-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
A genetic absence of the common IFN - α/β signaling receptor (IFNAR) in mice is associated with enhanced viral replication and altered adaptive immune responses. However, analysis of IFNAR-/- mice is limited for studying the functions of type I IFN at discrete stages of viral infection. To define the temporal functions of type I IFN signaling in the context of infection by West Nile virus (WNV), we treated mice with MAR1-5A3, a neutralizing, non cell-depleting anti-IFNAR antibody. Inhibition of type I IFN signaling at or before day 2 after infection was associated with markedly enhanced viral burden, whereas treatment at day 4 had substantially less effect on WNV dissemination. While antibody treatment prior to infection resulted in massive expansion of virus-specific CD8+ T cells, blockade of type I IFN signaling starting at day 4 induced dysfunctional CD8+ T cells with depressed cytokine responses and expression of phenotypic markers suggesting exhaustion. Thus, only the later maturation phase of anti-WNV CD8+ T cell development requires type I IFN signaling. WNV infection experiments in BATF3-/- mice, which lack CD8-α dendritic cells and have impaired priming due to inefficient antigen cross-presentation, revealed a similar effect of blocking IFN signaling on CD8+ T cell maturation. Collectively, our results suggest that cell non-autonomous type I IFN signaling shapes maturation of antiviral CD8+ T cell response at a stage distinct from the initial priming event.
Published in the journal: . PLoS Pathog 7(12): e32767. doi:10.1371/journal.ppat.1002407
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002407Summary
A genetic absence of the common IFN - α/β signaling receptor (IFNAR) in mice is associated with enhanced viral replication and altered adaptive immune responses. However, analysis of IFNAR-/- mice is limited for studying the functions of type I IFN at discrete stages of viral infection. To define the temporal functions of type I IFN signaling in the context of infection by West Nile virus (WNV), we treated mice with MAR1-5A3, a neutralizing, non cell-depleting anti-IFNAR antibody. Inhibition of type I IFN signaling at or before day 2 after infection was associated with markedly enhanced viral burden, whereas treatment at day 4 had substantially less effect on WNV dissemination. While antibody treatment prior to infection resulted in massive expansion of virus-specific CD8+ T cells, blockade of type I IFN signaling starting at day 4 induced dysfunctional CD8+ T cells with depressed cytokine responses and expression of phenotypic markers suggesting exhaustion. Thus, only the later maturation phase of anti-WNV CD8+ T cell development requires type I IFN signaling. WNV infection experiments in BATF3-/- mice, which lack CD8-α dendritic cells and have impaired priming due to inefficient antigen cross-presentation, revealed a similar effect of blocking IFN signaling on CD8+ T cell maturation. Collectively, our results suggest that cell non-autonomous type I IFN signaling shapes maturation of antiviral CD8+ T cell response at a stage distinct from the initial priming event.
Introduction
Type I interferons (IFN) comprise a family of cytokines that that were identified originally for their ability to render cells resistant to virus infection [1]. Type I IFN binds to a common IFN-αβ receptor (IFNAR), which initiates a signaling cascade that results in phosphorylation and nuclear translocation of STAT1 and STAT2, and induction of expression of hundreds of interferon-stimulated genes (ISG) [2]. These ISG control viral infections through a diverse range of direct antiviral effector functions [3] and by modulating adaptive immune responses [4].
Type I IFN responses are essential for the controlling infection by West Nile virus (WNV) [5], [6], an encephalitic positive strand RNA virus of the Flaviviridae family that has emerged over the past decade as a significant cause of neuroinvasive disease [7]. IFNAR-/- mice are exquisitely vulnerable to WNV infection, with expanded tissue tropism, uncontrolled viral replication, and rapidly uniform death, with all animals succumbing within four days of infection after inoculation with a single plaque forming unit (PFU) of virus [8].
Apart from its function in controlling viral infection through cell-intrinsic antiviral gene induction, type I IFN has an established role in priming of B and T cell responses (reviewed in [9], [10]). Signaling through IFNAR regulates early innate and adaptive B cell activation in the lymph node and spleen [11]–[13] and induces dendritic cells to mature, express higher levels of co-stimulatory molecules, and present antigen more efficiently, which is required for optimal induction of a functional T cell response (reviewed in [14]). Diminished effector functions of memory CD8+ T cells in IFNAR-/- mice have been described after infection with influenza and vaccinia (VV) viruses [15], [16]. This could be due in part, to defects in cross-priming of CD8+ T cells, which is believed to require both virus-induced type I IFN [9], [13], [17] and CD8-α dendritic cells [18].
Although cell-type and tissue-specific conditional deletions of IFNAR have been described [19]–[22], the function of type I IFN at discrete stages of viral infection remains unknown. To define the temporal functions of type I IFN signaling in the context of infection by WNV, we utilized a previously reported blocking anti-IFNAR monoclonal antibody (MAb MAR1-5A3), which prevented type I IFN-induced intracellular signaling in vitro, was non-cell-depleting, and inhibited antiviral, antimicrobial, and antitumor responses in mice [23].
By administering MAR1-5A3 antibody at different times after viral inoculation, we separated the early innate from the later innate-adaptive functions of type I IFN. Treatment prior to WNV infection resulted in massive expansion of virus-specific CD8+ T cells by day 9. However, blockade of type I IFN signaling beginning at day 4 after WNV infection was associated with defects in virus-specific effector CD8+ T cells at day 9 including depressed IFN-γ and TNF-α responses and changes in phenotypic markers suggesting altered activation status and CD8+ T cell exhaustion that is usually seen during chronic viral infection [24]. This phenotype was not due to direct signaling effects through IFNAR on CD8+ T cells and was also observed after vaccinia virus (VV) infection under similar experimental conditions. Experiments in BATF3-/- mice, which lack CD8-α dendritic cells and have impaired antigen cross-presentation and CD8+ T cell priming capacity, showed a similar effect of temporal blockade of type I IFN signaling on CD8+ T cell maturation. Collectively, our results suggest that cell non-autonomous type I IFN signaling shapes maturation of antiviral CD8+ T cell response at a stage distinct from the initial priming event.
Results
Blocking the type I IFN receptor at different times results in enhanced susceptibility to WNV
Previous studies established a critical requirement for type I IFN in controlling WNV-NY (strain New York, 2000) as infected IFNAR-/- mice showed expanded tissue tropism, uncontrolled viral replication, and rapidly uniform death within four days [8]. While these experiments suggested a dominant antiviral function of type I IFN in vivo, key roles in modulating adaptive B and T cell responses against viruses also have been described [13], [17]. One caveat of the antiviral and immunologic studies is that they have been performed primarily in complete or cell-type IFNAR-/- mice, which limits insight into the temporal function of IFN signaling in modulating immune responses. Also, because many viruses replicate to substantially higher levels in IFNAR-/- mice, it can be difficult to separate how enhanced antigen burden and lack of type I IFN signaling differentially impact adaptive immune responses in the context of live virus infection. To begin to define the temporal functions of type I IFN signaling, we utilized MAR1-5A3, a previously described MAb that potently blocks type I IFN receptor signaling and is non cell-depleting [23].
IFNAR-/- mice succumb to lethal WNV-NY infection within 4 days of infection after a dose of 102 PFU of virus [8]. We assessed whether treatment with the MAR1-5A3 MAb recapitulated this phenotype. We performed a dose titration of MAR1-5A3, in which mice were treated one day prior to infection with 102 PFU of WNV-NY and monitored for survival (Figure 1A). Similar to IFNAR-/- mice, all wild type mice treated with a single dose of MAR1-5A3 but not the isotype control GIR-208 MAb ranging from 0.3 to 2.5 mg succumbed to WNV-NY infection, although the mean time of death (MTD) was delayed (6.5 days versus 4 days, P<0.0001). Given this data, we chose a MAR1-5A3 dose of 1 mg per mouse for the remainder of the study. The difference in MTD was not unexpected as MAR1-5A3 is not expected to cross the blood-brain-barrier efficiently and type I IFN has direct antiviral effects on neurons in the central nervous system [8], [25], [26].
