-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDefective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
Proteins involved in membrane remodeling play an essential role in a plethora of cell functions including endocytosis and intracellular transport. Defects in several of them lead to human diseases. Myotubularins, amphiphysins, and dynamins are all proteins implicated in membrane trafficking and/or remodeling. Mutations in myotubularin, amphiphysin 2 (BIN1), and dynamin 2 lead to different forms of centronuclear myopathy, while mutations in myotubularin-related proteins cause Charcot-Marie-Tooth neuropathies. In addition to centronuclear myopathy, dynamin 2 is also mutated in a dominant form of Charcot-Marie-Tooth neuropathy. While several proteins from these different families are implicated in similar diseases, mutations in close homologues or in the same protein in the case of dynamin 2 lead to diseases affecting different tissues. This suggests (1) a common molecular pathway underlying these different neuromuscular diseases, and (2) tissue-specific regulation of these proteins. This review discusses the pathophysiology of the related neuromuscular diseases on the basis of animal models developed for proteins of the myotubularin, amphiphysin, and dynamin families. A better understanding of the common mechanisms between these neuromuscular disorders will lead to more specific health care and therapeutic approaches.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002595
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1002595Summary
Proteins involved in membrane remodeling play an essential role in a plethora of cell functions including endocytosis and intracellular transport. Defects in several of them lead to human diseases. Myotubularins, amphiphysins, and dynamins are all proteins implicated in membrane trafficking and/or remodeling. Mutations in myotubularin, amphiphysin 2 (BIN1), and dynamin 2 lead to different forms of centronuclear myopathy, while mutations in myotubularin-related proteins cause Charcot-Marie-Tooth neuropathies. In addition to centronuclear myopathy, dynamin 2 is also mutated in a dominant form of Charcot-Marie-Tooth neuropathy. While several proteins from these different families are implicated in similar diseases, mutations in close homologues or in the same protein in the case of dynamin 2 lead to diseases affecting different tissues. This suggests (1) a common molecular pathway underlying these different neuromuscular diseases, and (2) tissue-specific regulation of these proteins. This review discusses the pathophysiology of the related neuromuscular diseases on the basis of animal models developed for proteins of the myotubularin, amphiphysin, and dynamin families. A better understanding of the common mechanisms between these neuromuscular disorders will lead to more specific health care and therapeutic approaches.
Introduction
Membrane remodeling occurs in diverse and essential cellular processes, including endocytosis, intracellular transport, and synaptic vesicle fusion. There are numerous proteins related to membrane remodeling that have diverse functions, including regulation of lipids, membrane adaptor proteins, or cytoskeletal organization. Several genes implicated in membrane remodeling and trafficking are mutated in different forms of human neuropathies (DNM2, MTMR2, MTMR13, NEFL, RAB7A, FGD4, FIG4, SH3TC2, LITAF/SIMPLE) and myopathies (MTM1, BIN1, DNM2, CAV3, DYSF) (reviewed in [1]).
The purpose of this review is to discuss how several protein families with functions in membrane remodeling act in the same pathway and how defects of several of these proteins can induce similar human diseases. We consider here animal models of the myotubularin/amphiphysin/dynamin pathway, highlighted by their common implication in both centronuclear myopathies (CNM) and peripheral Charcot-Marie-Tooth neuropathies (CMT). Myotubularins are phosphoinositides phosphatases, amphiphysins are BAR (BIN1, Amphiphysin and RVS167) proteins sensing membrane curvature and regulating membrane remodeling, and dynamins are large GTPases able to tubulate and eventually cleave membranes. Members of these protein families are commonly mutated in several neuromuscular diseases affecting different tissues, suggesting a common pathological pathway and tissue-specific regulations. As other neuromuscular disease genes encode for proteins implicated in membrane transport, this proposed pathological pathway may link together a larger number of membrane trafficking proteins.
The Myotubularin Family
Myotubularin proteins are mutated in two human diseases, centronuclear myopathy (XLCNM, OMIM 310400) and CMT neuropathy. MTM1 is mutated in the X-linked, most severe form of CNM [2], [3]. Boys with MTM1 mutations causing X-linked CNM present a very severe and generalized muscle weakness at birth (Figure 1). Death normally occurs within the first year of life due to respiratory failure. Centralized nuclei in hypotrophic fibers are a prominent feature in muscle [2], [3].
Fig. 1. Protein domains and disease-causing mutations in the myotubularin, amphiphysin, and dynamin families. 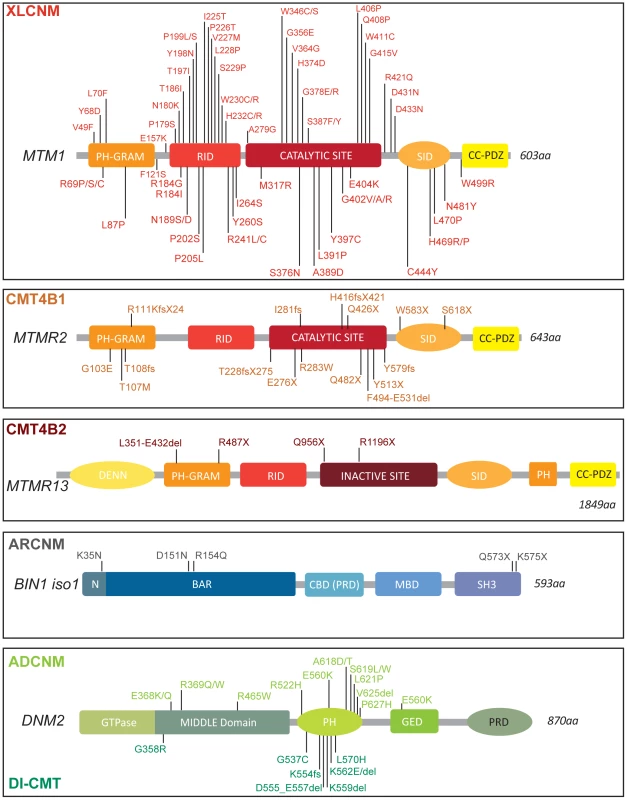
Myotubularin contains a PH-GRAM domain that may bind lipids and a coil-coiled-PDZ binding site to form homo- and hetero-dimers with other members of the myotubularin family. Only the disease-causing missense mutations in MTM1 are represented, based on the international UMD-MTM1 database, existing currently in a local version in Strasbourg (France). MTM1 mutations identified in more than two patients are R69C(9 families), P205L(5), V227M(3), R241C(13), G378R(4), E404K(4), and Y397C(5). AMPH1 and BIN1 possess an N-BAR domain able to sense and eventually curve membrane and a C-terminal SH3 domain binding to proteins with proline-rich domains, such as dynamins [48], [88]. In addition some isoforms have clathrin-binding and Myc-binding domains (CBD, MBD); a phosphoinositide-binding motif is present between the BAR and MBD domains specifically in skeletal muscle. DNM2 contains a GTPase domain, a central middle (MID) domain, a Pleckstrin Homology (PH) domain, a GTPase Effector Domain (GED), and a C-terminal Proline Rich Domain (PRD). Dominant mutations in DNM2 lead to either centronuclear myopathy (above), or peripheral CMT neuropathy (below). Only coding mutations are listed for all genes. CMT comprises a genetically heterogeneous group of inherited disorders affecting myelinated axons in the peripheral nervous system. The disease is characterized by progressive distally accentuated muscle weakness and atrophy. CMT has been subdivided into demyelinating, axonal and intermediate forms on the basis of clinical, electrophysiological, and histological data. CMT4B are severe demyelinating autosomal recessive inherited neuropathies. They are divided in two subgroups (Figure 1; Table S1), CMT4B1 (MTMR2 mutations, OMIM 601382) and CMT4B2 (MTMR13 mutations, OMIM 604563) [4]–[7].
