-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system (CNS) can lead to the development of HIV-1-associated dementia (HAD). We examined the virological characteristics of HIV-1 in the cerebrospinal fluid (CSF) of HAD subjects to explore the association between independent viral replication in the CNS and the development of overt dementia. We found that genetically compartmentalized CCR5-tropic (R5) T cell-tropic and macrophage-tropic HIV-1 populations were independently detected in the CSF of subjects diagnosed with HIV-1-associated dementia. Macrophage-tropic HIV-1 populations were genetically diverse, representing established CNS infections, while R5 T cell-tropic HIV-1 populations were clonally amplified and associated with pleocytosis. R5 T cell-tropic viruses required high levels of surface CD4 to enter cells, and their presence was correlated with rapid decay of virus in the CSF with therapy initiation (similar to virus in the blood that is replicating in activated T cells). Macrophage-tropic viruses could enter cells with low levels of CD4, and their presence was correlated with slow decay of virus in the CSF, demonstrating a separate long-lived cell as the source of the virus. These studies demonstrate two distinct virological states inferred from the CSF virus in subjects diagnosed with HAD. Finally, macrophage-tropic viruses were largely restricted to the CNS/CSF compartment and not the blood, and in one case we were able to identify the macrophage-tropic lineage as a minor variant nearly two years before its expansion in the CNS. These results suggest that HIV-1 variants in CSF can provide information about viral replication and evolution in the CNS, events that are likely to play an important role in HIV-associated neurocognitive disorders.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002286
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002286Summary
Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system (CNS) can lead to the development of HIV-1-associated dementia (HAD). We examined the virological characteristics of HIV-1 in the cerebrospinal fluid (CSF) of HAD subjects to explore the association between independent viral replication in the CNS and the development of overt dementia. We found that genetically compartmentalized CCR5-tropic (R5) T cell-tropic and macrophage-tropic HIV-1 populations were independently detected in the CSF of subjects diagnosed with HIV-1-associated dementia. Macrophage-tropic HIV-1 populations were genetically diverse, representing established CNS infections, while R5 T cell-tropic HIV-1 populations were clonally amplified and associated with pleocytosis. R5 T cell-tropic viruses required high levels of surface CD4 to enter cells, and their presence was correlated with rapid decay of virus in the CSF with therapy initiation (similar to virus in the blood that is replicating in activated T cells). Macrophage-tropic viruses could enter cells with low levels of CD4, and their presence was correlated with slow decay of virus in the CSF, demonstrating a separate long-lived cell as the source of the virus. These studies demonstrate two distinct virological states inferred from the CSF virus in subjects diagnosed with HAD. Finally, macrophage-tropic viruses were largely restricted to the CNS/CSF compartment and not the blood, and in one case we were able to identify the macrophage-tropic lineage as a minor variant nearly two years before its expansion in the CNS. These results suggest that HIV-1 variants in CSF can provide information about viral replication and evolution in the CNS, events that are likely to play an important role in HIV-associated neurocognitive disorders.
Introduction
Human immunodeficiency virus type 1 (HIV-1) infects CD4+ T cells in the blood and lymphoid organs. In addition, infection of the central nervous system (CNS) can result in mild to severe neurological disease, including HIV-1-associated dementia (HAD) [1]. Although the incidence of HAD and minor cognitive motor disorder have been significantly reduced following the introduction of highly active antiretroviral therapy (HAART), these disorders continue to affect a substantial proportion of the HIV-1-infected population [2], [3]. The insufficient CNS penetration of some antiretroviral drugs or viral resistance may allow HIV-1 to persist in the CNS during the course of therapy [4], [5], [6], [7]. The success of HAART has led to an increased lifespan and an older demographic of HIV-infected subjects, and these subjects in particular have an increased risk of developing HAD due to their enhanced age [8], [9]. Less severe neurological problems associated with HIV-1 infection such as minor cognitive impairments may also be increasing [10], [11], indicating that neurological disorders will remain a problem for HIV-1-infected subjects in the future. Finally, unequal access to HAART and the potential of CNS involvement prior to the initiation of HAART makes the question of HIV replication in the CNS relevant to many infected people.
Several lines of evidence suggest that some HAD subjects can harbor macrophage-tropic HIV-1 variants [12], [13], [14], [15], [16], [17], a distinct phenotype associated with the ability to infect cells with low surface expression of CD4. The initiation of antiretroviral therapy results in rapid decay of virus in the blood, which is associated with virus replicating in activated CD4+ T cells [18], [19]; however, HIV-1 in the cerebrospinal fluid (CSF) can decay slowly with the initiation of therapy in some subjects with HAD, suggesting a longer-lived cell type as the origin of this virus [20], [21], [22]. Macrophage tropism does not appear to be a feature of the transmitted variants of HIV-1 [23], [24], leaving open the question of when and where macrophage-tropic variants of HIV arise and their role in HIV-1-associated pathogenesis.
Previous studies have reported that HIV-1 populations in the CSF of HAD subjects have increased viral genetic compartmentalization compared to virus in the blood [25], [26], and genetically distinct HIV-1 variants have been detected at autopsy in the CNS of some subjects with HAD [27], [28], [29], suggesting that autonomous viral replication is occurring in the CNS of subjects with more severe neurological disease. We examined HIV-1 variants in the CSF of HAD subjects to determine the viral genotypes and phenotypes associated with the development of HAD. Here we show that genetically compartmentalized CCR5-tropic (R5) T cell-tropic and macrophage-tropic HIV-1 populations are independently found in the CSF of subjects diagnosed with HIV-1-associated dementia. Our results demonstrate that HIV-1 can replicate in at least two cell types within the CNS in association with the development of dementia. Macrophage-tropic viruses were poorly represented in the blood population, highlighting the restricted, tissue-specific host range of these variants.
Results
HIV-1 genetic compartmentalization and evolution of viral populations between the peripheral blood and CSF of HAD and non-HAD subjects were assessed using single genome amplification (SGA) of the viral env gene [30] and phylogenetic analyses (Table 1 and Table 2). Phenotypic characteristics of compartmentalized HIV-1 env genes from subjects with and without HIV-1-associated neurological disease were also measured to assess the entry phenotype of the compartmentalized virus. HIV-1 env genes were used to generate pseudotyped luciferase reporter viruses, which were then used to infect 293-Affinofile cells [31] with differential CD4 surface expression, and to infect monocyte-derived macrophages (MDM). Coreceptor tropism analysis revealed that most of the HIV-1 Env proteins were CCR5-tropic, including all of the Env proteins representing compartmentalized virus from the CSF (Table S1).
