-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAdult Circadian Behavior in Requires Developmental Expression of , But Not
Circadian clocks have evolved as internal time keeping mechanisms that allow anticipation of daily environmental changes and organization of a daily program of physiological and behavioral rhythms. To better examine the mechanisms underlying circadian clocks in animals and to ask whether clock gene expression and function during development affected subsequent daily time keeping in the adult, we used the genetic tools available in Drosophila to conditionally manipulate the function of the CYCLE component of the positive regulator CLOCK/CYCLE (CLK/CYC) or its negative feedback inhibitor PERIOD (PER). Differential manipulation of clock function during development and in adulthood indicated that there is no developmental requirement for either a running clock mechanism or expression of per. However, conditional suppression of CLK/CYC activity either via per over-expression or cyc depletion during metamorphosis resulted in persistent arrhythmic behavior in the adult. Two distinct mechanisms were identified that may contribute to this developmental function of CLK/CYC and both involve the ventral lateral clock neurons (LNvs) that are crucial to circadian control of locomotor behavior: (1) selective depletion of cyc expression in the LNvs resulted in abnormal peptidergic small-LNv dorsal projections, and (2) PER expression rhythms in the adult LNvs appeared to be affected by developmental inhibition of CLK/CYC activity. Given the conservation of clock genes and circuits among animals, this study provides a rationale for investigating a possible similar developmental role of the homologous mammalian CLOCK/BMAL1 complex.
Published in the journal: . PLoS Genet 7(7): e32767. doi:10.1371/journal.pgen.1002167
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002167Summary
Circadian clocks have evolved as internal time keeping mechanisms that allow anticipation of daily environmental changes and organization of a daily program of physiological and behavioral rhythms. To better examine the mechanisms underlying circadian clocks in animals and to ask whether clock gene expression and function during development affected subsequent daily time keeping in the adult, we used the genetic tools available in Drosophila to conditionally manipulate the function of the CYCLE component of the positive regulator CLOCK/CYCLE (CLK/CYC) or its negative feedback inhibitor PERIOD (PER). Differential manipulation of clock function during development and in adulthood indicated that there is no developmental requirement for either a running clock mechanism or expression of per. However, conditional suppression of CLK/CYC activity either via per over-expression or cyc depletion during metamorphosis resulted in persistent arrhythmic behavior in the adult. Two distinct mechanisms were identified that may contribute to this developmental function of CLK/CYC and both involve the ventral lateral clock neurons (LNvs) that are crucial to circadian control of locomotor behavior: (1) selective depletion of cyc expression in the LNvs resulted in abnormal peptidergic small-LNv dorsal projections, and (2) PER expression rhythms in the adult LNvs appeared to be affected by developmental inhibition of CLK/CYC activity. Given the conservation of clock genes and circuits among animals, this study provides a rationale for investigating a possible similar developmental role of the homologous mammalian CLOCK/BMAL1 complex.
Introduction
Circadian clocks are internal daily time keeping mechanisms that allow organisms to anticipate daily environmental rhythms as well as efficiently organize behavioral and physiological functions in a daily schedule. The molecular mechanisms that form the basis for circadian rhythmicity in animals involve interlocked feedback loops controlling gene expression as well as post-translational activities [1], [2]. In both insects and mammals a circadian transcription complex of two basic helix-loop-helix PAS domain transcription factors promotes the rhythmic expression of several of its negative feedback regulators. The fruit fly Drosophila melanogaster has emerged as a model system for animal circadian clocks that is both successful and representative. In the clock-bearing cells of Drosophila CLOCK/CYCLE (CLK/CYC) acts as the central circadian transcription complex and induces peak expression of a set of transcripts including those for the negative feedback regulators period (per), timeless (tim), vrille (vri), and clock work orange (cwo) just after dusk [3]–[9]. PER and TIM proteins form a complex with the casein kinase 1ε ortholog DOUBLETIME (DBT), in which TIM helps protect PER from destabilization by DBT-mediated phosphorylation [10]–[12]. PER-containing complexes enter the nucleus around midnight and trigger repression of CLK/CYC activity [5], [13]–[16], VRI acts as a transcriptional repressor for the Clk gene [9], [17], and CWO reduces CLK/CYC activity by competitively binding CLK/CYC-regulated promoter elements [4], [7], [8].
The circadian clock circuits are linked to synchronizing input pathways as well as output pathways that signal time-of-day information to downstream biological functions. The extensive interconnectedness of the molecular circadian cycle complicates identification of the order of its events. We reasoned that the development of transgenic flies with conditional circadian clock function, in which the circadian cycle could be arrested or started at will, would help distinguish direct from indirect effects and determine sequential steps in circadian pathways. Moreover, transgenic flies with conditionally titratable transcription of a clock component would allow molecular, cellular, and behavioral circadian phenotypes to be determined over a range of expression levels. Finally, flies with conditionally controlled clock function would allow separation of developmental and adult functions of clock genes. Based on these arguments we created conditionally rhythmic transgenic Drosophila strains.
In the present study, we describe the generation of transgenic flies in which clock function becomes conditional on account of temperature-dependent rescue of the per01 or cyc01 mutations or temperature-dependent mis-expression of per. Moreover, we made use of these flies to experimentally determine the developmental requirements for a functional circadian clock as well as the individual clock components PER and CYC. We confirmed and extended previously published observations [18], [19] indicating that developmental rescue of arrhythmia in per01 mutants is not needed for restoration of circadian rhythms in adults. However, developmental mis-expression of per or failure to developmentally rescue the cyc01 mutation led to persistent adult arrhythmia. In particular, CLK/CYC function during the pupal and pharate adult stages was associated with adult clock function. Our results suggest two distinct mechanisms underlying the developmental requirement for CLK/CYC function: (1) cyc expression contributes cell-type-autonomously in the ventral lateral neurons (LNvs) to the formation of peptidergic dorsal projections containing the neuropeptide PIGMENT DISPERSING FACTOR (PDF), which are thought to be important for adult circadian behavior and (2) CLK/CYC activity during development enables normal clock gene expression rhythms in the adult LNvs.
Results
Transgenic flies with conditional rescue of per01 in clock-bearing cells
We made use of the temporal and regional gene expression targeting (TARGET) system [20] to create transgenic flies in which the essential clock components CYC and PER were expressed conditionally in relevant spatiotemporal patterns. The TARGET system combines the binary GAL4/UAS system [21] that allows transgenic expression to be directed spatiotemporally by a promoter of interest via the intermediate regulator GAL4 with a ubiquitously expressed GAL80ts gene, which encodes a temperature sensitive inhibitor of GAL4. As a result, the TARGET system permits GAL4-mediated transgenic expression at high temperatures (e.g., 29°C), but progressively restricts it at lower temperatures. First, we generated transgenic flies that conditionally rescued the arrhythmic per01 phenotype [22] by introducing a GAL4-driver transgene directing expression in all clock-bearing cells (tim(UAS)-Gal4) [9] along with a GAL4-responsive per cDNA expression construct (UAS-per) [23] and a transgene ubiquitously expressing GAL80ts (tubP-Gal80ts) [20] in a per01 genetic background [22] (see Figure 1A). The resulting genotype is abbreviated, here, as per01[timP>per]ts. As expected, clock-controlled phenotypes such as behavioral rhythmicity, relative rhythmic power and period length were readily and significantly modulated by environmental temperature in these flies (Figure 1B–1D, Figures S1 and S2). Robust circadian rhythms in locomotor activity were virtually absent at a restrictive temperature (18°C), but rescued to varying degrees over a range of higher temperatures (21–29°C) (Figure 1B–1D, Figures S1 and S2). The circadian period length observed at 29°C was significantly longer than those at 25°C, 27°C, and 28°C for females and those at 23°C, 25°C and 28°C for males (Figure 1D, Figure S2A, S2C; Welch test and post-hoc Games-Howell analysis). It is noteworthy that the decrease in rhythmicity and relative rhythmic power and the increase in circadian period length found at the highest experimental temperature of transgenic induction (29°C) were also observed as a result of transgenic per over-expression in a wild-type background (see below). In comparison with wild-type controls per01[timP>per]ts flies were much less rhythmic at 18°C (or 29°C), but at 25°C both genotypes showed comparable percentages of rhythmic, weakly rhythmic, and arrhythmic flies (Figure S3). At permissive temperatures the most consistent difference in the behavior of per01[timP>per]ts flies relative to wild-type controls was a significantly longer circadian period length increased by 2 h or more (Figure S3B–S3D).
