-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
During the lytic phase of infection, the gamma herpesvirus Kaposi's Sarcoma-Associated Herpesvirus (KSHV) expresses a highly abundant, 1.1 kb nuclear noncoding RNA of unknown function. We observe that this polyadenylated nuclear (PAN) RNA avidly binds host poly(A)-binding protein C1 (PABPC1), which normally functions in the cytoplasm to bind the poly(A) tails of mRNAs, regulating mRNA stability and translation efficiency. During the lytic phase of KSHV infection, PABPC1 is re-localized to the nucleus as a consequence of expression of the viral shutoff exonuclease (SOX) protein; SOX also mediates the host shutoff effect in which host mRNAs are downregulated while viral mRNAs are selectively expressed. We show that whereas PAN RNA is not required for the host shutoff effect or for PABPC1 re-localization, SOX strongly upregulates the levels of PAN RNA in transient transfection experiments. This upregulation is destroyed by the same SOX mutation that ablates the host shutoff effect and PABPC1 nuclear re-localization or by removal of the poly(A) tail of PAN. In cells induced into the KSHV lytic phase, depletion of PAN RNA using RNase H-targeting antisense oligonucleotides reveals that it is necessary for the production of late viral proteins from mRNAs that are themselves polyadenylated. Our results add to the repertoire of functions ascribed to long noncoding RNAs and suggest a mechanism of action for nuclear noncoding RNAs in gamma herpesvirus infection.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002300
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002300Summary
During the lytic phase of infection, the gamma herpesvirus Kaposi's Sarcoma-Associated Herpesvirus (KSHV) expresses a highly abundant, 1.1 kb nuclear noncoding RNA of unknown function. We observe that this polyadenylated nuclear (PAN) RNA avidly binds host poly(A)-binding protein C1 (PABPC1), which normally functions in the cytoplasm to bind the poly(A) tails of mRNAs, regulating mRNA stability and translation efficiency. During the lytic phase of KSHV infection, PABPC1 is re-localized to the nucleus as a consequence of expression of the viral shutoff exonuclease (SOX) protein; SOX also mediates the host shutoff effect in which host mRNAs are downregulated while viral mRNAs are selectively expressed. We show that whereas PAN RNA is not required for the host shutoff effect or for PABPC1 re-localization, SOX strongly upregulates the levels of PAN RNA in transient transfection experiments. This upregulation is destroyed by the same SOX mutation that ablates the host shutoff effect and PABPC1 nuclear re-localization or by removal of the poly(A) tail of PAN. In cells induced into the KSHV lytic phase, depletion of PAN RNA using RNase H-targeting antisense oligonucleotides reveals that it is necessary for the production of late viral proteins from mRNAs that are themselves polyadenylated. Our results add to the repertoire of functions ascribed to long noncoding RNAs and suggest a mechanism of action for nuclear noncoding RNAs in gamma herpesvirus infection.
Introduction
Kaposi's Sarcoma-Associated Herpesvirus (KSHV) is the causative agent of several human cancers and immunoproliferative disorders, including Kaposi's Sarcoma, Multicentric Castleman's Disease and Primary Effusion Lymphoma [1], [2]. Like other herpesviruses, KSHV infection is characterized by two states: viral latency and lytic growth. During latency, very few viral genes are expressed, reducing the number of viral epitopes available to trigger a host immune response. Given appropriate but incompletely understood stimuli, the virus activates the lytic program of infection. This is characterized by three ordered waves of viral gene expression producing “immediate early,” “delayed early” and “late” proteins, as well as replication of the viral genome. Ultimately, the new genomes are packaged into virions, which are released from the cell for expansive host infection.
Upon KSHV entry into the lytic phase, an intronless viral noncoding (nc)RNA called polyadenylated nuclear (PAN) RNA, also known as T1.1 or nut-1, begins to be synthesized at unusually high levels [3], [4]. Although the 1.1 kb PAN RNA resembles an mRNA in being transcribed by RNA polymerase II, methyl-G capped at its 5′ end, and polyadenylated at its 3′ end, it is not exported to the cytoplasm for translation as are other viral transcripts. Instead, PAN RNA accumulates to astonishingly high levels, reaching ∼500,000 copies per nucleus and ultimately accounting for up to 80% of the total polyadenylated RNA in the cell [3]. Much has been learned regarding the mechanism that enables PAN RNA to accumulate to such high levels. Specifically, a 79-nucleotide element located near the 3′ end of the RNA, termed the expression and nuclear retention element (ENE), serves to stabilize the RNA in the nucleus [5], [6], [7]. Deletion of the ENE dramatically reduces the levels of transfected PAN RNA in HEK 293 cells, while insertion of the ENE into an intronless β-globin transcript significantly increases its nuclear levels. Insertion of the ENE has also been shown to enhance the abundance of nuclear pri-miRNAs [8]. It was hypothesized that a U-rich internal loop within the ENE engages the poly(A) tail, thereby sequestering it from deadenylases that initiate RNA decay [6], [7]. A recent x-ray crystal structure of the ENE complexed with oligo(A) reveals the formation of a triple helix that clamps the oligo(A) [9].
To address how PAN RNA contributes to lytic infection of KSHV, we began by investigating protein components of the PAN RNP and identified poly(A)-binding protein C1 (PABPC1). PABPC1 normally functions in the cytoplasm where it binds the poly(A) tails of mRNAs, regulating their stability by either antagonizing or enhancing the activity of cytoplasmic deadenylases [10], [11], [12], [13], [14]. PABPC1 also mediates circularization and enhances translation of mRNA via physical interactions with the translation initiation factor eIF4G, and assists in the export of mRNAs from the nucleus to the cytoplasm [14], [15], [16], [17]. However, since PAN RNA resides exclusively in the nucleus of KSHV-infected cells and does not shuttle (Conrad and Steitz, unpublished observations), re-localization of PABPC1 to the nucleus is a prerequisite for significant binding of PABPC1 to PAN RNA. Indeed, several groups have reported that re-localization of PABPC1 from the cytoplasm into the nucleus occurs during lytic KSHV infection of TIME endothelial cells and BC3 and BCBL1 TReX-RTA lymphoid cells [18], [19], [20]. The phenomenon is driven by the shutoff exonuclease (SOX) protein, as transient transfection of SOX into uninfected cells causes nuclear accumulation of PABPC1 in the absence of any other viral gene product [19]. SOX is also responsible for the host shutoff effect of the virus, which selectively downregulates host mRNAs while viral mRNAs persist [21], [22], [23]. SOX-mediated PABPC1 re-localization to the nucleus is critical for the host shutoff effect since knockdown of PABPC1 diminishes the ability of SOX to effect shutoff. GFP mRNA levels were downregulated in cells transfected with SOX, but less so in cells pre-treated with anti-PABPC1 siRNAs [19]. Furthermore, overexpression of PABPC1 targeted into the nucleus, using a nuclear retention signal from hnRNPC1, recapitulates aspects of host shutoff in the absence of any viral gene product [24]. Finally, a point mutation that disrupts the ability of SOX to re-localize PABPC1, but does not disrupt SOX's unrelated function as an alkaline DNase, ablates its ability to mediate host shutoff [19].
Here, we demonstrate that PAN RNA interacts with PABPC1 after it has re-localized to the nucleus during the lytic phase of KSHV infection. In transient transfection experiments, SOX strongly upregulates the levels of PAN RNA by a mechanism that is dependent on the ability of SOX to mediate the host shutoff effect, on the re-localization of PABPC1 into the nucleus and on the existence of a poly(A) tail on PAN RNA. In infected cultured cells activated into lytic phase, PAN RNA expression is coincident with the host shutoff effect, and correlates with PABPC1 re-localization from the cytoplasm into the nucleus. Yet, knockdown of PAN RNA using RNase H-targeting oligonucleotides shows that it is not required for the shutoff effect or the nuclear re-localization of PABPC1. Instead, the amount of virus released into the supernatant of cultured cells is severely reduced upon knockdown. This striking effect on viral titer is explained by our observation that knockdown of PAN RNA adversely affects the expression of a subset of viral genes during the late stage of lytic infection.
Materials and Methods
Cell lines, antibodies and transfection protocols
BCBL1 TReX-vector and TReX-RTA cells (gift from Jae Jung, USC) were maintained in RPMI supplemented with pen/strep, L-glutamine and 20% tetracycline-compatible FBS (Clonetech). iSLK.219, 293 DC-SIGN (gift from Robert Means and Sabine Lang, Yale University) and 293T cells were maintained in DMEM supplemented with pen/strep, L-glutamine and 10% FBS. 293 tet-on cells were maintained in DMEM supplemented with pen/strep, L-glutamine and 10% tetracycline-compatible FBS. Anti-orf6/SSB was a gift from G. Hayward (Johns Hopkins University), anti-LANA1 and anti-orf4 were gifts from Y. Chang (University of Pittsburg), anti-K8.1 antibody was from Advanced Biochemicals Incorporated, anti-orf65 antibody was a gift from G. Miller (Yale University), anti-myc antibody was from Santa Cruz Biotechnology. Electroporation was conducted in 0.4 cm electroporation cuvettes at 975 µF/220 mV for BCBL1 cells, and 975 µF/210 mV for iSLK.219 cells. 293T cells were transfected with MirusTransIT reagent according to the manufacturer's protocol. For some oligonucleotide knockdown experiments in BCBL1 TReX-RTA cells, cultures were first synchronized into S phase by serum starvation for 24 hours, followed by the re-addition of serum and incubation for 16 hours [25], [26]. Cells were then electroporated with RNase H-targeting modified oligonucleotides and allowed to recuperate overnight in RPMI supplemented with 20% tetracycline-compatible FBS (Clonetech), followed by induction the next day with 0.2 µg/mL doxycycline. Other transfection methods were tested, such as nucleofection with both Amaxa and Mirus reagents. Nucleofection with Amaxa reagent, as per the manufacturer's suggestions for BCBL1 cells, gave results comparable in terms of knockdown efficiency and cell death to the electroporation method used here.
