-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHemoglobin Promotes Nasal Colonization
Staphylococcus aureus nasal colonization is an important risk factor for community and nosocomial infection. Despite the importance of S. aureus to human health, molecular mechanisms and host factors influencing nasal colonization are not well understood. To identify host factors contributing to nasal colonization, we collected human nasal secretions and analyzed their ability to promote S. aureus surface colonization. Some individuals produced secretions possessing the ability to significantly promote S. aureus surface colonization. Nasal secretions pretreated with protease no longer promoted S. aureus surface colonization, suggesting the involvement of protein factors. The major protein components of secretions were identified and subsequent analysis revealed that hemoglobin possessed the ability to promote S. aureus surface colonization. Immunoprecipitation of hemoglobin from nasal secretions resulted in reduced S. aureus surface colonization. Furthermore, exogenously added hemoglobin significantly decreased the inoculum necessary for nasal colonization in a rodent model. Finally, we found that hemoglobin prevented expression of the agr quorum sensing system and that aberrant constitutive expression of the agr effector molecule, RNAIII, resulted in reduced nasal colonization of S. aureus. Collectively our results suggest that the presence of hemoglobin in nasal secretions contributes to S. aureus nasal colonization.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002104
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002104Summary
Staphylococcus aureus nasal colonization is an important risk factor for community and nosocomial infection. Despite the importance of S. aureus to human health, molecular mechanisms and host factors influencing nasal colonization are not well understood. To identify host factors contributing to nasal colonization, we collected human nasal secretions and analyzed their ability to promote S. aureus surface colonization. Some individuals produced secretions possessing the ability to significantly promote S. aureus surface colonization. Nasal secretions pretreated with protease no longer promoted S. aureus surface colonization, suggesting the involvement of protein factors. The major protein components of secretions were identified and subsequent analysis revealed that hemoglobin possessed the ability to promote S. aureus surface colonization. Immunoprecipitation of hemoglobin from nasal secretions resulted in reduced S. aureus surface colonization. Furthermore, exogenously added hemoglobin significantly decreased the inoculum necessary for nasal colonization in a rodent model. Finally, we found that hemoglobin prevented expression of the agr quorum sensing system and that aberrant constitutive expression of the agr effector molecule, RNAIII, resulted in reduced nasal colonization of S. aureus. Collectively our results suggest that the presence of hemoglobin in nasal secretions contributes to S. aureus nasal colonization.
Introduction
Staphylococcus aureus is a human commensal and the causative agent of many serious acute and chronic infections [1]. The primary reservoir for S. aureus is the nasal cavity [2], [3]. Asymptomatic colonization occurs in approximately 20% of the normal population, another 60% are transiently colonized and the remaining 20% appear to be rarely or never colonized [4], [5]. Why some individuals are prone to colonization while others resist colonization is not understood. S. aureus nasal colonization is a known risk factor for several infections including bacteremia [6], [7], postoperative infections [8], and diabetic foot ulcer infections [9]. Treatment with the topical antibiotic mupirocin has proven to be effective at reducing nasal colonization and the risk of postoperative infection [3], [6]. However the appearance of mupirocin resistance threatens this nasal eradication strategy [10]. Therefore an improved understanding of nasal carriage is needed to foster development of new strategies to reduce colonization and subsequent infection.
S. aureus nasal colonization likely involves both host and bacterial determinants. Studies analyzing patterns of nasal carriage suggest that host factors may influence S. aureus nasal colonization [3]. Different carriage rates have been observed among different ethnic groups and families [11], [12]. Furthermore, human nasal secretions show variability in supporting growth or having antimicrobial activity against S. aureus [13]. Nasal host receptors may also vary among individuals as S. aureus adherence to desquamated epithelial cells from carriers is significantly greater than for non-carriers [14]. Bacterial products influencing colonization have also been identified. These include sortase A, teichoic acid, clumping factor B, capsule, iron-regulated surface determinant A, alkyl hydroperoxide reductase, catalase, and the autolysin SceD [15]–[21]. In addition, recent evidence has shown that polymicrobial interactions likely play a role in S. aureus nasal colonization [22], [23]. Taken together these studies suggest that nasal carriage is a multifactorial process that is influenced by host determinants, bacterial products, and polymicrobial interactions.
The goal of this work was to determine if components of human nasal secretions are capable of promoting S. aureus colonization. Human nasal secretions were collected and examined for their ability to promote S. aureus surface colonization. Analysis of nasal secretions that promoted S. aureus surface colonization revealed that hemoglobin was both necessary and sufficient for this activity. Hemoglobin promoted surface colonization in a multitude of S. aureus strains. Furthermore, the addition of hemoglobin to a cotton rat model reduced the inoculum size necessary to establish S. aureus nasal colonization. Finally, hemoglobin was found to inhibit induction of the agr quorum sensing system and a construct expressing the agr effector molecule RNAIII displayed decreased nasal colonization. Our findings suggest that nasal secretions containing hemoglobin have the ability to modulate S. aureus gene expression and increase nasal colonization.
Materials and Methods
Ethics statement
Animal work in this study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on Use and Care of Animals (UCUCA) of the University of Michigan (Permit Number:10394). All efforts were made to minimize pain and discomfort during the procedure. Work involving collection of nasal secretions from human subjects was approved by the University of Michigan Institutional Review Board, approval number IRB00001996. Written informed consent was provided by study participants.
Strains, growth conditions and reagents
The bacterial strains used in this study are described in Table 1. Strains of Escherichia coli were grown in Luria-Bertani broth or Luria agar plates, and growth medium was supplemented with ampicillin (100 µg/ml) or chloramphenicol (10 µg/ml) as needed for maintenance of plasmids. Except where noted, S. aureus strains were grown in tryptic soy broth (TSB) or tryptic soy agar (TSA). For selection of chromosomal markers or maintenance of plasmids, S. aureus antibiotic concentrations were (in µg/ml): erythromycin (Erm) 10; chloramphenicol (Cam) 10. All reagents were purchased from Fisher Scientific (Pittsburg, PA) or Sigma (St. Louis, MO) unless otherwise indicated. The human proteins used were purchased from Sigma at the highest available purity: hemoglobin (H7379), IgK (K3502), carbonic anhydrase (C4396), orosomucoid (G9885), IgG (I4506), albumin (A4327), hemopexin (H9291), transferrin (90190), lactoferrin (L0520), plasminogen (P7999), myoglobin (M0630), fibronectin (F2006), and collagen (C7521). Apohemoglobin was prepared using the cold acid acetone precipitation method [24].
Tab. 1. Strain and plasmid list. 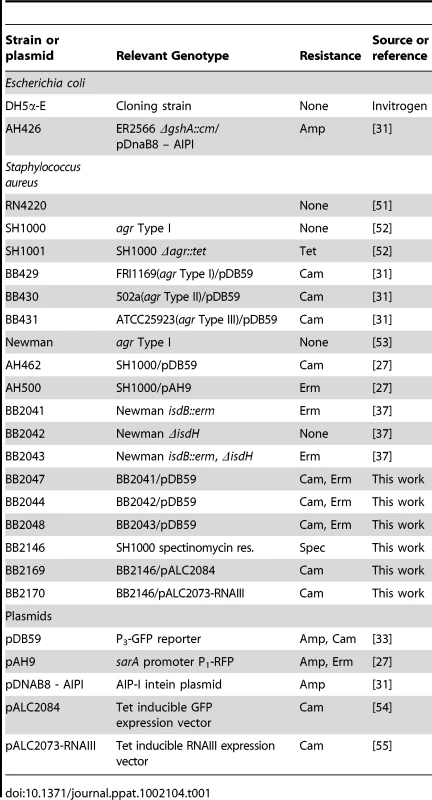
Recombinant DNA and genetic techniques
Restriction and modification enzymes were purchased from New England Biolabs (Beverly, MA). All DNA manipulations were performed in E. coli strain DH5α. Oligonucleotides were synthesized at Integrated DNA Technologies (Coralville, IA). Plasmids were transformed into S. aureus RN4220 by electroporation and then purified and moved to other indicated S. aureus strains by electroporation. Plasmid pALC2073-RNAIII was made by PCR amplification of RNAIII using primers GTTGTTGAATTCTTCATTACAAAAAAGGCCGCGAGCTTGGGA and GTTGTTGGTACCAGATCACAGAGATGTGATGGAAAATAGTTG. The PCR product was digested with KpnI and EcoRI and ligated into the pALC2084 vector that had been digested with the same restriction enzymes.