Fig. 1. Conditional transgenic rescue of per01 arrhythmic behavior. 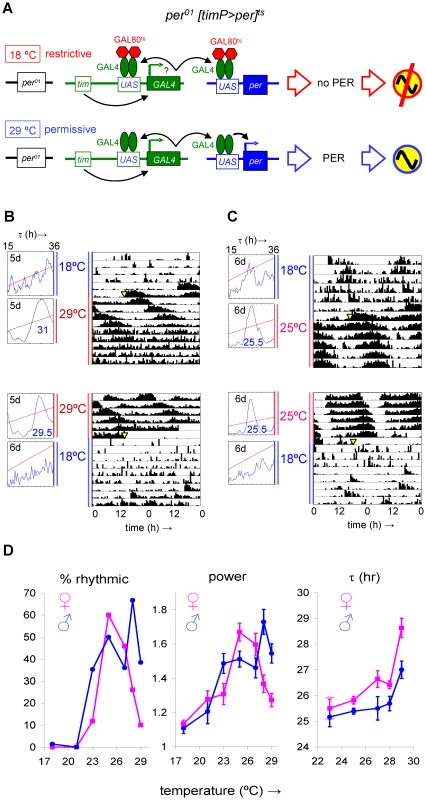
Introduction of tim(UAS)-Gal4, UAS-per, and tubP-Gal80ts transgenes in a per01 genetic background resulted in conditional circadian clock function, with virtually no rescue of circadian locomotor behavior at the restrictive temperature (18°C) and approximation of wild-type circadian behavior at permissive temperatures (23–29°C). (A) The lack of rescue at 18°C is explained by inhibition of GAL4-mediated expression of transgenic per mediated by the temperature sensitive GAL4 repressor GAL80ts. At permissive temperatures the modulating effect of GAL80ts is reduced allowing GAL4 expressed in clock-bearing cells to induce per and rescue circadian clock function. (B,C) Flies with a y per01 w; tim(UAS)-Gal4/tubPGal80ts; UAS-per/+ genotype, abbreviated as per01 [timP>per]ts, raised at ambient temperature (∼23°C) were monitored for adult locomotor activity sequentially at restrictive and permissive conditions (see Materials and Methods). The large diagrams in (B) and (C) are double-plotted actograms representing the median locomotor activity for 8 female flies. Yellow triangles indicate the time of the temperature shifts. The small diagrams are chi-square periodograms based on the first 5 or 6 full days at the first (top) or second (bottom) experimental condition. Circadian period lengths detected at the permissive conditions are indicated in large blue type-face. Note the absence of strong circadian rhythms at 18°C. The considerably lengthened period and progressive weakening of rhythms observed at 29°C are likely attributable to excessive per expression. (D) Temperature-dependent conditional rescue of the percentage of rhythmic flies, relative rhythmic power, and period length in per01 [timP>per]ts flies. Chi-square periodogram analysis of circadian locomotor behavior in DD was performed for 5-day intervals at the indicated temperatures. The three panels show changes as a function of environmental temperature in the percentage of rhythmic flies, relative rhythmic power (across both rhythmic and weakly rhythmic flies), and circadian period length for rhythmic flies. Error bars represent the Standard Error of the Mean (SEM). Next, we examined clock-controlled molecular responses in per01[timP>per]ts flies released from restrictive (17 or 18°C) to permissive conditions (25°C). As expected, transgenic per expression was strongly induced in adult fly heads following this transition (Figure S4A, S4B). In addition, the Clk, tim, vri, cwo, Par-domain Protein 1 (Pdp1), and Slow-poke binding protein (Slob) clock-controlled transcripts showed relative expression responses that appeared consistent with their circadian phase relationships in wild-type heads. Nevertheless, the amplitude of the observed expression responses in clock-controlled genes was reduced relative to previously reported amplitudes of circadian oscillation in wild-type heads [4], [7]–[9], [24]–[27]. Thus, upon transfer to permissive conditions, rescue of molecular circadian oscillations in adult per01[timP>per]ts heads, unlike behavioral rhythms, appeared to be incomplete. This discrepancy might be explained by a selective restoration of high-amplitude clock gene expression rhythms in clock neurons. We, therefore, examined circadian transcript responses in dissected adult brains of per01[timP>per]ts flies released under permissive conditions. However, molecular amplitudes in adult brains were comparable to those previously seen in adult heads (cf Figure S4A and S4C) suggesting incomplete restoration of molecular circadian rhythms in both peripheral clocks and the neural clock circuit. Since different time points in the Northern and Quantitative Reverse Transcriptase PCR (qRT-PCR) experiments of Figure S4 come from different samples of individual flies incomplete synchrony in the experimental population may have also contributed to the detection of relatively shallow transcript rhythms.
Adult circadian locomotor behavior does not require a functioning circadian clock or rescue of per01 during prior development
To test if developmental expression of per in clock-bearing cells was required for adult clock function, we raised per01[timP>per]ts flies at 17°C in constant light (LL) until adulthood and examined behavioral rhythms at restrictive (17°C) and subsequent permissive (25°C) conditions in constant darkness (DD). Consistent with the hypothesis that rescue of circadian clock function in per01 flies can be achieved when per expression is restricted to clock-bearing cells in the adult, we observed restoration of circadian locomotor rhythms immediately following transition to permissive conditions (Figure 2A, Figure S5). Although adult per01[timP>per]ts flies failed to show strongly rhythmic locomotor behavior at the restrictive temperature in DD, a subset of individual flies did exhibit residual weak rhythms under these conditions (Figure S2). Nevertheless, we do not believe that the observed behavioral rescue in adults depends on residual clock function during the prior exposure to the restrictive temperature for two reasons: (1) The phase of the restored rhythms is determined by the phase of the prior switch from restrictive to permissive conditions rather than the phase of the light/dark transition associated with the start of the behavioral experiment (Figure 2) and (2) our experiments included developmental exposure to LL, which is associated with both behavioral and molecular arrhythmia as well as severely reduced PER expression levels [13], [28]. Therefore, we conclude that there is no developmental requirement for either a functioning clock mechanism or expression of per in the clock-bearing cells in order to allow circadian clock function in adult flies.
Fig. 2. Adult circadian behavior does not require developmental rescue of per01. 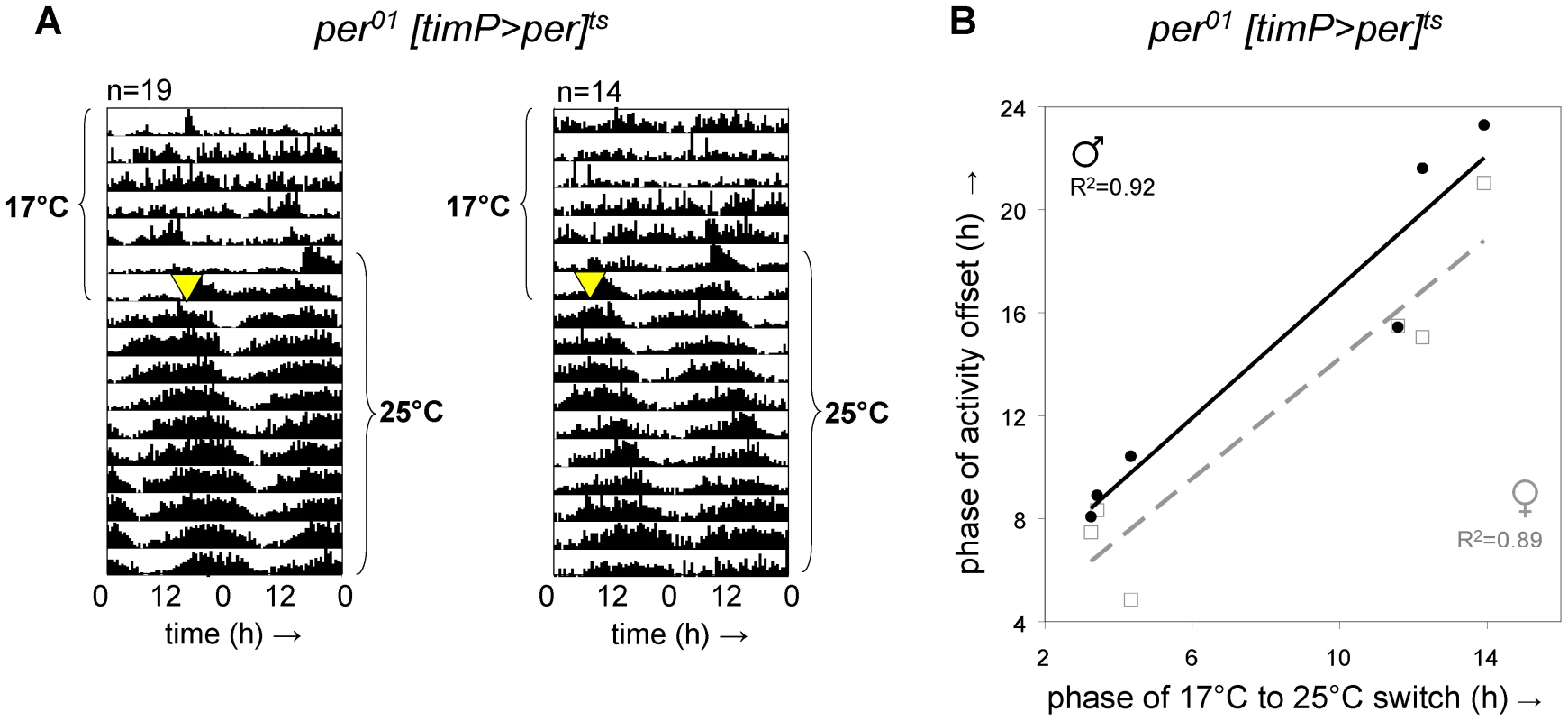
Circadian locomotor behavior was readily restored in per01 [timP>per]ts flies raised under restrictive conditions (17°C LL) upon transfer of adults from restrictive (17°C DD) to permissive conditions (25°C DD). Moreover, the phase of circadian behavior was determined by the phase of transfer. (A) Double-plotted actograms representing average locomotor activity at 17°C DD and subsequent 25°C DD. The left panel represents data for 19 female flies transferred to the permissive condition at reference phase hour 12.5, whereas the right panel corresponds to the activity of 14 female flies switched at reference phase 3.25. Note the phase relationship between subsequent behavioral rhythms and the time of transfer (marked by a yellow triangle). (B) The phase of the offset of circadian locomotor activity at 25°C is plotted separately for median data from 6 groups of male and 6 groups of female flies as a function of the phase of the 17°C to 25°C switch along with trend lines and associated correlation coefficients (2-tailed test significance: p<0.005 for females, p<0.003 for males). Over-expression of per during metamorphosis disrupts adult circadian behavior
Given the ability to restore adult clock function from a circadian cycle arrest due to PER depletion, we were wondering whether circadian cycle arrests associated with excess PER expression were equally reversible. This question was addressed experimentally with the help of transgenic flies, in which per was conditionally over-expressed to high levels in clock-bearing cells due to the introduction of the tim(UAS)-Gal4, UAS-per, and tubP-Gal80ts transgenes in the presence of a wild-type per gene. Flies homozygous for the autosomal tim(UAS)-Gal4 and UAS-per insertions with a single X-chromosomal tubP-Gal80ts transgene (abbreviated as [timP>per]ts) showed conditional clock function with robust rhythms, relative rhythmic power, and only marginally increased period lengths at permissive (17°C) conditions and behavioral arrhythmia (females) or dramatically reduced rhythms (males) at the restrictive (29°C) conditions (Figure 3, Figure 4, Figures S6 and S7). Loss of behavioral rhythms during prolonged exposure of adults to the restrictive condition could be reversed by returning the flies to the permissive (17°C) condition (Figure 3B, Figure S7). However, comparable exposure to restrictive conditions during development resulted in irreversible adult arrhythmia (Figure 3B, Figure 4, Figures S6 and S7) for both genders. To identify the developmental phase of sensitivity to PER over-expression flies were transferred from a permissive ambient temperature (∼23°C) to 29°C or vice versa at different points during development and then analyzed for behavioral rhythmicity as adults. When exposure to restrictive conditions occurred prior to the pupal stage it did not obviously affect the percentages of flies exhibiting rhythmic, weakly rhythmic or arrhythmic adult behavior or the relative power of the detected rhythms (Figure 4, Figure S6). However, when flies were exposed to the restrictive temperature throughout the pupal and pharate adult stages, adult locomotor rhythms were clearly inhibited (Figure 4, Figure S6). Therefore, it appears that per over-expression in pupal/pharate adult clock cells irreversibly affects adult circadian behavior. One possible explanation for the observed effect of developmental per mis-expression on adult behavior might be the persistence of abnormally high levels of PER protein into adulthood. However, PER is known to be an unstable protein and even a 7-d exposure to 12-h light/12-h dark/ (LD) cycles at the permissive (17°C) temperature did not allow subsequent restoration of behavioral rhythms in DD. Moreover, immunofluorescence analyses of clock neurons exposed to developmental PER over-expression did not reveal a continued increase in adult PER expression. Instead, the persistent behavioral arrhythmia of [timP>per]ts flies raised at 29°C and exposed to 17°C LD for 7 d as adults appeared to be matched by blunted circadian rhythms of PER expression in the PDF-expressing ventral lateral neurons (Figure 5). No gross morphological defects in clock neurons (including LNv, LNd, and DN cell bodies and LNv projections) were apparent in these experiments (see Figure S8). Thus, our results indicate that excess PER activity in clock cells during metamorphosis negatively affects both adult circadian locomotor activity and molecular rhythms in adult clock neurons.