Purification of PAN RNP
KSHV-infected BCBL1 TReX-vector or TReX-RTA cultures were grown to a density of 0.8 million cells/mL, and then treated with 2 ug/mL doxycycline for 24 hours. 100 million cells were pelleted, washed with PBS, resuspended in 500 uL 50 mM Tris-HCl, pH 7, 100 mM KCl, 10 mM MgCl2, 1 mM DTT, 10% glycerol, 1 mM ATP, protease inhibitor cocktail (CalbioChem) and RNase Inhibitor (Roche), and syringe lysed by 10 passages through a 20G needle, 15 passages through a 25G7/8 needle and 20 passages through a 27G needle. Lysate was clarified by spinning at 14,000 x g for 10 minutes at 4°C, before nutating with 500 µL bed volume DEAE-sepharose resin that had been pre-equilibriated with lysis buffer. After 4 hours at 4°C, the resin was pelleted at 3000 rpm for 1 minute at 4°C, washed twice with lysis buffer, and subjected to successive batch elutions with increasing concentrations of KCl in lysis buffer. Maximal yield of the PAN RNP was observed in the 0.3 M KCl fraction. 1 mM MgCl2, 1.5 mM ATP, 5 mM creatine phosphate and 2–5 pmol/µL of biotinylated 2′-O-methylated anti-PAN oligonucleotides, complementary to nucleotides 70 to 89 and 993 to 1012 of PAN RNA, was added to 35 µL (175 µg) of the 0.3 M KCl fraction in a 100 µL total volume. 150 µL bed volume of streptavidin beads was pre-blocked at 4°C for 15 minutes in 100 µg/mL E. coli tRNA, 100 µg/mL glycogen and 1 mg/mL BSA, washed three times in 20 mM Tris pH 7.6, 50 mM NaCl and 0.01% NP-40. Extract was nutated with streptavidin beads at 4°C for 1 hour, before pelleting, removing the supernatant, washing beads twice with lysis buffer and eluting bound proteins by treatment with micrococcal nuclease (Worthington Biochemical Corporation) supplemented with 2 mM CaCl2 at 37°C for 30 minutes. Proteins were fractionated by 10% SDS PAGE; the PAN RNP-specific bands were excised and identified by mass spectrometry (Columbia University). Further details on all peptides identified in each band can be found at links provided in Fig. S2C.
Immunofluorescence experiments
BCBL1 TReX-vector or TReX-RTA cells were fixed onto glass slides pre-treated with poly-L-lysine, fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton-X. Anti-PAN RNA oligonucleotides (SB2: ACAAATGCCACCTCACTTTGTCGC; SB85: CGCTGCTTTCCTTTCACATT; SB88: GTGAAGCGGCAGCCAAGGTGACTGG), which were labeled with digoxigenin-dUTP using the DIG oligonucleotide Tailing Kit (Roche), were hybridized with the samples in 50% formamide, 10% dextran sulfate, 2X SSC, 0.1% RNase-free BSA, 500 µg/mL salmon sperm DNA, 125 µg/mL E. coli tRNA and 1 mM vanadyl ribonucleoside complexes and detected using FITC-conjugated anti-DIG antibody (Jackson Lab Immunologicals). PABPC1 was visualized with anti-PABPC1 mouse monoclonal or rabbit polyclonal antibodies (gift of G. Dreyfuss, UPenn, and Abcam), and anti-mouse or anti-rabbit Alexa Fluor 594 antibody (Invitrogen). FLAG-PABPC1-NRS, a gift from B. Glaunsinger (UC Berkeley), was visualized with rabbit anti-FLAG antibody (Sigma) and anti-rabbit Alexa Fluor 594 (Invitrogen) [24]. rRNA was visualized with Y10B mouse monoclonal antibody [27] and anti-mouse Alexa Fluor 488 antibody (Invitrogen). Images were collected on a Leica TCS SP5 confocal microscope.
Modified oligonucleotides
RNase H-targeting oligonucleotide [28], [29] sequences used were as follows. SB215 anti-PAN RNA oligo 1 (complementary to nucleotides 70 to 89 of PAN RNA): mC*mC*mA*mA*mU*G*A*A*A*A*C*C*A*G*A*mA*mG*mC*mG*mG; SB216 anti-PAN RNA oligo 2 (complementary to nucleotides 993 to 1012 of PAN RNA): mU*mG*mA*mG*mC*T*C*T*A*G*G*C*A*C*G*mU*mU*mA*mA*mA; SB230 anti-GFP oligo: mC*mU*mG*mC*mC*A*T*C*C*A*G*A*T*C*G*mU*mU*mA*mU*mC; SB232anti-K7 oligo (complementary to nucleotides -305 to -285 of K7 mRNA, with respect to the PAN RNA transcriptional start site): mA*mA*mU*mC*mG*A*G*C*A*G*A*G*T*A*G*mC*mC*mA*fmA*mG, where m represents 2′-O-methyl substitutions of the ribose ring and * represents phosphorothioate substitutions.
Viral titer assays
BCBL1 TReX-RTA cells were induced with doxycycline for 8 days. Supernatant was collected, passed through a 0.45 micron filter, and incubated with 6.25 µg/mL RNase A (Sigma) and 20 units/mL DNase One (New England Biolabs) for 1 hour at 37°C to degrade extracellular RNA and any viral DNA that had not been properly packaged into viral capsid protein and released into the supernatant. The supernatant was then spun at 15,000 rpm using a Beckman TLA 100.2 rotor for 2 hours at 4°C, and the resulting pellet was resuspended in 600 µL of lysis buffer (20 mM Tris-HCl pH 8.0, 10 mM EDTA, 100 mM NaCl and 0.5% SDS) and incubated at room temperature for 10 minutes to inactivate the DNase. Following proteinase K treatment (0.1 mg/mL final concentration and 2 hours incubation at 37°C), 3 ng of a control plasmid DNA was added to each sample as a normalization control for loss of DNA during subsequent steps of the purification process. After extraction with phenol:chloroform:isoamyl alcohol (25∶24∶1), 600 µL of the aqueous phase was precipitated with isopropanol, sodium acetate and GlycoBlue (Ambion), and viral DNA levels were quantified using qRT-PCR. The viral DNA signal was normalized to signal from the control plasmid DNA (J. Ziegelbauer, personal communication).
For measuring virus production from iSLK.219 cells, cells were induced with doxycycline for 2-3 days, and supernatant was collected and passed through a 0.45 micron filter. 50 µL of supernatant was added to 300 µL of DMEM +10% FBS and placed on 293 DC-SIGN cells that had been grown in 12-well plates. Cells were then spun at 2000 rpm for 1 hour at room temperature in a Beckman Coulter J6-MI centrifuge fitted with a JS-4.2A rotor [30]. 48 hours later cells were harvested by trypsin, washed with PBS and fixed with 4% formaldehyde in PBS for 30 minutes at room temperature. GFP levels were quantified by flow cytometry using BD Biosciences' FACSCalibur platform.
Intracellular viral DNA replication assays
BCBL1 TReX-RTA cells were electroporated and induced as above for 3 days. 5 million live cells were washed with PBS, resuspended in 250 mM Tris pH 8.5, 125 mM EDTA, 1 mg/mL protease K and 1% SDS, and incubated at 60°C for 2 hours. 1/5th volume of 5 M potassium acetate pH 5.2 was added, lysate was incubated on ice for 20 minutes, and clarified by centrifugation at 12,000 rpm for 15 minutes at 4°C. Supernatant was transferred to a fresh tube, and incubated with 20 µg/mL RNase A at 37°C for 15 minutes. Then, 2.5-fold volume of ice-cold 100% ethanol was added, and DNA was precipitated overnight at −20°C. Pellets were resuspended in 100 µL water, and viral DNA levels were measured by qRT-PCR as above, normalizing to human DNA using GAPDH-specific primers.
Results
PABPC1 associates with PAN RNA in the nucleus of BCBL1 TReX-RTA cells
To determine what proteins bind to PAN RNA in the nucleus of lytically infected cells, we isolated the PAN RNP from a KSHV-infected cell line containing a doxycycline-inducible version of RTA (BCBL1 TReX-RTA), the viral transcription factor that is necessary and sufficient to promote entry into the lytic phase of viral infection [31], [32], [33]. After anion exchange and affinity chromatography using a selection oligonucleotide complementary to PAN RNA (Fig. 1), we identified both hnRNP C1/C2 and PABPC1 as specifically co-purifying with PAN RNA (see Fig. S1 and S2 for fractionation and mass spectrometry data). These proteins were not seen when extracts from the control doxycycline-treated BCBL1 TReX-vector cell line were used. hnRNP C1 has been previously observed to bind to PAN RNA, although the significance of this interaction is not understood [34].
Fig. 1. PAN RNA binds to PABPC1 in the nucleus of lytically infected BCBL1 TReX-RTA cells. 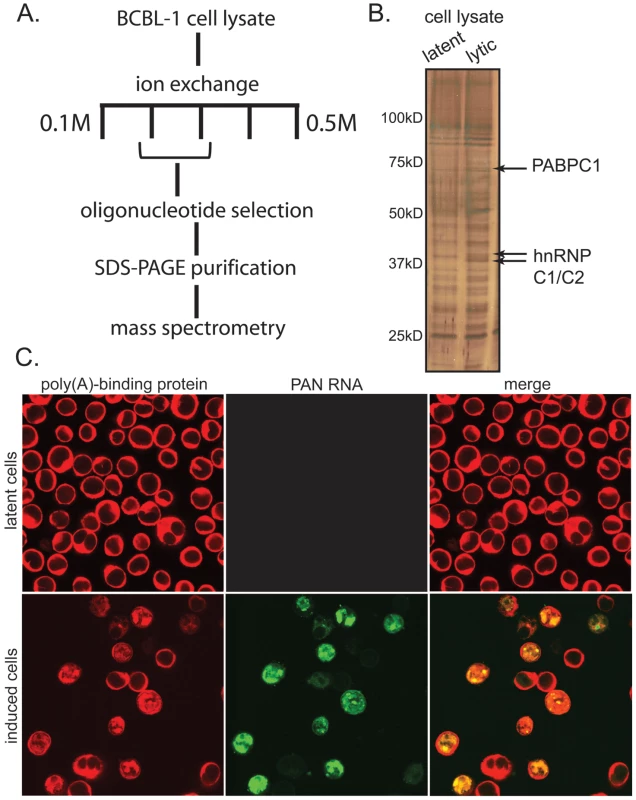
A. Scheme for biochemical purification of the PAN RNP. B. 10% SDS-PAGE gel silver-stained to show proteins co-purifying with PAN RNA from lysates of doxycycline-treated BCBL1 TReX-vector cells (left lane) or BCBL1 TReX-RTA cells (right lane). C. Dual immunofluorescence and in situ hybridization for PABPC1 (red) and PAN RNA (green) from BCBL1 TReX-vector cells (top panels) or BCBL1 TReX-RTA cells (bottom panels) treated with doxycycline. Since PAN RNA is exclusively nuclear, proteins that interact with PAN RNA must reside in the nucleus. While PABPC1 is essentially a cytoplasmic protein, nuclear re-localization of PABPC1 during KSHV lytic phase had been reported in the literature [18], [19], [20]. We therefore performed immunofluorescence experiments to confirm that PABPC1 re-localizes into the nucleus of the BCBL1 TReX-RTA cells [20] and to establish whether this re-localization correlates with the expression of PAN RNA. Cells were fixed and stained with primary and secondary antibodies to visualize PABPC1, and in situ hybridization probes and detection antibodies to visualize PAN RNA. As seen in Fig. 1C, PABPC1 indeed re-localizes into the nucleus during lytic infection, and the subnuclear distribution of PAN RNA and of re-localized PABPC1 appear strikingly similar. rRNA, however, remains predominantly cytoplasmic during both latent and lytic stages of infection (Fig. S3). We manually scored the immunofluorescence pattern of several hundred cells from a number of microscopic fields, and found that ∼80% of the cells in which PABPC1 had re-localized into the nucleus also expressed PAN RNA (representative images from the manual scoring experiment are provided in Fig. S4). The coincidence of PABPC1 re-localization and PAN RNA expression was further verified in the original BCBL1 cell line (data not shown). High throughput analysis, using multispectral imaging flow cytometry (Amnis Corporation), of PABPC1 nuclear re-localization and PAN RNA expression in BCBL1 cells has extended this correlation (S. Borah, L. Hassman, D.H. Kedes and J.A. Steitz, manuscript in preparation).
