-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
Genetic mapping studies have identified multiple cancer susceptibility regions at chromosome 8q24, upstream of the MYC oncogene. MYC has been widely presumed as the regulated target gene, but definitive evidence functionally linking these cancer regions with MYC has been difficult to obtain. Here we examined candidate functional variants of a haplotype block at 8q24 encompassing the two independent risk alleles for prostate and breast cancer, rs620861 and rs13281615. We used the mapping of DNase I hypersensitive sites as a tool to prioritise regions for further functional analysis. This approach identified rs378854, which is in complete linkage disequilibrium (LD) with rs620861, as a novel functional prostate cancer-specific genetic variant. We demonstrate that the risk allele (G) of rs378854 reduces binding of the transcription factor YY1 in vitro. This factor is known to repress global transcription in prostate cancer and is a candidate tumour suppressor. Additional experiments showed that the YY1 binding site is occupied in vivo in prostate cancer, but not breast cancer cells, consistent with the observed cancer-specific effects of this single nucleotide polymorphism (SNP). Using chromatin conformation capture (3C) experiments, we found that the region surrounding rs378854 interacts with the MYC and PVT1 promoters. Moreover, expression of the PVT1 oncogene in normal prostate tissue increased with the presence of the risk allele of rs378854, while expression of MYC was not affected. In conclusion, we identified a new functional prostate cancer risk variant at the 8q24 locus, rs378854 allele G, that reduces binding of the YY1 protein and is associated with increased expression of PVT1 located 0.5 Mb downstream.
Published in the journal: . PLoS Genet 7(7): e32767. doi:10.1371/journal.pgen.1002165
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002165Summary
Genetic mapping studies have identified multiple cancer susceptibility regions at chromosome 8q24, upstream of the MYC oncogene. MYC has been widely presumed as the regulated target gene, but definitive evidence functionally linking these cancer regions with MYC has been difficult to obtain. Here we examined candidate functional variants of a haplotype block at 8q24 encompassing the two independent risk alleles for prostate and breast cancer, rs620861 and rs13281615. We used the mapping of DNase I hypersensitive sites as a tool to prioritise regions for further functional analysis. This approach identified rs378854, which is in complete linkage disequilibrium (LD) with rs620861, as a novel functional prostate cancer-specific genetic variant. We demonstrate that the risk allele (G) of rs378854 reduces binding of the transcription factor YY1 in vitro. This factor is known to repress global transcription in prostate cancer and is a candidate tumour suppressor. Additional experiments showed that the YY1 binding site is occupied in vivo in prostate cancer, but not breast cancer cells, consistent with the observed cancer-specific effects of this single nucleotide polymorphism (SNP). Using chromatin conformation capture (3C) experiments, we found that the region surrounding rs378854 interacts with the MYC and PVT1 promoters. Moreover, expression of the PVT1 oncogene in normal prostate tissue increased with the presence of the risk allele of rs378854, while expression of MYC was not affected. In conclusion, we identified a new functional prostate cancer risk variant at the 8q24 locus, rs378854 allele G, that reduces binding of the YY1 protein and is associated with increased expression of PVT1 located 0.5 Mb downstream.
Introduction
Genome-wide association studies (GWAS) have identified multiple independent cancer susceptibility loci upstream of the MYC oncogene at chromosome 8q24 (reviewed in [1]): initial studies revealed one breast [2], three prostate [3], one bladder cancer [4] locus and one region conferring risk for multiple cancers including prostate, colon and ovarian cancer. More recently, a lymphocytic leukaemia [5] and five more prostate risk regions have been identified [3], [6], [7]. The 8q24 region is currently considered the most important susceptibility region for prostate cancer, accounting for about 8% of the two-fold increase in risk observed in first degree relatives [3]. MYC ranks as one of the most consistently overexpressed genes comparing prostate tumours to normal prostate tissue in a meta-analysis of five data sets [8], indicating its importance in prostate cancer. Breast tumours and metastatic prostate tumours carry frequent amplifications of the 8q24 region, spanning a large region covering both the MYC and the neighbouring PVT1 gene [9], [10]. Furthermore, both the MYC and the PVT1 genes are frequent targets for retroviral integration in mouse tumour assays [11]. The MYC oncogene functions as a transcriptional activator, and is part of a complex regulatory network controlling cell growth, apoptosis, differentiation and other cellular responses [12]. PVT1 encodes a non-coding RNA and is a host gene for several miRNAs, namely hsa-miR-1204, 1205, 1206 and 1207 [11], [13]. The targets and function of PVT1 and its embedded miRNAs are still largely unknown.
The study presented here focuses on a haplotype block containing a risk variant for prostate cancer (rs620861) [3] and a second independent risk variant for breast cancer (rs13281615) [2]. Other cancer risk loci at 8q24 have already been examined in some detail. For example, the colorectal and prostate cancer predisposition locus contains an enhancer that is able to interact with the MYC gene and it was suggested that rs6983267 within this enhancer alters a TCF7L2 (TCF4) binding site thus resulting in changed sensitivity to WNT signalling [14], [15]. Transgenic assays indicate that rs6983267 increases expression of a reporter gene in a pattern that mimics MYC activity in the mouse prostate [16]. Another prostate cancer-associated variant from the 8q24 region, rs11986220, has been shown to form a FoxA1 site, leading to an increased cancer risk because of stronger androgen responsiveness [17]. However, when examining gene expression in primary human prostate and colon tissue samples, no correlation between MYC expression and either of these SNPs was found [14], [15], [18], [19], but an allele-specific effect of rs6983267 was detectable in some colorectal cancer cell lines [20].
The majority of cancer susceptibility loci identified by GWAS do not affect coding regions of genes, and are thought to be regulatory. However, the identification of functional SNPs has been difficult since tagging SNPs employed in genetic mapping are in tight LD with many SNPs, often covering large haplotype blocks. In this study, we hypothesized that regulatory elements affected by SNPs are likely to be positioned in regions of active chromatin that are accessible for digestion by DNase I [21]. Thus, we mapped DNase I hypersensitive sites (DHSs) as means of prioritising regions for further functional analysis. Previously, this approach successfully showed that the likely causative SNPs in both the FGFR2 and TNRC9 susceptibility loci are in regions of open chromatin [22], [23]. Here, we find that rs378854, which is in perfect LD with the prostate cancer risk SNP rs620861, maps to a highly accessible site. We show that the cancer risk-associated allele of rs378854 decreases binding of the transcription factor YY1, activates in vitro expression of reporter constructs relative to the non-risk allele, and increases expression of PVT1 in primary normal human prostate tissue. Therefore, in addition to MYC, our study implicates PVT1, located almost 0.5 Mb from the functional variant, as a novel candidate cancer gene whose expression is influenced by 8q24 genetic variation.
