-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaQuorum Sensing in Fungi: Q&A
article has not abstract
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002301
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002301Summary
article has not abstract
I know that the accumulation of certain molecules called “quorum-sensing factors” in bacteria, as cultures grow dense, can control group behavior and that these include virulence traits and the formation of drug-resistant microbial biofilms. I am familiar with some of the molecules that mediate the sensing of organism density such as homoserine lactones and peptides. However, I don't know about such systems in fungi. What types of molecules are involved?
In the human commensal and pathogenic fungus Candida albicans, two molecules have been described: farnesol and tyrosol [1], [2]. Farnesol is a sesquiterpine alcohol, which is an alcohol made up of three isoprene units (Figure 1A). Tyrosol is an alcohol related to the amino acid tyrosine (Figure 1B). A completely different type of molecule, a peptide (Figure 1C), is made by the human opportunistic yeast Cryptococcus neoformans.
Fig. 1. Fungal quorum-sensing molecules and mechanisms. 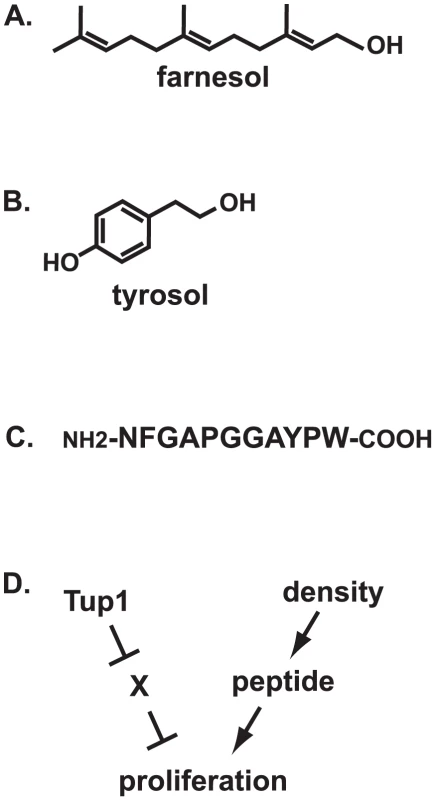
(A) Structure of farnesol; (B) structure of tyrosol; (C) sequence of mature Qsp1 peptide; (D) model for Qsp1 action in C. neoformans var. neoformans. How are farnesol and tyrosol made by C. albicans?
Farnesol pyrophosphate is an intermediate in sterol biosynthesis, and there is evidence that this is the source produced by C. albicans. However, the subsequent enzymatic pathway involved and the export pathways (if any) are unknown. Tyrosol synthesis requires an intact aromatic amino acid biosynthetic pathway, but again the detailed enzymology remains to be worked out [1].
Is the peptide in C. neoformans synthesized by nonribosomal synthases or by the ribosome?
Unlike some bacterial quorum-sensing peptides that are synthesized by nonribosomal synthases, the Cryptococcal peptide is synthesized from a larger precursor and is apparently processed and exported [3]. The enzymes and mechanisms are unknown.
Do these molecules function as sensors of culture density?
One definition of quorum sensing requires that the signal be synthesized at a fixed rate; thus, its concentration would be proportional to the concentration of the microbe in a liquid culture. Tyrosol is regulated by environmental conditions [4], and the QSP1 gene that encodes the Cryptococcal peptide is directly regulated by a signal-regulated DNA-binding regulator [5]. Therefore, these factors may be more accurately thought of as auto-regulatory molecules rather than pure sensors of culture density.
Does the accumulation of these factors promote cell division?
Tyrosol can overcome the lag experienced by sparse cultures of C. albicans [1]. Microarray expression analysis suggests that tyrosol induces the expression of genes involved in DNA replication [1]. The Cryptococcal peptide can also overcome an inhibition of growth at low culture density, but the story is more complex: only mutants lacking the Tup1 corepressor display a dependency on the peptide for growth [3]. One model to explain this observation is that Tup1 (presumably acting through a sequence-specific DNA binding repressor) turns off an inhibitor of growth that is redundantly inhibited by the quorum-sensing system (Figure 1D). Thus, the quorum system may only be required for growth normally when repression of a target by Tup1 is relieved in response to environmental signals.
I know that some bacteria regulate biofilm formation in response to quorum-sensing signals. Does this happen in fungi?
Yes. C. albicans biofilm formation is inhibited by farnesol [6]. This has been suggested to regulate the extent of biofilm formation. Microarray analysis indicates that farnesol activates hyphal-specific gene expression while inhibiting the expression of a cell surface hydrophobin [7]. Whether endogenous farnesol production controls biofilm formation will require mutants specifically defective in its synthesis.