Myotubularin (MTM1) is the founding member of a large family of phosphoinositide phosphatases (Figure 1). Myotubularins are 3-phosphatases that play an essential role in maintenance of the spatial and temporal equilibrium of phosphoinositides (PIs), molecular membrane flags that have key roles in membrane identity and protein recruitment [8], [9]. Via its tyrosine phosphatase-like (PTP) domain, MTM1 dephosphorylates phosphatidylinositol 3-phosphate (PtdIns3P) and PtdIns(3,5)P2, second messengers produced by PI 3-kinase (PI3K) and 5-kinase (PI5K) respectively, with important roles in endocytosis and membrane trafficking [10].
In mammals, the myotubularin family is composed of MTM1 and 13 MTM1-related (MTMR) proteins named MTMR1 to MTMR13 [11], [12] (Figure 2). All contain PTP-like and PH-GRAM domains. Six MTM family members contain an inactive catalytic site owing to conserved substitutions of several amino acids essential for activity [11], [13], [14]. These dead phosphatases, MTMR5 and MTMR9–13, heterodimerize with active myotubularins to regulate their phosphatase activity [12]. To date five pairs of active-dead phosphatases have been confirmed, MTM1-MTMR12, MTMR2-MTMR5, MTMR2-MTMR13, MTMR7-MTMR9, and MTMR6-MTMR9 [15]–[19]. Interestingly mutations in the coupled active and dead phosphatases, MTMR2 and MTMR13, lead to very similar neuropathies, distinguished only by the age of onset and the major involvement of proximal muscles in CMT4B1, often resulting in a more severe neuropathy.
Fig. 2. Phylogenetic relationships. 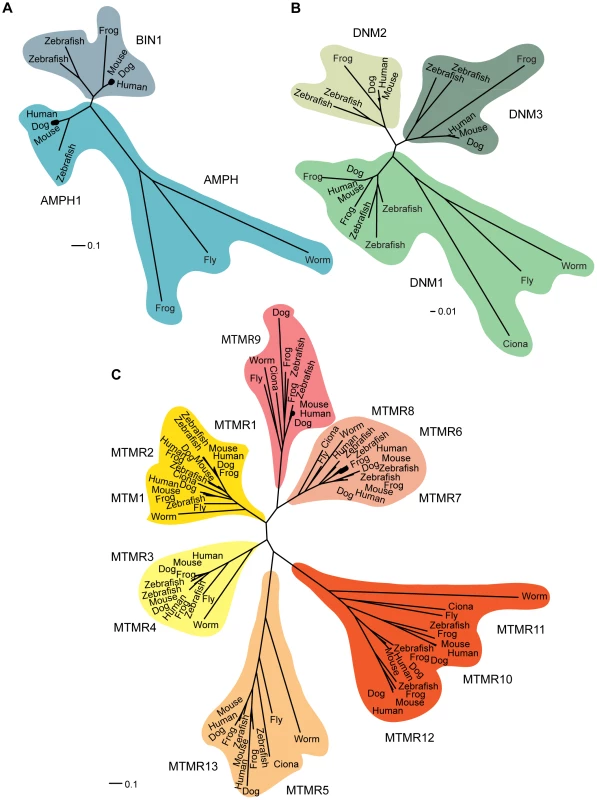
Phylogenetic relationships within the amphiphysin (A), dynamin (B), and myotubularin (C) protein families. Sequences were collected using the eggNOG database, which groups genes into families at different taxonomic levels. A high quality multiple sequence alignment was computed for each protein family on all proteins members including, respectively, 91 myotubularin protein sequences, 23 dynamin protein sequences, and 13 amphiphysin protein sequences. For a more detailed description, see Protocol S1. Scale represents the percentage of divergence. Myotubularin Drosophila Model
How the balance between specific kinases and phosphatases that regulate PI levels, and how disruption of this coregulation may lead to neuromuscular diseases remains unresolved. Studies using Drosophila have shown that Drosophila myotubularin (mtm, the ortholog of human MTM1 and MTMR2) coordinates with class II PI3K to modulate levels of PtdIns3P within the cell, which is important in regulating endolysosomal functions and cortical actin remodeling [20], and plays a role in integrin-mediated attachment of myofibers [21]. Integrin accumulated with PtdIns3P on endosomal vesicles when mtm was depleted, and integrin localization defects have also been observed in CNM patients [21]. This suggests that mtm/PI3K regulation may coordinate integrin trafficking and recycling to the plasma membrane. Modulating class II PI3K levels may therefore constitute a potential therapeutic target for CNM.
Myotubularin Caenorhabditis elegans Model
In C. elegans, six myotubularins have been identified, including three active phosphatases MTM-1, MTM-3, and MTM-6, and three dead phosphatases, MTM-5, MTM-9, and MTM-10 [11], [22]–[24], suggesting that the cooperation between active/dead phosphatases is conserved through evolution [17], [25], [26]. Specifically the MTM-6/MTM-9 complex regulated endosomal trafficking of the Wnt signaling complex [27]. However all myotubularins appear to be required for the endocytosis of fluid in coelomocytes from the pseudocoelome, a process called coelomocyte uptake (Table S1) [24], [26], [28]. Both mtm-1 and mtm-6 play a role in coelomocytes endocytosis, most likely by antagonizing Let-512 function (a PI3K in C. elegans, and a Vps34 homolog) [24]. A balance between mtm-1 and Vps-34 also regulates cell corpse engulfment of apoptotic cells, as MTM-1 inhibited this process via its phosphatase activity [28], [29]. In both mechanisms, MTM-1 regulation of PtdIns3P levels could be essential for membrane remodeling. Human MTM1 can substitute the worm MTM-1 function in cell corpse engulfment suggesting that MTM1, or its closer homologue MTMR2, can act similarly in human peripheral nerve or skeletal muscle, two tissues in which defects in MTM1 or MTMR2 induce diseases.
