-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNovel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
Riboswitches are regulatory elements modulating gene expression in response to specific metabolite binding. It has been recently reported that riboswitch agonists may exhibit antimicrobial properties by binding to the riboswitch domain. Guanine riboswitches are involved in the regulation of transport and biosynthesis of purine metabolites, which are critical for the nucleotides cellular pool. Upon guanine binding, these riboswitches stabilize a 5′-untranslated mRNA structure that causes transcription attenuation of the downstream open reading frame. In principle, any agonistic compound targeting a guanine riboswitch could cause gene repression even when the cell is starved for guanine. Antibiotics binding to riboswitches provide novel antimicrobial compounds that can be rationally designed from riboswitch crystal structures. Using this, we have identified a pyrimidine compound (PC1) binding guanine riboswitches that shows bactericidal activity against a subgroup of bacterial species including well-known nosocomial pathogens. This selective bacterial killing is only achieved when guaA, a gene coding for a GMP synthetase, is under the control of the riboswitch. Among the bacterial strains tested, several clinical strains exhibiting multiple drug resistance were inhibited suggesting that PC1 targets a different metabolic pathway. As a proof of principle, we have used a mouse model to show a direct correlation between the administration of PC1 and the reduction of Staphylococcus aureus infection in mammary glands. This work establishes the possibility of using existing structural knowledge to design novel guanine riboswitch-targeting antibiotics as powerful and selective antimicrobial compounds. Particularly, the finding of this new guanine riboswitch target is crucial as community-acquired bacterial infections have recently started to emerge.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000865
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000865Summary
Riboswitches are regulatory elements modulating gene expression in response to specific metabolite binding. It has been recently reported that riboswitch agonists may exhibit antimicrobial properties by binding to the riboswitch domain. Guanine riboswitches are involved in the regulation of transport and biosynthesis of purine metabolites, which are critical for the nucleotides cellular pool. Upon guanine binding, these riboswitches stabilize a 5′-untranslated mRNA structure that causes transcription attenuation of the downstream open reading frame. In principle, any agonistic compound targeting a guanine riboswitch could cause gene repression even when the cell is starved for guanine. Antibiotics binding to riboswitches provide novel antimicrobial compounds that can be rationally designed from riboswitch crystal structures. Using this, we have identified a pyrimidine compound (PC1) binding guanine riboswitches that shows bactericidal activity against a subgroup of bacterial species including well-known nosocomial pathogens. This selective bacterial killing is only achieved when guaA, a gene coding for a GMP synthetase, is under the control of the riboswitch. Among the bacterial strains tested, several clinical strains exhibiting multiple drug resistance were inhibited suggesting that PC1 targets a different metabolic pathway. As a proof of principle, we have used a mouse model to show a direct correlation between the administration of PC1 and the reduction of Staphylococcus aureus infection in mammary glands. This work establishes the possibility of using existing structural knowledge to design novel guanine riboswitch-targeting antibiotics as powerful and selective antimicrobial compounds. Particularly, the finding of this new guanine riboswitch target is crucial as community-acquired bacterial infections have recently started to emerge.
Introduction
Multiple drug resistance (MDR) has been a growing problem during the last decade, partly due to excessive use of antibiotics in human medicine and food animal production. MDR also stems from the fact that drug design has been largely based on limited chemical scaffolds leaving an opportunity for pathogens to circumvent antibiotic action mechanisms [1]. Staphylococcus aureus and Clostridium difficile are nosocomial pathogens responsible for a significant mortality rate in hospitals and increased health care costs [2]. Recently, community-acquired methicillin-resistant S. aureus (MRSA) infections have emerged and are commonly responsible for skin and soft-tissue infections that may rapidly evolve in severe and life-threatening infections [3], [4]. Moreover, some emerging clones were shown to be resistant to vancomycin, which is considered as the last chance antibiotic [5]. The pathogen C. difficile has also dramatically increased the hospital-associated deaths in recent years due to the MDR emergence and spreading of the hypervirulent and high toxin-producing strain BI/NAP1/027 [4], [5], [6]. This particular strain is spreading in North America and Europe with currently little therapeutic solutions besides the use of metronidazole and vancomycin, which are increasingly associated with relapses and poor treatment outcome [7].
