-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Regulation of Sulfur Metabolism in
Mycobacterium tuberculosis (Mtb) has evolved into a highly successful human pathogen. It deftly subverts the bactericidal mechanisms of alveolar macrophages, ultimately inducing granuloma formation and establishing long-term residence in the host. These hallmarks of Mtb infection are facilitated by the metabolic adaptation of the pathogen to its surrounding environment and the biosynthesis of molecules that mediate its interactions with host immune cells. The sulfate assimilation pathway of Mtb produces a number of sulfur-containing metabolites with important contributions to pathogenesis and survival. This pathway is regulated by diverse environmental cues and regulatory proteins that mediate sulfur transactions in the cell. Here, we discuss the transcriptional and biochemical mechanisms of sulfur metabolism regulation in Mtb and potential small molecule regulators of the sulfate assimilation pathway that are collectively poised to aid this intracellular pathogen in its expert manipulation of the host. From this global analysis, we have identified a subset of sulfur-metabolizing enzymes that are sensitive to multiple regulatory cues and may be strong candidates for therapeutic intervention.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002036
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1002036Summary
Mycobacterium tuberculosis (Mtb) has evolved into a highly successful human pathogen. It deftly subverts the bactericidal mechanisms of alveolar macrophages, ultimately inducing granuloma formation and establishing long-term residence in the host. These hallmarks of Mtb infection are facilitated by the metabolic adaptation of the pathogen to its surrounding environment and the biosynthesis of molecules that mediate its interactions with host immune cells. The sulfate assimilation pathway of Mtb produces a number of sulfur-containing metabolites with important contributions to pathogenesis and survival. This pathway is regulated by diverse environmental cues and regulatory proteins that mediate sulfur transactions in the cell. Here, we discuss the transcriptional and biochemical mechanisms of sulfur metabolism regulation in Mtb and potential small molecule regulators of the sulfate assimilation pathway that are collectively poised to aid this intracellular pathogen in its expert manipulation of the host. From this global analysis, we have identified a subset of sulfur-metabolizing enzymes that are sensitive to multiple regulatory cues and may be strong candidates for therapeutic intervention.
Introduction
Mycobacterium tuberculosis (Mtb), the bacterium that causes tuberculosis in humans, infects roughly 2 billion people worldwide [1]. However, less than 1% of those infected with Mtb show signs of active disease [2]. The majority of infected individuals have a latent infection characterized by dormant, nonreplicating bacteria that persist within a mass of immune cells in the lung [3]. These cells form a protective barrier between the bacteria and surrounding tissue known as the granuloma [4], [5]. When host immunity is compromised, the granuloma deteriorates, liberating the confined bacteria and reactivating the disease.
In order to mount a latent infection, Mtb must withstand phagocytosis by alveolar macrophages, the host's primary line of defense against airborne pathogens [5]. By evading typical bactericidal processes, Mtb is able to replicate and eventually induce granuloma formation [3]. The mechanisms by which Mtb persists in the hostile phagosomal environment and orchestrates the transition to latency are ill defined. Elucidating the molecular machinery and metabolic pathways that facilitate these pivotal events is crucial to identifying new therapeutic targets for this global human pathogen.
There is a growing body of evidence that supports a role for sulfur-containing metabolites in Mtb pathogenesis [6], [7]. Sulfated molecules from Mtb have been intimately linked to bacterial virulence (Figure 1A) [8]–[10]. For example, the prominent cell wall-associated glycolipid Sulfolipid-1 is only produced by pathogenic species of mycobacteria [8], [11], and its biosynthetic precursor SL1278, named for its observed mass, has been shown to elicit cytokine production in human tuberculosis patients [10]. On the contrary, biosynthesis of the sulfated menaquinone S881 suppresses bacterial virulence [9]. Reduced sulfur compounds (Figure 1B), such as cysteine and methionine, also contribute to Mtb pathogenesis [6]. Disabling their biosynthesis dramatically attenuates bacterial virulence and persistence during the chronic phase of infection in mice [12]. Mycothiol (MSH), the primary thiol-containing small molecule of mycobacteria [13], is instrumental in the detoxification of numerous bactericidal agents and confers protection against oxidative stress [14]–[16]. Biosynthesis of these important sulfur-containing metabolites relies on the sulfate assimilation pathway [17].
Fig. 1. Sulfur-containing metabolites from Mtb. 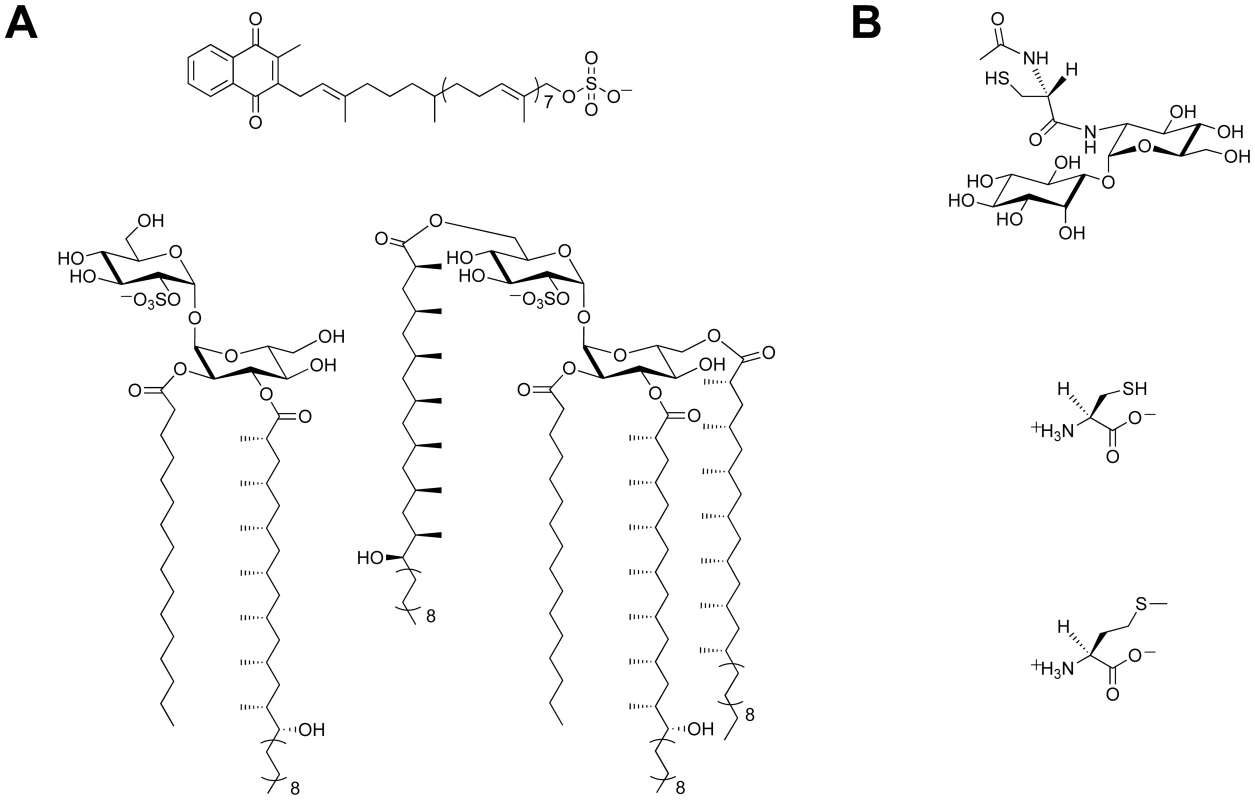
(A) Sulfated compounds, clockwise from top: S881, Sulfolipid-1, SL1278. (B) Reduced sulfur compounds, from top to bottom: MSH, cysteine, methionine. The sulfate assimilation pathway (Figure 2) is composed of a group of enzymes that catalyze the uptake and metabolism of inorganic sulfate from the host [7]. This pathway commences with the active import of sulfate, which is subsequently adenylated by ATP sulfurylase, an enzyme containing both GTPase and sulfurylase domains [18]. The resulting product, adenosine 5′-phosposulfate (APS), can be reduced to sulfite by APS reductase for the biosynthesis of reduced sulfur species via the reductive branch of the pathway [19]. Alternatively, because APS constitutes a metabolic branchpoint, it can be phosphorylated at the 3′-position by APS kinase to generate 3′-phosphoadenosine 5′-phosphosulfate (PAPS), the universal sulfate donor in the cell [17]. PAPS is a substrate for sulfotransferases, enzymes that catalyze the transfer of its sulfate group onto bacterial metabolites [11]. Collectively, these reactions constitute the sulfation branch of the sulfate assimilation pathway.
