-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSemen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
The mucosal events of HIV transmission have been extensively studied, but the role of infected cells present in the genital and rectal secretions, and in the semen, in particular, remains a matter of debate. As a prerequisite to a thorough in vivo investigation of the early transmission events through infected cells, we characterized in detail by multi-parameter flow cytometry the changes in macaque seminal leukocytes during SIVmac251 infection, focusing on T cells, macrophages and dendritic cells. Using immunocytofluorescence targeting SIV proteins and real-time quantitative PCR targeting SIV DNA, we investigated the nature of the infected cells on sorted semen leukocytes from macaques at different stages of infection. Finally, we cocultured semen CD4+ T cells and macrophages with a cell line permissive to SIV infection to assess their infectivity in vitro. We found that primary infection induced strong local inflammation, which was associated with an increase in the number of leukocytes in semen, both factors having the potential to favor cell-associated virus transmission. Semen CD4+ T cells and macrophages were productively infected at all stages of infection and were infectious in vitro. Lymphocytes had a mucosal phenotype and expressed activation (CD69 & HLA-DR) and migration (CCR5, CXCR4, LFA-1) markers. CD69 expression was increased in semen T cells by SIV infection, at all stages of infection. Macrophages predominated at all stages and expressed CD4, CCR5, MAC-1 and LFA-1. Altogether, we demonstrated that semen contains the two major SIV-target cells (CD4+ T cells and macrophages). Both cell types can be productively infected at all stages of SIV infection and are endowed with markers that may facilitate transmission of infection during sexual exposure.
Published in the journal: . PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003810
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003810Summary
The mucosal events of HIV transmission have been extensively studied, but the role of infected cells present in the genital and rectal secretions, and in the semen, in particular, remains a matter of debate. As a prerequisite to a thorough in vivo investigation of the early transmission events through infected cells, we characterized in detail by multi-parameter flow cytometry the changes in macaque seminal leukocytes during SIVmac251 infection, focusing on T cells, macrophages and dendritic cells. Using immunocytofluorescence targeting SIV proteins and real-time quantitative PCR targeting SIV DNA, we investigated the nature of the infected cells on sorted semen leukocytes from macaques at different stages of infection. Finally, we cocultured semen CD4+ T cells and macrophages with a cell line permissive to SIV infection to assess their infectivity in vitro. We found that primary infection induced strong local inflammation, which was associated with an increase in the number of leukocytes in semen, both factors having the potential to favor cell-associated virus transmission. Semen CD4+ T cells and macrophages were productively infected at all stages of infection and were infectious in vitro. Lymphocytes had a mucosal phenotype and expressed activation (CD69 & HLA-DR) and migration (CCR5, CXCR4, LFA-1) markers. CD69 expression was increased in semen T cells by SIV infection, at all stages of infection. Macrophages predominated at all stages and expressed CD4, CCR5, MAC-1 and LFA-1. Altogether, we demonstrated that semen contains the two major SIV-target cells (CD4+ T cells and macrophages). Both cell types can be productively infected at all stages of SIV infection and are endowed with markers that may facilitate transmission of infection during sexual exposure.
Introduction
More than 33 million people are currently living with HIV/AIDS worldwide. Almost 80% of new infections occur through sexual intercourse. Semen is thus one of the major factors in HIV transmission. Most studies on HIV sexual transmission have focused on the role of cell-free particles, and the underlying mechanisms of transmission have been extensively described. Moreover, most attempts to develop HIV vaccines and microbicides have focused on blocking cell-free virus transmission. The rectal and vaginal exposure of macaques to free SIV particles has been widely used in studies of the sexual transmission of HIV and evaluations of the efficacy of prophylactic strategies [1], [2]. Most challenge studies use viruses produced in vitro in the culture supernatants of human and nonhuman primate (NHP) cells.
However, genital secretions, including semen, contain HIV in both cell-free and cell-associated forms. The prevalence of proviral DNA in semen ranges from 21% to 65% in HIV-infected patients, and high levels of viral DNA have been associated with high leukocyte counts in semen [3]. Moreover, leukocytospermia, the incidence of which is higher in seropositive than in seronegative individuals [3], has been associated with a high degree of semen infectiousness [4]. This suggests that semen leukocytes may also be an important factor to be taken into account when considering the mucosal transmission of HIV. In the first few years of the HIV epidemic, the hypothesis that HIV could be efficiently transmitted by infected cells, through direct cell-to-cell contact, was proposed, as most retroviruses spread in this way [5].
It is now clearly established that HIV does indeed spread through cell contacts [6], [7]. Moreover, in reconstituted mucosal models, HIV-infected cells efficiently transmit the virus across epithelial barriers. If the epithelial surface is intact, viral translocation involves transcytosis, which is favored by viral synapses between productive cells and epithelial cells [8], [9], or direct uptake by local target cells, such as Langerhans cells, macrophages and intraepithelial T cells [10], [11]. We and others have demonstrated that the vaginal inoculation of humanized mice and macaques with infected leukocytes induces systemic infection. Inoculated CFSE-labeled cells were found in the vaginal tissue and the draining lymph nodes within 21 h, suggesting that these cells were able to migrate through the mucosal epithelium and to disseminate rapidly [12], [13].
We have reported that macrophages and CD4+ T cells present in the various secretory glands of the male NHP genital tract may efficiently seed the semen with free viral particles and infected cells [14]. However, semen from NHP is difficult to collect and process, so previous studies made use of spleen cells from infected animals (our reported work) or ex vivo infected PBMCs, as semen cell surrogates [12], [13], [15]. These cell-associated virus stocks may not be representative of leukocyte-infected populations in semen. Indeed, semen leukocytes, like mucosal cells, may have a distribution and differentiation and activation phenotypes different from those of blood and spleen cells. Therefore, it is of prime importance to thoroughly investigate the nature and characteristics of the potential cell transmitters in the semen of NHP, since this model is unique to decipher in vivo the early events of HIV sexual transmission.
Very few studies have focused on the nature and phenotype of the infected cells present in semen. There is still no formal proof that these cells can transmit HIV across mucosal barriers, and the mechanisms potentially involved have yet to be identified. An understanding of the contribution of semen leukocytes to the sexual transmission of HIV may have significant implications for prevention strategies.
We aimed to carry out a more detailed characterization of the infected cells present in semen, elucidating their role in mucosal transmission, in the experimental infection of macaques with pathogenic SIVmac251 model. We focused on characterization of the semen leukocyte populations, the nature of the infected cells and their capacity to transmit infection in vitro. We demonstrated that all major viral target cells, including CD4+ T cells and macrophages are productively infected at every stage of infection.
Results
Virus shedding in the semen of SIVmac251-infected macaques
We assumed that, similarly to men, macaque semen contains various types of infectious material, including free viral particles and infected leukocytes, and that current challenge studies in NHP models of HIV and AIDS do not fully reproduce the conditions of natural transmission. We analyzed the dynamics of free virus shedding in macaque semen, in longitudinal and transverse studies in cynomolgus macaques infected with pathogenic SIVmac251. As in HIV-infected patients, we found a strong positive correlation between blood plasma (PVL) and seminal plasma (SVL) viral RNA (vRNA) loads (Spearman r = 0.6381, p = 0.0001, n = 30) [16], [17], [18], [19]. After the first month of infection, SVL is variable and correlated with PVL (Fig. 1 A–B). Mean SVL remained systematically equal to, or lower than PVL (Fig. 1 B). However, as observed in humans, discordant profiles are also observed.
