-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLongevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
Cellular responses to Plasmodium falciparum parasites, in particular interferon-gamma (IFNγ) production, play an important role in anti-malarial immunity. However, clinical immunity to malaria develops slowly amongst naturally exposed populations, the dynamics of cellular responses in relation to exposure are difficult to study and data about the persistence of such responses are controversial. Here we assess the longevity and composition of cellular immune responses following experimental malaria infection in human volunteers. We conducted a longitudinal study of cellular immunological responses to sporozoites (PfSpz) and asexual blood-stage (PfRBC) malaria parasites in naïve human volunteers undergoing single (n = 5) or multiple (n = 10) experimental P. falciparum infections under highly controlled conditions. IFNγ and interleukin-2 (IL-2) responses following in vitro re-stimulation were measured by flow-cytometry prior to, during and more than one year post infection. We show that cellular responses to both PfSpz and PfRBC are induced and remain almost undiminished up to 14 months after even a single malaria episode. Remarkably, not only ‘adaptive’ but also ‘innate’ lymphocyte subsets contribute to the increased IFNγ response, including αβT cells, γδT cells and NK cells. Furthermore, results from depletion and autologous recombination experiments of lymphocyte subsets suggest that immunological memory for PfRBC is carried within both the αβT cells and γδT compartments. Indeed, the majority of cytokine producing T lymphocytes express an CD45RO+ CD62L- effector memory (EM) phenotype both early and late post infection. Finally, we demonstrate that malaria infection induces and maintains polyfunctional (IFNγ+IL-2+) EM responses against both PfRBC and PfSpz, previously found to be associated with protection. These data demonstrate that cellular responses can be readily induced and are long-lived following infection with P. falciparum, with a persisting contribution by not only adaptive but also (semi-)innate lymphocyte subsets. The implications hereof are positive for malaria vaccine development, but focus attention on those factors potentially inhibiting such responses in the field.
Published in the journal: . PLoS Pathog 7(12): e32767. doi:10.1371/journal.ppat.1002389
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002389Summary
Cellular responses to Plasmodium falciparum parasites, in particular interferon-gamma (IFNγ) production, play an important role in anti-malarial immunity. However, clinical immunity to malaria develops slowly amongst naturally exposed populations, the dynamics of cellular responses in relation to exposure are difficult to study and data about the persistence of such responses are controversial. Here we assess the longevity and composition of cellular immune responses following experimental malaria infection in human volunteers. We conducted a longitudinal study of cellular immunological responses to sporozoites (PfSpz) and asexual blood-stage (PfRBC) malaria parasites in naïve human volunteers undergoing single (n = 5) or multiple (n = 10) experimental P. falciparum infections under highly controlled conditions. IFNγ and interleukin-2 (IL-2) responses following in vitro re-stimulation were measured by flow-cytometry prior to, during and more than one year post infection. We show that cellular responses to both PfSpz and PfRBC are induced and remain almost undiminished up to 14 months after even a single malaria episode. Remarkably, not only ‘adaptive’ but also ‘innate’ lymphocyte subsets contribute to the increased IFNγ response, including αβT cells, γδT cells and NK cells. Furthermore, results from depletion and autologous recombination experiments of lymphocyte subsets suggest that immunological memory for PfRBC is carried within both the αβT cells and γδT compartments. Indeed, the majority of cytokine producing T lymphocytes express an CD45RO+ CD62L- effector memory (EM) phenotype both early and late post infection. Finally, we demonstrate that malaria infection induces and maintains polyfunctional (IFNγ+IL-2+) EM responses against both PfRBC and PfSpz, previously found to be associated with protection. These data demonstrate that cellular responses can be readily induced and are long-lived following infection with P. falciparum, with a persisting contribution by not only adaptive but also (semi-)innate lymphocyte subsets. The implications hereof are positive for malaria vaccine development, but focus attention on those factors potentially inhibiting such responses in the field.
Introduction
Malaria is caused by parasites of the genus Plasmodium that are transmitted from one human host to the next by Anopheline mosquitoes, putting an estimated 3.3 billion of the world's population at risk [1]. Upon inoculation by a mosquito, sporozoites initiate an asymptomatic infection of hepatocytes from which blood-stage forms emerge to invade and multiply exponentially within erythrocytes. The latter process underlies the full spectrum of morbidity and mortality associated with clinical malaria. Compounding this global public health burden is the fact that first infections do not immediately induce immunity. Instead, infants in endemic areas remain susceptible to multiple new symptomatic infections throughout childhood and early adulthood, and adults frequently still harbor sub-clinical parasitemia (reviewed in [2], [3]). Both poor induction (priming) of immune responses by the parasite and rapid attrition of such responses have been proposed as explanations, although the validity of both hypotheses has been brought into question (discussed in [4], [5], [6]).
Direct immunological evidence from studies in humans that support or reject these theories is limited. The commonly held view that immune responses to Plasmodium parasites are short-lived following exposure, is mainly based on the short half-life of specific antibodies (reviewed in [7]). It would appear that cellular responses to individual antigens are also either relatively short-lived, i.e. declining within a few years of exposure [8], [9], [10], or at least unstable [11], [12], [13], [14], [15], but may persist occasionally [16]. Many field studies, however, suffer from a profound difficulty in controlling for exposure amongst study subjects, limiting interpretation thereof. Anecdotal evidence from historical malaria-therapy studies suggests that cellular proliferative responses to crude whole parasite antigen can be detected in donors several years after a single infection [17]. More recently, robust cellular cytokine responses were detected three months post infection in previously naïve volunteers [18]. Within these cellular immune responses, interferon-gamma (IFNγ) in particular is considered to play a major role (reviewed in [19]).
Experimental human malaria infections by bites of P. falciparum infected mosquitoes offer a controlled measure of exposure and a safe and well-established model, and have been performed on hundreds of volunteers over the past two decades primarily for assessing the efficacy of candidate malaria vaccines [20]. This model allows controlled studies on the development and maturation of intrinsic immune responses in the course of a malaria infection, and on how (long) cellular memory is maintained. Here we conducted a comprehensive longitudinal study of cellular responses, focusing on IFNγ production by multiple subsets of innate and adaptive immune cells, induced by both P. falciparum sporozoites (PfSpz) and asexual blood-stage parasites (P. falciparum-infected red blood cells; PfRBC) in malaria-naïve volunteers undergoing single or multiple experimental infections with P. falciparum.
We show that even a single patent malaria episode induces robust cellular re-call responses to both parasite stages, persisting at almost undiminished levels at least 14 months post infection and involving both adaptive and innate compartments.
Results
Cellular IFNγ re-call responses to both sporozoites and blood-stage parasites are readily induced and long-lived following infection
In vitro parasite-specific responses were measured in peripheral blood mononuclear cells (PBMC) isolated from two sets of human volunteers prior to and at several time points after exposure to P. falciparum infection. Group A volunteers (n = 10) were exposed thrice to immunizing bites (I) of infected mosquitoes whilst under chloroquine prophylaxis and thereafter challenged (C) once again; Group B volunteers (n = 5) received only a single infection in parallel with Group A challenge (Figure 1). Total lymphocyte responses to PfSpz and PfRBC were barely detectable above background prior to exposure (day I-1) in both groups of volunteers (Figure 2). Re-call responses by lymphocytes to both PfSpz and PfRBC, as measured by IFNγ production following overnight re-stimulation, were detectable in Group A volunteers following exposure to immunizing bites (day C-1 compared to I-1, one-way ANOVA with Dunnet's post-test, p<0.05 for PfSpz and p<0.01 for PfRBC) and remained high after re-challenge until day C+35 (p<0.001 and p<0.01, respectively) (Figure 2.A+C). Of note, one volunteer displayed a disproportionally amplified IFNγ response to PfRBC at time point C+35. For this reason, this volunteer was left out of statistical analysis as an extreme outlier. Re-call responses to PfRBC (p<0.001, I-1 compared to C+35), and to a lesser extent also to PfSpz, became detectable in Group B volunteers following their first infection (Figure 2.B+D). This shows that cellular immune responses to whole parasites are readily inducible in previously-naïve human volunteers, following a small number of, or even a single P. falciparum infection. Most remarkably, in further experiments with samples collected at later time points (days C+140 and C+400), we found that parasite-specific cellular responses did not wane after exposure. Instead, they remained robust more than a year post-challenge, albeit with considerable inter-individual variation (Figure 2).