Fig. 2. The sulfate assimilation pathway of Mtb. 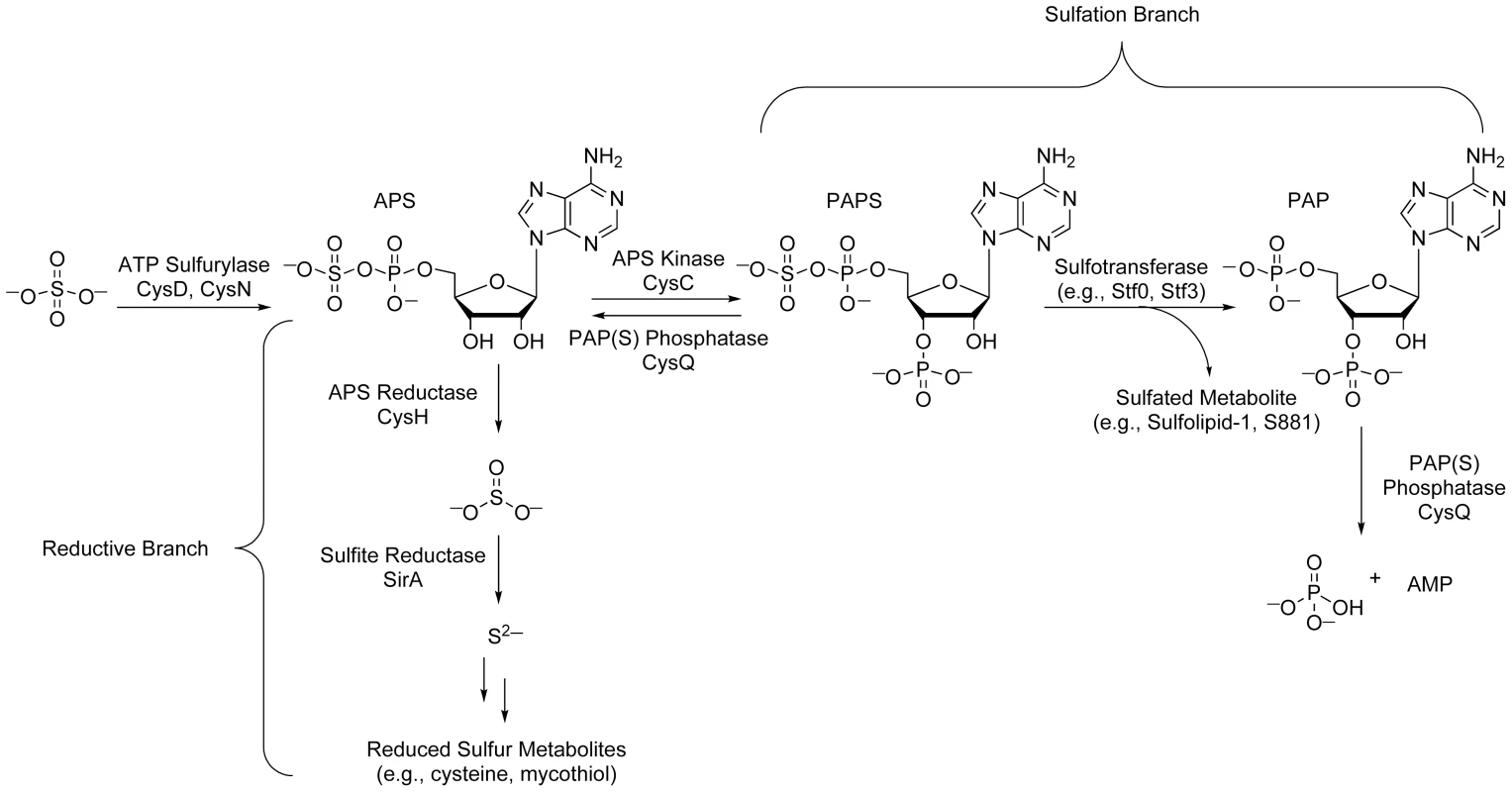
Inorganic sulfate from the host is converted to APS by ATP sulfurylase. APS is either sequentially reduced by APS reductase and sulfite reductase for the biosynthesis of essential reduced sulfur metabolites (reductive branch), or phosphorylated by APS kinase to generate PAPS, a substrate for sulfotransferases (sulfation branch). PAP, a byproduct of sulfotransferase reactions, is degraded by the PAP(S) phosphatase CysQ, which also converts PAPS to APS. The regulation of Mtb sulfur metabolism relies on the transcriptional response of sulfate assimilation enzymes to diverse environmental cues and regulatory proteins that influence flux through the sulfate assimilation pathway. Further, small molecule regulation of sulfur metabolism in other bacteria suggests similar mechanisms may modulate related transcriptional circuits in Mtb. Here, we review each of these regulatory elements in an effort to guide our understanding of how the sulfate assimilation pathway facilitates bacterial adaptation to the host.
Transcriptional Regulation of Sulfur Metabolism Genes
Transcriptional profiling has demonstrated that Mtb sulfur metabolism genes are dynamically regulated by diverse environmental cues (Table 1) [20]–[29]. This finding is perhaps not surprising, given the demonstrated importance of the sulfate assimilation pathway to bacterial survival [6] and the hostile conditions encountered by bacteria in the phagosome [30]. For example, upon phagocytosis, bacteria are exposed to oxidative stress and deprived of essential nutrients [30], [31]. Microarray analysis has shown that these conditions elicit the upregulation of key genes from the sulfate assimilation pathway (Table 1) [21], [22], [26], [27]. Genes encoding the primary sulfate transport complex of Mtb are induced following treatment with hydrogen peroxide (cysT) and nutrient starvation (cysA1, cysT, cysW, subI), conditions that also induce the ATP sulfurylase genes cysD and cysN, as well as the cysC gene that encodes APS kinase (in Mtb, cysC is fused to cysN, yielding a bifunctional cysNC gene [18]). Given that these genes coordinate the first few steps of the sulfate assimilation pathway, their induction is most likely accompanied by an increase in the biosynthesis of sulfur-containing metabolites that protect the pathogen during the course of infection, though this remains to be demonstrated. Notably, cysD and cysNC are also induced during macrophage infection, underscoring the importance of sulfur metabolism to intracellular survival [21], [25]. Indeed, reduced sulfur compounds have been shown to play a critical role in facilitating bacterial persistence in vivo [12]. Genes contributing to the biosynthesis of these metabolites, such as cysH and sirA, which direct the reduction of APS to sulfide [19], [32], and cysM, which is involved in cysteine biosynthesis [33], [34], are also among those induced by hydrogen peroxide and nutrient starvation. Collectively, these genes are likely to facilitate bacterial adaptation to the phagosomal environment.