Additionally, we noted that unique peptides in the mass spectrometry data also identified PABPC4 as a protein that co-purifies with PAN RNA (Fig. S2A). Recently, nuclear re-localization of this protein has also been reported to result from KSHV lytic infection; knockdown of both PABPC1 and PABPC4 in transient SOX-transfection experiments revealed that these proteins might have redundant functions with respect to SOX-mediated nuclear retention of polyadenylated RNA in 293T cells [24].
High levels of PAN RNA expression are dependent on the re-localization of PABPC1 into the nucleus, as directed by the SOX protein
Since SOX induces both host shutoff and the nuclear accumulation of PABPC1 [19], [22], and since PAN RNA binds re-localized PABPC1 in the nucleus (see Fig. 1), we hypothesized that PAN RNA might be involved in the host shutoff effect mediated by SOX. To elucidate the relationship between PAN RNA, the SOX protein and the host shutoff effect, we co-expressed PAN RNA and SOX protein in transient transfection experiments, in the absence of other viral genes. As seen in Fig. 2A, transfection of SOX protein into 293 tet-on cells had no effect on 18S rRNA or the RNase P RNA, which are normally unaffected during host shutoff, but resulted in a modest 2 - to 4-fold reduction in the level of endogenous GAPDH mRNA and a 2 - to 3-fold reduction in the level of co-transfected GFP mRNA. In contrast, transfection of SOX very strongly stimulated the level of PAN RNA (20 - to 30-fold). This stimulation was independent of the tetracycline-regulated promoter used to express PAN RNA, as upregulation was observed in experiments using PAN's endogenous RTA-dependent promoter (Fig. 2B and 2C) or a CMV promoter (data not shown).
Fig. 2. SOX stimulates PAN RNA expression in transient transfection assays. 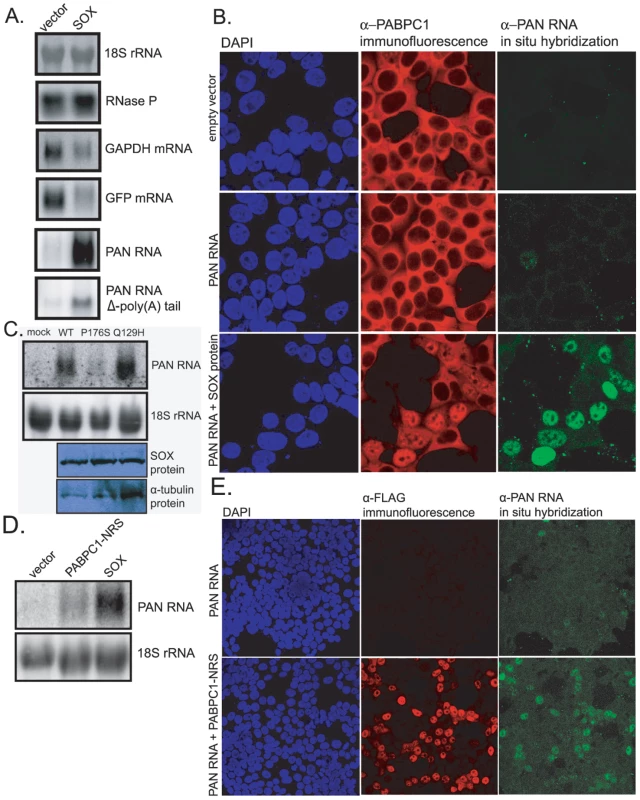
A. Northern blot analysis of total RNA extracted from 293 tet-on cells transfected with: 1) a GFP expression vector, 2) a PAN RNA or PAN/U7 3′-end (PAN RNA Δ-poly(A) tail) expression vector driven from a tetracycline-regulated promoter, and 3) either blank DNA (left lanes) or a SOX-expression vector (right lanes). The blot was sequentially probed for the RNAs listed on the right. B. Confocal images of dual immunofluorescence/in situ hybridization staining for PABPC1 (red) and PAN RNA (green) in transiently transfected 293T cells. Cells were transfected with: 1) empty plasmid (top panels), 2) a PAN RNA expression vector driven from the endogenous RTA-responsive PAN promoter and an RTA expression vector (middle panels), or 3) a PAN RNA expression vector driven from the endogenous RTA-responsive PAN promoter, an RTA expression vector and a SOX-expression vector (bottom panels). C. Top two panels: northern blot analysis for 18S or PAN RNA in total RNA extracted from 293T cells transfected with: 1) PAN RNA driven from its endogenous promoter, 2) an RTA expression vector, and 3) either empty plasmid, an expression vector for wild-type SOX, the P176S mutant SOX or the Q129H mutant SOX. Bottom two panels: western blots showing equal levels of expression of all three SOX proteins. D. Northern blot analysis of total RNA extracted from 293T cells transfected with an RTA expression vector, a PAN RNA expression vector driven from the endogenous PAN promoter and either blank DNA (left lane), a PABPC1-NRS expression vector (center lane) or a SOX expression vector (right lane). E. Confocal images of dual immunofluorescence/in situ hybridization staining for FLAG-tagged PABPC1-NRS (red) and PAN RNA (green) in transiently transfected 293T cells. The effect of SOX on PAN levels was also seen by immunofluorescence (Fig. 2B). When a PAN RNA expression vector that includes 1 kb of PAN's endogenous, RTA-responsive promoter was co-transfected into 293T cells with an RTA expression plasmid, only an occasional in situ hybridization signal for PAN RNA was observed (see Fig. 2B, middle right panel). However, when the SOX gene was co-transfected, the PAN RNA in situ hybridization signal was robust (bottom right panel). Furthermore, those cells in which a robust PAN RNA in situ signal was observed were the same cells in which PABPC1 had re-localized to the nucleus (Fig. 2B, middle column). This suggested that high levels of PAN RNA expression are not only dependent on SOX expression, but might also involve PABPC1 re-localization. Accordingly, when a mutant version of PAN RNA in which the poly(A) tail was replaced by a 3′ stem-loop structure derived from a histone mRNA [6] was transfected into these cells, SOX-mediated enhancement of PAN RNA levels was reduced by about 6-fold (see Fig. 2A, PAN RNA Δ-poly(A) tail, and Fig. S5).
The SOX protein is a dual-function protein, acting both as the mediator of the host shutoff effect and as an alkaline exonuclease that is important for processing the viral DNA [35]. These two functions are separable; point mutants that are unable to carry out the shutoff function are wild-type for exonuclease function, and vice versa. Moreover, mutations that inactivate only the shutoff function of the protein also ablate its ability to re-localize PABPC1 into the nucleus, arguing for a link between these phenomena. We therefore tested each of these mutants to determine which function of SOX is responsible for the strong stimulation of PAN RNA levels. As seen in Fig. 2C, the P176S SOX mutant, which retains exonuclease activity but does not mediate host shutoff, failed to enhance PAN RNA expression. Conversely, the Q129H mutant, which retains host shutoff but does not exhibit exonuclease activity, stimulated PAN RNA expression to the same extent as wild-type SOX. These results indicate that SOX's exonuclease activity is not required for its stimulatory effect on PAN RNA. They are also consistent with the possibility that upregulation of PAN is mediated by SOX's host shutoff function and ability to re-localize PABPC1.
To address whether nuclear localized PABPC1 alone can upregulate levels of PAN RNA, in the absence of SOX, we co-transfected genes for PAN RNA and a mutant PABPC1-NRS that is retained in the nucleus [24]. As seen in Fig. 2D, co-expression of PABPC1-NRS resulted in upregulation of PAN RNA. As with SOX expression, upregulation of PAN RNA levels were specific to those cells in which nuclear retention of PABPC1 had occurred (Fig. 2E). While this result clearly demonstrates that nuclear accumulation of PABPC1 results in increased PAN RNA levels, the levels of PAN RNA did not reach levels seen upon co-transfection with SOX (see Fig. 2D). This may be due to a different efficiency of PABPC1 nuclear re-localization in SOX-transfected versus PABPC1-NRS-transfected cells, or due to some other aspect of SOX expression that further augments PAN RNA expression, in addition to the nuclear localization of PABPC1.
A previous study showed that although SOX and its murine herpesvirus-68 homolog reside in both the nucleus and cytoplasm, it is the cytoplasmic fraction of these proteins that is responsible for the host shutoff effect and for PABPC1 re-localization [36]. A more recent study demonstrated that SOX promotes PABPC1 re-localization by depleting cytoplasmic polyadenylated RNA, which in turn allowed direct intereaction between PABPC1 and importin α [37]. The fact that upregulation of PAN by SOX (Fig. 2A and B) depends on the nuclear re-localization of PABPC1, as well as on the presence of PAN's poly(A) tail, suggests how cytoplasmic SOX protein strongly upregulates the levels of a nuclear RNA: SOX indirectly enhances PAN abundance through re-localizing PABPC1, which in turn stabilizes PAN RNA by binding to its poly(A) tail. This scenario is consistent with the role of poly(A) tails as stabilizing constituents of mRNAs by antagonizing deadenylases [14] and with the presence of nuclear degradation factors that destabilize PAN in the absence of its poly(A) tail [6].
PAN RNA expression is coincident with but nonessential for the KSHV host shutoff effect
If increased levels of PAN RNA in the nuclei of lytically reactivated cells are due to PABPC1 re-localization, as observed in transiently transfected cells (Fig. 2), then PAN RNA should accumulate at the same time during the course of KSHV infection that host mRNA levels decrease because of the shutoff effect. To test this, BCBL1 TReX-vector or TReX-RTA cells were induced into lytic phase, and total RNA was extracted from culture samples after different times. Northern blot and qRT-PCR analysis (Fig. 3A and B) showed that increases in SOX and PAN RNAs coincide with the decrease in β-actin and GAPDH mRNA (∼12–24 hours post-induction). Thus, PAN RNA expression correlates with the host shutoff effect in the context of actual viral infection. Furthermore, at approximately the same time during the course of lytic infection, PABPC1 re-localizes into the nucleus (data not shown and [19]).
