-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
Reklama—What Makes the Species a Ubiquitous Human Fungal Pathogen?
article has not abstract
Published in the journal: . PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003743
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003743Summary
article has not abstract
Introduction
Aspergillus fumigatus, the major cause of life threatening invasive aspergillosis (IA), is a ubiquitous saprophytic fungus to which humans are exposed daily in most parts of the world. The infection is initiated by inhalation of conidia, which are cleared quickly in a normal host but can cause invasive disease in immunocompromised individuals [1], [2]. The following features make A. fumigatus a ubiquitous pathogen: 1) survival and growth in a wide range of environmental conditions, 2) effective dispersal in the air, 3) physical characteristics that allow conidia to reach the distal airways, and 4) swift adaptability to the host environment. The biology, pathogenesis, molecular biology, and virulence factors of A. fumigatus have been exhaustively reviewed [2]–[8]. This brief article focuses on how A. fumigatus is equipped with the features necessary for a ubiquitous pathogen.
Aspergillus fumigatus Is Equipped to Survive and Propagate Successfully under a Wide Range of Environmental Conditions
In most parts of the world, Aspergillus fumigatus can be isolated from a wide variety of substrates throughout the year. Although A. fumigatus grows optimally at 37°C and a pH 3.7 to 7.6, it can be isolated wherever decaying vegetation and soil reach temperatures range between 12° and 65°C [9] and the pH ranges between 2.1–8.8 [10]. A. fumigatus was found to be the dominant fungus in garden and greenhouse soil that comprised 35 to 70 percent of the total numbers of colony-forming fungi [10]. As an efficient recycler in nature, A. fumigatus possesses a versatile metabolism that meets its nutritional requirements under different environmental conditions [11]. The presence of numerous glycosylhydrolases [6] and a group of extracellular proteinases in the A. fumigatus genome attest to the ability of the fungus to grow by degradation of polysaccharides from plant cell walls and acquire nitrogen sources made available by degradation of proteinacious substrates [8]. Self-heating compost heaps are major environmental sources of A. fumigatus due to its pronounced thermotolerance. One study found 100,000 colony-forming units (cfu)/gram/dry weight of compost [12], and compost piles of chipped leaves and branches may yield massive and almost pure cultures of A. fumigatus [1]. The thermotolerance of A. fumigatus is even more remarkable in the ascospores, the propagules produced in the sexual cycle. The ascospores of A. fumigatus (Figure 1A) are protected by an extraordinarily thick wall (Figure 1B) compared to those of other aspergilli such as A. nidulans [13]. The ascospores of A. fumigatus germinate after heating at 70°C for 30 min [14] (Figure 1C) and should survive at core temperatures of the compost pile that can reach ≥70°C [2].
Fig. 1. Aspergillus fumigatus ascospores. 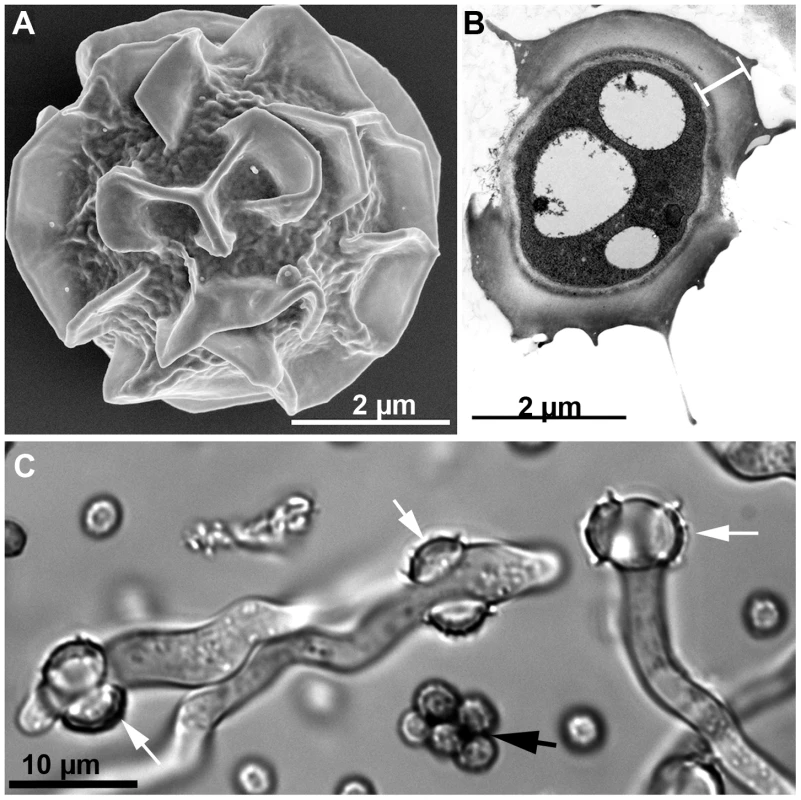
