-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
Rhinovirus infections are the major cause of asthma exacerbations. We hypothesised that IL-15, a cytokine implicated in innate and acquired antiviral immunity, may be deficient in asthma and important in the pathogenesis of asthma exacerbations. We investigated regulation of IL-15 induction by rhinovirus in human macrophages in vitro, IL-15 levels in bronchoalveolar lavage (BAL) fluid and IL-15 induction by rhinovirus in BAL macrophages from asthmatic and control subjects, and related these to outcomes of infection in vivo. Rhinovirus induced IL-15 in macrophages was replication-, NF-κB - and α/β interferon-dependent. BAL macrophage IL-15 induction by rhinovirus was impaired in asthmatics and inversely related to lower respiratory symptom severity during experimental rhinovirus infection. IL-15 levels in BAL fluid were also decreased in asthmatics and inversely related with airway hyperresponsiveness and with virus load during in vivo rhinovirus infection. Deficient IL-15 production in asthma may be important in the pathogenesis of asthma exacerbations.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002114
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002114Summary
Rhinovirus infections are the major cause of asthma exacerbations. We hypothesised that IL-15, a cytokine implicated in innate and acquired antiviral immunity, may be deficient in asthma and important in the pathogenesis of asthma exacerbations. We investigated regulation of IL-15 induction by rhinovirus in human macrophages in vitro, IL-15 levels in bronchoalveolar lavage (BAL) fluid and IL-15 induction by rhinovirus in BAL macrophages from asthmatic and control subjects, and related these to outcomes of infection in vivo. Rhinovirus induced IL-15 in macrophages was replication-, NF-κB - and α/β interferon-dependent. BAL macrophage IL-15 induction by rhinovirus was impaired in asthmatics and inversely related to lower respiratory symptom severity during experimental rhinovirus infection. IL-15 levels in BAL fluid were also decreased in asthmatics and inversely related with airway hyperresponsiveness and with virus load during in vivo rhinovirus infection. Deficient IL-15 production in asthma may be important in the pathogenesis of asthma exacerbations.
Introduction
Rhinovirus (RV) infections in healthy individuals manifest as common colds but in asthma are strongly associated with acute exacerbations [1], [2]. Type I (α/β), II (γ) and III (λ) interferon (IFN) responses are important in anti-viral immunity and increased susceptibility to RV infection has been demonstrated in asthma in vivo [2], [3]. RV infection of asthmatic bronchial epithelial cells (BEC) in vitro resulted in reduced IFN-β and -λ production and increased viral replication [4], [5]. Monocytes/macrophages are also infected and activated by RV [6] and macrophage IFN-λ production is also impaired in asthma and related to asthma exacerbation severity and virus load in vivo [5]. Bronchoalveolar lavage (BAL) cell IFN-γ induction by RV is also impaired in asthma and related to exacerbation severity in vivo [3].
IL-15 is important in linking innate and adaptive antiviral immune responses, promoting natural killer (NK) and memory CD8 T cell anti-viral immune responses [7], [8] and monocytes/macrophages produce IL-15 in response to virus infections [7]-[10]. There is little reported data on IL-15 in asthma. IL-15 mRNA expression levels in bronchial biopsies in asthma are not increased [11], while protein levels in sputum are undetectable in normal subjects and steroid naïve asthmatics, but detectable in steroid-treated asthmatics [12]. There is a single report that IL-15 gene expression is increased in asthma exacerbations in children [13] but no data on IL-15 in RV infections. Since (i) α/βIFNs are reported to induce IL-15 in dendritic cells and monocytes [14], [15], (ii) RV induction of IFN-β is reported deficient in asthma [4], (iii) IL-15 is important in innate and acquired antiviral immunity and (iv) there is increased susceptibility to RV infection in asthma [1]–[3] we hypothesized that IL-15 production may be deficient in asthma and related to asthma exacerbation pathogenesis.
We have therefore tested the hypotheses that RV infection of macrophages in vitro induces IL-15 production and that this is mediated by α/β IFNs and the transcription factor nuclear factor-κB (NF-κB). In addition, we determined whether RV induction of IL-15 ex vivo is deficient in macrophages from asthmatic subjects and whether IL-15 levels in BAL fluid are deficient in asthma. We also investigated whether IL-15 deficiency in asthma is related to parameters of severity and virus load during experimental RV16 infection in vivo.
Results
RV up-regulates IL-15 production in macrophages
To investigate whether RV induces IL-15 release from macrophages we used two models of monocyte-derived macrophages in which RV induces replication-dependent activation [6]. We did not measure virus replication in this study however we have previously reported that rhinovirus replication was productive in THP-1 macrophages, leading to release of infectious virus into supernatants, but was limited in monocyte-derived macrophages [6]. IL-15 protein release into supernatants was significantly induced by RV16, at 48 and 72 hours for THP-1-derived macrophages and between 24 and 72 hours for monocyte-derived macrophages (Figure 1A and B). RV9 (major group) and RV1B (minor group) also induced IL-15 release from THP-1-derived macrophages (Figure 1C) indicating that IL-15 induction was not RV serotype - or receptor-dependent. IL-15 release was reduced significantly by UV-inactivation, confirming that induction was largely replication-dependent (Figure 1C).
