-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
In humans, sexual dimorphism is associated with the presence of two X chromosomes in the female, whereas males possess only one X and a small and largely degenerate Y chromosome. How do men cope with having only a single X chromosome given that virtually all other chromosomal monosomies are lethal? Ironically, or even typically many might say, women and more generally female mammals contribute most to the job by shutting down one of their two X chromosomes at random. This phenomenon, called X-inactivation, was originally described some 50 years ago by Mary Lyon and has captivated an increasing number of scientists ever since. The fascination arose in part from the realisation that the inactive X corresponded to a dense heterochromatin mass called the “Barr body” whose number varied with the number of Xs within the nucleus and from the many intellectual questions that this raised: How does the cell count the X chromosomes in the nucleus and inactivate all Xs except one? What kind of molecular mechanisms are able to trigger such a profound, chromosome-wide metamorphosis? When is X-inactivation initiated? How is it transmitted to daughter cells and how is it reset during gametogenesis? This review retraces some of the crucial findings, which have led to our current understanding of a biological process that was initially considered as an exception completely distinct from conventional regulatory systems but is now viewed as a paradigm “par excellence” for epigenetic regulation.
Published in the journal: . PLoS Genet 7(7): e32767. doi:10.1371/journal.pgen.1002212
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1002212Summary
In humans, sexual dimorphism is associated with the presence of two X chromosomes in the female, whereas males possess only one X and a small and largely degenerate Y chromosome. How do men cope with having only a single X chromosome given that virtually all other chromosomal monosomies are lethal? Ironically, or even typically many might say, women and more generally female mammals contribute most to the job by shutting down one of their two X chromosomes at random. This phenomenon, called X-inactivation, was originally described some 50 years ago by Mary Lyon and has captivated an increasing number of scientists ever since. The fascination arose in part from the realisation that the inactive X corresponded to a dense heterochromatin mass called the “Barr body” whose number varied with the number of Xs within the nucleus and from the many intellectual questions that this raised: How does the cell count the X chromosomes in the nucleus and inactivate all Xs except one? What kind of molecular mechanisms are able to trigger such a profound, chromosome-wide metamorphosis? When is X-inactivation initiated? How is it transmitted to daughter cells and how is it reset during gametogenesis? This review retraces some of the crucial findings, which have led to our current understanding of a biological process that was initially considered as an exception completely distinct from conventional regulatory systems but is now viewed as a paradigm “par excellence” for epigenetic regulation.
A History of X-Inactivation: Early Studies (1950–1980)
The 1950s and the decades that followed provided much of the basis for present-day developmental biology and molecular genetics (Figure 1). It was a period of crucial advances in mammalian embryology (e.g., ex vivo growth of mouse embryos [1], [2] and transgenic experiments [3]). Contemporary description of the DNA double-helix [4], of homologous recombination [5], of cloning [6], and of the first DNA-based genetic markers [7] similarly opened up the path for genetic engineering, extensive genetic mapping, and seemingly extraordinary quirky observations such as those concerning Position Effect Variation (PEV) in Drosophila [8], [9]. McClintock's earlier work on transposable elements in maize [10] could, moreover, increasingly be assimilated and interpreted with reference to the intellectual context provided by work such as Jacob and Monod's on the genetic regulation of the lac operon [11]. The new and seemingly quirky kinds of gene regulation that could not be explained by Mendelian genetics per se laid the groundwork for the concept of epigenetics—a term derived from the fusion of “genetics”, referring to the primary DNA code, and “epigenesis”, referring to the differential interpretation of the hereditary material within different cell lineages—as being, at least in part, responsible for the relationship between genes and phenotypes [12].
Fig. 1. Timeline showing milestones in the history of X-inactivation (1950–1975). 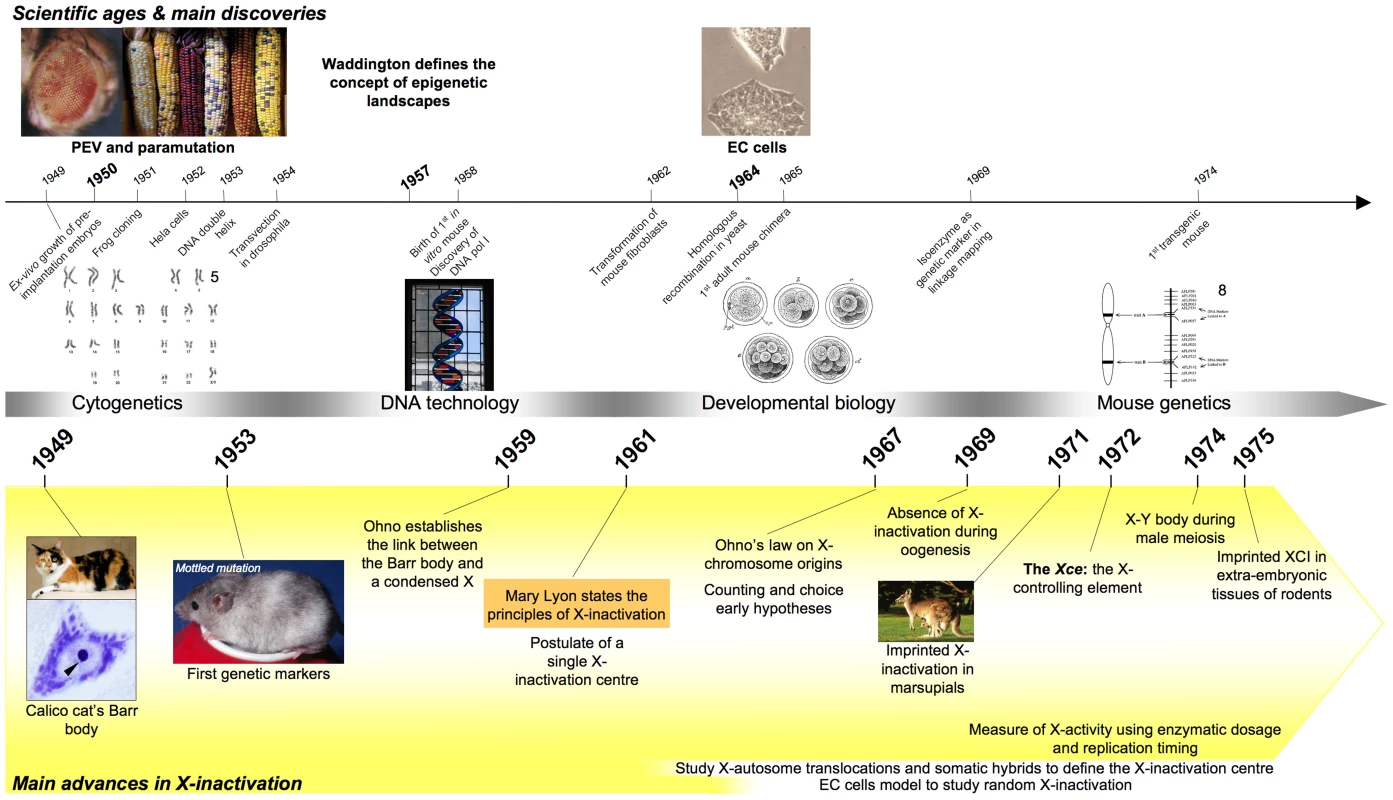
Images are taken from http://commons.wikimedia.org, are a courtesy of the corresponding authors, or are unpublished data. The conditions and nature of the discovery of X-inactivation in the early 1960s illustrate perfectly both the intellectual burgeoning that characterised these years and the emergence of the concept of epigenetics.
The Discovery of X-Inactivation
In 1949, the scrutiny of motoneurons of a female calico cat by Barr and his PhD student Bertram led to the identification of a dark, condensed structure situated close to the nucleolus [13]. Whilst Barr and Bertram did not realise at the time that they were looking at an inactive X chromosome (Xi)—the critical link between the “Barr” body and a condensed X chromosome was to be made only later by Susumu Ohno [14], [15]—their observation, along with that relating to the description of two X-linked loci, Tabby and Mottled, able to confer a mosaic coat colour to heterozygous females [16], and the realisation in 1959 that XO female mice were able both to develop normally and to reproduce [17], were critical to the formulation by Mary Lyon of the X-inactivation theory (for early reviews relating to the discovery of X-inactivation, see [18]–[20]).
In her key 1961 publication, Mary Lyon suggested that the heterochromatic X could correspond in different somatic cells of the same female mammal either to the maternally inherited or to the paternally inherited X chromosome, and proposed that a process leading to the global silencing of the genes of an entire X chromosome referred to as “X-inactivation” occurred during early embryogenesis and was clonally inherited thereafter, thus providing an explanation for the tortoiseshell pattern of Barr's calico cat [21]. Similar ideas were also advanced by Beutler and colleagues to account for their observation of the presence of two types of red cell in human females heterozygous for the X-linked deficiency in glucose-6-phosphate dehydrogenase (G6pdx gene) [22] and by Russell, who put forward a similar—if less elaborate—explanation for variegation in female mice carrying X-autosome translocations [23].
