-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaOne Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
Effective population size (Ne) determines the strength of genetic drift and the frequency of co-infection by multiple genotypes, making it a key factor in viral evolution. Experimental estimates of Ne for different plant viruses have, however, rendered diverging results. The independent action hypothesis (IAH) states that each virion has a probability of infection, and that virions act independent of one another during the infection process. A corollary of IAH is that Ne must be dose dependent. A test of IAH for a plant virus has not been reported yet. Here we perform a test of an IAH infection model using a plant RNA virus, Tobacco etch virus (TEV) variants carrying GFP or mCherry fluorescent markers, in Nicotiana tabacum and Capsicum annuum plants. The number of primary infection foci increased linearly with dose, and was similar to a Poisson distribution. At high doses, primary infection foci containing both genotypes were found at a low frequency (<2%). The probability that a genotype that infected the inoculated leaf would systemically infect that plant was near 1, although in a few rare cases genotypes could be trapped in the inoculated leaf by being physically surrounded by the other genotype. The frequency of mixed-genotype infection could be predicted from the mean number of primary infection foci using the independent-action model. Independent action appears to hold for TEV, and Ne is therefore dose-dependent for this plant RNA virus. The mean number of virions causing systemic infection can be very small, and approaches 1 at low doses. Dose-dependency in TEV suggests that comparison of Ne estimates for different viruses are not very meaningful unless dose effects are taken into consideration.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002122
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002122Summary
Effective population size (Ne) determines the strength of genetic drift and the frequency of co-infection by multiple genotypes, making it a key factor in viral evolution. Experimental estimates of Ne for different plant viruses have, however, rendered diverging results. The independent action hypothesis (IAH) states that each virion has a probability of infection, and that virions act independent of one another during the infection process. A corollary of IAH is that Ne must be dose dependent. A test of IAH for a plant virus has not been reported yet. Here we perform a test of an IAH infection model using a plant RNA virus, Tobacco etch virus (TEV) variants carrying GFP or mCherry fluorescent markers, in Nicotiana tabacum and Capsicum annuum plants. The number of primary infection foci increased linearly with dose, and was similar to a Poisson distribution. At high doses, primary infection foci containing both genotypes were found at a low frequency (<2%). The probability that a genotype that infected the inoculated leaf would systemically infect that plant was near 1, although in a few rare cases genotypes could be trapped in the inoculated leaf by being physically surrounded by the other genotype. The frequency of mixed-genotype infection could be predicted from the mean number of primary infection foci using the independent-action model. Independent action appears to hold for TEV, and Ne is therefore dose-dependent for this plant RNA virus. The mean number of virions causing systemic infection can be very small, and approaches 1 at low doses. Dose-dependency in TEV suggests that comparison of Ne estimates for different viruses are not very meaningful unless dose effects are taken into consideration.
Introduction
Viral evolutionary dynamics on different spatiotemporal scales will be largely determined and constrained by effective population size (Ne). For viruses, we define Ne as the average number of horizontally transmitted virions (i.e., reproducing individuals) in an idealized population [1], [2]. Here we focus exclusively on Ne at the level of an individual host organism, although it could also be considered at the metapopulation level (i.e., a population of infected hosts). Ne will determine how strong genetic drift – one of the key evolutionary forces – acts on viral populations [3]. Genetic drift can lead to decreases in fitness [4], [5] and virulence [6]. Ne will also be an important determinant of mixed-genotype infections, in collusion with the standing genetic variation within a population [7]. The number of mixed-genotype infections will in turn determine the extent to which recombination [8], complementation [9], and competition [10], [11] can occur. Moreover, as it can constrain within-host competition, Ne will also be important for determining the importance of different levels of selection [12], [13]. Estimates of Ne are therefore indispensable for understanding viral evolution.
Estimates of viral Ne would be simplified by understanding basic virus-host and virus-virus interactions during infection. The independent-action hypothesis (IAH) of micro-parasite infection [7], [14], [15], [16], [17] proposes that each virion has a non-zero probability of infection (virus-host interaction), and that the action of micro-parasite infectious entities (i.e., virions) is independent of the presence of other infectious entities (virus-virus interaction). We could therefore assign each virion a probability of infecting the host, which is fixed irrespective of the presence of other co-inoculated virions. The IAH model was first formulated for plant viruses [17], but has not been re-examined with more modern techniques in the past decades (reviewed in [18]). The model was used to better understand the number of local lesions in inoculated leaves [17], but the model is versatile and can be applied to systemic infection [7], [14] or other situations. For example, it has been shown that potyvirus inoculation by aphids is independent; the number of aphids the plant is exposed to does not affect the probability of infection per aphid [19]. Two important limitations of the IAH model are that it is strictly limited to: (i) challenge of the host at a single time point, and (ii) the virus-virus interactions between conspecific infectious entities. Dependency in infection probability between virions can occur. For example, synergistic interactions between virions have been reported for plant viruses [20], [21] and baculoviruses [22], [23], and cross-protection between plant viruses is a well-known antagonistic interaction [24]. However, these phenomena concern interactions between different genotypes, super-infection or both. The IAH model, on the other hand, considers only interactions between synchronously inoculated, conspecific virions.
If virus infection is characterized by independent action, Ne is simply the product of infection probability and the number of virions the host is challenged with. Under the IAH model, it would therefore be possible to predict Ne at multiple doses. Moreover, when IAH holds, viral population size is inextricably linked to the level of host infection and can be estimated directly from the rate of host infection [7]. However, no studies have addressed whether the IAH model pertains to the infection process of RNA viruses or plant viruses. Independent action has only been reported for an animal DNA virus, and was only applicable in two out of six pathosystems tested [7]. The generality of the independent-action model is therefore questionable.
Plant RNA viruses have been important model systems for empirical estimates of Ne. Moury et al. found an Ne of 0.5–3.2 individuals when considering transmission of Potato virus Y (PVY) between plants by aphids [25]. Ali et al. found that aphid transmission significantly reduced the genetic variation in an experimental Cucumber mosaic virus (CMV) population. Moreover, they identified that the reduction occurred not during acquisition of the virus by aphids, but rather during the infection of new plants [26]. Although estimates of the number of individuals were not made, 1–7 genotypes were recovered from infected plants. This suggests a small population size, but not on the order of magnitude of units. Finally, Betancourt et al. performed similar experiments with CMV, and estimated a population size of one to two individuals [27]. Low estimates of Ne appear to be the norm for transmission experiments.
Estimates of Ne have also been made when considering bottlenecks imposed by systemic colonization. Hall et al. found evidence for colonization of tillers by very small populations of Wheat streak mosaic virus (WSMV) [28], estimated at a size of four individuals [29]. Sacristan et al. found a similar result for the systemic colonization of plants by Tobacco mosaic virus (TMV) [30]. For CMV, a reanalysis of the data gathered by Li and Roossinck [31] lead to slightly larger population size estimates of 12–220 individuals [32]. More recently, Monsion et al. estimated that Ne for the pararetrovirus Cauliflower mosaic virus (CaMV), a dsDNA virus, was approx. 300–500 individuals [33]. Whereas studies with different viruses have somewhat similar estimates for Ne during horizontal transmission, there are discrepancies between estimates of Ne for systemic colonization. Although differences in experimental setup will undoubtedly contribute to the variation in estimates of Ne, what other factors contribute?
Here we address two related emerging questions about the infection biology of plant RNA viruses. First, can the infection process of a plant RNA virus be characterized by independent action? Second, could the variation in Ne estimates be due in part to dose-dependence? We hypothesize that the IAH model can characterize the infection process of a mono-partite RNA plant virus. As a corollary, we also predict that Ne will be dose-dependent. If Ne is dose-dependent, we may have uncovered an important potential source of variation between studies performed on different viruses. To test these hypotheses, we constructed two Tobacco etch virus (TEV, genus Potyvirus, family Potyviridae) genotypes, which differed only in the color of the incorporated fluorescent marker. We then challenged highly susceptible tobacco plants (Nicotiana tabacum), and less susceptible pepper plants (Capsicum annuum), with different viral doses. We determined the number of primary infection foci in the inoculated leaf, and which genotypes had systemically infected the plant, and compared these data to predictions of a simple IAH model.
Results/Discussion
The number of primary infection foci in inoculated leaves is dose dependent
N. tabacum and C. annuum plants were inoculated with a three-fold dilution series of purified TEV virions. These virions were a 1∶1 mix of TEV-GFP, a TEV infectious clone [34], tagged with a GFP (green fluorescent protein) marker inserted between P1 and HC-Pro cistrons, and TEV-mCherry, tagged with mCherry marker (a red fluorescent reporter protein [35]) in the same location. We confirmed the stability of these constructs after three serial passages in N. tabacum plants (see Materials and Methods). The fluorescent reporter proteins allowed us to quantify the number of primary infection foci in inoculated leaves. A time-course experiment was performed to determine when the number of foci could be quantified best. The data indicated that fluorescence could be quantified best at 84 hours post inoculation (hpi), for both plant species. At this time, virus-induced fluorescence was high and had not visibly spread from the site of primary infection in both N. tabacum (Fig. 1) and C. annuum (Fig. S1; see Supplementary Materials).
