-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLoss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
Ophthalmo-acromelic syndrome (OAS), also known as Waardenburg Anophthalmia syndrome, is defined by the combination of eye malformations, most commonly bilateral anophthalmia, with post-axial oligosyndactyly. Homozygosity mapping and subsequent targeted mutation analysis of a locus on 14q24.2 identified homozygous mutations in SMOC1 (SPARC-related modular calcium binding 1) in eight unrelated families. Four of these mutations are nonsense, two frame-shift, and two missense. The missense mutations are both in the second Thyroglobulin Type-1 (Tg1) domain of the protein. The orthologous gene in the mouse, Smoc1, shows site - and stage-specific expression during eye, limb, craniofacial, and somite development. We also report a targeted pre-conditional gene-trap mutation of Smoc1 (Smoc1tm1a) that reduces mRNA to ∼10% of wild-type levels. This gene-trap results in highly penetrant hindlimb post-axial oligosyndactyly in homozygous mutant animals (Smoc1tm1a/tm1a). Eye malformations, most commonly coloboma, and cleft palate occur in a significant proportion of Smoc1tm1a/tm1a embryos and pups. Thus partial loss of Smoc-1 results in a convincing phenocopy of the human disease. SMOC-1 is one of the two mammalian paralogs of Drosophila Pentagone, an inhibitor of decapentaplegic. The orthologous gene in Xenopus laevis, Smoc-1, also functions as a Bone Morphogenic Protein (BMP) antagonist in early embryogenesis. Loss of BMP antagonism during mammalian development provides a plausible explanation for both the limb and eye phenotype in humans and mice.
Published in the journal: . PLoS Genet 7(7): e32767. doi:10.1371/journal.pgen.1002114
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002114Summary
Ophthalmo-acromelic syndrome (OAS), also known as Waardenburg Anophthalmia syndrome, is defined by the combination of eye malformations, most commonly bilateral anophthalmia, with post-axial oligosyndactyly. Homozygosity mapping and subsequent targeted mutation analysis of a locus on 14q24.2 identified homozygous mutations in SMOC1 (SPARC-related modular calcium binding 1) in eight unrelated families. Four of these mutations are nonsense, two frame-shift, and two missense. The missense mutations are both in the second Thyroglobulin Type-1 (Tg1) domain of the protein. The orthologous gene in the mouse, Smoc1, shows site - and stage-specific expression during eye, limb, craniofacial, and somite development. We also report a targeted pre-conditional gene-trap mutation of Smoc1 (Smoc1tm1a) that reduces mRNA to ∼10% of wild-type levels. This gene-trap results in highly penetrant hindlimb post-axial oligosyndactyly in homozygous mutant animals (Smoc1tm1a/tm1a). Eye malformations, most commonly coloboma, and cleft palate occur in a significant proportion of Smoc1tm1a/tm1a embryos and pups. Thus partial loss of Smoc-1 results in a convincing phenocopy of the human disease. SMOC-1 is one of the two mammalian paralogs of Drosophila Pentagone, an inhibitor of decapentaplegic. The orthologous gene in Xenopus laevis, Smoc-1, also functions as a Bone Morphogenic Protein (BMP) antagonist in early embryogenesis. Loss of BMP antagonism during mammalian development provides a plausible explanation for both the limb and eye phenotype in humans and mice.
Introduction
Congenital absence of an eye (here termed anophthalmia) is a rare malformation in humans with a live birth prevalence of less than 1 in 10,000 [1]. Identifiable single gene disorders account for ∼25% of bilateral anophthalmia. The known genetic causes include compound heterozygous mutations in PAX6, de novo heterozygous loss-of-function mutations in SOX2 [2]–[4], inherited or de novo heterozygous loss-of-function mutations in OTX2 [5], [6], homozygous loss-of-function mutations in STRA6 [7] and possibly inherited, heterozygous loss-of-function mutations in BMP4 [8]. In most cases of anophthalmia no eye is visible on clinical examination but optic nerves, chiasm and optic tracts remnants are visible on magnetic resonance imaging. Absence of the eye with ipsilateral absence of optic nerves, chiasm and optic tracts is termed true anophthalmia and is taken to suggest very early failure of ocular development.
Ophthalmo–acromelic syndrome (OAS), also known as Waardenburg anophthalmia syndrome, is one of the most frequently reported causes of true anophthalmia, which occurs in association with a distinctive pattern of distal limb anomalies (Figure 1a–1c). OAS is an autosomal recessive disorder (MIM #206920) first reported 50 years ago by Waardenburg in two unrelated families [9]. This original report illustrated the phenotypic spectrum associated with this disorder. The first family was a sibship of nine and consisted of two sisters with unilateral anophthalmia, one of whom had coloboma in her contralateral eye. Both had bilateral synostosis of the 4th and 5th metacarpals and bilateral postaxial four-toe oligodactyly with soft tissue syndactyly. A brother had similar limb involvement but with normal eyes and the other siblings were reported as normal. In the second family, the proband was a girl with significant learning disability, bilateral anophthalmia, short fingers bilaterally and postaxial oligodactyly with four toes on both feet. Her younger sister was normal. 32 further cases of OAS in 23 different families have been reported [10]–[29]. Of the reported definite cases; 32/35 (91.4%) had anophthalmia (4∶28 for unilateral:bilateral), 29 (82.9%) had lower limb postaxial oligodactyly, 20 (57.1%) had fusion of metacarpals 4–5 and 13 (37.1%) had learning disability. Other recurrent features included orofacial clefts (4/35) and perinatal or early postnatal death (10/35) in the 25 families. At the point of submission of this paper, very little was known of the molecular basis of OAS. A locus on 10p11.23 with a reported LOD score >3 had been suggested on the basis of linkage analysis in three unrelated families. However, no pathogenic mutations could be identified in any of the genes in the linkage interval [17].
Fig. 1. Mapping ophthalmo-acromelic syndrome. 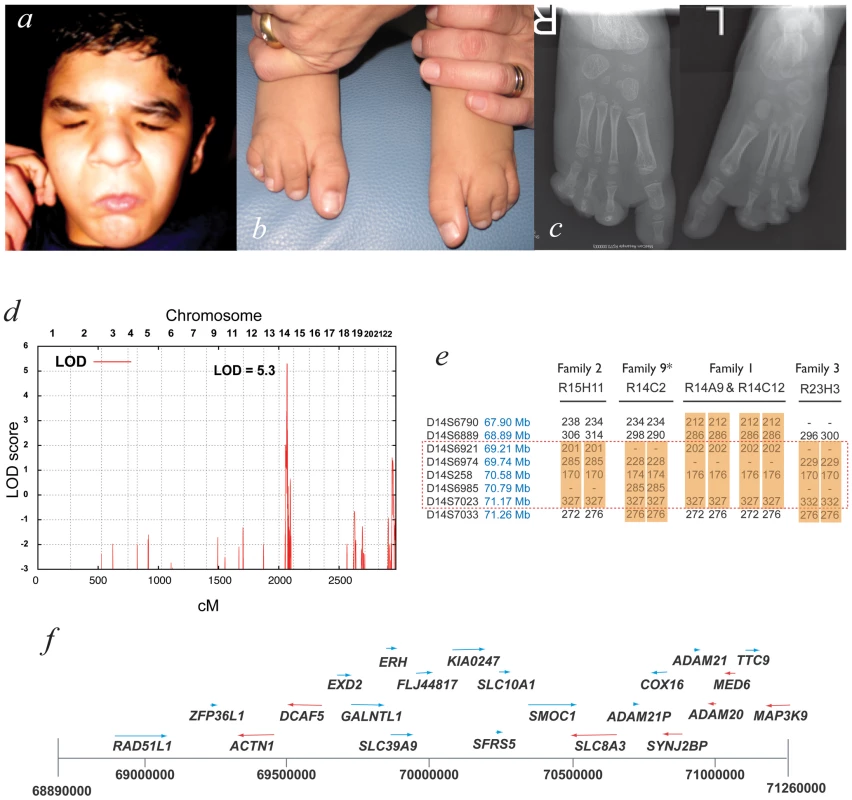
Clinical photographs (a,b) and radiographs (c) of patient R14C12 showing bilateral anophthalmia, in association with bilateral postaxial oligodactyly and cutaneous syndactyly of 2nd & 3rd toes. (d) Multipoint linkage analysis using 10K SNPchip data from families 1–3 showing a significant LOD score of Z = 5.3 at 14q22.3–24.2, a region also identified by autozygosity mapping (see Table 1). (e) Microsatellite marker analysis for affected individuals in Families 1–3 and Family 9 showing region of homozygosity, but no common haplotype. (f) The microsatellite data refined the OAS candidate interval to Chr14∶69.89–71.26 Mb which is shown diagrammatically with the 22 annotated genes that were sequenced in this study (UCSC assembly GRCh37). Bone morphogenetic proteins (BMPs) account for 10 of the 33 members of the transforming growth factor beta (TGFβ) superfamily of peptide growth factors in humans and are encoded by the genes BMP2-7, BMP8A, BMP8B, BMP10 and BMP15 (BMP1 does not encode a growth factor but a tolloid-like protease[30]). BMPs are secreted into the extracellular space where they bind to BMP type I serine-threonine kinase cell surface receptors encoded by BMPR1A and BMPR1B. The presence of BMP type I receptors appears sufficient for BMP binding, but a BMP type II receptor (encoded by BMPR2, ACVR2A and ACVR2B) is required for phosphorylation of the BMP type I receptors, endocytosis and activation of the signal transduction cascade [31]. The intracellular domain of the activated BMP type I receptors in turn phosphorylates a Ser-Ser-X-Ser (SSXS) motif at the C-terminal end of one of three homologous protein products of the human genes SMAD1, SMAD5 and SMAD9. Phosphorylated SMAD1/5/9 (pSMAD1/5/9 known as regulatory - or R-SMADs) then bind to the co-SMAD encoded by SMAD4. The co-SMAD/R-SMAD complex then translocates to the nucleus where it functions as a transcription factor mediating the activation of target genes [32]. It has recently become clear that BMP signaling can also directly induce the activation of the MAPK pathway [33].
