-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
In this study, B cell function in protective TH2 immunity against N. brasiliensis infection was investigated. Protection against secondary infection depended on IL-4Rα and IL-13; but not IL-4. Protection did not associate with parasite specific antibody responses. Re-infection of B cell-specific IL-4Rα−/− mice resulted in increased worm burdens compared to control mice, despite their equivalent capacity to control primary infection. Impaired protection correlated with reduced lymphocyte IL-13 production and B cell MHC class II and CD86 surface expression. Adoptive transfer of in vivo N. brasiliensis primed IL-4Rα expressing B cells into naïve BALB/c mice, but not IL-4Rα or IL-13 deficient B cells, conferred protection against primary N. brasiliensis infection. This protection required MHC class II compatibility on B cells suggesting cognate interactions by B cells with CD4+ T cells were important to co-ordinate immunity. Furthermore, the rapid nature of these protective effects by B cells suggested non-BCR mediated mechanisms, such as via Toll Like Receptors, was involved, and this was supported by transfer experiments using antigen pulsed Myd88−/− B cells. These data suggest TLR dependent antigen processing by IL-4Rα-responsive B cells producing IL-13 contribute significantly to CD4+ T cell-mediated protective immunity against N. brasiliensis infection.
Published in the journal: . PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003662
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003662Summary
In this study, B cell function in protective TH2 immunity against N. brasiliensis infection was investigated. Protection against secondary infection depended on IL-4Rα and IL-13; but not IL-4. Protection did not associate with parasite specific antibody responses. Re-infection of B cell-specific IL-4Rα−/− mice resulted in increased worm burdens compared to control mice, despite their equivalent capacity to control primary infection. Impaired protection correlated with reduced lymphocyte IL-13 production and B cell MHC class II and CD86 surface expression. Adoptive transfer of in vivo N. brasiliensis primed IL-4Rα expressing B cells into naïve BALB/c mice, but not IL-4Rα or IL-13 deficient B cells, conferred protection against primary N. brasiliensis infection. This protection required MHC class II compatibility on B cells suggesting cognate interactions by B cells with CD4+ T cells were important to co-ordinate immunity. Furthermore, the rapid nature of these protective effects by B cells suggested non-BCR mediated mechanisms, such as via Toll Like Receptors, was involved, and this was supported by transfer experiments using antigen pulsed Myd88−/− B cells. These data suggest TLR dependent antigen processing by IL-4Rα-responsive B cells producing IL-13 contribute significantly to CD4+ T cell-mediated protective immunity against N. brasiliensis infection.
Introduction
Parasitic nematode infections are a significant global public health burden. Infections with Ascaris lumbricoides, Trichuris trichiura and the hookworms Ancylostoma duodenale and Necator americanus occur in a third of the world's population [1]. Individuals frequently suffer from repeated infections and do not develop robust immunity against re-infection [2]. Such infections are significant causes of morbidity, with hookworm infections, for example, being a major cause of childhood anemia in many endemic areas [3]. Effects on cognitive development, as a result of repeated childhood infections have been reported [4], and parasitic larval migrations through the host may exacerbate chronic lung pathologies in endemic areas [5], [6]. To date no licensed vaccines exist against these parasites. To accelerate their development a detailed understanding of host immunity is essential, especially extra intestinal immunity against infective stage larvae [7]. Studies in humans and experimental models of infection have established that TH2 immune responses drive host resolution of primary infections [8], [9].
Key to effective expulsion of murine model parasites, such as Nippostrongylus brasiliensis, Heligmosomoides polygyrus and Trichuris muris, is host expression of IL-4Rα [10]. IL-4Rα is an essential component of the heterodimeric receptors required for IL-4 and IL-13 signalling, which ultimately drive host immune polarisation to TH2. Use of IL-4Rα−/− mice has clearly demonstrated an absolute requirement for IL-4Rα expression in resolving primary nematode infections. This is dependent on IL-4Rα expression on non-hematopoietic cells [11] including smooth muscle cells [12] and epithelial cells [13], [14]. However, IL-4Rα expression on hematopoietic cells does impact on the magnitude of the hosts TH2 response to N. brasiliensis. For example, disruption of IL-4Rα expression on CD4+ T-cells results in a significantly reduced TH2 response to primary N. brasiliensis infection [15] and contributes to optimal control of secondary infection [16]. However, it is not known how IL-4Rα expression on other hematopoietic cells contributes to protection from N. brasiliensis re-infection.
Our understanding of cellular mechanisms underlying protective immunity to helminth re-infection has, until recently, been limited. Protective immunity to nematode infection can occur both in the intestine, in the case of primary N. brasiliensis infection and both primary and secondary H. polygyrus infections, while immunity to secondary N. brasiliensis infections occurs in the lung. In the case of the strictly intestinal parasitic nematode H. polygyrus, rapid resolution of re-infection requires alternatively activated macrophages [17], CD4+ T cells [18], parasite specific type 2 antibody responses [19], [20] and B cell cytokine production [21]. In human infections such as A. lumbricoides and hookworms, which have some analogy to N. brasiliensis infections, the parasites are not confined to the intestine. Here larval migrations through the circulatory and pulmonary systems have resulted in these sites playing important roles in infection induced pathology and parasite killing [7]. Studies with N. brasiliensis show host responses in the lung play a key role in the rapid resolution of re-infection [7], [22]. Furthermore, roles for eosinophils [23], basophils [24] and CD4+ T cells [16], [25], but not B cells [20], in coordinating this immunity have also been demonstrated.
The work we present here addresses how B cells in secondary lymphoid organs (lymph nodes and spleen) can rapidly contribute to the control of recall immunity to N. brasiliensis re-infection. We then test if transfer of these potentially protective B cells can confer protection in a naïve mouse against primary exposure to N. brasiliensis infection. Using this approach, we identified key roles for B cells in immunity to the parasite. Strikingly, this was associated with production of IL-13 by IL-4Rα-sufficient B cells and also required MHCII compatibility and MyD88-expression. Thus, B cells can rapidly contribute to immunity to pathogens through multiple effector functions.
Results
IL-4Rα is essential for immunity against re-infection with N. brasiliensis
To identify a possible role for IL-4Rα in generating protective immunity IL-4Rα−/lox (IL-4Rα sufficient) and IL-4Rα−/− mice were infected with N. brasiliensis. Infection was subsequently cleared by drug treatment before re-infection with 500 L3 larva. Intestinal worm burden was quantified at different time points post-secondary infection (Figure 1A). IL-4Rα−/− mice had significantly higher intestinal worm burdens compared to IL-4Rα−/lox mice at day 5 or 7 post-secondary infection (Figure 1B). Loss of IL-4Rα was associated with multiple defects in known effectors of host TH2 immunity, including decreased mucus production in the lung (Supplementary Figure S1A), decreased IgG1 production and decreased IL-13 production by CD4 T cells and B cells in the lung draining lymph node (Figure 1C–E).