Tab. 1. Subject population clinical and virological characteristics. 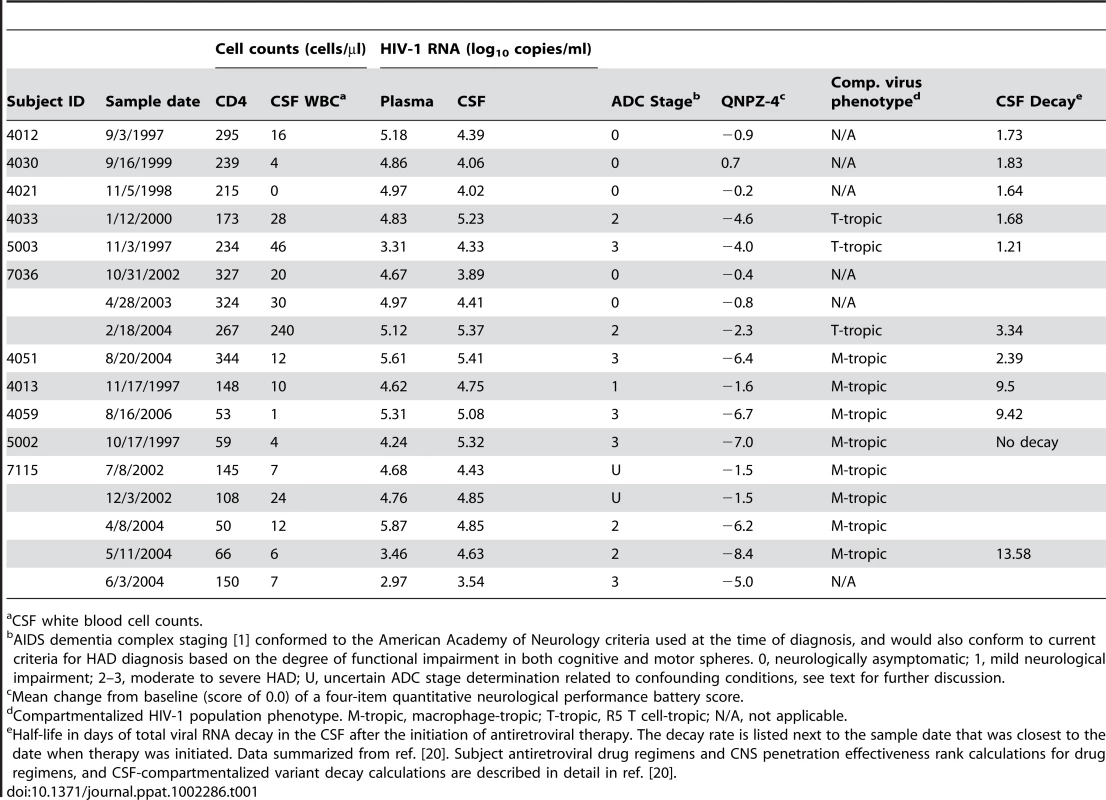
CSF white blood cell counts. Tab. 2. HIV-1 population characteristics in the CSF compartment. 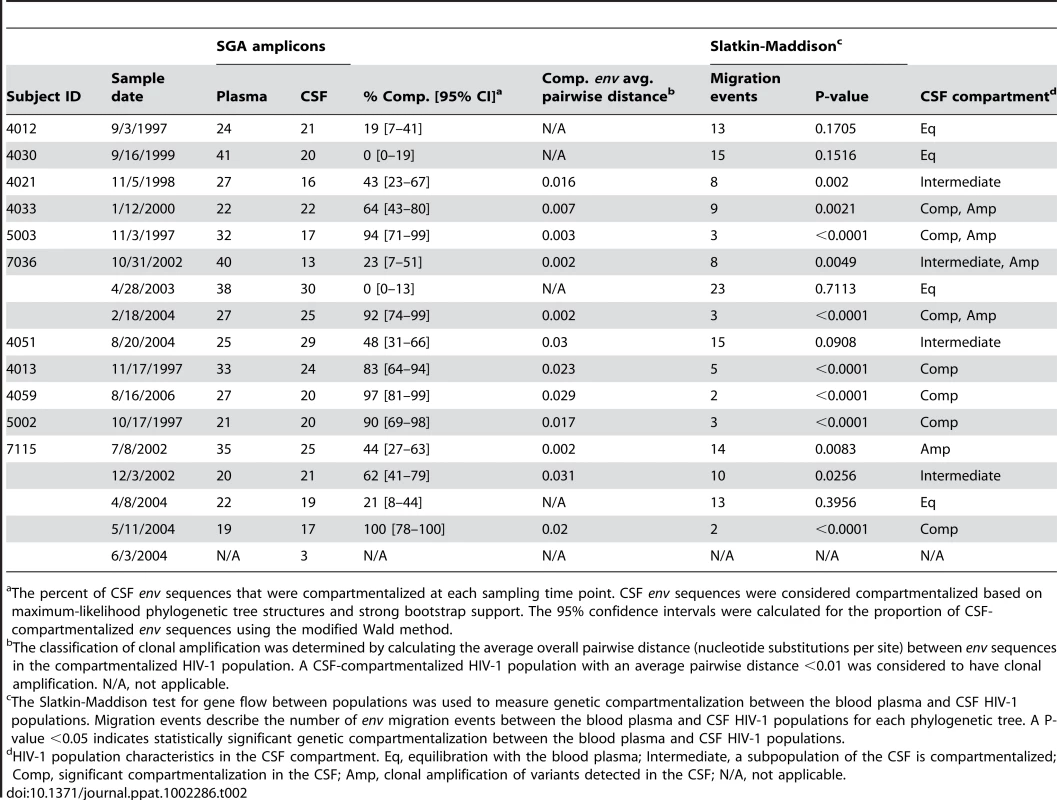
The percent of CSF env sequences that were compartmentalized at each sampling time point. CSF env sequences were considered compartmentalized based on maximum-likelihood phylogenetic tree structures and strong bootstrap support. The 95% confidence intervals were calculated for the proportion of CSF-compartmentalized env sequences using the modified Wald method. HIV-1 variants in the CSF of neurologically asymptomatic subjects can be R5 T cell-tropic
A range of genetic compartmentalization (0–43% of the total CSF sequences) was detected in the CSF HIV-1 populations of three neurologically asymptomatic subjects (Figure 1A and Table 2), which is consistent with reports from previous studies showing low but variable compartmentalization in asymptomatic subjects [26], [32]. Viruses pseudotyped with Env proteins derived from virus in the blood plasma and CSF of these neurologically asymptomatic subjects could only infect cells with high CD4 surface expression (Figure 1B; Table S1). In addition, most Env-pseudotyped viruses did not efficiently infect MDM (Figure 1C), although subject 4030 Env C23 infected about half as efficiently as the macrophage-tropic Ba-L envelope. This indicates that these viruses require the higher levels of surface CD4 found on activated CD4+ T cells for entry into target cells. While this represents a small sample size, this analysis demonstrates that T cell-tropic R5 viruses can be found in the CSF.
