-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
Eukaryotic cells respond to genomic and environmental stresses, such as DNA damage and heat shock (HS), with the synthesis of poly-[ADP-ribose] (PAR) at specific chromatin regions, such as DNA breaks or HS genes, by PAR polymerases (PARP). Little is known about the role of this modification during cellular stress responses. We show here that the nucleosome remodeler dMi-2 is recruited to active HS genes in a PARP–dependent manner. dMi-2 binds PAR suggesting that this physical interaction is important for recruitment. Indeed, a dMi-2 mutant unable to bind PAR does not localise to active HS loci in vivo. We have identified several dMi-2 regions which bind PAR independently in vitro, including the chromodomains and regions near the N-terminus containing motifs rich in K and R residues. Moreover, upon HS gene activation, dMi-2 associates with nascent HS gene transcripts, and its catalytic activity is required for efficient transcription and co-transcriptional RNA processing. RNA and PAR compete for dMi-2 binding in vitro, suggesting a two step process for dMi-2 association with active HS genes: initial recruitment to the locus via PAR interaction, followed by binding to nascent RNA transcripts. We suggest that stress-induced chromatin PARylation serves to rapidly attract factors that are required for an efficient and timely transcriptional response.
Published in the journal: . PLoS Genet 7(7): e32767. doi:10.1371/journal.pgen.1002206
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002206Summary
Eukaryotic cells respond to genomic and environmental stresses, such as DNA damage and heat shock (HS), with the synthesis of poly-[ADP-ribose] (PAR) at specific chromatin regions, such as DNA breaks or HS genes, by PAR polymerases (PARP). Little is known about the role of this modification during cellular stress responses. We show here that the nucleosome remodeler dMi-2 is recruited to active HS genes in a PARP–dependent manner. dMi-2 binds PAR suggesting that this physical interaction is important for recruitment. Indeed, a dMi-2 mutant unable to bind PAR does not localise to active HS loci in vivo. We have identified several dMi-2 regions which bind PAR independently in vitro, including the chromodomains and regions near the N-terminus containing motifs rich in K and R residues. Moreover, upon HS gene activation, dMi-2 associates with nascent HS gene transcripts, and its catalytic activity is required for efficient transcription and co-transcriptional RNA processing. RNA and PAR compete for dMi-2 binding in vitro, suggesting a two step process for dMi-2 association with active HS genes: initial recruitment to the locus via PAR interaction, followed by binding to nascent RNA transcripts. We suggest that stress-induced chromatin PARylation serves to rapidly attract factors that are required for an efficient and timely transcriptional response.
Introduction
The activity of eukaryotic genomes is regulated by dynamic changes in chromatin structure. A multitude of nucleosome remodeling enzymes, histone modifying activities and chromatin binding proteins cooperate to establish, maintain and reprogram chromatin structures that determine genome activity.
Drosophila heat shock (HS) genes provide a textbook example of how dramatic changes in the organismal and cellular environment affect chromatin structure in a manner that promotes transcriptional activation of genes coding for molecular chaperones required during the HS response. Upon temperature shift, the HS loci of polytene chromosomes form transcriptionally active “puffs”. This rapid chromatin decondensation correlates with a strong decrease in nucleosome density [1]. Puff formation can be uncoupled from transcription and much of the nucleosome loss at the hsp70 gene occurs prior to the first round of transcription [1], [2]. Recently, heat shock factor (HSF), GAGA factor and poly-[ADP-ribose] polymerase (PARP) have been shown to be required for the rapid removal of nucleosomes upon activation of the hsp70 gene [1]. In addition, HS puffs accumulate PARylated proteins and puff formation depends on PARP activity [3]. The mechanisms underlying PARP action during HS gene activation are not clear. It has been suggested that PARylation may be removing proteins, including histones - which are themselves a good PARP substrate - thereby promoting chromatin opening [1]. The accumulation of PARylated proteins at HS loci has recently been proposed to build up a “transcription compartment” which hinders the diffusion of proteins into and out of the compartment, thus favouring factor recycling [4]. In addition to histone displacement and transcription compartment formation at HS genes, recent evidence suggests that PARylation could also act as a signaling scaffold for the recruitment of PAR-sensing factors during DNA damage. In mammals PARylation at DNA damage sites can mediate the recruitment of several ATP-dependent nucleosome remodeling enzymes [5]–[10]. Here we sought to address whether and how nucleosome remodelers may be recruited to PARP activation sites upon environmental stresses other than DNA damage. We have investigated a paradigm of environmental stress, the activation of HS loci in Drosophila and have analyzed the mechanism through which the nucleosome remodeler dMi-2 is recruited to HS genes.
Mi-2 (CHD3/CHD4) is a conserved ATP-dependent nucleosome remodeler. In both vertebrates and invertebrates, it is a subunit of Nucleosome Remodeling and Deacetylation (NuRD) complexes. NuRD complexes repress cell type specific genes during differentiation [11]–[13]. dMi-2 is also a subunit of the Drosophila-specific Mep-1 complex (dMec) which represses neuron-specific genes during differentiation of the peripheral nervous system [12], [14].
Mi-2 containing complexes lack subunits with sequence-specific DNA binding activity. Two main mechanisms for their recruitment to chromatin have been suggested. First, NuRD complexes contain subunits with methylated DNA binding domains (MBD) which direct NuRD to methylated DNA [15], [16]. This is unlikely to be a major recruitment mechanism for Drosophila Mi-2 complexes, however, given the low and transient levels of DNA methylation in this organism [17]. A second mode of Mi-2 recruitment involves interactions with DNA bound transcription factors [11], [12], [14], [18]–[22]. In addition, SUMOylation of transcription factors can increase their affinity for Mi-2 complexes [21], [22].
Despite its well established role in repression, dMi-2 localises to actively transcribed chromosome regions suggesting an unexpected potential function of dMi-2 in transcription [23]. Here we sought to establish how dMi-2 is recruited to actively transcribed chromatin and to clarify its role in transcriptional activation using genetic, biochemical and pharmacological assays. We show that dMi-2 rapidly associates with activated HS loci, covering the entire transcribed region of the hsp70 gene. dMi-2 recruitment is not affected when transcriptional elongation is blocked but is abrogated when PARP is inhibited. Indeed, we find that dMi-2 specifically binds PARP's oligomeric product PAR in vitro. Significantly, a dMi-2 mutant unable to bind PAR is not recruited to active HS loci in vivo. We have identified several regions of dMi-2 that bind PAR in vitro. These include the chromodomains and a series of K/R-rich motifs near the N-terminus. Further, dMi-2 depletion or expression of an inactive enzyme greatly decreases transcript levels, suggesting that dMi-2 actively supports efficient HS gene expression. Indeed, dMi-2 associates with nascent hsp70 transcripts in vivo and ablation of dMi-2 function results in inefficient RNA processing. RNA and PAR compete for dMi-2 binding suggesting a two step process of dMi-2 association with HS genes: intial recruitment of dMi-2 is effected by its binding to PAR which is produced prior to the onset of transcription, dMi-2 then switches to interacting with the emerging nascent transcripts. Taken together, our results uncover PAR binding as a novel mechanism for the recruitment of the nucleosome remodeler dMi-2 to targeted sites of PARP activitation upon environmental stress and demonstrate that dMi-2 acts as a co-activator for the full transcriptional activation of HS genes. This study provides the first evidence for an in vivo function of PARylation in promoting the recruitment of a nucleosome remodeler to support the transcription of stress induced genes.
Results
dMi-2 is recruited to HS genes in a PARP–dependent manner
As shown previously, dMi-2 colocalised with active RNA polymerase II (Pol II) on polytene chromosomes [23] (Figure 1A). In addition, dMi-2 significantly colocalised with different forms of elongating Pol II (Ser2P and Ser5P) and elongation factors (Spt5). This suggests that dMi-2 may play an unanticipated role in active transcription. Upon HS, dMi-2 associated with the loci 87A and 87C which contain multiple copies of the hsp70 gene (Figure 1B), further strengthening a potential link between dMi-2 and active transcription.