Fig. 1. Fluorescence time course in inoculated N. tabacum leaves. 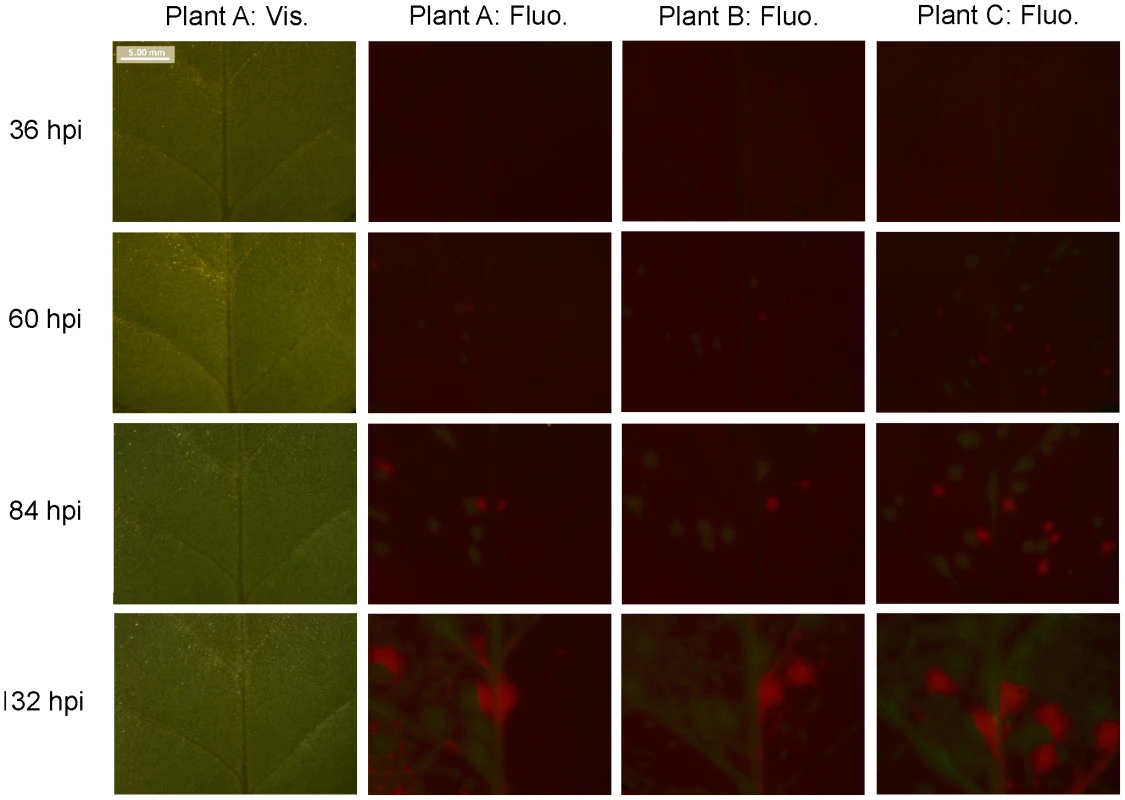
The development of foci of primary infection in the inoculated leaf of N. tabacum was followed over time. Patterns in vascular tissue, observed under visible light through a stereomicroscope (‘Plant A: Vis.’), were used to track fluorescence in the same region. GFP and mCherry signals were merged, and given for three example plants (‘Plant A: Fluo.’ through ‘Plant C: Fluo.’). The best time point for viewing foci of primary infection is 84 hpi: the foci have reasonably high levels of fluorescence, but the fluorescence has not spread. We then recorded the number of GFP and mCherry foci in the inoculated leaf (λ). Fifteen plants were used per replicate and dose, and four replicates were performed with N. tabacum and three with C. annuum. We fitted the expected dose-foci relationship (see Materials and Methods) and found a good fit of the IAH model for N. tabacum and C. annuum (Fig. 2), indicating that the number of foci increased linearly with dose, as would be expected under IAH. However, the slope of the relationship was different among the two hosts (ANCOVA, F1,33 = 70.385, P<0.001), being 36.4-times larger for N. tabacum. This result indicates that for a given increase in dose, the number of foci produced was disproportionately larger in N. tabacum leaves than in C. annuum leaves, thus confirming that tobacco is a much more susceptible host for TEV than pepper. We conclude that the number of foci of primary infection in the inoculated leaf is dose and host-species dependent, with a greater number of foci found at higher doses in the more susceptible host.
Fig. 2. Number of primary infection foci in inoculated leaves at different doses. 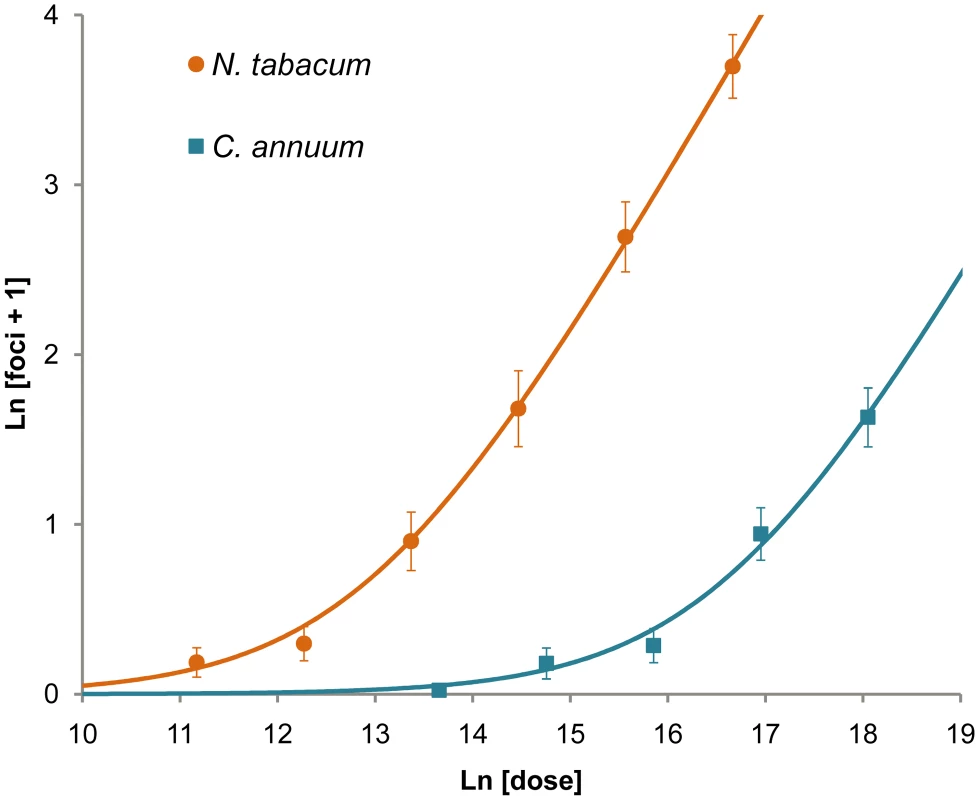
The ordinate is the mean ln-transformed number of foci of primary infection plus one, with error bars indicating 95% confidence intervals, while the abscise is ln-transformed dose (the number of virions per inoculated plant, as estimated by the number of RNA molecules measured by RT-qPCR). The orange circles and blue squares are data points for N. tabacum and C. annuum, respectively. The orange and blue lines are the fitted IAH dose-foci relationship. A good model fit for N. tabacum (R2 = 0.713, F1,21 = 52.091, P<0.001) and C. annuum (R2 = 0.799, F1,12 = 47.636, P<0.001) indicates number of foci increase linearly with dose. Note that the data and model taper off as the mean number of foci becomes low due to the ln transformation. Our results are similar to those obtained when quantifying the number of local lesions for plant viruses [15], [16], [17], [36], [37], [38]. However, care should be taken when comparing primary infection foci and local lesions, as there may not be one-to-one correspondence. Moreover, others have reported that the number of local lesions is not proportional to dose for relatively high doses, the number of foci being less than predicted [15], [35], [38]. We did not use extremely high doses here (>100 foci per leaf), perhaps explaining why this effect was not seen.
