-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaVaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
As is apparent in many fields of science and medicine, the new biology, and particularly new high-throughput genetic sequencing and transcriptomic and epigenetic technologies, are radically altering our understanding and views of science. In this article, we make the case that while mostly ignored thus far in the vaccine field, these changes will revolutionize vaccinology from development to manufacture to administration. Such advances will address a current major barrier in vaccinology—that of empiric vaccine discovery and development, and the subsequent low yield of viable vaccine candidates, particularly for hyper-variable viruses. While our laboratory's data and thinking (and hence also for this paper) has been directed toward viruses and viral vaccines, generalization to other pathogens and disease entities (i.e., anti-cancer vaccines) may be appropriate.
Published in the journal: . PLoS Pathog 7(12): e32767. doi:10.1371/journal.ppat.1002344
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002344Summary
As is apparent in many fields of science and medicine, the new biology, and particularly new high-throughput genetic sequencing and transcriptomic and epigenetic technologies, are radically altering our understanding and views of science. In this article, we make the case that while mostly ignored thus far in the vaccine field, these changes will revolutionize vaccinology from development to manufacture to administration. Such advances will address a current major barrier in vaccinology—that of empiric vaccine discovery and development, and the subsequent low yield of viable vaccine candidates, particularly for hyper-variable viruses. While our laboratory's data and thinking (and hence also for this paper) has been directed toward viruses and viral vaccines, generalization to other pathogens and disease entities (i.e., anti-cancer vaccines) may be appropriate.
Introduction
The goal in vaccinology is to discover, develop, and deploy highly immunogenic and safe vaccines that protect against infectious and non-infectious (i.e., cancers) diseases in essentially 100% of the population. While admirable, such a goal, to date, fails because of both pathogen and host variability. For hyper-variable viral pathogens like HIV, HCV, rhinovirus, and others, we have been unable to discover and develop highly immunogenic and protective vaccine candidates. This is true too for other highly complex pathogens such as bacteria (i.e., tuberculosis) and parasites (i.e., malaria). Host variability is evident in the multiplicity of immune response genes that encode >1012 products necessary for generating immune responses (i.e., antibodies, T cell receptors [TCRs], etc.), and the estimated diversity of human leukocyte antigen (HLA) haplotypes (estimated at >1013), allowing humans an almost limitless immune response capability [1].
Thus, both pathogen and host variability barriers make it difficult to induce protective immune responses to vaccine antigens in 100% of the population—at least for most of the pathogens of interest for vaccine public health needs such as HIV, HBV, HCV, measles, influenza, and others.
Current Vaccine Development
We propose that an additional approach to this dilemma resides in changing the paradigm and conceptual framework through which we develop new vaccines. For example, from the 1700s through the late 1990s, vaccine development was primarily characterized by an empiric “isolate – inactivate/attenuate – inject” approach. While successful in developing most of the vaccines we use today, it fails in the face of hyper-variable and highly complex pathogens and is an approach now limited by a lack of innovation, a predominant single mode of administration (injection), and a lack of directed adjuvants to overcome poor immunogenicity of the identified antigen. From a policy viewpoint, today's vaccines are administered to everyone at the same dose (“one dose fits all”) as a public health approach that assumes that everybody is at risk for every pathogen with equally devastating risks of complications. Too, our past and current approach to vaccines is prophylactic only (we have no therapeutic vaccines), is overwhelmingly aimed at childhood diseases (ignoring demographic trends of aging populations in every developed economy), and at least in the US, is exclusively a private sector, big Pharma manufacturing approach.
Vaccinomics and Directed Vaccine Development
Our laboratory has advocated for a new approach to vaccine discovery characterized as a “discover – validate – characterize – deploy” paradigm based on the foundations of vaccinomics and personalized vaccinology [2]–[4]. This approach moves away from a focus on the smaller details of immune function and advocates pursuing an understanding of the immune system as a whole in order to improve and expand upon empirical vaccine science. Furthermore, the approach is personalized in that it emphasizes a tiered risk and vaccination approach for new vaccines, multiple avenues of vaccine administration that take advantage of new findings (e.g., in mucosal immunology allowing for oral, transcutaneous, depot, and mucosal delivery), multiple highly specific vaccine adjuvants, directed vaccine development using systems biology and computational approaches, and private, public, and academic partnerships in the development of new vaccine candidates. An initial aspect of this new approach is the concept of reverse vaccinology, which uses sophisticated computer analysis of genomic data to characterize pathogen antigens and eliminate those with human homology. This is followed by careful screening of the remaining antigens for immunogenicity and eventual use in new vaccine products [5], [6]. For example, reverse immunology was used to create a recombinant protein containing nine different Th epitopes that has been used to enhance the hemophilus influenza type b oligosaccharide vaccine [7]. A large number of reverse immunology studies have focused on the characterization of T cell responses to vaccinia virus and have identified hundreds of CD4 and CD8 T cell epitopes. Other studies have carefully examined the vaccinia transciptome. [8]. Vaccinomics seeks to better and more fully integrate these findings, correlating humoral and cellular immune measures with transcriptomic, genomic, and proteomic data to gain a greater understanding of viral immunity. One such integrated study has uncovered complex interactions between CD4, CD8, and humoral responses to vaccinia virus [9].