Fig. 1. HIV-1 variants in the CSF of neurologically asymptomatic subjects can be R5 T cell-tropic. 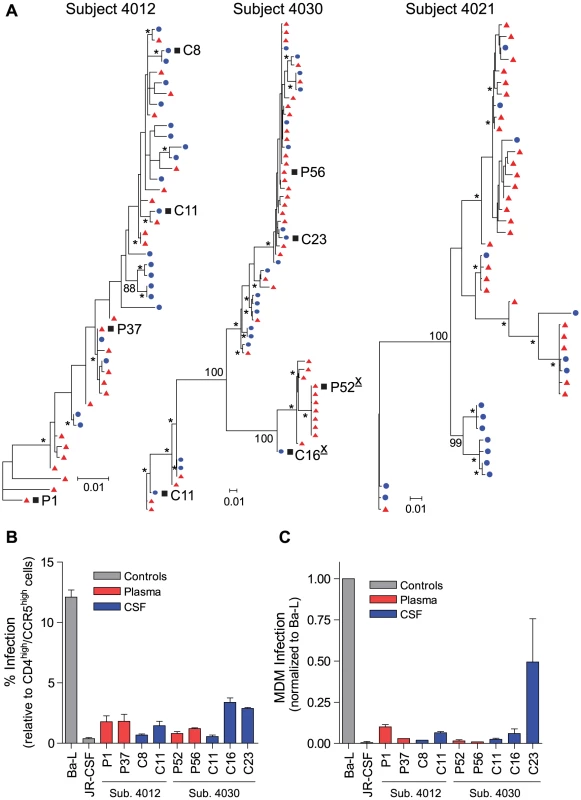
(A) Maximum-likelihood phylogenetic trees. env sequences from the CSF are labeled with solid blue circles, and plasma sequences are labeled with solid red triangles. Bootstrap numbers ≥70 are indicated with an asterisk at the appropriate nodes, and bootstrap values are listed at critical nodes in the trees. The genetic distance scale bar indicates the number of nucleotide substitutions per site between env sequences. HIV-1 env sequences selected for phenotypic analyses are indicated with a black square, and X4-tropic envelopes are indicated with an underlined, superscript “X” following the envelope name; all other envelopes were R5-tropic. (B) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on CD4lowCCR5high 293-Affinofile cells. Receptor expression was measured as follows: CD4low = 1,214 receptors/cell, CD4high = 97,003 receptors/cell, CCR5high = 34,431 receptors/cell. Data are expressed as means of quadruplicate wells ± s.d., and results are representative of two independent experiments. (C) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on primary human MDM. Data shown are means of duplicate wells ± s.d. for one donor, and were normalized to infection of the control macrophage-tropic HIV-1 Ba-L envelope. Compartmentalized R5 T cell-tropic and macrophage-tropic HIV-1 populations are independently found in the CSF of subjects diagnosed with HIV-1-associated dementia
Significant genetic compartmentalization was detected between the blood plasma and CSF HIV-1 populations of eight subjects diagnosed with HIV-1-associated neurological disease (Figure 2 and Figure 3; Table 2). We have previously shown that these subjects comprise two groups with respect to the rate of decay of compartmentalized virus in the CSF during the initiation of antiretroviral therapy: rapid decay in subjects 4033, 4051, 5003, 7036; and slow decay in subjects 4013, 4059, 5002, 7115 [20]. Phylogenetic analyses of the blood and CSF-derived virus in these subjects revealed significant compartmentalization and genetic distance between the blood plasma and CSF viral populations (Table 2; Figure 2A and Figure 3), indicating that sustained HIV-1 replication was likely occurring in the CNS of subjects with severe neurological disease. The detection of substantial compartmentalization in the HAD subjects was significant when compartmentalization versus an equilibrated population was compared to a model where each was equally likely (P = 0.03, Chi-squared test), or in comparison to the modest compartmentalization in the three asymptomatic subjects (P = 0.02, Fisher's Exact test).
Fig. 2. Compartmentalized R5 T cell-tropic HIV-1 populations are found in the CSF of subjects diagnosed with HAD. 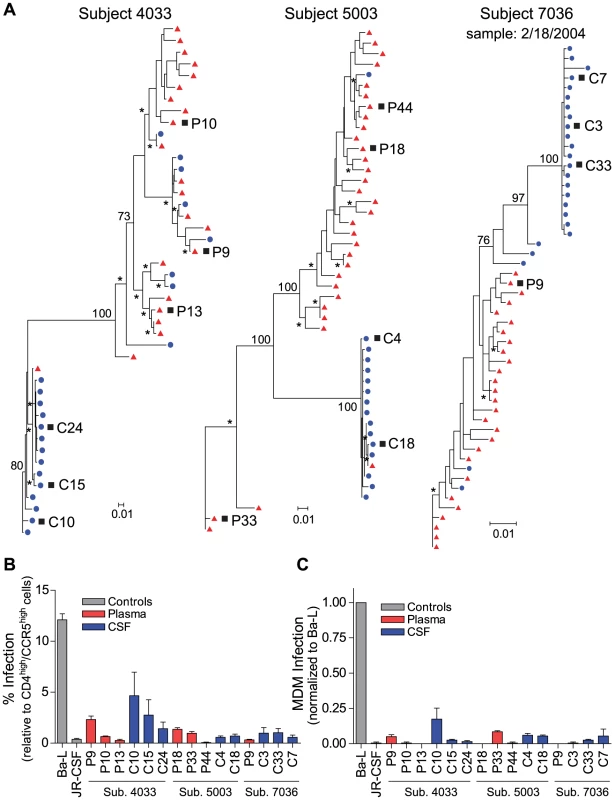
(A) Maximum-likelihood phylogenetic trees with compartmentalization and clonal amplification in the CSF. env sequences from the CSF are labeled with solid blue circles, and plasma sequences are labeled with solid red triangles. The phylogenetic tree characteristics are the same as those stated in Figure 1A. (B) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on CD4lowCCR5high 293-Affinofile cells. Receptor expression was measured as follows: CD4low = 1,214 receptors/cell, CD4high = 97,003 receptors/cell, CCR5high = 34,431 receptors/cell. Data are expressed as means of quadruplicate wells ± s.d., and results are representative of two independent experiments. (C) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on primary human MDM. Data shown are means of duplicate wells ± s.d. for one donor, and were normalized to infection of the control macrophage-tropic HIV-1 Ba-L envelope. Fig. 3. Compartmentalized HIV-1 populations in the CSF are associated with HAD. 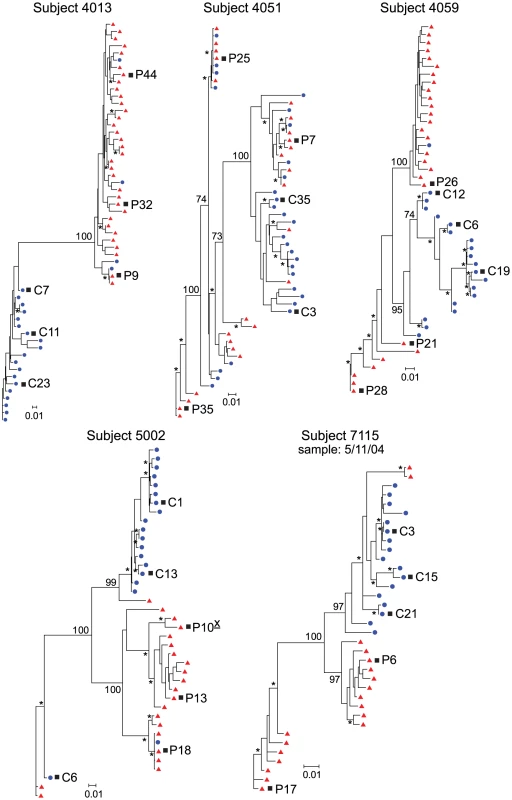
Maximum-likelihood phylogenetic trees are displayed for five HAD subjects with compartmentalization in the CSF. env sequences from the CSF are labeled with solid blue circles, and plasma sequences are labeled with solid red triangles. The phylogenetic tree characteristics are the same as those stated in Figure 1A. Clonal amplification of a specific viral lineage was identified for CSF variants that were separated phylogenetically from the plasma virus population in three HAD subjects (subjects 4033, 5003, and 7036; Figure 2) who also had rapid decay of compartmentalized virus in the CSF [20]. The clonal amplification was seen as decreased env diversity in the CSF viral population, defined as having an average env pairwise distance <0.01 (see Table 2), and short branch lengths in the phylogenetic tree, both indicative of a recent expansion of a single variant. Clonal amplification of the CSF-compartmentalized HIV-1 population was associated with elevated CSF pleocytosis (Table 1; P = 0.036 using a two-tailed Mann-Whitney test). We found that the clonally amplified, compartmentalized HIV-1 variants were not able to infect cells expressing a low surface density of CD4 (Figure 2B) and did not efficiently infect MDM (Figure 2C), indicating that for these subjects the CSF-compartmentalized viruses were replicating in activated T cells.