Fig. 1. Effect of blockade of type I IFN signaling on WNV-NY infection. 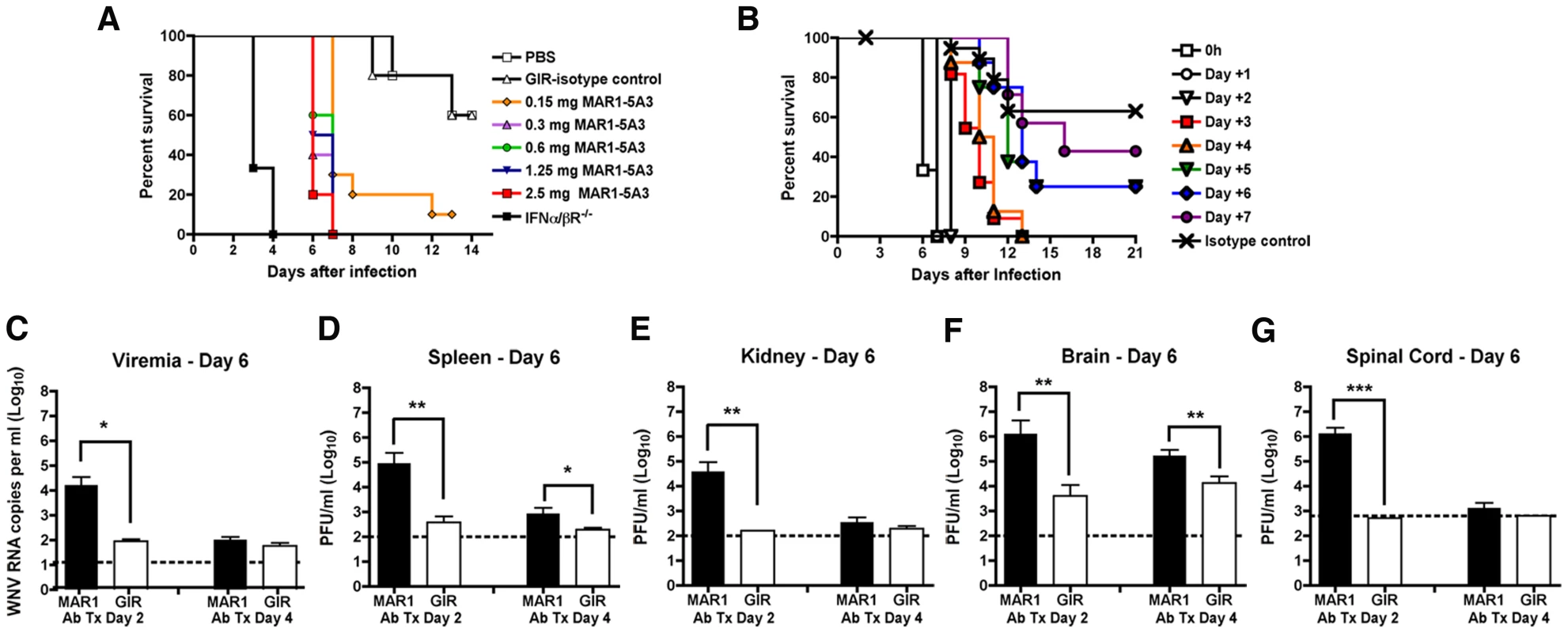
A. Dose titration of the MAR1-5A3 MAb in mice. Mice (n = 10 per group) were treated with increasing doses of MAR1-5A3 MAb, infected one day later with 102 PFU of WNV-NY, and survival was monitored. All MAR1-5A3 treatment doses shown were statistically different compared to GIR-208 treatment (P<0.05). B. Time course of MAR1-5A3 addition after WNV infection. Mice (n = 5 to 10 per group) were infected with 102 PFU of WNV-NY, treated with 1 mg of MAR1-5A3 or an isotype control (GIR-208 (GIR)) at different times after infection, and survival was determined. Treatment with MAR1-5A3 at days 0, 1, 2, 3, and 4 were statistically different (P<0.004) compared to treatment with GIR-208. C–G. Effect of MAR1-5A3 on viral burden. Mice (n = 5 to 10 per group) were infected with 102 PFU of WNV-NY, treated with 1 mg of MAR1-5A3 or GIR at day 2 or day 4 after infection. (C) Serum, (D) spleen, (E) kidney, (F) brain, and (G) spinal cord were harvested at day six and viral titers were determined by plaque assay or qRT-PCR. Asterisks indicate differences that are statistically significant (*, P<0.05; **, P<0.01, ***, P<0.001). We hypothesized that type I IFN signaling may have distinct functions at different stages of viral infections. To test this, mice were treated with a single 1 mg dose of MAb at different days after infection and survival was monitored (Figure 1B). We observed a significant difference (P<0.0003) in survival of mice treated with MAR1-5A3 as late as four days after infection as compared to the isotype control MAb treated mice. The MTD after WNV-NY infection for mice receiving MAR1-5A3 between days 0 and 2 was ∼8 days whereas those receiving MAb on days 3 or 4 survived on average between 10 and 11 days.
To further characterize the impact of type I IFN signaling at different stages, we compared viral titers from organs of mice at day 6 after infection in mice treated with the MAR1-5A3 or the control GIR-208 MAb at days 2 or 4 post infection (Figure 1C to G). In mice treated with MAR1-5A3 two days after infection, we observed an increase in viremia (739-fold, P<0.02), and infection in the spleen (242-fold, P<0.02) and kidney (240-fold, P<0.002) compared to the isotype control MAb. This corresponded with markedly higher viral titers in the brain (325-fold, P<0.006) and spinal cord (2,650-fold, P<0.001) compared to the control group. In contrast, mice treated with a single dose of MAR1-5A3 at day 4 after infection showed substantially smaller increases in the spleen (4.4-fold, P<0.03) and brain (13-fold, P<0.006) with no detectable elevation in serum, kidney, or spinal cord (P>0.19) at day 6. Thus, although the relative timing (day 2 or 4) of MAR1-5A3 administration did not differentially affect clinical outcome, it impacted viral spread and tropism; earlier blockade of type I IFN signaling resulted in enhanced replication in all tissues examined, whereas later administration had a small effect in only a subset of organs.
Temporal effects of MAR1-5A3 on adaptive immunity against WNV
Several groups have observed differences in antibody and CD8+ T cell responses in IFNAR-/- and STAT1-/- mice after infection or vaccination [13], [15]–[17], [19], [27], [28]. Because administration of MAR1-5A3 at day 4 had relatively minor effects on viral burden at day 6 (Figure 1) or day 8 (data not shown) yet still resulted in complete lethality, we hypothesized that blockade of type I IFN receptor signaling at later stages might impact early adaptive immune responses.
The development of an antibody response is critical for surviving WNV infection [29], [30]. To study the temporal effects of type I IFN signaling on the humoral response, wild type mice were infected with WNV-NY, treated with MAR1-5A3 or isotype control antibody two or four days later, and serum was harvested at day 6 or 9 after infection. We detected no statistically significant difference in WNV-specific IgM or IgG response between the MAR1-5A3 and control antibody-treated groups at any of the time points tested (Figure S1, P>0.2). Thus, blockade of type I IFN signaling at day 2 or 4 after infection had no major impact on induction of WNV-specific antibody responses during the acute phase of infection.
CD8+ T cells contribute to the rapid clearance of WNV infection from peripheral and central nervous system (CNS) tissues [31]–[34]. Analysis of CD8+ T cells at day 9 in the spleen of wild type mice treated with the MAR1-5A3 or control antibody at day 4 after infection showed a similar percentage and total number of WNV-specific CD8+ T cells when measured by intracellular IFN-γ and TNF-α staining after ex vivo incubation with an immunodominant Db-restricted NS4B peptide (Figure 2A) or direct tetramer staining (data not shown). Nonetheless, blockade of type I IFN signaling at day 4 resulted in a decrease in the amount of intracellular IFN-γ (P<0.0001) and TNF-α (P<0.006) produced by individual antigen-specific CD8+ T cells as judged by differences in the geometric mean fluorescence intensity of the positive cells. Correspondingly, the amount of granzyme B in NS4B tetramer positive cells was less (P<0.003) in MAR1-5A3 treated mice (Figure 2B). The differences in intracellular cytokines and granzyme B protease establish a late temporal role for type I IFN signaling in the maturation of the antigen-specific CD8+ T cells, even though initial priming, as reflected by the total percentage and number of WNV-specific IFN-γ+ CD8+ or TNF-α+ CD8+ T cells, remained intact.
Fig. 2. Effect of treatment of MAR1-5A3 on WNV-specific CD8+ T cell responses. 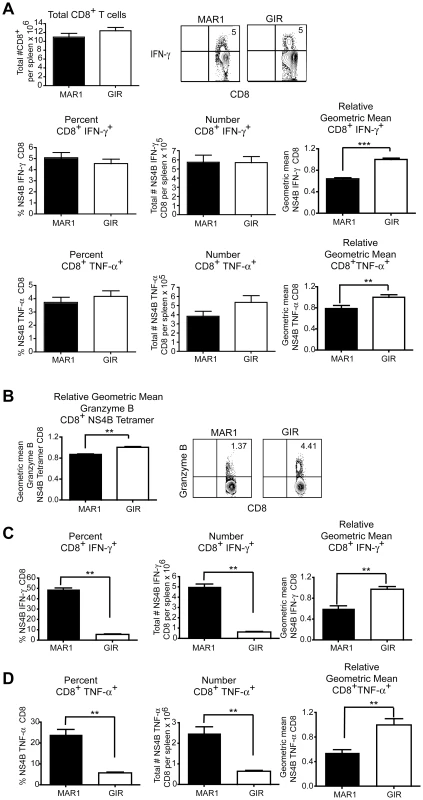
A-B. Mice were infected with 102 PFU of WNV-NY and treated with 1 mg of MAR1-5A3 or GIR-208 (GIR) at day 4 post infection. A. Analysis of the CD8+ T cell from the spleen of infected MAb-treated mice (n = 20 to 25 per group). Splenocytes were harvested on day 9 after WNV infection, and intracellular cytokine staining of IFN-γ and TNF-α was analyzed in CD8+ T cells after ex vivo restimulation with NS4B peptide. (Top left) Total number of splenic CD8+ T cells after infection and treatment with MAbs. (Top right) A representative contour plot showing intracellular IFN-γ levels on CD8+ T cells after MAb treatment is shown. The percentage, number, and relative staining of WNV-specific IFN-γ+ CD8+ T cells (middle panels) or WNV-specific TNF-α+ CD8+ T cells (bottom panels) are shown. Relative intracellular cytokine staining reflects pooling of data from independent experiments after normalization within a given experiment. B. The levels of intracellular granzyme B in WNV-specific CD8+ T cells were assessed by co-staining with Db-NS4B tetramer and antibodies to granzyme B (n = 6 mice per group). A representative contour plot showing intracellular granzyme B levels on CD8+ T cells after MAb treatment is shown. C–D. Mice were infected with 102 PFU of WNV-MAD and treated with 1 mg of MAR1-5A3 or GIR-208 (GIR) at day -1 and +4 relative to infection. The percentage, number, and relative staining of WNV-specific (C) IFN-γ+ CD8+ T cells or (D) TNF-α+ CD8+ T cells are shown (n = 5 mice per group). Asterisks indicate differences that are statistically significant (*, P<0.05; **, P<0.01, ***, P<0.001). To determine whether a similar effect on T cell maturation was observed if type I IFN was neutralized throughout infection, we pre-treated mice with MAR1-5A3 prior to infection with an attenuated lineage 2 WNV strain from Madagascar (WNV-MAD) [35], [36]. We used this less virulent WNV strain because mice treated with MAR1-5A3 and infected with WNV-NY did not survive past day 6 (see Figure 1). In comparison, mice treated with MAR1-5A3 before or after infection with attenuated WNV-MAD showed very limited mortality (data not shown). Accordingly, mice were treated with MAR1-5A3 or isotype control mAb one day prior to and four days after infection with WNV-MAD, and CD8+ T cells were analyzed at day 9. Notably, depletion of type I IFN signaling throughout the course of infection resulted in a substantial increase in the percentage (6 to 49%, P<0.008) and number (P<0.008) of WNV-specific IFN-γ+ CD8+ T cells (Figure 2C). Similar results were observed when intracellular TNF-α was measured (Figure 2D). The large increase in CD8+ T cell priming may be attributed to the greater WNV antigen burden in lymphoid tissues in mice lacking type I IFN signaling [8]. However, and consistent with that observed with MAR1-5A3 treatment at day 4 only with WNV-NY infection, the amounts of intracellular IFN-γ and TNF-α present in WNV-specific CD8+ T cells were lower (P<0.008) when type I IFN signaling was blocked throughout infection.
