-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaImmune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
article has not abstract
Published in the journal: . PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003784
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003784Summary
article has not abstract
More than 240 million people worldwide are infected with hepatitis B virus (HBV) and are at risk of developing liver cirrhosis and hepatocellular carcinoma (HCC). Reducing this pool of infected people is a necessity since despite an effective prophylactic vaccine, about 2% of the vaccinated individuals in high endemic areas still develop chronic HBV infection (CHB). A battery of antiviral drugs based on nucleoside or nucleotide analogues that target the HBV reverse transcriptase are available. They efficiently suppress HBV replication and reduce liver inflammation linked with cirrhosis but rarely achieve virus eradication. HBV is present in hepatocytes in a mini-chromosomal form (called cccDNA) that is untouched by reverse transcriptase inhibitors. As a consequence, response to the treatment is hardly durable and a majority of patients experience HBV reactivation when antiviral therapy is withdrawn [1]. Failure to achieve sustained control of HBV infection is linked with an inability to elicit an effective immune response that resembles one present in adult patients that resolve acute infection. However, since HBV is a non-cytopathic virus, immunological processes are also responsible for the chronic inflammatory events that cause cirrhosis and HCC [1]. Immunotherapeutic approaches primarily designed to control viral replication through the boosting of antiviral immunity or that aim to inhibit the liver inflammatory processes linked with cirrhosis and HCC development will be discussed.
What Is the Immunological Profile of HBV Control?
Efficient control of HBV infection is associated with the induction and persistence of helper and cytotoxic T cells targeting different HBV proteins and production of anti-HBV envelope antibodies. This antiviral immune response is composed of T cells able to secrete Th1 cytokines, proliferate and lyse HBV infected hepatocytes [1], and it is induced almost exclusively in adult patients after acute HBV infection. Chronic HBV patients fail to mount such an efficient antiviral response. HLA-class II genetic profile [2], dose of virus [3], and age at infection [4] influence the induction of a protective antiviral immunity, but a detailed discussion of the causes of HBV chronicity exceeds the focus of this brief review. What has instead been clearly demonstrated in animal models and in natural infection is that HBV-specific adaptive immunity occurs in the context of a peculiar innate immune response. This response is delayed four to six weeks post infection when HBV replication has already reached extremely high levels (>106 copies/ml) [1]. Innate immune activation is characterized by large production of IFN-γ rather than IFN-α/β [5]. Indeed, while HBV doesn't trigger high IFN-α production and also interferes with its antiviral effect [6], [7], a robust IFN-γ production precedes the detection of HBV-specific T cells in acute HBV and correlates with a significant drop in HBV replication and HBV antigens [5]. The innate immune cell components responsible for HBV sensing and IFN-γ production and ultimately induction of protective adaptive immunity have not been defined during natural infection [1]. Elegant work in mice showed that CD1-restricted NKT cells are activated by self-lipids induced by HBV replication [8], but in addition to the fact that HBV replication in this mouse model was mediated by an adenoviral vector, CD1-restricted NKT cells are abundant in mouse but not in human livers [9]. In human, the network of innate lymphocytes resident in the liver is mainly composed of NK CD56 bright cells and mucosal-associated invariant T (MAIT) cells that can produce large quantities of IFN-γ through cytokine-mediated (IL-12, IL-18) activation [10]. Activation of these innate cells might therefore be necessary for the successful control of HBV infection, but further studies are necessary to precisely define their role in the early stage of infection.
What Can Be the Strategies to Restore Adaptive Immunity in Chronic HBV Patients?
After years of exposure to HBV antigens, HBV-specific T cells are deleted or functionally exhausted in chronic patients. Virus-specific T cells express inhibitory molecules like PD-1 [11], CTLA-4 [12], SLAM [13], and TIM-3 [14], [15] and are defective in proliferation and cytokine production. Blocking inhibitory pathways, new aggressive vaccination regimens and experimental gene therapy strategies have been proposed to rebuild a functional HBV-specific T cell response in these patients (Figure 1).
