-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHomeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
Homeostatic proliferation ensures the longevity of central memory T-cells by inducing cell proliferation in the absence of cellular differentiation or activation. This process is governed mainly by IL-7. Central memory T-cells can also be stimulated via engagement of the T-cell receptor, leading to cell proliferation but also activation and differentiation. Using an in vitro model of HIV-1 latency, we have examined in detail the effects of homeostatic proliferation on latently infected central memory T cells. We have also used antigenic stimulation via anti-CD3/anti-CD28 antibodies and established a comparison with a homeostatic proliferation stimulus, to evaluate potential differences in how either treatment affects the dynamics of latent virus populations. First, we show that homeostatic proliferation, as induced by a combination of IL-2 plus IL-7, leads to partial reactivation of latent HIV-1 but is unable to reduce the size of the reservoir in vitro. Second, latently infected cells are able to homeostatically proliferate in the absence of viral reactivation or cell differentiation. These results indicate that IL-2 plus IL-7 may induce a detrimental effect by favoring the maintenance of the latent HIV-1 reservoir. On the other hand, antigenic stimulation efficiently reactivated latent HIV-1 in cultured central memory cells and led to depletion of the latently infected cells via virus-induced cell death.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002288
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002288Summary
Homeostatic proliferation ensures the longevity of central memory T-cells by inducing cell proliferation in the absence of cellular differentiation or activation. This process is governed mainly by IL-7. Central memory T-cells can also be stimulated via engagement of the T-cell receptor, leading to cell proliferation but also activation and differentiation. Using an in vitro model of HIV-1 latency, we have examined in detail the effects of homeostatic proliferation on latently infected central memory T cells. We have also used antigenic stimulation via anti-CD3/anti-CD28 antibodies and established a comparison with a homeostatic proliferation stimulus, to evaluate potential differences in how either treatment affects the dynamics of latent virus populations. First, we show that homeostatic proliferation, as induced by a combination of IL-2 plus IL-7, leads to partial reactivation of latent HIV-1 but is unable to reduce the size of the reservoir in vitro. Second, latently infected cells are able to homeostatically proliferate in the absence of viral reactivation or cell differentiation. These results indicate that IL-2 plus IL-7 may induce a detrimental effect by favoring the maintenance of the latent HIV-1 reservoir. On the other hand, antigenic stimulation efficiently reactivated latent HIV-1 in cultured central memory cells and led to depletion of the latently infected cells via virus-induced cell death.
Introduction
The existence of latent reservoirs of HIV-infected cells constitutes a major impediment to viral eradication. HIV-1 latent reservoirs are small, but extremely long-lived. Latent infection is associated with undetectable levels of viral gene expression and appears to be non-cytopathic. However, upon reactivation, latent viruses enter an active mode of replication in which they are fully competent for spread and induction of disease [1], [2], [3]. It is unclear which physiological stimuli may trigger or prevent viral reactivation in latently infected cells. Obvious possibilities include antigenic stimulation, inflammatory conditions, and, perhaps, certain immunological microenvironments. Regarding potential therapies, the current thinking in the field is that a combination of hypothetical drugs that will reactivate latent viruses (“anti-latency” drugs), with present-day antiretroviral drugs, will be an effective approach toward viral eradication [1], [4], [5]. However, we are limited by the lack of known drugs that can safely be used to induce viral reactivation in patients. We are also limited by our poor understanding of how cellular and viral factors govern the establishment of latency and the reactivation process.
Memory is a hallmark of the acquired immune system and results from the clonal expansion and differentiation of antigen-specific lymphocytes that persist for a lifetime. Memory T cells result from the activation and differentiation of naïve T cells and perform two indispensable and complementary functions, which are carried out by different cellular subsets [6]. Effector memory T cells (TEM) migrate to inflamed peripheral tissues and display immediate effector function. On the other hand, central memory T cells (TCM) home to areas of secondary lymphoid organs where, in response to antigenic stimulation, they can vigorously proliferate and differentiate to TEM. In the case of the CD4+ memory T cells, the effector subset is further subdivided into several T-helper types, such as TH1, TH2 and TH17, among others, which are characterized by the expression of specific chemokine receptors and the production of specific cytokines like IFNγ, IL-4 or IL-17, respectively [7].
The proliferation of memory T cells can be driven by antigenic stimulation (antigen-driven proliferation) or by cytokines (homeostatic proliferation). Through homeostatic proliferation, the immune system is able to maintain normal T-cell counts, and to correct for deviations due to expansion or depletion of the memory cell pool [8], [9], [10]. Homeostatic proliferation is governed by extrinsic cellular signals, typically γc-cytokines, in the absence of antigenic stimulation. For CD4+ memory T cells, IL-7 is key in governing homeostasis [8], [11], [12]. A role for IL-15, another γc-cytokine, has also been proposed [11].
