-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
article has not abstract
Published in the journal: . PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003612
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003612Summary
article has not abstract
Soon after birth, the human body establishes an intimate association with trillions of microbial cells belonging to numerous bacterial and fungal species [1]. These organisms coinhabit diverse microbial communities on cutaneous and mucosal surfaces such as the skin, the gastrointestinal tract, and the vaginal cavity [2]. Fungal and bacterial cells can interact in many ways, such as direct physical contact, secretion of toxins and signalling molecules, sharing or competing for metabolites and nutrients, and alteration of the environment [3]. These interactions can be antagonistic as well as mutually beneficial. Both bacteria and fungi have evolved sophisticated mechanisms to sense and respond to the presence and activity of other species nearby. In hospitals, fungi and bacteria are frequently isolated from the same site of infection. This raises the important question of whether fungi and bacteria interact in the process of infection, and whether the interaction dictates disease development and outcome, and if so, how they do so. This review summarizes recent discoveries in the study of signal sensing in the fungal pathogen Candida albicans. New findings support a model that adenylyl cyclases act as a hub of signal sensing and integration and may play a central role in bacterial sensing during fungal infection.
cAMP Signalling Plays a Major Role in Regulating Cellular Responses to Environmental Signals and in Virulence in C. albicans
C. albicans is frequently found as a benign member of the normal microflora of humans. However, when conditions are favourable, it can cause a range of localized superficial infections such as rash and thrush in otherwise healthy people. But in immunocompromised patients, C. albicans can initiate life-threatening invasive infections with mortality rates as high as 75% [4]. Several traits of this fungus determine its virulence, including its ability to switch growth forms between yeast, pseudohyphae, and hyphae, expression of surface adhesion proteins, and secretion of proteolytic enzymes. Importantly, these traits are coregulated primarily by the cAMP signalling pathway [5]. A central component of this pathway is the cell's sole adenylyl cyclase Cyr1 that catalyses the synthesis of the second messenger 3′-5′-cyclic adenosine monophosphate (cAMP). In response to inducing signals, Cyr1 increases cAMP synthesis that in turn activates protein kinase A (PKA), leading to the expression of virulence genes. cyr1Δ/Δ mutants cannot undergo the yeast-to-hyphae transition and are avirulent [6]. Many external signals of distinct nature such as peptidoglycan (PGN), CO2, pH, and temperature are known to stimulate Cyr1 activity. Then, how does Cyr1 distinguish different stimuli or sense and integrate multiple ones to initiate a proper physiological response?
Fungal Adenylyl Cyclases Are Large Proteins Containing Various Functional Domains Providing Multiple Points for Signal Sensing
Fungal Cyr1s contain several highly conserved domains (Figure 1), from the N - to C-terminus, including a Gα domain, a Ras-association (RA) domain, a leucine-rich repeat (LRR) domain, a protein phosphatase 2C (PP2C) domain, a cyclase catalytic (CYCc) domain, and a Cap1 (cyclase-associated protein 1) binding domain (CBD). In C. albicans, the small GTPase Ras1, when in the GTP-bound form, activates Cyr1 by binding to the RA domain [7]. ras1Δ/Δ mutants are severely compromised in virulence and hyphal growth [8]. Yeast-2-hybrid experiments demonstrated direct association of Ras1 with the RA domain, and mutating conserved residues in RA was shown to block Ras1-Cyr1 interaction and prevent adenylyl cyclase activation [7]. Ras1 is thought to be activated by the guanine nucleotide exchange factor Cdc25 and inactivated by the GTPase-activating protein Ira2 [9]. Currently, it remains unclear as to what regulates the Ras regulatory module. The Gα domain of Cyr1 is thought to be the binding site for a G-protein α subunit Gpa2 that is activated by the G-protein-coupled receptor Gpr1 in response to amino acids and glucose [10], [11]. Deleting either GPR1 or GPA2 caused defects in hyphal formation on solid media in a cAMP-dependent manner. Although Gpa2 has been shown to bind to the Gα domain in fission yeast [12], such interaction has not been demonstrated in C. albicans. Cap1 is a well-known Cyr1-associated and G-actin-binding protein and is required for the activation of fungal adenylyl cyclase. C. albicans cap1Δ/Δ mutants are unable to increase cAMP synthesis upon hyphal induction, fail to undergo the yeast-to-hyphae transition, and are avirulent [13]. Recently, Zou et al. [14] isolated a tripartite protein complex containing Cap1, Cyr1, and G-actin, in which Cap1 serves as a bridge by binding to Cyr1 and G-actin through its N - and C-terminus, respectively. This complex can enhance cAMP synthesis in response to hyphal-induction signals in vitro, and in a manner dependent on Cap1 interaction with G-actin. This study suggests that Cyr1 may be able to sense the status of the actin cytoskeleton, a central player in polarized growth, and influence the cyclase activity. The Cap1 binding site has been mapped to the C-terminal tail of Cyr1 [15]. Raising temperature to above 37°C is normally required for the yeast-to-hyphae transition. The mechanism of temperature sensing was found to involve the heat shock protein complex Hsp90/Sgt1 through physical association with Cyr1 [16], [17]. In S. cerevisiae, Sgt1 was shown to influence cAMP signalling via direct interaction with the LRR domain of Cyr1 [18]. High CO2 concentration, found in many host niches, is another promoter of hyphal growth. Klengel et al. [19] discovered that CO2/bicarbonate activates Cyr1 by targeting the catalytic domain. In summary, four distinct domains of Cyr1 serve as sensor for signals as diverse as sugar, gas, temperature, and actin.