Nasal colonization model and human nasal secretion collection
The cotton rat nasal colonization model described by Kokai-Kun was utilized in this study [25]. Briefly, S. aureus was grown overnight in TSB, harvested by centrifugation, washed and resuspended in phosphate buffered saline (PBS) or PBS supplemented with protein (hemoglobin, myoglobin, or apohemoglobin (5 mg/ml)) or/and anhydrotetracycline (200 ng/ml) as indicated. Cotton rats were anesthetized, and a 10-µl aliquot containing 1×108 or 1×105 colony forming units (CFUs) was intranasally instilled drop-wise equally between the two nostrils. After 5 days the animals were sacrificed and the noses were surgically removed. The noses were placed in 1 ml of PBS containing 0.5% Tween-20, homogenized, and dilution plated onto TSA supplemented with 7.5% NaCl and spectinomycin (200 µg/ml) to determine CFUs. All animal experiments were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the University of Michigan University Committee on Use and Care of Animals (UCUCA) approval number 10394.
Nasal secretions were collected from a convenience sample of 20 adult patients in a rhiniology clinic within the University of Michigan Department of Otolaryngology and 10 healthy volunteers not visiting a clinic (University of Michigan Institutional Review Board approval number IRB00001996). Written informed consent was provided by study participants. Clinic patients were seen for evaluation of a variety of concerns, including nosebleeds, nasal blockage, rhinitis, and sinusitis. Nasal examination was performed as part of the routine clinical evaluation. For experimental purposes, the anterior nare was swabbed and the swab was streaked onto Mannitol Salt Agar to determine colonization by S. aureus. Secretions were collected by thoroughly swabbing the anterior and posterior nasal passageways with a sterile cotton swab (Remel BactiSwab). The tip of the swab was cut from the shaft, placed in a ridged eppendorf tube and centrifuged to obtain secretions. Volumes of secretions obtained varied from ∼50 µl to ∼200 µl. Secretions were stored at −20°C. Collected secretions were vortexed and sonicated to break up clumps then passed through a 0.22 µm syringe filter. To determine major protein composition of the secretions, 15 µl of samples were separated by 10% sodium dodecyl sulfate (SDS)-PAGE and stained with Sypro Ruby (Biorad). Visible bands were identified by in gel trypsin digestion and subsequent LC-MS/MS analysis (MS Bioworks, Ann Arbor, MI). Statistical analysis was utilized to determine the major protein component of each excised band and the major protein constituents were used for further experiments. The value for the abundance measurement is the Normalized Spectral Abundance Factor (NSAF) [26]. Western blots to detect the presence of hemoglobin in nasal secretions were done using the primary antibody anti-Human Hemoglobin whole antiserum produced in rabbit (Sigma H4890) at a dilution of 1∶100 in 5% milk. The secondary antibody was anti-rabbit IgG produced in goat, conjugated to peroxidase (Sigma A0545), used at a concentration of 1∶10,000 diluted in 5% milk.
Biofilm experiments and adherence assays
Microtiter plate biofilms and flow cell biofilms were grown as previously described [27]. The growth medium for microtiter biofilms was 66% TSB supplemented with 0.2% glucose or with 10% (volume) nasal secretion or indicated concentration of protein. Microtiter plates used for this assay were cell culture treated 96 well (Costar 3596) or 384 well (Nunc 164688) plates. Flow cell biofilms were grown in 2% TSB with 0.2% glucose or 5 µg/ml hemoglobin. Confocal scanning laser microscopy and image analysis was performed as described previously [27]. Adherence assays to collagen and fibronectin were performed as previously described [28], [29]. Briefly, 96-well plates (Nunc 265301) were coated with the following proteins in PBS: Type IV collagen from human placenta (20 µg/ml; Sigma), fibronectin from human plasma (1 µg/ml; Sigma), or Bovine Serum Albumin (10 mg/ml; Fisher). 100 µl of protein was added to each well and incubated overnight at 4° with constant agitation. The wells were then washed three times with 1% BSA in PBS for 20 minutes at 37° with agitation. S. aureus was grown overnight in Brain Heart Infusion with 50 µg/ml of Human Hemoglobin. Following a one hour incubation at 37°C with agitation, wells were washed three times with PBS to remove non-adherent bacteria, then fixed with 2.5% glutaraldehyde in PBS for two hours at 4°C. Bacteria were stained with .1% crystal violet for 30 minutes at room temperature then washed three times with water. The stain was extracted by incubation with .2% Triton X-100 for 30 minutes at room temperature. The absorbance at 570 nm was read on a microtiter plate reader (Tecan Infinite M200).
RNAIII expression assays
To monitor expression from the RNAIII promoter (P3), an overnight culture of the appropriate reporter strain was inoculated into TSB with Cam and grown to an optical density at 600 nm of 0.05. In triplicate, 475 µl of reporter culture was aliquoted into test tubes and indicated amounts of hemoglobin (or other protein) were added. The tubes were shaken at 250 rpm at 37°C for 12 hours. Both cell density (optical density at 595 nm) and green fluorescent protein (GFP) fluorescence (excitation at 485 nm, emission at 535 nm) were measured in a Tecan Infinite M200 (Research Triangle Park, NC) microtiter plate reader by removing 100 µl from each tube and assaying in a microtiter plate (Corning 3606).
Autoinducing peptide activation assays with hemoglobin
S. aureus type 1 autoinducing peptide (AIP) was prepared as previously described using strain AH426 [27]. Briefly, an overnight preculture of expression strain AH426 was prepared and inoculated into 100 ml of Luria-Bertani broth with Amp. The culture was grown at 37°C with shaking until an optical density at 600 nm of 0.5 was reached, and IPTG (isopropyl-ß-d-thiogalactopyranoside) was added to a 0.5 mM final concentration. The culture was grown with shaking at 30°C for 3 h, and the cell pellets were stored at −70°C. Cell pellets were resuspended in 20 ml chitin binding buffer consisting of 100 mM phosphate buffer, pH 7.0, with 500 mM NaCl, 1 mM EDTA, 150 µl protease inhibitor cocktail (Sigma; catalog number P8465), and 0.5 mM phenylmethylsulfonyl fluoride. The cell suspension was lysed through two passes in a French press, and insoluble material was removed by centrifugation at 19,000 rpm for 30 min at 4°C in a Beckman JA-20 rotor. The supernatant was removed, 4 ml equilibrated 50% chitin beads (New England Biolabs) was added, and the resin suspension was mixed gently at room temperature for 30 min. The chitin resin was removed by centrifugation at 500×g for 5 min. The supernatant was removed, and the resin was washed three times for 5 min with 25 volumes of chitin binding buffer. The resin suspension was poured into a 10-ml column and allowed to settle by gravity (2-ml final resin volume), and the resin was equilibrated with three column volumes of elution buffer [100 mM phosphate, pH 7, 50 mM NaCl, 1 mM EDTA, 1 mM tris(2-carboxyethyl)phosphine (TCEP)]. Gravity flow from the column was stopped, and the resin was left sealed at room temperature for 15 h. Following incubation, fractions were eluted and assayed for activity or saved at −20°C. To determine AIP concentration the following was done: A Sep-Pak Plus cartridge (Waters, Milford, MA) was conditioned according to the manufacturer's instructions. To remove TCEP, an AIP sample from an intein purification was bound to the cartridge, washed with 20 ml of water with 0.1% trifluoroacetic acid (TFA), and eluted with 2 ml of 60% acetonitrile with 0.1% TFA. The concentration of the AIP was determined using assays with 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), also called Ellman's reagent (Pierce, Rockford, IL). The thiolactone ring was opened with 1 M (final concentration) NaOH and neutralized with HCl, and DTNB assays were performed before and after base treatment. For the assays, a 1-ml reaction mixture was prepared with 100 mM Tris-HCl, pH 8, and 0.1 mM DTNB (prepared fresh) and different amounts of untreated and base-treated AIP were added. The reaction mixtures were incubated for 10 min at room temperature, and the absorbance was measure at 412 nm. The concentrations were determined with an extinction coefficient of 13,600 M−1 cm−1, and the prebase reading was subtracted to get the final AIP concentration.