Fig. 1. dMi-2 is recruited to HS genes. 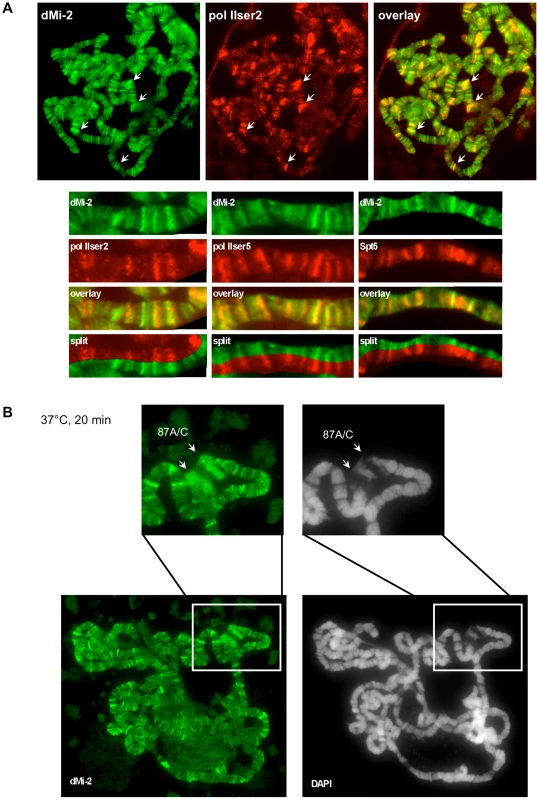
Immunofluorescence (IF) staining of polytene chromosomes with dMi-2, RNA polymerase II (pol IIser2 and pol IIser5), Spt5 antibodies and DAPI as indicated. (A) Untreated chromosomes. Arrows show prominent sites of colocalization. Lower panels show magnified sections of individual chromosome arms. (B) Heat shocked chromosomes. Upper panels show magnified section containing the hsp70 loci 87A and 87C (arrows). Chromatin immunoprecipitation (ChIP) analysis of dMi-2 binding to the activated hsp70 gene in Kc cells revealed an enrichment of dMi-2 in the transcribed region (Figure 2A and 2B). dMi-2 association was detected as early as 2 min after HS and progressively increased for 20 min (Figure S1).
Fig. 2. dMi-2 recruitment to HS genes requires PARP activity. 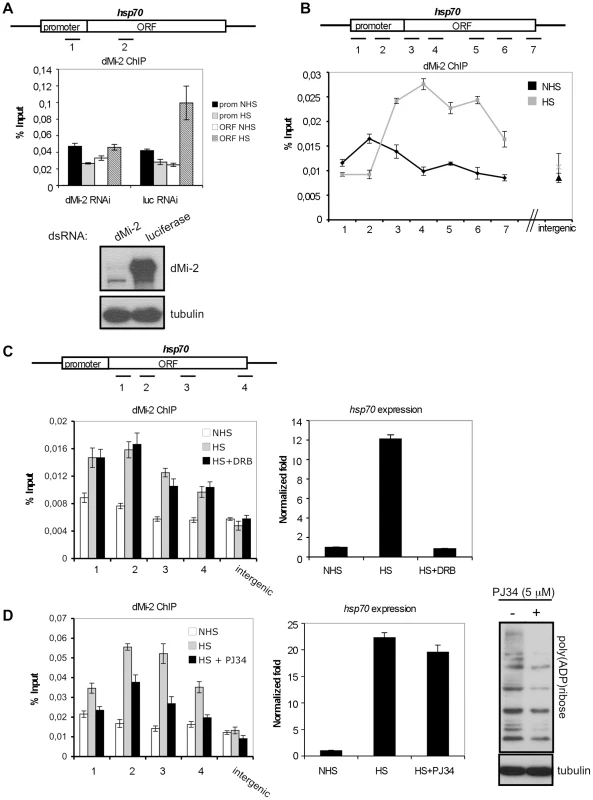
(A and B) ChIP analyses of dMi-2 binding to the hsp70 gene in Kc cells. (A) Upper panel: hsp70 gene and position of amplimers analysed (1: centred at -154, 2: +681). Middle panel: dMi-2 ChIP from cells treated with dsRNA against luciferase or dMi-2 as indicated. prom (amplimer 1): promoter; ORF (amplimer 2): open reading frame; NHS: non heat shock; HS: heat shock. Lower panel: Verification of RNAi knockdown by Western blot. (B) Upper panel: hsp70 gene and position of amplimers analysed (1: centred at -350; 2: -154; 3: +58; 4: +681; 5: +1702; 6: +2065; 7: +2549). Lower panel: dMi-2 ChIP from NHS (black graph) and HS (gray graph) cells. (C and D) Effect of elongation inhibitor DRB (C) and PARP inhibitor PJ34 (D) on dMi-2 recruitment to hsp70 gene. hsp70 gene and position of amplimers analysed are shown on top (1: centred at +58; 2: +681; 3: +1702; 4: +2549). Left panels: ChIP analyses of dMi-2 binding to hsp70 gene. Right panels: RT-QPCR analysis of hsp70 transcription. (D) Rightmost panel: anti-PAR Western blot of extracts from untreated and PJ34 treated Kc cells. We considered three recruitment mechanisms:
First, dMi-2 might bind histone modifications enriched in actively transcribed genes, such as H3K4me3 or H3K36me3. However, we did not find a methylation sensitive interaction of recombinant dMi-2 with histone peptides in pulldown assays (data not shown).
Second, dMi-2 might bind and travel with RNA Pol II or elongation factors. This hypothesis predicts that HS-dependent dMi-2 recruitment to the transcribed part of hsp70 is transcription-dependent. To test this hypothesis, we inhibited transcriptional elongation with DRB (Figure 2C). Although this treatment efficiently ablated production of hsp70 transcripts, it did not significantly reduce HS-dependent recruitment of dMi-2. In addition, we failed to detect robust biochemical interactions of dMi-2 with RNA Pol II or elongation factors in co-immunoprecipitation assays (data not shown). We conclude that the HS-dependent recruitment of dMi-2 to the hsp70 gene can be uncoupled from the transcriptional activity of hsp70.
Third, dMi-2 might be recruited by interaction with PAR, a modification that rapidly accumulates over the hsp70 locus upon HS [3]. We therefore treated Kc cells with the small molecule PARP inhibitor PJ34 (Figure 2D). This led to a significant decrease of global PARylation levels, but did not abrogate hsp70 transcription or nucleosome depletion (Figure 2D and Figure S2). Nevertheless, dMi-2 recruitment to hsp70 was severely decreased during HS, suggesting that efficient PARylation of the locus is a requirement for stress-dependent enrichment of dMi-2.
dMi-2 binds PAR
To determine whether dMi-2 binds PAR directly, we auto-PARylated PARP1 in vitro and incubated the reaction with immobilised dMi-2. mH2A1.1 which contains a macrodomain known to interact with PAR was used as a positive control in this assay. Western blot analysis revealed that dMi-2, like mH2A1.1, bound PARylated PARP1 efficiently (Figure 3A). We confirmed that dMi-2 also interacted with radioactively labeled PARylated PARP1 (Figure S3). To ensure that dMi-2 interacted directly with the PAR polymer, we assayed binding to purified PAR using a dot blot assay (Figure S4). This verified the apparent direct interaction between dMi-2 and PAR.
Fig. 3. dMi-2 binds PAR. 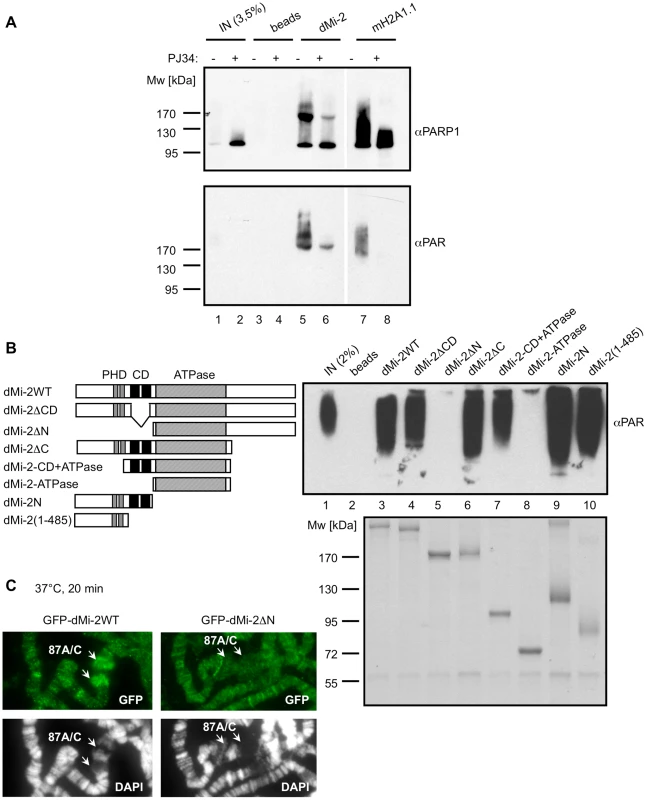
(A) PAR was synthesised in vitro by recombinant PARP1 in the presence (+) or absence (-) of PJ34. Reactions were incubated with control anti-Flag beads (beads) and beads loaded with dMi-2 or mH2a1.1 as indicated on top. Lanes 1, 2: input; Bound material was analysed by Western blot using PARP1 (upper panel) and PAR (lower panel) antibodies. (B) Mapping PAR binding regions. Left panel: Schematic representation of dMi-2 constructs used. Amino acid boundaries are as follows: dMi-2WT: dMi-2 1-1982; dMi-2ΔCD: dMi-2 Δ485-690; dMi-2ΔN: 691-1982; dMi-2ΔC: dMi-2 1-1271; dMi-2-CD+ATPase: dMi-2 484-1271; dMi-2-ATPase: dMi-2 691-1271; dMi-2N: dMi-2 1-690; dMi-2(1-485): dMi-2 1-485. Upper right panel: PAR binding assays with dMi-2 mutants were performed as in (A). dMi-2 mutants are shown on top. Bound material was analysed by anti-PAR Western blot. Lane 1: input. Lower right panel: Coomasie stained gel showing the dMi-2 constructs used. (C) Polytene chromosomes from transgenic larvae expressing GFP-dMi-2 transgenes (dMi-2WT and dMi-2ΔN) were analysed by IF using GFP antibody (green) and DAPI (gray). Arrows point to hsp70 HS loci 87A and 87C. Next, we sought to define the dMi-2 region required for PAR binding. We tested an array of dMi-2 truncation mutants for their ability to interact with PARylated PARP1 in vitro (Figure 3B). This revealed that the N-terminal region had a high affinity for PAR. Within this part of dMi-2, both the PHD finger containing region N-terminal of the chromodomains (aa 1-485) and (to a lesser extent) the chromodomains (aa 484-690) were capable of binding PAR. To verify these results we also tested binding of dMi-2 mutants to PAR in dot blot assays (Figure S4). We conclude that dMi-2 possesses at least two PAR-sensing regions that can function independently of each other.