Figure 1 outlines some of the important features that might be employed by a vaccinomics approach. It should be noted that not all of these features may be needed for a given vaccine, and those features that are needed may be prioritized differently depending on the unique constraints imposed by the disease, vaccine product, and/or population to be protected. For example, cancer vaccines have benefited greatly from advanced bioinformatics, reverse immunology, and epitope discovery to develop very personalized products. On the other hand, new vaccines against leishmaniasis or Japanese encephalitis for use in developing countries where distribution and inoculation are handled by public/private organizations with access to at-risk populations and familiarity with the local culture and society may require the development of a stable product not requiring a cold chain and transdermal application such that advanced training is not required for administration.
Fig. 1. New approach to vaccine discovery and development. 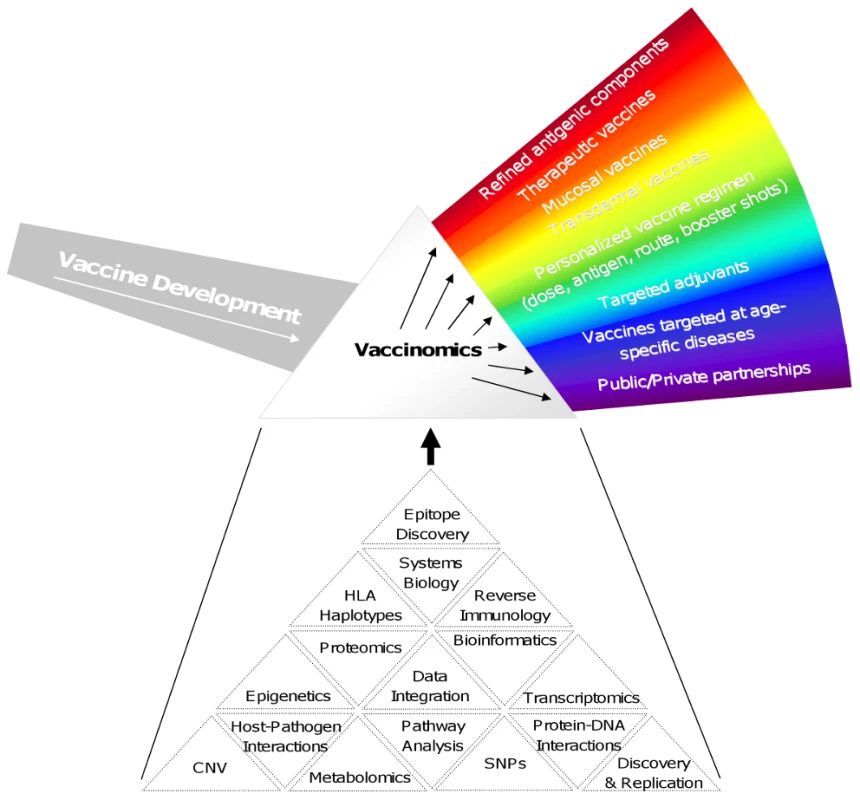
Figure 1 illustrates the differences between the one-size-fits-all approach of empiric vaccine development with a more directed and personal approach that relies upon vaccinomics and high-dimensional “omics” technologies. By analogy, empiric vaccine development represents the undifferentiated light entering the prism from the left. Individual aspects of directed vaccine development can be seen when viewed through the prism of vaccinomics. Several examples of these components are illustrated in the rainbow on the right side of the figure. These aspects may or may not be appropriate for all vaccines and are used here to illustrate the wide range of possibilities that a “discover – validate – characterize – deploy” approach allows one to independently investigate, optimize, and fully utilize. Below the vaccinomics prism are listed some examples (by no means complete or definitive) representing a range of potential components that can be assembled into a comprehensive, systems-level examination of infection/vaccination of a given pathogen. Please refer to the text for examples of how different components might be used in the development of specific vaccines. The need for and importance of new advances in vaccinology such as those above may not be apparent to all. Above and beyond the obvious value in decreasing (or eliminating in the case of smallpox, and hopefully soon polio and measles) morbidity and mortality due to infectious diseases, economic benefits accrue to healthy populations, national security may be enhanced, bioweapons development countered, and new insights into vaccine immunology generalized into other fields. As a result of these compelling arguments for vaccines, we deliver a series of vaccines to every human being on earth, multiple times over a lifetime. The importance of this is that there is nothing comparable in medicine that so touches every single human being. Indeed, this public health approach toward vaccine use has contributed to a doubling of the lifespan in the US over the last century by the control of infectious diseases, and the supportive role played by vaccines. But, as mentioned, it has been a one-size-fits-all approach. In the 21st century we may now ask, is such an approach, for our time and age, informed by science?
