-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIllumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
The parainfluenza viruses (PIVs) are highly contagious respiratory paramyxoviruses and a leading cause of lower respiratory tract (LRT) disease. Since no vaccines or antivirals exist, non-pharmaceutical interventions are the only means of control for these pathogens. Here we used bioluminescence imaging to visualize the spatial and temporal progression of murine PIV1 (Sendai virus) infection in living mice after intranasal inoculation or exposure by contact. A non-attenuated luciferase reporter virus (rSeV-luc(M-F*)) that expressed high levels of luciferase yet was phenotypically similar to wild-type Sendai virus in vitro and in vivo was generated to allow visualization. After direct intranasal inoculation, we unexpectedly observed that the upper respiratory tract (URT) and trachea supported robust infection under conditions that result in little infection or pathology in the lungs including a low inoculum of virus, an attenuated virus, and strains of mice genetically resistant to lung infection. The high permissivity of the URT and trachea to infection resulted in 100% transmission to naïve contact recipients, even after low-dose (70 PFU) inoculation of genetically resistant BALB/c donor mice. The timing of transmission was consistent with the timing of high viral titers in the URT and trachea of donor animals but was independent of the levels of infection in the lungs of donors. The data therefore reveals a disconnect between transmissibility, which is associated with infection in the URT, and pathogenesis, which arises from infection in the lungs and the immune response. Natural infection after transmission was universally robust in the URT and trachea yet limited in the lungs, inducing protective immunity without weight loss even in genetically susceptible 129/SvJ mice. Overall, these results reveal a dichotomy between PIV infection in the URT and trachea versus the lungs and define a new model for studies of pathogenesis, development of live virus vaccines, and testing of antiviral therapies.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002134
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002134Summary
The parainfluenza viruses (PIVs) are highly contagious respiratory paramyxoviruses and a leading cause of lower respiratory tract (LRT) disease. Since no vaccines or antivirals exist, non-pharmaceutical interventions are the only means of control for these pathogens. Here we used bioluminescence imaging to visualize the spatial and temporal progression of murine PIV1 (Sendai virus) infection in living mice after intranasal inoculation or exposure by contact. A non-attenuated luciferase reporter virus (rSeV-luc(M-F*)) that expressed high levels of luciferase yet was phenotypically similar to wild-type Sendai virus in vitro and in vivo was generated to allow visualization. After direct intranasal inoculation, we unexpectedly observed that the upper respiratory tract (URT) and trachea supported robust infection under conditions that result in little infection or pathology in the lungs including a low inoculum of virus, an attenuated virus, and strains of mice genetically resistant to lung infection. The high permissivity of the URT and trachea to infection resulted in 100% transmission to naïve contact recipients, even after low-dose (70 PFU) inoculation of genetically resistant BALB/c donor mice. The timing of transmission was consistent with the timing of high viral titers in the URT and trachea of donor animals but was independent of the levels of infection in the lungs of donors. The data therefore reveals a disconnect between transmissibility, which is associated with infection in the URT, and pathogenesis, which arises from infection in the lungs and the immune response. Natural infection after transmission was universally robust in the URT and trachea yet limited in the lungs, inducing protective immunity without weight loss even in genetically susceptible 129/SvJ mice. Overall, these results reveal a dichotomy between PIV infection in the URT and trachea versus the lungs and define a new model for studies of pathogenesis, development of live virus vaccines, and testing of antiviral therapies.
Introduction
The parainfluenza viruses (PIVs) are non-segmented, negative-strand RNA viruses of the family Paramyxoviridae. The paramyxoviruses include not only the PIVs but also a number of other important human pathogens transmitted via the respiratory route such as human respiratory syncytial virus (HRSV), metapneumovirus, measles virus, and mumps virus [1], [2]. The human PIVs (HPIVs) consist of four serotypes (HPIV1-4), are a common cause of upper respiratory tract (URT) infections, and are a leading cause of lower respiratory tract (LRT) disease in infants and children [3]. The HPIVs are efficiently transmitted by direct contact and exposure to nasopharyngeal secretions [4], and nearly all children are infected with HPIV3 by age 2 and with HPIV1 and HPIV2 by age 5 [5], [6]. No licensed anti-PIV vaccines or drugs are available, and therefore non-pharmaceutical interventions are currently the only means of control. In view of these facts, an understanding of how PIV infection spreads within the respiratory tract, promotes pathogenesis, elicits immunity, and is transmitted to naïve hosts would greatly advance the development of novel vaccines and therapeutics.
Experimental studies of HPIV infection in tissue culture and animal models have helped reveal basic replication mechanisms and evaluate preclinical vaccine candidates [7]–[9]. However, knowledge about the spread of PIV infection in individual, living animals that are fully susceptible to PIV-associated disease would allow more thorough investigations of PIV virus-host interactions and transmission. Mice are poorly permissive to infection by the HPIVs, and HPIV infection in cotton rats, hamsters, guinea pigs, and ferrets is usually asymptomatic with minimal or undetectable pathology [1]. As a result, a number of studies have used Sendai virus (SeV) infection of mice as a model to investigate pathogenesis in an experimental setting [10], [11]. SeV is the murine counterpart of HPIV1, the leading cause of laryngotracheobronchitis (pediatric croup) [12]. SeV and HPIV1 have 78% amino-acid sequence identity [13], elicit cross-protective immunity [14]–[16], and have similar tissue tropism and epidemiology [1], [11]. Moreover, SeV shows promise as a Jennerian vaccine for HPIV1 [17] and as a vaccine vector for HRSV, HPIV3, and HPIV2 [18]–[20].
Although SeV and the HPIVs were first isolated in the 1950s and have been studied for more than 50 years [1], fundamental aspects of PIV infection and immunity that remain unknown are directly relevant to our understanding of pathogenesis and transmission. For example, the spatial and temporal spread of natural infection in the respiratory tract after SeV transmission remains poorly understood because of the ambiguous results (marked inter-animal variability and error) of classical experiments measuring virus titers in sacrificed mice [21], [22]. It is also unknown how HPIV and SeV infection after transmission often results in immunity without causing severe pathology. The contribution of LRT infection to transmission is unknown. Finally, while infection of the lungs and the concomitant immune response are clearly associated with disease severity [1], [11], [23], [24], many questions remain about the contribution of infection in the URT and trachea to clinical outcome and protective immunity [25], [26]. For example, we are unaware of any reports of studies investigating the effect of the dose of virus inoculum, the replicative fitness of the virus, or the genetic susceptibility of the host on the growth and clearance of SeV in the URT and trachea.
To measure the dynamics of PIV infection in living animals, we generated three luciferase-expressing SeVs that allow non-invasive in vivo bioluminescence imaging in mice. Analogous systems have previously been reported for DNA and positive-strand RNA viruses [27] but have been elusive for negative-strand RNA viruses, largely due to virus attenuation [28] or genetic instability resulting from reporter gene insertion [29]. We considered SeV an ideal candidate for non-invasive imaging because (i) foreign-gene expression by paramyxovirus vectors is usually genetically stable [30], (ii) in vivo imaging of a non-replicating SeV in intact mice has been successfully demonstrated [31] and (iii) the match of SeV and the murine host allows pathogenesis studies [11]. The reporter virus rSeV-luc(M-F*) described here was found to express high levels of luciferase yet replicate and promote disease in mice similar to wild-type (WT) virus. We imaged the dynamics of SeV infection in living, intact mice after direct inoculation and after contact transmission, varying both the virus dose and mouse strain. Unexpectedly, we observed a dichotomous tissue tropism in which the URT and trachea supported robust virus growth, efficient transmission, and protective immunity even under conditions that resulted in little infection in the lungs.
Results
In vitro properties of luciferase-expressing viruses
To develop a model in which PIV infection could be visualized non-invasively in living, intact mice, we generated three recombinant Sendai viruses (rSeVs) in which a firefly luciferase reporter gene was inserted into the P-M, M-F, and F-HN gene junctions, respectively, of SeV (Figure 1A, Figure S1). Insertion of the firefly luciferase gene and gene junction into the SeV genome was expected to unacceptably decrease downstream viral gene expression and, consequently, virus replication [32]. To generate a luciferase-expressing SeV expected to suffer little or no attenuation, the rSeV-luc(M-F*) virus was constructed to contain both the luciferase reporter gene and a more efficient transcription start sequence AGGGTGAAAG upstream of the F gene (Figure S1). Therefore, the attenuating effects of reporter gene insertion could be counteracted by optimization of the naturally inefficient gene start sequence upstream of the F gene [33]. For the rSeV-luc(P-M) and rSeV-luc(F-HN) constructs, in which the luciferase gene was inserted into the P-M and F-HN gene junctions, respectively, the natural transcription start sequence upstream of the F gene was left intact (Figure S1).