Fig. 1. Viral shedding in blood and semen in macaques. 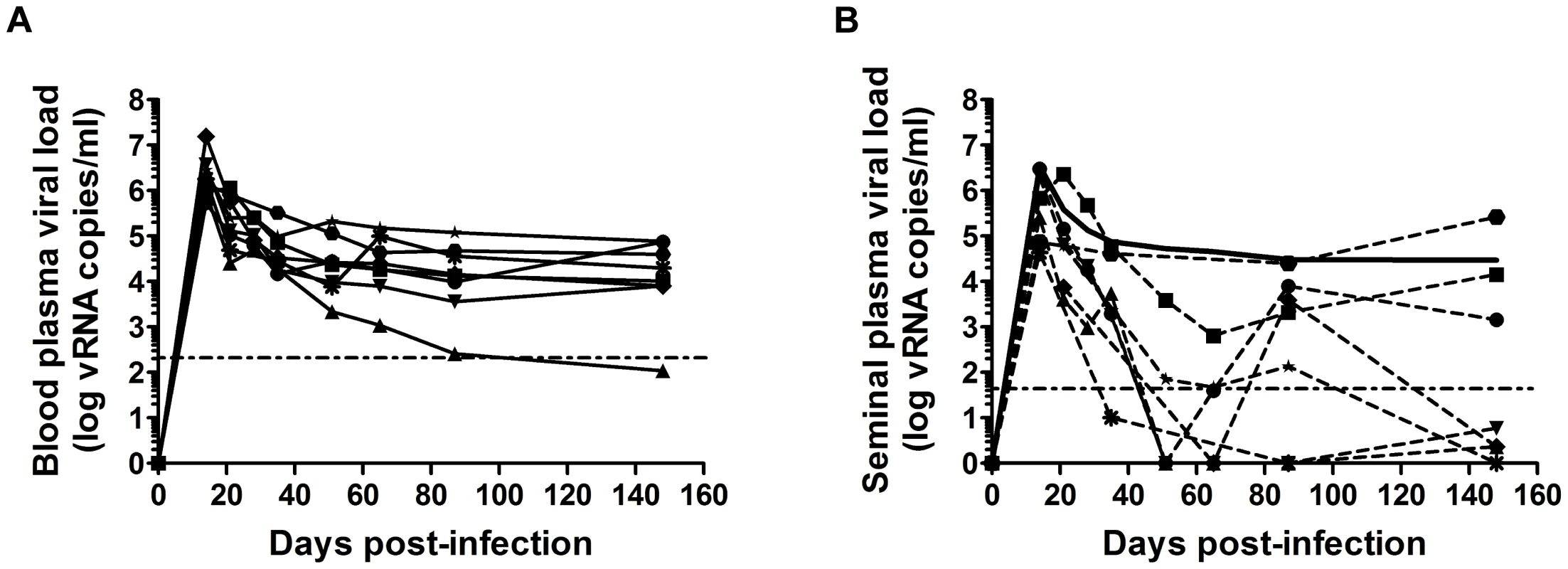
(A–B) Longitudinal follow-up of RNA viral loads in blood (A) and seminal plasma (B) in 8 macaques infected intravenously with high doses of SIVmac251 (5,000 AID50); each animal is represented by different dot. The solid black line indicates the mean PVL. The dotted horizontal line represents the limit of quantification (111 and 37 copies/ml in blood and semen plasma respectively). Changes in semen leukocytes in SIVmac251-infected macaques
We investigated whether semen leukocytes could be infected and play a role in the mucosal transmission of HIV/SIV, by carrying out a detailed characterization by multiple approaches. We first compared semen cells from uninfected and SIVmac251-infected cynomolgus macaques at various stages of infection.
In uninfected animals, the semen contained various amounts of leukocytes, the numbers of which were strongly correlated with markers of inflammation (Fig. 2 A). Indeed, leukocytospermic individuals had significantly higher concentrations of inflammatory cytokines, including IP-10 (p = 0.0157), MIP1β (p = 0.0002), IL-6 (p = 0.0004), RANTES (p = 0.0007), IL-8 (p = 0.0007) and MCP-1 (p = 0.0089), in seminal plasma. The levels of all these molecules, except MIP-1β, were affected by SIV infection (Table S1). IP10, Il-8 and RANTES concentrations were significantly higher in the seminal plasma of macaques during primary infection than in uninfected macaques. The concentrations of IL-8, RANTES, IL-6 and MCP-1 were correlated with PVL and/or SVL.
Fig. 2. Semen leukocytes in macaques. 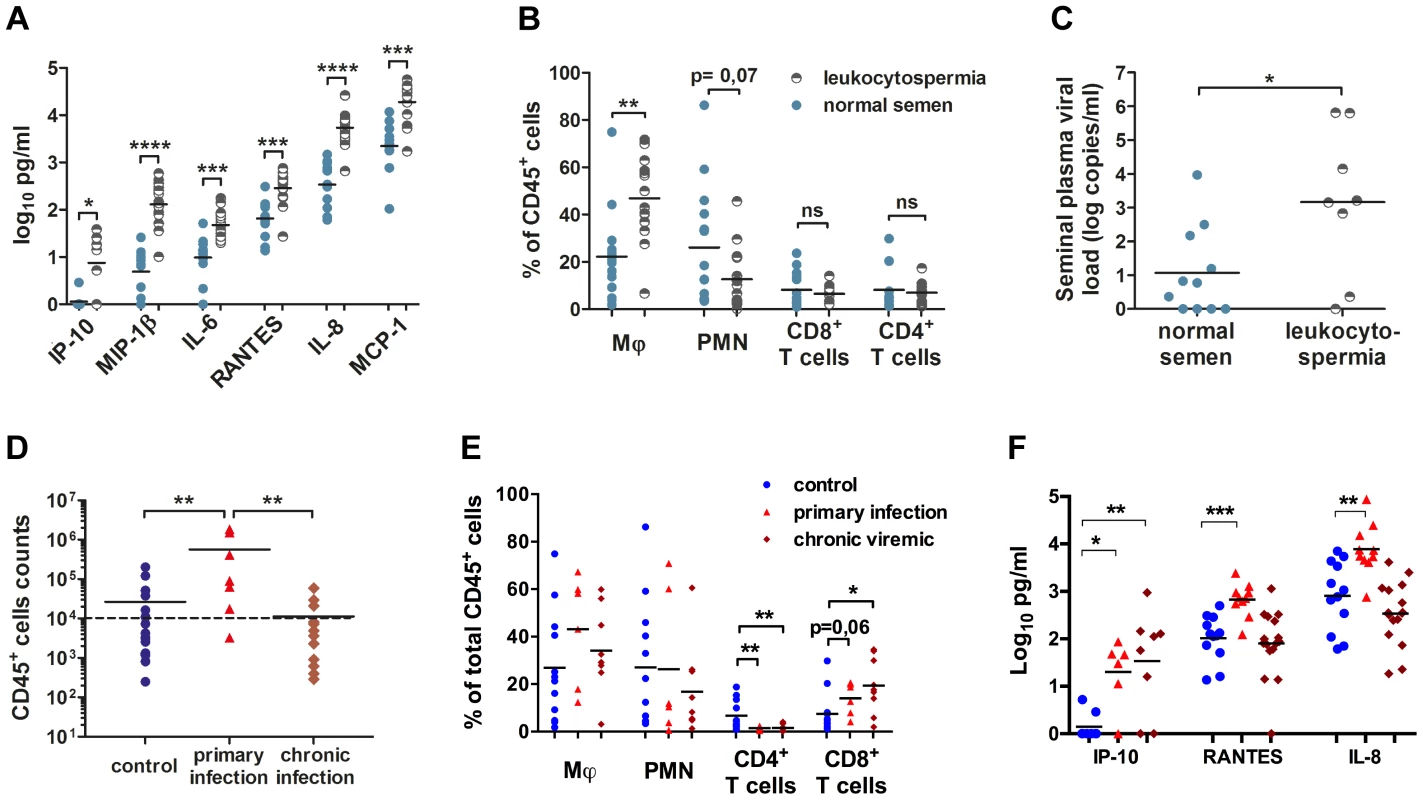
(A) Seminal concentrations of six pro-inflammatory molecules in normal and leukocytospermic animals. (B) Proportion of each studied leukocyte subset among total CD45+ cells. (C) Semen vRNA load in SIV+ macaques with normal (n = 11, blue circle) or leukocytospermic (n = 8, half plein black circles) semen. (D) Number of CD45+ events acquired by flow cytometry per sample since the total collected semen cells were analyzed, for control uninfected (n = 20), and SIV-infected macaques, at 10 and 14 dpi (n = 7) and during chronic infection. The dotted line represents the leukocytospermia threshold (10,000 CD45+ events acquired) (n = 13). (E) Proportion of each subset among total CD45+ events in SIV− macaques (n = 12, blue circle) and SIV+ macaques during primary infection (n = 7, red triangle) and chronic infection (n = 9, red diamond). (F) Seminal concentrations of IP-10, RANTES and IL-8 in SIV− macaques (n = 8 for IP-10 and n = 12 for RANTES and IL-8, blue circle) and SIV+ macaques during primary infection (n = 6 for IP-10 and n = 10 for RANTES and IL-8, red triangle) and chronic infection (n = 8 for IP-10 and n = 16 for RANTES and IL-8, red diamond). At steady state, macaque semen contains mostly polymorphonuclear cells (PMN, CD45+CD11b+HLA-DR−, 26.02%±6.70% of total CD45+ cells), macrophages (CD45+CD3−CD8−CD11b+HLA-DR+, 22.22%±5.06% of total CD45+ cells) and T cells (8.15%±1.94% CD4+ T cells and 8.11%±2.36% CD8+ T cells; Fig. 2 B, Fig. S1). A small proportion of dendritic cells was also identified, corresponding to a mean of 1.87%±1.20% of total CD45+ cells (Fig. S1). Inflammation affected the proportions of macrophages and PMN, with a significant increase of the proportion of macrophages among total CD45+ cells (Mann-Whitney test, p = 0.0022) and a relative decrease in the proportion of PMN (Mann-Whitney test, p = 0.0769; Fig. 2 B).