Myotubularin-Defective Animal Models for Muscle Pathologies
mtm-1 zebrafish morphants
Dowling et al. have generated a zebrafish model for X-linked CNM by morpholino knockdown of zebrafish MTM1 [30], which exhibited a delayed chorion hatching and a diminished touch-evoked escape behavior. Abnormally located nuclei, hypotrophy and organelle disruption, and neuromuscular junction disorganization were observed, as seen in patient muscle biopsies [30], [31]. The role of MTM1 as the primary PtdIns3P phosphatase in skeletal muscle development was reinforced by an increase of PtdIns3P level observed in the skeletal muscle of myotubularin morphants, but not in whole embryos. The rescue of the early muscle weakness by MTMR1 and MTMR2, the closest MTM1 paralogs, suggested that the specific muscle defect induced by a loss of MTM1 is due to the lack of compensation by other PtdIns3P myotubularin phosphatases in muscle [30]. Reduction of myotubularin in skeletal muscle affected the organization and the morphology of the T-tubules and sarcoplasmic reticulum (SR) and suggested that the regulation of PtdIns3P and PtdIns(3,5)P2, is essential for the excitation-contraction coupling machinery (Table S1). Indeed, impairment of MTMR14/JUMPY, a phosphatase sharing similar enzymatic activity to MTM1, led to T-tubule anomalies and PtdIns(3,5)P2 dependent defect of excitation-contraction coupling in mouse skeletal muscles [32], [33].
MTM1 mammalian models
The murine model of X-linked CNM generated by targeted mutagenesis of MTM1 [34] exhibited a progressive motor deficit and amyotrophy. Contrary to patients who display a strong muscle weakness from birth, knockout (KO) muscles appeared normal up to 2 wk of age. Mice then exhibited a progressive decrease in muscular strength with similar histopathology to patients [34], [35]. Death occurred around 7–12 wk probably because of cachexia and respiratory insufficiency. Tissue-specific excision of Mtm1 exon 4 confirmed that the muscle phenotype is due to loss of MTM1 function in skeletal muscle and not in nerves [34]. Altogether, this suggests that MTM1 is not essential for myogenesis but is important for muscle structural maintenance (Table 1). As in zebrafish myotubularin morphants, the loss of myotubularin in mouse skeletal muscle induced abnormal organization of the triads and T-tubules, which appeared prior to defects in excitation-contraction coupling [30], [35]. Similar triad defects have been noted in patients with mutations in MTM1 [36] and in Labrador retrievers with a mutation in MTM1, the latter providing a large animal model in which therapeutic trials could be envisaged [37]. MTM1 was shown to directly bind and regulate desmin localization, and MTM1 mutations causing CNM result in abnormal intermediate filament assembly and architecture, and perturbed mitrochondrial dynamics [38]. Re-expression of Mtm1 cDNA was sufficient to ameliorate the established muscle weakness in Mtm1 knockout mice and rescued desmin aggregation [38], [39]. Altogether, myotubularin might specifically regulate the appropriate organization and/or maintenance of triads through fine regulation of specific PI pools at the plasma and reticulum membranes, in skeletal muscle. These results provided new insight into the mechanisms causing CNM and indicated a common pathological link between CNM and desmin-related myopathies.
Tab. 1. Myotubularin/amphiphysin/dynamin protein functions in specific tissues, based on animal and cell models. 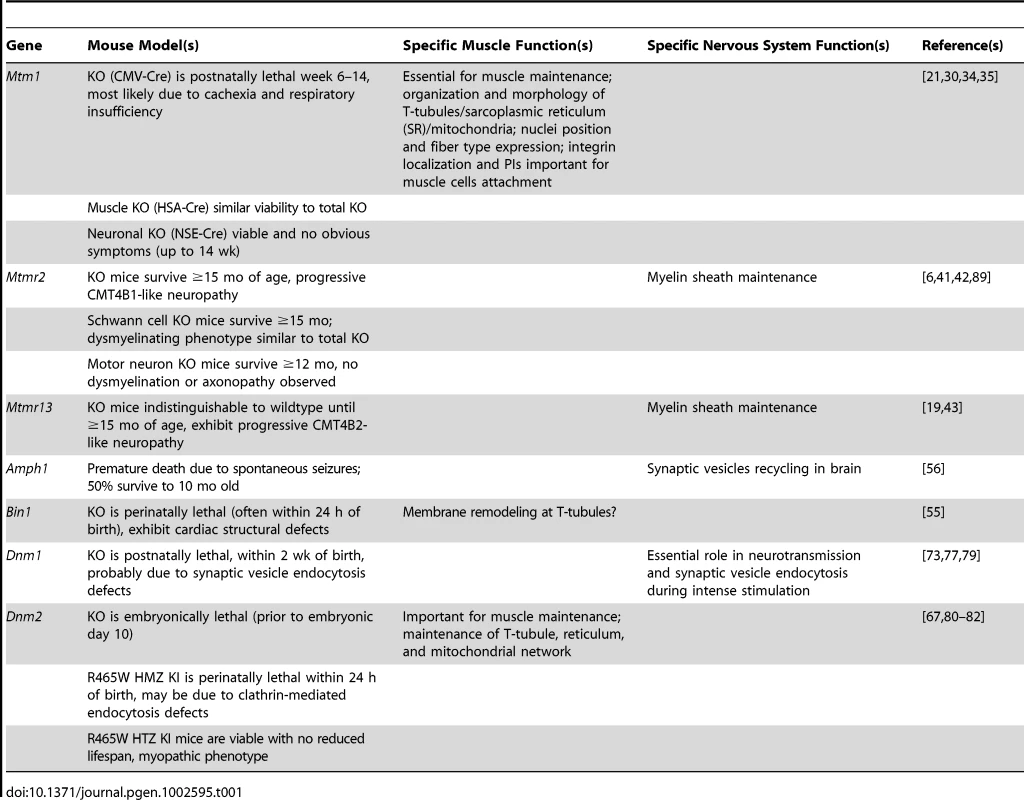
Myotubularin-Related Animal Models for Neuropathies
MTMR2 and MTMR13 murine models
Mtmr2-deficient mice start developing peripheral nerve abnormalities at 1 mo of age (Table 1) [40]–[42]. They present a dysmyelinating phenotype similar to CMT4B1 patients, consisting of motor and peripheral neuropathy with myelin outfoldings and redundant loops appearing as membrane extensions originating predominantly from paranodes, a region in between compact myelin and nodes of Ranvier. Expression of the MTMR2 binding protein Dlg-1/SAP97, a scaffolding protein belonging to the MAGUK protein family, decreased in Mtmr2-null mice, suggesting that loss of interaction between MTMR2 and Dlg-1/SAP97 disrupts cell polarity and cellular junctions or membrane remodeling in Schwann cells, leading to membrane outfoldings at paranodes. Two Mtmr13-deficient mouse models have been generated, both of which led to a loss of MTMR13 expression and reproduced the CMT4B2 phenotype, including myelin outfoldings and infoldings (Table 1) [43], [44]. As in Mtmr2-deficient mice, myelin outfoldings were found near nodes of Ranvier and increased in number and complexity with age [43], [44]. Double Mtmr2/Mtmr13-deficient mice presented no additional or more severe phenotype, suggesting MTMR13 regulates MTMR2 [43] and is consistent with data showing a strong increase of MTMR2 phosphatase activity when MTMR2 and MTMR13 formed a complex [19], [45].
