-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
Structural and biochemical studies have revealed the importance of a conserved, mobile domain of RNA Polymerase II (Pol II), the Trigger Loop (TL), in substrate selection and catalysis. The relative contributions of different residues within the TL to Pol II function and how Pol II activity defects correlate with gene expression alteration in vivo are unknown. Using Saccharomyces cerevisiae Pol II as a model, we uncover complex genetic relationships between mutated TL residues by combinatorial analysis of multiply substituted TL variants. We show that in vitro biochemical activity is highly predictive of in vivo transcription phenotypes, suggesting direct relationships between phenotypes and Pol II activity. Interestingly, while multiple TL residues function together to promote proper transcription, individual residues can be separated into distinct functional classes likely relevant to the TL mechanism. In vivo, Pol II activity defects disrupt regulation of the GTP-sensitive IMD2 gene, explaining sensitivities to GTP-production inhibitors, but contrasting with commonly cited models for this sensitivity in the literature. Our data provide support for an existing model whereby Pol II transcriptional activity provides a proxy for direct sensing of NTP levels in vivo leading to IMD2 activation. Finally, we connect Pol II activity to transcription start site selection in vivo, implicating the Pol II active site and transcription itself as a driver for start site scanning, contravening current models for this process.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002627
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002627Summary
Structural and biochemical studies have revealed the importance of a conserved, mobile domain of RNA Polymerase II (Pol II), the Trigger Loop (TL), in substrate selection and catalysis. The relative contributions of different residues within the TL to Pol II function and how Pol II activity defects correlate with gene expression alteration in vivo are unknown. Using Saccharomyces cerevisiae Pol II as a model, we uncover complex genetic relationships between mutated TL residues by combinatorial analysis of multiply substituted TL variants. We show that in vitro biochemical activity is highly predictive of in vivo transcription phenotypes, suggesting direct relationships between phenotypes and Pol II activity. Interestingly, while multiple TL residues function together to promote proper transcription, individual residues can be separated into distinct functional classes likely relevant to the TL mechanism. In vivo, Pol II activity defects disrupt regulation of the GTP-sensitive IMD2 gene, explaining sensitivities to GTP-production inhibitors, but contrasting with commonly cited models for this sensitivity in the literature. Our data provide support for an existing model whereby Pol II transcriptional activity provides a proxy for direct sensing of NTP levels in vivo leading to IMD2 activation. Finally, we connect Pol II activity to transcription start site selection in vivo, implicating the Pol II active site and transcription itself as a driver for start site scanning, contravening current models for this process.
Introduction
Cellular DNA-dependent RNA polymerases likely balance fidelity in substrate selection with synthesis speed to achieve appropriate transcriptome content and regulation in vivo. In multisubunit RNA polymerases (msRNAP) from archaea, bacteria and eukaryotes, a highly conserved subdomain known as the trigger loop (TL) is critical for rapid catalysis and selection of correct substrates [1]–[9]. The TL is present in the largest subunit of eukaryotic Pol II, generally referred to as Rpb1 (Rpo21 in Saccharomyces cerevisiae), and the analogous β′ subunit of bacterial RNAP, and A″ subunit of archaeal RNAP.
Similarly to mobile domains of other classes of nucleic acid polymerases, the TL undergoes conformational changes in conjunction with the presence of an NTP substrate complementary to the DNA template (matched) in the msRNAP active site [1], [3]. These conformational changes are proposed to link TL-substrate interactions to preferential catalysis of correctly matched substrates over mismatched substrates. The TL can be observed in distinct conformations depending on the presence of matched NTP substrate, natural product polymerase inhibitors, and msRNAP-interacting proteins, underscoring its flexibility [1]–[3], [10]–[15]. In addition to effects on phosphodiester bond catalysis, the TL has been implicated in polymerase pausing, intrinsic cleavage of RNA and translocation [7]–[9], [16], [17].
The TL comprises two mostly alpha-helical regions connected by a short loop (Figure 1A). Deletion or structural compromise of the TL in either E. coli (Eco) or T. thermophilus (Tth) strongly reduces catalytic activity, but to different extents depending on whether the substrate is matched to the template or not [3]–[5], [7]. Two specific regions of the TL appear important for control of TL function. First, conserved residues in the nucleotide interacting region (NIR) recognize specific features of matched NTP substrates and work in conjunction with non-TL residues, Rpb1 N479 and R446, positioned for interaction with hydroxyl moieties on the ribose of matched NTPs [1], [3](Figure 1A). Residues in the NIR showing NTP interactions in S. cerevisiae and Tth substrate-bound structures are (using S. cerevisiae Rpb1/Rpo21Ecoβ′/Tthβ′ numbering) Gln1078Gln930/Gln1236, Leu1081Met932/Met1238, Asn1082Arg933/Arg1239 and His1085His936/His1242.Second, in all kingdoms of life, substitutions in or near the helix distal to the NIR alter elongation rate, in some cases increasing elongation rate relative to WT (“superactivity”), [2], [16], [18], [19]. These substitutions may alter dynamics of TL movement between the substrate-interacting conformation and other conformations because they are adjacent to the hinge region in the C-terminal TL helix (another hinge is apparent in the N-terminal TL helix)(Figure 1B) [2], [9].
Fig. 1. S. cerevisiae Rpb1 trigger loop conformations and sequence. 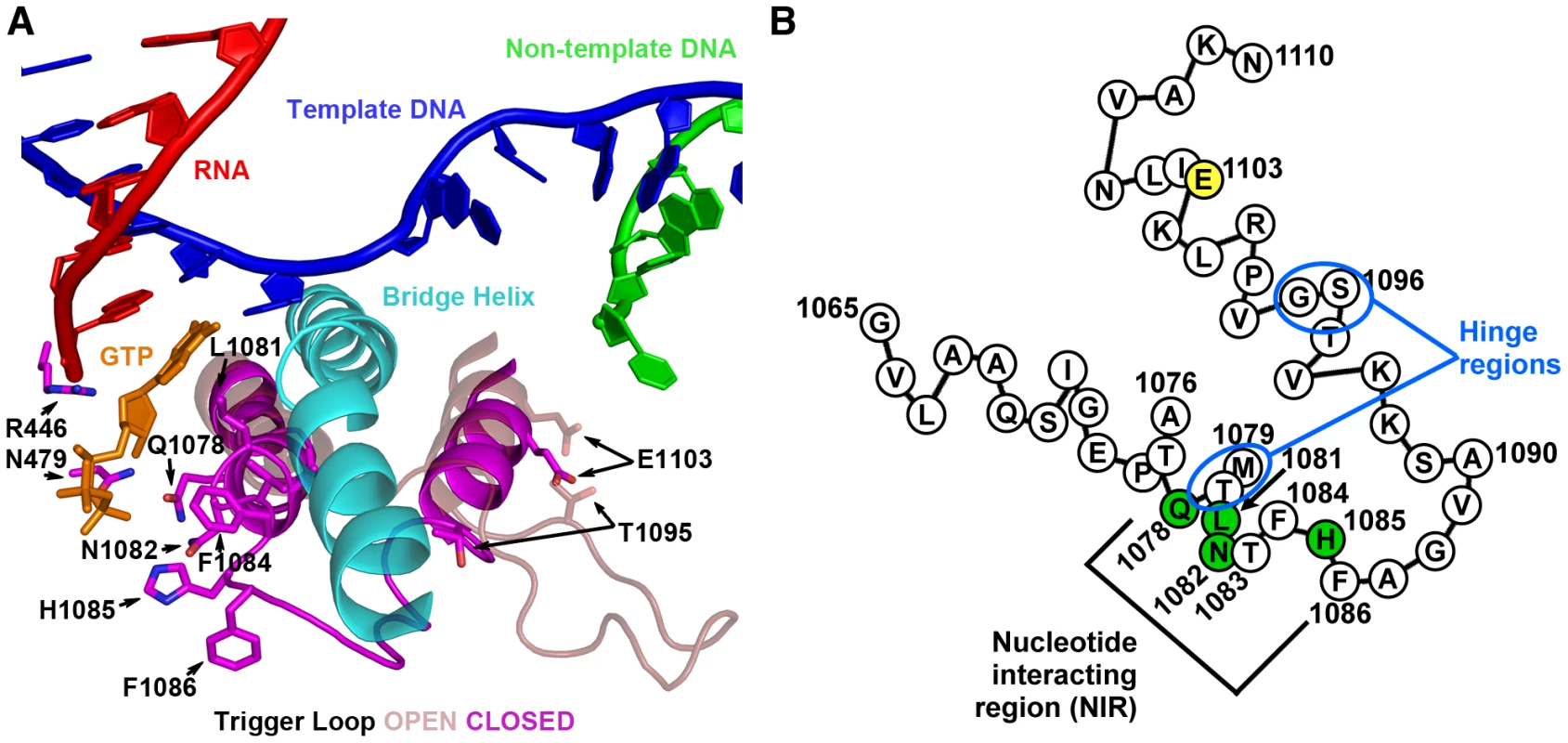
A. Cartoon representation of “closed” Pol II TL in relation to nucleic acids, Rpb1 bridge helix and a matched GTP substrate from structure PDB 2E2H [1] overlaid with TL constrained in open conformation by TFIIS (not shown) from structure PDB 1Y1V [69]. Amino acids (all derived from Rpb1) adjacent to the matched GTP substrate are indicated by numbers and single-letter amino acid codes. This figure was created with Pymol [111]. B. Schematic of TL showing amino acid sequence in single-letter code, with positions of interest numbered, residues with direct contact to GTP substrate in structure 2E2H shown in green, and position 1103 shown in yellow. Two hinge regions, about which the TL appears to change conformation from the open to closed positions are indicated. NIR residues of the Eco and Tth RNAPs have different degrees of contribution to catalytic activity, with individual Eco residue substitutions having smaller effects on activity than homologous substitutions in Tth, underscoring the diversity of TL residue functions in msRNAPs [5], [7]. A conserved histidine within the TL NIR has been proposed to have an important or essential catalytic role [1]. Many classes of structurally distinct nucleic acid polymerases utilize a basic residue located at a position in the active site analogous to that of the TL histidine and these residues function as a general acid in the catalytic cycle of the polymerase [20]. While clearly important for RNAP catalysis, the TL histidine appeared not to be functioning as a general acid based on pH-dependence curves for bacterial RNAPs [5], [7]. Experiments in the different bacterial systems show that a TL methionine residue (equivalent to S. cerevisiae Leu1081) packs against a base-paired NTP in the active site and has a greater contribution to activity than ArgEco933/Tth1239 or HisEco936/Tth1242, which interact with the triphosphate moiety of matched substrates [5], [7].
Genetic identification of mutants with substitutions in the TL region, first in S. cerevisiae Pol II, then in Eco RNAP, demonstrated that alteration of the TL could alter transcription in vivo [16], [18], [21]–[23]. How changes in TL function or msRNAP catalysis alter transcription in vivo are not well understood and to what extent polymerase activity defects may be tolerated in vivo is not clear. In S. cerevisiae, drugs that target nucleotide synthesis pathways such as mycophenolic acid (MPA, targets GTP synthesis) [24] and 6-azauracil (6-AU, targets UTP and GTP synthesis) [25], [26] have been shown to cause alterations in gene expression in vivo [27]–[30]. A large number of Pol II transcription-related mutant strains show altered sensitivities to these drugs, leading to the broadly utilized interpretation that these drugs are elongation inhibitors and that sensitivity to them suggests defective Pol II elongation [18], [31]–[54]. Notwithstanding the large number of mutants sensitive to these drugs that have no known transcriptional role, many MPA-sensitive mutants alter transcription of the gene IMD2 [35], [38], [47], which encodes an MPA-resistant form of IMPDH, the enzymatic activity targeted by the drug [55], [56]. This gene-specific transcription defect is not always considered when interpreting mutant phenotypes. Intriguingly, IMD2 transcription involves a switch between upstream transcription start sites and downstream productive start sites that differ in initiating NTPs (upstream: GTP, downstream: ATP) leading to the proposal that the initiation process for these different classes of transcript stems from GTP levels being sensed directly by Pol II [57], [58].