Fig. 3. Developmental over-expression of per disrupts adult circadian behavior, while the phenotype of adult per over-expression is reversible. 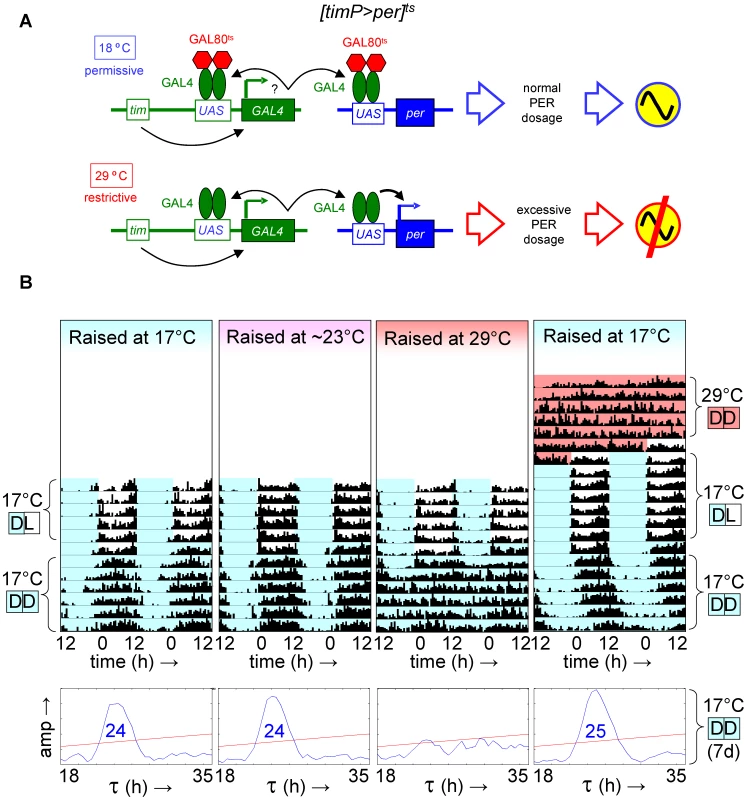
Transgenic flies with conditional over-expression of per exhibited a reversible loss of circadian behavior when temporarily shifted to restrictive conditions (29°C) as adults, but showed long-term behavioral arrhythmia when similarly exposed to restrictive conditions during development. (A) Flies with a y tubPGal80ts w/(FM7c or Y); tim(UAS)-Gal4; UAS-per genotype, abbreviated as [timP>per]ts, show rhythmic locomotor behavior at the permissive temperature when per over-expression is prevented by GAL80ts, but not at the restrictive temperature when GAL80ts is ineffective and excessive levels of PER prevent circadian clock function. (B) The top four panels are double-plotted actograms representing median locomotor activity data for groups of female [timP>per]ts flies during LD and subsequent DD conditions at the permissive temperature (17°C DD). The two actograms on the left illustrate that developmental exposure to 17°C or ∼23°C has no obvious effect on adult circadian behavior, while the data in the third panel from the left reflect the loss of adult locomotor activity rhythms following development at the restrictive temperature (29°C). The right-most actogram illustrates the reversible behavioral phenotype of 17°C-raised flies that where shifted to the restrictive temperature as adults (29°C DD data included at the top) prior to the rest of the experiment. The white, light blue, and red background colors in the actograms represent 17°C light, 17°C dark, and 29°C dark conditions, respectively. The lower four panels are chi-square periodograms for the median data reflecting circadian rhythmicity in 17°C DD. Single significant (p<0.01) period lengths in the circadian range (18–35 h) are indicated in large blue type-face. Fig. 4. Over-expression of per in clock-bearing cells during metamorphosis disrupts locomotor activity rhythms in adults. 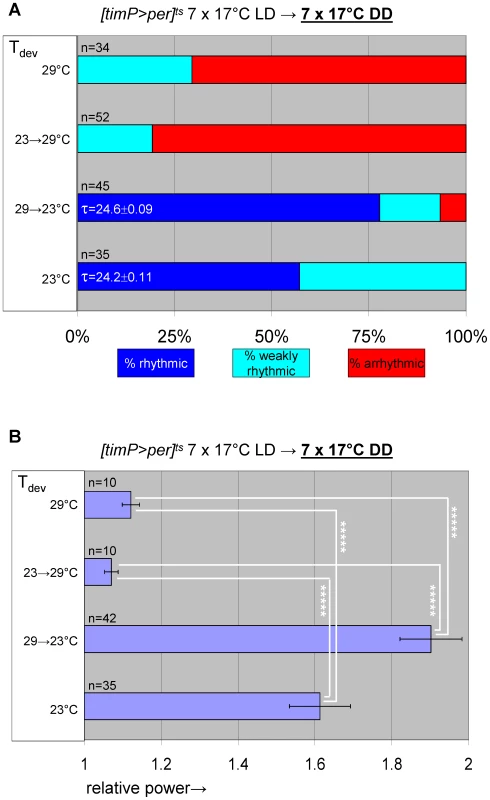
Circadian behavior of adult [timP>per]ts flies under permissive conditions (17°C DD) was strongly rhythmic for flies raised at ambient temperature (∼23°C) or flies transferred from 29°C to ambient temperature as wandering larvae or prepupae. However, flies exposed to restrictive (29°C DD) conditions throughout development or during the pupal and pharate adult stages exhibited mostly arrhythmic adult locomotor behavior. (A) Stacked bar diagram representing the percentages of female flies with rhythmic, weakly rhythmic, or arrhythmic adult behavior at permissive conditions (17°C DD). Prior to measurement of adult locomotor activity at 17°C LD and subsequent 17°C DD (analyzed here) flies were raised at the indicated temperatures (Tdev): 23°C, 23→29°C (transferred as wandering larvae or prepupae from 23°C to 29°C), 29→23°C (transferred as wandering larvae or prepupae 29°C to 23°C), and 29°C. The numbers (n) of flies included for each condition are indicated as well as the average (±SEM) circadian period length for rhythmic flies. Chi-square analysis indicated a highly significant (p<10−23) association between developmental temperature and the percentages of rhythmic, weakly rhythmic, and arrhythmic adults. (B) Bar diagram of the average (±SEM) relative rhythmic power observed among the rhythmic plus weakly rhythmic flies for each developmental condition. The number of flies included in this analysis (n) is indicated for each condition. The Welch test statistic indicated a highly significant association (p<10−13) of relative rhythmic power with developmental condition. Significant differences found by post-hoc Games-Howell tests for pairwise comparisons of developmental treatments indicated by (*****) represent p values smaller than 10−5. Fig. 5. Developmental over-expression of per affects molecular rhythms in adult clock neurons. 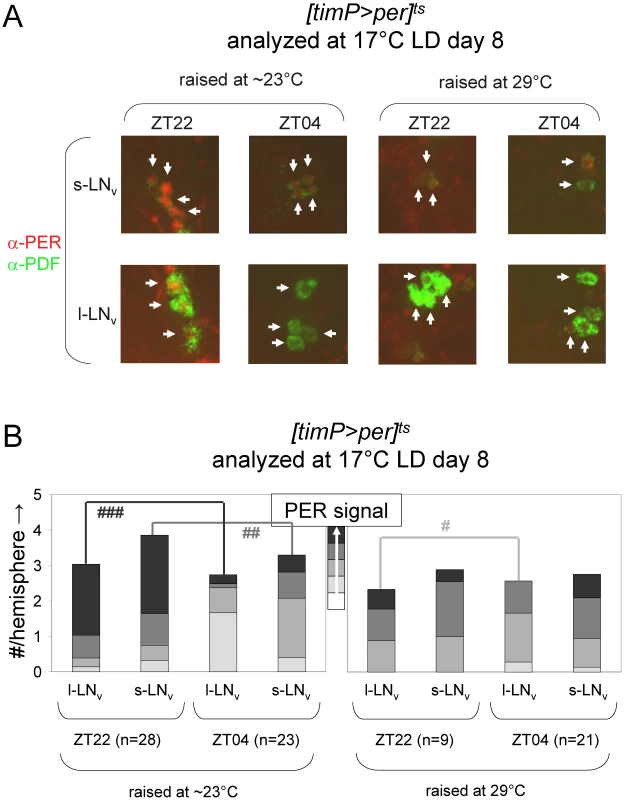
Comparative immunofluorescence analyses indicate that developmental per over-expression affects circadian PER protein rhythms in the PDF-expressing adult LNvs. (A) [timP>per]ts flies raised at either restrictive (29°C) or permissive (∼23°C) conditions were transferred as adults to 17°C LD conditions and harvested 2-h before lights-on (ZT22) on day 8 and 4-h after lights-on (ZT4) on day 9. Brains were dissected and subjected to immunofluorescence staining using antibodies directed against the PER and PDF proteins. Representative images illustrate the effects of both daily phase and developmental treatment on PER expression for PDF-expressing LNv cells (arrows). (B) Semi-quantitative analysis of the immunofluorescence signal for PER in adult PDF neurons. The cumulative height of the bars indicate the average number per brain hemisphere of l-LNv and s-LNv detected by PDF staining, whereas the segments with increasingly darker shades of gray represent the relative prevalence of cells with no, very faint, faint, moderate, or strong levels of PER signal, respectively. The numbers of brain hemispheres is indicated (n). Significant associations of PER signal level and daily phase found by Chi-square analyses indicated by (#), (##), and (###) represent p values smaller than 10−6, 10−10, and 10−17, respectively. Depletion of cyc expression during metamorphosis disrupts adult circadian behavior