Previous attempts to discover alternative antibacterial drugs targeting RNA were mainly based on a fortuitous interaction between an exogenous ligand and its RNA target [1], [8], [9], [10]. Metabolite-responsive riboswitches represent a novel solution to MDR since they could be considered as antimicrobial targets when agonistic ligands are employed as demonstrated for lysine, thiamine pyrophosphate (TPP), flavin mononucleotide (FMN) and guanine responsive riboswitches [1], [11], [12], [13], [14], [15]. In the case of lysine and TPP riboswitches, previously described ligand analogs were reported to have a multitude of cellular effects in addition to inhibition of gene expression via riboswitch binding [16], [17], [18], [19]. Pleiotropic effects were also observed for compounds targeting the guanine riboswitch and at least one analog was reported to be possibly incorporated in DNA during replication [15], [20]. Thus, while it is of interest to select antibiotics that are chemically distinct from natural ligands to avoid cellular efflux or chemical modification, it is important to consider that these chemical differences will potentially help avoid patient toxicity due to off-target binding. It is also important that the antibiotic provokes a bacteriostatic or bactericidal effect either by targeting a single gene, or a collection of genes, that is necessary for growth, or essential for bacterial survival or virulence. Thus, because modified pyrimidines can specifically bind the purine riboswitch with affinities in the low nanomolar range [21], they make excellent candidates to target purine riboswitches which are likely potent drug targets given their role in regulating purine metabolic pathways (Figure S1). For instance, the inactivation of the E. coli GMP synthetase guaA leads to guanine auxotrophy [22] whereas the inactivation of the B. subtilis IMP dehydrogenase guaB is lethal [23]. Here we show that the guanine riboswitch in S. aureus and C. difficile controls the expression of guaA and that this gene appears essential for virulence in a murine model.
Results
Pyrimidine-based antibiotics modulate the guanine riboswitch activity
Guanine-sensing riboswitches are members of the purine riboswitch class, which also comprises adenine and 2′-deoxyguanosine [24]. The guanine riboswitch negatively regulates transcription elongation at high guanine concentration in Bacillus subtilis [25] (Figure 1A). The guanine aptamer is organized around a three-way junction connecting three helices, in which a critically important nucleotide is involved in a Watson-Crick base pair interaction with the bound ligand [25] (Figure 1B). The ligand binding site contains a cavity in which the metabolite is completely surrounded by RNA contacts suggesting that most atomic positions are important for the formation of the native ligand-RNA complex [26], [27]. By using appropriate aminopyrimidines, it is also possible to recreate the correct network of hydrogen bonds required to ensure proper complex formation as previously shown for the adenine riboswitch [21]. Thus, by taking advantage of the fact that purine riboswitches efficiently bind pyrimidines, it may be possible to design novel antibiotics that bind to guanine riboswitches and therefore inhibit bacterial growth.