Fig. 1. Fungal adenylyl cyclases contain multiple domains acting as sensors for a diverse range of signals. 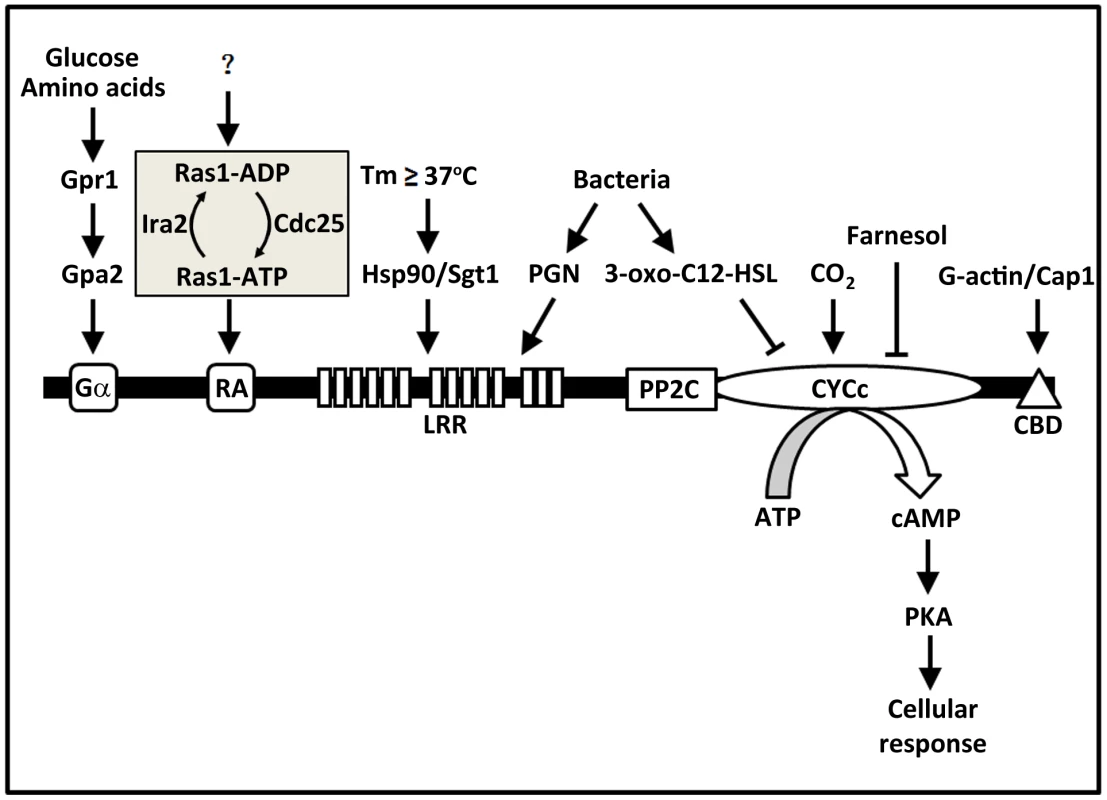
Evidence from many studies of the past decade or so supports a model in which fungal adenylyl cyclases function as a hub of signal sensing and integration. This figure illustrates all the conserved domains in fungal adenylyl cyclases and the external and internal signals each of the domains senses in C. albicans adenylyl cyclase Cyr1. For abbreviations and protein names, please refer to the text. C. albicans Cyr1 Directly Senses Bacterial PGN
Serum at 37°C is probably the strongest and physiologically relevant inducer of hyphal growth in C. albicans. Although the serum activity was first reported in 1956, the identity of the active molecule(s) remained as a mystery for decades. Recently, Xu et al. [20] discovered PGN fragments in serum fractions with high hyphal-inducing activity, and later confirmed that several muramyl dipeptides (MDPs), subunits of peptidoglycan, were indeed potent hyphal inducers. Mass spectrometry analysis detected ∼0.1 to 0.5 µM MDP in the blood of all healthy volunteers. As PGN is uniquely present in bacterial cell wall, the human microbiota is most likely the provider of PGN in the blood. Many bacteria are known to release a large amount of bioactive PGN subunits into the environment during cell wall remodelling [21]. Xu et al. [20] also demonstrated that MDP activates Cyr1 by binding to the LRR domain. Various mutations in the LRR domain completely abolished the hyphal growth induced by serum and MDP. This discovery has significant implications for the possible role of bacteria in C. albicans infection. As a commensal, C. albicans is constantly exposed to PGN fragments continuously released by trillions of bacterial cells. Although its effect on adenylyl cyclase activation may be balanced by other antagonistic factors, certain conditions may tip the balance in favour of C. albicans infection. For example, the use of broad-spectrum antibiotics, most of which inhibit PGN synthesis, may cause a massive release of PGN fragments. Together with antibiotic-associated colitis that damages the intestinal epithelium, PGN may enter the blood stream in large quantities, creating a window of opportunity for C. albicans to initiate systemic infection. This could be an important yet unappreciated factor underlying the high risk of candidemia in patients receiving high doses of broad-spectrum antibiotics.