Number of foci follows a Poisson distribution
If virions act independently and each focus of primary infection is typically initiated by a single virion, a point addressed below, the mean number of foci of primary infection will increase linearly with dose. Furthermore, if the probability of infection is also constant over host individuals (i.e., no heterogeneity in susceptibility) then, for any given dose, the number of foci (i.e., the number of infecting micro-parasites) is expected to follow a Poisson distribution [7], [15], [16]. We therefore performed a one-sample Kolmogorov-Smirnoff test to determine whether the distribution of foci of primary infection follows a Poisson distribution. As there was a block effect on the number of foci at the highest dose for both N. tabacum (ANOVA, F3,54 = 41.512, P<0.001) and C. annuum (ANOVA, F2,42 = 6.968, P = 0.002), we chose to analyze replicates separately. For both N. tabacum (Table 1) and C. annuum (Table 2), the data appear similar to a Poisson distribution. Only in N. tabacum do discrepancies exist at the highest dose, although we suspect that a similar pattern may emerge in C. annuum with even higher virion concentrations in the inoculum. Others have reported a distribution of the number of local lesions with more variance than a Poisson distribution, and concluded that this was due to differences in probability of infection between primary infection sites [36], [38]. However, for our experimental data, there is no reason to reject a fixed probability of infection per virion.
Tab. 1. Variance in the mean number of foci of primary infection for N. tabacum. 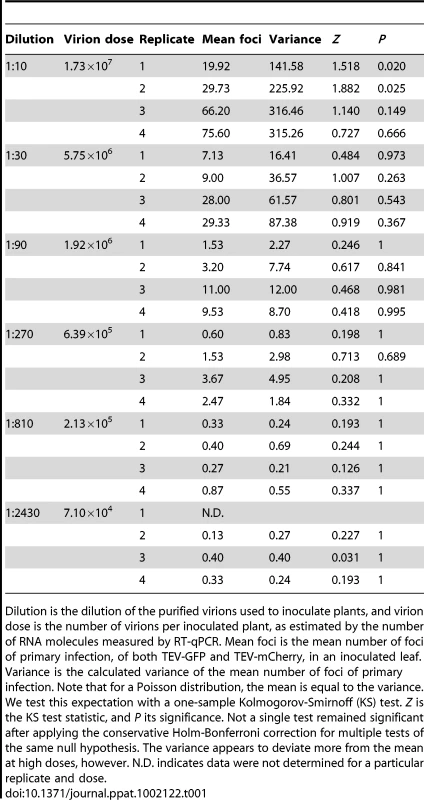
Dilution is the dilution of the purified virions used to inoculate plants, and virion dose is the number of virions per inoculated plant, as estimated by the number of RNA molecules measured by RT-qPCR. Mean foci is the mean number of foci of primary infection, of both TEV-GFP and TEV-mCherry, in an inoculated leaf. Variance is the calculated variance of the mean number of foci of primary infection. Note that for a Poisson distribution, the mean is equal to the variance. We test this expectation with a one-sample Kolmogorov-Smirnoff (KS) test. Z is the KS test statistic, and P its significance. Not a single test remained significant after applying the conservative Holm-Bonferroni correction for multiple tests of the same null hypothesis. The variance appears to deviate more from the mean at high doses, however. N.D. indicates data were not determined for a particular replicate and dose. Tab. 2. Variance in the mean number of foci of primary infection for C. annuum. 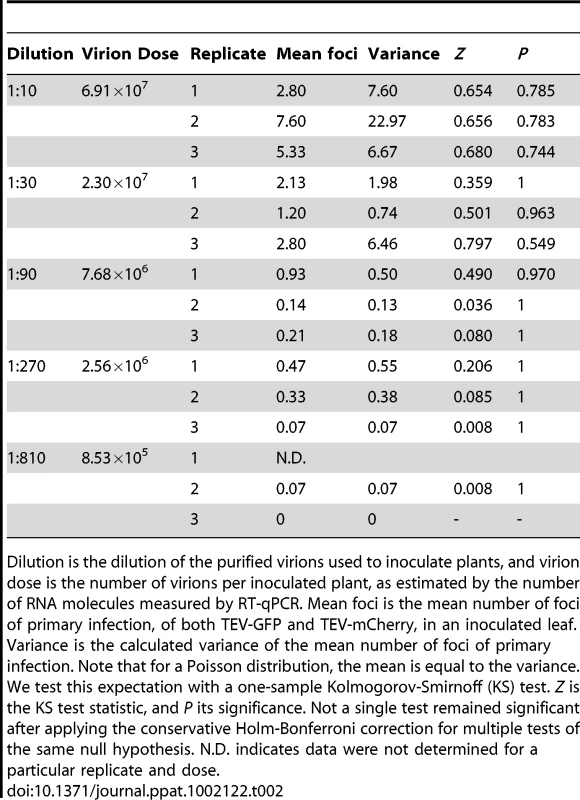
Dilution is the dilution of the purified virions used to inoculate plants, and virion dose is the number of virions per inoculated plant, as estimated by the number of RNA molecules measured by RT-qPCR. Mean foci is the mean number of foci of primary infection, of both TEV-GFP and TEV-mCherry, in an inoculated leaf. Variance is the calculated variance of the mean number of foci of primary infection. Note that for a Poisson distribution, the mean is equal to the variance. We test this expectation with a one-sample Kolmogorov-Smirnoff (KS) test. Z is the KS test statistic, and P its significance. Not a single test remained significant after applying the conservative Holm-Bonferroni correction for multiple tests of the same null hypothesis. N.D. indicates data were not determined for a particular replicate and dose. If the number of foci in inoculated leaves follows a Poisson distribution, then we should also be able to predict the zero term from the mean [7], [17]. As we expect single focus of primary infection to lead to systemic infection, we predicted the rate of systemic infection (I), for each experimental replicate, from λ:(1)Systemic infection status of a plant was determined by checking the plants for green or red fluorescence in non-inoculated leaves at 180 hpi. The data are in good agreement with the model predictions for both N. tabacum and C. annuum (Fig. 3; see also Tables S1 and S2 in Text S1), providing further evidence that the number of founders does indeed follow a Poisson distribution.
Fig. 3. The rate of infection and mixed-genotype infection predicted from the number of primary infection foci in the inoculated leaf. 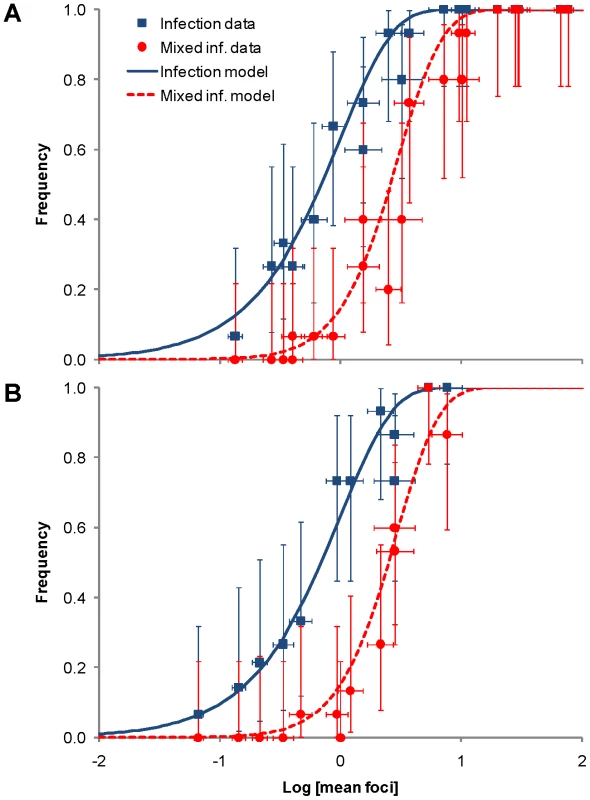
Dose-response curves are compatible with IAH predictions
We considered the relationship between the number of foci at the inoculated leaf and response (i.e., systemic infection). Dose response has, however, often been used as a test for IAH [14], [17], [39], [40], [41], [42] because the IAH model with a fixed probability of infection predicts a dose response with a fixed shape [42]. We therefore fit a general dose-response model [42] to the data from each experimental replicate (see Materials and Methods). Table 3 shows the estimated parameters as well as the results of the one-sample t-test evaluating whether the estimates of the κ parameter significantly deviate from the IAH expectation of κ = 1. In the case of N. tabacum, three estimates were smaller than one, although the difference was significant only in one case. Treating each estimate of κ as a replicate, we can test whether an overall trend exist to deviate from the IAH expectation. The average estimate of κ (± SEM) for N. tabacum was 0.931±0.144, a value that is not significantly different from one (t3 = 0.479, P = 0.663). In the case of C. annuum, κ was significantly larger for the third replicate (Table 3). The average estimate of κ for C. annuum experiments was 1.215±0.156, a value that does not deviate from the IAH expectation (t2 = 1.378, P = 0.302). Therefore, although heterogeneity among replicates may exist, on average, dose response is also in agreement with IAH predictions. This result is not surprising given that there is a linear relationship between dose and number of foci, and that the foci response was also similar to IAH predictions. Indeed, results are qualitatively the same if instead the number of foci is used as doses (data not shown).
