B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
B cells can contribute to acquired immunity against intracellular bacteria, but do not usually participate in primary clearance. Here, we examined the endogenous CD4 T cell response to genital infection with Chlamydia muridarum using MHC class-II tetramers. Chlamydia-specific CD4 T cells expanded rapidly and persisted as a stable memory pool for several months after infection. While most lymph node Chlamydia-specific CD4 T cells expressed T-bet, a small percentage co-expressed Foxp3, and RORγt-expressing T cells were enriched within the reproductive tract. Local Chlamydia-specific CD4 T cell priming was markedly reduced in mice lacking B cells, and bacteria were able to disseminate to the peritoneal cavity, initiating a cellular infiltrate and ascites. However, bacterial dissemination also coincided with elevated systemic Chlamydia-specific CD4 T cell responses and resolution of primary infection. Together, these data reveal heterogeneity in pathogen-specific CD4 T cell responses within the genital tract and an unexpected requirement for B cells in regulating local T cell activation and bacterial dissemination during genital infection.
Published in the journal:
. PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003707
Category:
Research Article
doi:
https://doi.org/10.1371/journal.ppat.1003707
Summary
B cells can contribute to acquired immunity against intracellular bacteria, but do not usually participate in primary clearance. Here, we examined the endogenous CD4 T cell response to genital infection with Chlamydia muridarum using MHC class-II tetramers. Chlamydia-specific CD4 T cells expanded rapidly and persisted as a stable memory pool for several months after infection. While most lymph node Chlamydia-specific CD4 T cells expressed T-bet, a small percentage co-expressed Foxp3, and RORγt-expressing T cells were enriched within the reproductive tract. Local Chlamydia-specific CD4 T cell priming was markedly reduced in mice lacking B cells, and bacteria were able to disseminate to the peritoneal cavity, initiating a cellular infiltrate and ascites. However, bacterial dissemination also coincided with elevated systemic Chlamydia-specific CD4 T cell responses and resolution of primary infection. Together, these data reveal heterogeneity in pathogen-specific CD4 T cell responses within the genital tract and an unexpected requirement for B cells in regulating local T cell activation and bacterial dissemination during genital infection.
Introduction
Chlamydia trachomatis is an obligate intracellular pathogen that causes the most prevalent bacterial sexual transmitted infection worldwide [1]. In the US, Chlamydia is now the most common notifiable disease reported to the US Centers for Disease Control (CDC). The 1.4 million cases of Chlamydia infection reported in 2011 represent an 8% increase over the previous year and is the largest number of annual infections ever reported to the CDC for any condition [2]. The introduction of a Chlamydia screening and control program in the mid-1990s has not prevented annual increases in infection, although a portion of this increase is due to improved disease surveillance [3]. Overall, the CDC reports a median 8.3% Chlamydia positivity test among women aged 15–24, making this one of the most prevalent bacterial infections in the US.
Most Chlamydia infections are initially asymptomatic and therefore unlikely to be treated. However, 5–15% of females with untreated infection will eventually develop serious pelvic inflammatory disease (PID) as a consequence. Furthermore, 1 in 6 women who develop PID will become infertile, and many others will develop chronic pelvic inflammation and pain, or suffer from ectopic pregnancy [4]–[6]. The combination of an extraordinarily high number of infections, the asymptomatic nature of initial disease, and the potential for serious reproductive pathology in young women, means that Chlamydia is now recognized as a growing health care problem in the US. The current consensus among scientists and clinicians is that an effective Chlamydia vaccine is urgently needed [7].
The development of an effective Chlamydia vaccine would likely alleviate the burden of Chlamydia on the public health care system. However, the rational design of a Chlamydia vaccine would be aided by improved understanding of the cellular immune response to infection of the female reproductive tract. As Chlamydia is an obligate intracellular pathogen, IFN-γ production by CD4 Th1 cells is essential for protective immunity to primary and secondary infection [8]–[13]. Unfortunately, we have at present only a rudimentary understanding of the development of protective Th1 responses in the context of the female upper reproductive tract and the extent of T helper heterogeneity is unclear. One of the major roadblocks to improving this situation is the lack of antigen-specific reagents that would allow detailed investigation of Chlamydia-specific CD4 T cell responses using a relevant genital challenge model.
Previous studies have demonstrated that antibody production by B cells can assist protective immunity during secondary Chlamydia infection [14]–[16]. In contrast, B cells are thought to be dispensable for resolving primary Chlamydia infection, and B cell-deficient and wild type mice shed similar numbers of Chlamydia, as measured by vaginal swabs [17], [18]. However, another study using the respiratory route of infection demonstrated that intranasal infection with Chlamydia requires B cells for efficient CD4 T cell activation [19]. Therefore, the issue of whether B cells contribute to initial CD4 T cell priming during vaginal infection requires additional analysis.