PAR–binding activity is required for dMi-2 recruitment to active HS loci
To assess the functional importance of dMi-2′s PAR binding activity, we compared recruitment of GFP-dMi-2 fusion proteins to the activated hsp70 loci in transgenic flies (Figure 3C). GFP fused to full length dMi-2 and a GFP-dMi-2 fusion lacking the N-terminal PAR-binding regions were expressed to similar levels in 3rd instar larvae and correctly localised to salivary gland nuclei (Figure S5). Full length GFP-dMi-2 was enriched at active HS loci, the PAR binding mutant, however, failed to accumulate. This supports the notion that dMi-2 binding to PAR makes an important contribution to the recruitment of this nucleosome remodeler to the stress-activated hsp70 gene.
Mapping of the PAR–binding regions
The N-terminal PAR binding region of dMi-2 contains two highly conserved domains, a pair of PHD fingers (residues 377 to 484) and a tandem chromodomain (residues 488 to 673). We generated GST fusions containing these domains and tested their ability to bind PAR in dot blot assays (Figure S6). This confirmed that the chromomodomains can bind PAR independently. However, the PHD fingers did not display PAR binding activity.
We next sought to better define the PAR binding region near the N-terminus of dMi-2. The N-terminal 375 residues of dMi-2 are characterised by a high content in charged residues (24% D/E, 21% R/K). This general feature is conserved between dMi-2 and mammalian CHD4 proteins (Figure 4A). In addition, these proteins share a region with high sequence similarity, the CHDNT domain (Pfam family PF08073). The function of this domain is not known. A number of diverse PAR binding motifs have recently been identified [24]–[26]. A common feature of these motifs is that they all contain several R/K residues that are interspersed by hydrophobic residues which often play critical roles in mediating PAR binding [24]–[26]. We subjected different dMi-2 fragments to the PAR binding assay, including four K/R-rich fragments (K/R I to IV in Figure 4A). This analysis revealed strong PAR binding activity for three of the four K/R-rich fragments (K/R I, K/R II and K/R IV; Figure 4B). By contrast, K/R-rich fragment II and a fragment encompassing the CHDNT domain failed to interact with PAR.
Fig. 4. PAR–binding regions of dMi-2. 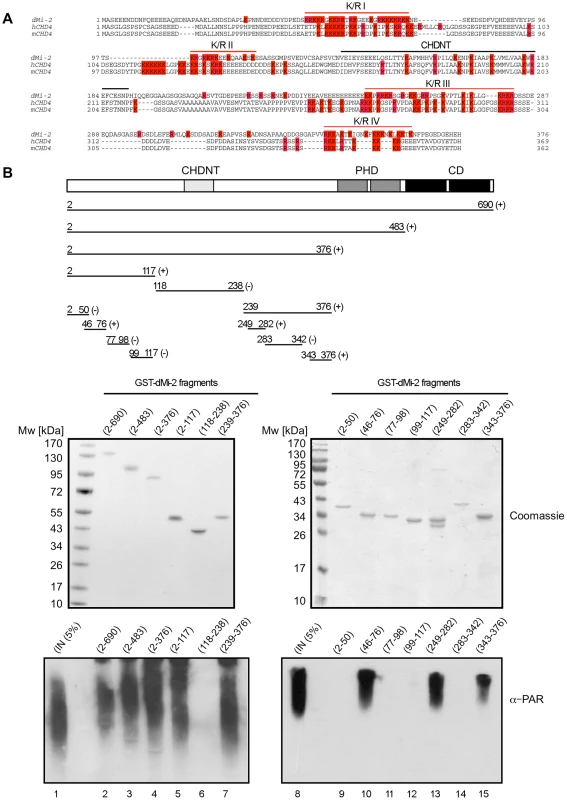
(A) Multiple sequence alignment of N-terminus of dMi-2 and human and mouse CHD4. All K and R amino acid residues are coloured in red. Red lines indicate the four K/R rich regions. The black line indicates the CHDNT domain. (B) Mapping of PAR binding regions in the N-terminal part of dMi-2. Upper panel: Schematic representation of dMi-2 constructs used. Numbers indicate the amino acid borders of the constructs. (+) and (-) indicate binding to PAR. Middle panel: Coomasie stained gels with purified GST-dMi-2 fragments used for PARP pulldown assays. Lower panel: PAR binding assays with GST-dMi-2 fragments were performed as in Figure 3B. Bound material was analysed by anti-PAR Western blot. Lanes 1 and 8: inputs. Taken together, our results suggest that dMi-2 contains multiple PAR binding regions in its N-terminus: three are characterised by a high content of basic amino acid residues (K/R I, K/R III and K/R IV) and one region containing the tandem chromodomain.
dMi-2 interacts with nascent HS gene transcripts
PARylation of the hsp70 locus has been proposed to assist in the opening of chromatin structure and to increase access of factors to DNA and nascent hsp70 transcripts [1]. Given that dMi-2 localises to the entire transcribed region and given that PAR exhibits chemical and structural similarity to RNA, we speculated that dMi-2, once recruited, might interact with nascent hsp70 RNA. We immunoprecipitated dMi-2 from nuclear extracts of heat shocked Kc cells and probed for the co-precipitation of nascent (unprocessed) hsp70 and hsp83 RNA (Figure 5A). Indeed, two independent dMi-2 antibodies precipitated these transcripts arguing for a physical, potentially direct interaction. In agreement with this, dMi-2 bound to single-stranded hsp70 RNA in an electrophoretic mobility shift assay in vitro (Figure 5B).