Fig. 1. Rhinovirus infection induces IL-15 protein and mRNA production in human monocyte-derived macrophages. 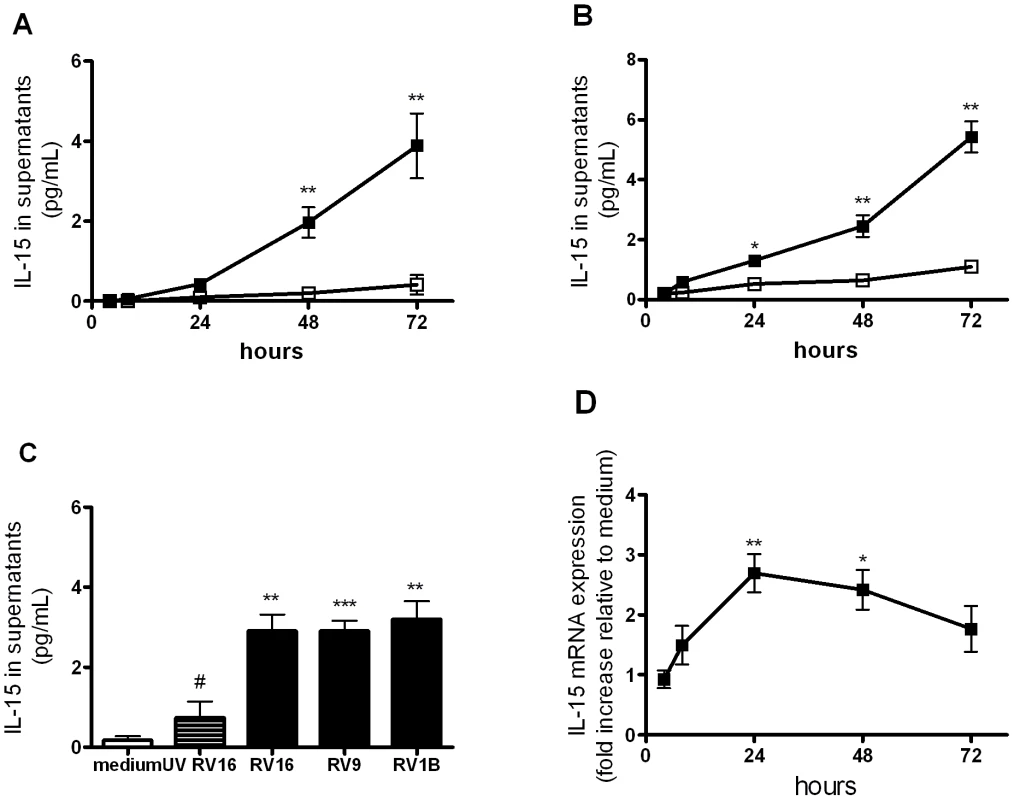
(A) THP-1-derived macrophages and (B) peripheral blood monocyte-derived macrophages were infected with RV16 (closed squares) or incubated with medium alone (open squares) at time 0. Supernatants were harvested at 4, 8, 24, 48 and 72 hours and levels of IL-15 released determined by ELISA. (C) THP-1-derived macrophages were exposed to major group rhinovirus (RV16 and RV9), minor group rhinovirus (RV1B), medium alone and UV-inactivated RV16 (UV RV16) at time 0 and supernatants harvested at 72 hours. (D) THP-1-derived macrophages were infected as for (A). Total RNA was extracted from cell lysates at 4, 8, 24, 48 and 72 hours post-infection. IL-15 mRNA was quantified by PCR and results normalised to constitutive 18S ribosomal RNA and expressed as fold induction over medium alone. Mean and SEM from at least four independent experiments (performed in triplicate) are shown. * P<0.05, ** P<0.01, *** P<0.001 for live virus compared to medium, and # P<0.05 for live RV16 compared to UV-inactivated. Because IL-15 protein can be stored intracellularly in macrophages and virus infection could simply trigger the release of preformed protein we next investigated whether RV infection up-regulated IL-15 mRNA expression and found that IL-15 mRNA was significantly induced by RV at 24 hours and 48 hours (Figure 1D).
NF-κB activation is required for IL-15 induction in RV-infected macrophages
The IL-15 promoter has been shown to have binding sites for nuclear factor (NF)-κB and interferon regulatory factors (IRFs) which are important for its activation by other stimuli [10], [16]–[18]. To assess the role of NF-κB in RV-induced IL-15 production we inhibited activation of the NF-κB pathway with a NF-κB pharmacological inhibitor (AS602868) [6] at the time point of maximal IL-15 production. We used the IKK2 inhibitor AS602868 at a concentration of 5 µM, which we have previously shown to be optimal for rhinovirus induced TNF-α inhibition and without cell toxicity [6].
RV16-induced IL-15 production was significantly decreased in THP-1-derived macrophages (p<0.01) and monocyte-derived macrophages (p<0.01) (Figure 2A and B) in the presence of the NF-κB inhibitor, confirming that RV-induction of IL-15 was NF-κB-dependent in both cell types.
Fig. 2. NF-kB-activation is required for rhinovirus induction of macrophage IL-15 production. 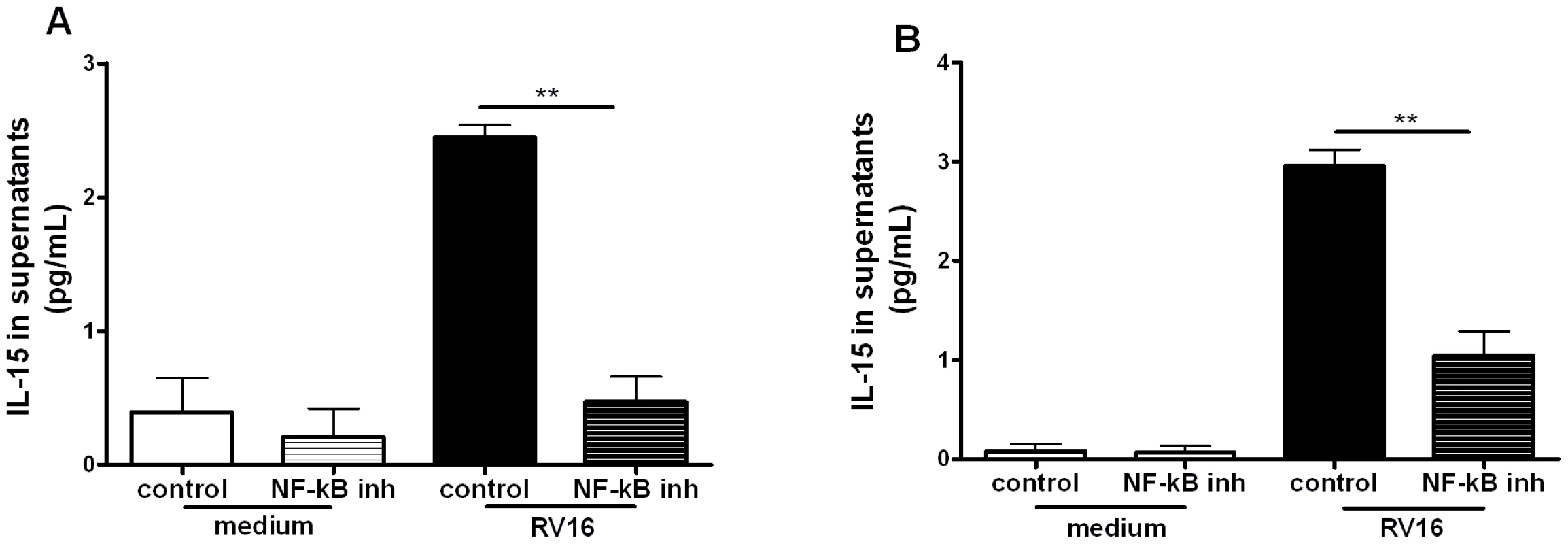
(A) THP-1-derived macrophages and (B) monocyte-derived macrophages were pre-treated for 1 hour with an inhibitor of NF-κB activation (the NF-κB inhibitor AS602868 5 µM) or diluent control, before infection with RV16. The same concentration of drug/diluent was added to the medium after infection. Supernatants were harvested at 72 hours and IL-15 release quantified by ELISA. Means and SEM from at least three independent experiments (performed in triplicate) are shown. ** P<0.01 for live virus infected cells, NF-κB inhibitor compared to diluent control. IFN-α/β induction in RV-infected macrophages is dependent on NF-κB
As α/β IFNs are induced during viral infections and are implicated in IL-15 induction in other systems, we next investigated RV induction of IFN-α/β in macrophages. RV16 infection induced IFN-β production at 8 and 48 hours (p<0.05), peaking at 24 hours (p<0.001, Figure 3A) while IFN-α was induced at 24 hours (p<0.001) and 48 hours (p<0.01, Figure 3B). RV9 and RV1B induced similar levels confirming that IFN-α/β production in macrophages is not serotype - or receptor-dependent and UV-inactivation completely abolished IFN-α/β induction demonstrating that induction is replication-dependent (Figure 3C and D). RV induction of IFN-α/β was also dependent on NF-κB activation as the NF-κB inhibitor markedly decreased RV-induction of IFN-β and IFN-α (Figure 3E and F, both p<0.01).