A variety of factors impact the heterogeneity and inter-individual variations in vaccine-induced immune responses. These include factors such as gender [10], age [11], ethnicity [12], vaccine dose [13], vaccine storage/cold chain [14], immune system function/integrity [15], size (body mass index [BMI]) [16], smoking [17], and others. Logically, genetics play an important—and defining—role in vaccine response. We increasingly understand the role of genetic causes of heterogeneity in treatment effects with drugs, but similar work in the field of vaccinology has lagged. One investigator has observed, “Just as pharmacogenetics has suggested ways of designing drugs to minimize population variability, understanding mechanisms of immunogenetic variation may lead to new vaccines designed to minimize immunogenetically based failure” [18]. This naturally leads to such questions as, “why do immune responses to biologics/vaccines vary among healthy individuals? “And what explains this heterogeneity?” “Could the answers to these questions be leveraged in reverse engineering new vaccine candidates?”
The Immune Response Network Theory
While we readily accept that genetic variation in TCR genes, antibody genes, and HLA loci all contribute to the differential ability of the host to respond to pathogens, these are not the only genes that impact vaccine immunity. Host genetic influences on inter-individual variability can also occur as a result of polymorphisms in genes involved in the generation of the immune response, including viral receptors, Toll-like and other pattern recognition receptors, signaling molecules, cytokine and cytokine receptor genes, Gm/Km genes, perforin and granzymes, and death receptors, as well as many others. In recognizing this, our laboratory developed the “Immune Response Network Theory,” which states that the response to a vaccine is “the cumulative result of interactions driven by a host of genes and their interactions, and is theoretically predictable” [19], [20]. This theory is different than Jerne's idiotype network theory stating that the antigen recognition site of one antibody can in turn serve as an antigen stimulating the production of anti-idiotype antibodies, and that these networks of antibodies/anti-idiotypic antibodies serve to positively and negatively regulate immune function [21]. “The basic genetic elements of the immune response network includes genes activating/suppressing immune responses, the dominance profile of a given gene or polymorphism, epigenetic modifications of genes, the influence of signaling genes, innate response genes, gene-gene interactions, and genes for other host response factors” [2]. Understanding the complex interplay of these networks and pathways as a coherent system allows one to build predictive models, anticipate possible side effects, and observe synergistic outcomes that cannot be foreseen with narrowly focused studies concentrating on single genes or proteins or even single cell types. Understanding the key initial events in the immune response to pathogen infection allows us to identify viral ligands responsible for cell binding and entry, innate receptors responsible for pathogen detection, innate pathways mediating protective responses specific for a given pathogen, host pathways usurped by viral machinery, and pathogen epitopes targeted by T and B cells, and the interplay between T helper lymphocytes and B cells or cytotoxic T cells necessary for optimal humoral and cell-mediated responses. In turn, this information allows for the identification of adjuvants stimulating the appropriate innate receptors and antiviral pathways, attenuation strategies for the pathogen of interest, the appropriate selection of viral epitopes for subunit vaccines, vaccine products that omit the viral proteins responsible for pathogen-induced damage and suppress the host pathways responsible for immunopathology, the effects that different routes of administration have on the immune response, and the appropriate dose/route/timing of immunizations to properly elicit strong immune memory.
As we have noted, the mechanisms for differential gene-based effects can include “differential binding, processing, and expression/presentation of antigenic peptides, a differential range of presented peptides (genetic restriction), altered secretion patterns (cytokines), altered transcription of important genes (signaling molecules) and gene products, altered binding of virus/antigens by membrane-based receptors (TLR, other), differential receptor function, expression, affinities, epigenetics, and of course, others” [22]. Further, our laboratory developed the term “vaccinomics” to encompass the integration of a systems biology approach with immunogenetics, immunogenomics, immune profiling, and functional SNP studies in order to understand and predict vaccine-induced immune responses. Using these concepts we have predicted “a new golden era of personalized Predictive Vaccinology” whereby we abandon a “one size and dose fits all vaccine approach,” predict whether to give a vaccine based on likelihood of response (and perhaps need), predict the likelihood of a significant adverse event to a vaccine, predict the number of doses likely to be needed to induce a response to a vaccine (HBV, HPV, measles examples), and design/develop new vaccines at the individual, gender-specific, race-specific, or sub-population levels for groups with identifiable and specific genetic restrictions [3], [4], [23].
Genetic Control of Measle Vaccine Response
As examples, we have focused our work in vaccinomics on the study of measles, rubella, smallpox, and influenza vaccines. In order to understand the role of genetic (host) variation in inter-individual vaccine-specific immune responses, we began by performing twin studies (n = 100 twins) to separate environmental and genetic influences, to determine the influence of genetic factors relating to variability in immune response, to determine the proportion of variation attributable to specific genes, and to determine heritability (the ratio of genetic variance to total variance) [24]. In this study we determined that the heritability of measles vaccine was 89% (p < 0.0001) [25]. We next studied a cohort of healthy schoolchildren, all immunized with one dose of MMR-II (medical record documentation), with no circulating measles in the community since 1980 (the earliest year of birth) [26], [27]. The results are noted in Figure 2, which demonstrates the diversity of inter-individual antibody response to vaccine among otherwise healthy schoolchildren.