Based on PER's known function as a negative regulator of CLK/CYC circadian transcription complexes the adult phenotypes associated with developmental PER over-expression are likely attributable to inhibition of CLK/CYC activity. We tested this hypothesis by determining whether adult circadian locomotor behavior required prior developmental expression of the essential clock component CYC. To this aim we generated transgenic flies that conditionally expressed cyc in postmitotic neurons by combining the elavC155::Gal4 driver element [29] with UAS-cyc [30], and tubP-Gal80ts transgenes in a cyc01 background. The resulting flies, here referred to as cyc01 [elav>cyc]ts, showed conditional rescue of rhythmic adult locomotor activity when raised at the permissive temperature for cyc01 rescue (29°C) (Figure 6, Figure S9). Ambient temperature (∼23°C), which acted as a mostly permissive condition for per01 [timP>per]ts flies (see Figure 1D, Figure S2, above) represented a restrictive condition for the cyc01 [elav>cyc]ts strain. This discrepancy is likely attributable to differences either in the amount of GAL4 protein produced in the relevant clock neurons in each of these strains or the level of GAL4-directed transgenic expression that is required to achieve behavioral rescue. Exposure of cyc01 [elav>cyc]ts flies to the restrictive temperature during metamorphosis, severely affected adult behavioral rhythms at the permissive temperature (Figure 6B, Figure 7, Figure S10). Therefore, depletion of cyc expression during metamorphosis, indeed, phenocopies the adult behavioral defects of per mis-expression during metamorphosis.
Fig. 6. Developmental depletion of cyc disrupts adult circadian behavior, while the phenotype of adult depletion of cyc is reversible. 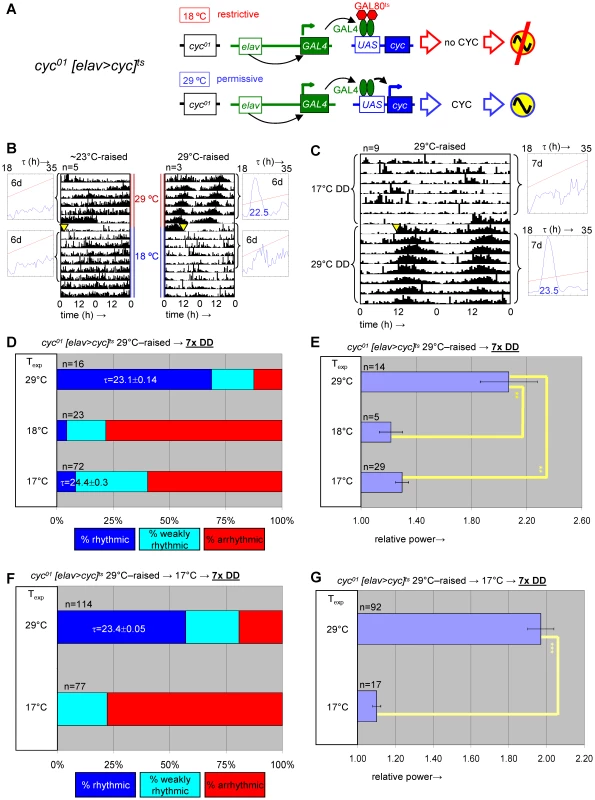
(A) elavC155::Gal4; UAS-cyc/CyO; cyc01 tubPGal80ts flies, here abbreviated as cyc01 [elav>cyc]ts, conditionally rescue cyc01 in postmitotic neurons at 29°C, but not lower temperatures (17–25°C). (B) Example actograms illustrate average locomotor activity at 29°C DD and subsequent 18°C DD conditions for females raised at restrictive (∼23°C) or permissive temperature (29°C), respectively. (C) The actogram shows behavioral arrhythmicity at 17°C for 29°C-raised females and subsequent rescue of circadian locomotor activity at 29°C. (B–C) Associated chi-square periodograms show strong behavioral rhythms only observed for 29°C-raised flies at 29°C. (D–G) Quantitative analysis of adult circadian behavior in 29°C-raised cyc01 [elav>cyc]ts flies at either permissive (29°C) versus two restrictive conditions (17°C, 18°C) (D,E) or permissive (29°C) versus restrictive (17°C) conditions following adult exposure to restrictive conditions (≥3 days 17°C) (F,G). The stacked bar diagrams (D,F) represent the percentages of 29°C-raised females with rhythmic, weakly rhythmic, or arrhythmic locomotor behavior. Rhythmicity was determined for individual flies by chi-square periodogram analysis of 7 d intervals at the indicated temperatures in constant darkness. The average (±SEM) circadian period length is indicated for rhythmic flies. Chi-square analyses indicated highly significant associations between experimental temperature and the percentages of rhythmic, weakly rhythmic, and arrhythmic adults [p<10−7 (D); p<10−17 (F)]. The bar diagrams (E,G) correspond to the average (±SEM) relative rhythmic power observed among the rhythmic plus weakly rhythmic flies for each experimental condition. The number of flies included in this analysis (n) is indicated for each condition. (E) The Welch test statistic indicated a significant association (p<10−2) of relative rhythmic power with experimental condition. Significant differences found by post-hoc Games-Howell tests for pairwise comparisons of developmental treatments indicated by (**) represent p values smaller than 10−2. (G) A significant association (p<10−3) of relative rhythmic power with experimental condition was found by the Mann-Whitney rank-sum test. Fig. 7. Transgenic rescue of cyc01 behavioral arrhythmia in adults depends on developmental cyc expression during metamorphosis. 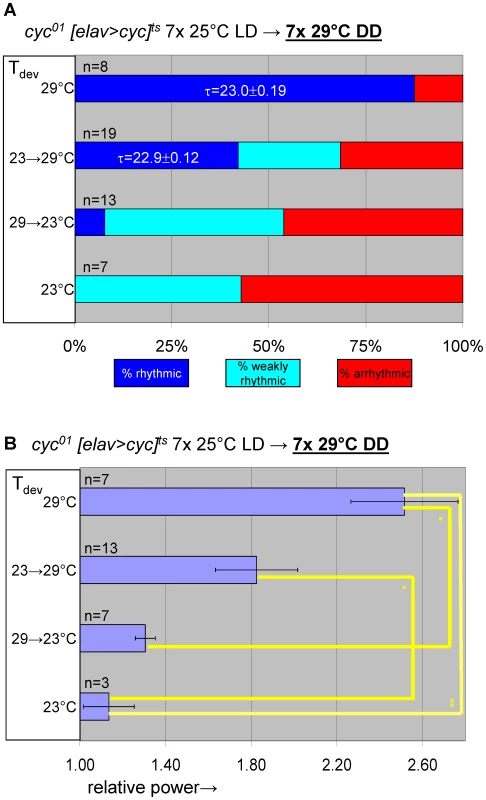
Adult circadian behavior of 29°C-raised cyc01 [elav>cyc]ts flies was strongly rhythmic under permissive conditions (29°C DD). Substantial rescue of adult locomotor rhythms was also observed for cyc01 [elav>cyc]ts flies raised at ambient temperature (∼23°C) and transferred to 29°C as wandering larvae or prepupae. However, flies exposed to restrictive (∼23°C) conditions throughout development or during the pupal and pharate adult stages exhibited weakly rhythmic or arrhythmic adult locomotor behavior. (A) Stacked bar diagram representing the percentages of female flies with rhythmic, weakly rhythmic, or arrhythmic adult behavior at permissive conditions (29°C DD) following prior exposure to 7 LD days at 25°C. Prior to measurement of adult locomotor activity during 25°C LD and subsequent 29°C DD (analyzed here) flies were raised at the indicated temperatures (Tdev): 23°C, 23→29°C (transferred as wandering larvae or prepupae from 23°C to 29°C), 29→23°C (transferred as wandering larvae or prepupae 29°C to 23°C), and 29°C. The numbers (n) of flies included for each condition are indicated as well as the average (±SEM) circadian period length for rhythmic flies. Chi-square analysis indicated a significant (p<10−2) association between developmental temperature and the percentages of rhythmic, weakly rhythmic, and arrhythmic adults. (B) Bar diagram of the average (±SEM) relative rhythmic power observed among the rhythmic plus weakly rhythmic flies for each developmental condition. The number of flies included in this analysis (n) is indicated for each condition. The Welch test statistic indicated a significant association (p<10−2) of relative rhythmic power with developmental condition. Significant differences found by post-hoc Games-Howell tests for pairwise comparisons of developmental treatments indicated by (*) and (**) represent p values smaller than 0.05 and 10−2, respectively. Selective inhibition of cyc01 rescue in the PDF-expressing clock neurons disrupts adult locomotor rhythms as well as cell-type-specific neuro-anatomy and molecular rhythms