Fig. 1. Flowchart of Experimental Human Malaria Infection study. 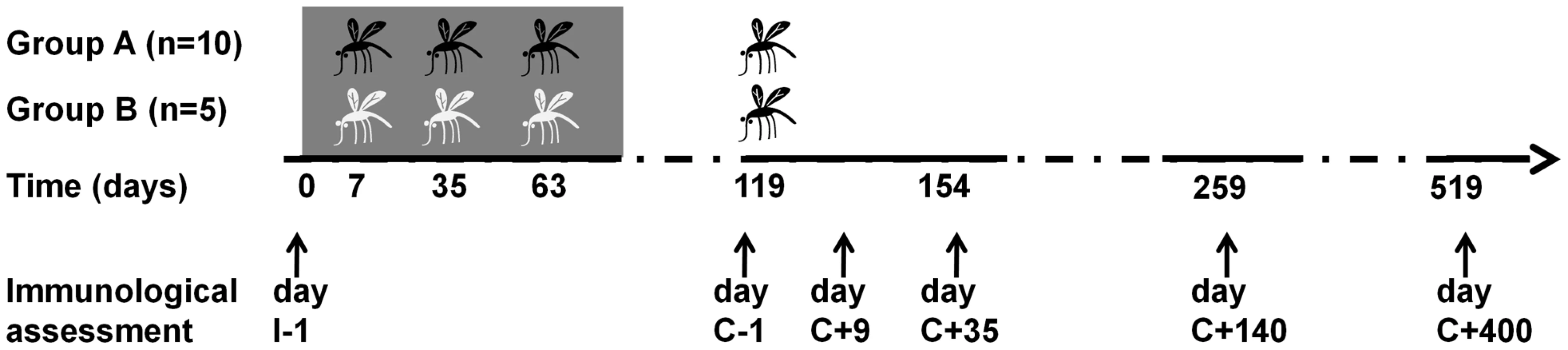
Black and white mosquito symbols indicate exposure to infected mosquito bites and uninfected mosquito bites, respectively. Development of patent blood-stage parasitemia following the first three inoculations was prevented by prophylactic chloroquine treatment, indicated by grey shading. Arrow heads indicate time points of immunological assessment: prior to immunization (I-1), prior to patent challenge (C-1), during expected blood-stage infection (C+9), two weeks after treatment (day C+35), 4.5 months post-challenge (day C+140) and again 1.1 year post-challenge (day C+400). Fig. 2. Induction and persistence of IFNγ responses to PfRBC and PfSpz during experimental malaria infection. 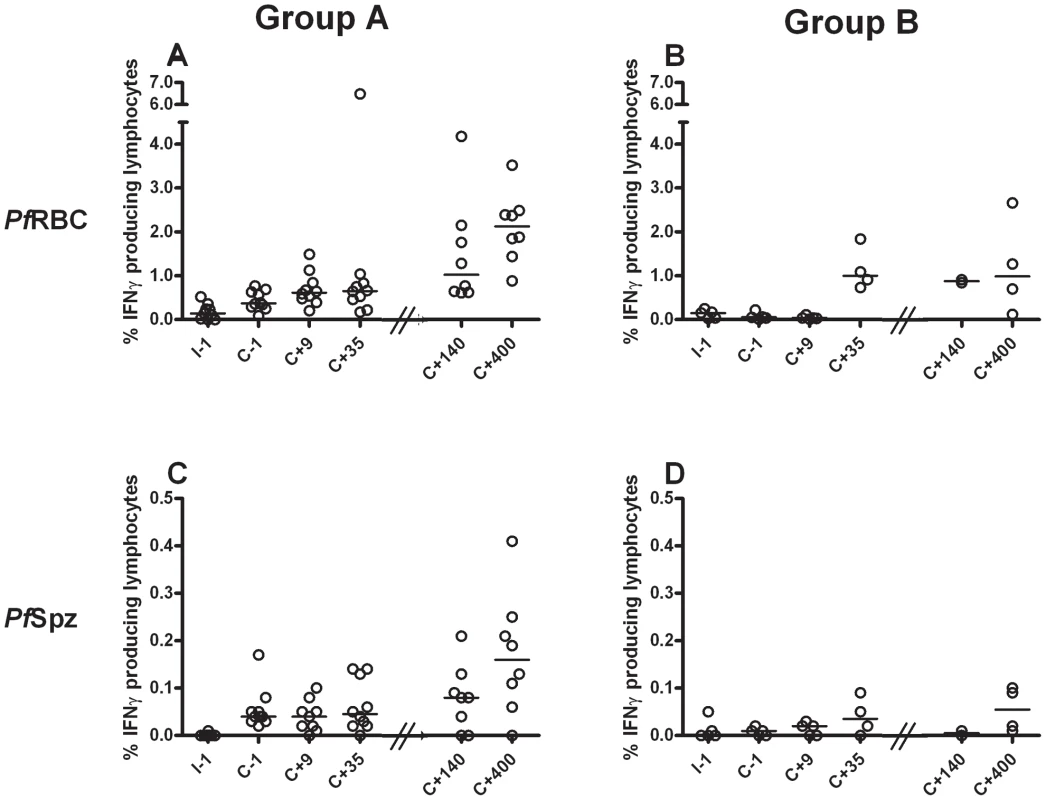
PBMC were isolated from volunteers prior to inclusion (day I-1), immediately prior to patent challenge (day C-1), during expected blood-stage malaria infection (day C+9), two weeks after treatment (day C+35), 4.5 months post-challenge (day C+140) and again 1.1 year post-challenge (day C+400). Note that Group A, but not Group B volunteers were exposed thrice to immunizing sub-patent infections between day I-1 and C-1 (Figure 1). PBMC of volunteers of Group A (A+C) and Group B (B+D) were stimulated in vitro for 24 hours with PfRBC (A+B) or PfSpz (C+D) or their respective uninfected red blood cells (uRBC) or salivary glands from uninfected mosquitoes (MSG) controls, then stained for intracellular IFNγ and analyzed by flow cytometry. Shown are the percentage of total lymphocytes staining positive for IFNγ at each time point. Background responses were subtracted from the responses to parasite stimuli for every individual volunteer at every individual time point. Symbols represents responses by individual Group A volunteers (n = 10) and Group B volunteers (n = 5) for whom sufficient cells were available. Horizontal lines represent group medians. Median background values for uRBC were 0.01% [0.01–0.03] (median [IQR]) on I-1 up to C+35 and 0.03% [0.01–0.16] on C+140 and C+400. Background values for MSG were 0.02% [0.01–0.02] on I-1 up to C+35 and 0.07% [0.03–0.25] on C+140 and C+400. Cellular responses to protein pools of either sporozoite-stage (CSP and TRAP), liver-stage (LSA-1 or Exp-1) or blood-stage (AMA-1, MSP-2, MSP-3 and GLURP) antigens (all leading malaria vaccine candidates), however, were never detectable above background.
αβT and γδT cells are the main in vitro IFNγ-producers in response to PfRBC following infection
Many different lymphocyte subsets, including αβT cells, γδT cells and NK cells, have variously been shown capable of responding to PfRBC. Therefore, we assessed IFNγ responses by those cell types to PfRBC prior to (I-1 for Group A, I-1 and C-1 for Group B) and post exposure (C-1 and later for Group A, C+9 and later for Group B; flow cytometry gating strategy illustrated in Figure S1). Relative proportions of lymphocyte subsets within the total peripheral population did not differ markedly over time at the various time points assessed (Table S1). The only exception were γδT cells, the relative numbers of which increased within the peripheral lymphocyte population post exposure in both sets of volunteers (p = 0.0013 for Group A; p = 0.029 for Group B, one-way ANOVA, I-1 to C+35). Response patterns in most lymphocyte subsets, including αβT cells, NKT cells and NK cells, mirrored the dynamics of the total lymphocyte response in relation to exposure: whereas almost no responses above background could be detected in volunteers at inclusion, IFNγ responses to PfRBC became clearly detectable following challenge (Figure S2). In contrast, a large proportion of γδT cells (median 7.9% and 6.8% for Group A and B, respectively) demonstrated the capacity to respond to PfRBC even prior to exposure. Following infection, this percentage increased still further (p = 0.013 Group A; p = 0.003 Group B, one-way ANOVA I-1 to C+35). Responses in ‘γδNKT’ cells, relatively infrequent in total number, resembled this pattern of regular γδT cells (Table S1 and Figure S2).
Next, we assessed the relative contribution of the different lymphocyte subsets to the total IFNγ response at various time points during the study (Figure 3) in volunteers of Group A. Few lymphocytes produced IFNγ in response to PfRBC prior to exposure (I-1), of which 63% (median) were γδT cells and 15% αβT cells, with γδNKT cells (11%) and NK cells (1.9%) making up most of the remainder. Interestingly, despite an increase in the overall proportion of responding cells over time, the relative contributions of the various lymphocyte subsets remained more or less stable following repeated exposure (C+35) (57%, 22%, 6.7% and 4.1%, respectively). By day 400 post-challenge, the dominating cell subsets contributing to overall IFNγ production remained αβT and γδT cells (35%, 25%, 11% and 17%, respectively). The contribution of the various cell subsets to responses in Group B volunteers also remained comparable over time (data not shown). Thus, not only ‘adaptive’ αβT cells and ‘semi-innate’ γδT cells, but clearly also ‘innate’ NK and NKT cells contributed to the overall increase in lymphocytes responding to P. falciparum by IFNγ production following exposure (Figure 3).