Previous work by several groups (reviewed in [13], [14]) suggested that in vivo, quiescent memory T-cells constitute a long-lived viral reservoir, whose decay constant ranges from months to years. A recent report has provided additional evidence to support the role of central memory T cells (TCM), along with transitional memory T cells, as the main latent viral reservoirs in vivo [15]. The Chomont et al. study also proposed that cells in the latent reservoir may be able to undergo homeostatic proliferation (as evidenced by the presence of the Ki67 marker), and this in turn would provide a means for the maintenance or even expansion of the HIV-1 latent reservoir [15]. While this idea could not be tested in patient cells due to the low abundance of latent infection, we were able to test the effect of homeostatic proliferation of latently infected cells in our cultured TCM model of latency [16]. Using this model, we confirm the ability of TCM cells to homeostatically proliferate in response to IL-2 plus IL-7, in a manner that is indistinguishable between uninfected and latently infected ones. We also demonstrate that proliferation of infected cells in response to IL-2 plus IL-7 stimulation is accompanied by inefficient viral reactivation. Because of the inefficient viral reactivation, many residual latently infected cells remain which can later be reactivated using antigenic stimulation.
Results
Antigenic stimulation of latently infected cultured TCM effectively depletes the latent reservoir whereas homeostatic proliferation fails to do so
To investigate the effect of homeostatic proliferation and antigen stimulation signals on the dynamics of a latent infection, we first established a population of latently infected cultured, non-polarized (NP) cells, as previously described [16]. NP cells are considered the in vitro equivalent of in vivo TCM [17], [18] and we will hereinafter refer to them as “cultured” TCM. Peripheral blood mononuclear cells (PBMC) were obtained from healthy human donors, and naïve CD4+ cells were isolated via negative selection [18]. Purified naïve CD4+ T cells were activated (day 0 to day 3) by incubation with beads coated with αCD3 and αCD28 antibodies in medium containing TGF-β1, αIL-12 and αIL-4 antibodies, and then cultured for 4 additional days with IL-2 (Figure 1A). Cells were then infected with 500 ng of p24 of the virus, DHIV/X4. DHIV/X4 is an envelope-defective molecular clone derived from HIV-1NL4-3, which is pseudotyped with the HIV-1LAI envelope glycoprotein. Because of the env deletion, this virus is only capable of a single round of infection.
Fig. 1. IL-7 induces partial reactivation of latent HIV-1 in cultured TCM. 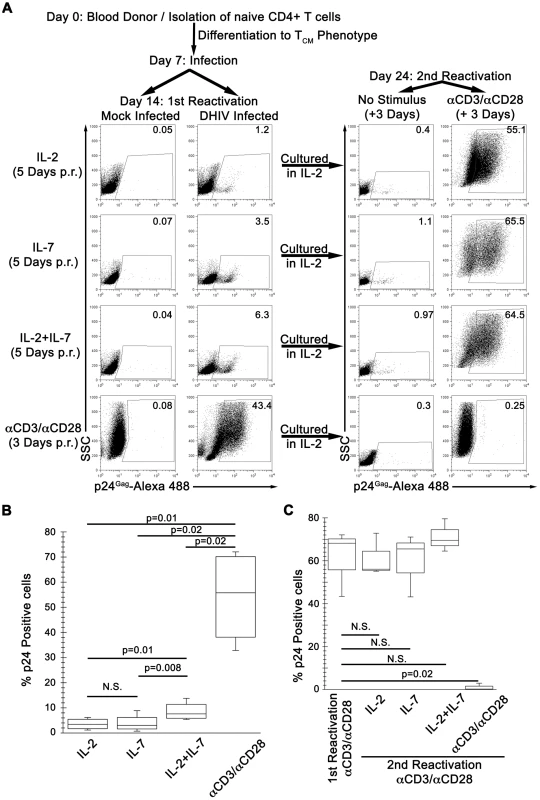
(A) Mock or DHIV latently infected, cultured TCM were incubated in the presence of IL-2, IL-7 or a combination of IL-2 and IL-7 (IL-2+IL-7) or costimulated with antibodies to CD3 and CD28 (αCD3/αCD28) and assessed for intracellular p24Gag by flow cytometry (1st Reactivation). The percentage of p24Gag positive cells is indicated in each panel. After reactivation, cells were kept in culture for an additional 7-day period in the presence of IL-2. At day 24, cells were incubated in the presence of IL-2 or costimulated with antibodies to CD3 and CD28 (αCD3/αCD28) and assessed for intracellular p24Gag by flow cytometry (2nd Reactivation). Data is representative of 4 donors for 1st reactivation and 3 donors for 2nd reactivation (p.r. post reactivation). (B) Box-plots corresponding to the 4 donors analyzed as in A, left panels. Horizontal lines indicate median values; statistical significance was assessed by 2-tailed paired-sample t test analysis (P values provided, N.S. not significant). (C) 3 of these previous donors were analyzed as in A (right panels, 2nd Reactivation), and compared with the first reactivation with αCD3/αCD28. Horizontal lines indicate median values; statistical significance was assessed by 2-tailed paired-sample t test analysis (P values provided, N.S. not significant). After infection, cells were cultured in medium containing IL-2 for an additional 7 days. Under these conditions, most of the cells returned to a quiescent state characterized as G0 as evidenced by low pyronin and 7-AAD staining intensities (Figure S1), where the vast majority of proviruses exist in a latent state [16]. The continuous presence of IL-2 in the culture did not interfere with either the establishment of latency or the return to quiescence of the cells [16]. IL-2 was, however, required for survival of the cells in culture (data not shown). At day 14 (first reactivation; Figure 1A), we tested the ability of IL-7 to induce viral reactivation in cultured TCM cells. To that end, cells were exposed to normal