The Amphiphysin Family
The autosomal recessive form of CNM (ARCNM, OMIM 255200) normally presents with muscle weakness typically appearing in infancy or early childhood, although cases were reported to present strong hypotonia at birth [46], [47]. The mutated gene associated with some cases of ARCNM is BIN1, encoding amphiphysin 2 (Figure 1) [46]. Amphiphysins belong to the BAR protein family [48]. They play roles in membrane remodeling and are conserved between humans and Drosophila, with functional orthologs found in yeast. In mammals, two genes encode for amphiphysins; amphiphysin1 (Amph1) and amphiphysin2 (BIN1) (Figure 2). Amph1 is expressed in brain and is implicated in synaptic endocytosis. BIN1, initially described as a tumor suppressor via its c-myc binding domain, is ubiquitously expressed although different isoforms are formed by complex alternative splicing that appears tissue specific [36], [49]. The muscle-specific isoform of BIN1 (iso8) contains a phosphoinositides-binding domain, and plays a role in T-tubule biogenesis [50], [51]. Nevertheless, mutations leading to CNM are found in common domains of the numerous BIN1 isoforms. Auto-antibodies against AMPH1 lead to a rare auto-immune disease called Stiff-man syndrome, involving chronic muscle rigidity and episodic spasms, sometimes with a paraneoplastic origin [52].
Amphiphysin Drosophila Model
The fly model has been generated by disruption of the only gene encoding amphiphysin (Amph) in Drosophila [53]. Adult Amph-deficient flies displayed no synaptic vesicle defects but were flightless and sluggish. Loss of Amph resulted in severe disorganization and reduction of the T-tubules, similar to the triads defect observed in CNM patients with BIN1 mutations [36] and in Mtm1-deficient mice and mtm1-deficient Zebrafish, reinforcing the hypothesis that MTM1 and amphiphysin 2 work in the same pathway in skeletal muscle.
Amphiphysin C. elegans Model
AMPH-1 is the only amphiphysin in C. elegans. AMPH-1 directly interacts and colocalizes with the membrane tubulating ATPase RME-1 (EHD ortholog) on membrane tubules from recycling endosomes [54]. Similarly to RME-1 mutants, loss of AMPH-1 induced a defect in basolateral recycling in the polarized intestinal epithelium. AMPH-1 may function in part to recruit RME to membrane of recycling endosomes [54]. All together these data suggest that AMPH-1 is implicated in recycling, probably through membrane remodeling and RME-1 recruitment (Table S1).
Amphiphysin 2/BIN1 Mouse Models
Deletion of the exon 3 in Bin1 led to loss of protein in homozygous mice [55]. Bin1-null embryos developed normally but died perinatally, potentially because of occlusion of the ventricular chambers. Unexpectedly there was no demonstrable impact of BIN1 loss on apoptosis, actin cytoskeletal organization, endocytosis, or on the specialized phagocytic endocytosis. Cardiac myofibrils were loosely packed and disorganized, with defective sarcomere units, diffuse Z-lines, and no apparent A-bands. Skeletal muscle was not thoroughly analyzed [55]. Moreover, as in Drosophila, Bin1-deficient mice presented normal brain architecture and synaptic vesicles, reinforcing the non-essential function of BIN1 in brain.
Amphiphysin 1 Murine Model
Amph1-deficient mice have been created by deletion of exon 1 (Table 1) [56]. AMPH1 and BIN1 form heterodimers in the brain, and absence of AMPH1 resulted in a strong reduction in BIN1 level in brain but not skeletal muscle [56]. Conversely, the expression level of Amph1 was not altered in the brain of Bin1-deficient mice, suggesting that Bin1 is not essential for Amph1 stability and function in brain [55]. Amph1-deficient mice suffered severe uncontrollable seizures, leading to reduced viability with 50% of mice dying by 40 wk of age. A reduction in synaptic vesicle recycling efficiency and reduced recruitment of AP-2 (alpha adaptin) and clathrin to membranes were detected [56]. AMPH1 is localized to the tubulobulbar complex (TBC) in Sertoli cells, and absence of AMPH1 led to a reduced number of plasma membrane invaginations and a subsequent increase number of unreleased spermatids [57].
According to the different animal models generated for amphiphysins and other cell studies, AMPH1 and BIN1 appear to have specialized functions depending on their splice isoforms and tissues where they are expressed. AMPH1 and brain isoforms of BIN1 might be dedicated to endocytosis and/or recycling, and non-neuronal AMPH1 and BIN1 proteins play a major role in plasma membrane remodeling and trafficking (Tables 1 and S1).
The Dynamin Family
Although ubiquitous expression and the central role of DNM2 in endocytosis suggested an essential function in all tissues, DNM2 was found mutated in two tissue-specific human diseases: autosomal dominant centronuclear myopathy (ADCNM, OMIM 160150) and dominant intermediate Charcot-Marie-Tooth neuropathy (DI-CMTB, OMIM 606482) (Figure 1) [58], [59]. The autosomal dominant form of CNM is generally clinically mild and usually appears in adults with a diffuse weakness that is slowly progressive and may be accompanied by muscle hypertrophy. However the severity varies from mild late-onset to severe neonatal onset [58], [60]–[63]. DNM2 mutations are also linked to either DI-CMTB with intermediate conduction velocity and associated neutropenia or axonal CMT. How different mutations in the same gene cause two diseases affecting different tissues (peripheral nerve and muscles) remains unexplained.
Dynamins belong to a large family of GTPases involved in formation and fission of budding vesicles from the plasma membrane and Golgi [64], [65]. In humans, the dynamin family comprises DNM1, DNM2, and DNM3 (Figure 2). DNM2 is ubiquitously expressed, DNM1 is present mainly in brain, and DNM3 is detected in brain and testis. Specific isoforms of each dynamin have been linked to specific subcellular localization and functions [66], [67]. Via the Pleckstrin homology (PH) and proline rich domains (PRD) domains, DNM2 binds respectively to the phosphoinositides PtdIns(4,5)P2, and to Src-Homology-3 (SH3) domain proteins including amphiphysins.
Drosophila Dynamin Models
In Drosophila the shibire (shi) gene encodes for the ortholog of mammalian dynamins. At non-permissive temperatures, the shits1 and shits2 mutations prevent GTP hydrolysis and shibire flies display disrupted synaptic vesicle endocytosis and depleted synaptic terminal vesicles [68]–[70]. Synaptic vesicles were trapped at a “collared-pit” stage in membrane internalization, and inactivation of the GTPase domain abolished vesicle translocation [70]. Dynamin regulates endocytosis in a variety of tissues [69], [71]. In cardiac tissue, recessive shits1 and shits2 mutations exhibited bradychardia and arrhythmia because of defects in electrical communication throughout the myocardium [72].