The formation of BMP signaling gradients is used extensively throughout vertebrate embryonic development. The formation and maintenance of stable developmental gradients appears to require multiple mechanisms to balance agonistic and antagonist effects on BMP signaling. The complexity of the system is demonstrated by the molecular basis of dorsal and ventral signaling centres in the gastrula of Xenopus laevis embryos [34]. The dorsal signaling centre (DSC; Spemann's organizer) has the general effect of antagonizing the Bmp gradient from the ventral signaling centre. The DSC secretes noggin and chordin, which (together with twisted-gastrulation [35]) bind to bmp in the extracellular space and prevents binding to the bmp type I receptor. The ventral signaling centre (VSC) secretes bmp4 and bmp7 but also bmper (bmp-binding endothelial regulator) [36] and sizzled, which inhibits tolloid-like 1, a zinc metalloproteinase that efficiently cleaves chordin [37]. The VSC also produces bambi (bmp and activin membrane-bound inhibitor), a bmp receptor that lacks the catalytic intracellular domain and thus acts dominant-negatively to inhibit bmp signaling [38].
SMOC-1 is encoded by the human gene SMOC1 (SPARC related modular calcium binding 1) and was initially characterised as a basement membrane protein with significant homology to BM-40 (also known as SPARC and osteonectin) [39]. The domain structure of the SMOC-1 peptide and the close homolog SMOC-2 [40], is evolutionarily conserved [41] and consists from N - to C-terminus of a follistatin-like domain, two thyroglobulin type I (Tg1) domains and an EF-hand calcium-binding domain. The ortholog of SMOC-1 in Xenopus laevis, XSMOC-1, has been shown to function as a BMP antagonist. Uniquely among the known peptide BMP antagonists, SMOC-1 was able to antagonize BMP activity in the presence of a constitutively active BMP receptor. The molecular basis of this antagonism is not yet clear but may function by stimulating MAPK-mediated phosphorylation of the linker (i.e. non-SSXS) region of the R-SMAD proteins [42].
We report the identification of a locus for OAS on 14q24.2 with subsequent identification of homozygous, predicted loss-of-function mutations in the SMOC1 gene in eight out of fourteen unrelated families with OAS. Whole mount in situ hybridisation (WISH) combined with optical projection tomography (OPT) shows site - and stage-specific developmental expression of the orthologous mouse gene, Smoc1, in embryonic limb bud and craniofacial structures. The phenotype associated with homozygosity for a targeted “pre-conditional” gene-trap mouse mutation of Smoc1 also shows significant overlap with the human disease. SMOC-1 and SMOC-2 appear to be the two vertebrate paralogs of the Drosophila protein Pentagone that has recently been shown to function as an antagonist of Decapentaplegic (Dpp) signaling in vivo [33]. We discuss the potential role for SMOC-1 in modulating BMP signaling during eye and limb development.
Results
Mapping and Mutation Analysis
A locus for OAS at 14q24.2 was identified using autozygosity mapping with 10K SNP chip data from multiple, apparently unrelated consanguineous pedigrees. Affected individuals from eight of the fourteen families showed tracks of >20 homozygous SNPs in a row at this locus (Dataset S1). This locus was confirmed with multipoint linkage analysis using data from three of these families giving a combined LOD score of 5.3 (Figure 1d). The critical region was narrowed to ∼1 Mb using microsatellite markers in four families (Figure 1e). To identify the causative gene, all coding exons for each gene in the critical region were sequenced (Figure 1f).
Potentially deleterious mutations were identified in only one gene: SMOC1, and independent homozygous SMOC1 mutations were found in eight out of fourteen families (Figure 2a, Table 1). Of these, 6 mutations predicted complete loss of protein function; 4 are nonsense mutations and 2 are single base deletions or insertions resulting in a frameshift. Two different missense changes were identified, both are in the C-terminal region of the second thyroglobulin type I domain of SMOC-1 (Figure 2b). No mutations were identified in sequence analysis of the SMOC1 coding region in 190 healthy blood donors.