medium containing IL-2, IL-7 or a combination of IL-2 plus IL-7 (IL-2+IL-7). As a positive control for virus reactivation, cells were incubated with beads coated with αCD3/αCD28 antibodies. In a representative donor shown in Figure 1A, latently infected cells stimulated with αCD3/αCD28 displayed 43.4% p24Gag+ cells, while incubation with IL-2+IL-7 only resulted in 6.3% p24Gag+ cells. Treatments with IL-2 or IL-7 alone reactivated latent viruses even less efficiently (1.2 and 3.5%, respectively). When we performed similar studies with cells from three additional healthy donors, IL-7 alone failed to reactivate latent HIV-1 in two donors. However, IL-2+IL-7 treatment reactivated latent HIV-1 in all four donors with a frequency that was, on average, one tenth of that obtained with αCD3/αCD28 incubation (Figure 1B). Therefore, IL-7 treatment reactivated latent viruses in 2 out of 4 donors and, when used in combination with IL-2, in 4 out of 4 donors; and always with a lower efficiency than that obtained with antigenic stimulation. Our results are in agreement with published reports showing that incubation with IL-7, either alone [19] or in combination with IL-2 [20] can reactivate latent HIV-1 in resting CD4+ T cells isolated from infected individuals. IL-7 also reactivated latent HIV-1 in thymocytes in a SCID-hu mouse model of HIV latency [21].
To explore the relatively low efficiency of viral reactivation induced by IL-7 alone or in combination with IL-2 we measured the levels of expression of the high-affinity alpha chain receptors for IL-2 or IL-7 (CD25 and CD127, respectively). As shown in Figure S2A, the frequencies of cultured TCM expressing CD25 and CD127 were high (81% and 24%, respectively), and similar to those observed in freshly isolated TCM (66% and 31%, respectively) although the mean fluorescence intensities (MFI) were low. For CD25, when cells were stimulated with αCD3/αCD28, MFI increased by almost two orders of magnitude (Figure S2B). In addition, a dose-response study indicated that the levels of IL-7 we utilized in the experiment shown in Figure 1 (50 ng/ml) and throughout the rest of the study produced near-maximal response in these cells (Figure S2C). Therefore, the low relative efficiency of viral reactivation induced by IL-2+IL-7 was also not due to suboptimal amounts of IL-7.
A minority of cells in the cytokine treatments shown in Figure 1A reactivated latent viruses as evidenced by intracellular p24 expression. The remainder of the cells failed to reactivate latent proviruses and, therefore, we predicted that they remained latently infected. To prove that residual latent viruses were indeed present after cytokine stimulation, we cultured these cells in IL-2 for an additional week, and then proceeded to stimulate with αCD3/αCD28 antibodies (day 24, second reactivation; Figure 1A, right panels). The latently infected culture that had previously been reactivated with αCD3/αCD28 antibodies (first reactivation) and produced 43.4% p24Gag+ cells, failed to produce additional p24Gag+ cells (0.25%) upon the second reactivation (Figure 1A, αCD3/αCD28 right panel).
The reasons for the lack of p24Gag+ cells after the second reactivation with αCD3/αCD28 are as follows. First, αCD3/αCD28 treatment induces reactivation of virtually all latently infected cells in the cultured TCM model [16]. Second, cells in which viruses are reactivated undergo cell death [16], [22]. Third, DHIV is a defective virus, which is unable to spread after the initial infection. Therefore, new infections are not generated when latent viruses reactivate. This simplifies the analysis because it rules out virus spread as a potential confounding factor upon reactivation. It also obviates the use of anti-retrovirals in the cell cultures in order to suppress viral spread. To ascertain whether, in this in vitro system, αCD3/αCD28 stimulation effectively depletes the latent reservoir, we performed a parallel experiment in which we also measured integrated HIV-1 DNA by Alu-LTR PCR prior to the first and the second reactivations. As shown in Figure S3B, the levels of integrated DNA in DHIV infected cells prior to the second reactivation decreased to background, undetectable levels, confirming the absence of residual latently infected cells.
Latently infected cells that had been exposed either to IL-2, IL-7, or IL-2+IL-7, when later exposed to αCD3/αCD28 antibodies, produced numerous p24Gag+ cells (55.1%, 65.5% and 64.5%, respectively, Figure 1B right panels). Therefore, latently infected cells that failed to reactivate in the presence of no stimulus (IL-2 alone) or a weak stimulus (IL-7 or IL-2+IL-7) remained as an inducible reservoir. Similar results were obtained in a total of three donors (Figure 1C).