Tab. 1. Sulfur metabolism genes from M. tuberculosis induced by various conditions of environmental stress. 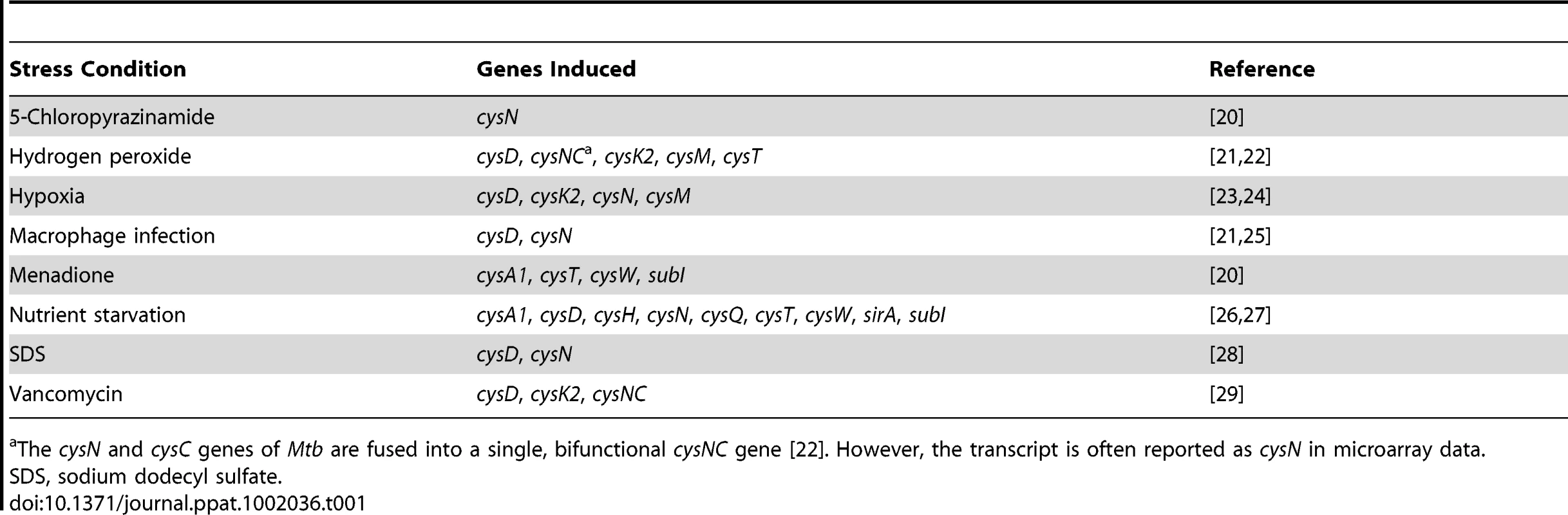
The cysN and cysC genes of Mtb are fused into a single, bifunctional cysNC gene [22]. However, the transcript is often reported as cysN in microarray data. Hypoxia is another environmental cue that has been correlated with the upregulation of genes from the sulfate assimilation pathway [23], [24]. Transcription of cysD, cysNC, cysK2, and cysM is induced by hypoxia, suggesting ATP sulfurylase and cysteine biosynthesis may facilitate bacterial adaptation to oxygen-limiting conditions. Because the granuloma has been shown to harbor regions of low oxygen tension [4], it is plausible that sulfur metabolism may be important in the transition to latency. The finding that reduced sulfur compounds are critical to the onset of chronic Mtb infection in mice lends additional support to this hypothesis [12].
Certain antibiotics have also been shown to affect the transcription of sulfur metabolism genes. Vancomycin, an inhibitor of peptidoglycan biosynthesis, induces expression of cysK2, cysD, and cysNC [29]. The cysNC gene is also induced by 5-chloropyrazinamide, a pyrazinamide analog that irreversibly inhibits fatty acid biosynthesis in vitro [20], [35]. Furthermore, cysA1, cysT, cysW, and subI are collectively induced by menadione, a synthetic vitamin K precursor that promotes the formation of reduced oxygen species [20], [36]. These data suggest that sulfur metabolism may modulate the bacterial response to certain drugs. Evaluating Mtb susceptibility to these drugs upon overexpression of related sulfur metabolism genes may reveal a more direct role for the encoded enzymes in antibiotic resistance.
Indeed, several studies point to a role for sulfur-containing compounds in bacterial detoxification pathways. In Salmonella typhimurium, cysteine biosynthesis is required for the enhanced antibiotic resistance of migrating swarm cells [37]. Similarly, dysregulation of cysteine metabolism in Bacillus subtilis and Staphylococcus aureus sensitizes the bacterial response to oxidative stress and tellurite exposure [38], [39]. Further, MSH exhibits well-documented protective effects against free radicals, potent oxidizing agents, alkylating agents, and certain antibiotics [14]–[16], [40], [41], though is essential for ethionamide susceptibility and was recently shown to be dispensable for Mtb growth [42]. Overall, these findings underscore the importance of bacterial sulfur metabolism to the detoxification of toxic species in the cell and are consistent with the enhanced expression of Mtb sulfur metabolism genes in response to oxidative stress and exposure to certain antibiotics.
Transcriptional Regulators of Bacterial Sulfur Metabolism
In all likelihood, one or more transcriptional regulators mediate the transcriptional response of the sulfate assimilation pathway to extracellular signals. The alternative sigma factor SigH, which is induced by phagocytosis and stress conditions, including acidic pH and exposure to diamide, a thiol oxidizer, has been shown to regulate the transcription of several sulfur metabolism genes (e.g., cysA1, cysT, cysW, cysD, and cysNC) following diamide treatment [43]. SigH activity is regulated by the redox-sensitive anti-sigma factor RshA [44]. Interestingly, the alternative sigma factor SigR from Streptomyces coelicolor, a related actinomycete, is also induced by diamide and regulated by the redox-sensitive anti-sigma factor RsrA (Figure 3) [45]. SigR and RsrA have been shown to modulate MSH production in S. coelicolor in response to intracellular MSH levels [45]. The strong dependence of MSH biosynthesis on the sulfate assimilation pathway suggests that SigH and RshA might similarly regulate the biosynthesis of this ubiquitous thiol in mycobacteria.