In this study, we generated MHC class-II tetramers to visualize the endogenous CD4 T cell response to systemic and genital tract Chlamydia infection. We show that, unlike intravenous infection, reproductive tract infection is associated with a short delay in the clonal expansion of Chlamydia-specific CD4 T cells in the local draining lymph node. While almost all expanded CD4 T cells expressed the Th1 marker, T-bet, we detected an expanded pool of Chlamydia-specific Tregs that co-expressed T-bet and Foxp3, and a population of Chlamydia-specific Th17 cells that were specifically enriched in the reproductive tract. In addition, we noted a surprising requirement for B cells in Chlamydia-specific CD4 cell priming within local draining lymph nodes. Loss of local priming in the absence of B cells coincided with bacterial dissemination to the peritoneal cavity inducing inflammatory infiltrate and ascites. Together, these data demonstrate heterogeneity in Chlamydia-specific T helper responses and an unexpected role for B cells in local CD4 T cell priming and bacterial containment within the upper reproductive tract.
Results
Visualization of Chlamydia-specific CD4 T cell expansion after intravenous infection
In order to develop an overview of the adaptive response to Chlamydia infection, we initially examined the kinetics of bacterial growth and Chlamydia-specific CD4 T cell expansion during systemic infection with Chlamydia. When C57BL/6 female mice were infected intravenously (i.v.) with 1×105 inclusion-forming units (IFUs) of C. muridarum, the bacterial burden in the spleen peaked around day 4 post-infection and decreased quickly thereafter (Fig. 1A). At day 20, no infectious Chlamydia was detected in the spleen (Fig. 1A). Consistent with previous findings [20], a small number of Chlamydia were found in the lung during the first week of systemic infection, but no bacteria were detected in kidney or heart at any time point (data not shown).
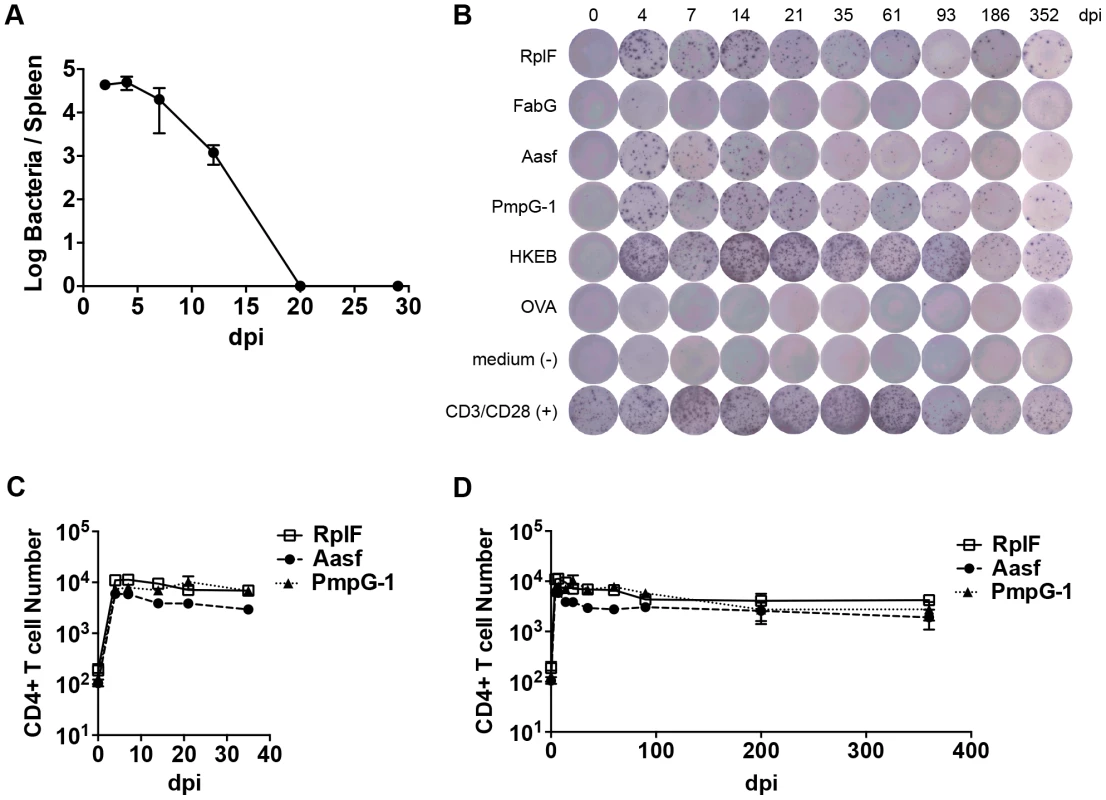
Numerous C. muridarum MHC class-II epitopes have been uncovered by Immunoproteomic analysis of infected APCs [21]. We used an ELISPOT assay to monitor the frequency of CD4 T cells responding to multiple C. muridarum epitopes after systemic infection. A population of IFN-γ-secreting CD4 T cells responding to RplF51–59, Aasf24–32, and PmpG-1303–311 was detected as early as 4 days after infection (Fig. 1B and C). Expansion of IFN-γ-secreting CD4 T cells peaked around day 4–7, and was followed by a slow contraction of the population over the next 90 days, before a plateau was reached that lasted for at least 352 days (Fig. 1B and 1D). Thus, peak expansion of IFN-γ-secreting CD4 cells closely mirrored peak bacterial burdens in vivo, and stable Chlamydia-specific CD4 T cell memory frequencies were maintained in the absence of active Chlamydia infection.