Fig. 1. In vitro and in vivo phenotypes of luciferase-expressing Sendai viruses. 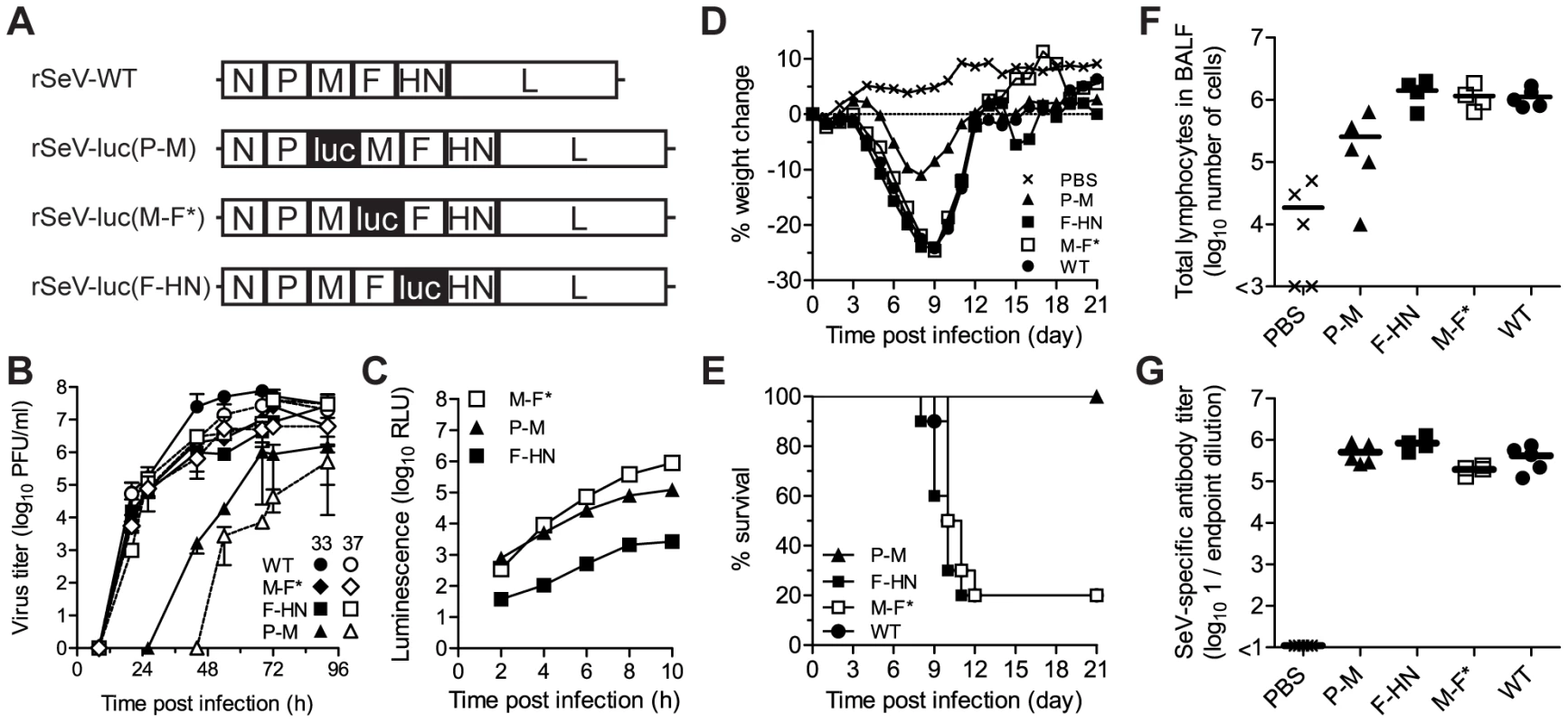
(A) Recombinant Sendai viruses that contain the firefly luciferase gene (luc) inserted into the P-M, M-F, and F-HN gene junctions were generated. (B) Multiple-step replication kinetics of wild-type (WT) and luciferase-expressing Sendai viruses in LLC-MK2 cell cultures infected at a multiplicity of infection (MOI) of 0.01 PFU/cell and incubated at 33°C (closed symbols) and 37°C (open symbols). (C) Kinetics of luciferase reporter gene expression in LLC-MK2 cells infected with recombinant Sendai viruses at an MOI of 5 PFU/cell, as measured by luminescence. (D) Changes in mean body weight after intranasal inoculation of Sendai viruses. (E) Percent survival after intranasal inoculation of Sendai viruses. (F) Total lymphocyte counts in bronchoalveolar lavage fluid (BALF) 10 days after infection. (G) Sendai virus-specific binding antibody titers in sera collected 10 days after infection, as measured by reciprocal endpoint dilutions in ELISA assays. For panels D–G, groups of five 8-week-old 129/SvJ-strain mice were intranasally inoculated with 7,000 PFU of recombinant Sendai virus or phosphate buffered saline (PBS) and the experiments were performed twice. Cumulative data are shown in panels D and E, and representative data are shown in panels F and G. Multiple-step growth curves were measured at 33°C and 37°C in LLC-MK2 cells that had been infected at a multiplicity of infection (MOI) of 0.01 PFU/cell (Figure 1B). Titers of rSeV-luc(M-F*) and rSeV-luc(F-HN) were similar at both temperatures and similar to SeV WT, showing that these two luciferase-expressing viruses were not substantially attenuated or temperature restricted. In contrast, the rSeV-luc(P-M) virus showed reduced growth kinetics at 33°C and grew even more slowly at 37°C. To compare luciferase reporter gene expression by the recombinant SeVs, we measured in vitro luciferase activity in LLC-MK2 cell lysates (MOI 5 PFU/cell) (Figure 1C). Upstream insertion of luciferase in rSeV-luc(P-M) resulted in greater luciferase activity than did downstream insertion in rSeV-luc(F-HN), consistent with the results of previous studies of SeVs using secreted alkaline phosphatase as the reporter gene [32]. Luciferase expression by rSeV-luc(M-F*) exceeded that of rSeV-luc(P-M) within 6 h post-infection (p.i.), showing that the enhanced gene start sequence engineered into the M-F* virus (Figure S1) increased reporter-gene transcription at later time points, perhaps due to greater downstream transcription of the L polymerase gene. To determine how the reporter gene insertions might have altered SeV protein expression, LLC-MK2 cells were infected at an MOI of 5 PFU/cell and lysates were subjected to radioimmunoprecipitation and SDS-PAGE analysis. Low levels of expression of the M, F, HN and presumably L proteins by the rSeV-luc(P-M) virus (Figure S2A) most likely contributed to the attenuation of this virus. Viral protein expression by rSeV-luc(M-F*) and rSeV-luc(F-HN) was sufficient to generate virions with WT-like compositions (Figure S2C), consistent with the in vitro growth of these two reporter viruses to levels similar to that of WT virus (Figure 1B).
Virulence of the luciferase-expressing viruses
An ideal luciferase-reporter virus for non-invasive bioluminescence imaging and pathogenesis studies would express high levels of luciferase without altering virus replication and disease severity. To assess the virulence of the luciferase-expressing SeVs, 129/SvJ mice were anesthetized with isoflurane and inoculated intranasally with 30 µl containing 7,000 PFU of virus. This method of inoculation delivers ∼1/3 of the volume to the nasopharynx and ∼1/2 to the lungs [34]. Infection with SeV WT, rSeV-luc(M-F*), and rSeV-luc(F-HN) resulted in a mean weight loss of ∼25% and mean mortality rates of 80% (Figure 1D,E). Thus, these two luciferase-expressing viruses remained fully virulent at a dose of 7,000 PFU. In contrast, 129/SvJ mice inoculated with 7,000 PFU of the attenuated rSeV-luc(P-M) virus experienced only 12% weight loss and no mortality. All mice inoculated with 70,000 or 700,000 PFU of rSeV-luc(P-M) also survived (data not shown), further demonstrating that the attenuated rSeV-luc(P-M) virus is avirulent.
Acute viral pneumonia by SeV induces high levels of lymphocyte infiltration that show a peak in bronchoalveolar lavage fluid (BALF) at ∼10 d p.i. [35]. To compare lymphocyte influx caused by the luciferase-expressing viruses and WT virus, we sacrificed 129/SvJ mice that had been infected with 7,000 PFU at 10 d p.i. and collected BALF. Similarly large total numbers of lymphocytes, CD4+ T-lymphocytes, and CD8+ T-lymphocytes were detected in BALF after infection with WT, rSeV-luc(M-F*), or rSeV-luc(F-HN) virus (Figure 1F; Figure S3A–B), whereas mice inoculated with the attenuated rSeV-luc(P-M) had total lymphocyte counts only 10% as high. To determine the extents to which the reporter viruses elicited SeV - or luciferase-binding antibodies, ELISA assays were performed on sera collected at 10 d p.i. The anti-SeV antibody titers elicited by all three rSeVs were similar to that induced by WT virus (Figure 1G). The three reporter viruses also induced similar anti-luciferase antibody titers (Figure S3C). Thus, despite being attenuated and avirulent, rSeV-luc(P-M) elicited a robust antibody response. rSeV-luc(M-F*), which induced WT-like morbidity and mortality while expressing high levels of luciferase, was identified as the best suited surrogate for WT virus for use in subsequent bioluminescence imaging experiments.
Dynamics of infection in living animals
In studies to determine whether non-invasive bioluminescence accurately reflected in vivo infection, 129/SvJ mice were intranasally inoculated with 7,000 PFU, imaged with a Xenogen IVIS instrument, and immediately euthanized. Respiratory tissues were promptly collected for ex vivo measurement of luminescence and viral titers. As in previous studies in immunocompetent mice [36], [37], viral titers and bioluminescence were limited to the respiratory tract. As shown in Figure S4, in vivo bioluminescence intensity levels in living animals were well correlated with ex vivo luminescence (R2 0.878) and with viral titers in the nasopharynx (R2 0.864), trachea (R2 0.915), and lungs (R2 0.961). The correspondence of these data validates the technique as a means of noninvasive measurement of infection in vivo. To determine whether the luciferase-reporter genes were genetically stable in the three rSeVs, we recovered lung tissues from 129/SvJ mice inoculated with 7,000 PFU of virus at 7 d p.i., homogenized the samples, and conducted plaque assays in LLC-MK2 cells. Five plaques of each of the three luciferase-expressing viruses were picked, amplified by one round of replication in eggs, RT-PCR transcribed, and sequenced. All of the individual viral clones contained the luciferase insert, which had no amino acid mutations, and expressed luciferase when grown in LLC-MK2 cells.