Reactivation of latent HIV-1 by IL-2+IL-7 in quiescent TCM is independent of entry into the cell cycle
Homeostatic proliferation of central memory T cells consists of entry into the cell division cycle, in the absence of cellular differentiation [12]. To confirm that IL-7 treatment alone, or in combination with IL-2, induces homeostatic proliferation, we determined the division status of the cells via staining for the nuclear antigen, Ki67. Ki67 is a nuclear marker that is present during all active phases of the cell cycle (G1, S, G2 and mitosis), but is absent in resting (G0) cells [23]. Ki67 staining was performed in conjunction with p24Gag staining, in order to evaluate whether viral reactivation was interdependent with entry into the cell cycle (Figure 2A). For the donor shown in Figure 2A, IL-2+IL7 incubation induced the strongest proliferation signal compared to other cytokine treatments or the no-cytokine control. The proportions of Ki67+ cells were similar in infected and mock-infected cultures (Figure 2A). Similar results were obtained in cells from four donors (Figure 2B). These observations indicate that the ability of cultured TCM cells to enter cell division upon IL-2+IL-7 stimulation is not affected by the presence of latent infections.
Fig. 2. IL-7 induces cell cycle entry of cultured TCM. 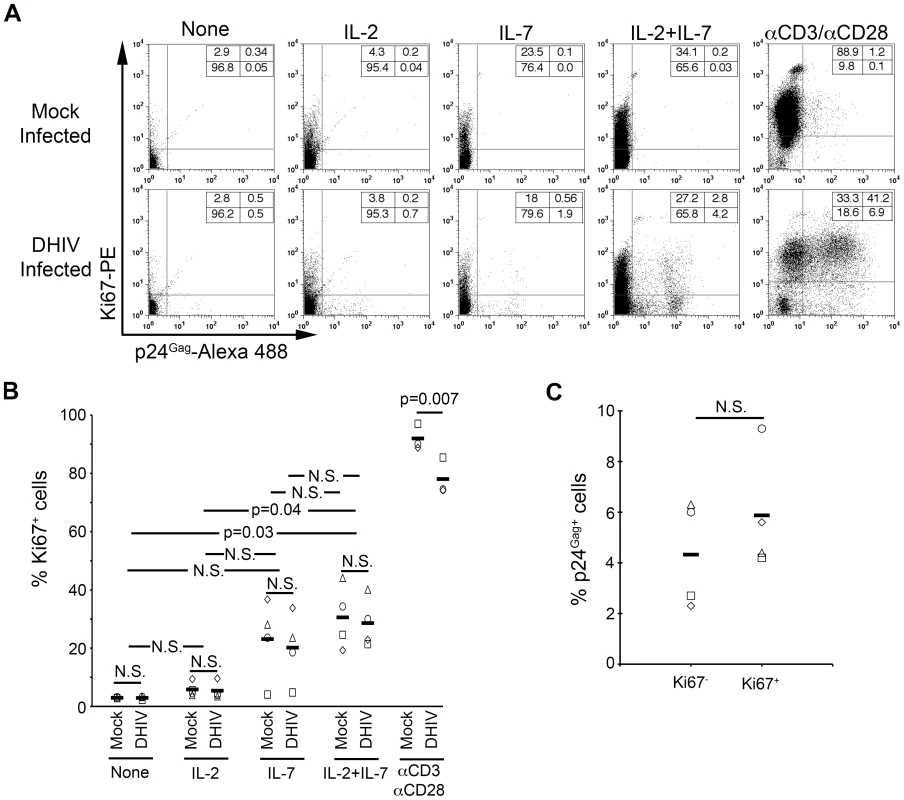
(A) Mock or DHIV latently infected, cultured TCM were left in the absence of cytokines (None) or in the presence of IL-2, IL-7 or a combination of IL-2 and IL-7 (IL-2+IL-7) or costimulated with antibodies to CD3 and CD28 (αCD3/αCD28) and assessed for intracellular p24Gag and Ki67 expression by flow cytometry. Numbers in boxes indicate percentages. (B) Analysis corresponding to 4 different donors analyzed as shown in panel A; each donor is represented with a different symbol and horizontal lines indicate media values. Statistical significance was calculated by 2-tailed paired-sample t test analysis (P values provided, N.S. not significant). (C) Analysis of the percentage of p24Gag+ cells in each compartment (Ki67− or Ki67+) after IL-2+IL-7 treatment in the four donors analyzed in B. Data was normalized as indicated in the text. Horizontal lines indicate media values. Statistical significance was calculated by 2-tailed paired-sample t test analysis (N.S. not significant). It is important to note that IL-2+IL-7 stimulation induced latently infected cells to express p24Gag both in the dividing, Ki67+ (2.8%), and in the non-dividing, Ki67− (4.2%) compartments (Figure 2A). We normalized the above frequencies of reactivation to the proportions of cells that were Ki67+ [2.8/(27.2+2.8)]×100 = 9.3% or Ki67− [4.2/(65.8+4.2)]×100 = 6.0%. We performed this analysis for the four donors shown in Figure 2B. The results showed not statistically significant differences (n = 4; p = 0.29; Figure 2C). Thus, we conclude, that entry into the cell cycle does not influence, positively or negatively, whether a latent virus will be reactivated by treatment with IL-2+IL-7.