Phylogenetic analysis showed significant compartmentalization between the blood plasma and CSF HIV-1 populations, and a more genetically complex viral CSF population, for the remaining five subjects with neurological disease (subjects 4013, 4051, 4059, 5002, and 7115; Figure 3). We demonstrated in a previous study that these subjects had slow decay of compartmentalized virus in the CSF after the initiation of antiretroviral therapy, although subject 4051 had rapid decay detected in the CSF due to a complex viral population and only partial compartmentalization of CSF virus [20]. Compartmentalized HIV-1 envelopes from the CSF of these five subjects efficiently infected cells with a low CD4 surface density (Figure 4A), and these Env proteins were macrophage-tropic based on their ability to infect MDM (Figure 4B). In contrast, most HIV-1 Env proteins derived from the blood of these subjects were not able to mediate infection of cells with low CD4 surface expression and could only infect cells with high CD4 levels (Figure 4), indicating adaptation for replication in activated T cells in the peripheral blood. However, a macrophage-tropic lineage was detected in the blood plasma of subject 4059 (Figure 3 and Figure 4), consistent with the previous observation that it is possible to isolate macrophage-tropic viruses from the blood of some subjects in late-stage disease [12], although this lineage was genetically distinct from that found in the CSF. The absence of the CNS macrophage-tropic virus lineage from the blood in the five subjects with macrophage-tropic virus in the CSF was statistically significant (P = 0.03, Chi-squared test). Finally, in the subjects with significant CSF compartmentalization there was a perfect correlation between the presence of R5 T cell-tropic virus and rapid viral load decay in the CSF, and with macrophage-tropic virus and slow viral decay in the CSF (P = 0.03, Fisher's Exact test).
Fig. 4. Compartmentalized HIV-1 populations in the CSF of some HAD subjects are macrophage-tropic. 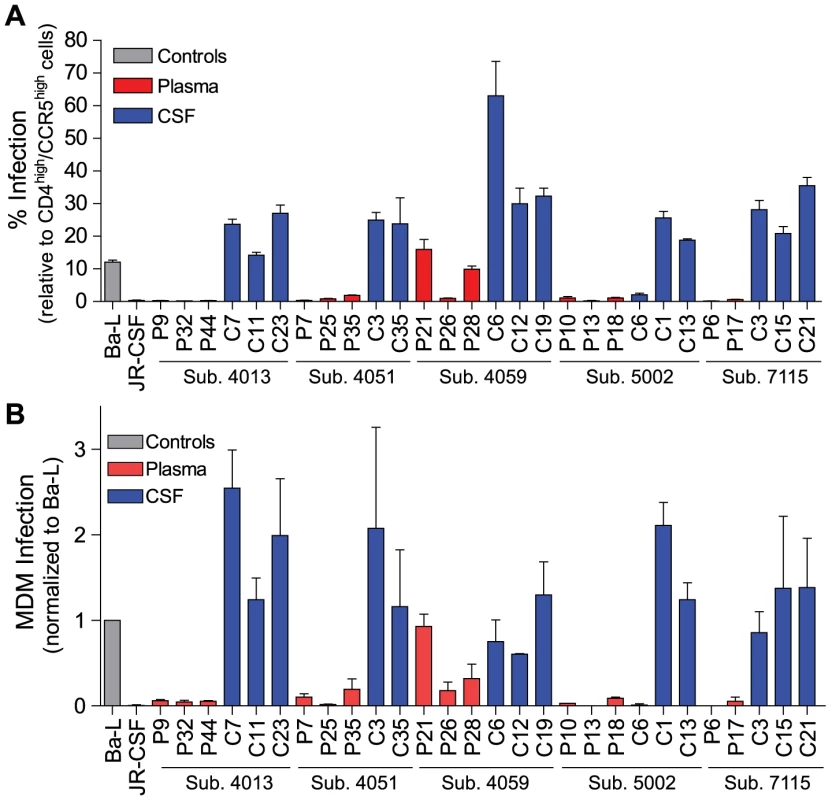
Phenotype data is displayed for HIV-1 env sequences from Figure 3. (A) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on CD4lowCCR5high 293-Affinofile cells. Receptor expression was measured as follows: CD4low = 1,214 receptors/cell, CD4high = 97,003 receptors/cell, CCR5high = 34,431 receptors/cell. Data are expressed as means of quadruplicate wells ± s.d., and results are representative of two independent experiments. (B) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on primary human MDM. Data shown are means of duplicate wells ± s.d. for one donor, and were normalized to infection of the control macrophage-tropic HIV-1 Ba-L envelope. Macrophage-tropic HIV-1 variants in the CSF/CNS viral population can be detected prior to the development of overt, severe dementia
We further examined macrophage-tropic and R5 T cell-tropic viral population dynamics in the CNS by conducting longitudinal env genotypic and phenotypic analyses for two subjects who progressed to HAD (Figure 5 and Figure S1). We detected a clonally amplified, compartmentalized R5 T cell-tropic population in the CSF of subject 7036 at the time of HAD diagnosis, but this population was not present in the CSF prior to the diagnosis of dementia (Figure S1), suggesting rapid expansion of a discrete viral population around the time of diagnosis. In addition, CSF viral populations at sampling time points prior to HAD diagnosis (10/31/2002 and 4/28/2003) were less compartmentalized, similar to CSF populations detected in neurologically asymptomatic subjects (Table 2). Clinical assessment also revealed a dramatic increase in CSF viral load and CSF neopterin, a pteridine associated with intrathecal immunoactivation [33], [34], over the study period (Table 1 and Figure S2A), which correlated with both the decline in neurological function from nearly asymptomatic to HAD (see QNPZ-4 scores in Table 1), and with the rapid expansion of a compartmentalized R5 T cell-tropic HIV-1 population in the CSF at this time-point.