AIP activation assays were performed by incubating 100 nM of AIP with a human hemoglobin solution (5 µg/ml in PBS) for 2 hours at 30°C. Hemoglobin was removed from indicated samples and nasal secretions by adding 2 µl anti-hemoglobin antibody produced in rabbit (Sigma) with a 1 hour incubation, followed by addition of 5 µg of goat anti-rabbit IgG Magnetic Beads (New England Biolabs) with a 1 hour incubation at room temperature with periodic rocking. Tubes were then placed in a magnetic tube separation rack for 30 minutes, after which time the supernatant was collected. The AIP activation assay was performed by growing overnight cultures of S. aureus reporter strain (AH462) in TSB and subculturing 1∶50 into TSB plus 0.2% glucose supplemented with AIP; AIP+hemoglobin; or AIP pretreated with hemoglobin that was removed by antibody pulldown as described above. Cultures were grown in test tubes at 37°C with shaking at 250 rpm. Cell density and fluorescence were monitored at 8 hours after inoculation.
Statistical analyses
Statistics were performed using a 1-way analysis of variance (ANOVA). Results are expressed as mean ± standard error of the mean, unless otherwise indicated.
Results
Nasal secretions from some S. aureus carriers have the ability to promote S. aureus surface colonization
To identify host factors that contribute to S. aureus nasal carriage, we collected nasal secretions from patients at the University of Michigan Otolaryngology Clinic and analyzed them for the ability to promote S. aureus surface colonization. Tryptic soy broth was supplemented with filtered nasal secretions (10% of total volume) from 9 subjects and analyzed for the ability to promote S. aureus surface colonization in triplicate wells of a 384 well plate. 384 well plates were utilized for this assay to reduce the amount of volume necessary, as a limited volume of secretion (∼50–200 µL) was collected from each subject. Secretions 1, 2, and 5 significantly increased S. aureus surface colonization (Figure 1). Protease treatment of secretions 1, 2, and 5 with pepsin-coated beads resulted in the loss of ability to promote surface colonization, suggesting a protein component was responsible for induction of surface colonization. Nasal swabs taken at the time of secretion collection revealed that individuals 1, 2, 3, 5, and 7 were colonized with S. aureus (Figure 2A).
Fig. 1. Effect of nasal secretions on S. aureus surface colonization. 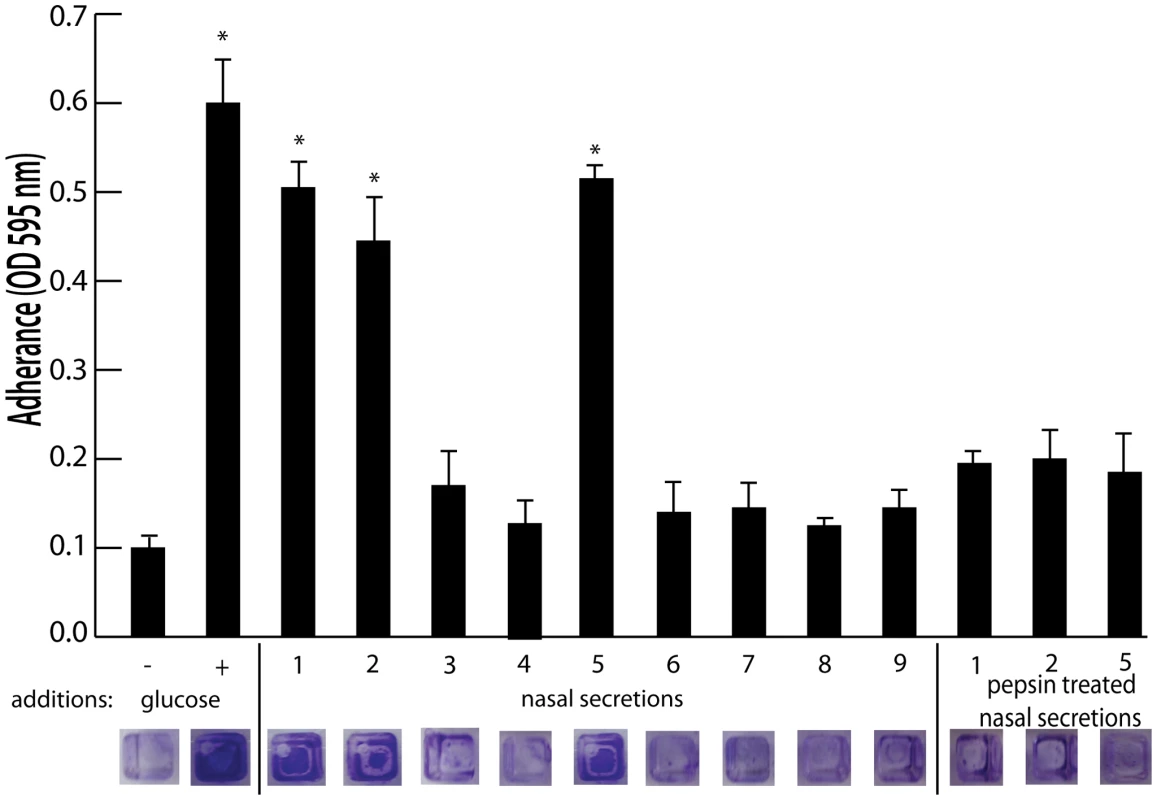
Shown is surface colonization in a 384 well plate with: 66% tryptic soy broth with or with out 0.2% glucose (columns 1 and 2); 66% tryptic soy broth supplemented with 10% filter sterilized nasal secretions from nine human volunteers (columns 3–11); and 66% tryptic soy broth supplemented with pepsin digested nasal secretions (secretions 1,2, and 5; columns 12–14). Graph shows quantitation of surface attached biomass and images below are crystal violet stained biomass attached to wells of a 384 well microtiter plate. Error bars show standard error of the mean; * P<0.005 versus unsupplemented tryptic soy broth (column 1). Fig. 2. Nasal secretion proteins and their ability to promote S. aureus surface colonization. 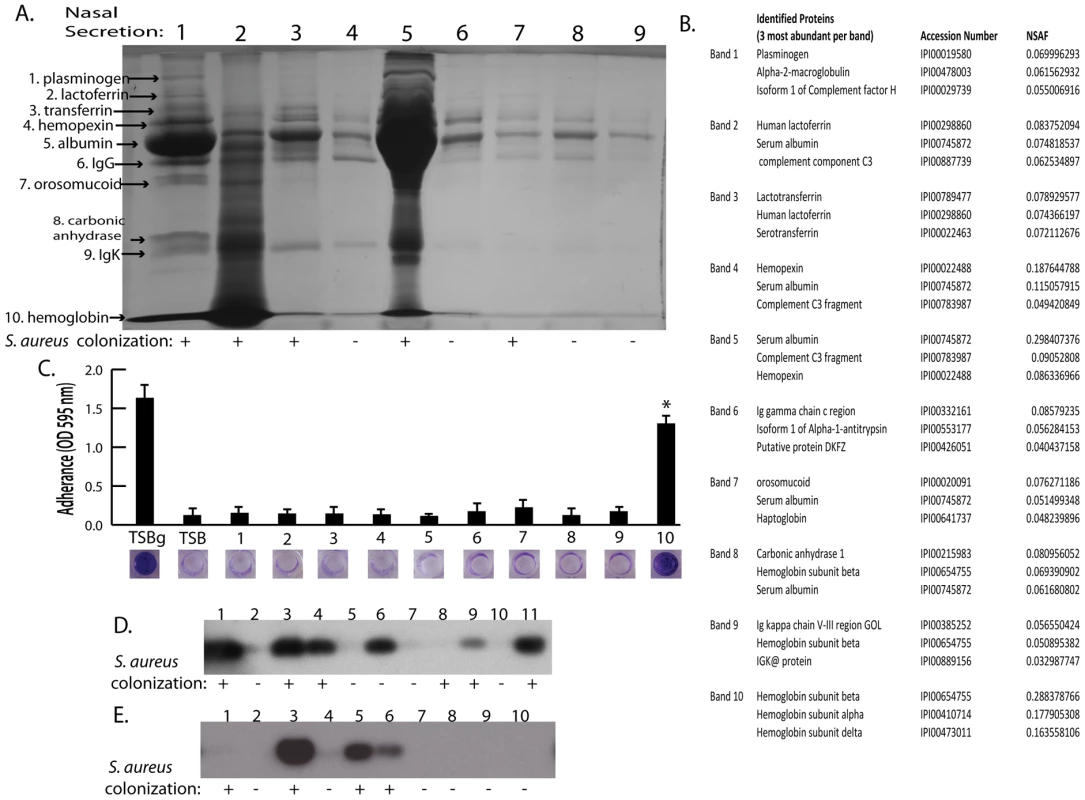