Dynamin C. elegans Models
Dyn-1 is the unique dynamin expressed in C. elegans. Dyn-1(ky51) mutant worms had uncoordinated movement, kinked posture, and were sluggish at non-permissive temperatures [73]. This is consistent with the enrichment of Dyn-1 in motor neurons that innervate bodywall muscles and head musculature, required respectively for locomotion and head movement [73]. Dyn-1 concentration in the nerve ring indicated a role in synaptic vesicle recycling [74]. In non-neuronal cells, Dyn-1 defects in coelomocytes led to decreased fluid-phase endocytosis. Dyn-1 (n4039) worms revealed that dynamin also has a role in engulfment and degradation of apoptotic cells during embryogenesis [75]. Interestingly, human DNM2 could perform a similar function to Dyn-1 in C. elegans [75]. These results suggest that dynamin proteins can have similar functions when expressed in the same tissue. In gonadal sheath cells, absence of Dyn-1 led to accumulation of enlarged vesicles, vesicles interconnected via lipid tubules, and plasma membrane-connected vesicles, all suggesting defects in fission [75]. A role for Dyn-1 in membrane transport has also been characterized for the maintenance of anterior polarity in C. elegans embryos (Table S1) [76].
Dynamin 1 Mammalian Models
Dnm1-deficient mice were indistinguishable from littermates at birth, however decreased ingestion of milk and poor motor coordination were detected, and mice usually died within 2 wk [77]. Analyses of the structure and function of synapses from Dnm1-deficient mice highlighted that DNM1 was dispensable for endocytic recycling of synaptic vesicles under basal conditions but became essential when intense stimulus imposed a heavy load of endocytosis [77]. Expression of DNM2 or DNM3 rescued partially or efficiently, respectively, the endocytic blockade in DNM1-defective cultured neurons, indicating that all dynamins could participate in synaptic vesicle endocytosis. This was recently confirmed in DNM1/DNM3 double KO [78].
An homozygous Arg256Leu DNM1 mutation was identified in Labrador retrievers affected with exercise-induced collapse [79]. A collapse episode lasts 5–10 min and complete recovery usually occurs after 30 min. Arg256Leu affects an amino acid conserved in DNM2 and DNM3, which is located at the boundary between the GTPase and the MID domains [79]. Similar to Drosophila and C. elegans temperature-sensitive phenotypes, the mutation in canine DNM1 may lead to a defect in synaptic vesicle recycling that sustains synaptic transmission during intensive exercise (Table S1).
Dynamin 2 Murine Models
Dnm2 KO mice died before embryonic day 10 [80]. In double Dnm1/Dnm2 null fibroblasts, clathrin-mediated endocytosis is arrested and endocytotic intermediates accumulated in the cytoplasm [80]. Heterozygous R465W-DNM2 knock-in mice, the most common mutation causing ADCNM, exhibited a fairly mild myopathic phenotype, with abnormalities in mitochondrial and reticular networks [81]. However homozygous mice died hours after birth, possibly owing to clathrin-mediated signaling defects, correlating with the above results from Dnm1/Dnm2 KO cells. A second model was created by intramuscular adeno-associated virus (AAV) injections of R465W-DNM2 into adult wild-type muscle. Mice exhibited muscle weakness associated with disruptions of the mitochondrial and T-tubule networks [82]. This model reproduced most ADCNM features in mice, and was akin to ADCNM patient biopsies [36]. These models provide evidence that ADCNM arises primarily from defects in skeletal muscle rather than peripheral nerves, and that dynamin 2 is crucial in the maintenance of adult muscle fiber structure and function.
A Common Molecular Mechanism?
Myotubularins are phosphatases regulating the level of PIs, amphiphysins can bend membranes, and dynamins oligomerize around the neck of vesicles to promote tubulation and eventually membrane fission. All these proteins fit in a membrane remodeling pathway (Figure 3) and are associated with myopathies and peripheral neuropathies or neurological syndromes. It is not yet clear if mutations causing more severely defective proteins correlate with earlier onset, or more severe forms of the disease, nor is it clear if the location of mutations within genes gives rise to tissue-specific disorders. A more detailed analysis of the impact of mutations on the protein functions is needed to answer these points. The above pathological pathway may also be expanded to include other genes mutated in CMT and other myopathies. This could include several genes mutated in different forms of CMT (e.g., NEFL, RAB7A, FGD4, FIG4, SH3TC2, LITAF/SIMPLE) (reviewed in [83]) encoding proteins regulating PIs metabolism and membrane trafficking; and myopathies involving mutations in caveolin 3 and dysferlin genes, which also lead to common defects in T-tubules and membrane transport in patients and animal models (Figure 3) [84], . While mutations in several membrane trafficking proteins described here are associated with neuromuscular disorders, in contrast mutations affecting normal lysosomal function induce a separate category of human diseases termed lysosomal storage diseases (LSD), which affect several tissues, most commonly causing progressive neurodegeneration. We propose a hypothesis to explain how different protein families are implicated in similar diseases while, conversely, the same protein families are associated with different neuromuscular diseases (Figure 3; Table 1).