Fig. 5. Macrophage-tropic HIV-1 variants in the CSF/CNS can be detected prior to the development of severe dementia. 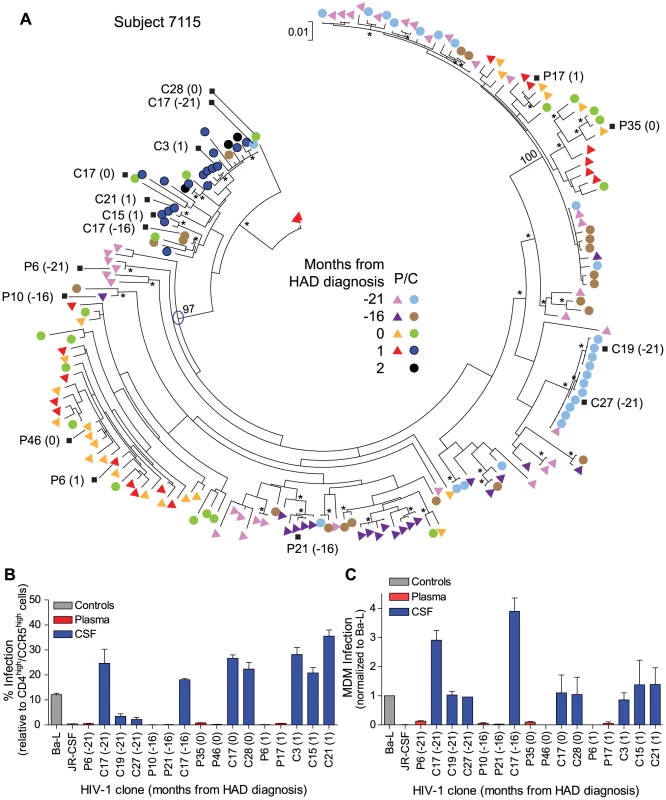
(A) Longitudinal phylogenetic analysis of env sequences from one subject with dementia. The genetic distance scale bar indicates the number of nucleotide substitutions per site between env sequences. HIV-1 env sequences selected for phenotypic analyses are indicated with a black square, and the node of divergence for the CSF M-tropic population is indicated with a blue open circle. Bootstrap numbers ≥70 are indicated with an asterisk at the appropriate nodes, and bootstrap values are listed at critical nodes in the tree. Months from HAD diagnosis correspond to the following sample dates: 7/8/2002 (−21), 12/3/2002 (−16), 4/8/2004 (0), 5/11/2004 (1), and 6/3/2004 (2). (B) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on CD4lowCCR5high 293-Affinofile cells. Receptor expression was measured as follows: CD4low = 1,214 receptors/cell, CD4high = 97,003 receptors/cell, CCR5high = 34,431 receptors/cell. Data are expressed as means of quadruplicate wells ± s.d., and results are representative of two independent experiments. (C) Single-cycle infection of HIV-1 Env-pseudotyped reporter viruses on primary human MDM. Data shown are means of duplicate wells ± s.d. for one donor, and were normalized to infection of the control macrophage-tropic HIV-1 Ba-L envelope. The other subject (7115) had a compartmentalized macrophage-tropic HIV-1 population present in the CSF at the time of dementia diagnosis. We identified a macrophage-tropic lineage in the CSF of this subject (Figure 5A) spanning a period of approximately two years prior to the diagnosis of severe dementia [see clones C17 (−21) and C17 (−16)]. All of the HIV-1 env genes tested from this lineage encoded Env proteins that were macrophage-tropic based on the ability to infect cells with a low CD4 surface expression (Figure 5B), and the ability to infect MDM (Figure 5C). HIV-1 env genes from the blood populations at each time-point encoded proteins that were T cell-tropic. The viral sequences in the CSF from the macrophage-tropic lineage increased as a fraction of the population over time, especially between the time of HAD diagnosis and one month later. Neurological assessment of subject 7115 several years prior to HAD diagnosis revealed slightly impaired neurological performance (see QNPZ-4 scores in Table 1), and CSF viral load and neopterin were fairly stable over the two-year course of the study (Table 1 and Figure S2B), perhaps indicating earlier onset of mild neurological disease, although this subject had other confounding conditions including drug use and psychiatric disease. These confounding factors precluded an AIDS dementia complex (ADC) stage determination at earlier sampling time-points (7/8/2002 and 12/3/2002), although this subject was considered ADC stage 0 if this could be applied. Neurological performance markedly declined at the time of dementia diagnosis (QNPZ-4 of −6.2) and continued to decline thereafter. Taken together, our data indicate that in this subject macrophage-tropic HIV-1 variants existed as minor variants within a specific evolutionary lineage of the CSF/CNS viral population and eventually became the dominant CSF population, suggesting that confounding factors may have obscured an understanding of the potential earlier contribution of HIV-related CNS dysfunction. Neurocognitive impairment can result from multiple factors, and knowledge about viral replication in the CNS, as viewed through virus in the CSF, can provide insight about the potential contribution of HIV-1 to the neurological status of the patient.
Discussion
We have demonstrated that genetically compartmentalized R5 T cell-tropic and macrophage-tropic HIV-1 populations are independently found in the CSF of subjects diagnosed with HIV-1-associated dementia. Our study was limited to a cohort of eight subjects with neurological disease who were receiving lumbar punctures at the time of HAD diagnosis and while initiating therapy, with samples available prior to therapy in two subjects followed longitudinally. In spite of a relatively small sample size we were able to link several features that distinguish two different virological states associated with severe neurological dysfunction. Compartmentalized HIV-1 populations in the CSF with R5 T cell-tropic entry phenotypes were separated phylogenetically from plasma virus populations, and were associated with clonal amplification of the CSF viral population. Replication in CD4+ T cells is consistent with both the rapid decay of compartmentalized virus in the CSF after the initiation of therapy in these subjects, and the migration of immune cells into the CNS/CSF as indicated by the presence of elevated CSF pleocytosis. Compartmentalized macrophage-tropic HIV-1 populations were associated with more genetically diverse viral populations in the CSF, and the slow decay of virus in the CSF after the initiation of therapy, indicative of viral replication in a long-lived cell. However, given the small sample size we cannot provide an accurate estimate of the relative frequency of each type of virologic state other than to note that they appeared with similar frequency in this cohort of eight subjects (three with compartmentalized R5 T cell-tropic virus, five with compartmentalized macrophage-tropic virus). Overall we detected a significantly compartmentalized CSF population in seven of these eight subjects suggesting that virus outgrowth in the CNS, whether macrophage-tropic or R5 T cell-tropic, will be a feature in a majority of HAD cases.