Farnesol and Bacterial Quorum-Sensing Molecules Inhibit C. albicans Hyphal Growth by Targeting the Catalytic Domain of Adenylyl Cyclase
Farnesol is a quorum-sensing molecule (QSM) produced by C. albicans that inhibits hyphal development and biofilm formation [22]. Early studies provided evidence suggesting that farnesol exerts its effect by interfering with the Ras/cAMP/PKA pathway [23], [24]. Hall et al. [25] later discovered that farnesol directly inhibits the cyclase activity of a truncated version of Cyr1 embracing the catalytic domain alone. Interestingly, the bacterial QSM 3-oxo-C12-homoserine lactone (HSL) secreted by Paseudomonas aeruginosa also inhibits C. albicans hyphal growth by a similar mechanism [25]. This mode of intertaxon chemical communication has important implications in the cause of microbial infections and ways to treat them. In the human microbiota, bacteria account for >99% of all microbial cells, which effectively checks fungal growth through secreting QSMs among other antagonistic mechanisms. However, disturbance of a microbial community by an antibacterial therapy may release the “brake” and create opportunities for commensal fungi such as C. albicans to initiate infection.
Future Directions
Currently, the evidence is strong for fungal adenylyl cyclases as a coincidence detector [26]. To understand how their activity is kept low in the absence of stimuli and is turned on by different ligands either individually or in combination, structural elucidation of fungal adenylyl cyclases is urgently needed particularly in complex with interacting proteins and ligands. Also, the role of the LRR domain in signal sensing deserves more attention. A long LRR domain is present in most pattern recognition receptors of the innate immune system in animals and plants that recognizes a wide range of microbe-associated molecular patterns to elicit immune response [27]. So far, little can be found in the literature on the role of the LRR domain in fungal adenylyl cyclases. In C. albicans Cyr1, the LRR domain senses PGN. It is important to know whether the LRR domain in other fungal adenylyl cylases also plays a role in bacterial sensing. As members of the class III adenylyl cyclases, dimerization is required for catalysis [28]. However, it remains entirely unknown whether there is a dynamic and regulated monomer-dimer interconversion in fungal adenylyl cyclases. Equally elusive is their cellular localization. Answers to these questions may unveil additional dimensions for regulation.
Zdroje
1. Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486 : 207–214.