We next measured the kinetics and tropism of bioluminescence in living 129/SvJ mice and compared the results to conventionally measured viral titers in dissected tissues (Figures 2 and 3). Just as rSeV-luc(M-F*) and rSeV-luc(F-HN) had in vitro replication rates and in vivo pathogenicity similar to those of WT virus, they also had WT-like titers in the nasal turbinates, trachea, and lungs. In the nasal turbinates, high virus titers (>105 PFU) were detected by day 2 p.i. and were maintained until day 9 p.i., after which rapid clearance occurred (Figure 3B). Between days 2 and 9 p.i., high levels of in vivo bioluminescence were similarly observed in the nasopharynx (>108 photons/s) of 129/SvJ mice infected with rSeV-luc(M-F); bioluminescence peaked at about 5 d p.i. (Figure 3A). In the lungs, the titers of all three luciferase-expressing viruses and of WT SeV peaked by day 5 p.i. and fell to low levels by day 9 p.i. Infection with the attenuated rSeV-luc(P-M) resulted in peak lung titers of ∼104 PFU (approximately 5% of the WT titer) at day 5 p.i. (Figure 3D). Similarly low levels of rSeV-luc(P-M) bioluminescence were observed in the lungs (Figure 3A), consistent with the attenuated and avirulent virus phenotype. On the other hand, rSeV-luc(P-M) reached high peak titers (∼105 PFU, similar to the WT titer) in the nasal turbinates at 7 d p.i. (Figure 3C), and high levels of bioluminescence were observed in the nasopharynx between days 3 and 7 p.i. (Figure 3A).
Fig. 2. Non-invasive bioluminescence imaging of Sendai virus infection in the respiratory tracts of living mice. 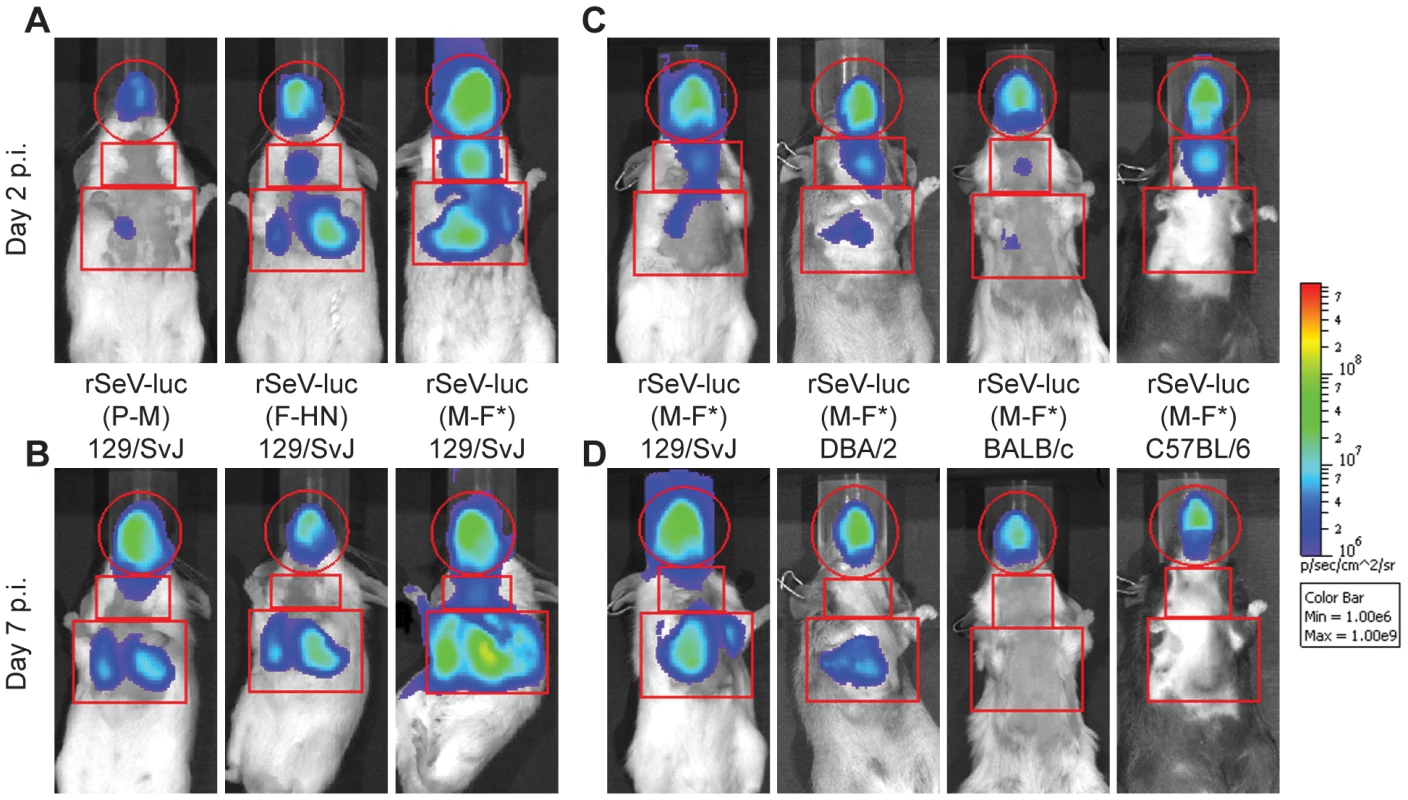
Eight-week-old mice were intranasally inoculated with 7,000 PFU of rSeV-luc(P-M), rSeV-luc(F-HN), or rSeV-luc(M-F*). Every 24 hours the mice were intraperitoneally injected with luciferin substrate, anesthetized with isoflurane, imaged with a Xenogen IVIS device, and then allowed to recover. Bioluminescence in one experiment is shown on day 2 (A) and day 7 (B) post-infection (p.i.) in 129/SvJ mice infected with rSeV-luc(P-M), rSeV-luc(F-HN), or rSeV-luc(M-F*). Bioluminescence in a second experiment is shown on day 2 (C) or day 7 (D) for 129/SvJ, DBA/2, BALB/c, or C57BL/6 mice infected with rSeV-luc(M-F*). The data are displayed as radiance (bioluminescence intensity) on a rainbow log scale with a range of 1×106 (blue) to 1×109 (red) photons/s/cm2/steradian. Red circles indicate regions of interest (ROI) used to calculate the total flux (photons/s) in the nasopharynx, and red rectangles indicate the ROI areas for the trachea and lungs. Fig. 3. Kinetics of Sendai virus spread and clearance in the respiratory tracts of 129/SvJ mice. 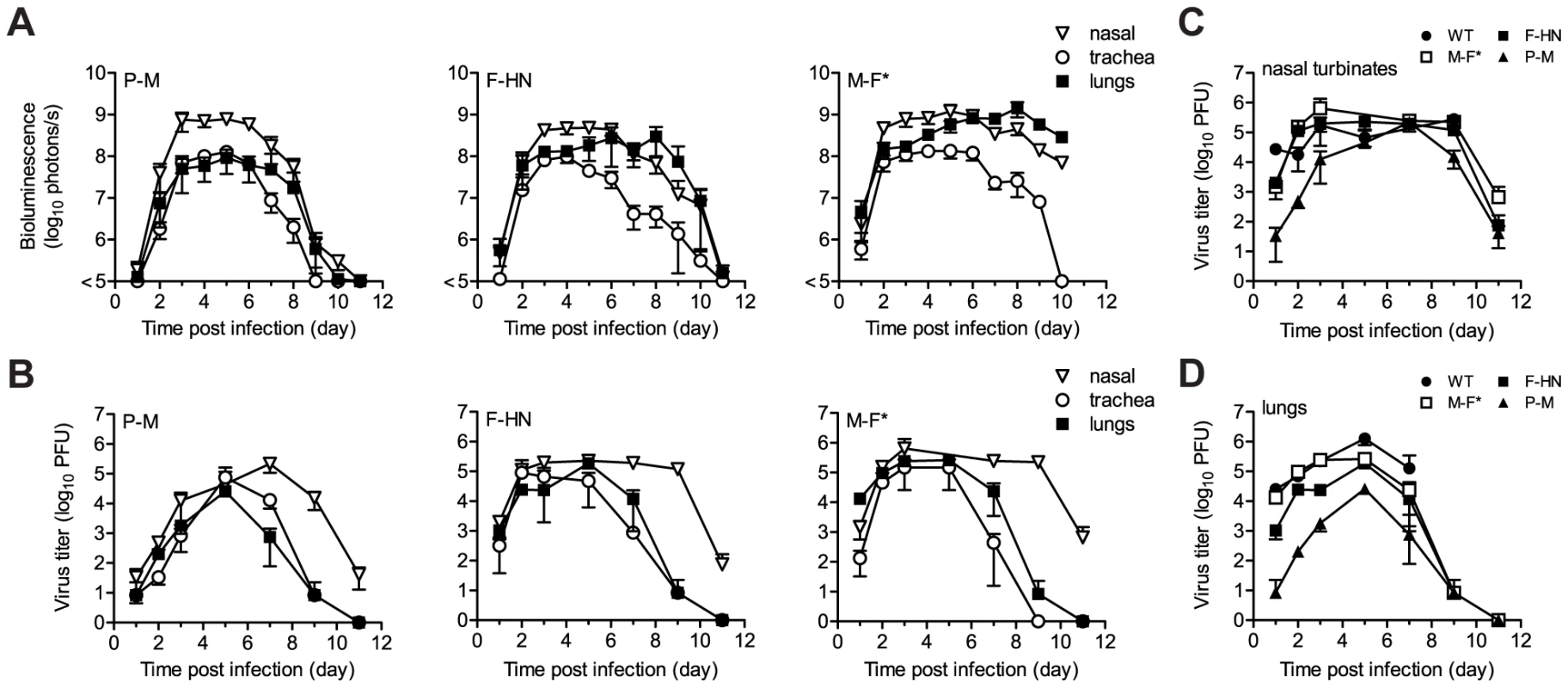
(A) The extent of infection was determined by non-invasive bioluminescence imaging of living, anesthetized mice every 24 h. Each data point represents the mean bioluminescence of 6 mice. The total flux (photons/s) of bioluminescence intensity is calculated as the sum of radiance in the region of interest. (B–D) Viral titers in the nasal turbinates, trachea, and lungs were determined by sacrificing groups of 3 mice at the reported days and performing plaque titrations of tissue homogenates in LLC-MK2 cells. Both experiments were repeated, and representative data are shown. Tissue tropism and viral dose
The high permissivity of the URT and trachea to infection by the attenuated rSeV-luc(P-M) virus was unexpected. We next investigated whether these tissues were also highly permissive to infection by the WT-like virus rSeV-luc(M-F*) at a low inoculating dose. Our preliminary studies showed that the 50% mouse infectious dose (MID50) of rSeV-luc(M-F*) was 9 PFU and that a 70-PFU dose resulted in 100% infection, similar to results obtained for WT SeV in mice [38] and HPIV1 in humans [39]. We inoculated 129/SvJ mice intranasally with 70, 700, or 7,000 PFU of rSeV-luc(M-F*) in equal 30 µl volumes and then measured bioluminescence and viral titers. After inoculation with 70 PFU, viral titers and bioluminescence in the lungs were ∼10% of that induced by a 7,000-PFU dose (Figure 4A,B), and weight loss was far less (Figure 4C). In contrast, infection in the nasopharynx and trachea after a 70-PFU inoculation was delayed ∼1 d compared to 7,000-PFU inoculation, reached a similar level by ∼5 d p.i. (Figure 4A,B), and induced relatively high titers of SeV-specific antibodies (>105) (Figure 4D). Therefore, low-dose inoculation of the WT-like rSeV-luc(M-F*) virus resulted in preferential infection of the URT and trachea, inducing a robust antibody response without causing much weight loss.