Fig. 1. The structure of the guanine riboswitch. 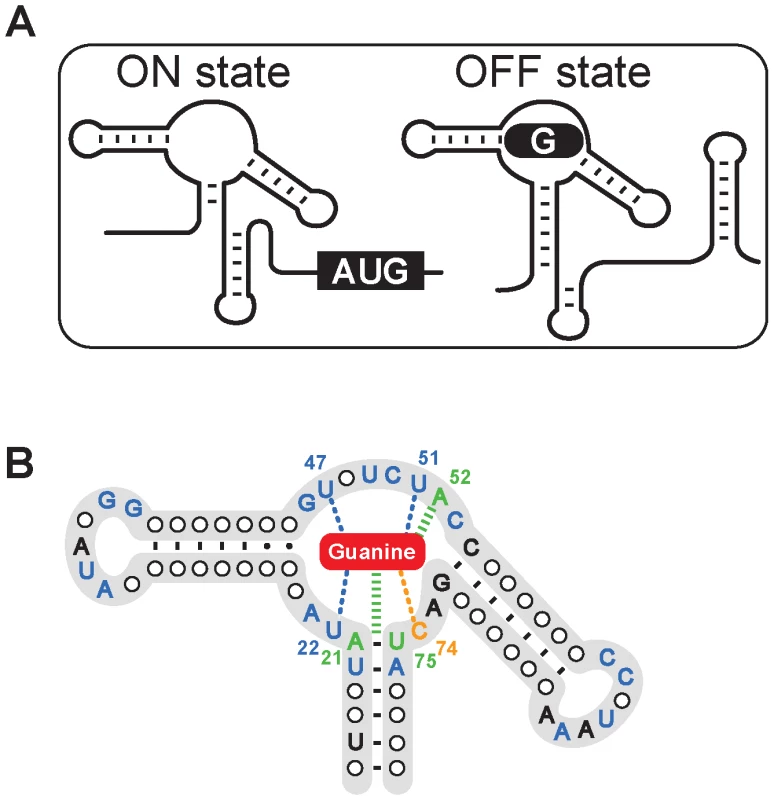
(A) Scheme representing the guanine riboswitch secondary structures in absence (ON state) or in presence (OFF state) of guanine. The formation of a guanine-riboswitch complex results in the adoption of an intrinsic terminator element that prematurely stops transcription. (B) Consensus sequence and secondary structure of the guanine riboswitch aptamer. Nucleotides indicated in blue, orange and green represent nucleotides that are conserved >90% and those colored in black are conserved >80% [28]. Nucleotides and lines in blue and in green indicate interactions with the ligand via hydrogen bonding and base stacking, respectively. The cytosine 74 which confers ligand binding specificity is shown in orange and the bound guanine is shown as a red rounded rectangle. Pyrimidine-based molecules that could fit into the guanine riboswitch aptamer binding site were selected based on molecular modeling of crystal structures [26], [27] (Figure 2A). Using this approach, we identified two pyrimidine compounds 2,5,6-triaminopyrimidin-4-one (PC1) and 2,6-diaminopyrimidin-4-one (PC2) that satisfied defined criteria such as geometrical constraint, hydrogen bonding pattern and molecule planarity (Figure 2B). As opposed to guanine, PC1 and PC2 lack one aromatic ring, making them electronically distinct from guanine despite their similarity to guanine in terms of H-bond donating and accepting potential. Next, using the established in-line probing assay [25], [28], we monitored PC1/PC2-induced riboswitch conformational changes (Figure 2C). In absence of ligand, several cleavage products that map to previously reported single-stranded regions were observed [25], [28]. However, a cleavage reduction consistent with a reorganization of the structure upon ligand binding was observed in the core domain in presence of guanine (Figure 2C). In-line probing assay with PC1 and PC2 instead of guanine showed an identical cleavage pattern for both pyrimidine compounds and guanine, suggesting that the core is reorganized similarly in presence of these compounds, consistent with the recently reported pyrimidine-bound riboswitch crystal structure [21].
Fig. 2. Guanine riboswitch agonists can be used to modulate gene expression. 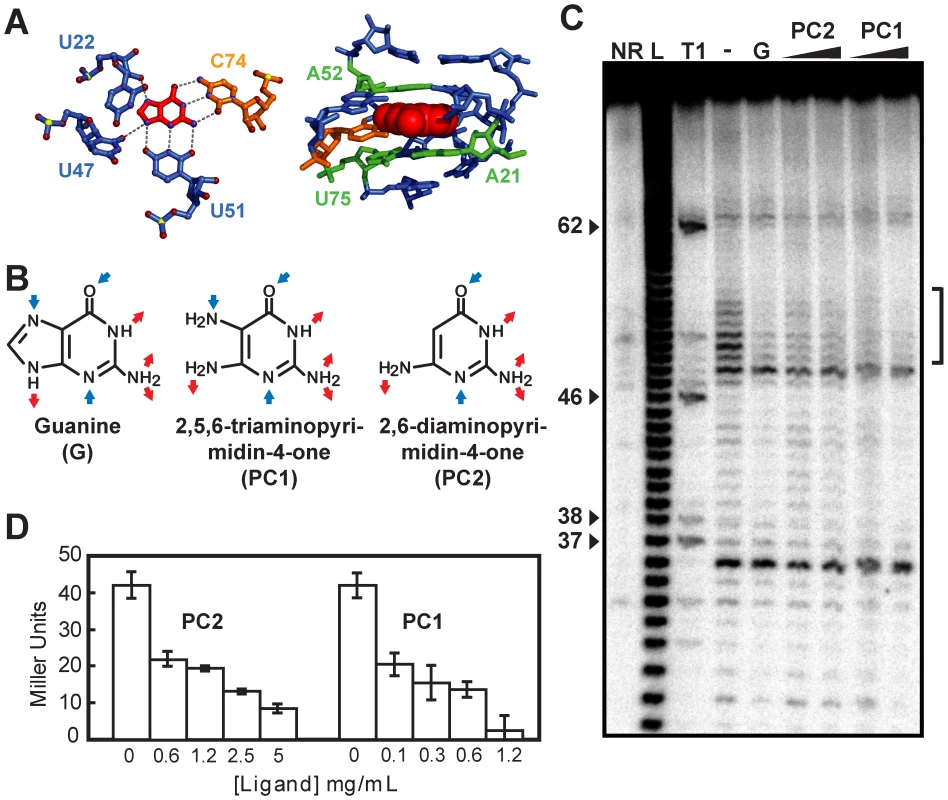
(A) Hydrogen bonds (left panel) and stacking interactions (right panel) formed between the bound guanine and the guanine riboswitch [26], [27]. Oxygen, nitrogen, and phosphorus atoms are in red, blue, and yellow, respectively (left panel). Nucleotides follow the color scheme used in Figure 1B. Figures were prepared using PyMol (DeLano Scientific, San Francisco, CA, USA). (B) Molecular recognition features for guanine (G) and predicted ones for PC1 and PC2. Blue and red arrows represent hydrogen bond acceptors and donors, respectively. (C) In-line probing assays of the B. subtilis xpt riboswitch in the absence (−) or in the presence (+) of 1 µM guanine (G), and 1 µM or 10 µM for both PC1 and PC2. Sites of substantial ligand-induced protections (positions 49–54) are assigned on the right by a vertical bracket. Lanes NR, L and T1 correspond to molecules that were not reacted or that were