Fig. 3. Contribution of innate, semi-innate and adaptive lymphocyte subsets to the total IFNγ+ response to PfRBC. 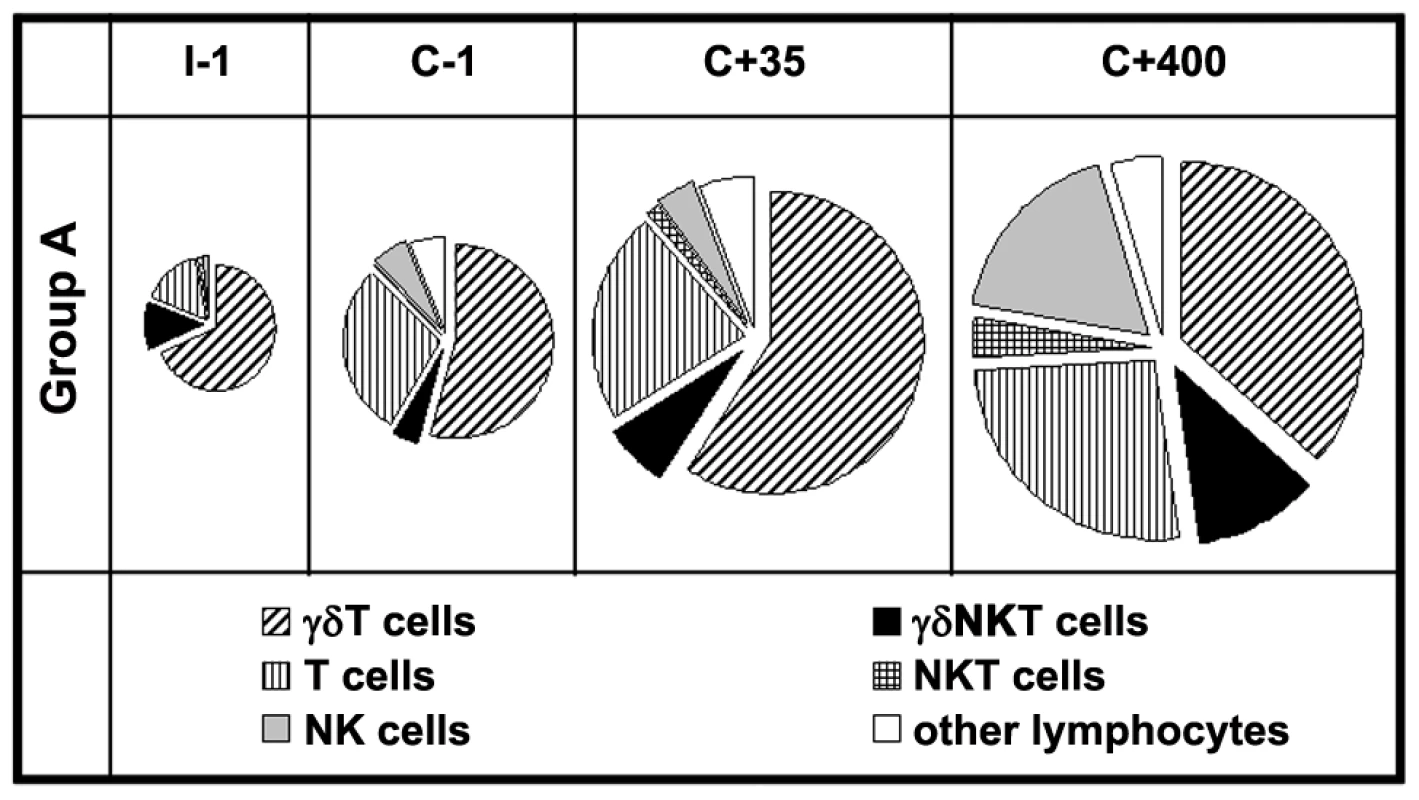
PBMC isolated from Group A volunteers at the respective study time points were stimulated with PfRBC or uRBC and stained for intracellular IFNγ and surface expression of CD3, γδT and CD56 (gating strategy shown in Figure S1.A). Pie charts show the relative contributions of αβT cells (CD3+γδ-CD56-), γδT cells (CD3+γδ+CD56-), NK cells (CD3-γδ-CD56+), NKT cells (CD3+γδ-CD56+), ‘γδNKT’ cells (CD3+γδ+CD56+) and other lymphocytes to the total number of IFNγ+ cells responding to PfRBC (corrected for uRBC background). Shown are median values for ten Group A volunteers; pie chart surface areas directly correlate with the magnitude of (total) IFNγ+ responses. At time point I-1 the median [IQR] contribution of γδT cells, γδNKT cells, αβT cells & NK cells to total IFNγ responses was 63% [45–74], 11% [6.4–15], 15% [5.1–37] & 1.9% [0.3–5.6], respectively; at C+35 57%[ 47–59], 6.7% [4.0–8.9], 22% [17–28] and 4.1% [3.0–7.1] and C+400 35% [29–44], 11% [8.0–16], 25% [20–28] & 17% [12–25]. A more in depth phenotypic analysis of responding T cell subsets in donors with sufficient responses at the latest time point (C+400, Figure S3) revealed that IFNγ-producing CD4+ T cells markedly outnumbered CD8+ T cells in response to both sporozoite and blood-stage parasites post-challenge. Following in vitro re-stimulation with PfRBC, 16% [13–22] (median [IQR]) and 26% [20–32] of IFNγ-producing T cells were of the CD4+CD8- T-helper phenotype in Group A and B volunteers, respectively. In contrast, only 4.5% [3.1–5.6] and 7.3% [4.7–8.8] were CD4-CD8+ cytotoxic T lymphocytes (CTLs). The majority of IFNγ-producing T cells in response to PfRBC, however, were CD4-CD8- cells. Analysis in a subset of donors showed that these cells were predominantly γδT cells (data not shown). The contribution of CD4+ T cells was even more pronounced for PfSpz-induced responses, with 70% [65–75] of IFNγ+ T cells belonging to the CD4+CD8- population in Group A volunteers, and only 1.7% [0.9–2.5] to the CD4-CD8+ population (day C+400, Figure S3). Thus, whereas both CD4-CD8- T cells and CD4+ T cells dominated responses to PfRBC, the IFNγ response to PfSpz was primarily mediated by CD4+ T cells only.
Cells showing in vitro parasite-specific IFNγ re-call responses predominantly display an effector memory phenotype both early and late after infection
Early after treatment (day C+35) in Group A volunteers, 84% [80–87] (median [IQR]) and 0.1% [0.0–0.4] of IFNγ-producing lymphocytes displayed effector memory (EM, CD45RO+CD62L-) and central memory (CM, CD45RO+CD62L+) phenotypes, respectively, following 24-hour in vitro PfRBC re-stimulation. Remarkably, despite an overall increase in the response to PfRBC in Group A volunteers on day C+400, the relative contributions of CD62L- EM and CD62L+ CM cells remained largely stable: 72% [67–75] and 0.6% [0.6–0.8], respectively (Figure 4.A). Corresponding values for Group B volunteers at day C+35 were 76% [74–79] and 0.5% [0.3–1.1] and remained constant over time, both in terms of percentage of responding cells and in EM/CM distribution (Figure 4.B). Responses to PfSpz stimulation showed an EM/CM pattern very similar to PfRBC responses as determined for group A volunteers (Figure 4.C). γδT cells also displayed an EM phenotype (CD45RO+CD62L- or CD62Lintermediate) as shown in Figure S1.C. Thus, in vitro parasite-specific re-call responses were primarily found in EM-type populations, which include both αβT cells and γδT cells, even months after infection. Cells of CD62L+ CM phenotype, in contrast, were detectable in only a negligible fraction of the total re-call response at all time points examined.
Fig. 4. Contribution of EM and CM cells to the total IFNγ response to PfRBC and PfSpz. 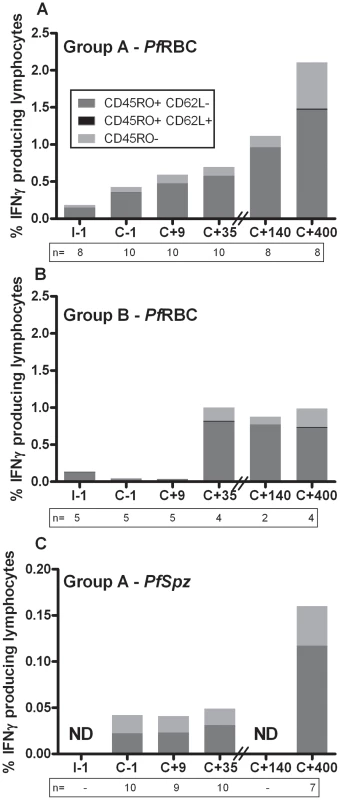
PBMC isolated from volunteers at various study time points were stimulated in vitro for 24 hours with PfRBC (A+B) or PfSpz (C) and stained for the memory marker CD45RO and the homing marker CD62L. Bars show the contributions of effector memory (EM, CD45RO+CD62L-), central memory (CM, CD45RO+CD62L+) and naive lymphocytes (CD45RO-) to the total percentage of IFNγ-producing cells over time. Height of bars represents median values for Group A (A+C) and Group B (B) volunteers for the different cell subsets. Donors with insufficient numbers of IFNγ responding cells to assess the relative contribution of cell subsets were excluded from this composition analysis. Numbers below the bars represent the number of donors included per time point. ND – not done: insufficient numbers to reliably assess group medians; this was similarly the case for anti-PfSpz responses in group B. Since only donors with sufficient numbers of responding cells to assess the relative contribution of lymphocyte subsets are represented, these distributions may appear biased towards patterns in relatively stronger responders. Immunological memory for PfRBC appears to be carried within both the αβT cell and γδT cell compartments
Since both αβT cells and γδT cells display memory phenotypes and can mount adaptive responses, we assessed their respective ability to initiate cellular re-call responses to PfRBC. To this end, we separated and re-combined γδT cells and other PBMC (consisting of approximately 80% αβT cells and 5% NK cells) from both inclusion (I; ‘Pf-naïve’) and 35 or 140 days post-challenge (C; ‘Pf-experienced’) of volunteers from both groups for whom sufficient cells were available (Figure 5.A). Following in vitro stimulation, total numbers of IFNγ+ lymphocytes in naïve PBMC populations supplemented with Pf-experienced γδT cells were significantly higher than in populations containing only Pf-naïve cells (I-I versus I-C; p<0.05, One-way ANOVA). This suggests that the γδT compartment carries some immunological memory for PfRBC (Figure 5.B). Indeed, the PfRBC response by Pf-experienced γδT cells in some donors was more than twice as high compared to that by Pf-naïve γδT cells, even in the presence of otherwise naïve PBMC populations (data not shown). IFNγ responses in PBMC populations containing Pf-experienced γδT-depleted cells (mainly αβT cells) also appeared higher than in populations containing only Pf-naïve cells (I-I versus C-I; not significant).