Tab. 3. Parameters estimated for the fitting of the dose response general infection model to each dataset. 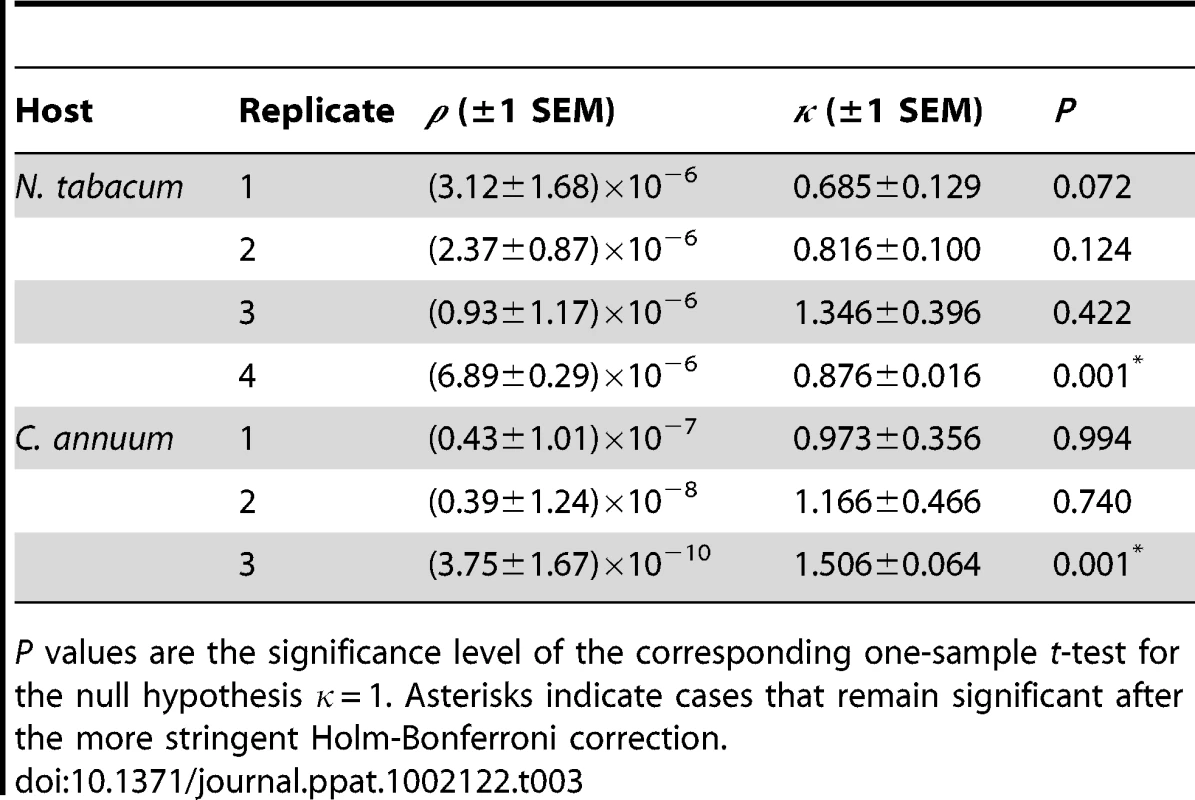
P values are the significance level of the corresponding one-sample t-test for the null hypothesis κ = 1. Asterisks indicate cases that remain significant after the more stringent Holm-Bonferroni correction. Foci with mixed TEV-GFP and TEV-mCherry infections occur at low frequency
Mixed TEV-GFP and TEV-mCherry foci of primary infection were not observed in the inoculated leaves when counting the number of foci. However, we wanted to ascertain whether multiple virions could in principle be responsible for inducing a single primary infection focus, because virions of plant viruses are known to aggregate [43]. We therefore amplified TEV-GFP and TEV-mCherry to obtain virus stocks with higher virion concentrations, and inoculated N. tabacum plants with a 1∶30 dilution of a 1∶1 mixture of these viruses (2.30×107 RNA molecules, as measured by RT-qPCR). Yellow primary infection foci – indicating mixed-genotype infection – were observed at a low frequency (Fig. 4). The frequency of mixed-genotype foci was estimated at 1.8% for this dose. The number of mixed-genotype foci may be greater at higher doses (1∶10 dilution of virions), but the number of foci could no longer be quantified (Fig. 4D). The same experiment was also performed on virions obtained from mixed-genotype infected plants, or virions extracted from a 1∶1 mixture of single-genotype infected tissues, with similar results and no differences in the frequency of mixed foci between the different treatments (data not shown). Our results have some similarities to those recently reported for Soil-borne wheat mosaic virus (SBWMV) [44], although this study explored co-infection at the cellular level. This work describes high levels of cellular co-infection upon saturation of the leaf with RNA, followed by lower levels of co-infection after each round of viral spread and new cellular infection. Similarly, we found much lower levels of co-infection in primary foci of infection, and we did not observe any co-infections in subsequently infected tissues, be it in the inoculated or systemically infected leaves.
Fig. 4. Mixed-genotype foci of primary infection in the inoculated leaf. 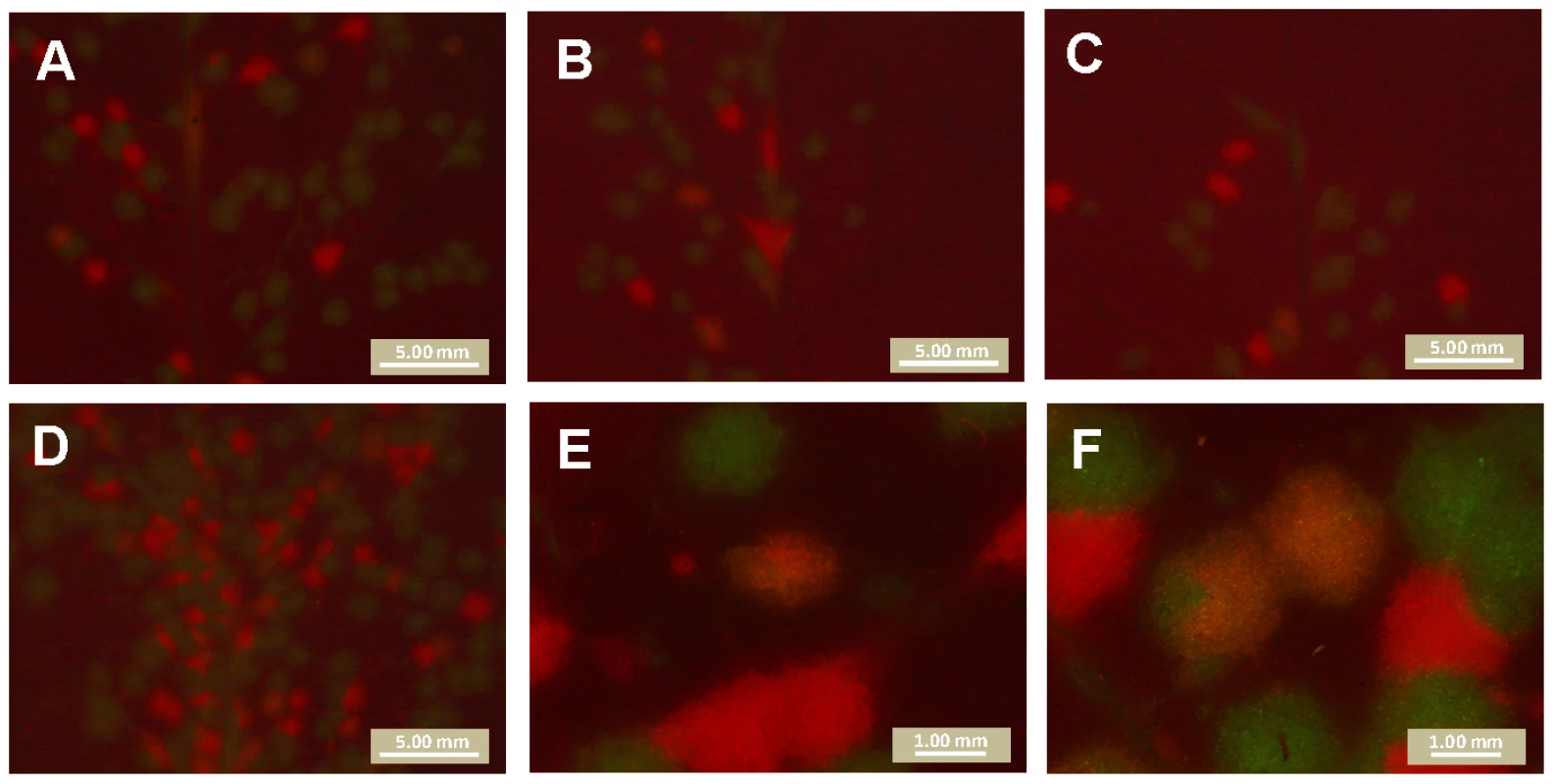
Plants were infected with 1∶30 dilution of a mixture of amplified TEV-GFP and TEV-mCherry virions, kept on ice for 0 h (panel A), 2 h (panel B) or 3 h (panel C). Yellow, mixed-genotype foci were observed at low frequency at all three time points (1.8% averaged for all three times), and the frequency of mixed-genotype foci did not change significantly over time (test for a trend in proportions: χ2 = 0.041, 1 d.f., P = 0.839). Plants were also infected with a 1∶10 dilution of the same mixture (Panel D): the number of mixed-genotype foci may be higher, but these leaves could not be quantitatively scored. High magnification pictures of yellow foci are also provided (Panels E and F). Note that the distribution of red and green fluorescence is not homogeneous within yellow foci. Our data show that multiple virions can in principle initiate a single locus of primary infection. On the other hand, the frequency at which these mixed-infections occur appears to be very low at the doses tested here. Most foci of primary infection are therefore probably initiated by a single virion, and we therefore conclude that the number of primary infection foci is, at all but extremely high doses (>100 foci per leaf), a very good approximation of the number of virions infecting the inoculated leaf. The number of virions infecting the inoculated leaf is, consequently, also dose dependent.