Fig. 2. Mutation analysis. 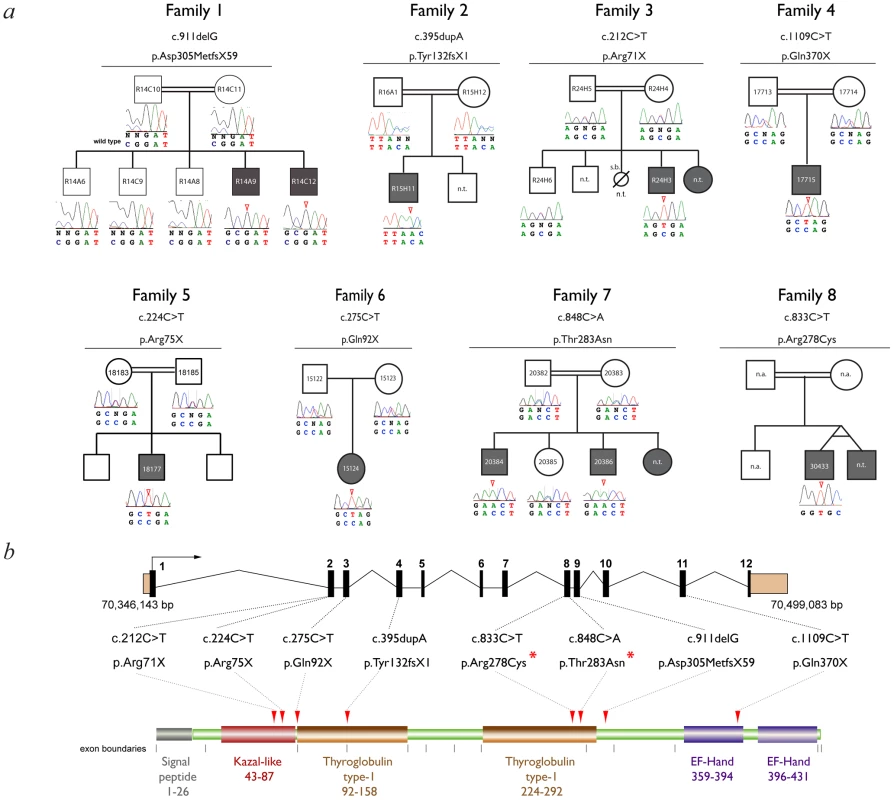
(a) Family pedigrees and associated SMOC1 mutations identified. The pedigree for Family 1 is representative and shows segregation of a homozygous SMOC1 mutation (c.911delG; p.Asp305MetfsX59) in affected individuals with both parents (and all unaffected sibs) being heterozygous carriers. (b) Schematic of the SMOC1 gene (top) and predicted protein (below), illustrating the exon positions for all eight mutations identified in the OAS families. Coding exons are coloured black and numbered, UTRs are brown, protein domains are labeled with amino acid residue numbers. Red arrowheads indicate the position of the mutations in the peptide. Red asterisks highlight the missense changes, which are located in the second thyroglobulin domain thought to be involved in the control of proteolytic degradation (n.t.- Sample not tested). Tab. 1. Clinical features and mutations in affected individuals with Ophthalmo-Acromelic Syndrome. 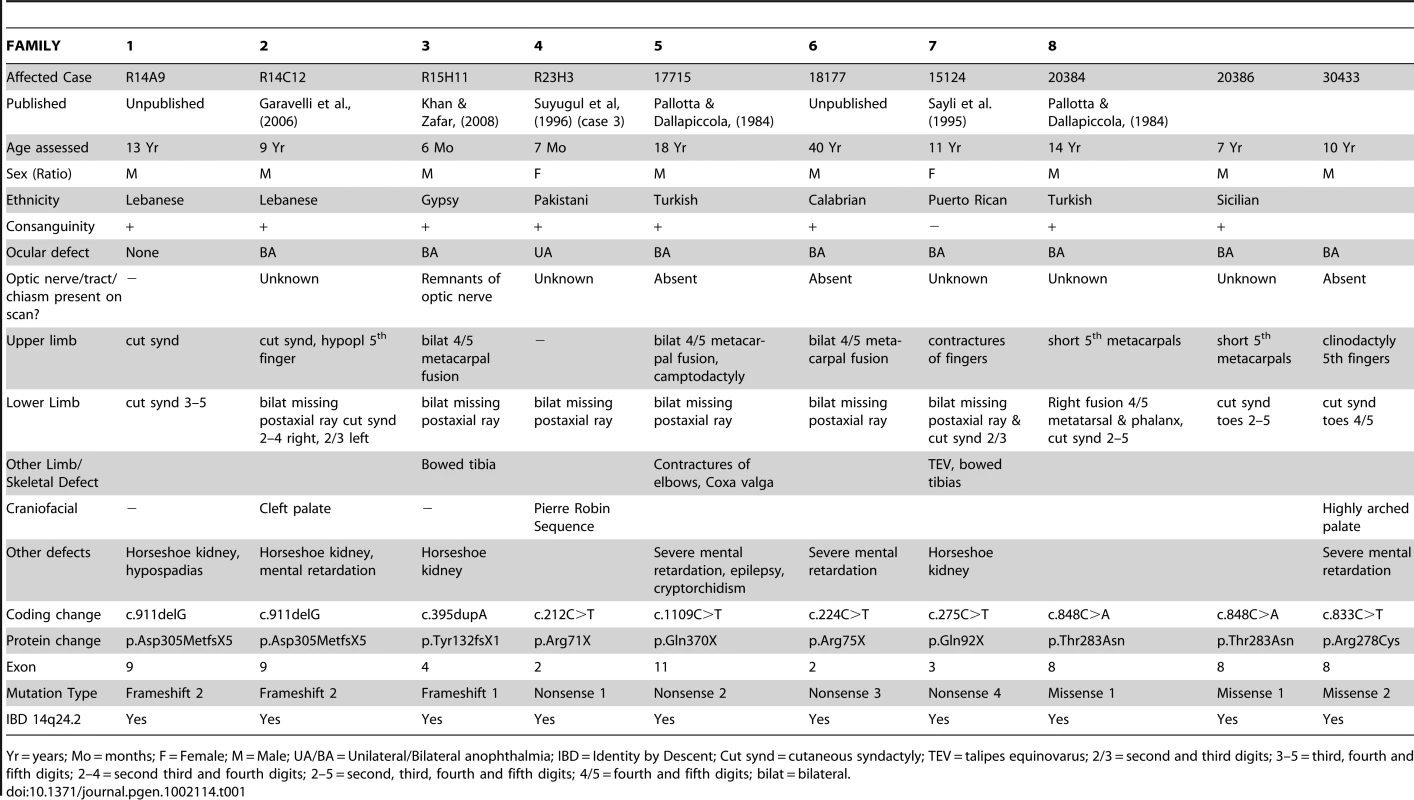
Yr = years; Mo = months; F = Female; M = Male; UA/BA = Unilateral/Bilateral anophthalmia; IBD = Identity by Descent; Cut synd = cutaneous syndactyly; TEV = talipes equinovarus; 2/3 = second and third digits; 3–5 = third, fourth and fifth digits; 2–4 = second third and fourth digits; 2–5 = second, third, fourth and fifth digits; 4/5 = fourth and fifth digits; bilat = bilateral. No mutations in SMOC1 could be identified in 6 of the 14 families. There were no obvious phenotypic differences between the cases with and without SMOC1 mutations; all have classical OAS. SNP and microsatellite data on two of the six families without detectable mutations showed large regions of homozygosity across the 14q24 region containing SMOC1. This suggests that we have missed a mutation within the transcription unit or that there may be a regulatory mutation impairing SMOC1 transcription. In the four remaining families no plausible locus could be identified using homozygosity mapping.
Expression Analysis of Smoc1
As a first step to determining the likely developmental role of SMOC1 we undertook developmental expression analysis of the orthologous gene Smoc1 in whole mouse embryos. WISH analysis with an antisense riboprobe specific to Smoc1 and optical projection tomography (OPT) were used to create a 3D representation of both the anatomy and colorimetric staining. We found site - and stage-specific expression of Smoc1 at all stages examined (Figure 3a–3b; Video S1; Video S2). Staining in the limb bud was particularly interesting with expression seen first in the very early limb bud anlage from 9.5 dpc (Figure 3a). At 10.5 dpc the limb expression distinctly localised to both the dorsal and ventral surfaces of the forelimb, but was predominantly dorsal in the hindlimb bud (Figure 3b, Figure S1). Strong expression was seen in the developing pharyngeal arches and the frontonasal region with low-level expression in the ectoderm overlying the developing optic vesicle (Figure S1). Using WISH and OPT no clear expression of Smoc1 was detected in the optic vesicle itself. There was clear expression in the developing somites at E9.5 and E10.5 (Figure 3a, 3b). At E9.5 there was also staining in the hindbrain (Figure 3a) and at E10.5 strong staining in the dorsal neural tube (Figure 3b).