The eukaryotic Pol II system provides an excellent model for in vivo studies of how the TL functions in transcription. Because nuclear transcription in eukaryotes is segregated among three essential polymerases instead of one, as in bacteria and archaea, strong defects may be more tolerated in Pol II than bacterial or archaeal RNAPs in vivo. We utilized extensive site-directed mutagenesis of the Pol II TL coupled with genetic screening to identify viable substitutions in TL NIR residues and substitutions conferring conditional growth phenotypes. We then used biochemical characterization together with a number of in vivo genetic and molecular phenotypes to probe the contributions of critical TL residues to transcription in vivo. We determined the relationship between NIR residues and superactivating TL substitutions and found that superactivating substitutions were mostly mutually suppressive with loss-of-function substitutions within the NIR in genetic and biochemical assays. These results indicated that NIR residues were not bypassed by superactivating substitutions and were still required for Pol II activity. Using a series of TL mutants that define a continuum of in vitro elongation rates, we demonstrated that a number of in vivo phenotypes correlated closely with Pol II activity in vitro. We provide support for models proposing that IMD2 transcription is directly sensitive to Pol II activity, therefore explaining the MPA-sensitivity of superactive Pol II mutants, which otherwise might have been expected to be resistant to reduced GTP levels due to increased elongation activity. Finally, we determined that start site selection at a number of other genes was similarly sensitive to alteration in Pol II activity leading to a new model for transcription-dependent polarity of start site selection in S. cerevisiae.
Results
Genetic Analyses of Rpb1 TL Mutants In Vivo
We have undertaken an extensive genetic dissection of the Pol II TL: specifically, we examined the contribution of TL residues to Pol II function, and how Pol II catalytic activity relates to transcription in vitro and in vivo. To examine a large number of Pol II mutants in vivo, we employed a yeast strain containing a deletion of the endogenous copy of RPO21 (the gene encoding Rpb1, which we henceforth refer to as RPB1) with RPB1 activity complemented by a low copy CEN plasmid containing RPB1 genomic DNA or mutant variants, allowing expression from the native RPB1 promoter. Site-directed mutagenesis was focused on TL NIR residues to identify viable substitutions and was combined with existing rpb1 TL alleles identified in genetic screens (Text S1, genetic screens to be described elsewhere). For any viable mutants, alteration in transcription in vivo was measured with two phenotypic reporters for transcriptional defects, the lys2-128∂ [59] and gal10Δ56 alleles [60], [61](Figure S1). These reporters are modulated by a number of transcription elongation factors and Pol II mutants with known transcription defects [2], [60], [62]–[66].
Previous analyses indicated that some TL substitutions cause increases in Pol II elongation rate in vitro (“superactivity” or gain of function (GOF))(e.g. E1103G) [2], [9], [18]; some of these increases in rates for misincorporation were greater than in rates for incorporation of templated NTPs, indicating possible loss of fidelity (F1084I, E1103G) [2], [9]. Other substitutions confer reduced elongation rate (loss of function (LOF))(H1085Y, F1086S) [2]. In vitro activity appeared to correlate with in vivo phenotypes for the few mutants examined [2]. Suppression of a growth defect on media lacking lysine conferred by lys2-128∂ (the Spt− phenotype) was observed for known TL GOF alleles and neighboring substitutions [2]. Lysine auxotrophy of lys2-128∂ relates to defective LYS2 transcription caused by a retrotransposon insertion in LYS2 [59]. Some of these GOF alleles are also sensitive to MPA or 6-AU, while the Spt− phenotype and strong MPA sensitivity have not been observed among known TL LOF alleles [2], [18]. Both known LOF and GOF alleles confer alteration of growth phenotypes relating to transcriptional interference at the GAL10-GAL7 locus when polyadenylation and termination at GAL10 are compromised in the gal10Δ56 allele [2].
In our current study, we found that the Spt− phenotype was concentrated in residue substitutions proximal to the two TL “hinges” (Figure 2A, 2E, see Figure 1B for positions of hinges). Growth defects were conferred by substitutions throughout the TL (Figure 2A, 2B). We found that MPA-sensitivity (MPAs) and Spt− phenotypes generally co-occurred although the relative strength of the two phenotypes varied among mutants (Figure 2A, 2D, 2E). Finally, we found that the strongest suppressors of gal10Δ56, with the exception of G1097D, were in TL NIR residues and were mostly Spt+ or MPA-resistant (MPAr), indicating a distinction between these phenotypes (Figure 2C). Taken together, these conditional plate phenotypes behave as sensitive readouts for likely complex sets of overlapping or distinct transcriptional defects in vivo.
Fig. 2. Genetic analyses of Pol II TL single substitution mutants. 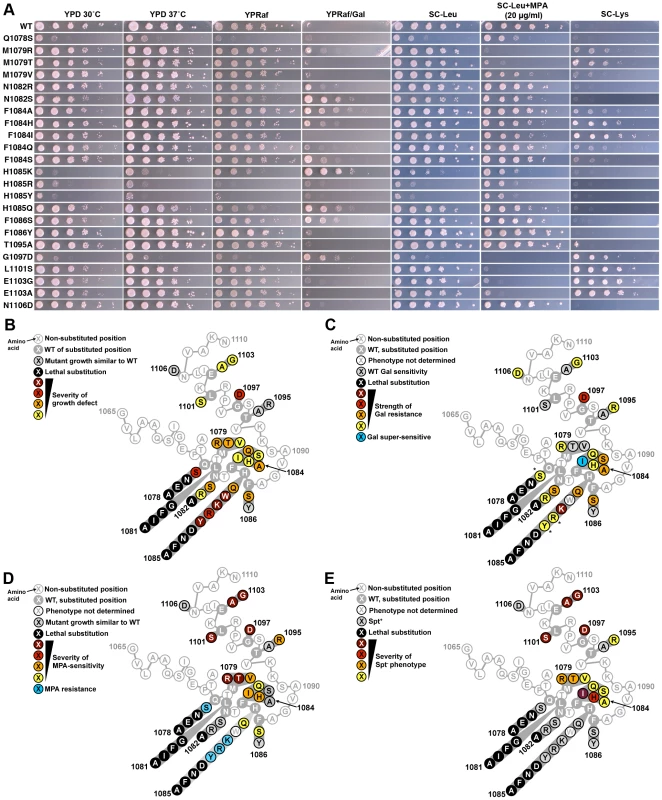
A. 10-fold serial dilutions of saturated cultures of Pol II TL mutant strains plated on different media. YPD is rich medium with dextrose as a carbon source. YPRaf is rich medium with raffinose as a carbon source. YPRaf/Gal has both raffinose and galactose, allowing assay of gal10Δ56-dependent galactose toxicity phenotypes. SC-Leu is defined, complete medium lacking leucine. MPA was added to this medium (SC-Leu+MPA) to 20 µg/ml final concentration, showing that a number of Pol II mutants are sensitive to this drug. SC-Lys is defined, complete medium lacking lysine, and detects the Spt− phenotype (Lys+) for strains containing lys2-128∂. WT strains grow robustly on most media, but will not grow on SC-Lys when lys2-128∂ is present and grow very poorly on YPRaf/Gal when gal10Δ56 is present. Mutant-dependent transcriptional phenotypes allow modulation of these specific growth defects. B. Schematic of TL (as diagrammed in Figure 1B) showing distribution of viable substitutions with their growth defects on YPD as well as lethal substitutions. All mutants were examined in the background of an Rpb1 T69 change, which is the allele present in the S288C reference genome as well as being normally found in our strains, representing a distinction for the subset of mutants described previously [2](see Materials and Methods, Text S1 and note concerning viability of Q1078S). Scoring of phenotypic strength is based on visual inspection of (A). C. Distribution of gal10Δ56 suppression (Gal resistance) or enhancement (Gal super-sensitivity) among TL substitutions. Scoring of phenotypic strength is based on visual inspection of (A). *Indicates strength of gal10Δ56 suppression is likely underestimated due to confounding generic growth defects. D. Distribution of MPA phenotypes among TL substitutions. Scoring of phenotypic strength is based on visual inspection of (A). E. Distribution of lys2-128∂ suppression (Spt− phenotype scored as Lys+) among TL substitutions. Scoring of phenotypic strength is based on visual inspection of (A). In Vitro Transcription Elongation Rates Correlate with In Vivo Growth Defects and Plate Phenotypes
As TL substitutions causing the Spt− phenotype included those with known TL GOF phenotypes (F1084I, E1103G) [2], we asked if our plate phenotypes were predictive of biochemical phenotypes. Analysis of TL substitutions in an in vitro transcription assay using reconstituted Pol II elongation complexes indicated a good correlation between elongation rate defect in vitro and growth defect in vivo (Figure 3, Figures S4 and S5). We note that we did not detect strong expression differences for tested mutant Rpb1 proteins (Figure S6). We previously showed that LOF substitution H1085Y and a putative GOF substitution (G1097D) caused severe growth defects in vivo [2]. In this study we find that these two mutants also represent extremes of Pol II activity defects, but interestingly at different ends of the activity spectrum. Biochemical analysis of Spt− and MPAs G1097D and L1101S shows that they are GOF substitutions conferring increased maximal elongation rate in vitro. In fact, G1097D elongates too quickly for accurate measurement in our short template run off assay (Figure 3)(Figures S4 and S5). We evaluated a number of other TL substitutions and found that WT elongation rate in vitro correlates with robust growth in vivo, and that the greater the deviation from WT for elongation rate in vitro, the greater the deviation from WT growth in vivo (Figure 3, right panel). We note that the non-TL LOF substitution, N479S, deviates slightly from this relationship as it has the most severe in vitro defect but has a less severe growth defect. This could be consistent with a factor(s) functioning in vivo that can compensate for the biochemical defect caused by the N479S substitution but not the H1085Y substitution; for example, a hypothetical factor that may work through the TL.