In infected macaques, leukocytospermia was associated with a higher SVL than in macaques with small numbers of semen leukocytes (Mann-Whitney test, p = 0.0372; Fig. 2 C). In these animals, the number of CD45+ events was strongly correlated with SVL (Spearman correlation, p = 0.0008, r = 0.6858). Interestingly, 85.7% (6/7) in the primary phase of infection (10–14 dpi) displayed leukocytospermia (Mann-Whitney test, p = 0.0062), whereas no significant difference in the number of CD45+ events acquired was found between uninfected and chronically infected macaques (Fig. 2 D). The percentages of macrophages and PMN were not significantly affected by infection, but the T–cell population was significantly modified (Fig. 2 E). Semen CD4+ T cells were strongly and persistently depleted (2.50%±0.78%), from primary infection onwards (Mann-Whitney test, p = 0.0076 and 0.0077, respectively; Fig. 3 C–D). In contrast, the proportion of CD8+ T cells tended to be increased by the infection.
Fig. 3. Changes in semen T cells, macrophages and DCs during SIV infection. 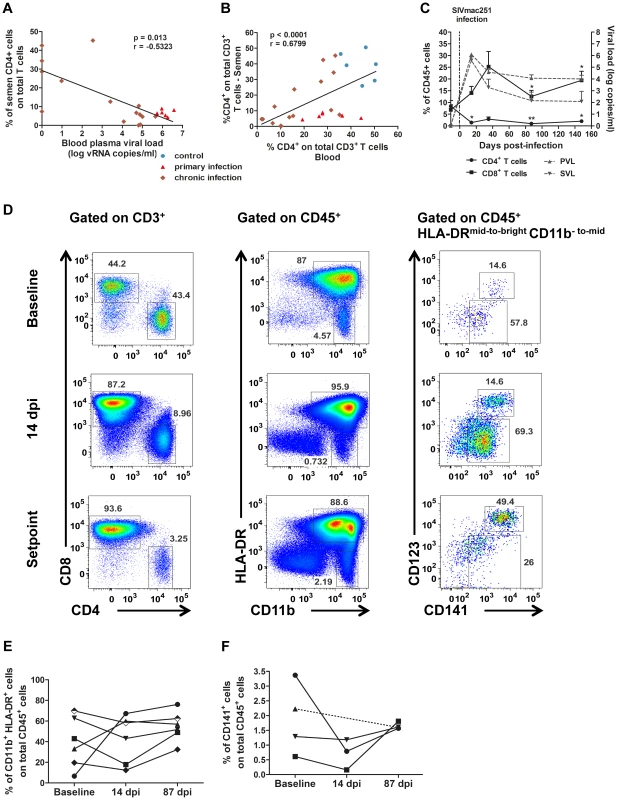
(A) Spearman's correlation between the proportion of CD4+ T cells among total T cells in the semen and PVL (n = 6 macaques at 14 dpi – red triangle, n = 15 at chronic stage – brown diamond). (B) Spearman's correlation between the proportions of CD4+ T cells among total T cells for semen and blood (n = 6 macaques at 14 dpi – red triangle, n = 15 at chronic stage – brown diamond, and n = 6 SIV− macaques). (C) RNA viral loads in blood (dotted line, grey triangle base down) and semen (dotted line, grey triangle base up) and changes in CD4+ (solid line, black circle) and CD8+ T-cell proportions (solid line, black box) during SIV infection; mean and SEM are represented. (D) Longitudinal follow-up of CD4+ and CD8+ T cells, CD11b+ HLA-DRbright macrophages, CD11bbright HLA-DR− polymorphonuclear cells and CD141+ dendritic cells, including CD123+ pDCs. Dot plots from a representative animal infected with 5,000 AID50 IV. (E) Longitudinal follow-up of CD11b+ HLA-DRbright macrophages in 6 macaques infected with 5,000 AID50 IV; each line represents an animal. (F) Longitudinal follow-up of CD123+ CD141+ pDCs in 4 SIV+ macaques. Interestingly, three of the inflammatory molecules increased by leukocytospermia were found also significantly increased in the semen of macaques during the primary infection (10–14 dpi): IP-10, RANTES and IL-8 (Mann-Whitney test, p = 0.01, 0.0007 and 0.0027 respectively; Fig. 2 F). Moreover, the seminal levels of RANTES and IL-8 significantly correlated to both PVL (Spearman correlation, p = 0.0004, r = 0.6857 and p<0.0001, r = 0.7445 respectively) and SVL (p = 0.0023, r = 0.6031 and p<0.0001, r = 0.7494 respectively). Their levels returned to baseline levels at chronic stages.
The percentage of CD4+ T cells among total semen T cells was negatively correlated with blood plasma viral load (Spearman correlation, p = 0.013, r = −0.5323; Fig. 3 A). Conversely the proportions of CD4+ T cells among total CD3+ T cells in semen and blood were positively correlated (Spearman correlation, p<0.0001, r = 0.679; Fig. 3 B).
Macrophages remained detectable in the semen at all stages of infection, with no significant change to the mean proportion among total CD45+ cells (Fig. 3 D–E). However, their frequency in semen was highly variable among individuals (Fig. 3 E).
Finally, CD141+ dendritic cells were also present in low quantity in the semen of SIV-infected macaques (Fig. 3 F). This population exhibited a tendency to decrease during primary infection (14 dpi), although non significantly, as this analysis could be performed only in 3 animals due to small proportion of these cells in semen (Wilcoxon test, p = 0.25, n = 3)
We can conclude from this first analysis that all cell types targeted by the virus are present in the semen and that significant changes in the numbers occur in macaques infected with SIV.
Infection of semen CD4+ T cells and macrophages
SIVmac251 DNA, despite being detected at low frequency in total semen DNA extracted from spermatozoa and semen leukocytes could be amplified by nested PCR with primer pairs binding to gag. During the first month of infection, SIV DNA was detected in 87.5% of the tested animals (7 of 8 macaques), whereas 66.67% macaques at chronic stage (10 of 15 macaques at 3 months of infection or after) were tested positive. Similar results have been reported for men infected with HIV [17], [20], [21], [22], [23], [24], [25], [26], [27], [28].
In macaques, sorted CD4+ T cells and macrophages contained SIV-DNA (Fig. S3), as demonstrated by quantitative real-time PCR (Table 1). SIV-DNA+ CD4+ T cells and macrophages were detected as early as 7 dpi, and in 100% of the sorted fractions at 10 dpi. During chronic infection (>90 dpi), SIV-DNA remained detectable but was below the quantification limit (<90 copies). In general, infected cells were more frequently found in the CD4+ T cells fraction and the proportion of infected cells among the total sorted cells was higher than the macrophages fraction.