To further explore the role of CLK/CYC expression in the PDF-positive LNvs in ensuring normal adult circadian behavior and neuro-anatomy we created flies in which rescue of cyc01 in postmitotic neurons (by elavC155::Gal4 and UAS-cyc) was selectively blocked in the PDF-expressing LNvs with the help of a Pdf-Gal80 transgene [31] that expresses the GAL4 inhibitor GAL80 specifically in these cells. The behavioral phenotype of the resulting transgenic flies, indicated as cyc01 (elav-Pdf)>cyc, consists of an altered daily locomotor activity profile in the presence of light/dark cycles that includes extended activity in anticipation of lights-on, but reduced activity in anticipation of lights-off (Figure 8A, Figure S11A) and a loss of sustained rhythmicity in constant darkness (Figure 8A–8C; Figure S11). In contrast, control cyc01 rescue flies lacking the Pdf-Gal80 element (cyc01 elav>cyc) showed strong behavioral rhythms in constant darkness as well as evening activity in anticipation of the lights-off transition (Figure 8A–8C; Figure S11). The cyc01 (elav-Pdf)>cyc phenotype is clearly different from that of flies with ablated PDF-expressing LNvs or defective expression of the PDF neuropeptide [32], which also lack consolidated rhythms in constant darkness but show the opposite effect on anticipation of the lights-on and lights-off transitions. The persistence of morning anticipation, which is thought to be attributable to PDF signaling from the s-LNvs [33] suggests that residual PDF expression and function persisted in s-LNvs with a cyc01 circadian cycle arrest. Moreover, it is insightful to compare the behavior of cyc01 (elav-Pdf)>cyc flies to previously published observations for flies, in which rescue of the per01 mutation in clock-bearing cells was blocked in the PDF-expressing clock neurons (per01 (elav-Pdf)>per) [31]. The behavior reported for per01 (elav-Pdf)>per flies resembles that of our cyc01 (elav-Pdf)>cyc flies with respect to the persistence of morning anticipation as well as reduced rhythmicity in constant darkness [31]. However, loss of evening anticipation appears to be unique to the cyc01-based as opposed to the per01-based arrest of the LNvs. Thus, cyc-depleted LNvs seemed to delay the generation of an evening activity signal by the neural clock circuits. It is possible that a slow rhythmic component in the disorganized clock circuit of cyc01 (elav-Pdf)>cyc flies is responsible for this delay in evening activity. Consistent with this notion, the few (weakly) rhythmic cyc01 (elav-Pdf)>cyc flies represented in Figure 8 and Figure S11 exhibited residual long period rhythms (τ for females: 25.1±1.24 h, n = 5; τ for males 24.4±0.83 h, n = 17).
Fig. 8. Selective inhibition of cyc01 rescue in the PDF-expressing clock neurons results in behavioral circadian phenotypes. 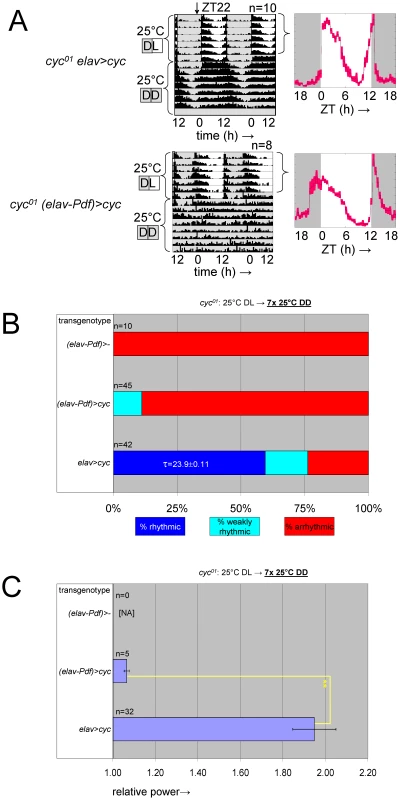
(A) Example actograms (left) and LD activity profiles (right) representing median locomotor behavior for female elavC155::Gal4; UAS-cyc/CyO; cyc01 flies (abbreviated as cyc01 elav>cyc), which are rescued for cyc expression in postmitotic neurons, versus female elavC155::Gal4; UAS-cyc/Pdf-Gal80; cyc01 flies (abbreviated as cyc01 (elav-Pdf)>cyc), in which transgenic cyc rescue is selectively blocked in the PDF-expressing clock neurons. The activity profiles represent average daily activity (±SEM) indicated by the black line and red shading for the median locomotor activity of cyc01 elav>cyc and cyc01 (elav-Pdf)>cyc flies in the presence of LD cycles. Note that cyc01 (elav-Pdf)>cyc flies exhibit loss of free running rhythms in DD as well as increased activity in anticipation of lights-on and loss of activity in anticipation of lights-off in LD. (B) Stacked bar diagram representing the percentages of female flies with rhythmic, weakly rhythmic, or arrhythmic adult behavior at 25°C DD. Along with cyc01 elav>cyc and cyc01 (elav-Pdf)>cyc flies, the experiment also included non-rescued control flies with an elavC155::Gal4; CyO/Pdf-Gal80; cyc01 genotype (abbreviated as cyc01 (elav-Pdf)>-). For each transgenic combination with cyc01 the number of flies (n) as well as the average (±SEM) circadian period length for rhythmic flies are indicated. Chi-square analysis indicated a highly significant (p<10−9) association between genotype and the percentages of rhythmic, weakly rhythmic, and arrhythmic adults. (C) Bar diagram of the average (±SEM) relative rhythmic power observed among the rhythmic plus weakly rhythmic female flies for each genotype. The number of flies included in this analysis (n) is indicated for each condition. Because all cyc01 (elav-Pdf)>- flies were arrhythmic, a Mann-Whitney rank-sum test was performed to compare the effect on relative rhythmic power of the other two genotypes. As indicated, relative rhythmic power was significantly reduced in cyc01 (elav-Pdf)>cyc flies compared to cyc01 elav>cyc flies (**; p<10−2). Next, we compared molecular and neuro-anatomical phenotypes of the PDF-expressing LNvs in cyc01 (elav-Pdf)>cyc flies with those of controls lacking either the Pdf-Gal80 (cyc01 elav>cyc) or the UAS-cyc transgenes (cyc01 (elav-Pdf)>-). Flies of these three genotypes were raised at ambient temperature and entrained as adults to LD cycles at 25°C. Brains were harvested 2 h prior to lights-on (ZT22) and stained using antibodies against PDF and the PDP1. The Pdp1 gene is a direct target gene for CLK/CYC and mutations in Clk or cyc strongly affect PDP1 protein expression in larvae and adults [26]. Indeed, cyc01 (elav-Pdf)>- flies, which completely lack CLK/CYC function, exhibited greatly reduced PDP1 expression in their LNvs at ZT22. Consistent with previous studies [34], s-LNvs in cyc01 (elav-Pdf)>- brains also showed a reduced PDF signal in the s-LNvs and mostly abnormal or missing PDF-positive dorsal projections (Figure 9). These phenotypes were to a large extent rescued in cyc01 elav>cyc flies, which not only exhibited PDP1 expression in virtually all PDF-expressing LNvs (and other clock neurons) at ZT22, but also presented with normal PDF-expression levels and dorsal LNv projections (Figure 9). The additional introduction of Pdf-Gal80 in the cyc01 (elav-Pdf)>cyc genotype, resulted in cell-type-specific phenotypes that included down-regulation of PDP1 in virtually all LNvs with detectable PDF expression as well as a reduction in the number of s-LNvs with detectable PDF expression (see Figure 9A, 9B). Moreover, PDF-positive sLNv dorsal projections were either abnormal or missing from most cyc01 (elav-Pdf)>cyc brains (see Figure 9C), although there is a formal possibility that PDF-negative sLNv projections, which would not have been detectable in these experiments, still extended to the dorsal protocerebrum. For other clock neurons no obvious differences were detected in numbers and PDP1 expression levels between the cyc01 (elav-Pdf)>cyc brains and the rescued cyc01 elav>cyc controls.
