-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
In female fruit flies, Sex-lethal (Sxl) turns off the X chromosome dosage compensation system by a mechanism involving a combination of alternative splicing and translational repression of the male specific lethal-2 (msl-2) mRNA. A genetic screen identified the translation initiation factor eif4e as a gene that acts together with Sxl to repress expression of the Msl-2 protein. However, eif4e is not required for Sxl mediated repression of msl-2 mRNA translation. Instead, eif4e functions as a co-factor in Sxl-dependent female-specific alternative splicing of msl-2 and also Sxl pre-mRNAs. Like other factors required for Sxl regulation of splicing, eif4e shows maternal-effect female-lethal interactions with Sxl. This female lethality can be enhanced by mutations in other co-factors that promote female-specific splicing and is caused by a failure to properly activate the Sxl-positive autoregulatory feedback loop in early embryos. In this feedback loop Sxl proteins promote their own synthesis by directing the female-specific alternative splicing of Sxl-Pm pre-mRNAs. Analysis of pre-mRNA splicing when eif4e activity is compromised demonstrates that Sxl-dependent female-specific splicing of both Sxl-Pm and msl-2 pre-mRNAs requires eif4e activity. Consistent with a direct involvement in Sxl-dependent alternative splicing, eIF4E is associated with unspliced Sxl-Pm pre-mRNAs and is found in complexes that contain early acting splicing factors—the U1/U2 snRNP protein Sans-fils (Snf), the U1 snRNP protein U1-70k, U2AF38, U2AF50, and the Wilms' Tumor 1 Associated Protein Fl(2)d—that have been directly implicated in Sxl splicing regulation.
Published in the journal: . PLoS Genet 7(7): e32767. doi:10.1371/journal.pgen.1002185
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002185Summary
In female fruit flies, Sex-lethal (Sxl) turns off the X chromosome dosage compensation system by a mechanism involving a combination of alternative splicing and translational repression of the male specific lethal-2 (msl-2) mRNA. A genetic screen identified the translation initiation factor eif4e as a gene that acts together with Sxl to repress expression of the Msl-2 protein. However, eif4e is not required for Sxl mediated repression of msl-2 mRNA translation. Instead, eif4e functions as a co-factor in Sxl-dependent female-specific alternative splicing of msl-2 and also Sxl pre-mRNAs. Like other factors required for Sxl regulation of splicing, eif4e shows maternal-effect female-lethal interactions with Sxl. This female lethality can be enhanced by mutations in other co-factors that promote female-specific splicing and is caused by a failure to properly activate the Sxl-positive autoregulatory feedback loop in early embryos. In this feedback loop Sxl proteins promote their own synthesis by directing the female-specific alternative splicing of Sxl-Pm pre-mRNAs. Analysis of pre-mRNA splicing when eif4e activity is compromised demonstrates that Sxl-dependent female-specific splicing of both Sxl-Pm and msl-2 pre-mRNAs requires eif4e activity. Consistent with a direct involvement in Sxl-dependent alternative splicing, eIF4E is associated with unspliced Sxl-Pm pre-mRNAs and is found in complexes that contain early acting splicing factors—the U1/U2 snRNP protein Sans-fils (Snf), the U1 snRNP protein U1-70k, U2AF38, U2AF50, and the Wilms' Tumor 1 Associated Protein Fl(2)d—that have been directly implicated in Sxl splicing regulation.
Introduction
Translation initiation is mediated by the binding of a pre-initiation complex to the 5′ cap of the mRNA (reviewed in [1], [2]) that in turn recruits the small subunit of the 40S ribosome to the mRNA. The pre-initiation complex consists of the cap binding protein, eIF4E, and a scaffolding protein, eIF4G, which mediates interactions with various components of the 40S initiation complex. In many organisms there is also a third protein in the complex, eIF4A, an ATP dependent RNA helicase. Modulating eIF4E activity appears to be a key control point for regulating translation. One of the most common mechanisms of regulation is by controlling the association eIF4E with eIF4G. Factors such as poly-A binding protein that promote the association between eIF4E and eIF4G activate translation initiation, while factors such as the 4E-binding proteins (4E-BPs) that block their association, inhibit initiation [3], [4].
Although eIF4E's primary function in the cell is in regulating translation initiation, studies over the past decade have revealed unexpected activities for eIF4E at steps prior to translation. Among the more surprising findings is that there are substantial amounts of eIF4E in eukaryotic nuclei [5]–[9]. One role for eIF4E in the nucleus is the transport of specific mRNAs, like cyclin D1, to the cytoplasm [10]. This eIF4E activity is distinct from translation initiation since an eIF4E mutation that prevents it from forming an active translation complex still allows cyclin D1 mRNA transport [8]. The transport function of eIF4E is modulated by at least two other proteins, PML and PRH [11], [12]. While PML seems to be ubiquitously expressed, PRH is found only in specific tissues [13]. In addition, the intracellular distribution of eIF4E exhibits dynamic changes during Xenopus development [9]. These observation raise the possibility that eIF4E might have additional functions in the nucleus during development. Consistent with this idea, we show here that eIF4E plays a novel role in the process of sex determination in Drosophila melanogaster.
Sex determination in the fly is controlled by the master regulatory switch gene Sex-lethal (Sxl) (reviewed in [14]–[16]). The activity state of the Sxl gene is selected early in development by an X chromosome counting system. The target for the X/A signaling system is the Sxl establishment promoter, Sxl-Pe [17]. When there are two X chromosomes, Sxl-Pe is turned on, while it remains off when there is a single X chromosome. Sxl-Pe mRNAs encode RRM type RNA binding proteins which mediate the transition from the initiation to the maintenance mode of Sxl regulation by directing the female-specific splicing of the first pre-mRNAs produced from a second, upstream promoter, the maintenance promoter, Sxl-Pm [18], [19]. Sxl-Pm is turned on before the blastoderm cellularizes, just as Sxl-Pe is being shut off. In the presence of Sxl-Pe proteins, the first Sxl-Pm transcripts are spliced in the female-specific pattern in which exon 2 is joined to exon 4 (see Figure 1A). The resulting Sxl-Pm mRNAs encode Sxl proteins that direct the female specific splicing of new Sxl-Pm pre-mRNAs and this establishes a positive autoregulatory feedback loop that maintains the Sxl gene in the “on” state for the remainder of development. In male embryos, which lack the Sxl-Pe proteins, the Sxl-Pm pre-mRNAs are spliced in the default pattern, incorporating the male specific exon 3 (Figure 1A). This exon has several in-frame stop codons that prematurely truncate the open reading frame so that male specific Sxl-Pm mRNAs produce only small non-functional polypeptides. As a consequence the Sxl gene remains off throughout development in males.
Fig. 1. Sx-N protein can repress the translation of endogenous Sxl-Pm mRNAs in an eif4e mutant background. 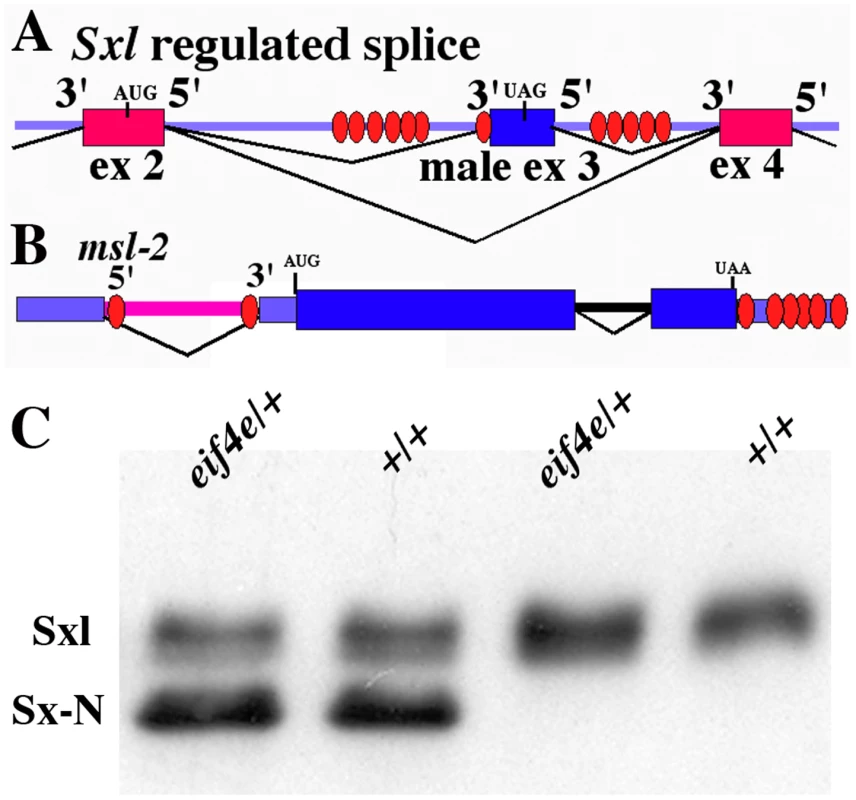
A) Model of the alternatively spliced region of Sxl (exons 2, 3 and 4). Sxl binding sites are shown as ovals. In males exon2 (ex2) is joined to exon3 (ex3) which is in turn joined to exon4 (ex4). The stop codon within exon 3 causes male transcripts to produce a truncated protein. In females Sxl protein prevents inclusion of exon3, and exon2 is joined directly to exon4. B) Model of the msl-2 gene. The Sxl binding sites are shown as ovals. In males the intron in the 5′UTR that contains the two Sxl sites is spliced out by the default splicing machinery. In females Sxl protein blocks the splicing of the 5′UTR intron and the two Sxl sites in the intron are retained. Binding of Sxl to these two sites and sites in the 3′UTR represses translation of msl-2 mRNA. C) Western blot of Sxl proteins from eif4e/+ hsp83:Sx-NΔ transgene females (lane 1), +/+ hsp83:Sx-NΔ transgene females (lane 2), eif4e/+ (lane 3) and +/+ (lane 4) females. The presence or absence of the eif4e mutation is indicated above each lane. Levels of both Sx-N protein and endogenous Sxl protein are unaffected by the presence of the eif4e mutation. In females, Sxl orchestrates sexual development by regulating the alternative splicing of transformer (tra) pre-mRNAs [20]–[23]. Like Sxl, functional Tra protein is only produced by female-specific tra mRNAs, while mRNAs spliced in the default, male pattern encode non-functional polypeptides. Sxl also negatively regulates the dosage compensation system, which is responsible for hyperactivating X-linked transcription in males, by repressing male-specific lethal-2 (msl-2). Sxl represses msl-2 by first blocking the splicing of an intron in the 5′ UTR of the msl-2 pre-mRNA (see Figure 1B), and then by inhibiting the translation of the mature mRNA [24]–[31]. In addition, there are two other known targets for Sxl translational repression. One is the Sxl mRNA itself. Sxl binds to target sequences in the Sxl 5′ and 3′ UTRs and downregulates translation. It is thought that this negative autoregulatory activity provides a critical homeostasis mechanism that prevents the accumulation of excess Sxl protein. This is important as too much Sxl can disrupt development and have female lethal effects [32]. The other known target is the Notch (N) mRNA [33]. Sxl-dependent repression of N mRNA translation is important for the elaboration of sexually dimorphic traits in females. Like msl-2 and Sxl, translational repression appears to be mediated by Sxl binding to sites in the N UTRs.
Translational repression of msl-2 mRNA by Sxl is thought to involve two separate mechanisms acting coordinately. Binding sites for Sxl in the unspliced intron in the 5′ UTR and in the 3′UTR of msl-2 are required for complete repression [25], [26]. Sxl binding to the 5′UTR blocks recruitment of the 40S pre-initiation complex [31], [34]. While factors that act with Sxl at the 5′UTR of msl-2 have yet to be identified, repression by the 3′UTR requires Sxl, PABP and a co-repressor UNR [35]–[37]. Somewhat unexpectedly, this complex does not affect recruitment of eIF4E or eIF4G to the 5′ end. Instead it prevents ribosomes that do manage to attach to the msl-2 mRNA from scanning [31], [38].
Although eIF4E does not appear to be a key player in the translational repression of msl-2 mRNAs, we report here that it has an important role in the process of sex determination in Drosophila. We find that eIF4E activity is required in females to stably activate and maintain the Sxl positive autoregulatory feedback loop and to efficiently repress msl-2. Surprisingly, this requirement for eIF4E activity in fly sex determination is in promoting the female-specific splicing of the Sxl and msl-2 transcripts, not in translational regulation.
Results
Mutations in eif4e rescue males expressing a Sxl transgene
In previous studies we examined the biological properties of a truncated Sxl protein, Sx-N, that contains both RRM RNA binding domains, but is missing 40 amino acids from the N-terminus [39]. We found that the splicing activity of Sx-N is impaired; it can not direct the female-specific splicing of tra and has substantially reduced autoregulatory activity. However, the truncated protein is able to inhibit the translation of msl-2 mRNA and kills males even in the absence of a wild type Sxl gene. As would be expected if the male lethal effects of Sx-N are due to repression of msl-2 mRNA translation, hsp83:Sx-NΔ males can be fully rescued by an hsp83:msl-2 transgene that lacks the Sxl binding sites in the 5′ and 3′ UTRs.
With the aim of discovering factors important for Sxl dependent repression of msl-2 we screened for deletions that dominantly suppress the male lethal effects (in a Sxl− background) of a transgene, hsp83:Sx-NΔ, that constitutively expresses the truncated Sx-N protein. We then identified the interacting locus by testing mutations mapping to the suppressing deletion. We anticipated that genes recovered in this screen would fall into two general classes. In the first would be genes required for efficient expression of Sx-N by the transgene. Consistent with this expectation, one of the suppressing mutations was the heat shock factor, hsf. Genes in the second class would be required for efficient repression of msl-2 by the truncated Sx-N protein. In this group we expected to find factors required by Sxl to inhibit msl-2 translation; however, since the Sxl binding sites in the msl-2 5′ UTR intron are needed to completely repress translation, we anticipated that we might also recover genes that collaborate with Sxl to block the removal of this intron [25], [26], [28], [31].