Results
The mapping of DHSs using DNase-seq in the breast cancer cell line MCF-7 across the entire 8q24 cancer susceptibility region revealed a prominent site of DNase I sensitivity within the haplotype block that carries a breast and a prostate cancer predisposition variant, rs13281615 and rs620861, respectively (Figure 1A, Figure S1). The strength of the signal for the DHS within this susceptibility block (S-DHS) is similar to that seen for promoter regions of the FAM84B, MYC and PVT1 genes (Figure 1A). In addition, a strong DHS was observed in a conserved region 60kb upstream of the MYC gene (MYC-DHS). The DNase-seq results were compared to results obtained in DNase-chip experiments (Figure S2). All strong DHSs, for example at the MYC promoter, were replicated. However, the S-DHS which maps to a repetitive element could not be detected by DNase-chip, as repetitive sequences are excluded from the array by design. Interestingly, the same DNase-seq approach applied to prostate cancer cell lines RWPE-1 and PC3 (Figure 1A) did not detect a signal at the S-DHS location, suggesting that transcription factor occupancy in this region may differ between breast and prostate cancer cell lines. Signals at other locations, for example at the MYC promoter are comparable in all three cell lines (Figure 1A). Figure S3A shows an enlarged view of the region, confirming that the S-DHS is in an open chromatin state in MCF-7 cells, but in a closed state in two prostate cell lines. Despite being located within a LINE element, the sequence of S-DHS is unique enough to specifically align it to this genomic locus (see Figure S3 and S4 for details). To confirm that the open chromatin at the S-DHS is indicative of the binding of regulatory nuclear proteins, we examined chromatin immunoprecipitation data (ChIP-seq) for MCF-7 cells [24]. Figure S5A and S5B show that S-DHS sequences are bound by the two cohesin subunits Rad21 and SA1, by CTCF and the transcription factor FoxA1, making it highly likely that this region acts as a regulatory element in MCF-7 cells. Sequence alignments for ChIP-seq, as for the DNase-seq, are sufficiently specific to uniquely map the signals to this region.
Fig. 1. Chromatin conformation at the 8q24 locus. 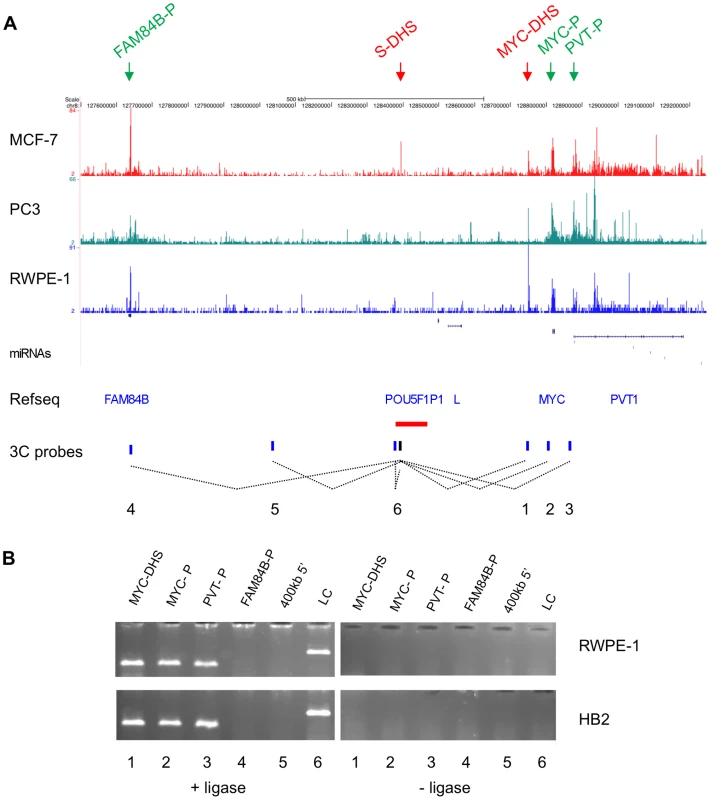
(A) DNase-seq track for the breast cancer cell line MCF-7, the prostate cancer cell line PC3 and the normal-like prostate cell line RWPE-1 displayed on the UCSC genome browser for a 1.7 MB region (chr8: 127,550,000-129,250,000) surrounding the 8q24 cancer susceptibility region, where peaks indicate regions of increased sensitivity to digestions with DNase I. Reads were adjusted for the total number of reads in each experiment. DHSs and promoters used in subsequent analysis are marked by arrows. RefSeq genes are shown, with L being LOC727677. The prostate and breast cancer susceptibility region is depicted by a horizontal red bar. Dotted lines show interactions tested in the chromatin conformation capture experiment shown in (B). (B) Chromatin conformation capture (3C) experiment using the S-DHS as bait in the HB2 breast and the RWPE-1 prostate cell lines. Target sequences are indicated above the lanes. A target sequence just upstream of the EcoRI site 5′ to the bait EcoRI site was used as ligation control (LC). Negative controls for each ligation reaction were cut but unligated DNA fragments. The numbers below the panel refer to interactions shown in (A). Next, we analysed the 1.2 kb S-DHS region for the presence of genetic variants that could be linked to variants previously reported to be associated with breast or prostate cancer. The 1000 Genomes database included 12 SNPs, of which only 5 were common (MAF >0.05) (Figure S2). Of these, rs378854 showed complete LD (r2 = 1) with rs620861, the most strongly associated prostate cancer SNP reported by Al Olama et al. [3] (independently confirmed by Yeager et al. [6]), and also with rs445114, reported in Gudmundsson et al. [7], while the other variants displayed only low LD with this SNP. There was no strong connection to breast cancer in this region, as rs378854 only ranks as number 23 of all SNPs tested in this haplotype block [25]. Previous work and LD analysis of this region (Figure S6) supports the presence of two independent functional variants within this haplotype block, one for prostate cancer and one for breast cancer [3], [8]. Thus, we conclude that the closed state of chromatin within the S-DHS might be associated with risk of prostate cancer, through rs378854.
Motif prediction algorithms suggest that the minor, non-risk allele (A) of rs378854 creates either a YY1 or a C/EBPα transcription factor binding site. Using electrophoretic mobility shift assays (EMSA) with nuclear extracts from breast and prostate cancer cells (MCF-7 and PC3) we demonstrate that the oligonucleotide probe overlapping rs378854 is able to interact with several nuclear proteins (Figure 2A). The low mobility band (black arrow) is not affected by the SNP. In contrast, the high mobility band (red arrow) binds the minor allele more strongly in both cell types. A third, more variable complex of intermediate mobility is formed, which is not affected by the presence of the SNP. The specificity of binding was confirmed by competition assays with self, non-self and unrelated Oct-1 probe at different concentrations (Figure 2A). Competition with known transcription factor binding sites suggest that the high mobility band contains the transcription factor YY1 (Figure 2B). This is confirmed by a supershift observed after including a YY1 antibody in the reaction (open red arrow, Figure 2C). The two upper complexes are sensitive to excess SP1 probe (Figure 2B), but only one of these complexes was supershifted by an SP1 antibody (black open arrow, Figure 2C). SP1 and YY1 are known to interact physically and function co-operatively to modify chromatin structure [26]. The SP1 binding site may therefore be able to enhance the allelic differences caused by YY1 binding to SNP rs378854. Using chromatin immunoprecipitation (ChIP) we also confirm that the identified YY1 site is occupied in vivo in the prostate cancer cell line 1542-CP, but not in MCF-7 breast cancer cells (Figure 2E). In 1542-CP cells YY1 occupancy at rs378854 is higher than that observed for a positive control fragment from the promoter region of the glucocorticoid receptor, known to contain three independent YY1 binding sites [27]. To test for allele-specific interaction between YY1 and rs378854 in 1542-CP, we attempted an allele-specific ChIP. Although there was an increase of binding towards the A allele in the majority of experiments, the effect was small and technical limitations prevent us from drawing definitive conclusions from this experiment.