Fig. 9. Selective inhibition of cyc01 rescue in the PDF-expressing clock neurons results in cell-type–specific neuro-anatomical and molecular circadian phenotypes. 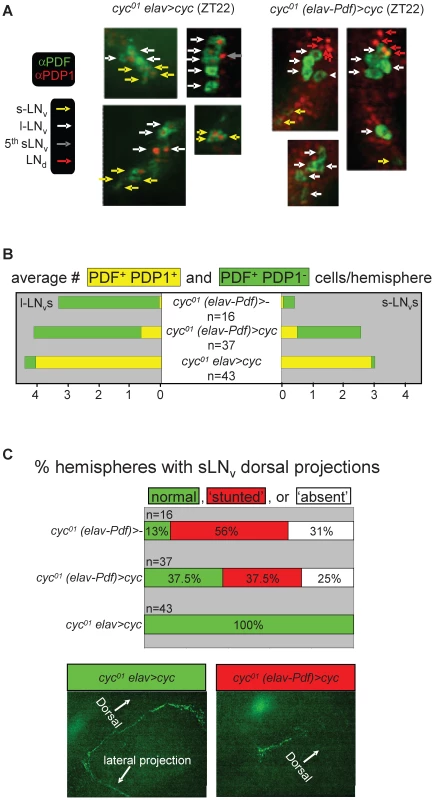
Comparative immunofluorescence analysis of PDF and PDP1 expression in the clock neurons of cyc01 elav>cyc and cyc01 (elav-Pdf)>cyc flies. (A) Example micrographs illustrating selective inhibition of PDP1 expression in the PDF-expressing LNvs of cyc01 (elav-Pdf)>cyc but not cyc01 elav>cyc flies at ZT22. Whole mount adult brains for both genotypes were stained with antibodies directed against PDF (green) and PDP1 (red). s-LNv, l-LNv, LNd, and 5th s-LNv clock neurons are indicated by yellow, white, red, and gray arrows, respectively. (B) Semi-quantitative analysis of the number of PDP1-positive and PDP1-negative PDF-expressing LNvs at ZT22 per brain hemisphere for cyc01 (elav-Pdf)>-, cyc01 (elav-Pdf)>cyc, and cyc01 elav>cyc flies. The numbers (n) of brain hemispheres analyzed are indicated for each genotype. Chi-square analyses found highly significant associations between genotype and PDP1 expression in both s-LNvs (p<10−31) and l-LNvs (p<10−55). (C) Semi-quantitative analysis of neuro-anatomical defects in the dorsal PDF-expressing LNv projections. Stacked bar diagram (top panel) representing the percentages of cyc01 (elav-Pdf)>-, cyc01 (elav-Pdf)>cyc, and cyc01 elav>cyc brain hemispheres with normal, stunted, or absent PDF-positive dorsal projections. The numbers (n) of brain hemispheres analyzed are indicated for each genotype. Chi-square analysis indicated a highly significant association between genotype and dorsal projection phenotype (p<10−5). The lower two panels represent examples of normal and stunted PDF-expressing dorsal projections, from cyc01 elav>cyc and cyc01 (elav-Pdf)>cyc brains, respectively. Discussion
We created transgenic flies with conditional clock function, in which expression of the essential clock components CYC and PER was induced or repressed in relevant spatiotemporal patterns. In per01 [timP>per]ts flies, which conditionally rescue the per01 mutation, clock function was conditional and readily reversible. Moreover, adult circadian behavior was restored in flies raised under restrictive conditions. In earlier studies conducted by Ewer and colleagues widespread transgenic expression of per under control of a heat-shock protein 70 (hsp70) promoter was shown to partially rescue the per01 mutation resulting in restoration of behavioral rhythms at an abnormally long period length. These long period rhythms could be generated in a conditional manner even when induction was restricted to the adult phase [18], [19]. In the present study we targeted expression of transgenic per specifically to clock-bearing cells and achieved a more complete conditional rescue of the per01 phenotype that did not require developmental per expression.
Although circadian behavior of per01 [timP>per]ts flies at 25°C showed rhythmicity comparable to that observed for wild-type flies, period lengths were at least 2 h longer than those of wild-type flies and molecular rhythms showed a relatively low amplitude. One key difference between the molecular clock circuits in per01 [timP>per]ts at the permissive temperature and those of wild-type flies is the constitutively high level of per mRNA expression in the transgenically rescued flies, which could contribute to the increased circadian period length and blunted molecular rhythms in per01 [timP>per]ts flies. Wild-type flies exhibit a trough in per transcript levels in the early morning that may facilitate subsequent down-regulation of PER protein levels and optimal induction of CLK/CYC-regulated genes [35]. The lack of a trough in per mRNA expression in the conditionally rescued flies could account for a delay in the turnover of PER protein in the morning and, therefore, a lengthened period and blunted CLK/CYC activity. This hypothesis also explains apparent discrepancies with previous reports, in which increased per gene dosage was associated with a shortened circadian period length [36] and decreased per dosage or expression resulted in longer circadian period lengths [22], [37], [38]. As long as per expression shows strong circadian regulation the timing of PER nuclear entry and PER-mediated transcriptional repression is predicted to be advanced by the introduction of one or two additional copies of the wild-type per gene and delayed by a reduction in per dosage, while neither manipulation is predicted to strongly affect subsequent PER turnover.
Adult circadian behavior was also conditional and reversible in [timP>per]ts flies, which exhibit temperature-dependent over-expression of per. However, developmental over-expression of per during metamorphosis was associated with irreversible behavioral arrhythmia in adults. Likewise, depletion of cyc expression during the metamorphosis in cyc01 [elav>cyc]ts flies resulted in disruption of adult circadian locomotor behavior under permissive conditions. Both increased levels of PER and decreased levels of CYC negatively regulate CLK/CYC activity. The CLK/CYC heterodimer functions as the central transcriptional regulator in the Drosophila clock and its activity critically depends on the presence of both CLK and CYC [3], [5], [6]. Loss of functional cyc expression in the cyc01 mutant results in both molecular and circadian arrhythmia and constitutively low expression levels for CLK/CYC-regulated target genes [6], whereas PER acts as a negative regulator of CLK/CYC activity by binding and inactivating the CLK/CYC complex [5], [15], [39]. The arrhythmic locomotor behavior and molecular arrhythmia in the clock neurons observed as a result of per over-expression [23], [40] are, therefore, interpreted to result from constitutive inhibition of CLK/CYC.
Adult behavioral arrhythmia in [timP>per]ts or cyc01 [elav>cyc]ts flies raised under permissive conditions was reversible (see Figure 3B, Figure 6C, 6F, 6G, Figures S7, S9C, S9D, above). However, exposures to restrictive conditions of comparable duration resulted in long-term after-effects only when they occurred during development and, particularly, during the pupal and pharate adult stages. We, therefore, attribute the effects of circadian arrests during development in [timP>per]ts or cyc01 [elav>cyc]ts flies on adult circadian behavior to a developmental requirement for CLK/CYC function beyond its immediate role in maintaining daily time keeping. The requirement for CLK/CYC activity, but not clock function per se may indicate that one or more transcriptional CLK/CYC targets play a role in enabling adult circadian locomotor behavior. Such targets would likely be expressed constitutively along with other CLK/CYC-regulated genes in conditionally arrested per01 [timP>per]ts flies, but constitutively down-regulated in circadian arrests due to low CLK/CYC activity.
A central question that remains is what mechanism links developmental CLK/CYC activity to adult circadian behavior. Our experiments indicate that both clock neuron anatomy and the molecular oscillator itself may be involved. Previously published studies of constitutively arrhythmic alleles of the Clk and cyc genes have documented a reduction in PDF expression as well as neuro-anatomical defects in the LNvs [34] that could be associated with a developmental role for the CLK/CYC transcription factor. By selectively blocking transgenic rescue of cyc01 in the PDF-expressing clock neurons we show, here, that the reduction of PDF expression and PDF-positive dorsal projections from the s-LNvs is a cell-type specific phenotype. PDF is known to play an important role in mediating clock-controlled behavior in both LD and DD conditions. The PDF-producing s-LNvs project towards the dorsal protocerebrum as do DN1, DN2, DN3, and LNd clock neurons, suggesting that the dorsal s-LNv projections may play an important part in signaling across the neural clock circuits [41]. In this context, it may be relevant that expression of the PDF RECEPTOR in ‘E’ cells, a subset of clock neurons including DN1s and LNds [31], has been associated with circadian control of locomotor activity [42]. Moreover, the axonal terminals of the dorsal s-LNv projections undergo clock-controlled rhythms in remodeling that may play a role in circadian signaling [43]. Nevertheless, the observed developmental requirement for CLK/CYC activity also appears to involve mechanisms other than PDF-mediated signaling for the following reasons. First, developmental over-expression of PER resulted in persistent adult arrhythmia, but did not lead to a loss of PDF-positive dorsal projections from the s-LNvs (see Figure S8). Second, while developmental suppression of CLK/CYC activity uniformly affected the behavior of adult flies (Figure 4, Figure 7, Figures S6, S7, S10) constitutive depletion of CYC from the PDF-expressing neurons resulted in a variable phenotype in the s-LNv dorsal projections (see Figure 9C). Third, the light/dark activity pattern of cyc01 (elav-Pdf)>cyc flies (Figure 8A, Figure S11A) was strikingly different from that of Pdf01 flies or flies from which the PDF-expressing cells have been ablated [32], suggesting that PDF signaling persisted in cyc-depleted LNvs in spite of the defects in PDF-positive dorsal projections.
In principle, neuro-anatomical defects affecting intercellular connectivity rather than cell-autonomous clock function could lead to behavioral phenotypes due to asynchrony among the clock neurons or the loss of output signals. Indeed, apparent separation of molecular and behavioral phenotypes has been reported previously for genetic manipulation of CLK/CYC function [44], [45]. It may be particularly relevant that rescue of the per01 phenotype in the PDF-expressing clock neurons restores rhythmic behavior [46], while rescue of cyc01 in the same cells restores molecular, but not behavioral rhythms [45]. However, our experimental results also provide support for developmental phenotypes at the level of the adult molecular clock circuits. Our immunofluorescence expression analyses indicated that the molecular clock circuits in the adult PDF-expressing clock neurons were affected by developmental over-expression of PER. PDF-expressing LNvs in adults that were behaviorally arrhythmic due to developmental PER over-expression exhibited adult PER expression with an altered daily profile, but not necessarily at excessively high levels. Future studies may determine the degree to which neuro-anatomical and molecular phenotypes are linked and help determine the effect of intercellular connectivity in the neural clock circuit on the function of molecular circadian rhythms in individual clock neurons.