Counting, Choosing, and Skewing
Mary Lyon's theory prompted researchers to study individuals carrying more than one X per set of autosomes. Surprisingly, independently of the configuration, all but one of the X chromosomes in the cell were observed to be condensed, suggesting that each cell could “count” the number of X chromosomes and accordingly inactivate (n−1) Xs per autosome set [20]. This presumed counting process would therefore be responsible for the absence of X-inactivation in male cells.
Other surprising observations concerned the concept of “choice” of active and inactive X(s) and the molecular mechanisms ensuring randomness. Non-randomness, or skewing, can be caused by secondary selection for or against cells carrying the active or the inactive X chromosome (for review see [24]) or alternatively by primary non-random choice occurring during the X-inactivation process itself. The latter implies that a distortion from the 1∶1 ratio of X-inactivation in diploid cells can be caused by factors/genomic region(s) implicated in the X-inactivation process itself. An example of primary skewing is the X-controlling element (Xce), a mouse locus defined in 1972 by Bruce Cattanach, after crosses of mice on different genetic backgrounds revealed that some Xs were more likely to resist X-inactivation than others depending on the Xce allele they carried [25]. No locus homologous to Xce has as yet been described in the human, possibly due to the difficulties of conducting similar analyses.
Developmental Regulation of X-Inactivation
Another key issue at this time was the establishment of where and when X-inactivation took place during development. In the mouse, the Xs that originate either from spermatogenesis, where the paternal X is sequestered within the “sex body” (for review see [26]), or from the female germline, where the maternal X undergoes reactivation at the onset of meiosis, were both shown to be active in the fertilised egg and to remain active until the 8-cell stage as measured by biochemical studies of the few available X-linked isoenzymes [27], [28]. Such early biallelic expression was suspected to concern only a few genes and/or to be of low level and therefore tolerated at these early embryonic stages. The first wave of X-inactivation was originally thought to occur around E3.5 in the extra-embryonic tissues of the trophectoderm and of the primitive endoderm and to consist in a preferential inactivation of the paternal X (imprinted X-inactivation) [29]. In contrast, random X-inactivation was identified as occurring around the time of implantation (E5.5) in cells of the epiblast that give rise to the embryo proper [30], [31]. Of note, the description of imprinting as part of the X-inactivation anticipated by several years the first reports of parental imprinting at autosomal loci [32], [33].
These early studies resulted in X-inactivation being firmly established as the major mechanism responsible for dosage compensation of X-linked gene expression between the sexes in mammals, with the characterisation of a small number of key characteristics such as late replication timing and condensed heterochromatic structure allowing the Xi to be reproducibly distinguished from its active homologue.
The X-Inactivation Centre and the Xist/XIST Gene (1970–2000)
Intuitively, both counting and choice had to require elaborate mechanisms of a new kind involving both the trans communication between Xs and between X chromosomes and autosomes and the cis propagation of the X-inactivation signal along the entire chromosome. Both functions were postulated to be controlled by a single X-linked region called the X-Inactivation Centre (Xic/XIC in mouse/human) from which the X-inactivation signal would then spread to the rest of the chromosome [34]. Retrospectively, it appears relatively visionary to have imagined such a region capable of chromosome-wide concerted gene silencing, especially considering that long-range cis-regulations such as the β-globin Locus Control Region were reported only considerably later [35], [36]. Paradoxically, the trans effect, which now seems particularly intriguing, may have appeared, at the time, as something relatively common given the fact that transvection in Drosophila had been described by Ed Lewis some 29 years earlier [37] (Figure 2; for review, see [38]).
Fig. 2. Main discoveries of the years 1975 to 2000. 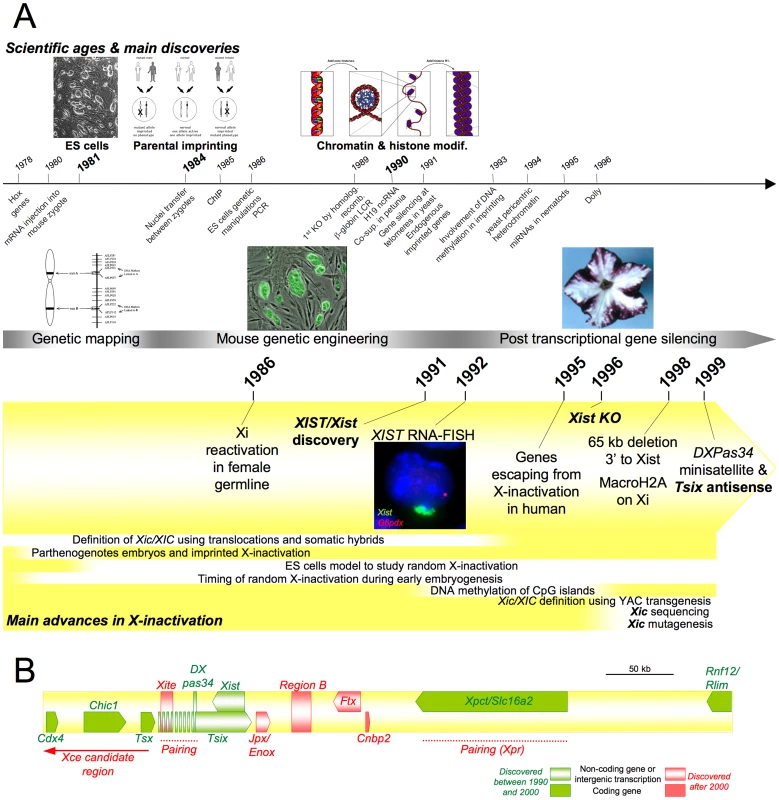
(A) Timeline showing milestones in the history of X-inactivation (1975–2000). Images are taken from http://commons.wikimedia.org, are a courtesy of the corresponding authors, or are unpublished data. (B) Map of the mouse Xic. Defining the X-Inactivation Centre (Xic/XIC) Using Chromosome Rearrangements and Transgenesis
The hunt for the Xic/XIC was initially engaged in the human by comparing a battery of X-autosome translocations that had been identified in clinical research centres. Translocation breakpoints were determined cytologically using chromosome banding patterns and X-inactivation profiles were assessed through replication timing. These experiments resulted in the human XIC candidate region being restricted to an interval of some 660–1,200 kb [39]. Similar approaches led to a much larger genetic interval of 8 CM being defined in the mouse [40], [41]. Importantly, both series of studies confirmed the original hypothesis that a single X-linked region—and not several interspersed loci—underlay Xic/XIC function. Other experiments using mouse translocations showed that inactivation was able to spread from the Xi into attached autosomal material, indicating that the propagation of X-inactivation probably involved mechanisms similar to PEV in Drosophila rather than mechanisms depending exclusively on X-specific sequences [42].
Early observations on female Embryonal Carcinoma (EC) cells [43] that had suggested that such cell lines might prove useful for X-inactivation studies [44] were confirmed and amplified by the derivation of male and female Embryonic Stem (ES) cells, which were shown to recapitulate, upon ex vivo differentiation, the steps leading to stable random X-inactivation. The concomitant development of large fragment transgenesis using these ES cells and embryos permitted the pursuit of Xic/XIC function using Yeast Artificial Chromosomes (YACs) first, then P1 phages and cosmids carrying different Xic formats [45]–[48]. These studies allowed the minimal Xic region necessary for both random X-inactivation and imprinted X-inactivation to be defined [45], [49]. An experimental rider to the 450-kb region defined as necessary for random X-inactivation is the multicopy nature of the transgene array used [50] (for review see [51]).
The Xist/XIST Non-Coding Gene
The search for an XIC candidate gene led to the isolation of the XIST gene based on its specific expression from the human Xi (hence its name, X-inactive specific transcripts) [52]. Though the human and mouse Xist homologues are relatively poorly conserved at the sequence level, both lie within the XIC/Xic and show similar overall genomic organisation [53]–[56]. Both XIST/Xist genes produced very large transcripts (15–17 kb) restricted to the nucleus that do not code for a protein. In this respect, Xist/XIST constituted one of the first large non-coding RNAs to be discovered, not long after the H19 RNA involved in the regulation of the imprinted locus Igf2/H19 was described [57].
The need to follow the behaviour of the inactive and active X chromosomes within the context of a single nucleus led to the rapid implementation of single cell analyses such as fluorescence in situ hybridisation (FISH) techniques. This allowed the visualisation of XIST RNAs within female somatic nuclei as an accumulation or decoration of the Xi, suggesting a possible structural role for the Xist/XIST transcripts [54], [58]. Additionally, kinetics of Xist expression during early mouse development revealed that Xist was expressed as early as the 4-cell stage from the paternal X, suggesting early onset of imprinted X-inactivation in the embryo [59], [60]. The lack of inactivation of an X chromosome mutated for Xist confirmed the major role of the gene in X-inactivation initiation [61], [62].
Xist/XIST Does Not Resume All Xic/XIC Functions
During this period, major positional cloning efforts using genetic and physical mapping resulted in the first large-scale sequencing of Xic subregions [63]. Several new genes and putative functional elements within the Xic/XIC interval were identified. Amongst them, the DXPas34 minisatellite lying 16 kb downstream of Xist appeared to share significant properties with imprinting centres governing the monoallelic expression of autosomal imprinted clusters such as differential DNA methylation profiles [64] and associated long-range non-coding transcription running antisense to Xist [65]. The Xce locus was also shown to map to the Xic region and to be distinct from Xist [66], although its precise location [67], nature, and action remain undetermined.