Fig. 3. A targeted Smoc1 mutation caused an ophthalmo-acromelic-like phenotype in mice. 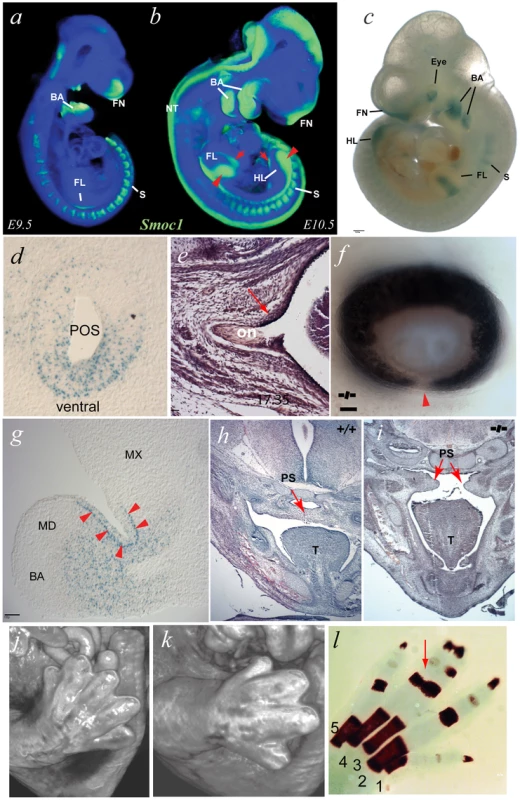
(a) OPT representation of wild type (WT) Smoc1 expression at embryonic day (E) 9.5 (green represents Smoc1 expression); Smoc1 is expressed in the pharyngeal (branchial) arches (BA), the rostral neural tube (NT), in the anlage of the forelimbs (FL), the fronto-nasal region (FN), and in the somites (S). (b) At E10.5, expression is maintained in the branchial arches, somites and in the frontal nasal processes, as well as extending caudally in the neural tube. (c) In E10.5 Smoc1tm1a/tm1a embryos, β-galactosidase activity was observed in tissues consistent with the OPT analysis of WT Smoc1 expression: in the dorsal hindlimbs; in the medial regions of dorsal and ventral forelimbs, the branchial arches, in the frontonasal processes, and in the somites. In addition, strong signal was observed in the eye region (scale bar = 500 µm). (d) X-gal stained sagittal sections of a representative E10.5 Smoc1tm1a/tm1a embryo in the developing eye showing that expression was restricted to ventral regions of the presumptive optic stalk (POS). (e) Examination of optic nerve morphology identified an extension of the RPE into the dorsal optic nerve in mutant animals compared to control. (f) Photographs of Smoc1tm1a/tm1a eye showing an optic fissure closure defect (arrowhead) consistent with coloboma (scale bars = 100 µm). (g) Expression in the 1st branchial arch mesenchyme was distributed in proximal regions and absent from distal areas, with positive signal also seen in the epithelial cells at the hinge region between maxilliary (MX) and mandibular (MD) components (arrowheads) (scale bar = 100 µm). (h,i) Pictomicrographs of sections through E14.5 heads showing a failure in palatal shelf (PS) fusion in the developing palate in the Smoc1tm1a/tm1a embryo (i) compared to the fully fused WT littermate (h). (j,k) Surface rendered visualization of OPT reconstructions of hindlimbs at E14.5. (j) WT embryo with normal arrangement of 5 digits in the hindlimb whereas the Smoc1tm1a/tm1a littermate (k) had hindlimb oligodactyly affecting the axial digits, with only 4 digits present. (l) Skeletal preparation of P0 Smoc1tm1a/tm1a hindlimb with osseous fusion of the phalanges of digits 3–4 (red arrow). Targeted Mutation in Mouse Smoc1
In order to determine if the non-redundant developmental role of SMOC1 is evolutionarily conserved we obtained mice with a targeted Pre-Conditional mutation in Smoc1 containing a LacZ reporter allele created as part of the EUCOMM project [43]. The integrated location and the details of the targeting construct are shown in Figure S1d. The Smoc1tm1a/tm1a mice have ∼10% of wild-type levels of Smoc1 mRNA during development (Figure S1f), presumably as a result of splicing across the gene-trap insertion.
Using the LacZ reporter in targeted animals, we were able to further assess the developmental expression of Smoc1 (Figure 3c, 3d, and 3g). Spatial and temporal expression data were similar between Smoc1+/tm1a and Smoc1tm1a/tm1a genotypes, but with less intense staining in Smoc1+/tm1a mice (data not shown). The X-gal staining at E10.5 of both whole mount embryos and cryosections was consistent with the wild type OPT data. Cryosections through the maxilliary-mandibular hinge region of the 1st pharyngeal (branchial) arch at E10.5 showed striking regional specificity of gene activation (Figure 3g). The expression was present within the mesenchyme or neural crest of both components of the first arch but is most intense in the sub-epithelial mesenchyme within the hinge region. Sharp boundaries of expression are evident in the subepithelial mesenchyme (Figure 3g). The only major difference between the riboprobe WISH analysis and the X-gal staining was the strong expression identified within the developing eye seen in the latter but not the former (Figure 3c). This staining was particularly strong at E10.5 within the ventral aspect of the developing optic nerve (Figure 3d).
Further phenotypic analysis of mutant animals showed that limb malformations were present in a high proportion of embryos and pups (Table 2). Oligodactyly (+/ − fibular agenesis) or osseous syndactyly of both hindlimbs was present in 11/12 of Smoc1tm1a/tm1a animals (Figure 3j–3k and Figure S1). Smoc1tm1a/tm1a embryos and neonates were also smaller than littermates but did not appear to have any other major malformations (Figure S2). To date all Smoc1tm1a/tm1a animals allowed to litter have died at or soon after birth. A proportion of this perinatal lethality is likely be related to the presence of cleft palate in a significant proportion (4 of 12) of the Smoc1tm1a/tm1a animals (Figure 3i). However it is not clear what accounts for the mortality in the non-cleft cases. Interestingly, unexplained perinatal lethality is reported in human OAS families (see Introduction).
Tab. 2. Phenotypes identified in Smoc1-targeted mice. 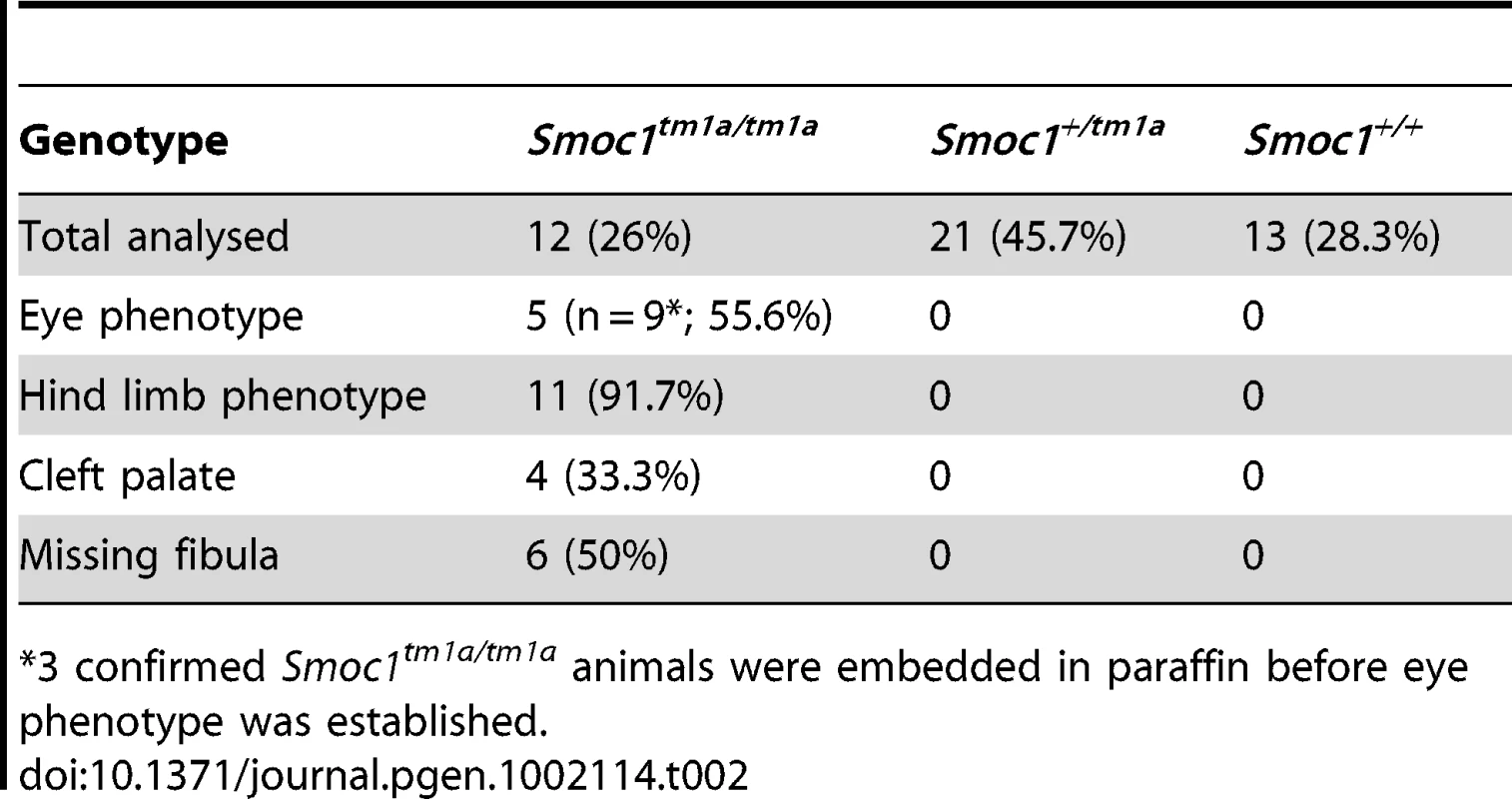
*3 confirmed Smoc1tm1a/tm1a animals were embedded in paraffin before eye phenotype was established. Eye malformations were apparent in a significant proportion of the homozygous embryos or pups. We observed iris and retinal coloboma in 55.6% of the homozygous animals that could be accurately phenotyped (Figure 3f). Extension of the retinal pigmented epithelium (RPE) into the optic nerve (figure 3e), and a reduction in optic nerve diameters (Figure S2) was also observed in these mice.
