-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCe-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
Infected animals will produce reactive oxygen species (ROS) and other inflammatory molecules that help fight pathogens, but can inadvertently damage host tissue. Therefore specific responses, which protect and repair against the collateral damage caused by the immune response, are critical for successfully surviving pathogen attack. We previously demonstrated that ROS are generated during infection in the model host Caenorhabditis elegans by the dual oxidase Ce-Duox1/BLI-3. Herein, an important connection between ROS generation by Ce-Duox1/BLI-3 and upregulation of a protective transcriptional response by SKN-1 is established in the context of infection. SKN-1 is an ortholog of the mammalian Nrf transcription factors and has previously been documented to promote survival, following oxidative stress, by upregulating genes involved in the detoxification of ROS and other reactive compounds. Using qRT-PCR, transcriptional reporter fusions, and a translational fusion, SKN-1 is shown to become highly active in the C. elegans intestine upon exposure to the human bacterial pathogens, Enterococcus faecalis and Pseudomonas aeruginosa. Activation is dependent on the overall pathogenicity of the bacterium, demonstrated by a weakened response observed in attenuated mutants of these pathogens. Previous work demonstrated a role for p38 MAPK signaling both in pathogen resistance and in activating SKN-1 upon exposure to chemically induced oxidative stress. We show that NSY-1, SEK-1 and PMK-1 are also required for SKN-1 activity during infection. Evidence is also presented that the ROS produced by Ce-Duox1/BLI-3 is the source of SKN-1 activation via p38 MAPK signaling during infection. Finally, for the first time, SKN-1 activity is shown to be protective during infection; loss of skn-1 decreases resistance, whereas increasing SKN-1 activity augments resistance to pathogen. Overall, a model is presented in which ROS generation by Ce-Duox1/BLI-3 activates a protective SKN-1 response via p38 MAPK signaling.
Published in the journal: . PLoS Pathog 7(12): e32767. doi:10.1371/journal.ppat.1002453
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002453Summary
Infected animals will produce reactive oxygen species (ROS) and other inflammatory molecules that help fight pathogens, but can inadvertently damage host tissue. Therefore specific responses, which protect and repair against the collateral damage caused by the immune response, are critical for successfully surviving pathogen attack. We previously demonstrated that ROS are generated during infection in the model host Caenorhabditis elegans by the dual oxidase Ce-Duox1/BLI-3. Herein, an important connection between ROS generation by Ce-Duox1/BLI-3 and upregulation of a protective transcriptional response by SKN-1 is established in the context of infection. SKN-1 is an ortholog of the mammalian Nrf transcription factors and has previously been documented to promote survival, following oxidative stress, by upregulating genes involved in the detoxification of ROS and other reactive compounds. Using qRT-PCR, transcriptional reporter fusions, and a translational fusion, SKN-1 is shown to become highly active in the C. elegans intestine upon exposure to the human bacterial pathogens, Enterococcus faecalis and Pseudomonas aeruginosa. Activation is dependent on the overall pathogenicity of the bacterium, demonstrated by a weakened response observed in attenuated mutants of these pathogens. Previous work demonstrated a role for p38 MAPK signaling both in pathogen resistance and in activating SKN-1 upon exposure to chemically induced oxidative stress. We show that NSY-1, SEK-1 and PMK-1 are also required for SKN-1 activity during infection. Evidence is also presented that the ROS produced by Ce-Duox1/BLI-3 is the source of SKN-1 activation via p38 MAPK signaling during infection. Finally, for the first time, SKN-1 activity is shown to be protective during infection; loss of skn-1 decreases resistance, whereas increasing SKN-1 activity augments resistance to pathogen. Overall, a model is presented in which ROS generation by Ce-Duox1/BLI-3 activates a protective SKN-1 response via p38 MAPK signaling.
Introduction
Infection by pathogenic microorganisms requires a coordinated response from the host to cope with the multitude of physiological challenges presented by the attack. In addition to producing compounds that have direct antimicrobial activity and countering pathogen virulence strategies, the host must also initiate stress responses to protect cellular resources and processes from the negative consequences of “friendly-fire.” Damage, disease, and sometimes death of the host can occur if immune responses are not controlled or protected against properly. Septic shock and various autoimmune diseases are examples of immune responses gone awry. In this work we explore the connections between infection, immune response and cellular stress response using the well-studied model host Caenorhabditis elegans.
A general response to microbial challenge that most animals possess is the production of reactive oxygen species (ROS). The best-studied example is the production of ROS as an antimicrobial response in the phagolysosome of phagocytic cells by the NADPH oxidase gp91phox. However, this response is not limited to phagocytes, and NADPH oxidases are present in the skin as well as the mucosal epithelium of the oral cavity, respiratory and gastrointestinal tracts of humans [1], [2]. Less complex organisms that lack innate immune cells, such as C. elegans, also encode for NADPH oxidases. For instance, the dual oxidase Ce-Duox1/BLI-3 is present in the hypodermis and in the intestine of C. elegans [3], [4]. Our laboratory and others recently demonstrated that an intestinal infection triggers the release of ROS by Ce-Duox1/BLI-3 in what appears to be a protective response [3], [5]. Presumably due to the production of ROS however, there was also evidence of cellular damage as shown by lipofuscin accumulation and loss of protein homeostasis, which was worsened by the knock-down of certain oxidative stress enzymes [6], [7]. The goal of this work was to determine if infection, by triggering ROS release by Ce-Duox1/BLI-3, induces an oxidative stress response in the host as part of the overall response to the pathogen.
SKN-1 is a transcription factor that senses oxidative stress and functionally affects resistance to oxidative stress and lifespan in C. elegans. It is an Nrf ortholog, a protein family found in all eukaryotes that upregulates the Phase 2 genes of the three-phase detoxification system [8]. Phase 2 genes encode enzymes that defend against ROS and other reactive compounds [9]. A large number of genes are regulated by SKN-1, including many glutathione-S-transferases that are important for detoxifying reactive compounds such as xenobiotics and peroxidized lipids by conjugating glutathione to electrophilic centers [10], [11].
SKN-1 transcriptional activity is regulated by phosphorylation by the p38 MAPK, PMK-1, which promotes its localization to the nucleus [12]. Interestingly, PMK-1 is a major regulator of C. elegans innate immunity and loss of this protein results in a strong susceptibility phenotype [13]. Work on PMK-1 showed that it is regulated by a phosphorylation cascade involving the activation of a Toll/IL-1 receptor (TIR) domain protein, TIR-1, which leads to the activation of a MAPKKK called NSY-1, which then activates a MAPKK called SEK-1, culminating in PMK-1 phosphorylation [13], [14]. Phosphorylation of the transcription factor ATF-7 by PMK-1 is thought to ultimately promote innate immune gene expression by turning this repressor of innate immune gene expression into an activator [15]. While SEK-1 is also required for activation of SKN-1 by oxidative stress [12], the roles of TIR-1 and NSY-1 are controversial. One report shows that NSY-1 and TIR-1 are dispensable for activation of PMK-1 in response to oxidative stress created by sodium arsenite [12], whereas two other publications show that NSY-1 is required for resistance to oxidative stress caused by paraquat [16]–[18].
In earlier studies, loss of SKN-1 was not observed to affect the overall susceptibility of the worm to infection by Pseudomonas aeruginosa, and it was postulated that the oxidative stress transcriptional response mediated by SKN-1 is not involved in pathogen defense [15], [19]. However, because our data suggested that oxidative stress is present during infection [3], [6], [7], we postulated that SKN-1 is activated and conducted experiments to investigate this hypothesis.
Specifically, this study examined SKN-1 directed gene expression and localization in C. elegans infected with Pseudomonas aeruginosa and Enterococcus faecalis. We establish that bacterial infection stimulates SKN-1 activity in a manner dependent on the p38 MAPK signaling pathway. We show that components of the p38 MAPK signaling pathway previously established as necessary for responding to pathogen are involved, including NSY-1, SEK-1 and PMK-1, but not TIR-1. In contrast to previous work, we find that loss of SKN-1 activity increases susceptibility to the pathogens whereas constitutive activation results in increased resistance. Of key significance is the demonstration that ROS produced by Ce-Duox1/BLI-3 is the source of oxidative stress triggering SKN-1 activity during infection. Overall, this work establishes that a protective SKN-1 response is activated during infection via p38 MAPK signaling as a result of the mucosal oxidative burst generated by the host.
Results
Pathogen Infection Induces Expression of SKN-1 Controlled Genes
Previous work in our laboratory demonstrated that C. elegans releases significant amounts of ROS during infection and that expression of oxidative stress response genes such as sod-3 is induced [6]. Additionally, we have shown that cells at the site of the infection, the intestine, display a loss of protein homeostasis indicative of cellular stress [7]. SKN-1 is a transcription factor that responds to cellular stressors, including ROS [8], and we predicted that its activity is likely to be induced as a result of pathogen exposure. We tested genes previously characterized as being regulated by SKN-1, including gst-4, gst-5, gst-7, gst-10 and gcs-1. The gst genes encode for glutathione-S-transferases, which are important for detoxifying reactive compounds by conjugating reduced glutathione to electrophilic centers, while gcs-1 encodes for gamma-glutamine cysteine synthetase heavy chain, a protein involved in glutathione biosynthesis [10], [11], [20]. Expression of these genes was examined by qRT-PCR following a 24-hour exposure of L4 worms to both P. aeruginosa and E. faecalis (Figure 1A). All of the reporter genes were induced between two and five-fold more when the animals were feeding on the pathogens, as compared to those feeding on their normal laboratory food source (E. coli OP50). As shown in Figure 1B, in which exposure of the animals to skn-1 RNAi preceded exposure to E. faecalis, the induction was prevented, indicating that expression of these genes retains dependence on SKN-1 under conditions of pathogen exposure.