Fig. 2. Protein DNA interactions at the sequences overlapping rs378854. 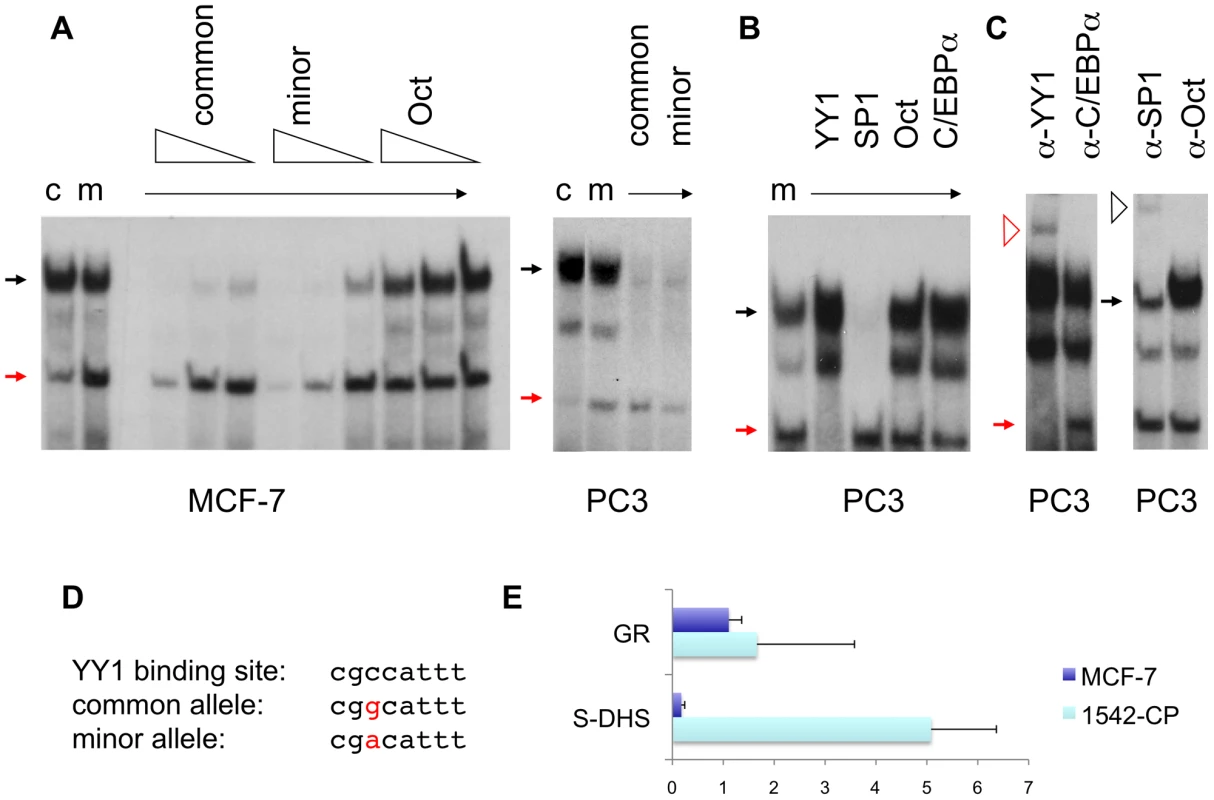
(A) Electrophoretic mobility shift assays (EMSA) were carried out with oligonucleotides containing the common (c) and minor (m) alleles of rs378854 and nuclear extracts from the breast cancer cell line MCF-7 and the prostate cancer cell line PC3. Competitor oligonucleotides were present at 100-, 30- and 10-fold excess for MCF-7 and 30-fold excess for PC3 and are indicated above the lanes. The red and black closed arrows denote the YY1 and SP1 complexes, respectively. (B) EMSAs of the oligonucleotide containing the minor allele were carried out with PC3 nuclear extract and 30-fold excess of competitor oligonucleotides as shown. (C) Supershift of the complex using polyclonal antibody against YY1, SP1, Oct-1 and C/EBPα with PC3 nuclear extracts. The red and black open arrows denote the YY1 and SP1 supershift complexes. (D) An alignment of the two alleles with a YY1 binding site [28] is shown. (E) Chromatin immunoprecipitation assay showing the fold-enrichment of the S-DHS and the positive control (glucocorticoid receptor, GR) sequences relative to a negative control (S-DHS-ve) after immunoprecipitation with YY1 antibody in MCF-7 breast 1542-CP prostate cancer cells. The S-DHS was identified in two breast cancer cell lines, T47 and MCF-7 (Figure S7). To examine the function of the S-DHS, we cloned a 395 bp fragment central to the S-DHS encompassing either the common (risk) or the minor (non-risk) allele of rs378854, into the pGL3-basic, pGL3-promoter and pGL3-enhancer vectors and assayed the ability of these allelic constructs to influence transcription in transient reporter assays. In the prostate cancer cell line PC3 the fragment containing the common (risk) allele has moderate ability to activate transcription in the context of the pGL3-enhancer construct, but for the minor allele there was statistically significant evidence for repression of transcription in the context of all constructs assayed (Figure 3). Our observation are consistent with previous reports that YY1 can act as a potent repressor of transcription [28]. In the breast cancer cell line MCF-7, the minor (protective) allele again displayed lower transcriptional activation than the common allele, but relative to the parental construct no repression was observed (Figure 2E). When the S-DHS was assayed in the pGL3-promoter vector the presence of the minor versus the common SNP had no effect in MCF-7 cells. Thus, rs378854 showed cell-type specific allelic effects on regulation of expression. The effect was evident in prostate cancer PC3 cells, with significant repressor activity of the protective minor allele. This may explain why rs378854 is associated with prostate, but not breast cancer susceptibility.
Fig. 3. Relative transcriptional activation by the common and minor alleles of rs378854. 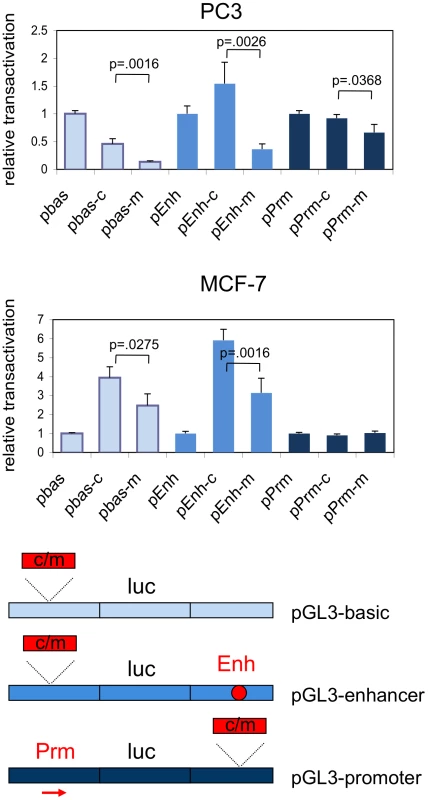
The 395 bp fragment overlapping the S-DHS was cloned into pGL3-basic (pbas), pGL3-enhancer (pEnh) and pGL3-promoter (pPrm) and transcriptional activation of the reporter luciferase gene by the two alleles (common and minor) assayed in the PC3 prostate cancer and the MCF-7 breast cancer cell lines. Activation by the insert is given relative to the parental vector. Averages of six independent transfections are shown and the standard deviation for each data set is given in the bar chart. Where significant, p-values are given for a Student's t-test comparing the values obtained with common versus the minor allele in each vector background. The S-DHS maps to a 1.2 MB region with very few annotated genes (Figure 1A). However, experiments for several 8q24 predisposition regions have indicated that this region is capable of undergoing long-range chromatin looping [14], [29]. We therefore used chromatin conformation capture (3C) to examine whether the DHS can physically interact with its neighbouring genes, MYC, PVT1 and FAM84B. The pseudogene POU5F1P1 and LOC727677 are not expressed in prostate cells at detectable levels [17] and were therefore not included in this analysis. These experiments showed that in both the normal-like breast cell line HB2 and in the prostate cell line RWPE-1 the region surrounding the S-DHS interacts with a DHS 60 kb upstream of the MYC gene (MYC-DHS) and with the MYC and PVT1 promoters, located 360 kb, 420 kb and 480 kb 3′ of the bait sequence, respectively (Figure 1). Similar results were obtained in MCF-7 cells (data not shown). There was no interaction with either the FAM84B promoter or with a negative control sequence 400 kb 5′ of the DHS (Figure 1B). Our results suggest that in prostate cells both MYC and PVT1 could be target genes of the S-DHS regulator element. We note that both cohesin and CTCF also bind to the MYC and PVT1 promoters in MCF-7 cells [24], suggesting a mechanism by which an interaction may occur.