Discussion
We report compelling evidence that loss-of-function mutations in SMOC1 cause a significant subset of OAS cases. No non-synonymous changes were found in any region of the SMOC1 coding region in the 190 control individuals that were fully sequenced but we identified eight mutations within OAS families that are all different and homozygous. Six of these mutations (four nonsense and two frame-shift) are very likely to result in severe or complete abrogation of protein function. The two missense mutations (p.Arg278Cys & p.Thr283Asn) we have identified are both located in the second thyroglobulin type-1 domain (Tg1) of SMOC-1. Tg1 domains are cysteine-rich motifs that were first identified in the C-terminal region of thyroglobulin which appear to function as peptidase inhibitors, specifically inhibitors of cysteine cathepsins [44], [45]. Neither Arg278 or Thr283 show identity at the equivalent residue within the first Tg1 in human SMOC-1. However, both residues show complete conservation with the second Tg1 in both mouse and Xenopus tropicalis Smoc-1 and Thr283 is conserved in the second Tg1 in Drosophila Pentagone (see Figure S3). The mutation of Arg278 to Cys may disrupt the highly conserved pattern of disulphide bonding within the second Tg1 [41]. Given that there is no obvious difference between the missense mutation cases and those with null mutations, it is reasonable to speculate that inhibition of a developmentally expressed peptidase, possibly a cysteine cathepsin, may be the non-redundant developmental function of SMOC-1. Interestingly, Cathepsin H has been shown to be involved in Bmp4 degradation during lung development [46]. It is also possible that a mutation resulting in constitutive activation of the target peptidase could phenocopy SMOC1 mutations.
The expression analysis and targeted partial gene inactivation in mouse embryos strongly supports the critical and non-redundant developmental role of SMOC-1 suggested by the human genetic analysis and that this role is conserved across evolutionary time. The hindlimb phenotype in Smoc1tm1a/tm1a homozygous mice was very similar to the lower limb phenotype in human OAS cases. The combination of osseous syndactyly and oligodactyly suggest that the mechanisms controlling digit number within the limb bud are significantly impaired. The control of digit number is critically dependent on correct dosage of sonic hedghog (Shh) [47], [48] and BMP4 & BMP7 signaling proteins [49], [50]. A significant proportion of the Smoc1tm1a/tm1a homozygous mice have cleft palate, a feature common to human OAS and consistent with the high level of expression of Smoc1 that was detected in the developing first pharyngeal arch. The eye malformations seen in the mouse were less severe than those seen in human OAS cases, being predominantly iris and retinal coloboma. This may relate to the difference in mutation type between the mouse and human cases: most human mutations are apparently null, whereas the mouse line had 10% of normal Smoc1 transcript levels present, most likely due to splicing across the gene trap. This level of Smoc-1 function could partially rescue the ocular phenotype in the mice. Analysis of the phenotype associated with a null allele in mice that we are currently making will answer this question. We will also screen a cohort of human patients to determine if hypomorphic mutations in SMOC1 may cause coloboma.
The formation of precise gradients of diffusible ligands is required during embryogenesis both for patterning - the formation of complex tissue structures from apparently homogenous populations of multipotent cells - and to control growth to achieve correct final organ size with appropriate symmetry of paired structures. BMPs represent an important class of diffusible ligands with roles in both patterning and control of organ size [51], [52]. Much of what we know about the formation, maintenance and function of BMP gradients derived from studies of Drosophila Decapentaplegic (Dpp) [53]. BMPs are considered to be the mammalian paralogs of Dpp. SMOC-1 and its close homolog SMOC-2 appear to be the mammalian paralogs of Drosophila Pentagone (Pent) [54]. Pent functions as an in vivo antagonist of Dpp by preventing receptor endocytosis close to its source thus allowing gradients to form over wider distances within the wing imaginal discs. Lack of Pent results in a very steep and narrow gradient of Dpp signaling, which in turn causes a relative deficiency of Dpp further from the source. In Drosophila the control of cell proliferation within the wing imaginal disc is dependent on Dpp signaling [55]. In the chick it has been shown that the level of cell proliferation within the limb bud must be precisely specified to in order to result in sufficient antero-posterior expansion to form the correct digit number [48]. If a similar mechanism exists in vertebrates then it may be that SMOC1/Smoc1 mutations could cause oligodactyly by altering the BMP gradient within the limb bud and thus alter anteroposterior expansion. Although the molecular basis of the developmental pathology associated with OAS remains to be elucidated, support for SMOC-1 mediated BMP antagonism as a component is provided by human and mouse genetic data that indicate the importance of BMP signalling in both limb [56], [57] and eye [8], [58], [59] development. Interestingly, heterozygous BMP4 mutations have been associated with microphthalmia, microcornea, coloboma, retinal dystrophy, and tilted optic disc [8]. In addition, BMP4 mutations are also associated with digital anomalies (polydactyly) and cleft lip/palate [60]. The partial overlap between the OAS phenotype and the phenotypes associated with BMP4 disruptions may reflect a functional relationship between SMOC-1 and BMP4.
Following submission of this paper two other groups have identified SMOC1 mutations as a cause of OAS [10], [61]. One group studied five affected individuals from four unrelated OAS families [61]. They identified the locus on 14q24 and then found SMOC1 mutations in three out of four of the families. Interestingly these were the same families in whom linkage to 10p11.23 had been previously reported by the same group [17]. The SMOC1 mutations were all homozygous and plausibly loss of function (one nonsense and two 5′ splice site mutations). This group also reported the phenotype in homozygous mice with Sleeping beauty transposon-induced gene trap mutations of Smoc1. The expression analysis and limb phenotype of the mice are very similar to that reported here. Their homozygous mice also showed unexplained uniform early lethality. Interestingly their mice had small eyes but they do not report coloboma. The optic nerves were shown to be significantly narrower than non-homozygous animals and they also showed extension of the RPE into the optic nerve. The second paper reports linkage to 14q24 and identification of a 5′ splice site mutation in SMOC1 in a single multiplex family with OAS [10]. This group also reports developmental expression analysis of the orthologous gene, smoc1, in zebrafish embryos. Expression was evident in the brain, choroid fissure and pharyngeal arches. “Knock-down” experiments using a morpholino targeted to smoc1 resulted in microphthalmia and brain abnormalities in the injected embryos. Taken together these papers strongly support loss of SMOC-1 function as the major cause of OAS and that this protein has a conserved non-redundant function during ocular and limb development.
Finally, in six families with typical OAS we could not identify SMOC1 mutations, including the original family described by Waardenburg in 1935 [9]. In two of these six families, affected individuals show homozygosity over the region of 14q24.2 suggesting that we have significant limitations in our current SMOC1 mutation analysis strategy. However, four families showed no apparent autozygosity around SMOC1, suggesting the likely existence of other OAS loci. Identifying causative genes at other loci is likely to help elucidate the embryopathology and is an active area of our future work.
Materials and Methods
Patient Recruitment and Ethics Approval
All patient related work was carried out with full written consent of the families. Details of the mutation positive cases are provided in Table 1. The informed consent process was reviewed and approved following consideration by national ethical committee systems in the UK and the Netherlands. Several of the cases have been previously published. [16], [19], [22], [25], [26]
Mapping and Linkage Analysis
Patient, parental and unaffected sib genomic DNA samples were run on Affymetrix GeneChip Human Mapping 10K Arrays (Xba131) and autozygosity mapping was performed using ExludeAR 1 with data subjected to multipoint linkage analysis using ALOHOMORA and Allegro (V1.2c). Gene data in the candidate interval were retrieved from the Ensembl Human genome (GRCh37 assembly; http://www.ensembl.org/Homo_sapiens/index.html). Microsatellites containing tri - or tetranucleotide repeats (Table 3) were identified from the UCSC browser (http://genome.ucsc.edu/index.html) and PCR primers were designed using Primer3 (http://frodo.wi.mit.edu/primer3/). FAM fluorescent labels were placed at the 5′-end of the forward primer. All microsatellites were tested for informativeness for each family.