Construction of Chlamydia-specific pMHCII tetramers
Previous studies have demonstrated that pMHC class-II tetramers can be used in conjunction with tetramer enrichment, to visualize low frequency endogenous antigen-specific CD4 T cells in infected and immunized mice [22], [23]. We constructed three distinct pMHC class-II tetramers, containing I-Ab with a Chlamydia-specific epitope (RplF51–59, Aasf24–32, or PmpG-1303–311) bound to the MHC class-II β chain. Uninfected C57BL/6 mice contained low frequency antigen-specific CD4 T cell population specific for each Chlamydia epitope (Fig. 2A). However, in mice immunized subcutaneously with peptide/CFA, or infected intravenously with C. muridarum, an expanded population of CD44hi CD4 T cells was detectable 7 days post immunization or infection (Fig. 2A). Tetramer staining was specific, as infection with Salmonella Typhimurium did not induce expansion of tetramer-specific CD4 T cells (Fig. 2A). Furthermore, no CD8 T cells were detected that bound to Chlamydia tetramers (Fig. 2A) Together, these results demonstrate that all three tetramers, RplF51–59:I-Ab, Aasf24–32:I-Ab, and PmpG-1303–311:I-Ab can be used to detect endogenous C. muridarum-CD4 T cells in vivo.
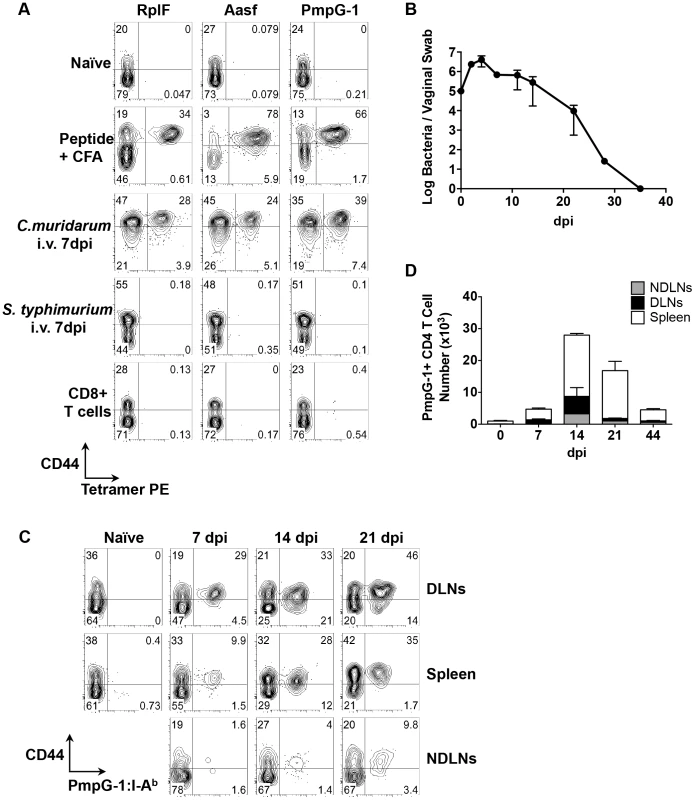
Visualization of Chlamydia-specific CD4 T cell expansion to intravaginal infection
We next used the PmpG-1303–311:I-Ab tetramer to visualize clonal expansion of antigen-specific CD4 T cells during intravaginal (i.vag.) infection. We focused on PmpG-1 because CD4 T cell clonal expansion against RplF, Aasf and PmpG-1 is similar (Fig. 1B, 1C and 1D) yet PmpG-1 is a promising Chlamydia vaccine candidate [24]. Consistent with previous findings, C. muridarum bacterial loads measured by vaginal swab peaked around day 4 post-infection and steadily decreased until clearance around day 35 (Fig. 2B). To visualize the primary site of endogenous T cell priming to Chlamydia infection, we examined PmpG-1-specific T cell activation in multiple secondary lymphoid tissues. One week after infection, PmpG-1-specific CD4 T cells expanded in iliac lymph nodes and spleen, but were barely detectable in other non-draining lymph nodes (Fig. 2C and 2D). At day 14, endogenous PmpG-1-specific CD4 T cell expansion peaked in all secondary lymphoid tissues, and was followed by a notable contraction phase (Fig. 2D). The kinetics of the CD4 T cell response to local Chlamydia genital tract infection was therefore markedly delayed in comparison to systemic infection with the same pathogen (Fig. 1).