Fig. 2. Distribution of measles vaccine–induced antibody levels. 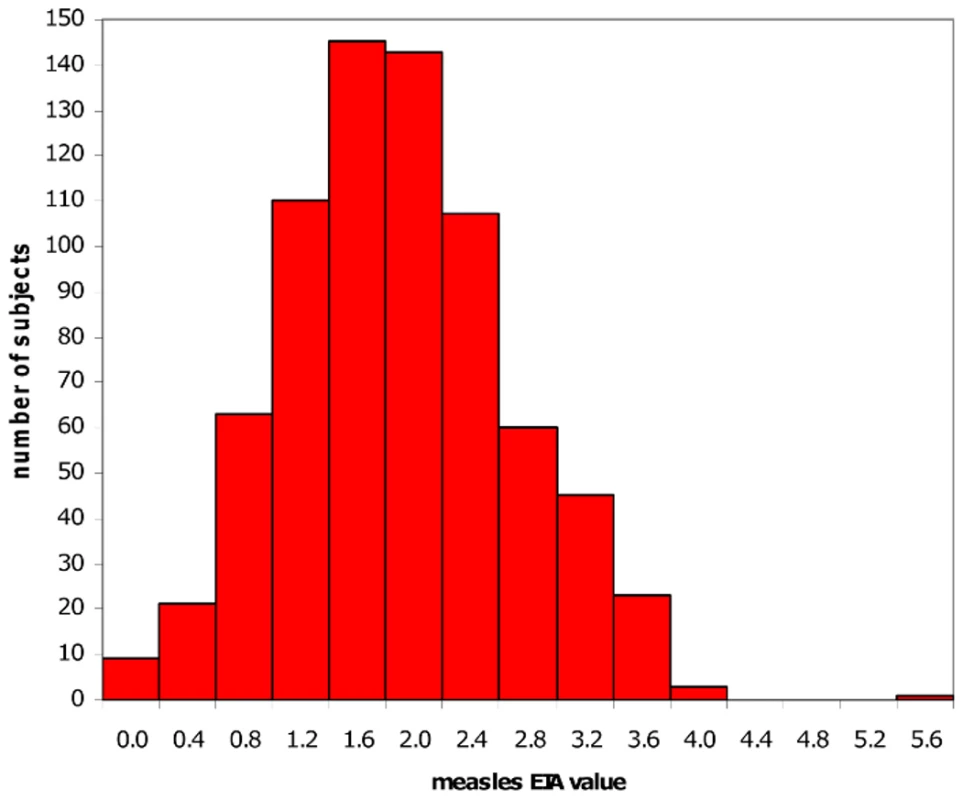
This graph represents the distribution of antibody levels determined by an EIA assay on healthy grade-school children immunized with a single dose of MMR-II vaccine. The inter-individual variation in antibody levels among this healthy cohort illustrates the importance of determining the mechanisms for heterogeneity in vaccine response. Among the above individuals who were vaccine non-responders, we re-immunized, and repeated antibody testing ≥ 6 weeks later. One hundred and six children (81.5%) became seropositive, and 24 (18.5%) remained seronegative [28]. We then re-examined our candidate gene associations in individuals who had received 2 doses of measles vaccine, and our previously detectable class I and II HLA effects were no longer detectable except for B*4403 [22]—a similar finding in studies of hepatitis B vaccine non-responders. Similarly, two doses of measles vaccine appeared to overcome HLA homozygosity associations with lower measles-specific antibody and cytokine levels detected following one dose of MMR vaccine [29], [30]. These findings illustrate that there is HLA-restricted recognition of measles virus epitopes with detectable impacts on immunity, and that through an as yet unclear mechanism, additional doses of vaccine may help to overcome this genetic restriction, including non-responsiveness to measles vaccine.
Our population-based MMR vaccine studies also determined that host gene polymorphisms are associated with measurable inter-individual variations in measles vaccine–induced immunity. Examples of such gene SNPs include HLA, measles virus binding CD46 and SLAM receptors, cytokine and vitamin receptors, as well as innate antiviral effector genes, including Toll-like receptors (TLRs) and their signaling genes, which play a significant role in contributing to variations in the immunity to measles due to genetic polymorphisms [31]–[34]. The adaptive immune response after measles vaccination is influenced in part by HLA gene polymorphisms. In fact, the occurrence or lack of specific HLA alleles and haplotypes (or supertypes) may significantly influence both humoral and cellular immune responses to a vaccine. Furthermore, our studies have demonstrated that genetic polymorphisms in measles virus receptor genes, pattern recognition receptor genes, genes controlling innate antiviral responses, and cytokines and cytokine receptor genes are associated with variations in measles vaccine–induced immune outcomes.
Associations with Measles-Specific Humoral Immunity
We found several HLA alleles (B*3503, DRB1*0701, and DQA1*0201) and haplotypes (A*29-C*16-B*44 and DRB1*15/15-DQB1*06-DPB1*03) with associations with measles-specific neutralizing antibody levels in two independent population-based studies. Individual genetic variants in the CD46 (rs11118580 and rs2724384) and SLAM (rs164288) genes that appear to modulate antibody responses to measles vaccine were also identified [33]. Increased carriage of major allele variants for coding SNPs in the TLR2 (rs3804100) and TLR4 (rs5030710) genes were associated with a dose-related increase and a dose-related decrease in measles antibody levels, respectively [34]. Recently, we also replicated a previously discovered association of a functional IL12B genetic variant rs3212227 with inter-individual variations in measles-specific antibody levels [35]. Genetic variants within the RIG-I gene, including a coding polymorphism (rs3205166), were associated as single-SNPs and in haplotype-level analysis, with measles antibody variations [36].