Fig. 4. Virus replication and pathogenesis as a function of virus dose and mouse strain. 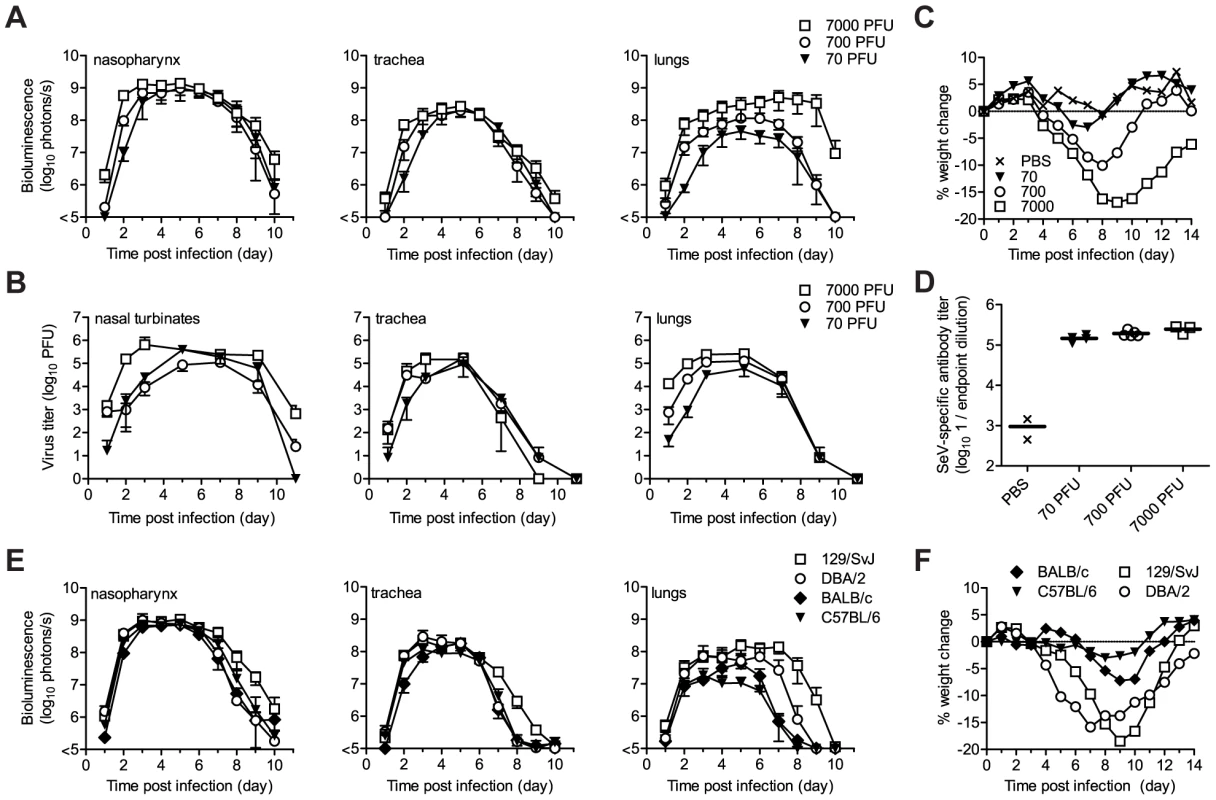
After intranasal inoculation of 129/SvJ mice with 70 to 7,000 PFU of rSeV-luc(M-F*), the total flux of bioluminescence intensity (A) and viral titers (B) were measured as described in Figure 3. (C) Percent body weight change was measured in groups of ten 129/SvJ mice after inoculation with 70 to 7,000 PFU. The experiment was performed in duplicate; representative data are shown. (D) Sendai virus-specific binding antibody titers in sera of 129/SvJ mice collected 10 days after inoculation with 70 to 7,000 PFU of rSeV-luc(M-F*). Titers are reported as the reciprocal endpoint dilutions in ELISA assays. Five infected and two control mice were used in the experiment, which was performed in duplicate. Representative data are shown. (E) The total flux of bioluminescence intensity in the nasopharynx, trachea, and lungs after 7,000-PFU intranasal inoculation of 129/SvJ, DBA/2, BALB/c, or C57BL/6-strain mice with rSeV-luc(M-F*). Values are the mean from six animals. The experiment was performed in duplicate, and the results from a representative experiment are shown. (F) Mean percent weight change in groups of 10 mice after infection with 7,000 PFU of rSeV-luc(M-F*). The experiment was performed in duplicate; representative data are shown. Tissue tropism and host genetics
While it is known that 129/SvJ and DBA/2 mice are highly susceptible to lung infection by SeV and BALB/c and C57BL/6 mice are highly resistant [40]–[43], the effect of host genetics on SeV replication in the URT and trachea has not been reported. Therefore, we measured the in vivo dynamics of SeV infection in 129/SvJ, DBA/2, C57BL/6, and BALB/c strains of mice that had been intranasally inoculated with 7,000 PFU of rSeV-luc(M-F*). As expected from previous studies, the extent of pulmonary infection and weight loss correlated with each other and followed the trend C57BL/6<BALB/c<<DBA/2<129/SvJ (Figure 4). In contrast, similarly high levels of bioluminescence were observed in the URT and trachea in all four strains of mice. The titers of rSeV-luc(M-F*) in BALB/c mice correlated with bioluminescence in intact mice (Figure S5A), as they were in 129/SvJ mice. Therefore, use of the bioluminescence technique to measure respiratory tract infection in living mice was validated in both 129/SvJ and BALB/c strains.
Dynamics of infection during contact transmission
SeV, the HPIVs, and HRSV are thought to be transmitted primarily via contact with respiratory secretions as opposed to long-range transmission of these secretions as small-particle aerosols [21], [22], [24], [44], [45]. It has also been shown that growth of SeV [21] and influenza virus [46] in the URT promotes transmission. Two fundamental questions about PIV transmission that have long remained unanswered are (i) how growth of virus in the lungs of donors influences transmission and (ii) how infection spreads in the respiratory tracts of contact animals after transmission. To address these fundamental questions about SeV transmission, we inoculated BALB/c or 129/SvJ donor mice with 70 or 7,000 PFU of rSeV-luc(M-F*) and then at 1 d p.i. we placed 1 donor mouse in a “clean” cage with 3 naïve contact mice. We measured bioluminescence daily in inoculated and contact mice until primary infection was cleared. We then collected sera on day 60, challenged the mice with 7,000 PFU of rSeV-luc(M-F*) on day 63, and subsequently imaged the mice daily to detect reinfection (Figures 5 and 6). Transmission to every naïve contact mouse was observed by nasopharyngeal bioluminescence and seroconversion, including resistant BALB/c mice exposed to donor animals inoculated at the lower dose of 70 PFU (Figure 5). The timing of transmission was not influenced by the extent of lung infection in donors, as lung titers were ∼10 times lower in BALB/c than in 129/SvJ donor mice after 7,000-PFU inoculation (Figure S5C), yet the timing of transmission (time between detection in inoculated animals and contact animals) was similar (3.3 and 3.4 days, respectively) (Figure 6F). While LRT infection occurred in both strains of mice and may have contributed to transmission, the primary determinant of transmission appeared to be virus shedding in the URT and trachea. For example, both high-titer (>105 PFU) shedding in the nasal cavities and trachea of 129/SvJ donor mice (Figure 4A,B) and contact transmission (Figure 6E,F) occurred ∼1 day earlier after 7,000-PFU inoculation than after 70-PFU inoculation.