Identification of Th1/Treg/Th17 cell subsets in Chlamydia-specific CD4 T cells
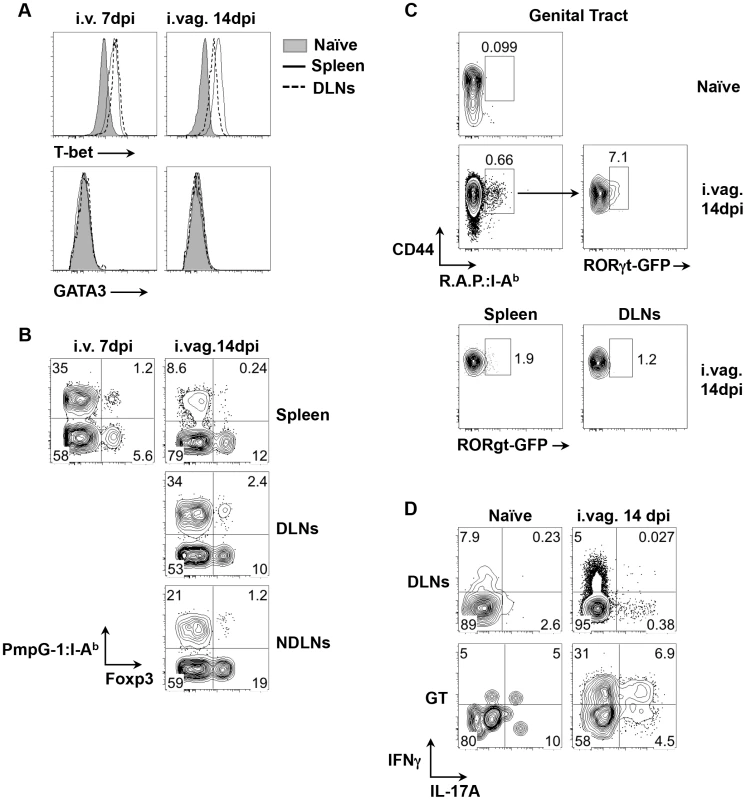
To examine CD4 T helper differentiation, the expression of lineage-specific transcription factors was examined in expanded CD44hi PmpG-1303–311:I-Ab+ CD4 T cells. Following either systemic or intravaginal infection, almost all PmpG-1-specific CD4 T cells expressed T-bet while no GATA3 expression was detected (Fig. 3A). This is consistent with previous reports that Th1 CD4 T cells are the dominant helper subset following Chlamydia infection [10], [11]. However, a distinct population of PmpG-1-specific CD4 T cells that co-expressed Foxp3 and T-bet was also detected after i.v. and i.vag. infection (Fig. 3B), suggesting that induced Chlamydia-specific Treg cells are also contained within the expanded CD4 pool. We also utilized RORγt-GFP reporter mice and combined staining with all three Chlamydia tetramers to examine the potential development of Chlamydia-specific Th17 cells after vaginal infection. While GFP-positive cells were undetectable among expanded tetramer-positive cells in the spleen or draining lymph nodes of infected mice, approximately 7% of CD4+CD44hitetramer+ T cells in infected non-lymphoid tissues expressed GFP (Fig. 3C). Furthermore, stimulation of lymphocytes purified from the genital tract confirmed the presence of T cells producing both IL-17A and IFN-γ (Fig. 3D). Thus, Chlamydia infection of the reproductive tract induces a heterogenous T helper response that comprises expanded T-bet+ Th1 cells, T-bet+Foxp3+ Tregs, and Th17 cells that are enriched in infected non-lymphoid tissues.
Reduced local CD4 expansion and Chlamydia dissemination in mice lacking B cells
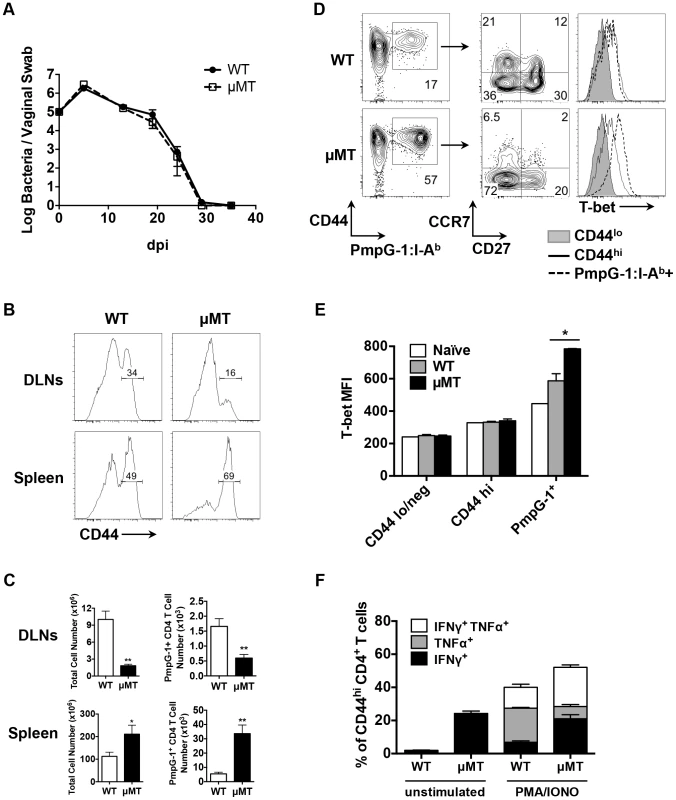
Next, we examined bacterial shedding after vaginal infection of WT and μMT mice with Chlamydia. Consistent with previous reports [17], [18], bacterial shedding was unaffected by the absence of B cells (Fig. 4A). However, more detailed analysis of the local draining lymph nodes of μMT mice suggested significantly reduced activation of CD4 T cells (Fig. 4B). Indeed, using the PmpG-1303–311:I-Ab tetramer, we detected much lower clonal expansion of Chlamydia-specific CD4 T cells in the local draining lymph node of infected μMT mice compared to WT mice (60±12 in μMT mice vs 166±36 in WT mice, p<0.01; Fig. 4C). This reduced local response was also accompanied by dissemination of Chlamydia to the spleen and peritoneal cavity, where ascites was noted 14 days post infection (Fig. 5A and 5B). Analysis of ascites fluid from μMT mice revealed a large proportion of macrophages (F4/80+), monocytes (Gr-1+) and T lymphocytes (Fig. 5C). In addition, PmpG-1-specific CD4 T cells were abundant in ascites, demonstrating that much of the lymphocyte infiltrate into the peritoneal cavity is likely to be Chlamydia-specific (Fig. 5C). Thus, the absence of B cells reduces local CD4 T cell priming and allows bacterial dissemination.