Associations with Measles-Specific Cellular Immunity
In a separate study we successfully replicated associations with two of the above mentioned measles virus receptor SNPs (CD46 rs2724384 and SLAM rs164288) and variations in measles antibody and IFN-γ Elispot responses, respectively [37]. A replicated CD46 polymorphism (rs2724384) also demonstrated associations with measles-specific IL-6 (p = 0.02), IFN-α (p = 0.007), and TNF-α (p = 0.0007) responses. Two previously reported promoter IL10 and IL2 SNPs (rs1800890 and rs2069762) demonstrated associations with measles-specific cellular response (p < 0.03) [38]. A different polymorphism (rs11265452) in the SLAM gene previously associated with measles antibody levels (p = 0.04) exhibited a significant association with measles-specific IL-10 production (p = 0.0008) [37]. Understanding the functional or mechanistic consequences of genetic variations such as those above on immune-response variations could assist in directing new vaccine design, and allows us to generate and test new hypotheses applicable to developing new measles vaccine candidates.
Additional Examples of Directed Vaccine Development
Taking these concepts further, hepatitis B vaccine serves as a useful example. Both HLA polymorphisms and cytokine SNPs have been found to be associated with hepatitis B vaccine non-response [39]. This information could be utilized by developing a candidate vaccine that included both cytokine adjuvants to overcome genetic restriction, and a peptide “cocktail” that could circumvent known immunogenetic restrictions, and investigators have begun such development [40], [41]. Similarly, we have previously reported a SNP in the SLAM receptor gene associated with a 4-fold decrease in measles antibody levels [33]. While mechanistic studies are ongoing, it is logical that this SNP may interfere with the ability of the measles vaccine virus to bind to its receptor, and thereby perturb the development of a protective immune response. One could imagine a candidate vaccine virus designed to allow binding regardless of the presence or absence of such a receptor polymorphism. Such a vaccinomics approach could result in a candidate vaccine that leads to protective immune responses regardless of the presence of such a polymorphism. As a further example, such an approach led to the identification of the CCR5 deletion mutation in the coding region of the CCR5 HIV receptor. This information can be utilized in the development of novel therapeutic drugs and vaccines [42]. For example, a subunit vaccine containing the CCR5 binding determinants of gp120 could be created to facilitate the formation of viral neutralizing antibody responses. In addition, adjuvants such as CpG or MPL-A could differentially activate TLRs to circumvent restrictions in other receptors [43].
Other limitations in the development of new vaccines for measles and other infectious pathogens include a lack of understanding of molecular mechanisms of vaccine-induced adaptive immunity. While we understand that viral peptides are processed and presented in the context of class I and II HLA molecules, this has generally not informed the specific design of new vaccine candidates. Our laboratory has used this information to successfully identify 13 novel naturally processed class II HLA-DRB1*0301 measles virus peptides [44], [45]. The development of a high performance mass spectrometry analytic approach also allowed us to identify 116 naturally processed and presented class I (A*0201, B*1501 and C*03) peptides derived from vaccinia virus [46], [47]. Recently, we also isolated 17 naturally processed avian influenza H5N1 peptides from the class I A*0201 peptide binding grove (P. Tosh, I. Ovsyannikova, G. Poland, unpublished data). Data on specific immunogenic peptides (and adjuvants) such as these become important in the design of future vaccines to combat infectious diseases, including measles, influenza, smallpox, and other pathogens [48].
Conclusion
Our laboratory has used the live, attenuated measles, mumps, rubella, and vaccinia vaccine viruses as models for our work and development of the vaccinomics approach. After two decades of work with measles vaccine virus we have determined that:
-
Almost 90% of measles vaccine response heterogeneity is explainable genetically
-
Polymorphisms of specific immune response genes significantly influence measles vaccine–induced immunity
-
Vaccine-induced immune responses can be profiled (and in the near future predicted)
-
Naturally processed and presented immunogenic peptides can be identified and sequenced, and represent a novel method of vaccine candidate discovery
The next step in the development of vaccinomics is to understand immune “signature profiles” from a systems biology perspective in order to develop vaccine response “markers” in support of personalized vaccinology, and to inform new vaccine development. An excellent example of this concept is the identification of a gene signature including C1QB and EIF2AK4, which correlated with and predicted CD8+ T cell responses to the yellow fever vaccine with a high degree of accuracy [49]. The authors also identified a separate predictive signature of neutralizing antibody response that included the B cell growth factor TNFRS17. Yet another example of predictive immune profiling has been demonstrated for influenza vaccination. The expression levels of CAMKIV (a calmodulin-dependent protein kinase involved in neural functions as well as stem cell maintenance and T cell development) at day 3 following vaccination with TIV is inversely correlated with antibody titers at the peak of the immune response [50]. The development of these predictive signatures provides significant insights into the generation of vaccine-induced immune responses, and may serve as useful biomarkers for the testing of novel vaccine candidates. The knowledge gained from these immune-profiling studies may indicate appropriate adjuvants or routes of administration that can be coupled with mass-spectrometry approaches to isolating and identifying highly immunogenic viral peptides, allow for peptide-based vaccine development (an area of vaccine research that currently suffers from poor immunogenicity), and allow us to anticipate novel, directed development (rather than an empiric approach) of a plethora of new candidate vaccines informed by genotype:phenotype associations, the role of epigenetics and complementarity, and other future advances.