The establishment of Xic physical maps and genomic sequencing also provided the tools to generate targeted mutations of specific Xic elements and regions. Such mutagenesis notably allowed the creation of a large deletion encompassing 65 kb of sequence 3′ to Xist, which resulted in a systematic inactivation of the mutated X regardless of the presence of another X chromosome in the cell [68]. At the time, this striking phenotype was interpreted as identifying a counting element within the deleted span, thereby irrevocably showing that Xist did not recapitulate all Xic functions.
Main Discoveries since the Year 2000 and Pending Questions (2000–Present)
During the new millennium, progress in gene targeting facilitated the creation of a large variety of novel mutations within the Xic that have considerably improved our understanding of X-inactivation initiation. In parallel, the emergence of a role for chromatin structures as putative transcription regulators [69], [70] and the development of Chromatin Immuno-Precipitation (ChIP) techniques allowing analysis of chromatin composition [71] has strongly impacted our ideas of the mechanisms involved in X-inactivation, building in this respect on earlier documented changes in Xi-associated global histone hypoacetylation [72] and CpG island methylation [73], [74]. These experiments have underlined the likely integrated multi-level and redundant nature of the mechanisms ensuring the stability of the inactive state. Additionally, the finding that lineage specific genome programmes could be efficiently reverted to the pluripotency state(s) as demonstrated, notably, by female induced Pluripotent Stem (iPS) cells [75] and that this was accompanied by Xi reactivation [76] has reinforced interest in the link(s) between cell differentiation and X-inactivation triggering suggested by ES cell differentiation studies. Finally, the many studies of gene nuclear organisation that have shown that chromatin fibres do not fold randomly but rather in a dynamic and directed manner that is correlated with gene expression status [77] have strongly encouraged the investigation of these topological and dynamic aspects of X-inactivation (Figure 3).
Fig. 3. Main discoveries of the years 2000 to 2011. 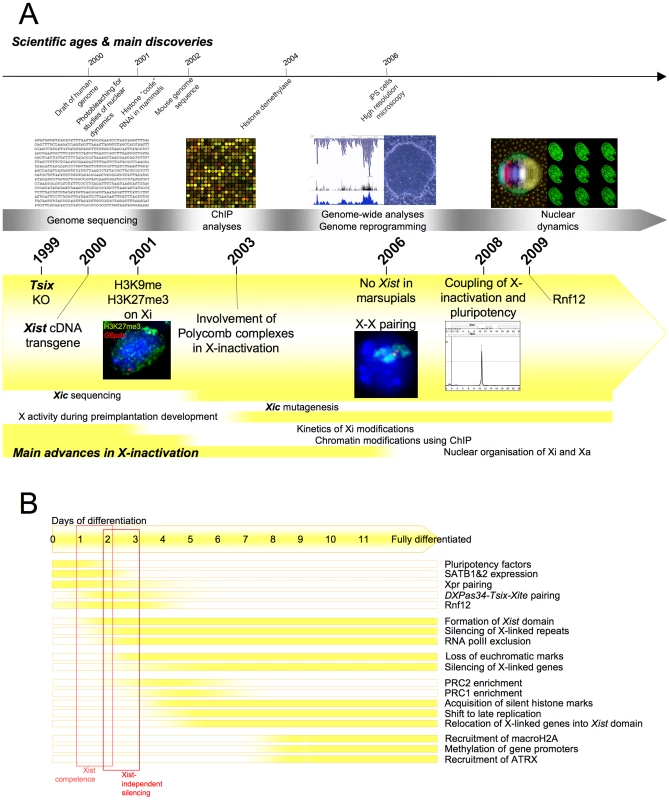
(A) Timeline showing milestones in the history of X-inactivation (2000–2011). Images are taken from http://commons.wikimedia.org, are a courtesy of the corresponding authors, or are unpublished data. (B) Kinetics of events leading to fully stable inactive state during the differentiation of female mouse ES cells. Tsix and the Transcription Antisense to Xist
In the mouse, the enigma of the transcription antisense to Xist was resolved with the description of Tsix, a non-coding gene whose major promoter is located just upstream of the DXPas34 minisatellite [78]. Interestingly, Tsix function does not seem to be conserved in other species (see below). The targeted deletion of Tsix [79]–[81] or of DXPas34 [82], [83] induced a drastic reduction of Tsix transcription that resulted in the preferential inactivation of the mutated X in differentiated female cells. This indicated that Tsix/DXPas34 is involved in the repression of Xist in pluripotent ES cells and in random choice during differentiation [84], [85]. The implication of Tsix in imprinted X-inactivation has also been inferred from the absence of apparent effect of paternally inherited Tsix mutations as opposed to ectopic Xist expression and embryonic lethality associated with maternal transmission [80], [86]. The role of Tsix in the counting process has been addressed by targeting Tsix mutations to XO or XY cells. In the majority of cases such mutations result in ectopic X-inactivation, thereby pointing to a role of Tsix in the counting process [68], [81], [82], [85], [87], although one report suggests otherwise [79]. The divergence in phenotypes in these studies has been suggested to be linked to variations in the differentiation protocols under use.
The emergence of regulatory antisense RNAs has raised a series of questions as to their underlying mechanism(s) of action. Does it necessarily involve RNA interference (RNAi) [88]–[90]? Or RNApolII activity across the target genes? Or the induction of local chromatin modifications? The investigation of these issues has implicated Tsix transcription in maintaining an open chromatin structure along the Xist gene [91]–[93] and in the setting up of a specific chromatin configuration at the Xist promoter [94]. This activity does not appear to be critically dependent in Tsix splicing [95]. Despite extensive community efforts, no conclusive evidence for a role of siRNAs involving the Xist/Tsix overlap has been adduced and the single report of such activity has yet to be confirmed [96]. The absence of an RNAi-based mechanism as the main mediator of Xist repression is in agreement with the absence of a drastic X-inactivation phenotype in ES cells mutated for an essential member of the RNAi machinery, Dicer [97], [98].
In-Depth Characterisation of Xist Expression and the Molecular Function(s) of Xist RNA
The fascinating visualisation of Xist/XIST RNAs “decorating” the Xi in cis but not in trans in a developmentally regulated manner has prompted researchers to investigate the molecular mechanisms behind Xist/XIST action. Keynote insights have come from a series of experiments based on the use of inducible Xist cDNA transgenes in male ES cells, a system that allowed the over-expression of Xist at different time points during differentiation. With the possible rider that these studies involve the generation of non-physiological Xist expression levels and the use of Xist as a spliced form, a major finding was that of a critical window of time during which Xist was competent to induce transcriptional repression and after which the chromosome becomes refractory to silencing and the maintenance of gene repression is Xist independent [99]. The existence of a “chromosomal memory” suggested by the observation of more efficient initiation of X-inactivation in cells that had experienced earlier Xist exposure was also postulated [99].
Using mutations within the Xist cDNA, the silencing function was attributed to the highly conserved repeat A located at the 5′ end of the transcript, whereas the rest of the molecule appears to participate in the coating of the Xi in a synergistic, if partially redundant, manner [100]. Another repeat (repeat C) also interacts with a nuclear matrix attachment protein—hnRNP-U/SAF-A—and this interaction is necessary for correct Xist coating [101]. These results may explain the long-standing observation that Xist RNAs remained attached to the nuclear matrix after chromatin extraction [58], suggesting that Xist transcripts interact with the nuclear scaffold rather than directly with the Xi (for review see [102], [103]). Xist-mediated mechanism(s) might also involve—albeit probably indirectly—the SATB1 and SATB2 nuclear matrix attachment proteins [104]–[106].
Chromatin Modifications, Chromatin Remodellers, and Their Role in the Establishment and Maintenance of Silencing
In the noughtie years, multiple experiments were aimed at indexing the chromatin modifications that characterise the Xi in the hope of reconstructing the chain of events leading to the fully locked inactive state. One of the strategies employed involved using immuno-fluorescence combined with Xist RNA-FISH at successive time points during female ES cell differentiation [107]. A sequential ordering was described with Xist coating of the Xi as the trigger rapidly followed by RNApolII exclusion, the loss of euchromatic marks and almost concomitantly the recruitment of the Polycomb group complex PRC2 [108]–[111], then PRC1 [112] with the consequent accumulation of the heterochromatin marks H3K27me3 and H2AK119ub. Other heterochromatic marks, histone variants such as macroH2A [113], chromatin remodellers (ATRX) [114], and CpG island methylation were other later apposed modifications (for details of the kinetics and the nature of the modifications see [115]).