Fig. 1. SKN-1 dependent genes are activated in response to pathogens. 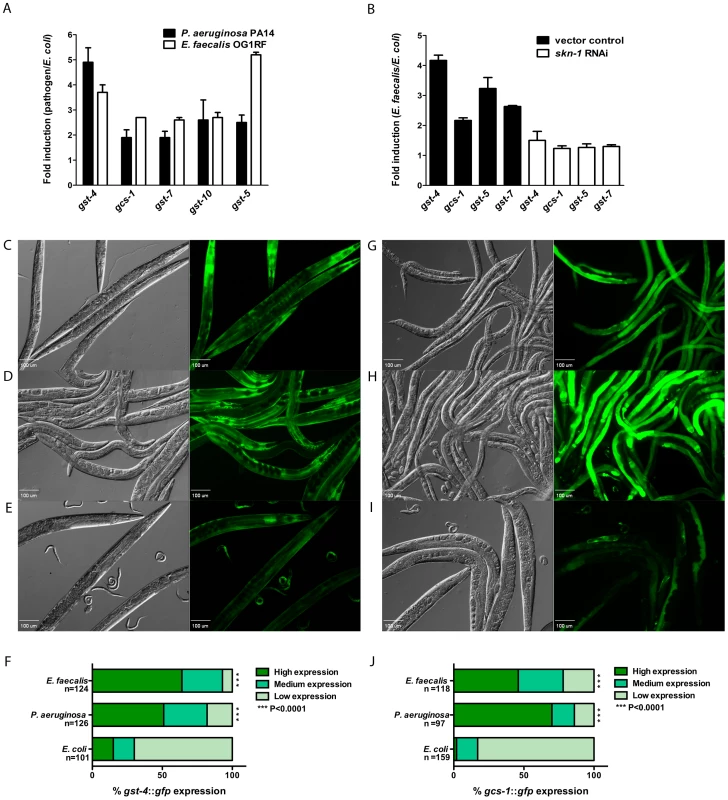
(A & B) qRT-PCR analysis of SKN-1 dependent genes induced in worms fed for 24 hours on E. faecalis OG1RF, P. aeruginosa PA14 and E. coli OP50, and in skn-1 and control RNAi worms fed on E. faecalis OG1RF and E. coli OP50. Experiments were performed with three separate replicates; each replicate was measured in duplicate and normalized to the control gene act-1. Error bars represent the standard error of the mean (SEM). (C - E) The expression pattern of gst-4::gfp in worms exposed to E. faecalis OG1RF, P. aeruginosa PA14 and E. coli OP50 for 18 hours. (F) The level of gst-4::gfp expression was scored as described in Materials and Methods and the percentage of worms in each category is indicated along with the number of worms observed (n). (G - I) The expression pattern of gcs-1::gfp in worms exposed to E. faecalis OG1RF, P. aeruginosa PA14 and E. coli OP50 for 18 hours. (J) The level of gcs-1::gfp expression was scored as described in Materials and Methods and the percentage of worms in each category is indicated along with the number of worms observed (n). In the micrographs, Normaski and fluorescent views of the worms are depicted. E. faecalis OG1RF and P. aeruginosa PA14 induced significantly higher levels of gst-4::gfp and gcs-1::gfp (P<0.0001) compared to E. coli OP50. The Blackwell and Johnson laboratories have generated several C. elegans strains containing GFP reporter fusions to genes that are markers for SKN-1 activity including gst-4 [21], gcs-1 [8], [22] and gst-7 [20]. We obtained these strains to further examine pathogen-induced SKN-1 activity. L4 animals were exposed to P. aeruginosa, E. faecalis or non-pathogenic E. coli for 18 hours. Fluorescent micrographs were taken, and the animals were scored for low, medium or high expression of the reporters as described in Methods. For each condition, several worms in a representative micrograph are shown (Figures 1C-E, 1G-I), and additionally the quantification of the scoring with statistical analysis is included (Figures 1F and 1J). Figures 1C-F show that significantly more animals had high levels of expression of gst-4::gfp when infected with E. faecalis (Figure 1C) and P. aeruginosa (Figure 1D) than when allowed to feed on E. coli (Figure 1E). Significantly higher expression of gcs-1::gfp was also observed on the pathogenic strains compared to the controls as shown in Figures 1G-J. We also examined the levels of GST-7::GFP using a transgenic strain containing a translational fusion of gfp to the gst-7 gene, and again we observed higher levels when the animals were exposed to the pathogens (Figure S1 in Text S1). To ensure that the expression was SKN-1 dependent, the levels of fluorescence were observed in strains exposed to skn-1 RNAi prior to pathogen exposure; the pathogen-induced increase in fluorescence was abolished, supporting the use of these reporters as read-outs for SKN-1 expression levels (see below, S2E in Text S1 and data not shown). In independent experiments looking at number of transcripts by RNAseq experiments that examine transcript numbers in worms exposed to another human pathogen, Staphylococcus aureus, both gcs-1 and gst-4 were expressed at higher levels compared to animals on E. coli (Javier Irazoqui, personal communication, data not shown). In conclusion, several different experimental methodologies indicate that SKN-1 regulated genes have increased levels of expression in C. elegans fed on pathogenic bacteria.
Attenuated Pathogen Mutants Reduce Induction of SKN-1 Controlled Genes
To investigate whether or not the strength of the SKN-1 response is affected by any known bacterial virulence factors, we tested some well-studied mutants. GacA is a response regulator in P. aeruginosa that strongly affects the virulence of this pathogen and a gacA mutant is greatly attenuated in a variety of hosts including C. elegans [23]. As shown in Figure 2B, a gacA deletion mutant significantly reduced the expression level of the gcs-1::gfp reporter as compared to the isogenic parental strain of P. aeruginosa shown in Figure 2A. One of the GacA-dependent virulence factors is pyocyanin [24], a secreted compound that is redox active and has been shown to oxidatively stress host cells [25]–[27]. We examined a pyocyanin-deficient mutant, which is deleted in phzM, a gene that encodes an enzyme critical for pyocyanin biosynthesis [28], [29]. As seen in Figure 2C, this mutant also results in less gcs-1::gfp reporter expression as compared to wild-type. The quantification of the effects of these P. aeruginosa mutants on gcs-1::gfp expression is shown in Figure 2D. In E. faecalis, FsrB is a component of a quorum sensing system that has been found to affect virulence in every animal model studied, including C. elegans [30], [31]. An fsrB deletion mutant also resulted in less SKN-1 activity, as measured by gst-4::gfp expression compared to the isogenic parental strain (Figure 2E-G). These results show that the level of SKN-1 activity is sensitive to the presence or absence of major virulence factors and that SKN-1 activity may be a way to discern the overall virulence of the infecting organism.
Fig. 2. Expression of SKN-1 regulated genes is reduced in response to attenuated mutants of P. aeruginosa PA14 and E. faecalis OG1RF. 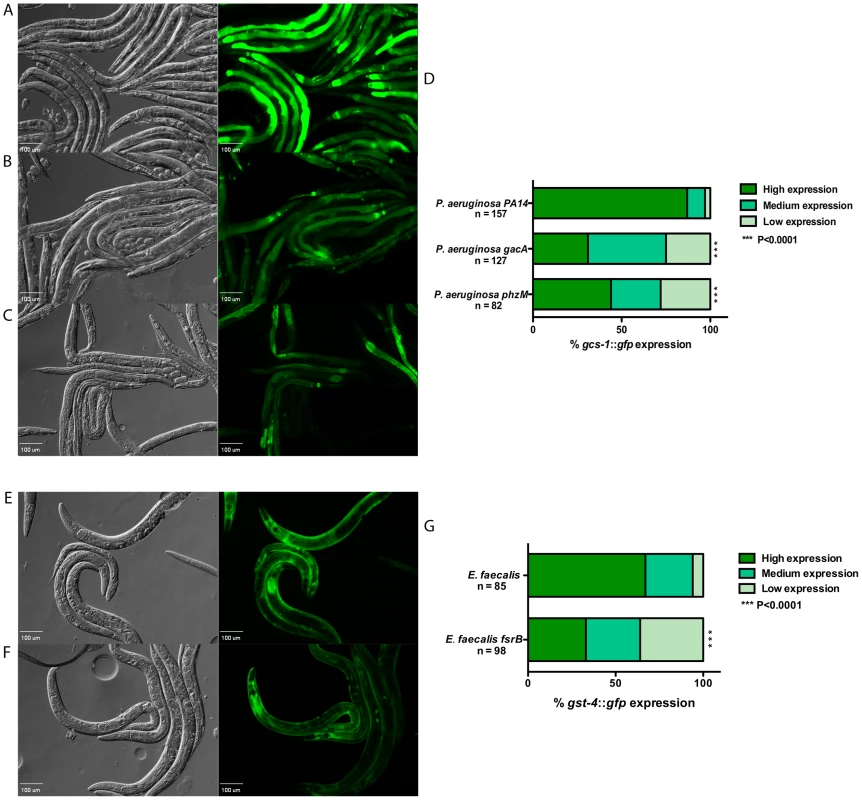
(A – C) The expression of gcs-1::gfp in worms fed on wild-type P. aeruginosa PA14, and mutant strains gacA and phzM for 18 hours. (D) The level of gcs-1::gfp expression was scored and the percentage of worms in each category is indicated along with the number of worms observed (n). (E & F) The expression of gst-4::gfp in worms fed on wild-type E. faecalis OG1RF and an fsrB mutant strain for 18 hours. (G) The level of gst-4::gfp expression was scored and the percentage of worms in each category is indicated along with the number of worms observed (n). In the micrographs, Normaski and fluorescent views of the worms are depicted. Wild-type strains induced significantly higher levels of gst-4::gfp and gcs-1::gfp expression (P<0.0001) compared to mutant strains of the pathogens. Pathogen Exposure Induces SKN-1 Nuclear Localization
SKN-1 activity is regulated by localization to the nucleus [8], [32]. If pathogen exposure increases SKN-1 activity, one would expect to see localization of this transcription factor to the nucleus. We obtained a SKN-1B/C::GFP transgenic line used in previous studies to examine SKN-1 activity and localization [8]. As shown in Figure 3, exposure to P. aeruginosa (Figure 3A) or E. faecalis (Figure 3B) causes significant nuclear localization of SKN-1B/C::GFP, similar to what is observed by exposure to paraquat (Figure 3C), a chemical that causes oxidative stress and is documented to promote SKN-1 nuclear localization [8]. In contrast, when feeding on their normal laboratory food source, the animals do not display significant SKN-1B/C::GFP nuclear localization (Figure 3D).