One of the candidate translation factors recovered in the screen was the eif4e gene, which encodes the cap binding protein. Three independent alleles of eif4e were tested. In an otherwise wild type background less than one in 103 Sxl− males carrying the hsp83:Sx-NΔ transgene survive. By contrast, when the hsp83:Sx-NΔ; Sxl− males were also heterozygous for an eif4e mutation, between 2% and 9% of the transgenic males survived depending upon the allele.
eif4e mutations do not impair the negative autoregulatory activity of the Sx-N protein
Since Sxl-dependent repression of msl-2 translation in vitro is independent of the cap and does not seem to be mediated through interactions with eIF4E [34], [38], it was surprising that eif4e was recovered in our screen. However, it seemed possible that an in vivo requirement for eif4e activity might be bypassed in in vitro translation systems. In this case, the levels of Msl-2 should increase in hsp83:Sx-NΔ transgene males when they are heterozygous for one of the eif4e mutations. However, testing whether eif4e mutations perturb Sx-N dependent translational repression of msl-2 mRNA in adults or at earlier stages of development is complicated by the male-lethal effects of the truncated Sxl protein.
To circumvent this complication, we tested the effects eif4e on Sxl negative autoregulation as this can be done in females where Sx-N doesn't have such deleterious consequences. The endogenous Sxl-Pm mRNAs have one Sxl binding site in the 5′ UTR, while there can be eight or more in the 3′ UTR. Sxl binds to these sites and downregulates translation. Though the truncated Sx-N protein can also repress translation of Sxl-Pm mRNAs, its inhibitory effects are somewhat weaker than the full-length protein [39]. However, it is possible to detect Sx-N repression of endogenous Sxl mRNAs using the hsp83:Sx-NΔ transgene. This transgene expresses Sxl mRNAs that lack the 5′ Sxl binding site and most of the 3′ UTR binding sites, and as a consequence are less sensitive to repression than the endogenous mRNAs [39]. For this reason, Sx-N protein produced by the transgene preferentially represses translation of the endogenous mRNAs and in hsp83:Sx-NΔ transgenic females the amount of Sx-N is typically greater than the two major endogenous Sxl proteins.
We compared the repression of the endogenous Sxl in hsp83:Sx-NΔ transgene females either wild type or heterozygous for eif4e. Figure 1C shows that in transgenic, wild type females the level of endogenous Sxl is less than Sx-N. Consistent with the results of the in vitro translation experiments, reducing eif4e activity does not have an obvious effect on repression of Sxl-Pm mRNAs by Sx-N and the ratio of the endogenous protein to Sx-N in eif4e/+ females remains similar to that in wild type females. With the caveat that Sxl may require a different set of accessory proteins to repress the translation of each of its target mRNAs, this finding does not support the idea that eIF4E functions as a co-factor in Sxl inhibition of msl-2 translation in vivo.
msl-2 mRNA splicing in eif4e/+ hsp83:Sx-NΔ transgene males
The alternative possibility is that eif4e rescues the male lethal effects of Sx-N because Sxl requires eif4e activity to effectively prevent the splicing of the intron in the 5′ UTR of msl-2 pre-RNA. To test this idea, we examined the splicing pattern of msl-2 mRNA in three surviving Sxl-;eif4e/+; hsp83:Sx-NΔ males. In wild type females, Sxl efficiently blocks the splicing of the msl-2 5′ UTR intron and in most female mRNAs the intron is unspliced. In wild type males the 5′ intron is spliced out of most msl-2 mRNAs. As expected, we found that ectopically expressed Sx-N protein blocks the splicing of the 5′ intron and as shown for one of the surviving Sxl−;eif4e/+; hsp83:Sx-NΔ males in Figure S1, msl-2 mRNA spliced in the female pattern is readily detected. However, we found that Sx-N wasn't able to fully inhibit the splicing of the 5′ intron, and roughly similar quantities of male spliced msl-2 mRNAs were also observed (Figure S1). Equivalent levels of male spliced msl-2 mRNAs were also found in both of the other Sxl−;eif4e/+; hsp83:Sx-NΔ males. Since the Sxl binding sites in the 5′ UTR are essential for efficient translational repression, Sx-N would not be able to completely block the translation of these male spliced msl-2 mRNAs.
eif4e is required for the stable activation of the Sxl positive autoregulatory feedback loop in early embryos
Though the results described in the previous section could explain why a small percentage of eif4e/+ males escape the lethal effects of Sx-NΔ, it is not possible to determine if the relative amount of male spliced msl-2 mRNA is increased compared to eif4e+ males because the controls don't survive. However, as it seemed possible that the effects of eif4e on Sxl dependent splicing might not be limited to msl-2, we took advantage of a simple genetic test for genes involved in Sxl positive autoregulation. The initial activation of the positive Sxl autoregulatory loop in female embryos is sensitive to alterations in the dose of gene products that play a critical role in promoting the female specific splicing of Sxl-Pm pre-mRNAs. Because of this sensitivity, mutations in splicing factors like the U1A/U2B” snRNP protein Snf often show dominant female lethal interactions with Sxl [40]–[46].
If eif4e is required for female specific splicing, then dominant female lethal interactions with Sxl might be observed. In contrast, if eif4e is needed to help repress the translation of Sxl target mRNAs, then reducing eif4e activity should increase the translation of Sxl mRNAs and would be expected to suppress rather than enhance any female specific lethality. The results in Table 1 show that the former prediction is correct. All three of the eif4e alleles we tested, eif4e568, eif4e587/11, and eif4e715, showed dominant female lethal interactions with the null mutation Sxlf1 (Table 1) [47]. These eif4e alleles are P-element insertions and are thought to be hypomorphic mutations [48]–[49]. The weakest allele, eif4e568, reduces female viability by a quarter, while female viability is reduced by a third to nearly a half for the two stronger alleles eif4e587/11 and eif4e715. Although the reductions in female viability seen for the three eif4e mutations are not as great as that observed for the snf null allele J210 or the dominant negative allele 1621, they are roughly equivalent to that seen for the hypomorphic allele JA2 (Table 1).
Tab. 1. eIF4e and snf interactions with Sxl. 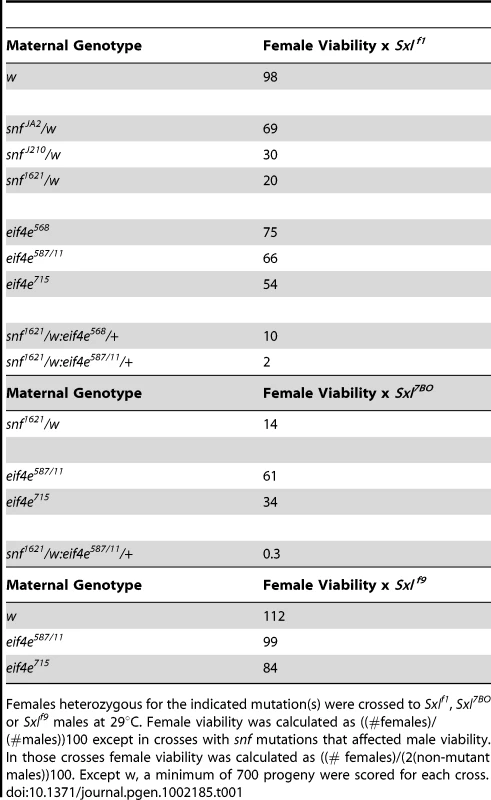
Females heterozygous for the indicated mutation(s) were crossed to Sxlf1, Sxl7BO or Sxlf9 males at 29°C. Female viability was calculated as ((#females)/(#males))100 except in crosses with snf mutations that affected male viability. In those crosses female viability was calculated as ((# females)/(2(non-mutant males))100. Except w, a minimum of 700 progeny were scored for each cross. In the experiments described above the eif4e/+ females were crossed to Sxlf1 males giving two classes of Sxlf1 progeny, those carrying the eif4e mutation and those with the wild type chromosome. We noticed that the viability of both classes of Sxlf1 progeny were affected equally (data not shown) suggesting that the lethality is predominantly the result of a lowered maternal contribution of eIF4E rather than a reduction in zygotic eIF4E. Consistent with this conclusion, when we did the reciprocal cross in which the eif4e mutation was introduced from the father and the Sxl mutation introduced from the mother, we found that the viability of Sxl−/+ females was close to that of wild type females (not shown).
To confirm that the female lethal interactions are due to a reduction in eif4e activity, we tested whether they can be rescued by an eif4e transgene. Two isoforms of eIF4E are expressed Drosophila. We introduced transgenes expressing each isoform into eif4e715/+ females and mated them to Sxlf1 males. We found that both could suppress the maternal effect lethal interactions between eif4E and Sxl (data not shown). We also tested a second independent Sxl allele, Sxl7B0 [50]. Like Sxlf1, Sxl7B0 exhibited dominant female lethal interactions with eif4e (Table 1).
eif4e mutations do not show dominant female lethal interactions with a mutation, Sxlf9, that only eliminates Sxl-Pe activity
The null mutations Sxlf1 and Sxl7B0 discussed above eliminate both early Sxl initiation functions provided by Sxl-Pe mRNAs and late Sxl sex determination functions (maintenance, sexual differentiation, and dosage compensation) provided by the Sxl-Pm mRNAs [47], [50]. While there are no known mutations that specifically eliminate only the late Sxl functions, the Sxlf9 mutation disrupts the initiation function of the Sxl-Pe transcripts [51]–[52]. If the reduction in eif4e activity impairs the female-specific splicing of Sxl-Pm pre-mRNAs, then eif4e mutations should have a smaller effect on the viability of flies carrying a Sxl mutation that only affects the Sxl-Pe pre-mRNAs as these transcripts do not require Sxl for proper splicing [53]–[54]. As can be seen in Table 1, Sxlf9 differs from Sxlf1 and Sxl7B0 in that it shows only a weak female lethal interaction with eif4e mutations. It also interacts much less strongly with snf1621 than either of the Sxl null alleles (data not shown).
Sxl protein expression is disrupted in progeny of snf and eif4e mothers
The female lethal interactions between Sxl and co-factors like snf that are critical for the female splicing of Sxl-Pm pre-mRNAs arise because the positive autoregulatory feedback loop is not properly set in motion [43]–[45]. However, there are no special requirements for these co - factors in the activation of Sxl-Pe by the X chromosome counting system or the splicing and translation of Sxl-Pe transcripts [53]–[54]. For these reasons, defects in Sxl accumulation are not observed in blastoderm stage embryos compromised for a sex-specific splicing co-factor. However, later in development, when protein expression depends upon female spliced Sxl-Pm mRNAs, the pattern of Sxl accumulation becomes abnormal. To determine if this is true for eif4e as well, we examined the expression of Sxl in blastoderm and post-blastoderm stage embryos.
Consistent with the idea that eif4e functions downstream of Sxl-Pe, eif4e mutations have no apparent effect on the expression of Sxl from the Sxl-Pe mRNAs. As shown in Figure 2 and Table S1, blastoderm stage progeny from eif4e−/+ and snf−/+ mothers crossed to Sxl−f1 fathers resemble wild type in that about 50% of the embryos (females) express Sxl protein (compare panels A & B with C & D). While reducing eif4e activity does not perturb activation of Sxl by the X chromosome counting system, it does have a significant effect on the expression of Sxl in older embryos. In the wild type controls (either w x w or w x Sxlf1), high uniform levels of Sxl protein are observed in about 50% of the embryos, while a equal number show no staining (panels E & F). For the dominant negative snf1621 allele only 11% of the embryos show the expected high uniform level of Sxl while Sxl expression in the remaining female embryos is either irregular or quite low (Table S1). As would be expected from the relative severity of the synthetic lethal interactions, the effects of the hypomorphic eif4e alleles on Sxl expression in post-cellular blastoderm embryos are not as strong as snf1621. For both eif4e587/11 and eif4e713 about one third of the embryos (or about two thirds of the females) show a high uniform level of Sxl accumulation (Table S1). The remaining female embryos show either a patchy pattern of Sxl protein accumulation or only low levels of protein (Figure 2G and 2H). These defects in Sxl expression in post-blastoderm embryos indicate that the Sxl autoregulatory feedback loop is not properly established in the female progeny of eif4e−/+ mothers.