Fig. 1. Different immune-based therapeutic strategies aiming to increase HBV control. 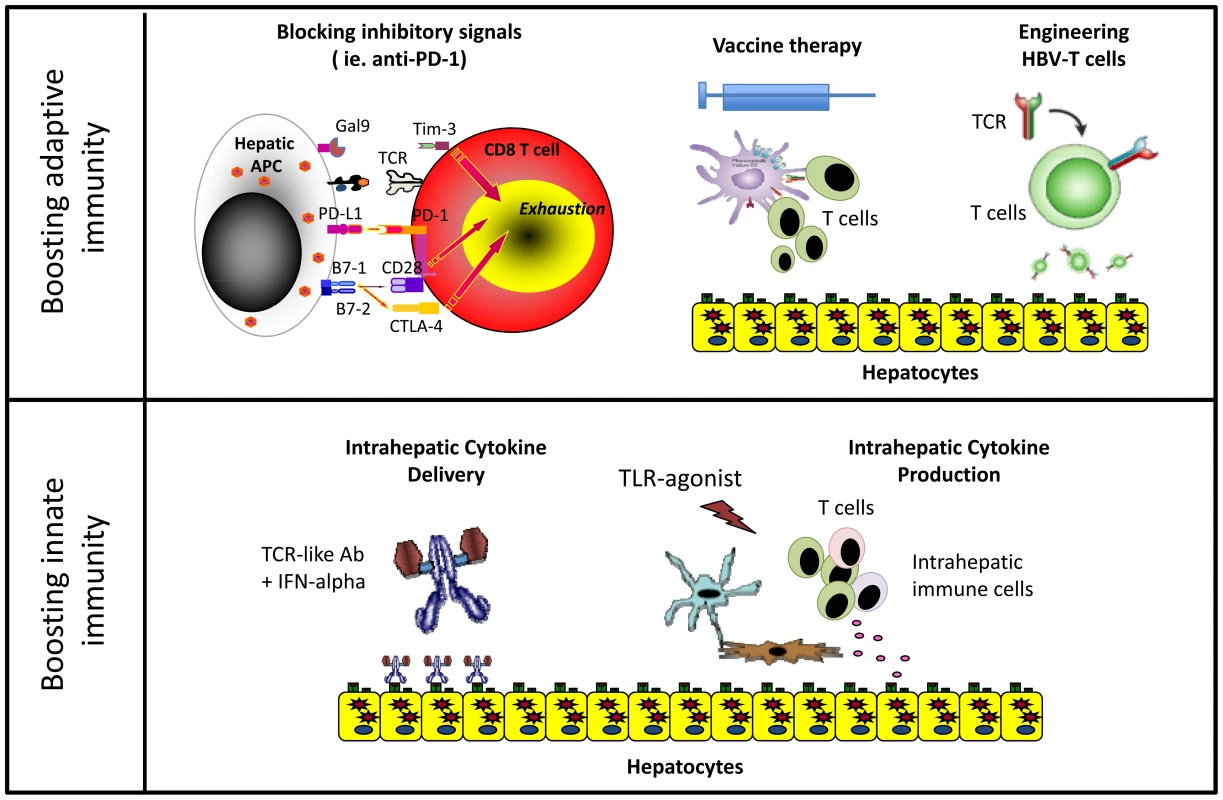
Blocking inhibitory pathways associated with T cell exhaustion has shown therapeutic efficacy in cancer patients [16]. Interfering with these pathways can also achieve partial functional recovery of HBV-specific T cells from CHB patients in vitro, but we still lack data in vivo evaluating the efficacy of this approach in CHB patients [16].