Tab. 3. Microsatellite repeat marker PCR and primer properties. 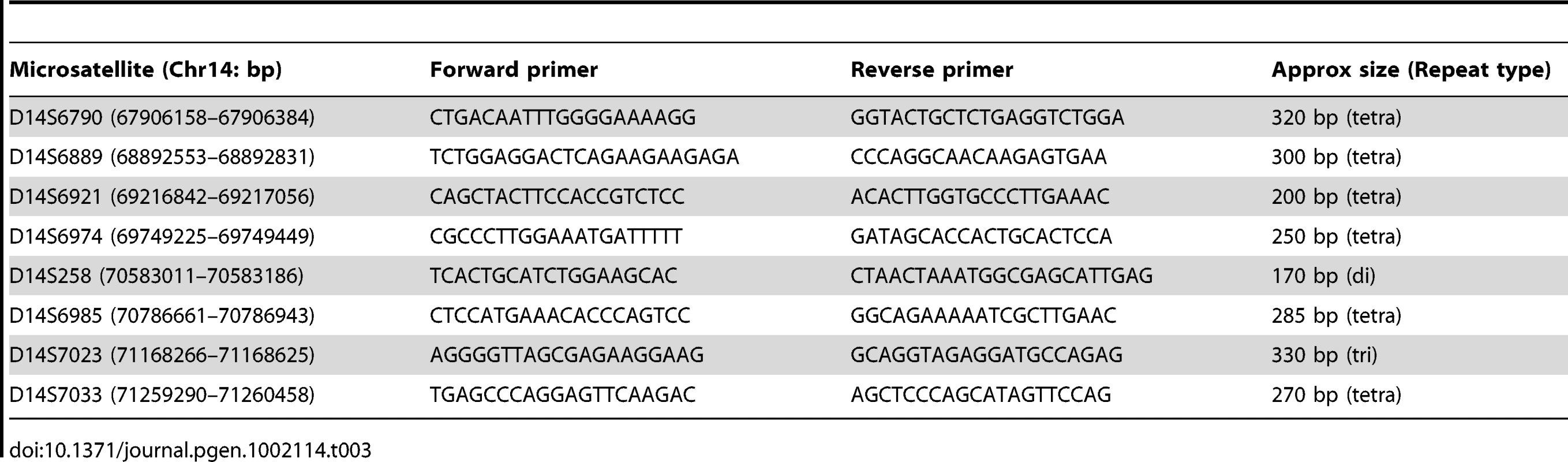
PCR and Sequence Analysis
Genomic DNA samples were Whole Genome Amplified (WGA) using the Illustra GenomiPhi V2 DNA Amplification Kit (GE Healthcare) according to the manufacturer's guidelines. For PCR, 50 ng WGA template DNA was amplified with 0.2 µM primers and 2x Custom Reddymix (Thermo Scientific) in H2O to 25 µl. Cycle conditions: 95°C×3 minutes; and 35×cycles of 95°C×1 minute, 56°C×45 seconds, and 72°C×1 minute; followed by a single step of 72°C×10 minutes. All mutations were confirmed by resequencing using non-WGA genomic DNA with identical reaction conditions. Sequencing was performed using Applied Biosystems 3130/3170 Genetic Analysers. Genbank sequences were downloaded from NCBI build 37.2 and mutation analysis was performed with Mutation Surveyor Software (SoftGenetics LLC, PA, USA) or Sequencher 4.8 (GeneCodes Corp. MI, USA).
In Situ Hybridisations
To generate a DNA tempate for production of Smoc1 riboprobe, PCR was performed from mouse genomic DNA targeting the 3′ untranslated region (UTR) of the Smoc1 gene using primers with T3 and T7 RNA polymerase sites at the 5′ ends of the forward and reverse primers respectively (underlined) (Smoc1 Forward 5′ - AATTAACCCTCACTAAAGGCGTGTGTGGTTTGTTTCTGG-3′; Smoc1 Reverse 5′ - TAATACGACTCACTATAGGTAGACTGCCAAGGGATCTGG-3′). Digoxigenin (DIG) labelled (Roche) sense and antisense riboprobes were generated by in vitro transcription using T3 and T7 RNA polymerase respectively. Whole-mount in situ hybridization to mouse embryos at 9.5 & 10.5 days post-coitum (dpc or E9.5/E10.5) was carried out as previously described [62]. Briefly embryos were fixed overnight in 4% paraformaldehyde (PFA) at 4°C, proteinase K (10 µg/ml) (Roche) treated for 15–35 minutes, depending on the stage then washed twice in 0.1 M triethanolamine, washed in phosphate-buffered saline (PBS) -Tween (0.1%) and refixed in 4% PFA/0.2% glutaraldehyde for 20 minutes. Prehybridisation was performed for 2 hours and hybridisation for 40 hours at 60°C in hybridisation buffer containing each DIG labelled probe. Washes were in 2×hybridisation buffer for 10 minutes, 3×2×SSC +0.1% Tween 20 for 20 minutes, 3×0.2×SSC +0.1% Tween 20 for 30 minutes, all at 60°C. At room temperature the embryos were washed in 1 M Malic Acid with 2% BMB (Boehringer - Mannheim blocking reagent) +20% heat-treated lamb serum solution for 2 hours and then in the same buffer containing a 1/2000 dilution of anti-DIG antibody coupled to alkaline phosphatase (Roche) overnight at 4°C. Embryos were washed 3×5 minutes and 3×1 hour in MAB. Colour detection was performed in 2 ml of BM purple precipitating solution (Roche).
Smoc1 Targeted Mouse
The Smoc1 pre-conditional knockout mouse (EUCOMM Project 48154; strain C57BL/6N-A-Smoc1tm1a(EUCOMM)WTSI, referred to as Smoc1tm1a in this manuscript) was generated by the International knockout Mouse Consortium (IKMC) under UK Home Office Project License 60/3785 (IJ Jackson, MRC Human Genetics Unit). Details of the allele and targeting strategy can be found at: http://www.eucomm.org/htgt/report/gene_report?project_id=48154 and in Figure S1. Genotypes were confirmed using the Smoc1 forward primer 5′-GGTCTGACTCGGTAGGCTTG - 3′ (positioned at mChr12∶82,236,250 bp) and Smoc1 reverse primer 5′-CCTCTCTCCAACCCTTTTCC-3′ (positioned at mChr12∶82,236,907 bp) which flank the targeted endogenous exon and produce a wild type PCR amplicon of 658 bp. Adding the targeting cassette specific primer 5′ - TTAGTCCCAACCCCTTCCTC-3′ to PCR mixes as multiplex reactions produced a targeted-allele specific amplicon of 250 bp. Ear-clip DNA was extracted by incubating in 50 µl of 25 mM NaOH 0.2 mM EDTA solution at 95oC for 1 hour, then adding 50 µl of 40 mM Trizma. 1 µl of template was used for PCR reactions with 0.2 µM primers and 2x Custom Reddymix (Thermo Scientific) in H2O to 50 µl. Cycle conditions: 95°C×3 minutes; and 31×cycles of 95°C×45 seconds, 56°C×40 seconds, and 72°C×1 minute; followed by a single step of 72°C×10 minutes. Products were run on 1% TBE agarose gels. For qRT-PCR, mouse hind limbs at stage 10.5 dpc were dissected and mRNA extracted using TRIzol (Invitrogen) according to the manufacturers instructions. Mice were genotyped by PCR using genomic DNA as described above with WT and Smoc1tm1a/tm1a samples carried on for testing. Samples were DNaseI treated and then cycled using Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems) using an ABI-HT7900 SDS instrument (Applied Biosystems) with the following conditions: 48°C×30 minutes, 95°C×10 minutes, followed by 40 cycles of 95°C×15 seconds and 60°C×1 minute. Each 10 µl reaction contained 0.08 µl of RT Enzyme Mix; 5 µl of RT-PCR Mix; 1 µl RNA sample and 1.92 µl sdH2O, with the inclusion of either Smoc1 forward 5′-GGATGGTTCCTTCACACAGG-3′ and reverse 5′ - TCATCTCCATCGAACACAGG-3′ primers or Hprt forward 5′ - CTGGTGAAAAGGACCTCTCG -3′ and reverse 5′ - CAAGGGCATATCCAACAACA-3′ primers. Each reaction was performed in triplicate in optical reaction plates (384-well, Applied Biosystems), and RNA samples were also run without RT Enzyme Mix for negative controls with the same reaction conditions.