The number and variety of epigenetic changes—including those still to be uncovered—highlights the extent and depth of the progressive metamorphosis that the presumptive X undergoes during X-inactivation. Although the regional organisation of these different marks along the length of the Xi remains to be established, some ChIP data have already revealed that some marks such as H3K27me3 are preferentially associated with promoters and gene bodies [116], and others, such as the macroH2A histone variants, are more globally distributed [117]. Interestingly, whilst DNA methylation was observed at Xi gene promoters—albeit quite heterogeneously—genes on the active X were hypomethylated at the promoter and hypermethylated in the body of the gene [118]. ChIP analyses on the Xic region have suggested that the presence of specific chromatin domains along the Tsix/Xist locus and upstream of Xist prior to the onset of differentiation is important for X-inactivation randomness [93], [119], [120], but stringent analysis of the specific function of the individual epigenetic marks is still mostly lacking.
Revisiting the Kinetics of X-Inactivation during Pre-Implantation Development
A fundamental question regarding the nature of the imprint on X chromosomes has been to clarify whether the paternal X enters the oocyte in an already “pre-inactivated” state that is subsequently maintained, implying that paternal genes would be silent from the zygotic stage onwards. This question has been the theatre of both lively debate and extensive work. RNA-FISH analysis of several genes interspersed along the paternal X during pre-implantation have now led to the consensual view that an additional reactivation of the paternal X must occur at some point between the onset of spermiogenesis and the 2 - to 4-cell embryo stage [121]–[123]. These analyses also revealed that genes on the paternal X were not silenced synchronously, suggesting that the initial repressive state involves genes or possibly region-specific mechanisms.
The evidence of de novo imprinted X-inactivation during pre-implantation development [111], [124], [125] favours the existence of a robust imprint acting to prevent the inactivation of the maternal X at these stages. This hypothesis is supported by previous observations on gynogenetic embryos where the absence of imprinted X-inactivation was accompanied by the death of the embryos around implantation, in contrast to androgenetic embryos, which were capable of achieving regular random X-inactivation and of surviving until E7.7 [59]. This imprint could be mediated by a strong repression of Xist (as illustrated by the total lack of expression from the maternal Xist locus compared to a pinpoint expression from the paternal locus [125]), although the requirement of Xist for the triggering of imprinted X-inactivation has recently been questioned [121].
Linking X-Inactivation to Pluripotency and Genome Reprogramming
The long-searched-for link between cellular differentiation and X-inactivation was recently established through the discovery that pluripotency factors Nanog, Oct3/4, and Sox2 bind to Xist intron 1 to prevent Xist upregulation in undifferentiated ES cells [126] whilst the pluripotency factors Rex1, Klf4, and c-myc occupied the Tsix promoter and activated Tsix expression [127]. As a consequence at the onset of differentiation, the loss of these pluripotency factors would be expected to be associated with the induction of Xist upregulation. Whilst it is clear that additional binding sites of pluripotency factors/developmentally regulated factors within the Xic remain to be uncovered [128], these important results suggest a direct connection between Xi reactivation during experimentally induced pluripotency and the molecular mechanisms responsible for the genome-wide resetting occurring in the inner cell mass (ICM) (for review see [129], [130]).
It is striking that Nanog has also been detected in female Primordial Germ Cells (PGCs) from E7.75 onwards, a time when Xi reactivation has been shown to initiate [131]–[133], indicating that Nanog might also be involved in Xi reprogramming in the female germline (for review see [134]). Intriguingly, however, Xi reactivation appears to occur progressively throughout the time of PGCs' migration to the genital ridge, thereby dramatically contrasting with the speed of reactivation occurring in the ICM. This suggests that slightly different and as yet uncharacterised mechanisms may be at work during one of the types of reactivation. Another related question concerns the absence of reactivation of the paternal Xi during early pre-implantation despite the expression of some of the key pluripotency factors. An attractive working hypothesis is that parental imprinting at these stages prevents the action of the pluripotency factors. The lack of Xi reactivation in the epiblast (and in derived female EpiStem Cells [135]) raises similar issues, although at this later stage, the absence of some pluripotency factors such as Nanog and Rex1 thought to be required for the initial Xist repression [126] may be sufficient explanation.
Nuclear Dynamics and trans-Communication between X-Chromosomes
Large-scale nuclear reorganisation has been shown to accompany the establishment of random X-inactivation. 3D-FISH analyses suggest that the core of the Xi chromosome territory is constituted of non-genic sequences, including LINE-1 repeats that provide the support for the initial coating by Xist RNAs [136]. This is followed by global chromatin changes and by the relocation of genes to within the Xist repressive compartment [137]. These observations favour another of Mary Lyon's hypotheses, who proposed, based on an enrichment of the X chromosome for LINE-1 elements, that the latter serve as “way-stations” facilitating the propagation of the inactivation signal [138], [139].
Nuclear dynamics may also be implicated in X chromosome counting and random choice. It has recently been observed that the two X chromosomes come into close nuclear proximity both before and at the very beginning of the differentiation process and that these X-X pairing events [61] involve two specific regions within the Xic, respectively: the Xpr, located within the Xpct gene [140], and the DXPas34-Tsix-Xite region [141], [142], which has long been suspected of participating in both counting and choice. Dynamic nuclear contacts between these regions are thought to mediate the trans-sensing of the two X chromosomes and to resolve through the apposition of distinct modifications on each allele, resulting in transient asymmetric Tsix expression [143]. This would then provide a window of opportunity for monoallelic Xist upregulation (for a review on nuclear organisation during X-inactivation, see [144]).
Changing Our Attitudes: The Evolution of X-Inactivation Mechanisms
X-inactivation in “ancient mammals” such as the marsupial is characterised by unstable imprinted inactivation of the paternal X, and, on this basis, imprinted X-inactivation was hypothesised until the mid-1990s to represent the ancestral form of X-inactivation [145]. This form of X-inactivation was thought to have been partly conserved in the mouse, which displays imprinted X-inactivation both during pre-implantation development, prior to the onset of random X-inactivation [111], [124], [146], and in extra-embryonic tissues [29], whereas hominids appear to have evolved towards the complete replacement of imprinted by random X-inactivation [147], [148] (reviewed in [149]). Crucial insights into our understanding of the evolution of X-inactivation mechanisms have come from recent sequence comparison of the X-inactivation centres of different species [150], [151]. These showed that Xist/XIST has evolved from a protein coding gene present in marsupials, indicating that other non-coding RNAs or totally different mechanisms must be at work in such “ancient mammals” [152]. Xic/XIC sequence comparisons had previously shown that the human TSIX was either completely absent or present in a truncated form, resulting in an absence of antisense transcription at the XIST promoter [150], [153], [154] (for review see also [155]). In parallel, other studies have led to the identification of several new non-coding genes (Jpx/Enox and Ftx) in the Xic, showing various degree of conservation [150]. Taken together, these analyses underline the surprising evolutionary instability of the master region controlling X-inactivation and of some of the key actors identified as critical in functional studies in the mouse.
Other important mechanistic differences have been identified through transgenic experiments. For instance, a YAC transgene containing the entire human XIST when integrated into the mouse genome, unlike the endogenous mouse Xist gene, initiated X-inactivation even before differentiation [156], [157]. This points to a conservation—totally or partially—of the mechanisms involved in the cis-spreading of X-inactivation between the two species together with a lack of conservation of the mechanisms acting to ensure XIST cis-repression prior to differentiation. The latter may be associated with the absence of human TSIX (see above). Interestingly, a recent comparison of X-inactivation profiles during pre-implantation development in humans and rabbits has found a late onset of X-inactivation in both species compared to mice and initial biallelic upregulation of Xist alleles prior to monoallelic resolution [158]. Additional species-specific differences include the recruitment of diverse heterochromatin marks in marsupials, mice, and humans [159]–[162].
A last but certainly not least difference between mice and human concerns X-linked genes escaping from X-inactivation. In humans, unlike mice [163], a large number (15%) of X-linked genes have been shown to escape from X-inactivation [164], offering a potential explanation of the severity of the phenotypic alterations observed in XO women (Turner Syndrome) compared to mice (for review see [165], [166]). A level of variability in the degree of escape has also been reported between individuals, between tissues, and even amongst cells of the same tissue. Interestingly, the distribution of the genes escaping from X-inactivation along the chromosome also differs between human and mouse. In mice, the few “escapees” are either embedded within regions undergoing X-inactivation or located within the single murine Pseudo-Autosomal Region (PAR) (shared with the Y chromosome). In humans, genes escaping from X-inactivation are similarly found in both human PARs but, in addition, exist within clusters in large genomic domains that may be several megabases in size. This suggests that large-scale chromatin remodelling as opposed to gene-based mechanisms is likely at work in humans [163], [164], [167]. In mice, LINE-1 transcription [136], the expression of other non-coding RNAs [168], and binding of the insulator CTCF [169] at the boundaries of escapees are associated with the looping out from the Xist-repressive compartment [137], which is thought to participate in preventing the spreading of heterochromatin into genes that escape from X-inactivation. Transgenesis approaches allowing the introduction of escapees into different genomic contexts should enable the further dissection of the molecular mechanisms underlying this phenomenon [170].