Fig. 3. Cellular functions of myotubularins, amphiphysins, and dynamins implicated in human diseases and their related pathological mechanisms. 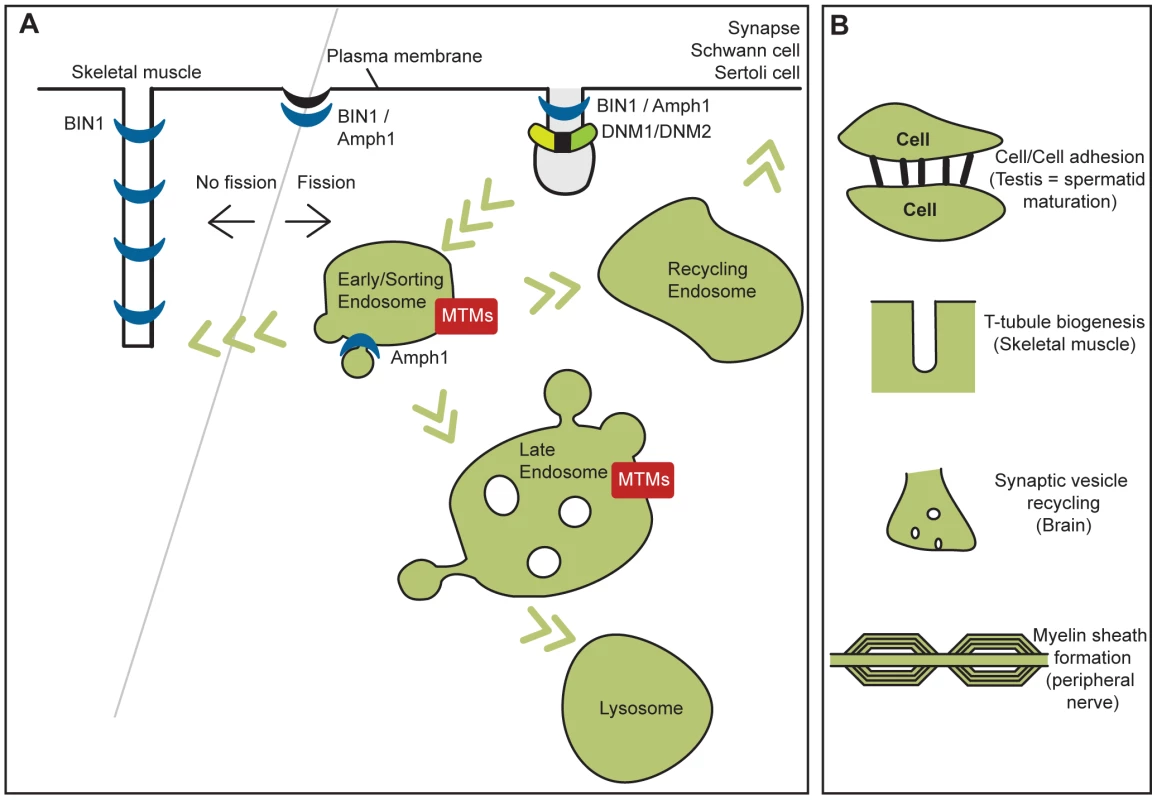
(A) Human diseases, (B) their related pathological mechanisms. Membrane fission is necessary for vesicle formation and subsequent trafficking, while inhibition of membrane fission or membrane addition at the T-tubules in muscle may be necessary for their formation and maintenance. Neurological Functions
These proteins could coordinate vesicle formation and endocytosis and recycling. Schwann cells require large amounts of plasma membrane to wrap around axons and may be more susceptible than other cell types to membrane transport defects. This pathway is also important at the synapse that requires very rapid trafficking and recycling through membrane fission, transport, and fusion. Amphiphysins and dynamins are well-known interactors and regulators of endocytosis, and dynamins are excellent candidates for membrane fission. Indeed dynamin inhibition leads to decreased separation of vesicles from the plasma membrane [70]. Several myotubularins have been implicated in endocytosis, through regulation of PtdIns3P and PtdIns(3,5)P2 levels in Drosophila, C. elegans, zebrafish, and mice models, and these PIs were shown to be key players in both endocytosis and exocytosis [8], [9]. Such membrane trafficking is implicated in synaptic vesicle recycling in brain, and in recycling of adhesion molecules that may be important for cell–cell contact and myelin sheath formation or maintenance. Defects in these pathways could explain the hallmarks of neurological syndromes and neuropathies associated with mutations in these protein families.
Skeletal Muscle Functions
In skeletal muscle, MTM1, BIN1, and DNM2 may coordinate the formation and/or organization of T-tubule/triad structures (Figure 3). Following induction of membrane curvature, the invagination may become elongated to form a T-tubule. BIN1 is highly expressed in skeletal muscle and was implicated in T-tubule biogenesis in Drosophila and muscle cells [51], [53], and T-tubule defects are seen in zebrafish and mouse animal models, and in CNM patients with mutations in these three proteins [36]. BIN1 contains a phosphoinositide-binding domain present only in the muscle isoform indicating PIs are involved in this process, however their role and the function of MTM1 in this process remain to be determined. During T-tubule biogenesis and maintenance, additional membrane may be acquired through fusion of endocytic vesicles (similar to the process proposed for Schwann cells in peripheral nerves); defective endocytosis and recycling may therefore contribute to the phenotype observed in patient muscle.
Tissue-Specific Function
Whilst MTM1 and MTMR2 have very similar sequences (90% identity) and ubiquitous expression, mutations induce two different diseases, highlighting that myotubularin functions are not redundant, and isoforms may perform tissue-specific functions. The membrane remodeling pathway may have different functions in different tissues, and mutations may affect different protein functions. Unlike the myotubularin family, for dynamin 2 the same gene is mutated in two tissue-specific disorders. The tissue-specific function may thus be dictated by the ability of dynamins to promote membrane fission or not. Inhibition or slow GTPase hydrolysis would favor membrane tubules, and fast GTP hydrolysis would lead to fission and vesicle formation. Inhibition of membrane fission will lead to the neurological and neuropathy phenotypes while increased fission may prevent formation of T-tubules and lead to muscle contraction defects. Supporting this theory DNM2 mutations causing CNM have been shown to have increased oligomer stability and GTPase activity [86], [87]. However in contrast to BIN1, DNM2 was not observed at T-tubules to date. Regulation of this balance can be due to tissue-specific isoforms, interactors, or post-translational regulation as most of these proteins are ubiquitous.
Conclusions and Pending Issues
Animal models were instrumental to decipher the cellular and physiological functions of the implicated proteins and will be an asset to further characterize the underlying pathological mechanisms and test therapeutic approaches. Myotubularins, amphiphysins, and dynamins play a key role in membrane remodeling. Mammalian models could now be used to follow the dynamics of membrane remodeling and trafficking in the affected tissues and to characterize the tissue-specific regulation of the membrane fission process. Phosphoinositides associated with these proteins are involved in membrane trafficking; however, the molecular link between phosphoinositides and membrane remodeling and how this relates to T-tubule formation and myelin maintenance remain to be deciphered.
A glossary and useful links can be found in Text S1.