Fig. 3. Pol II TL substitution mutants show a wide range of elongation rates in vitro. 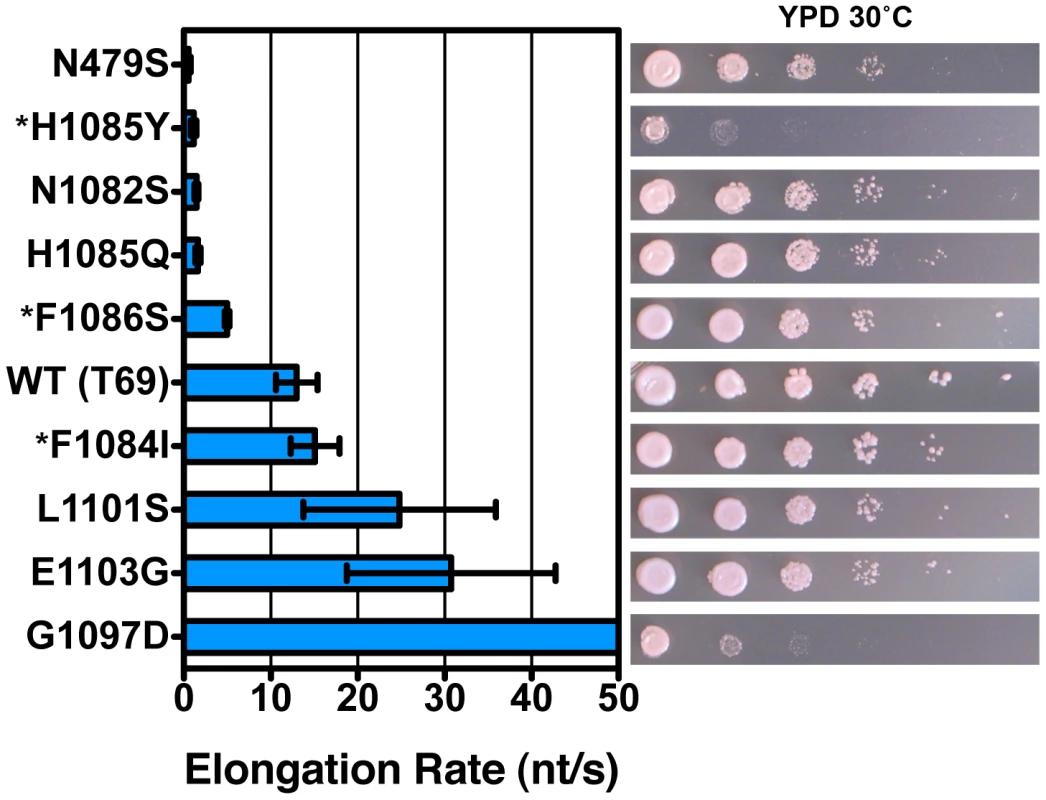
Pol II TL single substitution elongation rates as determined in vitro using reconstituted Pol II elongation complexes with synthetic oligonucleotides and an RNA primer (Materials and Methods). Raw data for some mutants (*) are from [2] and are shown for comparison. Plotted data are maximal elongation rates as determined by non-linear regression of elongation rates determined for different NTP substrate concentrations, and error bars indicate the range of the 95% confidence intervals (Figures S4, S5). Growth on rich medium of mutants is shown in the right panels for comparison with elongation defects (from Figure 2). Role of E1103-T1095 Interactions in Superactivation of E1103G
For msRNAPs from each kingdom of life, GOF substitutions have been reported in TL residues distal to the NIR and proximal to the C-terminal “hinge” region [2], [9], [16], [18], [19], which is the portion of the TL that changes conformation from open to the closed NTP-interacting orientation (Figure 1). There is evidence that the Pol II GOF substitution E1103G promotes a closed TL state [9]. An increased frequency or duration of this state could have consequences for catalysis (increased activity with all substrates and net increase in misincorporation) and translocation (reduced translocation rate likely due to stabilization of closed state after catalysis). The E1103 sidechain interacts with the T1095 sidechain and makes backbone contacts with K1112 in elongation complex crystal structures where the TL is in the open conformation or constrained by other factors [67]–[69]. Loss of this E1103-T1095 interaction has been posited to explain the effect of E1103G on Pol II transcription [9]. We found that the T1095A substitution was phenotypically indistinguishable from wild type (WT), indicating that loss of T1095-E1103 contacts are not responsible for E1103G growth alteration or, most likely, biochemical superactivity (Figure 2). T1095R, presenting a much longer sidechain that might disrupt “out conformation” hinge folding, only confers weak MPAs, Spt− and Galr phenotypes, consistent with C-terminal hinge function or TL dynamics being controlled more strongly by distal residues or other contacts of E1103 (Figure S3).
Combinatorial Analyses of TL Substitutions Reveal Functional Distinctions between Residues with Similar Single-Mutant Behavior
We next wished to probe genetic relationships between TL GOF and LOF substitutions, particularly those between TL hinge residues and TL NIR residues, as these relationships might provide mechanistic insight into TL function (Figure 4A, Figure S2, Figure S3, Text S1). Surprisingly, by combining TL GOF with TL NIR substitutions, we found that either E1103G or G1097D GOF mutations were able to suppress lethality of many, but not all, inviable (LOF) single substitution NIR mutants (Figure 4A, Figure S2, Table 1). Examination of double mutant plate phenotypes provided more evidence of mutual suppression in E1103G-NIR mutant combinations because Spt− and MPAs phenotypes of E1103G were suppressed together with moderate Galr phenotypes of individual NIR substitutions (Figure S2). In contrast, tested pairwise combinations of genetically and biochemically similar GOF substitutions, F1084I, G1097D and E1103G resulted in lethality (Figure 4A). Additionally, combination of viable LOF NIR-residue substitutions with the GOF substitution E1103G resulted in mutual suppression of conditional plate phenotypes and growth defects (Figure S2).
Fig. 4. Combination of TL substitutions alters in vivo growth and in vitro biochemical phenotypes. 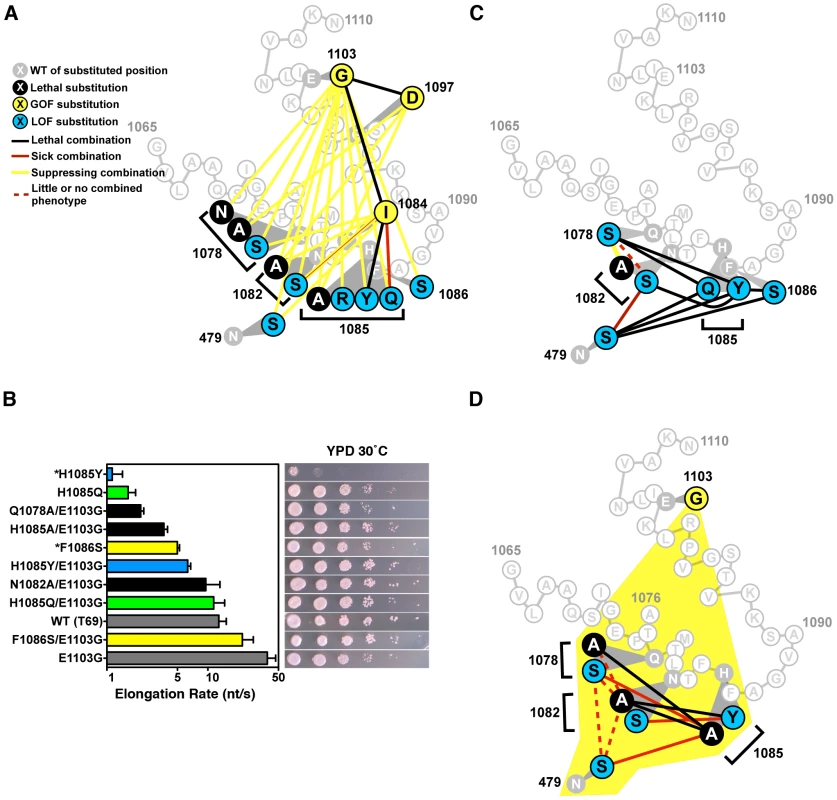
A. GOF-GOF and GOF-LOF genetic interactions among rpb1 TL and other substitutions. GOF (yellow) and LOF (blue) classifications were determined by biochemical and genetic phenotypes of single substitution mutants, with lethal single substitutions shown in black. Double mutant phenotypes are illustrated as the colored lines connecting colored nodes of particular single mutants. Schematic of TL sequence and orientation is as in Figure 1B. Data were compiled from Figure S2, Figure S3 and Table 1. N1082S/F1084I shows a complex interaction with exacerbation of growth defects on rich medium but mutual suppression of other conditional plate phenotypes. B. In vitro elongation rates determined as in Figure 3 for select Pol II TL double mutant enzymes. Single mutants and their relevant E1103G double mutants are indicated by color-coding: F1086 and F1086/E1103G, yellow; H1085Q and H1085Q/E1103G, green; H1085Y and H1085Y/E1103G, blue. Black bars indicate double mutants with E1103G for which the counterpart single substitution mutant is inviable (Q1078A, N1082A, H1085A). Single mutant data are from Figure 3. Raw data for some mutants (*) are from [2] and are shown for comparison. Error bars indicate the ranges of the 95% confidence intervals. Note that x-axis is logarithmic scale. Growth on rich medium of mutants is shown in the right panels for comparison with elongation defects (from Figure 2, Figure S2). C. Interaction diagram showing phenotypes of pairwise combinations of rpb1 LOF alleles (legend shown in (A)). D. Interaction diagram illustrating genetic interactions between pairs of Rpb1 substitution mutants each in the presence of the E1103G substitution (yellow shading encompassing all relevant residues, legend shown in (A)). Tab. 1. Mutational analysis of the Pol II TL. 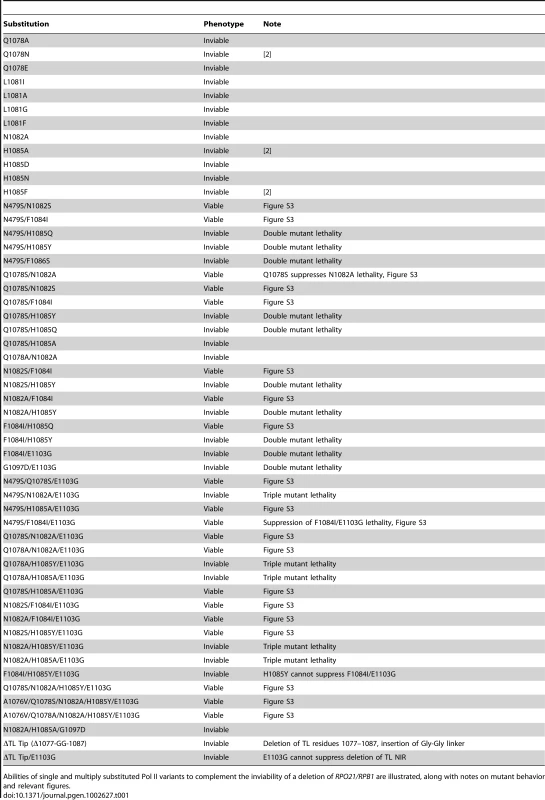
Abilities of single and multiply substituted Pol II variants to complement the inviability of a deletion of RPO21/RPB1 are illustrated, along with notes on mutant behavior and relevant figures. If observed plate phenotypes directly relate to elongation rate, as suggested by our analysis of single substitution mutations (Figure 3), the observations above predict that the double mutants' in vitro elongation rates might be closer to WT than those of the single substitution mutants. We therefore measured elongation rates of doubly substituted TL mutants to further examine the relationships between GOF and LOF variants (Figure 4B)(Figures S4 and S5). We found that combination of E1103G and individual viable LOF substitutions F1086S, H1085Q or H1085Y resulted in enzymes with activity intermediate between the relevant singly substituted enzymes. Combination of E1103G individually with the lethal single substitutions Q1078A, N1082A or H1085A resulted in viable strains and Pol II enzymes with activity reduced compared to E1103G alone. The fold enhancement of viable LOF substitutions F1086S, H1085Q and H1085Y by E1103G was roughly similar in each case.