Fig. 1. Protective immunity to N. brasiliensis re-infection is IL-13 and IL-4Rα dependent. 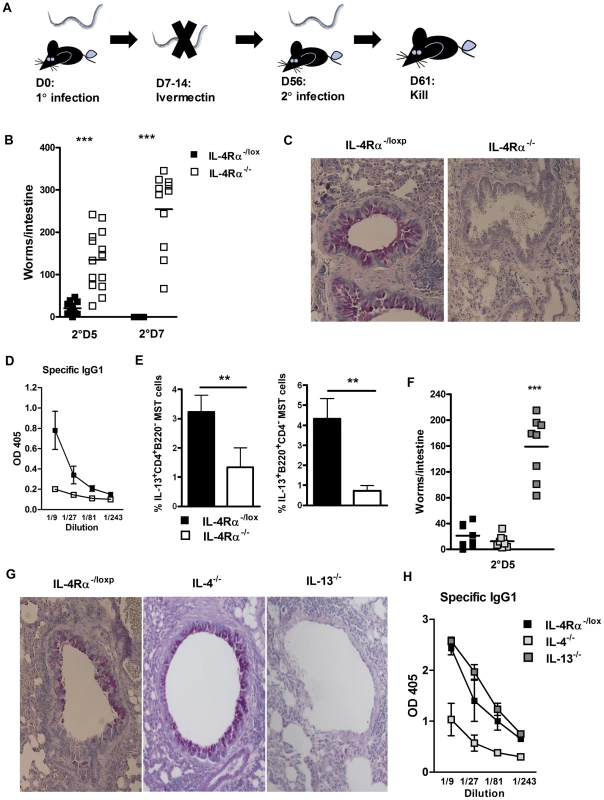
IL-4Rα−/− and IL-4Rα−/lox mice were infected for 5 or 7 days post-secondary N. brasiliensis infection (A). Intestinal worm burdens were then quantified (B). Pulmonary mucus production was established by PAS staining (C). Serum Antibody titers of N. brasiliensis specific IgG1 were determined by ELISA (D). Mediastinal lymph node IL-13 responses were established by intracellular FACS staining in CD4+ T-cell and B220+ B-cell populations (E). IL-4−/−, IL-13−/− and IL-4Rα−/lox mice were infected for 5 days post-secondary N. brasiliensis infection and intestinal worm burdens were then quantified (F). Pulmonary mucus production was established by PAS staining (G). Serum Antibody titers of N. brasiliensis specific IgG1 were determined by ELISA (H). Data is representative of 3–4 independent experiments. n = 4–6 mice per group. IL-4Rα dependent immunity to N. brasiliensis re-infection is driven by IL-13 signalling
To investigate whether resolution of secondary N. brasiliensis infection was dependent on IL-4 and/or IL-13 signalling via IL-4Rα re-infection studies were repeated in IL-4−/− and IL-13−/− mice. Here, the significantly higher intestinal worm burden at day 5 secondary infection in IL-13−/− mice compared to IL-4−/− mice (Figure 1F), demonstrated IL-13 signalling through IL-4Rα is essential for immunity against re-infection with N. brasiliensis. This higher worm burden could was also be associated with an absence in goblet cell mucus production in IL-13−/− mice, but not IL-4−/− mice (Figure 1G and Figure S1B). IL-13−/− mice demonstrated equivalent N. brasiliensis specific IgG1 antibody titers as IL-4Rα−/lox mice. Conversely, IL-4−/− mice demonstrated reduced specific IgG1 responses (Figure 1H). Taken together, these data (Figures 1) indicate that IL-4-depedent antigen specific IgG1 antibody responses may not be required for optimal immunity to re-infection, but that IL-4Rα-mediated IL-13 signalling is required.
IL-4Rα-responsive B cells producing IL-13 are required for effective immunity to N. brasiliensis re-infection
IL-4Rα-mediated effects on B cell function during N. brasiliensis re-infection were investigated in MB1CreIL-4Rα−/lox BALB/c mice, which have B cell-specific abrogation of IL-4Rα expression [26]. Secondary infection resulted in a significantly higher intestinal worm burden in MB1CreIL-4Rα−/lox mice when compared to IL-4Rα−/lox mice (Figure 2A). Whilst goblet cell hyperplasia in MB1CreIL-4Rα−/lox mice was equivalent to that seen in control IL-4Rα−/lox mice (Figure 2B and Figure S1C). Antigen specific IgG1 was significantly drastically reduced (Figure 2C). Interestingly, IL-13 cytokine production by T and B cells was also reduced in MB1CreIL-4Rα−/lox mice, when compared to IL-4Rα−/lox mice (Figure 2D and Figure S2). Together, these studies show that a loss of IL-4Rα on B cells is sufficient to impair immunity to N. brasiliensis re-infection.
