-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAre We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
.
Published in the journal: . PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003642
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003642Summary
article has not abstract
Are we there yet? Every parent has heard this inexorable question, but it is especially wrenching to hear it from a patient with a fatal illness. When someone, or someone's child, is diagnosed with invasive aspergillosis (IA) caused by Aspergillus fumigatus, the outcome has historically been bleak. In 2013, the field is erupting with exciting advances on multiple scientific fronts. But whether those research findings will improve clinical outcomes is still unclear—does your spouse, child, or neighbor now live or die with this disease? Medical breakthroughs over the last few decades surrounding cancer and transplantation, the main underlying conditions of those afflicted with IA, have been nothing short of astonishing. However, this revolution has also spawned a plethora of immunocompromised patients to inhale fungal spores and develop IA. For many patients with an absent immune system, the second leading cause of death, just behind cancer remission or transplant failure, is infection. The leading infectious cause of death is invasive fungal infections, and the most common mortal fungal infection is A. fumigatus. So have we sufficiently advanced scientifically for today's clinical needs in order to diagnose and treat IA—are we there yet?
A. fumigatus is both a beautiful and frustrating fungus. The beauty lies in the pathogen's ability to readily adapt its niche based on the host. The same fungus that lives in compost piles can shear pulmonary blood vessels in immunosuppressed patients, masquerade as an allergic trigger in an ectopic individual, or hide as a saprophyte and slowly sap the strength from an unsuspecting normal host. The frustration with this fungus is the current difficulty in early and accurate diagnosis, a limited number of effective antifungal treatments coupled with the growing emergence of antifungal resistance, and some persistent basic mechanistic questions surrounding its growth and regulation.
What Is the Current Diagnostic Method for Invasive Aspergillosis?
Clinically diagnosing invasive aspergillosis is often the most difficult of the common human pathogenic fungi triumvirate (Candida, Cryptococcus, and Aspergillus). For years, invasive aspergillosis remained recalcitrant to facile or proper diagnosis. Clinicians were relegated to ionizing radiation-laden CT scans to interpret small pixel changes, or dangerous biopsies in fragile patients. In May 2003, the US Food and Drug Administration approved the galactomannan assay (Platelia® Aspergillus EIA), a double-sandwich ELISA to detect galactomannan (GM) using a rat monoclonal antibody (Mab EB-A2) directed against tetra(1→5)-β-D-galactofuranoside, the immunodominant epitope of the Aspergillus cell wall antigen (Figure 1). This assay afforded the ability for noninvasive diagnosis, forever altering the diagnostic landscape. While GM has been validated as an effective tool, it is by no means a panacea to the diagnostic dilemma. It performs poorly in the setting of solid organ transplantation, use of mold-active antifungals drops its sensitivity, and there is cross-reactivity with certain beta-lactam antibiotics. More recently, testing bronchoalveolar lavage fluid with the GM assay reveals generally greater sensitivity and specificity than serum testing [1]. Another set of commercially available assays (Fungitell® in the United States) detect (1–3)-β-D-glucan in the cell wall of most fungi, including Aspergillus. Two consecutive positive assays have a high specificity for an invasive fungal infection, although the infecting fungal genus cannot be depicted, but sensitivity is lower and there are numerous cross-reactive concerns so results need to be carefully interpreted [2].
Fig. 1. Recent diagnostic and therapeutic target advances against Aspergillus fumigatus. 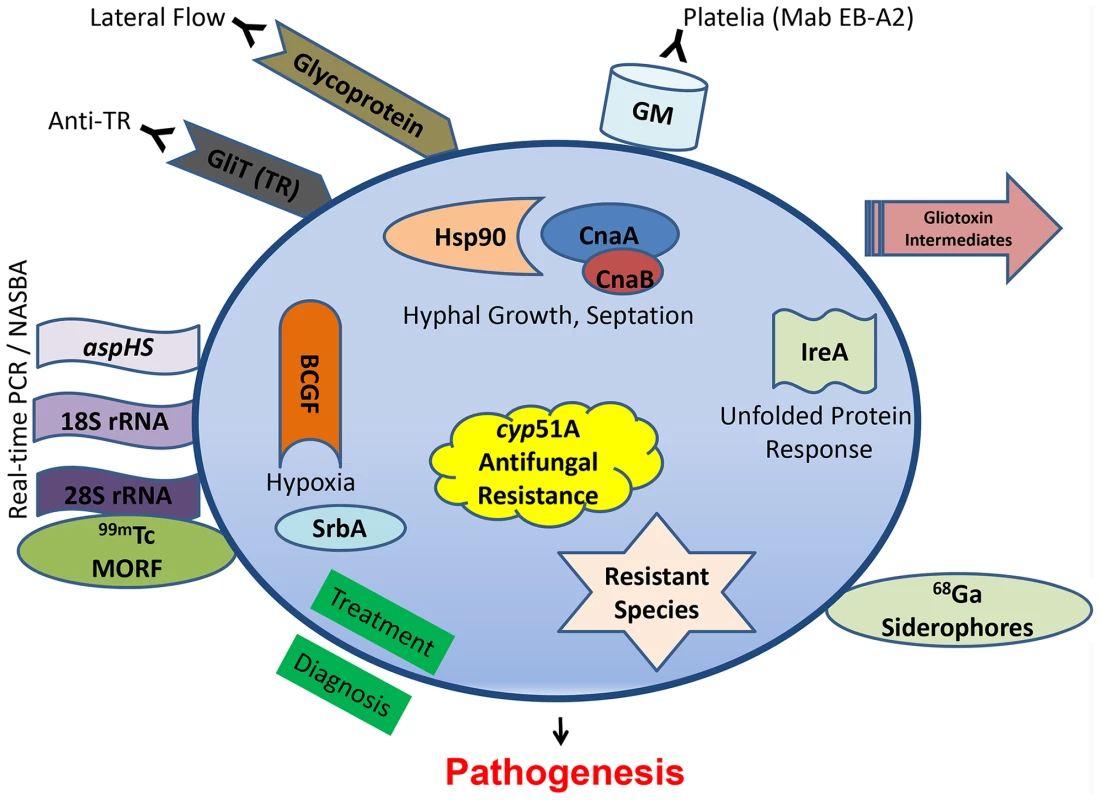