Fig. 5. Sendai virus infection and immune response after contact transmission. 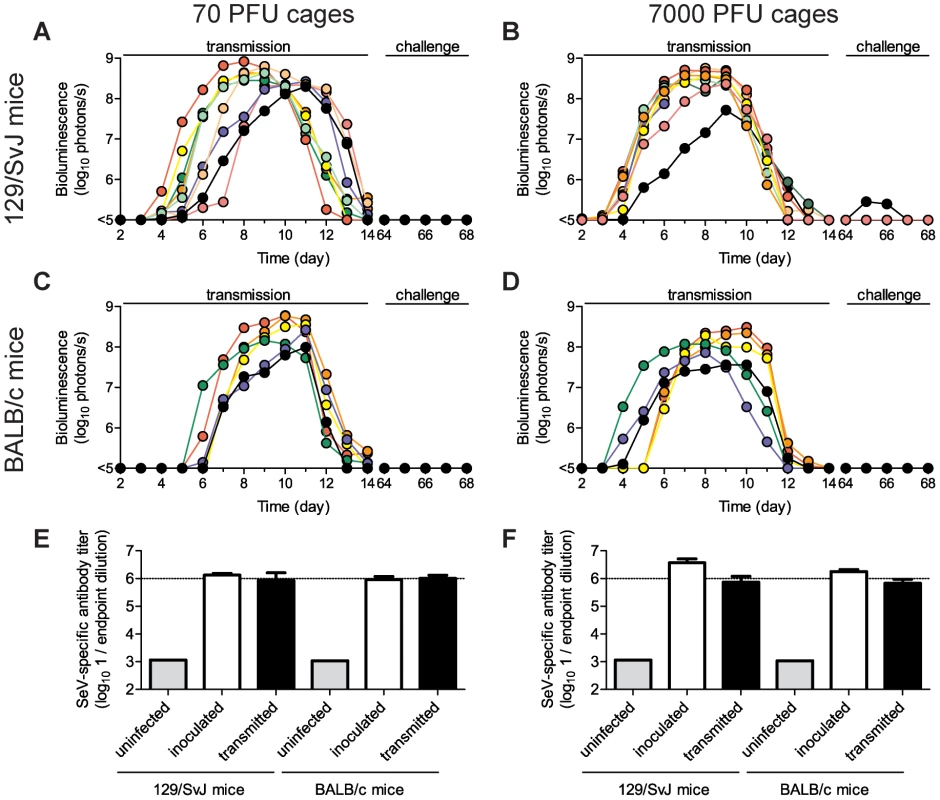
Donor mice were directly inoculated with 70 PFU (A,C,E) or 7,000 PFU (B,D,F) of rSeV-luc(M-F*) and then introduced into a cage with 3 naïve animals one day later. The total flux of bioluminescence intensity in the nasopharyngeal cavities of individual 129/SvJ (A–B) and BALB/c (C–D) mice are shown. Serum was collected on day 60 and contact mice were challenged with 7,000 PFU of rSeV-luc(M-F*) on day 63 to monitor for re-infection by bioluminescence. Sendai virus-specific binding antibody titers were measured as reciprocal endpoint dilutions of sera collected on day 60 from mice co-housed with animals inoculated with 70 PFU (E) or 7,000 PFU (F). Open bars represent mice inoculated on day 0 and solid bars represent the contact mice. The experiment was performed in triplicate for 129/SvJ mice (3 donor and 9 contact mice) and duplicate for BALB/c mice (2 donor and 6 contact mice). Fig. 6. Timing and tissue-tropic spread of Sendai virus infection after contact transmission. 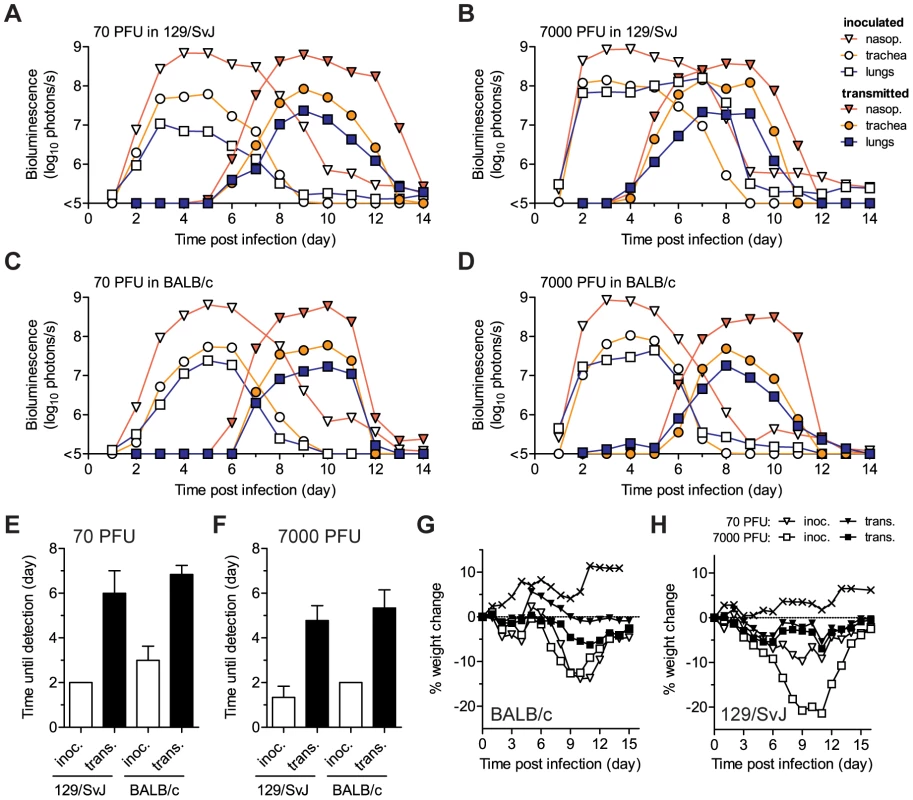
The co-housing of contact mice with mice inoculated with rSeV-luc(M-F*) is described in Figure 5. The total flux of bioluminescence intensity in individual, representative 129/SvJ (A–B) and BALB/c (C–D) mice is shown for the nasopharynx (triangles), trachea (circles), and lungs (squares). Time to detection of bioluminescence (>6 log10 photons/s) in the nasopharynx after inoculation of donors with either 70 PFU (E) or 7,000 PFU (F) of virus. Mean percent weight change in BALB/c (G) and 129/SvJ (H) mice. The contact transmission experiment was performed in triplicate for 129/SvJ mice and in duplicate for BALB/c mice. Open symbols and bars represent directly inoculated mice and solid symbols and bars represent contact mice. In panels G and H, the symbol X indicates PBS-inoculated control mice. Under all four conditions tested (129/SvJ or BALB/c donor mice infected with 70 or 7,000 PFU of virus), the tropism and magnitude of infection in contact animals was similar to that observed after direct intranasal inoculation with 70 PFU of rSeV-luc(M-F*). After contact transmission, bioluminescence was first observed in the nasopharynx and then spread to the trachea and lungs an average of 0.8 and 1.0 days later, respectively (Figure S6A–D). Robust infection was observed in the nasopharynx and trachea after transmission (Figure 6A–D, Figure S6E–H). In contrast, low levels of infection in the lungs were observed after transmission, consistent with low weight loss (Figure 6G–H). In all four groups of mice, SeV-specific antibody titers on day 60 were similarly high (∼106) and all animals were protected from challenge on day 63 (Figure 5). After challenge, a low level of bioluminescence (<106 photons/s), but no weight loss, was detected in only 1 of the 30 contact mice; this animal had shown the lowest level of bioluminescence on days 5–12 after primary infection (Figure 5B, solid black circles). As this animal also had the lowest level of SeV-specific antibodies at day 60 before challenge, a threshold level of infection may be required to induce the highest levels of protective immunity. Overall, SeV infection after transmission was observed to be sufficiently robust in the URT and trachea, yet sufficiently limited in the lungs, to induce protective immunity without causing severe pathogenesis.
Discussion
In this study, we generated and used luciferase-reporter viruses to study the kinetics of SeV infection in living mice after direct inoculation or contact transmission. WT SeV virus and the luciferase-expressing virus rSeV-luc(M-F*) had a similar replication rate in vivo and elicited similar levels of weight loss, mortality, bronchoalveolar lymphocyte influx, and serum antibody titers. Both susceptible (129/SvJ) and resistant (BALB/c) strains of mice were intranasally inoculated with 70 - and 7,000-PFU doses of rSeV-luc(M-F*), and the spread of infection was measured by both in vivo bioluminescence in intact mice and ex vivo virus titers in the tissues of sacrificed animals. The consequences of infection in the URT and trachea were found to be distinct from those of infection in the lungs. Unexpectedly, under all conditions tested, including 70-PFU inoculation of resistant BALB/c mice, the URT and trachea supported robust SeV growth, efficient contact transmission, and protective immunity independently of the extent of infection in the lungs. In contrast, the extent of infection in the lungs varied with the virus dose and mouse strain and was highly correlated with weight loss and mortality. Overall, the results reported here reveal a tissue-specific dichotomy in the respiratory tract in which robust infection in the URT and trachea supports efficient transmission while the extent of infection in the lungs and the host response determines disease severity.