Fig. 2. B cell IL-4Rα expression is required for optimal immunity to N. brasiliensis re-infection. 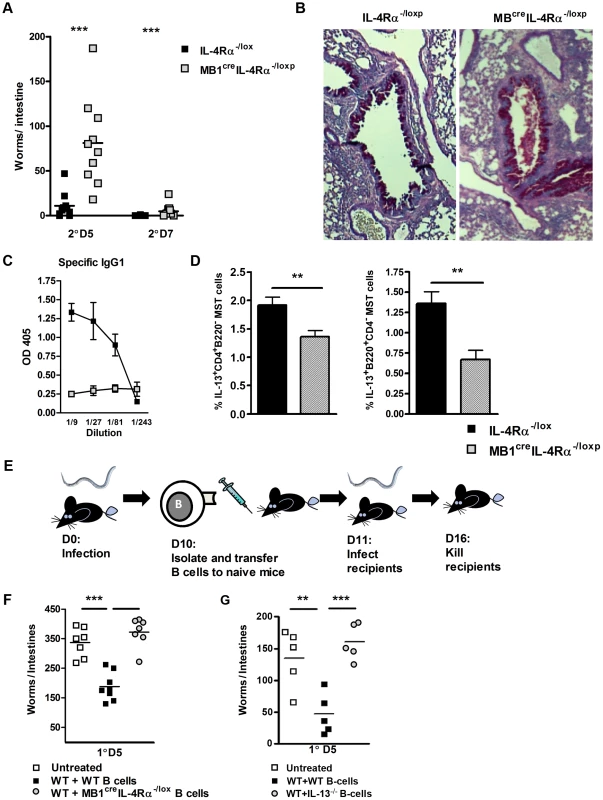
MB1CreIL-4Rα−/lox and IL-4Rα−/lox mice were infected for 5 or 7 days post-secondary N. brasiliensis infection and intestinal worm burdens were then quantified (A). Pulmonary mucus production was established by PAS staining (B). Serum Antibody titers of N. brasiliensis IgG1 were determined by ELISA (C). Mediatstinal lymph node IL-13 responses were established by intracellular FACS staining in CD4+ T-cell and B220+ B cell populations (D). B cells were isolated from N. brasiliensis infected BALB/c, MBcreIL-4Rα−/lox and IL-13−/− and transferred into naïve BALB/c mice (E). Mice were then infected with 500xL3 N. brasiliensis larvae and worm burdens were then established at day 5 post infection (F & G). The results shown represent 2–4 independent experiments. n = 4–7 mice per group. To demonstrate if IL-4Rα responsive and IL-13 competent B cells can directly confer protection against primary N. brasiliensis infection, we adoptively transferred B cells isolated from infected IL-4Rα−/lox, IL-13−/− or MB1CreIL-4Rα−/lox mice into naïve BALB/c mice (Figure 2E and Figure S3). Transfer of antigen-experienced IL-4Rα-responsive B cells into naïve BALB/c mice (WT+WT B-cells) reduced intestinal worm burdens. In contrast, transfer of primed B cells deficient for either the IL-4Rα or IL-13 did not reduce intestinal worm burden (Figure 2F, G). These results support our previous observations (Figures 1 & 2A) that IL-4Rα-responsive and IL-13 competent B cells contribute to protective immunity against N. brasiliensis.
Previous studies have shown that in the absence of B cells control of N. brasiliensis re-infection is similar to WT mice [20]. Our experiments also found that the host's ability to control N. brasiliensis re-infection did not require B cells (Figure 3A). Nevertheless, this does not exclude the possibility that antigen-experienced B cells are able to modulate the response in normal mice. To test this experimentally, we transferred B cells from infected IL-4Rα−/lox or infected MB1CreIL-4Rα−/lox mice into naive μMT mice (Figure 3B). Transferred N. brasiliensis primed IL-4Rα responsive B cells augmented protection in μMT mice (Figure 3B), whereas transferred B cells from infected MB1CreIL-4Rα−/lox mice did not. This B cell IL-4Rα-dependent protection correlated with significant increases in MST CD4+ T cell IL-13 production (Figures 3C). Together these results show that the immune response can compensate for the absence of B cells, but the introduction of pathogen-experienced IL-4Rα-responsive B cells can accelerate protective immunity against N. brasiliensis.
Fig. 3. Transfer of N. brasiliensis experienced B cells enhances immunity to N. brasiliensis independently of endogenous B cell populations. 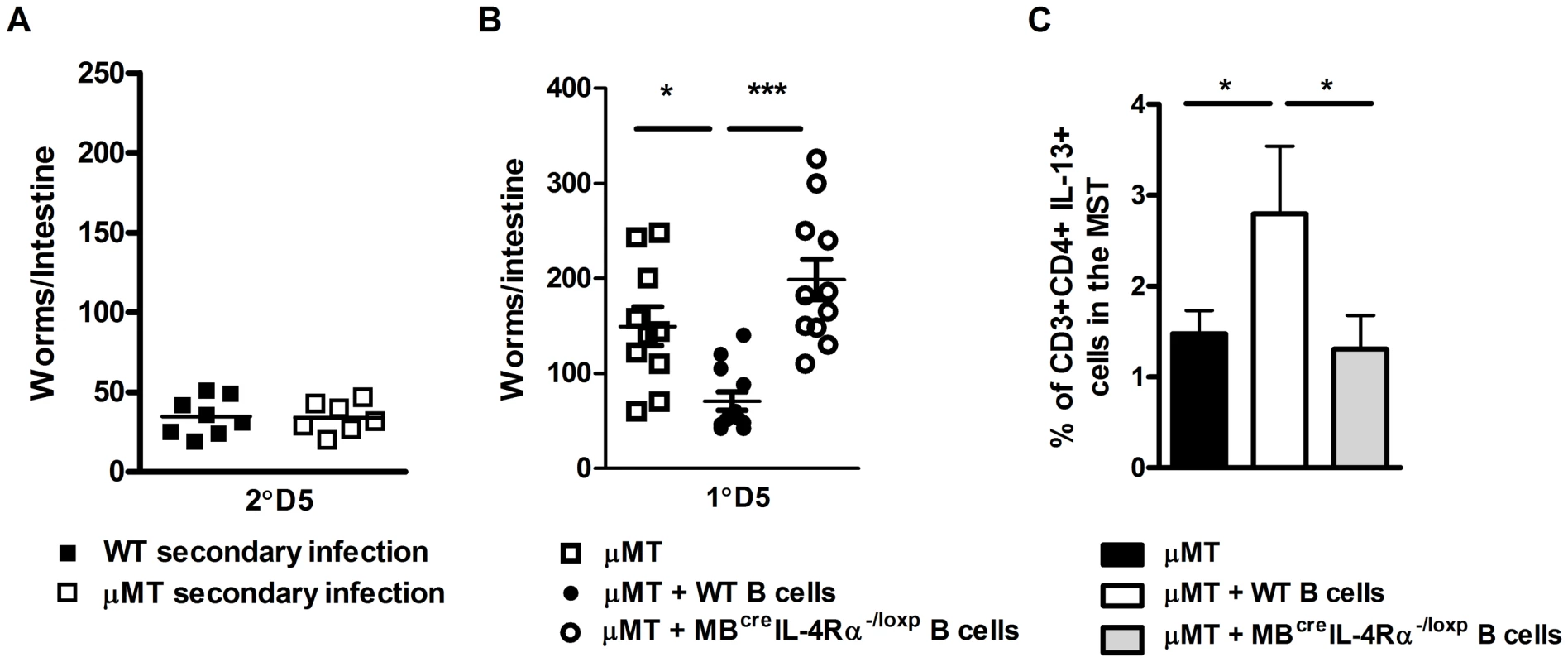
N. brasiliensis infected μMT and BALB/c mice were re-infected with 500xL3 larvae and at day 5 post-secondary infection, the intestinal worm burdens was quantified (A). The possible role for IL-4Rα expressing B cells in boosting immunity independently of endogenous B cells was determined by transfer of B cells isolated from N. brasiliensis infected IL-4Rα−/lox (WT B cells) or MB1Cre IL-4Rα−/lox (IL-4Rα−/− B cells) into naïve μMT mice. These mice were then infected with 500xL3 N. brasiliensis and worm burdens quantified at day 5 post infection (B). Mediastinal lymph node CD3+CD4+ T cell populations IL-13 responses (C) were established by FACS staining. Results shown represent 2 independent experiments. n = 4–7 mice per group. Enhanced B cell ability to interact with T helper cells is required for optimal immunity to N. brasiliensis re-infection
Optimal host control of N. brasiliensis re-infection was associated with B cell IL-13 production and enhanced CD4+ T cell TH2 responses. A further way B cells could contribute to T cell responses is through cognate, physical interactions that are associated with antigen-presentation through MHCII and co-stimulatory molecule expression. As a first step in demonstrating, if such an interaction is also a feature of IL-4Rα-dependent immunity to N. brasiliensis re-infection, we initially assessed expression of CD86 and MHCII on CD19+ B cells from naive and N. brasiliensis re-infected mice. CD86 and MHCII expression was equivalent between naive mice of both groups (Figure 4A, lower panel). In re-infected mice, CD86 and MHCII surface expression in MB1CreIL-4Rα−/lox mice was reduced when compared with IL-4Rα−/lox mice (Figure 4B, lower panel). CD4+ T cells showed no differences between the mouse strains in expression of CD28 and TCR. (Figure 4A and B, upper panel). Thus, IL-4Rα expression in B cells can help enhance the expression of B cell markers of activation after N. brasiliensis infection.