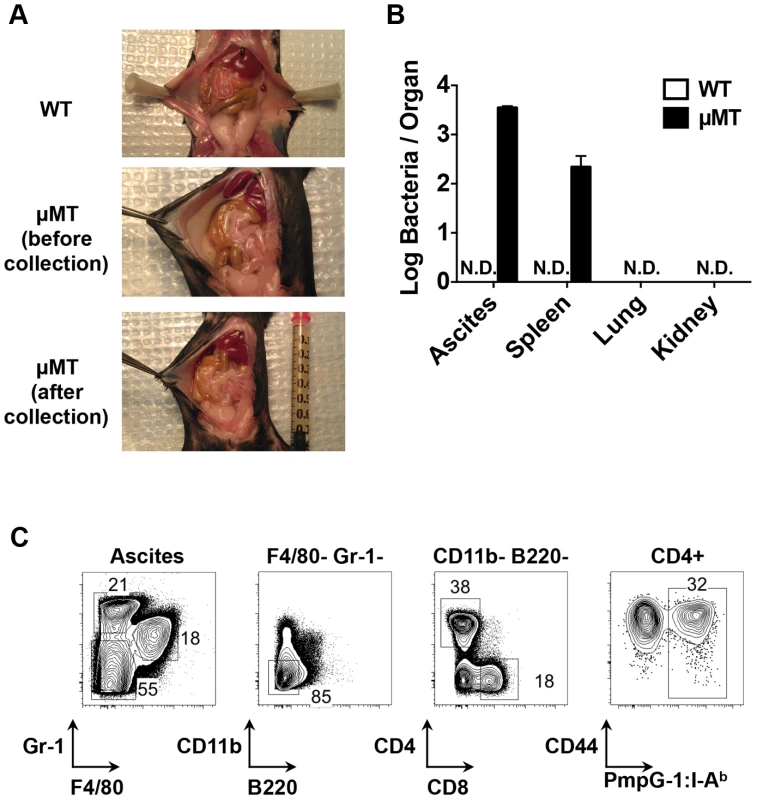
Enhanced systemic CD4 T cell priming in B cell deficient mice
In contrast to the local response, polyclonal CD4 T cells in the spleen of μMT mice displayed evidence of increased activation (Fig. 4B). Consistent with increased systemic activation, expansion of PmpG-1-specific CD4 T cells was markedly increased in the spleen of μMT mice (33600±6044 in μMT mice vs 5494±1164 in WT mice, p<0.001; Fig. 4C). Furthermore, Chlamydia-specific CD4 T cells in μMT mice expressed higher levels of T-bet and produced more IFN-γ than CD4 T cells from WT mice (Fig. 4D and E). A greater percentage of multifunctional CD4 T cells producing IFN-γ and TNF-α was also detected (Fig. 4F). Consistent with an enhanced effector response, the percentage of Chlamydia-specific CD4 T cells expressing CCR7 was considerably lower in μMT mice (Fig. 4D). These data suggest a compensatory systemic T cell response is induced to clear the disseminated bacteria that accompany B cell deficiency.
Discussion
In vivo visualization studies of pathogen-specific CD4 T cell responses have typically involved adoptive transfer of monoclonal TCR transgenic T cells and have rarely focused on sexually transmitted infections [25], [26]. Here, we describe the generation of peptide-MHC class II tetramers that allow direct visualization of endogenous, polyclonal antigen-specific CD4 T cell responses to Chlamydia infection. Using Chlamydia tetramers and ELISPOT assays, we detected differences in the tempo of the initial CD4 T cell responses to systemic or vaginal challenge with Chlamydia. Unlike other mucosal tissues, the female genital mucosa lacks defined lymphoid structures [7], which may explain the delayed clonal expansion of CD4 T cells after Chlamydia genital tract infection. Alternatively, this delay may represent an unappreciated virulence mechanism of Chlamydia to delay early T cell priming, as has been noted during respiratory infection with MTB [27]. Thus, delayed CD4 T cell priming in the draining lymph node may be due to limited access to bacterial antigen by tissue dendritic cells or impeded migration to the local lymph nodes. Further studies will be required to examine this issue directly.
Our studies show that Chlamydia-specific memory CD4 T cells persist for at least one year after infection, which may suggest the presence of persistent antigen and/or persistent Chlamydia without culturable Chlamydia organisms [28], after the clearance of infectious bacteria. Interestingly, a recent study by Johnson et al suggested that the PmpG-1303–311 epitope can be detected on splenic APC for at least 6 months after the clearance of primary genital tract infection [29]. Our data, together with others, also support the potential role of PmpG-1 specific CD4 T cells in Chlamydia protective immunity [24], [29].