Histological and Histochemical Analysis
Embryos were fixed in 4% PFA; dehydrated through graded alcohol series and xylene; and embedded in paraffin. Microtome sections were cut at 6 µm and rehydrated through ethanol series and stained with haematoxylin and eosin. For skeletal preparations the animals were dehydrated in 95% ethanol for 24 hours; followed by 72 hours in 100% acetone; 3 days in stain solution (1 part 0.3% alcian blue in 70% ethanol; 1 part 0.1% alazarin red in 95% ethanol; and 1 part acetic acid, in 17 parts 70% ethanol); followed by 3 days clearing in 1% KOH; 3 days in 1% KOH/30% glycerol; and two 24 hour periods in 1% KOH/50% glycerol; in 1% KOH/70% glycerol and were analysed in 100% glycerol. X-gal (5-bromo-4-chloro-3-indolyl-β-D - galactopyranoside) staining was performed as follows: targeted mouse embryos were dissected at 10.5 dpc and rinsed in PBS, then fixed for 1 hour in 4% PFA at 4oC, rinsed again in PBS and then washed for 3x 20 minutes in detergent wash (2 mM MgCl2, 0.1% Sodium Deoxycholate, 0.02% NP-40 [Igepal CA 630], in PBS). Detection was performed in β-galactosidase stain (0.085% NaCl, 5 mM K3 [Fe(CN)6], 5 mM K4 [Fe(CN)6], 200 µl/ml X-gal (Promega), in detergent wash), followed by a brief final stain fixation in 4% PFA for 30 minutes. For cryosection analysis, embryos were dissected and fixed as above, then incubated overnight in 20% sucrose/PBS at 4°C, transferred into OCT solution and frozen embedded on dry ice. Sections of 25 µm thickness were cut at −20°C, air dried and rinsed briefly in PBS. X-gal staining was then performed as described above.
Optical Projection Tomography
For optical projection tomography (OPT) analysis In Situ stained embryos were mounted in 1% agarose, dehydrated in methanol and then cleared overnight in BABB solution (1 part Benzyl Alcohol: 2 parts Benzyl Benzoate). Samples were then imaged using a Bioptonics OPT Scanner 3001 (Bioptonics, UK) using brightfield analysis to detect tissue autofluorescence for capture of anatomical and signal data (wavelengths: excitation at 425 nm, emission: 475 nm). The resulting data were reconstructed using Bioptonics proprietary software (Bioptonics, MRC Technology, Edinburgh, UK), then automatically thresholded to remove background and finally merged into a single 3D image output using Bioptonics Viewer software.
Supporting Information
Zdroje
1. MorrisonDFitzPatrickDHansonIWilliamsonKvan HeyningenV 2002 National study of microphthalmia, anophthalmia, and coloboma (MAC) in Scotland: investigation of genetic aetiology. J Med Genet 39 16 22
2. FantesJRaggeNKLynchSAMcGillNICollinJR 2003 Mutations in SOX2 cause anophthalmia. Nat Genet 33 461 463
3. RaggeNKLorenzBSchneiderABushbyKde SanctisL 2005 SOX2 anophthalmia syndrome. Am J Med Genet A 135 1 7 discussion 8
4. WilliamsonKAHeverAMRaingerJRogersRCMageeA 2006 Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum Mol Genet 15 1413 1422
5. RaggeNKBrownAGPoloschekCMLorenzBHendersonRA 2005 Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet 76 1008 1022
6. HendersonRHWilliamsonKAKennedyJSWebsterARHolderGE 2009 A rare de novo nonsense mutation in OTX2 causes early onset retinal dystrophy and pituitary dysfunction. Mol Vis 15 2442 2447
7. PasuttoFStichtHHammersenGGillessen-KaesbachGFitzpatrickDR 2007 Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 80 550 560
8. BakraniaPEfthymiouMKleinJCSaltABunyanDJ 2008 Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet 82 304 319
9. WaardenburgPJ 1961 Autosomally-recessive anophthalmia with malformations of the hands and feet. P. J. WaardenburgAFaDK Vol I. Assen, The Netherlands: Royal Van Gorcum 773
10. AbouzeidHBoissetGFavezTYoussefMMarzoukI 2011 Mutations in the SPARC-related modular calcium-binding protein 1 gene, SMOC1, cause waardenburg anophthalmia syndrome. Am J Hum Genet 88 92 98
11. LIAGSabarinathan DK AK 1994 Microphthalmia and distal limb abnormalities in a child of consanguineous parents. Clin Dysmorphol 3 258 262
12. CaksenHOdabasDOnerAFAbuhandanMCalebiV 2002 Ophthalmo-acromelic syndrome in a Turkish infant: case report. East Afr Med J 79 339 340
13. CoguluOOzkinayFGunduzCSapmazGOzkinayC 2000 Waardenburg anophthalmia syndrome: report and review. Am J Med Genet 90 173 174
14. GalassoCBombardieriRCerminaraCStranciGCuratoloP 2007 Anophthalmia-Waardenburg syndrome with expanding phenotype: does neural crest play a role? J Child Neurol 22 1252 1255
15. GambhirPSGambhirSPBankarSM 2010 Ophthalmoacromelic syndrome: two further cases expanding the phenotype. Clin Dysmorphol 19 91 94
16. GaravelliLPedoriSDal ZottoRFranchiFMarinelliM 2006 Anophthalmos with limb anomalies (Waardenburg opththalmo-acromelic syndrome): report of a new Italian case with renal anomaly and review. Genet Couns 17 449 455
17. HamanoueHMegarbaneATohmaTNishimuraAMizuguchiT 2009 A locus for ophthalmo-acromelic syndrome mapped to 10p11.23. Am J Med Genet A 149A 336 342
18. KaraFYesildaglarNTuncerRASemerciNOnatN 2002 A case report of prenatally diagnosed ophthalmo-acromelic syndrome type Waardenburg. Prenat Diagn 22 395 397
19. KhanAZafarSN 2008 Pierre Robin sequence with unilateral anophthalmia and lower limb oligodactyly: an unusual presentation of ophthalmoacromelic syndrome? Clin Dysmorphol 17 187 188
20. Le MerrerMNessmannCBriardMLMaroteauxP 1988 Ophthalmo-acromelic syndrome. Ann Genet 31 226 229
21. MegarbaneASouratyNTamrazJ 1998 Ophthalmo-acromelic syndrome (Waardenburg) with split hand and polydactyly. Genet Couns 9 195 199
22. PallottaRDallapiccolaB 1984 A syndrome with true anophthalmia, hand-foot defects and mental retardation. Ophthalmic Paediatr Genet 4 19 23
23. QuarrellOW 1995 Ophthalmo acromelic syndrome. Clin Dysmorphol 4 272 273
24. Richieri-CostaAGollopTROttoPG 1983 Brief clinical report: autosomal recessive anophthalmia with multiple congenital abnormalities–type Waardenburg. Am J Med Genet 14 607 615
25. SayliBSAkarsuANAltanS 1995 Anophthalmos-syndactyly (Waardenburg) syndrome without oligodactyly of toes. Am J Med Genet 58 18 20
26. SuyugulZSevenMHacihanefiogluSKartalASuyugulN 1996 Anophthalmia-Waardenburg syndrome: a report of three cases. Am J Med Genet 62 391 397
27. TeiberMLGarridoJABarreiroCZ 2007 Ophthalmo-acromelic syndrome: report of a case with vertebral anomalies. Am J Med Genet A 143A 2460 2462
28. TekinMTutarEArsanSAtayGBodurthaJ 2000 Ophthalmo-acromelic syndrome: report and review. Am J Med Genet 90 150 154
29. TraboulsiEINasrAMFahdSDJabbourNMDer KaloustianVM 1984 Waardenburg's recessive anophthalmia syndrome. Ophthalmic Paediatr Genet 4 13 18
30. HopkinsDRKelesSGreenspanDS 2007 The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 26 508 523
31. SieberCKopfJHiepenCKnausP 2009 Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 20 343 355
32. MiyazonoKKamiyaYMorikawaM 2010 Bone morphogenetic protein receptors and signal transduction. J Biochem 147 35 51
33. HuMCWassermanDHartwigSRosenblumND 2004 p38MAPK acts in the BMP7-dependent stimulatory pathway during epithelial cell morphogenesis and is regulated by Smad1. J Biol Chem 279 12051 12059
34. De RobertisEM 2006 Spemann's organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol 7 296 302
35. WillsAHarlandRMKhokhaMK 2006 Twisted gastrulation is required for forebrain specification and cooperates with Chordin to inhibit BMP signaling during X. tropicalis gastrulation. Dev Biol 289 166 178
36. AmbrosioALTaelmanVFLeeHXMetzingerCACoffinierC 2008 Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell 15 248 260
37. DaleLEvansWGoodmanSA 2002 Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev 119 177 190
38. YanXLinZChenFZhaoXChenH 2009 Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem 284 30097 30104
39. VannahmeCSmythNMiosgeNGoslingSFrieC 2002 Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem 277 37977 37986