A) SEM image of an ascospore produced by mating between two compatible strains. Courtesy of Bryan Hansen. B) TEM image of an ascospore cross-section showing an unusually thick wall (white bar) composed of an electron-dense inner wall covered by a thick outer wall. Courtesy of Mones Abu-Asab. C) DIC image of germinating ascospores (white arrows) and dead conidia (black arrow) after 30 min incubation at 70°C. Although A. fumigatus fails to grow at temperatures below 12°C, its conidia can tolerate stresses imposed by freezing for prolonged periods. Depending on the strain, conidia can survive in liquid nitrogen for up to 18 years [9]. Although a few genes associated with fungal growth at ≥48°C have been characterized, the genetic systems involved in survival and growth under extreme temperatures remain unidentified [15]. A. fumigatus conidia can also tolerate dehydration for prolonged periods, surviving for more than 60 years when lyophilized, and the conidia that had been maintained in anhydrous silica gel survived for more than 25 years (unpublished data).
The wide distribution of A. fumigatus in nature may also be due to the presence of successful defense systems such as the production of potent secondary metabolites and efflux pumps. The A. fumigatus genome contains 22 secondary metabolism gene clusters [11] and 16 different secondary metabolites have been identified [16], including gliotoxin, a broad range antimicrobial [17]. A. fumigatus possesses a higher number of ABC transporters than its close genetic relative, Aspergillus fischerianus [15]. The A. fumigatus genome is also rich in specific enzymes such as catalases, superoxide dismutases, and glutathione transferases for the detoxification of reactive oxygen species (ROS) [8], [18]. All these features equip A. fumigatus to survive and propagate in conditions that are detrimental to a broad range of other environmental organisms.
Aspergillus fumigatus Conidia Are Dispersed More Efficiently in the Air Than Those of Most Other Molds
Aspergillus fumigatus conidia accumulate 1,8-dihydroxynaphthalene melanin in their cell wall, have a blue-green color [19], [20], and are notorious for their high dispersibility. The slightest air current can cause conidia to disperse due to their remarkable hydrophobicity, and these airborne conidia are protected from ultraviolet irradiation due to the melanin in their cell wall [20]. One study has estimated the emission rate of A. fumigatus conidia from an undisturbed compost pile to be 8–11×103 cfu/m2/s at the mean wind speed of 1 m/s [21], which indicates how efficiently conidia are dispersed with the slightest agitation. Figure 2A shows an aerosol cloud over a disturbed compost pile. A majority of the microbial growth on a plate of agar medium briefly exposed to the air at the site was that of A. fumigatus (Figure 2B). Although all fungal spores produced on aerial hyphae or conidiophores are hydrophobic, the degree varies from mild to highly hydrophobic [22] which impacts the efficiency of spore dispersibility. A. fumigatus conidia are considerably more hydrophobic than those of other aspergilli such as A. nidulans. This requires more caution in the handling of A. fumigatus cultures than other fungi to prevent contamination of surrounding areas in the laboratory (Figure 2C, D). Conidial hydrophobicity is conferred by the surface rodlet layer encoded by the rodA gene [23]. In addition to dispersal of airborne conidia, conidia imbedded in soil may also be effectively transported from one place to another by swarming soil bacteria such as Paenibacillus vortex. P. vortex facilitates the dispersal of A. fumigatus more efficiently than other fungal species that have similarly sized conidia such as Penicillium expansum or P. citrinum [24]. Conidial surface proteins are crucial for the passive dispersal of A. fumigatus by the bacteria since proteinase K treatment of conidia abolished the conidia-bacterial interaction. Undoubtedly, A. fumigatus conidia are also being passively dispersed via rodents, insects, and worms but the impact of A. fumigatus spread by these means has not been studied.