(A) SDS-PAGE analysis on 15 microliters of nasal secretions from the nine human volunteers (lanes 1–9). Protein names and arrows on left indicate protein identity determined by LC-MS/MS. S. aureus colonization status of each individual is indicated below gel. (B) Data set for the MS-based protein identifications from nasal secretions. The three most abundant proteins detected from each excised band are shown. (C) S. aureus surface colonization assay in the presence of individual proteins identified in nasal secretions. Columns 1 and 2 are positive and negative controls grown 66% TSB with (TSBg) or without (TSB) 0.2% glucose. Columns 3–12 were grown in 66% TSB supplemented with 10 µg/ml of each individual protein found in nasal secretions, 1: plasminogen, 2: lactoferrin, 3: transferring, 4: hemopexin, 5: albumin, 6: IgG, 7: orosomucoid, 8: carbonic anhydrase, 9: IgK, 10: hemoglobin. Graph shows quantitation of surface attached biomass and images below are crystal violet stained biomass attached to wells of a 96 well microtiter plate. Error bars show standard error of the mean; * P<0.001 versus unsupplemented tryptic soy broth. (D and E) Western blot analysis on nasal secretions using anti-hemoglobin antibody to detect the presence or absence of hemoglobin. (D) is a western blot on 11 nasal secretions from 11 clinic patient volunteers distinct from those in Figure 2A. (E) is a western blot on 10 nasal secretions from 10 healthy volunteers. S. aureus nasal colonization status is indicated below each blot. Hemoglobin can promote S. aureus surface colonization
The nasal secretions were next examined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and all visible protein bands from secretion 1 were identified by mass spectrometry (Figure 2A). Secretion 1 contained plasminogen, lactoferrin, transferrin, hemopexin, albumin, IgG, orosomucoid, carbonic anhydrase, IgK, and hemoglobin (alpha and beta subunits) (Figure 2B). Next, these proteins were analyzed individually to determine if any were capable of promoting S. aureus surface colonization (Figure 2C). We found that purified hemoglobin promoted S. aureus surface colonization. None of the other proteins tested promoted S. aureus surface colonization.
Hemoglobin concentrations of 10 µg/ml and higher were found to significantly increase S. aureus surface colonization in microtiter plate assays and flow cell assays (Figure 3A and 3B). Other heme containing proteins, such as myoglobin, or heme itself did not possess the ability to promote S. aureus surface colonization (Figure 3A). In addition, apohemoglobin promoted surface colonization in the same concentration range as hemoglobin, suggesting iron is not the cause of this phenomenon.
Fig. 3. The effect of hemoglobin on S. aureus surface colonization. 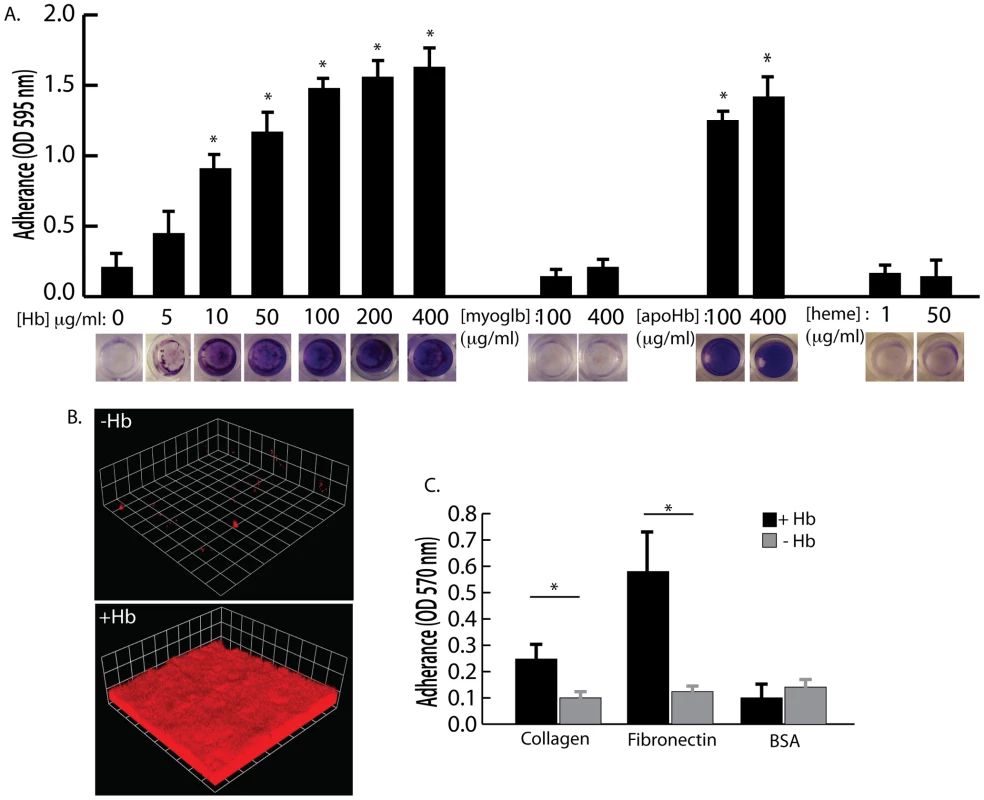
(A) S. aureus surface colonization in 66% TSB supplemented with human hemoglobin, myoglobin, apohemoglobin, or heme (concentrations indicated in graph). Graph shows quantitation of surface attached biomass and images below are crystal violet stained biomass attached to wells of a 96 well microtiter plate. Error bars show standard error of the mean; * P<0.005 versus unsupplemented tryptic soy broth (B) Confocal laser scanning microscopy (CLSM) of S. aureus grown in flow cells with tryptic soy broth without (−Hb) or with 5 µg/ml hemoglobin (+Hb). Shown are three dimensional image reconstructions of a z series created with Velocity software. CLSM images are representative of three separate experiments and each side of a grid square represents 10 micrometers. (C) Adherence assay of S. aureus grown in the presence or absence of 50 µg/ml hemoglobin to collagen and fibronectin. Error bars show standard error of the mean; * P<0.01. Adherence assays were also performed to determine if growth in the presence of hemoglobin could influence initial attachment to the human extracellular matrix proteins collagen and fibronectin (Figure 3C). S. aureus grown in the presence of hemoglobin attached to both collagen and fibronectin at significantly higher levels than S. aureus grown in the absence of hemoglobin. These results are consistent with previous reports suggesting that growth on blood agar plates is ideal for expression of surface binding proteins by S. aureus [29], [30].