Vaccine therapy aims to induce functionally efficient HBV-specific T cells on the background of virus-specific T cell exhaustion. Several strategies have been tested in clinical trials with disappointing results. Often, vaccine therapy did not induce an HBV-specific T cell response, or when such response was boosted, it did not have a therapeutic effect [17]. However, it was recently demonstrated that the use of new highly immunogenic vaccine preparations in combination with antiviral treatment showed immunological and therapeutic efficacy in the woodchuck model of chronic HBV infection [18]. In addition, intriguing data was reported from a phase III clinical trial of a therapeutic vaccine. A significant virological response was observed in 20% of patients treated not only with the vaccine but also with adjuvant alone [19]. The immunological mechanisms responsible were not characterized but plausible hypotheses can be derived from recent studies. Intrahepatic activation of the myeloid compartment with TLR agonists [20] results in effective T cell expansion in murine systems. Agonistic anti-CD40 [21] activation of dendritic cells rescues naïve CD8 T cell priming to antigens produced in the liver of HBV transgenic mice, which is otherwise aborted by PD-1/PD-L1 interactions. These data together with our recent demonstration that monocytes internalize HBV antigens in the circulation of chronic patients and activate autologous HBV-specific T cells after maturation with inflammatory stimuli [22] suggest that repetitive injections of adjuvants alone could induce the inflammatory environment capable of stimulating intrahepatic HBV-specific T cells (Figure 2). This could provide a clear advantage to current vaccines that use a single recombinant antigen and do not account for the 8% of genetic diversity among HBV genotypes [23]. Capitalizing on the antigen present in infected patients could provide the full repertoire of antigens personalized to the infecting virus. If this can be confirmed in vivo, CHB vaccine therapy could be performed using adjuvants alone and the “personalized antigenic depot” within the patients to overcome the problem of viral diversity.
Fig. 2. Schematic representation of the potential ability of HBV-loaded monocytes [22] to stimulate HBV-specific T cells trough TLR [20] or anti-CD40 agonists [21]. ![Schematic representation of the potential ability of HBV-loaded monocytes <em class="ref">[22]</em> to stimulate HBV-specific T cells trough TLR <em class="ref">[20]</em> or anti-CD40 agonists <em class="ref">[21]</em>.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/67d519b6d5a1718b0ff190e8da662ae8.png)
MN = monocytes, DC = dendritic cells. However, more radical approaches could be needed to circumvent HBV-specific T cell deletion in patients with high viral loads. Engineering HBV-specific T cells through transfer of HBV-specific T cell receptors or HBV-specific chimeric antigen receptors (CAR) showed encouraging results in vitro and in animal models [24], [25]. The concept of adoptively transferring a functionally efficient HBV-specific immune system is not new in HBV. CHB patients receiving bone marrow transplants from HBV-immune donors were cured. Likewise, HBV+ livers transplanted in HBV-immune donors resulted in viral control [17]. Thus, gene therapy approaches might have potential, but safety concerns, cost, and ethical issues related to viral vector use need to be addressed.
Several other factors have also hampered the restoration of efficient adaptive immunity in CHB. Epigenetic programs were suggested to preserve the T cell exhaustion state in chronic viral infection [26]. Also, in many CHB patients, HBV-specific T cells are not only dysfunctional but can be physically deleted, adding obstacles to vaccination approaches. Furthermore, IL-10 [16], TGF-β [27], arginase [28], and T regulatory cells [29] are increased in the chronically inflamed livers. Even if it is possible to genetically engineer HBV-specific immunity, the suppressive microenvironment could hinder HBV clearance.
The duration of exposure and antigen burden in chronic HVB infection are believed to be the primary factors affecting virus-specific T cell function. Thus, modulating the quantity of viral antigens secreted by HBV might have a beneficial effect as has been observed in HBV transgenic mice [30]. It should also be considered that adolescent and young CHB patients display a less compromised HBV-specific antiviral immune response than their adult counterparts [31]; therefore, they may be more responsive to immunotherapeutic strategies.
How Can We Directly Stimulate Intrahepatic Innate Immunity?
The important role played by innate immunity in the early stages of infection has stimulated therapeutic strategies to specifically target this branch. IFN-α therapeutic efficacy is associated with NK cell activation [32]. Therefore, increasing intrahepatic IFN-α levels could be clinically beneficial (Figure 1). In one approach, we have developed T cell receptor–like antibodies conjugated with IFN-α that specifically target HBV-infected hepatocytes to increase intrahepatic IFN-α delivery [33].