Fig. 3. Expression of PAN RNA is coincident with the host shutoff effect. 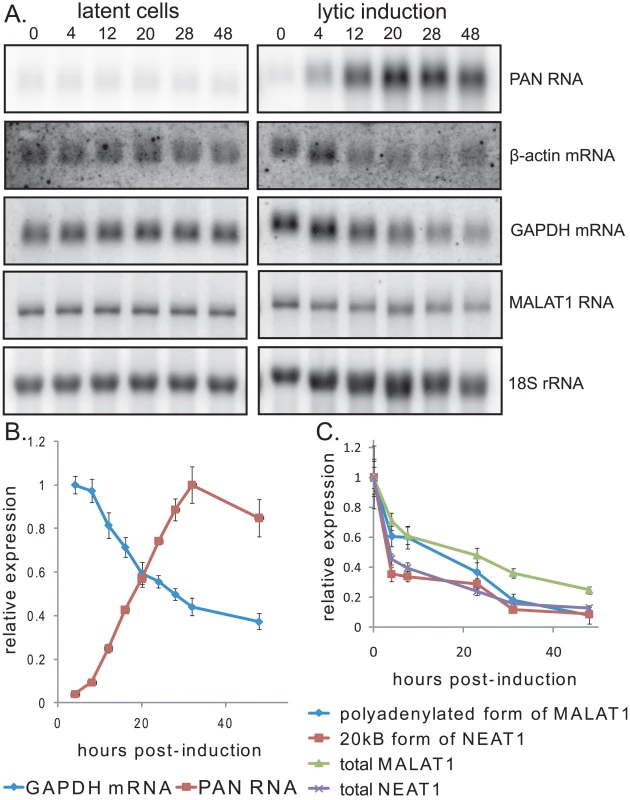
A. Northern blot showing host and viral RNA expression during progression of lytic reactivation by doxycycline treatment of BCBL1 TReX-vector (left) or BCBL1 TReX-RTA (right) cells. B. qRT-PCR data from a representative experiment showing increased expression of PAN RNA versus the decline in host GAPDH mRNA. C. qRT-PCR data from a representative experiment showing that host MALAT1 and NEAT1 RNAs decline during lytic infection. qRT-PCR signals were normalized to endogenous 18S rRNA. To ask whether lytic induction results in the accumulation of other abundant, polyadenylated noncoding RNAs coincident with PABPC1 re-localization, we measured the levels of host MALAT1 and NEAT1 RNAs by northern blot and qRT-PCR. MALAT1 RNA exists either as a long polyadenylated transcript that localizes to nuclear speckles, or a shorter transcript that localizes to the cytoplasm [38]. Similarly, NEAT1 RNA exists either as a shorter polyadenylated transcript or a longer transcript whose 3′ end is generated by endonucleolytic cleavage by RNase P [39]. We observed that the levels of the nuclear, polyadenylated forms of MALAT1 or NEAT1 RNA did not increase dramatically (Fig. 3A and C) but rather declined in abundance during lytic infection, after normalization to 18S rRNA levels. Thus, PAN RNA selectively accumulates in the nucleus during the KSHV lytic phase, while two other similar noncoding polyadenylated RNAs, MALAT1 RNA and NEAT1 RNA, do not.
To investigate whether PAN RNA expression directly contributes to the host shutoff effect and/or PABPC1 re-localization during the KSHV lytic phase, we knocked down the levels of PAN RNA using 2′-O-methylated and phosphorothioate-substituted antisense oligonucleotides that target endogenous RNase H to cleave PAN RNA (α-PAN oligos 1 and 2 in Fig. 4A) [28]. Interpretation of PAN knockdown experiments is complicated by the fact that the region of the KSHV genome from which PAN RNA is transcribed is included in the overlapping K7/survivin transcript (Fig. 4A) [40]. To differentiate between the involvement of PAN RNA and the K7/survivin protein, we designed an additional antisense oligonucleotide that targets the K7 transcript in a region not included in the sequence of PAN RNA (α-K7 oligo in Fig. 4A). We reasoned that any results of transfection of oligonucleotides targeting both PAN RNA and K7 mRNA (Fig. 4A, α-PAN oligos 1 and 2) that are not seen upon transfection of the K7-specific oligonucleotide (α-K7 oligo) would be specifically due to PAN RNA knockdown. First, we showed by qRT-PCR that the K7-specific oligonucleotide selectively lowered the level of the K7 mRNA ∼20-fold (Fig. 4B). However, the K7-specific oligonucleotide did not downregulate levels of PAN RNA, as seen in the northern blot of Fig. 4C. The combination of the two anti-PAN RNA oligonucleotides decreased not only PAN RNA to 5%–10% of wild-type levels in BCBL1 TReX-RTA cells (see Fig. 4C), but also the K7 mRNA, as expected (see Fig. 4B). Thus, differential knockdown of the overlapping K7 and PAN transcripts can be achieved by use of modified oligonucleotides.
Fig. 4. PAN RNA and K7 mRNA levels are reduced by transfection of RNase H-targeting oligonucleotides. 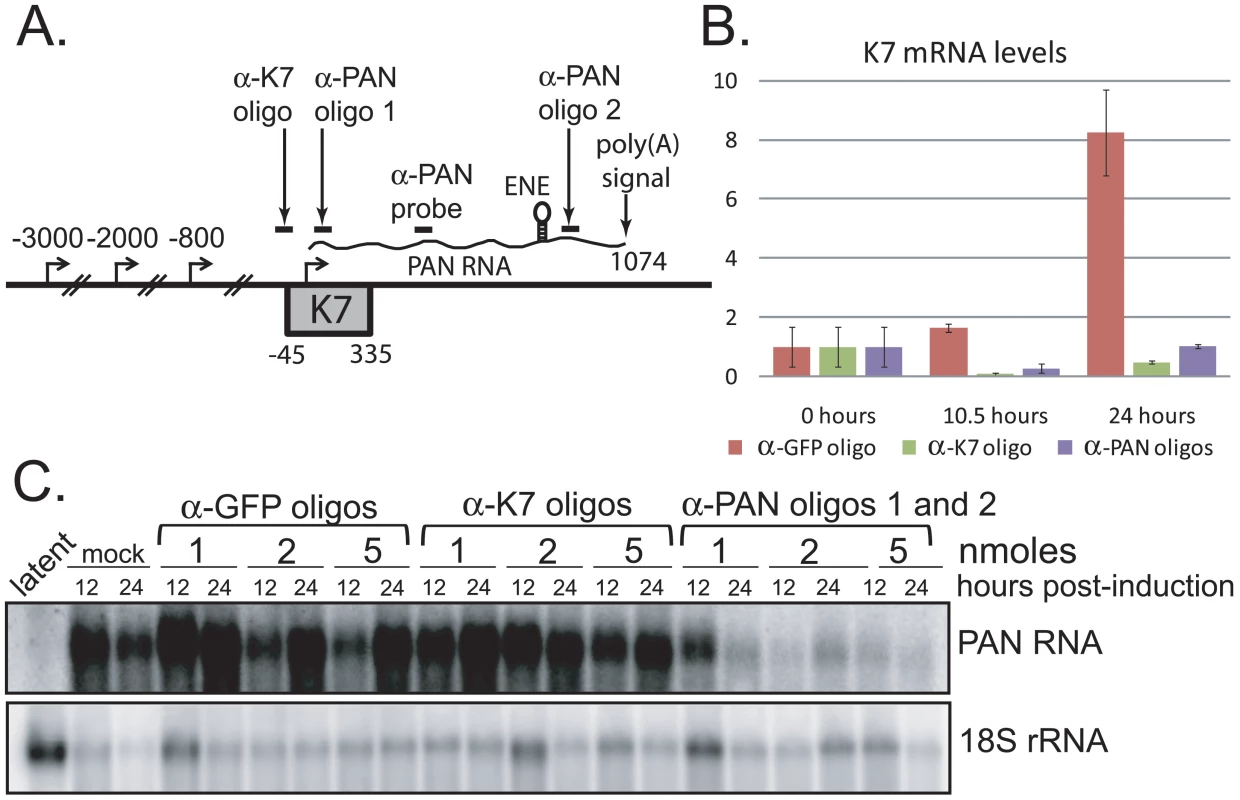
A. Region of the KSHV genome from which K7 mRNA and PAN RNA are transcribed. Alternative transcriptional start sites for the K7 mRNA isoforms [40], regions targeted by anti-K7 (α-K7) and anti-PAN RNA (α-PAN) 1 and 2 modified oligonucleotides, as well as the oligonucleotide probe used to detect PAN RNA by northern blot, are indicated. The K7 ORF, ENE and poly(A) signal shared by all four RNA species are shown. B. K7 mRNA levels at 0, 10.5 or 24 hours post-induction of BCBL1 TReX-RTA cells after recovery from electroporation with α-GFP, α-K7 or α-PAN RNA 1 and 2 oligonucleotides. qRT-PCR signals were normalized to endogenous 18S rRNA. C. Northern blot analysis of total RNA extracted from BCBL1 TReX-RTA cells electroporated with different concentrations of α-GFP, α-K7 or α-PAN RNA 1 and 2 oligonucleotides and at 0, 12 or 24 hours post-induction. Revealingly, the anti-PAN oligonucleotides had no effect on the host shutoff effect, as indicated by GAPDH and β-actin mRNA levels, despite a knockdown of PAN RNA levels by ∼90%–95% (Fig. 5). Transfection with a GFP - or K7-specific control oligonucleotide also did not alter the magnitude of host shutoff in BCBL1 TReX-RTA cells (Fig. S6). Confocal analysis indicated that PABPC1 was re-localized into the nucleus of BCBL1 TReX-RTA cells upon lytic induction despite PAN RNA knockdown (data not shown). We conclude that the expression of PAN RNA may be related to but is not required for SOX-dependent host shutoff during the KSHV lytic phase.