A mechanism of dependence between virions in systemic infection
We determined which virus genotypes had systemically infected the plant by checking for red and green fluorescence in all leaves other than the inoculated leaf at 180 hpi. When red or green fluorescent foci of primary infection were observed in the inoculated leaf, the respective virus was almost always found systemically. The probability of establishing systemic infection after primary infection is therefore 1, or nearly 1, as demonstrated by Fig. 3. Including a pilot experiment, a total of 523 N. tabacum plants were inoculated, and 275 became infected. Of these 275 infected plants, there were only 3 plants in which a genotype was present in the primary infection foci, but not in the systemically infected leaves. In all three cases, the single locus of primary infection in the inoculated leaf was completely surrounded by the other genotype, probably cutting off access to the vascular tissue and preventing further spread of the virus in the inoculated leaf and systemically (Fig. 5). 265 C. annuum plants were inoculated in total, and 114 became infected; of these, there was only 1 plant in which a genotype was present in the primary infection foci, but not in the systemically infected leaves. However, in this one case there were three GFP foci of primary infection in the inoculated leaf, which were completely surrounded by the other genotype (Fig. 6). The host does not have any significant effect on the frequency at which foci are surrounded and precluded to move systemically among host (Fisher's exact test P = 0.664).
Fig. 5. A mechanism of dependence between virions during infection of N. tabacum. 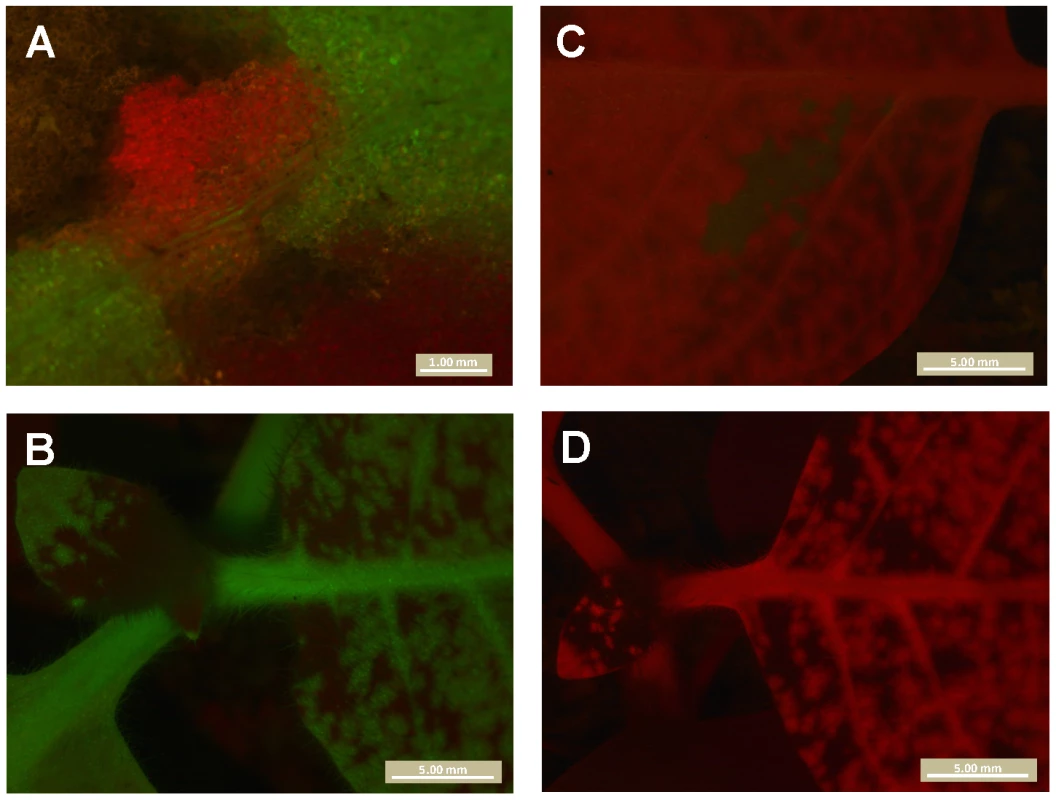
All panels are merges of red and green fluorescence observed in N. tabacum plants inoculated with TEV-GFP and TEV-mCherry. Panel A is the only TEV-mCherry primary infection focus observed in the inoculated leaf, which at 132 hpi is surrounded by TEV-GFP and tissue damaged by the inoculation. Note that vascular tissue is present directly below the focus, but that only the TEV-GFP virus is visible there. In B, a view of systemically infected leaves is shown. No red fluorescence was seen in systemically infected leaves, indicating TEV-mCherry was trapped in the primary infection focus. In Panel C is the only green fluorescence observed in another plant. Although the TEV-GFP virus appears to have spread locally in the inoculated leaf, it is entirely surrounded by TEV-mCherry, and no green fluorescence was visible in systemically infected leaves (Panel D). Although these observations represent a mechanism of dependence between virions in the infection process (antagonism), ‘trapping’ of one of the two viruses in the inoculated leaf was observed only in 1.8% of the infected N. tabacum plants. Fig. 6. Dependence of virions during infection of C. annuum. 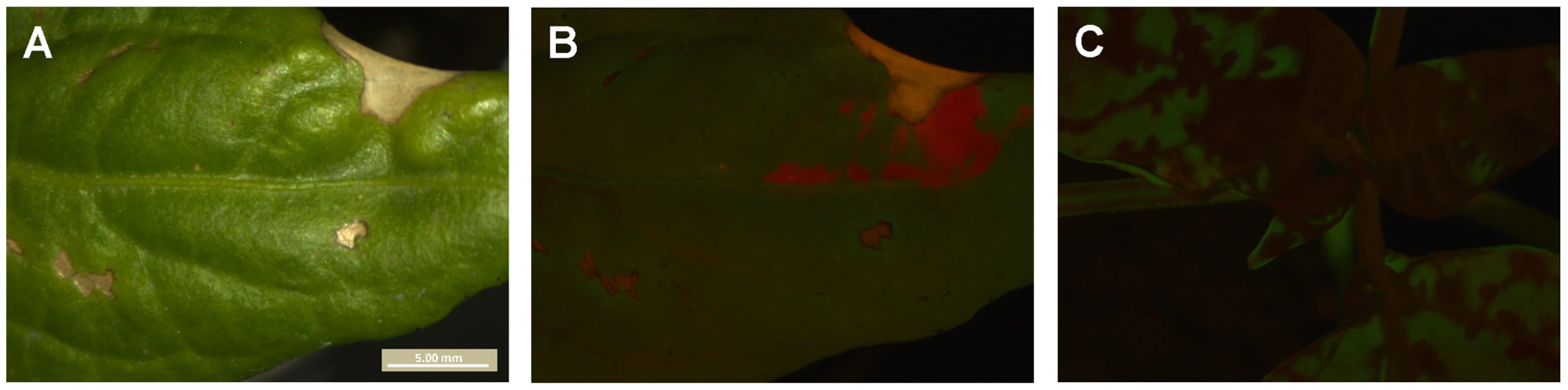
In panel A, the inoculated leaf of a C. annuum plant is shown. Note the areas of the leaf damaged due to inoculation. In panel B, the same region of the leaf is shown but with merged GFP and mCherry signal. mCherry is restricted to small portion of the leaf, probably also in part due to leaf damage from inoculation. On day three, there were three mCherry foci visible in the inoculated leaf. Note that the yellow signal in this panel is due to leaf damage, and not co-infection of tissue. In panel C, the rest of the plant is shown, and only GFP signal is present. TEV-mCherry has therefore been ‘trapped’ in the inoculated leaf. This exclusion was observed in only one infected C. annuum plant (0.9%). Interference between genotypes has been described for super-infection of different strains or co-inoculation of different plant viruses [24], but to the best of our knowledge not for the synchronous co-infection of multiple genotypes. Although we have identified potential mechanism of dependence between virions, the low frequency (1.8% of infected N. tabacum plants, and 0.9% of infected C. annuum plants) at which one genotype blocked systemic infection by the other genotype make it negligible in this experimental setup. However, when a large number of genotypes are present in the viral inoculum, it becomes more likely that one or more genotypes may be blocked from systemic infection. The same holds for de novo genetic variation arising early in the infection process.