Supporting Information
Zdroje
1. De MatteisMALuiniA 2011 Mendelian disorders of membrane trafficking. N Engl J Med 365 927 938
2. van WijngaardenGKFleuryPBethlemJMeijerAE 1969 Familial “myotubular” myopathy. Neurology 19 901 908
3. LaporteJHuLJKretzCMandelJLKioschisP 1996 A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13 175 182
4. SenderekJBergmannCWeberSKetelsenUPSchorleH 2003 Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum Mol Genet 12 349 356
5. AzzedineHBolinoATaiebTBiroukNDi DucaM 2003 Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-ooth disease associated with early-onset glaucoma. Am J Hum Genet 72 1141 1153
6. BolinoAMugliaMConfortiFLLeGuernESalihMA 2000 Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet 25 17 19
7. SuterUSchererS 2003 Disease mechanisms in inherited neuropathies. Nat Rev Neurosci 4 714 726
8. NicotASLaporteJ 2008 Endosomal phosphoinositides and human diseases. Traffic 9 1240 1249
9. Di PaoloGDe CamilliP 2006 Phosphoinositides in cell regulation and membrane dynamics. Nature 443 651 657
10. VicinanzaMD'AngeloGDi CampliADe MatteisMA 2008 Phosphoinositides as regulators of membrane trafficking in health and disease. Cell Mol Life Sci 65 2833 2841
11. LaporteJBedezFBolinoAMandelJ-L 2003 Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum Mol Genet 12 285 292
12. BegleyMJDixonJE 2005 The structure and regulation of myotubularin phosphatases. Curr Opin Struct Biol 15 614 620
13. CuiXDe VivoISlanyRMiyamotoAFiresteinR 1998 Association of SET domain and myotubularin-related proteins modulates growth control. Nat Genet 18 331 337
14. LaporteJBlondeauFBuj-BelloATentlerDKretzC 1998 Characterization of the myotubularin dual specificity phosphatase gene family from yeast to human. Hum Mol Genet 7 1703 1712
15. ZouJChangSMarjanovicJMajerusP 2009 MTMR9 increases MTMR6 enzyme activity, stability, and role in apoptosis. J Biol Chem 2009 284 2064 2071
16. NandurkarHHLaytonMLaporteJSelanCCorcoranL 2003 Identification of myotubularin as the lipid phosphatase catalytic subunit associated with the 3-phosphatase adapter protein, 3-PAP. Proc Natl Acad Sci U S A 100 8660 8665
17. MochizukiYMajerusPW 2003 Characterization of myotubularin-related protein 7 and its binding partner, myotubularin-related protein 9. Proc Natl Acad Sci U S A 100 9768 9773
18. KimSAVacratsisPOFiresteinRClearyMLDixonJE 2003 Regulation of myotubularin-related (MTMR)2 phosphatidylinositol phosphatase by MTMR5, a catalytically inactive phosphatase. Proc Natl Acad Sci U S A 100 4492 4497
19. RobinsonFLDixonJE 2005 The phosphoinositide-3-phosphatase MTMR2 associates with MTMR13, a membrane-associated pseudophosphatase also mutated in type 4B Charcot-Marie-Tooth disease. J Biol Chem 280 31699 31707
20. VelichkovaMJuanJKadandalePJeanSRibeiroI 2010 Drosophila Mtm and class II PI3K coregulate a PI(3)P pool with cortical and endolysosomal functions. J Cell Biol 190 407 425
21. RibeiroIYuanLTanentzapfGDowlingJJKigerA 2011 Phosphoinositide regulation of integrin trafficking required for muscle attachment and maintenance. PLoS Genet 7 e1001295 doi:10.1371/journal.pgen.1001295
22. LaporteJBlondeauFBuj-BelloAMandelJL 2001 The myotubularin family: from genetic disease to phosphoinositide metabolism. Trends Genet 17 221 228
23. WishartMJTaylorGSSlamaJTDixonJE 2001 PTEN and myotubularin phosphoinositide phosphatases: bringing bioinformatics to the lab bench. Curr Opin Cell Biol 13 172 181
24. XueYFaresHGrantBLiZRoseAM 2003 Genetic analysis of the myotubularin family of phosphatases in caenorhabditis elegans. J Biol Chem 278 34380 34386
25. FaresHGreenwaldI 2001 Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics 159 133 145
26. DangHLiZSkolnikEYFaresH 2004 Disease-related myotubularins function in endocytic traffic in Caenorhabditis elegans. Mol Biol Cell 15 189 196
27. SilhankovaMPortFHarterinkMBaslerKKorswagenHC 2010 Wnt signalling requires MTM-6 and MTM-9 myotubularin lipid-phosphatase function in Wnt-producing cells. EMBO J 29 4094 4105
28. ZouWLuQZhaoDLiWMapesJ 2009 Caenorhabditis elegans myotubularin MTM-1 negatively regulates the engulfment of apoptotic cells. PLoS Genet 5 e1000679 doi:10.1371/journal.pgen.1000679
29. NeukommLJNicotASKinchenJMAlmendingerJPintoSM 2011 The phosphoinositide phosphatase MTM-1 regulates apoptotic cell corpse clearance through CED-5-CED-12 in C. elegans. Development 138 2003 2014
30. DowlingJJVreedeAPLowSEGibbsEMKuwadaJY 2009 Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet 5 e1000372 doi:10.1371/journal.pgen.1000372
31. RobbSASewryCADowlingJJFengLCullupT 2011 Impaired neuromuscular transmission and response to acetylcholinesterase inhibitors in centronuclear myopathies. Neuromuscul Disord 21 379 386
32. ShenJYuWMBrottoMSchermanJAGuoC 2009 Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca(2+) homeostasis. Nat Cell Biol 11 769 776
33. HniaKKretzCAmoasiiLBohmJLiuX 2011 Primary T-tubule and autophagy defects in the phosphoinositide phosphatase Jumpy/MTMR14 knockout mice muscle. Adv Enzyme Regul In press
34. Buj-BelloALaugelVMessaddeqNZahreddineHLaporteJ 2002 The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci U S A 99 15060 15065
35. Al-QusairiLWeissNToussaintABerbeyCMessaddeqN 2009 T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc Natl Acad Sci U S A 106 18763 18768
36. ToussaintACowlingBSHniaKMohrMOldforsA 2011 Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol 121 253 266
37. BeggsAHBohmJSneadEKozlowskiMMaurerM 2010 MTM1 mutation associated with X-linked myotubular myopathy in Labrador Retrievers. Proc Natl Acad Sci U S A 107 14697 14702
38. HniaKTronchereHTomczakKKAmoasiiLSchultzP 2011 Myotubularin controls desmin intermediate filament architecture and mitochondrial dynamics in human and mouse skeletal muscle. J Clin Invest 121 70 85
39. Buj-BelloAFougerousseFSchwabYMessaddeqNSpehnerD 2008 AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis. Hum Mol Genet 17 2132 2143
40. BolinoABolisAPrevitaliSCDinaGBussiniS 2004 Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J Cell Biol 167 711 721
41. BolisACovielloSBussiniSDinaGPardiniC 2005 Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings. J Neurosci 25 8567 8577
42. BonneickSBoentertMBergerPAtanasoskiSManteiN 2005 An animal model for Charcot-Marie-Tooth disease type 4B1. Hum Mol Genet 14 3685 3695
43. TersarKBoentertMBergerPBonneickSWessigC 2007 Mtmr13/Sbf2-deficient mice: an animal model for CMT4B2. Hum Mol Genet 16 2991 3001
44. RobinsonFLNiesmanIRBeiswengerKKDixonJE 2008 Loss of the inactive myotubularin-related phosphatase Mtmr13 leads to a Charcot-Marie-Tooth 4B2-like peripheral neuropathy in mice. Proc Natl Acad Sci U S A 105 4916 4921
