digests 5′ ended ssDNA at a rate of about 1 kb per minute in vitro [42]. A RecBCD mutant complex, in which the helicase function of the RecD subunit is inactivated, is three times slower than the intact enzyme in vitro [49]. This may explain why RecJ, and RecBC in the absence of RecD, do not catch up with the RuvAB-migrated HJ prior to resolution by RuvC. In addition RecJ and RecBC are less processive than RecBCD [42], [49] and the dsDNA degradation that we observe in this work is likely to result from multiple DNA binding events. Why this DNA degradation slows down at ribosomal operons remains an open question. Some features of the rrn locus, either structural (the operon contains DNA sequences prone to the formation of secondary structures), or functional (the operon might be expressed, even on a broken DNA arm), might trigger the dissociation of RecJ or RecBC from DNA.
Enzymes that catalyze replication fork reversal
We have not identified the enzymatic function(s) that reverse forks in Inv strains, as the inactivation of the enzymes previously shown to reverse forks in vivo or in vitro, RecA, RuvAB or RecG, did not prevent RFR. The Rep, UvrD, and DinG helicases, which act at blocked forks in Inv mutants, are not responsible for RFR since the inactivation of any of them aggravated rather than relieved the requirement for RecBC (our unpublished results). Similarly in a dnaNts mutant, impaired for the β-clamp subunit of DNA polymerase III, forks are reversed by a yet unknown function, indicating that more RFR-catalyzing enzymes remain to be identified in E. coli [50].
Recently, replication fork reversal was proposed to occur in cells where the Rho terminator of transcription is inactivated by a specific agent, bicyclomycin [51]. Inactivating Rho-dependent transcription termination is thought to cause replication-transcription conflicts, and bicyclomycin treatment killed recB mutants but did not kill recA mutants. In the recB mutants, a high level of chromosome breakage was detected, suggestive of the occurrence of RFR. Chromosome breakage was attributed to the resolution by RuvABC of reversed forks, but the role of RuvABC in bicyclomycin-induced chromosome breakage was not tested. Because the amount of linear DNA measured by PFGE was strongly decreased in a recA mutant, it was proposed that RecA was the enzyme responsible for fork reversal. This interpretation of the data is questionable since in recA cells the very low level of detectable linear chromosomes could reasonably be ascribed to their degradation by RecBCD [22], [52]. Actually, we observed here that fork breakage at replication-transcription collision sites is RecA-independent.
RFR is caused by the encounter of replication forks with RNA polymerases
Two kinds of obstacles can impede replication progression across highly transcribed regions: collisions with R-loops, and collisions with DNA-bound, transcribing (or arrested) RNA polymerases. R-loops have been shown to cause replication arrest in several organisms and to stimulate DNA rearrangements, often associated with replication fork blockage ([53], [54] and Ref therein). However, R-loops are unlikely to be the cause of replication fork arrest and reversal in Inv recB mutants, because over-expression of RNaseH does not suppress the sensitivity to rich medium of these strains. DinG plays two roles in Inv mutants, as it participates to RNA Pol removal together with Rep and UvrD, and it is the helicase responsible for the removal of R-loops [8]. In vitro studies showed that DinG can remove R-loops by directly recognizing them, independently of the presence of a replication fork [55]. The lack of effects of RNaseH over-expression in the Inv recB mutants leads us to conclude that the presence of DinG actually prevents the accumulation of R-loops in these mutants, and, in turn, that forks are mostly arrested at highly expressed inverted rrn genes by trains of RNA polymerases transcribing the rDNA.
The occurrence of RFR at inverted rrn genes is in agreement with the idea that replication arrest is mainly due to collisions of replication forks with RNA polymerases in a rep single mutant, where RFR was first reported. However, in the rep mutant the conversion of forks into reversed forks was partly catalyzed by RuvAB and partly by an unidentified function [56]. Here we have no evidence for RuvAB catalyzing some of the RFR reactions, since inactivating ruvAB does not rescue the Inv recB or Inv recA recD mutants, possibly because the alternative function can reverse all forks at the inverted rrn locus in the absence of RuvAB.