Fig. 3. Feedback regulation of RsrA activity and SigR (σR)-mediated transcription by MSH in S. coelicolor. 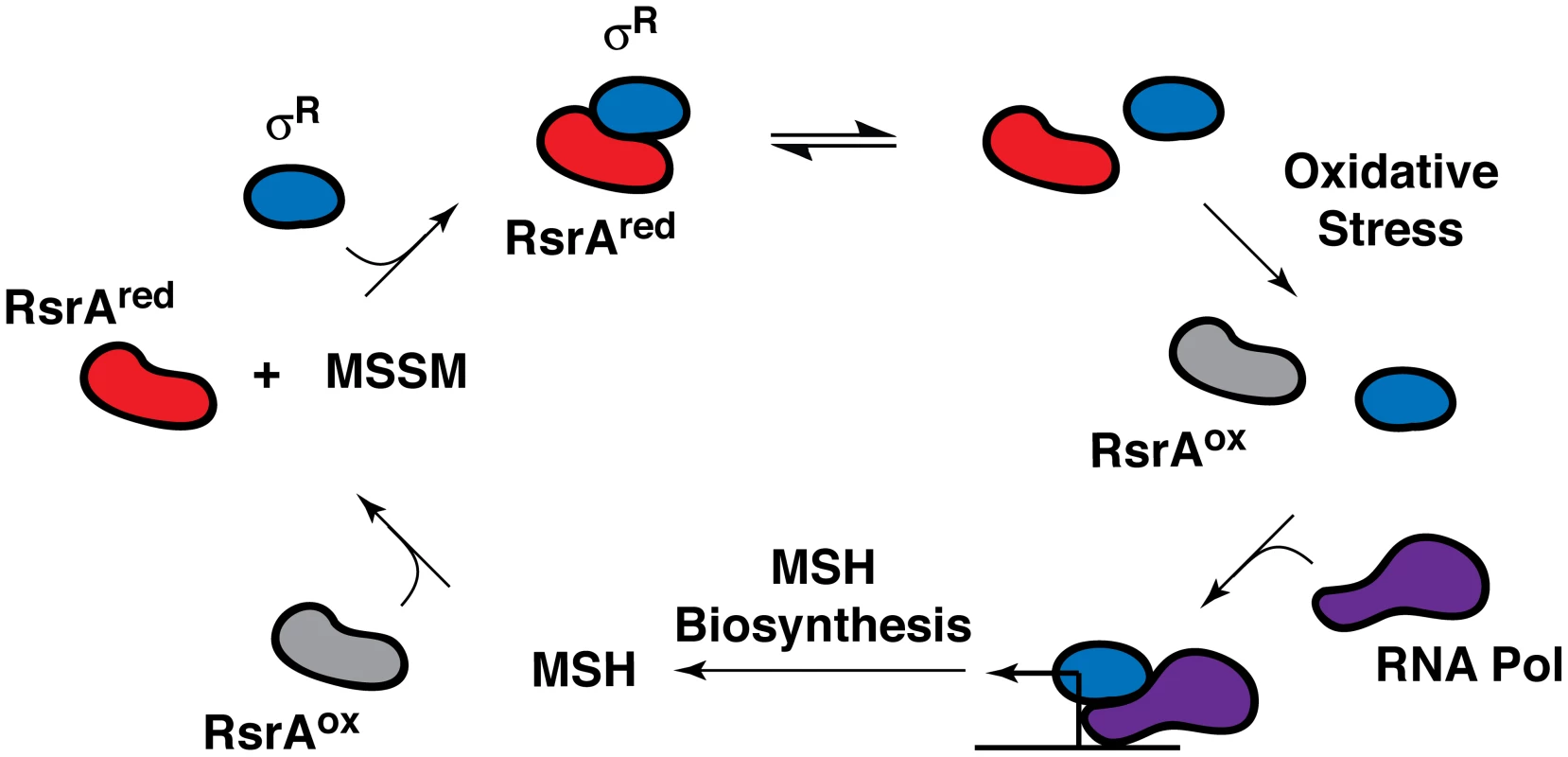
The anti-sigma factor RsrA binds the alternative sigma factor σR in its reduced state (RsrAred), preventing the association of σR with RNA polymerase (RNA Pol). Under conditions of oxidative stress (e.g., hydrogen peroxide or diamide treatment), RsrA is oxidized (RsrAox) and no longer binds σR, enabling it to form a complex with RNA polymerase, which induces the transcription of MSH biosynthetic genes. The reduction of oxidized RsrA by MSH facilitates sequestration of σR, which, in turn, suppresses MSH biosynthesis. Importantly, when oxidizing or alkylating agents deplete MSH levels, RsrA remains oxidized, which stimulates σR-mediated transcription and MSH biosynthesis [45], [67]. In Escherichia coli, the majority of sulfate assimilation genes belong to the cysteine (cys) regulon that is positively regulated by the transcription factor CysB [46]. In the absence of inorganic sulfate, a second transcription factor, Cbl, induces a series of sulfate starvation-inducible (SSI) genes that coordinate the uptake and subsequent metabolism of organosulfur compounds for the generation of sulfite [47], [48]. Comparable transcriptional regulators have not yet been identified in mycobacteria.
Though the enzymes involved in sulfur metabolism are largely conserved between these two prokaryotes, there are some notable differences in the organization of their respective sulfate assimilation pathways that caution against using the transcriptional regulatory networks of E. coli as a blueprint for those of Mtb. For example, the reductive branch of the Mtb sulfate assimilation pathway emanates from APS, whereas E. coli initiates the biosynthesis of reduced sulfur metabolites from PAPS [17]. Mtb also encodes four eukaryotic-like sulfotransferases (stf0, stf1, stf2, and stf3), which are absent from E. coli [11], [49]. Further, Mtb encodes two independent pathways for cysteine biosynthesis: the canonical pathway, which utilizes L-serine (as in E. coli), and a novel route that utilizes O-phospho-L-serine [33], [50], [51]. While these distinctions do not rule out the possibility of a CysB-like master regulator in Mtb, they do suggest that the transcriptional regulation of mycobacterial sulfur-metabolizing enzymes may diverge considerably from that of E. coli. Additional studies are needed to elucidate the transcriptional machinery mediating the expression of these enzymes in Mtb. Notably, a functional analog of CysB was recently identified in Corynebacterium glutamicum, a gram-positive bacterium belonging to the same suborder as Mtb, which will likely facilitate the search for related proteins in mycobacteria [52].
Biochemical Regulation of Sulfur Metabolism
Sulfate Transporters
In addition to the various environmental conditions that modulate sulfur metabolism at the transcriptional level, several proteins regulate the metabolic pipeline by mediating the availability of sulfate and its flux through the sulfate assimilation pathway. Most notable are the sulfate transporters, which enable the uptake of extracellular sulfate. The primary sulfate permease of Mtb is an ABC transporter encoded by the subI-cysTWA1 operon [53]. Genetic disruption of the cysA1 gene in M. bovis completely inhibits sulfate uptake in vitro and renders the mutant auxotrophic for methionine, yet does not impair bacterial survival in mice [53]. This implies either a strong reliance on methionine transport in vivo, or the presence of other sulfate transporters that can compensate for the loss of SubI-CysTWA1 activity during infection. Notably, Mtb encodes two additional putative sulfate transporters, Rv1739c and Rv1707, whose genes are induced 24 h postinfection of activated macrophages [21], [54]. Rv1739c expression in E. coli has been shown to enhance sulfate uptake, though complementation of the M. bovis cysA1 mutant with the Rv1739c gene is not sufficient to restore sulfate prototrophy [54]. In contrast, little is known about the Rv1707 gene product. Assuming all three of these transporters are functional, it is possible they modulate sulfate uptake in response to discrete environmental signals.