45. BergerPBergerISchaffitzelCTersarKVolkmerB 2006 Multi-level regulation of myotubularin-related protein-2 phosphatase activity by myotubularin-related protein-13/set-binding factor-2. Hum Mol Genet 15 569 579
46. NicotASToussaintAToschVKretzCWallgren-PetterssonC 2007 Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet 39 1134 1139
47. BohmJYisUOrtacRCakmakciHKurulSH 2010 Case report of intrafamilial variability in autosomal recessive centronuclear myopathy associated to a novel BIN1 stop mutation. Orphanet J Rare Dis 5 35
48. PeterBJKentHMMillsIGVallisYButlerPJ 2004 BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303 495 499
49. Wechsler-ReyaRSakamuroDZhangJDuhadawayJPrendergastGC 1997 Structural analysis of the human BIN1 gene. Evidence for tissue-specific transcriptional regulation and alternate RNA splicing. J Biol Chem 272 31453 31458
50. Wechsler-ReyaRJElliottKJPrendergastGC 1998 A role for the putative tumor suppressor Bin1 in muscle cell differentiation. Mol Cell Biol 18 566 575
51. LeeEMarcucciMDaniellLPypaertMWeiszOA 2002 Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297 1193 1196
52. De CamilliPThomasACofiellRFolliFLichteB 1993 The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. J Exp Med 178 2219 2223
53. RazzaqARobinsonIMMcMahonHTSkepperJNSuY 2001 Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev 15 2967 2979
54. PantSSharmaMPatelKCaplanSCarrC 2009 AMPH-1/Amphiphysin/Bin1 functions with RME-1/Ehd1 in endocytic recycling. Nat Cell Biol 11 1399 1410
55. MullerAJBakerJFDuHadawayJBGeKFarmerG 2003 Targeted disruption of the murine Bin1/Amphiphysin II gene does not disable endocytosis but results in embryonic cardiomyopathy with aberrant myofibril formation. Mol Cell Biol 23 4295 4306
56. Di PaoloGSankaranarayananSWenkMRDaniellLPeruccoE 2002 Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron 33 789 804
57. KusumiNWatanabeMYamadaHLiSAKashiwakuraY 2007 Implication of amphiphysin 1 and dynamin 2 in tubulobulbar complex formation and spermatid release. Cell Struct Funct 32 101 113
58. BitounMMaugenreSJeannetPYLaceneEFerrerX 2005 Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet 37 1207 1209
59. ZuchnerSNoureddineMKennersonMVerhoevenKClaeysK 2005 Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet 37 289 294
60. BitounMBevilacquaJAEymardBPrudhonBFardeauM 2009 A new centronuclear myopathy phenotype due to a novel dynamin 2 mutation. Neurology 72 93 95
61. BitounMBevilacquaJAPrudhonBMaugenreSTaratutoAL 2007 Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol 62 666 670
62. Echaniz-LagunaANicotASCarreSFranquesJTranchantC 2007 Subtle central and peripheral nervous system abnormalities in a family with centronuclear myopathy and a novel dynamin 2 gene mutation. Neuromuscul Disord 17 955 959
63. SchesslJMedneLHuYZouYBrownMJ 2007 MRI in DNM2-related centronuclear myopathy: evidence for highly selective muscle involvement. Neuromuscul Disord 17 28 32
64. PraefckeGJMcMahonHT 2004 The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5 133 147
65. JonesSMHowellKEHenleyJRCaoHMcNivenMA 1998 Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science 279 573 577
66. McNivenMACaoHPittsKRYoonY 2000 The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem Sci 25 115 120
67. DurieuxACPrudhonBGuicheneyPBitounM 2010 Dynamin 2 and human diseases. J Mol Med 88 339 350
68. RamaswamiMKrishnanKRB.K 1994 Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron 13 363 375
69. KosakaTIkedaK 1983 Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1. J Cell Biol 97 499 507
70. EstesPSRoosJvan der BliekAKellyRBKrishnanKS 1996 Traffic of dynamin within individual Drosophila synaptic boutons relative to compartment-specific markers. J Neurosci 16 5443 5456
71. TsuruharaTKoenigJIkedaK 1990 Synchronized endocytosis studied in the oocyte of a temperature-sensitive mutant of Drosophila melanogaster. Cell Tissue Res 259 199 207
72. JohnsonERingoJDowseH 2001 Dynamin, encoded by shibire, is central to cardiac function. J Exp Zool 289 81 89
73. ClarkSGShurlandDLMeyerowitzEMBargmannCIvan der BliekAM 1997 A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proc Natl Acad Sci U S A 94 10438 10443
74. LabrousseAShurlandDvan der BliekA 1998 Contribution of the GTPase domain to the subcellular localization of dynamin in the nematode Caenorhabditis elegans. Mol Biol Cell 9 3227 3239
75. YuXOderaSChuangCHLuNZhouZ 2006 C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev Cell 10 743 757
76. NakayamaYShivasJPooleDSquirrellJKulkoskiJ 2009 Dynamin participates in the maintenance of anterior polarity in the Caenorhabditis elegans embryo. Dev Cell 16 889 900
77. FergusonSMBrasnjoGHayashiMWolfelMCollesiC 2007 A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316 570 574
78. RaimondiAFergusonSMLouXArmbrusterMParadiseS 2011 Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron 70 1100 1114
79. PattersonEEMinorKMTchernatynskaiaAVTaylorSMSheltonGD 2008 A canine DNM1 mutation is highly associated with the syndrome of exercise-induced collapse. Nat Genet 40 1235 1239
80. FergusonSMRaimondiAParadiseSShenHMesakiK 2009 Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17 811 822
81. DurieuxACVignaudAPrudhonBViouMTBeuvinM 2010 A centronuclear myopathy-dynamin 2 mutation impairs skeletal muscle structure and function in mice. Hum Mol Genet 19 4820 4836
82. CowlingBSToussaintAAmoasiiLKoebelPFerryA 2011 Increased expression of wild-type or a centronuclear myopathy mutant of dynamin 2 in skeletal muscle of adult mice leads to structural defects and muscle weakness. Am J Pathol 178 2224 2235
83. ReillyMMMurphySMLauraM 2011 Charcot-Marie-Tooth disease. J Peripher Nerv Syst 16 1 14
84. Hernandez-DeviezDJMartinSLavalSHLoHPCooperST 2006 Aberrant dysferlin trafficking in cells lacking caveolin or expressing dystrophy mutants of caveolin-3. Hum Mol Genet 15 129 142
85. KlingeLHarrisJSewryCCharltonRAndersonL 2010 Dysferlin associates with the developing T-tubule system in rodent and human skeletal muscle. Muscle Nerve 41 166 173
86. WangLBarylkoBByersCRossJAJamesonDM 2010 Dynamin 2 mutants linked to centronuclear myopathies form abnormally sTable polymers. J Biol Chem 285 22753 22757
87. KennistonJALemmonMA 2010 Dynamin GTPase regulation is altered by PH domain mutations found in centronuclear myopathy patients. Embo J 29 3054 3067
88. OwenDJWiggePVallisYMooreJDEvansPR 1998 Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation. Embo J 17 5273 5285
89. BolinoAMarigoVFerreraFLoaderJRomioL 2002 Molecular characterization and expression analysis of Mtmr2, mouse homologue of MTMR2, the Myotubularin-related 2 gene, mutated in CMT4B. Gene 283 17 26
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