Here we describe for the first time the development of a non-invasive bioluminescence imaging system to visualize negative-strand RNA virus infection throughout the bodies of living animals, using the respiratory paramyxovirus SeV as a model. The development of a non-attenuated paramyxovirus that expresses sufficiently high levels of a reporter gene to allow non-invasive imaging of small animals has been challenging because of the polarized transcription mechanism of these non-segmented negative-strand RNA viruses [2]. A significant advance described here is the generation of the rSeV-luc(M-F*) virus, in which the attenuating effect of reporter-gene insertion [32] is counteracted by enhancement of the naturally occurring but suboptimal start sequence upstream of the F gene [33]. Expression of the F gene, a virulence factor [47], [48], is also downregulated by HPIV1 [49], HPIV3 [50], PIV5 [51], measles virus [52] and canine distemper virus (CDV) [47] by readthrough transcription or long untranslated regions. Therefore, we predict that other WT-like reporter paramyxoviruses that express high levels of luciferase can be engineered by inserting the reporter gene into the M-F junction and maintaining F gene expression through compensating mutations. Reporter gene expression without attenuation of SeV has also been achieved by construction of a bicistronic gene that contains an internal ribosome entry site [53], although it is not yet clear whether this approach yields sufficient luciferase expression to allow non-invasive imaging of in vivo infection.
The use of the luciferase reporter gene in the present work enabled the measurement of infection throughout the entire respiratory tracts of intact animals, allowing us to measure the spread and clearance of infection after direct inoculation or transmission. eGFP-expressing reporter viruses have also been used to study the dynamics of CDV infection in ferrets [54], [55] and measles virus infection in monkeys [56], [57]. The eGFP reporter gene provides the advantage of allowing the tropism of infection to be studied at the cellular level in dissected tissues. Moreover, eGFP-expressing viruses can also be used to quantify and type infected cells in the peripheral blood, skin, and mouths of living animals. eGFP-expressing HPIV3 and Sendai viruses have been used to study the cellular tropism of PIV infection in well differentiated, primary epithelial cultures. In the case of HPIV3, infection was found to be restricted to ciliated epithelial cells and to cause little cytopathology [58]. In contrast, SeV was found to infect ciliated and non-ciliated cells, but not goblet cells, and was observed to induce ciliostasis, cell sloughing, apoptosis, and cellular degeneration [59]. It is unknown whether cell-free virus or cell-associated virus is associated with SeV transmission.
A major finding reported here is that the efficiency and timing of SeV transmission are independent of the extent of pulmonary infection, clinical symptoms, and host genetics. HPIV1 transmission from asymptomatic human donors has also been observed in an experimental setting [39] and is consistent with epidemiological observations for PIV outbreaks in general [23], [24]. These observations suggest that LRT infection and the severity of clinical symptoms are poor predictors of transmission potential in surveillance and infection control efforts. As in previous work [21], [38], we observed that SeV transmission coincides with high-titer virus growth in the URT and is remarkably efficient because of the high infectivity of the virus (e.g., the MID50 of SeV is <10 PFU). HPIV1, HPIV3, and HRSV are similarly highly infectious and also transmit predominantly by direct contact or indirect exposure to nasal secretions [44], [45], [60]–[62]. In the absence of an available prophylactic drug for uninfected individuals in high-risk groups (e.g., premature infants and the immunocompromised), the results described here suggest that efforts to control PIV infection should focus on reducing URT shedding from infected individuals, disinfecting contaminated surfaces, and hand washing. In contrast to infection control, which would be best served by limiting URT infection, therapeutic antivirals would be better targeted to the LRT to control clinical manifestations of PIV-associated disease.
Genetic factors have been identified that modulate viral susceptibility and disease severity in humans [63]–[65] and in the lungs of mice [40], [42], [43], [66]–[70]. Our results show for the first time that genetic factors limiting virus growth in the lungs of resistant BALB/c mice, compared to susceptible 129/SvJ mice, do not limit robust virus growth in the URT and trachea and, consequently, do not limit transmission. Furthermore, BALB/c and 129/SvJ mice showed a similarly high extent of infection in the URT and trachea and a similarly low extent of infection in the lungs after exposure to cagemates inoculated with high or low virus doses. This finding shows that host genetics do not play a major role in SeV transmission, at least in these strains of mice. These observations reinforce our inference that transmission and pathogenesis are independent consequences of URT versus LRT infection, respectively. Additional experiments are needed to delineate the mechanisms responsible for the higher permissivity of the URT and trachea than of the lungs to SeV infection. Possible mechanisms include the site of inoculation in the nasal cavity, lower temperature in the URT, tissue-specific differences in virus replication and innate immunity, and antiviral mechanisms, such as surfactant proteins in the lungs. Reduced replication in the lungs may be associated with lower levels of the secreted tryptase Clara, which is required for cleavage of the F protein to allow viral entry [71], [72].
Asymptomatic infection that promotes immunity and transmission represents a balanced relationship that benefits both the virus and the host. Such has been the case in several enzootic (clinically unapparent) epidemics of SeV in which subclinical infections were maintained in mouse and hamster colonies for years with no increase in pathogenicity, causing apparent disease only occasionally in suckling and old animals [73], [74]. These epidemiological observations are reminiscent of the low virulence yet high transmissibility of the reverse-genetics engineered SeV described here, which was derived from a modified Enders strain that had been passaged in embryonated chicken eggs. Our results show that the level of virus replication in the lungs affects neither the timing nor the efficiency of transmission; thus, SeV replication in the lungs may offer no selective advantage. Instead, we suggest the following mechanism for symbiotic virus-host interplay in enzootic epidemics of SeV: natural infection after transmission is sufficiently limited in the lungs to avoid clinical signs of disease yet is sufficiently robust in the nasopharynx and trachea to promote efficient transmission and induce protective immunity.
Epizootic (clinically apparent) outbreaks of SeV have caused morbidity and high rates of mortality in mouse colonies [75]–[77]. Two closely related, highly pathogenic field isolates of SeV are the Ohita and Hamamatsu strains [78], [79]. While inoculation with only a few PFU of unpassaged Hamamatsu strain SeV results in mortality in mice, the MLD50 of the virus was attenuated by as much as 400-fold after 50 passages in eggs [80]. When the highly pathogenic Ohita and Hamamatsu strains were adapted to LLC-MK2 cells and chicken eggs, they were found to have selected for mutations in the C protein and untranslated leader region, respectively, which increase replication in cultured cells but attenuate replication and pathogenesis in the lungs of mice [81]–[83]. The bioluminescence imaging system described here would be useful in determining whether the mutations that attenuate replication in the lungs also attenuate replication in the URT and trachea, thereby reducing transmission, or whether they actually promote sustained transmission by supporting nasal and tracheal shedding of virus while reducing pathogenesis in the lungs. Such experiments may also reveal whether our observations about the spread and transmission of the egg-adapted SeV extend to unpassaged, highly pathogenic field isolates.
SeV is a promising Jennerian vaccine against HPIV1 [1], [13], and recombinant SeV vaccine vectors containing an envelope gene from HRSV, HPIV3, or HPIV2 inserted into the F-HN gene junction have been shown to elicit both B - and T-cell responses that lead to protection from challenge in small-animal models [18]–[20]. While SeV is pathogenic in mice, an ongoing clinical trial has demonstrated SeV to be well tolerated in humans [17]. In non-human primates, SeV has been shown to protect against HPIV1 challenge with no associated adverse events [15], [84]. This result is likely due in part to the sensitivity of SeV to human IFN-mediated innate immunity [85]. As SeV is developed further as a vaccine vector, the luciferase-expressing SeVs, imaging system, and methods described here will be useful in investigating the effect of vaccine dose, volume, and position of foreign antigen insertion in the SeV genome on tissue-specific vector growth and the immune response in small animal models. Of course, replacing the luciferase reporter gene in SeV with a vaccine antigen could alter in vivo replication of the vector. For example, three different recombinant HPIV3 vectors expressing HPIV1 HN, HPIV2 HN, or measles virus HA inserted into the P-M gene junction were found to replicate to different levels in hamsters [86].
In summary, we have described the development of the non-attenuated reporter virus rSeV-luc(M-F*), which can be used to quantify tissue-specific SeV infection in living mice. Our results reveal how infection by SeV spreads in individual, living animals after direct intranasal inoculation and after transmission. Importantly, infection in the URT and trachea were found to be associated with contact transmission while infection in the lungs was found to be associated with pathogenesis. The imaging tools developed here will provide a method to study the effect of viral factors, host genetics, host age, immune status, environmental conditions, and inoculation mode on the dynamics of infection and transmission. For example, infection can be tracked non-invasively in WT and knockout mice before immune responses are measured ex vivo and then interpreted in light of the preceding infection. Methods similar to those reported here could also be developed to image infection by other paramyxoviruses in small-animal models. Overall, our model system and results suggest tissue-targeted approaches to PIV infection control and vaccine development, while our non-invasive bioluminescence imaging technique is expected to advance the preclinical testing of candidate vaccine vectors and experimental therapies.