Tab. 1. Quantification of SIV DNA copies in sorted semen CD4+ T cells and macrophages at various stages of infection. 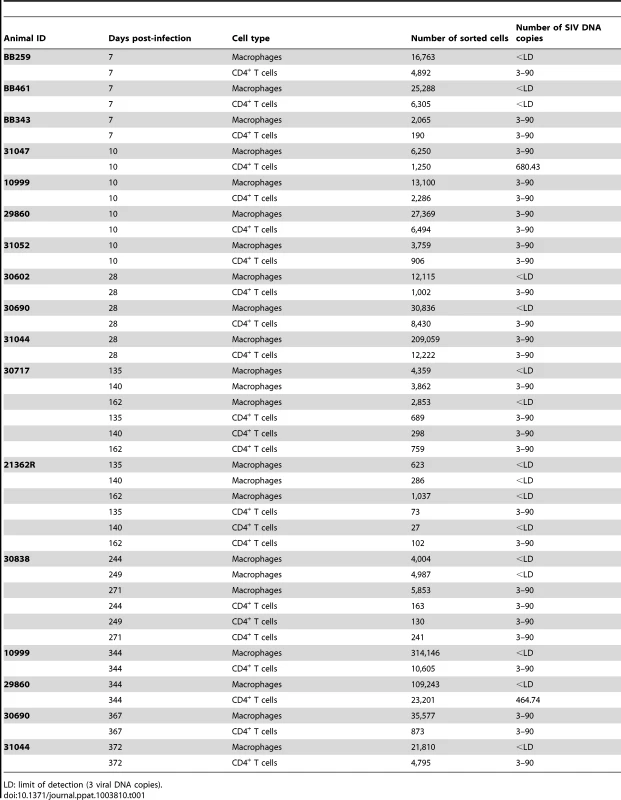
LD: limit of detection (3 viral DNA copies). We also demonstrated, by immunocytofluorescence, that CD45+-enriched fractions of semen leukocytes contained T cells (CD3+) and macrophages (CD163+) harboring SIV proteins in macaques at 28 and 65 dpi (Fig. 4). These results are consistent with real-time PCR results for sorted cells, confirming that both CD4+ T cells and macrophages in semen can be infected with SIVmac251.
Fig. 4. SIV antigens in semen CD4+ T cells and macrophages. 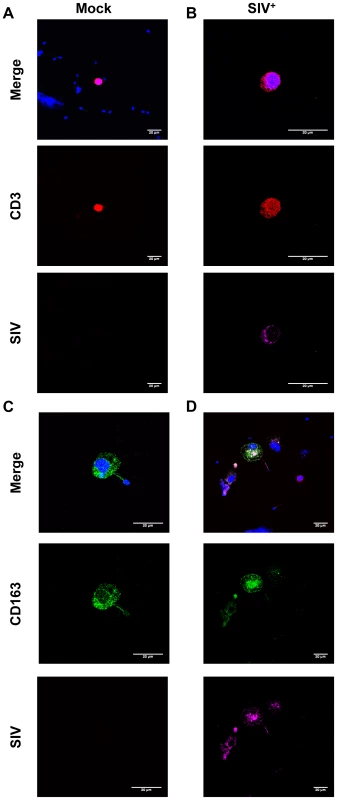
(A–D) Immunocytofluorescence staining targeting CD3 (red), CD163 (green) and SIV Nef (pink) on cytospun CD45+-enriched semen cells from SIV+ (B,D) and uninfected macaques (A,C). Nuclei are stained using DAPI (blue), visible on merged figures (B) SIV+ macaque at 28 dpi. (D) SIV+ macaque at 65 dpi. Finally, semen leukocytes were found to be productively infected with SIVmac251 during both primary and chronic infection. Transmission could be achieved, albeit with various degrees of efficacy, with both CD4+ T cells and macrophages cocultured with CEMx174 susceptible cells (Fig. S3 A–D).
Semen CD4+ T-cell phenotype
As semen CD4+ T cells were found to be productively infected, we investigated their differentiation, activation and migratory profiles, with a view to determining their potential contribution to the mucosal transmission of HIV.
At steady state, most semen CD4+ T cells (94.57%±4.57%) co-expressed CD95+ and CD28+ and could therefore be considered to have a central memory phenotype (Tcm cells, Fig. 5 A), whereas such co-expression was less frequent in peripheral blood CD4+ T cells (58.02%±8.10, Mann-Whitney test, p<0.0001, Fig. 5B). Interestingly, memory CD4+ T cells are the preferential targets of HIV, and these cells are also the principal producers of viral particles [29], [30], [31].
Fig. 5. Semen CD4+ T-cell phenotype. 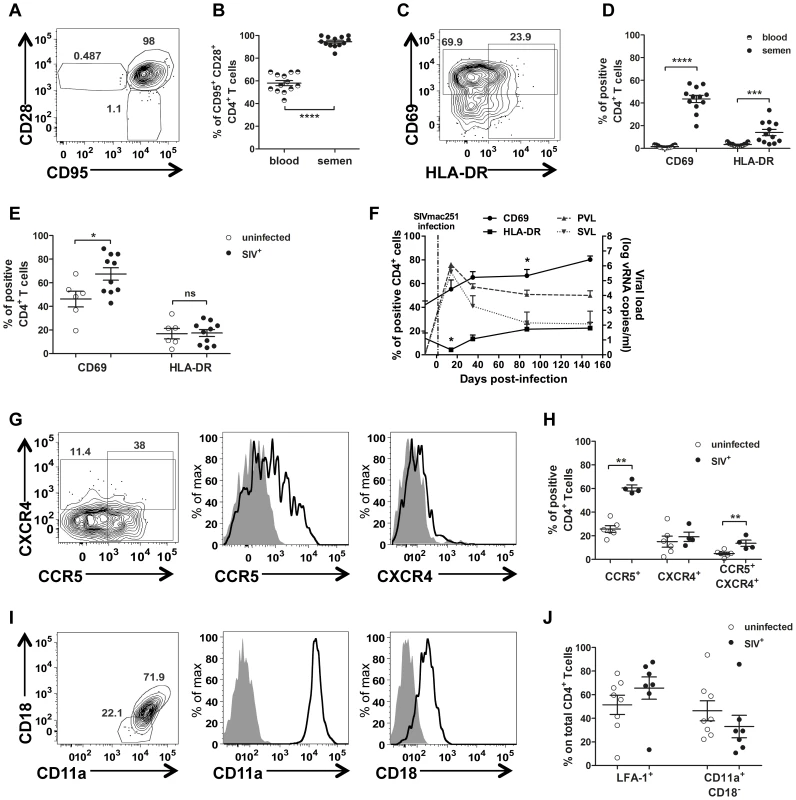
(A) Gating of central memory CD4+ T cells (CD95+CD28+) (B) Comparison of CD4+ T-cell differentiation markers between blood (half plain dot) and semen (plain dot) (n = 13 uninfected macaques). (C) Gating strategy for CD69 and HLA-DR expression. (D) CD69 and HLA-DR expression in CD4+ T cells in blood (half plain dots) and semen (plain dots) from SIV− macaques (n = 13). (E) CD69 and HLA-DR expression in semen CD4+ T cells from SIV− (n = 6, empty dots) and SIV+ macaques, at different stages of infection (n = 10, plain dots). (F) Longitudinal follow-up of CD69 (solid line, plain dot) and HLA-DR (solid line, plain box) expression in semen CD4+ T cells during infection (n = 6). Dotted lines represent PVL (plain grey triangle base down) and SVL (plain grey triangle base up) (G). CCR5 and CXCR4 expression in CD4+ T cells from a representative uninfected macaque. From left to right: contour plots representing outliers, overlay of CCR5 and CXCR4 staining (black line) and isotype control (solid gray curve). (H) Comparison of CCR5+, CXCR4+ and CCR5+ CXCR4+ CD4+ T cells between SIV− (n = 6, open dot) and chronically SIV-infected macaques (n = 6, plain dot). (I) Gating strategy for LFA-1 integrin expression on CD4+ T cells. From left to right: contour plots representing the outliers, overlay of CD11a and CD18 staining (black line) and isotype control (solid gray curve) (J) Comparison of CD11a+ CD18+ (LFA-1) CD4+ T cells between SIV− (n = 6, open dot) and chronically SIV-infected macaques (n = 7, plain dot). Mean and SEM are represented. CD69 expression on Tcm CD4+ cells is an early marker of activation and proliferation and HLA-DR is a late activation marker. In uninfected macaques, the percentage of CD69+ cells among semen CD4+ T lymphocytes was 26.8 times higher than that among blood cmCD4+ T cells, and the percentage of HLA-DR+ cells among semen CD4+ T cells was four times that for the corresponding lymphocytes in blood (Mann-Whitney test p<0.0001 and p = 0.0002 respectively; Fig. 5 D). Infection with SIV resulted in a significant increase in the proportion of CD69-expressing CD4+ T cells, beginning in primary infection (Mann-Whitney test, p = 0.042; Fig. 5 F). We observed a significant, transient decrease in the proportion of HLA-DR+ cells among semen CD4+ T lymphocytes at 14 dpi (Wilcoxon matched paired rank test, p = 0.031), followed by a rapid return to baseline levels.