Sulfatases
The sulfatases are a second class of proteins that likely contribute to the intracellular concentration of free sulfate. These enzymes catalyze the hydrolysis of sulfate esters from sulfated proteins, peptides, and small molecules (Figure 4A) [55]. Type I sulfatases are characterized by an active-site formylglycine residue that is critical for catalysis [56]. The formylglycine is either co - or post-translationally installed by a formylglycine-generating (FGE) enzyme via oxidation of a conserved cysteine (Figure 4B). The Mtb genome encodes six type I sulfatases, yet little is known about their biochemistry [56]. One of these, AtsG, was recently shown to possess arylsulfatase activity, but its native substrate has not been identified [57]. In the absence of FGE, Mtb exhibits residual sulfatase activity that may be attributed to FGE-independent type II and type III sulfatases [56]. It is possible that some of these enzymes are secreted and facilitate sulfate scavenging from the host. Alternatively, they may hydrolyze sulfate from endogenous metabolites to redirect the biosynthesis of sulfur-containing compounds in response to evolving cellular needs. In either scenario, these sulfatases are likely to regulate sulfur metabolism by influencing sulfate availability.
Fig. 4. Sulfatase biochemistry. 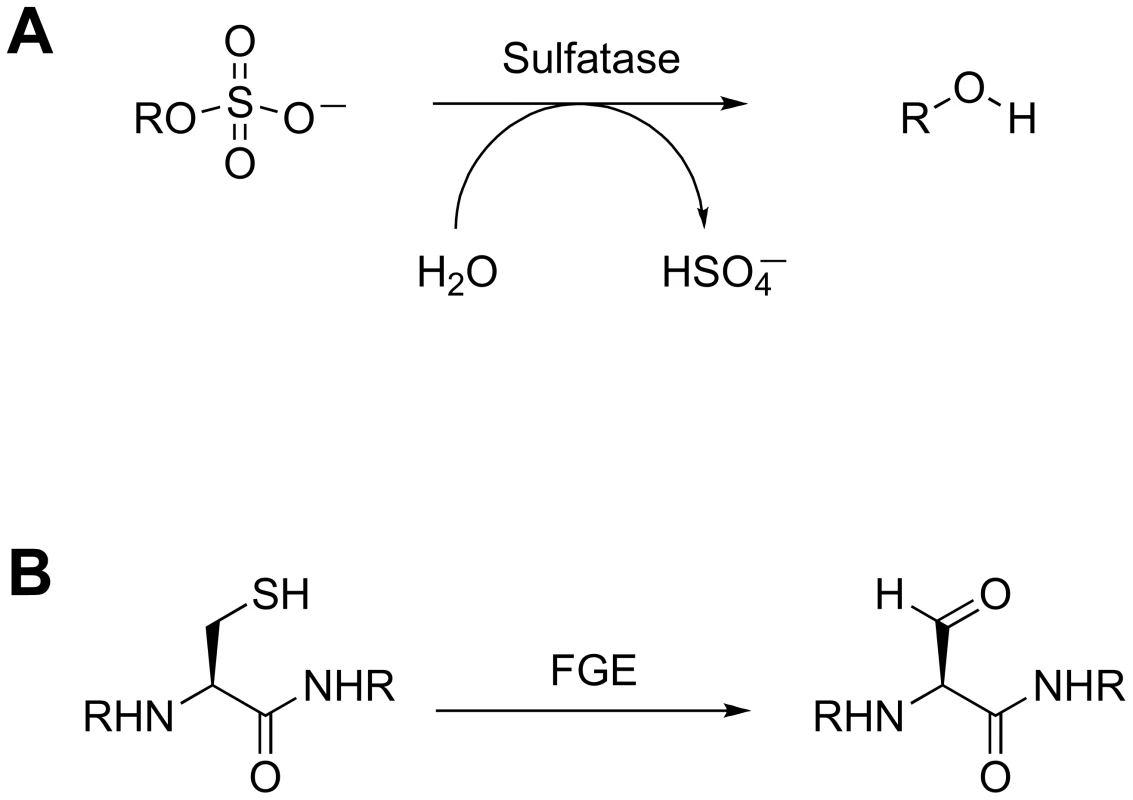
(A) Sulfatase-catalyzed hydrolysis of a sulfated metabolite. (B) Modification of the active site cysteine of type I sulfatases by FGE. The 3′-Phosphoadenosine-5′-Phosphatase CysQ
Another regulator of Mtb sulfur metabolism is CysQ, a 3′-phosphoadenosine-5′-phosphatase. Unlike sulfate permeases and sulfatases, whose activity directly influences the availability of free sulfate, CysQ has the ability to modulate the levels of intermediates that may affect flux through the sulfate assimilation pathway. Specifically, CysQ dephosphorylates 3′-phosphoadenosine 5′-phosphate (PAP), a byproduct of sulfotransferase reactions, and its sulfated counterpart, PAPS [58]. Since PAP inhibits at least one Mtb sulfotransferase [59], and PAPS accumulation is believed to be cytotoxic [60], the degradation of these pathway intermediates by CysQ is likely to promote sulfation and balance sulfur transactions in the cell. Interestingly, CysQ activity is inhibited by alkali metal cations in vitro, including physiological concentrations of sodium, and a homologous enzyme from Streptococcus mutans confers resistance to superoxide stress [58], [61]. These findings suggest that CysQ may be sensitive to changes in the ionic composition of the cytosol or to oxidative stress encountered during the course of infection. If so, CysQ may modulate sulfur metabolism in response to evolving environmental conditions.
Molecular Mechanisms of Sulfur Metabolism Regulation
Small molecule regulation of sulfur metabolism is another important mechanism directing the biosynthesis of sulfur-containing metabolites in bacteria. Though this phenomenon has not been thoroughly investigated in Mtb, several paradigms have emerged from other bacteria that may prove functional in mycobacteria. While it is important to consider genus - and species-specific variations in sulfur metabolism when drawing such parallels [62], regulatory networks from other bacteria have often guided the discovery of comparable pathways in Mtb [63]–[65] and thus warrant further discussion.
In S. typhimurium, cysteine, sulfide, and thiosulfate have all been shown to inhibit sulfate assimilation [46]. Cysteine does so by inhibiting serine acetyltransferase, which catalyzes the formation of O-acetylserine, the biosynthetic precursor of cysteine. In contrast, sulfide and thiosulfate bind the CysB transcriptional regulator, repressing the transcription of genes that facilitate sulfide biosynthesis or in the case of thiosulfate, its uptake from outside the cell. In E. coli, APS has been shown to inhibit Cbl-mediated transcription, preventing the induction of SSI genes that facilitate sulfur metabolism in the absence of inorganic sulfate [66]. Thus, APS may serve as a molecular barometer of sulfate assimilation that modulates gene expression in response to sulfate availability. While similar feedback mechanisms remain to be elucidated in Mtb, they are likely to play an important role in regulating sulfur metabolism. Consistent with this hypothesis, transcription of the cysDNC operon of Mtb increases in response to sulfur limitation and decreases following treatment with exogenous cysteine [22].