Blockade of type I IFN receptor at day 4 also modulated the CD4+ T response after WNV-NY infection. The percentage of IFN-γ+ or TNF-α+ CD4+ T cells, as measured after ex vivo stimulation with anti-CD3 antibodies, was decreased (P<0.007) in mice receiving MAR1-5A3 compared to the GIR-208 isotype control MAb (Figure S2). While we observed a significant decrease (P<0.004) in the total number of TNF-α+ producing CD4+ T cells in MAR1-5A3 treated mice, this was not observed in IFN-γ+ CD4+ T cells. Analogous to that seen with WNV-specific CD8+ T cells, decreased amounts (P<0.01) of IFN-γ and TNF-α were produced by activated CD4+ T cells in animals treated with MAR1-5A3.
Temporal effect of MAR1-5A3 on regulatory T cell induction
Given that a blockade of type I IFN signaling resulted in WNV-specific CD8+ T cells that expressed lower levels of intracellular cytokines, we speculated that this could be due to an increase in CD4+CD25+FoxP3+ regulatory T cells. Type I IFN has been reported to alter regulatory T cell activity, which impacts CD8+ T cell function [37], [38], and decreased regulatory T cell levels augment WNV-specific CD8+ T cell responses [39]. However, we observed no difference in the percentage or numbers of CD4+CD25+FoxP3+ cells at day 9 in the blood (data not shown) or spleen (P>0.6), when MAR1-5A3 or control GIR-208 MAb was administered at day 4 after WNV-NY infection (Figure S3).
Temporal effect of MAR1-5A3 on CD8+ T cell responses after VV infection
Our data suggested that type I IFN signaling at a later stage modulated WNV-specific T cell responses despite having limited effects on viral replication or initial priming. To determine whether this finding was typical of other viral infections, we repeated MAR1-5A3 treatments at day 4 after infection with an unrelated DNA (VV, Western reserve strain) virus. Mice were harvested eight days after infection (four days after treatment) and T cell populations were analyzed. Similar to that seen with WNV, the amount of intracellular IFN-γ produced by CD8+ T cells from the MAR1-5A3 treated mice was lower (P<0.02) after re-stimulation ex vivo with two different VV-peptides (A47L or B8R) compared to the isotype control GIR-208 treated mice (Figure 3A and B). Notably, and in contrast to WNV infection, we also detected a decrease in the percentage (P<0.02) and number (P<0.04) of IFN-γ producing VV-specific CD8+ T cells, suggesting that for VV infection, type I IFN signaling at day 4 or after also contributed to initial priming. Similar results were observed with TNF-α production with VV-specific CD8+ T cells after MAR1-5A3 treatment (Figure 3A and B). Thus, a temporal blockade of type I IFN signaling impairs antigen-specific CD8+ T cell maturation in the context of infection by WNV and VV, two unrelated RNA and DNA viruses.
Fig. 3. Effect of MAR1-5A3 on VV-specific CD8+ T cell responses in the spleen. 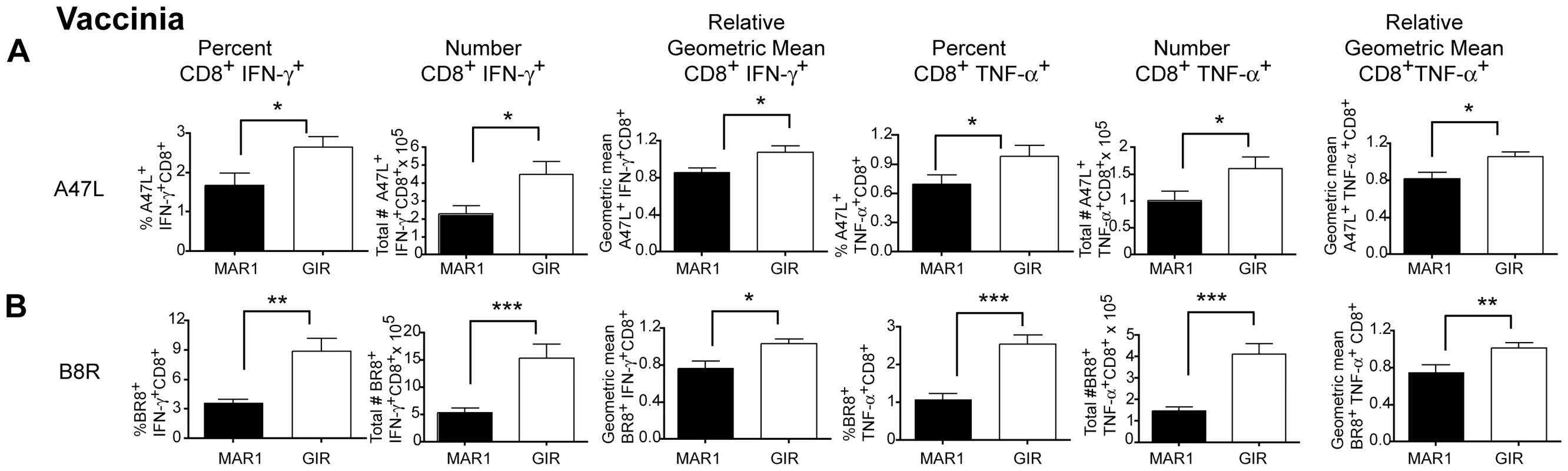
Mice were infected with 104 PFU of VV (Western reserve strain), treated at day 4 with 1 mg of MAR1-5A3 or GIR-208 MAb, and splenocytes were harvested on at day 9 for intracellular cytokine staining of IFN-γ and TNF-α of CD8+ T cells after peptide restimulation with immunodominant A47L (A) or B8R (B) peptides (n = 9 mice per group). Relative intracellular cytokine staining reflects pooling of data from independent experiments after normalization within a given experiment. Asterisks indicate differences that are statistically significant (*, P<0.05; **, P<0.01, ***, P<0.001). Type I IFN receptor signaling, CD8-α dendritic cells, and CD8+ T cell responses
Recent studies have suggested that type I IFN enhances the CD8+ T cell response during antigen cross-presentation [17], [22], [40], [41]. To evaluate whether the temporal effect of type I IFN signaling on CD8+ T cell responses occurred in mice with impaired cross-presentation capacity, we utilized BATF3-/- mice, which lack CD8-α and CD103+ dendritic cells [18], [42]. Consistent with earlier results from BATF3-/- 129SvEv mice [18], we observed a decrease in the percentage and number (P<0.008) WNV-specific CD8+ T cells in BATF3-/- mice on the C57BL/6 background although no substantive difference (P>0.06) in intracellular IFN-γ levels was detected (Figure 4A).