Fig. 3. IFN-β and IFN-α are induced by rhinovirus infection of macrophages via NF-κB-dependent mechanisms. 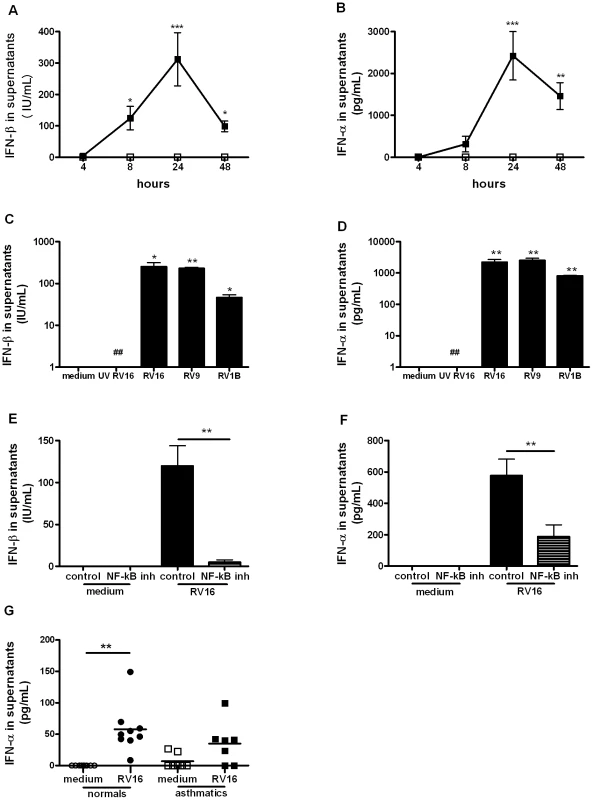
(A–B) Monocyte-derived macrophages were infected with RV16 (closed squares) or incubated with medium alone (open squares) at time 0, supernatants were harvested at 4, 8, 24 and 48 hours and levels of IFN-β (A) and IFN-α (B) quantified by ELISA. Means and SEM from at least four independent experiments (performed in triplicate) are shown. (C–D) Monocyte-derived macrophages were exposed to medium alone, UV-inactivated RV16 (UV RV16) or infected with RV16, RV9 or RV1B, cultured for 24 hours, supernatants harvested and IFN-β (C) and IFN-α (D) quantified by ELISA. Means and SEM from at least five independent experiments (performed in triplicate) are shown. P<0.05, ** P<0.01, *** P<0.001 for live virus compared to medium, and ## P<0.01 for live RV16 compared to UV-inactivated RV16. (E–F) Monocyte-derived macrophages were pre-treated for 1 hour with an inhibitor of NF-κB activation (the NF-κB inhibitor AS602868 5 µM) or diluent control, before infection with RV16. The same concentration of drug/diluent was added to the medium after infection. Supernatants were harvested at 24 hours and IFN-β (E) and IFN-α (F) quantified by ELISA. Means and SEM from at least five independent experiments (performed in triplicate) are shown. ** P<0.01 for live virus infected cells, NF-κB inhibitor compared to diluent control. G. BAL cells from the baseline bronchoscopy of normal (circles, n = 9) or asthmatic (squares, n = 7) subjects were incubated for 48 hours with medium alone (open symbol, medium) or live rhinovirus (closed symbol, RV16) and IFN-α and IFN-β release into supernatants assessed by ELISA. Bars are median values, ** P<0.01, RV16 vs medium. We previously reported that there is deficient IFN-β production in bronchial epithelial cells in asthmatics upon RV infection [4] and that macrophage production of IFN-λ was similarly deficient [5]. Therefore we wished to investigate if type I IFN production is also deficient in alveolar macrophages from asthmatics. Cells were obtained with bronchoalveolar lavage and the composition of the lavage was ∼90% macrophages in all subjects with no differences in cellular composition between the asthmatics and the non-asthmatics [5]. In vitro RV16 infection of BAL cells induced significantly higher IFN-α levels compared with medium in normal subjects (p<0.01) but infection of BAL cells from asthmatics did not result in significantly up-regulated IFN-α levels (Figure 3G). IFN-α levels produced by BAL cells from normals were higher than levels from asthmatics but this difference was not statistically significant (p = 0.09). No IFN-β could be detected in supernatants of BAL cells at the 48 hour time point studied in either normal or asthmatic subjects.
We attempted to confirm deficient production of these IFNs in vivo, by measuring IFN-α and IFN-β directly in BAL fluid. Despite using up to 30x concentrated BAL fluid, no IFN-α or IFN-β could be detected in BAL fluid taken at the baseline, day 4 post-RV16 infection or the convalescence bronchoscopies.