Analysis of the cell surface phenotype of the cytokine-treated cultures revealed that the majority of the cells maintained the phenotype of central memory cells, characterized by dual expression of CCR7 and CD27. Therefore, the IL-7 or IL-2+IL-7 induced cellular proliferation did not change the differentiation status of cultured TCM cells, a hallmark of homeostatic proliferation (Figure S4).
Latently infected, cultured TCM undergo homeostatic proliferation in the absence of viral reactivation
The above experiments focused on the fraction of cells that reactivated virus under homeostatic proliferation, but did not reveal whether residual proviruses that remain latent after IL-7 or IL-2+IL-7 stimulation may be present within the proliferating (Ki67+) or non-proliferating (Ki67−) populations. Ki67 and p24Gag co-staining require fixation and permeabilization of the cells, which precludes further culture and reactivation of cells. Therefore, to more closely examine the fate of the latently infected population, we resorted to a different experimental scenario, as follows. To track cell division, we used Cell Proliferation Dye eFluor 670 (CPe670), a red-fluorescent dye that is analogous to the well-known carboxyfluorescein succinimidyl ester (CFSE). The progressive 2-fold dilution of CPe670 fluorescence within daughter cells following each cell division is used as an easy and quantitative means for measuring cell division in individual cells. The use of the red CPe670 allowed us to use a virus that expresses GFP in place of nef (DHIV-GFP). The combined use of CPe670 and GFP was designed to allow us to monitor and sort infected cells, assess cell division status, and continue to culture the cells thereafter.
We previously showed that GFP expression driven by DHIV-GFP closely parallels that of p24Gag and that the absence of nef did not modify the ability of HIV-1 to establish latent infection or to reactivate in cultured TCM [16]. A latent infection was established using DHIV-GFP (day 7), and at day 14, cells were labeled with CPe670; cells were then treated with either IL-2 alone or IL-2+IL-7. We decided to use the combination of both cytokines and not IL-7 alone because the combination induced a stronger and more consistent proliferative effect among different donors (Figures 2A and 2B) without altering the phenotype of the cells (Figure S4).
Five days after the above treatment (day 19; Figure 3A), cells were sorted by flow cytometry in order to (a) remove the GFP+ fraction; and (b) separate the GFP− fraction into CPe670High (having undergone no cell divisions) or CPe670Low (having undergone one or more cell divisions). As shown in Figure 3B, only 10.7% of the IL-2-cultured cells underwent cell division, whereas 31.6% of the cells did so when incubated with IL-2+IL-7. After sorting, the four cultures shown in Figure 3B (IL-2, IL-2+IL-7 [unsorted], IL-2+IL-7 [CPe670High] and IL-2+IL-7 [CPe670Low]) were placed back in culture and reactivated with αCD3/αCD28 to reactivate latent viruses; or they were cultured in IL-2 for 3 days. Cells were then analyzed for GFP expression. As shown in Figure 3B (right panels), all four cultures were able to produce GFP+ cells after αCD3/αCD28 reactivation. The frequencies of GFP+ cells induced by the αCD3/αCD28 treatment correspond to the frequencies of latency prior to reactivation. The IL-2+IL-7 stimulated, unsorted culture revealed 24.4% GFP+ cells after αCD3/αCD28 reactivation. Cells that underwent no divisions (CPe670High) sorted from the previous culture produced 7.03% GFP+ events, whereas those that had divided at least once (CPe670Low) produced 21.7% GFP+. Therefore, we conclude that cells that fail to reactivate latent HIV-1 in response to homeostatic proliferation signals manage to preserve latent infections, and this occurs whether cells divide or not.