We believe that the future of vaccine development, utilizing the tools of vaccinomics and predictive vaccinology, is such that the science will move us to abandon a “one size and dose fits all empiric vaccine approach,” predict vaccine response and the possibility of a significant adverse response to a vaccine, predict the number of doses likely to be needed to induce a response to a vaccine, and direct us toward a science-based directed design/develop paradigm for novel vaccine candidates. In turn, abandoning the empiric approach of vaccine development, and moving toward a new paradigm of “discover – validate – characterize – deploy” is likely to hold promise in the development of vaccine candidates for hyper-variable pathogens, and overcome the current one-size-fits-all approach that leads to substantial inter-individual vaccine responses, vaccine non-response, increased costs, and substantial barriers to the development of novel vaccine candidates.
Zdroje
1. BrusicVAugustJT 2004 The changing field of vaccine development in the genomics era. Pharmacogenomics 5 597 600
2. PolandGAOvsyannikovaIGJacobsonRMSmithDI 2007 Heterogeneity in vaccine immune response: The role of immunogenetics and the emerging field of vaccinomics. Clinical Pharmacology and Therapeutics 82 653 664
3. PolandGAOvsyannikovaIGJacobsonRM 2008 Personalized vaccines: The emerging field of vaccinomics. Expert Opinion on Biological Therapy 8 1659 1667
4. PolandGA 2007 Pharmacology, vaccinomics, and the 2nd golden age of vaccinology. Clinical Pharmacology and Therapeutics 82 623 626
5. SetteARappuoliR 2010 Reverse vaccinology: developing vaccines in the era of genomics. Immunity 33 530 541
6. HeYRappuoliRDe GrootASChenRT 2010 Emerging vaccine informatics. J Biomed Biotechnol 2010 218590
7. FalugiFPetraccaRMarianiMLuzziEManciantiS 2001 Rationally designed strings of promiscuous CD4(+) T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: a model for new conjugate vaccines. Eur J Immunol 31 3816 3824
8. AssarssonEGreenbaumJASundstromMSchafferLHammondJA 2008 Kinetic analysis of a complete poxvirus transcriptome reveals an immediate-early class of genes. Proc Natl Acad Sci U S A 105 2140 2145
9. MoutaftsiMTscharkeDCVaughanKKoelleDMSternL 2010 Uncovering the interplay between CD8, CD4 and antibody responses to complex pathogens. Future Microbiol 5 221 239
10. GreenMSShohatTLermanYCohenDSleponR 1994 Sex differences in the humoral antibody response to live measles vaccine in young adults. International Journal of Epidemiology 23 1078 1081
11. NairNGansHLew-YasukawaLLong-WagarACArvinA 2007 Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J Infect Dis 196 1339 1345
12. HsuLCLinSRHsuHMChaoWHHsiehJT 1996 Ethnic differences in immune responses to hepatitis B vaccine. American Journal of Epidemiology 143 718 724
13. CouchRBWinokurPBradyRBelsheRChenWH 2007 Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 25 7656 7663
14. HaworthEABooyRStirzakerLWilkesSBattersbyA 1993 Is the cold chain for vaccines maintained in general practice? British Medical Journal 307 242 244
15. GaucherDTherrienRKettafNAngermannBRBoucherG 2008 Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 205 3119 3131
16. MiddlemanABAndingRTungC 2010 Effect of needle length when immunizing obese adolescents with hepatitis B vaccine. Pediatrics 125 e508 e512
17. WinterAPFollettEAMcIntyreJStewartJSymingtonIS 1994 Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine 12 771 772
18. SpielbergSP 1998 Therapeutics and toxicology. Current Opinion in Pediatrics 10 201 202
19. PolandGAOvsyannikovaIGJacobsonRMSmithDI 2007 Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin Pharmacol Ther 82 653 664
20. PolandGAOvsyannikovaIGJacobsonRM 2009 Application of pharmacogenomics to vaccines. Pharmacogenomics 10 837 852
21. JerneNK 1974 Towards a network theory of the immune system. Ann Immunol (Paris) 125C 373 389
22. PolandGAOvsyannikovaIGJacobsonRM 2008 Vaccine immunogenetics: bedside to bench to population. Vaccine 26 6183 6188
23. HaralambievaIHPolandGA 2010 Vaccinomics, predictive vaccinology and the future of vaccine development. Future Microbiol 5 1757 1760 10.2217/fmb.10.146 [doi]
24. JacobsonRMOvsyannikovaIGTargonskiPVPolandGA 2007 Studies of twins in vaccinology. Vaccine 25 3160 3164
25. TanPLJacobsonRMPolandGAJacobsenSJPankratzSV 2001 Twin studies of immunogenicity - determining the genetic contribution to vaccine failure. Vaccine 19 2434 2439
26. PolandGAJacobsonRMVierkantRAColbourneSAThampyAM 2002 Effect of differing immunization policies on circulating measles antibody levels in US and Canadian children. Mayo Clin Proc 77 446 451
27. PolandGAJacobsonRMColbourneSAThampyAMLipskyJJ 1999 Measles antibody seroprevalence rates among immunized Inuit, Innu and Caucasian subjects. Vaccine 17 1525 1531
28. PolandGAJacobsonRMThampyAMColbourneSAWollanPC 1997 Measles re-immunization in children seronegative after initial immunization. Journal of the American Medical Association 277 1156 1158
29. St.SauverJLDhimanNOvsyannikovaIGJacobsonRMVierkantRA 2005 Extinction of the human leukocyte antigen homozygosity effect after two doses of the measles-mumps-rubella vaccine. Human Immunology 66 788 798