Fig. 2. Dispersibility of A. fumigatus conidia. 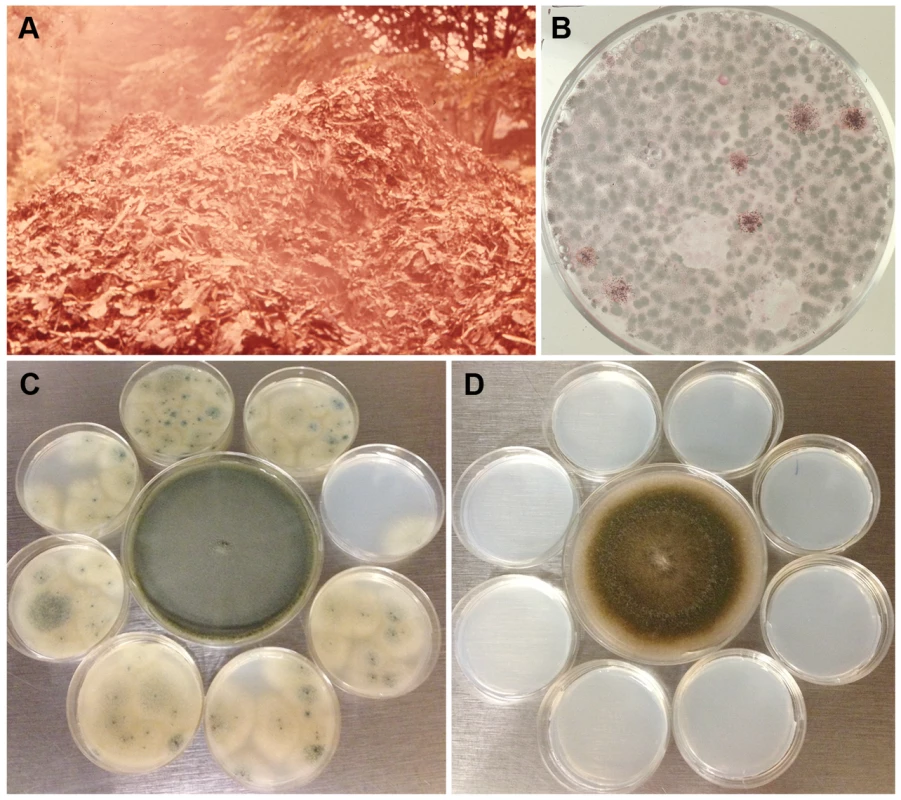
A) A cloud of aerosol released in the air after turning of a compost pile located in Maryland, USA. B) A malt extract agar plate exposed to the air for a minute at the site and incubated for a few days at 37°C grew predominantly A. fumigatus colonies (both pictures were taken by the late Dr. Chester Emmons). C) Eight small sterile agar plates of Aspergillus minimal medium were placed around a seven-day-old culture of A. fumigatus strain B-5233 (center) in a class 2 biosafety cabinet. In the absence of air flow the lids of all the plates were removed for 24 h. The small plates were then incubated for three days at 37°C. D) The same procedure as in C except that the small plates were exposed to the culture of a ten-day-old A. nidulans strain RYC13B (center). A. fumigatus conidia dispersed to the surrounding small agar plates while none was evident for the A. nidulans strain. Physical Characteristics of Conidia That Contribute to Respiratory Tract Disease
Fungal spores account for a significant proportion of the aerosol particle mass that the human respiratory system is exposed to daily. Airborne fungal spores exist in various sizes and any spore with a size of ≥5.0 µm (diameter) is too large to reach the lower airways [25] where systemic infection is primarily initiated. A. fumigatus conidia are globose to subglobose with a size (2.0–3.0 µm in diameter with extremes up to 3.5 µm) adequate to bypass mucociliary clearance and reach the lower airways. The average adult inhales more than 100 A. fumigatus conidia daily since the conidial concentration in the air indoors or outdoors is estimated to be 1–100 conidia/m3 [4]. Conidial size does not change significantly with increased relative humidity from 30% to 90% [26], and so airborne conidia maintain an optimum size for reaching the lower airways regardless of the relative humidity. Melanin in the conidial wall offers protection from ROS while also enabling resistance to lysis by host cells [4]. A. fumigatus conidial surface contains more exposed negatively charged sialic acid residues than other Aspergillus species and sialic acid partly mediates binding to basal lamina proteins of the host [27]. A. fumigatus conidia, therefore, may adhere to the epithelium of airways and alveoli more effectively than other fungal species with similarly sized airborne spores.