2. FindleyK, OhJ, YangJ, ConlanS, DemingC, et al. (2013) Topographic diversity of fungal and bacterial communities in human skin. Nature 498 : 367–370.
3. PelegAY, HoganDA, MylonakisE (2010) Medically important bacterial-fungal interactions. Nat Rev Microbiol 8 : 340–349.
4. BrownGD, DenningDW, GowNAR, LevitzSM, NeteaMG, et al. (2012) Hidden killers; human fungal infections. Sci Transl Med 4 : 165rv13.
5. KumamotoCA, VincesMD (2005) Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol 7 : 1546–1554.
6. RochaCR, SchröppelK, HarcusD, MarcilA, DignardD, et al. (2001) Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell 12 : 3631–3643.
7. FangHM, WangY (2006) RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol Microbiol 61 : 484–496.
8. FengQ, SummersE, GuoB, FinkG (1999) Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol 181 : 6339–6346.
9. HoganDA, SundstromP (2009) The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol 4 : 1263–1270.
10. MaidanMM, TheveleinJM, Van DijckP (2005) Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem Soc Trans 33 : 291–293.
11. MaidanMM, De RopL, SerneelsJ, ExlerS, RuppS, et al. (2005) The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell 16 : 1971–1986.
12. IveyFD, HoffmanCS (2005) Direct activation of fission yeast adenylate cyclase by the Gpa2 Galpha of the glucose signaling pathway. Proc Natl Acad Sci U S A 102 : 6108–6113.
13. BahnYS, SundstromP (2001) CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J Bacteriol 183 : 3211–3223.
14. ZouH, FangHM, ZhuY, WangY (2009) A sensor/effector apparatus for activating cAMP synthesis in Candida albicans hyphal growth. Mol Microbiol 75 : 579–591.
15. BaiC, XuXL, WangHS, WangYM, ChanFY, et al. (2011) Characterization of a hyperactive Cyr1 mutant reveals new regulatory mechanisms for cellular cAMP levels in Candida albicans. Mol Microbiol 82 : 879–893.
16. ShapiroRS, UppuluriP, ZaasAK, CollinsC, SennH, et al. (2009) Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signalling. Curr Biol 19 : 621–629.
17. ShapiroRS, ZaasAK, Betancourt-QuirozM, PerfectJR, CowenLE (2012) The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PLoS ONE 7: e44734 doi:10.1371/journal.pone.0044734
18. DubacqC, GueroisR, CourbeyretteR, KitagawaK, MannC (2002) Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot Cell 1 : 568–582.
19. KlengelT, LiangWJ, ChaloupkaJ, RuoffC, SchröppelK, et al. (2005) Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15 : 2021–2026.
20. XuXL, LeeRTH, FangHM, WangYM, LiR, et al. (2008) Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 4 : 28–39.
21. Cloud-HansenKA, PetersonSB, StabbEV, GoldmanWE, McFall-Ngai, et al. (2006) Breaching the great wall: peptidoglycan and microbial interactions. Nat Rev Microbiol 4 : 710–716.
22. LangfordML, AtkinAL, NickersonKW (2009) Cellular interactions of farnesol, a quorum-sensing molecule produced by Candida albicans. Future Microbiol 4 : 1353–1362.
23. Davis-HannaA, PiispanenAE, StatevaLI, HoganDA (2008) Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol 67 : 47–62.
24. LindsayAK, DeveauA, PiispanenAE, HoganDA (2012) Farnesol and cyclic AMP signaling effects on the hypha-to-yeast transition in Candida albicans. Eukaryot Cell 11 : 1219–1225.
25. HallRA, TurnerKJ, ChaloupkaJ, CottierF, De SordiL, et al. (2011) The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans. Eukaryot Cell 10 : 1034–1042.
26. HoganDA, MuhlschlegelFA (2011) Candida albicans developmental regulation: adenylyl cyclase as a coincidence detector of parallel signals. Curr Opin Microbiol 14 : 682–686.
27. SoanesDM, TalbotNJ (2010) Comparative genome analysis reveals an absence of leucine-rich repeat pattern recognition receptor proteins in the kingdom fungi. PLoS ONE 5: e12725 doi:10.1371/journal.pone.0012725
28. LinderJU (2006) Class III adenylyl cyclases: molecular mechanisms of catalysis and regulation. Cell Mol Life Sci 63 : 1736–1751.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