Expression of MYC has been analysed extensively in human primary prostate and colon samples, but no correlation between its expression and the genotype of six different risk SNPs was identified [18], [19]. In contrast, our study revealed that in a set of 59 normal prostate samples mRNA expression level of PVT1 increased with the presence of the risk allele at rs378854 (p = 0.025) (Figure 4). The trend was similar for individuals of European-American and African-American origin, even though the frequency of the risk G allele was 0.66 in Europeans and 0.90 in African-Americans (data not shown). As PVT1 is located in a region of frequent genomic amplification, we examined copy-number variation (CNV) for intron 1 of PVT1 on DNA from all tissue samples used for expression studies. PVT1 CNV was not significantly associated with PVT1 mRNA expression and the association for rs378854 was not affected by the CNV adjustment (Figure S8). At the same time, there was no evidence for association between MYC expression and rs378854 in these samples (p = 0.274). While this analysis will have to be repeated in larger sample sets, our results are consistent with a model in which disruption of YY1 binding at the common risk allele of rs378854 is associated with transcriptional activation of PVT1. We also examined expression of miRNAs embedded within the PVT1 locus (hsa-mir-1204, hsa-mir-1205, hsa-mir-1206, hsa-mir-1207-3p and hsa-mir-1207-5p), but the expression was found to be very low or undetectable in both normal and tumour prostate samples and no clear pattern of association emerged (data not shown). However, we found hsa-mir-1208, located 50 kb downstream of PVT1, to be expressed in all prostate samples tested. In samples homozygous for the risk allele the expression was borderline increased in normal samples (n = 58, p = 0.042), while being decreased in tumour samples (n = 17, p = 0.068), demonstrating significant interaction effect for hsa-mir-1208 expression dependent on tissue status (normal/tumour) and rs378854 genotype (n = 75, p_int = 0.020, Figure S9). Similar analysis of hsa-mir-1208 expression in relation to previously reported rs6983267 did not reveal any significant associations in sets of normal and tumour samples, and in interaction (data not shown). The role of hsa-mir-1208 expression in prostate cancer and its long-distance regulation by rs378854 warrant further studies. In summary, we observe both a physical interaction between the risk SNP rs378854 and the PVT1 promoter and an association between genotype and PVT1 expression.
Fig. 4. Association of PVT1 gene expression with rs378854 genotype in 59 normal prostate samples. 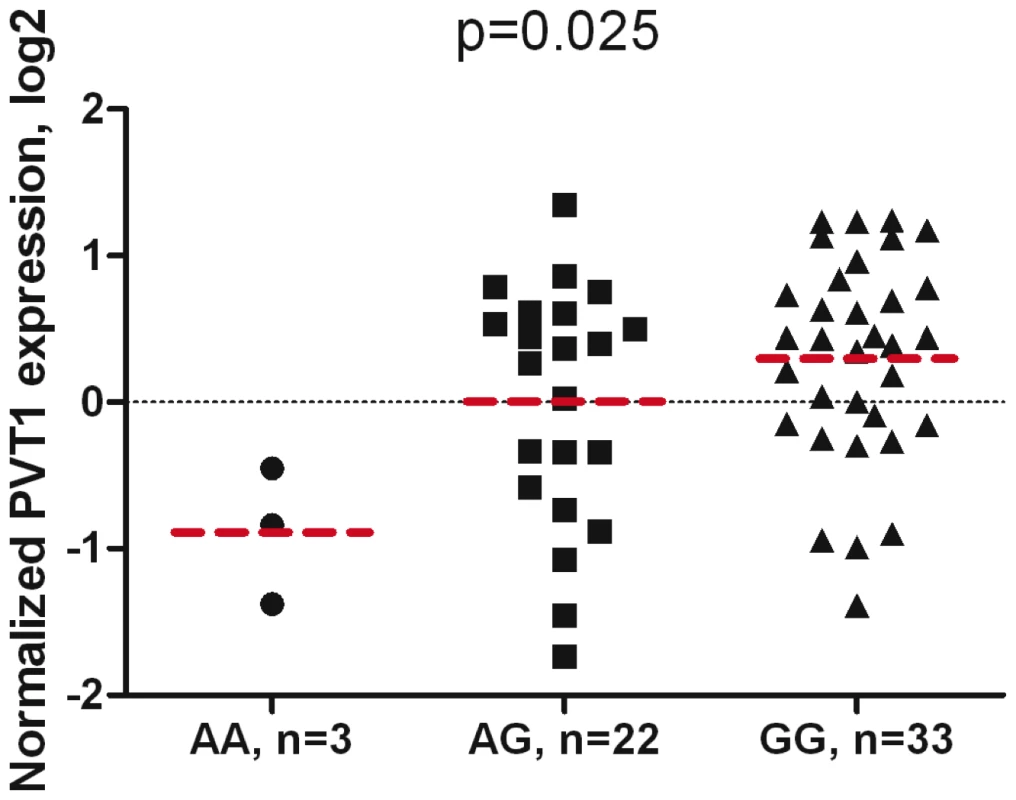
P-values are for univariate linear regression analysis of PVT1 expression in relation to 0, 1 and 2 risk allele (G) of rs378854, adjusted for race. Expression values are shown on log2 scale relative to a mean value of all samples. Expression is lowest in carriers of non-risk AA genotypes and highest in carriers of risk GG genotypes. Mean expression values of each group are shown as bars. Discussion
We describe here the identification of a novel transcriptional regulator element within the 8q24 region that is associated with expression of PVT1. The risk region is marked by a strong DHS in breast cancer MCF-7 cells and is able to interact physically with both the MYC and the PVT1 promoter, but only PVT1 and not MYC gene expression correlates with the presence of the risk allele. Within the S-DHS we identify rs378854 as a functional variant for prostate cancer susceptibility. We demonstrate that, when tested in vitro, using nuclear extracts from breast and prostate cancer cell lines, the YY1 protein was able to bind the non-risk allele (G) of 378854, a perfect proxy for the initially reported prostate cancer signal rs620861 [3], [6], more strongly than the risk allele. However, when tested in vivo, occupancy by YY1 was only observed in a prostate cancer cell line (1542-CP) but not in a breast cancer cell line (MCF-7) raising the possibility that the presence of YY1 prevents the establishment of a DHS. YY1, a zinc-finger transcription factor, is known to interact with chromatin remodelling enzymes which are thought to mediate some of YY1's functions [22]. Furthermore we observed that, in the context of the S-DHS, the protective allele of rs378854 was able to repress transcription in the prostate cell line PC3, but not in breast cancer MCF-7 cells. Therefore, we suggest that differential binding of YY1 to the prostate-cancer associated variant rs378854 might be functionally important for the regulation of MYC and/or PVT1 expression. While MYC expression was not associated with rs378854, PVT1 expression did correlate with the presence of the risk allele of rs378854 (p = 0.025). The absence of an association between MYC expression and the risk genotype may indicate that either the physical interaction between the risk region and the MYC promoter is functionally less important or secondary to the effect of PVT1, or that MYC expression is subject to additional control mechanisms, such as cell cycle or developmental regulation, which may mask the underlying association.