Diagnostic modalities are outside the circle, while novel antifungal targeting concepts, all shown to be significantly related to pathogenesis, are shown inside. A newer antibody-based approach involves a lateral flow device, akin to a home pregnancy test, that offers ease of diagnosis and potential cost savings. First reported in 2008 [3], it is an immunochromotographic assay based on a mouse Mab that binds to a yet-unknown protein epitope present on an extracellular glycoprotein antigen. The assay is quite specific for Aspergillus spp., but a recent study in 101 patients showed inferior sensitivity to the GM assay [4]. Another antibody, found in the sera of non-neutropenic patients with invasive aspergillosis, reacted to the A. fumigatus secretory protein thioredoxin reductase GLiT (TR). Western analysis showed that the recombinant His6-tagged TR protein could be recognized in sera from infected animals and non-neutropenic patients via ELISA-detecting anti-TR antibodies [5], but unfortunately not earlier in the infection than the GM assay.
Does PCR Improve Diagnosis?
PCR is an ultrasensitive diagnostic platform; however, PCR detection of Aspergillus remains limited by a lack of standardization, a problem undertaken by the European Aspergillus PCR Initiative (EAPCRI). Recently, the group tested 29 combinations of PCR variables used in 21 centers (showcasing the current wide variation), and excluded nested-PCR assays as too prone to false-positive results and unlikely to be used outside of referral centers [6]. Despite continued efforts, standardized and validated procedures have not been developed. A refinement to the Aspergillus PCR approach is real-time nucleic acid sequence–based amplification (NASBA), which can allow single-stranded RNA to be detected by molecular beacon probes [7]. This technology, targeting the 28S rRNA, offers robust sensitivity and specificity, but it is unclear if it can be translated to routine clinical laboratory practice. The commercially available Myconostica MycAssay Aspergillus PCR, targeting the 18S rRNA gene, is a real-time PCR with promise for detection of Aspergillus DNA in respiratory tract samples, but there are no reports yet in serum or blood [8]. Using a different genetic target, real-time PCR detection of aspHS, encoding for an A. fumigatus–secreted hemolysin, afforded detection in murine samples but unfortunately not consistently in clinical bronchoalveolar lavage samples due to inhibitors [9].
In general, PCR seems to be more sensitive than GM, but requires specialized equipment and laboratory technologists and a standardized protocol. Additionally, the potential PCR inhibitors present in clinical samples needs to be better addressed. The benefit of PCR is that while GM cannot identify infecting Aspergillus species, PCR could be tailored to the species level and also possibly infer general antifungal susceptibility patterns.
What about Other Diagnostic Strategies?
There seems to be less recent focus on metabolomics, but infection with an overexpression strain for RsmA, a Yap-like basic leucine zipper protein found to regulate gliotoxin in A. fumigatus, reveals gliotoxin intermediates seen in murine lung infection [10]. Other new diagnostic approaches have focused on radiopharmaceutical imaging. The isotope carrier molecule Gallium (68Ga) radiolabeled to A. fumigatus siderophores demonstrated high and specific uptake by A. fumigatus using positron emission tomography (PET) imaging in vitro and in a rat model [11]. Iron plays an important role during infection and another imaging strategy used technetium (99mTc)-labeled morpholino oligomers (MORF), DNA analogs in which the sugar is replaced by a morpholino moiety that binds to the complimentary DNA or RNA. Using 99mTc-labeled 28S rRNA, Aspergillus genus-specific and A. fumigatus species-specific probes confirmed the infectious biodistribution via single-photon emission computed tomography (SPECT) imaging of murine lungs [12]. These imaging-based diagnostic tools are useful for locating infection, or possible monitoring response to therapy, but their sensitivity in various patient populations and settings, as well as cross-reactivity with other pathogens, remains unclear.