RV induction of IL-15 in macrophages is IFN-α/β dependent
Having demonstrated that RV induces IL-15 and IFN-α/β production we next investigated whether IFN-α/β signalling is required for RV induction of IL-15 in macrophages. Firstly we investigated whether IFN-β could induce IL-15 secretion in macrophages and found that recombinant human IFN-β induced IL-15 secretion from both THP-1-derived and monocyte-derived macrophages in a dose dependent manner (Figure 4A). Using a blocking antibody against the IFN-α-receptor subunit 2 (IFNAR2) we found that RV16-induced IL-15 production was almost completely inhibited by blocking antibody, but not isotype control in both THP-1-derived and monocyte-derived macrophages (Figure 4B and C, p<0.01 and p<0.001 respectively). Since interferon regulatory factor-1 (IRF-1) is induced by type I IFN [19] and regulates IL-15 gene transcription in other systems [20] we next investigated IRF-1 protein induction by RV. Unstimulated THP-1-derived macrophages expressed low levels of IRF-1, however both IFN-β and RV16 increased IRF-1 intracellular protein levels as early as 4 hours, both remaining elevated to 24 hours (Figure 4D). Blocking the IFNAR, but not isotype control markedly inhibited IRF-1 induction by RV16 (Figure 4E), confirming that the IFN-α/β-IFNAR pathway was also required for IRF-1 activation by RV in macrophages.
Fig. 4. IFN-β induces IL-15 in macrophages and rhinovirus induction is via IFN-αβ receptor signalling. 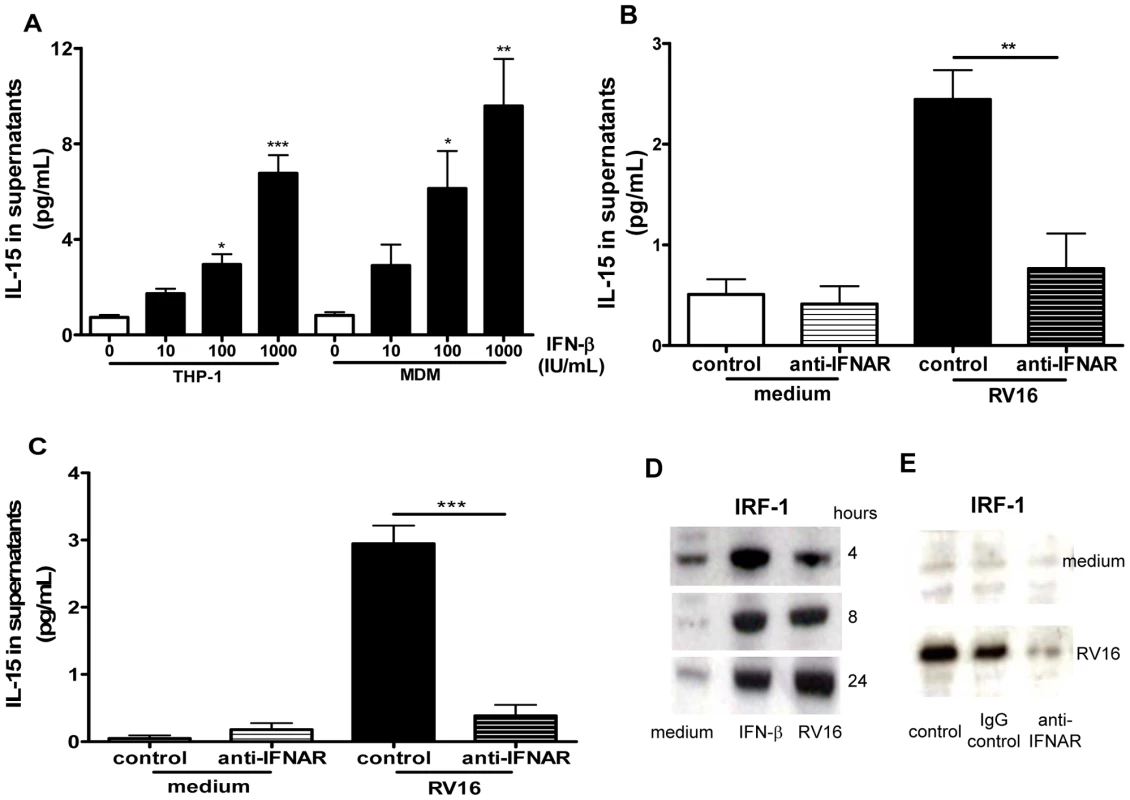
(A) THP-1-derived macrophages (THP-1) or monocyte-derived macrophages were stimulated with diluent control (0), or recombinant IFN-β at concentrations of 10, 100 and 1000 IU/mL, supernatants harvested at 72 hours and IL-15 quantified by ELISA. Means and SEM from at least three independent experiments (performed in triplicate) are shown. * P<0.05, ** P<0.01 and *** P<0.001, compared with diluent control. (B) THP-1-derived macrophages and (C) monocyte-derived macrophages were pre-treated for 1 hour with IFN-αβ receptor blocking antibody (anti-IFNAR) or isotype control (both at 5 µg/mL), the same concentration of antibody was added to the medium after infection with RV16, supernatants were harvested at 24 hours (C) and 72 hours (B) and IL-15 quantified by ELISA. Means and SEM from at least four independent experiments (performed in triplicate) are shown. ** P<0.01 and *** P<0.001 compared with isotype control. (D) Lysates of THP-1-derived macrophages incubated with medium alone (medium), or stimulated with IFN-β (IFN-β) at 1000 IU/mL or RV16 (RV16) were analysed for the presence of IRF-1 by Western blot at 4, 8 and 24 hours. A representative image of three independent experiments with similar results is shown. (E) Monocyte-derived macrophages treated as in (C) were lysed after 24 hours and analysed for IRF-1 by Western blot. RV16 induction of IRF-1 protein over medium control (medium) was clearly inhibited by prevention of αβ IFN signalling with an IFN-αβ receptor blocking antibody (anti-IFNAR), but not by isotype control (IgG control), or diluent alone (control). A representative image of three independent experiments with similar results is shown. RV induces IL-15 production in alveolar macrophages ex vivo; induction is deficient in asthma and related to lower respiratory symptom severity during RV infection
Having shown that RV infection induces IL-15 in two macrophage models in vitro we next investigated if induction is observed in alveolar macrophages infected ex vivo and whether induction is deficient in asthmatics. Supernatants from BAL cells (>90% alveolar macrophages) from normal and asthmatic subjects exposed ex vivo to RV16 for 48 hours were assessed for levels of IL-15 by ELISA. IL-15 levels were significantly increased by RV16 in BAL cells from normal subjects (p<0.05), but not in cells obtained from asthmatics, and levels in supernatants from RV16-infected cells were significantly higher in normal compared to asthmatic subjects (Figure 5A, p<0.01). IL-15 production by RV infected macrophages was inversely related to lower respiratory symptom severity on subsequent RV16 experimental infection in the same subjects in vivo (r = −0.6, p = 0.022, Figure 5B). Although IL-15 levels were very low, they were above the lower limit of detection (0.25 pg/mL) and differences observed were both statistically significant and related to a number of different clinical parameters.