An unexpectedly large variety of mechanisms involved in the initiation, spreading, and stabilisation of X-inactivation therefore probably exist in the mammalian kingdom. This suggests that “a la carte” mechanisms most likely evolved to adapt to, and cope with, the developmental and gestational specificities of each species. The original observation of the dense Barr body led researchers to postulate a chromosome-wide process that would affect the entire X chromosome uniformly. The more recent findings suggest that gene - or gene cluster-based mechanisms allow the fine tuning of X-inactivation to cope with the specific requirements of development and/or tissue/lineage functionalities. Such mechanisms may be related to systems used in other phyla to compensate sex chromosome dosage, as in birds, where only few genes are subject to dosage compensation [171], [172], or in Drosophila, where X over-expression in males is initially established preferentially and locally at entry sites scattered all along the X [173].
Concluding Remarks
As the inactivation traveller looks back over the 50 years since Mary Lyon's original hypothesis was published, it seems that quite a long—if winding—road has been covered and some great achievements made. Raising our eyes, however, reveals the extent of the path still in front of us.
Moreover, earlier X-inactivation travellers, like Himalayan climbers, have left their load of unresolved issues. For instance, despite intense scrutiny and in-depth mutagenesis studies, we still mostly ignore how the XIC/Xic exerts its function, and even Xist's mode of action remains rather obscure. A role for Xist in recruiting the chromatin remodeller PRC2 [174], which, in turn, triggers H3K27 trimethylation, has found support from similar results obtained with other large non-coding RNAs such as Air/AIR, Kcnq1ot1/KCNQ1OT1 (regulation of imprinted genes at the Igf2r/IGF2R and at the Cdkn1c/CDKN1C loci), and HOTAIR (developmental regulation of HOXD gene cluster in human) [175], [176]. The recent observation that the mutation of the mouse Hotair was without dramatic impact on the regulation of the mouse Hoxd cluster [177] provides a welcome cautionary reminder of the need to cross-reference such studies to in vivo functional approaches. We also still ignore how the original euchromatic marks are removed from the Xi. Does this require the association of Xist RNAs with specific histone demethylases, or does it depend solely on the passive dilution occurring via DNA replication and/or successive mitoses? Other Xist/XIST-related questions concern the potential role of Xist/XIST splice variants—are they just relics of evolution? Or integral to the resetting of the Xist/XIST domain after DNA replication or mitosis?
Within the Xic, the function of many of the more recently discovered non-coding RNAs such as Jpx/Enox [178], [179] and Ftx [180] and of sites of intergenic transcription such as Xite [181] and the Region B [150] remains to be fully elucidated, as does the role of actors lying outside of the immediate Xic/XIC interval, which are involved in the counting process. The U3 ubiquitin ligase produced by the X-linked Rnf12 gene, which was recently shown to act on the initiation of X-inactivation in a dose-dependent manner, is the first of such actors to be characterised [182]–[184].
The concentration of research into understanding how the Xic/XIC operates to count, choose, and initiate X-inactivation has led to a relative neglect of other topics such as that concerning the re-equilibration of levels of expression between the single Xa and autosome pairs. The latter has been suggested to involve the global upregulation of genes on the Xa in both males and females, inducing an increase of 1.4 - to 2-fold in expression levels of the X chromosome during the time course of differentiation [185], [186], although a later study involving high-throughput RNA sequencing failed to confirm these observations [187]. Clarification of this important point and a more detailed understanding of the underlying mechanisms are likely to impact largely on current models of both dosage compensation and of the evolution of the sex chromosomes.
The molecular processes responsible for the individualisation of the establishment of a heterochromatin structure on a gene-by-gene basis and the nature of the mechanism(s) rendering “escapees” resistant to global heterochromatinisation or sensitive to reactivation similarly remain, for the most part, unknown. Some of these studies will clearly benefit from the single-cell analyses that will be required to follow in real time the chromatin dynamics occurring during embryogenesis and to capture the putative furtive nuclear interactions and changes in large-scale chromatin organisation that are likely to be part and parcel of the initiation of X-inactivation. Clearly, integrating chromosome-wide and Xic nuclear dynamics to transcriptional regulation is but one step in this process. The development of in vivo systems allowing the specific perturbation of some of these features/mechanisms during early embryogenesis will, almost certainly, be critical to a complete understanding of how a fully stable Xi is established and how Xi and Xa epigenetic features are transmitted during the formation of mosaic cell populations making up the pre-implantation embryo.
Zdroje
1. HammondJJr 1949 Recovery and culture of tubal mouse ova. Nature 163 28
2. McLarenABiggersJD 1958 Successful development and birth of mice cultivated in vitro as early as early embryos. Nature 182 877 878
3. JaenischRMintzB 1974 Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A 71 1250 1254
4. WatsonJDCrickFH 1953 Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171 737 738
5. HollidayR 1964 The induction of mitotic recombination by mitomycin C in Ustilago and Saccharomyces. Genetics 50 323 335
6. BriggsRKingTJ 1952 Transplantation of living nuclei from blastula cells into enucleated frogs' eggs. Proc Natl Acad Sci U S A 38 455 463
7. MorganTHSturtevantAHBridgeCB 1920 The Evidence for the linear order of the genes. Proc Natl Acad Sci U S A 6 162 164
8. LewisEB 1950 The phenomenon of position effect. Adv Genet 3 73 115
9. SpoffordJB 1959 Parental Control of Position-Effect Variegation: I. Parental Heterochromatin and Expression of the White Locus in Compound-X Drosophila Melanogaster. Proc Natl Acad Sci U S A 45 1003 1007
10. McClintockB 1950 The origin and behavior of mutable loci in maize. Proc Natl Acad Sci U S A 36 344 355
11. JacobFMonodJ 1961 Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol 3 318 356
12. WaddingtonCH 1953 Epigenetics and evolution. Symp Soc Exp Biol 7 186 199
13. BarrMLBertramEG 1949 A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis. Nature 163 676
14. OhnoSKaplanWDKinositaR 1959 Formation of the sex chromatin by a single X-chromosome in liver cells of Rattus norvegicus. Exp Cell Res 18 415 418
15. OhnoSHauschkaTS 1960 Allocycly of the X-chromosome in tumors and normal tissues. Cancer Res 20 541 545
16. FraserASSobeySSpicerCC 1953 Mottled: a sex-modified lethal in the house mouse. J Genet 51 217 221
17. RussellWLRussellLBGowerJS 1959 Exceptional inheritance of a sex-linked gene in the mouse explained on the basis that the X/O sex-chromosome constitution is female. Proc Natl Acad Sci U S A 45 554 560
18. GrantSGChapmanVM 1988 Mechanisms of X-chromosome regulation. Annu Rev Genet 22 199 233
19. LyonMF 1992 Some milestones in the history of X-chromosome inactivation. Annu Rev Genet 26 16 28
20. GartlerSMRiggsAD 1983 Mammalian X-chromosome inactivation. Annu Rev Genet 17 155 190
21. LyonMF 1961 Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190 372 373
22. BeutlerEYehMFairbanksVF 1962 The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker. Proc Natl Acad Sci U S A 48 9 16
23. RussellLB 1961 Genetics of mammalian sex chromosomes. Science 133 1795 1803
24. MoreyCAvnerP 2010 Genetics and epigenetics of the X chromosome. Ann N Y Acad Sci 1214 E18 33
25. CattanachBMWilliamsCE 1972 Evidence of non-random X chromosome activity in the mouse. Genet Res 19 229 240
26. TurnerJM 2007 Meiotic sex chromosome inactivation. Development 134 1823 1831
27. MonkMMcLarenA 1981 X-chromosome activity in foetal germ cells of the mouse. J Embryol Exp Morphol 63 75 84
28. AndinaRJ 1978 A study of X chromosome regulation during oogenesis in the mouse. Exp Cell Res 111 211 218
29. TakagiNSasakiM 1975 Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256 640 642
30. NesbittMN 1971 X chromosome inactivation mosaicism in the mouse. Dev Biol 26 252 263
31. McMahonAMonkM 1983 X-chromosome activity in female mouse embryos heterozygous for Pgk-1 and Searle's translocation, T(X; 16) 16H. Genet Res 41 69 83
32. McGrathJSolterD 1984 Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37 179 183
33. SuraniMABartonSCNorrisML 1984 Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308 548 550