With respect to clinical prognosis, it is interesting to note that lower expression of nuclear YY1 correlates with a poorer outcome for prostate cancer [30], with the data suggesting that decreased YY1 levels give metastatic cells a survival advantage. YY1 has been reported to control many aspects of cancer biology through its interaction with cell cycle genes, association with p53 and other oncogenes as well as its regulation of key apoptosis-related molecules [28]. Furthermore, YY1 expression may play a role in both sensitivity and resistance to chemo - and immunotherapy [28].
Although risk intervals at 8q24 can affect the development of many different cancer types, it is striking that individual risk loci predispose to only a specific type of cancer. We observe that the enhancer identified within the colon/prostate/ovarian susceptibility region (rs696983267, shown in red in Figure S1) maps to a DHS in the prostate and colon, but not in the breast cancer cell lines analysed (Figure S10). However, the situation for the risk SNP we examine here is more complicated. We describe a DHS likely to mark a transcriptional enhancer in breast cancer cell lines, but suggest that a SNP lying within this element increases susceptibility to prostate cancer. We find that the YY1 site overlapping the risk SNP is occupied in a prostate but not a breast cancer cell line and propose that this binding of YY1 in prostate cells mediates transcriptional repression that is influenced by the presence of the SNP. Figure 5 depicts our model of action of rs378854 in the different cell lines. Interestingly, binding of YY1 does not appear to cause the formation of a DHS in the prostate cell lines. The available ENCODE data suggests that the majority of transcription factors bind to open chromatin. However, there is precedent for transcription factor binding to regions of closed chromatin. For example, approximately 40% of genome-wide FOXA1 binding sites map to regions of closed chromatin, where presumably the factor can exert changes in the chromatin structure in response to cell signalling or developmental cues [31]. For many transcription factors, including YY1, the correlation between site occupancy and chromatin accessibility has not yet been examined. Furthermore, the S-DHS is clearly subject to regulation since we detect it only in breast cancer cell lines. Histone modification data (UCSC genome browser) suggests that this region may also be accessible in human embryonic stem cells. In contrast, the majority of DHS elements are accessible across different tissue types. The regulatory elements in the 8q24 desert are clearly highly complex, tissue-specific and may be affected by the presence of SNPs. Here we have indentified one such variant, rs378854, but additional variants and other mechanisms, such as chromosome amplification and/or rare variants, may also contribute to cancer risk.
Fig. 5. Schematic representation of the mode of action of rs378854. 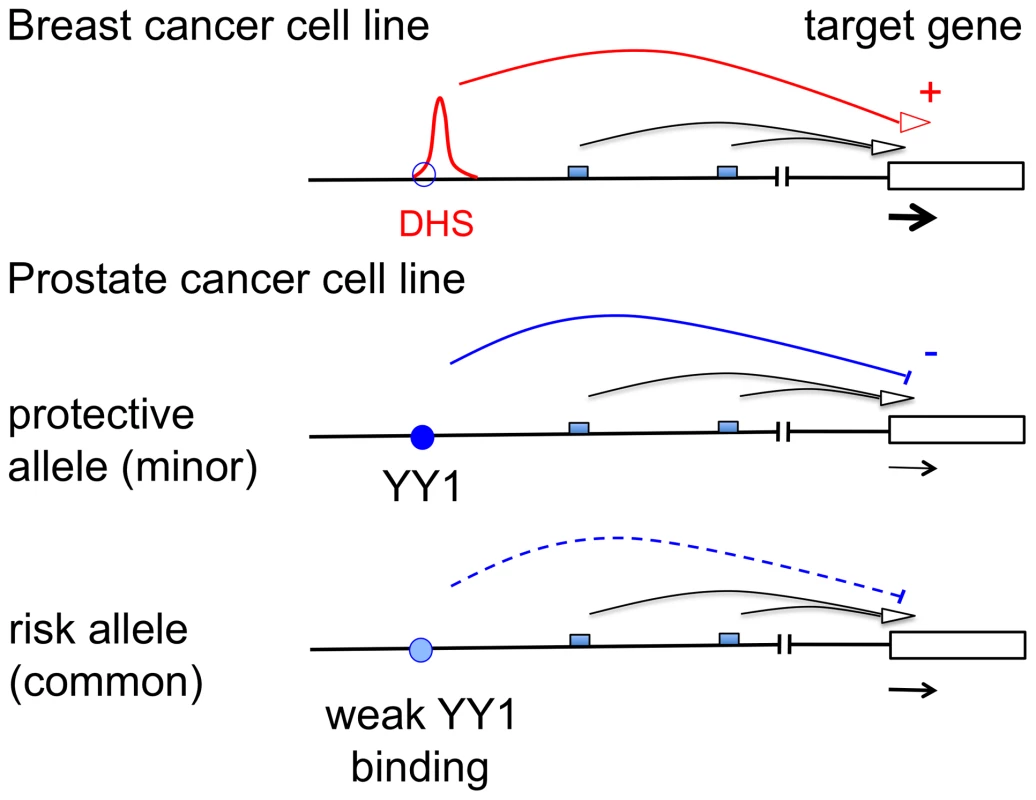
In a breast cancer cell line rs378854 is not bound by YY1 (site shown as empty blue circle) and the DHS present in this region is likely to act as a transcriptional regulator. In contrast, in a prostate cancer cell line YY1 binds (filled blue circle) and represses transcription of a target gene. The level of repression is influenced by the presence of the SNP in the YY1 binding site. Small boxes represent multiple other regulatory elements known to exist in the region. Previous experiments have implicated MYC as the target gene of the 8q24 susceptibility region [14]–[16], [18], [19]. However, in many primary tissue samples examined the presence of the risk allele does not affect MYC expression [18], [19]. Using allelic expression it has been possible to establish such a correlation, but only for some colon cancer cell lines [20]. Here we present evidence that one of the 8q24 risk loci for prostate cancer is able to interact with the promoter of PVT1 and that the presence of the risk allele correlates with increased PVT1 gene expression in normal prostate tissue. The PVT1 gene encodes a non-coding transcript implicated in tumourigenesis [32], [33]. PVT1 was identified as a locus co-amplified with MYC in human Burkitt's lymphomas and found to be a MYC activator [34]. In fact, we observed significant correlation between MYC and PVT1 expression, r2 = 0.54 (p = 0.021 in 18 prostate tumours) and r2 = 0.34 (p = 0.0073 in 59 normal prostates). Therefore, the cancer-associated variants might eventually affect MYC expression, but indirectly, through PVT1. Furthermore, miRNAs encoded within PVT1 may regulate MYC expression either directly or indirectly, by regulating factors that activate MYC [11]. However, the expression of these miRNAs appears to be low or undetectable in normal and tumour prostate tissue samples. On the other hand, a miRNA located 50 kb downstream of PVT1, hsa-mir-1208, showed an interesting pattern of expression and interaction between sample status (normal/tumour) and genotype of rs378854. This interesting association warrants further studies. Biochemical analysis of the PVT1 promoter suggests that it is a target gene of MYC [35]. Importantly, silencing of PVT1 transcripts activates apoptosis in cell lines with PVT1 amplifications, while silencing of MYC in the same cell lines has no effect, suggesting that PVT1 may play a direct role in tumourigenesis that is independent of MYC [10]. In this respect, it is interesting to note that PVT1 shows increased expression in prostate cell lines compared to normal prostate tissue [17]. Furthermore, genetic variants within PVT1 have been associated with Hodgkin's lymphoma [36].