Fig. 5. Rhinovirus induction of IL-15 release from alveolar macrophages; induction is inversely related to severity of lower respiratory symptoms following rhinovirus infection in vivo. 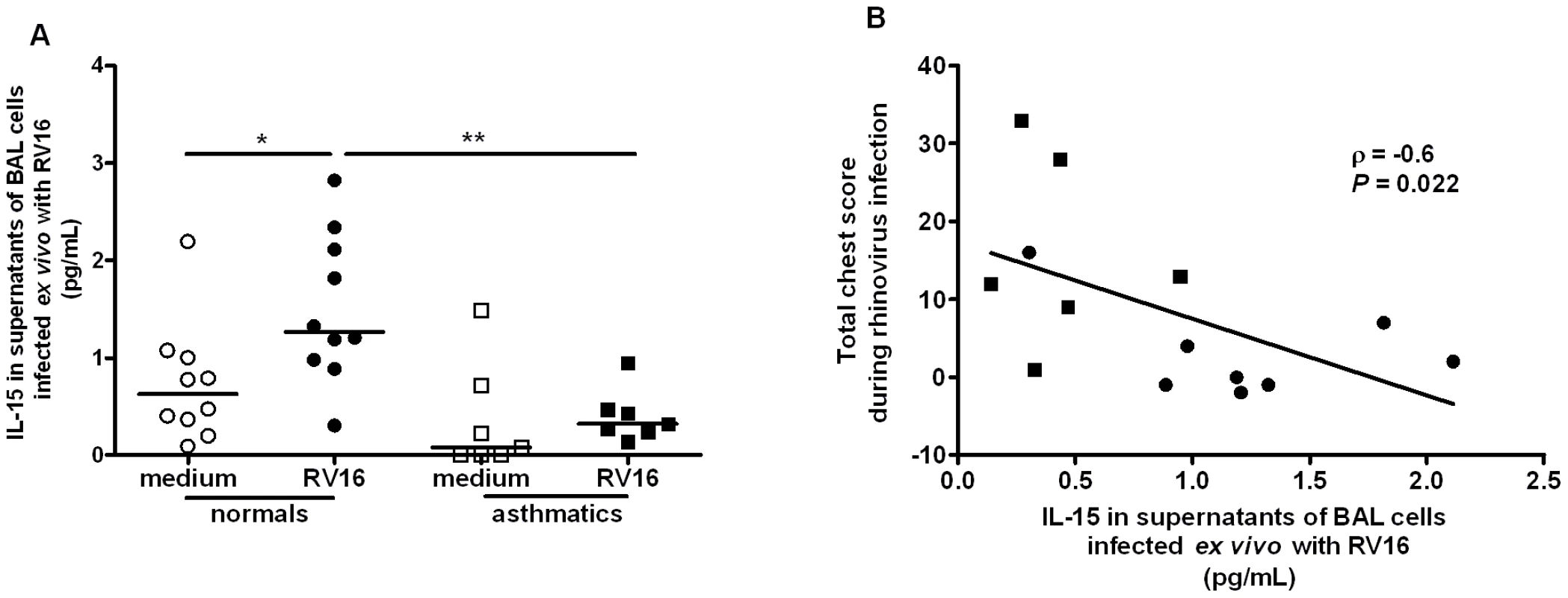
(A) BAL cells were collected at bronchoscopy from normal (circles, n = 10) or asthmatic (squares, n = 7) subjects. Cells were incubated for 48 hours with medium alone (open symbol, medium) or live rhinovirus (closed symbol, RV16) and IL-15 release into supernatants assessed by ELISA. Bars are median values, * P<0.05, RV16 vs medium and ** P<0.01 RV16 asthmatic vs. RV16 normal subjects. (B) Levels of IL-15 released ex vivo in RV16 infected cultures from normal (closed circles, n = 8) and asthmatic (closed squares, n = 6) subjects were significantly related to severity of total lower respiratory symptoms during the 2 weeks following experimental infection with RV16 in vivo. BAL IL-15 levels are deficient in asthmatics and related to airway hyperresponsiveness and virus load during subsequent RV infection in vivo
To investigate whether IL-15 levels are deficient in asthma in vivo, IL-15 was measured in BAL fluid collected from asthmatic and normal subjects when clinically stable prior to experimental RV16 infection. The study recruited 15 non-atopic healthy and 10 atopic mild asthmatic subjects, all rhinovirus-16 serum neutralizing antibody negative and all non-smokers. The healthy control group had a median age of 24, sex ratio 8 male/7 female and a median baseline FEV1% predicted of 99% and the asthmatic subjects were inhaled steroid naïve, median age of 22, sex ratio 2 male/8 female and a median baseline FEV1% predicted of 104% as previously reported [3].
IL-15 levels were significantly lower in asthmatic compared to normal subjects (Figure 6A, p<0.05) and were significantly correlated with airway hyperresponsiveness as measured by baseline PC10 histamine (r = 0.47, p = 0.021, Figure 6B). IL-15 levels in vivo were also significantly related to virus loads in nasal lavage (r = −0.57, p = 0.005), induced sputum (r = −0.44, p = 0.041) and BAL (r = −0.61, r = 0.024) upon subsequent in vivo RV16 experimental infection in the same subjects (Figure 6C–E).