HIV-1 replication in the CNS is thought to occur in perivascular macrophages and/or microglia within the brain parenchyma [13], [35], [36]. We found that both R5 T cell-tropic and macrophage-tropic HIV-1 populations are independently associated with clinical dementia. This indicates a more complex interaction between HIV-1 and the CNS since the genetically compartmentalized R5 T-cell tropic viruses are unlikely to be replicating in macrophages or microglia given their requirement for high levels of CD4 to enter target cells. During simian immunodeficiency virus (SIV) infection of macaques, CNS infection is associated with the presence of infiltrating SIV-specific CD8+ T cells in the brain, but infiltrating CD4+ T cells have not been detected [37]. Trafficking of CD4+ T cells has been reported in the CNS during infection of other neurotropic viruses [38], [39]. We propose that the presence of viral antigen, especially during periods of increased HIV-1 replication in the CNS/CSF compartment, could drive the migration of both CD8+ and CD4+ T cells into the CNS/CSF (resulting in elevated CSF pleocytosis) and lead to persistence of compartmentalized virus through replication in the CD4+ T cells, and thus the apparent loss of this cell type. Consistent with the loss of these cells is the rapid decay of virus in the CSF during the initiation of therapy, which is considered a marker of viral replication in activated T cells [18], [19].
The pathological determinants of HAD are poorly understood. Some subjects with dementia exhibit HIV-1 encephalitis (HIVE) characterized by the presence of multinucleated giant cells of the macrophage/microglia origin and immunohistochemical evidence of viral replication [40], [41]. Although the incidence of HIVE has decreased during the HAART era, neuropathological changes in brain tissue, including glial activation and monocyte/macrophage infiltration [3], [42], [43], are still common. Future studies examining HIV-1 populations in paired blood, CSF, and brain tissue from HAD subjects with and without neuropathological findings will help determine whether there are physiological differences in brain pathology between subjects with R5 T cell-tropic versus macrophage-tropic HIV-1 variants as the predominant CSF population. Also, the appearance of macrophage-tropic viruses largely restricted to the CNS/CSF compartment is most consistent with the appearance of these viruses late in the infection time course of HIV-1, representing an expanded host range of the virus that is initially replicating in activated T cells. Although severe neurological disease associated with HIV-1 infection has declined in the HAART era, milder forms of neurological disease are increasing. In this study we detected a significantly compartmentalized macrophage-tropic HIV-1 population in the CSF of one subject with more mild neurological dysfunction (subject 4013; Table 1), illustrating the potential importance of understanding the correlates of HIV-1-associated neurological dysfunction with CNS/CSF viral population phenotypes.
HIV replication in the CNS can contribute to neurocognitive decline, so the ability to detect features of the CSF viral population associated with viral replication in the CNS may provide new opportunities to guide interventions prior to the development of overt neurological disease. In our study, one subject with longitudinal sampling (subject 7115) had macrophage-tropic variants present as a minor CSF population prior to the diagnosis of severe dementia (Figure 5). The application of sequencing technologies with greater capacity to sample a large number of viral genomes would allow the identification of minor CSF population variants, but this approach would rely on genotypic determinants of macrophage tropism rather than the phenotypic determinants used in our study. Several sequence determinants in env have been reported to be associated with macrophage tropism [27], [44], [45]; however, none of these determinants distinguishes the CSF-derived macrophage-tropic viruses from the paired blood-derived T cell-tropic viruses for the subjects in our study. Thus, the evolution of macrophage-tropic virus likely occurs through multiple pathways that will require a larger catalog of env sequences to allow reliable genotypic identification. It remains a possibility that the clonal amplification of R5 T cell-tropic viruses we detected in three HAD subjects is obscuring a smaller population of macrophage-tropic CNS virus, a question that could be addressed using more sensitive sampling methods. Developing a more complete understanding of the virological markers of CNS replication, and utilizing deep sequencing technologies to find minor populations, will provide opportunities to examine the use of CSF for information about viral replication in the CNS as a potential predictor of neurological involvement in the pathogenic process.
Materials and Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The studies were approved by the Committee for Human Research at the University of California at San Francisco, and written informed consent was obtained for the collection of samples from all subjects or their health care surrogates when informed consent was not considered possible.
Study subject population
All subjects included in this study were HIV-1-infected individuals that eventually initiated highly-active antiretroviral therapy. The subject samples used for viral genetic compartmentalization and Env protein phenotypic analyses were collected during previous studies carried out at the University of California at San Francisco [46]. Serial blood plasma and cerebrospinal fluid (CSF) samples were collected from subjects at baseline prior to the start of therapy and during the initiation of antiretroviral therapy, and samples were collected longitudinally from subjects 7036 and 7115 for several years prior to the initiation of therapy. Plasma and CSF HIV-1 RNA concentrations were determined using the Amplicor HIV Monitor kit (Roche). Blood CD4 and CSF white blood cell (WBC) counts were performed by the San Francisco General Hospital Clinical Laboratory using routine methods. Subjects all underwent standardized neurological testing, including clinical criteria for diagnosis and staging of ADC. They also underwent brief quantitative neurological testing using four tests to derive a normalized score, the QNPZ-4 [47].
Single genome amplification
HIV-1 RNA was isolated from blood plasma and CSF samples as previously described [20]. Viral RNA was reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) with oligo (dT) as the primer per the manufacturer's instructions. Single genome amplification of the full-length HIV-1 env gene through the 3′ U3 region was conducted as previously described [30]. Briefly, cDNA was diluted to endpoint, and nested PCR was conducted using the Platinum Taq High Fidelity polymerase (Invitrogen). The primers B5853 UP0 and LTR DN1, and B5957 UP1 and LTR DN1, were used for the first and second rounds of PCR, respectively [48]. The SGA amplicons were sequenced from the start of V1 through the ectodomain of gp41 [Hxb2 numbering of positions 6600–8000].
Phylogenetic and compartmentalization analyses
Nucleotide sequences of the env genes were aligned using Clustal W [49], [50] or MAFFT software [51]. Sequences from each subject were codon aligned using GeneCutter (Los Alamos HIV-1 database; http://www.hiv.lanl.gov/content/sequence/GENE_CUTTER/cutter.html). Maximum likelihood phylogenetic trees were generated using PhyML [52] with the following parameters: HKY85 nucleotide substitution model, four substitution rate categories, estimation of the transition/transversion rate ratio, estimation of the proportion of invariant sites, and estimation of the gamma distribution parameter [53]. Compartmentalization of CSF viral populations by sequence was determined using the Slatkin-Maddison test for compartmentalization [54] by HyPhy software [55] using 10,000 permutations. Pairwise distance was calculated for HIV-1 env sequences in the CSF-compartmentalized population using MEGA 4.1 software [56], [57], [58].
Construction of HIV-1 env clones
The SGA amplicons used in the cloning procedure were selected based on each subjects' phylogenetic tree structure and sequenced from the start of gp120 to the end of gp41. An additional PCR was conducted to amplify only the full-length HIV-1 env gene using the Phusion hot start high-fidelity DNA polymerase (Finnzymes) and the primers B5957F-TOPO (5′-CACCTTAGGCATCTCCTATGGCAGGAAGAAG-3′) and B8904R-TOPO (5′-GTCTCGAGATACTGCTCCCACCC-3′) following the manufacturer's instructions. HIV-1 env amplicons were then gel purified using the QIAquick gel extraction kit (Qiagen). The purified HIV-1 env genes were cloned into the pcDNA 3.1D/V5-His-TOPO expression vector (Invitrogen) using the pcDNA 3.1 directional TOPO expression kit (Invitrogen) and MAX Efficiency Stbl2 competent cells (Invitrogen) as per the manufacturer's instructions.