Other approaches in clinical development seek to induce intrahepatic IFN-α production through oral administration of TLR agonists. TLR7 agonists stimulate robust IFN-α production in plasmacytoid dendritic cells. However, prolonged efficacy in TLR-7 agonist–treated, HBV-infected chimpanzees was not only related to the production of IFN-α, as its production was transient and not liver specific. Antiviral efficacy was more consistent with a boost of intrahepatic NK, NKT, and T cell responses associated with production of IFN-γ [34]. Since, as reported previously, intrahepatic NK and MAIT cells can be activated by IL-12 and IL-18 cytokines [10], TLR agonists triggering IL-12 and IL-18 hepatic secretion might be particularly important. These cytokines selectively activate innate immune cells within the liver compartment and can induce partial functional recovery of exhausted HBV-specific CD8+ T cells [35].
Is the Reduction of Liver Inflammation a Valuable Therapeutic Strategy for CHB Infection?
The immunotherapeutic strategies we reviewed aim to control HBV infection by increasing antiviral immunity and as such terminate the chronic inflammatory process. However, activation of intrahepatic adaptive or innate cellular immunity requires tight control because it could also exacerbate liver inflammation. Even though mechanisms like IL-10 production, release of arginase from hepatocytes, and even the dampening of T cell responses by activated NK cells [36] are in place to control excessive activation of intrahepatic immunity, hepatocyte lysis and increased IFN-γ production can still trigger inflammatory chemokines (e.g., CXCL10) responsible for recruiting inflammatory cells (macrophages, non-HBV-specific T cells) that cause the bulk of liver damage [1].
A radically different perspective in chronic HBV treatment is to consider CHB a necro-inflammatory rather than viral disease. Recent data in HBV transgenic mice clearly indicate that suppressing intrahepatic CTL activity in the liver using anti-platelet therapy prevents hepatocellular carcinoma. Platelets promote the accumulation of CD8 T cells in the liver and anti-platelet therapy blocks this process, reducing hepatocellular injury and fibrosis [37]. In addition to these experimental data, observations that HBsAg quantity is associated with liver fibrosis protection [38] introduced a further level of uncertainty about the beneficial effect of HBV suppression.
In conclusion, the current immunotherapeutic strategies designed to suppress and control HBV replication have strong scientific support. However, they are restricted by our limited knowledge, and further understanding of the relationship with the virus in the unique environment of the human liver will almost certainly provide opportunities to enhance them or perhaps develop totally novel approaches.
Zdroje
1. BertolettiA, FerrariC (2012) Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 61 : 1754–1764.
2. KamataniY, WattanapokayakitS, OchiH, KawaguchiT, TakahashiA, et al. (2009) A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet 41 : 591–595.
3. AsabeS, WielandSF, ChattopadhyayPK, RoedererM, EngleRE, et al. (2009) The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol 83 : 9652–9662.
4. PublicoverJ, GaggarA, NishimuraS, Van HornCM, GoodsellA, et al. (2013) Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest 123 : 3728–3739.
5. WielandS, ThimmeR, PurcellRH, ChisariFV (2004) Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A 101 : 6669–6674.
6. LutgehetmannM, BornscheuerT, VolzT, AllweissL, BockmannJH, et al. (2011) Hepatitis B virus limits response of human hepatocytes to interferon-alpha in chimeric mice. Gastroenterology 140 : 2074–2083.
7. TianY, ChenW-l, OuJ-hJ (2011) Effects of interferon-α/β on HBV replication determined by viral load. PLoS Pathog 7: e1002159 doi:10.1371/journal.ppat.1002159
8. ZeissigS, MurataK, SweetL, PublicoverJ, HuZ, et al. (2012) Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med 18 : 1060–1068.
9. TangXZ, JoJ, TanAT, SandalovaE, ChiaA, et al. (2013) IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol 190 : 3142–3152.
10. TuZ, BozorgzadehA, PierceRH, KurtisJ, CrispeIN, et al. (2008) TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med 205 : 233–244.
11. BoniC, FisicaroP, ValdattaC, AmadeiB, Di VincenzoP, et al. (2007) Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 81 : 4215–4225.
12. SchurichA, KhannaP, LopesAR, HanKJ, PeppaD, et al. (2011) Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 53 : 1494–1503.
13. RaziorrouhB, SchrautW, GerlachT, NowackD, GrunerNH, et al. (2010) The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 52 : 1934–1947.