Fig. 2. eif4e mutations alter expression of Sxl from the late, but not the early, promoter. 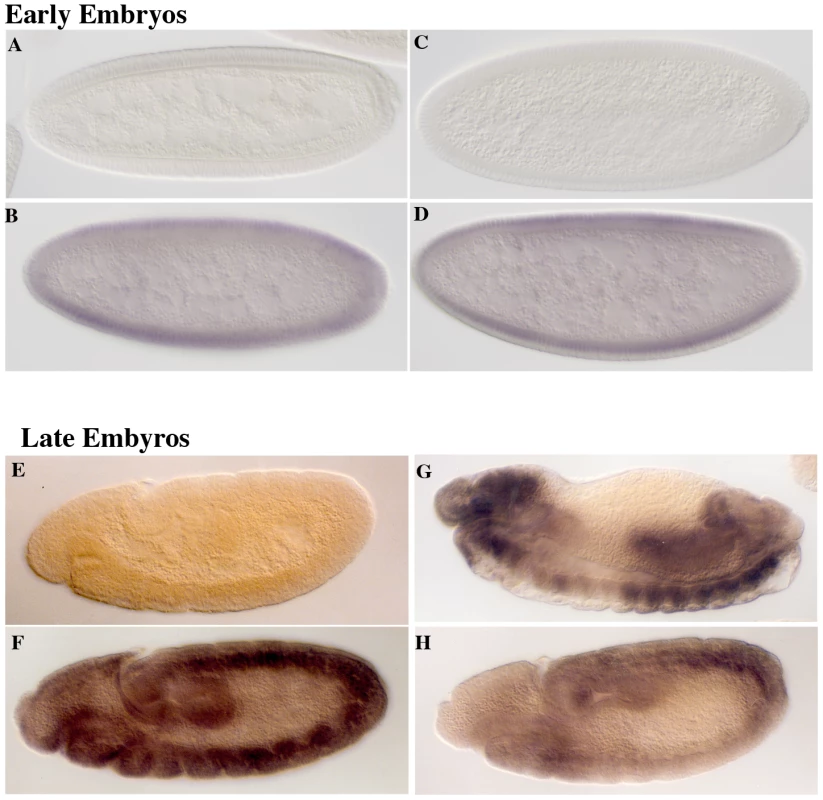
Embryos from wild type (A, B, E, F) and eif4e/+ (C, D, G, H) mothers crossed to Sxlf1/Y fathers were stained with antibody to Sxl. Male embryos from either cross do not express Sxl protein (A, C, E). Female embryos from wild type mothers express Sxl evenly throughout the embryo both early (B) and late (F). Female embryos from eif4e/+ mothers express Sxl normally early (D), but often display patchy expression late (G,H). The constitutively active SxlM mutations suppress the dominant female lethal interactions between eif4e and Sxl
To confirm that the female lethal effects of eif4e are due to a failure to activate the Sxl positive autoregulatory loop we tested whether Sxl−/+ female progeny of eif4e−/+mothers can be rescued by three different gain-of-function Sxl alleles, SxlM1, SxlM4, and SxlM6, that constitutively splice Sxl-Pm transcripts in the female mode [55]. As a positive control we generated an equivalent combination of SxlM1 and snf1621. Females trans-heterozygous for each combination were mated with Sxlf1 males. As can be seen in Table S2 for the positive control, SxlM1 suppresses the maternal effect female lethal interactions between snf and Sxlf1. Similarly, SxlM1 and both of the other gain-of-function alleles also suppress the maternal effect lethal interactions between eif4e587/11 and Sxlfl. In these crosses only half of the female progeny inherit the Sxl gain-of-function allele. As expected, most of the surviving females are the ones that carry the gain-of-function allele.
Female embryos from eif4e−/+ mothers produce male Sxl transcripts
If the positive autoregulatory loop is not properly activated when eif4e is compromised, we would expect to find male spliced Sxl transcripts in female blastoderm/early gastrula embryos. To examine the splicing pattern of Sxl-Pm transcripts specifically in female embryos during this period we took advantage of an X-linked Sxl-Pm splicing reporter. The splicing reporter has a Sxl genomic fragment extending across the regulated splice sites from exon 2 to exon 4 while exon 4 is fused to β-galactosidase sequences (see Figure 3A: [56]). Expression of the fusion gene is driven by the hsp83 promoter. This promoter is activated in the zygote during the late syncytial blastoderm stage around the time when Sxl-Pm transcription commences [57]. Figure 3B shows that the transcripts spanning the regulated Sxl exon2-exon3-exon4 splicing cassette are spliced in the appropriate sex-specific pattern in control adult flies collected from a stock homozygous for the transgene: exon 2–4 in females and exons 2–3–4 in male.
Fig. 3. Female progeny of eif4e/+ mothers produce male transcripts during early embryogenesis and in splicing compromised backgrounds. 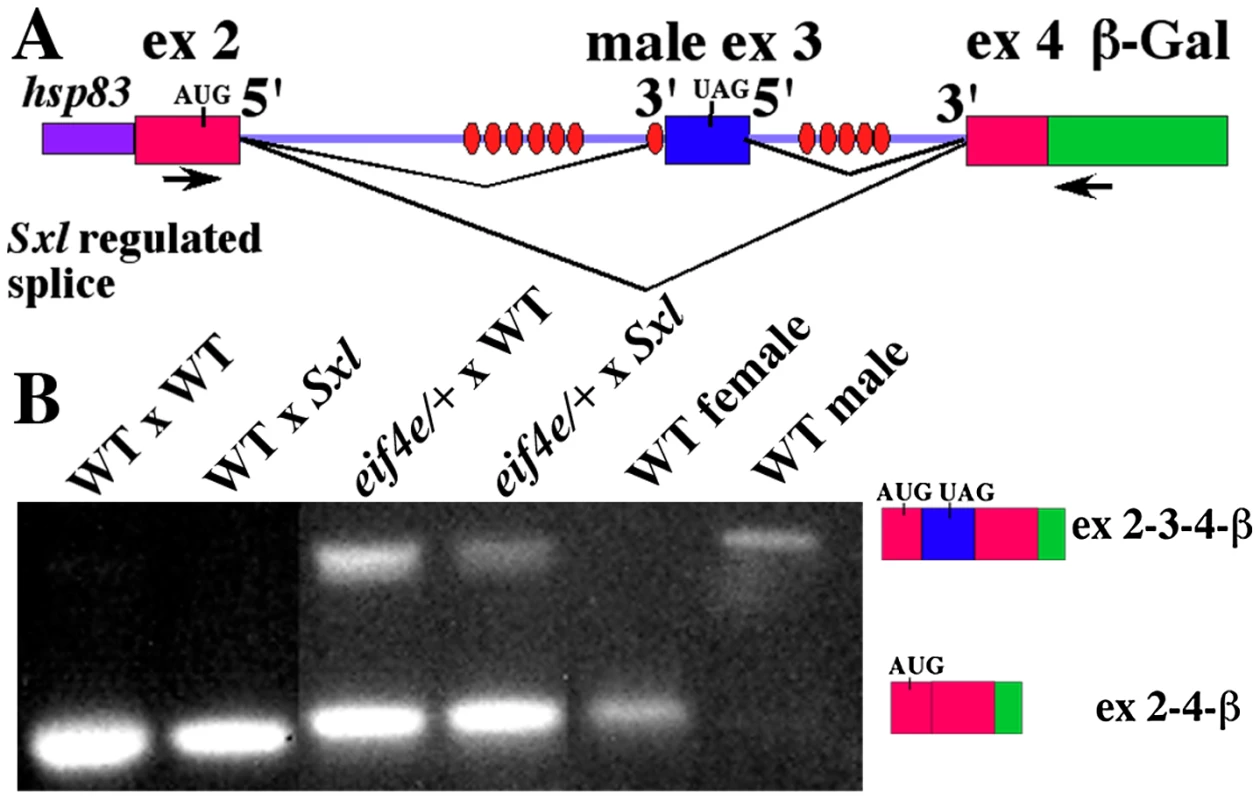
A) Model of the Sxl splicing reporter. Sxl binding sites are shown as ovals. Primers for PCR are indicated as arrows below the gene model. As indicated next to the gel, the female splice pattern skips exon3 (lane 5), while the male splice pattern includes exon3 (lane 6). B) RT-PCR was performed to analyze the products of an X-linked Sxl splice reporter brought from the male parent. Results were visualized with ethidium bromide. Female blastoderm stage embryos from wild type females express only female transcripts even when heterozygous for the Sxlf1 mutation (lane 1, 2). Female blastoderm stage embryos from eif4e/+ females express both the male and female transcripts (lane 3, 4). Sxlf1 or Sxl+ males carrying the splicing reporter were crossed to eif4e587/11/+ or control wild type females. To visualize the splicing of the regulated exon2-exon3-exon4 cassette when the autoregulatory feedback loop is first activated, we isolated RNA from 1–3 hr embryos and analyzed the structure of the transcripts expressed from the reporter by RT-PCR. When the mother is wild type we find that transcripts spanning the exon2-exon3-exon4 cassette are spliced exclusively in the female pattern (Figure 3B). This is true not only for female embryos that have two wild type copies of Sxl (fathers are Sxl+/Y), but also for female embryos that are heterozygous for the Sxlfl mutation (fathers are Sxlf1/Y). A different result is obtained when the mother is heterozygous for eif4e587/11 (Figure 3B). In this case, we detect not only female but also male spliced reporter RNAs. With this allele, male spliced RNAs are observed in both Sxlfl/+ embryos and in embryos that are wild type for Sxl. Similar results were obtained for snf1621 (not shown). We also observed male spliced reporter RNAs in the female progeny of mothers heterozygous for two other eif4e alleles. However, for both of these eif4e alleles the male transcripts were only present when the female embryos were heterozygous for the Sxl mutation (not shown).
Does eIF4E function in Sxl-dependent splicing regulation?
Two general mechanisms, one direct and the other indirect, could potentially account for the effects of eif4e on Sxl activation. In the direct mechanism, eif4e would function as a Sxl co-factor in the female specific processing of Sxl-Pm pre-mRNAs. In this case, reducing eif4e activity would compromise the female specific splicing of Sxl-Pm pre-mRNAs and prevent full activation of the positive autoregulatory feedback loop when the loop is first being initiated. In the second, eif4e would be required at a point subsequent to the splicing of the Sxl-Pm pre-mRNAs. For example, it may be needed in the cytoplasm for the efficient translation of Sxl-Pm mRNAs, or it might function in their nuclear export. In this scenario, the expression of Sxl proteins from the newly synthesized Sxl-Pm mRNAs would be impaired and sub-optimal levels of Sxl-Pm proteins would be produced. As a consequence, when the Sxl-Pe proteins decay, there would be an insufficient amount of Sxl remaining to stably maintain the positive autoregulatory feedback loop, and splicing would gradually switch from the female to the male pattern. Though our experiments with the splicing reporter suggest an immediate rather than a gradual effect on splicing of the Sxl-Pm transcripts, we cannot rule out the possibility that there is some disruption in the export or translation of Sxl-Pm mRNAs during the initial activation of the positive autoregulatory feedback loop. Moreover, consistent with the possible importance of post-splicing steps in Sxl activation, Stitzinger et al [58] found female lethal interactions with Sxl when mothers are simultaneously heterozygous for mutations in aspartyl tRNA synthetase and snf. Although the aspartyl tRNA synthetase mutants differ from eif4e in that they do not show female lethal interactions with Sxl on their own, the fact that reductions in the maternal dose of this synthetase can affect the activation of the autoregulatory loop lends credence to a post-splicing function. For these reasons we sought experimental paradigms in which we could assay for eif4e induced perturbations in Sxl dependent female-specific splicing under conditions in which the autoregulatory loop had already been “fully” activated and Sxl proteins were present at wild type levels.
Effects of eif4e mutations on Sxl pre-mRNA splicing in a sensitized background
In previous studies on snf we found that though there is substantial female lethality when snf1621/+ mothers are mated to Sxl− fathers, the surviving snf1621/Sxl− trans-heterozygous females are morphologically normal, fertile, and express wild type levels of Sxl protein. When we examined the splicing of the Sxl-Pm mRNAs in these surviving females using RT-PCR primer sets that give products spanning the regulated exon2-exon3-exon4 cassette, we found that unlike wild type females (which give only female spliced transcripts: exons 2–4) we could often detect a very low level of male spliced transcripts (exons 2–3–4) in these snf1621/Sxl− trans-heterozygous adult females (not shown: see snf1621 Sxlf1/++ in Figure 4B). We reasoned that the snf1621Sxlf1/++ heterozygous mutant combination might provide a suitable sensitized background to test whether eif4e activity is required for Sxl dependent pre-mRNA splicing.
Fig. 4. eif4e mutations shift Sxl regulated splicing toward male mode although Sxl protein levels are normal. 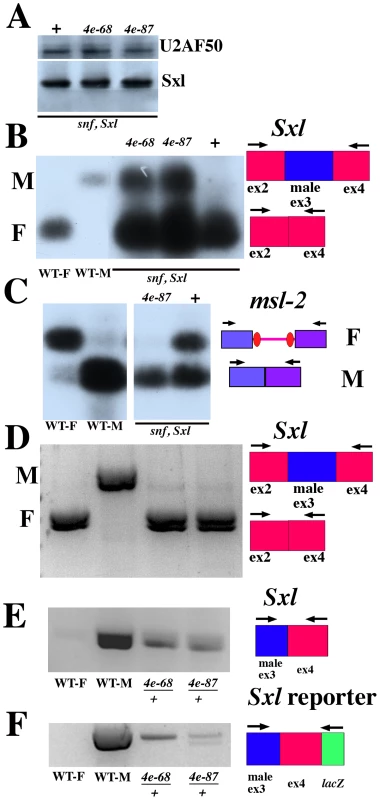
A) Western blot of control snf1621Sxlf1/++ (lane 1) females and of snf1621Sxlf1/++;eif4e568/+lane 2) and snf1621Sxlf1/++;eif4e587/11/+ (lane 3) females probed with antibodies to U2AF50 and Sxl. B, C, D) RT-PCR was performed on adult females to analyze the products of the Sxl (B, C) or msl-2 (D) gene. Presence or absence of an eif4e mutation is indicated above the relevant lanes. Results were visualized with by Southern blot (B,C) or ethidium bromide (D,E & F). Wild-type males (WT-M) produce male (ex3 included) but no female (ex3 excluded) Sxl mRNAs when assayed with primers that amplify only the male transcript (E.F) or primers that amplify both the male and female mRNAs (B,D). Wild-type females (WT-F) express no male Sxl mRNA. Females heterozygous for mutations in snf and Sxl (snf Sxl) express a small amount of male Sxl mRNA (B). Females triply heterozygous for mutations in snf, Sxl and eif4e express significantly more male Sxl mRNA. Similarly, addition of an eif4e mutation increases the amount of male (intron removed) msl-2 mRNA (C). Though all of the msl-2 mRNA in these triply heterozygous females appears to be spliced in the male pattern, there is not an obvious effect on their viability. This is not altogether surprising as females can tolerate an hsp83 transgene that expresses an msl-2 mRNA lacking not only the 5′ but also the 3′ Sxl binding sites (26). Panels D, E and F show that male spliced Sxl and Sxl reporter mRNAs are present in female heterozygous for two different hypomorphic eif4e alleles while they absent in wild type females (WT-F). In D, primers in exon2 and exon4 that amplify both male and female spliced mRNAs were used for the PCR. In E we used primers in exon3 and exon4 that amplify male spliced Sxl mRNAs. For the splicing reporter in F, we did two PCR reactions using nested primers in LacZ. eif4e alleles: 4e-68: eif4e568; 4e-87: eif4e587/11. Before assaying the splicing of Sxl-Pm transcripts in adult females triply heterozygous for snf1621, Sxlf1, and eif4e, we examined Sxl protein expression in these females. We anticipated that as long as the level of female spliced Sxl mRNAs remained well above some threshold critical for maintaining the positive autoregulatory feedback loop, the homeostasis mechanism provided by Sxl negative autoregulation of Sxl mRNA translation would ensure that Sxl levels would be maintained close to that in wild type. With the possible caveat that there may be tissue specific variations in Sxl levels that can't be detected by this assay, Figure 4A shows that this expectation is correct. We find that the level of Sxl protein in the triple mutant combinations with two different eif4e alleles is equivalent to that seen in control snf1621 Sxlf1/++ (ane +) adult females.