Fig. 3. Most latently infected cells can proliferate in response to IL-2 plus IL-7 without inducing viral reactivation. 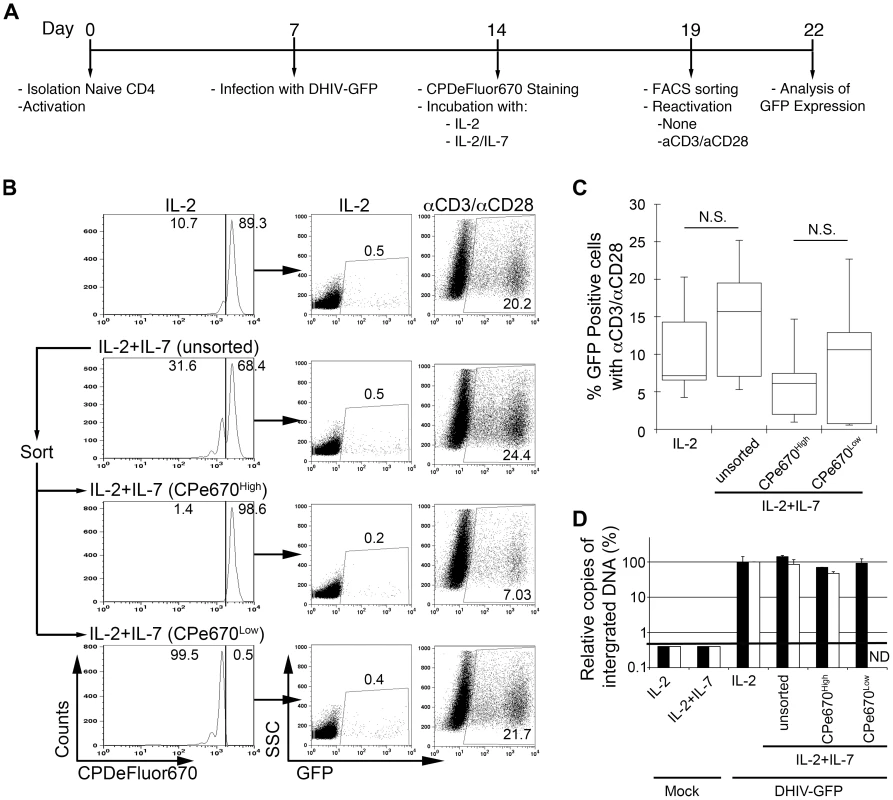
(A) Timeline of the experiment. (B) At day 19, cells treated with IL-2 plus IL-7 (unsorted) were subjected to cell sorting into two populations: CPe670High (undivided cells) or CPe670Low (divided cells). The percentages of CPe670High and CPe670Low cells are indicated in the histograms. After sorting, cells were incubated in the presence of IL-2 or costimulated with antibodies to CD3 and CD28 (αCD3/αCD28) and assessed for GFP expression by flow cytometry. The three groups of cells treated with IL-2+IL-7 were compared with the same cells treated with IL-2 alone (IL-2). The percentages of GFP positive cells are indicated in each panel. (C) Box-plots corresponding to the 5 donors analyzed as in B. Horizontal lines indicate median values; statistical significance was assessed by 2-tailed paired-sample t test analysis (N.S. not significant). (D) Integrated HIV-1 DNA was analyzed by Alu-LTR PCR in duplicates in two donors from panel C. Results were normalized for each donor relative to the levels of integration in cells treated with IL-2. Black bars correspond to a donor with 6.5% of GFP positive cells after reactivation with αCD3/αCD28 of cells treated with IL-2. White bars correspond to a donor with 4.2% of GFP positive cells after reactivation with αCD3/αCD28 of cells treated with IL-2. Mock infected cells retrieved a value below the threshold of detection of the technique (ND not determined). The results from the above analysis was extended to a total of 5 different donors, and the results are summarized in Figure 3C. The frequencies of latent proviruses present in the dividing versus the non-dividing cell populations are not statistically significant (n = 5; p = 0.36). To directly determine the levels of latently infected cells in these subsets in a manner that does not relay on viral reactivation, Alu-LTR PCR was performed in cells from two donors (white and black bars in Figure 3D) after the cell sorting. As shown in Figure 3D, the levels of integrated DNA remained similar in cells treated with IL-2 or IL-2+IL-7; and between cells that had divided (CPe670High) versus those that had not (CPe670Low). Therefore, we conclude that latently infected cells can proliferate in response to homeostatic proliferation signals, allowing for mitotic spread of the latent proviruses within.
Discussion
Previous studies have shown that IL-7 or IL-2+IL-7 stimulation is able to induce HIV-1 reactivation in latently infected, resting cells isolated from infected individuals [19], [20] and in thymocytes from a SCID-hu mouse model of HIV-1 latency [21]. However, the above studies did not analyze the effect of homeostatic proliferation on the size of the latent reservoir in TCM. In the present study, we show that, although homeostatic proliferation signals are able to induce partial viral reactivation from HIV-1 latency in TCM cells, cell proliferation may nullify the potential benefits of IL-7 as a natural “anti-latency” treatment. It is noteworthy that while incubation with IL-2 alone induced very low levels of reactivation in our system, others have reported significant effects of IL-2 [24]. Two crucial differences come to mind when trying to reconcile our study with that of Gondois-Rey et al. [24]. First, Gondois-Rey et al. used PHA instead of αCD3/αCD28 for T-cell stimulation. Second, our studies isolate cells with a pure central memory phenotype, whereas Gondois-Rey et al. did not discriminate between central, transitional and effector memory cells. These discrepancies may underlie dramatic differences in the responsiveness of various T cell subsets to IL-2 and should be further examined.
Because HIV-1 latently infected TCM cells do not detectably express viral antigens, there is no reason to suspect that homeostatic proliferation will occur at a different rate in infected versus uninfected cells. In fact, our results using cultured TCM indicate that there is not a statistically significant difference between latently infected cells and uninfected ones (Figures 3C) in their abilities to proliferate. We surmise that the normal ability of latently infected cells to proliferate is an important contributor toward their long-term maintenance. This result also indicates that the resting state is not an absolute requirement toward establishing latency. A clear precedent for this notion is the well known fact that certain immortalized cell lines can harbor latent viruses indefinitely, which are capable of being reactivated [25], [26]. Therefore, cell division status can be independent of latency, and we here extended this notion to primary TCM cells.