We also studied the expression of CCR5 and CXCR4, two major coreceptors for HIV/SIV which are also markers of cell migration (Fig. 5 G–H). In uninfected macaques, semen T cells expressed both CCR5 and CXCR4 on their surface, with CCR5+ cells more numerous than CXCR4+ cells. A small proportion of double-positive CCR5+CXCR4+ T cells was also detected (5.512%±3.301). In infected macaques, the proportion of CCR5+ cells was significantly higher among CD4+ T cells (1.98 times higher, Mann-Whitney test, p = 0.0022; Fig. 5 H). This difference probably reflects the activation of T cells. Intracellular staining showed that all cells were positive for CCR5 and CXCR4, providing evidence of highly regulated surface expression (Fig. S4 A).
Finally, we studied LFA-1 expression in semen T cells. This integrin plays a crucial role in T-cell adhesion to epithelial cells and migration through mucosal tissues. Moreover, LFA-1 has been shown to be an important component of the virological synapse, which mediates the efficient cell-to-cell transmission of HIV-1 [32], [33]. LFA-1 is formed by the α-chain CD11a and the β-chain CD18. All CD4+ T cells express CD11a, but only a fraction of these cells express the complete form of LFA-1 (CD11a/CD18; Fig. 5 I–J). In our study, most of the CD4+ T cells in semen were CD11a+CD18+ (60.60%±6.18 at steady state), and the proportion of these cells was not affected by SIV infection (Fig. 5 J).
Thus, semen CD4+ T cells were highly activated. They expressed CCR5 and had a memory phenotype. This profile is typical of mucosal cells [34], and makes semen T cells a potential target for virus infection and replication. These lymphocytes also express migratory and adhesion factors, which might strengthen virological synapse formation and increase the capacity of the cells to migrate towards chemokine-producing tissues and, therefore, the capacity to transmit infection.
Semen antigen-presenting cells phenotype
Macaque semen contains a population of antigen-presenting cells (APCs) that is heterogeneous in terms of morphology (using CD45 versus SSC-A) and HLA-DR expression (Fig. 3 and Fig. S1). We identified two major populations of APCs on the basis of the intensity of CD11b expression: a) a population of HLA-DRbright/CD11bmid-to-bright cells, consisting mostly of macrophages, and b) a population of HLA-DRmid-to-bright/CD11b- to mid cells, including dendritic cells.
Macrophages were the most abundant HIV/SIV target cell in semen. Variable intensities of CD163, CD14 and CD11b expression were observed (Fig. 6 A), resulting in the definition of three different subsets. The most frequent of these subsets was CD163brightCD14bright cells (31.59%±3.51 of CD11b+ HLA-DR+ cells), 74.27%±5.01 of which were CD11bbright (Fig. 6 B–C). This profile is typical of activated macrophages. The second subset was CD163midCD14low cells (27.24%±2.39), and the third was CD163lowCD14− cells (mean 29.26%±3.84). This last subset, which may also include dendritic cells, contained equal proportions of CD11bbright and CD11bmid cells (57.44%±6.80 and 53.11%±7.06, respectively). The proportion of CD11bbright cells in subset 1 was significantly different from those in subsets 2 and 3 (Mann-Whitney test, p = 0.011 and p = 0.003, respectively). All semen macrophages expressed CD4 (Fig. 6 D–E). We also found no significant difference in macrophages CD4 expression between uninfected and SIV-infected macaques (Fig. 6 E).
Fig. 6. Semen macrophage phenotype. 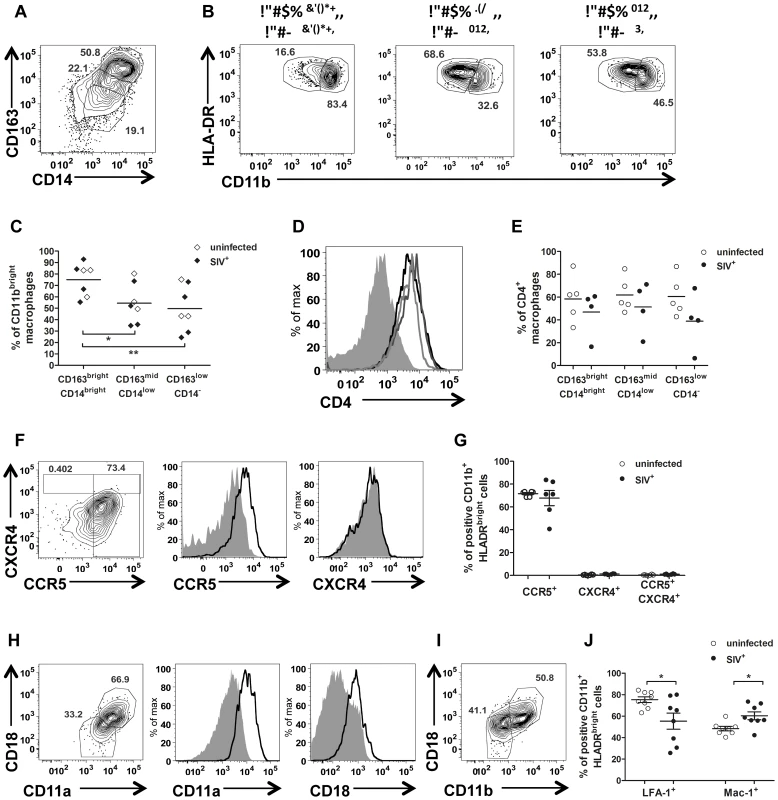
(A) Gating strategy for CD163 and CD14 expression in CD11b+ HLA-DRbright cells. (B) Intensity of CD11b expression by subset (CD163brightCD14bright, CD163brightCD14low and CD163lowCD14− respectively), in a representative animal. (C) Proportion of CD11bbright cells in each subset in 5 SIV− (open dot) and 4 SIV+ animals (plain dot). (D) CD4 expression in each subset from a representative uninfected animal (CD163brightCD14bright: black line, CD163brightCD14low: dark grey line and CD163lowCD14−: light grey line). The solid grey curve represents the negative control (no anti-CD4 staining). (E) Comparison of CD4 expression in each subset between 4 SIV− (open dot) and 6 SIV+ macaques (plain dot). (F) CCR5 and CXCR4 expression in a representative SIV− macaque. From left to right: contour plots representing the outliers, overlay of CCR5 and CXCR4 staining (black line) and isotype control (solid gray curve). (G) Comparison of CCR5+, CXCR4+ and CCR5+ CXCR4+ macrophages between SIV− (n = 6, open dot) and SIV+ macaques (n = 6, plain dot). (H) Gating strategy for LFA-1 integrin expression. From left to right: contour plots representing outliers, overlay of CCR5 and CXCR4 staining (black line) and isotype control (plain gray curve). (I) Gating strategy for Mac-1 integrin expression. (J) Comparison of LFA-1 and Mac-1 expression by macrophages between 6 SIV− (open dot) and 8 SIV+ (plain dot) macaques. SIV+ macaques were all at chronic stage of infection. Mean and SEM are represented. Interestingly, HLA-DR expression was found to be decreased at 14 dpi, both on semen macrophages and on CD4+ T cells (Fig. S5 A, Fig. 3 D). By contrast, CD11b had increased (83.70%±2.03% of CD11bbright; Wilcoxon test, p = 0.0313, Fig. S5 B). The concentrations of the pro-inflammatory chemokines MCP-1, MIP-1β and IL-8, which are produced principally by macrophages, also decreased transiently at this time point (Fig. S5 C). These observations indicate that the acute peak of SVL is accompanied by major changes to the activation and maturation profiles of semen macrophages.
Semen macrophages strongly expressed CCR5 at the membrane, and this expression was not affected by SIV infection (Fig. 6 F–G). By contrast, the proportion of CXCR4+ cells was significantly higher in infected than in uninfected macaques (Mann-Whitney test, p = 0.011), although this proportion remained low (2.96%±0.31). As for CD4+ T cells, almost 100% of semen macrophages contained intracellular CCR5 and CXCR4, indicating high levels of turnover for these molecules (Fig. S5 B).