We next asked if a reduction in eif4e activity in the sensitized snf1621Sxlfl/++ background had any effect on the splicing of Sxl-Pm pre-mRNAs. For this purpose, we used a primer set that simultaneously amplifies both the male (exon 2–3–4) and female (exon 2–4) spliced Sxl mRNAs. This allows us to directly compare the relative ratio of female to male spliced mRNAs in each genetic background. Figure 4B shows that the very modest defects in the female specific splicing of Sxl-Pm pre-mRNAs evident in snf1621Sxlf1/++ females are clearly exacerbated when eif4e activity is reduced. For both eif4e alleles there is a marked increase in the amount of male-spliced Sxl-Pm mRNAs compared to the snf1621Sxlf1/++ control.
Effects of eif4e mutations on msl-2 pre-mRNA splicing in a sensitized background
We used this same sensitized background to examine the effects of reducing eif4e activity on the splicing of the intron in the 5′ UTR of msl-2 mRNAs. As illustrated in Figure 4C, Sxl blocks the splicing of the 5′ UTR intron so that it is retained in most msl-2 mRNAs in females, while this intron is spliced out efficiently in males. In control snf1621Sxlf1/++ females the female-specific splicing of the msl-2 mRNA is partially compromised and, we observe a nearly equal mixture of female and male spliced transcripts. As observed for Sxl-Pm splicing, reducing eif4e activity in this sensitized background further disrupts the female specific splicing of msl-2 mRNAs. In addition to demonstrating a role for eif4e in the splicing of a second Sxl target pre-mRNA, these findings provide additional evidence that the male lethal effects of the hsp83:Sx-NΔ transgene are suppressed because eif4e mutations perturb the female specific splicing of msl-2 mRNAs.
Male spliced Sxl mRNAs are also observed in eif4e/+ females
The results in the previous sections demonstrate that the modest defects in Sxl and msl-2 pre-mRNA splicing evident in a sensitized snf1621 Sxlf1/++ background are significantly enhanced by reducing eif4e activity. We wondered whether splicing defects are also observed in eif4e/+ females that are wild type for both snf and Sxl. To test this possibility, we examined the splicing of transcripts from the endogenous Sxl gene and the Sxl splicing reporter in females heterozygous for two different eif4e alleles. When we used primers that allow us to visualize simultaneously both the male and female spliced Sxl mRNAs from either the endogenous gene (Figure 4D) or from the splicing reporter (not shown), only female spliced Sxl mRNAs were observed in wild type females. In contrast, a very small amount of Sxl mRNA spliced in the male pattern could be detected from the endogenous gene (Figure 4D) and also from the splicing reporter (not shown) in females heterozygous for eif4e568 or for eif4e587/11. To confirm that male spliced Sxl mRNAs from the endogenous gene are present in these eif4e/+ females we used RT primers from exon 5 and then PCR amplified using a primer from the male exon and a primer from exon4. Figure 4E shows that male spliced Sxl mRNAs from the endogenous gene are readily evident in both eif4e568/+ and eif4e587/1/+ females, but not in wild type. Figure 4F shows that male spliced Sxl mRNAs from the reporter are also present in these eif4e heterozygous females, while there is little male spliced reporter mRNAs in control wild type females.
Mutations in eif4E do not affect the alternative splicing of dsx mRNA
To determine whether the effects of eif4e on sex-specific splicing are general or only restricted to Sxl dependent alternative splicing we examined the splicing of doublesex (dsx) mRNAs. The dsx gene is downstream of Sxl and like Sxl its transcripts are sex-specifically spliced. However, female-specific splicing of dsx mRNA is dependent upon tra and tra-2, not Sxl (reviewed in [14]–[16]). We used primer sets that would RT-PCR amplify either female or male spliced dsx mRNAs isolated from either wild type or eif4e/+ females. As expected, wild type females produce only female, not male products (Figure S2). Significantly, females heterozygous for eif4e also produce only female dsx mRNAs.
Could eIF4E play a direct role in Sxl-dependent alternative splicing?
The results described in the previous sections show that eif4e is required for Sxl splicing. Since eif4e is known to function in translation initiation, it might be needed for the synthesis of some limiting Sxl co-factor. In this scenario, the amount of this splicing co-factor would drop below some critical threshold when eif4e activity is reduced, and this would impair the ability of Sxl to regulate splicing. Alternatively, eif4e itself could be the Sxl splicing co-factor. This latter model makes several predictions that we have tested below.
eif4e mutations enhance the female specific lethality of dominant negative snf1621 and fl(2)d1
If eif4e functions in Sxl dependent alternative splicing, we might expect genetic interactions between eif4e and genes like snf that are required for female specific splicing of Sxl pre-mRNAs. To test this possibility females trans-heterozygous for different eif4e alleles and snf1621 were mated to Sxlf1 or Sxl7BO males. When combined with the Sxlf1, the weaker eif4e568 allele reduces the viability of female progeny of snf1621/+ mothers two-fold, while the stronger eif4e587/11allele reduces female viability ten-fold (see Table 1). An equivalent synergistic maternal effect female lethality is observed in progeny of snf1621/+; eif4e587/11/+ mothers mated to fathers carrying the deletion allele Sxl7B0. We also observed weak, female lethal interactions when eif4e was combined with a mutation in another Sxl splicing co-factor fl(2)d, which encodes the fly Wilm's Tumor 1 Associated Protein (WTAP) [51], [59]–[61].
eIF4E is localized in the nucleus of somatic cells but not germ cells
A splicing function requires that some eIF4E protein be present in the nucleus. To test this we probed late pre-cellular and cellular blastoderm embryos with antibodies against eIF4E and the germline marker Vasa. This is the stage in development when the first Sxl-Pm transcripts are expressed and the positive autoregulatory feedback loop must be set in motion in females [62]. There are also marked differences in RNA polymerase activity between the soma and germline. In the soma, transcription is substantially upregulated following the midblastula transition. By contrast, newly formed germ cells are transcriptionally quiescent and genes specifying somatic development, including Sxl, are off. Figure 5 shows that as expected for a translation factor, most of the eIF4E in soma is localized in the cytoplasm. However, as has been reported for Drosophila S2 tissue culture cells [8], there is a small but readily detectable amount of eIF4E in somatic nuclei. Interestingly, the transcriptionally quiescent germ cells differ from the somatic cells in that eIF4E is exclusively cytoplasmic and is not observed in their nuclei.
Fig. 5. Some eIF4E protein is located in the nucleus. 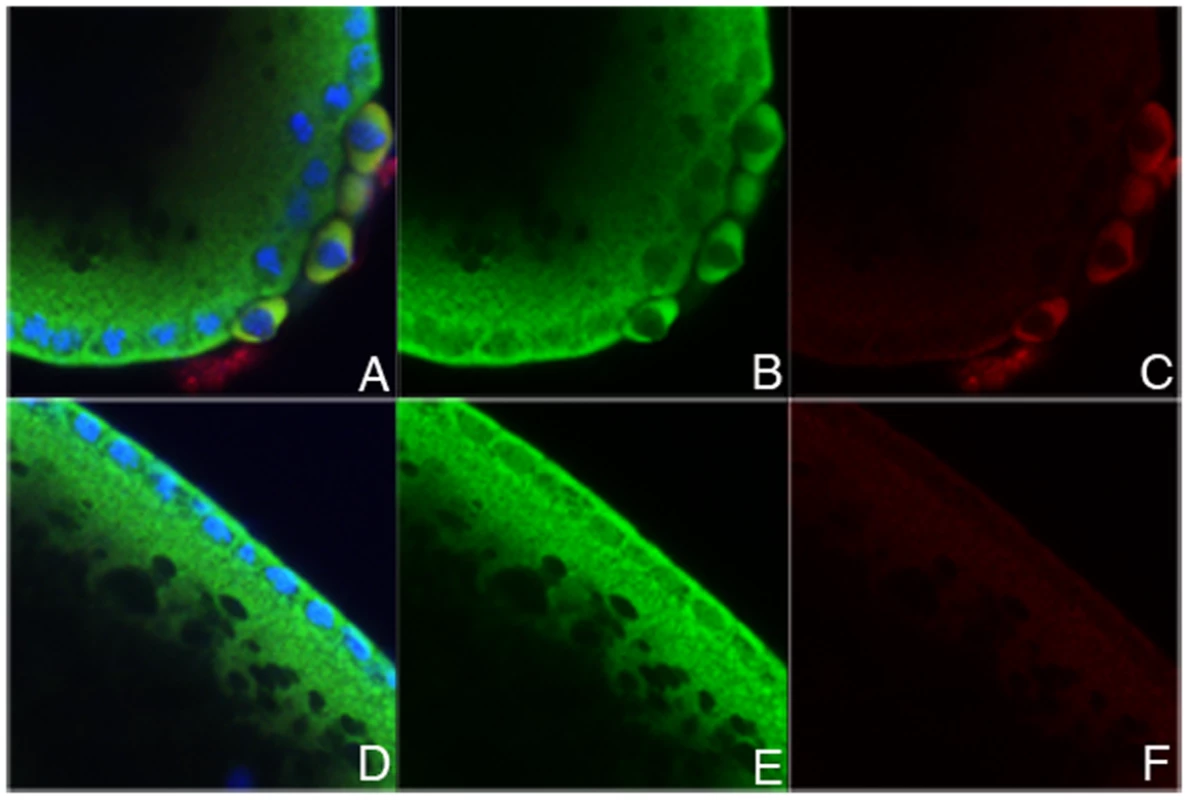
Wild type pre-cellular and cellular blastoderm stage embryos were stained with eIF4E (green) or Vasa (red) antibodies and hoechst (blue) to label the DNA. The embryo shown here is a late pre-cellular blastoderm embryo. A, D: All three channels. B,E: eIf4e only. C, F: Vasa only. Note the high levels of cyoplasmic eIF4E in the soma and in the Vasa positive germline pole cells. eIF4E can also be readily detected in the somatic nuclei, though the levels are less than in the somatic cytoplasm (see panel E). By contrast, there is only little eIF4E in the pole cell nuclei (Vasa plus cells at posterior in panel B). eIF4E is bound to Sxl pre-mRNAs
To function in Sxl dependent alternative splicing, eIF4E has to be bound to incompletely spliced Sxl transcripts. We first tested for the binding of Sxl and eIF4E to nuclear Sxl RNAs that had undergone the first splice of exon 1 to exon 2. As shown in the top panel of Figure 6, exon 1–2 spliced Sxl RNAs are found associated with both Sxl and eIF4E in nuclear extracts. Since splicing of the regulated sex-specific exons in the Sxl-Pm pre-mRNA is known to occur more slowly than the splicing of the non-regulated exons in the transcript [63], we next assayed the immunoprecipitates for Sxl-Pm RNAs in which exon1 has been spliced to exon2, but the Sxl regulated splice between exon2 and either exon3 or exon4 has not yet occurred (see 2nd panel in Figure 6). Consistent with previous studies which have shown that Sxl binds to partially spliced RNAs [43], exon1-exon2-intron2 Sxl-Pm RNAs are found in Sxl immunoprecipitates. Consistent with a function in the sex-specific splicing of Sxl-Pm pre-mRNAs, exon1-exon2-intron2 Sxl-Pm RNAs are also found in eIF4E immunoprecipitates, but not in control Scute immunoprecipitates. To exclude the possibility that Sxl and eIF4E associate non-specifically with any pre-mRNA in nuclear extracts, we assayed for the presence of incompletely processed tango transcripts; however, unspliced tango RNAs were not detected in either Sxl or eIF4E immunoprecipitates (data not shown). Since we were able to detect tango pre-mRNAs in U2UF50 immunoprecipitates, it would appear that eIF4E does not bind to all pre-mRNAs.