RFR precedes and may facilitate the action of accessory replicative helicases
We have shown that after replication-transcription collisions, replication restart requires the action of the accessory replicative helicases Rep, UvrD and DinG, which presumably remove RNA polymerases from DNA [8]. The occurrence of RFR indicates that these helicases do not act first, since their action would allow forks to progress unimpeded across the inverted rrn, preventing RFR. In other words, why would RecBC be essential for growth in the presence of Rep, UvrD and DinG, if these helicases could directly act at blocked forks to remove obstacles? Therefore, we propose that RFR takes place first and that helicases act at forks that are reset after reversal (Figure 7). We can envision different reasons why RFR would take place first: either the enzyme that promotes the reaction may have more affinity for blocked forks than the accessory replicative helicases, or there may be too many RNA polymerases on highly expressed rrn operons for the helicases to deal with them within the time frame that is required to initiate RFR, or RFR may be required for the action of these helicases. In support for the latter hypothesis, UvrD was previously shown to act in conjunction with RecBC in two cases of replication fork restart. Firstly, UvrD acts together with homologous recombination for the removal of Tus from extra Ter sites. UvrD is then required for viability, and does not directly remove Tus from Tus/Ter-blocked forks, since it does not bypass the need for homologous recombination [57]. Secondly, UvrD is essential for viability in the rep mutant, where it necessarily acts after RFR, since it does not bypass the need for RecBC. These observations suggest that UvrD might more easily find its target on PriA-dependent restarted forks formed after homologous recombination, or after degradation of reversed forks. At forks blocked by an inverted rrn locus, the concerted action of two accessory helicases is required for restart, which are Rep, and either UvrD or DinG [8], [32]. However, in contrast with UvrD, Rep acts without homologous recombination or RFR, since it acts in wild-type E. coli where RecBC is not essential for viability (RecBC becomes essential only in its absence, when UvrD is required). Rep may be directly targeted to blocked forks by its interaction with the DnaB helicase [33], [35]. We propose that RFR occurs at forks blocked by an inverted rrn locus to promote the action of UvrD or DinG, required here in addition to Rep (Figure 7). Forks that have been reversed, and then converted back to a fork structure either by homologous recombination or by degradation of the DNA double-strand end in the reversed fork may be easier targets for UvrD and DinG than the original blocked forks. Although the molecular mechanism by which fork reversal facilitates replication restart is not known at present, we can speculate that replication fork reversal may trigger the disassembly of the blocked replisome and thereby facilitates access to DNA for the accessory helicases, and/or allow the targeting of these helicases to restarting forks by some interaction with a recombination or replication restart protein.
Materials and Methods
Strains and constructions
Strains and plasmids used in this work are described in Table S3 (Ref. in Text S1). They were constructed by P1 transduction. The recB mutation was introduced in the presence of the wild-type recBCD genes carried on an IPTG-dependent plasmid pAM-RecBCD. A pAM-RecA plasmid was used for propagation and P1 transduction of recA mutants. Plasmids were cured prior to each experiment by growing cells in the absence of IPTG and plasmid-less colonies were isolated on minimal medium (MM). All other mutations were directly introduced in the Inv mutants by P1 transduction on MM. For insertion of the I-Sce1 site into the chromosome, the following double-stranded sequence 5′ GCATGCTAGGGATAACAGGGTAATATCGAT 3′ carrying the I-Sce1 cleavage site (in italics) was inserted between the Cla1 and Sph1 sites of plasmid pKD3 [58]. Then the site was amplified by PCR together with the adjacent FRT-CmR-FRT region of pKD3 and the PCR product was inserted into the chromosome as described [58].