To determine if TL NIR residues function individually or in a coupled fashion, we constructed pairwise combinations using TL NIR LOF alleles and a substitution in the TL-adjacent Rpb1 NTP-interacting residue, N479S (Figure 4C). We observed lethality when H1085 substitutions were combined with substitutions in other TL NIR residues or with N479S, consistent with H1085 functioning non-redundantly or distinctly from other TL NIR residues or N479 in the Pol II nucleotide addition cycle. Surprisingly, when we examined combinations among N479, Q1078, and N1082 substitutions we did not observe lethality (Figure 4C). In fact, we observed suppression or epistasis between Q1078 and N1082 substitutions. These observations suggested a functional distinction between N-terminal TL NIR residues (Q1078, N1082) and H1085. Viable substitutions in these residues maintain sidechains that might still confer some interaction between the TL and NTPs, however alanine substitutions in key residues Q1078, N1082, and H1085 were inviable and could not be directly assessed genetically. Because all LOF TL residues, including these lethal substitutions, were suppressed by E1103G, we examined triple mutants that contained pairwise substitutions in the TL NIR in the background of E1103G (Figure 4D). These triple mutant combinations revealed similar functional distinctions between two sets of Pol II active site residues, with the set of residues containing N479, Q1078 and N1082 being distinct from H1085. These results may be interpreted in light of structural information and have implications for the mechanism of TL function (see Discussion).
In Vivo Transcription Defects of Pol II TL Mutants
To examine our Pol II TL mutants for in vivo transcription defects, we analyzed expression of IMD genes and URA2 (Figure 5A, 5B), which function in GTP and UTP synthesis, respectively. Expression of one IMD gene, IMD2, and URA2, have been linked to GTP or UTP levels, respectively, possibly directly through alteration of Pol II function by changes in levels of these transcription substrates [57], [58], [70], [71]. Expression of IMD2 is required for WT yeast to be resistant to the drug MPA [46], [56]. IMD2 transcripts are increased in the presence of MPA due to reduced GTP levels that result from inhibition of MPA-sensitive Imd2 homologs, Imd3 and Imd4 [55], [56]. A number of transcription mutants are defective for IMD2 upregulation upon MPA treatment [35], [38], [47] while some Pol II mutants have been reported to show constitutive expression of IMD2 [18], [57]. As IMD2 has a high level of sequence similarity to the pseudogene IMD1 and to IMD3 and IMD4, and because of possible cross-hybridization [56], we do not specify that observed changes in our Northern blotting are solely due to IMD2 changes. However, it has been demonstrated that the majority of MPA-induced IMD expression comes from IMD2 [56].
Fig. 5. Pol II TL mutants alter transcription of IMD2 and URA2, genes required for GTP and UTP synthesis, respectively. 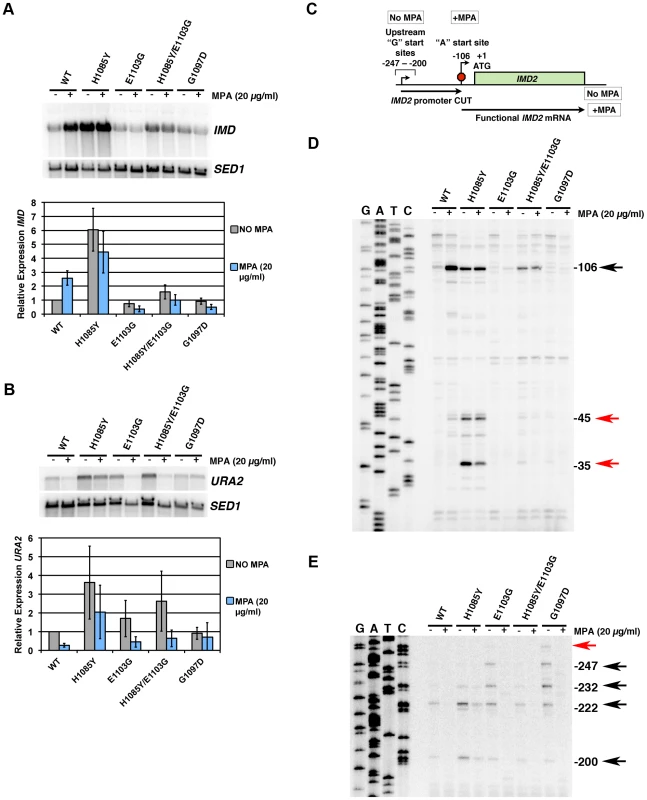
A. Northern blotting for different Pol II mutants in the presence or absence of 20 µg/ml MPA (2 hours treatment) for expression of IMD gene(s) using IMD2 DNA probe (top). SED1 was probed as a loading control. Values for IMD normalized to the WT ratio of IMD/SED1 are shown in the graph and represent the mean relative expression for IMD transcript(s) +/− the standard deviation for at least three independent experiments. B. Northern blotting for URA2 expression in the presence or absence of 20 µg/ml MPA (2 hours treatment) (top). SED1 was probed as a loading control. Values for URA2 normalized to the WT ratio of URA2/SED1 are shown in the graph and represent the mean relative expression for URA2 +/− the standard deviation for at least three independent experiments. C. Schematic of IMD2 gene transcription in the absence or presence of MPA. IMD2 is not functionally expressed in the absence of MPA or low GTP because upstream transcriptional starts are terminated at a terminator element (stop sign) that can be bypassed by utilization of downstream start sites. D. Primer extension analysis for downstream IMD2 start site usage in Pol II TL mutants in presence or absence of 20 µg/ml MPA (2 hours treatment). Numbers on right indicate position of RNA terminus relative to the A of the IMD2 ATG codon. Sequence ladder on right is derived from the primer used in (E) and has been cross-referenced with the primer used here. E. Primer extension analysis for upstream IMD2 start site usage in Pol II TL mutants as in (D). Numbers on right indicate position of RNA terminus relative to the A of the IMD2 ATG codon. Sequence ladder on right is derived from the same primer used. The examples in D and E are representative of at least three independent experiments. We observed a constitutive upregulation of IMD transcripts in LOF mutant H1085Y and loss of MPA-responsiveness in GOF mutants E1103G and G1097D as well as in the H1085Y/E1103G double mutant (Figure 5A). E1103G appeared to have reduced expression of IMD genes in the absence of MPA treatment, and importantly, E1103G and G1097D showed reduced IMD expression upon MPA treatment. These results are consistent with the MPA-sensitivity of E1103G and G1097D and the apparent MPA-resistance of H1085Y (Figure 2) [2]. They also indicate that defects in IMD expression may fully explain the MPA-sensitivity of Pol II TL mutants and need not relate to global elongation defects due to reduced GTP levels, which is the commonly invoked mechanism for MPA-sensitivity. The results, however, are consistent with a model in which IMD2 regulation occurs directly through Pol II-nucleotide sensing.
Activation of URA2 has also been proposed to result from a Pol II-dependent NTP-sensing event because a class of Pol II mutants causes constitutive URA2 expression [70], [71]. URA2 is normally controlled by a change in start site selection from upstream start sites, which produce transcripts that are prematurely terminated and degraded, to downstream start sites that allow production of full-length mRNA. The Pol II mutants that lead to URA2 expression are located in the “switch 1” region of Rpb1 [72], near a residue linked to start site selection [73]. We reasoned that if URA2 were directly responsive to Pol II-sensing of UTP levels, then a Pol II TL LOF mutant should also show constitutive upregulation of URA2, as a reduction of Pol II activity through TL defects should mimic reduction in substrate levels. We found that H1085Y caused URA2 to be upregulated (Figure 5B). GOF TL mutants E1103G and G1097D were closer to WT for URA2 expression, while H1085Y/E1103G showed an increase in URA2 expression. Furthermore, URA2 was not responsive to MPA treatment, an expected result if URA2 were specifically responsive to UTP depletion as opposed to GTP depletion.
IMD2, like URA2, is regulated by a shift in start sites from upstream starts that lead to premature termination, to downstream starts upon nucleotide limitation [57], [58]. The start site changes in IMD2 are proposed to relate to concentration of initiating NTP, with upstream starts initiating with GTP, whereas under GTP-limiting conditions, the major downstream start initiates with ATP (Figure 5C). We examined this phenomenon in Pol II TL mutants by primer extension (Figure 5D, 5E). Examination of downstream start sites at IMD2 showed that the major ATP start site at −106 from the IMD2 start codon is induced by MPA treatment in WT cells (Figure 5D) [74]. TL LOF H1085Y showed constitutive expression from this start regardless of MPA treatment, and, interestingly, apparent usage of downstream cryptic starts at approximately −35 and −45. Although mRNA processing in theory could generate these shorter new transcripts, it is unlikely as processed transcripts lacking a 5′-cap should be fairly unstable. In contrast, for TL GOF mutants E1103G and G1097D little or no usage of the −106 start was observed, regardless of MPA treatment, consistent with MPA-sensitivity of these strains and Northern blotting showing no MPA induction of IMD transcripts. In the H1085Y/E1103G mutant that showed mutual suppression of respective single mutant phenotypes but reduced activity from WT in vitro, we observed constitutive usage of the −106 start, but at apparent lower levels than H1085Y. In addition, we observed MPA-dependent loss of upstream start site usage in this set of mutants, suggesting maintained responsiveness to MPA-effects for these starts, even though H1085Y showed constitutive use of downstream starts. In contrast to the observed H1085Y usage of new −35 and −45 downstream start sites (Figure 5D), we observed increased E1103G and G1097D usage of upstream starts relative to WT (Figure 5E), suggesting altered start site selection in Pol II TL mutants.
Activity-Dependent Control of Pol II Start Site Selection In Vivo
A number of Pol II subunit and general transcription factor (GTF) mutants have been found to alter start site selection in S. cerevisiae [73], [75]–[84], but it is unclear how Pol II activity might relate to these start site defects. For example, deletion of the Rpb9 subunit of Pol II confers many similar activity defects as Pol II TL GOF substitutions such as increased rate of elongation and misincorporation [85]–[87] and is defective for start site switching at IMD2 in response to MPA [58]. Additionally, rpb9Δ has long been known to exhibit upstream shifts in start site selection [77], [79], [84], while many mutations throughout Pol II can cause downstream shifts in start site selection (references above). Models for Pol II residue function in start site selection have previously focused on physical or functional interactions between these residues and GTFs. We propose that the Pol II TL primarily or exclusively functions in controlling the Pol II nucleotide addition cycle (substrate selection, catalysis and translocation), which, for this work, we define as Pol II activity. Therefore we used our TL mutants to interrogate the relationship between Pol II biochemical activity and start site selection in vivo, following our observations of downstream start site usage at IMD2 in TL LOF H1085Y and apparent upstream shifts in TL GOF E1103G and G1097D mutants. We examined start site selection at a number of other genes by primer extension analysis (Figure 6).
Fig. 6. Pol II TL contributes to start site selection at a number of genes. 