Fig. 5. PAN RNA does not contribute to the host shutoff effect. 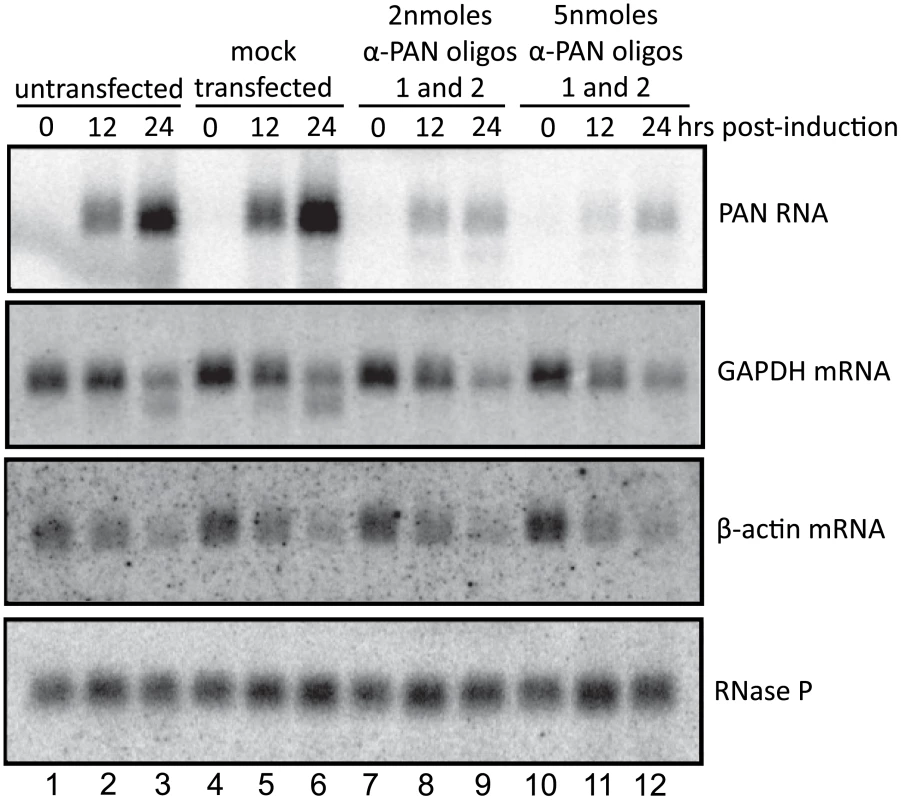
Northern blot of total RNA harvested from BCBL1 TReX-RTA cells electroporated with increasing concentrations of α-PAN RNA 1 and 2 oligonucleotides at various times after doxycycline-mediated induction, probed for the RNAs indicated on the right. The faster migrating band in lanes 3 and 6 of the GAPDH panel is residual 32P-labeled anti-PAN RNA probe. PAN RNA is required for expression of a subset of viral genes
Since knockdown of PAN RNA does not appear to affect the SOX-induced shutoff of host protein synthesis, we asked whether PAN RNA might alter the expression of viral proteins instead. We compared the effects of knockdown of PAN RNA on viral protein expression in two different cells lines: BCBL1 TReX-RTA cells, which harbor the authentic KSHV virus, and iSLK.219 cells, in which the recombinant rKSHV.219 virus has been re-introduced into a KS cell line that has lost the original KSHV genome [41], [42]. In both these cell lines, the virus is induced into lytic phase by overexpression of RTA controlled by a tetracycline-responsive promoter.
Western blot analyses of lysates from BCBL1 TReX-RTA that had been transfected with no (mock), anti-GFP, anti-K7 or anti-PAN RNA oligonucleotides revealed that knockdown of PAN RNA affects late rather than early KSHV proteins (Fig. 6). Several early viral proteins, such as Orf50/RTA, vIL-6 and Orf6/ssDNA-binding protein (Fig. 6A), as well as the latency-associated nuclear antigen Orf73/LANA1 (data not shown), accumulate normally. In contrast, the levels of the late viral proteins K8.1 and Orf65/small viral capsid antigen (Fig. 6A), as well as Orf4/complement-binding protein (data not shown) were significantly lowered upon knockdown of PAN RNA, compared to the control oligonucleotides against GFP or K7 mRNAs. Densitometric quantifications by two different methods (see Fig. S7 legend for description of methods) of the immunoblot signals for the immediate early protein RTA, early protein vIL-6 and late protein K8.1 from 9 independent experiments are averaged in Fig. 6B (7 independent experiments for signal from cells transfected with anti-K7 oligonucleotide). The efficiency of PAN RNA knockdown ranged from 75% to 95% (data not shown) and the immunoblots from all 9 experiments are presented in Fig. S7. Together these data demonstrate the selective downregulation of the late protein K8.1 upon knockdown of PAN RNA.
Fig. 6. Knockdown of PAN RNA expression adversely affects late gene expression in BCBL1 TReX-RTA cells. 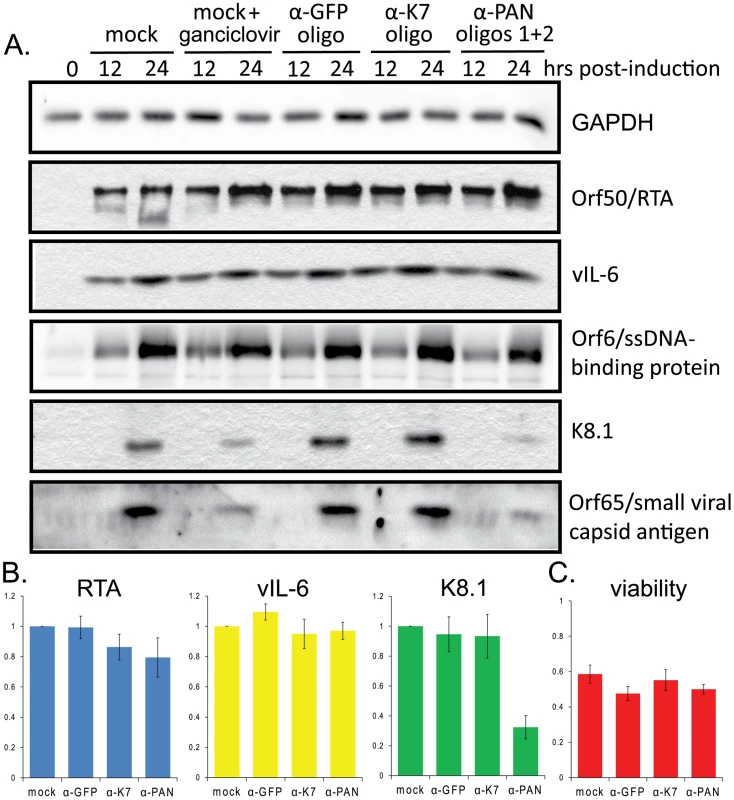
A. BCBL1 TReX-RTA cells were electroporated with the indicated modified oligonucleotides and induced with doxycycline 12–16 hours later. After various times, lysates from equivalent numbers of living cells were loaded in each lane, and blotted proteins were probed sequentially with antibodies against the indicated proteins. B. Densitometric analysis of immunoblot signals (see Fig. S6 legend) for RTA (immediate early protein), vIL-6 (early protein) and K8.1 (late protein) after knockdown with control or α-PAN RNA oligonucleotides. Standard error of the mean from 9 experiments (7 experiments for α-K7 knockdown) is shown. C. Cell viability was measured by trypan blue staining 24 hours post-induction. Averages and standard error of the mean from 7 independent experiments are shown. Although non-specific toxic effects were experienced upon electroporation followed by lytic induction, particularly in the BCBL1 TReX-RTA cells (see Materials and Methods), similar levels of cell death were seen regardless of the oligonucleotide transfected (Fig. 6C). Cultures were routinely stained by trypan blue and scored for percent viability prior to harvest in order to analyze lysate from the same number of living cells for each condition. Thus, the loss of late gene expression in PAN knockdown cells is not due simply to increased levels of cell death.
Likewise, several lines of evidence indicate that the decreased levels of late protein expression observed upon knockdown of PAN RNA are not simply due to a non-specific decrease in RTA expression. Although some of the 9 immunoblot experiments revealed a decrease in RTA expression upon transfection with the K7 and PAN RNA oligonucleotides (Fig. 6B), in others a significant decrease in K8.1 expression was observed despite robust expression of RTA (see Fig. S7, panels A, D, E, G and H). Moreover, an independent assay was devised to test whether the effect of PAN RNA knockdown on K8.1 was due to decreased RTA expression. The expression levels of both K8.1 and myc-tagged RTA were measured by dual immunofluorescence staining and analysis by confocal microscopy. Manual scoring of RTA-positive BCBL1 TReX-RTA cells revealed that fewer cells expressed K8.1 upon knockdown of PAN RNA (Fig. S8). Importantly, since K8.1 expression was scored only in cells that also expressed RTA, the observed effect could not be due to decreased RTA expression. We conclude that the effect of PAN RNA knockdown on K8.1 expression is independent of any effect on RTA.
The results of PAN RNA knockdown in iSLK.219 cells were also assessed (Fig. 7A). Knockdown efficiency in this cell line was 90%–95% (data not shown). In iSLK.219 cells, knockdown of PAN RNA resulted in some decrease in all viral proteins tested, including the early proteins Orf6/ssDNA binding protein and vIL-6 (Fig. 7A). However, the effect on the late K8.1 protein was much more pronounced: K8.1 protein levels dropped 12.5-fold in cells transfected with anti-PAN oligonucleotides compared to cells transfected with anti-K7 oligonucleotide (Fig. 7B), whereas vIL-6 protein levels decreased only 2.5-fold. We conclude that PAN RNA preferentially enhances the expression of late viral proteins in KSHV-infected cells.
Fig. 7. Knockdown of PAN RNA adversely affects gene expression in iSLK.219 cells, with a more pronounced effect on late genes. 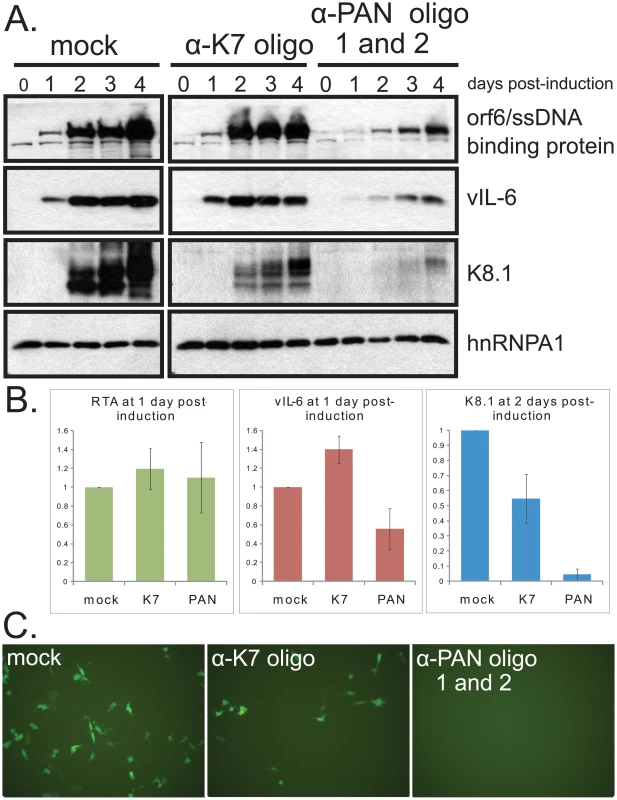
A. Procedures were the same as in Fig. 6 B. Densitometric analyses of immunoblot signals of RTA (immediate early protein), vIL-6 (early protein) and K8.1 (late protein) after knockdown with control or α-PAN RNA oligonucleotides. Standard error of the mean from 4 experiments is shown. C. GFP expression in target 293 DC-SIGN cells after inoculation with media from iSLK.219 cells treated with no oligonucleotide (mock), α-K7 oligonucleotide or α-PAN RNA oligonucleotides 48 hours after induction with doxycycline. FACS sorting of cells indicated mean fluorescence intensities (in arbitrary units) of 0.07+/− 0.04 for uninfected cells, 1.00 for cells infected with supernatant from mock-transfected iSLK.219 cells, 0.14+/− 0.08 for cells infected with supernatant from anti-K7-transfected iSLK.219 cells and 0.05+/− 0.03 for cells infected with supernatant from anti-PAN-transfected iSLK.219 cells. Standard error of the mean for 3 independent experiments is given. Knockdown of PAN RNA decreases viral titers
Since knockdown of PAN RNA adversely affects expression of important late viral genes, we predicted that its knockdown would affect overall viral yield. To assess the role of PAN RNA in the production of new virus, we harvested virus from the supernatant of induced BCBL1 TReX-RTA cells 8 days post-induction. As seen in Fig. 8A, knockdown of PAN RNA significantly reduced viral production, as assayed by qPCR measurement of DNase resistant, encapsulated viral DNA released into the supernatant. The effect was comparable to treatment with ganciclovir, an inhibitor of viral DNA replication [43], [44].