Cells
293T and TZM-bl cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 µg/ml of penicillin and streptomycin. 293-Affinofile cells [31] were maintained in DMEM supplemented with 10% dialyzed FBS (12–14 kD dialyzed; Atlanta biologicals) and 50 µg/ml blasticidin (D10F/B). The Affinofile cell line was generously provided by Dr. Ben-Hur Lee. Monocyte-derived macrophages (MDM) were isolated from Ficoll-purified PBMCs (Biological Specialty Corporation, Colmar, PA) using the human monocyte enrichment kit without CD16 depletion (Stemcell Technologies) as per the manufacturer's instructions. Following isolation, MDM were seeded in 48-well tissue culture plates and cultured for 6 days. MDM were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 µg/ml of penicillin and streptomycin, and 10 ng/ml recombinant human macrophage colony stimulating factor (M-CSF; Gibco).
Env-pseudotyped viruses
Env-pseudotyped luciferase reporter viruses were generated by co-transfection of 293T cells with 3 µg HIV-1 env expression vector and 3 µg of the pNL4-3.LucR-E - plasmid (obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) [59], [60] using the FuGENE 6 transfection reagent and protocol (Roche). Five hours post-transfection the medium was changed and the cells were incubated at 37°C for an additional 48 hours, and viral supernatants were harvested.
Coreceptor tropism analysis
Two hours prior to infection the coreceptor inhibitors TAK-779 [61], [62] and bicyclam JM-2987 (hydrobromide salt of AMD-3100) [63], [64], [65] (both obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) were added to TZM-bl cells at concentrations of 2.5 µM and 5 µM using the following conditions for each virus: no drug, TAK-779 only, AMD-3100 only, and both drugs. Cells were infected in the presence of drug using 50 µl of viral supernatant per well and spinoculated (2,000 rpm) for 2 hours at 37°C. Infections were incubated for 48 hours at 37°C, and then the cells were harvested and luciferase activity was assayed using the Luciferase assay system (Promega). All infections and conditions were conducted in triplicate.
293-Affinofile cellular surface expression of CD4 and CCR5
293-Affinofile cell [31] CD4 and CCR5 receptor expression was induced with tetracycline and ponasterone A (ponA; Invitrogen), respectively. Cells were induced for 18 hours at 37°C in a matrix format for a total of 24 induction levels with varying amounts of tetracycline (0–0.1 µg/ml) and ponA (0–2 µM/ml). Receptor expression was measured using quantitative fluorescence-activated cytometry (qFACS). Cells were stained with either phycoerythin (PE)-conjugated anti-human CD4 antibody (clone Q4120, BD Biosciences) or PE-conjugated mouse anti-human CCR5 antibody (clone 2D7, BD Biosciences). CD4 and CCR5 receptor levels were quantified using QuantiBRITE beads (BD Biosciences).
Single-cycle infection of 293-Affinofile cells
Env-pseudotyped luciferase reporter viruses were initially titered on 293-Affinofile cells expressing the highest induction levels for CD4 (0.1 µg/ml tetracycline) and CCR5 (2 µM ponA) surface expression. The amount of pseudotyped virus used in the single-cycle Affinofile infection assay was normalized to 1×106 relative light units for infection at the highest drug levels tested. All pseudotyped viruses were used within the linear range of the assay, and all infection conditions were assayed in quadruplicate.
Two days prior to infection, 96-well black tissue culture plates were coated with 10% poly-lysine in PBS and seeded with 293-Affinofile cells (2.5×104 cells/well). Expression of CD4 and CCR5 was induced the following day by adding varying concentrations of tetracycline and ponasterone A as described above. Eighteen hours later, the induction medium was removed and fresh culture medium containing Env-pseudotyped virus was gently added to the cells. The infection plates were spinoculated [66] at 2,000 rpm for 2 hours at 37°C, and incubated for an additional 48 hours at 37°C. Infection medium was then removed and the cells harvested, and luciferase activity was assayed using the Luciferase assay system (Promega).
Single-cycle infection of MDM
Env-pseudotyped luciferase reporter viruses were initially titered on 293-Affinofile cells expressing the highest induction levels for CD4 (0.1 µg/ml tetracycline) and CCR5 (2 µM ponA) surface expression. The amount of pseudotyped virus used in the single-cycle MDM infection assay was normalized to 1×107 relative light units for infection at the highest 293-Affinofile drug levels tested. All pseudotyped viruses were used within the linear range of the assay, and all infection conditions for MDM were assayed in duplicate wells.
Env-pseudotyped virus was gently added to MDM in culture and the infection plates were spinoculated at 1,200×g for 2 hours at 37°C [66]. Unattached virus was removed from the MDM cultures by removing the medium gently without disturbing the cells. The MDM were then washed once with warm PBS supplemented with 1% fetal bovine serum to remove any residual virus, and once with warm RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 µg/ml of penicillin and streptomycin to remove any remaining PBS/FBS. After the second wash, RPMI 1640 medium supplemented with 10% FBS, 100 µg/ml of penicillin and streptomycin, and 10 ng/ml M-CSF was added, and the cells were cultured for 5 days at 37°C. The culture medium was then removed and the cells were harvested and luciferase activity was assayed using the Luciferase assay system (Promega).
Nucleotide sequence accession numbers
The HIV-1 env nucleotide sequences determined in this study have been deposited in GenBank under accession numbers JN562755-JN563605.