Fig. 5. dMi-2 binds RNA. 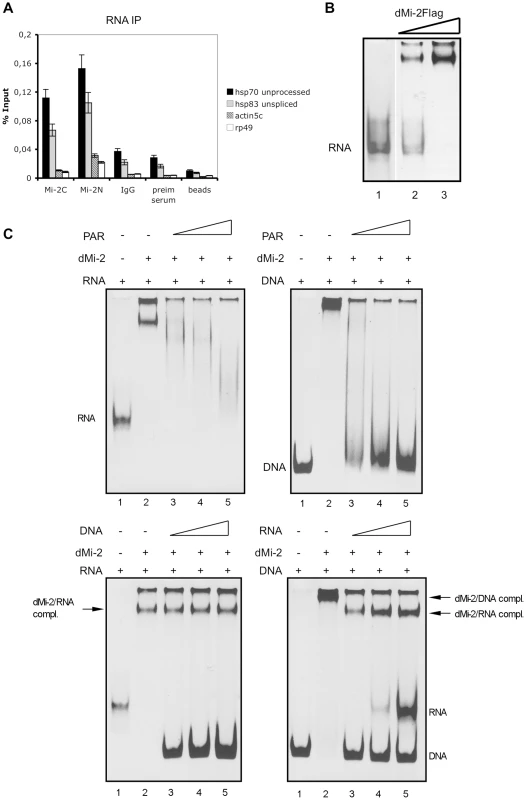
(A) RNA immunoprecipitation (RIP) of hsp70 and hsp83 unprocessed transcripts from heat shocked Kc cells. RIP was performed with two independent dMi-2 antibodies (dMi-2C and dMi-2N), IgG, preimmune serum and protein G beads as indicated. Primer pairs that specifically amplify actin5c, rp49 and unprocessed hsp70 and hsp83 transcripts (see Figure 7) were used for RT-QPCR. (B) RNA electrophoretic mobility shift assay. Single stranded hsp70 RNA was incubated with recombinant dMi-2. Lane 2: 0.1 µg dMi-2, lane 3: 0.2 µg dMi-2, lane 1: no protein. RNA and RNA:protein complexes were resolved by electrophoresis and visualized with ethidium bromide. Position of unbound RNA probe is indicated. (C) Competition mobility shift assays. Upper left panel: Single stranded hsp70 RNA was incubated with 0.2 µg of recombinant dMi-2 in the absence or in the presence of increasing amounts of PAR polymer, as indicated. Upper right panel: hsp70 DNA was incubated with 0.2 µg of recombinant dMi-2 in the absence or in the presence of increasing amounts of PAR polymer, as indicated. The following mass ratios of RNA to PAR or DNA to PAR were used: lane 3 - 1∶1, lane 4 -1∶2, lane 5 -1∶4. Positions of unbound RNA and DNA probes are indicated. Lower left panel: Single stranded hsp70 RNA was incubated with 0.2 µg of recombinant dMi-2 in the absence or in the presence of increasing amounts of DNA, as indicated. Lower right panel: hsp70 DNA was incubated with 0.2 µg of recombinant dMi-2 in the absence or in the presence of increasing amounts of RNA, as indicated. The following weight ratios of RNA to DNA or DNA to RNA were used: line 3 - 1∶1, lane 4 -1∶2, lane 5 -1∶4. Positions of unbound RNA and DNA probes and dMi-2/DNA and dMi-2/RNA complexes are shown on the right. Next, we performed competition assays to gain insight into the relative affinities of dMi-2 for DNA, RNA and PAR and to determine if dMi-2 can bind to several types of nucleic acid simultaneously or if binding is competitive. First, we tested dMi-2 binding to RNA and DNA, respectively, in the presence of increasing amounts of PAR in electrophoretic mobility shift assays (mass ratios 1∶1, 1∶2 and 1∶4; Figure 5C). In this assay, PAR was able to compete with RNA and DNA for dMi-2 binding. However, whereas dMi-2 no longer bound to DNA at a DNA:PAR mass ratio of 1∶2, residual dMi-2/RNA complexes were still detectable at an RNA:PAR mass ratio of 1∶4. This suggests that dMi-2 has a higher binding affinity for RNA than for DNA. We confirmed this hypothesis by incubating dMi-2 with different mass ratios of RNA and DNA (Figure 5C): At a DNA:RNA mass ratio of 1∶1, dMi-2/RNA complexes formed readily but dMi-2/DNA complexes were not detected. dMi-2/RNA complexes formed even at DNA:RNA mass ratios of 4∶1.
To test if RNA or DNA can compete with dMi-2 for binding to the branched PAR polymer we performed dot blot assays (Figure S7). RNA competed with immobilised PAR for binding to dMi-2 whereas DNA failed to do so.
Taken together, our results suggest that dMi-2 has a higher affinity for binding to RNA and PAR than for binding to DNA. In addition, dMi-2 appears to bind RNA and PAR in a mutually exclusive manner. These results are consistent with the hypothesis that dMi-2 is first recruited to HS loci by interaction with PAR (which is produced prior to and independent of transcription) and, once RNA synthesis has been strongly activated, switches to binding the nascent RNA.
dMi-2 is required for efficient HS gene transcription and processing
We hypothesised that dMi-2 binding to nascent RNA might influence hsp70 transcription or processing. We used transgenic fly lines to deplete dMi-2 by RNAi (Figure 6A). We subjected transgenic larvae to HS and determined the HS gene transcription by RT-QPCR. Although hsp70, hsp26 and hsp83 genes were all activated by HS, transcript levels were severely reduced in dMi-2 depleted larvae compared to controls. Importantly, transcription of a housekeeping gene was not significantly affected. We conclude that dMi-2 makes a positive contribution to transcription and is essential for full HS gene activation in larvae.
Fig. 6. dMi-2 is required for efficient HS gene transcription. 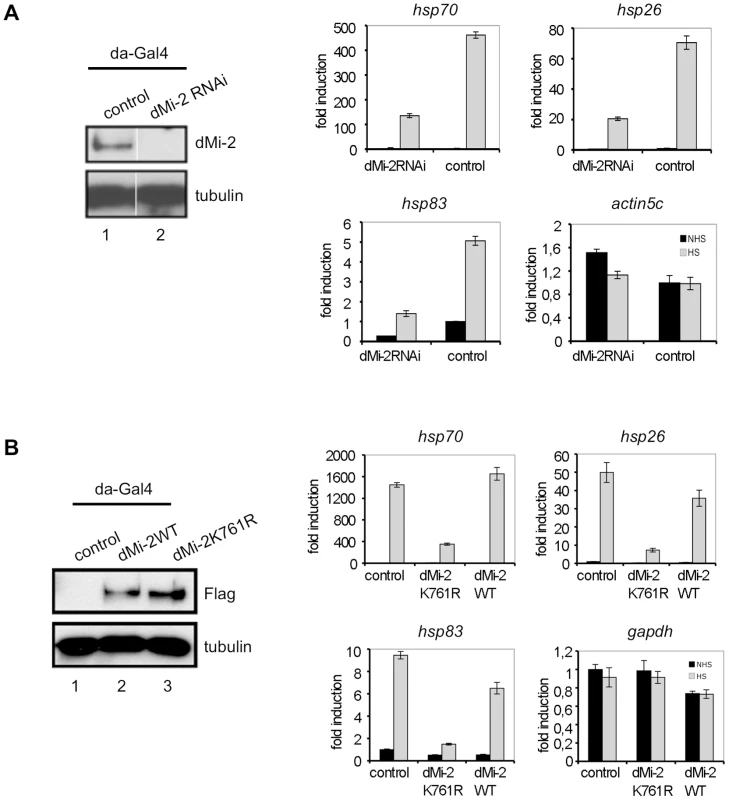
(A) Left panel: Verification of dMi-2 knockdown in control and dMi-2 RNAi larvae. Control flies and flies carrying an dMi-2 RNAi transgene under UAS control were crossed with a da-GAL4 driver strain. Extracts from third instar larvae were subjected to Western Blot. Antibodies used are indicated on the right. Right panel: RT-QPCR analysis of hsp70, hsp26, hsp83 and actin5c expression in control and dMi-2 RNAi larvae. Values are expressed relative to the value in NHS control larvae. (B) Left panel: Verification of dMi-2 transgene expression in larvae by anti-Flag Western blot. Right panel: RT-QPCR analysis of hsp70, hsp26, hsp83 and GAPDH expression in control and transgenic larvae. We next determined whether dMi-2 enzymatic activity was required to activate HS genes. We generated transgenic fly lines overexpressing wild type dMi-2 or a dMi-2 mutant carrying a point mutation in the ATP binding site (K761R) predicted to prevent ATP binding (Figure 6B). Indeed, dMi-2K761R could not hydrolyse ATP in vitro (Figure S8). We subjected 3rd instar larvae to HS and determined effects on HS gene transcription as before. Whereas overexpression of wild type dMi-2 had little effect, levels of HS gene transcripts were greatly reduced in larvae overexpressing the enzymatically inactive dMi-2 (Figure 6B). We conclude that the ATPase activity of dMi-2 is essential for full HS gene activation.
Next, we sought to assess whether dMi-2 influences RNA processing. Because dMi-2 depletion and expression of enzymatically inactive dMi-2 resulted in an overall reduction of hsp70 transcript levels we determined the ratio of 3′ unprocessed to total hsp70 RNA as a measure of RNA processing efficiency. We reasoned that a mere reduction in hsp70 activation (e.g. a reduction in the number of initiation events per time) would not change the ratio of unprocessed to total hsp70 RNA. By contrast, processing defects might give rise to a higher relative proportion of unprocessed RNA and, therefore, to a higher unprocessed:total RNA ratio. Depletion of dMi-2 increased the relative proportion of unprocessed hsp70 RNA (Figure 7A). An even more striking effect was observed in larvae overexpressing inactive dMi-2, whereas overexpression of wild type dMi-2 was of little consequence. Similar effects on 3′ RNA processing were observed with the hsp83 gene (data not shown). Hsp83 is one of the few HS genes possessing an intron. Therefore, we determined the ratio of unspliced to total hsp83 transcripts in transgenic larvae (Figure 7B). Again, we observed a significant increase in the relative proportion of unspliced RNA in dMi-2-depleted larvae and in larvae overexpressing inactive enzyme. This suggests that dMi-2 activity is required for the efficient processing of HS gene transcripts and that dMi-2 affects both RNA 3′ end cleavage and splicing.