30. OvsyannikovaIGDhimanNJacobsonRMVierkantRAPankratzVS 2007 HLA homozygosity does not adversely effect measles vaccine-induced cytokine responses. Virology 364 87 94
31. OvsyannikovaIGPankratzVSVierkantRAJacobsonRMPolandGA 2006 Human leukocyte antigen haplotypes in the genetic control of immune response to measles-mumps-rubella vaccine. J Infect Dis 193 655 663
32. OvsyannikovaIGJacobsonRMVierkantRAPankratzVSPolandGA 2007 HLA supertypes and immune responses to measles-mumps-rubella viral vaccine: Findings and implications for vaccine design. Vaccine 25 3090 3100
33. DhimanNCunninghamJMJacobsonRMVierkantRAWuY 2007 Variations in measles vaccine-specific humoral immunity by polymorphisms in SLAM and CD46 measles virus receptors. Journal of Allergy and Clinical Immunology 120 666 672
34. OvsyannikovaIGHaralambievaIHVierkantRAPankratzVSPolandGA 2011 The role of polymorphisms in Toll-like receptors and their associated intracellular signaling genes in measles vaccine immunity. Hum Genet 130 547 561
35. DhimanNOvsyannikovaIGCunninghamJMVierkantRAKennedyRB 2007 Associations between measles vaccine immunity and single nucleotide polymorphisms in cytokine and cytokine receptor genes. J Infect Dis 195 21 29
36. HaralambievaIHOvsyannikovaIGUmlaufBJVierkantRAPankratzSV 2011 Genetic polymorphisms in host antiviral genes: associations with humoral and cellular immunity to measles vaccine. Vaccine 29 8988 8997
37. OvsyannikovaIGHaralambievaIHVierkantRAO'ByrneMMJacobsonRM 2011 The association of CD46, SLAM, and CD209 cellular receptor gene SNPs with variations in measles vaccine-induced immune responses--a replication study and examination of novel polymorphisms. Hum Hered 72 306 323
38. HaralambievaIHOvsyannikovaIGKennedyRBVierkantRAPankratzSV 2011 Associations between single nucleotide polymorphisms and haplotypes in cytokine and cytokine receptor genes and immunity to measles vaccination. Vaccine 29 7883 7895
39. WangCTangJSongWLobashevskyEWilsonCM 2004 HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology 39 978 988
40. KimMJNafzigerANHarroCDKeyserlingHLRamseyKM 2003 Revaccination of healthy nonresponders with hepatitis B vaccine and prediction of seroprotection response. Vaccine 21 1174 1179
41. LeeHGLimJSLeeKYChoiYKChoeIS 1997 Peptide-specific CTL induction in HBV-seropositive PBMC by stimulation with peptides in vitro: novel epitopes identified from chronic carriers. Virus Research 50 185 194
42. DeanMCarringtonMO'BrienSJ 2002 Balanced polymorphism selected by genetic versus infectious human disease. Annu Rev Genomics Hum Genet 3 263 292
43. FitzgeraldKAGolenbockDT 2007 Immunology. The shape of things to come. Science 316 1574 1576
44. OvsyannikovaIGJohnsonKLMuddimanDCVierkantRAPolandGA 2004 Identification and characterization of novel, naturally processed measles virus class II HLA-DRB1 peptides. Journal of Virology 78 42 51
45. JohnsonKLOvsyannikovaIGPolandGMuddimanDC 2005 Identification of class II HLA-DRB1*03-bound measles virus peptides by 2D-liquid chromatography tandem mass spectrometry. Journal of Proteome Research 4 2243 2249
46. JohnsonKLOvsyannikovaIGMaddenBJPolandGAMuddimanDC 2005 Accurate mass precursor ion data and tandem mass spectrometry identify a class I Human Leukocyte Antigen A*0201-presented peptide originating from vaccinia virus. Journal of American Society for Mass Spectrometry 16 1812 1817
47. JohnsonKLOvsyannikovaIGMasonCJBergen HRIIIPolandGA 2009 Discovery of naturally processed and HLA-presented class I peptides from vaccinia virus infection using mass spectrometry for vaccine development. Vaccine 28 38 47
48. OvsyannikovaIGJohnsonKLBergenHRIIIPolandGA 2007 Mass spectrometry and peptide-based vaccine development. Clinical Pharmacology and Therapeutics 82 644 652
49. QuerecTDAkondyRSLeeEKCaoWNakayaHI 2009 Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10 116 125
50. NakayaHIWrammertJLeeEKRacioppiLMarie-KunzeS 2011 Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 12 786 795
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion DiseaseČlánek Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) VirusesČlánek Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance toČlánek Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a TombusvirusČlánek Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic PhenotypesČlánek Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence
- Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion Disease
- Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis
- Nutritional Immunology: A Multi-Dimensional Approach
- Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) Viruses
- Vaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
- Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly
- Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance to
- Computational and Biochemical Analysis of the Effector AvrBs2 and Its Role in the Modulation of Type Three Effector Delivery
- Granzyme B Inhibits Vaccinia Virus Production through Proteolytic Cleavage of Eukaryotic Initiation Factor 4 Gamma 3