Fig. 4. Effect of deletion of BATF3 and loss of CD8-α dendritic cells on CD8+ T cell responses after WNV infection. 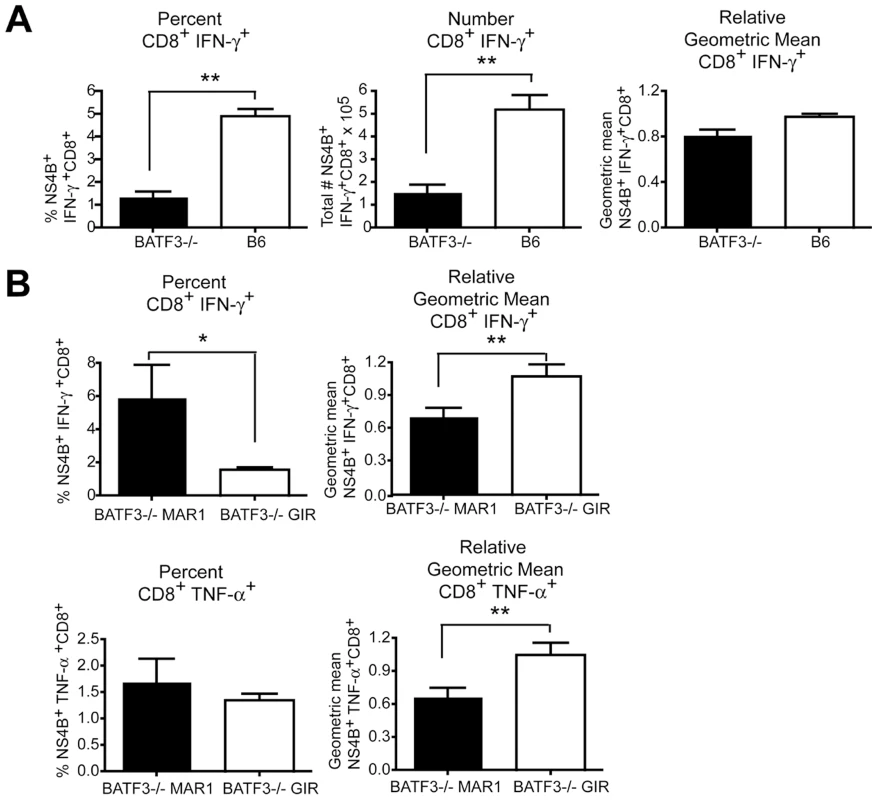
A. Wild type or BATF3-/- mice were infected with 102 PFU of WNV-NY, spleens were harvested on day 9 after infection, and intracellular IFN-γ responses in CD8+ T cells were measured by flow cytometry after ex vivo stimulation with NS4B peptide (n = 5 mice per group). B. Wild type or BATF3-/- mice were treated with MAR1-5A3 or GIR-208 (1 mg per dose) at day 4 after infection with 102 PFU of WNV-NY. Spleens were harvested on day 9 after infection, and intracellular IFN-γ (top panels) and TNF-α (bottom panels) responses in CD8+ T cells were measured by flow cytometry after ex vivo stimulation with NS4B peptide (n = 5 mice per group). Relative intracellular cytokine staining reflects pooling of data from independent experiments after normalization within a given experiment. Asterisks indicate differences that are statistically significant (*, P<0.05; **, P<0.01, ***, P<0.001). To determine whether mice with priming defects due to impaired cross-presentation still required late stage type I IFN for CD8+ T cell maturation, MAR1-5A3 or control GIR-208 MAb was administered to wild type or BATF3-/- mice at day 4 after WNV-NY infection. As expected, associated with the absence of CD8-α dendritic cells, the magnitude (percentage and number) of IFN-γ+ and TNF-α+ NS4B-specific CD8+ T cells at day 9 was markedly lower in MAR1-5A3 or GIR-208 MAb treated BATF3-/- mice compared to wild type animals (data not shown). Nonetheless, reduced intracellular levels of IFN-γ and TNF-α (P<0.009) in WNV-specific CD8+ T cells were still observed in BATF3-/- mice treated with MAR1-5A3 at day 4 compared to control MAb-treated animals (Figure 4B). Thus, the temporal effect of type I IFN blockade on CD8+ T cell maturation occurred both in the presence or absence of CD8-α dendritic cells and efficient antigen cross-presentation.
Cell-extrinsic effect of type I IFN modulates CD8+ T cell functional development
Studies with IFNAR-/- bone marrow chimera or conditionally deleted IFNAR on T cells showed reduced cross-presentation of ovalbumin peptides to CD8+ T cells, suggesting that direct stimulation of T cells by type I IFN enhances the antigen-specific CD8+ T cell response, at least for soluble antigens [22]. Blockade of type I IFN signaling four days after WNV infection results in a dysfunctional antigen-specific CD8 T cell population that nonetheless appeared to undergo a relatively normal priming phase. In comparison, MAR1-5A3 treatment at days -1 and 4 (essentially throughout infection) resulted in a dysfunctional antigen-specific CD8+ T cell population, but with a massive increase in the fraction and number of antigen-specific T cells. To establish whether the effect of type I IFN on CD8+ T cell functional development was cell-intrinsic in the context of viral infection, we adoptively transferred naïve purified IFNAR-/- (CD45.2) or B6.SJL (CD45.1) CD8+ T cells into RAG1-/- recipient mice. Immediately after WNV infection, blood was sampled to confirm transfer of T cell populations in the recipient mice (data not shown). At day nine after infection, spleens were harvested and the CD8+ T cell activation profiles analyzed. Notably, we did not detect a significant difference (P>0.06) in the intracellular levels of IFN-γ or TNF-α between the IFNAR-/- (CD45.2) and B6.SJL (CD45.1) CD8+ T cells donor cells in the IFNAR+/+ RAG1-/- mice (Figure S4). This result suggests that at least in the context of WNV infection, the effect of type I IFN on the development of a functional CD8+ T cell response is largely T cell non-autonomous in nature.
Effect of MAR1-5A3 on antigen-presenting cells
As our adoptive transfer experiments suggested that efficient WNV-specific CD8+ T cell activation did not require cell autonomous type I IFN signaling in CD8+ T cells, we assessed whether antigen-presenting cells in the spleen were differentially affected by MAR1-5A3 treatment. MAR1-5A3 was administered 2 or 4 days after WNV infection and APC were examined on days 6 and 9 after infection (Figure 5A, B, and C). When MAR1-5A3 was given on day 2 and splenocytes analyzed on day 6, no difference was observed in the percentage of CD11c+ cells or their relative expression of the co-stimulatory molecules CD80 and CD86 (Figure 5B). We did however, observe an increased percentage of CD11b+ splenocytes at this time point, and this was associated with reciprocal decreases and increases in expression of CD80 and CD86, respectively. In comparison, when MAR-5A3 was administered on day 4 after WNV-NY infection and splenocytes analyzed on day 6, we observed a reduced percentage of CD11b+ (P<0.01) and CD11c+ (P<0.02) cells, and this was associated with decreased expression of CD86 only on CD11c+ cells (Figure 5B, P<0.008). When MAR-5A3 was administered on day 4 after WNV-NY infection and splenocytes analyzed on day 9, we also observed a decrease in surface expression of CD86 on CD11b+ (P<0.05) and CD11c+ (P<0.007) cells relative to the control MAb treatment (Figure 5A and C). In comparison, MAR1-5A3 treatment had no effect on CD80 expression on CD11c+ cells although an increase (P<0.005) was noted in CD11b+ cells at this time. Thus, blockade of type I IFN signaling at day 4 after infection resulted in a distinct antigen-presenting cell activation phenotype compared to MAR1-5A3 treatment at day 2; this suggests that disruption of type I IFN signaling pathways at particular stages of infection might limit the ability of antigen-presenting cells to provide key temporal signals that allow optimal generation of antigen-specific effector CD8+ T cells.
Fig. 5. Effect of MAR1-5A3 treatment on costimulatory molecule expression of antigen-presenting cells. 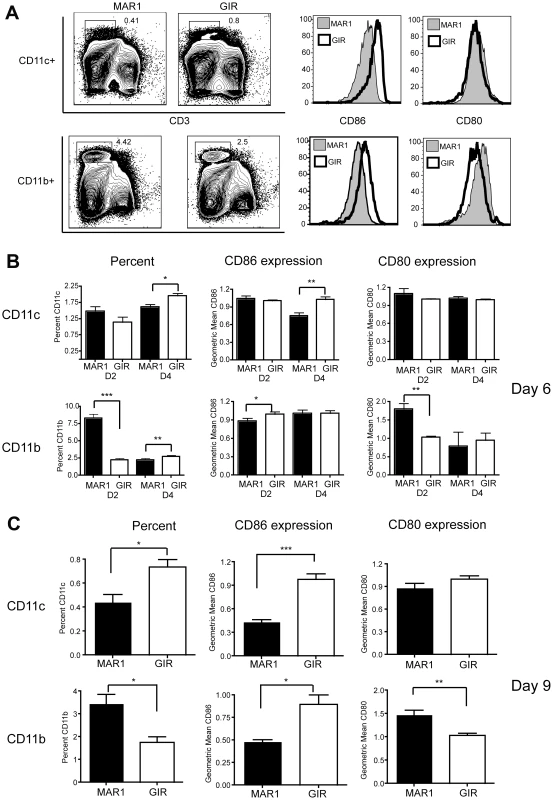
Mice were infected with 102 PFU of WNV-NY and treated with 1 mg of MAR1-5A3 or GIR-208 at days 2 or 4 post infection. At days 6 or 9 after infection, CD11b+ and CD11c+ splenocytes were analyzed for expression of CD80 and CD86 by flow cytometry. A. Gating strategy and representative histograms are shown from animals treated with MAR1-5A3 or GIR-208 at day 4 and harvested at day 9. B-C. Summary of data showing the percentage of CD11c+ and CD11b+ splenocytes and the mean fluorescence intensity of CD80 and CD86 staining (n = 7 to 9 mice per group) from animals (B) treated at days 2 or 4 and harvested at day 6 or (C) treated at day 4 and harvested at day 9. Asterisks indicate differences that are statistically significant (*, P<0.05; **, P<0.01, ***, P<0.001). Effect of MAR1-5A3 on cytokine levels
We speculated that a specific absence of type I IFN signaling in amtigen-presenting cells impaired development of a WNV-specific CD8+ T cell response because of an altered production of cytokines required for maturation. To assess this, we measured the cytokine levels in mice that were treated with MAR1-5A3 at day 4 after WNV-NY infection. Two or five days after MAb treatment (day 6 or 9 after infection), serum was harvested and levels of relevant cytokines (IFN-γ, TNF-α, IL-10, IL-12 p40, IL-17, and IL-18) were measured by bioplex assay (Figure 6A–F). Two days after MAR1-5A3 treatment, significantly (P<0.04) reduced levels of IL-12 p40 were observed (Figure 6D). Within five days of MAR1-5A3 treatment, serum levels of IFN-γ, TNF-α, and IL-12 p40 were reduced significantly (P<0.01) and IL-10 was elevated (P<0.02). The increased level of IL-10 in mice treated with the blocking type I IFN MAb may be particularly relevant as IL-10 negatively impacts CD8+ T cell activation and function [37], [38].