Fig. 8. Knockdown of PAN RNA reduces production of encapsulated viral DNA without affecting levels of intracellular viral DNA. 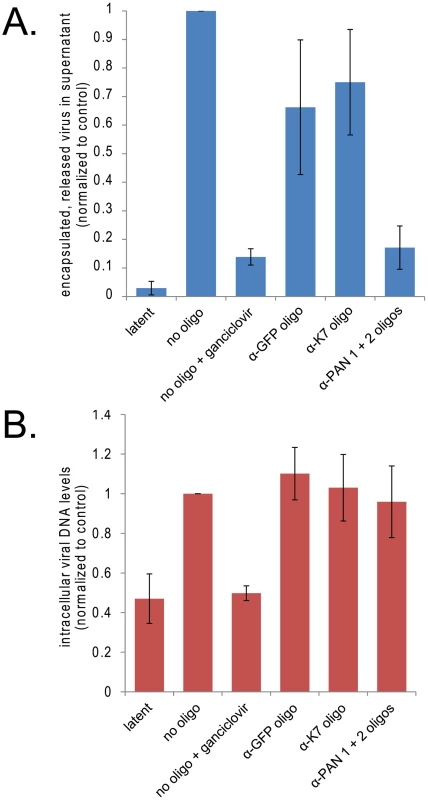
A. Knockdown of PAN RNA lowers viral yield in cell culture supernatant. BCBL1 TReX-RTA cells were electroporated with the indicated modified oligonucleotides, allowed to recover overnight, and then induced with doxycycline. 8 days later, encapsulated viral DNA was harvested from the supernatant and quantified by qPCR, normalizing to control plasmid exogenously added at onset of purification. Values are the average of 4 independent experiments. Standard error of the mean is shown. Note that electroporation of all oligonucleotides decreased virus production to some degree, most probably because of cell death experienced upon electroporation of nucleic acids followed by lytic induction. B. Knockdown of PAN RNA does not decrease intracellular viral DNA. BCBL1 TReX-RTA cells were treated as above and intracellular DNA was collected 3 days post-induction and quantified by qPCR, normalizing to the amount of host DNA, as measured by GAPDH DNA signal. Values are the average of 3 independent experiments. Standard error of the mean is shown. The effect of PAN RNA knockdown on production of infectious virus from iSLK.219 cells was also assayed by harvesting the supernatant from induced iSLK.219 cells and infecting target 293 cells that stably express the DC-SIGN receptor. Infection was assessed by visualization (Fig. 7C) and by FACS analysis of GFP expression arising from the recombinant KSHV genome in target cells 48 hours later. Fig. 7C reveals that treatment of iSLK.219 cells with anti-PAN RNA oligonucleotides resulted in virtually no GFP-positive 293 DC-SIGN cells upon infection with virus harvested from cells treated with anti-PAN oligonucleotides. However, GFP-positive cells were detected among 293 DC-SIGN cells incubated with virus harvested from cells treated with the anti-K7 oligonucleotide. Thus, release of infectious virus as well as encapsulated viral DNA levels were both diminished by knocking down PAN RNA.
Since viral DNA replication is required for the expression of herpesvirus late proteins [45], [46], [47], the effect of PAN RNA knockdown on the accumulation of intracellular viral DNA during the lytic phase was also measured by qPCR. Increases in intracellular viral DNA levels 4 days post-induction in BCBL1 TReX-RTA cells were very modest (∼5–7 fold), in agreement with other reports [48], [49]; this level was further decreased when cells were electroporated prior to induction. The low levels of intracellular viral DNA make it difficult to conclude definitively whether the knockdown of PAN RNA specifically inhibits viral DNA replication. However, no dramatic differences in intracellular viral DNA levels were detectable in cells transfected with control versus anti-PAN RNA oligonucleotides (Fig. 8B), suggesting that PAN RNA is not directly involved in viral DNA replication.
Discussion
A noncoding RNA is important for viral gene expression during the KSHV lytic phase
The data presented here indicate that the highly abundant PAN RNA binds to the normally cytoplasmic poly(A) binding protein PABPC1 once it has been re-localized to the nucleus of KSHV-infected cells (Fig. 1). This is supported by both the composition of the PAN RNP and the observation that the detailed patterns of PAN RNA and PABPC1 concentration within the nucleus coincide. The abundance of PAN RNA (upwards of 0.5×106 transcripts per nucleus) corresponds well to the abundance of PABPC1 (estimated at 7×106 copies in the average HeLa cell), given that the length of PAN RNA's poly(A) tail is estimated to be similar to that of the average host mRNA (Conrad and Steitz, data not shown) and that each PAN RNA thus likely binds 8–10 PABPC1 molecules [3], [50]. Our results extend prior reports of PABPC1 re-localization by SOX protein alone and the concurrent host shutoff effect [19] by showing that that the level of PAN RNA is likewise dependent on expression of the SOX protein in transient transfection assays (Fig. 2). In infected cells, PAN RNA is not required for the re-localization of PABPC1 nor for the host shutoff effect (Fig. 5), which promotes this re-localization.
Since PAN RNA expression is concurrent with the host shutoff effect in the context of viral lytic infection (Fig. 3), PAN RNA likely functions downstream of SOX action. An alternative possibility-that RTA levels (and therefore all late functions) are compromised by treatment with anti-PAN oligonucleotides-is not consistent with several observations. Because lytic reactivation is dependent on RTA expression, an across-the-board reduction in all lytic-related events would be expected but was not observed. Specifically, PAN RNA knockdown did not affect: 1) the host shutoff effect and PABPC1 nuclear re-localization (Figs. 5 and S6 and data not shown), 2) accumulation of intracellular viral DNA (Fig. 8B), and 3) expression of the viral lytic marker vIL-6 (Fig. 6A, B). Instead, knockdown of PAN RNA adversely affected the expression of late viral genes in cells of both lymphoid (BCBL1, Figs. 6, S7 and S8) and endothelial (iSLK.219, Fig. 7) origin, which are two major KSHV targets in vivo.
Ultimately, knockdown of PAN RNA adversely affects release of new infectious virus into the supernatant from BCBL1 TReX-RTA cells (Fig. 8A), as would be expected from the downregulation of late viral gene expression. This effect appears to be independent of viral DNA replication (Fig. 8B). We conclude that PAN RNA plays an important role in the expression of a subset of viral genes, perhaps related to the nuclear re-localization of PABPC1. Further work is needed to establish whether this effect is at the level of transcription, translation or mRNA stability, although selective downregulation of mRNAs for the late genes K8.1, Orf18 and Orf29 was observed using qRT-PCR analysis (data not shown). It is also possible that the effects seen on virus production and late viral protein expression result from dual knockdown of both PAN RNA and K7. However, the fact that the knockdown of PAN RNA is required to observe these effects, as K7 knockdown alone is insufficient, underscores the importance of PAN RNA.
KSHV targets host gene expression at several levels during the lytic phase. First, host mRNA levels are specifically downregulated by the SOX protein, a phenomenon that is itself related to nuclear re-localization of PABPC1, a highly abundant translation factor that displays nanomolar affinity for the polyadenylate tails of mRNAs [24], [50]. Rowe and colleagues have found that the Epstein Barr virus SOX homolog BGLF5 mediates host shutoff in EBV-infected cells [51], and Glaunsinger and colleagues have extended their findings of SOX function to the homologous murine herpesvirus-68 (MHV-68) protein [36]. Both EBV and MHV-68 SOX homologs drive PABPC1 re-localization into the nucleus, like their KSHV-counterpart [19], and we have extended the observation of PABPC1 nuclear re-localization to EBV-infected HH514-16 cells upon lytic activation (S. Borah, R. Park, G. Miller and J.A. Steitz, unpublished observations). Second, in addition to the downregulation of host mRNA levels, other changes in host translation during the KSHV lytic phase [20] have been reported to include increased levels of 4E-BP1 and eIF4E phosphorylation, which are expected to enhance rates of eIF4F assembly onto and of translation of viral mRNAs. Third, KSHV mRNAs, many of which are unspliced, are preferentially exported by the viral export factor Orf57, which tethers the nuclear export factor TAP protein to viral mRNAs and enhances their translation [52], [53], [54].
Our data support a model in which the highly abundant PAN RNA contributes importantly to the viral manipulation of gene expression. Aspects of viral infection that downregulate host gene expression, such as host shutoff and PABPC1 re-localization, do not require PAN RNA, but instead affect the expression level of PAN RNA itself both in transiently transfected cells and in bona fide infected cells. PAN RNA then impacts the expression of at least a subset of viral genes; possible molecular mechanisms for this regulation are presented below.
Viral targeting of PABP
Poly(A) binding protein is a central player in cellular gene expression and is targeted by a number of viruses, usually as a means to achieve shutdown of host gene expression. Some, such as picornaviruses and caliciviruses, have evolved specific proteases that cleave PABPC1 [55], [56], [57]. Rotavirus instead expresses the NSP3 protein, which displaces PABPC1 from binding to eIF4G. This reduces the translational efficiency of host mRNAs, whereas viral mRNAs, which lack a poly(A) tail, are efficiently translated [58]. Expression of NSP3 also leads to nuclear accumulation of PABPC1, although the significance of this re-localization is not fully understood [59], [60]. Nuclear re-localization of PABPC1 is also observed during infection with Bunyamwera virus. Furthermore, although siRNA-mediated knockdown of PABPC1 decreased translation of a polyadenylated luciferase reporter, translation of a reporter whose 3′ end was derived from Bunyamwera virus mRNA, and thus lacked a poly(A) tail, was unaffected by knockdown [61].