Fig. 7. dMi-2 is required for efficient RNA processing. 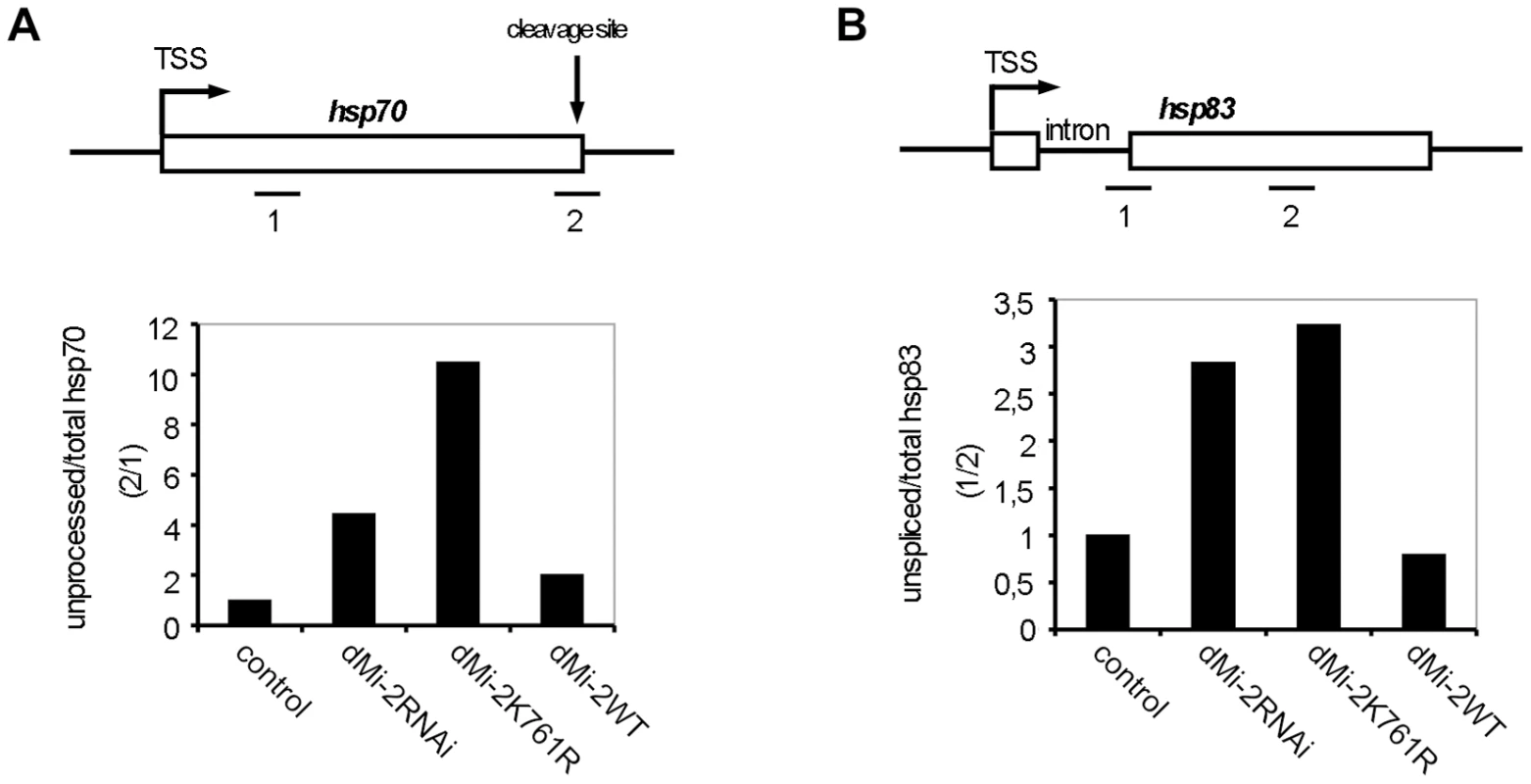
(A and B) Upper panels: Schematic representations of the hsp70 and hsp83 genes. RT-QPCR amplimers, hsp70 cleavage site, hsp83 intron and transcriptional start sites (TSS) are shown. (A) Lower panel: RT-QPCR from control and transgenic larvae. The ratio between 3′ unprocessed and total hsp70 RNA was determined (hsp70: amplimer 2/amplimer 1). The ratio obtained for control larvae was set to 1, other ratios were expressed relative to this. (B) Lower panel: The ratio between unspliced and total hsp83 RNA was determined (hsp83: amplimer 1/amplimer 2) and plotted as in (A). Discussion
dMi-2 associates with active genes
Mi-2 is strongly linked to transcriptional repression in both vertebrate and invertebrate organisms. Within NuRD and dMec complexes it contributes to the repression of cell type-specific genes [11], [14], [19]–[21]. Therefore, the widespread colocalisation of dMi-2 with active Pol II and elongation factors at many chromosomal sites is surprising and suggests that dMi-2 might play an unappreciated role during active transcription, at least (or specifically) during environmental stresses such as HS. Indeed, dMi-2 is recruited to HS genes within minutes of HS. This property is not shared by other chromatin remodelers: Brahma (BRM) is not enriched at HS puffs and HS gene activation is independent of BRM function ([27] and data not shown). Moreover, although imitation switch (ISWI) containing complexes are important for HS gene transcription, ISWI does not accumulate to high levels at active HS loci ([28], [29] and data not shown). Recruitment to HS puffs has previously been reported for Drosophila CHD1 [30]. Thus, accumulation at active HS genes is shared by at least two members of the CHD family of nucleosome remodelers but not by SWI/SNF and ISWI proteins.
dMi-2 contributes to efficient HS gene transcription
Depletion of dMi-2 or a reduction of dMi-2 recruitment does not significantly perturb hsp70 transcription in Kc cells and, therefore, dMi-2 is dispensable for HS gene activation in this system (Figure 2D and data not shown). By contrast, depletion of dMi-2 in larvae strongly decreases hsp70, hsp26 and hsp83 activation (Figure 6A). It is possible, that the RNAi-mediated depletion of dMi-2 is more efficient in transgenic flies compared to cell lines. In addition, it is believed that several factors contributing to HS gene activation are highly abundant or redundant in Kc cells but more limiting in other contexts. Accordingly, FACT and Spt6 are required for a HS gene activation in flies but are not essential in Kc cells [31], [32].
The strong decrease of HS gene activation in dMi-2 RNAi larvae indicates a positive contribution of dMi-2 to transcription in vivo. Overexpression of inactive dMi-2 also results in reduced HS gene transcription implying that its enzymatic activity is critical (Figure 6B). It is presently unclear whether this reflects a requirement for dMi-2 catalysed nucleosome remodeling or whether its activity is directed towards different substrates.
dMi-2 contributes to efficient RNA processing
While dMi-2 could indirectly influence transcription by remodeling nucleosomes within the transcribed part of hsp70, its physical association with nascent HS gene transcripts argues for a more direct effect. Indeed, dMi-2 is not only required for high HS gene mRNA levels, but also affects the efficiency of co-transcriptional 3′ end formation and splicing. A role of chromatin remodelers in splicing has been suggested before: Both CHD1 and BRG1 bind components of the splicing apparatus [33], [34]. CHD1 associates with Pol II and binds nucleosomes containing H3K4me3, which are enriched near the 5′ end of active genes [34], [35]. BRG1 is present at the coding region of genes and influences splice site choice [33], [36]. It has been proposed that CHD1 and BRG1 physically recruit splicing factors but it is unclear if their ATPase activities play a role. Indeed, inactive BRG1 retains the ability to affect exon choice [33], [34]. Inefficient processing of the hsp70 and hsp83 transcripts is not only observed in larvae expressing reduced levels of dMi-2. Importantly, even stronger processing defects are generated by overexpression of inactive dMi-2 (Figure 7). This strongly suggests, for the first time, that the catalytic activity of a chromatin remodeler is required for correct co-transcriptional RNA processing. It remains to be determined whether dMi-2 nucleosome remodeling activity influences RNA processing indirectly, e.g. by altering Pol II elongation rates, or whether it has a more direct role.