A. Primer extension analysis of RNA 5′-ends at ADH1 in Pol II TL mutants. Sequence ladder at left is derived from primer used in Figure 5E but has been cross-referenced with ADH1 primer used for primer extension here. Numbers indicate positions of putative start sites in relation to the ADH1 ATG (A is +1). Right panel quantifies start site usage at ADH1 as determined by primer extension with a radiolabeled oligo. Signals in each lane were divided into bins based on positions of start sites relative to ADH1 ATG sequence (A is +1) and normalized to total signal per lane. WT start site fractions were subtracted from mutant start site fractions for particular regions to determine the relative alteration in start site distribution in Pol II mutant strains. A negative value indicates that the mutant has relatively lower usage for that particular group of start sites and a positive value indicates a relatively higher usage for that particular group of start sites. Values shown are the average of at least four independent experiments +/− standard deviation. B. Primer extension analysis of RNA 5′-ends at HIS4 and PMA1 in Pol II TL mutants. C. Primer extension analysis of RNA 5′-ends at GAL1 in Pol II TL mutants. Cells are grown in medium lacking raffinose and then GAL1 is induced by addition of galactose to 2% for two hours. Sequence ladder at left is derived from the same primer used for primer extension. Numbers indicate positions of putative start sites in relation to the GAL1 ATG (A is +1). We found that TL LOF H1085Y caused downstream shift in start site distribution at ADH1, similar to reported defects of a number of other Pol II mutants and GTF mutants (Figure 6A). In contrast, TL GOF E1103G and G1097D substitutions confer upstream defects similar to those that occur in rpb9Δ (references above), a number of TFIIF mutants [78], [80]–[83], and novel insertion mutants within TFIIB [88]. As with other in vivo and in vitro phenotypes, combination of H1085Y and E1103G leads to mutual suppression, extending the correlation between observed phenotypes and in vitro activity. For transcripts from HIS4 and PMA1, genes known to be sensitive to start site alterations in mutant strains [73], [79], we also observed upstream shifts in E1103G and G1097D-bearing strains (Figure 6B). E1103G-dependent start site changes were suppressed by H1085Y for these genes as well. It is not obvious how the alteration of TL function would impact start site selection so strongly, as we expect TL function to be downstream of initiation events important for start site selection, such as pre-initiation complex formation and promoter melting. The strong upstream starts observed for GOF mutants led us to predict that these positions should already be accessible to Pol II in WT cells, because TL function in controlling Pol II activity should be downstream of promoter melting. Using permanganate footprinting to identify regions of single-stranded DNA in the GAL1 and GAL10 promoters, Giardina and Lis observed strong permanganate reactivity at greater than 60 basepairs (bp) upstream of the major transcriptional start sites, indicating promoter melting far upstream [89]. We reasoned that GAL1 should show far upstream start site shifts in TL GOF mutants within this melted region though it does not normally support productive start sites. Indeed, we observed new start site usage more than 40 bp upstream of the major GAL1 start sites in TL GOF mutants, and a slight shift in starts sites downstream in TL LOF H1085Y (Figure 6C)(Figure S7).
Discussion
The contributions of TL residues to the Pol II nucleotide addition cycle (“activity” for the purposes of this discussion) are critical for transcription in vitro and in vivo. The catalytic contribution of the TL has been proposed to function through minimization of NTP dynamics within the Pol II active site, which in turn could increase the probability of catalysis [90]. Our biochemical experiments coupled with extensive genetic analyses provide insight into the function of TL residues. We calculated an approximate 6-fold suppression effect on elongation rate conferred by the E1103G GOF substitution when it was combined with LOF substitutions. We used this fold enhancement to infer the putative defects of inviable TL LOF substitution mutants for which we were unable to measure elongation rate directly. Assuming an approximate 6-fold increase in rate contributed by E1103G to the values of E1103G-suppressed inviable substitution elongation rates, we infer 7–30 fold defects for N1082A, H1085A and Q1078A single substitutions (Figure 4B). Examination of analogous substitutions in E. coli or T. thermophilus RNAPs has revealed large differences in the contribution of these residues to catalysis with NTP substrates (4 and 6 fold for Eco R933ASceN1082A and H936ASceH1085A, respectively [7] vs. 50 and 100 fold for Tth R1239ASceN1082A and H1242ASceH1085A, respectively [5]). Our results indicate that S. cerevisiae Pol II TL residues have contributions intermediate to TL residues from different bacteria and suggest that contributions of TL residues to activity cover a range of values in homologous enzymes.
Our data also support a functional relationship between regions adjacent to TL hinges, where the TL has been observed to deviate from its fully folded, NTP-bound conformation in crystal structures. These regions are the location of most of the strong Spt− and MPAs TL substitutions, as well as the strongest GOF alleles tested. These results underscore the idea that the TL is finely balanced between functional states or conformations, and that the hinges are sensitive to changes that in some cases can promote Pol II activity. These changes likely occur at the expense of fidelity, as has been shown for E1103G [2], [9]. Alteration of distal TL residues (e.g. E1103, G1097D) conferred broad suppression on a number of TL NIR substitutions. Thus, it appears that loss of NIR function can be compensated by alteration in trigger loop dynamics. Importantly, functions of TL NIR residues are not completely bypassed, as activities of tested double mutants are intermediate between the single mutants, and not all NIR mutants are suppressible (Table 1, Text S1).
Functional distinctions between TL residues and genetic epistasis between some residue combinations have been hinted at in the case of residues analogous to Q1078 and N1082 in T. thermophilus experiments [5](Text S1), but our combinatorial genetic analyses reveal this more clearly (Figure 4). Examination of different Pol II crystal structures shows the TL in a number of states between partially and stably folded in the presence or absence of matched NTP or NTP analog [1], [13], [67](Figure 7). These structures suggest a TL folding pathway wherein N-terminal TL residues that are proximal to the N-terminal TL hinge (primarily Q1078), along with other active site residues (for example, Rpb1 R446 and N479), are positioned essentially at all times for NTP interaction. Biochemical experiments indicate that these residues function in ribose 2′ - and 3′-hydroxyl (OH) recognition, with the 2′-OH of course critical for selection of NTPs over 2′-dNTPs [1], [5], [91]. Upon matched NTP basepairing with the DNA template, NTP interactions with R446, N479 and Q1078 may stabilize or begin to promote TL-folding in an N-terminal to C-terminal fashion, where L1081 and N1082 next come into position via N-terminal TL hinge movement. Supporting such a step, in some Pol II crystal structures, these residues are observed adjacent to a matched NTP basepairing with the template, but without complete folding or stabilization of the TL (Figure 7). Our genetic analyses suggest that Q1078 and N1082 (along with N479) are functionally interdependent (see also Text S1). Following initial hinge movement and TL folding, H1085 and the rest of the TL loop tip must fold into the closed conformation, and genetic analyses of H1085 substitutions suggest this step is distinguishable from functions of other TL NIR residues. It is important to note that we have not been able to evaluate contributions of residues L1081 and R446 in our genetic system because all tested substitutions in these residues were inviable and these substitutions were generally not suppressed by E1103G (Table 1, Table S1, Text S1).
Fig. 7. Model for stepwise function of TL residues in contributing to TL folding and function. 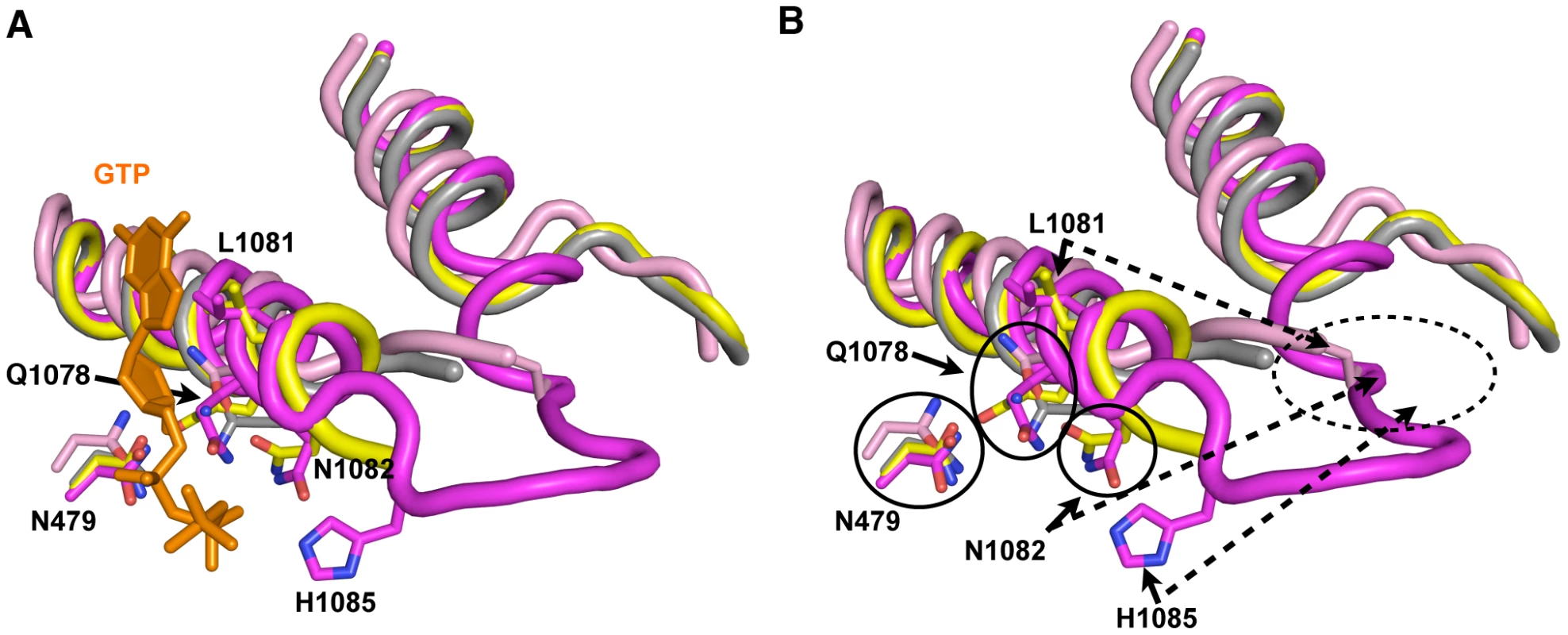
A. Structural cartoon showing overlay of TLs and Rpb1 N479 from a number of Pol II elongation complex/initial transcribing complex crystal structures with TLs in different states of folding/NTP interaction. Matched GTP nucleotide (orange) and TL and N479 (magenta) are from PDP 2E2H [1], which shows TL in a closed conformation. Partially folded TL and N479 (yellow) from PDB 4A3F [13], which contains a matched NTP analog (not shown) and short RNA (not shown). Related structure to PDB 4A3F from PDB 4A3D [13], but without matched NTP analog is shown in gray. Partially folded TL from PDB 1R9T, containing a mismatched NTP (not shown) is shown in pink. B. Same figure as in (A) but with GTP omitted. Certain residues are relatively closely positioned in all structures overlaid (N479, Q1078), while other residues are observed stabilized in partially folded structures directly adjacent to the active site (L1081, N1082). H1085 is observed positioned adjacent to the active site only upon complete folding/stabilization of the TL. Dashed oval indicates likely location of TL NIR residues when in out conformation where the TL is either mobile or unfolded. This figure was created with Pymol [111]. The Relationship between MPA Sensitivity and Pol II Elongation Defects
In vivo it has been observed that some Pol II mutants constitutively express IMD2 in the absence of drug-induced stimulation [18], [47]. It is unclear whether this constitutive expression represents a mutant-induced alteration in Pol II start site specificity or a generic reduction of Pol II activity that mimics a drug-induced nucleotide depleted state, which itself leads to altered start site selection. We show here that an MPA-resistant LOF TL allele caused constitutive IMD2 expression, and in other work we found that this expression is likely a generic hallmark for reduced Pol II activity (Braberg et al, in preparation), and not necessarily due to a specific defect in initiation as previously proposed [57]. These results support published models that IMD2 regulation is directly responsive to reduction in nucleotide levels as reduction or increase in Pol II catalytic rates have opposing effects on the ability of IMD2 to be induced. Our observations that reduced Pol II activity does not correlate with sensitivity to MPA suggest that generic Pol II catalytic defects do not confer sensitivity to reduced nucleotide levels. Given the fact that LOF TL mutants are highly likely to confer elongation defects in vivo, the absence of MPA-sensitivity raises the question of whether reduction of GTP levels sensitizes cells to generic elongation defects caused by any transcription factor proposed to be acting through Pol II elongation. MPA sensitivity of any transcription factor mutant should not be used to infer presence or absence of an elongation defect because actual nucleotide levels in different mutant strains may vastly differ due to IMD2 transcriptional effects.