Fig. 3. SKN-1 is localized to the nuclei of the intestinal cells in response to the pathogens. 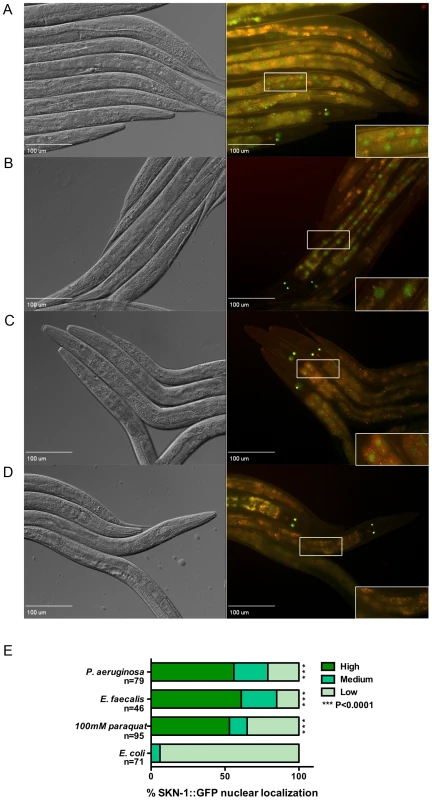
Worms integrated with SKN-1B/C::GFP transgene were exposed to (A) P. aeruginosa PA14 for 6 hrs, (B) E. faecalis OG1RF for 24 hrs, (C) 100 mM paraquat for 30 minutes and (D) E. coli OP50 for 24 hrs. SKN-1B/C::GFP localization was observed by fluorescence microscopy. Close-ups of the boxed region are shown in the lower right-hand corner of each fluorescent image. (E) The degree of nuclear localization was scored as described in Materials and Methods and is given as a percentage for each category. The number of worms used in scoring each experimental condition is indicated (n). Significantly higher nuclear localization of SKN-1B/C::GFP was observed in worms exposed to the pathogens and paraquat (P<0.0001) compared to E. coli OP50. Pathogen-Induced Expression of SKN-1 Genes Is Dependent on p38 MAPK Signaling
Utilizing chemicals known to generate oxidative stress (paraquat, sodium arsenite and t-butyl peroxide), previous work demonstrated that activation of SKN-1 is dependent on p38 MAPK signaling components [12], [16], [17]. We asked whether or not pathogen-induced activation of SKN-1 is also dependent on p38 MAPK signaling. To investigate this question, the levels of GFP were scored in the gst-4::gfp transgenic worms after RNAi knock-down of the genes-of-interest followed by 18 hours of exposure to E. faecalis (Figure 4). Additionally, we assayed gcs-1::gfp transgenics exposed to P. aeruginosa following RNAi (Figure S2 in Text S1). As a control, the effects of RNAi knock-down on animals exposed to the non-pathogenic control, E. coli, were also assayed and found to be minimal (Table S1 in Text S1).
Fig. 4. Activation of SKN-1 in response to pathogens is dependent on the p38-MAPK pathway. 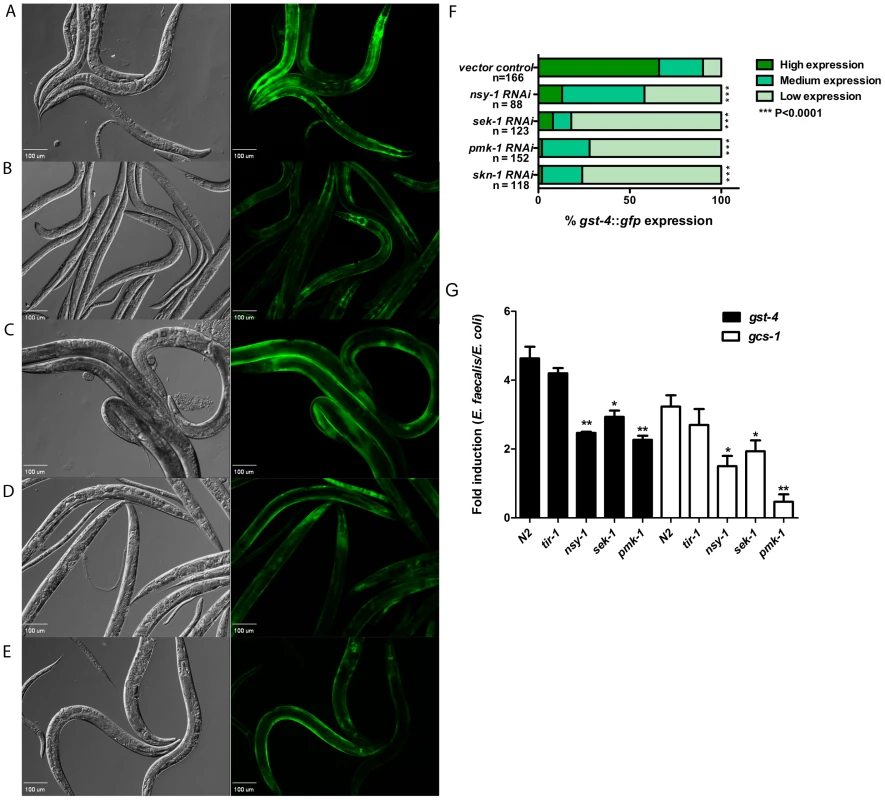
Representative Nomarski and fluorescent views of worms expressing gst-4::gfp exposed to (A) control, (B) nsy-1, (C) sek-1, (D) pmk-1, and (E) skn-1 RNAi prior to feeding E. faecalis OG1RF for 18 hours. (F) The level of gst-4::gfp expression was scored and the percentage of worms in each category is indicated along with the number of worms observed (n). Significantly higher levels of expression of gst-4::gfp were observed in worms exposed to the vector control RNAi (P<0.0001) as compared to nsy-1, sek-1, pmk-1 and skn-1 RNAi. (G) qRT-PCR analysis of SKN-1 dependent genes gst-4 and gcs-1 induced in N2, tir-1(qd4)III, nsy-1(ag3)II, sek-1(ag1)X and pmk-1(km25)IV worms exposed to E. faecalis OG1RF and E. coli OP50 for 24 hours. Significantly lower levels of gst-4 and gcs-1 gene expression were observed in the nsy-1(ag3)II, sek-1(ag1) and pmk-1(km25) compared to the N2 strain. * P<0.01 and ** P<0.001 respectively. The p38 MAPK, PMK-1, and the upstream MAPKK, SEK-1, are absolutely necessary to activate SKN-1 as a result of oxidative stress [12]. As shown in Figure 4, reduction in the expression of sek-1 (Figure 4C) or pmk-1 (Figure 4D) by RNAi prior to exposure to E. faecalis resulted in significantly less fluorescence of the SKN-1 dependent reporter, gst-4::gfp, in the worm intestine, with similar levels to the skn-1 RNAi control (Figure 4E). These data suggest that PMK-1 and SEK-1 are also crucial for SKN-1 activation as a result of pathogen exposure. RNAi of these genes had similar effects on gcs-1::gfp transgenics exposed to P. aeruginosa (Figure S2C and S2D in Text S1).
In addition to its role in responding to oxidative stress, it was previously demonstrated that PMK-1 is activated by pathogen exposure and plays a very important role in host defense. The infection protective activity of PMK-1 is dependent not only on SEK-1, but also on the upstream MAPKKK NSY-1 and the Toll/IL-1 receptor (TIR) domain protein, TIR-1 [13], [14]. The role of NSY-1 and TIR-1 in PMK-1 activation under conditions of oxidative stress is less clear and may be dependent on the stressor utilized [12], [16], [17]. To investigate the possible roles of NSY-1 and TIR-1 on SKN-1 activation during pathogen exposure, we exposed the animals to nsy-1 or tir-1 RNAi prior to placing them on pathogen. Knock-down of nsy-1 caused loss of SKN-1 activity on both pathogens as assayed by the gst-4::gfp and gcs-1::gfp reporters, (Figure 4B, Figure S2B in Text S1). In contrast, knock-down of tir-1, resulted in only a non-statistically significant trend towards less expression, suggesting no, to minimal involvement, at best (Figure S3B in Text S1 and data not shown).