Using cultured TCM cells, we were also able to model the dramatic effect of antigenic stimulation on the size of the latent reservoir in TCM. The results indicate that antigenic stimulation can deplete the latent reservoir via viral reactivation, whereas weak stimuli, such as IL-2 or IL-2+IL-7, are unable to do so (Figure 1).
In this report, we carefully analyze the effect of homeostatic proliferation and antigen stimulation on HIV-1 latently infected TCM in vitro. As this reservoir in vivo is extremely long-lived and impervious to conventional anti-retroviral treatment, analysis of other factors that may impact the latent reservoir in patients is extremely compelling. Such factors will likely include a plethora of immunological mediators, general and specific inflammatory conditions, and the presence of other infectious agents.
In addition to the documented ability of IL-7 to induce reactivation of latent HIV-1 in resting cells from patients [19], [20], recombinant IL-7 (rIL-7) has shown efficacy in combating lymphopenia when administered to HIV-1 patients [27]. These benefits, combined with the low toxicity observed in the setting of rIL-7 administration, have prompted several clinical trials with the goal of eradicating the HIV-1 reservoir with rIL-7 administration. In the past, potential anti-latency drugs were evaluated for their ability to induce virus replication in the face of minimal cellular activation [28], [29]. Given the results presented here, we propose that potential “anti-latency” drugs should also be examined for the undesired ability to induce cellular proliferation in the presence of incomplete viral reactivation.
Methods
Reagents
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID: Human rIL-2 from Dr. Maurice Gately, Hoffman-La Roche Inc. [30]; and Monoclonal Antibody to HIV-1 p24 (AG3.0) from Dr. Jonathan Allan [31].
Generation of cultured TCM cells and their latent infection
Peripheral blood mononuclear cells were obtained from Leukopaks from unidentified, healthy donors. Cultured TCM and latently infected cultured TCM were generated as previously described [16], [18].
Ethics statement
Cells from uninfected blood donors. Human subjects 18 years and older serve as blood donors. Written informed consent was obtained from all donors. These studies are covered under the IRB #392 protocol approved by the University of Utah Institutional Review Board. The amount of blood drawn varies, depending on the experiments to be done. This ranges from as little as 50 milliliters (ml) to as much as 500 ml in young, healthy donors.
Reactivation assays
2.5×105 cells were reactivated with beads coated with αCD3 and αCD28 (1 bead per cell, Dynal/Invitrogen, Carlsbad, CA) during 3 days in the presence of 30 IU/ml IL-2; with IL-2 alone; with 50 ng/ml of IL-7 alone or with a combination of IL-2 and IL-7 (Peprotech, Rocky Hill, NJ) for 5 days.
Flow cytometry
To assess intracellular p24Gag expression, 2.5×105 cells were fixed, permeabilized, and stained as previously described [16].
To phenotype the cells, 2.5×105 cells were stained with surface marker-specific mAb specific for human: PE-anti-CD25 (Caltag, Burlingame, CA), FITC-anti-CD127 (eBioscience, San Diego, CA), PE-anti-CD27 (Caltag, Burlingame, CA) or FITC-anti-CCR7 (R&D Systems, Minneapolis, MN) followed by flow cytometric analysis in a BD Facscanto II flow cytometer using the FACSDiva software (Becton Dickinson, Mountain View, CA) and analyzed using FlowJo (Tree Star Inc., Ashland, OR). To analyze the expression of CD25 and CD127 in ex vivo isolated TCM, memory CD4+ T cells were isolated by MACS microbead-negative sorting using the memory CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA). After sorting, cells were stained with FITC-anti-CD127, PE-anti-CD27 and APC-anti-CCR7 (R&D Systems, Minneapolis, MN); or FITC-anti-CD27 (Caltag, Burlingame, CA), PE-annti-CD25 and APC-anti-CCR7 followed by flow cytometric analysis as described above.
To analyze the dual expression of Ki67 and p24Gag, 5×105 cells were fixed, permeabilized and stained for p24Gag as previously described [16]. After p24Gag staining, cells were washed with Perm/Wash Buffer (BD Biosciences) and were incubated with 1/100 dilution of Mouse IgG1,κ (MOPC-21, Sigma, Saint Louis, MO) in 100 µl of Perm/Wash Buffer for 30 min at 4°C to remove excess of secondary antibody. Cells were then washed with Perm/Wash Buffer and incubated with a 1/20 dilution of anti-Ki67-PE (BD Biosciences) in 100 µl of Perm/Wash Buffer for 30 min at 4°C. Cells were washed with Perm/Wash Buffer and samples were analyzed on a FACSCalibur flow cytometer. Forward versus side scatter profiles were used to define the live population. In all the experiments, HIV p24Gag negative staining regions were set with uninfected cells treated in parallel and Ki67 negative staining regions were set with the corresponding IgG-PE.