PAR–dependent recruitment of dMi-2
A series of complementary results support our hypothesis that dMi-2 interacts with PAR polymers that are rapidly synthesized at activated HS loci. First, the broad distribution of dMi-2 over the entire transcribed region correlates with the distribution of PAR polymer [3]. Second, pharmacological inhibition of PARP greatly decreases dMi-2 binding to activated hsp70. Third, dMi-2 directly binds PAR polymers in vitro. Fourth, an dMi-2 mutant unable to bind PAR also fails to localise to active HS loci. As discussed above, dMi-2 physically associates with nascent HS gene transcripts and binds RNA in vitro. While this interaction is potentially important for the efficiency of transcription and processing, it likely plays a minor role in dMi-2 targeting. Accordingly, inhibition of transcriptional elongation has no significant effect on dMi-2 recruitment (Figure 2C).
It is important to note, that while our results argue for an important role of PAR binding in the recruitment of dMi-2 to HS loci, we cannot exclude that protein-protein interactions with histone or non-histone proteins also play a role.
dMi-2 PAR–binding
Our analysis indicates that dMi-2 harbours several PAR binding motifs in its N-terminal region. Polo and colleagues have recently demonstrated that human CHD4 is recruited to double stranded DNA breaks in a PARP-dependent manner [10]. They have mapped PAR binding activity to the region N-terminal of the ATPase domain of CHD4. This agrees well with our data and suggests that the PAR binding function of CHD4/dMi-2 has been conserved in evolution.
Two structural protein modules directly interact with PAR, the macrodomain and the PBZ domain; however, these domains are not present in dMi-2 [5], [7], [37]. In addition, several shorter PAR binding motifs have been identified [5], [26]. These motifs bear little sequence similarity but share the presence of several K/R residues which are interspersed by hydrophobic residues. Our results have uncovered three K/R-rich regions with PAR binding activity near the N-terminus of dMi-2. Two of these three K/R-rich regions (K/R III and K/R IV) consist of interspersed basic and hydrophobic residues and are therefore reminiscent of the previously described PAR binding motifs [24], [25], the third (K/R I) lacks hydrophobic residues completely. None of the three K/R regions matches the consensus PAR binding motifs. It is possible that a consensus motif should generally be chosen less stringently and that a high content of K and R-residues in these regions is sufficient to provide PAR binding activity in vitro. Further characterisation of these regions will be required to resolve this issue. In addition to the K/R regions, the tandem chromodomains of dMi-2 bind PAR in vitro. We have previously shown that the chromodomains are required for interacting with nucleosomal DNA in vitro [38]. Our new data suggests that these domains can interact with different nucleic acids.
Different functions of PAR in HS gene transcription
Several potential molecular functions of PARylation at HS genes have been suggested. First, PARP activity is required for the rapid loss of nucleosomes at hsp70 within the first two minutes after HS [1]. It has been suggested that PARylation of histones aids rapid nucleosome disassembly [1]. Second, at later stages of the HS response (20–60 minutes after HS), PARP activity is required to establish a compartment which restricts the diffusion of factors such as Pol II and Spt6 and promotes efficient factor recycling [4]. Our results suggest that PARylation carries out a third task, namely, to recruit factors via their direct interaction with PAR. The earliest time point when we can detect dMi-2 binding to hsp70 is between 2 and 5 minutes after HS. This places dMi-2 recruitment between the early PARP-dependent nucleosome removal (0–2 minutes after HS) and effects of the transcription compartment (20–60 minutes after HS).
PAR versus RNA binding
The ability of dMi-2 to bind both PAR and RNA and the finding that RNA can compete for PAR binding to dMi-2 is consistent with the hypothesis that dMi-2 association with active HS genes is a two step process (Figure 8). We propose that dMi-2 is initially recruited via interaction with PAR polymers. Synthesis of these starts prior to the onset of hsp70 transcription [1]. This results in a rapid local increase of the dMi-2 concentration. In the second step, when hsp70 transcripts are produced by elongating RNA polymerase II at high rates, dMi-2 can switch from binding PAR to interacting with nascent transcripts.
Fig. 8. Model. 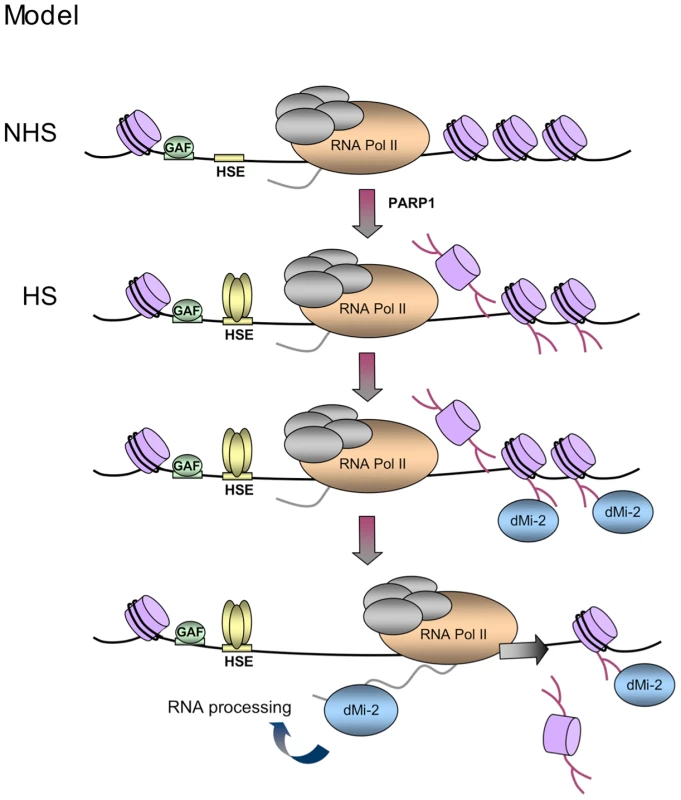
Upon HS, PARylation of the locus creates binding sites for PAR-sensing regions of dMi-2. dMi-2 is recruited and, subsequently, interacts with nascent transcripts to support transcription and processing. GAF: GAGA Factor, HSE: HS elements. PAR signals rapid and efficient factor recruitment to chromatin during stress
Severe cellular stresses, such as DNA strand breaks and acute HS, must be dealt with quickly and efficiently. In both cases, a multitude of factors are rapidly recruited to orchestrate the repair of DNA and the massive transcriptional activation of HS genes, respectively. We postulate that rapid synthesis of PAR polymers at both DNA damage sites and HS genes affords an efficient mechanism to recruit chromatin remodelers and other factors. It has recently been shown that PARylation of DNA breaks is instrumental in recruiting chromatin remodelers, including mammalian dMi-2 homologs, to damaged sites [8], [9], [10], [39], [40], [41]. Here, we show that dMi-2′s recruitment to activated HS genes requires PARP activity and that dMi-2 binds PAR directly. The high local concentration of PAR polymers at DNA breaks and HS genes might exploit the general affinity of dMi-2 for nucleic acids. Indeed, dMi-2 binds both DNA and RNA as well as PAR in vitro ([38] and this study). In this manner, PAR polymers might act as a scaffold to redirect dMi-2 to chromatin regions where high levels of dMi-2 activity are required, thus acting as a stress-dependent, transient affinity site for chromatin remodeling and possibly RNA processing activities (Figure 8). Our results highlight a signaling and scaffolding function for PARP activity during transient environmental stresses other than DNA damage, suggesting that PARylation carries out important modulatory functions in the stress-dependent reprogramming of nuclear activities.
Materials and Methods
Chromatin immunoprecipitation (ChIP)
Kc cell HS treatment and ChIP was performed as decribed using dMi-2C antibody [14], [42]. For primer sequences see Dataset S2. Triplicate mean values of percentage input DNA and standard deviations are plotted. dMi-2 knockdown by RNAi was described previously [23]. For RNAi primer sequences see Dataset S4.
Pharmacological treatments
Kc cells were treated with 125 µM DRB (Sigma) to inhibit transcription and with 5 µM PJ34 (Alexis) to inhibit PARP activity for 20 min before subjecting cells to HS.
Polytene chromosomes
Chromosomes were prepared as before [23]. The following antibodies were used: Primary antibodies: anti-dMi-2N (rabbit) 1∶200, anti-pol II (mouse H5, Covance) 1∶50, anti-GFP (rabbit, Abcam) 1∶50, anti-Spt5 (guinea pig) 1∶200. Secondary antibodies: Alexa Fluor 488 goat anti-rabbit 1∶200, Alexa Fluor 546 goat anti-mouse or anti-guinea pig 1∶200 (Invitrogen).
Analysis was performed with a Zeiss fluorescence microscope (Axioplan).