Fig. 5. Immunological memory carriage by the γδT compartment vs other PBMC. 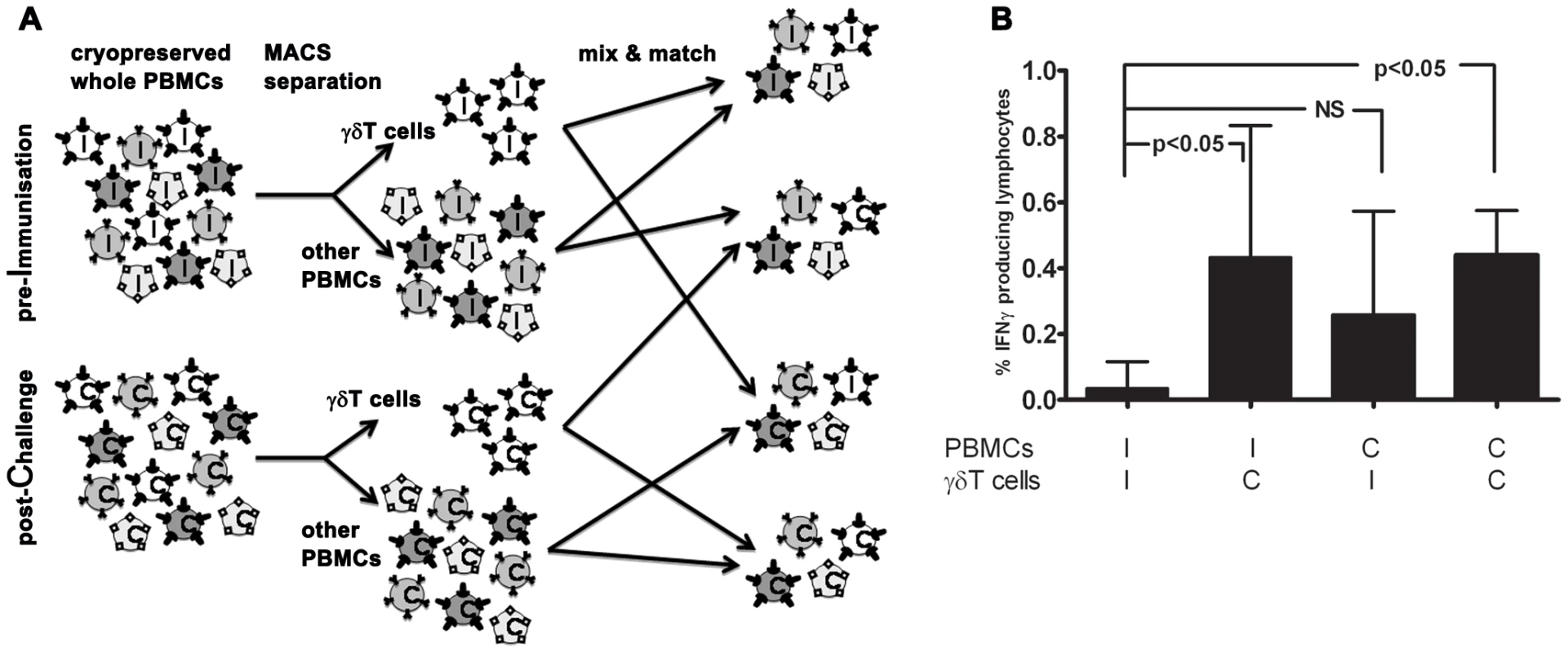
(A) Cryopreserved PBMC isolated from volunteers at inclusion (I) or 35 or 140 days post-challenge (C), were thawed and separated by magnetic beads into γδT+ lymphocytes (white) and remaining γδT- PBMC (shades of grey, e.g. αβT cells, NK cells, B cells, monocytes). Following autologous re-combination at original ratios, PBMC were stimulated, stained and measured as for Figure 3. (B) Shown are percentages of total lymphocytes staining IFNγ+ following incubation with PfRBC (corrected for uRBC background). Data represent median+IQR of seven volunteers from whom sufficient cells were available for the assay. Long-lived polyfunctional memory re-call responses to malaria parasites are more prominent in anti-PfSpz compared to anti-PfRBC responses
Whereas IFNγ has many direct effector functions, IL-2 is important for T cell proliferation and induction of cellular memory responses. In a final set of experiments, we therefore explored the dynamics of EM lymphocytes producing either IL-2 or IFNγ alone (unifunctional), or both cytokines simultaneously (polyfunctional cells), in response to PfRBC and PfSpz. In Group A volunteers, the percentage of total IL-2+ EM cells responding to PfRBC, although low in absolute numbers, increased significantly from 0.08% [0.04–0.12] (median [IQR]) of EM cells at day I-1, to 0.31% [0.17–0.45] at day C-1 (p<0.001, one-way ANOVA with Dunnett's post-test) and 0.22% [0.19–0.42] at day C+35 (p<0.01, Figure S4.A) and remained clearly detectable at day C+140 and day C+400. This was in line with the increase in total lymphocyte IFNγ responses to PfRBC after immunization (Figure 2). Similarly to both total IFNγ and total IL-2 responses, the percentage of EM-type cells producing both IFNγ and IL-2 in response to PfRBC increased from 0.025% [0.003–0.078] on day I-1 to 0.14% [0.09–0.22] on C-1 (p<0.01) and 0.13% [0.10–0.18] on day C+35 (p<0.01, Figure S4.B) and remained present up to day C+400. The relative contribution of such polyfunctional cells to the overall number of cytokine producing EM cells, however, remained relatively stable with an apparent slight, but non-significant increase on day C-1 and C+9 (Figure S5).
Total IL-2 and polyfunctional responses to PfSpz by EM cells from Group A volunteers remained low up to C+35 (p = 0.8 and 0.1, respectively, compared to I-1). IL-2 increased from C+140 to C+400 (p = 0.039, paired Student's t-test; Figure S4.A). A similar trend was seen for polyfunctional responses (p = 0.15; Figure S4.B). Interestingly, months after malaria infection, the contribution of IFNγ+IL-2+ EM cells to the total EM cytokine response towards PfSpz was relatively more pronounced than to that against PfRBC. Specifically, on day C+140 and C+400 the relative contribution of polyfunctional EM cells was 37% [25–62] and 19.2% [16–30] in response to PfSpz, compared to 3.3% [1.5–4.3] and 3.4% [2.0–5.3] in response to PfRBC (p<0.001 and p<0.05 respectively; two-way ANOVA with Bonferroni post-test; data not shown and Figure 6). Thus, although infrequent in total number, polyfunctional EM cells with specificity for both PfRBC and PfSpz were readily induced upon exposure, and formed a greater relative contribution to PfSpz than to PfRBC responses.
Fig. 6. Uni- and polyfunctional EM T cell responses to PfRBC and PfSpz one year post-infection. 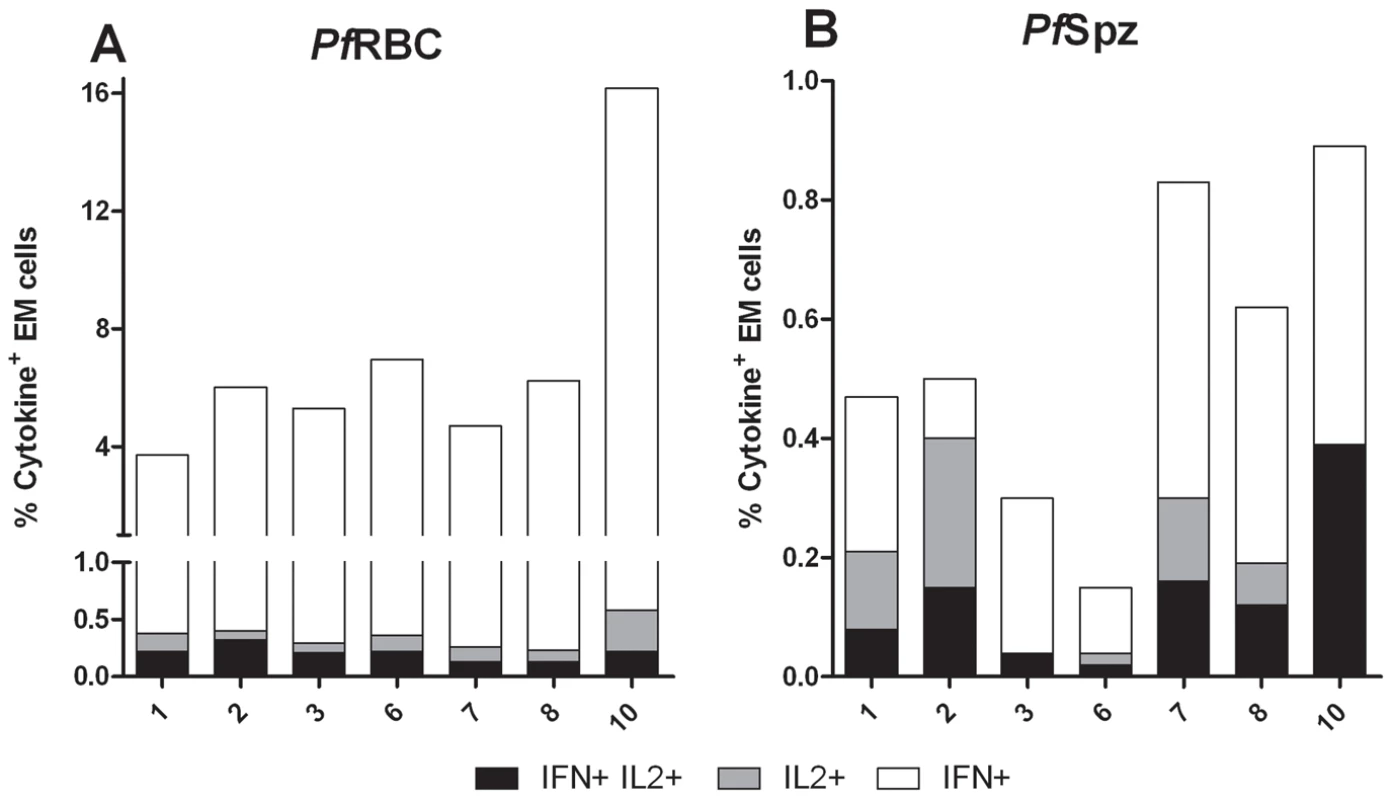
Data represent percentage of effector memory (EM) cells producing either IFNγ alone, IFNγ and IL-2, or IL-2 alone, following 24 hours in vitro stimulation with (A) PfRBC or (B) PfSpz at 400 days after challenge (C+400) for seven individual volunteers of Group A. Discussion
In this study we delineate the dynamics and composition of cellular immune responses to both sporozoites and asexual blood-stage Plasmodium falciparum parasites following infection in previously-naïve individuals. We demonstrate unequivocally that specific IFNγ responses to both stages of the malaria parasite are not only readily induced following infection, but also persist more or less undiminished over at least 14 months in the absence of further exposure. The main contributors to these whole parasite-specific IFNγ responses are γδT cells and CD4+ EM T cells, with NK cells making up a smaller remaining fraction of responding cells. We show that not only adaptive, but also semi-innate and innate lymphocytes responses exhibit an immunological re-call pattern and present evidence suggesting that immunological memory for PfRBC is carried within both the αβT cell and γδT cell compartments.