Fig. 6. Effect of MAR1-5A3 treatment on serum inflammatory cytokines. 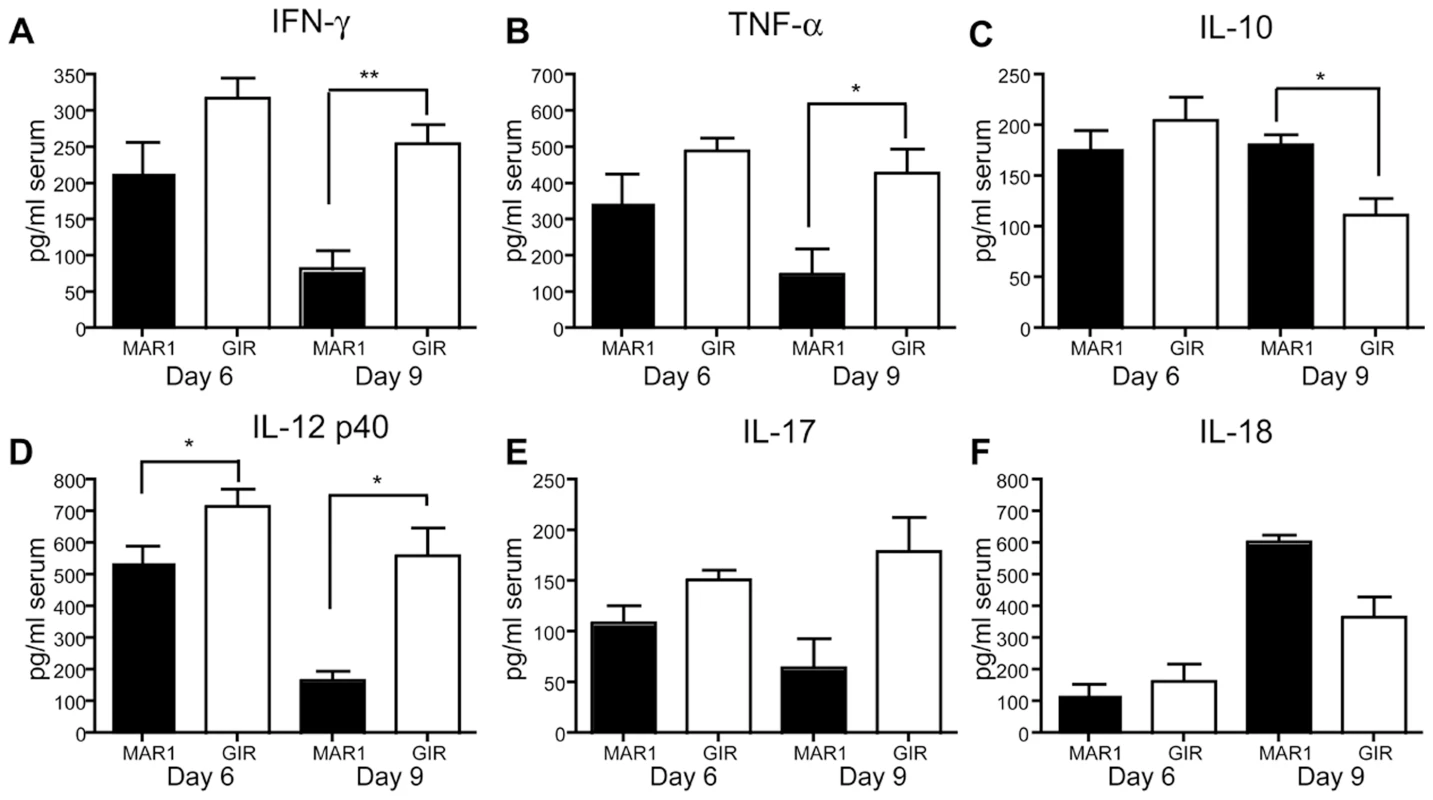
Mice were infected with 102 PFU of WNV and treated with 1 mg of MAR1-5A3 or GIR-208 at day 4 post infection. Serum was harvested at day 6 or 9 after infection and analyzed for the (A) IFN-γ, (B) TNF-α, (C) IL-10, (D) IL-12 p40, (E) IL-17, and (F) IL-18 using a Bio-Plex pro cytokine assay (n = 6 mice per group). Asterisks indicate differences that are statistically significant (*, P<0.05; **, P<0.01, ***, P<0.001). Phenotype of CD8+ T cells in MAR1-5A3 treated mice
Because blockade of IL-10 in chronic lymphocytic choriomengitis virus (LCMV) infection prevents functional exhaustion of CD8+ T cells and promotes viral clearance [43], [44], we hypothesized that the increased IL-10 levels in serum of MAR1-5A3 treated mice after WNV-NY infection might cause the CD8+ T cells to acquire an exhausted phenotype. To assess this, at day 5 after MAb treatment (day 9 after infection), we profiled Db-NS4B-tetramer+ CD8+ T cells for expression of PD-1, CTLA-4, CD43, CD44, CD127, and CD11a (Figure 7A). Notably, treatment with MAR1-5A3 compared to the control MAb resulted in reduced expression of CD11a (P<0.001) and increased expression of CD127, CD43, CD44, CTLA-4 and PD-1 (P<0.007). Thus, CD8+ T cells from mice treated at day 4 with MAR1-5A3 not only showed altered intracellular cytokine patterns (see Figure 2) but also displayed some of the phenotypic hallmarks of exhaustion. Similarly, BATF3-/- mice treated with MAR1-5A3 at day 4 after WNV infection also expressed elevated (P<0.02) levels of the exhaustion markers CTLA-4 and PD-1 on WNV-specific CD8+ T cells at day 9 compared to control MAb (Figure 7B). Whereas prior studies described CD8+ T cell exhaustion at later time points during chronic LCMV infection [24], [45], blockade of type I IFN signaling independent of the mode of priming appears to exhaust WNV-specific CD8+ T cells during the acute effector phase.
Fig. 7. Effect of MAR1-5A3 treatment on expression of markers of CD8+ T cell activation and exhaustion. 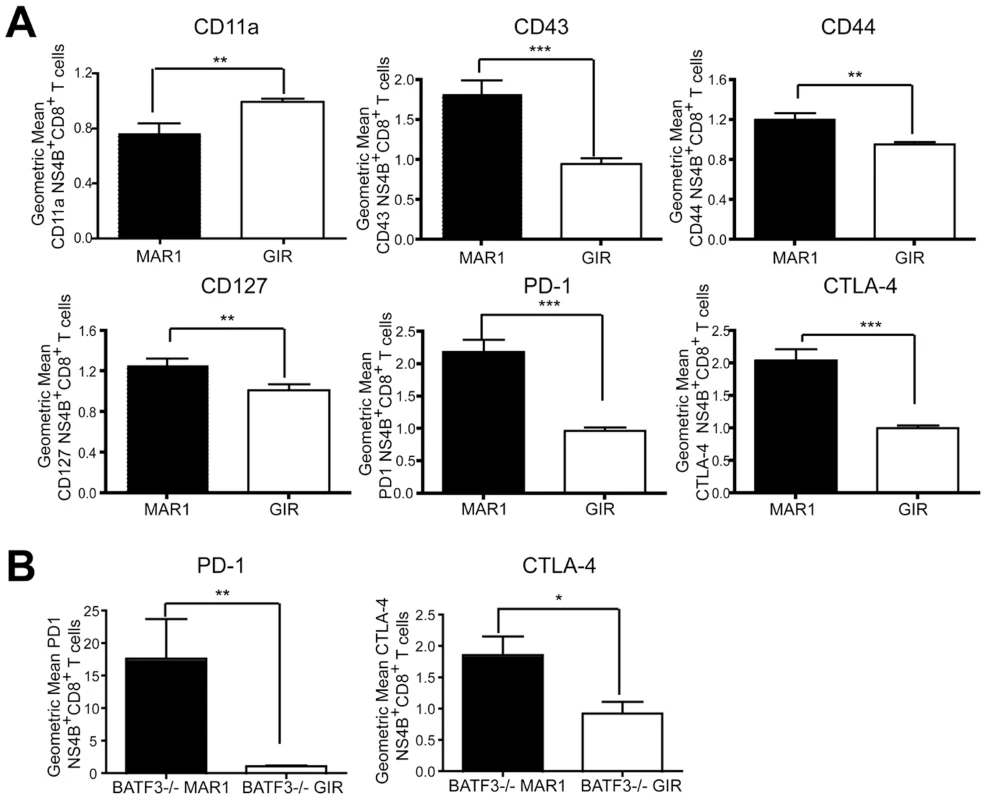
A. Wild type mice were infected with 102 PFU of WNV-NY and treated with 1 mg of MAR1-5A3 or GIR-208 at day 4 post infection. Splenocytes were harvested on day 9, co-stained for CD8β and Db-NS4B tetramer, and the gated cells analyzed by flow cytometry for relative expression of PD-1, CTLA-4, CD44 CD127, CD11a and CD43 (n = 18 mice per group). B. BATF3-/- mice were treated with MAR1-5A3 or GIR-208 at day 4 after infection with 102 PFU of WNV-NY. Spleens were harvested on day 9 after infection, co-stained for CD8β and Db-NS4B tetramer, and the gated cells analyzed by flow cytometry for relative expression of PD-1 and CTLA-4 (n = 5 mice per group). Relative staining reflects pooling of data from independent experiments after normalization within a given experiment. Asterisks indicate differences that are statistically significant (*, P<0.05; **, P<0.01, ***, P<0.001). One of the earliest stages of CD8+ T cell exhaustion is characterized by a reduced capacity to lyse target cells [45], [46]. Although we observed reduced levels of granzyme B in Db-NS4B tetramer positive CD8+ T cells (Figure 2), we questioned whether WNV-specific effector cells during the acute immune response displayed a fully exhausted phenotype. We assessed how MAR1-5A3 treatment affected CD8+ T cells ability to lyse peptide pulsed targets in vivo (Figure S5). Splenocytes from naïve B6.SJL (CD45.1) mice were divided into two groups: one group was pulsed with NS4B immunodominant peptide and labeled with 500 nM carboxyfluorescein diacetate succinimidyl ester (CFDA), and the other was not pulsed with peptide and labeled with 5 nM CFDA. The two groups were mixed in equal numbers and injected into WNV-infected C57BL/6 (CD45.2) mice at day 9 that had undergone treatment with either MAR1-5A3 or control GIR-208 MAb at day 4. Six hours after labeled cells were transferred, splenocytes were harvested and in vivo killing was assessed. Notably, we observed no difference in killing between the MAR1-5A3 and the control MAb-treated mice (P>0.3). Thus, type I IFN blockade at a later stage of WNV infection produces an intermediate exhaustion phenotype with skewed cytokine production, surface expression of exhaustion markers, yet relatively intact cytolytic potential.