Fig. 4. B cell MHCII antigen presentation mediates optimal immunity to N. brasiliensis. 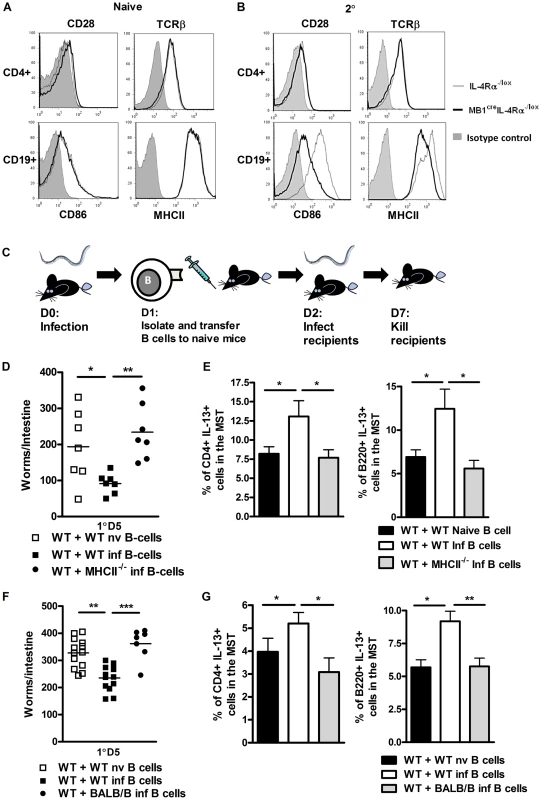
Surface expression of CD28 and TCR on CD4+ T cells and CD86 and MHCII on B cells in naive (A) and N. brasiliensis re-infected (B) IL-4Rα−/lox mice and MB1CreIL-4Rα−/lox mice was established by FACS analysis. Histograms: filled gray: isotype control, thin line: IL-4Rα−/lox, thick black line: MB1CreIL-4Rα−/lox. Contributions by MHCII dependent antigen presentation were demonstrated by isolating WT or MHCII−/− B cells from naive or infected mice then adoptively transferring into naive C57BL/6 mice (C). Mice were then infected with 500xL3 N. brasiliensis larvae and worm burdens were established at day 5 post infection (D). Mediastinal lymph node IL-13 responses were established by intracellular FACS staining in CD4+ T-cell and B220+ B cell populations (E). MHC dependent antigen presentation was confirmed by isolating WT and BALB/b B cells from naive or infected mice adoptively transferring into naive BALB/c mice. Mice were then infected with 500xL3 N. brasiliensis larvae and worm burdens were established at day 5 post infection (F). Mediastinal lymph node IL-13 responses were established by intracellular FACS staining in CD4+ T-cell and B220+ B cell populations (G). Data is representative of 2 independent experiments. n = 4–6 mice per group. These findings indicated B cell cognate interactions with T cells and also antigen presentation may contribute to optimal immunity against N. brasiliensis re-infection. Our data presented in Figures 1 and 2 also indicates that B cell immunity may be independent of antibody class switching. This may exclude involvement of highly specific clonally expanded populations of B cells. We therefore hypothesized that protection may instead be mediated by a rapidly modified B cell antigen presenting response to N. brasiliensis infection. To demonstrate the possible role for antigen presentation, we adoptively transferred MHCII−/− B cells from 1 day N. brasiliensis infected mice (Figure 4C) into naive mice. At the next day, mice were infected and days 5 post infection, recipients of MHCII−/− B cells showed significantly higher worm burdens than mice, which received control WT B cells (Figure 4D). Protection was associated with increased IL-13 production by both B and T cell populations in the mediastinal lymph node (Figure 4E). To further control MHCII dependency, similar infection experiments were carried out in BALB/b mice, which are unable to present antigen via MHCII to BALB/c B cells. BALB/c recipients of adoptively transferred N. brasiliensis-experienced BALB/b B cells showed also significantly higher worm burdens, when compared to mice which received BALB/c B cells from N. brasiliensis infected mice (Figure 4F). These results further support that MHCII-dependent antigen presentation by B cells does contribute to host immunity to N. brasiliensis. Again, protection was associated with increased IL-13 production by both B and T cell populations in the mediastinal lymph node (Figure 4G). Together, these results suggest that MHCII expression contributes to the B cell protective response to N. brasiliensis re-infection.