Generation of baculovirus and GST-fusion vectors
For baculovirus production, dMi-2 mutants (aa 1-691) and (aa 1-485) were generated by PCR using appropriate sets of primers and cloned with NotI and XbaI into the pVL1392 transfer vector. Vectors for dMi-2 WT and other mutants were described previously [38]. dMi-2 GST-fusion fragments were generated by PCR using appropriate sets of primers and cloned with NotI and SalI into the pGEX4T1 vector. All constructs were verified by DNA sequencing. For primer sequences see Dataset S1.
Preparation of protein extracts, purification of recombinant proteins, and ATPase assay
Protein extracts from 3rd instar larvae were prepared as described in [14].
Purification of recombinant dMi-2 and ATPase assays are described [23]. Recombinant mH2A1.1 was purified as in [43]. GST-fusion proteins were expressed in E.coli BL21(DE3) and purified with Glutathione Sepharose 4 Fast flow (GE Healthcare) according to the manufacturer's instructions.
Electrophoretic mobility shift assays (EMSA)
A typical DNA or RNA binding reaction (25 µl) was performed in the presence of 0.2 µg of dMi-2F and 80 ng of nucleic acid (DNA or ssRNA) in 40 mM KCl, 20 mM Tris pH 7.6, 1.5 mM MgCl2, 0.5 mM EGTA, 10% glycerol, BSA (200 ng/µl), 1 mM DTT (supplemented with 0.4 units of RNAsin). For competition assays, samples were preincubated for 15 min at 26°C before the different amounts of competitor (PAR or DNA or RNA) were added. Reactions were further incubated at 26°C for 75 min. Products were analyzed on 6% native PAA gel and visualized with ethidium bromide (EtBr) staining. ssRNA was synthesized by in vitro transcription using a fragment of hsp70 DNA as a template. This template (also used for the DNA bandshift assays) was produced by PCR amplification of cDNA derived from heat shocked Kc cells using the following primers: T7-hsp70_f - TAATACGACTCACTATAGGGCCTACGGACTGGACAAGAAC and hsp70_r -AGGGTTGGAGCGCAGATCCTTCTTGTAC.
RNA isolation and RT-QPCR
Total RNA was isolated from 3rd instar larvae using PeqGold total RNA Kit (PeqLab). 10-12 larvae from each cross were pestled in 400 µl of lysis buffer before loading the material on the column. 1 µg of RNA was reverse transcribed by incubation with 0.3 µg of random primers (Invitrogene) and 100 U of M-MLV reverse transcriptase (Invitrogen). cDNA synthesis was performed according to the manufacturer's protocol. cDNA was analyzed by QPCR using Absolute SybrGreen Mix (Thermo Fisher) and the Mx3000P real-time detection system (Agilent). For primer sequences used in RT-QPCR see Dataset S3. All amplifications were performed in triplicate using 0.6 µl of cDNA per reaction. Triplicate mean values were calculated according to the ΔΔCt quantification method using rp49 gene transcription as reference for normalization. Relative mRNA levels in uninduced control larvae were set to 1 and other values were expressed relative to this. The RT-QPCR results were reproduced several times using independent fly crosses and representative data sets are shown.
RNA immunoprecipitation
RNA immunoprecipitation was performed as described previously [44]. Briefly, Kc cells were crosslinked as for ChIP. Cells were washed once with PBS buffer and lysed on ice for 15 min in FA buffer (50 mM Hepes - KOH, pH 7,6, 140 mM NaCl, 1% Triton X-100, 0,1% sodium deoxycholate, proteinase inhibitors, RNAsin (100 u/ml of buffer)). Cells were sonicated, spun down and chromatin was digested with DNAse I. The chromatin containing solution was adjusted to 25 mM MgCl2 and 5 mM CaCl2. 1 ul of DNAse I (Qiagen) was added and reactions were incubated for 10 min at room temperature and then stopped with 20 mM EDTA. Chromatin was spun down for 10 min (13000 rpm) at 4°C. 300 µl of chromatin was used for IP. 2 µl of anti-dMi-2(C) and anti-dMi-2(N) antibodies, 2 µl rabbit IgG, 2 µl rabbit preimmuneserum and beads only (control) and were used for IP. Samples were incubated over night at 4°C. RNA-protein complexes were precipitated with 30 ul of 50% protein G Sepharose beads for 2 hr at 4°C. IPs were washed 5 times in FA buffer, twice with TE buffer and eluted twice with 100 µl of elution buffer (100 mm Tris HCl, pH 8,0, 10 mM EDTA, 1% SDS) – once at room temperature and once at 65°C. All buffers were supplemented with RNAse inhibitor (RNAsin, Promega). All samples were digested with proteinase K for 1 hr at 42°C and decrosslinking was performed at 65°C over night. Immunoprecipitated RNA was purified using PeqGold total RNA Kit (PeqLab), digested with DNAse on the column and eluted with 30 µl of RNAse free dH20. cDNA was synthesized with 10 µl of eluted RNA and 2 µl of input with random hexamers and analysed by Q-PCR with appropriate primer pairs.
PARP/PAR pulldowns
Non-radioactive PAR synthesis was performed according to the standard protocol [45]. Briefly, PARP reactions were set up in a final volume of 0.5 ml: 2 µg recombinant Parp1, 100 mM Tris-HCl, pH 7.5, 50 mM NaCl, 10 mM MgCl2, 2 µg/ml DNA oligonucleotides, 1 mM NAD+, 1 mM DTT. Reactions were incubated at 37°C for 25 min. PJ34 inhibitor was added before the reaction to control samples to a final concentration of 5 µM. All reactions were stopped with PJ34. Control beads and beads with bound proteins (dMi-2, dMi-2 mutants and mH2A1.1 or GST fusions) were equilibrated in binding buffer (50 mM Tris, pH 8.0, 0,2 mM DTT, 4 mM MgCl2, 200 mM NaCl, 0,1% NP-40). 10 µl of bead-bound proteins were used for each pulldown. Pulldowns were performed with the whole PARP reaction (0.5 ml) and 500 µl of binding buffer (for baculovirus expressed proteins) or in 250 µl of PARP reaction and 250 µl of binding buffer (for GST fusions) for 1 hr at 4°C. After extensive washing (5 times), beads were boiled in SDS loading buffer, loaded on 4–12% gradient SDS-Page gels and analysed by Western blot. For Western blot anti-PAR (10H, 1∶500) antibodies were used. mH2A1.1 was used as a positive control. Radioactive pulldown reactions were prepared in the same way, in the presence of 2 µl of radioactive NAD+ (PerkinElmer). After washing, samples were resuspended in 30 µl of SDS-loading buffer and 10 µl was resolved by SDS PAGE. The gel was dried and and subjected to autoradiography.
Generation of transgenic fly strains and the PAR dot blot assay are described in Text S1.