To confirm the RNAi results using another methodology, we also examined expression in genetic mutants using qRT-PCR to measure the expression of gst-4 and gcs-1. In null mutants of nsy-1, sek-1 and pmk-1, expression of these two genes was down relative to wild-type worms on both E. faecalis and P. aeruginosa (Figure 4G). In contrast, expression levels were not significantly different in a tir-1 mutant.
Pathogen-Induced Expression of SKN-1 Genes Is Dependent on Ce-Duox1/BLI-3
Our previous studies demonstrated that part of the response of the worm to pathogen challenge is the release of ROS into the intestinal lumen by the dual oxidase Ce-Duox1/BLI-3. Rather than a tangential consequence of cell death, this response is purposeful and protective [3], [6]. Since SKN-1 is known to respond to oxidative stress, and the above-described experiments conducted on the p38 MAPK signaling pathway are most consistent with an oxidative stress response, we postulated that the ROS generated by Ce-Duox1/BLI-3 during pathogen exposure may trigger signaling through the p38 MAPK pathway, resulting in SKN-1 activation. To test this, we knocked down the expression of bli-3 by RNAi in the gst-4::gfp background and then exposed the strain to E. faecalis. As shown in Figure 5A-C, SKN-1 activity is reduced to a level comparable to worms exposed to skn-1 RNAi (Figure 4E), suggesting that the presence of Ce-Duox1/BLI-3 is necessary for SKN-1 activity. We propose that the ROS produced by Ce-Duox1/BLI-3 in response to pathogens and the resulting oxidative stress activates SKN-1 activity.
Fig. 5. SKN-1 dependent genes are induced in response to ROS produced by Ce-Duox1/BLI-3 during infection. 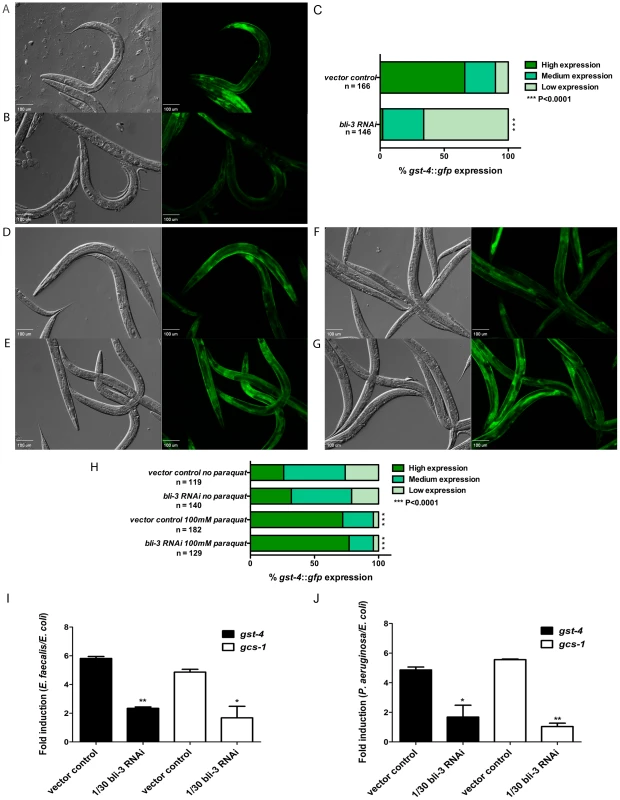
Expression of gst-4::gfp in worms exposed to vector control (A) and 1/30 bli-3 (B) RNAi prior to feeding on E. faecalis OG1RF for 18 hours. Representative Normaski and fluorescent views of the worms are depicted. (C) The level of gst-4::gfp expression was scored and the percentage of worms in each category is indicated along with the number of worms observed (n). Significantly higher levels of expression of gst-4::gfp were observed in worms exposed to the control (P<0.0001) compared to 1/30 bli-3 RNAi prior to exposure to the pathogen. Images of vector control (D and E) and 1/30 bli-3 (F and G) RNAi worms expressing gst-4::gfp in the presence of M9 (D and F) and paraquat (E and G) for 30 minutes. (H) The level of gst-4::gfp expression was scored and the percentage of worms in each category is indicated along with the number of worms observed (n). No significant difference in expression was observed between the control and the 1/30 bli-3 RNAi worms when exposed to paraquat (P = 0.5694). Similar results were obtained in three independent replicates of each experiment. qRT-PCR analysis of SKN-1 dependent genes gst-4 and gcs-1 induced in 1/30 bli-3 RNAi and control worms when exposed to E. faecalis OG1RF (I) and P. aeruginosa PA14 (J) compared to E. coli OP50 for 6 hours. Significantly lower levels of gst-4 and gcs-1 expression were observed in 1/30 bli-3 knockdown animals on both pathogens compared to the control. * P<0.01 and ** P<0.001 respectively. To test if SKN-1 activity could be rescued in the bli-3 knock-down worms by providing ROS from an alternative source, we exposed the animals to paraquat. Paraquat generates ROS by redox cycling in vivo and has been used in previous studies as a trigger for SKN-1 activity in C. elegans [8]. L4 worms were exposed to either 1/30 bli-3 RNAi or vector control RNAi and were placed for 30 minutes in M9 solution with or without 100 mM of paraquat. As shown in Figure 5E, SKN-1 activity, as measured by fluorescence of the gst-4::gfp fusion, was activated in response to paraquat in worms fed vector control RNAi, in agreement with previous work [8]. Knock-down of bli-3 had no effect on activation of SKN-1 by paraquat (Figure 5G). These data indicate that SKN-1 activation induced by a chemical ROS generator does not require Ce-Duox1/BLI-3, unlike activation by pathogen exposure.
We were unable to perform these experiments on P. aeruginosa using the gfp-expressing transgenics because we discovered that the bli-3 knock-down caused a severe susceptibility phenotype on P. aeruginosa, much more severe than on E. faecalis, and more than half the worms were dead by the 24 hour time point (see below). Instead we used qRT-PCR to look at SKN-1 regulated genes on both E. faecalis and P. aeruginosa at an earlier time point (6 hours). By this methodology, we observed that both gst-4 and gcs-1 expression was significantly reduced in the bli-3 knock-downs compared to wild-type on both pathogens (Figure 5I and 5J). In conclusion, our results support a model in which ROS generated by Ce-Duox1/BLI-3 as a result of pathogen exposure trigger SKN-1 activity.
SKN-1 Influences Pathogen Susceptibility
In previous investigations, loss of skn-1 did not influence susceptibility to P. aeruginosa [15], [19]. One study examined loss of skn-1 by RNAi [19]. However, in this work the animals were not exposed to RNAi until the L4 stage, at which point RNAi is likely less effective since the SKN-1 protein is produced in significant quantities during larval development [8]. We reduced skn-1 expression using RNAi, but began the exposure at the L1 stage and continued exposure through the L4 stage. Following this experimental procedure, the animals exhibited a statistically significant and reproducible susceptibility phenotype to both E. faecalis (Figure 6C, Table S2A in Text S1) and P. aeruginosa (Table S2B in Text S1). We additionally tested the possible role in susceptibility of some individual SKN-1 targets such as gst-4, gst-7 and gcs-1 by reducing the expression of these genes by RNAi. A significant difference compared to control RNAi was not observed, suggesting that these are not the critical SKN-1 targets or more than one gene contributes to SKN-1 pathogen resistance (data not shown).