Many pathogenic fungi are dimorphic, switching between yeast and hyphal forms. Does quorum-sensing control this transition?
Indeed, this is how farnesol was isolated: dense cultures of C. albicans display a reduced propensity for the yeast-to-hyphal switch, and this is mediated by the accumulation of farnesol. This may limit nutrient foraging behavior under conditions that are nutrient replete.
Do we know anything about the receptors or downstream signaling pathways that respond to fungal quorum-sensing molecules?
We are just beginning to answer this question. A receptor histidine kinase homolog called Chk1 in C. albicans has been implicated in the inhibition of hyphal growth by farnesol, but the story is not simple [8]. More recently, it has been shown that that farnesol reception requires a signaling pathway that includes the conserved small GTPase Ras and its downstream effector, adenylate cyclase, and a DNA-binding repressor called Nrg1 [9]–[11]. We know nothing of the reception mechanism for tyrosol and the Cryptococcal peptide.
You have told me about C. albicans and C. neoformans, but is there evidence for quorum-sensing phenomenon in other pathogenic fungi?
Yes [12]. Inoculum size affects the yeast–hyphal transition in the human pathogens Histoplasma capsulatum and Mucor rouxii. The factors involved remain to be identified.
Is there evidence for cross-species communication via fungal quorum-sensing molecules?
It has been reported that cocultivation of C. albicans and the nonpathogenic filamentous fungus Aspergillus nidulans results in farnesol-dependent inhibition of growth of the latter [13]. Moreover, farnesol can inhibit growth, biofilm formation, and virulence factors by some bacteria [14]–[17], which could be relevant for the lifestyle of C. albicans as a human gut commensal.
Do quorum-sensing signaling mechanisms play a role in pathogenesis?
Given the abundant evidence that the yeast-to-hyphal switch is important for C. albicans virulence, farnesol production could play a role in pathogenesis, but this remains to be proven.
Where do we go from here?
Identifying additional quorum-sensing molecules in more pathogenic fungi would be a good start. In C. albicians and C. neoformans, defining the molecules responsible for export as well as receptors and intracellular signal transduction mechanisms would allow the construction of mutants to test the biological role of these systems.
Zdroje
1. ChenHFujitaMFengQClardyJFinkGR 2004 Tyrosol is a quorum-sensing molecule in Candida albicans. Proc Natl Acad Sci U S A 101 5048 5052
2. HornbyJMJensenECLisecADTastoJJJahnkeB 2001 Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67 2982 2992
3. LeeHChangYCNardoneGKwon-ChungKJ 2007 TUP1 disruption in Cryptococcus neoformans uncovers a peptide-mediated density-dependent growth phenomenon that mimics quorum sensing. Mol Microbiol 64 591 601
4. GhoshSKebaaraBWAtkinALNickersonKW 2008 Regulation of aromatic alcohol production in Candida albicans. Appl Environ Microbiol 74 7211 7218
5. ChunCDBrownJCMadhaniHD 2011 A major role for capsule-independent phagocytosis-inhibitory mechanisms in mammalian infection by Cryptococcus neoformans. Cell Host Microbe 9 243 251
6. RamageGSavilleSPWickesBLLopez-RibotJL 2002 Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 68 5459 5463
7. CaoYYCaoYBXuZYingKLiY 2005 cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob Agents Chemother 49 584 589
8. KruppaMKromBPChauhanNBambachAVCihlarRL 2004 The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot Cell 3 1062 1065
9. DeveauAPiispanenAEJacksonAAHoganDA 2010 Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway. Eukaryot Cell 9 569 577
10. HallRATurnerKJChaloupkaJCottierFDe SordiL 2011 The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans. Eukaryot Cell 10 1034 1042
11. LuYSuCWangALiuH 2011 Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol 9 e1001105 doi:10.1371/journal.pbio.1001105
12. HoganDA 2006 Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot Cell 5 613 619
13. SemighiniCPHornbyJMDumitruRNickersonKWHarrisSD 2006 Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol Microbiol 59 753 764
14. Jabra-RizkMAMeillerTFJamesCEShirtliffME 2006 Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother 50 1463 1469
15. KooHHayacibaraMFSchobelBDCuryJARosalenPL 2003 Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother 52 782 789
16. CuginiCCalfeeMWFarrowJM3rdMoralesDKPesciEC 2007 Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol Microbiol 65 896 906
17. CuginiCMoralesDKHoganDA 2010 Candida albicans-produced farnesol stimulates Pseudomonas quinolone signal production in LasR-defective Pseudomonas aeruginosa strains. Microbiology 156 3096 3107
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