Because nasal secretions are complex mixtures of protein, sugars, and salts, hemoglobin was specifically depleted by immunoprecipitation to test the hypothesis that hemoglobin was a necessary factor in inducing bacterial surface colonization. Additional nasal secretions were collected and one that contained hemoglobin and promoted S. aureus surface colonization was processed as described above to remove clumps and debris. Half of this secretion was left untreated while the other half was incubated with anti-hemoglobin antibody that was then immunoprecipitated with a secondary antibody conjugated to magnetic beads. Figure 4A shows SDS-PAGE analysis of the untreated (lane 1) and hemoglobin immunoprecipitated nasal secretion (lane 2). Immunoprecipitation of hemoglobin significantly reduced the ability of this nasal secretion to promote S. aureus surface colonization (Figure 4B). Of note, one protein band that was not hemoglobin nonspecifically immunoprecipitated in this experiment so we cannot rule out the possibility that this unidentified protein could have an impact on the colonization assay.
Fig. 4. Depletion of hemoglobin from a nasal secretion results in reduced S. aureus surface colonization. 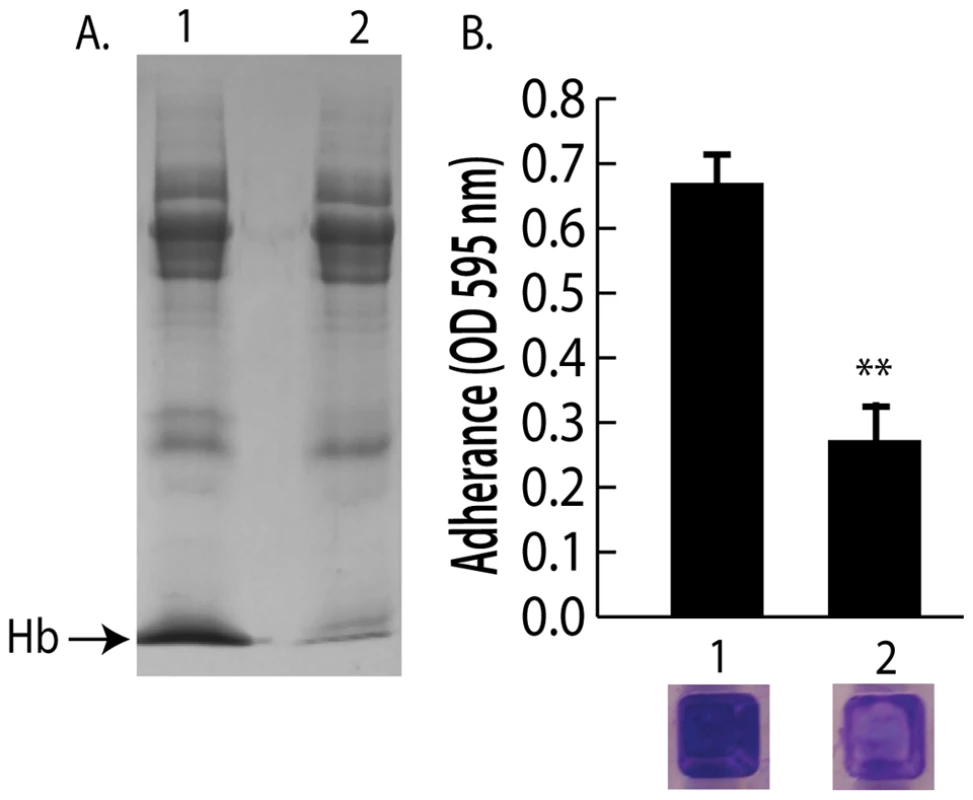
(A) A single hemoglobin containing secretion collected from one volunteer was split in half; one half was untreated and the other half was depleted of hemoglobin by immunoprecipitation (A) lane 1 (untreated) versus lane 2 (hemoglobin IP). (B) Promotion of surface colonization by nasal secretion shown in (A); 1 is untreated and 2 is hemoglobin depleted secretion. Error bars show standard error of the mean; ** P<0.01 versus Hb containing secretion (column 1). We next collected nasal secretions and performed nasal swabs on 11 additional individuals visiting the clinic and 10 healthy individuals to determine if a correlation between the presence of hemoglobin in nasal secretions and S. aureus colonization persist in an additional sampling (Figure 2D and 2E). Six of the secretions from clinic patients (Figure 2D lanes 1,3,4,6,9,11) had detectable hemoglobin by western blotting and five of these secretions came from individuals determined to be nasally colonized with S. aureus. Analysis of healthy volunteers revealed detectable hemoglobin in nasal secretions from three individuals and all three of these volunteers were nasally colonized with S. aureus (Figure 2E lanes 3,5,6).
The presence of hemoglobin promotes S. aureus nasal colonization
To determine if the presence of hemoglobin could influence S. aureus nasal colonization, we utilized the cotton rat model [25]. Nasal instillation with 10 µl of a S. aureus suspension at a density of 1×108 colony forming units resulted in reproducible colonization after 5 days (Figure 5). A reduced inoculum, 10 µl of a S. aureus suspension at a density of 1×105 colony forming units, resulted in no isolation of nasal S. aureus after 5 days. However, if the reduced inoculum (1×105) was suspended in a solution of hemoglobin at 5 mg/ml, nasal colonization was reproducibly observed 5 days after instillation. This high concentration of hemoglobin was utilized in an attempt to keep hemoglobin present in the nasal passageway for as long as possible. Myoglobin supplementation of the reduced inoculum (1×105) did not significantly increase S. aureus nasal colonization. Supplementation with apohemoglobin resulted in robust nasal colonization similar to the hemoglobin condition. These results suggest that the presence of hemoglobin in nasal secretions, but not all heme containing proteins, can increase the likelihood of S. aureus nasal colonization given a small inoculum.