Fig. 6. SKN-1 is required for survival of worms during infection. 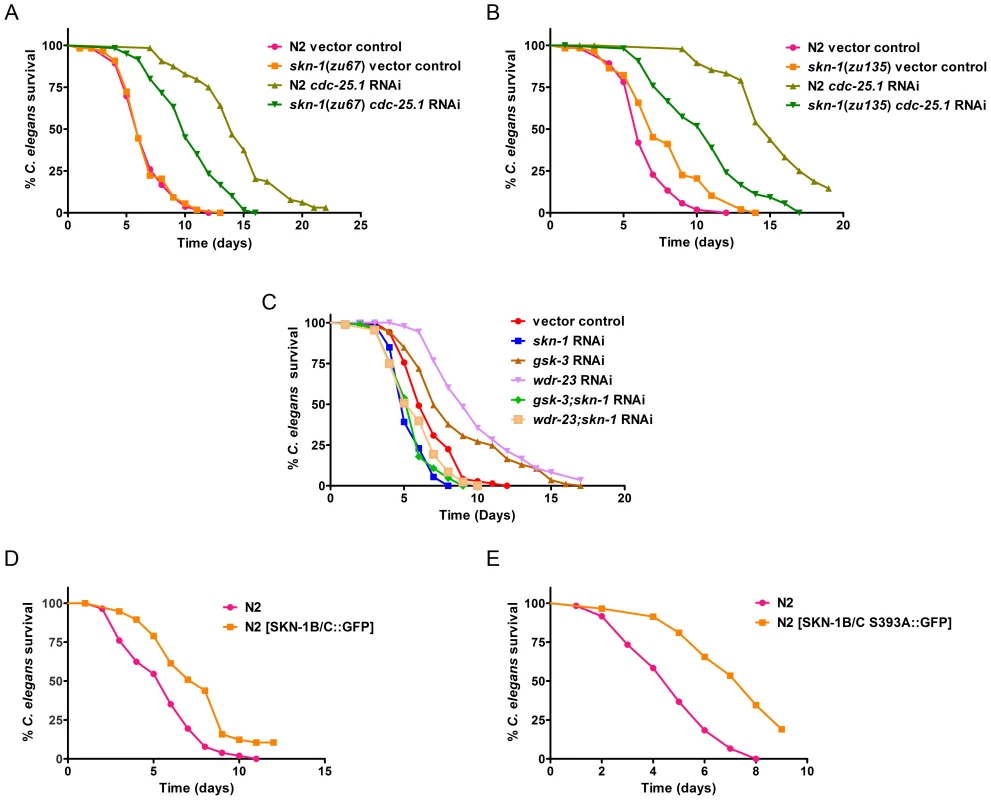
Animals were exposed to E. faecalis in all panels of this figure. (A) N2 and skn-1(zu67) worms exposed to vector control RNAi, and cdc-25.1 RNAi, prior to pathogen exposure. (B) N2 and skn-1(zu135) worms exposed to vector control RNAi, and cdc-25.1 RNAi, prior to pathogen exposure. (C) N2 animals exposed to vector control, skn-1, gsk-3, wdr-23, gsk-3;skn-1 and wdr-23;skn-1 RNAi. (D) Survival of N2 [rol-6(su1006)] and N2 [SKN-1B/C::GFP[rol-6(su1006)]] and (E) N2 [rol-6(su1006)] and N2 [SKN-1B/C S393A::GFP[rol-6(su1006)]]. Median survival and P-values are listed in Table S2A in Text S1 along with replicas of all experiments. The results of the same experiments performed on P. aeruginosa are shown in Table S2B in Text S1. The data are representative of experiments repeated two or more times with an n of 60 – 90 worms for each condition. Another study examined loss of skn-1 by mutation using loss of function alleles zu67 and zu135 [15]. Using these same strains, we also examined susceptibility to P. aeruginosa and additionally E. faecalis. In a wild-type background, there was no significant difference in susceptibility, as previously reported; in fact zu135 was slightly more resistant (Figure 6A, 6B, Table S2 in Text S1). However, the zu67 and zu135 strains are sterile and do not produce viable embryos. This eliminates a major mechanism of killing, the internal hatching of the embryos during exposure to pathogen, a process called “bagging” [33], [34]. We found that RNAi of skn-1 did not cause a severe sterility phenotype until the second generation, likely due to its maternal effect [35], so the RNAi experiments described above were not affected by this problem. To render all the strains equally sterile so that they would be directly comparable, we exposed them to cdc-25.1 RNAi prior to pathogen exposure [36], [37]. Under these conditions, both skn-1 mutants were significantly more susceptible compared to wild-type (Figure 6A, 6B, Table S2 in Text S1). Note that the cdc-25.1 RNAi targets germline mitosis/meiosis, while a skn-1 mutation affects cell division within the embryo. Preventing development of the germline is known to increase resistance [34] and likely accounts for the increase in susceptibility observed when the already sterile skn-1 mutants are exposed to cdc-25.1 RNAi. Shivers et al. also attempted to control for sterility by adding FUDR to their assay plates, a compound that prevents cell division [15]. We speculate that this procedure may have affected the virulence of the pathogen, as noted in previous reports [36], [38]. Since the activation of skn-1 is very sensitive to the overall virulence of the pathogen (see Figure 2), this may have confounded the results.
To further test the role of SKN-1 on pathogen susceptibility, we used another approach. If loss of SKN-1 causes a susceptibility phenotype, constitutively active SKN-1 is predicted to increase resistance. We increased SKN-1 activity by two known means. First, we reduced expression of gsk-3 by RNAi, which causes constitutive nuclear localization of SKN-1 and therefore, greater transcriptional activity. By phosphorylation, GSK-3 normally inhibits nuclear localization of SKN-1 [39]. Secondly, we reduced expression of wdr-23, which encodes a WD40 repeat protein that targets SKN-1 to an ubiquitin ligase for proteasomal degradation. Loss of WDR-23 results in increased SKN-1 protein levels and greater output of its transcriptional program [40]. Reducing the expression of both of these genes significantly increased resistance to E. faecalis and P. aeruginosa (Figure 6C, Table S2 in Text S1). As a control, the expression of skn-1 was additionally reduced by RNAi, which abrogated the phenotypes, confirming that the increased resistance was dependent on skn-1. In previous work, loss of gsk-3 or wdr-23 both increased survival upon exposure to oxidative stress, however there was little (wdr-23) to no (gsk-3) concomitant increase in longevity when lifespan was assayed on non-pathogenic E. coli [39], [40]. Therefore, the significant increase in pathogen resistance observed in Figure 6A is not just a byproduct of causing a long-lived phenotype in general. To look at the effect of increased SKN-1 activity by another means, we examined two strains in which SKN-1 is over-produced because the strains carry extra copies of SKN-1::GFP. One strain carried wild-type SKN-1, fused to GFP (Figure 6D, Table S2 in Text S1), whereas the second expressed a mutant form of SKN-1, which is constitutively active (Figure 6E, Table S2 in Text S1) [8], [20]. Both strains exhibited increased resistance to both E. faecalis and P. aeruginosa. The effect was stronger utilizing the strain that produces constitutively active SKN-1 (Figure 6E, Table S2 in Text S1). Overall the data in this section demonstrates that that the level of SKN-1 activity significantly influences how long the worm survives on pathogen; less SKN-1 activity reduces resistance whereas more SKN-1 activity increases resistance.
Epistasis Analysis of skn-1 and bli-3
In previous work, loss of bli-3 was shown to increase susceptibility to E. faecalis [3]. To determine if this effect could be completely dependent on skn-1, phenotypic analysis of the loss of both genes on susceptibility was performed. In a skn-1 background, loss of bli-3 by RNAi caused an increase in susceptibility to E. faecalis compared to skn-1 animals not exposed to bli-3 RNAi (P<0.0001). These data suggest that skn-1 is not completely epistatic to bli-3, ie not all of Ce-Duox1/BLI-3's protective effects are mediated through SKN-1 (Figure 7A, Table S2A in Text S1). However, on P. aeruginosa, we observed some differences (Figure 7B, Table S2B in Text S1). First, we discovered that a bli-3 knock-down caused a profound susceptibility phenotype – much more severe than that observed on E. faecalis in this work and in our previous publication [3]. Loss of skn-1 ameliorated this severe phenotype and showed the same level of susceptibility as a skn-1 mutant plus bli-3 RNAi (P = 0.3429). The data is consistent with skn-1 being epistatic to bli-3 on P. aeruginosa, despite the difference in phenotypic consequences compared to E. faecalis. In this case, loss of skn-1 protects against the severe susceptibility phenotype caused by the loss of bli-3 on P. aeruginosa, even though the skn-1 mutant is still more susceptible than the wild-type strain (P<0.0001). Understanding why there is a difference in the bli-3 phenotypes of animals exposed to these two different pathogens will require further investigation. Overall, the results are consistent with the model shown in Figure 8 that postulates that SKN-1 acts downstream of BLI-3. However, the experiments do not rule out the possibility that SKN-1 and BLI-3 have other roles independent of each other.