MSH is also likely to trigger transcriptional regulation of sulfur-metabolizing enzymes in Mtb. As mentioned previously, the alternative sigma factor SigR from S. coelicolor has been shown to modulate the biosynthesis of MSH in response to the intracellular MSH concentration [45], [67]. The current model of MSH regulation revolves around the redox-sensitive anti-sigma factor RsrA, which binds SigR in its reduced state, sequestering the sigma factor and preventing it from binding RNA polymerase (Figure 3). MSH facilitates this activity by maintaining RsrA in its reduced state. Thus, when MSH is abundant, RsrA remains active, suppressing MSH biosynthesis. However, when thiol-reactive toxins overwhelm the cell's MSH supply, RsrA is no longer maintained in its reduced form, freeing SigR to induce MSH biosynthesis. Given the importance of MSH in maintaining the reducing environment of the cytosol and promoting resistance to toxic oxidants [68], it is likely that a similar regulatory network is in place to regulate its biosynthesis in mycobacteria.
Identifying Nodes of Regulatory Convergence in Mtb Sulfur Metabolism
A global assessment of the transcriptional, biochemical, and molecular mechanisms of sulfur metabolism regulation described herein suggests nodes of regulatory convergence, or enzymes that are sensitive to multiple regulatory cues. These enzymes are apparently poised to tune flux through the sulfate assimilation pathway in response to substrate availability and a broad range of environmental signals. While many sulfur-metabolizing enzymes are promising drug targets (please see the review by Bhave et al. [6] for a comprehensive discussion), those that occupy key positions in the regulatory landscape may be particularly strong candidates for therapeutic intervention.
ATP sulfurylase and APS kinase, encoded by the cysDNC operon, are the most prominent regulatory nodes to emerge from this analysis. As illustrated in Figure 5, the cysD and cysNC genes are consistently upregulated by diverse environmental cues, including nutrient starvation [26], [27], hypoxia [23], [24], oxidative stress [21], [22], and cell wall stress [28], [29]. These genes are also induced upon phagocytosis [21], [25], a process that integrates many of these signals in vivo. Moreover, the catalytic activities of ATP sulfurylase and APS kinase make them particularly susceptible to biochemical regulatory cues. Together, these enzymes form the sulfate-activating complex (SAC) of Mtb, which converts free sulfate into PAPS [18]. Sulfate transporters, and possibly sulfatases, indirectly regulate this complex by determining sulfate availability in the cell. CysQ may also influence SAC activity by affecting the equilibrium between PAPS and APS. Finally, both cysteine and sulfate have been shown to modulate cysDNC transcription [22], indicating these genes are subject to small molecule regulation. In sum, ATP sulfurylase and APS kinase are highly regulated enzymes whose inhibition may critically impair Mtb sulfur metabolism.
Fig. 5. Venn diagram illustrating the convergent transcriptional regulation of <i>Mtb</i> sulfur metabolism genes by various conditions of environmental stress. 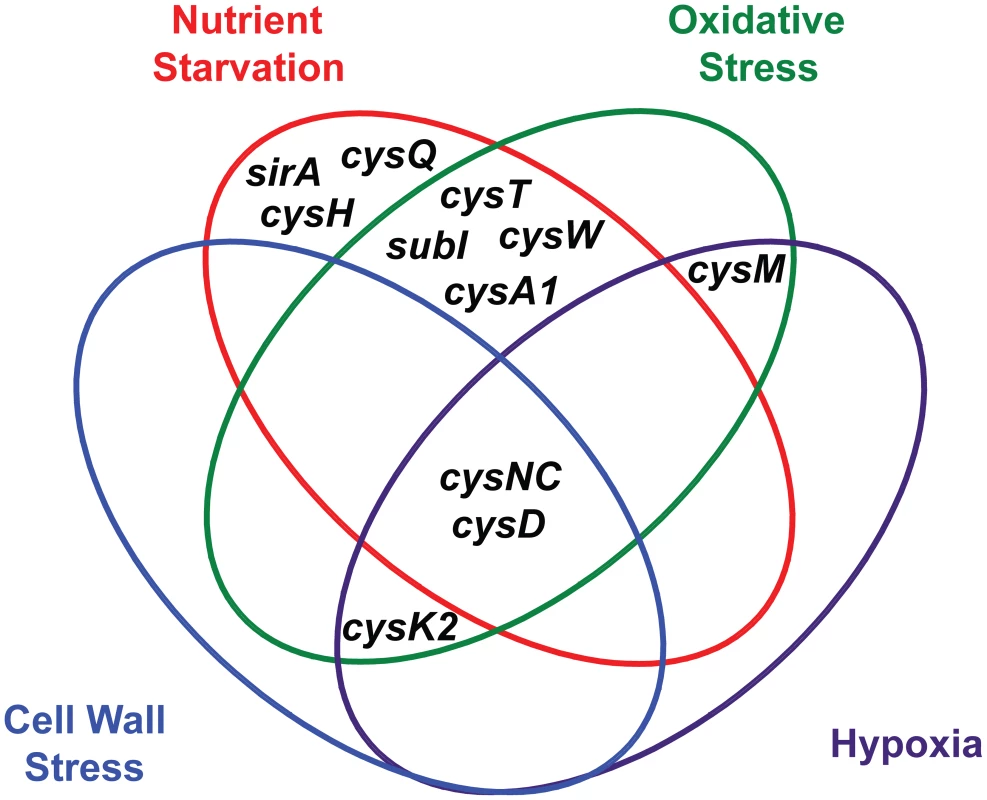
Notably, ATP sulfurylase, a heterodimeric enzyme with both GTPase (CysN) and sulfurylase (CysD) domains, possesses several unique features that make it a particularly compelling drug target [6]. Since this enzyme catalyzes the first committed step in the Mtb sulfate assimilation pathway [18], its inhibition would eliminate flux through the pathway. Further, significant structural, mechanistic, and genetic features (e.g., the presence of a GTPase domain; the fusion of cysN with cysC) distinguish ATP sulfurylase from its human counterpart, which may facilitate the discovery of selective inhibitors [6], [18], [69]. Consistent with these findings, CysD was classified as a high-confidence drug target by an in silico target identification program following an extensive series of systems-, sequence-, and structure-based analyses [70].
Additional regulatory nodes suggested by the combined transcriptional data (Figure 5) are cysK2, cysM, and the subI-cysTWA1 operon. Both cysK2 and cysM are annotated as putative O-acetyl-L-serine sulfhydrylases (OAS), enzymes that catalyze the PLP-dependent biosynthesis of L-cysteine from O-acetyl-L-serine and a sulfur donor such as sulfide [34]. In fact, a third gene, cysK1, encodes the true OAS of Mtb [71]. CysM has been shown to catalyze an alternate cysteine biosynthetic pathway that uses O-phosphoserine as a substrate [33], [51], while the role of CysK2 in sulfur metabolism remains an enigma. Both cysK2 and cysM are transcriptionally regulated by multiple environmental cues (Table 1) [21], [24], [29], [72]. Similarly, the subI-cysTWA1 operon, which encodes the primary sulfate transport system of Mtb, is sensitive to diverse stress conditions [20], [21], [26], [27]. CysT, CysW, and CysK2 have also been classified as high-confidence drug targets [70]. Thus, these enzymes warrant further biochemical investigation and may prove attractive targets for the inhibition of Mtb sulfur metabolism.