34. RussellLB 1963 Mammalian X-chromosome action: inactivation limited in spread and region of origin. Science 140 976 978
35. GreavesDRAntoniouMvan AssendelftGBCollisPDillonN 1989 The beta-globin dominant control region. Prog Clin Biol Res 316A 37 46
36. JacksonPDEvansTNickolJMFelsenfeldG 1989 Developmental modulation of protein binding to beta-globin gene regulatory sites within chicken erythrocyte nuclei. Genes Dev 3 1860 1873
37. LewisEB 1954 The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am Naturalist 88 225 239
38. HeardEClercPAvnerP 1997 X-chromosome inactivation in mammals. Annu Rev Genet 31 571 610
39. BrownCJLafreniereRGPowersVESebastioGBallabioA 1991 Localization of the X inactivation centre on the human X chromosome in Xq13. Nature 349 82 84
40. RastanS 1983 Non-random X-chromosome inactivation in mouse X-autosome translocation embryos–location of the inactivation centre. J Embryol Exp Morphol 78 1 22
41. RastanSRobertsonEJ 1985 X-chromosome deletions in embryo-derived (EK) cell lines associated with lack of X-chromosome inactivation. J Embryol Exp Morphol 90 379 388
42. LyonMF 1989 X-chromosome inactivation as a system of gene dosage compensation to regulate gene expression. Prog Nucleic Acid Res Mol Biol 36 119 130
43. MartinGREvansMJ 1974 The morphology and growth of a pluripotent teratocarcinoma cell line and its derivatives in tissue culture. Cell 2 163 172
44. MartinGREpsteinCJTravisBTuckerGYatzivS 1978 X-chromosome inactivation during differentiation of female teratocarcinoma stem cells in vitro. Nature 271 329 333
45. HeardEKressCMongelardFCourtierBRougeulleC 1996 Transgenic mice carrying an Xist-containing YAC. Hum Mol Genet 5 441 450
46. LeeJTJaenischR 1997 Long-range cis effects of ectopic X-inactivation centres on a mouse autosome. Nature 386 275 279
47. HerzingLBRomerJTHornJMAshworthA 1997 Xist has properties of the X-chromosome inactivation centre. Nature 386 272 275
48. LeeJTLuNHanY 1999 Genetic analysis of the mouse X inactivation center defines an 80-kb multifunction domain. Proc Natl Acad Sci U S A 96 3836 3841
49. LeeJTStraussWMDausmanJAJaenischR 1996 A 450 kb transgene displays properties of the mammalian X-inactivation center. Cell 86 83 94
50. HeardEMongelardFArnaudDAvnerP 1999 Xist yeast artificial chromosome transgenes function as X-inactivation centers only in multicopy arrays and not as single copies. Mol Cell Biol 19 3156 3166
51. MinksJBrownCJ 2009 Getting to the center of X-chromosome inactivation: the role of transgenes. Biochem Cell Biol 87 759 766
52. BrownCJBallabioARupertJLLafreniereRGGrompeM 1991 A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349 38 44
53. BorsaniGTonlorenziRSimmlerMCDandoloLArnaudD 1991 Characterization of a murine gene expressed from the inactive X chromosome. Nature 351 325 329
54. BrownCJHendrichBDRupertJLLafreniereRGXingY 1992 The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71 527 542
55. BrockdorffNAshworthAKayGFMcCabeVMNorrisDP 1992 The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71 515 526
56. BrockdorffNAshworthAKayGFCooperPSmithS 1991 Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351 329 331
57. BrannanCIDeesECIngramRSTilghmanSM 1990 The product of the H19 gene may function as an RNA. Mol Cell Biol 10 28 36
58. ClemsonCMMcNeilJAWillardHFLawrenceJB 1996 XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol 132 259 275
59. KayGFBartonSCSuraniMARastanS 1994 Imprinting and X chromosome counting mechanisms determine Xist expression in early mouse development. Cell 77 639 650
60. KayGFPennyGDPatelDAshworthABrockdorffN 1993 Expression of Xist during mouse development suggests a role in the initiation of X chromosome inactivation. Cell 72 171 182
61. MarahrensYPanningBDausmanJStraussWJaenischR 1997 Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev 11 156 166
62. PennyGDKayGFSheardownSARastanSBrockdorffN 1996 Requirement for Xist in X chromosome inactivation. Nature 379 131 137
63. SimmlerMCCunninghamDBClercPVermatTCaudronB 1996 A 94 kb genomic sequence 3′ to the murine Xist gene reveals an AT rich region containing a new testis specific gene Tsx. Hum Mol Genet 5 1713 1726
64. CourtierBHeardEAvnerP 1995 Xce haplotypes show modified methylation in a region of the active X chromosome lying 3′ to Xist. Proc Natl Acad Sci U S A 92 3531 3535
65. DebrandEChureauCArnaudDAvnerPHeardE 1999 Functional analysis of the DXPas34 locus, a 3′ regulator of Xist expression. Mol Cell Biol 19 8513 8525
66. SimmlerMCCattanachBMRasberryCRougeulleCAvnerP 1993 Mapping the murine Xce locus with (CA)n repeats. Mamm Genome 4 523 530
67. ChadwickLHPertzLMBromanKWBartolomeiMSWillardHF 2006 Genetic control of X chromosome inactivation in mice: definition of the Xce candidate interval. Genetics 173 2103 2110
68. ClercPAvnerP 1998 Role of the region 3′ to Xist exon 6 in the counting process of X-chromosome inactivation. Nat Genet 19 249 253
69. WolffeAP 1991 Developmental regulation of chromatin structure and function. Trends Cell Biol 1 61 66
70. StrahlBDAllisCD 2000 The language of covalent histone modifications. Nature 403 41 45
71. SolomonMJVarshavskyA 1985 Formaldehyde-mediated DNA-protein crosslinking: a probe for in vivo chromatin structures. Proc Natl Acad Sci U S A 82 6470 6474
72. JeppesenPTurnerBM 1993 The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell 74 281 289
73. NorrisDPBrockdorffNRastanS 1991 Methylation status of CpG-rich islands on active and inactive mouse X chromosomes. Mamm Genome 1 78 83
74. TribioliCTamaniniFPatrossoCMilanesiLVillaA 1992 Methylation and sequence analysis around EagI sites: identification of 28 new CpG islands in XQ24–XQ28. Nucleic Acids Res 20 727 733
75. TakahashiKIchisakaTYamanakaS 2006 Identification of genes involved in tumor-like properties of embryonic stem cells. Methods Mol Biol 329 449 458
76. MaheraliNSridharanRXieWUtikalJEminliS 2007 Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1 55 70
77. FraserPBickmoreW 2007 Nuclear organization of the genome and the potential for gene regulation. Nature 447 413 417
78. LeeJTDavidowLSWarshawskyD 1999 Tsix, a gene antisense to Xist at the X-inactivation centre. Nat Genet 21 400 404
79. LeeJTLuN 1999 Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99 47 57
80. SadoTWangZSasakiHLiE 2001 Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development 128 1275 1286
81. LuikenhuisSWutzAJaenischR 2001 Antisense transcription through the Xist locus mediates Tsix function in embryonic stem cells. Mol Cell Biol 21 8512 8520
82. VigneauSAuguiSNavarroPAvnerPClercP 2006 An essential role for the DXPas34 tandem repeat and Tsix transcription in the counting process of X chromosome inactivation. Proc Natl Acad Sci U S A 103 7390 7395
83. CohenDEDavidowLSErwinJAXuNWarshawskyD 2007 The DXPas34 repeat regulates random and imprinted X inactivation. Dev Cell 12 57 71
84. MoreyCArnaudDAvnerPClercP 2001 Tsix-mediated repression of Xist accumulation is not sufficient for normal random X inactivation. Hum Mol Genet 10 1403 1411
85. MoreyCNavarroPDebrandEAvnerPRougeulleC 2004 The region 3′ to Xist mediates X chromosome counting and H3 Lys-4 dimethylation within the Xist gene. EMBO J 23 594 604
86. LeeJT 2000 Disruption of imprinted X inactivation by parent-of-origin effects at Tsix. Cell 103 17 27
87. SadoTLiESasakiH 2002 Effect of TSIX disruption on XIST expression in male ES cells. Cytogenet Genome Res 99 115 118
88. JorgensenR 1990 Altered gene expression in plants due to trans interactions between homologous genes. Trends Biotechnol 8 340 344
89. GuoSKemphuesKJ 1995 par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81 611 620
90. FireAXuSMontgomeryMKKostasSADriverSE 1998 Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 806 811
91. SadoTHokiYSasakiH 2005 Tsix silences Xist through modification of chromatin structure. Dev Cell 9 159 165
92. NavarroPPichardSCiaudoCAvnerPRougeulleC 2005 Tsix transcription across the Xist gene alters chromatin conformation without affecting Xist transcription: implications for X-chromosome inactivation. Genes Dev 19 1474 1484
93. NavarroPChantalatSFoglioMChureauCVigneauS 2009 A role for non-coding Tsix transcription in partitioning chromatin domains within the mouse X-inactivation centre. Epigenetics Chromatin 2 8
94. NavarroPPageDRAvnerPRougeulleC 2006 Tsix-mediated epigenetic switch of a CTCF-flanked region of the Xist promoter determines the Xist transcription program. Genes Dev 20 2787 2792
95. SadoTHokiYSasakiH 2006 Tsix defective in splicing is competent to establish Xist silencing. Development 133 4925 4931
96. OgawaYSunBKLeeJT 2008 Intersection of the RNA interference and X-inactivation pathways. Science 320 1336 1341
97. NesterovaTBPopovaBCCobbBSNortonSSennerCE 2008 Dicer regulates Xist promoter methylation in ES cells indirectly through transcriptional control of Dnmt3a. Epigenetics Chromatin 1 2
98. KanellopoulouCMuljoSADimitrovSDChenXColinC 2009 X chromosome inactivation in the absence of Dicer. Proc Natl Acad Sci U S A 106 1122 1127