Fig. 7. Epistasis analysis of skn-1 and bli-3. 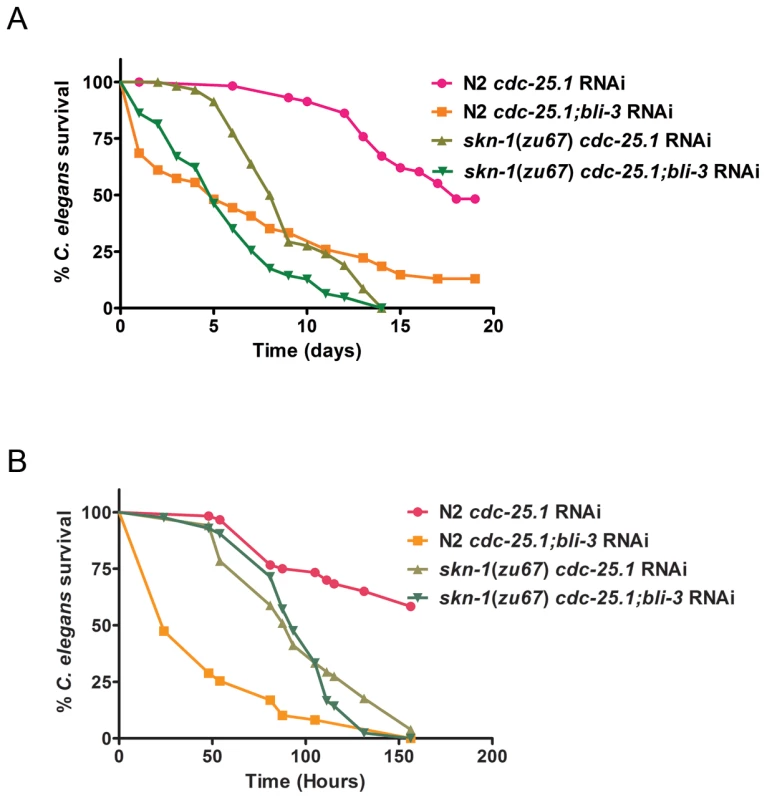
Survival curves of N2 and skn-1(zu67) exposed to cdc-25.1 RNAi and cdc-25.1;bli-3 RNAi on (A) E. faecalis OG1RF and (B) P. aeruginosa PA14. Median survival and P-values are listed in Table S2 in Text S1 along with replicas of the experiments. The data shown is representative of experiments repeated two or more times with an n of 60 – 90 worms for each condition. Discussion
We have shown for the first time that the oxidative stress response transcription factor, SKN-1, plays an important role in C. elegans innate immunity. A model for the activation of SKN-1 is shown in Figure 8. Upon exposure to intestinal pathogens, Ce-Duox1/BLI-3 is activated by an unknown mechanism to produce extracellular ROS. In addition to possibly having direct antimicrobial properties, ROS generated by Ce-Duox1/BLI-3 (likely in the form of membrane diffusible H2O2) activates the p38 MAPK signaling cascade, which results in the phosphorylation and nuclear localization of SKN-1. SKN-1 carries out a transcriptional program to produce proteins with detoxification functions that eliminate ROS and help repair or recycle damaged molecules. Overall, we demonstrated that this response is functionally important during infection; loss of skn-1 increased susceptibility of the worms to pathogen, whereas increasing SKN-1's activity increased resistance
Fig. 8. Proposed model for SKN-1 activation during infection. 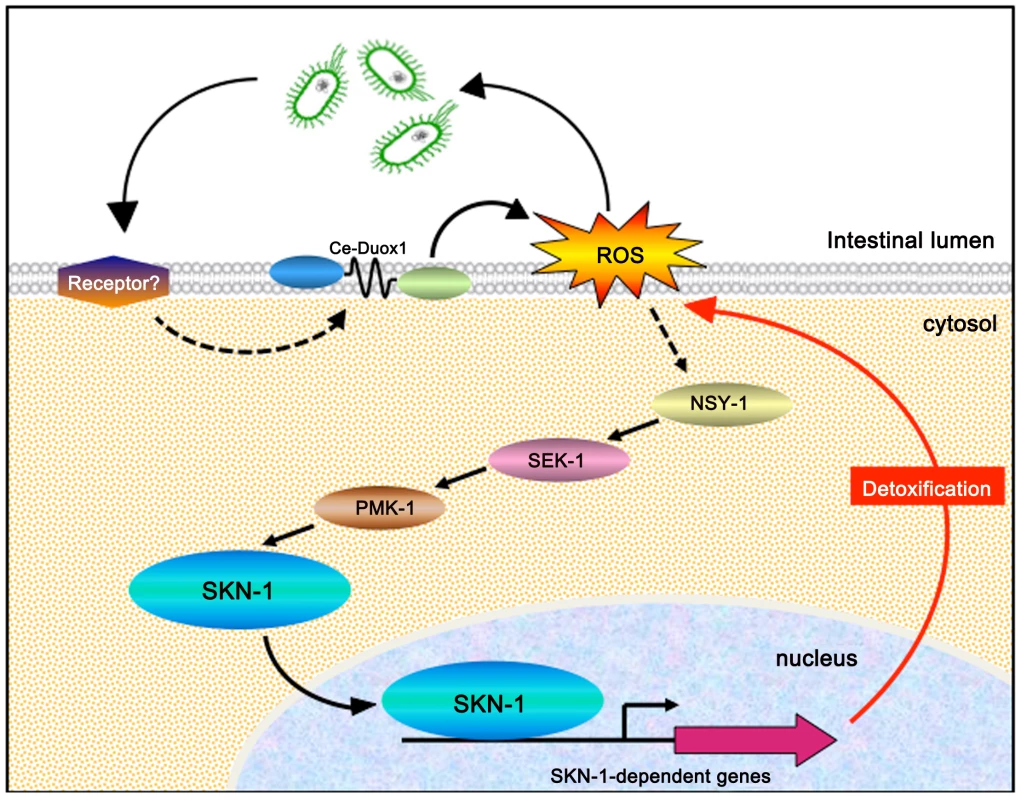
See the main text for a description. Interestingly, there is evidence for Nrf-related transcription factors like SKN-1 protecting against immune-derived oxidative stress in other systems, suggesting broad conservation of this protective mechanism. A study by Jain et al. showed that the SKN-1 ortholog, Yap1 in Saccharomyces cerevisae, protected this microbe against Ce-Duox1/BLI-3-derived ROS produced by C. elegans. Specifically, it was demonstrated that a dar (distended anal region) phenotype caused by colonization with S. cerevisae was abrogated by a Yap1 mutation. The dar phenotype was dependent on bli-3 [5]. Therefore it appears that Nrf-related transcription factors not only protect the host against oxidative stress during host-pathogen interactions, but also protect eukaryotic pathogens and enable their virulence. In higher animals, there is also evidence that Nrf-related transcription factors respond to immune-induced oxidative stress. For example, increasing concentrations of HOCl were shown to increase activation of Nrf2 and immune responsive genes in mouse macrophages [41]. Because Nrf transcription factors generally upregulate the production of “Phase 2” detoxification enzymes with activities such as metabolizing free radicals and conjugating xenobiotics and peroxidized lipids [10], [11], it is logical that their activity would be helpful during pathogen attack when there is often excess ROS production and cellular stress. It is therefore not surprising that one central signaling pathway, p38 MAPK signaling, has a crucial role in the C. elegans response to both pathogen and oxidative stress.
When considering the components of the p38 MAPK pathway that govern oxidative stress response, SEK-1 and PMK-1 have well-established functions [12]. The importance of two upstream components, NSY-1 and TIR-1 is less clear and may depend on the oxidative stressor used to assay function [12], [16], [17]. On pathogen, we observed that loss of nsy-1 significantly reduced SKN-1 activity (Figure 4B, 4G, S2B in Text S1). The loss of tir-1 did not cause a significant change in expression, suggesting that it is not involved (Figure 4G, S3B in Text S1). These data suggest that other components may feed into the pathway upstream of SEK-1 to activate SKN-1. Though we tested several potential candidate kinases with previously established roles in p38 MAPK signaling including MEK-1, MKK-4 and IKKε-1 [42], [43], none of them reduced SKN-1 activity (data not shown). In mammalian systems, a thioredoxin redox sensor inhibits the mammalian homolog of NSY-1, ASK-1 [44], [45]. ROS production causes the disassociation of the thioredoxin and allows an active signaling complex to form with other adaptor molecules enabling p38 MAPK signaling [46]. We are actively exploring if a thioredoxin is involved in activating NSY-1 in C. elegans.
In addition to SKN-1, p38 MAPK signaling in C. elegans was previously shown to regulate another bZIP transcription factor, ATF-7. Loss of atf-7 enhances susceptibility to pathogen. However, rather than simply acting to induce immune activation, ATF-7 normally represses innate immune activation. Phosphorylation by PMK-1 turns ATF-7 from a repressor into an activator [15]. Since ROS released from Ce-Duox1/BLI-3 activates SKN-1 activity via p38 MAPK signaling, one could postulate that ROS also activates ATF-7. Alternatively, the signaling cascade might have some way of distinguishing between different inputs to selectively activate these transcription factors depending on the stimulus. Such a model might allow better coordination of gene expression to meet specific challenges. One gene shown to be regulated by ATF-7, T24B8.5, encodes a ShK-like toxin peptide which has predicted antimicrobial activity [15]. Perhaps SKN-1 coordinates a “defensive” arm of the innate immune response by regulating genes encoding enzymes involved in protecting against and repairing cellular damage, whereas ATF-7 regulates an “offensive” arm by controlling genes encoding for activities that are directly antimicrobial. A more thorough study of the genes regulated by ATF-7 and SKN-1 on pathogen would need to be carried out to investigate this hypothesis.
The precise role(s) of ROS generated by Ce-Duox1/BLI-3 in protecting the worm from infection is not yet completely understood, though this study implicates an important signaling function. On E. faecalis, reducing expression of bli-3 increased the susceptibility phenotype of skn-1. The incomplete epistasis suggests that Ce-Duox1 has additional roles in protecting the worm from the pathogen independent of activating skn-1. One role could be activating other transcription factors regulated by p38 MAPK signaling such as ATF-7, as mentioned above. A potential role, independent of signaling, is in host physical barrier function. The ROS generated by Ce-Duox1/BLI-3 could be utilized by peroxidases to increase the impermeability of the ECM (extracellular matrix) in the worm intestine, analogous with how peroxidases use ROS generated by Ce-Duox1/BLI-3 to cross-link the cuticle [47]. There is some evidence for NADPH oxidases contributing to barrier function in the mosquito gut [48]. Another possibility is that ROS generated by Ce-Duox1/BLI-3, is turned into a more potent antimicrobial, as is known to happen in other systems, including the oral and respiratory mucosa of animals, in which DUOXs generate the H2O2 necessary for lactoperoxidase (LPO) to oxidize thiocyanate to create the microcidal compound hypothiocyanite [49], [50].