Our demonstration of lengthy persistence of cellular immunological responses following P. falciparum infection in humans stands in contrast to the popularly held perception that clinical immunity to malaria is short-lived. As discussed previously by Struik et al. [6] studies reporting such short-lived immunity are mainly anecdotal and few consistent data pro or contra this hypothesis have been published. Our current findings prove that long-lived cellular responses can be adequately maintained, at least when induced under experimental conditions.
A central mediator of such cellular immunological responses to the malaria parasite is the cytokine IFNγ (reviewed in [19]). In vitro parasite-specific IFNγ responses have been shown by us and others to associate with protection against malaria both amongst volunteers undergoing experimentally induced infection [21], [22], [23] and naturally-exposed human populations [24], [25], [26], [27]. Phenotypic characterization of the in vitro IFNγ response to whole blood-stage parasites (PfRBC) in malaria-naive donors has variously implicated ‘innate’ natural killer (NK) cells [28], [29], [30], ‘semi-innate’ γδT cells [31], [32] (including NK-like γδT cells [33]) and ‘adaptive’ αβT cells [32], [34], [35]. It remains unknown, however, how these intrinsic responses develop and mature in the course of a malaria infection and how (long) cellular memory is maintained.
Consistent with findings by others [31], [33], we show that ‘semi-innate’ γδT cells comprise the largest population of lymphocytes responding with IFNγ production to PfRBC in malaria-naïve donors. Interestingly, this remains largely true following exposure, despite the obvious increase in ‘adaptive’ responses. Two factors may contribute to the overall increase in responding γδT cell numbers: i) The overall proportion of γδT cells in the PBMC pool increases following exposure to parasites, which persists for at least a year. A transient dip in circulating γδT cells during infection, followed by reactive increase afterwards, has been observed before in primary [36], [37] but not in repeated malaria infections [38]. ii) A slightly increased proportion of these γδT cells responds to PfRBC following infection. This increase may represent the recruitment of PfRBC-specific γδT clones to the peripheral circulation or a non-specific bystander effect, since γδT cells can readily respond to P. falciparum lysate by proliferation in a polyclonal fashion [39], [40]. Whatever the underlying mechanism, our data suggest that the γδT compartment does contribute autonomously to cellular immunological memory up to 14 months post infection, independently of other PBMC including αβT cells. In contrast, we and others have recently shown that the ‘re-call-like’ response observed in NK cells post infection is in fact fully dependent on αβT cells [30], [41]. These data can be combined into a model in which ‘semi-innate’ γδT cells, ‘adaptive’ αβT cells and ‘innate’ NK cells all contribute to a robust and long-lived IFNγ response following infection with P. falciparum, although through different mechanisms. For γδT cells this is largely through an overall expansion of this compartment in peripheral blood, in addition to a minor increase in the proportion of responding γδT cells. For αβT cells the proportional increase in response is also relatively small, but in absolute terms these lymphocytes already make up the vast majority of PBMC populations. NK cells finally, although fewer in absolute terms, show a much larger proportional increase in response, albeit dependent on the increase in T cell responses [30].
The majority of responding T cells displays an EM (CD45RO+ CD62L-) phenotype, even over a year post infection, at least in donors with sufficient numbers of responding cells to assess this. Whether such a composition is also representative of extremely low responders, or whether those donors exhibit a relative response deficit in a particular lymphocyte sub-set, cannot as yet be determined.
The apparent scarcity of responding CD62L+ CM cells may be partly due to the fact that CM cells by definition form only a minor population within the peripheral blood, residing primarily in ‘target’ tissues (e.g., skin and liver) and lymph nodes. Another possibility is that this pattern is inherent to short-term in vitro assays such as ours where within the short timeframe of 24 hours, effector memory cells, which are defined by their ability to perform immediate (cytokine producing) effector function, will preferentially respond. Finally, the low number of CD62L expressing responding lymphocytes could be due to loss of CD62L expression, since following antigenic stimulation CM cells can differentiate into an effector memory phenotype and subsequently acquire effector function [42], [43]. Thus the formal compartmental origin of responding cells cannot be determined with certainty from this assay.
The importance of polyfunctional lymphocytes in immunological protection is believed to depend on i) their higher cytokine production [23] and hence more potent effector capability compared to monofunctional cells [44] and ii) their role in the induction and persistence of T cell memory [45]. We recently showed that the development of protection against infection with P. falciparum in human volunteers is associated with the induction of IFNγ+IL-2+ double-positive (polyfunctional) EM T cells in response to PfRBC [22], [23]. Despite an overall increase in the number of responding lymphocytes up to one year post infection, we show here that the relative contribution of polyfunctional cells to the total response remains roughly constant. This may indicate that little differentiation takes place in the functionality of cellular immune responses to PfRBC following exposure [46]. It will be of obvious interest to explore this further in future studies and to determine whether such responses genuinely afford protection.
In contrast to responses to the asexual stage of the malaria parasite, sporozoite-specific cytokine responses have received little attention to date. We find that similar to PfRBC responses, IFNγ responses to PfSpz are readily induced and persist following exposure to infected mosquito bites. Furthermore, as for PfRBC responses, IFNγ production dominates the total cytokine response. A striking feature of the anti-PfSpz response, however, is that polyfunctional IFNγ+/IL-2+ cells form a relatively larger component of this compared to PfRBC. Whether this represents a genuine acquisition of effector function of the anti-PfSpz response or conversely a failure of these lymphocytes to terminally differentiate into IFNγ single producers [46] remains to be determined.
Our data demonstrate that there is no intrinsic deficit in either the induction or persistence of cellular responses to P. falciparum after experimental infection. This raises the obvious question as to why clinical immunity to malaria develops so slowly amongst naturally exposed populations [2], [4]. More specifically, why do cellular responses to P. falciparum antigens in naturally exposed donors appear to be so transient/unstable [8], [10], [11], [12], [13], [14], [15] and tend in fact to be lower than in non-exposed donors [47], [48]? Several lines of reasoning may help to explain this paradox.
Firstly, by the time treatment is sought by and initiated in patients in resource-poor endemic settings, their parasitemia is typically higher compared to that in our strictly-observed volunteers. High parasitemia has been shown to inhibit the development of immunity both in mice [49] and in humans [11]. This may be due to active suppression or elimination of responding T cells [50], [51] by P. falciparum, resulting in reduced Pf-specific cellular responses following repeated or chronic infection [11], [47], [48], [52]. Obvious accomplices are regulatory T cells [53], [54], [55], [56], and a comparison of the dynamics of regulatory T cells in natural and experimental infections would be informative in this regard. Secondly, underlying differences in the status of the immune system of inhabitants of the rural tropics may predispose to tolerant, as opposed to sterilizing, immune responses [57]. This may be due to e.g. malnutrition [58] or helminth co-infections [59], [60]. Another factor may be the inherent immaturity in the immune systems of infants and young children, the stage in life at which malaria infections are typically first experienced in endemic settings [61], [62], as well as prior in utero exposure [63]. Indeed, IFNγ responses to P. falciparum antigens in children tend to be weaker than in adults [14], [64], [65], [66], [67], although of course the effect of prior exposure in these studies cannot be distinguished from that of age per sé. In addition, immunization and in vitro PBMC re-stimulation in our experimental infection model were performed with homologous strain parasites, whereas in field studies prior strain exposure varies. Well-described target antigens for protective immunity exhibit high rates of genetic variation, hindering cross-protective immunity in the field [68]. Finally, the immune modulating effects of chloroquine might have enhanced the development of immune responses during the immunization process [69], possibly contributing to the persisting immune responses in Group A.
Despite these caveats in extrapolating our findings to the situation in endemic areas, we show that robust long-lasting cellular immune responses to malaria parasites can be readily induced under experimental conditions, and extend our understanding of how cellular immunological memory to P. falciparum develops and is maintained following exposure.