Aspergillus fumigatus Conidia Germinate and Adapt Readily to the Immunocompromised Host Environment
Aspergillus fumigatus conidia that reach the alveoli are unable to withstand the immune assault mounted by normal hosts because the fungus lacks specialized virulence factors [6]. However, patients who are undergoing organ transplantation, cancer chemotherapy, or have chronic granulomatous disease (CGD) as an underlying condition are highly susceptible to infection by the fungus because the inhaled conidia can efficiently adapt their physiology to the altered host environment. A review of 146 autopsies at the National Institutes of Health over a 22-year period showed no firm link between hospital exposure and an increased incidence of invasive aspergillosis. There was, however, a clear link between cancer chemotherapy regimens and increased incidence [28]. This indicates that adaptability of A. fumigatus to the human environment, though successful, is secondary to the host immune status. Inhaled conidia readily germinate at the mammalian body temperature since 37°C is the optimum temperature for both germination and growth. Conidia shed the hydrophobin layer and swell in 4 h to germinate into short hyphae by 6–8 h at 37°C in vitro as well as in immunocompromised mammalian tissue [29]. During this early growth period, A. fumigatus responds to the stress imposed by the host environment by utilizing a highly coordinated gene expression program that enables adaptation to iron limitation, nitrogen and glucose deprivation, alkaline stress, and other unfavorable conditions [29]. One of the features during early infection in mice is the activation of gliotoxin biosynthesis [29]. Since gliotoxin is immunosuppressive and cytocidal [17], it can be speculated that the fungus benefits from nutrients released by the gliotoxin-destroyed host cells. Presence of the toxin in sera of patients infected with A. fumigatus suggests its involvement in the adaptation to the host environment [17]. How efficiently A. fumigatus cells can sense and respond to the host environment has been shown by clear differences in transcriptional profiles between conidia exposed to the neutrophils of normal host compared to those from patients with CGD, which are defective in ROS production [30]. All these features indicate that, being equipped to grow in a wide range of unfavorable conditions in nature, A. fumigatus finds the immunocompromised host environment just another adverse condition to which it can successfully adapt.
Concluding Remarks
Among the over 200 species of Aspergillus, A. fumigatus is the best at meeting the four features discussed in this review. Since innate immunity protects against Aspergillus, the reason for the wide spread of IA caused by A. fumigatus is due to the global distribution of both the fungus and an increase in susceptible hosts. However, only a portion of the high-risk population, such as those with stem cell transplantation or CGD, develop IA despite daily exposure to the fungus. This suggests that a genetic risk associated with aspergillosis may exist in IA patients in addition to their underlying immunosuppressive condition. Although several studies on the role of immune-related gene SNPs of both donors and recipients of stem cell transplant have been conducted, the genetic factors that confer increased susceptibility to IA have yet to be validated. In light of the high fatality rate of IA, identification of such factors would improve prophylactic measures against not only IA but invasive infection by other mold species.
Zdroje
1. Kwon-Chung KJ, Bennett JE (1992) Medical mycology. Philadelphia: Lea & Febiger. 823 p.
2. LatgéJ-P (1999) Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12 : 310–350.
3. LatgéJ-P (2001) The pathobiology of Aspergillus fumigatus. Trends Microbiol 9 : 382–389.
4. BrakhageAA, LangfelderK (2002) Menacing mold: the molecular biology of Aspergillus fumigatus. Annu Rev Microbiol 56 : 433–455.
5. DagenaisTR, KellerNP (2009) Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 22 : 447–465.
6. TekaiaF, LatgéJ-P (2005) Aspergillus fumigatus: saprophyte or pathogen? Curr Opin Microbiol 8 : 385–392.
7. HohlTM, FeldmesserM (2007) Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell 6 : 1953–1963.
8. AbadA, Fernandez-MolinaJV, BikandiJ, RamirezA, MargaretoJ, et al. (2010) What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27 : 155–182.