Model for Start Site Selection in Budding Yeast
The observation that TL mutants show polar effects on start site selection in vivo correlating with in vitro catalytic defects suggests a model for how Pol II activity may directly influence initiation. Start site selection in S. cerevisiae is not restricted to a short window a defined distance from the TATA box, but instead can take place over a longer region downstream [92]. It has been proposed that Pol II scans for sequences permissive for productive elongation [89]. We propose that our mutants primarily affect the nucleotide addition cycle and not some other step in initiation, therefore we expect promoter melting is already occurring in regions where start sites are not normally utilized in WT cells, and that scanning occurs subsequent to template melting, as has been proposed for GAL genes [89]. Continuous RNA synthesis during the scanning process does not appear to be required for scanning, as experiments show that chain-terminating nucleotides do not abrogate scanning in vitro [93], [94]. In light of these results, models for start site scanning in yeast that do not invoke transcription have been proposed (i.e. [95], also reviewed in [96]). We show that substitutions within the Pol II TL that have increased activity in vitro shift start sites upstream at ADH1, HIS3, PMA1 and GAL1, and those with reduced in vitro activity shift start sites downstream at ADH1 and GAL1 (Figure 6 and Figure S8). To explain these data, we invoke a new transcription-assisted model for start site scanning (Figure 8). Inefficiently or apparently unused start sites may allow undetected abortive but not productive initiation. Such abortive transcription may support Pol II downstream translocation, providing an explanation how Pol II catalytic mutants alter start site selection in a polar fashion, which is indicative of altered scanning as described in the flux model proposed by the Brow lab [97]. In the flux model, Pol II scanning from upstream to downstream will utilize starts in a polar fashion, and a decrease in usage of an upstream start will lead to increased usage of a downstream start. In our model, upstream shifts in start sites are due to increased Pol II catalytic activity, which in turn increases the probability of normally abortive initiation events becoming productive at the expense of downstream events. Conversely, downstream shifts in start sites are due to decreased Pol II activity, which increases the probability that abortive initiation will occur at upstream positions at the expense of productive initiation from those positions. Competition between abortive and productive initiation may occur at key transitions for early elongation complex stability [98], or by modulating stability of RNA:DNA hybrid in early transcription complexes as suggested by recent structural studies from the Kornberg lab [99] and the Cramer lab [13]. These new structures demonstrated a role for a matched NTP substrate in the Pol II active site in stabilization of short nascent RNAs. As GOF Pol II TL alleles have been implicated in extending the duration of substrate-bound complexes [9], these GOF alleles may also stabilize early elongation complexes in addition to increasing elongation rate. Any increase in the probability of next nucleotide incorporation at the expense of transcript release would lead to a net increase in productive elongation and a net decrease in downstream scanning by our model.
Fig. 8. Model for transcription-assisted start site scanning through abortive initiation. 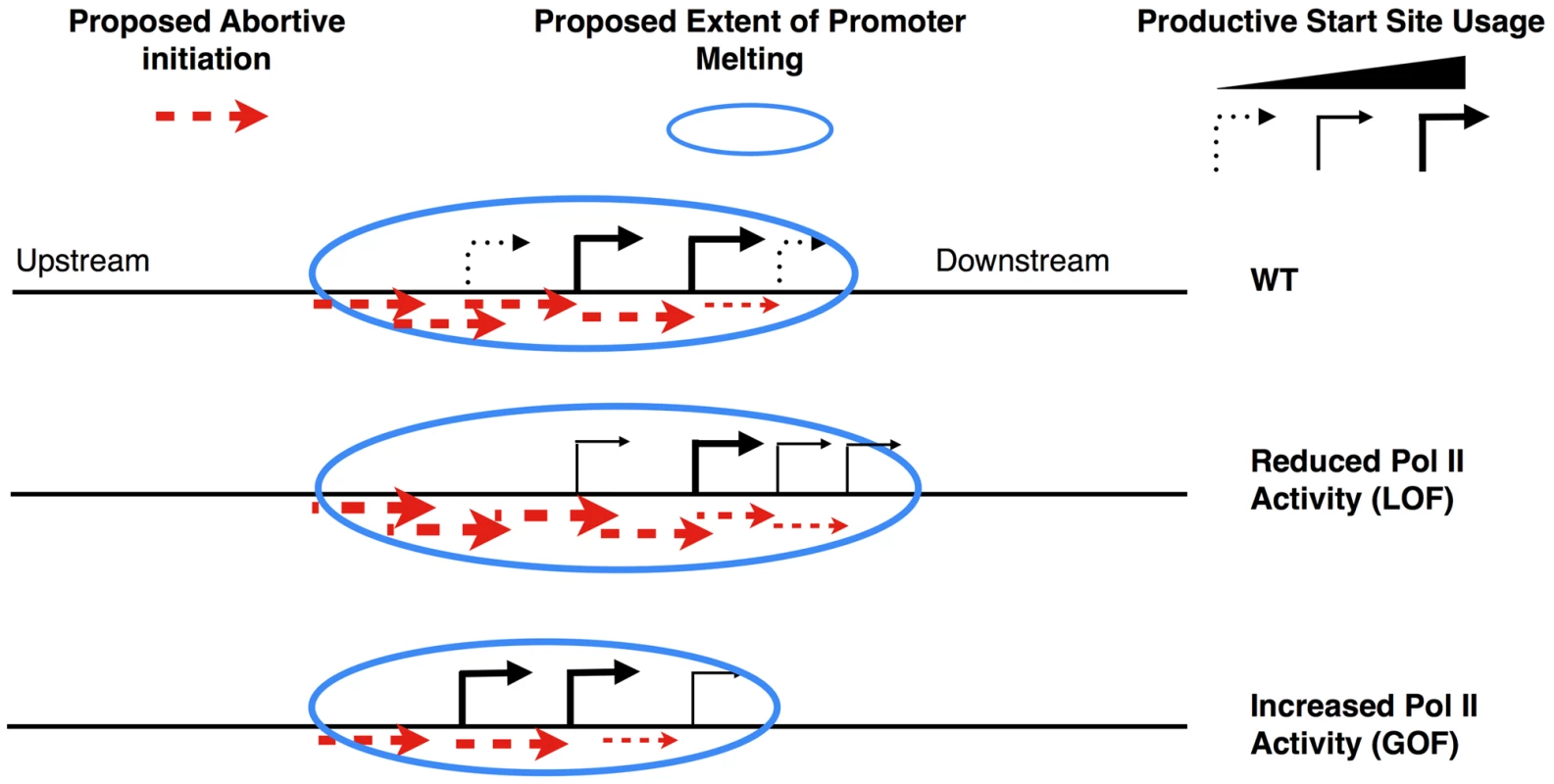
Cartoon showing predicted start site distribution for an ADH1-like gene in WT cells (top) or alteration in distribution in the presence of LOF (middle) or GOF (bottom) Pol II TL mutants. Previously described start site-altering mutants in Pol II general factors and other Pol II subunits have activities that are entirely consistent with this model. rpb9Δ shows upstream shifts in start sites at ADH1 (and other genes) [77], [79], inability to shift to downstream IMD2 starts upon MPA treatment, and causes Pol II to be superactive in vitro [85], similarly to superactive TL mutants E1103G and G1097D. An allele of the TFIIF subunit-encoding TFG1 gene causes upstream start site shifts in vivo while increasing efficiency of Pol II initiation in an abortive initiation assay, consistent with this allele conferring an increase in Pol II activity [94]. This increase in Pol II activity may be due to a gain of function in TFIIF or a loss of a negative function, under our activity-dependent framework for interpreting start site defects. Consistent with the latter, a recent in vitro study shows that TFIIF can inhibit initiation at specific template positions on a modified HIS4 template [100]. On some of the templates used in this study, TFIIF inhibits transcription initiation from upstream positions, consistent with polarity of most start site shifting mutants of TFIIF subunits in vivo. A number of sua7 (TFIIB) alleles show downstream shifts in start site selection [76], [101], [102], and in the case of the highly studied E62K allele, this mutant causes a decrease in Pol II transcription efficiency in vitro [103]. We provide a framework for interpreting the relationship between start site selection changes and the nature of alteration of the initiation process. Increasing or decreasing efficiency of productive initiation from particular nucleotide positions may relate to upstream or downstream shifts in start site selection, which appear to correlate with increases or decreases in Pol II activity, respectively. While start site defects strongly correlate with Pol II activity, their contribution to the growth defects of Pol II TL mutants remains to be determined.
Materials and Methods
Genetic Analyses
To examine mutant polymerase alleles encoding substitutions in the TL and elsewhere in Rpb1, we perform a plasmid shuffle with rpb1 CEN LEU2 plasmids by assaying ability of rpb1Δ cells to grow without an RPB1 CEN URA3 plasmid. This is accomplished by treatment of cells with the drug 5-fluoroorotic acid (5-FOA, Gold Biotechnology), which is toxic to Ura3+ cells [104], thus selecting cells containing solely an rpb1 CEN LEU2 plasmid so we might assess the ability of mutant plasmids to complement the essential function of RPB1. During the course of our experiments, we identified a previously unreported polymorphism in our RPB1 plasmid encoding substitution of isoleucine at position 69 for the threonine reported for this position in the Yeast Genome Database (T69I). This substitution was tracked as far back as the pRP112 subclone derived from an original RPO21 genomic clone from the Young lab [105]. Our analyses indicate that this substitution is phenotypically inert (Figure S8), yet all genetic data shown are for plasmids with the Ile69 corrected to threonine (I69T).
Yeast Strains, Plasmids, and Media
Yeast media are prepared in standard fashion as described in [106] with minor alterations. Yeast extract (1%)(BD), peptone (2%)(BD), dextrose (2%)(“YPD”) solid medium (2% bacto-agar, BD) is supplemented with adenine (0.15 mM final) and tryptophan (0.4 mM final)(Sigma-Aldrich). YP plates with alternate carbon sources such as raffinose (2%, USB) or raffinose (2%) plus galactose (1%, Sigma-Aldrich) also contain antimycin A (1 µg/ml, Sigma-Aldrich). Minimal media plates are synthetic complete (“SC”/“Hopkins mix”) with amino-acids dropped out as appropriate as described in [106] with minor alterations: per standard batch formulation Adenine Hemisulfate was 2 g, Uracil was 2 g, myo-inositol was 0.1 g, p-Aminobenzoic acid (PABA) was 0.2 g. For studies with mycophenolic acid (MPA, Sigma-Aldrich), MPA was added to minimal SC-Leucine medium at 20 µg/ml from a 10 mg/ml stock in ethanol. Construction of mutants is described in Text S1. Yeast strain genotypes, numbers and plasmid descriptions are found in Tables S1 and S2.