14. NebbiaG, PeppaD, SchurichA, KhannaP, SinghHD, et al. (2012) Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS ONE 7: e47648 doi:10.1371/journal.pone.0047648
15. WuW, ShiY, LiS, ZhangY, LiuY, et al. (2012) Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol 42 : 1180–1191.
16. MainiMK, SchurichA (2010) The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J Hepatol 52 : 616–619.
17. MichelML, DengQ, Mancini-BourgineM (2011) Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: perspectives and challenges. J Hepatol 54 : 1286–1296.
18. KosinskaAD, ZhangE, JohrdenL, LiuJ, SeizPL, et al. (2013) Combination of DNA prime–adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog 9: e1003391 doi:10.1371/journal.ppat.1003391
19. XuDZ, WangXY, ShenXL, GongGZ, RenH, et al. (2013) Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol 59 : 450–456.
20. HuangLR, WohlleberD, ReisingerF, JenneCN, ChengRL, et al. (2013) Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 14 : 574–583.
21. IsogawaM, ChungJ, MurataY, KakimiK, ChisariFV (2013) CD40 activation rescues antiviral CD8+ T cells from PD-1-mediated exhaustion. PLoS Pathog 9: e1003490 doi:10.1371/journal.ppat.1003490
22. GehringAJ, HaniffaM, KennedyPT, HoZZ, BoniC, et al. (2013) Mobilizing monocytes to cross-present circulating viral antigen in chronic infection. J Clin Invest 123 : 3766–3776.
23. Kidd-LjunggrenK, MiyakawaY, KiddAH (2002) Genetic variability in hepatitis B viruses. J Gen Virol 83 : 1267–1280.
24. KohS, ShimasakiN, SuwanaruskR, HoZZ, ChiaA, et al. (2013) A practical approach to immunotherapy of hepatocellular carcinoma using T cells redirected against hepatitis B virus. Mol Ther Nucleic Acids 2: e114.
25. KrebsK, BottingerN, HuangLR, ChmielewskiM, ArzbergerS, et al. (2013) T cells expressing a chimeric antigen receptor that binds hepatitis b virus envelope proteins control virus replication in mice. Gastroenterology 145 : 456–465.
26. SchietingerA, DelrowJJ, BasomRS, BlattmanJN, GreenbergPD (2012) Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 335 : 723–727.
27. SunC, FuB, GaoY, LiaoX, SunR, et al. (2012) TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 8: e1002594 doi:10.1371/journal.ppat.1002594
28. DasA, HoareM, DaviesN, LopesAR, DunnC, et al. (2008) Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 205 : 2111–2124.
29. XuD, FuJ, JinL, ZhangH, ZhouC, et al. (2006) Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol 177 : 739–747.
30. LanP, ZhangC, HanQ, ZhangJ, TianZ (2013) Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology 58 : 73–85.
31. KennedyPT, SandalovaE, JoJ, GillU, Ushiro-LumbI, et al. (2012) Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 143 : 637–645.
32. MiccoL, PeppaD, LoggiE, SchurichA, JeffersonL, et al. (2013) Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol 58 : 225–233.
33. JiC, SastryKS, TiefenthalerG, CanoJ, TangT, et al. (2012) Targeted delivery of interferon-alpha to hepatitis B virus-infected cells using T-cell receptor-like antibodies. Hepatology 56 : 2027–2038.
34. LanfordRE, GuerraB, ChavezD, GiavedoniL, HodaraVL, et al. (2013) GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 144 : 1508–1517.
35. SchurichA, PallettLJ, LubowieckiM, SinghHD, GillUS, et al. (2013) The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog 9: e1003208 doi:10.1371/journal.ppat.1003208
36. PeppaD, GillUS, ReynoldsG, EasomNJ, PallettLJ, et al. (2013) Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med 210 : 99–114.
37. SitiaG, AiolfiR, Di LuciaP, MainettiM, FiocchiA, et al. (2012) Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A 109: E2165–2172.
38. ShouvalD (2013) Focus: quantitative HBsAg measurement as a new surrogate marker for assessment of hepatic fibrosis in HBeAg+ chronic hepatitis B. J Hepatol 58 : 1063–1064.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