Discussion
In this study, we evaluated the antiviral and immunomodulatory roles of type I IFN signaling after viral infection. While past studies in IFNAR-/- mice with virulent or attenuated WNV strains revealed enhanced susceptibility, dissemination, and lethality compared to congenic wild type mice [8], [36], [47], they did not address the temporal functions of type I IFN during infection. While administration of MAR1-5A3 at day 2 after infection resulted in markedly enhanced viral burden in multiple tissues as seen in IFNAR-/- mice [8], treatment at day 4 had more subtle effects on viral replication. Instead, detailed analysis established a key role for later type I IFN signaling in the maturation of effector CD8+ T cells. Blockade of type I IFN signaling at day 4 after infection with WNV resulted in depressed cytokine responses and changes in phenotypic markers suggesting altered activation and exhaustion.
Prior studies have reported that type I IFN signaling primes adaptive immune functions including cross-presentation of CD8+ T cells, enhancement of antibody responses, and maintenance of dendritic cells in a state competent for antigen-presentation [9], [13], [17], [48]. Depending on the experimental system, type I IFN can act directly on CD8+ T cells or indirectly on antigen-presenting cells to influence the fate of CD8+ T cells during the initial phases of antigen recognition (reviewed in [49]). Many of these studies used IFNAR-/- mice [50], adoptive transfer of wild type or IFNAR-/- immune cells into IFNAR-/- or wild type mice [27], or cell-type specific deletion of IFNAR [51]. While they have provided significant insight into the immunomodulatory effects of type I IFN and defined key cells involved in priming, they have not elucidated the stage-specific effects of type I IFN. In our experiments, when type I IFN signaling was blocked with MAR1-5A3 prior to infection with an attenuated WNV strain, we observed at day 9 paradoxically enhanced numbers of antigen-specific effector CD8+ T cell responses that had deficits in IFN-γ or TNF-α production, results that are consistent with prior infection experiments [52]. The increased numbers of WNV-specific CD8+ T cells in mice treated with MAR1-5A3 at day -1 could be due to increased antigen burden or a failure to produce IL-10 and negatively regulate T cell expansion [37].
Administration of a single dose of MAR1-5A3 at day 4 after infection with virulent or attenuated WNV strains revealed a distinct phenotype. Although the absolute percentage and number of NS4B-specific CD8+ T cells was similar compared to isotype MAb-treated or unmanipulated animals, the geometric mean fluorescence intensity of IFN-γ or TNF-α was consistently lower. Thus, in the context of WNV infection, the initial priming phase of virus-specific CD8+ T cells does not absolutely require type I IFN signaling whereas the later maturation phase does. In addition, MAR1-5A3 treatment on day 4 was associated with lower granzyme B expression, decreased surface levels of the adhesion molecule CD11a (LFA-1), and increased expression of CD44, CD127 (IL-7R α-chain), and CD43 on WNV-specific CD8+ T cells. These markers are significant because in mice activated, lytic CD8+ T cells display a CD44hi CD43hi CD127lo granzyme Bhi phenotype whereas memory CD8+ T cells express a CD44hi CD43lo/int CD127hi granzyme Blo phenotype [53]-[55]. Thus, stage-specific blockade of type I IFN signaling alters intracellular cytokine production of antigen-specific CD8+ T cells and promotes a transitional phenotype during the acute (day 9) phase that appears to fall somewhere between effector and memory populations.
Consistent with functionally dysregulated CD8+ T cells when type I IFN signaling was blocked at day 4, we observed increased expression of PD-1 and CTLA-4, two markers of T cell exhaustion [24], [56], which were originally described in the context of chronic, persistent infection of LCMV [46]. In chronic LCMV infection, there is a hierarchy to CD8+ T cell exhaustion with some functions exhausted early (IL-2 production, cytotoxicity, and proliferation) and others persisting longer (intracellular pro-inflammatory cytokines) [45]. In comparison, blockade of type I IFN signaling at day 4 resulted in WNV-specific CD8+ T cells at day 9 that retained the ability to kill targets in vivo although they expressed lower quantities of IFN-γ and TNF-α. Thus, stage-specific blockade of type I IFN results in dysfunctional CD8+ T cells with loss of some but not all effector functions during the acute phase. Although we cannot address what happens during later stages (evolution and maintenance of memory CD8+ T cells) in the context of type I IFN blockade and virulent WNV-NY infection because of complete lethality in the model, kinetic studies are planned with the attenuated WNV-MAD strain and MAR1-5A3 to determine how and when type I IFN signaling affects the transition to and establishment of memory phenotypes.
The dysfunctional CD8+ T cell phenotype observed after MAR1-5A3 treatment and WNV infection also was observed after VV infection. The change in CD8+ T cell profile with type I IFN blockade at day 4 was even more marked after VV infection, as the percentage, number, and mean fluorescence intensity of antigen-specific CD8+ T cells were all significantly reduced at day 9 for two independent immunodominant epitopes. Thus, for VV, late stage type I IFN blockade affected both priming and subsequent maturation.
Cross-priming of CD8+ T cells occurs after dendritic cells pick up soluble molecules or cellular debris [57] and are licensed by additional cellular or inflammatory signals [58]. Although type I IFN can license dendritic cells for cross-priming of CD8+ T cells with soluble ovalbumin [17], it remains unknown if it is essential in the context of the inflammatory milieu associated with viral infection. We speculated that stage-specific blockade of type I IFN signaling might have dominant effects on CD8+ T cells maturation if CD8-α dendritic cells and cross-presentation were required for priming and activation. To evaluate this, we infected BATF3-/- mice, which lack CD8-α dendritic cells, are defective in antigen cross-presentation, and fail to optimally prime CD8+ T cell responses [18]. While the percentage and number of WNV-specific IFN-γ+ CD8+ T cells was blunted in BATF3-/- mice, the remaining CD8+ T cells that were presumably primed by a distinct antigen presentation pathway showed reduced intracellular cytokine levels and enhanced expression of CTLA-4 and PD-1. Thus, at least during WNV infection, the temporal effects of type I IFN signaling on effector CD8+ T cell maturation occur regardless of the initial priming pathway.
Although prior studies have suggested that direct stimulation of T cells by type I IFN enhances ovalbumin-specific CD8+ T cell responses during cross-priming [22], we did not observe this in the context of WNV infection. CD45.2 CD8+ T cells lacking IFNAR showed roughly equivalent WNV-specific responses compared to congenic CD45.1 CD8+ T cells after transfer into and infection of RAG1-/- recipient mice. An analogous small impact of direct stimulation by type I IFN on CD8+ T cells was observed after infection with VV [59] but not with LCMV [27], [50]. The differential requirement for direct signaling on CD8+ T cells may be due to differences in local and systemic type I IFN production during infection with different pathogens [50].
Blockade of type I IFN at day 4 after WNV infection was associated with decreased expression of CD86 on antigen-presenting cells, which likely influences optimal antigen presentation to CD8+ T cells [14], [60]. Indeed, lower levels of pro-inflammatory dendritic cell-produced cytokines (IL-12) [61] that regulate CD8+ T cell expansion and activation state were observed in mice treated with MAR1-5A3 at day 4. Alternatively, blockade of type I IFN signaling at day 4 could affect CD8+ T cell activation because of the elevated levels of the inhibitory cytokine IL-10. Although our results point to a critical temporal role of type I IFN signaling in the functional activation of CD8+ T cells in the context of infection by WNV, future studies are required to define the precise spatial and cell-type specific cues that govern this process.
The administration of a neutralizing anti-IFNAR antibody at day 2 after infection limited the ability of the host to control WNV replication and spread to target tissues, thus confirming a dominant antiviral effect of type I IFN during the early stages of pathogenesis. In comparison, administration of the anti-IFNAR antibody at day 4 after WNV infection had marginal effects on viral replication, no effect on the magnitude of CD8+ T cell priming, yet profoundly impacted the functional CD8+ T cell responses during the acute effector phase, resulting in blunted cytokine production, and changes in phenotypic markers associated with altered activation status and CD8+ T cell exhaustion. Given that several studies have established a protective clearance role for CD8+ T cells in the brain after WNV infection with virulent North American strains [31], [33], [34], [62], it is not surprising that a temporally defective type I IFN response that affects optimal CD8+ T cell maturation resulted in enhanced lethality.
Future studies that administer neutralizing antibodies against IFNAR, other individual IFN subtypes, or other anti - or pro-inflammatory cytokines at different phases of acute virus infection may reveal stage-specific requirements for shaping effector CD8+ T cells, the contraction phase, and the transition to central and effector memory. Such studies, coupled with experiments in mice with cell-type specific deletions of IFNAR, will provide new insight into the spatial-temporal dynamics of CD8+ T cell expansion and development during infection by different viruses.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (Assurance Number: A3381-01). All inoculation and experimental manipulation was performed under anesthesia that was induced and maintained with ketamine hydrochloride and xylazine, and all efforts were made to minimize suffering.
Viruses and cells
The lineage 1 WNV strain 3000.0259 (WNV-NY) was isolated in New York in 2000 [63] and passaged twice in C6/36 Aedes albopictus cells. The lineage 2 WNV strain from Madagascar (DakAnMg798, WNV-MAD) was isolated in 1978 and passaged on C6/36 cells [35]. BHK21-15 cells were used for plaque assay experiments with WNV. VV (Western Reserve) was grown in Vero cells and purified by ultracentrifugation through a 36% sucrose cushion.