Frequency of mixed-genotype infections is predicted by an independent-action model
Although the number of primary infection foci appears to be dose-dependent (Fig. 2), we also sought to test whether the Ne for the whole infection process is also dose-dependent. We used the frequency of mixed-genotype infection in systemically infected leaves as an indicator for population size in systemic infection. If both genotypes had systemically infected the plant, then both genotypes were usually present in all infected leaves as well. We predicted the frequency of mixed-genotype systemic infection based on the mean number of primary infection foci (λ) per replicate. We partitioned λ into λR and λG, the number of infecting virions for TEV-mCherry and TEV-GFP, based on the proportion of foci of each genotype at the 1∶10 virion dilution. Following Zwart et al. [7], we estimated the frequency of mixed-genotype systemic infection in all hosts P(R ∩ G), both infected and non-infected, as:(2)Note that this model again assumes that each focus of primary infection will lead to systemic infection of the plant. The data corresponded well to model predictions (Fig. 3; see also Tables S1 and S2 in Text S1). The IAH model of infection, with a fixed probability of infection, is therefore well supported by the mixed-genotype systemic infection data.
The high probability of systemic infection following primary infection, and the fact that the mean number of primary infection foci (Fig. 3) predicted the frequency of mixed-genotype infection, support the hypothesis that Ne at the level of systemic infection is also dose-dependent. The probability of systemic infection following primary infection is approximately 1, and the size of Ne for systemic infection is therefore very similar to Ne in the inoculated leaf (Fig. 2). As an estimate of Ne at different doses, we therefore use the mean number of foci of primary infection in infected plants (Table 4). The similarity of Ne in the inoculated and systemically infected leaves contrasts with previous work, in which there is a bottleneck between primary and systemic infection [28], [29], [30], [31], [33], and presumably a low probability of causing systemic infection after having caused primary infection (summarized in Table 4). This difference is undoubtedly associated to plant immune defenses, although the underlying mechanism is unclear. One way to approach this question would be to consider whether the probability of causing systemic infection is independent for each virion in these pathosystems. If there is independence between infecting virions, non-adaptive – and probably local – defense mechanisms in the leaf would appear to be responsible. If there is dependence between infecting virions, an adaptive immune defense – perhaps acting at the systemic level – would be implicated.
Tab. 4. Comparison of estimates of Ne. 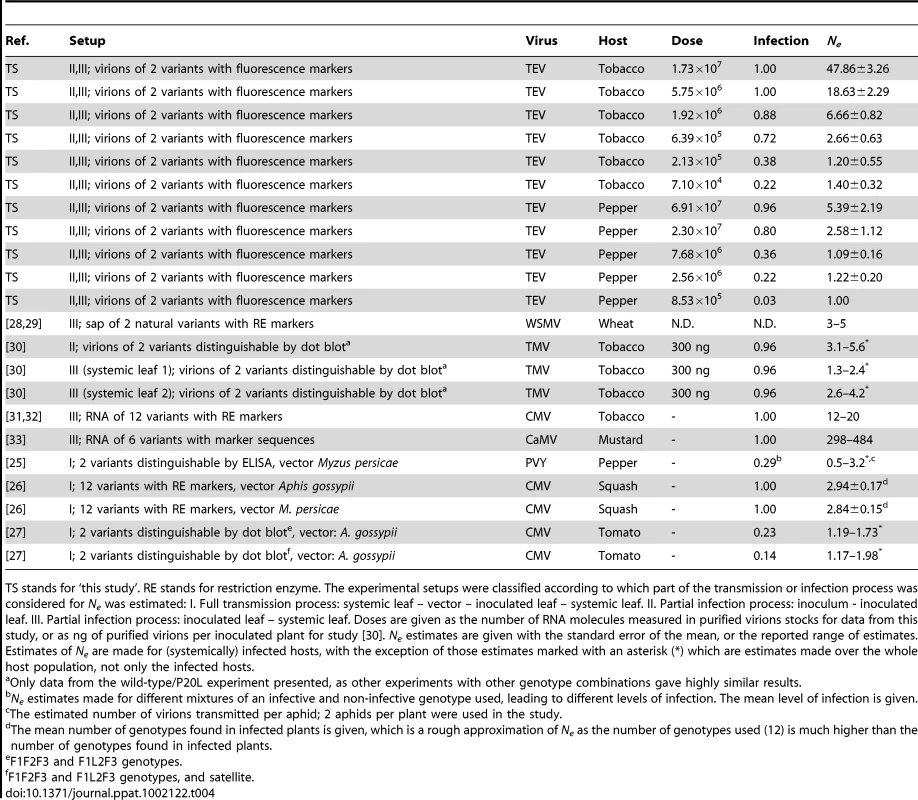
TS stands for ‘this study’. RE stands for restriction enzyme. The experimental setups were classified according to which part of the transmission or infection process was considered for Ne was estimated: I. Full transmission process: systemic leaf – vector – inoculated leaf – systemic leaf. II. Partial infection process: inoculum - inoculated leaf. III. Partial infection process: inoculated leaf – systemic leaf. Doses are given as the number of RNA molecules measured in purified virions stocks for data from this study, or as ng of purified virions per inoculated plant for study [30]. Ne estimates are given with the standard error of the mean, or the reported range of estimates. Estimates of Ne are made for (systemically) infected hosts, with the exception of those estimates marked with an asterisk (*) which are estimates made over the whole host population, not only the infected hosts. Concluding remarks
We provide strong evidence from different experimental approaches that Ne is dose-dependent for TEV, a plant RNA virus. Moreover, the relationship between Ne and dose can be described with a simple mathematical model. This means that comparison of estimates of Ne for different plant viruses (Table 4) is incomplete without taking into account dose effects as discrepancies between estimates of Ne at a single dose may be due in part to dose-dependence. In previous work estimating Ne for systemic colonization, most or all plants were infected (Table 4), meaning that the actual doses used to achieve these high levels of systemic infection could therefore have varied widely, despite causing high levels of systemic infection in all cases. Note that all other estimates of Ne fall within the range of estimates we made (approx. 1 to 48, see Table 4), with the exception of the dsDNA pararetrovirus CaMV [33]. We speculate that the independent-action model may very well apply to many mono-partite plant viruses, but this will require experiments considering the frequency of mixed-genotype infection at different doses, as we have done here.
Dose-dependent Ne was observed in host plants with a high (N. tabacum) and low susceptibility (C. annuum). Therefore, the only appreciable difference between TEV infections of these two plant species was the probability that a virion causes a focus of primary infection in the inoculated leaf. This probability was estimated to be ∼53 times lower for C. annuum than for N. tabacum when considering the number of foci in the inoculated leaf, and ∼52 times lower when considering mean values of ρ. It has been suggested the failure of the IAH may be linked to resistance of the host [7], while here we show that this does not, in principle, have to be the case. There are of course important differences between the infection process in these two hosts (i.e., note the differences in viral expansion in the inoculated leaf between Fig. 1 and Fig. S1), but basic infection parameters of the number of foci of primary infection and the frequency of mixed-genotype infections are adequately described by the IAH model.
At low doses, we regularly observed systemic infection caused by a single focus of primary infection. Although leaves were exposed to a moderate number of virions (∼105 RNA molecules, see Table 4), only a very small number of foci, approaching 1, were observed. As our data also indicate that most foci of primary infection are initiated by a single virion, we conclude that systemic infection initiated by a single virion will regularly occur at low doses. Therefore, plant RNA virus Ne can be extremely small and the impact of genetic drift on virus populations can be tremendous. The fact that Ne is dose-dependent and can be very small is relevant for experimental evolution with plant viruses. If the typical dose of virions transmitted in the field by vectors also proves to be small, as shown for another plant RNA virus [25], [27], this will demonstrate the dominant role genetic drift plays in plant virus evolution and highlight the importance of mechanisms to buffer its effects [45].