Fig. 5. Nasal colonization of S. aureus in the cotton rat model. 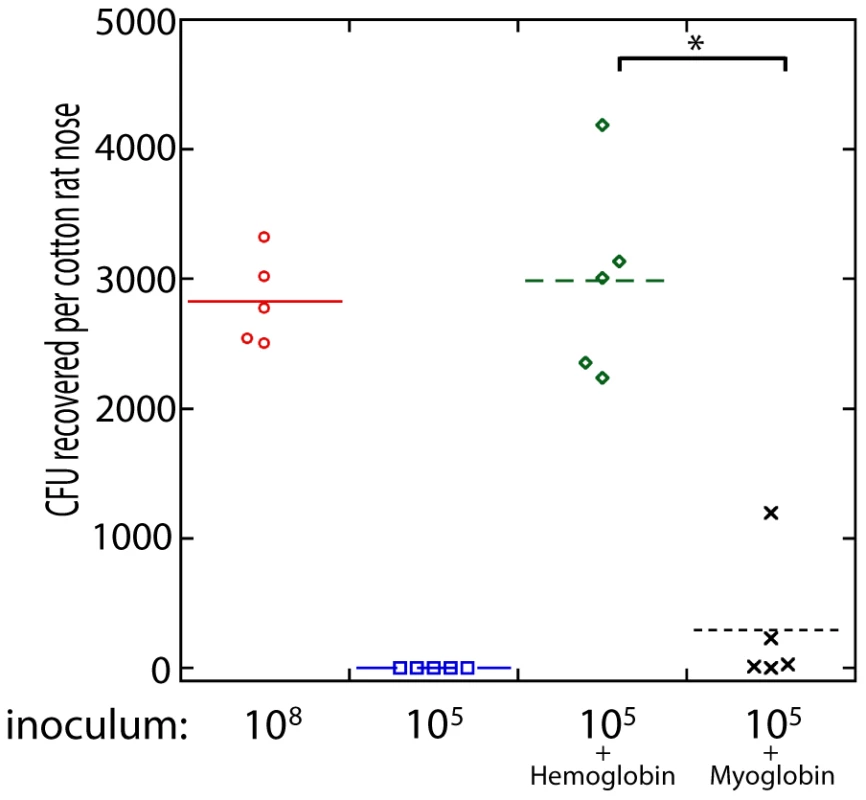
The inoculum (in colony forming units) used to initially colonize the rat nose is shown below the graph. Bacteria were suspended in phosphate buffered saline (PBS) or PBS with hemoglobin (5 mg/ml), myoglobin (5 mg/ml), or apohemoglobin (5 mg/ml) as indicated. Bacterial colony forming units in the cotton rat nose 5 days after instillation are shown in the graph. Medians are given as horizontal lines. * P<0.001, ** P<0.01. Hemoglobin inhibits expression of the agr quorum sensing system
Biofilm formation and lack of biofilm dispersal often correlate with reduced expression of the agr quorum sensing system [27], [31], [32], [33]. Induction of the agr system results in the increased production of several secreted virulence factors including proteases, hemolysins, and toxins [34], [35]. Because a recent report by Schlievert et. al. [36] demonstrated that hemoglobin found in menses inhibits production of secreted exotoxins, we hypothesized that hemoglobin would inhibit agr expression. To determine if hemoglobin was affecting agr expression we followed expression of the quorum sensing responsive promoter fusion, P3-GFP, in the presence of increasing concentrations of hemoglobin (Figure 6A). Hemoglobin significantly inhibited expression from the P3 promoter measured after 12 hours of growth at concentrations from 10–100 µg/ml. Control experiments with myoglobin did not result in reduced expression from the P3 promoter but supplementation with apohemoglobin inhibited P3 expression, suggesting the activity is specific to the hemoglobin peptide rather than any heme containing protein.
Fig. 6. agr activity in the presence of hemoglobin. 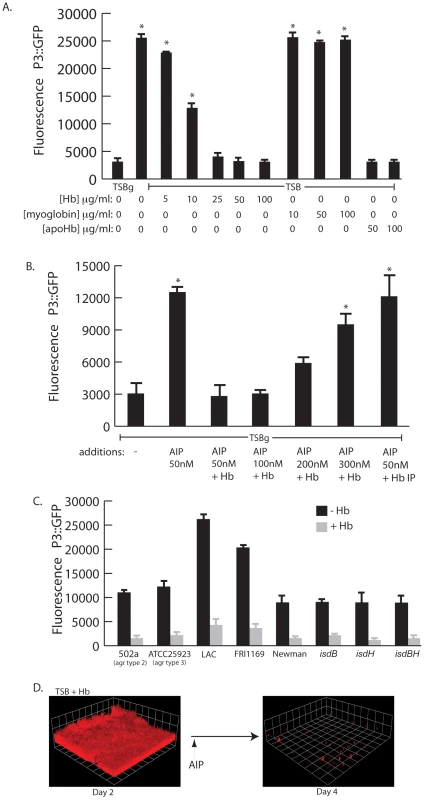
(A) Measurement of agr P3::GFP reporter activity in wildtype S. aureus (strain AH462) grown for 12 hours in TSBg or TSB supplemented with indicated concentrations of hemoglobin, myoglobin, or apohemoglobin. Error bars show standard error of the mean; * P<0.001 versus TSBg condition. (B) agr activation assay in the presence of hemoglobin. Reporter strain (AH462) was grown in 66% TSB with 0.2% glucose. At the beginning of logarithmic phase the cultures were supplemented with: 50 nM AIP, 50–300 nM AIP+5 ug/ml hemoglobin, or 50 nM AIP preincubated with hemoglobin that was subsequently immunoprecipitated. Error bars show standard error of the mean; * P<0.01 versus no addition control. (C) Effect of hemoglobin on agr activation on different agr types (502a – type 2, ATCC25933 –type 3), multiple S. aureus agr type 1 strains (LAC, FRI1164, Newman) and isd mutants. Error bars show standard deviation. (D) AIP induced biofilm detachment in the presence of hemoglobin. Biofilms were grown for 48 hours in TSB supplemented with 2.5 µg/ml of hemoglobin. After 48 hours, 2 ml of 25 µM AIP was added into the biofilm growth media. We reasoned that hemoglobin could antagonize activation of the agr P3 promoter either by sequestering autoinducing peptide (AIP) or directly binding to the cell surface and interfering with the agr activation process. To delineate between these two possibilities we incubated purified AIP with hemoglobin, removed the hemoglobin by immunoprecipitation, and assayed the ability of the remaining solution to activate expression from the P3 promoter (Figure 6B). A mixture of hemoglobin and AIP at a concentration of 50 nM was unable to activate expression of the P3 promoter compared to AIP alone. However, when AIP concentration levels were increased this inhibition was overcome. If hemoglobin was depleted by immunoprecipitation from a mixture containing 50 nM AIP the remaining solution possessed P3 activation activity, suggesting that hemoglobin does not sequester AIP. In addition, S. aureus biofilms grown in the presence of hemoglobin dispersed upon addition of AIP (Figure 6D).
There are four types of agr quorum sensing systems among S. aureus strains. Each agr system (agr-I through agr-IV) recognizes a unique autoinducing peptide structure (AIP-1 through AIP-4). These agr types can be divided into three cross-inhibitory groups: agr-I/IV, agr-II, and agr-III. Hemoglobin was found to inhibit induction of the P3 promoter in each agr class and in multiple strains (Figure 6C). We also examined mutants in receptors known to bind hemoglobin (isdB and isdH) [37] and found no difference in the ability of hemoglobin to inhibit agr quorum sensing this these genetic backgrounds. These results suggest the ability of hemoglobin to inhibit quorum sensing is spread across all agr types and does not occur by binding of the AIP peptide or via binding to known hemoglobin receptors (IsdB or IsdH).
Constitutive expression of the agr quorum sensing effector molecule, RNAIII, inhibits S. aureus nasal colonization
Recent work by Burian et. al. has shown that expression of the agr quorum sensing system is minimal during nasal colonization [38], [39]. Based on this finding and the knowledge that hemoglobin can promote S. aureus nasal colonization and inhibit the expression of the agr quorum sensing system, we hypothesized that expression of RNAIII, the agr quorum sensing system effector molecule, would reduce nasal colonization. To test this hypothesis we utilized a plasmid containing promoterless RNAIII cloned behind a tetracycline inducible promoter, pALC2073-RNAIII. A similar construct has previously been shown to reduce expression of surface associated adhesins and reduce biofilm formation in the presence of tetracycline [40]. In the rat nasal colonization model, presence of pALC2073-RNAIII resulted in reduced nasal colonization, even in the presence of hemoglobin (Figure 7). These results suggest that repression of the agr quorum sensing system is necessary for efficient S. aureus nasal colonization and factors capable of inhibiting agr expression may promote nasal colonization.