Fig. 6. eIF4E co-immunoprecipitates with Sxl pre-mRNAs. 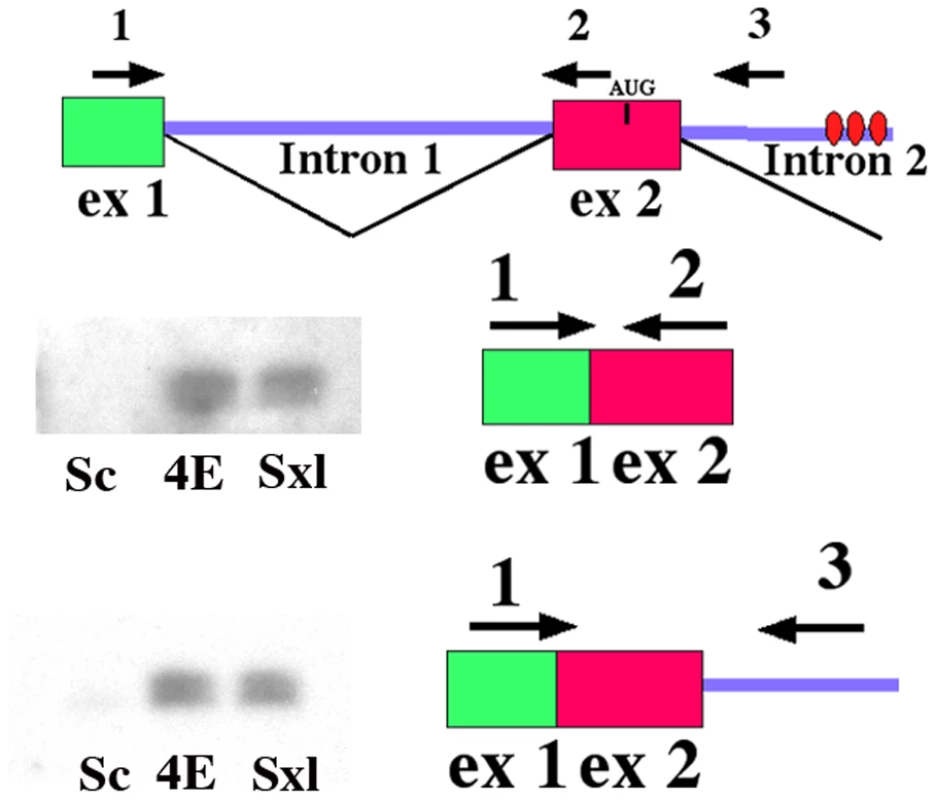
Nuclear extracts were incubated with antibodies to Scute (Sc), Sxl or eIF4E (4E). RNA was isolated from the immunoprecipitates and used for RT-PCR reactions. Top: Diagram of the 5′ region of the Sxl-Pm transcription unit showing the exon-intron structure and the position of primers used for PCR. Bottom: Southern blots of RT-PCR products that are amplified from the immunoprecipitates using the indicated primers. Next to the blots is a diagram of the amplification product. Antibodies to eIF4E and Sxl immunoprecipitate both spliced and partially spliced Sxl-Pm mRNAs. Antibodies to Sc do not immunoprecipitate any Sxl-Pm mRNAs. eIF4E is associated with Sxl and Snf in nuclear extracts
If eIF4E participates in Sxl dependent splicing regulation, it should be associated not only with Sxl but also with the U1/U2 snRNP protein that has been implicated Sxl splicing regulation. As can be seen in Figure 7A, eIF4E is present in Sxl, but not control immunoprecipitates of nuclear extracts. Similarly, a small but readily detectable amount of eIF4E is found in the Snf immunoprecipitates (Figure 7B). Though recombinant Sxl and Snf are able to interact directly with each other in vitro, the complexes between these two proteins in nuclear extracts are disrupted by RNase digestion [43]. Figure 7A and 7B show that like nuclear Sxl:Snf complexes, both the eIF4E:Sxl and eIF4E:Snf complexes are also RNase sensitive.
Fig. 7. eIF4E co-immunoprecipitates with several splicing factors. 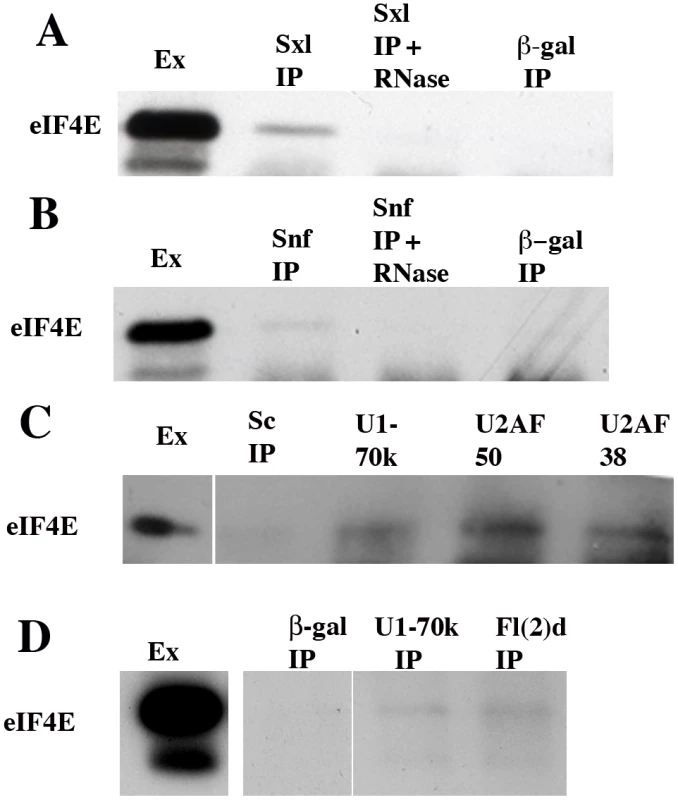
Western blots of immunoprecipitates isolated using antibodies to splicing factors (Sxl, Snf, U1-70K, U2AF50, U2AF38, and Fl(2)d) or negative controls (β-Galactosidase or Scute (Sc)) were probed with antibodies against eIF4E. Nuclear extract (lane 1 all blots) contains substantial amounts of eIF4E. Two isoforms are usually observed in nuclear extracts; however, the lower isoform is often obscured by the immunoglobulin light chain in the IPs. A, B) eIF4E is present in Sxl and Snf immunoprecipitates (2nd lane from left in panels A and B as indicated), but is released from the Sxl and Snf complexes by pre-treatment with RNase (3rd lane from left as indicated). eIF4E is not immunoprecipitated by antibodies to β-galactosidase (lane 4 from left). Note that though Snf and Sxl interact directly with each other in vitro and in vivo, Sxl protein is typically not detected in Snf immunoprecipitates of total nuclear extracts (37) whereas Snf is readily detected in Sxl IPs. The reason for this discrepancy is that only a small amount of the Snf protein is associated with Sxl. As we are able to recover only a fraction of the total Snf in the IPs, there is probably too little Sxl to be detected. On the other hand, Sxl can be readily detected in the Snf IPs when Sxl:Snf complexes are first partially purified away from bulk Snf protein on sucrose gradients and then immunoprecipitated. (C) eIF4E is not present in Scute (Sc) immunoprecipitates (2nd lane), but is present in the U1-70K, U2AF50 and U2AF38 immunoprecipitates (lanes 3–5 from left as indicated). (D) eIF4E is not present in β-galactosidase immunoprecipitates (2nd lane), but is present in the U1-70k (3rd lane from left) and Fl(2)d (4th lane from left) immunoprecipitates. Band visible at very bottom of the IP lanes in panels A, B and also C is the immunoglobulin light chain. eIF4E is associated with splicing factors that function in the assembly of the spliceosome complexes E and A
We [43], [61] and Nagengast et al [45] have presented genetic and biochemical evidence that Sxl autoregulation depends upon interactions between Sxl and components of the splicing machinery that are involved in the initial assembly of the U1 snRNP on the 5′ splice sites of the Sxl-Pre mRNAs and the U2 snRNP on the 3′ splice sites. If eIF4E plays a role in Sxl autoregulation, it should also be present in RNP complexes that contain factors that function at these early steps in the splicing reaction. Both U1-70K, which is a component of the U1 snRNP and the U2AF proteins, U2AF38 and U2AF50 play important roles in Sxl autoregulation and are found associated with Sxl protein in nuclear extracts [45]. U1-70K/U1 snRNP and the U2AF heterodimer function in one of the first steps in the splicing reaction, the formation of the E complex. This complex is formed by the binding of the U1-70K/U1 snRNP to the 5′ splice site and the interaction of the U2AF heterodimer with the polypyrimidine tract at the 3′ splice junction. U2AF at the 3′ splice site then recruits the U2 snRNP which becomes loosely associated with the pre-mRNA [64]–[69]. The E spliceosome complex then undergoes an ATP dependent rearrangement that stabilizes the pairing interactions between the U2 snRNP and the pre-mRNA to form the A complex [70]–[73]. U1-70K/U1 snRNP and U2AF remain associated with the splicing complex when the three other snRNPs, U4/U6 and U5, are recruited to give spliceosomal complex B [74]; however, when the B complex rearranges during formation of the activated complex B* both U1-70K and U2AF dissociate from the splicesome along with the U1 and U4 snRNPs [75]–[77]. To determine if eIF4E is associated with these early acting splicing factors, we immunoprecipitated nuclear extracts with antibodies against U1-70K and the two U2AF subunits dU2AF38 and dU2AF50. Figure 7C and 7D show that eIF4E is in complexes in the nucleus with the U1-70K protein and with both of the U2AF subunits.
Another factor required for Sxl regulated splicing is the fly WTAP protein Fl(2)d [59]–[60], [77]. The interaction of Fl(2)d with the spliceosomal apparatus more closely parallels that seen for Sxl than U1-70K, U2AF or Snf. Like Sxl, Fl(2)d is found associated with splicing factors that are present during the formation of the spliceosomal E and A complexes which define the 5′ and 3′ exon-intron junctions and position the U2 snRNP, but appears to disassociate from the spliceosome before the tripartite snRNPs, U4/U6 and U5, are recruited to the pre-mRNA to form pre-catalytic complex B. The available evidence indicates that Sxl:Fl(2)d interactions may facilitate the incorporation of Sxl into pre-mRNA spliceosome complexes and perhaps mediate its interactions with Snf [61]. If eIF4E is important for Sxl dependent alternative splicing, we would expect to find it associated not only with Sxl but also with Fl(2)d in nuclear extracts. Figure 7D shows that this prediction is correct: eIF4E can be co-immunoprecipitated with Fl(2)d.
Discussion
The RNA binding protein Sxl orchestrates sexual development by controlling gene expression post-transcriptionally at the level of splicing and translation. To exert its different regulatory functions Sxl must collaborate with sex-non-specific components of the general splicing and translational machinery. In the studies reported here we present evidence that one of the splicing co-factors is the cap binding protein eIF4E. We initially identified eif4e in a screen for mutations that dominantly suppress the male lethal effects induced by ectopic expression of a mutant Sxl protein, Sx-N, which lacks part of the N-terminal domain. The Sx-N protein is substantially compromised in its splicing activity, but appears to have closer to wild type function in blocking the translation of the Sxl targets msl-2 and Sxl-Pm. As the male lethal effects of Sx-N (in an Sxl- background) are due to its inhibition of Msl-2 expression [39] we anticipated that general translation factors needed to help Sxl repress msl-2 mRNA would be recovered as suppressors in our screen. Indeed, one of the suppressors identified was eif4e. However, consistent with in vitro experiments, which have shown that Sxl dependent repression of msl-2 mRNA translation is cap independent [34], we found that eif4e does not function in Sxl mediated translational repression of at least one target mRNA in vivo. Instead, our results indicate that eif4e is needed for Sxl dependent alternative splicing and argue that it is this splicing activity that accounts for the suppression of male lethality by eif4e mutations. In wild type females, Sxl protein blocks the splicing of a small intron in the 5′ UTR of the msl-2 pre-mRNA. This is an important step in msl-2 regulation because the intron contains two Sxl binding sites that are needed by Sxl to efficiently repress translation of the processed msl-2 mRNA. When this intron is removed repression of msl-2 translation by Sxl is incomplete [25]–[28] and this would enable eif4e/+ males to escape the lethal effects of the Sx-N transgene.
Several lines of evidence support the conclusion that eif4e is required for Sxl dependent alternative splicing. One comes from our analysis of the dominant maternal effect female lethal interactions between eif4e and Sxl. The initial activation of the Sxl positive autoregulatory feedback loop in early embryos can be compromised by a reduction in the activity of splicing factors like Snf, Fl(2)d, and U1-70K, and mutations in genes encoding these proteins often show dose sensitive maternal effect, female lethal interactions with Sxl. Like these splicing factors, maternal effect female lethal interactions with Sxl are observed for several eif4e alleles. Moreover, these female lethal interactions can be exacerbated when the mothers are trans-heterozygous for mutations in eif4e and the splicing factors snf or fl(2)d. Genetic and molecular experiments indicate that female lethality is due to a failure in the female specific splicing of Sxl-Pm mRNAs. First, female lethality can be rescued by gain-of-function Sxl mutations that are constitutively spliced in the female mode. Second, transcripts expressed from a Sxl-Pm splicing reporter in the female Sxl−/+ progeny of eif4e/+ mothers are inappropriately spliced in a male pattern at the time when the Sxl positive autoregulatory loop is being activated by the Sxl-Pe proteins. While splicing defects are evident in these embryos at the blastoderm/early gastrula stage, obvious abnormalities in expression of Sxl protein are not observed until several hours later in development.
Though this difference in timing would favor the idea that eif4e is required for splicing of Sxl-Pm transcripts rather than for the export or translation of the processed Sxl-Pm mRNAs, we can not exclude the possibility that there are subtle defects in the expression of Sxl protein at the blastoderm/early gastrula stage that are sufficient to disrupt splicing regulation during the critical activation phase yet aren't detectable in our antibody staining experiments. However, evidence from two different experimental paradigms using adult females indicates that this is likely not the case. In the first, we found that reducing eif4e activity in a sensitized snf1621 Sxlf1/++ background can compromise Sxl dependent alternative splicing even though there is no apparent reduction in Sxl protein accumulation. In this experiment we took advantage of the fact that once the positive autoregulatory feedback loop is fully activated a homeostasis mechanism (in which Sxl negatively regulates the translation of Sxl-Pm mRNAs) ensures that Sxl protein is maintained at the same level even if there are fluctuations in the amount of female spliced mRNA. While only a small amount of male spliced Sxl-Pm mRNAs can be detected in snf1621 Sxlf1/++ females, the level increases substantially when eif4e activity is reduced. Since these synergistic effects occur even though Sxl levels in the triply heterozygous mutant females are the same as in the control snf1621 Sxlf1/++ females, we conclude that the disruption in Sxl dependent alternative splicing of Sxl-Pm transcripts in this context (and presumably also in early embryos) can not be due to a requirement for eif4e in either the export of Sxl mRNAs or in their translation. Instead, eif4e activity must be needed specifically for Sxl dependent alternative splicing of Sxl-Pm pre-mRNAs. Consistent with a more general role in Sxl dependent alternative splicing, there is a substantial increase in msl-2 mRNAs lacking the first intron when eif4e activity is reduced in snf1621 Sxlf1/++ females. In the second experiment we examined the splicing of pre-mRNAs from the endogenous Sxl gene and from a Sxl splicing reporter in females heterozygous for two hypomorphic eif4e alleles. Male spliced mRNAs from the endogenous gene and from the splicing reporter are detected the eif4e/+ females, but not in wild type females. Moreover, the effects on sex-specific alternative splicing seem to be specific for transcripts regulated by Sxl as we didn't observe any male spliced dsx mRNAs in eif4e/+ females.