Supporting Information
Zdroje
1. PriceRWBrewBSidtisJRosenblumMScheckAC 1988 The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 239 586 592
2. BoisseLGillMJPowerC 2008 HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin 26 799 819
3. BellJE 2004 An update on the neuropathology of HIV in the HAART era. Histopathology 45 549 559
4. GisolfEHEntingRHJurriaansSde WolfFvan der EndeME 2000 Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS 14 1583 1589
5. Gonzalez-ScaranoFMartin-GarciaJ 2005 The neuropathogenesis of AIDS. Nat Rev Immunol 5 69 81
6. PomerantzRJ 2003 Reservoirs, sanctuaries, and residual disease: the hiding spots of HIV-1. HIV Clin Trials 4 137 143
7. SchragerLKD'SouzaMP 1998 Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA 280 67 71
8. BhaskaranKMussiniCAntinoriAWalkerASDorrucciM 2008 Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol 63 213 221
9. JevtovicDVanovacVVeselinovicMSalemovicDRaninJ 2009 The incidence of and risk factors for HIV-associated cognitive-motor complex among patients on HAART. Biomed Pharmacother 63 561 565
10. AncesBMEllisRJ 2007 Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol 27 86 92
11. Fischer-SmithTRappaportJ 2005 Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med 7 1 26
12. GorryPRBristolGZackJARitolaKSwanstromR 2001 Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol 75 10073 10089
13. KoenigSGendelmanHEOrensteinJMDal CantoMCPezeshkpourGH 1986 Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233 1089 1093
14. RossiFQueridoBNimmagaddaMCocklinSNavas-MartinS 2008 The V1–V3 region of a brain-derived HIV-1 envelope glycoprotein determines macrophage tropism, low CD4 dependence, increased fusogenicity and altered sensitivity to entry inhibitors. Retrovirology 5 89
15. ThomasERDunfeeRLStantonJBogdanDTaylorJ 2007 Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology 360 105 119
16. GorryPRTaylorJHolmGHMehleAMorganT 2002 Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J Virol 76 6277 6292
17. PetersPJBhattacharyaJHibbittsSDittmarMTSimmonsG 2004 Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol 78 6915 6926
18. HoDDNeumannAUPerelsonASChenWLeonardJM 1995 Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373 123 126
19. WeiXGhoshSKTaylorMEJohnsonVAEminiEA 1995 Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373 117 122
20. SchnellGSpudichSHarringtonPPriceRWSwanstromR 2009 Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog 5 e1000395
21. HaasDWCloughLAJohnsonBWHarrisVLSpearmanP 2000 Evidence of a source of HIV type 1 within the central nervous system by ultraintensive sampling of cerebrospinal fluid and plasma. AIDS Res Hum Retroviruses 16 1491 1502
22. EllisRJGamstACCapparelliESpectorSAHsiaK 2000 Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology 54 927 936
23. AlexanderMLynchRMulengaJAllenSDerdeynCA 2010 Donor and recipient envs from heterosexual human immunodeficiency virus subtype C transmission pairs require high receptor levels for entry. J Virol 84 4100 4104
24. Salazar-GonzalezJFSalazarMGKeeleBFLearnGHGiorgiEE 2009 Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206 1273 1289
25. RitolaKRobertsonKFiscusSAHallCSwanstromR 2005 Increased human immunodeficiency virus type 1 (HIV-1) env compartmentalization in the presence of HIV-1-associated dementia. J Virol 79 10830 10834
26. HarringtonPRSchnellGLetendreSLRitolaKRobertsonK 2009 Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS 23 907 915
27. DunfeeRLThomasERGorryPRWangJTaylorJ 2006 The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc Natl Acad Sci U S A 103 15160 15165
28. OhagenADevittAKunstmanKJGorryPRRosePP 2003 Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol 77 12336 12345
29. PowerCMcArthurJCJohnsonRTGriffinDEGlassJD 1994 Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol 68 4643 4649
30. Salazar-GonzalezJFBailesEPhamKTSalazarMGGuffeyMB 2008 Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol 82 3952 3970
31. JohnstonSHLobritzMANguyenSLassenKDelairS 2009 A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. J Virol 83 11016 11026
32. HarringtonPRHaasDWRitolaKSwanstromR 2005 Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. J Virol 79 7959 7966
33. BrewBJBhallaRBPaulMGallardoHMcArthurJC 1990 Cerebrospinal fluid neopterin in human immunodeficiency virus type 1 infection. Ann Neurol 28 556 560
34. HagbergLCinquePGisslenMBrewBJSpudichS 2010 Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 7 15
35. BagasraOLaviEBobroskiLKhaliliKPestanerJP 1996 Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10 573 585
36. TakahashiKWesselinghSLGriffinDEMcArthurJCJohnsonRT 1996 Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol 39 705 711
37. von HerrathMOldstoneMBFoxHS 1995 Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J Immunol 154 5582 5589
38. PharesTWKeanRBMikheevaTHooperDC 2006 Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol 176 7666 7675
39. ZhaoJPerlmanS 2009 De novo recruitment of antigen-experienced and naive T cells contributes to the long-term maintenance of antiviral T cell populations in the persistently infected central nervous system. J Immunol 183 5163 5170
40. BudkaH 1986 Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS). Acta Neuropathol 69 253 258
41. NaviaBAChoESPetitoCKPriceRW 1986 The AIDS dementia complex: II. Neuropathology. Ann Neurol 19 525 535
42. EverallIPHansenLAMasliahE 2005 The shifting patterns of HIV encephalitis neuropathology. Neurotox Res 8 51 61
43. Kraft-TerrySDStothertARBuchSGendelmanHE 2010 HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis 37 542 548
44. DunfeeRLThomasERWangJKunstmanKWolinskySM 2007 Loss of the N-linked glycosylation site at position 386 in the HIV envelope V4 region enhances macrophage tropism and is associated with dementia. Virology 367 222 234
45. MusichTPetersPJDuenas-DecampMJGonzalez-PerezMPRobinsonJ 2011 A conserved determinant in the V1 loop of HIV-1 modulates the V3 loop to prime low CD4 use and macrophage infection. J Virol 85 2397 2405
46. StapransSMarloweNGliddenDNovakovic-AgopianTGrantRM 1999 Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS 13 1051 1061
47. PriceRWYiannoutsosCTCliffordDBZaborskiLTselisA 1999 Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. AIDS 13 1677 1685
48. SchnellGPriceRWSwanstromRSpudichS 2010 Compartmentalization and Clonal Amplification of HIV-1 Variants in the Cerebrospinal Fluid during Primary Infection. J Virol 84 2395 2407
49. ChennaRSugawaraHKoikeTLopezRGibsonTJ 2003 Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31 3497 3500
50. ThompsonJDHigginsDGGibsonTJ 1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
51. KatohKTohH 2008 Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9 286 298
52. GuindonSLethiecFDurouxPGascuelO 2005 PHYML Online–a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 33 W557 559
53. GuindonSGascuelO 2003 A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52 696 704
54. SlatkinMMaddisonWP 1989 A cladistic measure of gene flow inferred from the phylogenies of alleles. Genetics 123 603 613
55. PondSLFrostSDMuseSV 2005 HyPhy: hypothesis testing using phylogenies. Bioinformatics 21 676 679
56. KumarSNeiMDudleyJTamuraK 2008 MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9 299 306
57. KumarSTamuraKNeiM 1994 MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput Appl Biosci 10 189 191
58. TamuraKDudleyJNeiMKumarS 2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
59. ConnorRIChenBKChoeSLandauNR 1995 Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206 935 944
60. HeJChoeSWalkerRDi MarzioPMorganDO 1995 Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 69 6705 6711
61. BabaMNishimuraOKanzakiNOkamotoMSawadaH 1999 A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A 96 5698 5703
62. DragicTTrkolaAThompsonDACormierEGKajumoFA 2000 A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci U S A 97 5639 5644
63. BridgerGJSkerljRTThorntonDPadmanabhanSMartellucciSA 1995 Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J Med Chem 38 366 378
64. De ClercqEYamamotoNPauwelsRBalzariniJWitvrouwM 1994 Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother 38 668 674
65. HendrixCWFlexnerCMacFarlandRTGiandomenicoCFuchsEJ 2000 Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother 44 1667 1673
66. O'DohertyUSwiggardWJMalimMH 2000 Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol 74 10074 10080
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