9. Kozakiewicz Z, Smith D (1994) Physiology of Aspergillus. In: Smith JE, editor. Biotechnology handbooks - 7: Aspergillus. New York: Plenum Press. pp. 23–40.
10. JensenHL (1931) The fungus flora of the soil. Soil Science 31 : 123–158.
11. GibbonsJG, BeauvaisA, BeauR, McGaryKL, LatgéJ-P, et al. (2012) Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot Cell 11 : 68–78.
12. AnastasiA, VareseGC, MarchisioVF (2005) Isolation and identification of fungal communities in compost and vermicompost. Mycologia 97 : 33–44.
13. Egel-MitaniM, OlsonLW, EgelR (1982) Meiosis in Aspergillus nidulans: another example for lacking synaptonemal complexes in the absence of crossover interference. Hereditas 97 : 179–187.
14. SuguiJA, LosadaL, WangW, VargaJ, NgamskulrungrojP, et al. (2011) Identification and characterization of Aspergillus fumigatus ‘supermater’ pair. mBio 2: e00234–11 doi:10.1128/mBio.00234-11
15. NiermanWC, MayG, KimHS, AndersonMJ, ChenD, et al. (2005) What the Aspergillus genomes have told us. Med Mycol 43 Suppl 1: S3–5.
16. Frisvad JC, Samson RA (1990) Chemotaxonomy and morphology of Aspergillus fumigatus and related taxa. In: Samson RA, Pitt JI, editors. Modern concepts in Penicillium and Aspergillus classification. New York: Plenum Press. pp. 201–208.
17. SuguiJA, PardoJ, ChangYC, ZaremberKA, NardoneG, et al. (2007) Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 6 : 1562–1569.
18. BurnsC, GeraghtyR, NevilleC, MurphyA, KavanaghK, et al. (2005) Identification, cloning, and functional expression of three glutathione transferase genes from Aspergillus fumigatus. Fungal Genet Biol 42 : 319–327.
19. TsaiHF, WheelerMH, ChangYC, Kwon-ChungKJ (1999) A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 181 : 6469–6477.
20. BrakhageAA, LiebmannB (2005) Aspergillus fumigatus conidial pigment and cAMP signal transduction: significance for virulence. Med Mycol 43: S75–82.
21. TahaMP, PollardSJ, SarkarU, LonghurstP (2005) Estimating fugitive bioaerosol releases from static compost windrows: feasibility of a portable wind tunnel approach. Waste Manag 25 : 445–450.
22. BeeverRE, DempseyGP (1978) Function of rodlets on the surface of fungal spores. Nature 272 : 608–610.
23. BayryJ, AimaniandaV, GuijarroJI, SundeM, LatgéJ-P (2012) Hydrophobins—unique fungal proteins. PLoS Pathog 8: e1002700 doi:10.1371/journal.ppat.1002700
24. InghamCJ, KalismanO, FinkelshteinA, Ben-JacobE (2011) Mutually facilitated dispersal between the nonmotile fungus Aspergillus fumigatus and the swarming bacterium Paenibacillus vortex. Proc Natl Acad Sci U S A 108 : 19731–19736.
25. CohenJ, PostmaDS, DoumaWR, VonkJM, De BoerAH, et al. (2011) Particle size matters: diagnostics and treatment of small airways involvement in asthma. Eur Respir J 37 : 532–540.
26. ReponenT, WillekeK, UleviciusV, ReponenA, GrinshpunSA (1996) Effect of relative humidity on the aerodynamic diameter and respiratory deposition of fungal spores. Atmos Environ 30 : 3967–3974.
27. WasylnkaJA, SimmerMI, MooreMM (2001) Differences in sialic acid density in pathogenic and non-pathogenic Aspergillus species. Microbiology 147 : 869–877.
28. HospenthalDR, Kwon-ChungKJ, BennettJE (1998) Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol 36 : 165–168.
29. McDonaghA, FedorovaND, CrabtreeJ, YuY, KimS, et al. (2008) Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog 4: e1000154 doi:10.1371/journal.ppat.1000154
30. SuguiJA, KimHS, ZaremberKA, ChangYC, GallinJI, et al. (2008) Genes differentially expressed in conidia and hyphae of Aspergillus fumigatus upon exposure to human neutrophils. PLoS ONE 3: e2655 doi:10.1371/journal.pone.0002655
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