Materials and Methods
Parasites
NF54 strain P. falciparum asexual blood-stage parasites, regularly screened for mycoplasma contamination, were grown in RPMI-1640 medium containing 10% human A+ serum at 5% hematocrit in a semi-automated suspension culture system, in the absence of antibiotics and in an atmosphere containing 3% CO2 and 4% O2. For in vitro stimulation experiments, asynchronous asexual-stage cultures of NF54 strain parasites were harvested at a parasitemia of approximately 5–10% and mature asexual stages purified by centrifugation on a 27% and 63% Percoll density gradient [70]. This purification step results in preparations of 80-90% parasitemia, consisting of more than 95% schizonts/mature trophozoites. Preparations of parasitized red blood cells (PfRBC) were washed twice in PBS and cryopreserved at 150x106/ml in 15% glycerol/PBS in aliquots for use in individual stimulation assays. Cryopreserved PfRBC form almost as strong a stimulus as freshly-prepared PfRBC and have identical stimulatory characteristics (Figure S6). Their use in large experiments has logistical advantages, in addition to reducing confounding influences due to inter-batch variation. Mock-cultured uninfected erythrocytes (uRBC) were obtained similarly and served as controls.
Sporozoites were obtained from Anopheles stephensi mosquitoes that were reared according to standard procedures in our insectary. Infected mosquitoes were obtained by feeding on gametocyte-containing cultures of NF54 strain P. falciparum, as described previously [71]. On day 21–28 after infection, the salivary glands of the mosquitoes were collected by hand-dissection. Salivary glands were collected in RPMI-1640 medium (Gibco) and homogenized in a custom glass grinder. Sporozoites were counted in a Bürker-Türk counting chamber using phase-contrast microscopy. Sporozoites were cryopreserved at 16×106/ml in 15% glycerol/PBS in aliquots for use in individual stimulation assays. Sporozoites that had undergone one freeze-thaw cycle were determined microscopically to be still intact, but were no longer able to glide (assay described in [72]). To control for a possible immune-stimulatory effect of salivary gland remnants in the sporozoite preparation, salivary glands from an equal number of uninfected mosquitoes (MSG) were obtained similarly and served as a background control.
Human ethics statement
All volunteers were recruited after giving written informed consent. The study was approved by the Institutional Review Board of the Radboud University Nijmegen Medical Centre (CMO 2006/207).
Human infections
The basic design and outcome of experimental human malaria infections at our centre has been described before [73]. For the study presented here [23], 15 healthy malaria naïve Dutch volunteers were recruited and randomized double-blind to either Group A (n = 10) or Group B (n = 5). Group A volunteers were immunized by exposure on three occasions, at monthly intervals, to the bites of 12-15 NF54 strain P. falciparum-infected mosquitoes, whilst continuously taking a standard prophylactic regimen of chloroquine (300mg base per week). Group B volunteers similarly took chloroquine and were exposed to the same number of bites, but from uninfected mosquitoes. Two months after the final exposure and one month after discontinuation of chloroquine prophylaxis, all 15 volunteers were challenged by exposure to the bites of 5 P. falciparum-infected mosquitoes and followed-up closely for symptoms and signs of malaria. As soon as they were found to be thick blood-smear positive, volunteers were treated with a standard curative regimen of artemether/lumefantrine (AL) consisting of six doses of 13 80/480 mg over three days. Duration and peak height of parasitemia in volunteers following each round of infection, as measured retrospectively by PCR [23], is shown in Table S2.
Cellular immunology
Venous whole blood was collected into citrated CPT vacutainers (Becton and Dickinson, Basel) at inclusion (day I-1), and immediately prior to challenge (day C-1), during expected blood-stage malaria infection (day C+9), two weeks after treatment with AL (day C+35) and again 4.5 months (day C+140) and 1.1 year (day C+400) after challenge (Figure 1). Peripheral Blood Mononuclear Cells (PBMC) were obtained by density gradient centrifugation, washed three times in cold PBS, enumerated, frozen down in fetal-calf serum containing 10% dimethylsulfoxide and stored in liquid nitrogen. Immediately prior to use, cells were thawed, washed twice in RPMI and re-suspended in complete culture medium (RPMI 1640 Dutch modification (Gibco) containing 2 mM glutamine, 1mM pyruvate, 50 µg/ml gentamycine and 10% human A+ serum, (Sanquin, Nijmegen)) for a final concentration of 2.5×106/ml. PBMC were transferred into 96-well round-bottom plates and stimulated in duplicate wells with either 5x106/ml (final concentration) cryopreserved PfRBC or uRBC, or 5.6×105/ml cryopreserved sporozoites or the extract of an equivalent number of uninfected mosquito salivary glands in a total volume of 200 µl/well for 24 hours at 37°C/5%CO2. Dose and duration of stimulation were chosen based on earlier optimization assays. Initial experiments included samples from time points I-1 through C+35; in a later set of experiments, time points C+140 and C+400 were compared. In a subset of experiments, PBMC from time points I-1 through C+35 were instead stimulated with protein pools of individual purified sprorozoite-stage (CSP and TRAP), liver-stage (LSA-1 or Exp-1) or blood-stage (AMA-1, MSP-2, MSP-3 and GLURP) antigens in concentrations of both 5 and 30 µg/ml per antigen. Full length CSP [74] was kindly provided by A. Birkett, TRAP MR149A, MSP-2 MR141 [75], PfExp-1 MR95 [76] by G. Corradin, MSP-3 [77] by C. Oeuvray, GLURP [78] by M. Theisen, LSA-1 [79] by T. Richie and AMA-1 [80] by A. Thomas. In these latter experiments 60 IU human recombinant IL-2 (Proleukin, Novartis) was added to the culture medium for optimal cellular responses. In all experiments, 100μL/well supernatant was removed 4 hours prior to cell harvest and replaced with 100μL/well fresh culture medium containing Brefeldin A (Sigma) with a final concentration of 10μg/ml.
Depletion/recombination
For recombination experiments, PBMC collected at inclusion (I) and post-challenge (C) from seven donors from Group A and B for whom sufficient cells were available, were divided into two aliquots. For two of these donors, cells from day 35 post-challenge were used and for the other five donors C+140 cells. One aliquot of each sample was depleted of γδT cells by magnetic beads, whereas untouched γδT+ cells were isolated from the second aliquot by negative selection (Anti-TCR γ/δ MicroBead Kit and TCRγ/δ+T Cell Isolation Kit, respectively, both from Miltenyi Biotech), according to the manufacturer’s instructions. Following separation, autologous I/C γδT- and γδT+ cells were recombined at their original ratios. Purity of depletion was consistently >90%, whereas purity of negative selected untouched γδT+ cells varied between 40–80%. The majority of contaminating non-γδT cells in these negatively selected populations consisted of NK and other non-T lymphocytes. Since the proportion of γδT+ cells added directly reflected the proportion of these cells in the PBMC population (I or C) from which they were derived (see also Table S1), this proportion was higher in wells containing C γδT+ cells than in wells containing I γδT+ cells : 1.5 [1.1–2.1], 4.7 [2.6–9.1], 1.4 [1.1–1.8] and 3.9 [2.8–4.0] (% of lymphocytes [IQR]) respectively for I+I, I+C, C+I and C+C.
Intracellular staining for flow cytometry
CD3-CD56-γδT stain (all time points): Following 24 hour of in vitro stimulation, PBMC were harvested and transferred to FACS tubes (250,000 cells/tube), washed once in FACS buffer (0.5% BSA/PBS) and incubated for 15 minutes in 100 µl FACS buffer with fluorochrome-labelled mAbs against the cell-surface markers CD3-PerCP (clone CK7, BD Biosciences), TCR Pan γ/δ-PE (clone IMMU510, Beckman Coulter, Fullerton, CA, USA) and CD56-APC (clone MEM188, eBioscience San Diego, CA, USA). Cells were washed again in FACS buffer and incubated for 15 minutes in 100 µl fixation medium A (Caltag Laboratories, Carlsbad CA) according to the manufacturer's instructions, washed and incubated for 15 minutes with IFNγ-FITC (clone 4S.B3, eBioscience) in 100 µl permeabilization medium B. After a final wash step, cells were re-suspended in FACS buffer and acquired on a FACScalibur flow cytometer (Becton Dickenson). Figure S1.A shows the gating strategy for this staining.
Effector memory phenotyping stain (I-1, C-1, C+9, C+35): Following the procedure described above, cells were stained with CD45RO-PE (clone UCHL1), CD62L-PE-Cy7 (clone DREG56), IFNγ-FITC (clone 4S.B3) and IL-2-APC (clone MQ1-17H12, all eBioscience). Figure S1.B shows the gating strategy for this staining.
Additional T cell phenotyping stain for C+140 and C+400: Following 24 hour of in vitro stimulation, PBMC were harvested and transferred to 96 wells V-plate (500,000 cells/well), washed once in PBS and incubated with 50 µl Live/Dead fixable dead cell stain kit Aqua (Invitrogen, Carlsbad, CA, USA) in PBS for 30 min on 4°C. Cells were washed in PBS and for a second time with FACS buffer (PBS containing 0.5% albumin for bovine serum (Sigma Chemical Co.)), and stained in 50 µl FACS buffer with anti-TCR Pan γ/δ-PE (clone IMMU510, Beckman-Coulter), CD45RO-ECD (clone UCHL1, Beckman-Coulter), CD3-PerCP (Clone UCHT1, BioLegend, San Diego, CA, USA), CD62L-PE-Cy7 (Clone DREG56, eBioscience), CD4-Pacific Blue (Clone OKT4, eBioscience) and CD8a-Alexa-fluor 700 (clone HIT8a, BioLegend) for 20 min at 4°C. After washing, cells were incubated with 50 µl fixation Medium A (Caltag, S. San Francisco, CA, USA) and subsequently, incubated with anti-IFNγ-FITC (clone 4S.B3, eBioscience) and IL-2-APC (Clone MQ1-17H12, eBioscience) in 50 µl permeabilization Medium B (Caltag) for 20 min at 4°C. Lymphocytes (100,000) gated by forward - and side-scatter characteristics were acquired on a CyAn ADP 9-color flow cytometer (Beckman - Coulter). Figure S1.C and S1.D show the gating strategy for this staining.