Our susceptibility analysis indicated that the genetic interactions between skn-1 and bli-3 are different on P. aeruginosa than on E. faecalis. The data in Figure 7 demonstrated that in the absence of Ce-Duox1/BLI-3, and only on P. aeruginosa, SKN-1 is activating a transcriptional program that is harmful to the worm, but in the presence of Ce-Duox1/BLI-3, SKN-1 is protective, as expected. On E. faecalis, in contrast, the presence of SKN-1 is protective in both the bli-3 and wild-type backgrounds. Perhaps the explanation for the difference lies in the fact that P. aeruginosa is actively manipulating the host innate immune response through production of redox-active factors such as pyocyanin. In previous work using human respiratory epithelial cells, pyocyanin production by P. aeruginosa was shown to cause increased oxidative stress by potentiating the intracellular production of superoxide in the host cells. Pyocyanin, like Ce-Duox1/BLI-3 uses NADPH and molecular oxygen to create ROS, so these host and pathogen factors are potentially competing for the same substrates [27]. We postulate that intracellular superoxide production by pyocyanin, in contrast to the extracellular production of H2O2 by Ce-Duox1/BLI-3, activates a different SKN-1 transcriptional program that is very harmful to the worm. In a previous investigation, different oxidative stressors caused significant differences in SKN-1's transcriptional program, so this idea is not with out precedent [10]. This hypothesis could explain the results in Figure 7B. When Ce-Duox1/BLI-3 is present, the H2O2 produced activates SKN-1 to carry out a protective response. It may also inhibit pyocyanin activity by decreasing availability of NADPH and oxygen. Loss of skn-1 is detrimental, but loss of bli-3 when skn-1 is present allows this transcription factor to be influenced by the ROS generated by the pathogen resulting in a transcriptional program that is actively harmful to the host. Testing this hypothesis will require further study.
In conclusion, we have demonstrated for the first time that the Nrf-family transcription factor SKN-1 is induced by exposure to pathogen and has a protective function during infection. We additionally showed that components of the p38 MAPK pathway, including NSY-1, SEK-1 and PMK-1, are necessary for this response. Finally, ROS produced by the dual oxidase Ce-Duox1/BLI-3 in response to pathogen was shown to trigger SKN-1 activity. Because Ce-Duox1/BLI-3 plays an important role in activating p38 MAPK signaling and SKN-1 activity, defining how this dual oxidase's activity is triggered will be an important area of future investigation.
Materials and Methods
Strains
C. elegans strains were grown and maintained as previously described [51]. The following bacterial strains were used in this study: E. coli OP50 [52], E. faecalis OG1RF [53], P. aeruginosa PA14 [23]. C. elegans strains used in this study are indicated in Table S3 in Text S1.
RNA Isolation and Quantitative Real Time PCR (qRT-PCR) Analysis
In Figure 1A and 4G, RNA was extracted from L4 larvae exposed to E. faecalis OGIRF, P. aeruginosa PA14 and E. coli OP50 for 24 hours. In Figure 5I and 5J, RNAi treated eri-1(mg336) worms were exposed to E. faecalis OGIRF, P. aeruginosa PA14 and E. coli OP50 for 6 hours. The RNA was extracted using Trizol (Invitrogen) as indicated by the manufacturer. Samples were treated with DNase I to remove DNA contamination using the Turbo DNA free kit (Applied Biosystems) as described by the manufacturer. qRT-PCR was performed on an ABI 7500 instrument using the Power SYBR Green RNA-to-CT 1 step kit (Applied Biosystems). Comparative CT method was used to determine fold changes in gene expression normalized to act-1 [20]. Primers used are listed in Table S4 in Text S1.
RNA Interference
RNAi was induced by feeding L1 worms through L4 stage with bacteria producing dsRNA to target genes. RNAi expressing clones were obtained from the C. elegans library (Geneservices, UK) [54], [55]. All clones were verified by sequencing. Clones absent in the library were constructed as follows. Briefly, RNA was extracted from C. elegans L4 larvae using Trizol (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized using SuperscriptII reverse transcriptase (Invitrogen) with oligodT and random hexamer primers. Gene specific primers were used to amplify regions of target genes, cloned into the vector pL4440 [56] and transformed into E. coli HT115(DE3). Constructs were verified by sequencing. Sequences of gene specific primers are listed in Table S5 in Text S1. To induce bli-3 knockdown, the bacterial strain expressing bli-3 RNAi was diluted in a 1∶30 ratio using the vector control. Double RNAi knockdowns were obtained by mixing bacteria expressing dsRNA to each target gene in a 1∶1 ratio. To render worms sterile prior to killing assays, larvae were exposed to cdc-25.1 RNAi.
Fluorescence Microscopy
To investigate the expression of gst-4::gfp, gcs-1::gfp and GST-7::GFP, worms were exposed to E. faecalis, P. aeruginosa and E. coli strains for 24 hours at 25°C and paralyzed with 1mM levamisole. Anesthetized worms were mounted on 2% agarose pads and imaged using an Olympus IX81 automated inverted microscope and Slidebook (version 5.0) software. The levels of GFP expression were scored as previously described [8], [12]. Briefly, little or no expression of GFP, expression of GFP in the anterior or posterior of the worm and expression throughout the intestine of the worm are categorically indicated by low, medium and high respectively for gst-4, gcs-1 and gst-7. To determine the effect of hydrogen peroxide and paraquat on gst-4::gfp expression, worms were exposed to 5mM hydrogen peroxide or 100mM paraquat in M9 for 20 and 30 minutes respectively, then transferred to seeded NG plate to recover for 4 hours before imaging. As controls, worms were exposed to the same period of time in M9. Higher background expression was observed in the control worms using the latter procedures.
SKN-1B/C::GFP expression was analyzed by fluorescence microscopy in worms exposed to E. faecalis for 24 hours and P. aeruginosa for 6 hours. Imaging was performed using the FITC, TRITC, DAPI and YFP filter sets to exclude the signal from autofluorescence in the worms. Percentages of worms indicating the degree of nuclear localization in the intestinal cells were scored as previously described [8], [12]. Briefly, no nuclear localization, localization of SKN-1B/C::GFP in the anterior or posterior of the worm and nuclear localization of SKN-1B/C::GFP in all intestinal cells are categorically indicated by low, medium and high, respectively. All fluorescence microscopy experiments shown were independently repeated at least three times.
Killing Assays
Killing assays were generally performed as previously described [28], [30], [57]. Briefly, for E. faecalis killing assays, E. faecalis OG1RF grown in Brain Heart Infusion (BHI) medium for 5 hours was seeded on BHI plates and incubated at 37°C for 24 hours. While for P. aeruginosa killing assays, P. aeruginosa PA14 was cultured in Luria broth (LB), seeded on slow-killing plates and incubated first for 24 hours at 37°C and then for 24 hours at 25°C. A total of 90 L4 larvae were transferred to three replica plates. Worms were scored as live and dead at various points along the time course.
Statistical Analysis
After scoring the fluorescent micrographs, statistical differences were determined by Chi square and Fisher's exact tests using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Each experimental condition was compared pairwise to the control condition. P-values of <0.05 were considered to be statistically significant. Statistically significant differences are indicated in the figures with asterisks next to the experimental condition. Kaplan-Meier log rank analysis was used to compare survival curves pairwise and to calculate the median survival. P-values <0.05 were considered to be statistically significant.