Most semen macrophages expressed LFA-1 (76.93%±2.79 at steady state). The expression of this molecule was significantly decreased by SIV infection (Mann-Whitney test, p = 0.049; Fig. 6 H, J). CD11b+ macrophages also expressed Mac-1 (49.08%±2.678, Fig. 6 I). This integrin is formed by the α-chain CD11b and the β-chain CD18. The mean proportion of double-positive cells was increased significantly (p = 0.028) by SIV infection (Fig. 6 J).
Discussion
We report here in a macaque model of transmission the first comprehensive characterization of semen leukocytes with the potential to transmit HIV/SIV infection across mucosal barriers. This study was carried out with cynomolgus macaques infected with pathogenic SIVmac251, which is recognized as the most relevant experimental model for studies of HIV infection pathogenesis and mucosal transmission [13], [35], [36], [37]. However, the fact that we used a unique virus isolate, does not allow full representation of the diversity of the HIV-1 populations found in the semen of infected men. Although it is dual-tropic, it is well established that SIVmac251 shows a high tropism for macrophages [38] which is not the case for a significant proportion of founder HIV. Therefore we cannot exclude that our model over estimates the role of macrophage-tropic strains of HIV-1 in transmission.
Macaque semen was found to be very similar to human semen, with similar distributions and phenotypes of semen leukocytes in these two species. Moreover, the dynamics of viral RNA shedding in the semen of SIVmac251-infected macaques was similar to that reported for men. Seminal plasma viral load was correlated with blood plasma vRNA, although some discrepancies were observed in untreated animals.
Leukocytospermia is frequent in humans and macaques and has been reported to be associated with higher levels of HIV DNA in humans, suggesting that semen leukocytes may contribute to virus transmission across mucosal surfaces [3]. We show here that the presence of large numbers of leukocytes in semen is associated with high levels of inflammation markers (IL-6, IL-8, MIP-1β, MCP-1, RANTES and IP-10). Concentrations of these cytokines were correlated with blood and/or seminal plasma viral load. Interestingly, in SIV-infected macaques, leukocytospermic animals had higher seminal vRNA. Moreover, during primary SIV infection, the semen is highly inflammatory and contains large numbers of macrophages, a major target of HIV.
As in other compartments, CD4+ T cells were rapidly and profoundly depleted in the semen of SIV-infected macaques. These cells have a central memory phenotype (CD95+CD28+) and express CCR5, a profile typical of resident mucosal T cells [31], [39], [40]. A large proportion of cells expressed CD69, an activation marker observed at all stages of infection. If infected, semen CD4+ T cells should be able to produce replicative viral particles.
Despite the high degree of CD4+ T-cell depletion in semen, SIV DNA was detected in these cells at all stages of infection. Only a few CD4+ T cells could be sorted from semen, but these cells transmitted the infection when cocultured in vitro with a permissive cell line, demonstrating their considerable capacity to produce infectious SIV. However, in the context of chronic infection, CD4+ T cells are present in very small numbers and therefore probably have a very small impact on virus transmission. Further studies are required to confirm this hypothesis.
In our study, semen macrophages formed a heterogeneous population, with different levels of CD163, CD14 and CD11b expression. This population also expressed markers of activation and migration. Interestingly, semen HLA-DR+ APCs contain SIV DNA at all stages of infection, including the first weeks after infection. Moreover, like T cells, these cells were able to transmit infection to CEMx174 cells when collected at the primary and chronic stages of infection. Thus, semen APCs are also productively infected and produce replication-competent viral particles. Macrophages from chronically infected and leukocytospermic individuals are therefore major candidates for involvement in cell-associated virus transmission.
We also identified other types of APC in semen. This is the first study to report the presence of dendritic cells (DCs), including pDCs, in the semen of infected macaques. More studies are required to characterize semen DCs, particularly for the CD141+ CD123− subpopulation, and their role in the dissemination of viral particles requires investigation.
We found that a large proportion of semen CD4+ T cells and macrophages expressed CCR5, one of the co-receptors of HIV/SIV. The expression of this coreceptor was significantly stronger in infected than in uninfected macaques. This predominance of CCR5+ viral target cells might also account for most of the transmitted viral founders, after mucosal exposure, being R5 strains. It also provides support for the role of cell-associated virus transmission, because semen leukocytes have a migratory and mucosal profile, and are therefore able to migrate to tissues producing RANTES, MIP-1α and MIP-1β, such as the cervico-vaginal mucosa [41]. Furthermore, semen T cells and macrophages express high levels of LFA-1 and/or Mac-1 integrins. Both play an important role in leukocyte adhesion to epithelial cells and transmigration. LFA-1 has also been described as a key player in virological synapse formation and virus transmission [32], [33].
Taken together, these data suggest that infected semen leukocytes may play a role in mucosal transmission, even if present in very small numbers. This hypothesis is supported by the observation that infection may be initiated by a very small number of transmission events [42], [43], [44], [45]. Mucus, low pH and natural microbicidal molecules secreted by the genital and rectal mucosa may be less effective against infected cells than against free viral particles. In this scenario, semen leukocytes may act as Trojan horses, protecting cell-associated virus from host mucosal defenses [3].
It remains nevertheless hard to evaluate the relative impact of cell-associated HIV in mucosal transmission in comparison to free viral particles. Although viral DNA is detected in semen leukocytes at all stages of infection, in our model, SIV-DNA is present in quantifiable and substantial amounts only during the acute phase of infection. The first weeks of infection may therefore represent a restricted window of opportunity for cell-associated virus transmission, whereas infected semen leukocytes may not play a substantial role during the chronic stage.
Materials and Methods
Ethics statement
Adult cynomolgus macaques (Macaca fascicularis) were imported from Mauritius and housed in the facilities of the “Commissariat à l'Energie Atomique et aux Energies Alternatives” (CEA, Fontenay-aux-Roses, France). Non-human primates (NHP, which includes M. fascicularis) are used at the CEA in accordance with French national regulation and under national veterinary inspectors (CEA Permit Number A 92-032-02). The CEA is in compliance with Standards for Human Care and Use of Laboratory of the Office for Laboratory Animal Welfare (OLAW, USA) under OLAW Assurance number #A5826-01. All experimental procedures were also conducted accordingly to European guidelines for animal care (European directive 86/609, “Journal Officiel des Communautés Européennes”, L358, December 18, 1986). The use of NHP at CEA is also in accordance with recommendation with the newly published European Directive (2010/63, recommendation N°9). No suffering was specifically associated with vaginal treatment of macaques. The animals were used under the supervision of the veterinarians in charge of the animal facility. This study was part of the European microbicides project Combined Highly Active Anti-Retroviral Microbicides (CHAARM), which NHP studies were accredited by ethical committee “Comité Régional d'Ethique pour l'Expérimentation Animale Ile-De-France Sud” under statement numbers 12-048 (December 6th 2012) and 12-103 (December 31st 2012).
Animals, infection and sample collection
Adult male macaques were infected by intravenous or intrarectal inoculation with a single dose of 50–5,000 50% animal infectious doses (AID50) of SIVmac251 [46]. Semen and blood were collected from animals sedated by a 5 mg/kg intramuscular injection of chlorhydrate Tiletamine (50 mg) combined with chlorhydrate Zolazepan (50 mg) (Virbac, Carros, France).
Ejaculation was performed by intrarectal electrostimulation of nervous centers near the prostate, with a probe (12.7 mm diameter) lubrified with conductor gel, and an AC-1 electroejaculator (Beltron Instrument, Longmont, USA). Sequential stimulations were performed, with a pattern of 6 cycles, each cycle consisting of nine two-second stimulations followed by a tenth stimulation lasting 10 seconds. The voltage was increased every two cycles (1–3 V for the first two cycles, 2–4 V volts for the third and fourth cycles and 6–8 V for the last two cycles). If a complete ejaculate had not been obtained after six cycles of stimulation, a 7th cycle of stimulation at 7–10 V was performed. The complete ejaculate was immediately diluted in 1.2 ml of phosphate-buffered saline (PBS) and centrifuged.
Blood sample were collected into K3EDTA tubes (BD Biosciences, Le Pont de Claix, France).