It is striking that the S-DHS element potentially exerts its effect over large genomic distances, being located approximately 420 kb and 480 kb from the MYC and PVT1 promoters, respectively. There are two large “gene deserts” upstream and downstream of the MYC-PVT1 gene cluster (Figure S11), both containing extensive sequence conservation as well as multiple cancer predisposition loci, suggesting that these gene deserts are in fact highly complex regulatory regions of DNA, as supported by recent findings of multiple prostate enhancer elements within the MYC upstream region [17]. The identification of the repressor protein YY1 as a mediator of protection from cancer susceptibility is a step towards a better understanding of the function of this genomic region.
Materials and Methods
Ethics
Anonymised tumour and normal adjacent to tumour prostate tissue samples were either received from the National Cancer Institute (NCI) Cooperative Prostate Cancer Tissue Resource (CPCTR) or from the University of Maryland under protocols reviewed and approved by Institutional Review Boards.
Cell culture
Breast cancer cell lines MCF-7, MDA-MB-134 (also referred to as MDA134), PMC42, T47D, normal-like breast cell line HB2, prostate cancer cell lines PC3 and 1542-CP, normal-like prostate cell line RWPE-1 and the HCT116 colon cancer cell line were from the Cambridge Research Institute culture collection. T47D, MDA134, PMC42, PC3 and HCT116 were maintained in RPMI, 10% foetal calf serum (FCS) and antibiotics; MCF-7 and HB2 in DMEM, 10% FCS and antibiotics, but HB2 cell medium was supplemented with insulin (5 µg/ml) and hydrocortisone (1 µg/ml) (Sigma, UK). RWPE-1 cells were cultured in Keratinocyte Serum Free Medium (Gibco) and supplemented with 5 ng/ml human recombinant EGF and 0.05 mg/ml bovine pituitary extract (both from Sigma). 1542-CP cells were cultured in keratinocyte serum-free medium (Keratinocyte-SFM, Life Technologies, Grand Island, NY) containing 25 µg/ml bovine pituitary extract, 5 ng/ml epidermal growth factor, 2 mM L-glutamine, 10 mM HEPES buffer, antibiotics, and 5% heat-inactivated FCS.
DNase I hypersensitivity assay
Nuclei of all cell lines were harvested and digested with DNase I as previously described [22]. For the DNase-seq experiment DNA from DNase-digested nuclei was isolated by standard procedures, separated on a 1.4% agarose gel and gel-purified DNA fragments between 125 and 500 bp were used directly in Illumina pre-amplification. The amplified library was again resolved on an agarose gel to select 200–300 bp fragments that were gel-purified and sequenced using an Illumina Genome Analyser II, according to manufacturer's instructions. MCF-7 DNase-seq was carried out as part of our analysis of ER responses. Multiple MCF-7 aliquots were transfected with and without a siRNA to GATA3, but no differences in the DHS profile were detected. BWA software [37] was used for sequence alignment by the CRI Bioinformatics core facility. A representative experiment with low background is shown. Two independent DNase-seq experiments for PC3 and RWPE-1 gave very similar results and again a representative example is shown. Sequence reads were displayed on the UCSC genome browser. The presence of the S-DHS DNase-seq peak was verified in T47D cells (Figure S4). For DNase-chip the generation of libraries of DNase hypersensitive fragments has previously been described [22]. Libraries were hybridised to Agilent custom tiling arrays covering 2Mb at 8q24.
Chromatin conformation capture (3C)
The 3C method [38] was applied to detect physical interactions between genomic regions (such as between promoters and distant enhancers [39]) using a sequence of interest (bait) from 8q24 region. The experimental procedure for this technique is outlined in Figure S12. The 3C experiments were carried out in the normal-like cell lines HB2 and RWPE-1 as many cancer cell lines such as PC3 and MCF-7 carry amplifications across the 8q24 region [9], [10]. Briefly, exponentially growing cells were cross-linked with 1% formaldehyde for 10 min at room temperature, washed 3x with cold PBS and scraped into microcentrifuge tubes. Cells were spun at 3000 rpm for 5 mins and resuspended in 1 ml lysis buffer (50 mM Tris-HCl pH 8.0, 1% SDS, 10 mM EDTA). After 10 min cells were centrifuged and resuspended in lysis buffer supplemented with 0.4% NP-40 and 1.8% Triton-X. After centrifugation for 20 seconds at full speed 1.5×106 nuclei were resuspended in 300 µl EcoRI digestion buffer and 1.8% Triton-X. Nuclei were incubated for 45 minutes at 37°C in a cell shaker and subsequently digested with 1000 U EcoRI (NEB) overnight. The enzyme was heat-inactivated and the sample diluted in 1 ml. Ligation was carried out overnight using 4000 U T4 ligase at 16°C. A second digestion step was carried out using 1000 U BamHI (NEB). Genomic DNA was purified after proteinase K treatment using standard protocols. PCRs were carried out using Power SYBR Green Mastermix (Applied Biosystems), 100 ng template DNA, 5 pmol of each primer in a volume of 20 µl (initial 95°C denaturation step, then 1 min at 60°C and 20 secs at 92°C for 40 cycles). Products were separated on a 3% NuSieve agarose gel. The identity of all PCR products was verified by direct sequencing of PCR products.
Electrophoretic mobility shift assay (EMSA)
EMSAs were carried out as previously described [40]. Oligonucleotide sequences used in the assays are provided in Table S1. Antibodies for supershifts were obtained from Santa Cruz Biotech: αYY1 (sc1703x), αC/EBPα (sc9314x), αSP1 (sc14027x) and αOct-1 (sc232x) and 2 µl were used per reaction.
Chromatin Immunoprecipitation (ChIPs)
ChIP reactions were carried out as described by Schmidt et al. [41] using 10 µg αYY1 (sc7341x, Santa Cruz Biotech). DNA was purified from the precipitates and used in RT-PCR reactions with primers listed in Table S1. Primer design was carried out using the UCSC genome browser mapability plot (Figure S3B) in conjunction with Primer 3 software. The primer pairs used gave rise to a single reproducible band whose identity was confirmed by sequencing. The RT-PCR protocol includes a dissociation step to confirm that the reaction gave rise to only a single product. Results were normalised against input and normal mouse IgG (sc2025, Santa Cruz Biotech). As positive control the promoter region of the glucocorticoid receptor [42] was chosen, as this contains three YY1 sites. Results are given relative to S-DHS-ve, a sequence 5 kb upstream of the S-DHS which we have shown to be in inaccessible chromatin (Figure 1). 1542-CP cells and MCF-7 cells are heterozygous for the risk SNP rs378854. Consistent results were obtained in three biological repeats. Error bars denote the technical error in a representative experiment.