Conclusions and Perspectives
The sulfate assimilation pathway of Mtb is responsible for the biosynthesis of sulfur-containing metabolites that influence bacterial pathogenesis. The transcriptional regulation of this pathway in response to multifarious environmental cues, including those typically encountered in the phagosome, likely facilitates adaptation to host immune cells. In addition, several proteins that mediate the flux of sulfate through the pathway, such as sulfate permeases, sulfatases, and the phosphatase CysQ, modulate the biosynthesis of sulfur-containing compounds in response to the evolving metabolic demands of the cell. Finally, the potential for small molecule regulation of Mtb sulfur metabolism abounds; the metabolites produced by the sulfate assimilation pathway are themselves candidate regulators of this network, as evidenced by cysteine and MSH in other bacteria. Realizing the extent of this regulation remains an outstanding challenge in the field, as does identifying the transcriptional proteins that orchestrate this pathway's response to the bacterial environment. Characterization of CysK2 activity and the design of selective inhibitors targeting it and other nodes of regulatory convergence, particularly ATP sulfurylase, also warrant exploration. Addressing these aims will not only advance our understanding of sulfur metabolism in Mtb, but may also reveal a molecular linchpin whose inhibition could dismantle an essential metabolic pathway in this ubiquitous human pathogen.
Zdroje
1. World Health Organization Tuberculosis (TB). Available: www.who.int/tb/en/
2. GinsbergAMSpigelmanM 2007 Challenges in tuberculosis drug research and development. Nat Med 13 290 294
3. LinPLFlynnJL 2010 Understanding latent tuberculosis: a moving target. J Immunol 185 15 22
4. RussellDG 2007 Who puts the tubercle in tuberculosis? Nat Rev Microbiol 5 39 47
5. RussellDG 2001 Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol 2 569 577
6. BhaveDPMuseWB3rdCarrollKS 2007 Drug targets in mycobacterial sulfur metabolism. Infect Disord Drug Targets 7 140 158
7. SchelleMWBertozziCR 2006 Sulfate metabolism in mycobacteria. Chembiochem 7 1516 1524
8. GangadharamPRCohnMLMiddlebrookG 1963 Infectivity, pathogenicity and sulpholipid fraction of some Indian and British strains of tubercle bacilli. Tubercle 44 452 455
9. MougousJDSenaratneRHPetzoldCJJainMLeeDH 2006 A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103 4258 4263
10. GilleronMStengerSMazorraZWittkeFMariottiS 2004 Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med 199 649 659
11. MougousJDGreenREWilliamsSJBrennerSEBertozziCR 2002 Sulfotransferases and sulfatases in mycobacteria. Chem Biol 9 767 776
12. SenaratneRHDe SilvaADWilliamsSJMougousJDReaderJR 2006 5′-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol Microbiol 59 1744 1753
13. NewtonGLArnoldKPriceMSSherrillCDelcardayreSB 1996 Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J Bacteriol 178 1990 1995
14. BuchmeierNANewtonGLKoledinTFaheyRC 2003 Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. Mol Microbiol 47 1723 1732
15. JothivasanVKHamiltonCJ 2008 Mycothiol: synthesis, biosynthesis and biological functions of the major low molecular weight thiol in actinomycetes. Nat Prod Rep 25 1091 1117
16. RawatMJohnsonCCadizVAv-GayY 2007 Comparative analysis of mutants in the mycothiol biosynthesis pathway in Mycobacterium smegmatis. Biochem Biophys Res Commun 363 71 76
17. WilliamsSJSenaratneRHMougousJDRileyLWBertozziCR 2002 5′-Adenosinephosphosulfate lies at a metabolic branch point in mycobacteria. J Biol Chem 277 32606 32615
18. SunMAndreassiJL2ndLiuSPintoRTriccasJA 2005 The trifunctional sulfate-activating complex (SAC) of Mycobacterium tuberculosis. J Biol Chem 280 7861 7866
19. CarrollKSGaoHChenHStoutCDLearyJA 2005 A conserved mechanism for sulfonucleotide reduction. PLoS Biol 3 e250 doi:10.1371/journal.pbio.0030250
20. BoshoffHIMyersTGCoppBRMcNeilMRWilsonMA 2004 The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 279 40174 40184
21. SchnappingerDEhrtSVoskuilMILiuYManganJA 2003 Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med 198 693 704
22. PintoRTangQXBrittonWJLeyhTSTriccasJA 2004 The Mycobacterium tuberculosis cysD and cysNC genes form a stress-induced operon that encodes a tri-functional sulfate-activating complex. Microbiology 150 1681 1686
23. VoskuilMIViscontiKC Schoolnik GK 2004 Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb) 84 218 227
24. RustadTRHarrellMILiaoRShermanDR 2008 The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE 3 e1502 doi:10.1371/journal.pone.0001502
25. FontanPArisVGhannySSoteropoulosPSmithI 2007 Global transcriptional profile of Mycobacterium tuberculosis during THP-1 human macrophage infection. Infect Immun 76 717 725
26. HampshireTSonejiSBaconJJamesBWHindsJ 2004 Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis (Edinb) 84 228 238
27. BettsJCLukeyPTRobbLCMcAdamRADuncanK 2002 Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43 717 731
28. ManganelliRVoskuilMI Schoolnik GK, Smith I 2001 The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol Microbiol 41 423 437
29. ProvvediRBoldrinFFalcianiFPaluGManganelliR 2009 Global transcriptional response to vancomycin in Mycobacterium tuberculosis. Microbiology 155 1093 1102
30. RohdeKYatesRMPurdyGERussellDG 2007 Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev 219 37 54
31. AppelbergR 2006 Macrophage nutriprive antimicrobial mechanisms. J Leukoc Biol 79 1117 1128
32. PintoRHarrisonJSHsuTJacobsWRJrLeyhTS 2007 Sulfite reduction in mycobacteria. J Bacteriol 189 6714 6722
33. O'LearySEJurgensonCTEalickSEBegleyTP 2008 O-phospho-L-serine and the thiocarboxylated sulfur carrier protein CysO-COSH are substrates for CysM, a cysteine synthase from Mycobacterium tuberculosis. Biochemistry 47 11606 11615
34. SchnellRSchneiderG 2010 Structural enzymology of sulphur metabolism in Mycobacterium tuberculosis. Biochem Biophys Res Commun 396 33 38
35. BoshoffHIMizrahiVBarryCE3rd 2002 Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J Bacteriol 184 2167 2172
36. CriddleDNGilliesSBaumgartner-WilsonHKJaffarMChinjeEC 2006 Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem 281 40485 40492
37. TurnbullALSuretteMG 2008 L-Cysteine is required for induced antibiotic resistance in actively swarming Salmonella enterica serovar Typhimurium. Microbiology 154 3410 3419