- Association of Activating KIR Copy Number Variation of NK Cells with Containment of SIV Replication in Rhesus Monkeys
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa
- Follicular Dendritic Cell-Specific Prion Protein (PrP) Expression Alone Is Sufficient to Sustain Prion Infection in the Spleen
- Autophagy Protein Atg3 is Essential for Maintaining Mitochondrial Integrity and for Normal Intracellular Development of Tachyzoites
- Longevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
- Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence
- Acquisition of Pneumococci Specific Effector and Regulatory Cd4 T Cells Localising within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue
- The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites
- Deficiency of a Niemann-Pick, Type C1-related Protein in Is Associated with Multiple Lipidoses and Increased Pathogenicity
- Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
- Eight RGS and RGS-like Proteins Orchestrate Growth, Differentiation, and Pathogenicity of
- Prion Uptake in the Gut: Identification of the First Uptake and Replication Sites
- Nef Decreases HIV-1 Sensitivity to Neutralizing Antibodies that Target the Membrane-proximal External Region of TMgp41
- Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a Tombusvirus
- A Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
- The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
- HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency
- Neonatal CD8 T-cell Hierarchy Is Distinct from Adults and Is Influenced by Intrinsic T cell Properties in Respiratory Syncytial Virus Infected Mice
- Two Novel Transcriptional Regulators Are Essential for Infection-related Morphogenesis and Pathogenicity of the Rice Blast Fungus
- Five Questions about Non-Mevalonate Isoprenoid Biosynthesis
- The Human Cytomegalovirus UL11 Protein Interacts with the Receptor Tyrosine Phosphatase CD45, Resulting in Functional Paralysis of T Cells
- Wall Teichoic Acids of Limit Recognition by the Drosophila Peptidoglycan Recognition Protein-SA to Promote Pathogenicity
- A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen
- Inflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
- CNS Recruitment of CD8+ T Lymphocytes Specific for a Peripheral Virus Infection Triggers Neuropathogenesis during Polymicrobial Challenge
- Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic Phenotypes
- A Receptor-based Switch that Regulates Anthrax Toxin Pore Formation
- Targeting of Heparin-Binding Hemagglutinin to Mitochondria in Macrophages
- Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants
- Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
- Structural Elucidation and Functional Characterization of the Effector Protein ATR13
- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection
- Acid Stability of the Hemagglutinin Protein Regulates H5N1 Influenza Virus Pathogenicity
- Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons
- A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection
- Juxtamembrane Shedding of AMA1 Is Sequence Independent and Essential, and Helps Evade Invasion-Inhibitory Antibodies
- Pathogenesis and Host Response in Syrian Hamsters following Intranasal Infection with Andes Virus
- IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network
- Epstein-Barr Virus Evades CD4 T Cell Responses in Lytic Cycle through BZLF1-mediated Downregulation of CD74 and the Cooperation of vBcl-2
- Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction
- Late Repression of NF-κB Activity by Invasive but Not Non-Invasive Meningococcal Isolates Is Required to Display Apoptosis of Epithelial Cells
- Polar Flagellar Biosynthesis and a Regulator of Flagellar Number Influence Spatial Parameters of Cell Division in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- The Enteropathogenic (EPEC) Tir Effector Inhibits NF-κB Activity by Targeting TNFα Receptor-Associated Factors
- Toward an Integrated Model of Capsule Regulation in
- A Systematic Screen to Discover and Analyze Apicoplast Proteins Identifies a Conserved and Essential Protein Import Factor
- A Host Small GTP-binding Protein ARL8 Plays Crucial Roles in Tobamovirus RNA Replication
- Comparative Pathobiology of Fungal Pathogens of Plants and Animals
- Synergistic Roles of Eukaryotic Translation Elongation Factors 1Bγ and 1A in Stimulation of Tombusvirus Minus-Strand Synthesis
- Engineered Immunity to Infection
- Inflammatory Monocytes and Neutrophils Are Licensed to Kill during Memory Responses
- Sialidases Affect the Host Cell Adherence and Epsilon Toxin-Induced Cytotoxicity of Type D Strain CN3718
- Eurasian-Origin Gene Segments Contribute to the Transmissibility, Aerosol Release, and Morphology of the 2009 Pandemic H1N1 Influenza Virus
- SARS Coronavirus nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of mRNAs: Viral mRNAs Are Resistant to nsp1-Induced RNA Cleavage
- Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus
- Functional Analysis of the Kinome of the Wheat Scab Fungus
- Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- Engineered Immunity to Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