Cloning and transfection
S-DHS_cloning-forward and reverse primers (Table S1) were used to amplify a DNA fragment from genomic DNA of PMC42 breast cancer cell line (heterozygous for rs378854) and cloned using the TopoTA cloning system (Invitrogen). Plasmids were sequenced and clones carrying either the minor or the common allele of rs378854 were selected. The inserts were excised using SalI and BamHI (resulting fragment: chr8 : 128,392,958-128,393,353) and cloned into pGL3-basic or pGL3-enhancer vector (Promega), cut with XhoI and BglII. The same fragments were inserted into pGL3-promoter vector (Promega) cut with BamHI and SalI. Plasmids were prepared with an endotoxin-free kit (Qiagen) and transiently transfected into PC3 and MCF-7 cells grown in 24 well plates. 0.5 µg reporter plasmid and 0.1 µg β-galactoside transfection control plasmid in 30 µl OptiMEM (Gibco) were incubated for 20 min with 4.5 µl Fugene (Roche) before being added to 50% confluent wells. 10% FCS was added after 2 hours. Cells were harvested in 100 µl lysis buffer after 24 hours and luciferase and β-galatosidase activity was assayed using Promega kits. The data is averaged from two independent transfection experiments, each carried out in triplicate.
Ethics statement and clinical data
Anonymised tumour and normal adjacent to tumour prostate tissue samples were either received from the National Cancer Institute (NCI) Cooperative Prostate Cancer Tissue Resource (CPCTR) or from the University of Maryland under protocols reviewed and approved by Institutional Review Boards. We also obtained clinical information including age at diagnosis, race, Gleason score and PSA level at diagnosis.
Analysis of gene expression in prostate samples
The frozen samples were homogenized with Tissue Lyser (Qiagen) and divided into two fractions from which total RNA was prepared with MirVana kit (Applied Biosystems) and DNA was prepared with Gentra (Qiagen). The integrity of RNA was confirmed by Bioanalyzer (Agilent).
mRNA expression analysis
cDNA was prepared from 800 ng of total RNA with Superscript III kit and random hexamers (Invitrogen). All expression assays were first evaluated in pooled prostate cDNA samples containing 25 ng, 5 ng, or 1 ng of total RNA per reaction. Genomic DNA and water were used as negative controls for each assay. Based on this test, endogenous controls Beta-2 microglobulin (B2M, assay HS_00187842_m1), Cyclophilin (PPIA, assay 4326316E) and MYC expression (assay Hs00905030) were tested using 5 ng of total RNA per reaction, while PVT1 expression (assay Hs00413039) was evaluated with 20 ng of total RNA per reaction. Expression of alternative splicing forms of PVT1 measured by assay Hs01069044 was beyond confident detection level even with 20 ng of total RNA and was not used on individual tissue samples.
The expression was tested in 4 technical replicates for each sample and assay, technical replicates were averaged and standard deviations were determined.
miRNA expression
TaqMan miRNA expression assays for target miRNAs from PVT1 gene (002872 for hsa-miR-1204, 002778 for hsa-miR-1205, 002878 for hsa-miR-1206, 002826 for hsa-miR-1207-3p, 241060-mat for hsa-miR-1207-5p, 002880 for hsa-mir-1208) and 3 endogenous controls (001973 for U6 snRNA, 001094 for RNU44 and 001006 for RNU48) as well as other reagents for miRNA expression were purchased from Applied Biosystems. cDNA was prepared from experimentally determined amount of total RNA with TaqMan MicroRNA reverse transcription kit. All assays were first tested on pooled cDNA samples using 10, 20 and 100 ng of total RNA per reaction as input. Based on this test, U6, RNU44 and RNU48 assays were run on all individual prostate samples starting from 20 ng of total RNA per reaction. For hsa-miR-1204, 1205, 1206 and 1207-3p reactions were run starting from 100 ng of total RNA per reaction. Expression of hsa-miR-1207-5p was below detection level and was not tested further. All expression assays were run in technical duplicates and target assays were normalized by a geometric mean of U6, RNU44 and RNU48.
CNV analysis in PVT1 region
A custom-designed assay was used to quantify copy number variation (CNV) within intron 1 of PVT1 gene (PVT1-CNV-F and PVT1-CNV-R, 173 bp) (Table S1). The assay was run on DNA prepared from the prostate tissue samples with 5 ng of DNA, 2x Power SYBR Green master mix (Applied Biosystems) and primers. The results were normalized to copy number of control RNaseP assay quantified in the same DNA samples. All assays were run in four technical replicates on 7900 Sequence Detection System (Applied Biosystems).
Statistical analysis
Statistical significance of differences in the reporter assays was determined using a Student's two-sided t-test with a Bonferroni correction for multiple comparisons. The expression values of mRNA and miRNA target assays were normalized by corresponding endogenous controls according to dCt method of relative quantification and tested for normality of distribution. Univariate linear regression was used to analyze expression values in relation to 0, 1 or 2 copies of risk alleles of rs378854. Age, race and PVT1-CNV values were tested as possible covariates but were found to have no significant effect. The analysis was performed with SPSS 16.0. Normalized expression values were centered to the mean of the samples heterozygous for rs378854 and plotted with GraphPad Prism5 software.
Supporting Information
Zdroje
1. GhoussainiMSongHKoesslerTAl OlamaAAKote-JaraiZ 2008 Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst 100 962 966
2. EastonDFPooleyKADunningAMPharoahPDThompsonD 2007 Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447 1087 1093
3. Al OlamaAAKote-JaraiZGilesGGGuyMMorrisonJ 2009 Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet 41 1058 1060
4. KiemeneyLAThorlaciusSSulemPGellerFAbenKK 2008 Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet 40 1307 1312
5. Crowther-SwanepoelDBroderickPDi BernardoMCDobbinsSETorresM 2010 Common variants at 2q37.3, 8q24.21, 15q21.3 and 16q24.1 influence chronic lymphocytic leukemia risk. Nat Genet 42 132 136
6. YeagerMChatterjeeNCiampaJJacobsKBGonzalez-BosquetJ 2009 Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat Genet 41 1055 1057
7. GudmundssonJSulemPGudbjartssonDFBlondalTGylfasonA 2009 Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet 41 1122 1126
8. LinSDingJ 2009 Integration of ranked lists via cross entropy Monte Carlo with applications to mRNA and microRNA Studies. Biometrics 65 9 18
9. LiuWXieCCZhuYLiTSunJ 2008 Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia 10 897 907
10. GuanYKuoWLStilwellJLTakanoHLapukAV 2007 Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res 13 5745 5755
11. Beck-EngeserGBLumAMHuppiKCaplenNJWangBB 2008 Pvt1-encoded microRNAs in oncogenesis. Retrovirology 5 4
12. AdhikarySEilersM 2005 Transcriptional regulation and transformation by myc proteins. Nature Reviews Molecular Cell Biology 6 635 645
13. HuppiKVolfovskyNRunfolaTJonesTLMackiewiczM 2008 The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res 6 212 221
14. PomerantzMMAhmadiyehNJiaLHermanPVerziMP 2009 The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet 41 882 884
15. TuupanenSTurunenMLehtonenRHallikasOVanharantaS 2009 The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat Genet 41 885 890
16. WassermanNFAneasINobregaMA 2010 An 8q24 gene desert variant associated with prostate cancer risk confers differential in vivo activity to a MYC enhancer. Genome Res 20 1191 1197
17. JiaLLandanGPomerantzMJaschekRHermanP 2009 Functional enhancers at the gene-poor 8q24 cancer-linked locus. PLoS Genet 5 e1000597 doi:10.1371/journal.pgen.1000597
18. PomerantzMMBeckwithCAReganMMWymanSKPetrovicsG 2009 Evaluation of the 8q24 prostate cancer risk locus and MYC expression. Cancer Res 69 5568 5574