Pol II Purification and In Vitro Transcription Assays
Pol II enzymes were purified from yeast strains expressing mutant rpb1 genes from a low copy plasmid from the endogenous RPO21 promoter via a tandem-affinity tag (TAP) on Rpb3, in a procedure derived from [107] and as described in [2]. All mutant enzymes are Rpb1 I69 variants, except H1085Q/E1103G, F1086S/E1103G and Q1078A/E1103G, which are T69 variants. Transcription reactions of Pol II elongation complexes formed on nucleic acid scaffolds follow the approach described by Komissarova et al [108], and are performed as in [2] with the following modifications: for some experiments, amounts of all nucleic acids were reduced to 1/10th the amount stated in [2] (to 30 pmol from 300 pmol, etc.)(Text S1). Reactions were separated on 13.5% acrylamide-bisacrylamide gels (19∶1 ratio) containing 1× TBE and 7 M urea and quantitated as previously described [2].
Primer Extension and Northern Blotting Analysis
Total yeast RNA was purified as described [109]. Primer extension analysis was performed exactly as described (http://labs.fhcrc.org/hahn/Methods/mol_bio_meth/primer_ext.html) [110] with the following modifications. Total RNA used was 30 µg, and in the case of more dilute RNA samples, volumes of reactions were increased by 50% to accommodate greater volume of sample. Reverse transcriptase (RT) was M-MLV RT from either Life Technologies or Fermentas. RT synthesis reactions were supplemented with 1 µl RNAse Inhibitor (Fermentas). Extension products were separated on either 7% or 10% acrylamide-bisacrylamide gels (19∶1 ratio) containing 1× TBE and 7 M Urea. Northern blotting was performed essentially as described in manual for GeneScreen hybridization membranes (Perkin-Elmer) with the following modifications. RNA samples (20 µg) were prepared in NorthernMax loading buffer (Ambion/AB). Prehybridization solution did not contain SSPE or SSC buffers, but contained 5× Denhardt's solution, 50 mM Tris-HCl pH 7.5, 1 M NaCl, 0.1% sodium pyrophosphate, 0.1% SDS instead of 1%, 10% Dextran Sulfate, 50% formamide, and 500 µg/ml sheared/denatured salmon sperm DNA. Probes for northern blots were radiolabeled using α-32P-dATP by random priming using the Decaprime II kit (Ambion) according to manufacturer's instructions. Blots were washed at twice for 10 minutes each wash at 42°C with 2× SSC, 0.5% SDS, then twice at 67°C with 5× SSC, 0.5% SDS for 30 minutes each wash, then twice in 0.2× SSC for 30 minutes each wash at room temperature. Primer extension gels and northern blots were visualized by phosphorimaging (GE Healthcare or Bio-Rad) and quantified using ImageQuant 5.0 (GE) or Quantity One (Bio-Rad) software, with data exported to Microsoft Excel for management. Oligo sequences for site-directed mutagenesis, primer extension analysis, amplification of DNA for Northern blotting and in vitro transcription are available upon request.
Supporting Information
Zdroje
1. WangDBushnellDAWestoverKDKaplanCDKornbergRD 2006 Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell 127 941 954
2. KaplanCDLarssonKMKornbergRD 2008 The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin. Molecular cell 30 547 556
3. VassylyevDGVassylyevaMNZhangJPalangatMArtsimovitchI 2007 Structural basis for substrate loading in bacterial RNA polymerase. Nature 448 163 168
4. TemiakovDZenkinNVassylyevaMNPerederinaATahirovTH 2005 Structural basis of transcription inhibition by antibiotic streptolydigin. Mol Cell 19 655 666
5. YuzenkovaYBochkarevaATadigotlaVRRoghanianMZorovS 2010 Stepwise mechanism for transcription fidelity. BMC Biol 8 54
6. KaplanCD 2010 The architecture of RNA polymerase fidelity. BMC Biol 8 85
7. ZhangJPalangatMLandickR 2010 Role of the RNA polymerase trigger loop in catalysis and pausing. Nat Struct Mol Biol 17 99 104
8. ToulokhonovIZhangJPalangatMLandickR 2007 A central role of the RNA polymerase trigger loop in active-site rearrangement during transcriptional pausing. Mol Cell 27 406 419
9. KireevaMLNedialkovYACremonaGHPurtovYALubkowskaL 2008 Transient reversal of RNA polymerase II active site closing controls fidelity of transcription elongation. Mol Cell 30 557 566
10. SydowJFBruecknerFCheungACDamsmaGEDenglS 2009 Structural basis of transcription: mismatch-specific fidelity mechanisms and paused RNA polymerase II with frayed RNA. Mol Cell 34 710 721
11. BruecknerFCramerP 2008 Structural basis of transcription inhibition by alpha-amanitin and implications for RNA polymerase II translocation. Nat Struct Mol Biol 15 811 818
12. SpahrHCaleroGBushnellDAKornbergRD 2009 Schizosacharomyces pombe RNA polymerase II at 3.6-A resolution. Proceedings of the National Academy of Sciences of the United States of America 106 9185 9190
13. CheungACSainsburySCramerP 2011 Structural basis of initial RNA polymerase II transcription. The EMBO journal 30 4755 4763
14. TagamiSSekineSKumarevelTHinoNMurayamaY 2010 Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein. Nature 468 978 982
15. KettenbergerHArmacheKJCramerP 2003 Architecture of the RNA polymerase II-TFIIS complex and implications for mRNA cleavage. Cell 114 347 357
16. Bar-NahumGEpshteinVRuckensteinAERafikovRMustaevA 2005 A ratchet mechanism of transcription elongation and its control. Cell 120 183 193
17. YuzenkovaYZenkinN 2010 Central role of the RNA polymerase trigger loop in intrinsic RNA hydrolysis. Proc Natl Acad Sci U S A 107 10878 10883
18. MalagonFKireevaMLShaferBKLubkowskaLKashlevM 2006 Mutations in the Saccharomyces cerevisiae RPB1 gene conferring hypersensitivity to 6-azauracil. Genetics 172 2201 2209
19. TanLWieslerSTrzaskaDCarneyHCWeinzierlRO 2008 Bridge helix and trigger loop perturbations generate superactive RNA polymerases. J Biol 7 40
20. CastroCSmidanskyEDArnoldJJMaksimchukKRMoustafaI 2009 Nucleic acid polymerases use a general acid for nucleotidyl transfer. Nat Struct Mol Biol 16 212 218
21. HekmatpanahDSYoungRA 1991 Mutations in a conserved region of RNA polymerase II influence the accuracy of mRNA start site selection. Mol Cell Biol 11 5781 5791
22. ArchambaultJJansmaDBKawasoeJHArndtKTGreenblattJ 1998 Stimulation of transcription by mutations affecting conserved regions of RNA polymerase II. J Bacteriol 180 2590 2598
23. WeilbaecherRHebronCFengGLandickR 1994 Termination-altering amino acid substitutions in the beta' subunit of Escherichia coli RNA polymerase identify regions involved in RNA chain elongation. Genes Dev 8 2913 2927
24. SweeneyMJ 1977 Mycophenolic acid and its mechanism of action in cancer and psoriasis. Jpn J Antibiot 30 Suppl 85 92
25. ExingerFLacrouteF 1992 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr Genet 22 9 11
26. HandschumacherREPasternakCA 1958 Inhibition of orotidylic acid decarboxylase, a primary site of carcinostasis by 6-azauracil. Biochimica et biophysica acta 30 451 452
27. MasonPBStruhlK 2005 Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol Cell 17 831 840
28. KulishDStruhlK 2001 TFIIS enhances transcriptional elongation through an artificial arrest site in vivo. Molecular and cellular biology 21 4162 4168
29. HoweKJKaneCMAresMJr 2003 Perturbation of transcription elongation influences the fidelity of internal exon inclusion in Saccharomyces cerevisiae. Rna 9 993 1006
30. GrigullJMnaimnehSPootoolalJRobinsonMDHughesTR 2004 Genome-wide analysis of mRNA stability using transcription inhibitors and microarrays reveals posttranscriptional control of ribosome biogenesis factors. Molecular and cellular biology 24 5534 5547
31. ArchambaultJLacrouteFRuetAFriesenJD 1992 Genetic interaction between transcription elongation factor TFIIS and RNA polymerase II. Mol Cell Biol 12 4142 4152
32. PowellWReinesD 1996 Mutations in the second largest subunit of RNA polymerase II cause 6-azauracil sensitivity in yeast and increased transcriptional arrest in vitro. J Biol Chem 271 6866 6873
33. WuJAwreyDEEdwardsAMArchambaultJFriesenJD 1996 In vitro characterization of mutant yeast RNA polymerase II with reduced binding for elongation factor TFIIS. Proc Natl Acad Sci U S A 93 11552 11557
34. HemmingSAJansmaDBMacgregorPFGoryachevAFriesenJD 2000 RNA polymerase II subunit Rpb9 regulates transcription elongation in vivo. J Biol Chem 275 35506 35511
35. ShawRJReinesD 2000 Saccharomyces cerevisiae transcription elongation mutants are defective in PUR5 induction in response to nucleotide depletion. Mol Cell Biol 20 7427 7437
36. KeoghMCPodolnyVBuratowskiS 2003 Bur1 kinase is required for efficient transcription elongation by RNA polymerase II. Mol Cell Biol 23 7005 7018
37. KimHJJeongSHHeoJHJeongSJKimST 2004 mRNA capping enzyme activity is coupled to an early transcription elongation. Mol Cell Biol 24 6184 6193
38. RilesLShawRJJohnstonMReinesD 2004 Large-scale screening of yeast mutants for sensitivity to the IMP dehydrogenase inhibitor 6-azauracil. Yeast 21 241 248
39. XiaoTKaoCFKroganNJSunZWGreenblattJF 2005 Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol Cell Biol 25 637 651
40. DuHNFingermanIMBriggsSD 2008 Histone H3 K36 methylation is mediated by a trans-histone methylation pathway involving an interaction between Set2 and histone H4. Genes Dev 22 2786 2798
41. SongYHAhnSH 2010 A Bre1-associated protein, large 1 (Lge1), promotes H2B ubiquitylation during the early stages of transcription elongation. J Biol Chem 285 2361 2367
42. HartzogGAWadaTHandaHWinstonF 1998 Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev 12 357 369
43. OrphanidesGWuWHLaneWSHampseyMReinbergD 1999 The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature 400 284 288
44. OteroGFellowsJLiYde BizemontTDiracAM 1999 Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Molecular cell 3 109 118
45. WittschiebenBOOteroGde BizemontTFellowsJErdjument-BromageH 1999 A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Molecular cell 4 123 128
46. ShawRJWilsonJLSmithKTReinesD 2001 Regulation of an IMP dehydrogenase gene and its overexpression in drug-sensitive transcription elongation mutants of yeast. J Biol Chem 276 32905 32916
47. DesmoucellesCPinsonBSaint-MarcCDaignan-FornierB 2002 Screening the yeast “disruptome” for mutants affecting resistance to the immunosuppressive drug, mycophenolic acid. The Journal of biological chemistry 277 27036 27044
48. ReinesD 2003 Use of RNA yeast polymerase II mutants in studying transcription elongation. Methods Enzymol 371 284 292
49. GinsburgDSGovindCKHinnebuschAG 2009 NuA4 lysine acetyltransferase Esa1 is targeted to coding regions and stimulates transcription elongation with Gcn5. Mol Cell Biol 29 6473 6487