Materials and Methods
Drosophila stocks
Flies were raised on standard yeast cornmeal agar food either at ambient temperature (observed to range between 22°C and 24°C) or other experimental temperatures as specified. The conditional per01 rescue flies indicated as per01 [timP>per]ts in Figure 1, Figure 2 and Figures S1, S2, S3, S5 consisted of male and female y per01 w; tim(UAS)-Gal4/tubPGal80ts; UAS-per/+ offspring from a cross between stable lines y per01 w; tim(UAS)-Gal4 and y per01 w; tubPGal80ts; UAS-per. These stocks were created by combining the previously described per01 [22], tim(UAS)-Gal4 [9], tubPGal80ts [20], and UAS-per [23] genetic elements. Flies with conditional over-expression of per in clock-bearing cells ([timP>per]ts in Figure 3, Figure 4, Figure 5, and Figures S6, S7, S8) were obtained from a genetically stable y tubPGal80ts w/FM7c; tim(UAS)-Gal4; UAS-per stock as females heterozygous for FM7c and non-FM7c males. The insertion site of the tubPGal80ts transgene in this stock appears to be associated with homozygous female lethality. An X-chromosomal period-lengthening allele present in the genetic background of the original tubPGal80ts stocks was avoided during the creation of the [timP>per]ts stock by recombination with a control y w chromosome. Flies with conditional rescue of cyc01 (cyc01 [elav>cyc]ts in Figure 6, Figure 7 and Figures S9, S10) were obtained from a stable elavC155::Gal4; UAS-cyc/CyO; cyc01 tubPGal80ts stock as males and females heterozygous for CyO. The elavC155::Gal4 [29], UAS-cyc [30], cyc01 [6], and tubPGal80ts [20] elements used to create this stock had al been described previously. The cyc01 rescue line elavC155::Gal4; UAS-cyc/CyO; cyc01 (cyc01 elav>cyc in Figure 8, Figure 9 and Figure S11) was created as a stable stock, whereas the selective cyc01 rescue genotype elavC155::Gal4; UAS-cyc/Pdf-Gal80; cyc01 and the unrescued control genotype elavC155::Gal4; CyO/Pdf-Gal80; cyc01 (respectively, cyc01 (elav-Pdf)>cyc and cyc01 (elav-Pdf)>- in Figure 8, Figure 9 and ) were obtained in offspring from a cross of elavC155::Gal4; UAS-cyc/CyO; cyc01 flies with elavC155::Gal4; Pdf-Gal80; cyc01 flies. The Pdf-Gal80 element used in the latter two genotypes has also been characterized previously [31].
Locomotor behavior assays
Using previously described protocols [47], locomotor activity was monitored for individual adult flies of both genders in glass tubes on standard sugar agar media including 0.07% Tegosept (Genesee Scientific) using the Drosophila Activity Monitoring System (TriKinetics). Experiments were conducted in incubators kept at 70% relative humidity in 12 h L∶ 12 h D or DD conditions using white fluorescent light with an approximate intensity of 450 µW/cm2 during the L condition. Due to lack of space only analyses for female flies are shown in Figure 1BC, Figure 4, Figure 6D–6G, Figure 7, and Figure 8; the corresponding analyses for male flies are found in Figures S1, S6, S9, S10, and S11, respectively.
Statistical analyses of locomotor activity data
Individual, experimental average, and experimental median activity records, as well as periodic activity profiles, and chi-square periodograms were generated using ClockLab Software (ActiMetrics). Actograms (Figure 1BC, Figure 2A, Figure 3B, Figure 6B and 6C, Figure 8A, Figures S1, S3A–S3C, S11A) were double-plotted with a resolution of half-hour intervals. Each row represents a 2-day interval of Zeitgeber Time (ZT, with ZT0 as the time of lights-on; during LD) or Circadian Time (CT; during DD), of which the second day is repeated as the first day on the next row. Chi-square periodograms (Figure 1BC, Figure 3B, Figure 6B and 6C, Figures S1 and S3A–S3C) were used to represent the experimental signal (amplitude) observed for a range of period lengths (τ, x-axis) relative to threshold values associated with a p<0.01 significance (red line). Analyses of the percentages of rhythmic, weakly rhythmic, and arrhythmic flies (Figure 1D, Figure 4A, Figure 6D and 6F, Figure 7A, Figure 8B, Figures S2AC, S3D, S5AC, S6A, S7A, S7B, S7D, S7E, S9A, S9C, S10A, S11B) were based on chi-square periodogram statistics for locomotor activity rhythms of individual flies. For period lengths in the circadian range (∼15–36 h) detected with a significance of p<0.01 relative rhythmic power was calculated by dividing the detected peak amplitude by the significance threshold value at the same period length. Flies were classified based on their values of relative rhythmic power as rhythmic (>1.5) or weakly rhythmic ([1,1.5]) and flies without significant periodicity in the circadian range were considered arrhythmic. Chi-square analyses for association of genotype or experimental protocol with the relative distribution of rhythmic, weakly rhythmic, or arrhythmic behavior were conducted using Microsoft Excel (Microsoft). Next, statistical analyses were performed using SPSS software (IBM) to detect associations between the relative rhythmic power values of rhythmic and weakly rhythmic flies with experimental conditions (Figure 4B, Figure 6E and 6G, Figure 7B, Figures S2BD, S5BD, S6B, S7CF, S9BD, S10B) or genotypes (Figure 8C, Figure S11C). In virtually all cases Levene's test indicated that homogeneous variances could not be assumed. Therefore, the Welch test statistic with Games-Howell post-hoc analysis was used to test for significant differences in relative rhythmic power among different genotypes and treatments. When only two conditions were compared the non-parametric Mann-Whitney rank-sum test was performed. Average (Figure 2A, Figure 6B and 6C, Figure S3A, S3B, S3C) and median (Figure 1B and 1C, Figure 3B, Figure 8A, Figures S1, S11A) activity records that emphasize reproducible features of rhythmic locomotor activity measured in individual flies were created without prior normalization from the raw individual activity records on a point-by-point basis. For representation in illustrative double-plotted actograms we generally used median activity records, which are less susceptible to skewing by outliers and show discrete numbers of events per half-hour bin, but when a better resolution of data with relatively low activity counts was preferred average activity records were used instead. Average daily or circadian activity profiles representing records of median or average activity ± the Standard Error of the Mean (SEM) were generated across included days under entraining (Figure 8A, Figure S11A) or free running conditions (S3, for phase determination in Figure 2B), respectively. The phase of the offset of circadian activity (Figure 2B) was determined from the activity profiles by interpolation as described previously [47]. Error bars throughout the manuscript represent SEM, except in cases where less than three observations were made. The parentheses surrounding individual error bars in Figure 1B (right-hand panel), Figure S7F, and Figure S9B indicate that these represent the range of two observations, instead.
Northern analysis
Extraction of total RNA from approximately 100 µl adult heads per time point using the guanidinium thiocyanate/cesium chloride method and subsequent Northern analysis were conducted according to previously published protocols [48], [49]. Quantitative analysis of the radioactive signals on the blots was conducted with a Storm 840 Phosphorimager (GE healthcare) and the resulting data was graphed using Microsoft Excel (Microsoft Corporation). Five independent time course experiments were conducted addressing the transcript responses observed in the adult head upon transfer of per01 [timP>per]ts flies from restrictive to permissive conditions. A representative example is shown in Figure S4A.
Quantitative reverse transcriptase PCR (qRT-PCR) analysis
Flies for the conditions of interest were harvested onto ice, and either adult heads or brains were dissected on a chilled platform and transferred to guanidinium thiocyanate buffer. DNAse I-digested total RNA was obtained from the heads or brains using the RNAqueous4PCR kit (Ambion). Aliquots of the RNA samples were then analyzed with the SuperScript III Platinum SYBR Green One-Step qPCR Kit (Invitrogen) using experimental primer pairs designed to specifically amplify fragments of the circadian per, tim, vri, and cwo transcripts, the transgenic UAS-per transcript or the rp49 control transcript. Expression levels measured on a SmartCycler system (Cepheid) relative to rp49 were determined using the comparative Cycle threshold (Ct) method [50].
Immunofluorescence analysis
Adult brains were dissected, fixed, and stained for immunofluorescence analysis according to standard protocols [51]. Imaging was conducted with a spinning disk confocal microscope. Brains from [timP>per]ts flies raised under restrictive versus permissive conditions were probed with primary antibodies against PDF (mouse monoclonal; DSHB) as well as PER (rabbit polyclonal; [52]), whereas brains from cyc01 elav>cyc and cyc01 (elav-Pdf)>cyc flies were stained with antibodies to PDF as well as PDP1 (rabbit polyclonal;[26]) and then visualized with fluorescently labeled secondary antibodies (Alexa-488 for PDF, Alexa-568 for PER or PDP1).