Flow cytometry analysis
Flow cytometry analysis was performed using Cell Quest and FlowJo V9.1 software. Gating of lymphocytes and subsequent subgroups was performed as shown in Figure S1. Gating of cells positive for IFNγ and/or IL2 was performed using a cut-off based on the geometric mean of cells cultured in medium only.
Statistical analysis
Statistical analysis were performed using GraphPad Prism 5. Differences in responses within volunteers between multiple time points or between stimuli were analyzed by repeated measures one-way ANOVA with Dunnett's or Bonferroni post-hoc test, as appropriate. Paired/repeated measures analysis was carried out exclusively on complete data sets obtained within a single experiment. Two-way analysis with Bonferroni post-test was performed in order to analyze data sets with multiple variables (both time points and stimuli). One donor had to be excluded from all statistical analysis due to an extreme, but highly variable, outlying IFNγ response to PfRBC at time point C+35. All statistical analyses were performed on data corrected for background: background responses were subtracted from the responses to parasite stimuli for every volunteer at every time point individually (PfRBC - uRBC; sporozoite – mosquito salivary gland; parasite antigens – medium only); negative values were set to zero. P-values <0.05 were considered statistically significant in all analyses.
Supporting Information
Zdroje
1. World Health Organization 2008 World Malaria Report 2008. WHO/HTM/GMP/2008.1
2. MarshKKinyanjuiS 2006 Immune effector mechanisms in malaria. Parasite Immunol 28 51 60
3. SchofieldLMuellerI 2006 Clinical immunity to malaria. Curr Mol Med 6 205 221
4. CockburnIAZavalaF 2007 T cell memory in malaria. Curr Opin Immunol 19 424 429
5. DoolanDLDobanoCBairdJK 2009 Acquired immunity to malaria. Clin Microbiol Rev 22 13 36
6. StruikSSRileyEM 2004 Does malaria suffer from lack of memory? Immunol Rev 201 268 290
7. AchtmanAHBullPCStephensRLanghorneJ 2005 Longevity of the immune response and memory to blood-stage malaria infection. Curr Top Microbiol Immunol 297 71 102
8. MigotFChougnetCRaharimalalaLAstagneauPLepersJP 1993 Human immune responses to the Plasmodium falciparum ring-infected erythrocyte surface antigen (Pf155/RESA) after a decrease in malaria transmission in Madagascar. Am J Trop Med Hyg 48 432 439
9. WipasaJOkellLSakkhachornphopSSuphavilaiCChawansuntatiK 2011 Short-lived IFN-gamma effector responses, but long-lived IL-10 memory responses, to malaria in an area of low malaria endemicity. PLoS Pathog 7 e1001281
10. ZeveringYKhamboonruangCRungruengthanakitKTungviboonchaiLRuengpipattanapanJ 1994 Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax. Proc Natl Acad Sci U S A 91 6118 6122
11. BejonPMwacharoJKaiOTodrykSKeatingS 2007 The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol 179 4193 4201
12. DentAEChelimoKSumbaPOSpringMDCrabbBS 2009 Temporal stability of naturally acquired immunity to Merozoite Surface Protein-1 in Kenyan adults. Malar J 8 162
13. FlanaganKLMwangiTPlebanskiMOdhiamboKRossA 2003 Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am J Trop Med Hyg 68 421 430
14. MoormannAMSumbaPOTischDJEmburyPKingCH 2009 Stability of interferon-gamma and interleukin-10 responses to Plasmodium falciparum liver stage antigen 1 and thrombospondin-related adhesive protein immunodominant epitopes in a highland population from Western Kenya. Am J Trop Med Hyg 81 489 495
15. RileyEMMorris-JonesSBlackmanMJGreenwoodBMHolderAA 1993 A longitudinal study of naturally acquired cellular and humoral immune responses to a merozoite surface protein (MSP1) of Plasmodium falciparum in an area of seasonal malaria transmission. Parasite Immunol 15 513 524
16. HviidLTheanderTGJakobsenPHbu-ZeidYAAbdulhadiNH 1990 Cell-mediated immune responses to soluble Plasmodium falciparum antigens in residents from an area of unstable malaria transmission in the Sudan. APMIS 98 594 604
17. WylerDJOppenheimJJ 1974 Lymphocyte transformation in human Plasmodium falciparum malaria. J Immunol 113 449 454
18. TodrykSMWaltherMBejonPHutchingsCThompsonFM 2009 Multiple functions of human T cells generated by experimental malaria challenge. Eur J Immunol 39 3042 3051
19. McCallMBSauerweinRW 2010 Interferon-gamma--central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol 88 1131 1143
20. SauerweinRWRoestenbergMMoorthyVS 2011 Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol 11 57 64
21. PomboDJLawrenceGHirunpetcharatCRzepczykCBrydenM 2002 Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360 610 617
22. RoestenbergMTeirlinckACMcCallMBTeelenKMakamdopKN 2011 Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377 1770 1776
23. RoestenbergMMcCallMHopmanJWiersmaJLutyAJv etal 2009 Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361 468 477
24. DodooDOmerFMToddJAkanmoriBDKoramKA 2002 Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 185 971 979
25. D'OmbrainMCRobinsonLJStanisicDITaraikaJBernardN 2008 Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 47 1380 1387
26. LutyAJLellBSchmidt-OttRLehmanLGLucknerD 1999 Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 179 980 988
27. McCallMBHopmanJDaouMMaigaBDaraV 2010 Early interferon-gamma response against Plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric Fulani and Dogon in Mali. J Infect Dis 201 142 152
28. Artavanis-TsakonasKRileyEM 2002 Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol 169 2956 2963
29. BaratinMRoetynckSLepolardCFalkCSawadogoS 2005 Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc Natl Acad Sci U S A 102 14747 14752
30. McCallMBRoestenbergMPloemenITeirlinckAHopmanJ 2010 Memory-like IFN-gamma response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum. Eur J Immunol 40 3472 3477
31. HensmannMKwiatkowskiD 2001 Cellular basis of early cytokine response to Plasmodium falciparum. Infect Immun 69 2364 2371
32. WaterfallMBlackARileyE 1998 Gammadelta+ T cells preferentially respond to live rather than killed malaria parasites. Infect Immun 66 2393 2398
33. D'OmbrainMCHansenDSSimpsonKMSchofieldL 2007 gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-gamma response to Plasmodium falciparum malaria. Eur J Immunol 37 1864 1873
34. CurrierJSattabongkotJGoodMF 1992 ‘Natural’ T cells responsive to malaria: evidence implicating immunological cross-reactivity in the maintenance of TCR alpha beta+ malaria-specific responses from non-exposed donors. Int Immunol 4 985 994
35. ZeveringYAmanteFSmillieACurrierJSmithG 1992 High frequency of malaria-specific T cells in non-exposed humans. Eur J Immunol 22 689 696
36. RoussilhonCAgrapartMGuglielmiPBensussanABrasseurP 1994 Human TcR gamma delta+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol 95 91 97
37. RzepczykCMStamatiouSAndersonKStowersAChengQ 1996 Experimental human Plasmodium falciparum infections: longitudinal analysis of lymphocyte responses with particular reference to gamma delta T cells. Scand J Immunol 43 219 227
38. HviidLKurtzhalsJADodooDRodriguesORonnA 1996 The gamma/delta T-cell response to Plasmodium falciparum malaria in a population in which malaria is endemic. Infect Immun 64 4359 4362
39. BehrCDuboisP 1992 Preferential expansion of V gamma 9 V delta 2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol 4 361 366
40. BehrCPoupotRPeyratMAPoquetYConstantP 1996 Plasmodium falciparum stimuli for human gammadelta T cells are related to phosphorylated antigens of mycobacteria. Infect Immun 64 2892 2896
41. HorowitzANewmanKCEvansJHKorbelDSDavisDM 2010 Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 184 6043 6052
42. JungTMGallatinWMWeissmanILDaileyMO 1988 Down-regulation of homing receptors after T cell activation. J Immunol 141 4110 4117
43. SallustoFGeginatJLanzavecchiaA 2004 Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22 745 763
44. DarrahPAPatelDTDe LucaPMLindsayRWDaveyDF 2007 Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13 843 850
45. BejonPKeatingSMwacharoJKaiOKDunachieS 2006 Early gamma interferon and interleukin-2 responses to vaccination predict the late resting memory in malaria-naive and malaria-exposed individuals. Infect Immun 74 6331 6338
46. SederRADarrahPARoedererM 2008 T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8 247 258
47. ChizzoliniCGrauGEGeinozASchrijversD 1990 T lymphocyte interferon-gamma production induced by Plasmodium falciparum antigen is high in recently infected non-immune and low in immune subjects. Clin Exp Immunol 79 95 99
48. RheeMSAkanmoriBDWaterfallMRileyEM 2001 Changes in cytokine production associated with acquired immunity to Plasmodium falciparum malaria. Clin Exp Immunol 126 503 510
49. Ocana-MorgnerCMotaMMRodriguezA 2003 Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med 197 143 151
50. KempKAkanmoriBDAdabayeriVGokaBQKurtzhalsJA 2002 Cytokine production and apoptosis among T cells from patients under treatment for Plasmodium falciparum malaria. Clin Exp Immunol 127 151 157
51. RiccioEKJuniorINRiccioLRdas GracasAMCorte-RealS 2003 Malaria associated apoptosis is not significantly correlated with either parasitemia or the number of previous malaria attacks. Parasitol Res 90 9 18
52. OtooLNRileyEMMenonAByassPGreenwoodBM 1989 Cellular immune responses to Plasmodium falciparum antigens in children receiving long term anti-malarial chemoprophylaxis. Trans R Soc Trop Med Hyg 83 778 782
53. BrustoskiKMollerUKramerMHartgersFCKremsnerPG 2006 Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J Infect Dis 193 146 154
54. FinneyOCNwakanmaDConwayDJWaltherMRileyEM 2009 Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur J Immunol 39 1288 1300
55. TorciaMGSantarlasciVCosmiLClementeAMaggiL 2008 Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc Natl Acad Sci U S A 105 646 651
56. WaltherMTongrenJEAndrewsLKorbelDKingE 2005 Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23 287 296