50. SchwabishMAStruhlK 2007 The Swi/Snf complex is important for histone eviction during transcriptional activation and RNA polymerase II elongation in vivo. Mol Cell Biol 27 6987 6995
51. SunMLariviereLDenglSMayerACramerP 2010 A tandem SH2 domain in transcription elongation factor Spt6 binds the phosphorylated RNA polymerase II C-terminal repeat domain (CTD). The Journal of biological chemistry 285 41597 41603
52. SchneiderSPeiYShumanSSchwerB 2010 Separable functions of the fission yeast Spt5 carboxyl-terminal domain (CTD) in capping enzyme binding and transcription elongation overlap with those of the RNA polymerase II CTD. Molecular and cellular biology 30 2353 2364
53. LindstromDLHartzogGA 2001 Genetic interactions of Spt4–Spt5 and TFIIS with the RNA polymerase II CTD and CTD modifying enzymes in Saccharomyces cerevisiae. Genetics 159 487 497
54. Pascual-GarciaPGovindCKQueraltECuenca-BonoBLlopisA 2008 Sus1 is recruited to coding regions and functions during transcription elongation in association with SAGA and TREX2. Genes Dev 22 2811 2822
55. JenksMHReinesD 2005 Dissection of the molecular basis of mycophenolate resistance in Saccharomyces cerevisiae. Yeast 22 1181 1190
56. HyleJWShawRJReinesD 2003 Functional distinctions between IMP dehydrogenase genes in providing mycophenolate resistance and guanine prototrophy to yeast. J Biol Chem 278 28470 28478
57. KuehnerJNBrowDA 2008 Regulation of a eukaryotic gene by GTP-dependent start site selection and transcription attenuation. Mol Cell 31 201 211
58. JenksMHO'RourkeTWReinesD 2008 Properties of an intergenic terminator and start site switch that regulate IMD2 transcription in yeast. Mol Cell Biol 28 3883 3893
59. SimchenGWinstonFStylesCAFinkGR 1984 Ty-mediated gene expression of the LYS2 and HIS4 genes of Saccharomyces cerevisiae is controlled by the same SPT genes. Proc Natl Acad Sci U S A 81 2431 2434
60. KaplanCDHollandMJWinstonF 2005 Interaction between transcription elongation factors and mRNA 3′-end formation at the Saccharomyces cerevisiae GAL10-GAL7 locus. J Biol Chem 280 913 922
61. GregerIHProudfootNJ 1998 Poly(A) signals control both transcriptional termination and initiation between the tandem GAL10 and GAL7 genes of Saccharomyces cerevisiae. Embo J 17 4771 4779
62. BucheliMEHeXKaplanCDMooreCLBuratowskiS 2007 Polyadenylation site choice in yeast is affected by competition between Npl3 and polyadenylation factor CFI. Rna 13 1756 1764
63. BucheliMEBuratowskiS 2005 Npl3 is an antagonist of mRNA 3′ end formation by RNA polymerase II. Embo J 24 2150 2160
64. MaloneEAClarkCDChiangAWinstonF 1991 Mutations in SPT16/CDC68 suppress cis - and trans-acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol Cell Biol 11 5710 5717
65. WinstonFChaleffDTValentBFinkGR 1984 Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics 107 179 197
66. PrelichGWinstonF 1993 Mutations that suppress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics 135 665 676
67. WestoverKDBushnellDAKornbergRD 2004 Structural basis of transcription: nucleotide selection by rotation in the RNA polymerase II active center. Cell 119 481 489
68. GnattALCramerPFuJBushnellDAKornbergRD 2001 Structural basis of transcription: an RNA polymerase II elongation complex at 3.3 A resolution. Science 292 1876 1882
69. KettenbergerHArmacheKJCramerP 2004 Complete RNA polymerase II elongation complex structure and its interactions with NTP and TFIIS. Mol Cell 16 955 965
70. KwapiszMWeryMDespresDGhavi-HelmYSoutourinaJ 2008 Mutations of RNA polymerase II activate key genes of the nucleoside triphosphate biosynthetic pathways. The EMBO journal 27 2411 2421
71. ThiebautMColinJNeilHJacquierASeraphinB 2008 Futile cycle of transcription initiation and termination modulates the response to nucleotide shortage in S. cerevisiae. Molecular cell 31 671 682
72. CramerPBushnellDAKornbergRD 2001 Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292 1863 1876
73. BerroteranRWWareDEHampseyM 1994 The sua8 suppressors of Saccharomyces cerevisiae encode replacements of conserved residues within the largest subunit of RNA polymerase II and affect transcription start site selection similarly to sua7 (TFIIB) mutations. Mol Cell Biol 14 226 237
74. SteinmetzEJWarrenCLKuehnerJNPanbehiBAnsariAZ 2006 Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol Cell 24 735 746
75. PappasDLJrHampseyM 2000 Functional interaction between Ssu72 and the Rpb2 subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol Cell Biol 20 8343 8351
76. PintoIWareDEHampseyM 1992 The yeast SUA7 gene encodes a homolog of human transcription factor TFIIB and is required for normal start site selection in vivo. Cell 68 977 988
77. SunZWTessmerAHampseyM 1996 Functional interaction between TFIIB and the Rpb9 (Ssu73) subunit of RNA polymerase II in Saccharomyces cerevisiae. Nucleic acids research 24 2560 2566
78. SunZWHampseyM 1995 Identification of the gene (SSU71/TFG1) encoding the largest subunit of transcription factor TFIIF as a suppressor of a TFIIB mutation in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America 92 3127 3131
79. HullMWMcKuneKWoychikNA 1995 RNA polymerase II subunit RPB9 is required for accurate start site selection. Genes Dev 9 481 490
80. GhazyMABrodieSAAmmermanMLZieglerLMPonticelliAS 2004 Amino acid substitutions in yeast TFIIF confer upstream shifts in transcription initiation and altered interaction with RNA polymerase II. Mol Cell Biol 24 10975 10985
81. EichnerJChenHTWarfieldLHahnS 2010 Position of the general transcription factor TFIIF within the RNA polymerase II transcription preinitiation complex. EMBO J 29 706 716
82. Freire-PicosMAKrishnamurthySSunZWHampseyM 2005 Evidence that the Tfg1/Tfg2 dimer interface of TFIIF lies near the active center of the RNA polymerase II initiation complex. Nucleic acids research 33 5045 5052
83. MajovskiRCKhaperskyyDAGhazyMAPonticelliAS 2005 A functional role for the switch 2 region of yeast RNA polymerase II in transcription start site utilization and abortive initiation. J Biol Chem 280 34917 34923
84. Furter-GravesEMHallBDFurterR 1994 Role of a small RNA pol II subunit in TATA to transcription start site spacing. Nucleic Acids Res 22 4932 4936
85. WalmacqCKireevaMLIrvinJNedialkovYLubkowskaL 2009 Rpb9 subunit controls transcription fidelity by delaying NTP sequestration in RNA polymerase II. The Journal of biological chemistry 284 19601 19612
86. NesserNKPetersonDOHawleyDK 2006 RNA polymerase II subunit Rpb9 is important for transcriptional fidelity in vivo. Proceedings of the National Academy of Sciences of the United States of America 103 3268 3273
87. KoyamaHItoTNakanishiTSekimizuK 2007 Stimulation of RNA polymerase II transcript cleavage activity contributes to maintain transcriptional fidelity in yeast. Genes to cells : devoted to molecular & cellular mechanisms 12 547 559
88. ZhangDYCarsonDJMaJ 2002 The role of TFIIB-RNA polymerase II interaction in start site selection in yeast cells. Nucleic Acids Res 30 3078 3085
89. GiardinaCLisJT 1993 DNA melting on yeast RNA polymerase II promoters. Science 261 759 762
90. HuangXWangDWeissDRBushnellDAKornbergRD 2010 RNA polymerase II trigger loop residues stabilize and position the incoming nucleotide triphosphate in transcription. Proc Natl Acad Sci U S A 107 15745 15750
91. SvetlovVVassylyevDGArtsimovitchI 2004 Discrimination against deoxyribonucleotide substrates by bacterial RNA polymerase. J Biol Chem 279 38087 38090
92. StruhlK 1989 Molecular mechanisms of transcriptional regulation in yeast. Annual review of biochemistry 58 1051 1077
93. LueNFFlanaganPMSugimotoKKornbergRD 1989 Initiation by yeast RNA polymerase II at the adenoviral major late promoter in vitro. Science 246 661 664
94. KhaperskyyDAAmmermanMLMajovskiRCPonticelliAS 2008 Functions of Saccharomyces cerevisiae TFIIF during transcription start site utilization. Mol Cell Biol 28 3757 3766
95. MillerGHahnS 2006 A DNA-tethered cleavage probe reveals the path for promoter DNA in the yeast preinitiation complex. Nature structural & molecular biology 13 603 610
96. CordenJL 2008 Yeast Pol II start-site selection: the long and the short of it. EMBO reports 9 1084 1086
97. KuehnerJNBrowDA 2006 Quantitative analysis of in vivo initiator selection by yeast RNA polymerase II supports a scanning model. The Journal of biological chemistry 281 14119 14128
98. KugelJFGoodrichJA 2002 Translocation after synthesis of a four-nucleotide RNA commits RNA polymerase II to promoter escape. Molecular and cellular biology 22 762 773
99. LiuXBushnellDASilvaDAHuangXKornbergRD 2011 Initiation complex structure and promoter proofreading. Science 333 633 637
100. FishburnJHahnS 2011 Architecture of the yeast RNA polymerase II open complex and regulation of activity by TFIIF. Molecular and cellular biology
101. WuWHPintoIChenBSHampseyM 1999 Mutational analysis of yeast TFIIB. A functional relationship between Ssu72 and Sub1/Tsp1 defined by allele-specific interactions with TFIIB. Genetics 153 643 652
102. PintoIWuWHNaJGHampseyM 1994 Characterization of sua7 mutations defines a domain of TFIIB involved in transcription start site selection in yeast. J Biol Chem 269 30569 30573
103. ChoEJBuratowskiS 1999 Evidence that transcription factor IIB is required for a post-assembly step in transcription initiation. J Biol Chem 274 25807 25813
104. BoekeJDTrueheartJNatsoulisGFinkGR 1987 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol 154 164 175
105. NonetMSweetserDYoungRA 1987 Functional redundancy and structural polymorphism in the large subunit of RNA polymerase II. Cell 50 909 915
106. AmbergDCBurkeDJStrathernJN 2005 Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 2005 Edition Cold Spring Harbor, NY Cold Spring Harbor Press 230
107. PuigOCasparyFRigautGRutzBBouveretE 2001 The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24 218 229
108. KomissarovaNKireevaMLBeckerJSidorenkovIKashlevM 2003 Engineering of elongation complexes of bacterial and yeast RNA polymerases. Methods Enzymol 371 233 251
109. SchmittMEBrownTATrumpowerBL 1990 A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic acids research 18 3091 3092
110. RanishJAHahnS 1991 The yeast general transcription factor TFIIA is composed of two polypeptide subunits. The Journal of biological chemistry 266 19320 19327
111. Schrodinger, LLC 2010 The PyMOL Molecular Graphics System, Version 1.3r1
112. GregerIHArandaAProudfootN 2000 Balancing transcriptional interference and initiation on the GAL7 promoter of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 97 8415 8420
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