40. VannahmeCGoslingSPaulssonMMaurerPHartmannU 2003 Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J 373 805 814
41. NovinecMKordisDTurkVLenarcicB 2006 Diversity and evolution of the thyroglobulin type-1 domain superfamily. Mol Biol Evol 23 744 755
42. ThomasJTCanelosPLuytenFPMoosMJ 2009 Xenopus SMOC-1 Inhibits bone morphogenetic protein signaling downstream of receptor binding and is essential for postgastrulation development in Xenopus. J Biol Chem 284 18994 19005
43. FriedelRHSeisenbergerCKaloffCWurstW 2007 EUCOMM–the European conditional mouse mutagenesis program. Brief Funct Genomic Proteomic 6 180 185
44. HitzelCKanzlerHKonigAKummerMPBrixK 2000 Thyroglobulin type-I-like domains in invariant chain fusion proteins mediate resistance to cathepsin L digestion. FEBS Lett 485 67 70
45. PungercicGDolencIDolinarMBevecTJenkoS 2002 Individual recombinant thyroglobulin type-1 domains are substrates for lysosomal cysteine proteinases. Biol Chem 383 1809 1812
46. LuJQianJKepplerDCardosoWV 2007 Cathespin H is an Fgf10 target involved in Bmp4 degradation during lung branching morphogenesis. J Biol Chem 282 22176 22184
47. StopperGFWagnerGP 2007 Inhibition of Sonic hedgehog signaling leads to posterior digit loss in Ambystoma mexicanum: parallels to natural digit reduction in urodeles. Dev Dyn 236 321 331
48. TowersMMahoodRYinYTickleC 2008 Integration of growth and specification in chick wing digit-patterning. Nature 452 882 886
49. KatagiriTBoorlaSFrendoJLHoganBLKarsentyG 1998 Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet 22 340 348
50. RobertB 2007 Bone morphogenetic protein signaling in limb outgrowth and patterning. Dev Growth Differ 49 455 468
51. AsheHLBriscoeJ 2006 The interpretation of morphogen gradients. Development 133 385 394
52. SchwankGBaslerK 2010 Regulation of organ growth by morphogen gradients. Cold Spring Harb Perspect Biol 2 a001669
53. AffolterMBaslerK 2007 The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet 8 663 674
54. VuilleumierRSpringhornAPattersonLKoidlSHammerschmidtM 2010 Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol 12 611 617
55. RoguljaDIrvineKD 2005 Regulation of cell proliferation by a morphogen gradient. Cell 123 449 461
56. MaatoukDMChoiKSBouldinCMHarfeBD 2009 In the limb AER Bmp2 and Bmp4 are required for dorsal-ventral patterning and interdigital cell death but not limb outgrowth. Dev Biol 327 516 523
57. Pajni-UnderwoodSWilsonCPElderCMishinaYLewandoskiM 2007 BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development 134 2359 2368
58. ChangBSmithRSPetersMSavinovaOVHawesNL 2001 Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet 2 18
59. FurutaYHoganBL 1998 BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12 3764 3775
60. SuzukiSMarazitaMLCooperMEMiwaNHingA 2009 Mutations in BMP4 are associated with subepithelial, microform, and overt cleft lip. Am J Hum Genet 84 406 411
61. OkadaIHamanoueHTeradaKTohmaTMegarbaneA 2011 SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet 88 30 41
62. HarewoodLLiuMKeelingJHowatsonAWhitefordM 2010 Bilateral renal agenesis/hypoplasia/dysplasia (BRAHD): postmortem analysis of 45 cases with breakpoint mapping of two de novo translocations. PLoS ONE 5 e12375 doi:10.1371/journal.pone.0012375
Štítky
Genetika Reprodukční medicína
Článek Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal MutationsČlánek Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene ExpressionČlánek Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary IntegrityČlánek A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with ExpressionČlánek Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line PedigreeČlánek Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 7
-
Všechny články tohoto čísla
- Gene-Based Tests of Association
- The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
- Variants in and Underlie Natural Variation in Translation Termination Efficiency in
- SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
- Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations
- Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer
- Identification of a Mutation Associated with Fatal Foal Immunodeficiency Syndrome in the Fell and Dales Pony
- Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
- An Epigenetic Switch Involving Overlapping Fur and DNA Methylation Optimizes Expression of a Type VI Secretion Gene Cluster
- Recombination and Population Structure in
- A Rice Plastidial Nucleotide Sugar Epimerase Is Involved in Galactolipid Biosynthesis and Improves Photosynthetic Efficiency
- A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets
- Colon Stem Cell and Crypt Dynamics Exposed by Cell Lineage Reconstruction
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Interactions between Glucocorticoid Treatment and Cis-Regulatory Polymorphisms Contribute to Cellular Response Phenotypes
- Translation Reinitiation Relies on the Interaction between eIF3a/TIF32 and Progressively Folded -Acting mRNA Elements Preceding Short uORFs
- DAF-12 Regulates a Connected Network of Genes to Ensure Robust Developmental Decisions
- Adult Circadian Behavior in Requires Developmental Expression of , But Not
- Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary Integrity
- Proteins in the Nutrient-Sensing and DNA Damage Checkpoint Pathways Cooperate to Restrain Mitotic Progression following DNA Damage
- Complex Evolutionary Events at a Tandem Cluster of Genes Resulting in a Single-Locus Genetic Incompatibility
- () and Its Regulated Homeodomain Gene Mediate Abscisic Acid Response in
- A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
- LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs
- Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications
- The Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
- Somatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
- Molecular Mechanisms Generating and Stabilizing Terminal 22q13 Deletions in 44 Subjects with Phelan/McDermid Syndrome
- Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line Pedigree
- Mechanisms of Chromosome Number Evolution in Yeast
- Regulatory Cross-Talk Links Chromosome II Replication and Segregation
- Ancestral Genes Can Control the Ability of Horizontally Acquired Loci to Confer New Traits
- Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
- Rare and Common Regulatory Variation in Population-Scale Sequenced Human Genomes
- The Epistatic Relationship between BRCA2 and the Other RAD51 Mediators in Homologous Recombination
- Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy
- NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
- Araucan and Caupolican Integrate Intrinsic and Signalling Inputs for the Acquisition by Muscle Progenitors of the Lateral Transverse Fate
- Pathologic and Phenotypic Alterations in a Mouse Expressing a Connexin47 Missense Mutation That Causes Pelizaeus-Merzbacher–Like Disease in Humans
- Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination
- Epistatic Relationships in the BRCA1-BRCA2 Pathway
- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium
- Fine Mapping of Five Loci Associated with Low-Density Lipoprotein Cholesterol Detects Variants That Double the Explained Heritability
- CHD1 Remodels Chromatin and Influences Transient DNA Methylation at the Clock Gene
- Nonlinear Fitness Landscape of a Molecular Pathway
- Genome-Wide Scan Identifies , , and as Novel Risk Loci for Systemic Sclerosis
- Quantitative and Qualitative Stem Rust Resistance Factors in Barley Are Associated with Transcriptional Suppression of Defense Regulons
- A Systematic Screen for Tube Morphogenesis and Branching Genes in the Tracheal System
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Gene-Based Tests of Association
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