99. WutzAJaenischR 2000 A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell 5 695 705
100. WutzARasmussenTPJaenischR 2002 Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet 30 167 174
101. HasegawaYBrockdorffNKawanoSTsutuiKNakagawaS 2010 The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev Cell 19 469 476
102. LeebMSteffenPAWutzA 2009 X chromosome inactivation sparked by non-coding RNAs. RNA Biol 6 94 99
103. PontierDBGribnauJ 2011 Xist regulation and function eXplored. Hum Genet E-pub ahead of print 28 May 2011
104. AgreloRSouabniANovatchkovaMHaslingerCLeebM 2009 SATB1 defines the developmental context for gene silencing by Xist in lymphoma and embryonic cells. Dev Cell 16 507 516
105. WutzA 2007 Xist function: bridging chromatin and stem cells. Trends Genet 23 457 464
106. SavareseFDavilaANechanitzkyRDe La Rosa-VelazquezIPereiraCF 2009 Satb1 and Satb2 regulate embryonic stem cell differentiation and Nanog expression. Genes Dev 23 2625 2638
107. ChaumeilJOkamotoIGuggiariMHeardE 2002 Integrated kinetics of X chromosome inactivation in differentiating embryonic stem cells. Cytogenet Genome Res 99 75 84
108. PlathKFangJMlynarczyk-EvansSKCaoRWorringerKA 2003 Role of histone H3 lysine 27 methylation in X inactivation. Science 300 131 135
109. PlathKTalbotDHamerKMOtteAPYangTP 2004 Developmentally regulated alterations in Polycomb repressive complex 1 proteins on the inactive X chromosome. J Cell Biol 167 1025 1035
110. SilvaJMakWZvetkovaIAppanahRNesterovaTB 2003 Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev Cell 4 481 495
111. OkamotoIOtteAPAllisCDReinbergDHeardE 2004 Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303 644 649
112. de NapolesMMermoudJEWakaoRTangYAEndohM 2004 Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 7 663 676
113. CostanziCPehrsonJR 1998 Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393 599 601
114. BaumannCDe La FuenteR 2009 ATRX marks the inactive X chromosome (Xi) in somatic cells and during imprinted X chromosome inactivation in trophoblast stem cells. Chromosoma 118 209 222
115. NoraEPHeardE 2011 Chromatin Structure and Nuclear Organization Dynamics during X-Chromosome Inactivation Cold Spring Harb Symp Quant Biol
116. MarksHChowJCDenissovSFrancoijsKJBrockdorffN 2009 High-resolution analysis of epigenetic changes associated with X inactivation. Genome Res 19 1361 1373
117. MiettonFSenguptaAKMollaAPicchiGBarralS 2009 Weak but uniform enrichment of the histone variant macroH2A1 along the inactive X chromosome. Mol Cell Biol 29 150 156
118. HellmanAChessA 2007 Gene body-specific methylation on the active X chromosome. Science 315 1141 1143
119. HeardERougeulleCArnaudDAvnerPAllisCD 2001 Methylation of histone H3 at Lys-9 is an early mark on the X chromosome during X inactivation. Cell 107 727 738
120. RougeulleCChaumeilJSarmaKAllisCDReinbergD 2004 Differential histone H3 Lys-9 and Lys-27 methylation profiles on the X chromosome. Mol Cell Biol 24 5475 5484
121. KalantrySPurushothamanSBowenRBStarmerJMagnusonT 2009 Evidence of Xist RNA-independent initiation of mouse imprinted X-chromosome inactivation. Nature 460 647 651
122. NamekawaSHPayerBHuynhKDJaenischRLeeJT 2010 Two-step imprinted X inactivation: repeat versus genic silencing in the mouse. Mol Cell Biol 30 3187 3205
123. PatratCOkamotoIDiabangouayaPVialonVLe BacconP 2009 Dynamic changes in paternal X-chromosome activity during imprinted X-chromosome inactivation in mice. Proc Natl Acad Sci U S A 106 5198 5203
124. MakWNesterovaTBde NapolesMAppanahRYamanakaS 2004 Reactivation of the paternal X chromosome in early mouse embryos. Science 303 666 669
125. OkamotoIArnaudDLe BacconPOtteAPDistecheCM 2005 Evidence for de novo imprinted X-chromosome inactivation independent of meiotic inactivation in mice. Nature 438 369 373
126. NavarroPChambersIKarwacki-NeisiusVChureauCMoreyC 2008 Molecular coupling of Xist regulation and pluripotency. Science 321 1693 1695
127. NavarroPOldfieldALegoupiJFestucciaNDuboisA 2010 Molecular coupling of Tsix regulation and pluripotency. Nature 468 457 460
128. NavarroPMoffatMMullinNPChambersI 2011 The X-inactivation trans-activator Rnf12 is negatively regulated by pluripotency factors in embryonic stem cells. Hum Genet
129. MakhloufMRougeulleC 2011 Linking X chromosome inactivation to pluripotency: necessity or fate? Trends Mol Med 17 329 336
130. NavarroPAvnerP 2010 An embryonic story: analysis of the gene regulative network controlling Xist expression in mouse embryonic stem cells. Bioessays 32 581 588
131. de NapolesMNesterovaTBrockdorffN 2007 Early loss of Xist RNA expression and inactive X chromosome associated chromatin modification in developing primordial germ cells. PLoS ONE 2 e860 doi:10.1371/journal.pone.0000860
132. SugimotoMAbeK 2007 X chromosome reactivation initiates in nascent primordial germ cells in mice. PLoS Genet 3 e116 doi:10.1371/journal.pgen.0030116
133. Chuva de Sousa LopesSMHayashiKShovlinTCMifsudWSuraniMA 2008 X chromosome activity in mouse XX primordial germ cells. PLoS Genet 4 e30 doi:10.1371/journal.pgen.0040030
134. SennerCEBrockdorffN 2009 Xist gene regulation at the onset of X inactivation. Curr Opin Genet Dev 19 122 126
135. TesarPJChenowethJGBrookFADaviesTJEvansEP 2007 New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448 196 199
136. ChowJCCiaudoCFazzariMJMiseNServantN 2010 LINE-1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell 141 956 969
137. ChaumeilJLe BacconPWutzAHeardE 2006 A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev 20 2223 2237
138. LyonMF 2000 LINE-1 elements and X chromosome inactivation: a function for “junk” DNA? Proc Natl Acad Sci U S A 97 6248 6249
139. RiggsAD 1975 X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet 14 9 25
140. AuguiSFilionGJHuartSNoraEGuggiariM 2007 Sensing X chromosome pairs before X inactivation via a novel X-pairing region of the Xic. Science 318 1632 1636
141. BacherCPGuggiariMBrorsBAuguiSClercP 2006 Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat Cell Biol 8 293 299
142. XuNTsaiCLLeeJT 2006 Transient homologous chromosome pairing marks the onset of X inactivation. Science 311 1149 1152
143. MasuiOBonnetILe BacconPBritoIPollexT 2011 Live-Cell Chromosome Dynamics and Outcome of X Chromosome Pairing Events during ES Cell Differentiation. Cell 145 447 458
144. ChowJCHeardE 2010 Nuclear organization and dosage compensation. Cold Spring Harb Perspect Biol 2 a000604
145. CooperDW 1971 Directed genetic change model for X chromosome inactivation in eutherian mammals. Nature 230 292 294
146. HuynhKDLeeJT 2003 Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature 426 857 862
147. MigeonBR 2002 X chromosome inactivation: theme and variations. Cytogenet Genome Res 99 8 16
148. Moreira de MelloJCde AraujoESStabelliniRFragaAMde SouzaJE 2010 Random X inactivation and extensive mosaicism in human placenta revealed by analysis of allele-specific gene expression along the X chromosome. PLoS ONE 5 e10947 doi:10.1371/journal.pone.0010947
149. van den BergIMGaljaardRJLavenJSvan DoorninckJH 2011 XCI in preimplantation mouse and human embryos: first there is remodelling. Hum Genet E-pub ahead of print 7 June 2011. doi:10.1007/s00439-011-1014-9
150. ChureauCPrissetteMBourdetABarbeVCattolicoL 2002 Comparative sequence analysis of the X-inactivation center region in mouse, human, and bovine. Genome Res 12 894 908
151. NesterovaTBSlobodyanyukSYElisaphenkoEAShevchenkoAIJohnstonC 2001 Characterization of the genomic Xist locus in rodents reveals conservation of overall gene structure and tandem repeats but rapid evolution of unique sequence. Genome Res 11 833 849
152. DuretLChureauCSamainSWeissenbachJAvnerP 2006 The Xist RNA gene evolved in eutherians by pseudogenization of a protein-coding gene. Science 312 1653 1655
153. MigeonBRChowdhuryAKDunstonJAMcIntoshI 2001 Identification of TSIX, encoding an RNA antisense to human XIST, reveals differences from its murine counterpart: implications for X inactivation. Am J Hum Genet 69 951 960
154. MigeonBRLeeCHChowdhuryAKCarpenterH 2002 Species differences in TSIX/Tsix reveal the roles of these genes in X-chromosome inactivation. Am J Hum Genet 71 286 293
155. YangCChapmanAGKelseyADMinksJCottonAM 2011 X-chromosome inactivation: molecular mechanisms from the human perspective. Hum Genet E-pub ahead of print 7 May 2011
156. HeardEMongelardFArnaudDChureauCVourc'hC 1999 Human XIST yeast artificial chromosome transgenes show partial X inactivation center function in mouse embryonic stem cells. Proc Natl Acad Sci U S A 96 6841 6846
157. MigeonBRKaziEHaisley-RoysterCHuJReevesR 1999 Human X inactivation center induces random X chromosome inactivation in male transgenic mice. Genomics 59 113 121
158. OkamotoIPatratCThepotDPeynotNFauqueP 2011 Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature 472 370 374