Measures of viability
Appropriate dilutions of overnight cultures in MM were plated on MM and LB plates. Plates were incubated at 37°C and the number of colony forming units was counted after 16–24 hours of incubation for LB plates and after 48 hours of incubation for MM plates.
Fork breakage analysis
For each experiment, freshly isolated colonies of recB or recA recD mutants cured of the pAM-RecBCD plasmid were used for over-night cultures. However, for some mutants that grow slowly in MM, overnight cultures of cells carrying pAM-RecBCD were grown in the absence of IPTG. These cultures were plated on plates with or without Ap and IPTG and they contained less than 5% of plasmid carrying cells. This protocol was used for the following mutants: InvBE and InvA recB recJ, InvBE and InvA recA recD ruvAB, and InvA recB ruvAB. Plugs preparation, Not1 restriction, and PFGE of Not1 digested DNA were performed as described in [8]. Cleavage of DNA by I-Sce1 was performed in plugs as recommended by the supplier, with 1 hr incubation at 37°C in the presence of 2.5 units of enzyme. PFGE of I-Sce1 restricted chromosome was as for Not1, but with a ramp of pulses from 40 s to 70 s and migration for 18 hours. Transfer, hybridization, probe preparation, and quantification with Image Quant were performed as described in [8]. In Figure 3 and Figure 4 the origin proximal probe is in the slp gene and the origin distal probe in the cycA gene. Probes used in Figure 5 are in the following genes: probe 1 - origin-proximal probe: slp gene, probe 2 - rrnC promoter probe: yieP gene, probe 3 - rrnC terminator probe: hdfR gene, probe 4 - rrnA promoter probe: hemG gene, probe 5 - rrnA terminator probe: mobA-yihE genes, probe 6 - origin-distal probe: cycA gene. Probes used in Figure 6 are rrnC promoter probe: yieP gene, and rrnC terminator probe: hdfR gene.
Supporting Information
Zdroje
1. BranzeiDFoianiM 2007 Interplay of replication checkpoints and repair proteins at stalled replication forks. DNA Repair (Amst) 6 994 1003
2. LambertSFrogetBCarrAM 2007 Arrested replication fork processing: interplay between checkpoints and recombination. DNA Repair (Amst) 6 1042 1061
3. HellerRCMariansKJ 2006 Replisome assembly and the direct restart of stalled replication forks. Nat Rev Mol Cell Biol 7 932 943
4. GabbaiCBMariansKJ 2010 Recruitment to stalled replication forks of the PriA DNA helicase and replisome-loading activities is essential for survival. DNA Repair (Amst) 9 202 209
5. MichelBGromponeGFloresMJBidnenkoV 2004 Multiple pathways process stalled replication forks. Proc Natl Acad Sci USA 101 12783 12788
6. MichelBBoubakriHBaharogluZLemassonMLestiniR 2007 Recombination proteins and rescue of arrested replication forks. DNA Repair (Amst) 6 967 980
7. McGlynnP 2011 Helicases that underpin replication of protein-bound DNA in Escherichia coli. Biochem Soc Trans 39 606 610
8. BoubakriHde SeptenvilleALVigueraEMichelB 2010 The helicases DinG, Rep and UvrD cooperate to promote replication across transcription units in vivo. EMBO J 29 145 157
9. FrenchS 1992 Consequences of Replication Fork Movement Through Transcription Units In Vivo. Science 258 1362 1365
10. BrewerBJ 1988 When polymerases collide: replication and the transcriptional organization of the E. coli chromosome. Cell 53 679 686
11. LiuBAlbertsBM 1995 Head-on collision between a DNA replication apparatus and RNA polymerase transcription complex. Science 267 1131 1137