Two models could potentially explain why eif4e is needed for Sxl dependent alternative splicing. In the first, eif4e would be required for the translation of some critical and limiting splicing co-factor. When eif4e activity is reduced, insufficient quantities of this splicing factor would be produced and this, in turn, would compromise the fidelity of Sxl dependent alternative splicing. In the second, the critical splicing co-factor would be eif4e itself. It is not possible to conclusively test whether there is a dose sensitive requirement for eif4e in the synthesis of a limiting splicing co-factor. Besides the fact that the reduction in the level of this co-factor in flies heterozygous for hypomorphic eif4e alleles is likely to be rather small, only a subset of the Sxl co-factors have as yet been identified (unpublished data). For these reasons, the first model must remain a viable, but in our view, unlikely possibility. As for the second model, the involvement of a translation factor like eif4e in alternative splicing is unexpected if not unprecedented. For this to be a viable model, a direct role for eif4e must be consistent with what is known about the dynamics of Sxl pre-mRNA splicing and the functioning of the Sxl protein. The evidence that the second model is plausible is detailed below.
Critical to the second model is both the nuclear localization of eIF4E and an association with incompletely spliced Sxl pre-mRNAs. Nuclear eIF4E has been observed in other systems, and we have confirmed this for Drosophila embryos. We also found that eIF4E is bound to Sxl transcripts in which the regulated exon2-exon3-exon4 cassette has not yet been spliced. In contrast, it is not associated with incompletely processed transcripts from the tango gene, which are constitutively spliced. With the caveat that we have only one negative control, it is not surprising that Sxl transcripts might be unusual in this respect. There is growing body of evidence that splicing of constitutively spliced introns is co-transcriptional [78]–[83]. However, recent in vivo imaging experiments have shown that the splicing of the regulated Sxl exon2-exon3-exon4 cassette is delayed until after the Sxl transcript is released from the gene locus in female, but not in male cells [84]. These in vivo imaging studies also show that, like bulk pre-mRNAs, the 1st Sxl intron is spliced co-transcriptionally in both sexes. Consistent with a delay in the splicing of the regulated cassette, we've previously reported that polyadenylated Sxl RNAs containing introns 2 and 3 can be readily detected by RNase protection, whereas other Sxl intron sequences are not observed [19]. The delay in the splicing of the regulated Sxl cassette until after transcription is complete and the RNA polyadenylated could provide a window for exchanging eIF4E for the nuclear cap binding protein.
To function as an Sxl co-factor, eIF4E would have to be associated with the pre-mRNA-spliceosomal complex before or at the time of the Sxl dependent regulatory step. There is still a controversy as to exactly which step in the splicing pathway Sxl exerts its regulatory effects on Sxl-Pm pre-mRNAs and two very different scenarios have been suggested. The first is based on an in vitro analysis of Sxl-Pm splicing using a small hybrid substrate consisting of an Adenovirus 5′ exon-intron fused to a short Sxl-Pm sequence spanning the male exon 3′ splice site [85]. These in vitro studies suggest that Sxl acts very late in the splicing pathway after the 1st catalytic step, which is the formation of the lariat intermediate in the intron between exon 2 and the male exon. According to these experiments Sxl blocks the 2nd catalytic step, the joining of the free exon 2 5′ splice site (or Adeno 5′ splice site) to the male exon 3′ splice site (see Figure 1A). It is postulated that this forces the splicing machinery to skip the male exon altogether and instead join the free 5′ splice site of exon 2 to the downstream 3′ splice site of exon 4. Since we have shown that eIF4E binds to Sxl-Pm pre-mRNAs that have not yet undergone the 1st catalytic step (Figure 6), it would be in place to influence the splicing reaction if this scenario were correct.
The second scenario is more demanding in that it proposes that Sxl acts during the initial assembly of the spliceosome. Evidence for Sxl regulation early in the pathway comes from the finding that Sxl and the Sxl co-factor Fl(2)d show physical and genetic interactions with spliceosomal proteins like U1-70K, Snf, U2AF38 and U2AF50 that are present in the early E and A complexes and are important for selecting the 5′ and 3′ splice sites [45], [61], [64]–[71]. In addition to these proteins, Sxl can also be specifically cross-linked in nuclear extracts to the U1 and U2 snRNAs [43]. Formation of the E complex depends upon interactions of the U1 snRNP with the 5′ splice site, and this is thought to be one of the first steps in splicing. The other end of the intron is recognized by U2AF, which recruits the U2 snRNP to the 3′ splice site. After the base pairing of the U2 snRNP with the branch-point to generate the A complex the next step is the addition of the U4/U5/U6 snRNPs to form the B complex. However, Sxl and Fl(2)d are not found associated with components of the splicing apparatus like U5-40K, U5-116K or SKIP that are specific for complexes B and B*, or the catalytic C complex [70]–[71], [74]–[75], [86]–[88]. Nor can Sxl be cross-linked to the U4, U5 or U6 snRNAs [43]. If Sxl and Fl(2)d dissociated from the spliceosome before U4/U5/U6 are incorporated into the B complex, then they must influence splice site selection during the formation/functioning of the E and/or A complex. (Since the transition from the E to the A complex has been shown to coincide with an irreversible commitment to a specific 5′—3′ splice site pairing, Sxl would likely exerts its effects in the E complex when splice site pairing interactions are known to still be dynamic [89].) If this is scenario is correct, eIF4E would have to be associated with factors present in the earlier complexes in order to be able to promote Sxl regulation. This is the case. Thus, eIF4E is found in complexes containing the U1 snRNP protein U1-70K, the U1/U2 snRNP protein Snf, and the two U2AF proteins, U2AF38 and U2AF50. With the exception of the Snf protein bound to the U2 snRNP, all of these eIF4 associated factors are present in the early E or A complexes, but are displaced from the spliceosome together with the U1 and U4 snRNPs when the B complex is rearranged to form the activated B* complex. This would imply that eIF4E is already in place either before or at the time of B complex assembly. Arguing that eIF4E associates with these E/A components prior to the assembly of the B complex is the finding that eIF4E is also in complexes with both Sxl and Fl(2)d. Thus, even in this more demanding scenario for Sxl dependent splicing, eIF4E would be present at a time when it could directly impact the regulatory activities of Sxl and its co-factor Fl(2)d.
Taken together these observations would be consistent with a Sxl co-factor model. While further studies will be required to explain how eIF4E helps promote female specific processing, an intriguing possibility is suggested by the fact that hastening the nuclear export of msl-2 in females would favor the female splice (which is no splicing at all). Hence, one idea is that eIF4E binding to the pre-mRNA provides a mechanism for preventing the Sxl regulated splice sites from re-entering the splicing pathway, perhaps by constituting a “signal” that blocks the assembly of new E/A complexes. A similar post-transcriptional mechanism could apply to female-specific splicing of the regulated Sxl exon2-exon3-exon4 cassette. The binding of eIF4E (and PABP) to incompletely processed Sxl transcripts after transcription has terminated in females would prevent the re-assembly of E/A complexes on the two male exon splice sites, and thus promote the formation of an A complex linking splicing factors assembled on the 5′ splice sites of exons 2 and on the 3′ splice site of exon 4.
Materials and Methods
Fly culture
Flies were raised at room temperature on a standard Drosophila media. Crosses were performed at 29°C unless otherwise indicated with 3–7 females and 2–4 males per vial. Crosses were transferred to new vials every 2–3 days. Similar crosses were performed at 25°C, but the effects were significantly weaker.
Stocks
Unless otherwise noted stocks are referenced by Lindsley & Zimm [89]. w; eif4eSO587/11/TM3Sb (eif4e587, FBal0129763), w;eif4eEP568/TM3Sb (eif4e568, FBal0122994), w;eif4eSO715/TM3Sb(eif4e715, FBal0175695), y1w67c23, w cm Sxlf1 ct/Bincinscy, y w (FBal0016680), Sxl7BO/Bincinscy (FBal0016694), y pn SxlM1/Bincinscy (FBal0016703), y pn SxlM4/Bincinscy (FBal0016710), y pn SxlM6/Bincinscy (FBal0103944), cm Sxlf9/Bincinscy (FBal0016686), y w snf1621 ct/Bincinscy, y w snf1621 Sxlf1 ct/Bincinscy.
Screen for suppressors of hsp83:Sx-NΔ transgene
To identify suppressors of the dominant male lethality conferred by Sx-N, we crossed w Sxl7B0/Bin; hsp83:Sx-NΔ transgene mothers to Deficiency/Balancer fathers and scored for viable, non-Balancer males containing the transgene. The 67A8-A9 region was one of the chromosomal intervals that was found to contain a suppressor. The eif4e gene mapped to this region and was a strong candidate gene for the dominant suppressor. Four independent eif4e alleles suppressed the male lethal effects of hsp83:Sx-NΔ transgene as indicated in the text. All crosses for both screens were conducted in vials with five females and three males of the appropriate genotype. Matings were allowed to occur for three days at 25°C, at which time the parents were transferred to new vials to ensure that larvae were not crowded.
Immunohistochemical staining
Embryos were collected on apple juice plates sprinkled with yeast at 29°C. They were dechorionated in bleach and fixed in 4% paraformaldehyde:heptane for 20–25 minutes. The fix was removed and embryos devitilinized and stored in methanol at −20°C. To stain, embryos were stepped into PBS, incubated for 1 hour in PAT (PBS with 1% BSA, 1%Triton-X100) and blocked for 30 minutes in PBT (PBS with 5% BSA). Embryos were incubated overnight at 4°C with primary antibody at the appropriate concentration in PBT. The next day the embryos were washed with PBS-T (PBS with 1% Triton-X100) then, incubated for 2 hours at room temperature with secondary antibody at the appropriate concentration in PBT. Embryos were washed with PBS-T, then with PBS. For embryos with fluorescently tagged secondary antibodies, the embryos were incubated for 5 minutes with a 1∶1000 dilution of Hoescht, rinsed twice with PBS, then mounted in Aquamount (Polysciences, Inc.). For embryos with HRP conjugated secondary antibodies, embryos were incubated with 400 ul of 0.4 mg/ml DAB in PBS, 1 ul of 3% hydrogen peroxide and 0.6 ul of 1 M NiCl2 until the embryos appeared fully stained. To prepare for mounting embryos were stepped into 100% ethanol, then incubated overnight in methyl salicylate. The following morning, embryos were mounted in Permount (Fisher Scientific). Primary antibodies used were: anti-Sxl18 monoclonal at 1∶10, anti-snf 9G3 monoclonal at 1∶10 and anti-eIF4E polyclonal at 1∶500 (gift from Paul Lasko). Secondary antibodies used were: HRP conjugated goat anti-mouse (Jackson ImmunoResearch) at 1∶500, rhodamine conjugated goat anti-rabbit (Alexa) at 1∶500, fluorescence conjugated goat anti-mouse (Alexa) at 1∶500.
RT-PCR analysis and Southern blotting
Embryonic RNA was prepared as described by Bell et al [90]. Adult RNA from 33 flies was prepared using GE Healthcare mini-spin columns. Reverse transcription was performed according to the procedure of Frohman et al. [91]. 1.5–3% of the cDNA was used as template. PCR cycles for embryonic cDNAs were 1X 95°C 4 minutes, 30X 95°C 1 minute, 60–65°C 45 seconds, 72°C 30 seconds, 1X 72°C 10 minutes. If re-amplification was needed, only 10 cycles were performed in the first PCR. Up to 40% of the first PCR was used as template for the second PCR. Primers and temperatures were the same for the second reaction as in the first and 10–30 cycles were performed as needed. Number of cycles needed was evaluated by examining 10 ul samples with EtBr. For adult cDNAs PCR cycles were as follows: 2X 95°C 1 minute, 70–72°C 45 seconds, 7°C 1 minute, 2-4X 95°C 1 minute, 68–70°C 45 seconds, 72°C 1 minute, 2-4X 95°C 1 minute, 66–67°C 45 seconds, 72°C 1 minute, 2-4X 95°C 1 minute, 65–66°C 45 seconds, 72°C 1 minute, 10X (first PCR) or 5-30X (second PCR) 95°C 1 minute, 65–67°C 45 seconds, 72°C 1 minute. 5 ul of the first PCR diluted 1/100 was used as template for the second PCR. For Southern blotting DNA was run on 1–1.2% agarose gels, and Southern blotted to Zeta-Probe membrane or nitrocellulose. For Sxl reactions blots were hybridized with randomly primed Sxl 3B1Δ cDNA [39]. For msl-2 mRNAs the membrane was hybridized to randomly primed msl-2 5′UTR PCR product. Primers used are described in Figure 6 and listed in Table 2.