Fig. 6. IL-15 levels in BAL fluid in asthma are inversely related to airway hyperresponsiveness and virus load on subsequent in vivo rhinovirus infection. 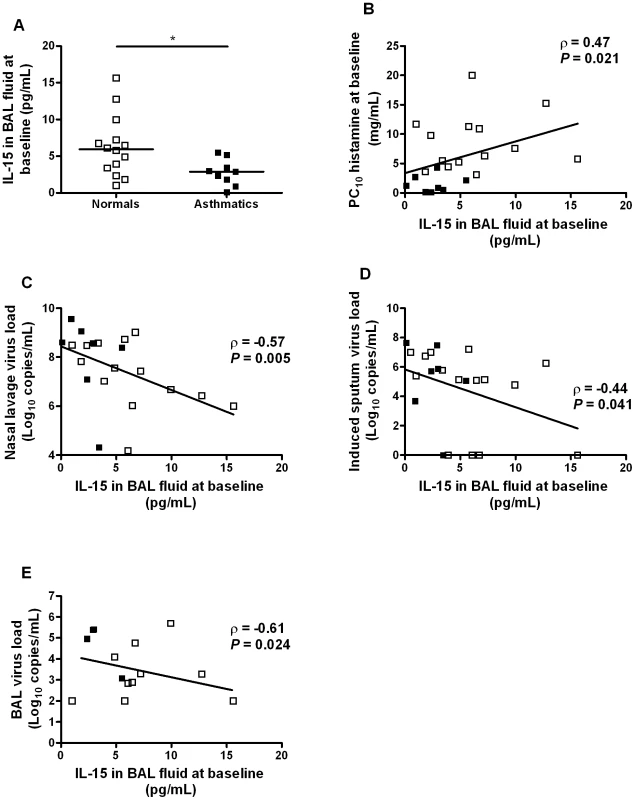
Airway hyperresponsiveness was assessed and BAL fluid collected at a baseline bronchoscopy from normal volunteers and asthmatic subjects. Two weeks later, the same subjects were experimentally infected with RV16 and virus load measured in nasal lavage, sputum and BAL. (A) IL-15 levels were measured by ELISA in BAL fluid collected at bronchoscopy from normal (n = 14) and asthmatic subjects (n = 9). Bars are median values, * P<0.05 for comparison between groups. (B) Levels of IL-15 in BAL fluid in asthmatic (n = 8) and normal subjects (n = 15) were inversely related to bronchial hyperresponsiveness. (C–E) Levels of IL-15 in BAL fluid in asthmatic and normal subjects were inversely related to virus load during a subsequent experimental RV16 infection in vivo: (C) peak virus load in nasal lavage, (D) virus load in induced sputum on day 3 post infection, (E) virus load in BAL on day 4 post infection. Discussion
We report herein the first investigation of the role of IL-15 in the pathogenesis of RV-induced asthma exacerbations. IL-15 production was induced by RV in macrophage cell lines in vitro, as well as in primary alveolar macrophages. IL-15 induction by RV was deficient in cells from asthmatic compared to normal subjects and levels in BAL fluid deficient in asthmatics. IL-15 induction ex vivo and levels in vivo were both related to airway hyperresponsiveness, lower respiratory symptom severity and virus load following experimental RV16 infection in vivo. Finally, IL-15 induction was dependent on IFN-α/β and both IL-15 and IFN-α/β induction were dependent on NF-κB.
These findings have important implications as they suggest IL-15 as a novel candidate for development for treatment or prevention of asthma exacerbations. As IL-15 is deficient in asthma this suggests either or both of prophylactic or therapeutic treatment approaches may have value. Further, IL-15 inducers such as IFN-β may have additional benefit beyond directly enhancing anti-viral immunity in bronchial epithelium, since, via induction of IL-15 production, they may enhance other aspects of both innate and acquired anti-viral immunity. Finally, NF-κB inhibitors are in development as possible therapies for asthma exacerbations, our data that IFN-α, IFN-β and IL-15 induction by RV are all NF-κB dependent suggest that such approaches may further impair already deficient responses in asthma.
Rhinoviruses are the respiratory viruses most commonly associated with asthma exacerbations. Asthma is the most common chronic respiratory disease, and it is increasing in many countries. The major cause of asthma related morbidity and mortality are acute exacerbations and current asthma treatments are only partially effective at preventing asthma exacerbations. Therefore, new approaches to treatment and prevention are urgently required and these are likely to stem from a better understanding of the pathogenesis of asthma exacerbations.
Asthma exacerbation pathogenesis is poorly understood, however, it is clear that people with asthma have increased susceptibility to respiratory virus infections [2], [3]. We have previously reported deficient IFN-β and IFN-λ production in response to RV infection of bronchial epithelial cells in asthmatics [4], [5], further these deficiencies were related to increased RV replication in the same cells, and replacement with exogenous IFN-β restored normal resistance to RV infection. Deficiency of IFN-λ induction by RV was observed in macrophages from asthmatic subjects, and this was related to virus load, asthma exacerbation severity and severity of airway inflammation in vivo [5]. These findings led to inhaled IFN-β being developed as a possible novel therapy to treat/prevent asthma exacerbations – currently in a Phase II clinical trial.
We have also recently reported deficient IFN-γ production in asthma and related impaired IFN-γ production to greater virus loads and reductions in lung function in a human model of asthma exacerbation in vivo [3]. IFN-γ is produced by both innate and acquired arms of the antiviral immune system. As IL-15 is also important in linking innate and adaptive antiviral immune responses, and in other systems, has been shown to be induced by type I IFNs we wished to investigate the hypothesis that IL-15 might also be deficient in asthma, and that deficiency might be related to the pathogenesis of asthma exacerbations.
Alveolar macrophages are important immune cells in lung antiviral immune responses and we previously reported that monocytes/macrophages support RV replication, become activated and secrete immunomodulatory cytokines [3], [6], [21], [22]. However, there are no published data on IL-15 induction in macrophages by RV, nor on its possible importance in the pathogenesis of asthma exacerbations. Consequently, we investigated macrophage IL-15 and type I IFN responses during RV infection in vitro and in vivo, in both asthmatic and normal subjects.
The most important findings of this study are the deficient induction of IL-15 by RV in alveolar macrophages from asthmatic subjects in vitro, deficient IL-15 levels in asthma in BAL fluid in vivo, and the relationships of these to airway hyperresponsiveness, severity of symptoms and virus load on subsequent RV infection in vivo. The correlations between IL-15 levels in BAL fluid at baseline and PC10 histamine at baseline and virus load during an experimental rhinovirus infection were only statistically significant when all subjects were included. There were no significant correlations within the group of asthmatic subjects alone, presumably due to the low numbers of asthmatic patients included, and larger studies are required to confirm the link between asthma exacerbation pathogenesis, virus replication and IL-15. However these findings taken together suggest IL-15 as a promising candidate for development as a novel therapy to enhance deficient antiviral immunity in asthma, to correct the increased susceptibility to virus infection present in this condition [2], [3] and in particular as a potential treatment to prevent/treat asthma exacerbations.