B cells can rapidly launch protective antigen-dependent responses to N. brasiliensis infection
Our results presented in Figure 4 suggested that antigen-experienced B cells can rapidly contribute to protection. We therefore tested in vivo whether immunity induced early in infection with N. brasiliensis is dependent on IL-4Rα-responsive B cells. Adoptive transfer of IL-4Rα responsive B cells from IL-4Rα−/loxp mice isolated from mice 1 day post N. brasiliensis infection into wild type mice (Figure 4C) [27], [28] enhanced protection (Figure 5A), but not adoptive transfer of IL-4Rα unresponsive B cells from MB1creIL-4Rα−/loxp mice. Furthermore, only transfer of IL-4Rα responsive B cells enhanced B and CD4+ T cell IL-13 responses in the lung (Figure 5B, C) and mediastinal lymph node (Figure 5D), strengthen the necessity of IL-4Rα-responsive B cells for protective immunity.
Fig. 5. Rapid IL-Rα dependent B cell mediated protection against N. brasiliensis occurs in the lung. 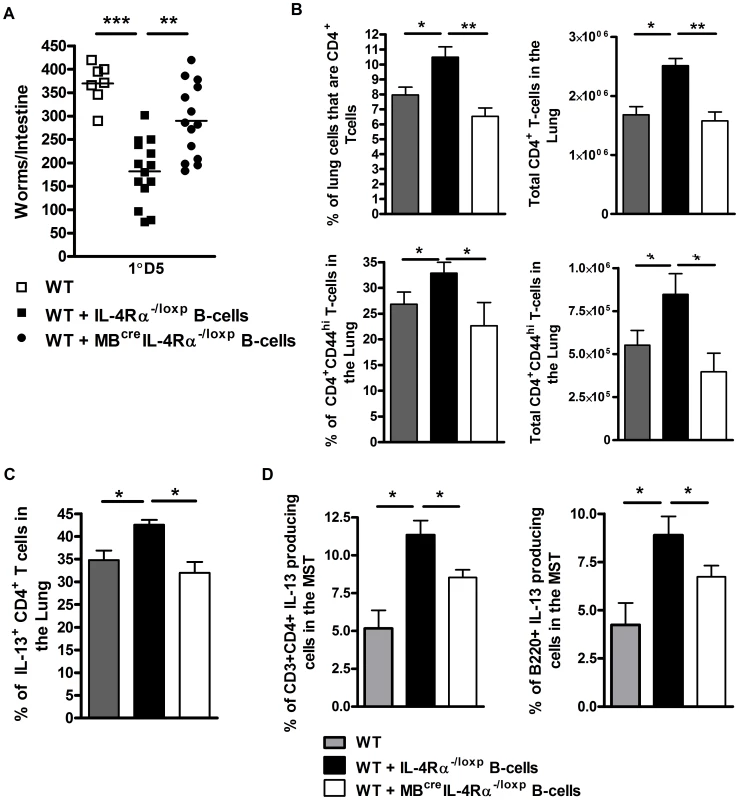
MBcreIL-4Rα−/lox and IL-4Rα−/lox mice were infected for 1 day with N. brasiliensis before spleen B cells were isolated and transferred into naive wild type mice (As in Figure 4C). These were infected with 500xL3 N. brasiliensis and intestinal worm burdens were quantified at day 5 post infection (A). Lung CD3+CD4+ and CD3+CD4+CD44hi T cell populations were analysed by FACS staining (B). Lung CD4+ T cell (C) and mediastinal lymph node CD4+ T cell and B220+ B cell population (D) IL-13 responses were established by intracellular FACS staining. Data is representative of 2 independent experiments. n = 4–6 mice per group. This ability of B cells to confer protection so rapidly after parasite exposure further supports this response being independent of BCR. Other mechanisms of more rapid and possibly less stringent/polyfunctional antigen recognition by B cells may therefore play a role. Initial analysis does not support these transferred B cells conferring protection via an early production of IL-13 (Figure S5). However, rapid antigen processing and presentation may be mediated by B cells directly loading soluble peptide onto MHCII [29], [30] or via antigen internalisation and processing by Toll like receptors (TLR) [31], [32], [33].
To assess if rapid TLR mediated antigen processing contributed to reduced worm burdens, we repeated transfer experiments using B cells isolated from MyD88−/− mice at one day post infection. Mice, which received MyD88−/− B cells displayed significantly higher worm burdens than those which received B cells from WT 1 day infected recipients (Figure 6A). Protection was associated with increased IL-13 production by T cell populations in the mediastinal lymph node (Figure 6B). We then examined whether these effects were due to direct exposure of antigen by B cells by pulsing naive WT or MyD88−/− B cells overnight with N. brasiliensis antigen (Figure 6C). Transfer of B cells from MyD88−/− mice into naive mice resulted in impaired control of infection (Figure 6D), associated with lower IL-13 production by T cells in the mediastinal lymph node (Figure 6E). To rule out non-specific effects, we also pulsed wild type B cells with a range of antigens. We found that only B cells pulsed with N. brasiliensis antigen conferred a reduction in host intestinal worm burdens. Recipients of B cells pulsed with LPS (a potential bacterial contaminant during N. brasiliensis infection), ovalbumin and soluble Leishmania major antigen did not show any reduction in intestinal worm burden when compared to wild type controls (Figure S6). This data indicated that the reduction in worm burden that we see in recipients of N. brasiliensis pulsed B cells is pathogen specific. Moreover, pulsing of MHCII−/− B cells with N. brasiliensis also resulted in impaired reduction in worm burdens (Figure 6F). Together these data suggest an association between rapid pathogen specific MyD88 dependent antigen processing and MHCII antigen presentation by B cells underlying the accelerated host immunity to N. brasiliensis infection.