Tab. 2. Primers used for RT-PCR experiments. 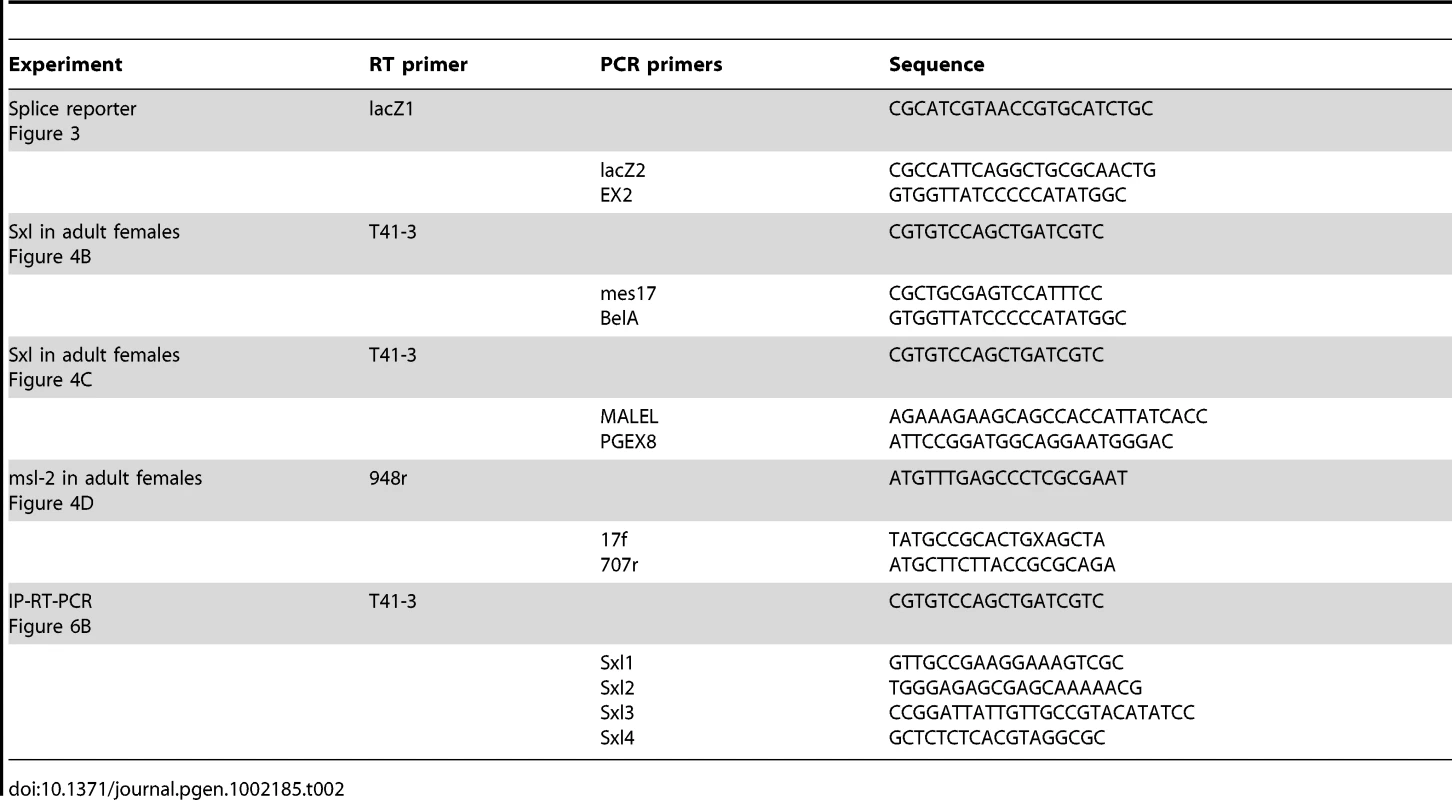
Immunoprecipitation
Nuclear extract was prepared by collecting embryos laid by w1 stock overnight (<24 hours). Embryos were washed with distilled water and 0.12 M NaCl, 0.04% Triton-X 100, then dechorionated in 100% bleach for 3 minutes. Dechorionated embryos were rinsed with NaCl, Triton, then NaCl, blotted dry and collected. Embryos were homogenized at 4°C in buffer 1(15 mM HEPES-KOH pH 7.6, 10 mM KCl, 5 mM MgCl2, 0.1 mMEDTA, 0.5 mM EGTA, 0.35 M sucrose, with 1 mM DTT, 1 mMNa2S2O5, protease inhibitors, benzamidine and 1mMPMSF), using 4 ml buffer/ml lightly packed embryos. The homogenate was filtered through three layers of Mira-cloth, then centrifuged at 2000 xg for 10 minutes at 4°C. Supernatant was removed with a pipet. The pellet was re-suspended in 4 ml buffer/ml embryos, and overlaid onto an equal volume of buffer 2 (same as buffer 1 except 0.8 M sucrose), then spun 10 minutes at 2000 xg, at 4°C. The supernatant was removed. The pellet was resuspended in TEN (10 mM Tris-HCl pH 7.8–8, 1.5 mM EDTA, 100 mM NaCl), 2 ml TEN/ml embryos, and sonicated. 20 ul–40 ul of 50% antibody linked protein AG beads, 350 ul co-immunoprecipitation buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 250 mM sucrose, 0.05% (w/v) Tergitol NP-40, 0.5% (v/v) Triton-X 100 plus 1 mM DTT, 1 mMNa2S2O5, protease inhibitors, benzamidine and 1mMPMSF) and 12.5 ul RNAsin were added to a 150 ul aliquot of sonicate. The mixture was rocked at 4°C overnight, then washed 5 times with co-IP buffer. The beads were boiled for 5–10 minutes with 20 ul protein sample buffer, then spun for 5–10 minutes. 5–10 ul of sample was loaded onto a 12% polyacrylimide gel. The proteins were transferred to Immobilon-P or nitrocellulose. Blots were prehybridized in PBS-5% nonfat dry milk and probed with primary antibody overnight at 4°C. Antibodies used include: mouse anti-Sxl 104 and 114, mouse anti snf 9G3 [41], rabbit anti-eIF4e antibody at 1∶1000 [92], rabbit anti-U170K (gift of Helen Salz; [45]) at 1/5000, rabbit anti-U2AF50 (gift of Don Rio; [93])at 1/5000, rabbit anti-U2F38 1/5000 (gift of Don Rio; [94]) or mouse anti-Fl(2)d9G2 [60] at 1/10, mouse anti-scute 5A10 [95]. Blots were washed three times for 10 minutes each in PBST and hybridized with horseradish peroxidase-conjugated secondary antibody (Goat anti-rabbit (1∶10,000) or Goat anti-mouse (1/1000−1/10,000) from Jackson ImmunoResearch) in PBST-5% milk for two hours at room temperature. Blots were again washed three times for 10 minutes each in PBST and visualized with an enhanced chemiluminescent agent.
Immunoprecipitation, RT-PCR
Nuclear extract was prepared essentially as above except, after the first centrifugation, the pellet was resuspended in 1 ml buffer/ml embryos and sonicated. 20 ul of 50% antibody linked protein A beads were added to a 150 ul aliquot of sonicate. The mixture was allowed to rock 1 hour at room temperature, then washed as above. RNA was isolated using TRIreagent (Molecular Research Center, Inc.) then, treated with DNAse 1. Reverse transcription, PCR and Southern blotting conditions were as described above with primers as indicated in Figure 6 and Table 2. Southern blotting conditions were as described above using randomly primed Sxl 3B1Δ cDNA [39) as the probe. Antibodies used for immunoprecipitation were; anti-scute SA10, anti-Sxl 104 and 114 mixed 1∶1, and anti-eIF4E.
Western blotting
2–5 flies of each genotype were collected and frozen at −80°C. 10 ul of 2x Laemmli sample buffer per fly was added to the flies, which were then homogenized with a hand held Dounce homogenizer. Samples were boiled for 5 minutes and spun for three minutes at 14,000 rpm. Samples were diluted as needed with 2x Laemmli sample buffer and up to 10 ul of sample were loaded onto sodium dodecyl sulfate (SDS)-12% acrylamide gels, run out and transferred to Immobilon-P or nitrocellulose. Blots were incubated for 60 minutes in PBST (PBS with 1% Triton-X100), with 10% dry milk then, incubated overnight at 4°C with primary antibody at the appropriate concentration in PBST with 10% dry milk. The next day the blots were washed with PBST for at least an hour, then incubated for 2–4 hours at room temperature with secondary antibody at the appropriate concentration in PBST with 10 mg/ml BSA. Blots were washed with PBST then, developed with ECL Plus (Amersham). Primary antibodies used were: a 1∶1 mixture of anti-SXL104 and 114 at 1/10−1/1000, anti-eIF4E 1739 at 1/1000, anti-U2AF50 at 1/50,000, and anti-dFMR J11 at 1/1000. HRP conjugated goat anti-mouse and goat anti-rabbit (Jackson ImmunoResearch) secondary anti-bodies were used at 1/2500 or 1/5000.
Supporting Information
Zdroje
1. MerrickWCHersheyJWB 1996 Translational Control. Cold Spring Harbor, NY Cold Spring Harbor Laboratory
2. GingrasACRaughtBSonenbergN 1999 eIF4 Initiation Factors: Effectors of mRNA Recruitment to Ribosomes and Regulators of Translation. Annu Rev Biochem 68 913 63
3. SachsABSarnowPHentzeMW 1997 Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell 89 831 838
4. SonenbergNGingrasAC 1998 The mRNA 5' cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol 10 268 275
5. LejbkowiczFGoyerCDarveauANeronSLemieuxR 1992 A fraction of the mRNA 5' cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc Natl Acad Sci U S A 89 9612 9616
6. LangVZanchinNILunsdorfHTuiteMMcCarthyJE 1994 Initiation factor eIF-4E of Saccharomyces cerevisiae. Distribution within the cell, binding to mRNA, and consequences of its overproduction. J Biol Chem 269 6117 6123
7. DostieJLejbkowiczFSonenbergN 2000 Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J Cell Biol 148 239 247
8. CohenNSharmaMKentsisAPerezJMStrudwickS 2001 PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J 20 4547 4559
9. StrudwickSBordenKL 2002 The emerging roles of translation factor eIF4E in the nucleus. Differentiation 70 10 22
10. RousseauDKasparRRosenwaldIGehrkeLSonenbergN 1996 Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci U S A 93 1065 1070
11. TopisirovicICapiliADBordenKL 2002 Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E-dependent mRNA transport of cyclin D1 in a PML-dependent manner. Mol Cell Biol 22 6183 6198
12. TopisirovicICuljkovicBCohenNPerezJMSkrabanekL 2003 The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J 22 689 703
13. HromasRRadichJCollinsS 1993 PCR cloning of an orphan homeobox gene (PRH) preferentially expressed in myeloid and liver cells. Biochem Biophys Res Commun 195 976 983
14. ClineTWMeyerBJ 1996 Vive la difference: males vs females in flies vs worms. Annu Rev Genet 30 637 702
15. PenalvaLOSanchezL 2003 RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev 67 343 359
16. PennKMJGrahamPSchedlP 2004 Alternative Splicing: Regulation of Sex Determination in Drosophila melanogaster: Elsevier Inc
17. KeyesLNClineTWSchedlP 1992 The primary sex determination signal of Drosophila acts at the level of transcription. Cell 68 933 943
18. BellLRMaineEMSchedlPClineTW 1988 Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell 55 1037 1046
19. SamuelsMESchedlPClineTW 1991 The complex set of late transcripts from the Drosophila sex determination gene sex-lethal encodes multiple related polypeptides. Mol Cell Biol 11 3584 3602
20. SosnowskiBABeloteJMMcKeownM 1989 Sex-specific alternative splicing of RNA from the transformer gene results from sequence -dependent splice site blockage. Cell 3 449 459
21. InoueKHoshijimaKSakamotoHShimuraY 1990 Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature 344 461 463
22. ValcarcelJSinghRZamorePDGreenMR 1993 The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature 362 171 175
23. GranadinoBPenalvaLOGreenMRValcarcelJSanchezL 1997 Distinct mechanisms of splicing regulation in vivo by the Drosophila protein Sex-lethal. Proc Natl Acad Sci U S A 94 7343 7348
24. ZhouSYangYScottMJPannutiAFehrKC 1995 Male-specific lethal 2, a dosage compensation gene of Drosophila, undergoes sex-specific regulation and encodes a protein with a RING finger and a metallothionein-like cysteine cluster. EMBO J 14 2884 2895
25. BashawGJBakerBS 1997 The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell 89 789 798
26. KelleyRLWangJBellLKurodaMI 1997 Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature 387 195 199
27. GebauerFMerendinoLHentzeMWValcarcelJ 1998 The Drosophila splicing regulator sex-lethal directly inhibits translation of male-specific-lethal 2 mRNA. RNA 4 142 150
28. GebauerFCoronaDFPreissTBeckerPBHentzeMW 1999 Translational control of dosage compensation in Drosophila by Sex-lethal: cooperative silencing via the 5' and 3' UTRs of msl-2 mRNA is independent of the poly(A) tail. EMBO J 18 6146 6154
29. MerendinoLGuthSBilbaoDMartinezCValcarcelJ 1999 Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3' splice site AG. Nature 402 838 841
30. ForchPMerendinoLMartinezCValcarcelJ 2001 Modulation of msl-2 5' splice site recognition by Sex-lethal. RNA 7 1185 1191
31. BeckmannKGrskovicMGebauerFHentzeMW 2005 A dual inhibitory mechanism restricts msl-2 mRNA translation for dosage compensation in Drosophila. Cell 122 529 540
32. SuissaYKalifaYDinurTGrahamPDeshpandeG 2010 Hrp48 attenuates Sxl expression to allow for proper notch expression and signaling in wing development. PNAS 107 6930 6935
33. PennJKMSchedlP 2007 The master switch gene Sex-lethal promotes female development by negatively regulating the N-signaling pathway. Dev. Cell 12 275 286
34. GebauerFGskovicMHentzeM 2003 Drosophila Sex-lethal inhibits the stable association if the 40S ribosomal subunit with msl-2 mRNA. Mol Cell 11 1397 1404
35. AbrazaIGebauerF 2005 Functional domains of Drosophila UNR in translational control. RNA 14 482 490
36. AbrazaICollOPatalanoSGebauerF 2006 Drosophila UNR is required for translational repression of male-specific lethal 2 mRNA during regulation of X-chromosom dosage compensation. Genes Dev 20 380 389
37. DuncanKGrskovicMStreinCBeckmannKNiggewegR 2006 Sex-lethal imparts a sex-specific function to UNR by recruiting it to the msl-2 mRNA 3’UTR: translational repression for dosage compensation. Genes Dev 20 368 379
38. DuncanKEStreinCHentzeMW 2009 The SXL-UNR co-repressor complex uses a PABP-mediated mechanism to inhibit ribosome recruitment to msl-2 mRNA. Mol. Cell 36 571 582
39. YanowitzJLDeshpandeGCalhounGSchedlPD 1999 An N-terminal truncation uncouples the sex-transforming and dosage compensation functions of sex-lethal. Mol Cell Biol 19 3018 3028
40. HarperDSFrescoLDKeeneJD 1992 RNA binding specificity of a Drosophila snRNP protein that shares sequence homology with mammalian U1-A and U2-B" proteins. Nucleic Acids Res 20 3645 3650
41. FlickingerTWSalzHK 1994 The Drosophila sex determination gene snf encodes a nuclear protein with sequence and functional similarity to the mammalian U1A snRNP protein. Genes Dev 8 914 925
42. Polycarpou-SchwarzMGundersonSIKandels-LewisSSeraphinBMattajIW 1996 Drosophila SNF/D25 combines the functions of the two snRNP proteins U1A and U2B' that are encoded separately in human, potato, and yeast. RNA 2 11 23
43. DeshpandeGSamuelsMESchedlPD 1996 Sex-lethal interacts with splicing factors in vitro and in vivo. Mol Cell Biol 16 5036 5047
44. SalzHKFlickingerTW 1996 Both loss-of-function and gain-of-function mutations in snf define a role for snRNP proteins in regulating Sex-lethal pre-mRNA splicing in Drosophila development. Genetics 144 95 108
45. NagengastAAStitzingerSMTsengCHMountSMSalzHK 2003 Sex-lethal splicing autoregulation in vivo: interactions between SEX-LETHAL, the U1 snRNP and U2AF underlie male exon skipping. Development 130 463 471
46. OliverBPauliD 1998 Suppression of distinct ovo phenotypes in the Drosophila female germline by maleless - and Sex-lethal. Dev Genet 23 335 346
47. MaineEMSalzHKSchedlPClineTW 1985 Sex-lethal, a link between sex determination and sexual differentiation in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol 50 595 604