Materials and Methods
Ethics statement
All animal studies were approved by the Animal Care and Use Committee of St. Jude Children's Research Hospital and were performed in compliance with relevant institutional policies, the Association for the Accreditation of Laboratory Animal Care guidelines, the National Institutes of Health regulations, and local, state, and federal laws.
Cell culture
Monolayer cultures of LLC-MK2 cells were grown in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum, 1% L-glutamine, 1% penicillin, and 1% streptomycin at 37°C, 5% CO2.
Recombinant Sendai viruses
Unique NotI recognition sites were cloned into the P-M, M-F, and F-HN intergenic junctions of an Enders-based pSeV viral genome plasmid using cloning sites described previously [32]. The firefly luciferase gene was amplified by PCR using the pGL3 Basic vector (Promega) and a pair of AscI tagged primers, subcloned into a shuttle plasmid containing a SeV intergenic junction and flanking NotI restriction sites [32], and then subcloned into the unique NotI site of each of the pSeV viral genome plasmids. Within the pSeV-luc(M-F*) plasmid, the start signal upstream of the F protein was changed from AGGGATAAAG to AGGGTGAAAG by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene Corp). The rSeVs were rescued from the pSeV genome plasmids as described previously [20].
Luciferase expression in vitro
rSeV-infected LLC-MK2 cells (MOI, 5 PFU/cell) were incubated at 33°C, 5% CO2, and lysates were collected at various times p.i.. Luciferase assays were performed using the Luciferase Assay System (Promega) and expression was measured on an automated luminometer (Turner Biosystems, Inc.) as described previously [87].
Viral titers and bioluminescence imaging
Virus titers from multistep growth curves (MOI of 0.01 PFU/cell) and homogenized tissues were determined by plaque titration in LLC-MK2 cells as described previously [48]. Eight week-old female 129/SvJ mice or BALB/c mice (Jackson Laboratories) were anesthetized with isoflurane (Baxter Health Care Corp.) and inoculated intranasally (i.n.) with 30 µl of PBS or PBS containing virus. Animals were monitored daily for weight loss, morbidity, and mortality. Before imaging, mice were injected intraperitoneally with luciferin (Xenogen Corp) at a dose of 150 mg/kg of body weight and anesthetized with isoflurane for 5 min. In vivo images were acquired with a Xenogen IVIS CCD camera system (Caliper Life Sciences) and analyzed with Living Image 3.2 software (Caliper Life Sciences) using an exposure of 60s, 30s, or 5s (binning 4; f/stop 1). Pseudocolor images (representative of bioluminescence) of mice were displayed using a binning of 4 on a colorimetric scale ranging from 1×106 to 1×109 surface radiance (photons/s/cm2/steradian), which is defined as the number of photons that leave a cm2 of tissue and radiate into a solid angle of one steradian. To quantify bioluminescence, regions of interest (ROI) were defined manually and graphed data were expressed as total flux (photons/s), which is defined as the radiance within each pixel summed over the ROI area (cm2)×4π. For experiments shown in Figures 1D, 1E, 2, 3, 4E, 4F, and S4, mice were anesthetized by IP injection of 300 µl 2,2,2-Tribromoethanol (300 mg/kg) and chest hair was removed by shaving and application of a depilatory cream 3 d before inoculation.
Immunology
Sera and BALF were collected from euthanized animals on day 10 or day 60 p.i.. BALF samples (3 ml) were centrifuged to collect cellular material and plated in a tissue culture dish for 1 h at 37°C to remove adherent cells. Suspension cells were harvested, total lymphocytes were counted microscopically, and red blood cells were lysed. For analyses by flow cytometry, cells were stained with FITC-conjugated anti-CD4 (RM4-4) and PE-conjugated anti–CD8b (53-5.8) antibodies (BD Biosciences Pharmingen). Lymphocytes were gated based on forward and side scatter, and the percentages of CD4+ and CD8+ T cell populations were measured within this gate. ELISAs were used to measure the levels of SeV-specific or luciferase-specific antibodies present in the sera. Briefly, 96-well plates were coated overnight with disrupted, purified SeV (10 µg/ml) or firefly luciferase (1 µg/ml, Abcam). Plates were blocked with PBS containing 1% BSA and then incubated with 10-fold serially diluted serum samples. After incubation, plates were washed, incubated with HRP-Goat anti mouse IgG (Southern Biotechnologies) and then washed further. To quantify levels of antibodies, TMB substrate (Kirkegaard and Perry Laboratories) was added to the wells followed by stop solution and absorbance was read at a wavelength of 450 nm. GraphPad Prism non-linear regression software was used to calculate antibody titers.
Contact transmission
Donor animals were inoculated intranasally with 30 µL of rSeV-luc(M-F*) and were individually placed into cages containing 3 naïve contact mice at 24 h p.i. Bioluminescence was monitored daily until it remained consistently at background levels (∼15 days). Sera were collected on day 60 so that SeV-specific antibody levels could be measured as described above. On day 63, mice were challenged with 7000 PFU rSeV-luc(M-F*) administered i.n. and bioluminescence was measured daily.
Supporting Information
Zdroje
1. KarronRACollinsPL 2007 Parainfluenza Viruses. 5 ed Philadelphia Lippincott, Williams and Wilkins 1497 1526
2. LambRAParksGD 2007 Paramyxoviridae: The Viruses and Their Replication. 5 ed Philadelphia Lippincott, Williams and Wilkins 1449 1496
3. WilliamsJVEdwardsKMWeinbergGAGriffinMRHallCB 2010 Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis 201 1890 1898
4. ChanockRMParrottRHJohnsonKMKapikianAZBellJA 1963 Myxoviruses: Parainfluenza. Am Rev Respir Dis 88 S152 S166
5. ParrottRHVargoskoAJKimhwBellJAChanockRM 1962 Acute respiratory diseases of viral etiology. III. parainfluenza. Myxoviruses. Am J Public Health Nations Health 52 907 917
6. ParrottRHVargoskoALuckeyAKimHWCummingC 1959 Clinical features of infection with hemadsorption viruses. N Engl J Med 260 731 738
7. MurphyBRCollinsPL 2002 Live-attenuated virus vaccines for respiratory syncytial and parainfluenza viruses: applications of reverse genetics. J Clin Invest 110 21 27
8. MosconaA 2005 Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest 115 1688 1698
9. Schaap-NuttAScullMASchmidtACMurphyBRPicklesRJ 2010 Growth restriction of an experimental live attenuated human parainfluenza virus type 2 vaccine in human ciliated airway epithelium in vitro parallels attenuation in African green monkeys. Vaccine 28 2788 2798
10. NagaiY 1999 Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev Med Virol 9 83 99
11. FaiscaPDesmechtD 2007 Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res Vet Sci 82 115 125
12. DennyFWMurphyTFClydeWAJrCollierAMHendersonFW 1983 Croup: an 11-year study in a pediatric practice. Pediatrics 71 871 876
13. TakimotoTHurwitzJLZhanXKrishnamurthySProuserC 2005 Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol 18 255 266
14. DaveVPAllanJESlobodKSSmithFSRyanKW 1994 Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology 199 376 383
15. HurwitzJLSoikeKFSangsterMYPortnerASealyRE 1997 Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15 533 540
16. SangsterMHylandLSealyRColecloughC 1995 Distinctive kinetics of the antibody-forming cell response to Sendai virus infection of mice in different anatomical compartments. Virology 207 287 291
17. SlobodKSShenepJLLujan-ZilbermannJAllisonKBrownB 2004 Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 22 3182 3186
18. JonesBZhanXMishinVSlobodKSSurmanS 2009 Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine 27 1848 1857
19. ZhanXHurwitzJLKrishnamurthySTakimotoTBoydK 2007 Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine 25 8782 8793
20. ZhanXSlobodKSKrishnamurthySLuqueLETakimotoT 2008 Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine 26 3480 3488
21. IidaT 1972 Experimental study on the transmission of Sendai virus in specific pathogen-free mice. J Gen Virol 14 69 75
22. van der VeenJPoortYBirchfieldDJ 1970 Experimental transmission of Sendai virus infection in mice. Arch Gesamte Virusforsch 31 237 246
23. HallCB 2001 Respiratory syncytial virus and parainfluenza virus. N Engl J Med 344 1917 1928
24. HenricksonKJ 2003 Parainfluenza viruses. Clin Microbiol Rev 16 242 264
25. SealyRJonesBGSurmanSLHurwitzJL 2010 Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine 28 6749 6756
26. RudrarajuRSurmanSJonesBSealyRWoodlandDL 2011 Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology 410 429 436
27. LukerKELukerGD 2008 Applications of bioluminescence imaging to antiviral research and therapy: multiple luciferase enzymes and quantitation. Antiviral Res 78 179 187
28. HasanMKKatoAShiodaTSakaiYYuD 1997 Creation of an infectious recombinant Sendai virus expressing the firefly luciferase gene from the 3′ proximal first locus. J Gen Virol 78 Pt 11 2813 2820
29. ManicassamyBManicassamySBelicha-VillanuevaAPisanelliGPulendranB 2010 Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A 107 11531 11536