IL-15, via the IL-2/IL-15 receptor β-chain and common γ chain receptor, promotes NK cell activation, as well as enhancing memory CD8 T cell antiviral immunity [7], [8], [23]. In addition it enhances type I (α/β) IFN production by dendritic cells and macrophages [24], [25] as well as IFN-γ production by NK and CD8 T cells [7], [8], [26]. Since induction of both IFN types is deficient in asthma and related to asthma exacerbation severity, IL-15 has potential to correct deficiencies present in antiviral immunity in asthma.
Our findings also have important implications for development of type I IFNs as potential therapies for asthma exacerbations, as we show that RV infection of macrophages induces α/β IFNs, that IL-15 is induced by IFN-β stimulation and that RV induction of IL-15 in macrophages is dependent on IFN-α/β receptor signalling. These data suggest that administration of α/β IFNs in asthma is likely to enhance IL-15 baseline levels, as well as enhancing induction upon RV infection, both of which we have shown to be deficient in this report. Type I IFN administration might therefore have benefits well beyond correcting the specific deficit in anti-viral immunity in bronchial epithelial cells, indirectly enhancing deficient IFN-γ production, as well as other NK and CD8 responses via correction of deficient IL-15 production.
These findings also have important implications for development programmes for inhibitors of NF-κB [27]. This transcription factor is implicated in many pro-inflammatory responses linked with the pathogenesis of asthma exacerbations and many pharmaceutical companies have active NF-κB inhibitor development programmes. However we find that RV induction of IFN-α, IFN-β and IL-15 are all profoundly suppressed by inhibition of NF-κB. These data suggest that administration of such inhibitors in asthma, while inhibiting pro-inflammatory mediator production, is likely to further impair deficient type I IFN and IL-15 production, and therefore might have potential to increase rather than decrease severity of virus induced asthma exacerbations. Careful investigation of these outcomes, as well as their relationships with clinical outcomes in human models of RV-induced asthma exacerbations [3] may shed further light on these possibilities.
Because signalling by IL-15 occurs via the IL-2/IL-15 receptor β-chain (CD122) and common γ chain receptor (CD132) expressed primarily by NK and memory CD8 T cells [7], [8], [23], [26], it will be of great interest to determine the levels of these receptors on airway NK and CD8 T cells in virus-induced asthma.
Finally, our findings of deficient IL-15 production in asthma, combined with previous reports of deficient IFN-β [4], IFN-λ [5], IFN-γ and IL-12 [3], [22], suggest complex impairment of anti-viral immune responses in asthma. The mechanisms behind these complex deficiencies clearly require urgent investigation. These findings also have major implications for management of pandemic influenza, where asthma is a major risk factor for severe disease and death [28], as replacing deficient anti-viral immune proteins such as IFN-β and now IL-15, may have therapeutic potential to ameliorate severity of disease and perhaps prevent death.
Materials and Methods
Cells, viruses and cell infection/stimulation
HeLa and THP-1 cell lines (ECCC) were cultured in E-MEM and RPMI-1640 (Invitrogen, Paisley, UK) respectively with 10% foetal calf serum (FCS). RV serotype 16, 9 (major group) and 1B (minor group) stocks were prepared and their identities confirmed by neutralisation using serotype-specific antibodies (ATCC), UV-inactivation was performed as previously described [29]. Peripheral blood monocyte-derived macrophages and THP-1-derived macrophages were generated and infected with RV at a multiplicity of infection (MOI) of 1 as previously described [6]. Recombinant human IFN-β (10–1000 IU/mL, R&D, Abingdon, UK) was added to wells. Supernatants, RNA or protein lysates were harvested and stored at −80°C.
IL-15 mRNA quantification
Total RNA was extracted with RNeasy Kit (Qiagen) and 2 µg was used for cDNA synthesis (Omniscript RT Kit, Qiagen). Quantitative RT-PCR was performed using specific primers and probe for IL-15 (forward GGGAAAGTGATGTTCACCCC, reverse CATCTCCGGACTCAAGTGAAATAA, probe ATCTGGATGCAAAGAATGTGAGGAACTGGA). IL-15 gene expression was normalized to 18S rRNA and presented as fold induction relative to medium [30].
Ethics statement
Ethics approval (No 99/BA/345) was obtained from St Mary's Local Research Ethics Committee, London, UK. All study participants gave written informed consent.
Human experimental model of RV-induced asthma exacerbation
The exacerbation model, clinical details including allergy testing and lung function, sampling and analysis are described in detail elsewhere [3]. The study recruited 15 non-atopic healthy control and 10 atopic, inhaled steroid naïve asthmatic subjects; all were non-smokers. Assessment of airway hyperresponsiveness by determination of the provocative concentration of histamine inducing a 10% fall in FEV1 (PC10 histamine) and BAL were performed at baseline ∼2 weeks prior to experimental RV infection as described [3]. Baseline BAL fluid was collected in a single plastic chamber and transferred immediately to polypropylene tubes on ice, transported to the laboratory, filtered (100 µm), centrifuged at 1500 rpm for 10 min at 4°C, the fluid decanted, aliquoted and stored at −80°C till analysed. Because we could not detect IL-15 in un-concentrated BAL fluid, we concentrated the BAL fluid 30 times as described below. Baseline BAL cells were cultured and exposed to RV16 MOI 5/medium as described [3]. After 48 hours, supernatants were harvested and stored at −80°C.
Virus load on days 1–8 and 11 post-infection for nasal lavage, on day 3 post-infection for sputum and on day 4 post-infection for BAL were determined using quantitative PCR as described [3]. Lower respiratory symptom severity during the two weeks post infection was derived using a chest symptom score as described [3].
BAL fluid concentration for IL-15 ELISA
IL-15 levels were measured after concentrating the baseline BAL fluid up to 30 times using a centrifugal filter with a nominal molecular weight value of 3000 kD (Millipore, Centriplus YM-3). Values were corrected for variability in dilution during lavage, and during subsequent concentration using total protein concentration of the concentrated BAL (Bradford method, Sigma) [31].