159. ChowJCHallLLBaldrySEThorogoodNPLawrenceJB 2007 Inducible XIST-dependent X-chromosome inactivation in human somatic cells is reversible. Proc Natl Acad Sci U S A 104 10104 10109
160. ChadwickBPWillardHF 2003 Chromatin of the Barr body: histone and non-histone proteins associated with or excluded from the inactive X chromosome. Hum Mol Genet 12 2167 2178
161. KoinaEChaumeilJGreavesIKTremethickDJGravesJA 2009 Specific patterns of histone marks accompany X chromosome inactivation in a marsupial. Chromosome Res 17 115 126
162. ChaumeilJWatersPDKoinaEGilbertCRobinsonTJ 2011 Evolution from XIST-independent to XIST-controlled X-chromosome inactivation: epigenetic modifications in distantly related mammals. PLoS ONE 6 e19040 doi:10.1371/journal.pone.0019040
163. YangFBabakTShendureJDistecheCM 2010 Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res 20 614 622
164. CarrelLWillardHF 2005 X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434 400 404
165. BerletchJBYangFXuJCarrelLDistecheCM 2011 Genes that escape from X inactivation. Hum Genet E-pub ahead of print 26 May 2011. doi:10.1007/s00439-011-1011-z
166. ProtheroKEStahlJMCarrelL 2009 Dosage compensation and gene expression on the mammalian X chromosome: one plus one does not always equal two. Chromosome Res 17 637 648
167. TsuchiyaKDGreallyJMYiYNoelKPTruongJP 2004 Comparative sequence and x-inactivation analyses of a domain of escape in human xp11.2 and the conserved segment in mouse. Genome Res 14 1275 1284
168. ReiniusBShiCHengshuoLSandhuKSRadomskaKJ 2010 Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse. BMC Genomics 11 614
169. FilipovaMBujdakovaH 2005 [Factors of virulence and mechanisms of resistance to aminoglycosides in clinical isolates of Enterococcus faecalis and Enterococcus faecium with high-level gentamicin resistance]. Epidemiol Mikrobiol Imunol 54 65 74
170. LiNCarrelL 2008 Escape from X chromosome inactivation is an intrinsic property of the Jarid1c locus. Proc Natl Acad Sci U S A 105 17055 17060
171. WolfJBBrykJ 2011 General lack of global dosage compensation in ZZ/ZW systems? Broadening the perspective with RNA-seq. BMC Genomics 12 91
172. ArnoldAPItohYMelamedE 2008 A bird's-eye view of sex chromosome dosage compensation. Annu Rev Genomics Hum Genet 9 109 127
173. StraubTBeckerPB 2011 Transcription modulation chromosome-wide: universal features and principles of dosage compensation in worms and flies. Curr Opin Genet Dev 21 147 153
174. MaennerSBlaudMFouillenLSavoyeAMarchandV 2010 2-D structure of the A region of Xist RNA and its implication for PRC2 association. PLoS Biol 8 e1000276 doi:10.1371/journal.pbio.1000276
175. ClercPAvnerP 2011 New lessons from random x-chromosome inactivation in the mouse. J Mol Biol 409 62 69
176. ArtholdSKurowskiAWutzA 2011 Mechanistic insights into chromosome-wide silencing in X inactivation. Hum Genet E-pub ahead of print 13 May 2011. doi:10.1007/s00439-011-1002-0
177. SchorderetPDubouleD 2011 Structural and functional differences in the long non-coding RNA hotair in mouse and human. PLoS Genet 7 e1002071 doi:10.1371/journal.pgen.1002071
178. JohnstonCMNewallAEBrockdorffNNesterovaTB 2002 Enox, a novel gene that maps 10 kb upstream of Xist and partially escapes X inactivation. Genomics 80 236 244
179. TianDSunSLeeJT 2011 The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell 143 390 403
180. ChureauCChantalatSRomitoAGalvaniADuretL 2011 Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum Mol Genet 20 705 718
181. OgawaYLeeJT 2003 Xite, X-inactivation intergenic transcription elements that regulate the probability of choice. Mol Cell 11 731 743
182. BarakatTSGunhanlarNPardoCGAchameEMGhazviniM 2011 RNF12 activates Xist and is essential for X chromosome inactivation. PLoS Genet 7 e1002001 doi:10.1371/journal.pgen.1002001
183. ShinJBossenzMChungYMaHByronM 2010 Maternal Rnf12/RLIM is required for imprinted X-chromosome inactivation in mice. Nature 467 977 981
184. JonkersIBarakatTSAchameEMMonkhorstKKenterA 2009 RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell 139 999 1011
185. LinHGuptaVVermilyeaMDFalcianiFLeeJT 2007 Dosage compensation in the mouse balances up-regulation and silencing of X-linked genes. PLoS Biol 5 e326 doi:10.1371/journal.pbio.0050326
186. NguyenDKDistecheCM 2006 Dosage compensation of the active X chromosome in mammals. Nat Genet 38 47 53
187. XiongYChenXChenZWangXShiS 2010 RNA sequencing shows no dosage compensation of the active X-chromosome. Nat Genet 42 1043 1047
Štítky
Genetika Reprodukční medicína
Článek Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal MutationsČlánek Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene ExpressionČlánek Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary IntegrityČlánek A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with ExpressionČlánek Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line PedigreeČlánek Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 7
-
Všechny články tohoto čísla
- Gene-Based Tests of Association
- The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
- Variants in and Underlie Natural Variation in Translation Termination Efficiency in
- SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
- Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations
- Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer
- Identification of a Mutation Associated with Fatal Foal Immunodeficiency Syndrome in the Fell and Dales Pony
- Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
- An Epigenetic Switch Involving Overlapping Fur and DNA Methylation Optimizes Expression of a Type VI Secretion Gene Cluster
- Recombination and Population Structure in
- A Rice Plastidial Nucleotide Sugar Epimerase Is Involved in Galactolipid Biosynthesis and Improves Photosynthetic Efficiency
- A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets
- Colon Stem Cell and Crypt Dynamics Exposed by Cell Lineage Reconstruction
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Interactions between Glucocorticoid Treatment and Cis-Regulatory Polymorphisms Contribute to Cellular Response Phenotypes
- Translation Reinitiation Relies on the Interaction between eIF3a/TIF32 and Progressively Folded -Acting mRNA Elements Preceding Short uORFs
- DAF-12 Regulates a Connected Network of Genes to Ensure Robust Developmental Decisions
- Adult Circadian Behavior in Requires Developmental Expression of , But Not
- Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary Integrity
- Proteins in the Nutrient-Sensing and DNA Damage Checkpoint Pathways Cooperate to Restrain Mitotic Progression following DNA Damage
- Complex Evolutionary Events at a Tandem Cluster of Genes Resulting in a Single-Locus Genetic Incompatibility
- () and Its Regulated Homeodomain Gene Mediate Abscisic Acid Response in
- A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
- LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs
- Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications
- The Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
- Somatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
- Molecular Mechanisms Generating and Stabilizing Terminal 22q13 Deletions in 44 Subjects with Phelan/McDermid Syndrome
- Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line Pedigree
- Mechanisms of Chromosome Number Evolution in Yeast
- Regulatory Cross-Talk Links Chromosome II Replication and Segregation
- Ancestral Genes Can Control the Ability of Horizontally Acquired Loci to Confer New Traits
- Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
- Rare and Common Regulatory Variation in Population-Scale Sequenced Human Genomes
- The Epistatic Relationship between BRCA2 and the Other RAD51 Mediators in Homologous Recombination
- Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy
- NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
- Araucan and Caupolican Integrate Intrinsic and Signalling Inputs for the Acquisition by Muscle Progenitors of the Lateral Transverse Fate
- Pathologic and Phenotypic Alterations in a Mouse Expressing a Connexin47 Missense Mutation That Causes Pelizaeus-Merzbacher–Like Disease in Humans
- Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination
- Epistatic Relationships in the BRCA1-BRCA2 Pathway
- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium
- Fine Mapping of Five Loci Associated with Low-Density Lipoprotein Cholesterol Detects Variants That Double the Explained Heritability
- CHD1 Remodels Chromatin and Influences Transient DNA Methylation at the Clock Gene
- Nonlinear Fitness Landscape of a Molecular Pathway
- Genome-Wide Scan Identifies , , and as Novel Risk Loci for Systemic Sclerosis
- Quantitative and Qualitative Stem Rust Resistance Factors in Barley Are Associated with Transcriptional Suppression of Defense Regulons
- A Systematic Screen for Tube Morphogenesis and Branching Genes in the Tracheal System
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Gene-Based Tests of Association
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