How Do We Improve Current Antifungal Treatment Options?
Antifungal discovery is tricky—it is complicated to design an agent to selectively kill one eukaryote (pathogen) while not harming the larger infected eukaryote (host). In pharmaceutical development lingo, treating Aspergillus is the gold standard. Designing a new antifungal with activity only against yeasts would be welcome, but without the ability to kill molds it would only be an incremental advance. Current guideline-recommended therapy against invasive aspergillosis is the triazole voriconazole, superior to the now generally antiquated polyene amphotericin B deoxycholate [13]. While a 2002 landmark clinical trial found an improved response with voriconazole (52.8%) versus amphotericin B (31.6%), the field is still in need of improved antifungal targets. Many felt the answer was a combination antifungal approach, akin to other medical disciplines where agents with different mechanisms are employed for synergistic response. This had been reported in Aspergillus with mixed results in in vitro, animal model, and small clinical studies. A recently completed multinational randomized clinical trial comparing voriconazole with and without the echinocandin anidulafungin showed no clear and definitive benefit, and only statistical trends in improvement in certain groups [14]. This is likely the end of that discussion, whereby currently available antifungal agents have now been tested and retested in combinations and show limited benefit. It is unlikely that the optimal therapeutic answer is an existing antifungal, or even a combination of existing possibilities; what is needed is an entirely new approach with a new target or pathway uncovered from solid basic pathogenesis studies. This is even more pressing with the increasing development of antifungal azole resistance amongst A. fumigatus isolates, generally through one of several cyp51A mutations [15], and also the emerging non-fumigatus Aspergillus species with antifungal resistance, such as Aspergillus lentulus, Aspergillus calidoustus, Aspergillus udagawae, Neosartorya pseudofisheri, and others [16].
How Do We Target a More Promising Regulatory Pathway?
Decades of Aspergillus pathogenesis research have confirmed its multifactorial nature. There are myriad studies highlighting A. fumigatus virulence factors, all of which are possible antifungal drug targets and could not be reviewed here. Some of the more recent promising approaches target not specific putative virulence genes, but instead overarching pathways or regulatory circuits (Figure 1) for a more pleiotropic effect.
Calcineurin is a conserved protein phosphatase important in stress response, and deletions of multiple components of the pathway (cnaA, cnaB, crzA, cbpA, and pmrA) all lead to various growth and virulence defects. Recently, targeted mutations of fungal-specific calcineurin A residues, completely absent in humans, blocked phosphorylation and activation of this enzyme and led to a significant virulence defect [17]. Similarly, repression of calcineurin's molecular chaperone heat shock protein 90 (Hsp90) led to a substantial growth and virulence defect [18]. The unfolded protein response is also critical, and an ΔireA strain, defective in the endoplasmic reticulum transmembrane sensor, is avirulent [19]. LaeA, which serves as a master regulator governing numerous aspects of secondary metabolite production, has also been shown to be involved in development, toxin production, and correlated to virulence in several Aspergillus species.
A. fumigatus invades and occludes blood vessels, causing tissue hypoxia. Basic fibroblast growth factor (BCGF) significantly potentiated the antifungal effects of amphotericin B through increasing neutrophil influx into infected murine tissue and reversed the antiangiogenic activity of A. fumigatus [20]. Angioinvasion is an important and understudied hallmark of Aspergillus pathogenesis, as its presence also prevents delivery of antifungals to the site of infection. Hypoxia adaptation itself has also been studied, and an ΔsrbA mutant, defective in a sterol regulatory element–binding protein transcription factor, demonstrates the importance of this pathway for survival in the areas of inflammation [21].
Unfortunately, to move from “virulence factor” to drug target requires more than an impressive Kaplan-Meier curve and histology evidence. A “druggable” target is required, whereby molecules can be generated to target that entity. Therefore, the next critical steps for these exciting putative targets listed still remain. Similar to the future of molecular diagnosis, the therapeutic answer might be a new composite strategy—attacking known biosynthesis targets with adjunctive stress response inhibitors. While we are not there yet, we are moving faster than ever before to improve our diagnosis and treatment of invasive aspergillosis.
Zdroje
1. GuoYL, ChenYQ, WangK, QinSM, WuC, et al. (2010) Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: a bivariate metaanalysis and systematic review. Chest 138 : 817–824.
2. LamothF, CrucianiM, MengoliC, CastagnolaE, LortholaryO, et al. (2012) β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis 54 : 633–643.