12. ViletteDUzestMEhrlichSDMichelB 1992 DNA transcription and repressor binding affect deletion formation in Escherichia coli plasmids. EMBO J 11 3629 3634
13. ViletteDEhrlichSDMichelB 1995 Transcription-induced deletions in Escherichia coli plasmids. Mol Microbiol 17 493 504
14. AzvolinskyADunawaySTorresJZBesslerJBZakianVA 2006 The S. cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev 20 3104 3116
15. MirkinEVMirkinSM 2007 Replication fork stalling at natural impediments. Microbiol Mol Biol Rev 71 13 35
16. WangJDBerkmenMBGrossmanAD 2007 Genome-wide coorientation of replication and transcription reduces adverse effects on replication in Bacillus subtilis. Proc Natl Acad Sci U S A 104 5608 5613
17. MerrikhHMachonCGraingerWHGrossmanADSoultanasP 2011 Co-directional replication-transcription conflicts lead to replication restart. Nature 470 554 557
18. CourcelleJHanawaltPC 2003 RecA-dependent recovery of arrested DNA replication forks. Annu Rev Genet 37 611 646
19. SeigneurMBidnenkoVEhrlichSDMichelB 1998 RuvAB acts at arrested replication forks. Cell 95 419 430
20. GuarinoEJimenez-SanchezAGuzmanEC 2007 Defective ribonucleoside diphosphate reductase impairs replication fork progression in Escherichia coli. J Bacteriol 189 3496 3501
21. SalgueroIGuarinoEGuzmanEC 2011 RecA-dependent replication in the nrdA101(Ts) mutant of Escherichia coli under restrictive conditions. J Bacteriol 193 2851 2860
22. KuzminovA 1999 Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol Mol Biol Rev 63 751 813
23. LukasLKuzminovA 2006 Chromosomal fragmentation is the major consequence of the rdgB defect in Escherichia coli. Genetics 172 1359 1362
24. TingHKouzminovaEAKuzminovA 2008 Synthetic lethality with the dut defect in Escherichia coli reveals layers of DNA damage of increasing complexity due to uracil incorporation. J Bacteriol 190 5841 5854
25. FloresMJBierneHEhrlichSDMichelB 2001 Impairment of lagging strand synthesis triggers the formation of a RuvABC substrate at replication forks. EMBO J 20 619 629
26. McGlynnPLloydRG 2000 Modulation of RNA polymerase by (P)ppGpp reveals a RecG-dependent mechanism for replication fork progression. Cell 101 35 45
27. McGlynnPLloydRG 1999 RecG helicase activity at three - and four-strand DNA structures. Nucleic Acids Res 27 3049 3056
28. DonaldsonJRCourcelleCTCourcelleJ 2004 RuvAB and RecG are not essential for the recovery of DNA synthesis following UV-induced DNA damage in Escherichia coli. Genetics 166 1631 1640
29. RudolphCJUptonALHarrisLLloydRG 2009 Pathological replication in cells lacking RecG DNA translocase. Mol Microbiol 73 352 366
30. RudolphCJUptonALBriggsGSLloydRG 2010 Is RecG a general guardian of the bacterial genome? DNA Repair (Amst) 9 210 223
31. KhanSRKuzminovA 2012 Replication forks stalled at UV-lesions are rescued via RecA - and RuvABC-catalyzed disintegration in Escherichia coli. J Biol Chem 287 6250 6265
32. BaharogluZLestiniRDuigouSMichelB 2010 RNA polymerase mutations that facilitate replication progression in the rep uvrD recF mutant lacking two accessory replicative helicases. Mol Microbiol 77 324 336
33. GuyCPAtkinsonJGuptaMKMahdiAAGwynnEJ 2009 Rep provides a second motor at the replisome to promote duplication of protein-bound DNA. Mol Cell 36 654 666
34. LaneHEDenhardtDT 1975 The rep mutation. IV. Slower movement of replication forks in Escherichia coli rep strains. J Mol Biol 97 99 112
35. AtkinsonJGuptaMKRudolphCJBellHLloydRG 2010 Localization of an accessory helicase at the replisome is critical in sustaining efficient genome duplication. Nucleic Acids Res 39 949 957