19. Prokunina-OlssonLHallJL 2009 No effect of cancer-associated SNP rs6983267 in the 8q24 region on co-expression of MYC and TCF7L2 in normal colon tissue. Mol Cancer 8 96
20. WrightJBBrownSJColeMD 2010 Upregulation of c-MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol Cell Biol 30 1411 1420
21. BoyleAPDavisSShulhaHPMeltzerPMarguliesEH 2008 High-resolution mapping and characterization of open chromatin across the genome. Cell 132 311 322
22. UdlerMSMeyerKBPooleyKAKarlinsEStruewingJP 2009 FGFR2 variants and breast cancer risk: fine-scale mapping using African American studies and analysis of chromatin conformation. Hum Mol Genet 18 1692 1703
23. UdlerMSAhmedSHealeyCSMeyerKStruewingJ 2010 Fine scale mapping of the breast cancer 16q12 locus. Hum Mol Genet 19 2507 2515
24. SchmidtDSchwaliePCRoss-InnesCSHurtadoABrownGD 2010 A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res 20 578 588
25. TurnbullCAhmedSMorrisonJPernetDRenwickA 2010 Genome-wide association study identifies five new breast cancer susceptibility loci. (2010). Nat Genet 42 504 507
26. ThomasMJSetoE 1999 Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene 236 197 208
27. BreslinMBVedeckisWV 1998 The human glucocorticoid receptor promoter upstream sequences contain binding sites for the ubiquitous transcription factor, Yin Yang 1. J Steroid Biochem Mol Biol 67 369 381
28. GordonSAkopyanGGarbanHBonavidaB 2006 Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene 25 1125 1142
29. AhmadiyehNPomerantzMMGrisanzioCHermanPJiaL 2010 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proc Natl Acad Sci U S A 107 9742 9746
30. SeligsonDHorvathSHuerta-YepezSHannaSGarbanH 2005 Expression of transcription factor Yin Yang 1 in prostate cancer. Int J Oncol 27 131 141
31. HurtadoAHolmesKARoss-InnesCSSchmidtDCarrollJS 2011 FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43 11 12
32. AskerCMareniCCovielloDIngvarssonSSessaregoM 1988 Amplification of c-myc and pvt-1 homologous sequences in acute nonlymphatic leukemia. Leuk Res 12 523 527
33. BakkusMHBrakel-van PeerKMMichielsJJvan 't VeerMBBennerR 1990 Amplification of the c-myc and the pvt-like region in human multiple myeloma. Oncogene 5 1359 1364
34. GrahamMAdamsJM 1986 Chromosome 8 breakpoint far 3′ of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J 5 2845 2851
35. CarramusaLContinoFFerroAMinafraLPercontiG 2007 The PVT-1 oncogene is a Myc protein target that is overexpressed in transformed cells. J Cell Physiol 213 511 518
36. Enciso-MoraVBroderickPMaYJarrettRFHjalgrimH 2010 A genome-wide association study of Hodgkin's lymphoma identifies new susceptibility loci at 2p16.1 (REL), 8q24.21 and 10p14 (GATA3). Nat Genet 42 1126 1130
37. LiHDurbinR 2009 Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 1754 1760
38. DekkerJRippeKDekkerMKlecknerN 2002 Capturing chromosome conformation. Science 295 1306 1311
39. WestAGFraserP 2005 Remote control of gene transcription. Hum Mol Genet 14 Spec No 1 R101 111
40. MeyerKBMaiaATO'ReillyMTeschendorffAEChinSF 2008 Allele-specific up-regulation of FGFR2 increases susceptibility to breast cancer. PLoS Biol 6 e108 doi:10.1371/journal.pbio.0060108
41. SchmidtDWilsonMDSpyrouCBrownGDHadfieldJ 2009 ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods 48 240 248
42. KimJKollhoffABergmannAStubbsL 2003 Methylation-sensitive binding of transcription factor YY1 to an insulator sequence within the paternally expressed imprinted gene, Peg3. Hum Mol Genet 12 233 245
Štítky
Genetika Reprodukční medicína
Článek Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal MutationsČlánek Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene ExpressionČlánek Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary IntegrityČlánek Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line PedigreeČlánek Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 7
-
Všechny články tohoto čísla
- Gene-Based Tests of Association
- The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
- Variants in and Underlie Natural Variation in Translation Termination Efficiency in
- SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
- Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations
- Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer
- Identification of a Mutation Associated with Fatal Foal Immunodeficiency Syndrome in the Fell and Dales Pony
- Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
- An Epigenetic Switch Involving Overlapping Fur and DNA Methylation Optimizes Expression of a Type VI Secretion Gene Cluster
- Recombination and Population Structure in
- A Rice Plastidial Nucleotide Sugar Epimerase Is Involved in Galactolipid Biosynthesis and Improves Photosynthetic Efficiency
- A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets
- Colon Stem Cell and Crypt Dynamics Exposed by Cell Lineage Reconstruction
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Interactions between Glucocorticoid Treatment and Cis-Regulatory Polymorphisms Contribute to Cellular Response Phenotypes
- Translation Reinitiation Relies on the Interaction between eIF3a/TIF32 and Progressively Folded -Acting mRNA Elements Preceding Short uORFs
- DAF-12 Regulates a Connected Network of Genes to Ensure Robust Developmental Decisions
- Adult Circadian Behavior in Requires Developmental Expression of , But Not
- Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary Integrity
- Proteins in the Nutrient-Sensing and DNA Damage Checkpoint Pathways Cooperate to Restrain Mitotic Progression following DNA Damage
- Complex Evolutionary Events at a Tandem Cluster of Genes Resulting in a Single-Locus Genetic Incompatibility
- () and Its Regulated Homeodomain Gene Mediate Abscisic Acid Response in
- A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
- LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs
- Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications
- The Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
- Somatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
- Molecular Mechanisms Generating and Stabilizing Terminal 22q13 Deletions in 44 Subjects with Phelan/McDermid Syndrome
- Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line Pedigree
- Mechanisms of Chromosome Number Evolution in Yeast
- Regulatory Cross-Talk Links Chromosome II Replication and Segregation
- Ancestral Genes Can Control the Ability of Horizontally Acquired Loci to Confer New Traits
- Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
- Rare and Common Regulatory Variation in Population-Scale Sequenced Human Genomes
- The Epistatic Relationship between BRCA2 and the Other RAD51 Mediators in Homologous Recombination
- Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy
- NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
- Araucan and Caupolican Integrate Intrinsic and Signalling Inputs for the Acquisition by Muscle Progenitors of the Lateral Transverse Fate
- Pathologic and Phenotypic Alterations in a Mouse Expressing a Connexin47 Missense Mutation That Causes Pelizaeus-Merzbacher–Like Disease in Humans
- Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination
- Epistatic Relationships in the BRCA1-BRCA2 Pathway
- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium
- Fine Mapping of Five Loci Associated with Low-Density Lipoprotein Cholesterol Detects Variants That Double the Explained Heritability
- CHD1 Remodels Chromatin and Influences Transient DNA Methylation at the Clock Gene
- Nonlinear Fitness Landscape of a Molecular Pathway
- Genome-Wide Scan Identifies , , and as Novel Risk Loci for Systemic Sclerosis
- Quantitative and Qualitative Stem Rust Resistance Factors in Barley Are Associated with Transcriptional Suppression of Defense Regulons
- A Systematic Screen for Tube Morphogenesis and Branching Genes in the Tracheal System
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Gene-Based Tests of Association
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