Fig. 7. Nasal Colonization of S. aureus containing a RNAIII expression vector (BB2170) or vector control (BB2169) in the cotton rat model. 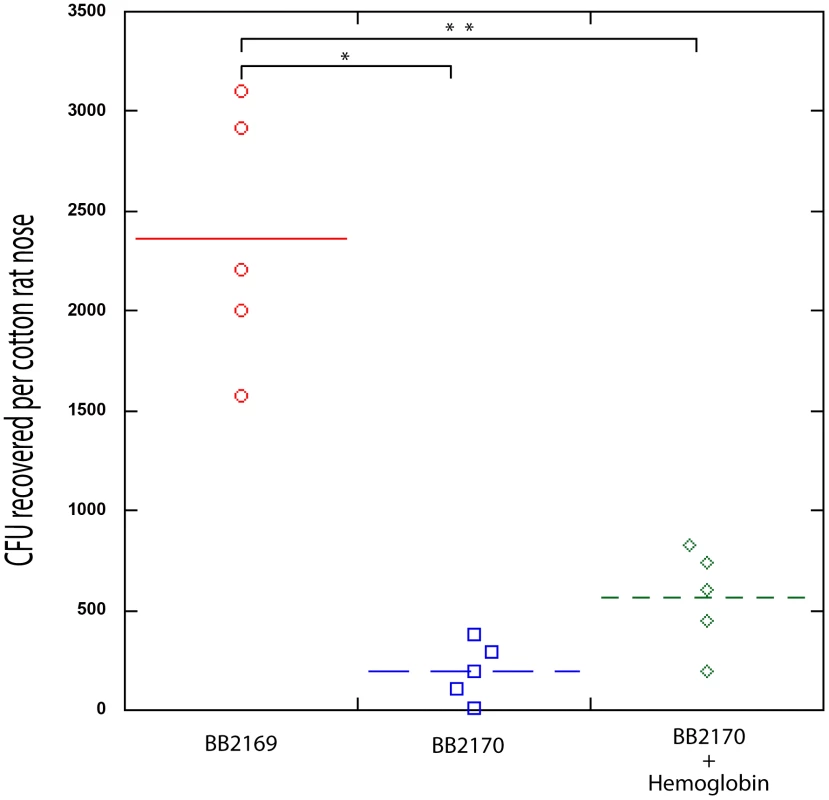
Bacterial colony forming units in the cotton rat nose 5 days after instillation are shown in the graph. Medians are given as horizontal lines. *P<0.0001; ** P<0.001. Discussion
Nasal carriage of S. aureus is a major risk factor for developing a range of infections in both clinical and community settings [6]–[9]. Nasal colonization is multifactorial, likely involving both host and bacterial determinants [11]–[21], [41], [42]. Current strategies for eradication of S. aureus nasal carriage include the use of topical mupirocin; however mupirocin resistance is appearing suggesting that susceptibility may not be long lasting [43], [44]. Therefore, further advances in the control of S. aureus colonization are needed and will depend on an in-depth understanding of both host and bacterial determinants of carriage. In this work, we describe a host factor, hemoglobin, and a bacterial molecular mechanism, expression of the agr quorum sensing system, that influence S. aureus nasal colonization.
The collection and analysis of human nasal secretions from nine volunteers revealed that secretions from three subjects had the ability to significantly promote S. aureus surface colonization (Figure 1). Protease digestion of these secretions eliminated their ability to promote surface colonization, suggesting the involvement of proteinaceous factors. Major protein components of one of the secretions were identified and individual analysis of each protein revealed that hemoglobin possessed the ability to promote S. aureus surface colonization (Figures 2 and 3). Depletion of hemoglobin from a nasal secretion resulted in reduced S. aureus surface colonization (Figure 4). Furthermore, in a rat model the presence of hemoglobin reduced the size of the inoculum necessary to achieve nasal colonization (Figure 5). Finally, we found that hemoglobin prevented expression of the agr quorum sensing system (Figure 6) and the aberrant constitutive expression of RNAIII resulted in reduced nasal colonization (Figure 7). Collectively these results suggest that hemoglobin is a host factor whose presence in nasal secretions contributes to S. aureus nasal colonization.
Several S. aureus factors have been described as determinants for nasal colonization, including: wall teichoic acid, the iron-regulated surface determinant A, catalase, alkyl hydroperoxide reductase, clumping factor B, sortase A, and the autolysin SceD [15]–[21]. Here we show that the aberrant constitutive expression of RNAIII, the agr quorum sensing effector molecule, results in reduced nasal colonization. This finding correlates well with recent data that agr is not expressed during nasal colonization in the cotton rat model or in humans [38], [39]. At this time it is unclear which agr regulated factors influence nasal colonization. Recent work has demonstrated that the Staphylococcus epidermidis secreted protease Esp is capable of preventing and eradicating S. aureus nasal colonization [22]. Considering that the expression of several secreted proteases are up-regulated by the agr regulatory system and the expression of agr-regulated proteases results in biofilm dispersal [27], [35], we speculate that S. aureus protease expression may result in reduced nasal colonization. Further work is needed to elucidate the role of agr-regulated factors in nasal colonization.
The mechanism by which hemoglobin influences S. aureus agr expression and nasal colonization is not clear. Hemoglobin is an iron (heme) containing protein, but other iron or heme containing proteins did not produce the same effect and apohemoglobin elicited the same responses as hemoglobin. Our data suggest that hemoglobin is not able to bind and sequester autoinducing peptides, so hemoglobin likely acts by binding to the cell surface. Mutations of known hemoglobin binding proteins, IsdB and IsdH, exhibited no difference in their response to hemoglobin, suggesting they are not involved in this phenomenon. Agr inhibition also occurs in multiple strains and agr types. Hemoglobin has regions of high positive charge that could promote interaction with negatively charged phospholipids and interfere with activation of membrane histidine kinases such as AgrC. Indeed, work by Scheilvert et al. demonstrated that exotoxin regulation via the SrrA-SrrB two component system was affected by the presence of hemoglobin [36].
Our findings are consistent with a recent study that found individuals experiencing epistaxis (nose bleeds) are more likely to be nasally colonized with S. aureus than control individuals [45]. Some nasal secretions used in our study were collected from patients experiencing epistaxis, nasal blockage, rhinitis, or sinusitis, which presumably increased the likelihood of hemoglobin being present in their secretions. However microepistaxis is thought to be common and can result from digital trauma (i.e., nose picking), nasal or sinus infections, dry ambient air, and from the use of topical nasal medications such as antihistamines and corticosteroids [46], [47]. Sampling of nasal secretions from 10 healthy individuals revealed the presence of hemoglobin in secretions from 3 individuals, all of whom were nasally colonized by S. aureus. Therefore it seems possible that hemoglobin is commonly present in nasal secretions and we propose this could contribute to S. aureus nasal colonization. A large sampling of healthy individuals is needed to determine if there is a significant correlation between S. aureus nasal colonization and the presence of hemoglobin in nasal secretions.
The presence of hemoglobin may also play an important role in S. aureus colonization of other body sites. Recent work has demonstrated that staphylococcal exotoxins were not produced when the organism was cultured in human menses and hemoglobin was identified as the inhibitory factor [36]. It seems plausible that hemoglobin present in vaginal secretions and menses could have profound effects on S. aureus vaginal colonization. Exposure to hemoglobin could also influence S. aureus bloodstream infections by limiting exotoxin production and promoting biofilm infections such as infective endocarditis.
Why would S. aureus have evolved to down regulate the agr quorum sensing system and colonize surfaces in the presence of hemoglobin? Since S. aureus preferentially uses hemoglobin as an iron source [48], it is likely advantageous to form a surface associated community where the scarce resource, iron, is available. Another non-exclusive possibility is that the production of some agr regulated secreted proteases could cleave hemoglobin, releasing antimicrobial peptides. Others have shown hemoglobin peptide fragments found in menses and ticks possess antimicrobial activity against S. aureus [49], [50]. Therefore by not producing agr-regulated proteases when hemoglobin is present, S. aureus may avoid the generation of these antimicrobials. In any case, S. aureus has evolved to respond to one of the most abundant proteins found in humans and this interaction could have profound effects on S. aureus colonization and pathogenesis.