36. Taucher-ScholtzGAbdel-MonemMHoffmann-BerlingH 1983 Functions of helicases in E. coli. CozzarelliNR Mechanisms of DNA replication and recombination New york Alan Liss Inc 65 76
37. SelbyCPSancarA 1993 Molecular Mechanism of Transcription-Repair Coupling. Science 260 53 58
38. ParkJSMarrMTRobertsJW 2002 E. coli Transcription repair coupling factor (Mfd protein) rescues arrested complexes by promoting forward translocation. Cell 109 757 767
39. PomerantzRTO'DonnellM 2010 Direct restart of a replication fork stalled by a head-on RNA polymerase. Science 327 590 592
40. MasseEPhoenixPDroletM 1997 DNA topoisomerases regulate R-loop formation during transcription of the rrnB operon in Escherichia coli. J Biol Chem 272 12816 12823
41. PostowLUllspergerCKellerRWBustamanteCVologodskiiAV 2001 Positive torsional strain causes the formation of a four-way junction at replication forks. J Biol Chem 276 2790 2796
42. HanESCooperDLPerskyNSSuteraVAJrWhitakerRD 2006 RecJ exonuclease: substrates, products and interaction with SSB. Nucleic Acids Res 34 1084 1091
43. KouzminovaEAKuzminovA 2008 Patterns of chromosomal fragmentation due to uracil-DNA incorporation reveal a novel mechanism of replication-dependent double-stranded breaks. Mol Microbiol 68 202 215
44. BidnenkoVEhrlichSDMichelB 2002 Replication fork collapse at replication terminator sequences. EMBO J 21 3898 3907
45. NordmanJSkovgaardOWrightA 2007 A novel class of mutations that affect DNA replication in E. coli. Mol Microbiol 64 125 138
46. DermicD 2006 Functions of multiple exonucleases are essential for cell viability, DNA repair and homologous recombination in recD mutants of Escherichia coli. Genetics 172 2057 2069
47. RinkenRThomsBWackernagelW 1992 Evidence That recBC-Dependent Degradation of Duplex DNA in Escherichia-Coli recD Mutants Involves DNA Unwinding. Journal of Bacteriology 174 5424 5429
48. HandaNMorimatsuKLovettSTKowalczykowskiSC 2009 Reconstitution of initial steps of dsDNA break repair by the RecF pathway of E. coli. Genes Dev 23 1234 1245
49. SpiesMAmitaniIBaskinRJKowalczykowskiSC 2007 RecBCD enzyme switches lead motor subunits in response to chi recognition. Cell 131 694 705
50. GromponeGSeigneurMEhrlichSDMichelB 2002 Replication fork reversal in DNA polymerase III mutants of Escherichia coli: a role for the beta clamp. Mol Microbiol 44 1331 1339
51. WashburnRSGottesmanME 2011 Transcription termination maintains chromosome integrity. Proc Natl Acad Sci U S A 108 792 797
52. SkarstadKBoyeE 1993 Degradation of Individual Chromosomes in RecA Mutants of Escherichia-coli. J Bacteriol 175 5505 5509
53. GanWGuanZLiuJGuiTShenK 2011 R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev 25 2041 2056
54. Gomez-GonzalezBGarcia-RubioMBermejoRGaillardHShirahigeK 2011 Genome-wide function of THO/TREX in active genes prevents R-loop-dependent replication obstacles. EMBO J 30 3106 3119
55. VoloshinONCamerini-OteroRD 2007 The DinG protein from Escherichia coli is a structure-specific helicase. J Biol Chem 282 18437 18447
56. BaharogluZPetranovicMFloresMJMichelB 2006 RuvAB is essential for replication forks reversal in certain replication mutants. EMBO J 25 596 604
57. BidnenkoVLestiniRMichelB 2006 The Escherichia coli UvrD helicase is essential for Tus removal during recombination-dependent replication restart from Ter sites. Mol Microbiol 62 382 396
58. DatsenkoKAWannerBL 2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 6640 6645
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