Seminal plasma and cell preparation
Seminal plasma was isolated from total semen immediately after collection, by centrifugation for 15 minutes at 775× g. The seminal cell pellet was resuspended in 14 ml of complete medium, consisting of RPMI-1640 medium enriched in glutamine (Invitrogen, Carlsbad, USA) supplemented with a mixture of penicillin, streptomycin and neomycin (Invitrogen) and 10% FCS (Lonza, Allendale, USA), and kept at room temperature for no more than one hour. Cells were then centrifuged for 10 min at 1,500× g, filtered through a sieve with 70 µM pores and washed with 5 ml of PBS supplemented with 10% FCS.
Blood and semen RNA viral load quantification
Blood plasma was isolated from EDTA-treated blood samples by centrifugation for 10 min at 1,500× g, and stored frozen at −80°C. Seminal plasma samples were maintained on ice for no more than one hour and were frozen at −80°C. Semen vRNA was prepared from 500 µl of seminal plasma with commercial kit (QIAamp UltraSens, Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. RT-PCR on blood and seminal plasma RNA was performed as previously described (Karlsson, 2007, J Virol). The quantification limit (QL) was estimated at 111 copies/ml and the detection limit (DL) was estimated at 37 copies/ml. Samples from chronically infected macaques on antiretroviral treatment (ART) were treated in the same way, increasing the amount of plasma to increase sensitivity (QL and DL of 37 and 12.3 copies of vRNA/ml, respectively).
Phenotypic characterization of semen leukocytes
Staining was performed on either whole blood or PBMC. Except for whole-blood assays, staining was performed after the saturation of Fc receptors by incubation with healthy macaque serum (produced in-house) for 1 h at 4°C. Amine-reactive blue dye (Live/dead Fixable, Life Technologies) was used to assess cell viability and to exclude dead cell from the analysis. Cells were stained with monoclonal antibodies by incubation for 30 min at 4°C, washed in PBS/10% FCS and fixed in commercial fixation solution (CellFIX, BD Biosciences). Five different antibody panels were used (see Protocol S1). Corresponding isotype controls for CD163, CD14, CCR5, CXCR4, CD11a and CD18 were used at the same concentrations as the reference antibody. Acquisition was performed on a BD LSRII machine equipped with four lasers (355, 405, 488 and 633 nm), with Flowjo v9 (Tree Star, Ashland, OR) used for analysis.
Semen sample were considered leukocytospermic if it was possible to acquire a minimum of 50,000 CD45+ events per ml on flow cytometry (consisting of 10,000 events per ejaculate, Fig. S6). This cut-off was defined as the minimal required number of acquired CD45+ events to perform statistically relevant analysis of the different populations of leukocytes.
Sorting of semen CD4+ T cells and antigen-presenting cells
Total semen cells were filtered and washed (see above), and then incubated for 15 min at 4°C with 20 µl of anti-CD45 magnetic beads (Miltenyi Biotec) and washed once with 2 ml of cold PBS supplemented with 0.5% BSA and 2 mM EDTA (sorting buffer). The CD45+ cell fraction was then enriched by magnetic bead sorting, with LS columns (Miltenyi Biotec), used according to the manufacturer's instructions. Cells were eluted in 4 ml of sorting buffer. Cells were stained as described above, using amine-reactive blue dye (Life Technologies) to identify the dead cells, and the same antibodies as for leukocyte phenotyping: CD45, CD3, CD8, CD4 and HLA-DR. Cells were washed twice and stored at 4°C in PBS/10% FCS. Following the magnetic bead-based enrichment process, CD4+ T cells and HLA-DR+ APCs were sorted by simultaneous four-way sorting on a FACSAria flow cytometer (BD Biosciences). The enrichment of cell fractions was estimated by flow cytometry with BDDiva (BD Biosciences) and FlowJo software (see Fig. S2).
Detection of SIV DNA in the cellular components
After semen collection and the separation of the cellular components from the seminal plasma, the cells were centrifuged and the cell pellet was kept at −80°C until further tests. Nested PCR targeting SIVmac251 gag was performed as previously described [47]. Twenty tests were carried out for each semen DNA sample. A second amplification was then carried out, as we previously described [14].
After the sorting of CD4+ T cells and macrophages, the cells were washed once in PBS and centrifuged for 10 min at 1,500× g. The supernatant was discarded and the dry cell pellets were frozen at −80°C. PCR was directly performed on cell lysates as we previously described [37]. The number of cells was determined by amplifying the CCR5 gene (see Protocol S2) with serial 10-fold dilutions of SIV-negative PBMCs (starting with 1 million cells) as a standard and a lysate of SIV-negative PBMCs and nuclease-free water as the negative control. SIV DNA was quantified with serial dilutions over five orders of magnitude of a pCR4-TOPO plasmid (Invitrogen) containing the SIVmac251 gag cDNA sequence and diluted in SIV-negative PBMCs as the standard. The quantification threshold was 90 SIV-DNA copies and the detection threshold varied between 1 and 3 copies of SIV-DNA (see Figure S7).
Coculture of sorted semen leukocytes with CEMx174
After sorting, semen CD4+ T cells and macrophages were washed once with 10% FCS in PBS and transferred to a U-bottomed 96-well plate. CEMx174 cells were added to each well, the number of cells added being three times the number of sorted cells. If this number was below 10,000 cells, we added a minimum of 50,000 CEMx174 cells per well. Cells were cultured in a final volume of 250 µl of complete medium (see above), at 37°C, under an atmosphere containing 5% CO2. The positive control was CEMx174 cells cultured with 50 µl of highly concentrated SIVmac251 particles, and the negative control was CEMx174 cells alone. Cells were passaged three or four days after the initiation of the coculture and then every two or three days thereafter. Coculture was stopped after 8 days. At each passage, half the cell suspension was replaced with fresh medium. Supernatants and cell pellets were cryopreserved at −80°C until further analysis. SIV-DNA and SIV-RNA were quantified as described above.
Immunofluorescence staining of cytospun semen cells
Cells were washed once in PBS, then diluted in a maximal volume of 450 µl and cytospun on Superfrost slides at 500T for 10 min, in a Cytospin 4 Cytocentrifuge (Thermo Shandon, Thermo Fisher Scientific, Waltham, USA), at a concentration of 200,000 cells per spot. Cytospun cells were allowed to dry at room temperature for two hours and were then fixed by incubation in a mixture of 50% ice-cold methanol and 50% ice-cold acetone for 15 min. Slides were then allowed to dry at room temperature for 20 min and frozen at −20°C for later use.
Before staining, the slides were thawed and allowed to dry at room temperature for 20 min. They were then washed three times, for 5 min each, in PBS. Cells were permeabilized by incubation with 0.025% Triton in PBS for 5 min and the slides were then washed three times, for five minutes each, in PBS. Non specific binding sites were saturated by incubation for 1 hour at room temperature with 5% BSA and 10% macaque serum in PBS. Immunofluorescence staining was performed with the following antibodies: anti-CD3 Alexa Fluor 700 (BD Biosciences, clone SP34-2), anti-CD163 Alexa Fluor 488 (BD Biosciences, clone GHI/61) and anti-SIV nef (NIBSC, Center for AIDS Reagents, clone KK75) and anti-SIV gp41 (NIBSC, Center for AIDS Reagents, clone KK7a) both coupled with Alexa Fluor 594, in 10% BSA/0.05% Tween 20 in PBS, for 30 min, at room temperature, in a dark chamber. Slides were washed three times with 0.05% Tween 20 in PBS and three times in PBS alone. Finally, slides were mounted in anti-fade mounting medium which stained nuclei using DAPI (ProLong, Invitrogen) and stored at 4°C until further analysis.
Images were acquired with a Leica confocal microscope (equipped with 4 lasers) and analyzed with ImageJ software. The final images were generated with Adobe Photoshop.
Cytokine quantification in semen
Concentrations of IL-6, IL-8, MIP-1β and MCP-1 in seminal plasma were determined with the Milliplex Map Non-Human Primate Cytokine Magnetic Bead Panel - Premixed 23-plex (Merck Millipore, Darmstadt, Germany). The concentrations of RANTES and IP-10 were determined with a 2-plex Milliplex kit. Assays were performed in duplicate, with 25 µl of seminal fluid. Samples were thawed at room temperature and centrifuged for 10 min at 1,500× g to harvest any cellular components. Immunoassays were performed according to the manufacturer's instructions. Data were acquired with a Bio-Plex Instrument 200 and analyzed with Bio-Plex Manager Software version 6.1 (Bio-Rad, Hercules, USA).