Western blot for IRF-1 expression
THP-1-derived macrophages were lysed into SDS sample buffer (Invitrogen). Western blot was performed as previously described [6]. After blocking, membranes were incubated with rabbit anti-human IRF-1 (1/500) followed by HRP swine anti-rabbit antibody (1/4000) (AbD Serotec). Bands were visualized by chemiluminescence with the ECL Western blotting detection reagent (GE Healthcare).
IL-15, IFN-α and IFN-β ELISA
Levels of IFN-α and IFN-β were measured using ELISA kits (BioSource International) (sensitivity 15 pg/mL and 5 IU/mL respectively). For the IL-15 ELISA we used commercially available paired antibodies (R&D Systems) at concentrations recommended by the manufacturer and for the standard curve recombinant human IL-15 (Biosource). The protocol recommended by the manufacturer was modified by incubating the plates for 10 minutes with streptavidin-HRP (Biosource) at a concentration of 0.5 µg/mL. A TMB containing substrate solution was used to develop the colour. Once optimised, we could reproducibly detect levels of IL-15 over 0.25 pg/mL.
NF-κB and IFN - IFN-receptor pathway inhibition
The effect of NF-κB activation on IL-15 production was evaluated using a NF-κB inhibitor (AS602868) as previously described [6]. The role of type I interferons in IL-15 production was assessed using a blocking antibody to IFNAR2, a matched isotype antibody was used as control (Merck Chemicals). Cells were pre-treated for 1 hour before infection with IFNAR2 blocking antibody/isotype control at a concentration of 5 µg/mL. The same concentration of antibody was added to the medium after infection.
Statistical analysis
The results were analyzed using GraphPad Prism version 4.00 for Windows (GraphPad Software, California, USA). For in vitro experiments results expressed as mean±standard error of the mean (SEM) and analyzed using ANOVA for multiple comparisons followed where appropriate by paired Student's t tests for paired comparisons. Differences between normal and asthmatic groups were analysed using Mann Whitney tests and correlations using Spearman's rank correlation.
Zdroje
1. JohnstonSLPattemorePKSandersonGSmithSLampeF 1995 Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 310 1225 1229
2. CorneJMMarshallCSmithSSchreiberJSandersonG 2002 Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 359 831 834
3. MessageSDLaza-StancaVMalliaPParkerHLZhuJ 2008 Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A 105 13562 13567
4. WarkPAJohnstonSLBucchieriFPowellRPuddicombeS 2005 Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201 937 947
5. ContoliMMessageSDLaza-StancaVEdwardsMRWarkPA 2006 Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12 1023 1026
6. Laza-StancaVStanciuLAMessageSDEdwardsMRGernJE 2006 Rhinovirus replication in human macrophages induces NF-kappaB-dependent tumor necrosis factor alpha production. J Virol 80 8248 8258
7. CarsonWERossMEBaiocchiRAMarienMJBoianiN 1995 Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest 96 2578 2582
8. FawazLMSharif-AskariEMenezesJ 1999 Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J Immunol 163 4473 4480
9. MussoTCalossoLZuccaMMillesimoMRavarinoD 1999 Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood 93 3531 3539
10. EnnaciriJAhmadRMenezesJ 2007 Interaction of monocytic cells with respiratory syncytial virus results in activation of NF-kappaB and PKC-alpha/beta leading to up-regulation of IL-15 gene expression. J Leukoc Biol 81 625 631
11. MuroSTahaRTsicopoulosAOlivensteinRTonnelAB 2001 Expression of IL-15 in inflammatory pulmonary diseases. J Allergy Clin Immunol 108 970 975
12. Komai-KomaMMcKayAThomsonLMcSharryCChalmersGW 2001 Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clin Exp Allergy 31 1441 1448
13. BoscoAEhteshamiSSternDAMartinezFD 2010 Decreased activation of inflammatory networks during acute asthma exacerbations is associated with chronic airflow obstruction. Mucosal Immunol 3 399 409
14. MatteiFSchiavoniGBelardelliFToughDF 2001 IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol 167 1179 1187
15. Gary-GouyHLebonPDalloulAH 2002 Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J Interferon Cytokine Res 22 653 659
16. WashizuJNishimuraHNakamuraNNimuraYYoshikaiY 1998 The NF-kappaB binding site is essential for transcriptional activation of the IL-15 gene. Immunogenetics 48 1 7
17. TaniguchiTOgasawaraKTakaokaATanakaN 2001 IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19 623 655
18. AhmadREnnaciriJCordeiroPEl BassamSMenezesJ 2007 Herpes simplex virus-1 up-regulates IL-15 gene expression in monocytic cells through the activation of protein tyrosine kinase and PKC zeta/lambda signaling pathways. J Mol Biol 367 25 35
19. SirenJPirhonenJJulkunenIMatikainenS 2005 IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol 174 1932 1937
20. TamuraTYanaiHSavitskyDTaniguchiT 2008 The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 26 535 584
21. PapadopoulosNGStanciuLAPapiAHolgateSTJohnstonSL 2002 Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin Exp Allergy 32 537 542
22. PapadopoulosNGStanciuLAPapiAHolgateSTJohnstonSL 2002 A defective type 1 response to rhinovirus in atopic asthma. Thorax 57 328 332
23. BeckerTCWherryEJBooneDMurali-KrishnaKAntiaR 2002 Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195 1541 1548
24. JinushiMTakeharaTTatsumiTKantoTGrohV 2003 Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol 171 5423 5429
25. FoongYYJansDARolphMSGahanMEMahalingamS 2009 Interleukin-15 mediates potent antiviral responses via an interferon-dependent mechanism. Virology 393 228 237
26. KutzlerMARobinsonTMChattergoonMAChooDKChooAY 2005 Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol 175 112 123
27. EdwardsMRBartlettNWClarkeDBirrellMBelvisiM 2009 Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther 121 1 13
28. JainSKamimotoLBramleyAMSchmitzAMBenoitSR 2009 Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 361 1935 1944
29. JohnstonSLPapiABatesPJMastronardeJGMonickMM 1998 Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol 160 6172 6181
30. HewsonCAEdbrookeMRJohnstonSL 2004 PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol 344 683 695
31. AghanouriRGhaneiMAslaniJKeivani-AmineHRastegarF 2004 Fibrogenic cytokine levels in bronchoalveolar lavage aspirates 15 years after exposure to sulfur mustard. Am J Physiol Lung Cell Mol Physiol 287 L1160 1164
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