To analyze cell division with Cell Proliferation Dye eFluor 670 (eBioscience, San Diego, CA), cells were stained as indicated by the manufacturer.
To analyze DNA and RNA content, samples were stained with 7-aminoactinomycin D (7-AAD) and pyronin Y (PY) as previously described [32].
Integration analysis
Genomic DNA was isolated with DNeasy Tissue Kit (QIAGEN, Valencia, CA). Genomic DNA (250 ng) was subjected to quantitative Alu-LTR polymerase chain reaction (PCR) for integrated proviruses as previously described [33], [34].
Supporting Information
Zdroje
1. RichmanDDMargolisDMDelaneyMGreeneWCHazudaD 2009 The challenge of finding a cure for HIV infection. Science 323 1304 1307
2. ChunTWStuyverLMizellSBEhlerLAMicanJA 1997 Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94 13193 13197
3. FinziDHermankovaMPiersonTCarruthLMBuckC 1997 Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278 1295 1300
4. TronoDVan LintCRouziouxCVerdinEBarre-SinoussiF 2010 HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science 329 174 180
5. ShenLSilicianoRF 2008 Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol 122 22 28
6. SallustoFLenigDForsterRLippMLanzavecchiaA 1999 Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401 708 712
7. ZhuJYamaneHPaulWE 2010 Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28 445 489
8. BoymanOPurtonJFSurhCDSprentJ 2007 Cytokines and T-cell homeostasis. Curr Opin Immunol 19 320 326
9. MichieCAMcLeanAAlcockCBeverleyPC 1992 Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature 360 264 265
10. ToughDFSprentJ 1994 Turnover of naive - and memory-phenotype T cells. J Exp Med 179 1127 1135
11. SurhCDSprentJ 2008 Homeostasis of naive and memory T cells. Immunity 29 848 862
12. SeddonBTomlinsonPZamoyskaR 2003 Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol 4 680 686
13. PersaudDZhouYSilicianoJMSilicianoRF 2003 Latency in human immunodeficiency virus type 1 infection: no easy answers. J Virol 77 1659 1665
14. DouekDCPickerLJKoupRA 2003 T cell dynamics in HIV-1 infection. Annu Rev Immunol 21 265 304
15. ChomontNEl-FarMAncutaPTrautmannLProcopioFA 2009 HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15 893 900
16. BosqueAPlanellesV 2009 Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood 113 58 65
17. MessiMGiacchettoINagataKLanzavecchiaANatoliG 2003 Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol 4 78 86
18. BosqueAPlanellesV 2011 “Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells”. Methods 53 54 61
19. WangFXXuYSullivanJSouderEArgyrisEG 2005 IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest 115 128 137
20. LehrmanGYlisastiguiLBoschRJMargolisDM 2004 Interleukin-7 induces HIV type 1 outgrowth from peripheral resting CD4+ T cells. J Acquir Immune Defic Syndr 36 1103 1104
21. Scripture-AdamsDDBrooksDGKorinYDZackJA 2002 Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J Virol 76 13077 13082
22. AndersenJLDeHartJLZimmermanESArdonOKimB 2006 HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog 2 e127
23. ScholzenTGerdesJ 2000 The Ki-67 protein: from the known and the unknown. J Cell Physiol 182 311 322
24. Gondois-ReyFBiancottoAPionMChenineALGluschankofP 2001 Production of HIV-1 by resting memory T lymphocytes. AIDS 15 1931 1940
25. JordanABisgroveDVerdinE 2003 HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. Embo J 22 1868 1877
26. DuvergerAJonesJMayJBibollet-RucheFWagnerFA 2009 Determinants of the establishment of human immunodeficiency virus type 1 latency. J Virol 83 3078 3093
27. SeretiIDunhamRMSpritzlerJAgaEProschanMA 2009 IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113 6304 6314
28. YangHCXingSShanLO'ConnellKDinosoJ 2009 Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest 119 3473 86
29. ArchinNMEspesethAParkerDCheemaMHazudaD 2009 Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses 25 207 212
30. LahmHWSteinS 1985 Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr 326 357 361
31. SimmMShahabuddinMChaoWAllanJSVolskyDJ 1995 Aberrant Gag protein composition of a human immunodeficiency virus type 1 vif mutant produced in primary lymphocytes. J Virol 69 4582 4586
32. SchmidIColeSWKorinYDZackJAGiorgiJV 2000 Detection of cell cycle subcompartments by flow cytometric estimation of DNA-RNA content in combination with dual-color immunofluorescence. Cytometry 39 108 116
33. VandegraaffNKumarRHockingHBurkeTRJrMillsJ 2001 Specific inhibition of human immunodeficiency virus type 1 (HIV-1) integration in cell culture: putative inhibitors of HIV-1 integrase. Antimicrob Agents Chemother 45 2510 2516
34. ButlerSLHansenMSBushmanFD 2001 A quantitative assay for HIV DNA integration in vivo. Nat Med 7 631 634
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