Our data confirm directly the previous notion that T-bet expressing Th1 cells are the predominant CD4 effector lineage among Chlamydia-specific CD4 T cells. However, we also identify small populations with the phenotypic marker of Tregs in the lymph node and spleen following both systemic and mucosal infection, as well as the enrichment of Chlamydia-specific Th17 cells at the genital mucosa itself. These data suggest that Chlamydia-specific Tregs are expanded to regulate the large Th1 response that develops during infection [30]. The enrichment of Th17 cells at mucosal surfaces has also been detected in other infection models [23], [31]–[33]. For example, flagellin-specific Th17 cells are predominantly enriched in the gut mucosal sites after Salmonella oral infection [23]. Further experimentation is required to determine whether these pathogen-specific Th17 cells contribute to Chlamydia clearance and/or the induction of upper genital tract pathology.
The presence of large numbers of B cells in the lower and upper genital tract during Chlamydia infection, as shown by both immunohistochemical staining and flow cytometry [34] (and data not shown), led us to speculate that B cells play an important role during primary Chlamydia infection. Indeed, our data show that in the absence of B cells, there is a marked reduction in antigen-specific CD4 T cell priming within the draining iliac lymph nodes. Given this effect of early CD4 T cell expansion it is possible that B cells participate directly in antigen presentation during the early stages of primary infection. Although this has not previously been observed during genital tract infection, a similar finding was reported after C. muridarum lung infection [19]. A recent study has suggested that macrophage deficiency could also account for protective defects of μMT mice against viral infections [35]. However, our observations that ascites did not occur at early time point (7 days post infection, data not shown) suggested that the phenotype we observed is mediated by altered adaptive immune mechanisms.
Although mild Chlamydia dissemination to other mucosal sites has been reported previously in C57BL/6 mice [36], a large number of disseminated Chlamydia has only previously been found in IFN-γ-deficient and SCID mice and largely attributed to deficient Th1 development [8]. B cell deficient mice therefore provide an unexpected additional model where Chlamydia also disseminate to non-mucosa tissues. Notably, our data provides a clear example where the marked pathology of Chlamydia infection does not always correlate with the inability of host to clear the infection. A likely explanation for highly efficient bacteria clearance in μMT mice is the robust systemic CD4 T cell response that may compensate for the loss of initial CD4 T cell priming within the local draining lymph nodes of the genital tract. The fact that Chlamydia genital tract infection can lead to ascites in the absence of B cells is also clinically relevant: Chlamydia infection induces ascites in patients with salpingitis and peritonitis [37]–[40], although it is unclear whether Chlamydia dissemination in mouse models reflects the same mechanism that leads to symptoms in human such as PID and Fitz-Hugh-Curtis syndrome. Further pathological studies are needed to understand differences of mouse and human infections.
Overall, our data demonstrate unappreciated heterogeneity of the CD4 T cell response to genital tract infection in a model where Th1 cells are essential for protective immunity. In addition, our data uncover a surprising involvement of B cells in local expansion of effector Chlamydia-specific T cells in the genital tract and prevention of bacterial dissemination. Greater understanding of the mechanism of Chlamydia dissemination from the genital tract and the T and B cell responses that restrain this spread of bacteria may reduce the risk of sequelae after Chlamydia genital infection in infected women.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The University of California Davis is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animal experiments were approved by University of California Davis Institutional Animal Care and Use Committee (IACUC) (Protocol number 16612).
Mice
C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD) and The Jackson Laboratory (Bar Harbor, ME). μMT mice were purchased from The Jackson Laboratory (Bar Harbor, ME). RORγt-GFP reporter mice were obtained from Dr. Marc Jenkins (University of Minnesota). Mice used for experiments were 6–12 weeks old, unless otherwise noted. All mice were maintained in accordance with University of California Davis Research Animal Resource guidelines.
Bacteria
Chlamydia muridarum strain Nigg II was purchased from ATCC (Manassas, VA). The organism was cultured in HeLa 229 cells in Dulbecco's Modified Eagle Medium (DMEM) (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS). Elementary bodies (EBs) were purified by discontinuous density gradient centrifugation as previously described and stored at −80 degree [41]. The number of IFUs of purified EBs was determined by infection of HeLa 229 cells and enumeration of inclusions that were stained with anti-Chlamydia MOMP antibody. Heat-killed EBs (HKEBs) were prepared by heating at 56°C for 30 min. A fresh aliquot was thawed and used for every infection experiment. Salmonella enterica serovar Typhimurium strains BRD509 (AroA−D−) were kindly provided by Dr. D. Xu (University of Glasgow, Glasgow, U.K).
Chlamydia infection and enumeration
For systemic infection, mice were injected intravenously in the lateral tail vein with 1×105 C. muridarum. To enumerate the bacteria burden in tissues, the spleen, liver and kidney, were crushed in 5 mL SPG buffer and tissue homogenate was placed in a tube with glass beads to disrupt cells. After shaking for 5 min, and centrifugation at 500 g for 10 minutes, supernatants were collected and serial dilutions were plated on HeLa 229 cells. For intravaginal infections, estrus was synchronized by subcutaneous injection of 2.5 mg medroxyprogesterone acetate (Greenstone, NJ) 7 days before infection. 1×105 C. muridarum in 5 µL SPG buffer were then deposited into vaginal vaults. To enumerate bacteria, vaginal swabs were collected, shaken with glass beads, and serial dilutions were plated on HeLa 229 cells.