57. BairdJK 1998 Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol 92 367 390
58. SchaibleUEKaufmannSH 2007 Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4 e115
59. HartgersFCObengBBKruizeYCDijkhuisAMcCallM 2009 Responses to malarial antigens are altered in helminth-infected children. J Infect Dis 199 1528 1535
60. WammesLJHamidFWiriaAEdeGBSartonoE 2010 Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur J Immunol 40 437 442
61. SiegristCA 2001 Neonatal and early life vaccinology. Vaccine 19 3331 3346
62. ZolaH 1997 The development of antibody responses in the infant. Immunol Cell Biol 75 587 590
63. BroenKBrustoskiKEngelmannILutyAJ 2007 Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol Biochem Parasitol 151 1 8
64. BucciKKastensWHollingdaleMRShankarAAlpersMP 2000 Influence of age and HLA type on interferon-gamma (IFN-gamma) responses to a naturally occurring polymorphic epitope of Plasmodium falciparum liver stage antigen-1 (LSA-1). Clin Exp Immunol 122 94 100
65. JohnCCMoormannAMSumbaPOOfullaAVPregibonDC 2004 Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect Immun 72 5135 5142
66. RamharterMWinklerHKremsnerPGAdegnikaAAWillheimM 2005 Age-dependency of Plasmodium falciparum-specific and non-specific T cell cytokine responses in individuals from a malaria-endemic area. Eur Cytokine Netw 16 135 143
67. WinklerSWillheimMBaierKSchmidDAichelburgA 1999 Frequency of cytokine-producing T cells in patients of different age groups with Plasmodium falciparum malaria. J Infect Dis 179 209 216
68. KidgellCVolkmanSKDailyJBorevitzJOPlouffeD 2006 A systematic map of genetic variation in Plasmodium falciparum. PLoS Pathog 2 e57
69. SauerweinRWBijkerEMRichieTL 2010 Empowering malaria vaccination by drug administration. Curr Opin Immunol 22 367 373 S0952-7915(10)00065-8 [pii];10.1016/j.coi.2010.04.001 [doi]
70. RivadeneiraEMWassermanMEspinalCT 1983 Separation and concentration of schizonts of Plasmodium falciparum by Percoll gradients. J Protozool 30 367 370
71. PonnuduraiTLensenAHvan GemertGJBensinkMPBolmerM 1989 Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98 Pt 2 165 173
72. StewartMJVanderbergJP 1988 Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool 35 389 393
73. VerhageDFTelgtDSBousemaJTHermsenCCvan GemertGJv etal 2005 Clinical outcome of experimental human malaria induced by Plasmodium falciparum-infected mosquitoes. Neth J Med 63 52 58
74. NardinEHOliveiraGACalvo-CalleJMWetzelKMaierC 2004 Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect Immun 72 6519 6527
75. FlueckCFrankGSmithTJafarshadANebieI 2009 Evaluation of two long synthetic merozoite surface protein 2 peptides as malaria vaccine candidates. Vaccine 27 2653 2661
76. MeraldiVNebieIMoretRCuzin-OuattaraNThioconeA 2002 Recognition of synthetic polypeptides corresponding to the N - and C-terminal fragments of Plasmodium falciparum Exp-1 by T-cells and plasma from human donors from African endemic areas. Parasite Immunol 24 141 150
77. AudranRCachatMLuratiFSoeSLeroyO 2005 Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect Immun 73 8017 8026
78. HermsenCCVerhageDFTelgtDSTeelenKBousemaJT 2007 Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine 25 2930 2940
79. HillierCJWareLABarbosaAAngovELyonJA 2005 Process development and analysis of liver-stage antigen 1, a preerythrocyte-stage protein-based vaccine for Plasmodium falciparum. Infect Immun 73 2109 2115
80. RoestenbergMRemarqueEdeJEHermsenRBlythmanH 2008 Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS One 3 e3960
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion DiseaseČlánek Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) VirusesČlánek Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance toČlánek Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a TombusvirusČlánek Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic PhenotypesČlánek Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence
- Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion Disease
- Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis
- Nutritional Immunology: A Multi-Dimensional Approach
- Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) Viruses
- Vaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
- Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly
- Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance to
- Computational and Biochemical Analysis of the Effector AvrBs2 and Its Role in the Modulation of Type Three Effector Delivery
- Granzyme B Inhibits Vaccinia Virus Production through Proteolytic Cleavage of Eukaryotic Initiation Factor 4 Gamma 3
- Association of Activating KIR Copy Number Variation of NK Cells with Containment of SIV Replication in Rhesus Monkeys
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa
- Follicular Dendritic Cell-Specific Prion Protein (PrP) Expression Alone Is Sufficient to Sustain Prion Infection in the Spleen
- Autophagy Protein Atg3 is Essential for Maintaining Mitochondrial Integrity and for Normal Intracellular Development of Tachyzoites
- Longevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
- Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence
- Acquisition of Pneumococci Specific Effector and Regulatory Cd4 T Cells Localising within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue
- The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites
- Deficiency of a Niemann-Pick, Type C1-related Protein in Is Associated with Multiple Lipidoses and Increased Pathogenicity
- Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
- Eight RGS and RGS-like Proteins Orchestrate Growth, Differentiation, and Pathogenicity of
- Prion Uptake in the Gut: Identification of the First Uptake and Replication Sites
- Nef Decreases HIV-1 Sensitivity to Neutralizing Antibodies that Target the Membrane-proximal External Region of TMgp41
- Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a Tombusvirus
- A Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
- The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
- HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency
- Neonatal CD8 T-cell Hierarchy Is Distinct from Adults and Is Influenced by Intrinsic T cell Properties in Respiratory Syncytial Virus Infected Mice
- Two Novel Transcriptional Regulators Are Essential for Infection-related Morphogenesis and Pathogenicity of the Rice Blast Fungus
- Five Questions about Non-Mevalonate Isoprenoid Biosynthesis
- The Human Cytomegalovirus UL11 Protein Interacts with the Receptor Tyrosine Phosphatase CD45, Resulting in Functional Paralysis of T Cells
- Wall Teichoic Acids of Limit Recognition by the Drosophila Peptidoglycan Recognition Protein-SA to Promote Pathogenicity
- A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen
- Inflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
- CNS Recruitment of CD8+ T Lymphocytes Specific for a Peripheral Virus Infection Triggers Neuropathogenesis during Polymicrobial Challenge
- Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic Phenotypes
- A Receptor-based Switch that Regulates Anthrax Toxin Pore Formation
- Targeting of Heparin-Binding Hemagglutinin to Mitochondria in Macrophages
- Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants
- Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
- Structural Elucidation and Functional Characterization of the Effector Protein ATR13
- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection
- Acid Stability of the Hemagglutinin Protein Regulates H5N1 Influenza Virus Pathogenicity
- Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons
- A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection
- Juxtamembrane Shedding of AMA1 Is Sequence Independent and Essential, and Helps Evade Invasion-Inhibitory Antibodies
- Pathogenesis and Host Response in Syrian Hamsters following Intranasal Infection with Andes Virus
- IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network
- Epstein-Barr Virus Evades CD4 T Cell Responses in Lytic Cycle through BZLF1-mediated Downregulation of CD74 and the Cooperation of vBcl-2
- Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction
- Late Repression of NF-κB Activity by Invasive but Not Non-Invasive Meningococcal Isolates Is Required to Display Apoptosis of Epithelial Cells
- Polar Flagellar Biosynthesis and a Regulator of Flagellar Number Influence Spatial Parameters of Cell Division in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- The Enteropathogenic (EPEC) Tir Effector Inhibits NF-κB Activity by Targeting TNFα Receptor-Associated Factors
- Toward an Integrated Model of Capsule Regulation in
- A Systematic Screen to Discover and Analyze Apicoplast Proteins Identifies a Conserved and Essential Protein Import Factor
- A Host Small GTP-binding Protein ARL8 Plays Crucial Roles in Tobamovirus RNA Replication
- Comparative Pathobiology of Fungal Pathogens of Plants and Animals
- Synergistic Roles of Eukaryotic Translation Elongation Factors 1Bγ and 1A in Stimulation of Tombusvirus Minus-Strand Synthesis
- Engineered Immunity to Infection
- Inflammatory Monocytes and Neutrophils Are Licensed to Kill during Memory Responses
- Sialidases Affect the Host Cell Adherence and Epsilon Toxin-Induced Cytotoxicity of Type D Strain CN3718
- Eurasian-Origin Gene Segments Contribute to the Transmissibility, Aerosol Release, and Morphology of the 2009 Pandemic H1N1 Influenza Virus
- SARS Coronavirus nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of mRNAs: Viral mRNAs Are Resistant to nsp1-Induced RNA Cleavage
- Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus
- Functional Analysis of the Kinome of the Wheat Scab Fungus
- Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- Engineered Immunity to Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