3. ThortonCR (2008) Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol 15 : 1095–1105.
4. HeldJ, SchmidtT, ThorntonCR, KotterE, BertzH (2013) Comparison of a novel Aspergillus lateral-flow device and the Platelia galactomannan assay for the diagnosis of invasive aspergillosis following haematopoietic stem cell transplantation. Infection E-pub ahead of print. doi:10.1007/s15010-013-0472-5
5. ShiLN, LiFQ, LuJF, KongXX, WangSQ, et al. (2012) Antibody specific to thioredoxin reductase as a new biomarker for serodiagnosis of invasive aspergillosis in non-neutropenic patients. Clin Chim Acta 413 : 938–943.
6. WhitePL, MengoliC, BretagneS, Cuenca-EstrellaM, FinnstromN, et al. (2011) Evaluation of Aspergillus PCR protocols for testing serum specimens. J Clin Microbiol 49 : 3842–3848.
7. ZhaoY, ParkS, WarnP, ShriefR, HarrisonE, et al. (2010) Detection of Aspergillus fumigatus in a rat model of invasive pulmonary aspergillosis by real-time nucleic acid sequence-based amplification. J Clin Microbiol 48 : 1378–1383.
8. WhitePL, PerryMD, MoodyA, FollettSA, MorganG, et al. (2011) Evaluation of analytical and preliminary clinical performance of Myconostica MycAssay Aspergillus when testing serum specimens for diagnosis of invasive aspergillosis. J Clin Microbiol 49 : 2169–2174.
9. Abad-Diaz-De-CerioA, Fernandez-MolinaJV, Ramirez-GarciaA, SendinoJ, HernandoFL, et al. (2013) The aspHS gene as a new target for detecting Aspergillus fumigatus during infections by quantitative real-time PCR. Med Mycol 51 : 545–554.
10. SekonyelaR, PalmerJM, BokJ-W, JainS, BerthierE, et al. (2013) RsmA regulates Aspergillus fumigatus gliotoxin cluster metabolites including cyclo(L-Phe-L-Ser), a potential new diagnostic marker for invasive aspergillosis. PLoS ONE 8: e62591 doi:10.1371/journal.pone.0062591
11. PetrikM, FranssenGM, HaasH, LavermanP, HörtnaglC, et al. (2012) Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur J Nucl Med Mol Imaging 39 : 1175–1183.
12. WangY, ChenL, LiuX, ChengD, LiuG, et al. (2013) Detection of Aspergillus fumigatus pulmonary fungal infections in mice with (99m)Tc-labeled MORF oligomers targeting ribosomal RNA. Nucl Med Biol 40 : 89–96.
13. HerbrechtR, DenningDW, PattersonTF, BennettJE, GreeneRE, et al. (2002) Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347 : 408–415.
14. Marr KA, Schlamm H, Rottinghaus ST, Jagannatha S, Bow EJ, et al.. (2012) A randomised, double-bind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherpay for primary treatment of invasive aspergillosis; 22nd European Congress of Clinical Microbiology and Infectious Diseases, March 31–April 3, 2012; London. Abstract LB2812.
15. AlbarragAM, AndersonMJ, HowardSJ, RobsonGD, WarnPA, et al. (2011) Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob Agents Chemother 55 : 5113–5121.
16. Van Der LindenJW, WarrisA, VerweijPE (2011) Aspergillus species intrinsically resistant to antifungal agents. Med Mycol 49 (suppl 1) S82–89.
17. JuvvadiPR, GehrkeC, FortwendelJR, LamothF, SoderblomEJ, et al. (2013) Phosphorylation of calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus. PLoS Pathog 9: e1003564 doi:10.1371/journal.ppat.1003564
18. LamothF, JuvvadiPR, GehrkeC, AsfawYG, SteinbachWJ (2013) Transcriptional activation of heat-shock protein 90 (Hsp90) mediated via a proximal promoter region confers caspofungin resistance in Aspergillus fumigatus. J Infect Dis In press.
19. FengX, KrishnanK, RichieDL, AimaniandaV, HartlL, et al. (2011) HacA-independent functions of the ER stress sensor IreA synergize with the canonical UPR to influence virulence traits in Aspergillus fumigatus. PLoS Pathog 7: e1002330 doi:10.1371/journal.ppat.1002330
20. Ben-AmiR, AlbertND, LewisRE, KontoyiannisDP (2013) Proangiogenic growth factors potentiate in situ angiogenesis and enhance antifungal drug activity in murine invasive aspergillosis. J Infect Dis 207 : 1066–1074.
21. WillgerSD, PuttikamonkulS, KimK-H, BurrittJB, GrahlN, et al. (2008) A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathogens 4: e1000200 doi:10.1371/journal.ppat.1000200
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