Supporting Information
Zdroje
1. BedardKKrauseKH 2007 The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87 245 313
2. LambethJDKawaharaTDieboldB 2007 Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43 319 331
3. ChavezVMohri-ShiomiAGarsinDA 2009 Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. PMCID: 2772517. Infect Immun 77 4983 4989
4. EdensWASharlingLChengGShapiraRKinkadeJM 2001 Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 154 879 891
5. JainCYunMPolitzSMRaoRP 2009 A pathogenesis assay using Saccharomyces cerevisiae and Caenorhabditis elegans reveals novel roles for yeast AP-1, Yap1, and host dual oxidase BLI-3 in fungal pathogenesis. Eukaryot Cell 8 1218 1227
6. ChavezVMohri-ShiomiAMaadaniAVegaLAGarsinDA 2007 Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 176 1567 1577
7. Mohri-ShiomiAGarsinDA 2008 Insulin Signaling and the Heat Shock Response Modulate Protein Homeostasis in the Caenorhabditis elegans Intestine during Infection. J Biol Chem 283 194 201
8. AnJHBlackwellTK 2003 SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev 17 1882 1893
9. XuCLiCYKongAN 2005 Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28 249 268
10. OliveiraRPPorter AbateJDilksKLandisJAshrafJ 2009 Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 8 524 541
11. ParkSKTedescoPMJohnsonTE 2009 Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell 8 258 269
12. InoueHHisamotoNAnJHOliveiraRPNishidaE 2005 The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19 2278 2283
13. KimDHFeinbaumRAlloingGEmersonFEGarsinDA 2002 A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 623 626
14. LiberatiNTFitzgeraldKAKimDHFeinbaumRGolenbockDT 2004 Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc Natl Acad Sci U S A 101 6593 6598
15. ShiversRPPaganoDJKooistraTRichardsonCEReddyKC 2010 Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet 6 e1000892
16. HayakawaTKatoKHayakawaRHisamotoNMatsumotoK 2011 Regulation of Anoxic Death in Caenorhabditis elegans by Mammalian Apoptosis Signal-regulating Kinase (ASK) Family Proteins. Genetics 187 785 792
17. KondoMYanaseSIshiiTHartmanPSMatsumotoK 2005 The p38 signal transduction pathway participates in the oxidative stress-mediated translocation of DAF-16 to Caenorhabditis elegans nuclei. Mech Ageing Dev 126 642 647
18. LiXMatilainenOJinCGlover-CutterKMHolmbergCI 2011 Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity. PLoS Genet 7 e1002119
19. KawliTWuCTanMW 2010 Systemic and cell intrinsic roles of Gqalpha signaling in the regulation of innate immunity, oxidative stress, and longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A 107 13788 13793
20. TulletJMHertweckMAnJHBakerJHwangJY 2008 Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132 1025 1038
21. LeiersBKampkotterAGreveldingCGLinkCDJohnsonTE 2003 A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic Biol Med 34 1405 1415
22. WangJRobida-StubbsSTulletJMRualJFVidalM 2010 RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet 6 e1001048
23. TanMWMahajan-MiklosSAusubelFM 1999 Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A 96 715 720
24. ReimmannCBeyelerMLatifiAWintelerHFoglinoM 1997 The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol 24 309 319
25. LauGWHassettDJRanHKongF 2004 The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med 10 599 606
26. Price-WhelanADietrichLENewmanDK 2006 Rethinking 'secondary' metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol 2 71 78
27. RadaBLekstromKDamianSDupuyCLetoTL 2008 The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol 181 4883 4893
28. Mahajan-MiklosSTanMWRahmeLGAusubelFM 1999 Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96 47 56
29. MavrodiDVBonsallRFDelaneySMSouleMJPhillipsG 2001 Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183 6454 6465
30. GarsinDASifriCDMylonakisEQinXSinghKV 2001 A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A 98 10892 10897
31. SifriCDMylonakisESinghKVQinXGarsinDA 2002 Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect Immun 70 5647 5650
32. BowermanBDraperBWMelloCCPriessJR 1993 The maternal gene skn-1 encodes a protein that is distributed unequally in early C. elegans embryos. Cell 74 443 452
33. AballayAYorgeyPAusubelFM 2000 Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol 10 1539 1542
34. MiyataSBegunJTroemelERAusubelFM 2008 DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics 178 903 918
35. BowermanBEatonBAPriessJR 1992 skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell 68 1061 1075
36. IrazoquiJENgAXavierRJAusubelFM 2008 Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc Natl Acad Sci U S A 105 17469 17474
37. ShapiraMHamlinBJRongJChenKRonenM 2006 A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci U S A 103 14086 14091
38. AballayA 2009 Neural regulation of immunity: role of NPR-1 in pathogen avoidance and regulation of innate immunity. Cell Cycle 8 966 969
39. AnJHVranasKLuckeMInoueHHisamotoN 2005 Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci U S A 102 16275 16280
40. ChoeKPPrzybyszAJStrangeK 2009 The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol 29 2704 2715
41. WoodsCGFuJXuePHouYPlutaLJ 2009 Dose-dependent transitions in Nrf2-mediated adaptive response and related stress responses to hypochlorous acid in mouse macrophages. Toxicol Appl Pharmacol 238 27 36
42. KellAVenturaNKahnNJohnsonTE 2007 Activation of SKN-1 by novel kinases in Caenorhabditis elegans. Free Radic Biol Med 43 1560 1566
43. KimDHLiberatiNTMizunoTInoueHHisamotoN 2004 Integration of Caenorhabditis elegans MAPK pathways mediating immunity and stress resistance by MEK-1 MAPK kinase and VHP-1 MAPK phosphatase. Proc Natl Acad Sci U S A 101 10990 10994
44. LiuHNishitohHIchijoHKyriakisJM 2000 Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol 20 2198 2208
45. SaitohMNishitohHFujiiMTakedaKTobiumeK 1998 Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17 2596 2606
46. MatsuzawaASaegusaKNoguchiTSadamitsuCNishitohH 2005 ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol 6 587 592
47. TheinMCWinterADStepekGMcCormackGStapletonG 2009 Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. J Biol Chem 284 17549 17563
48. KumarSMolina-CruzAGuptaLRodriguesJBarillas-MuryC 2010 A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327 1644 1648
49. FortezaRSalatheMMiotFFortezaRConnerGE 2005 Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 32 462 469
50. GeisztMWittaJBaffiJLekstromKLetoTL 2003 Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. Faseb J 17 1502 1504
51. HopeIA 1999 C. elegans A Practical Approach. HamesBD Oxford: Oxford University Press
52. BrennerS 1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
53. DunnyGMBrownBLClewellDB 1978 Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A 75 3479 3483
54. FraserAGKamathRSZipperlenPMartinez-CamposMSohrmannM 2000 Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 325 330
55. KamathRSFraserAGDongYPoulinGDurbinR 2003 Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231 237
56. TimmonsLCourtDLFireA 2001 Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 103 112
57. GarsinDAVillanuevaJMBegunJKimDHSifriCD 2003 Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300 1921
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion DiseaseČlánek Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) VirusesČlánek Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance toČlánek Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a TombusvirusČlánek Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic PhenotypesČlánek Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence
- Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion Disease
- Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis
- Nutritional Immunology: A Multi-Dimensional Approach
- Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) Viruses
- Vaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
- Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly
- Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance to
- Computational and Biochemical Analysis of the Effector AvrBs2 and Its Role in the Modulation of Type Three Effector Delivery
- Granzyme B Inhibits Vaccinia Virus Production through Proteolytic Cleavage of Eukaryotic Initiation Factor 4 Gamma 3
- Association of Activating KIR Copy Number Variation of NK Cells with Containment of SIV Replication in Rhesus Monkeys
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa
- Follicular Dendritic Cell-Specific Prion Protein (PrP) Expression Alone Is Sufficient to Sustain Prion Infection in the Spleen
- Autophagy Protein Atg3 is Essential for Maintaining Mitochondrial Integrity and for Normal Intracellular Development of Tachyzoites
- Longevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
- Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence
- Acquisition of Pneumococci Specific Effector and Regulatory Cd4 T Cells Localising within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue
- The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites
- Deficiency of a Niemann-Pick, Type C1-related Protein in Is Associated with Multiple Lipidoses and Increased Pathogenicity
- Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
- Eight RGS and RGS-like Proteins Orchestrate Growth, Differentiation, and Pathogenicity of
- Prion Uptake in the Gut: Identification of the First Uptake and Replication Sites
- Nef Decreases HIV-1 Sensitivity to Neutralizing Antibodies that Target the Membrane-proximal External Region of TMgp41
- Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a Tombusvirus
- A Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
- The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
- HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency
- Neonatal CD8 T-cell Hierarchy Is Distinct from Adults and Is Influenced by Intrinsic T cell Properties in Respiratory Syncytial Virus Infected Mice
- Two Novel Transcriptional Regulators Are Essential for Infection-related Morphogenesis and Pathogenicity of the Rice Blast Fungus
- Five Questions about Non-Mevalonate Isoprenoid Biosynthesis
- The Human Cytomegalovirus UL11 Protein Interacts with the Receptor Tyrosine Phosphatase CD45, Resulting in Functional Paralysis of T Cells
- Wall Teichoic Acids of Limit Recognition by the Drosophila Peptidoglycan Recognition Protein-SA to Promote Pathogenicity
- A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen
- Inflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
- CNS Recruitment of CD8+ T Lymphocytes Specific for a Peripheral Virus Infection Triggers Neuropathogenesis during Polymicrobial Challenge
- Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic Phenotypes
- A Receptor-based Switch that Regulates Anthrax Toxin Pore Formation
- Targeting of Heparin-Binding Hemagglutinin to Mitochondria in Macrophages
- Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants
- Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
- Structural Elucidation and Functional Characterization of the Effector Protein ATR13
- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection
- Acid Stability of the Hemagglutinin Protein Regulates H5N1 Influenza Virus Pathogenicity
- Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons
- A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection
- Juxtamembrane Shedding of AMA1 Is Sequence Independent and Essential, and Helps Evade Invasion-Inhibitory Antibodies
- Pathogenesis and Host Response in Syrian Hamsters following Intranasal Infection with Andes Virus
- IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network
- Epstein-Barr Virus Evades CD4 T Cell Responses in Lytic Cycle through BZLF1-mediated Downregulation of CD74 and the Cooperation of vBcl-2
- Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction
- Late Repression of NF-κB Activity by Invasive but Not Non-Invasive Meningococcal Isolates Is Required to Display Apoptosis of Epithelial Cells
- Polar Flagellar Biosynthesis and a Regulator of Flagellar Number Influence Spatial Parameters of Cell Division in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- The Enteropathogenic (EPEC) Tir Effector Inhibits NF-κB Activity by Targeting TNFα Receptor-Associated Factors
- Toward an Integrated Model of Capsule Regulation in
- A Systematic Screen to Discover and Analyze Apicoplast Proteins Identifies a Conserved and Essential Protein Import Factor
- A Host Small GTP-binding Protein ARL8 Plays Crucial Roles in Tobamovirus RNA Replication
- Comparative Pathobiology of Fungal Pathogens of Plants and Animals
- Synergistic Roles of Eukaryotic Translation Elongation Factors 1Bγ and 1A in Stimulation of Tombusvirus Minus-Strand Synthesis
- Engineered Immunity to Infection
- Inflammatory Monocytes and Neutrophils Are Licensed to Kill during Memory Responses
- Sialidases Affect the Host Cell Adherence and Epsilon Toxin-Induced Cytotoxicity of Type D Strain CN3718
- Eurasian-Origin Gene Segments Contribute to the Transmissibility, Aerosol Release, and Morphology of the 2009 Pandemic H1N1 Influenza Virus
- SARS Coronavirus nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of mRNAs: Viral mRNAs Are Resistant to nsp1-Induced RNA Cleavage
- Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus
- Functional Analysis of the Kinome of the Wheat Scab Fungus
- Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- Engineered Immunity to Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