Zdroje
1. LowyFD 1998 Staphylococcus aureus infections. N Engl J Med 339 520 532
2. KluytmansJvan BelkumAVerbrughH 1997 Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10 505 520
3. PeacockSJde SilvaILowyFD 2001 What determines nasal carriage of Staphylococcus aureus? Trends Microbiol 9 605 610
4. KuehnertMJKruszon-MoranDHillHAMcQuillanGMcAllisterSK 2006 Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis 193 172 179
5. WertheimHFMellesDCVosMCvan LeeuwenWvan BelkumA 2005 The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5 751 762
6. von EiffCBeckerKMachkaKStammerHPetersG 2001 Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 344 11 16
7. WertheimHFVosMCOttAvan BelkumAVossA 2004 Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers. Lancet 364 703 705
8. MuñozPHortalJGiannellaMBarrioJMRodríguez-CréixemsM 2008 Nasal carriage of Staphylococcus aureus increases the risk of surgical site infection after major heart surgery. J Hosp Infect 68 25 31
9. StanawaySJohnsonDMoulikPGillG 2007 Methicillin-resistant Staphylococcus aureus (MRSA) isolation from diabetic foot ulcers correlates with nasal MRSA carriage. Diabetes Res Clin Pract 75 47 50
10. CaffreyARQuilliamBJLaPlanteKL 2010 Risk factors associated with mupirocin resistance in methicillin-resistant Staphylococcus aureus. J Hosp Infect 76 206 210
11. NobleWC 1974 Carriage of Staphylococcus aureus and beta haemolytic streptococci in relation to race. Acta Derm Venereol 54 403 405
12. NobleWCValkenburgHAWoltersCH 1967 Carriage of Staphylococcus aureus in random samples of a normal population. J Hyg (Lond) 65 567 573
13. ColeAMDewanPGanzT 1999 Innate antimicrobial activity of nasal secretions. Infect Immun 67 3267 3275
14. AlyRShinefieldHIStraussWGMaibachHI 1977 Bacterial adherence to nasal mucosal cells. Infect Immun 17 546 549
15. WeidenmaierCKokai-KunJFKulauzovicEKohlerTThummG 2008 Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Microbiol 298 505 513
16. WeidenmaierCKokai-KunJFKristianSAChanturiyaTKalbacherH 2004 Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med 10 243 245
17. WertheimHFWalshEChoudhurryRMellesDCBoelensHA 2008 Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5 1 e17
18. KiserKBCantey-KiserJMLeeJC 1999 Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun 67 5001 5006
19. ClarkeSRBrummellKJHorsburghMJMcDowellPWMohamadSA 2006 Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis 193 1098 1108
20. CosgroveKCouttsGJonssonIMTarkowskiAKokai-KunJF 2007 Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol 189 1025 35
21. StapletonMRHorsburghMJHayhurstEJWrightLJonssonIM 2007 Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J Bacteriol 189 7316 7325
22. IwaseTUeharaYShinjiHTajimaASeoH 2010 Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465 346 349
23. ParkBNizetVLiuGY 2008 Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J Bacteriol 190 2275 2278
24. FanelliRAntoniniECaputoA 1958 Studies on the structure of hemoglobin I. Physicochemical properties of human globin. Biochim Biophys Acta 30 608 615
25. Kokai-KunJF 2008 The Cotton Rat as a Model for Staphylococcus aureus nasal colonization in humans: cotton rat S. aureus nasal colonization model. Methods Mol Biol 431 241 254
26. MosleyALFlorensLWenZWashburnMP 2009 A label free quantitative proteomic analysis of the Saccharomyces cerevisiae nucleus. Journal of Proteomics 72 110 120
27. BolesBRHorswillAR 2008 Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4 4 e1000052
28. de BentzmannSTristanAEtienneJBrousseNVandeneschF 2004 Staphylococcus aureus isolates associated with necrotizing pneumonia bind to basement membrane type I and IV collagens and laminin. J Infect Dis 190 8 1506 15
29. PaulssonMLiangODAscencioFWadstromT 1992 Vitronectin binding surface proteins of Staphylococcus aureus. Zbl Bakt 277 54 64
30. LjunghAHjertenSWadstromT 1985 High surface hydrophobicity of autoaggregating Staphylococcus aureus strains isolated from human infections studied with the salt aggregation test. Infection and Immunity 47 522 526
31. MaloneCLBolesBRHorswillAR 2007 Biosynthesis of Staphylococcus aureus autoinducing peptides by using the synechocystis DnaB mini-intein. Appl Environ Microbiol 73 6036 6044
32. VuongCSaenzHLGotzFOttoM 2000 Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis 182 1688 1693
33. YarwoodJMBartelsDJVolperEMGreenbergEP 2004 Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 186 1838 1850
34. NovickRP 2003 Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48 1429 1449
35. DunmanPMMurphyEHaneySPalaciosDTucker-KelloggG 2001 Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol 183 7341 7353
36. SchlievertPMCaseLCNemethKADavisCCSunY 2007 Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry 46 14349 58
37. TorresVJPishchanyGHumayunMSchneewindOSkaarEP 2006 Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol 188 8421 8429
38. BurianMWolzCGoerkeC 2010 Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5 4 e10040
39. BurianMRautenbergMKohlerTFritzMKrismerB 2010 Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis 201 9 1414 21
40. Vergara-IrigarayMValleJMerinoNLatasaCGarcíaB 2009 Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect Immun 77 3978 3991
41. SivaramanKVenkataramanNTsaiJDewellSColeAM 2008 Genome sequencing and analysis reveals possible determinants of Staphylococcus aureus nasal carriage. BMC Genomics 9 433
42. SivaramanKVenkataramanNColeAM 2009 Staphylococcus aureus nasal carriage and its contributing factors. Future Microbiol 4 999 1008
43. MongkolrattanothaiKMankinPRajuVGrayB 2008 Surveillance for mupirocin resistance among methicillin-resistant Staphylococcus aureus clinical isolates. Infect Control Hosp Epidemiol 29 993 994
44. PatelJBGorwitzRJJerniganJA 2009 Mupirocin resistance. Clin Infect Dis 49 935 941
45. WhymarkADCrampseyDPFraserLMoorePWilliamsC 2008 Childhood epistaxis and nasal colonization with Staphylococcus aureus. Otolaryngol Head Neck Surg 138 307 310
46. SchlosserRJ 2009 Epistaxis. N Engl J Med 360 784 789
47. DamroseJFMaddalozzoJ 2006 Pediatric epistaxis. Laryngoscope 116 3 387 93
48. SkaarEPHumayunMBaeTDeBordKLSchneewindO 2004 Iron-source preference of Staphylococcus aureus infections. Science 305 5690 1626 8
49. MakPWójcikKWicherekLSuderPDubinA 2004 Antibacterial hemoglobin peptides in human menstrual blood. Peptides 25 11 1839 47
50. MakPWójcikKSilberringJDubinA 2000 Antimicrobial peptides derived from heme-containing proteins: hemocidins. Antonie Van Leeuwenhoek 77 3 197 207
51. NovickRP 1991 Genetic systems in staphylococci. Methods Enzymol 204 587 636
52. HorsburghMJAishJLWhiteIJShawLLithgowJK 2002 sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184 5457 5467
53. DuthieESLorenzLL 1952 Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol 6 95 107
54. BatemanBTDoneganNPJarryTMPalmaMCheungAL 2001 Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect Immun 69 7851 7
55. BensonMALiloSWassermanGASmithAMcDonaldWH 2010 Staphylococcus aureus compenstates for agr dysfunction by coordinating an immunomodulatory response. Submitted
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