Fig. 6. B cell MyD88 expression dependent protection against N. brasiliensis infection. 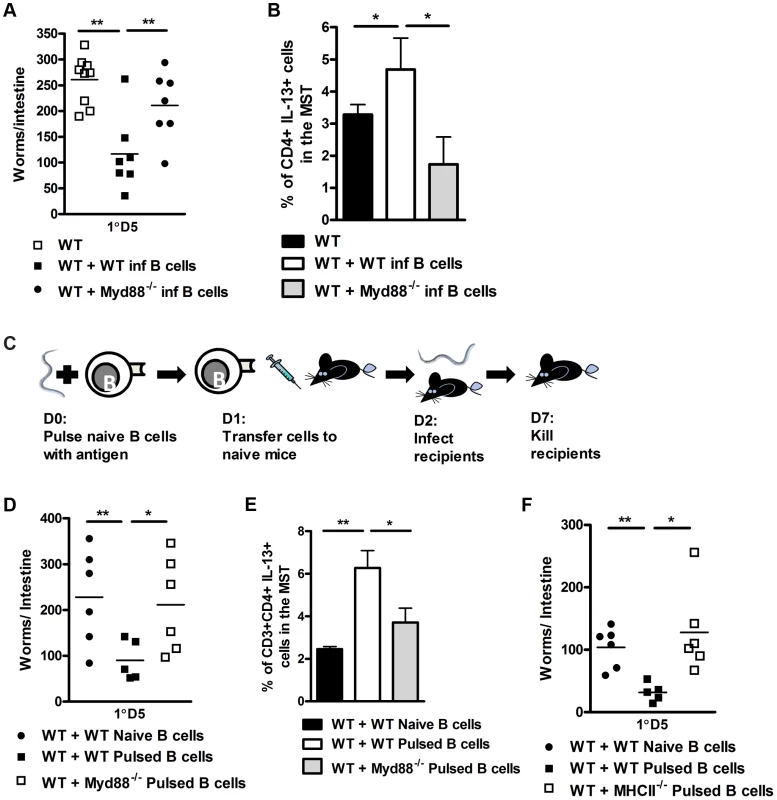
B cells were isolated from WT or MyD88−/− mice 24 hours post N. brasiliensis infection and adoptively transferred into naive WT mice (As in Figure 4C). 24 hours later these mice were infected with 500xL3 N. brasiliensis and subsequently killed 5 days post infection and worm burdens were counted (A). The mediastinal lymph node CD4+ T-cell IL-13 response was established (B). B cells were isolated from naive C57/BL6, MyD88−/− or MHCII−/− mice and pulsed with N. brasiliensis antigen overnight. These were then washed and transferred into naive C57/BL6 mice 24 h prior to infection (C). D5 PI intestinal worm counts are shown (D & F). The mediastinal lymph node CD4+ T-cell IL-13 response was established (E). Data is representative of 2 experiments, n = 5–7 mice per group. Discussion
This study demonstrated that IL-4Rα responsive B cells co-ordinate optimal immunity to secondary N. brasiliensis infection. This was related to B cell IL-13 expression, not IL-4 expression. B cell IL-4Rα mediated protection was associated with increased B cell and CD4 T cell IL-13 production. MHCII dependent B cell priming of T cells associated with this effect. Our data also demonstrated a rapid poly-functional antigen processing associated with B cell Myd88 expression.
B cell responses to N. brasiliensis have been suggested to be largely redundant [20]. Both our current study and that of Liu et al (18) demonstrate an absence of B cells per se does not alter host ability to control N. brasiliensis infection. However, we now show that a molecular change in B cell function, such as cell specific disruption of IL-4Rα expression on B cells, significantly impairs host ability to resolve N. brasiliensis infection. These findings also demonstrate important differences between B cell dependent immunity to N. brasiliensis and H. polygyrus. B cell Be2 immunity to H. polygyrus is dependent on B cell IL-4 production, B cell IL-4Rα expression and antigen presentation. As with H. polygyrus, N. brasiliensis re-infection is also dependent on B cell IL-4Rα expression and antigen presentation, however, B cell IL-13 production appears to play a functional role and not IL-4.
The protective B cell response we demonstrate may be independent of antibody and instead mediated through a B effector response. Such responses are particularly important in controlling CD4+ T cell driven immunity [34] via direct B and T cell interactions [35] as well as B cell cytokine production [36]. These B effector responses have an equivalent diversity in immune polarisation as T cells; producing Be1 (TH1) [35], Be2 (TH2) [36] and Breg (Treg) [37], [38] effector B cells respectively. Functionally Be1 cells contribute significantly to immunity to bacterial infections, such as Salmonella [39], [40], [41]. In helminth infections Be2 cells have been demonstrated to be important for immunity to H. polygyrus [21], although humoral contributions also play a significant role [19], [20]. Evidence of Bregs induced by both Heligmosomoides polygyus and Schistosoma mansoni infection have elegantly shown helminth elicited B cell control of allergy [38], [42].
In this study we show that B cells develop a rapid and potent protective response against N. brasiliensis infection. This rapid protection precludes BCR-dependent clonal expansion following antigen exposure. Instead, it appears B cells are capable of responding to antigen via less stringent mechanisms than the BCR, such as direct peptide loading and Toll like receptors [29], [30]. Antigen binding by TLRs is established as an important regulator of B cell function [32]. TLR-dependent B cell responses can increase BCR-dependent antigen presentation [31], B cell cytokine production [39], [40] and play pivotal roles in B cell ability to interact with T cells [33]. These TLR mediated responses to antigen by B cells can be rapid and may not require clonal expansion of B cells [27].
In addition to antigen presentation we also demonstrate IL-4Rα-dependent increases in IL-13 production by endogenous B cells to be associated with control of secondary infection. This along with B cell-dependent induction of IL-13 production by endogenous CD4+ T cell and B cells would provide an important source of IL-13 to activate potential effector cell populations, including epithelial [13], [43], smooth muscle [12], [44] and innate immune cells [24], [45], [46].
In summary this study demonstrates IL-4Rα-responsive B cells playing an important role resolving secondary N. brasiliensis infection. We suggest the protective role played by B cells develops from antigen encounter with TLR driving an increase in CD86 and MHCII dependent interactions with CD4 T cells. This drives increased IL-13 production by CD4 T cells and B cells, facilitating host launching of protective mechanisms against N. brasiliensis infection.
Methods
Animals used
In this study the following BALB/c background mice were used: BALB/c, BALB/b, IL-4Rα−/− [described as Il4ratm1Fbb/Il4ratm1Fbb], IL-13−/−, IL-4−/−and μMT. BALB/c background B cell specific IL-4Rα deficient MB1CreIL-4Rα−/lox [described as Il4ratm1Fbb/Il4ratm2FbbTg (Cd79atm1(cre)Reth)] were generated as previously described [26]. MHCII−/−, MyD88−/− and C57BL/6 mice were on C57BL/6 genetic background. Mice were bred and housed in specific pathogen–free conditions at the University of Cape Town, South Africa, and used in accordance with University Ethical Committee guidelines. All experimental mice were sex matched and used between 6–12 weeks of age with appropriate littermate controls of the same generation.
Ethics statement
All studies were carried out under protocol 008/019 approved by the University of Cape Town Faculty of Health Sciences Animal Ethics Committee in accordance national guidelines laid down by the South African Board of Standards.
N. brasiliensis infection
Mice were initially inoculated subcutaneously with 500 N. brasiliensis L3 larvae. At day 7 post infection worms were cleared by treatment with 10 mg/ml Ivermectin in drinking water for 7 days. Mice were then shelved for 21 days prior to a secondary subcutaneous infection with 500 N. brasiliensis L3 larvae. Mice were killed at day 5 post-secondary infection by CO2 inhalation. Adult worm burdens were determined as previously described [12]. Briefly, intestines were removed from infected mice and the lumen exposed by dissection. Intestines were then incubated at 37°C for 4 h in 0.65% NaCl. Intestinal tissue was then removed and the worms in the remaining saline solution counted.