Materials and Methods
Construction of TEV-mCherry and TEV-GFP
Plasmid pMTEV is a transcription vector containing a TEV infectious clone (Genbank accession DQ986288) [34] and was used as starting point to construct the TEV-GFP and TEV-mCherry genotypes in which these two fluorescent marker genes were inserted between TEV P1 and HC-Pro cistrons. GFP or mCherry cDNA [46] was amplified by PCR using primers forward 5′-ATGGTGAGCAAGGGCGAGGAGCTG-3′ and reverse 5′-TTGGAAGTACAAGTTTTCTCCGCCGAGGTCTGAGTACTTGTAC-3′ (GFP), or forward 5′-ATGGTTAGCAAAGGCGAGG-3′ and reverse 5′-TTGGAAGTACAAGTTTTCTCCGCCCTTATACAGCTCATCCATG-3′ (mCherry). The reverse primers inserted two glycines, as spacers, and a partial NIa-Pro proteolytic site downstream of GFP or mCherry sequences. This partial proteolytic site (ENLYFQ) is complemented by the first contiguous serine in HC-Pro cistron to mediate marker release from the viral polyprotein. The pTV1a vector, which contains the first 3221 nt of the TEV genome including the complete P1 to HC-Pro cistrons, was amplified using the forward primer 5′-AGCGACAAATCAATCTCTGAGGC-3′ and reverse primer 5′-TTTGTCGCTATAATGTGTCATTGAG-3′. The PCR-amplified GFP and mCherry sequences were then ligated into the amplified vector sequence, and transformed into electrocompetent Escherichia coli DH5α. The identity of the resulting pTV1a-GFP and pTV1a-mCherry plasmids was checked by restriction digests and sequencing. Finally, PauI-AatII restriction fragments from pTV1a-GFP and pTV1a-mCherry were ligated into PauI-AatII digested pMTEV to construct pTEV-GFP and pTEV-mCherry. All PCR reactions were performed with high fidelity Phusion DNA polymerase (Finnzymes).
Reconstitution, purification and quantification of virions
We required virions of TEV-mCherry and TEV-GFP at equal concentrations in order to make stoichiometric virion mixes. To this end, RNA was transcribed from pMTEV-GFP and pMTEV-mCherry as described elsewhere [47]. Four-week-old Nicotiana tabacum var. Xanthi plants were inoculated with ca. 5 µg of RNA in the third true leaf, and systemically infected leaves harvested 9 days post inoculation (dpi). Virions were extracted from leaves as described elsewhere [47], although half the amount of infected tissue was used as starting material, and all volumes in the procedure halved. Virions were resuspended in 100 µL 0.05 M borate buffer (pH 8.0, 5 mM EDTA) with 20% glycerol, and stored as 15 µL aliquots.
In order to quantify virion concentration, we used a RT-qPCR assay. The assay was similar to that described by Carrasco et al. [47], with a number of modifications. 5×105 copies of in vitro transcribed full-length Turnip mosaic virus (TuMV) genomic RNA were added to each sample at the start of RNA extraction, as an internal control. RNA was extracted using the RNeasy Plant kit (Qiagen), following the manufacturer's instructions. 10 µL of the purified virions was used as starting material for the extraction. RT was performed with the TaqMan kit (Applied Biosystems), using an oligo(dT)16 primer. qPCR amplification was performed separately for TEV (forward primer: 5′-TTGGTCTTGATGGCAACGTG-3′ and reverse primer 5′-TGTGCCGTTCAGTGTCTTCCT-3′) and TuMV (5′-GGCACTCAAGAAAGGCAAGG-3′ and 5′-TTGTCGCGTTTTCCCTCTTC-3′). 20 µL total reaction volume was used, with 10 µL 2× SYBR Green PCR Master Mix (Applied Biosystems), 2 µL cDNA template and 300 nM of each primer. RT and qPCR were performed in triplicate for each sample. For the standard curve, serially diluted quantified DNA of pMTEV and p35Tunos [48] plasmids were used. An ABI PRISM Sequence Analyzer 7000 was used to amplify the DNA with the following thermal profile: 10 min at 95°C; 40 cycles of 30 s at 95°C and 1 min at 60°C. Concentrations of TEV and TuMV cDNA were calculated with SDS7000 software version 1.2.3 (Applied Biosystems), and TuMV concentrations were used to normalize TEV concentrations. We did not find appreciable differences in concentration between virions simultaneously extracted, and therefore TEV-GFP and TEV-mCherry virions were mixed 1∶1 for inoculation experiments.
TEV-GFP and TEV-mCherry were amplified in 3-week-old N. tabacum plants, following inoculation of a 1∶10 dilution of sap by abrasion. Viruses were amplified separately, or by a 1∶1 mixture of virions. For 1∶1 mixtures, plants that showed equal levels of systemic infection for both viruses, and the highest levels of mixing of fluorescence in the leaves were selected (‘mixed-infected plant virions’). Systemically infected leaves were harvested 9 dpi, and virions purified as described above. We also purified virions from a 1∶1 mixture (by weight) of tissues (‘mixed-tissue virions’). RNA extractions and RT-qPCR were performed, and no appreciable differences were found between samples. TEV-GFP and TEV-mCherry virions were mixed 1∶1 (‘mixed virions’) treatment, and equivalent volumes of ‘mixed-infected plant’ and ‘mixed-tissue’ virions used for experiments. Note that the concentration of these virions was higher than those originating from plants infected with RNA.
Inoculation experiments with mixtures of TEV-GFP and TEV-mCherry
N. tabacum plants that were 25 days old were used for inoculation experiments, and a selection of plants to be included in the experiment was made based on size. Plants were inoculated by abrasion of the third true leaf with a mixture of virions, with dilutions made in the 0.05 M borate buffer. Five µL of virion dilution were added to each leaf. Plants were subsequently kept in a growth chamber at 24°C. Fluorescence was observed with a Leica MZ16F stereomicroscope, using a 0.5× objective lens, and GFP2 and DSR filters (Leica) to view GFP and mCherry, respectively.
For a pilot experiment in N. tabacum, 15 plants were inoculated with the following virion dilutions: 1∶10, 1∶30, 1∶90, 1∶270, 1∶810, 1∶2430, 1∶7290 and a non-virus control. No infections were observed with a 1∶2430 and 1∶7290 dilutions of the pilot experiment. The number of TEV-GFP foci was ca. two-fold higher than the number of TEV-mCherry foci in the pilot experiment, so the dose of TEV-mCherry was doubled for the definitive experiment. For the definitive experiment, four replicates were performed. For replicate 1, 15 plants each were used with three-fold dilutions from 1∶10 until 1∶810, and a non-virus control. For replicates 2 to 4, 15 plants each were used with three-fold dilutions from 1∶10 until 1∶2430, and a non-virus control.
For experiments in Capsicum annuum var. Marconi, 32 day old plants were selected based on size. TEV-GFP and TEV-mCherry virions that had undergone one round of amplification in N. tabacum (i.e. ‘mixed virions’) were used for the inoculum, again with double the dose of TEV-mCherry. Inoculation and experimental conditions used were otherwise identical to those for N. tabacum. For a pilot experiment, 12 plants were inoculated with a 1∶10 mixture. For replicate 1 of the definitive experiment, 15 plants were inoculated with the following virion dilutions: 1∶10, 1∶30, 1∶90, 1∶270, 1∶810, and a non-virus control. For replicates 2 and 3, a 1∶2430 dilution was also included.
Testing reporter gene stability
To test the stability of the reporter genes, four-week-old N. tabacum plants were infected with TEV-mCherry or TEV-GFP virions by abrasion, with five replicates for each virus. One week post inoculation, infected tissues were collected and a 1∶10 dilution of sap was inoculated into new plants by abrasion. After one week, infected tissues were collected, and fluorescence was observed by stereomicroscope to be equivalent to that in plants infected with TEV-GFP or TEV-mCherry RNA (data not shown). RNA was extracted with the RNeasy plant kit (Qiagen), and RT was performed using M-MuLV (Fermentas) and random hexamers as a primer. A Taq polymerase (Roche) based PCR was performed with two sets of specific primers flanking the reporter gene (set 1, forward primer: 5′-CAATTGTTCGCAAGTGTGC-3′, reverse primer: 5′-ATGGTATGAAGAATGCCTC-3′; set 2, forward primer: 5′ - GCAATCAAGCATTCTACTTC-3′, reverse primer: 5′ - CCTGATATGTTTCCTGATAAC-3′). PCR products were resolved on a 1% agarose gel, and only the amplicon corresponding to TEV-GFP or TEV-mCherry was observed for the passaged viruses (data not shown). To test the sensitivity of the assay for genomes with the reporter gene deleted, we mixed TEV and TEV-mCherry RNA in different ratios. Primer set 1 could detect the small amplicon corresponding to TEV in at a ratio 1∶10,000, and primer set 2 could detect the TEV-derived amplicon at 1∶100.
Statistical analysis
All statistical analysis was performed in SPSS version 16.0 (SPSS Inc.) unless otherwise noted.
To analyze the dose foci relationship, the foci data λ were transformed as y = ln(λ+1) and the relationship y = ln(φd+1) fitted using non-linear regression, where φ is the probability that a unit dose results in a focus of primary infection. One was added to λ because some leaves had zero foci; d stands for the dose.
The general model of dose-response relationships [42] is given by:where I is the fraction of systemically infected plants, d is the virion dose as estimated by RT-qPCR, ρ is the average infection probability and κ determines what the effect of dose on the rate of infection is. When κ = 1, the dose-response relationship corresponds to the IAH. When κ>1, there is synergism between virions (the dose-response is steeper than predicted for the IAH), whilst when κ<1, there is antagonism (the dose-response is shallower than expected for the IAH). This model was fitted to the data using non-linear regression to estimate ρ and κ. For dose in N. tabacum, we divided the dilution by a constant, chosen for convenience (10−4). This constant is arbitrary because we are not interested in valid estimates of ρ, but rather we want an indication of the fit of the model. However, we wanted to compare these results to those in C. annuum. The number of foci of primary infection in the inoculated leaf of N. tabacum was therefore compared for the two virions stocks used for experiments in N. tabacum (virions purified from transfected N. tabacum plants) and C. annuum (virions purified from N. tabacum plants in the virus resulting from transfection was amplified). There were four-fold more foci generated by the amplified virion stock.