Mice
Wild type and RAG1-/- C57BL/6 mice were obtained commercially (Jackson Laboratories). C57BL/6.SJL-Ptprca/BoyAiTac (B6.SJL, CD45.1) mice were purchased (Taconic). IFNAR-/- mice were obtained from J. Sprent (Scripps Institute, San Diego CA) and were backcrossed ten times onto the C57BL/6 background. BATF3-/- mice [18] were backcrossed onto a C57BL/6 background for ten generations. All mice were housed in the pathogen-free mouse facility at the Washington University School of Medicine. Mice (8 to 12 week-old) were inoculated subcutaneously via footpad injection with 102 plaque-forming units (PFU) of WNV-NY or WNV-MAD. VV (104 PFU) was injected via an intraperitoneal route. MAR1-5A3 (mouse anti-mouse IFNAR, IgG1) or isotype control GIR-208 (mouse anti-human IFN-γ receptor 1, IgG1) MAbs [23] were administered as a single dose at 1 mg per mouse unless otherwise indicated by intraperitoneal (IP) injection at specific times with respect to viral infection. MAR-5A3 and GIR-208 MAbs were purchased (Leinco Technologies) and certified as free of endotoxin contamination and aggregates. The half-life of MAR1-5A3 is reported as 5.2 days when a sufficient amount is administered to saturate the receptor pool [23].
Quantification of viral burden
For analysis of viral burden MAR1-5A3 or GIR-208 was administered two or four days after infection, and organs were recovered on day 6 after cardiac perfusion with 10 ml of PBS. Tissues were weighed, homogenized using a bead-beater apparatus, and titrated for WNV by plaque assay on BHK21-15 cells as described previously [29]. Serum was obtained from whole blood after phlebotomy of the axillary vein immediately before sacrifice and viremia was measured by analyzing WNV RNA levels using fluorogenic quantitative RT-PCR (qRT-PCR) as described [25].
WNV-specific antibody analysis
WNV-specific IgM and IgG levels were determined using an envelope (E) protein–specific ELISA as described [64].
CD4+ and CD8+ T cell analysis
Intracellular staining of TNF-α and IFN-γ from splenocytes was performed as described previously [33]. Briefly, spleens were harvested and homogenized to form a single cell suspension. Cells (2×106 cells) were added to a 96 well plate and incubated with 2 µg/ml brefeldin A (Sigma) for 6 h at 37°C with 10−6 M of immunodominant T cell peptides (WNV: Db-restricted NS4B 2488–2496 (SSVWNATTA) [33]; and VV: Kb-restricted A47L 138–146 (AAFEFINSL) and B8R 20–27 (TSYKFESV) [65]) or 2 µg/ml anti-CD3 (145-2C11) (BD Biosciences). After incubation, the cells were stained with directly labeled antibodies (all from BD Biosciences unless indicated) against CD4 (GK1.5), CD19 (6D5), CD43 (1B11), CD127 (SB/199), CD8β (YTS156.7.7), CD44 (MI7), PD-1 (RMP1-30), and CTLA-4 (UC10-4B9, Biolegend). Db-NS4B tetramer was obtained from the NIH tetramer core facility. Cells were washed, fixed, and permeabilized with FixPerm Buffer (eBioscience), and stained intracellularly for anti-IFN-γ (XMG1.2), anti-TNF-α (MP6-XT22, eBioscience), or anti-granzyme B (GB12, Invitrogen). Lymphocytes were processed on an LSRII (BD Bioscience) using FACSDiva 6.1.1 software (BD Bioscience) and analyzed with FlowJo (Treestar). The total numbers of IFN-γ or TNF-α expressing CD4+ or CD8+ T cells was determined by multiplying the percentage of IFN-γ+ or TNF-α+ CD4+ or CD8+ T cells by the total numbers of splenocytes. CD4+CD25+FoxP3+ regulatory T cells were measured using a specific staining kit (eBioscience) following manufacturer's protocol.
Adoptive transfer experiments
Splenocytes from naïve wild type (CD45.1) or IFNAR-/- (CD45.2) mice were harvested. CD8+ T cells were isolated by negative selection after mixing splenocytes with biotinylated antibodies specific for CD4, NK1.1, B220, and MHC class II (eBioscience). After incubation with anti-biotin beads (Miltenyi Biotec), CD8+ T cells were collected (∼85 percent purity) in the flow-through fraction. Wild type or IFNAR-/- CD8+ T cells (3×106) were transferred into RAG1-/- recipient mice. One day later, mice were inoculated with 102 PFU of WNV, and nine days post-infection, splenocytes were harvested and analyzed by flow cytometry as described above.
Cytokine bioplex assay
The cytokine bioplex assay was performed on serum samples from mice at day 6 and day 9 post-infection from WNV-infected mice that had received either MAR1-5A3 or GIR-208 (1 mg/mouse) at day 4 after infection. The BioPlex Pro Assay was performed according to the manufacturer's protocol (BioRad). The cytokine screen included IL-2, IL-4, IL-10, IL-12p40, IL-12p70, IL-15, IL-17, IL-18, IFN-γ, and TNF-α.
In vivo cytolysis assay
In vivo killing of target cells was performed as previously described [66]. Briefly, splenocytes from B6.SJL (CD45.1) mice were isolated. Half of the cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFDA) at 500 nM and the remainder was labeled with 5 nM CFDA. After labeling, cells labeled with 500 nM CFDA were pulsed for one hour at 37°C with 1 µM NS4B 2488–2496 peptide, whereas the 5 nM CFDA cells were not pulsed with peptide. Both sets of cells were counted and equal numbers were mixed and injected intravenously (107 cells total per mouse) into recipient WNV-infected (at day 9 after infection) or naïve mice that had received either MAR1-5A3 or GIR-208 (1 mg/mouse) at day 4 post-infection. After 8 hours, the mice were sacrificed and splenocytes were gated on CD45.1 cells (donor cells). The percent killing of target cells was calculated: (1 – (ratio immune/ratio naive)) x 100. Ratio equals the number of NS4B peptide-coated targets/number of reference targets [67].
Statistical analysis
For survival analysis, Kaplan-Meier curves were analyzed by the log rank test. Statistical significance of viral burden, antiviral antibody titers, and number of activated T cells were analyzed by the Mann-Whitney test. All statistical analysis was performed using Prism software (GraphPad Prism).
Supporting Information
Zdroje
1. IsaacsALindenmannJ 1957 Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 147 258 267
2. StarkGRKerrIMWilliamsBRSilvermanRHSchreiberRD 1998 How cells respond to interferons. Annu Rev Biochem 67 227 264
3. SchogginsJWWilsonSJPanisMMurphyMYJonesCT 2011 A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472 481 485
4. ColonnaMTrinchieriGLiuYJ 2004 Plasmacytoid dendritic cells in immunity. Nat Immunol 5 1219 1226
5. SamuelMADiamondMS 2006 Pathogenesis of West Nile virus infection: A balance between virulence, innate and adaptive immunity, and viral evasion. J Virol 80 9349 9360
6. DaffisSSutharMSSzretterKJGaleMJrDiamondMS 2009 Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog 5 e1000607
7. MackenzieJSGublerDJPetersenLR 2004 Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10 S98 109
8. SamuelMADiamondMS 2005 Type I IFN protects against lethal West Nile Virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol 79 13350 13361
9. Le BonAToughDF 2002 Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol 14 432 436
10. Hervas-StubbsSPerez-GraciaJLRouzautAFernandez de SanmamedMLe BonA 2011 Direct Effects of type I IFNs on cells of the immune system. Clin Cancer Res 17 2619 2627
11. PurthaWEChachuKAVirginHWDiamondMS 2008 Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J Virol 82 10964 10974
12. Le BonASchiavoniGD'AgostinoGGresserIBelardelliF 2001 Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14 461 470
13. Le BonAThompsonCKamphuisEDurandVRossmannC 2006 Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol 176 2074 2078
14. BanchereauJBriereFCauxCDavoustJLebecqueS 2000 Immunobiology of dendritic cells. Annu Rev Immunol 18 767 811
15. KohlmeierJECookenhamTRobertsADMillerSCWoodlandDL 2010 Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity 33 96 105
16. QuigleyMHuangXYangY 2008 STAT1 signaling in CD8 T cells is required for their clonal expansion and memory formation following viral infection in vivo. J Immunol 180 2158 2164
17. Le BonAEtchartNRossmannCAshtonMHouS 2003 Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 4 1009 1015
18. HildnerKEdelsonBTPurthaWEDiamondMMatsushitaH 2008 Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322 1097 1100
19. FrenzTWaiblerZHofmannJHamdorfMLantermannM 2010 Concomitant type I IFN receptor-triggering of T cells and of DC is required to promote maximal modified vaccinia virus Ankara-induced T-cell expansion. Eur J Immunol 40 2769 2777
20. PrinzMSchmidtHMildnerAKnobelochKPHanischUK 2008 Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28 675 686
21. DetjeCNMeyerTSchmidtHKreuzDRoseJK 2009 Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol 182 2297 2304
22. Le BonADurandVKamphuisEThompsonCBulfone-PausS 2006 Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol 176 4682 4689
23. SheehanKCLaiKSDunnGPBruceATDiamondMS 2006 Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res 26 804 819
24. WherryEJHaSJKaechSMHainingWNSarkarS 2007 Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27 670 684
25. SamuelMAWhitbyKKellerBCMarriABarchetW 2006 PKR and RNAse L contribute to protection against lethal West Nile virus infection by controlling early viral spread in the periphery and replication in neurons. J Virol 80 7009 7019
26. DelhayeSPaulSBlakqoriGMinetMWeberF 2006 Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A 103 7835 7840
27. KolumamGAThomasSThompsonLJSprentJMurali-KrishnaK 2005 Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 202 637 650
28. CoroESChangWLBaumgarthN 2006 Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol 176 4343 4351
29. DiamondMSShresthaBMarriAMahanDEngleM 2003 B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol 77 2578 2586
30. DiamondMSSitatiEFriendLShresthaBHiggsS 2003 A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med 198 1853 1862
31. ShresthaBDiamondMS 2004 The role of CD8+ T cells in the control of West Nile virus infection. J Virol 78 8312 8321
32. WangYLobigsMLeeEMullbacherA 2003 CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol 77 13323 13334
33. PurthaWEMyersNMitaksovVSitatiEConnollyJ 2007 Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur J Immunol 37 1845 1854
34. BrienJDUhrlaubJLNikolich-ZugichJ 2007 Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol 37 1855 1863
35. BeasleyDWLiLSudermanMTBarrettAD 2002 Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology 296 17 23
36. KellerBCFredericksenBLSamuelMAMockREMasonPW 2006 Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. J Virol 80 9424 9434