Histology
Tissue samples were fixed in a neutral buffered formalin solution. Following embedding in paraffin, samples were cut into 5 µm sections. Sections were stained with periodic acid-Schiff reagent (PAS) in order to visualise goblet cell hyperplasia [15]. The Histological Mucus Index (HMI) was used to quantify PAS positive airway epithelial cells. Sections photographed at 100× were overlaid with a standard grid. The number of grid units containing PAS positive epithelial goblet cells were divided by all units containing epithelial cells to establish the HMI.
Determination of antibody titres
Parasite specific serum antibody levels from infected animals were determined as previously described [15]. Briefly, flat-bottom 96-well plates were coated overnight with 10 µg/ml of N. brasiliensis antigen. The plates were then washed and incubated in PBS containing 2% milk powder v/v for 1 h at 37°C. Following this, the plates were washed, samples loaded and incubated overnight at 4°C. Appropriate biotinylated secondary antibodies were then added following further washing and incubated overnight at 4°C. The plates were then washed, and antibody titres were determined using streptavidin-coupled horseradish peroxidase. The plates were developed with the TMB microwell peroxidase substrate system, and the reaction was stopped with 1 M H3PO4. The absorbance at 450 nm was determined with a Versamax microplate spectrophotometer (Molecular Devices, Germany).
FACS analysis
The expression of surface receptors involved in B and T cell interactions were measured on mediastinal lymph node cells. Essentially, CD40-PE (clone 3/23), CD28-PE (clone 37.51) and TCRβ-biotin (clone H57-597) antibodies were used to detect receptors on CD4+ (clone GK1.5) T cells, while CD40L-PE (clone MR1), CD86-PE (clone GL1) and MHCII-bio (clone M5/114) were used to detect receptors on CD19+ (clone 1D3) B cells in IL-4Rα−/lox and mb1CreIL-4Rα−/lox mice. Biotin-labeled antibodies were detected by streptavidin-APC, anti-FcR (clone 2.4G2) was used to block non-specific binding of immunoglobulins to the FcγII/III receptors and dead cells were excluded from analysis by 7-AAD staining (Sigma). Antibodies were from BD Pharmingen (San Diego, CA). Cells were acquired using a FACSCalibur (Beckton-Dickinson, Ferndale, South Africa) and data were analysed with Flowjo software (Treestar).
Intracellular cytokine staining was performed on mediastinal lymph node cells re-suspended in complete media (IMDM (GIBCO/Invitrogen; Carlsbad, CA), 10% FCS, P/S) at 2.5×107/ml and stimulated with 10 µg/ml of N. brasiliensis antigen and GolgiStop (as per manufacturer's protocol; BD Pharmingen) at 37°C for 4 hours. After re-stimulation, cells were surface stained for CD3 (clone 500A2), CD4 (clone GK1.5) and B220 (clone RA3-6B2), then fixed and permeabilized with Cytofix/Cytoperm Plus (as per manufacturer's instructions; BD Pharmingen). Intracellular staining was performed by staining cells with IL-13-PE (ebio 13a) or appropriately labelled isotype control (eBioscience) [47].
Sorting of B cells for adoptive transfer
Single cell suspensions from spleen were surface labelled with CD19 and B220 antibodies described above, re-suspended at 1×107 cells/ml in media and sorted with a BD FACSARIA cell sorter (Supplementary Figure S3A). The purity of the isolated population was confirmed by flow cytometry, and samples showing <95% positive cells were discarded (Supplementary Figure S3B). Then the isolated B cells from naïve or infected mice were adoptively transferred to naive mice. The cells were re-suspended at 2.5×106 cells/ml in media. Each mouse received 0.5×106 B cells injected intravenously into the tail vein 24 h prior to infection with N. brasiliensis.
In vitro B cell antigen pulsing
Naïve B cells were isolated from a single splenocyte suspension by FACSARIA as described above. Purity was confirmed by flow cytometry, samples showing <95% B220 positive cells were discarded (Supplementary Figure S3). Cells were incubated (pulsed) with 10 µg/ml N. brasiliensis antigen, ovalbumin, LPS or soluble Leishmania antigen for 16 h at 37°C. Cells were then washed 3× in media by centrifugation and then re-suspended in media at 2.5×107/ml. 0.5×106 cells were then transferred intravenously into naïve mice 24 h prior to infection with N. brasiliensis [27], [28].
Generating N. brasiliensis somatic antigen
L3 larvae were washed from filter paper into H2O/50 µg/ml Penicillin+Streptomycin and allowed to stand for an hour during which the larvae settle to the bottom of the container, after which the larvae washed once more in H2O/Pen./Strep and once in H2O. Then the larvae were concentrated into 2 ml of distilled H2O and snap frozen in liquid nitrogen. Following this the preparation was homogenized for 5 to 10 minutes before the whole solution is centrifuged at 10 000 rpm for 10 minutes. The supernatant contains the soluble fraction of the L3 larvae proteins which is measured and standardised using a BCA protein assay (Pierce; Chicago, IL). Antigen was added to the cells in solution at 10 µg/ml.
Statistics
Values are expressed below as means ± standard deviations and significant differences were determined using either Mann-Whitney U test or ANOVA (GraphPad Prism4).
Supporting Information
Zdroje
1. HotezPJ, BrindleyPJ, BethonyJM, KingCH, PearceEJ, et al. (2008) Helminth infections: the great neglected tropical diseases. J Clin Invest 118 : 1311–1321.
2. BethonyJ, BrookerS, AlbonicoM, GeigerSM, LoukasA, et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367 : 1521–1532.
3. HotezPJ (2007) Hookworm and Poverty. Ann N Y Acad Sci 1136 : 38–44.
4. Jardim-BotelhoA, RaffS, Rodrigues RdeA, HoffmanHJ, DiemertDJ, et al. (2008) Hookworm, Ascaris lumbricoides infection and polyparasitism associated with poor cognitive performance in Brazilian schoolchildren. Trop Med Int Health 13 : 994–1004.
5. MarslandBJ, KurrerM, ReissmannR, HarrisNL, KopfM (2008) Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur J Immunol 38 : 479–488.