30. BukreyevASkiadopoulosMHMurphyBRCollinsPL 2006 Nonsegmented negative-strand viruses as vaccine vectors. J Virol 80 10293 10306
31. GriesenbachUMengCFarleyRChengSHScheuleRK 2008 In vivo imaging of gene transfer to the respiratory tract. Biomaterials 29 1533 1540
32. TokusumiTIidaAHirataTKatoANagaiY 2002 Recombinant Sendai viruses expressing different levels of a foreign reporter gene. Virus Res 86 33 38
33. KatoAKiyotaniKHasanMKShiodaTSakaiY 1999 Sendai virus gene start signals are not equivalent in reinitiation capacity: moderation at the fusion protein gene. J Virol 73 9237 9246
34. SouthamDSDolovichMO'ByrnePMInmanMD 2002 Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol 282 L833 839
35. MoXYSarawarSRDohertyPC 1995 Induction of cytokines in mice with parainfluenza pneumonia. J Virol 69 1288 1291
36. TashiroMPritzerEKhoshnanMAYamakawaMKurodaK 1988 Characterization of a pantropic variant of Sendai virus derived from a host range mutant. Virology 165 577 583
37. MiyamaeT 2005 Differential invasion by Sendai virus of abdominal parenchymal organs and brain tissues in cortisone - and cyclophosphamide-based immunosuppressed mice. J Vet Med Sci 67 369 377
38. KiyotaniKSakaguchiTFujiiYYoshidaT 1993 F0-containing noninfectious Sendai virus can initiate replication in mouse lungs but requires a relatively long incubation period. J Virol 67 7618 7622
39. ReichelderferTEChanockRMCraigheadJEHuebnerRJTurnerHC 1958 Infection of human volunteers with type 2 hemadsorption virus. Science 128 779 780
40. BrownsteinDG 1987 Resistance/susceptibility to lethal Sendai virus infection genetically linked to a mucociliary transport polymorphism. J Virol 61 1670 1671
41. BrownsteinDGSmithALJohnsonEA 1981 Sendai virus infection in genetically resistant and susceptible mice. Am J Pathol 105 156 163
42. BrownsteinDGWinklerS 1986 Genetic resistance to lethal Sendai virus pneumonia: virus replication and interferon production in C57BL/6J and DBA/2J mice. Lab Anim Sci 36 126 129
43. FaiscaPAnhDBDesmechtDJ 2005 Sendai virus-induced alterations in lung structure/function correlate with viral loads and reveal a wide resistance/susceptibility spectrum among mouse strains. Am J Physiol Lung Cell Mol Physiol 289 L777 787
44. HallCBDouglasRGJr 1981 Modes of transmission of respiratory syncytial virus. J Pediatr 99 100 103
45. McLeanDMBannatyneRMGivanKF 1967 Myxovirus dissemination by air. Can Med Assoc J 96 1449 1453
46. LowenACMubarekaSSteelJPaleseP 2007 Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog 3 1470 1476
47. AndersonDEvon MesslingV 2008 Region between the canine distemper virus M and F genes modulates virulence by controlling fusion protein expression. J Virol 82 10510 10518
48. LuqueLEBridgesOAMasonJNBoydKLPortnerA 2010 Residues in the heptad repeat A region of the fusion protein modulate the virulence of Sendai virus in mice. J Virol 84 810 821
49. BousseTMatrosovichTPortnerAKatoANagaiY 2002 The long noncoding region of the human parainfluenza virus type 1 f gene contributes to the read-through transcription at the m-f gene junction. J Virol 76 8244 8251
50. SpriggsMKCollinsPL 1986 Human parainfluenza virus type 3: messenger RNAs, polypeptide coding assignments, intergenic sequences, and genetic map. J Virol 59 646 654
51. RassaJCParksGD 1998 Molecular basis for naturally occurring elevated readthrough transcription across the M-F junction of the paramyxovirus SV5. Virology 247 274 286
52. CattaneoRRebmannGBaczkoKter MeulenVBilleterMA 1987 Altered ratios of measles virus transcripts in diseased human brains. Virology 160 523 526
53. TouzeletOLoukiliNPeletTFairleyDCurranJ 2009 De novo generation of a non-segmented negative strand RNA virus with a bicistronic gene. Virus Res 140 40 48
54. von MesslingVMilosevicDCattaneoR 2004 Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc Natl Acad Sci U S A 101 14216 14221
55. RuddPACattaneoRvon MesslingV 2006 Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J Virol 80 9361 9370
56. LemonKde VriesRDMesmanAWMcQuaidSvan AmerongenG 2011 Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog 7 e1001263
57. de SwartRLLudlowMde WitteLYanagiYvan AmerongenG 2007 Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog 3 e178
58. ZhangLBukreyevAThompsonCIWatsonBPeeplesME 2005 Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 79 1113 1124
59. VillenaveRTouzeletOThavagnanamSSarlangSParkerJ 2010 Cytopathogenesis of Sendai virus in well-differentiated primary pediatric bronchial epithelial cells. J Virol 84 11718 11728
60. HallCBDouglasRGJrSchnabelKCGeimanJM 1981 Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun 33 779 783
61. ParrottRHKimHWBrandtCDChanockRM 1975 Potential of attenuated respiratory syncytial virus vaccine for infants and children. Dev Biol Stand 28 389 399
62. TyrrellDABynoeMLPetersenKBSuttonRNPereiraMS 1959 Inoculation of human volunteers with parainfluenza viruses types 1 and 3 (HA 2 and HA 1). Br Med J 2 909 911
63. StephensHA 2010 HLA and other gene associations with dengue disease severity. Curr Top Microbiol Immunol 338 99 114
64. ZhangLKatzJMGwinnMDowlingNFKhouryMJ 2009 Systems-based candidate genes for human response to influenza infection. Infect Genet Evol 9 1148 1157
65. ArkwrightPDAbinunM 2008 Recently identified factors predisposing children to infectious diseases. Curr Opin Infect Dis 21 217 222
66. SimonAYMoritohKTorigoeDAsanoASasakiN 2009 Multigenic control of resistance to Sendai virus infection in mice. Infect Genet Evol 9 1253 1259
67. BoonACdeBeauchampJHollmannALukeJKotbM 2009 Host genetic variation affects resistance to infection with a highly pathogenic H5N1 influenza A virus in mice. J Virol 83 10417 10426
68. AnhDBFaiscaPDesmechtDJ 2006 Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am J Physiol Lung Cell Mol Physiol 291 L426 435
69. ItohTIwaiHUedaK 1991 Comparative lung pathology of inbred strain of mice resistant and susceptible to Sendai virus infection. J Vet Med Sci 53 275 279
70. StarkJMMcDowellSAKoenigsknechtVProwsDRLeikaufJE 2002 Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J Med Virol 67 92 100
71. KidoHYokogoshiYSakaiKTashiroMKishinoY 1992 Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J Biol Chem 267 13573 13579
72. TashiroMYokogoshiYTobitaKSetoJTRottR 1992 Tryptase Clara, an activating protease for Sendai virus in rat lungs, is involved in pneumopathogenicity. J Virol 66 7211 7216
73. ZurcherCBurekJDVan NunenMCMeihuizenSP 1977 A naturally occurring epizootic caused by Sendai virus in breeding and aging rodent colonies. I. Infection in the mouse. Lab Anim Sci 27 955 962
74. ProfetaMLLiefFSPlotkinSA 1969 Enzootic sendai infection in laboratory hamsters. Am J Epidemiol 89 316 324
75. BhattPNJonasAM 1974 An epizootic of Sendai infection with mortality in a barrier-maintained mouse colony. Am J Epidemiol 100 222 229
76. IshidaNHommaM 1978 Sendai virus. Adv Virus Res 23 349 383
77. NakagawaMSaitoMKinoshitaKSuzukiEImaizumiK 1980 Pathogenicity of Sendai virus in mice cage-mated with infectors and their offsprings. Nippon Juigaku Zasshi 42 337 344
78. SakaguchiTKiyotaniKSakakiMFujiiYYoshidaT 1994 A field isolate of Sendai virus: its high virulence to mice and genetic divergence form prototype strains. Arch Virol 135 159 164
79. ItohMIsegawaYHottaHHommaM 1997 Isolation of an avirulent mutant of Sendai virus with two amino acid mutations from a highly virulent field strain through adaptation to LLC-MK2 cells. J Gen Virol 78 Pt 12 3207 3215
80. KiyotaniKSakaguchiTFujiiYYoshidaT 2001 Attenuation of a field Sendai virus isolate through egg-passages is associated with an impediment of viral genome replication in mouse respiratory cells. Arch Virol 146 893 908
81. GarcinDItohMKolakofskyD 1997 A point mutation in the Sendai virus accessory C proteins attenuates virulence for mice, but not virus growth in cell culture. Virology 238 424 431
82. FujiiYSakaguchiTKiyotaniKHuangCFukuharaN 2002 Involvement of the leader sequence in Sendai virus pathogenesis revealed by recovery of a pathogenic field isolate from cDNA. J Virol 76 8540 8547
83. SakaguchiTKiyotaniKWatanabeHHuangCFukuharaN 2003 Masking of the contribution of V protein to Sendai virus pathogenesis in an infection model with a highly virulent field isolate. Virology 313 581 587
84. SkiadopoulosMHSurmanSRRiggsJMElkinsWRSt ClaireM 2002 Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology 297 153 160
85. BousseTChambersRLScroggsRAPortnerATakimotoT 2006 Human parainfluenza virus type 1 but not Sendai virus replicates in human respiratory cells despite IFN treatment. Virus Res 121 23 32
86. SkiadopoulosMHSurmanSRRiggsJMOrvellCCollinsPL 2002 Evaluation of the replication and immunogenicity of recombinant human parainfluenza virus type 3 vectors expressing up to three foreign glycoproteins. Virology 297 136 152
87. LuqueLERussellCJ 2007 Spring-loaded heptad repeat residues regulate the expression and activation of paramyxovirus fusion protein. J Virol 81 3130 3141
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