48. KrautRMenonKZinnK 2001 A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Curr Biol 11 417 30
49. LachancePEMironMRaughtBSonenbergNLaskoP 2002 Phosphorylation of Eukaryotic Translation Initiation Factor 4E Is Critical for Growth. Molec Cell Biol 22 1656 1663
50. SalzHKClineTWSchedlP 1987 Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics 117 221 231
51. AlbrechtEBSalzHK 1993 The Drosophila sex determination gene snf is utilized for the establishment of the female-specific splicing pattern of Sex-lethal. Genetics 134 801 807
52. GranadinoBTorresMBachillerDTorrojaEBarberoJL 1991 Genetic and molecular analysis of new female-specific lethal mutations at the gene Sxl of Drosophila melanogaster. Genetics 129 371 383
53. HorabinJISchedlP 1996 Splicing of the Drosophila Sex-lethal early transcripts involves exon skipping that is independent of Sex-lethal protein. RNA 2 1 10
54. ZhuCUranoJBellLR 1997 The Sex-lethal early splicing pattern uses a default mechanism dependent on the alternative 5’ splice sites l997 Mol Cell Biol 17 1674 81
55. BernsteinMLerschRASubrahmanyanLClineTW 1995 Transposon insertions causing constitutive Sex-lethal activity in Drosophila melanogaster affect Sxl sex-specific transcript splicing. Genetics 139 631 648
56. HorabinJISchedlP 1993 Sex-lethal autoregulation requires multiple cis-acting elements upstream and downstream of the male exon and appears to depend largely on controlling the use of the male exon 5' splice site. Mol Cell Biol 13 7734 7746
57. DingDParkhurstSMHalsellSRLipshitzHD 1993 Dynamic Hsp83 RNA localization during Drosophila oogenesis and embryogenesis. Mol Cell Biol 13 3773 3781
58. StitzingerSMPellicena-PalleAAlbrechtEBGajewskiKMBeckinghamKM 1999 Mutations in the predicted aspartyl tRNA synthetase of Drosophila are lethal and function as dosage sensitive maternal modifiers of the sex determination gene Sex-Lethal. Mol. Gen. Genet. 261 142 151
59. PenalvaLORuizMFOrtegaAGranadinoBVicenteL 2000 The Drosophila fl(2)d gene, required for female-specific splicing of Sxl and tra pre-mRNAs, encodes a novel nuclear protein with a HQ-rich domain. Genetics 155 : 129-39. Erratum in: Genetics 2000 Aug;155(4):following 2020
60. OretgaANiksicMBachiAWilmMSanchezL 2003 Biochemical function of female lethal 2D/Wilms’ tumor suppressor 1 associated proteins in alternative pre-mRNA splicing. J Biol Chem 278 3040-7
61. PennJKMGrahamPDeshpandeGCalhounGChaoukiAS 2008 Functioning of the Drosophila Wilms’-Tumor-1-Associated Protein Homolog, Fl(2)d, in Sex-Lethal Dependent Alternative Splicing. Genetics 178 737 48
62. GonzalazANLuHEricksonJW 2008 A shared enhancer controls a temporal switch between promoters during Drosophila primary sex determination. PNAS 105 18436 18441
63. SamuelsMEBoppDColvinRARoscignoRFGarcia-BlancoMA 1994 RNA binding by Sxl proteins in vitro and in vivo. Mol Cell Biol 14 4975 4990
64. DasRReedR 1999 Resolution of the mammalian E complex and the ATP-dependent spliceosomal complexes on native agarose mini-gels. RNA 5 1504 1508
65. DasRShouSReedR 2000 Functional association of U2 snRNP with the ATP independent splicosomal complex E. Mol Cell 5 779 787
66. KentOARitchieDBMacMillanAM 2005 Characterization of a U2AF-independent commitment complex (E') in the mammalian spliceosome assembly pathway. Mol Cell Biol 25 233 40
67. SharmaSFalickAMBlackDL 2005 Polypyrimidine tract binding protein blocks the 5' splice site-dependent assembly of U2AF and the prespliceosomal E complex. Mol Cell 19 19 485 96
68. BlackDL 2003 Mechanisms of alternative pre-messenger RNA splicing. Ann. Rev. Biochem. 72 291 336
69. HouseAELynchKW 2008 Regulation of alternative splicing: more than just the ABCs J Biol Chem 283 1217 1221
70. JuricaMSMooreMJ 2003 Pre-mRNA splicing: awash in a sea of proteins. Mol Cell 12 5 14
71. StarkHLuhrmannR 2006 Cryo-electron microscopy of spliceosomal components. Annu Rev Biophys Biomol Struct 35 435 57
72. DonmezGHartmuthKKastnerBWillCLLuhrmannR 2007 The 5' end of U2 snRNA is in close proximity to U1 and functional sites of the pre-mRNA in early spliceosomal complexes. Mol Cell 25 399 411
73. SpadacciniRReidtUDybkovOWillCFRStierG 2006 Biochemical and NMR analyses of an SF3b155-p14-U2AF-RNA interaction network involved in branch point definition during pre-mRNA splicing. RNA 12 410 25
74. DeckertJHartmuthKBoehringerDNastaranBWillCL 2006 Protein composition and electron microscopy structure of affinity-purified human spliceosomal B complexes isolated under physiological conditions. Mol Cell Biol 26 5528 5543
75. StaleyJPGuthrieC 1998 Mechanical devices of the spliceosome: motors, clocks, springs and things. Cell 92 315 326
76. MakarovEMMakarovaOVUrlaubHGentzeMWillCL 2002 Small nuclear ribonucleoprotein remodeling during catalytic activation of the spliceosome. Science 298 2205 2208
77. GrandinoBCampuzanoSSanchezL 1990 The Drosophila melanogaster fl(2)d gene is needed for the female-specific splicing of Sex-lethal RNA. EMBO J 9 2597 602
78. ZhangGTanejaKLSingerRHGreenMR 1994 Localization of pre-mRNA splicing in mammalian nuclei. Nature 372 809 812
79. BeyerLOsheimYN 1988 Splice site selection, rate of splicing and alternative splicing on nascent transcripts. Genes Dev 2 754 765
80. BaurenGWieslanderL 1994 Splicing of Balbiani ring 1 gene pre-mRNA occurs simultaneously with transcription Cell 76 183 192
81. ListermanISapraAKNeugebauerKM 2006 Cotranscriptional coupling of splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. Nat Struct Mol Biol 13 815 822
82. Pandya-JonesABlackD 2009 Co-transcriptional splicing of constitutive and alternative exons. RNA 15 1896 1908
83. SinghJPadgettRA 2009 Rates of in situ transcription and splicing in large human genes. Nat Struct Mol Biol 16 1128 1133
84. VargasDYShahKSinhaSMarras SalvatoreAE 2010 Single Molecule Imaging of Transcriptionally Coupled and Uncoupled Splicing. Cell In press
85. LallenaMJChalmersKJLlamazaresSLamondAIValcarcelJ 2002 Splicing regulation at the second catalytic step by Sex-lethal involves 3' splice site recognition by SPF45. Cell 109 285 96
86. HarmuthKUrlaubHVornlocherHPWillCLGentzelM 2002 Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc Natl Acad Sci U S A 99 16719 16724
87. JuricaMSLickliderLJGygiSRGrigorieffNMooreMJ 2002 Purification and characterization of native spliceosomes suitable for native three dimensional structural analysis. RNA 8 426 439
88. Lim SR, HertelKJ 2004 Commitment to splice site pairing coincides with A complex formation. Mol. Cell 15 477 483
89. Lindsley DL, ZimmGG 1992 The genome of Drosophila melanogaster. San Diego, CA Academic Press
90. BellLRHorabinJISchedlPClineTW 1991 Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 65 229 239
91. FrohmanMADushMKMartinGR 1988 Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A 85 8998 9002
92. LavoieCALachancePEDSonenbergNLaskoP 1996 Alternatively spliced transcripts from the Drosophila eIF4E gene produce two different cap-binding proteins. J Biol Chem 271 16393 16398
93. RudnerDZKanaarRBregerKSRioD 1998 Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol Cell Biol 18 1765 1773
94. RudnerDZKanaarRBregerKSRioD 1996 Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects. Proc. Natl. Acad. Sci. USA 93 10333 10337
95. DeshpandeGStukeyJSchedlP 1995 scute (sis-b) function in Drosophila sex determination. Mol Cell Biol 15 4430 40
Štítky
Genetika Reprodukční medicína
Článek Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal MutationsČlánek Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene ExpressionČlánek Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary IntegrityČlánek A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with ExpressionČlánek Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line PedigreeČlánek Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 7
-
Všechny články tohoto čísla
- Gene-Based Tests of Association
- The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
- Variants in and Underlie Natural Variation in Translation Termination Efficiency in
- SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
- Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations
- Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer
- Identification of a Mutation Associated with Fatal Foal Immunodeficiency Syndrome in the Fell and Dales Pony
- Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
- An Epigenetic Switch Involving Overlapping Fur and DNA Methylation Optimizes Expression of a Type VI Secretion Gene Cluster
- Recombination and Population Structure in
- A Rice Plastidial Nucleotide Sugar Epimerase Is Involved in Galactolipid Biosynthesis and Improves Photosynthetic Efficiency
- A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets
- Colon Stem Cell and Crypt Dynamics Exposed by Cell Lineage Reconstruction
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Interactions between Glucocorticoid Treatment and Cis-Regulatory Polymorphisms Contribute to Cellular Response Phenotypes
- Translation Reinitiation Relies on the Interaction between eIF3a/TIF32 and Progressively Folded -Acting mRNA Elements Preceding Short uORFs
- DAF-12 Regulates a Connected Network of Genes to Ensure Robust Developmental Decisions
- Adult Circadian Behavior in Requires Developmental Expression of , But Not
- Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary Integrity
- Proteins in the Nutrient-Sensing and DNA Damage Checkpoint Pathways Cooperate to Restrain Mitotic Progression following DNA Damage
- Complex Evolutionary Events at a Tandem Cluster of Genes Resulting in a Single-Locus Genetic Incompatibility
- () and Its Regulated Homeodomain Gene Mediate Abscisic Acid Response in
- A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
- LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs
- Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications
- The Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
- Somatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
- Molecular Mechanisms Generating and Stabilizing Terminal 22q13 Deletions in 44 Subjects with Phelan/McDermid Syndrome
- Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line Pedigree
- Mechanisms of Chromosome Number Evolution in Yeast
- Regulatory Cross-Talk Links Chromosome II Replication and Segregation
- Ancestral Genes Can Control the Ability of Horizontally Acquired Loci to Confer New Traits
- Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
- Rare and Common Regulatory Variation in Population-Scale Sequenced Human Genomes
- The Epistatic Relationship between BRCA2 and the Other RAD51 Mediators in Homologous Recombination
- Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy
- NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
- Araucan and Caupolican Integrate Intrinsic and Signalling Inputs for the Acquisition by Muscle Progenitors of the Lateral Transverse Fate
- Pathologic and Phenotypic Alterations in a Mouse Expressing a Connexin47 Missense Mutation That Causes Pelizaeus-Merzbacher–Like Disease in Humans
- Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination
- Epistatic Relationships in the BRCA1-BRCA2 Pathway
- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium
- Fine Mapping of Five Loci Associated with Low-Density Lipoprotein Cholesterol Detects Variants That Double the Explained Heritability
- CHD1 Remodels Chromatin and Influences Transient DNA Methylation at the Clock Gene
- Nonlinear Fitness Landscape of a Molecular Pathway
- Genome-Wide Scan Identifies , , and as Novel Risk Loci for Systemic Sclerosis
- Quantitative and Qualitative Stem Rust Resistance Factors in Barley Are Associated with Transcriptional Suppression of Defense Regulons
- A Systematic Screen for Tube Morphogenesis and Branching Genes in the Tracheal System
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Gene-Based Tests of Association
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