Data visualization and statistical analysis
All data visualization and statistical analyses were carried out with GraphPad Prism 5.03 software (GraphPad software, La Jolla, USA). Nonparametric Spearman's rank correlation tests were used to investigate the relationships between parameters. The nonparametric Mann-Whitney test was used to compare groups of macaques, and the nonparametric Wilcoxon rank sum test was used to compare data from same macaques at different time points, before and after SIV infection. P values of 0.05 or less in two-tailed tests were considered significant, *:p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001.
Supporting Information
Zdroje
1. HaigwoodNL (2004) Predictive value of primate models for AIDS. AIDS Rev 6 : 187–198.
2. VeazeyRS, ShattockRJ, KlassePJ, MooreJP (2012) Animal models for microbicide studies. Curr HIV Res 10 : 79–87.
3. AndersonDJ, PolitchJA, NadolskiAM, BlaskewiczCD, PudneyJ, et al. (2010) Targeting Trojan Horse leukocytes for HIV prevention. AIDS 24 : 163–187.
4. PolitchJA, MayerKH, AndersonDJ (2009) Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J Acquir Immune Defic Syndr 50 : 283–289.
5. AndersonDJ, YunisEJ (1983) “Trojan Horse” leukocytes in AIDS. N Engl J Med 309 : 984–985.
6. AlfsenA, YuH, Magerus-ChatinetA, SchmittA, BomselM (2005) HIV-1-infected blood mononuclear cells form an integrin - and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell 16 : 4267–4279.
7. GrootF, WelschS, SattentauQJ (2008) Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111 : 4660–4663.
8. BomselM (1997) Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 3 : 42–47.
9. GanorY, ZhouZ, TudorD, SchmittA, Vacher-LavenuMC, et al. (2010) Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol 3 : 506–522.
10. GanorY, ZhouZ, BodoJ, TudorD, LeibowitchJ, et al. (2012) The adult penile urethra is a novel entry site for HIV-1 that preferentially targets resident urethral macrophages. Mucosal Immunol 6 : 776–86.
11. HladikF, SakchalathornP, BallweberL, LentzG, FialkowM, et al. (2007) Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26 : 257–270.
12. Di FabioS, GianniniG, LapentaC, SpadaM, BinelliA, et al. (2001) Vaginal transmission of HIV-1 in hu-SCID mice: a new model for the evaluation of vaginal microbicides. AIDS 15 : 2231–2238.
13. SalleB, BrochardP, BourryO, ManniouiA, AndrieuT, et al. (2010) Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis 202 : 337–344.
14. Le TortorecA, Le GrandR, DenisH, SatieAP, ManniouiK, et al. (2008) Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS One 3: e1792.
15. KaizuM, WeilerAM, WeisgrauKL, VielhuberKA, MayG, et al. (2006) Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis 194 : 912–916.
16. BaetenJM, KahleE, LingappaJR, CoombsRW, Delany-MoretlweS, et al. (2011) Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 3 : 77ra29.
17. TachetA, DulioustE, SalmonD, De AlmeidaM, RivallandS, et al. (1999) Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS 13 : 823–831.
18. CoombsRW, SpeckCE, HughesJP, LeeW, SampoleoR, et al. (1998) Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis 177 : 320–330.
19. VernazzaPL, DyerJR, FiscusSA, EronJJ, CohenMS (1997) HIV-1 viral load in blood, semen and saliva. AIDS 11 : 1058–1059.
20. Van VoorhisBJ, MartinezA, MayerK, AndersonDJ (1991) Detection of human immunodeficiency virus type 1 in semen from seropositive men using culture and polymerase chain reaction deoxyribonucleic acid amplification techniques. Fertil Steril 55 : 588–594.
21. MerminJH, HolodniyM, KatzensteinDA, MeriganTC (1991) Detection of human immunodeficiency virus DNA and RNA in semen by the polymerase chain reaction. J Infect Dis 164 : 769–772.
22. HamedKA, WintersMA, HolodniyM, KatzensteinDA, MeriganTC (1993) Detection of human immunodeficiency virus type 1 in semen: effects of disease stage and nucleoside therapy. J Infect Dis 167 : 798–802.
23. QuayleAJ, XuC, MayerKH, AndersonDJ (1997) T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis 176 : 960–968.
24. XuC, PolitchJA, TuckerL, MayerKH, SeageGR3rd, et al. (1997) Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J Infect Dis 176 : 941–947.
25. KriegerJN, NirapathpongpornA, ChaiyapornM, PetersonG, NikolaevaI, et al. (1998) Vasectomy and human immunodeficiency virus type 1 in semen. J Urol 159 : 820–825; discussion 825–826.
26. MayerKH, BoswellS, GoldsteinR, LoW, XuC, et al. (1999) Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis 28 : 1252–1259.
27. VernazzaPL, TroianiL, FleppMJ, ConeRW, SchockJ, et al. (2000) Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS 14 : 117–121.
28. GhosnJ, ViardJP, KatlamaC, de AlmeidaM, TubianaR, et al. (2004) Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. AIDS 18 : 447–457.
29. MattapallilJJ, DouekDC, HillB, NishimuraY, MartinM, et al. (2005) Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434 : 1093–1097.
30. BrenchleyJM, HillBJ, AmbrozakDR, PriceDA, GuenagaFJ, et al. (2004) T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol 78 : 1160–1168.
31. VeazeyRS, MansfieldKG, ThamIC, CarvilleAC, ShvetzDE, et al. (2000) Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol 74 : 11001–11007.
32. JollyC, KashefiK, HollinsheadM, SattentauQJ (2004) HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med 199 : 283–293.
33. RudnickaD, FeldmannJ, PorrotF, WietgrefeS, GuadagniniS, et al. (2009) Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol 83 : 6234–6246.
34. VeazeyRS, ThamIC, MansfieldKG, DeMariaM, ForandAE, et al. (2000) Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol 74 : 57–64.
35. MillerCJ (1994) Animal models of viral sexually transmitted diseases. Am J Reprod Immunol 31 : 52–63.
36. LacknerAA, VeazeyRS (2007) Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med 58 : 461–476.
37. ManniouiA, BourryO, SellierP, DelacheB, BrochardP, et al. (2009) Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology 6 : 106.
38. MillerCJ, MarthasM, GreenierJ, LuD, DaileyPJ, et al. (1998) In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol 72 : 3248–3258.
39. HladikF, LentzG, DelpitE, McElroyA, McElrathMJ (1999) Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol 163 : 2306–2313.
40. PrakashM, KapembwaMS, GotchF, PattersonS (2001) Higher levels of activation markers and chemokine receptors on T lymphocytes in the cervix than peripheral blood of normal healthy women. J Reprod Immunol 52 : 101–111.
41. LehnerT, MitchellE, BergmeierL, SinghM, SpallekR, et al. (2000) The role of gammadelta T cells in generating antiviral factors and beta-chemokines in protection against mucosal simian immunodeficiency virus infection. Eur J Immunol 30 : 2245–2256.
42. KeeleBF, GiorgiEE, Salazar-GonzalezJF, DeckerJM, PhamKT, et al. (2008) Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105 : 7552–7557.
43. LiH, BarKJ, WangS, DeckerJM, ChenY, et al. (2010) High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS Pathog 6: e1000890.
44. MillerCJ, LiQ, AbelK, KimEY, MaZM, et al. (2005) Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol 79 : 9217–9227.
45. Salazar-GonzalezJF, SalazarMG, KeeleBF, LearnGH, GiorgiEE, et al. (2009) Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206 : 1273–1289.
46. KarlssonI, MalleretB, BrochardP, DelacheB, CalvoJ, et al. (2007) Dynamics of T-cell responses and memory T cells during primary simian immunodeficiency virus infection in cynomolgus macaques. J Virol 81 : 13456–13468.
47. Benlhassan-ChahourK, PenitC, DioszeghyV, VasseurF, JanvierG, et al. (2003) Kinetics of lymphocyte proliferation during primary immune response in macaques infected with pathogenic simian immunodeficiency virus SIVmac251: preliminary report of the effect of early antiviral therapy. J Virol 77 : 12479–12493.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