ELISPOT assay
Spleen and lymph nodes (axillary, brachial, inguinal and mesenteric) were harvested and a single-cell suspension prepared. After RBC lysis, CD4+ T cells were enriched using LS MACS columns and anti-CD4 magnetic beads (Miltenyi Biotec, Auburn, CA). Enriched CD4+ T cells were incubated with irradiated APCs from naive mice in the presence of 10 µM Chlamydia peptide (RplF51–59, FabG157–165, Aasf24–32 or PmpG-1303–311) in 96-well ELISPOT plates (Millipore, Billerica, MA) that had been pre-coated with purified anti-IFN-γ (BD Biosciences, San Diego, CA). The RplF51–59, FabG157–165, Aasf24–32 and PmpG-1303–311 epitopes used for stimulation have been described previously [21], [42]. After 20 h of incubation at 37°C, cells were washed and cytokine spots developed using biotinylated anti-IFN-γ (BD Biosciences), AKP Streptavidin (BD Biosciences), and 1-Step NBT/BCIP substrate (Thermo Scientific, Waltham, MA). Cytokine spots were counted using an ImmunoSpot S5 Core Analyzer (C.T.L., Shaker Heights, OH), and the total number of IFN-γ-producing CD4+ T cells per spleen was calculated.
Construction of pMHCII tetramers
The methodology for construction of pMHCII tetramers has been described in detail [22]. Briefly, biotinylated I-Ab monomers containing a covalently linked C. muridarum peptide (RplF51–59, Aasf24–32 or PmpG-1303–311) were expressed by S2 Drosophila insect cell lines and cultured in a Wave Bioreactor (GE Healthcare Biosciences, Pittsburgh, PA). After purification, I-Ab monomers were tetramerized by co-incubated with fluorochrome-conjugated streptavidin at a pre-determined optimal ratio at room temperature for 30 min [22]. To test for tetramer specificity, C57BL/6 mice were immunized with each of the three peptides and CFA (Sigma-Aldrich, St. Louis, MO). Seven days post-immunization, draining lymph nodes were isolated and tetramer positive cells enriched using the methodology described below.
Tetramer enrichment and flow cytometry
Spleen and LNs were harvested from naïve or infected mice. Single cell suspensions were prepared in FACS buffer (PBS with 2% FCS) and stained with tetramers in Fc block (culture supernatant from the 24G2 hybridoma, 2% mouse serum, 2% rat serum, and 0.01% sodium azide) for 1 h at room temperature in the dark. Cells were then washed and tetramer positive cells enriched via LS MACS columns and anti-fluorochrome magnetic beads (Miltenyi Biotec, Auburn, CA). Bound and unbound fractions were stained with a panel of monoclonal antibodies (listed below) and analyzed on a FACSCanto or an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA). To stain for intracellular transcription factors and cytokines, cells were left untreated or stimulated with phorbol 12-myristate 13-acetate (PMA, 50 ng/ml), ionomycin (200 ng/ml) in the presence of brefeldin A (10 µg/ml) for 4 h at 37°C. After surface staining, cells were fixed, permeablized and stained using the Foxp3 staining Kit (eBioscience, San Diego, CA). Antibodies for staining included FITC-CD11b, CD11c, F4/80, B220, TNFα; PerCP-eFlour710-CD4; APC-CCR7; eFlour660-T-bet; Alexa700-CD44; eFlour450-CD3, Foxp3, IFN-γ (eBioscience, San Diego, CA); FITC-IL-17A and APC-Cy7-CD8 (BD Biosciences, San Diego, CA). Flow data were analyzed using FlowJo software (Tree Star, Ashland, OR) and endogenous, tetramer-specific CD4 T cells were identified using a previously published gating strategy [22].
Statistical analysis
All data sets were analyzed by unpaired Student's t-test using Prism (GraphPad Software, La Jolla, CA). A p value<0.05 were considered statistically significant.
Zdroje
1. StarnbachMN, RoanNR (2008) Conquering sexually transmitted diseases. Nature reviews Immunology 8: 313–317.
2. US Department of Health and Human Services (2012) Sexually Transmitted Disease Surveillance 2011. Available: http://www.cdc.gov/std/stats11/Surv2011.pdf
3. GottliebSL, BermanSM, LowN (2010) Screening and treatment to prevent sequelae in women with Chlamydia trachomatis genital infection: how much do we know? The Journal of infectious diseases 201 Suppl 2: S156–167.
4. GottliebSL, MartinDH, XuF, ByrneGI, BrunhamRC (2010) Summary: The natural history and immunobiology of Chlamydia trachomatis genital infection and implications for Chlamydia control. The Journal of infectious diseases 201 Suppl 2: S190–204.
5. HaggertyCL, GottliebSL, TaylorBD, LowN, XuF, et al. (2010) Risk of sequelae after Chlamydia trachomatis genital infection in women. The Journal of infectious diseases 201 Suppl 2: S134–155.
6. GottliebSL, BrunhamRC, ByrneGI, MartinDH, XuF, et al. (2010) Introduction: The natural history and immunobiology of Chlamydia trachomatis genital infection and implications for chlamydia control. The Journal of infectious diseases 201 Suppl 2: S85–87.
7. BrunhamRC, Rey-LadinoJ (2005) Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nature reviews Immunology 5: 149–161.