6. MuroA, Perez-ArellanoJL (2010) Nitric oxide and respiratory helminthic diseases. J Biomed Biotechnol 2010 : 958108.
7. HarvieM, CamberisM, TangSC, DelahuntB, PaulW, et al. (2010) The lung is an important site for priming CD4 T cell mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 78 (9) 3753–62.
8. WrightV, BickleQ (2005) Immune responses following experimental human hookworm infection. Clin Exp Immunol 142 : 398–403.
9. TurnerJD, FaulknerH, KamgnoJ, CormontF, Van SnickJ, et al. (2003) Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J Infect Dis 188 : 1768–1775.
10. FinkelmanFD, UrbanJFJr (2001) The other side of the coin: the protective role of the TH2 cytokines. J Allergy Clin Immunol 107 : 772–780.
11. UrbanJFJr, Noben-TrauthN, SchopfL, MaddenKB, FinkelmanFD (2001) Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol 167 : 6078–6081.
12. HorsnellWG, CutlerAJ, HovingJC, MearnsH, MyburghE, et al. (2007) Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog 3: e1.
13. HerbertDR, YangJQ, HoganSP, GroschwitzK, KhodounM, et al. (2009) Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med 206 : 2947–2957.
14. NieuwenhuizenN, HerbertDR, BrombacherF, LopataAL (2009) Differential requirements for interleukin (IL)-4 and IL-13 in protein contact dermatitis induced by Anisakis. Allergy 64 : 1309–1318.
15. MearnsH, HorsnellWG, HovingJC, DewalsB, CutlerAJ, et al. (2008) Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology. Infect Immun 76 : 5535–5542.
16. ThawerSG, HorsnellWG, DarbyM, HovingJC, DewalsB, et al. (2013) Lung-resident CD4 T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol [epub ahead of print] doi:10.1038/mi.2013.40
17. AnthonyRM, UrbanJFJr, AlemF, HamedHA, RozoCT, et al. (2006) Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 12 : 955–960.
18. MorimotoM, WhitmireJ, XiaoS, AnthonyRM, MirakamiH, et al. (2004) Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol 172 : 2424–2430.
19. McCoyKD, StoelM, StettlerR, MerkyP, FinkK, et al. (2008) Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4 : 362–373.
20. LiuQ, KreiderT, BowdridgeS, LiuZ, SongY, et al. (2010) B cells have distinct roles in host protection against different nematode parasites. J Immunol 184 : 5213–5223.
21. WojciechowskiW, HarrisDP, SpragueF, MousseauB, MakrisM, et al. (2009) Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity 30 : 421–433.
22. KnottML, MatthaeiKI, GiacominPR, WangH, FosterPS, et al. (2007) Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol 37 : 1367–1378.
23. GiacominPR, GordonDL, BottoM, DahaMR, SandersonSD, et al. (2008) The role of complement in innate, adaptive and eosinophil-dependent immunity to the nematode Nippostrongylus brasiliensis. Mol Immunol 45 : 446–455.
24. OhnmachtC, VoehringerD (2009) Basophil effector function and homeostasis during helminth infection. Blood 113 : 2816–2825.
25. VoehringerD, ShinkaiK, LocksleyRM (2004) Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20 : 267–277.
26. HovingJC, KirsteinF, NieuwenhuizenNE, FickLC, HobeikaE, et al. (2011) B Cells That Produce Immunoglobulin E Mediate Colitis in BALB/c Mice. Gastroenterology 142 (1) 96–108.
27. ChappellCP, DravesKE, GiltiayNV, ClarkEA (2012) Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J Exp Med 209 : 1825–1840.
28. Flores-LangaricaA, MarshallJL, BobatS, MohrE, HitchcockJ, et al. (2011) T-zone localized monocyte-derived dendritic cells promote Th1 priming to Salmonella. Eur J Immunol 41 : 2654–2665.
29. BernasconiNL, TraggiaiE, LanzavecchiaA (2002) Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298 : 2199–2202.
30. JellisonER, GuayHM, Szomolanyi-TsudaE, WelshRM (2007) Dynamics and magnitude of virus-induced polyclonal B cell activation mediated by BCR-independent presentation of viral antigen. Eur J Immunol 37 : 119–128.
31. PasareC, MedzhitovR (2005) Control of B-cell responses by Toll-like receptors. Nature 438 : 364–368.
32. RuprechtCR, LanzavecchiaA (2006) Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol 36 : 810–816.
33. SayiA, KohlerE, TollerIM, FlavellRA, MullerW, et al. (2011) TLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J Immunol 186 : 878–890.
34. LundFE (2008) Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol 20 : 332–338.
35. HarrisDP, HaynesL, SaylesPC, DusoDK, EatonSM, et al. (2000) Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1 : 475–482.
36. HarrisDP, GoodrichS, MohrsK, MohrsM, LundFE (2005) Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol 175 : 7103–7107.
37. BouazizJD, YanabaK, TedderTF (2008) Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 224 : 201–214.
38. AmuS, SaundersSP, KronenbergM, ManganNE, AtzbergerA, et al. (2010) Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol 125 : 1114–e1118, 1114-1124, e1118.
39. BarrTA, BrownS, MastroeniP, GrayD (2009) B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol 183 : 1005–1012.
40. BarrTA, BrownS, MastroeniP, GrayD (2010) TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol 185 : 2783–2789.
41. MorrisonVL, BarrTA, BrownS, GrayD (2010) TLR-mediated loss of CD62L focuses B cell traffic to the spleen during Salmonella typhimurium infection. J Immunol 185 : 2737–2746.
42. WilsonMS, TaylorMD, O'GormanMT, BalicA, BarrTA, et al. (2010) Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol 40 : 1682–1696.
43. CliffeLJ, HumphreysNE, LaneTE, PottenCS, BoothC, et al. (2005) Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308 : 1463–1465.
44. HorsnellWG, ViraA, KirsteinF, MearnsH, HovingJC, et al. (2010) IL-4Ralpha-responsive smooth muscle cells contribute to initiation of T(H)2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol 4 (1) 83–92.
45. VoehringerD, ReeseTA, HuangX, ShinkaiK, LocksleyRM (2006) Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med 203 : 1435–1446.
46. ZhaoA, UrbanJFJr, AnthonyRM, SunR, StiltzJ, et al. (2008) Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135 : 217–e211, 217-225, e211.
47. MohrE, CunninghamAF, ToellnerKM, BobatS, CoughlanRE, et al. (2010) IFN-{gamma} produced by CD8 T cells induces T-bet-dependent and -independent class switching in B cells in responses to alum-precipitated protein vaccine. Proc Natl Acad Sci U S A 107 : 17292–17297.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