PABPN1, a distinct nuclear poly(A) binding protein [14], is also targeted by viruses. Influenza NS1 protein binds both PABPN1 and the cleavage and polyadenylation stimulation factor (CPSF) within the assembled 3′-end processing machinery [62]. Thus, poly(A) polymerase does not processively extend the poly(A) tail of nascent mRNAs past ∼12 residues, and multiple PABPN1 molecules do not assemble onto the mRNA. This failure to properly form a poly(A) tail [63] and interact with PABPN1 are thought to underlie the lack of host mRNA export during influenza virus infection [64]. NS1 has other effects on PABPN1 as well, including inhibition of PABPN1 shuttling and re-distribution of PABPN1 from nuclear speckles to a uniform pattern throughout the nucleoplasm [62]. However, to date, no role for a noncoding RNA has been identified in the mechanisms by which these viruses target PABPC1 for host shutoff.
Homologs of PAN RNA?
Given the striking abundance of PAN RNA and the role that it plays in KSHV gene expression within the context of PABPC1 re-localization, it might be expected that homologs of this RNA would be found in related herpesviruses in which re-localization of PABPC1 occurs. Viruses whose mRNAs lack poly(A) tails, such as rotavirus and Bunyamwera virus, would be exceptions. Indeed, the EBV homolog of the SOX protein, BGLF5, has been shown to mediate host shutoff in cells infected with EBV and to drive PABPC1 re-localization [36], [51]. Although no PAN-like RNA has yet been reported in EBV or other herpesviruses, we have recently discovered putative homologs in several members of the gamma herpesvirus family. Indeed, expression of a PAN homolog has been verified in the closely related Rhesus Rhadinovirus (RRV), even though the overlapping K7 open reading frame does not appear to be conserved (K. Tycowski, S. Borah, M. Shu and J.A. Steitz, manuscript in preparation) [65], [66]. Additional studies are required to explore the possibility that PAN RNA-like RNAs also exist in more distantly-related viruses such as EBV and MHV-68. Interestingly, the sequence of PAN-like RNAs may not be conserved, whereas their abundance, nuclear localization and the presence of a poly(A) tail may be critical for function. Furthermore, there is no reason to expect that the role of PAN RNA be fulfilled by a single RNA transcript. Perhaps in other viruses, PAN RNA is functionally replaced by two or three different polyadenylated, nuclear RNAs, which together sequester PABPC1. Immunoprecipitation of PABPC1 from the nuclei of other herpesviruses during the lytic phase and sequencing the RNAs that co-precipitate might identify such PAN-like RNAs.
Interestingly, the best-characterized viral noncoding RNAs, the VA RNAs of adenovirus, which facilitate viral translation by suppressing protein kinase R and competitively inhibiting miRNA export and processing [67], [68], are not found outside of adenoviruses. Perhaps their biological functions are fulfilled by alternative mechanisms in other viruses.
Potential molecular mechanisms for the role of PAN RNA in the nucleus
By what molecular mechanism might PAN RNA enhance the expression of late viral genes, and could this role be related to its ability to bind re-localized PABPC1 in the nucleus of KSHV-infected cells? It is first critical to understand why PABPC1 re-localization might be harmful to the cell's normal functioning. PABPC1 is proposed to have three major functions: 1) to synergistically enhance mRNA translation by interaction with eIF4G, 2) to regulate mRNA fate by inhibiting deadenylation of the poly(A) tail or promoting interaction with deadenylating factors, and 3) to facilitate nuclear export of mRNA [14]. It seems likely that loss of PABPC1 from the cytoplasm would be detrimental to any or all of these functions in uninfected cells. In the context of viral infection, re-localization of PABPC1 appears to be important for the shutoff effect since siRNA-mediated knockdown of PABPC1 diminishes the ability of SOX to target GFP mRNA for degradation, and since point mutations in SOX that ablate its effect on PABPC1 re-localization also abolish its downregulation of host mRNAs [19]. Importantly, aspects of host shutoff have been recapitulated by transient transfection of a nuclear targeted PABPC1 protein [24]. These results argue that it is not merely the absence of PABPC1 from the cytoplasm that is detrimental for host mRNA stability, but that its presence in the nucleus is pivotal.
How might nuclear re-localized PABPC1 contribute to host mRNA degradation? Under normal conditions, PABPN1 is required for the synthesis of and co-transciptionally binds to the newly-extended poly(A) tails of mRNAs. It has been suggested that PABPN1 is further required for proper export of mRNA via its interaction with nuclear export factors [63]. PABPC1, which shuttles in and out of the nucleus, is also thought to aid in mRNA export by interacting with polyadenylated mRNA at an early stage in maturation [69], [70], [71], [72], [73]. The dramatic influx of PABPC1 into the nucleus during KSHV lytic infection might therefore perturb the process of mRNA nuclear export. Given the extreme abundance of PABPC1 and its higher affinity for poly(A) tails, nuclear PABPC1 (Kd ∼7 nM) could displace PABPN1 (Kd ∼555 nM) from polyadenylated mRNAs and interfere with PABPN1's role in export [50], [74]. As mRNAs that are not properly processed and exported from the nucleus become hyperadenylated and degraded [19], [75], [76], [77], the re-localization of PABPC1 to the nucleus could be the initiating step in their degradation during shutoff (Fig. 9A and B). Similar ideas are discussed in two recent studies, by Glaunsinger and colleagues, on the inhibition of nuclear PABPC1 on mRNA export [24], [37].
Fig. 9. Model for PAN RNA sequestering nuclear re-localized PABPC1. 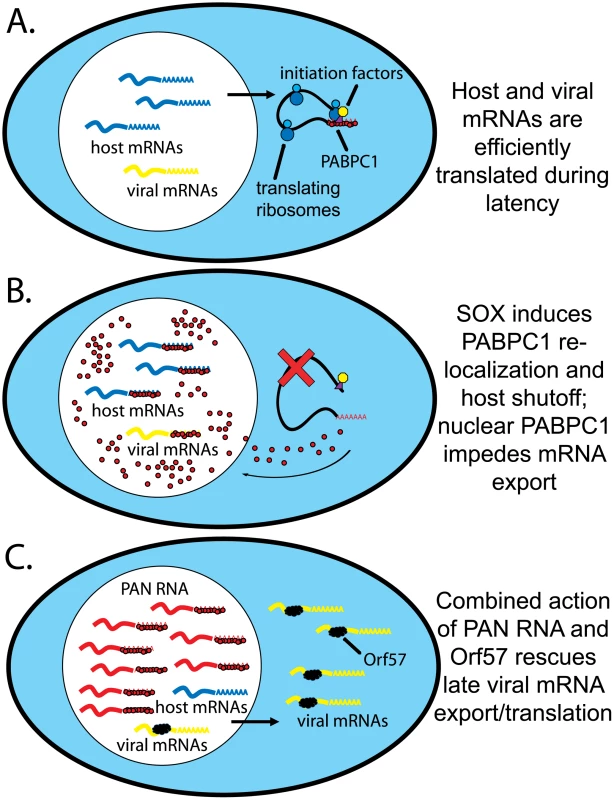
A. mRNA transcription, export and translation during latent infection. Host mRNAs (blue) are exported and bound by initiation factors and PABPC1. B. Viral infection in the absence of PAN RNA. SOX action mediates host shutoff and PABPC1 nuclear re-localization. Host and viral mRNAs are bound by PABPC1, leading to inefficient processing and/or export and resulting degradation. C. Viral infection in the presence of PAN RNA. As in B, PABPC1 is re-localized but bound by PAN RNA. Viral, but not host, mRNAs are bound by Orf57, which mediates their efficient export into the cytoplasm. If a highly abundant, inert, polyadenylated RNA were expressed in the nucleus, then the re-localized PABPC1 would be stoichiometrically bound, or nearly so. Thus, PAN RNA could serve as a “buffer” against changes in nuclear PABPC1 concentration (Fig. 9C). The fact that host-cell encoded abundant, nuclear polyadenylated RNAs, such as MALAT1 and NEAT1 RNA, do not accumulate to exceptionally high levels in response to PABPC1 re-localization (Fig. 3) suggests that there is something unique about PAN RNA. It should be noted that PABPN1 knockdown also diminishes SOX-mediated mRNA degradation, so it may be the balance of the two proteins on the poly(A) tail that is critical [19]. A comparison of intranuclear PABPC1 and PABPN1 immunofluorescence signals relative to PAN RNA in situ hybridization signal should be performed.
A challenge for the model (Fig. 9) is explaining how PAN RNA specifically protects viral mRNAs. Should not host mRNAs be equally protected from the negative effects of PABPC1 re-localization by the abundant expression of PAN RNA? Some possible explanations derive from fundamental differences between viral and host mRNAs in the nucleus. First, the viral Orf57 protein selectively binds intronless viral mRNAs, mediating their export by anchoring the human transcription and export complex (hTREX) [52]. Since most KSHV genes lack introns, Orf57 is not only important, but in fact essential for virus replication [53]. Second, transcription rates of viral mRNAs appear to greatly outpace those of host mRNAs during the lytic phase [23]. Thus, viral mRNAs may be preferentially exported and translated during the late lytic phase simply because they outcompete their host counterparts. PAN RNA would therefore function in concert with several other proteins (PABPN1 and PABPC1, SOX and Orf57) to create an environment that favors the export and expression of viral but not host mRNAs (Fig. 9C).