37. DikopoulosNBertolettiAKrogerAHauserHSchirmbeckR 2005 Type I IFN negatively regulates CD8+ T cell responses through IL-10-producing CD4+ T regulatory 1 cells. J Immunol 174 99 109
38. LiuXSXuYHardyLKhammanivongVZhaoW 2003 IL-10 mediates suppression of the CD8 T cell IFN-gamma response to a novel viral epitope in a primed host. J Immunol 171 4765 4772
39. LanteriMCO'BrienKMPurthaWECameronMJLundJM 2009 Tregs control the development of symptomatic West Nile virus infection in humans and mice. J Clin Invest 119 3266 3277
40. LapentaCSantiniSMSpadaMDonatiSUrbaniF 2006 IFN-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8(+) T cells against exogenous viral antigens. Eur J Immunol 36 2046 2060
41. WeiJWaithmanJLataRMifsudNACebonJ 2010 Influenza A infection enhances cross-priming of CD8+ T cells to cell-associated antigens in a TLR7 - and type I IFN-dependent fashion. J Immunol 185 6013 6022
42. EdelsonBTKcWJuangRKohyamaMBenoitLA 2010 Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med 207 823 836
43. EjrnaesMFilippiCMMartinicMMLingEMTogherLM 2006 Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med 203 2461 2472
44. BrooksDGTrifiloMJEdelmannKHTeytonLMcGavernDB 2006 Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12 1301 1309
45. WherryEJBlattmanJNMurali-KrishnaKvan der MostRAhmedR 2003 Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77 4911 4927
46. ZajacAJBlattmanJNMurali-KrishnaKSourdiveDJSureshM 1998 Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188 2205 2213
47. DaffisSLazearHMLiuWJAudsleyMEngleM 2011 The naturally attenuated Kunjin strain of West Nile virus shows enhanced sensitivity to the host type I interferon response. J Virol 85 5664 5668
48. ZietaraNLyszkiewiczMGekaraNPuchalkaJDos SantosVA 2009 Absence of IFN-beta impairs antigen presentation capacity of splenic dendritic cells via down-regulation of heat shock protein 70. J Immunol 183 1099 1109
49. HuberJPFarrarJD 2011 Regulation of effector and memory T-cell functions by type I interferon. Immunology 132 466 474
50. ThompsonLJKolumamGAThomasSMurali-KrishnaK 2006 Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol 177 1746 1754
51. KamphuisEJuntTWaiblerZForsterRKalinkeU 2006 Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 108 3253 3261
52. CousensLPPetersonRHsuSDornerAAltmanJD 1999 Two roads diverged: interferon alpha/beta - and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med 189 1315 1328
53. HarringtonLEGalvanMBaumLGAltmanJDAhmedR 2000 Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med 191 1241 1246
54. KaechSMTanJTWherryEJKoniecznyBTSurhCD 2003 Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4 1191 1198
55. SarkarSKaliaVHainingWNKoniecznyBTSubramaniamS 2008 Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 205 625 640
56. BarberDLWherryEJMasopustDZhuBAllisonJP 2006 Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439 682 687
57. HeathWRCarboneFR 2001 Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol 19 47 64
58. BennettSRCarboneFRKaramalisFFlavellRAMillerJF 1998 Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393 478 480
59. XiaoZCaseyKAJamesonSCCurtsingerJMMescherMF 2009 Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol 182 2786 2794
60. ToughDF 2004 Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk Lymphoma 45 257 264
61. KochFStanzlUJenneweinPJankeKHeuflerC 1996 High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med 184 741 746
62. BrienJDUhrlaubJLHirschAWileyCANikolich-ZugichJ 2009 Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med 206 2735 2745
63. EbelGDDupuisAPIIINgoKNicholasDKauffmanE 2001 Partial genetic characterization of West Nile Virus strains, New York State, 2000. Emerg Inf Dis 7 650 653
64. MehlhopEDiamondMS 2006 Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Exp Med 203 1371 1381
65. TscharkeDCKarupiahGZhouJPalmoreTIrvineKR 2005 Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med 201 95 104
66. ByersAMKemballCCMoserJMLukacherAE 2003 Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol 171 17 21
67. JellisonERKimSKWelshRM 2005 Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol 174 614 618
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion DiseaseČlánek Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) VirusesČlánek Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance toČlánek Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a TombusvirusČlánek Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic PhenotypesČlánek Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence
- Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion Disease
- Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis
- Nutritional Immunology: A Multi-Dimensional Approach
- Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) Viruses
- Vaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
- Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly
- Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance to
- Computational and Biochemical Analysis of the Effector AvrBs2 and Its Role in the Modulation of Type Three Effector Delivery
- Granzyme B Inhibits Vaccinia Virus Production through Proteolytic Cleavage of Eukaryotic Initiation Factor 4 Gamma 3
- Association of Activating KIR Copy Number Variation of NK Cells with Containment of SIV Replication in Rhesus Monkeys
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa
- Follicular Dendritic Cell-Specific Prion Protein (PrP) Expression Alone Is Sufficient to Sustain Prion Infection in the Spleen
- Autophagy Protein Atg3 is Essential for Maintaining Mitochondrial Integrity and for Normal Intracellular Development of Tachyzoites
- Longevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
- Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence
- Acquisition of Pneumococci Specific Effector and Regulatory Cd4 T Cells Localising within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue
- The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites
- Deficiency of a Niemann-Pick, Type C1-related Protein in Is Associated with Multiple Lipidoses and Increased Pathogenicity
- Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
- Eight RGS and RGS-like Proteins Orchestrate Growth, Differentiation, and Pathogenicity of
- Prion Uptake in the Gut: Identification of the First Uptake and Replication Sites
- Nef Decreases HIV-1 Sensitivity to Neutralizing Antibodies that Target the Membrane-proximal External Region of TMgp41
- Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a Tombusvirus
- A Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
- The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
- HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency
- Neonatal CD8 T-cell Hierarchy Is Distinct from Adults and Is Influenced by Intrinsic T cell Properties in Respiratory Syncytial Virus Infected Mice
- Two Novel Transcriptional Regulators Are Essential for Infection-related Morphogenesis and Pathogenicity of the Rice Blast Fungus
- Five Questions about Non-Mevalonate Isoprenoid Biosynthesis
- The Human Cytomegalovirus UL11 Protein Interacts with the Receptor Tyrosine Phosphatase CD45, Resulting in Functional Paralysis of T Cells
- Wall Teichoic Acids of Limit Recognition by the Drosophila Peptidoglycan Recognition Protein-SA to Promote Pathogenicity
- A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen
- Inflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
- CNS Recruitment of CD8+ T Lymphocytes Specific for a Peripheral Virus Infection Triggers Neuropathogenesis during Polymicrobial Challenge
- Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic Phenotypes
- A Receptor-based Switch that Regulates Anthrax Toxin Pore Formation
- Targeting of Heparin-Binding Hemagglutinin to Mitochondria in Macrophages
- Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants
- Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
- Structural Elucidation and Functional Characterization of the Effector Protein ATR13
- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection
- Acid Stability of the Hemagglutinin Protein Regulates H5N1 Influenza Virus Pathogenicity
- Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons
- A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection
- Juxtamembrane Shedding of AMA1 Is Sequence Independent and Essential, and Helps Evade Invasion-Inhibitory Antibodies
- Pathogenesis and Host Response in Syrian Hamsters following Intranasal Infection with Andes Virus
- IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network
- Epstein-Barr Virus Evades CD4 T Cell Responses in Lytic Cycle through BZLF1-mediated Downregulation of CD74 and the Cooperation of vBcl-2
- Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction
- Late Repression of NF-κB Activity by Invasive but Not Non-Invasive Meningococcal Isolates Is Required to Display Apoptosis of Epithelial Cells
- Polar Flagellar Biosynthesis and a Regulator of Flagellar Number Influence Spatial Parameters of Cell Division in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- The Enteropathogenic (EPEC) Tir Effector Inhibits NF-κB Activity by Targeting TNFα Receptor-Associated Factors
- Toward an Integrated Model of Capsule Regulation in
- A Systematic Screen to Discover and Analyze Apicoplast Proteins Identifies a Conserved and Essential Protein Import Factor
- A Host Small GTP-binding Protein ARL8 Plays Crucial Roles in Tobamovirus RNA Replication
- Comparative Pathobiology of Fungal Pathogens of Plants and Animals
- Synergistic Roles of Eukaryotic Translation Elongation Factors 1Bγ and 1A in Stimulation of Tombusvirus Minus-Strand Synthesis
- Engineered Immunity to Infection
- Inflammatory Monocytes and Neutrophils Are Licensed to Kill during Memory Responses
- Sialidases Affect the Host Cell Adherence and Epsilon Toxin-Induced Cytotoxicity of Type D Strain CN3718
- Eurasian-Origin Gene Segments Contribute to the Transmissibility, Aerosol Release, and Morphology of the 2009 Pandemic H1N1 Influenza Virus
- SARS Coronavirus nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of mRNAs: Viral mRNAs Are Resistant to nsp1-Induced RNA Cleavage
- Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus
- Functional Analysis of the Kinome of the Wheat Scab Fungus
- Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- Engineered Immunity to Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