8. CotterTW, RamseyKH, MiranpuriGS, PoulsenCE, ByrneGI (1997) Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infection and immunity 65: 2145–2152.
9. PerryLL, SuH, FeilzerK, MesserR, HughesS, et al. (1999) Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. Journal of immunology 162: 3541–3548.
10. PerryLL, FeilzerK, CaldwellHD (1997) Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. Journal of immunology 158: 3344–3352.
11. CainTK, RankRG (1995) Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infection and immunity 63: 1784–1789.
12. MorrisonRP (2000) Differential sensitivities of Chlamydia trachomatis strains to inhibitory effects of gamma interferon. Infection and immunity 68: 6038–6040.
13. CohenCR, KoochesfahaniKM, MeierAS, ShenC, KarunakaranK, et al. (2005) Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon- gamma. J Infect Dis 192: 591–599.
14. CotterTW, MengQ, ShenZL, ZhangYX, SuH, et al. (1995) Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infection and immunity 63: 4704–4714.
15. MorrisonSG, SuH, CaldwellHD, MorrisonRP (2000) Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infection and immunity 68: 6979–6987.
16. MorrisonSG, MorrisonRP (2005) A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. Journal of immunology 175: 7536–7542.
17. SuH, FeilzerK, CaldwellHD, MorrisonRP (1997) Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infection and immunity 65: 1993–1999.
18. RamseyKH, SoderbergLS, RankRG (1988) Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infection and immunity 56: 1320–1325.
19. YangX, BrunhamRC (1998) Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. Journal of immunology 161: 1439–1446.
20. BrunhamRC, KuoC, ChenWJ (1985) Systemic Chlamydia trachomatis infection in mice: a comparison of lymphogranuloma venereum and trachoma biovars. Infection and immunity 48: 78–82.
21. KarunakaranKP, Rey-LadinoJ, StoynovN, BergK, ShenC, et al. (2008) Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. Journal of immunology 180: 2459–2465.
22. MoonJJ, ChuHH, HatayeJ, PaganAJ, PepperM, et al. (2009) Tracking epitope-specific T cells. Nature protocols 4: 565–581.
23. LeeSJ, McLachlanJB, KurtzJR, FanD, WinterSE, et al. (2012) Temporal expression of bacterial proteins instructs host CD4 T cell expansion and Th17 development. PLoS pathogens 8: e1002499.
24. YuH, JiangX, ShenC, KarunakaranKP, JiangJ, et al. (2010) Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infection and immunity 78: 2272–2282.
25. RoanNR, GierahnTM, HigginsDE, StarnbachMN (2006) Monitoring the T cell response to genital tract infection. Proceedings of the National Academy of Sciences of the United States of America 103: 12069–12074.
26. McSorleySJ, AschS, CostalongaM, ReinhardtRL, JenkinsMK (2002) Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity 16: 365–377.
27. BoldTD, BanaeiN, WolfAJ, ErnstJD (2011) Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS pathogens 7: e1002063.
28. BeattyWL, ByrneGI, MorrisonRP (1993) Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proceedings of the National Academy of Sciences of the United States of America 90: 3998–4002.
29. JohnsonRM, YuH, KerrMS, SlavenJE, KarunakaranKP, et al. (2012) PmpG303-311, a protective vaccine epitope that elicits persistent cellular immune responses in Chlamydia muridarum-immune mice. Infection and immunity 80: 2204–2211.
30. KochMA, Tucker-HeardG, PerdueNR, KillebrewJR, UrdahlKB, et al. (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature immunology 10: 595–602.
31. WoolardMD, HensleyLL, KawulaTH, FrelingerJA (2008) Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun 76: 2651–2659.
32. PepperM, LinehanJL, PaganAJ, ZellT, DileepanT, et al. (2010) Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology 11: 83–89.
33. ZhangZ, ClarkeTB, WeiserJN (2009) Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119: 1899–1909.
34. MorrisonSG, MorrisonRP (2000) In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infection and immunity 68: 2870–2879.
35. MosemanEA, IannaconeM, BosurgiL, TontiE, ChevrierN, et al. (2012) B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 36: 415–426.
36. PerryLL, HughesS (1999) Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infection and immunity 67: 3686–3689.
37. Votte-LambertA, JolyJP, BecuweC, EbF, CapronJP, et al. (1990) Chlamydia trachomatis peritonitis: another cause of protein-rich lymphocytic ascites. Journal of clinical gastroenterology 12: 341–343.
38. WallaceTM, HartWR (1991) Acute chlamydial salpingitis with ascites and adnexal mass simulating a malignant neoplasm. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 10: 394–401.
39. GuagentiRC, BermanAL, CohenNN (1989) Chlamydial ascites. Digestive diseases and sciences 34: 139–141.
40. PunnonenR, TerhoP, KlemiPJ (1982) Chlamydial pelvic inflammatory disease with ascites. Fertility and sterility 37: 270–272.
41. ScidmoreMA (2005) Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Current protocols in microbiology Chapter 11: Unit 11A 11.
42. YuH, JiangX, ShenC, KarunakaranKP, BrunhamRC (2009) Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. Journal of immunology 182: 1602–1608.
Štítky
Hygiena a epidemiologie Infekční lékařství LaboratořČlánek vyšel v časopise
PLOS Pathogens
2013 Číslo 10
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
Nejčtenější v tomto čísle
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