Supporting Information
Zdroje
1. PeteschSJLisJT 2008 Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell 134 74 84
2. WinegardenNAWongKSSoptaMWestwoodJT 1996 Sodium salicylate decreases intracellular ATP, induces both heat shock factor binding and chromosomal puffing, but does not induce hsp 70 gene transcription in Drosophila. J Biol Chem 271 26971 26980
3. TulinASpradlingA 2003 Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science 299 560 562
4. ZobeckKLBuckleyMSZipfelWRLisJT 2010 Recruitment Timing and Dynamics of Transcription Factors at the Hsp70 Loci in Living Cells. Molecular Cell 40 965 975
5. KrishnakumarRKrausWL 2010 The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell 39 8 24
6. TillSLadurnerAG 2009 Sensing NAD metabolites through macro domains. Front Biosci 14 3246 3258
7. TiminszkyGTillSHassaPOHothornMKustatscherG 2009 A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat Struct Mol Biol 16 923 929
8. AhelDHorejsiZWiechensNPoloSEGarcia-WilsonE 2009 Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325 1240 1243
9. GottschalkAJTiminszkyGKongSEJinJCaiY 2009 Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc Natl Acad Sci U S A 106 13770 13774
10. PoloSEKaidiABaskcombLGalantyYJacksonSP 2010 Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. Embo J 29 3130 3139
11. FujitaNJayeDLGeigermanCAkyildizAMooneyMR 2004 MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell 119 75 86
12. MurawskyCMBrehmABadenhorstPLoweNBeckerPB 2001 Tramtrack69 interacts with the dMi-2 subunit of the Drosophila NuRD chromatin remodelling complex. EMBO Rep 2 1089 1094
13. MarfellaCGImbalzanoAN 2007 The Chd family of chromatin remodelers. Mutat Res 618 30 40
14. KunertNWagnerEMurawskaMKlinkerHKremmerE 2009 dMec: a novel Mi-2 chromatin remodelling complex involved in transcriptional repression. Embo J 28 533 544
15. FengQZhangY 2001 The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes. Genes Dev 15 827 832
16. Le GuezennecXVermeulenMBrinkmanABHoeijmakersWACohenA 2006 MBD2/NuRD and MBD3/NuRD, two distinct complexes with different biochemical and functional properties. Mol Cell Biol 26 843 851
17. LykoFBeiselCMarholdJParoR 2006 Epigenetic regulation in Drosophila. Curr Top Microbiol Immunol 310 23 44
18. KehleJBeuchleDTreuheitSChristenBKennisonJA 1998 dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science 282 1897 1900
19. KimJSifSJonesBJacksonAKoipallyJ 1999 Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 10 345 355
20. KoipallyJRenoldAKimJGeorgopoulosK 1999 Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. Embo J 18 3090 3100
21. ReddyBABajpePKBassettAMoshkinYMKozhevnikovaE 2010 Drosophila transcription factor Tramtrack69 binds MEP1 to recruit the chromatin remodeler NuRD. Mol Cell Biol 30 5234 5244
22. StielowBSapetschnigAKrugerIKunertNBrehmA 2008 Identification of SUMO-dependent chromatin-associated transcriptional repression components by a genome-wide RNAi screen. Mol Cell 29 742 754
23. MurawskaMKunertNvan VugtJLangstGKremmerE 2008 dCHD3, a novel ATP-dependent chromatin remodeler associated with sites of active transcription. Mol Cell Biol 28 2745 2757
24. GagneJPHunterJMLabrecqueBChabotBPoirierGG 2003 A proteomic approach to the identification of heterogeneous nuclear ribonucleoproteins as a new family of poly(ADP-ribose)-binding proteins. Biochem J 371 331 340
25. PleschkeJMKleczkowskaHEStrohmMAlthausFR 2000 Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J Biol Chem 275 40974 40980
26. ZhangYLiuSMickaninCFengYCharlatO RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol 13 623 629
27. ArmstrongJAPapoulasODaubresseGSperlingASLisJT 2002 The Drosophila BRM complex facilitates global transcription by RNA polymerase II. Embo J 21 5245 5254
28. BadenhorstPXiaoHCherbasLKwonSYVoasM 2005 The Drosophila nucleosome remodeling factor NURF is required for Ecdysteroid signaling and metamorphosis. Genes Dev 19 2540 2545
29. DeuringRFantiLArmstrongJASarteMPapoulasO 2000 The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol Cell 5 355 365
30. KelleyDEStokesDGPerryRP 1999 CHD1 interacts with SSRP1 and depends on both its chromodomain and its ATPase/helicase-like domain for proper association with chromatin. Chromosoma 108 10 25
31. ArdehaliMBYaoJAdelmanKFudaNJPeteschSJ 2009 Spt6 enhances the elongation rate of RNA polymerase II in vivo. Embo J 28 1067 1077
32. SaundersAWernerJAndrulisEDNakayamaTHiroseS 2003 Tracking FACT and the RNA polymerase II elongation complex through chromatin in vivo. Science 301 1094 1096
33. BatscheEYanivMMuchardtC 2006 The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Struct Mol Biol 13 22 29
34. SimsRJ3rdMillhouseSChenCFLewisBAErdjument-BromageH 2007 Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell 28 665 676
35. SrinivasanSArmstrongJADeuringRDahlsveenIKMcNeillH 2005 The Drosophila trithorax group protein Kismet facilitates an early step in transcriptional elongation by RNA Polymerase II. Development 132 1623 1635
36. TyagiARymeJBrodinDOstlund FarrantsAKVisaN 2009 SWI/SNF associates with nascent pre-mRNPs and regulates alternative pre-mRNA processing. PLoS Genet 5 e1000470 doi:10.1371/journal.pgen.1000470
37. AhelIAhelDMatsusakaTClarkAJPinesJ 2008 Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 451 81 85
38. BouazouneKMitterwegerALangstGImhofAAkhtarA 2002 The dMi-2 chromodomains are DNA binding modules important for ATP-dependent nucleosome mobilization. Embo J 21 2430 2440
39. ChouDMAdamsonBDephoureNETanXNottkeAC 2010 A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci U S A 107 18475 18480
40. LarsenDHPoinsignonCGudjonssonTDinantCPayneMR 2010 The chromatin-remodeling factor CHD4 coordinates signaling and repair after DNA damage. J Cell Biol 190 731 740
41. SmeenkGWiegantWWVrolijkHSolariAPPastinkA 2010 The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. J Cell Biol 190 741 749
42. BoehmAKSaundersAWernerJLisJT 2003 Transcription factor and polymerase recruitment, modification, and movement on dhsp70 in vivo in the minutes following heat shock. Mol Cell Biol 23 7628 7637
43. KustatscherGHothornMPugieuxCScheffzekKLadurnerAG 2005 Splicing regulates NAD metabolite binding to histone macroH2A. Nat Struct Mol Biol 12 624 625
44. GilbertCSvejstrupJQ 2006 RNA immunoprecipitation for determining RNA-protein associations in vivo. Curr Protoc Mol Biol Chapter 27 Unit 27 24
45. KarrasGIKustatscherGBuhechaHRAllenMDPugieuxC 2005 The macro domain is an ADP-ribose binding module. Embo J 24 1911 1920
Štítky
Genetika Reprodukční medicína
Článek Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal MutationsČlánek Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary IntegrityČlánek A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with ExpressionČlánek Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line PedigreeČlánek Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 7
-
Všechny články tohoto čísla
- Gene-Based Tests of Association
- The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
- Variants in and Underlie Natural Variation in Translation Termination Efficiency in
- SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
- Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations
- Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer
- Identification of a Mutation Associated with Fatal Foal Immunodeficiency Syndrome in the Fell and Dales Pony
- Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
- An Epigenetic Switch Involving Overlapping Fur and DNA Methylation Optimizes Expression of a Type VI Secretion Gene Cluster
- Recombination and Population Structure in
- A Rice Plastidial Nucleotide Sugar Epimerase Is Involved in Galactolipid Biosynthesis and Improves Photosynthetic Efficiency
- A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets
- Colon Stem Cell and Crypt Dynamics Exposed by Cell Lineage Reconstruction
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Interactions between Glucocorticoid Treatment and Cis-Regulatory Polymorphisms Contribute to Cellular Response Phenotypes
- Translation Reinitiation Relies on the Interaction between eIF3a/TIF32 and Progressively Folded -Acting mRNA Elements Preceding Short uORFs
- DAF-12 Regulates a Connected Network of Genes to Ensure Robust Developmental Decisions
- Adult Circadian Behavior in Requires Developmental Expression of , But Not
- Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary Integrity
- Proteins in the Nutrient-Sensing and DNA Damage Checkpoint Pathways Cooperate to Restrain Mitotic Progression following DNA Damage
- Complex Evolutionary Events at a Tandem Cluster of Genes Resulting in a Single-Locus Genetic Incompatibility
- () and Its Regulated Homeodomain Gene Mediate Abscisic Acid Response in
- A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
- LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs
- Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications
- The Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
- Somatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
- Molecular Mechanisms Generating and Stabilizing Terminal 22q13 Deletions in 44 Subjects with Phelan/McDermid Syndrome
- Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line Pedigree
- Mechanisms of Chromosome Number Evolution in Yeast
- Regulatory Cross-Talk Links Chromosome II Replication and Segregation
- Ancestral Genes Can Control the Ability of Horizontally Acquired Loci to Confer New Traits
- Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
- Rare and Common Regulatory Variation in Population-Scale Sequenced Human Genomes
- The Epistatic Relationship between BRCA2 and the Other RAD51 Mediators in Homologous Recombination
- Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy
- NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
- Araucan and Caupolican Integrate Intrinsic and Signalling Inputs for the Acquisition by Muscle Progenitors of the Lateral Transverse Fate
- Pathologic and Phenotypic Alterations in a Mouse Expressing a Connexin47 Missense Mutation That Causes Pelizaeus-Merzbacher–Like Disease in Humans
- Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination
- Epistatic Relationships in the BRCA1-BRCA2 Pathway
- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium
- Fine Mapping of Five Loci Associated with Low-Density Lipoprotein Cholesterol Detects Variants That Double the Explained Heritability
- CHD1 Remodels Chromatin and Influences Transient DNA Methylation at the Clock Gene
- Nonlinear Fitness Landscape of a Molecular Pathway
- Genome-Wide Scan Identifies , , and as Novel Risk Loci for Systemic Sclerosis
- Quantitative and Qualitative Stem Rust Resistance Factors in Barley Are Associated with Transcriptional Suppression of Defense Regulons
- A Systematic Screen for Tube Morphogenesis and Branching Genes in the Tracheal System
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Gene-Based Tests of Association
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