Supporting Information
Zdroje
1. AlladaRChungBY 2010 Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol 72 605 624
2. ZhangEEKaySA 2010 Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol 11 764 776
3. AlladaRWhiteNESoWVHallJCRosbashM 1998 A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93 791 804
4. LimCChungBYPitmanJLMcGillJJPradhanS 2007 Clockwork orange encodes a transcriptional repressor important for circadian-clock amplitude in Drosophila. 17 1082 1089
5. DarlingtonTKWager-SmithKCerianiMFStaknisDGekakisN 1998 Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 280 1599 1603
6. RutilaJESuriVLeMSoWVRosbashM 1998 CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93 805 814
7. MatsumotoAUkai-TadenumaMYamadaRGHoulJUnoKD 2007 A functional genomics strategy reveals clockwork orange as a transcriptional regulator in the Drosophila circadian clock. 21 1687 1700
8. KadenerSStoleruDMcDonaldMNawatheanPRosbashM 2007 Clockwork Orange is a transcriptional repressor and a new Drosophila circadian pacemaker component. 21 1675 1686
9. BlauJYoungMW 1999 Cycling vrille expression is required for a functional Drosophila clock. Cell 99 661 671
10. KlossBPriceJLSaezLBlauJRothenfluhA 1998 The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94 97 107
11. PriceJLBlauJRothenfluhAAbodeelyMKlossB 1998 double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94 83 95
12. KlossBRothenfluhAYoungMWSaezL 2001 Phosphorylation of period is influenced by cycling physical associations of double-time, period, and timeless in the Drosophila clock. Neuron 30 699 706
13. ZerrDMHallJCRosbashMSiwickiKK 1990 Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci 10 2749 2762
14. CurtinKDHuangZJRosbashM 1995 Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron 14 365 372
15. LeeCBaeKEderyI 1999 PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: a basis for circadian transcription. Mol Cell Biol 19 5316 5325
16. ShaferOTRosbashMTrumanJW 2002 Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J Neurosci 22 5946 5954
17. GlossopNRHoulJHZhengHNgFSDudekSM 2003 VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron 37 249 261
18. EwerJRosbashMHallJC 1988 An inducible promoter fused to the period gene in Drosophila conditionally rescues adult per-mutant arrhythmicity. Nature 333 82 84
19. EwerJHamblen-CoyleMRosbashMHallJC 1990 Requirement for period gene expression in the adult and not during development for locomotor activity rhythms of imaginal Drosophila melanogaster. J Neurogenet 7 31 73
20. McGuireSELePTOsbornAJMatsumotoKDavisRL 2003 Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302 1765 1768
21. BrandAHPerrimonN 1993 Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. 118 401 415
22. KonopkaRJBenzerS 1971 Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 68 2112 2116
23. YangZSehgalA 2001 Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron 29 453 467
24. BaeKLeeCSidoteDChuangKYEderyI 1998 Circadian regulation of a Drosophila homolog of the mammalian Clock gene: PER and TIM function as positive regulators. Mol Cell Biol 18 6142 6151
25. SehgalARothenfluh-HilfikerAHunter-EnsorMChenYMyersMP 1995 Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science 270 808 810
26. CyranSABuchsbaumAMReddyKLLinMCGlossopNR 2003 vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112 329 341
27. Claridge-ChangAWijnenHNaefFBoothroydCRajewskyN 2001 Circadian regulation of gene expression systems in the Drosophila head. Neuron 32 657 671
28. PriceJLDembinskaMEYoungMWRosbashM 1995 Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. Embo J 14 4044 4049
29. LinDMGoodmanCS 1994 Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron 13 507 523
30. TanoueSKrishnanPKrishnanBDryerSEHardinPE 2004 Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol 14 638 649
31. StoleruDPengYAgostoJRosbashM 2004 Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431 862 868
32. RennSCParkJHRosbashMHallJCTaghertPH 1999 A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99 791 802
33. ShaferOTTaghertPH 2009 RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: the anatomical basis of a neuropeptide's circadian functions. PLoS ONE 4 e8298 doi:10.1371/journal.pone.0008298
34. ParkJHHelfrich-ForsterCLeeGLiuLRosbashM 2000 Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A 97 3608 3613
35. HardinPEHallJCRosbashM 1990 Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343 536 540
36. SmithRFKonopkaRJ 1982 Effects of dosage alterations at the per locus on the period of the circadian clock of Drosophila. Molecular & general genetics 185 30 36
37. BayliesMKBargielloTAJacksonFRYoungMW 1987 Changes in abundance or structure of the per gene product can alter periodicity of the Drosophila clock. Nature 326 390 392
38. MartinekSYoungMW 2000 Specific genetic interference with behavioral rhythms in Drosophila by expression of inverted repeats. Genetics 156 1717 1725
39. MenetJSAbruzziKCDesrochersJRodriguezJRosbashM 2010 Dynamic PER repression mechanisms in the Drosophila circadian clock: from on-DNA to off-DNA. Genes Dev 24 358 367
40. KanekoMParkJHChengYHardinPEHallJC 2000 Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol 43 207 233
41. Helfrich-ForsterCShaferOTWulbeckCGrieshaberERiegerD 2007 Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol 500 47 70
42. LearBCZhangLAlladaR 2009 The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol 7 e1000154 doi:10.1371/journal.pbio.1000154
43. FernandezMPBerniJCerianiMF 2008 Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol 6 e69 doi:10.1371/journal.pbio.0060069
44. AlladaRKadenerSNandakumarNRosbashM 2003 A recessive mutant of Drosophila Clock reveals a role in circadian rhythm amplitude. Embo J 22 3367 3375
45. PengYStoleruDLevineJDHallJCRosbashM 2003 Drosophila free-running rhythms require intercellular communication. PLoS Biol 1 e13 doi:10.1371/journal.pbio.0000013
46. GrimaBChelotEXiaRRouyerF 2004 Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431 869 873
47. CurrieJGodaTWijnenH 2009 Selective entrainment of the Drosophila circadian clock to daily gradients in environmental temperature. BMC biology 7 49
48. WijnenHNaefFYoungMW 2005 Molecular and statistical tools for circadian transcript profiling. Methods Enzymol 393 341 365
49. BoothroydCEWijnenHNaefFSaezLYoungMW 2007 Integration of light and temperature in the regulation of circadian gene expression in Drosophila. 3 e54
50. SchmittgenTDLivakKJ 2008 Analyzing real-time PCR data by the comparative C(T) method. Nature protocols 3 1101 1108
51. WuJSLuoL 2006 A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nature protocols 1 2110 2115
52. StanewskyRFrischBBrandesCHamblen-CoyleMJRosbashM 1997 Temporal and spatial expression patterns of transgenes containing increasing amounts of the Drosophila clock gene period and a lacZ reporter: mapping elements of the PER protein involved in circadian cycling. J Neurosci 17 676 696
Štítky
Genetika Reprodukční medicína
Článek Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal MutationsČlánek Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene ExpressionČlánek Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary IntegrityČlánek A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with ExpressionČlánek Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line PedigreeČlánek Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 7
-
Všechny články tohoto čísla
- Gene-Based Tests of Association
- The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
- Variants in and Underlie Natural Variation in Translation Termination Efficiency in
- SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
- Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations
- Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer
- Identification of a Mutation Associated with Fatal Foal Immunodeficiency Syndrome in the Fell and Dales Pony
- Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
- An Epigenetic Switch Involving Overlapping Fur and DNA Methylation Optimizes Expression of a Type VI Secretion Gene Cluster
- Recombination and Population Structure in
- A Rice Plastidial Nucleotide Sugar Epimerase Is Involved in Galactolipid Biosynthesis and Improves Photosynthetic Efficiency
- A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets
- Colon Stem Cell and Crypt Dynamics Exposed by Cell Lineage Reconstruction
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Interactions between Glucocorticoid Treatment and Cis-Regulatory Polymorphisms Contribute to Cellular Response Phenotypes
- Translation Reinitiation Relies on the Interaction between eIF3a/TIF32 and Progressively Folded -Acting mRNA Elements Preceding Short uORFs
- DAF-12 Regulates a Connected Network of Genes to Ensure Robust Developmental Decisions
- Adult Circadian Behavior in Requires Developmental Expression of , But Not
- Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary Integrity
- Proteins in the Nutrient-Sensing and DNA Damage Checkpoint Pathways Cooperate to Restrain Mitotic Progression following DNA Damage
- Complex Evolutionary Events at a Tandem Cluster of Genes Resulting in a Single-Locus Genetic Incompatibility
- () and Its Regulated Homeodomain Gene Mediate Abscisic Acid Response in
- A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
- LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs
- Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications
- The Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
- Somatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
- Molecular Mechanisms Generating and Stabilizing Terminal 22q13 Deletions in 44 Subjects with Phelan/McDermid Syndrome
- Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line Pedigree
- Mechanisms of Chromosome Number Evolution in Yeast
- Regulatory Cross-Talk Links Chromosome II Replication and Segregation
- Ancestral Genes Can Control the Ability of Horizontally Acquired Loci to Confer New Traits
- Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
- Rare and Common Regulatory Variation in Population-Scale Sequenced Human Genomes
- The Epistatic Relationship between BRCA2 and the Other RAD51 Mediators in Homologous Recombination
- Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy
- NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
- Araucan and Caupolican Integrate Intrinsic and Signalling Inputs for the Acquisition by Muscle Progenitors of the Lateral Transverse Fate
- Pathologic and Phenotypic Alterations in a Mouse Expressing a Connexin47 Missense Mutation That Causes Pelizaeus-Merzbacher–Like Disease in Humans
- Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination
- Epistatic Relationships in the BRCA1-BRCA2 Pathway
- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium
- Fine Mapping of Five Loci Associated with Low-Density Lipoprotein Cholesterol Detects Variants That Double the Explained Heritability
- CHD1 Remodels Chromatin and Influences Transient DNA Methylation at the Clock Gene
- Nonlinear Fitness Landscape of a Molecular Pathway
- Genome-Wide Scan Identifies , , and as Novel Risk Loci for Systemic Sclerosis
- Quantitative and Qualitative Stem Rust Resistance Factors in Barley Are Associated with Transcriptional Suppression of Defense Regulons
- A Systematic Screen for Tube Morphogenesis and Branching Genes in the Tracheal System
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Gene-Based Tests of Association
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