Supporting Information
Zdroje
1. VermaSCRobertsonES 2003 Molecular biology and pathogenesis of Kaposi sarcoma-associated herpesvirus. FEMS Microbiol Lett 222 155 163
2. GanemD 2006 KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu Rev Pathol 1 273 296
3. SunRLinSFGradovilleLMillerG 1996 Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A 93 11883 11888
4. ZhongWGanemD 1997 Characterization of ribonucleoprotein complexes containing an abundant polyadenylated nuclear RNA encoded by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). J Virol 71 1207 1212
5. ConradNKSteitzJA 2005 A Kaposi's sarcoma virus RNA element that increases the nuclear abundance of intronless transcripts. EMBO J 24 1831 1841
6. ConradNKMiliSMarshallELShuMDSteitzJA 2006 Identification of a rapid mammalian deadenylation-dependent decay pathway and its inhibition by a viral RNA element. Mol Cell 24 943 953
7. ConradNKShuMDUyhaziKESteitzJA 2007 Mutational analysis of a viral RNA element that counteracts rapid RNA decay by interaction with the polyadenylate tail. Proc Natl Acad Sci U S A 104 10412 10417
8. PawlickiJMSteitzJA 2008 Primary microRNA transcript retention at sites of transcription leads to enhanced microRNA production. J Cell Biol 182 61 76
9. Mitton-FryRMDeGregorioSJWangJSteitzTASteitzJA 2010 Poly(A) tail recognition by a viral RNA element through assembly of a triple helix. Science 330 1244 1247
10. BernsteinPPeltzSWRossJ 1989 The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol 9 659 670
11. FordLPBaggaPSWiluszJ 1997 The poly(A) tail inhibits the assembly of a 3′-to-5′ exonuclease in an in vitro RNA stability system. Mol Cell Biol 17 398 406
12. KornerCGWormingtonMMuckenthalerMSchneiderSDehlinE 1998 The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. EMBO J 17 5427 5437
13. WiluszCJGaoMJonesCLWiluszJPeltzSW 2001 Poly(A)-binding proteins regulate both mRNA deadenylation and decapping in yeast cytoplasmic extracts. RNA 7 1416 1424
14. MangusDAEvansMCJacobsonA 2003 Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol 4 223
15. TarunSZJrSachsAB 1996 Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J 15 7168 7177
16. TarunSZJrWellsSEDeardorffJASachsAB 1997 Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc Natl Acad Sci U S A 94 9046 9051
17. WellsSEHillnerPEValeRDSachsAB 1998 Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell 2 135 140
18. KannoTSatoYSataTKatanoH 2006 Expression of Kaposi's sarcoma-associated herpesvirus-encoded K10/10.1 protein in tissues and its interaction with poly(A)-binding protein. Virology 352 100 109
19. LeeYJGlaunsingerBA 2009 Aberrant herpesvirus-induced polyadenylation correlates with cellular messenger RNA destruction. PLoS Biol 7 e1000107
20. AriasCWalshDHarbellJWilsonACMohrI 2009 Activation of host translational control pathways by a viral developmental switch. PLoS Pathog 5 e1000334
21. GlaunsingerBGanemD 2004 Highly selective escape from KSHV-mediated host mRNA shutoff and its implications for viral pathogenesis. J Exp Med 200 391 398
22. GlaunsingerBGanemD 2004 Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol Cell 13 713 723
23. ChandrianiSGanemD 2007 Host transcript accumulation during lytic KSHV infection reveals several classes of host responses. PLoS ONE 2 e811
24. KumarGRGlaunsingerBA 2010 Nuclear import of cytoplasmic poly(A) binding protein restricts gene expression via hyperadenylation and nuclear retention of mRNA. Mol Cell Biol 30 4996 5008
25. BryanBADysonOFAkulaSM 2006 Identifying cellular genes crucial for the reactivation of Kaposi's sarcoma-associated herpesvirus latency. J Gen Virol 87 519 529
26. McAllisterSCHansenSGMessaoudiINikolich-ZugichJMosesAV 2005 Increased efficiency of phorbol ester-induced lytic reactivation of Kaposi's sarcoma-associated herpesvirus during S phase. J Virol 79 2626 2630
27. LernerEALernerMRJanewayCAJrSteitzJA 1981 Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A 78 2737 2741
28. IdeueTHinoKKitaoSYokoiTHiroseT 2009 Efficient oligonucleotide-mediated degradation of nuclear noncoding RNAs in mammalian cultured cells. RNA 15 1578 1587
29. SasakiYTIdeueTSanoMMituyamaTHiroseT 2009 MENepsilon/beta noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc Natl Acad Sci U S A 106 2525 2530
30. YooSMAhnAKSeoTHongHBChungMA 2008 Centrifugal enhancement of Kaposi's sarcoma-associated virus infection of human endothelial cells in vitro. J Virol Methods 154 160 166
31. SunRLinSFGradovilleLYuanYZhuF 1998 A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A 95 10866 10871
32. StaudtMRDittmerDP 2007 The Rta/Orf50 transactivator proteins of the gamma-herpesviridae. Curr Top Microbiol Immunol 312 71 100
33. LukacDMRenneRKirshnerJRGanemD 1998 Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252 304 312
34. ConradNK 2008 Chapter 15. Co-immunoprecipitation techniques for assessing RNA-protein interactions in vivo. Methods Enzymol 449 317 342
35. GlaunsingerBChavezLGanemD 2005 The exonuclease and host shutoff functions of the SOX protein of Kaposi's sarcoma-associated herpesvirus are genetically separable. J Virol 79 7396 7401
36. CovarrubiasSRichnerJMClydeKLeeYJGlaunsingerBA 2009 Host shutoff is a conserved phenotype of gammaherpesvirus infection and is orchestrated exclusively from the cytoplasm. J Virol 83 9554 9566
37. KumarGRShumLGlaunsingerBA 2011 Importin α-mediated nuclear import of cytoplasmic poly(A) binding protein occurs as a direct consequence of cytoplasmic mRNA depletion. Mol Cell Biol 31 3113 3125
38. WiluszJEFreierSMSpectorDL 2008 3′ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 135 919 932
39. SunwooHDingerMEWiluszJEAmaralPPMattickJS 2009 MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res 19 347 359
40. WangHWSharpTVKoumiAKoentgesGBoshoffC 2002 Characterization of an anti-apoptotic glycoprotein encoded by Kaposi's sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J 21 2602 2615
41. VieiraJO'HearnPM 2004 Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325 225 240
42. MyoungJGanemD 2011 Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: Maintenance of tight latency with efficient reactivation upon induction. J Virol Methods 174 12 21
43. KedesDHGanemD 1997 Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Invest 99 2082 2086
44. FauldsDHeelRC 1990 Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 39 597 638
45. HollandLEAndersonKPShipmanCJrWagnerEK 1980 Viral DNA synthesis is required for the efficient expression of specific herpes simplex virus type 1 mRNA species. Virology 101 10 24
46. PowellKLPurifoyDJCourtneyRJ 1975 The synthesis of herpes simplex virus proteins in the absence of virus DNA synthesis. Biochem Biophys Res Commun 66 262 271
47. JonesPCRoizmanB 1979 Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J Virol 31 299 314
48. ZhangYJBonaparteRSPatelDSteinDAIversenPL 2008 Blockade of viral interleukin-6 expression of Kaposi's sarcoma-associated herpesvirus. Mol Cancer Ther 7 712 720
49. GregorySMWestJADillonPJHilscherCDittmerDP 2009 Toll-like receptor signaling controls reactivation of KSHV from latency. Proc Natl Acad Sci U S A 106 11725 11730
50. GorlachMBurdCGDreyfussG 1994 The mRNA poly(A)-binding protein: localization, abundance, and RNA-binding specificity. Exp Cell Res 211 400 407
51. RoweMGlaunsingerBvan LeeuwenDZuoJSweetmanD 2007 Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc Natl Acad Sci U S A 104 3366 3371
52. BoyneJRJacksonBRWhitehouseA 2010 ORF57: Master regulator of KSHV mRNA biogenesis. Cell Cycle 9 2702 2703
53. BoyneJRColganKJWhitehouseA 2008 Recruitment of the complete hTREX complex is required for Kaposi's sarcoma-associated herpesvirus intronless mRNA nuclear export and virus replication. PLoS Pathog 4 e1000194
54. SahinBBPatelDConradNK 2010 Kaposi's sarcoma-associated herpesvirus ORF57 protein binds and protects a nuclear noncoding RNA from cellular RNA decay pathways. PLoS Pathog 6 e1000799
55. JoachimsMVan BreugelPCLloydRE 1999 Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J Virol 73 718 727
56. KerekatteVKeiperBDBadorffCCaiAKnowltonKU 1999 Cleavage of Poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: another mechanism for host protein synthesis shutoff? J Virol 73 709 717
57. Kuyumcu-MartinezMBelliotGSosnovtsevSVChangKOGreenKY 2004 Calicivirus 3C-like proteinase inhibits cellular translation by cleavage of poly(A)-binding protein. J Virol 78 8172 8182
58. PironMVendePCohenJPoncetD 1998 Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J 17 5811 5821
59. HarbMBeckerMMVitourDBaronCHVendeP 2008 Nuclear localization of cytoplasmic poly(A)-binding protein (PABP-C1) upon rotavirus infection involves interaction of NSP3 with eIF4G and RoXaN. J Virol
60. MonteroHRojasMAriasCFLopezS 2008 Rotavirus infection induces the phosphorylation of eIF2alpha but prevents the formation of stress granules. J Virol 82 1496 1504
61. BlakqoriGvan KnippenbergIElliottRM 2009 Bunyamwera orthobunyavirus S-segment untranslated regions mediate poly(A) tail-independent translation. J Virol 83 3637 3646
62. ChenZLiYKrugRM 1999 Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J 18 2273 2283
63. HuangYCarmichaelGG 1996 Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol Cell Biol 16 1534 1542
64. FortesPBelosoAOrtinJ 1994 Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J 13 704 712
65. AlexanderLDenekampLKnappAAuerbachMRDamaniaB 2000 The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J Virol 74 3388 3398
66. DittmerDPGonzalezCMVahrsonWDeWireSMHines-BoykinR 2005 Whole-genome transcription profiling of rhesus monkey rhadinovirus. J Virol 79 8637 8650
67. SchneiderRJWeinbergerCShenkT 1984 Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell 37 291 298
68. LuSCullenBR 2004 Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and MicroRNA biogenesis. J Virol 78 12868 12876
69. AfoninaEStauberRPavlakisGN 1998 The human poly(A)-binding protein 1 shuttles between the nucleus and the cytoplasm. J Biol Chem 273 13015 13021
70. BruneCMunchelSEFischerNPodtelejnikovAVWeisK 2005 Yeast poly(A)-binding protein Pab1 shuttles between the nucleus and the cytoplasm and functions in mRNA export. RNA 11 517 531
71. ChekanovaJABelostotskyDA 2003 Evidence that poly(A) binding protein has an evolutionarily conserved function in facilitating mRNA biogenesis and export. RNA 9 1476 1490
72. HosodaNLejeuneFMaquatLE 2006 Evidence that poly(A) binding protein C1 binds nuclear pre-mRNA poly(A) tails. Mol Cell Biol 26 3085 3097
73. ThakurtaAGHo YoonJDharR 2002 Schizosaccharomyces pombe spPABP, a homologue of Saccharomyces cerevisiae Pab1p, is a non-essential, shuttling protein that facilitates mRNA export. Yeast 19 803 810
74. MeyerSUrbankeCWahleE 2002 Equilibrium studies on the association of the nuclear poly(A) binding protein with poly(A) of different lengths. Biochemistry 41 6082 6089
75. HillerenPParkerR 2001 Defects in the mRNA export factors Rat7p, Gle1p, Mex67p, and Rat8p cause hyperadenylation during 3′-end formation of nascent transcripts. RNA 7 753 764
76. JensenTHPatricioKMcCarthyTRosbashM 2001 A block to mRNA nuclear export in S. cerevisiae leads to hyperadenylation of transcripts that accumulate at the site of transcription. Mol Cell 7 887 898
77. WyersFRougemailleMBadisGRousselleJCDufourME 2005 Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121 725 737
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