A two-tailed exact binomial test (R version 2.10.1, The R Foundation) was used to test whether the frequency of infections and mixed-genotype infections were significantly different from model predictions.
Supporting Information
Zdroje
1. WrightS 1930 Evolution in Mendelian populations. Genetics 16 97 159
2. NijhuisMBoucherCABSchipperPLeitnerTSchuurmanR 1998 Stochastic processes strongly influence HIV-1 evolution during suboptimal protease-inhibitor therapy. Proc Natl Acad Sci USA 95 14441 14446
3. MoyaAElenaSFBrachoAMirallesRBarrioE 2000 The evolution of RNA viruses: A population genetics view. Proc Natl Acad Sci USA 97 6967 6973
4. ChaoL 1990 Fitness of RNA virus decreased by Muller's Ratchet. Nature 348 454 455
5. de la IglesiaFElenaSF 2007 Fitness declines in Tobacco etch virus upon serial bottleneck transfers. J Virol 81 4941 4947
6. BergstromCTMcElhanyPRealLA 1999 Transmission bottlenecks as determinants of virulence in rapidly evolving pathogens. Proc Natl Acad Sci USA 96 5095 5100
7. ZwartMPHemerikLCoryJSde VisserJBianchiF 2009 An experimental test of the independent action hypothesis in virus-insect pathosystems. Proc R Soc B 276 2233 2242
8. FroissartRRozeDUzestMGalibertLBlancS 2005 Recombination every day: Abundant recombination in a virus during a single multi-cellular host infection. PLoS Biol 3 389 395
9. VignuzziMStoneJKArnoldJJCameronCEAndinoR 2006 Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439 344 348
10. ZwartMPvan der WerfWvan OersMMHemerikLvan LentJMV 2009 Mixed infections and the competitive fitness of faster-acting genetically modified viruses. Evol Appl 2 209 221
11. MartinSElenaSF 2009 Application of game theory to the interaction between plant viruses during mixed infections. J Gen Virol 90 2815 2820
12. TaylorDRZeylCCookeE 2002 Conflicting levels of selection in the accumulation of mitochondrial defects in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 99 3690 3694
13. ZwartMPvan der WerfWGeorgievskaLvan OersMMVlakJM 2010 Mixed-genotype infections of Trichoplusia ni larvae with Autographa californica multicapsid nucleopolyhedrovirus: Speed of action and persistence of a recombinant in serial passage. Biol Control 52 77 83
14. DruettHA 1952 Bacterial invasion. Nature 170 288 288
15. FurumotoWAMickeyR 1967 A mathematical model for infectivity-dilution curve of Tobacco mosiac virus - experimental tests. Virology 32 224 233
16. FurumotoWAMickeyR 1967 A mathematical model for infectivity-dilution curve of Tobacco mosaic virus - theoretical considerations. Virology 32 216 223
17. BaldJG 1937 The use of numbers of infections for comparing the concentration of plant virus suspensions I. Dilution experiments with purified suspensions. Ann Appl Biol 24 33 55
18. García-ArenalFFraileA 2011 Population dynamics and genetics of plant infection by viruses. CarantaCArandaMATepferMLópez-MoyaJJ Recent advances in plant virology Norfolk, UK Caister Academic Press 263 281
19. WatsonMA 1972 Transmission of plant viruses by aphids. KadoCIAgrawalHO Principles and techniques in plant virology New York Van Nostrand Reinhold Co 131 167
20. GomezPSempereRNElenaSFArandaMA 2009 Mixed infections of Pepino mosaic virus strains modulate the evolutionary dynamics of this emergent virus. J Virol 83 12378 12387
21. HammondJLecoqHRaccahB 1999 Epidemiological risks from mixed virus infections and transgenic plants expressing viral genes. Adv Vir Res 54 189 314
22. Lopez-FerberMSimonOWilliamsTCaballeroP 2003 Defective or effective? Mutualistic interactions between virus genotypes. Proc R Soc B 270 2249 2255
23. ClavijoGWilliamsTMunozDCaballeroPLopez-FerberM 2010 Mixed genotype transmission bodies and virions contribute to the maintenance of diversity in an insect virus. Proc R Soc B 277 943 951
24. BennettCW 1953 Interactions between viruses and virus strains. Adv Vir Res 1 39 67
25. MouryBFabreFSenoussiR 2007 Estimation of the number of virus particles transmitted by an insect vector. Proc Natl Acad Sci USA 104 17891 17896
26. AliALiHYSchneiderWLShermanDJGrayS 2006 Analysis of genetic bottlenecks during horizontal transmission of Cucumber mosaic virus. J Virol 80 8345 8350
27. BetancourtMFereresAFraileAGarcia-ArenalF 2008 Estimation of the effective number of founders that initiate an infection after aphid transmission of a multipartite plant virus. J Virol 82 12416 12421
28. HallJSFrenchRHeinGLMorrisTJStengerDC 2001 Three distinct mechanisms facilitate genetic isolation of sympatric Wheat streak mosaic virus lineages. Virology 282 230 236
29. FrenchRStengerDC 2003 Evolution of Wheat streak mosaic virus: Dynamics of population growth within plants may explain limited variation. Ann Rev Phytopathol 41 199 214
30. SacristanSMalpicaJMFraileAGarcia-ArenalF 2003 Estimation of population bottlenecks during systemic movement of Tobacco mosaic virus in tobacco plants. J Virol 77 9906 9911
31. LiHYRoossinckMJ 2004 Genetic bottlenecks reduce population variation in an experimental RNA virus population. J Virol 78 10582 10587
32. ElenaSFBedhommeSCarrascoPCuevasJMde la IglesiaF 2011 The evolutionary genetics of emerging plant RNA viruses. Mol Plant Microbe In 24 287 293
33. MonsionBFroissartRMichalakisYBlancS 2008 Large bottleneck size in Cauliflower mosaic virus populations during host plant colonization. PloS Pathog 4 7
34. BedoyaLCDarosJA 2010 Stability of Tobacco etch virus infectious clones in plasmid vectors. Vir Res 149 234 240
35. ShanerNCCampbellRESteinbachPAGiepmansBNPalmerAE 2004 Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22 1567 1572
36. FurumotoWAMickeyR 1970 Mathematical analyses of interference phenomenon of Tobacco mosiac virus - theoretical considerations. Virology 40 316 321
37. KleczkowskiA 1949 The transformation of local lesion counts for statistical analysis. Ann Appl Biol 36 139 152
38. KleczkowskiA 1950 Interpreting relationships between the concentrations of plant viruses and numbers of local lesions. J Gen Microbiol 4 53 69
39. Ben-AmiFRegoesRREbertD 2008 A quantitative test of the relationship between parasite dose and infection probability across different host–parasite combinations. Proc R Soc B 275 853 859
40. RidoutMSFenlonJSHughesPR 1993 A generalized one-hit model for bioassays of insect viruses. Biometrics 49 1136 1141
41. DieuBTMZwartMPVlakJM 2010 Can VNTRs be used to study genetic variation within white spot syndrome virus isolates? J Fish Dis 33 689 693
42. RegoesRRHottingerJWSygnarskiLEbertD 2003 The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass-action principle. Epidemiol Infect 131 957 966
43. ShallaTA 1964 Assembly and aggregation of Tobacco mosaic virus in Tomato leaflets. J Cell Biol 21 253 264
44. MiyashitaSKishinoH 2010 Estimation of the size of genetic bottlenecks in cell-to-cell movement of Soil-borne wheat mosaic virus and the possible role of the bottlenecks in speeding up selection of variations in trans-acting genes or elements. J Virol 84 1828 1837
45. CodonerFMDarosJASoleRVElenaSF 2006 The fittest versus the flattest: Experimental confirmation of the quasispecies effect with subviral pathogens. PLoS Pathog 2 1187 1193
46. BedoyaLMartinezFRubioLDarosJA 2010 Simultaneous equimolar expression of multiple proteins in plants from a disarmed potyvirus vector. J Biotechnol 150 268 275
47. CarrascoPDarosJAAgudelo-RomeroPElenaSF 2007 A real-time RT-PCR assay for quantifying the fitness of Tobacco etch virus in competition experiments. J Virol Meth 139 181 188
48. SanchezFMartinez-HerreraDAguilarIPonzF 1998 Infectivity of Turnip mosaic potyvirus cDNA clones and transcripts on the systemic host Arabidopsis thaliana and local lesion hosts. Vir Res 55 207 219
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