38. HulloMFMartin-VerstraeteISoutourinaO 2010 Complex phenotypes of a mutant inactivated for CymR, the global regulator of cysteine metabolism in Bacillus subtilis. FEMS Microbiol Lett 309 201 207
39. SoutourinaODubracSPoupelOMsadekTMartin-VerstraeteI 2010 The pleiotropic CymR regulator of Staphylococcus aureus plays an important role in virulence and stress response. PLoS Pathog 6 e1000894 doi:10.1371/journal.ppat.1000894
40. MillerCCRawatMJohnsonTAv-GayY 2007 Innate protection of Mycobacterium smegmatis against the antimicrobial activity of nitric oxide is provided by mycothiol. Antimicrob Agents Chemother 51 3364 3366
41. NewtonGLBuchmeierNFaheyRC 2008 Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol Mol Biol Rev 72 471 494
42. VilchezeCAv-GayYAttarianRLiuZHazbonMH 2008 Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol Microbiol 69 1316 1329
43. MehraSKaushalD 2009 Functional genomics reveals extended roles of the Mycobacterium tuberculosis stress response factor sigmaH. J Bacteriol 191 3965 3980
44. ParkSTKangCMHussonRN 2008 Regulation of the SigH stress response regulon by an essential protein kinase in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 105 13105 13110
45. ParkJHRoeJH 2008 Mycothiol regulates and is regulated by a thiol-specific antisigma factor RsrA and sigma(R) in Streptomyces coelicolor. Mol Microbiol 68 861 870
46. KredichNM 1992 The molecular basis for positive regulation of cys promoters in Salmonella typhimurium and Escherichia coli. Mol Microbiol 6 2747 2753
47. Iwanicka-NowickaRHryniewiczMM 1995 A new gene, cbl, encoding a member of the LysR family of transcriptional regulators belongs to Escherichia coli cys regulon. Gene 166 11 17
48. StecEWitkowska-ZimnyMHryniewiczMMNeumannPWilkinsonAJ 2006 Structural basis of the sulphate starvation response in E. coli: crystal structure and mutational analysis of the cofactor-binding domain of the Cbl transcriptional regulator. J Mol Biol 364 309 322
49. MougousJDPetzoldCJSenaratneRHLeeDHAkeyDL 2004 Identification, function and structure of the mycobacterial sulfotransferase that initiates Sulfolipid-1 biosynthesis. Nat Struct Mol Biol 11 721 729
50. BurnsKEBaumgartSDorresteinPCZhaiHMcLaffertyFW 2005 Reconstitution of a new cysteine biosynthetic pathway in Mycobacterium tuberculosis. J Am Chem Soc 127 11602 11603
51. AgrenDSchnellROehlmannWSinghMSchneiderG 2008 Cysteine synthase (CysM) of Mycobacterium tuberculosis is an O-phosphoserine sulfhydrylase: evidence for an alternative cysteine biosynthesis pathway in mycobacteria. J Biol Chem 283 31567 31574
52. RuckertCMilseJAlbersmeierAKochDJPuhlerA 2008 The dual transcriptional regulator CysR in Corynebacterium glutamicum ATCC 13032 controls a subset of genes of the McbR regulon in response to the availability of sulphide acceptor molecules. BMC Genomics 9 483
53. WooffEMichellSLGordonSVChambersMABardarovS 2002 Functional genomics reveals the sole sulphate transporter of the Mycobacterium tuberculosis complex and its relevance to the acquisition of sulphur in vivo. Mol Microbiol 43 653 663
54. ZolotarevASUnnikrishnanMShmuklerBEClarkJSVandorpeDH 2008 Increased sulfate uptake by E. coli overexpressing the SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis. Comp Biochem Physiol A Mol Integr Physiol 149 255 266
55. BojarovaPWilliamsSJ 2008 Sulfotransferases, sulfatases and formylglycine-generating enzymes: a sulfation fascination. Curr Opin Chem Biol 12 573 581
56. CarlsonBLBallisterERSkordalakesEKingDSBreidenbachMA 2008 Function and structure of a prokaryotic formylglycine generating enzyme. J Biol Chem 283 20117 20125
57. HossainMMKawarabayasiYKimuraMKakutaY 2009 Expression and functional analysis of a predicted AtsG arylsulphatase identified from Mycobacterium tuberculosis genomic data. J Biochem 146 767 769
58. HatziosSKIavaroneATBertozziCR 2008 Rv2131c from Mycobacterium tuberculosis is a CysQ 3′-phosphoadenosine-5′-phosphatase. Biochemistry 47 5823 5831
59. PiNHoangMBGaoHMougousJDBertozziCR 2005 Kinetic measurements and mechanism determination of Stf0 sulfotransferase using mass spectrometry. Anal Biochem 341 94 104
60. NeuwaldAFKrishnanBRBrikunIKulakauskasSSuziedelisK 1992 cysQ, a gene needed for cysteine synthesis in Escherichia coli K-12 only during aerobic growth. J Bacteriol 174 415 425
61. ZhangJBiswasI 2009 3′-Phosphoadenosine-5′-phosphate phosphatase activity is required for superoxide stress tolerance in Streptococcus mutans. J Bacteriol 191 4330 4340
62. UngKSAv-GayY 2006 Mycothiol-dependent mycobacterial response to oxidative stress. FEBS Lett 580 2712 2716
63. RodriguezGMSmithI 2003 Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol Microbiol 47 1485 1494
64. KrawczykJKohlTAGoesmannAKalinowskiJBaumbachJ 2009 From Corynebacterium glutamicum to Mycobacterium tuberculosis--towards transfers of gene regulatory networks and integrated data analyses with MycoRegNet. Nucleic Acids Res 37 e97
65. FanFVettingMWFrantomPABlanchardJS 2009 Structures and mechanisms of the mycothiol biosynthetic enzymes. Curr Opin Chem Biol 13 451 459
66. BykowskiTvan der PloegJRIwanicka-NowickaRHryniewiczMM 2002 The switch from inorganic to organic sulphur assimilation in Escherichia coli: adenosine 5′-phosphosulphate (APS) as a signaling molecule for sulphate excess. Mol Microbiol 43 1347 1358
67. NewtonGLFaheyRC 2008 Regulation of mycothiol metabolism by sigma(R) and the thiol redox sensor anti-sigma factor RsrA. Mol Microbiol 68 805 809
68. BuchmeierNANewtonGLFaheyRC 2006 A mycothiol synthase mutant of Mycobacterium tuberculosis has an altered thiol-disulfide content and limited tolerance to stress. J Bacteriol 188 6245 6252
69. MougousJDLeeDHHubbardSCSchelleMWVocadloDJ 2006 Molecular basis for G protein control of the prokaryotic ATP sulfurylase. Mol Cell 21 109 122
70. RamanKYeturuKChandraN 2008 targetTB: a target identification pipeline for Mycobacterium tuberculosis through an interactome, reactome and genome-scale structural analysis. BMC Syst Biol 2 109
71. SchnellROehlmannWSinghMSchneiderG 2007 Structural insights into catalysis and inhibition of O-acetylserine sulfhydrylase from Mycobacterium tuberculosis. Crystal structures of the enzyme alpha-aminoacrylate intermediate and an enzyme-inhibitor complex. J Biol Chem 282 23473 23481
72. VoskuilMI 2004 Mycobacterium tuberculosis gene expression during environmental conditions associated with latency. Tuberculosis (Edinb) 84 138 143
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