partially digested by alkali or by RNase T1, respectively. Guanines are identified on the left as molecular weight markers. (D) The beta-galactosidase activity of a xpt-lacZ transcriptional fusion construct integrated in the genome of B. subtilis by recombination was assayed after 4 h of growth at 37°C in minimal medium in presence of the indicated ligand concentrations. Each experiment was performed three times and the average as well as standard deviations are shown. To determine whether PC1 and PC2 repress gene expression, we transformed B. subtilis with transcriptional fusions in which a guanine riboswitch was fused to a lacZ reporter gene (Figure 2D). When cells were grown in minimal medium with increasing concentrations of PC1 and PC2, beta-galactosidase activity was clearly repressed in a dose-dependent manner suggesting a modulation of the guanine riboswitch gene regulation by both molecules. We also performed growth inhibition experiments using various concentrations of both PC1 and PC2 (Figure S2). While growth inhibition was observed in minimal medium, no such inhibition was observed using a richer medium such as cation-adjusted Muller-Hinton broth (CAMHB). This selective growth inhibition can be explained by PC1/PC2 inhibiting the biosynthesis or transport of essential metabolites, which are present in CAMHB but not in minimal medium. For instance, it was recently shown that guanine-related compounds can only inhibit B. subtilis growth in a minimal medium but not in Luria broth; the growth inhibition was partly attributed to the riboswitch-mediated repression of de novo purine synthesis [15].
PC1-dependent bacterial growth inhibition requires guaA to be riboswitch-regulated
The S. aureus ATCC 29213 genome contains a unique guanine riboswitch located immediately upstream of the xpt gene (Figure S3). Very interestingly, RT-PCR experiments identified that the riboswitch controls a four-gene operon consisting of xpt, pbuX, guaB and guaA, thus placing guaA and guaB under the control of a riboswitch in S. aureus (Figure S3). To determine if PC1 and PC2 have antibiotic activities by targeting the guanine riboswitch in S. aureus, we performed antibiograms with PC1 and PC2 as well as with three additional molecules having similar structures (compounds 3, 4 and 5). While compounds 3 and 5 are structurally very close to PC1 and PC2, compound 4 is a guanine analog (Figure 3A). Surprisingly, among the five compounds tested, only PC1 inhibited bacterial growth in Muller-Hinton agar, which is consistent with its ability to modulate riboswitch gene expression in B. subtilis (Figure 2D). The absence of PC2 antibiotic activity is consistent with the ∼5-fold lower PC2-mediated gene expression modulation in B. subtilis, which may result from the lower number of riboswitch-ligand interactions (Figure 2B). The binding affinity of PC1 suggests that the guanine riboswitch can tolerate modifications on the ligand pyrimidine ring that are not strongly deleterious for complex formation (∼100 nM vs ∼5 nM for PC1 and guanine, respectively). The binding affinity of PC1 is very similar to that of hypoxanthine, which is a naturally occurring guanine analog [25].
Fig. 3. S. aureus growth inhibition requires guaA to be under a riboswitch control. 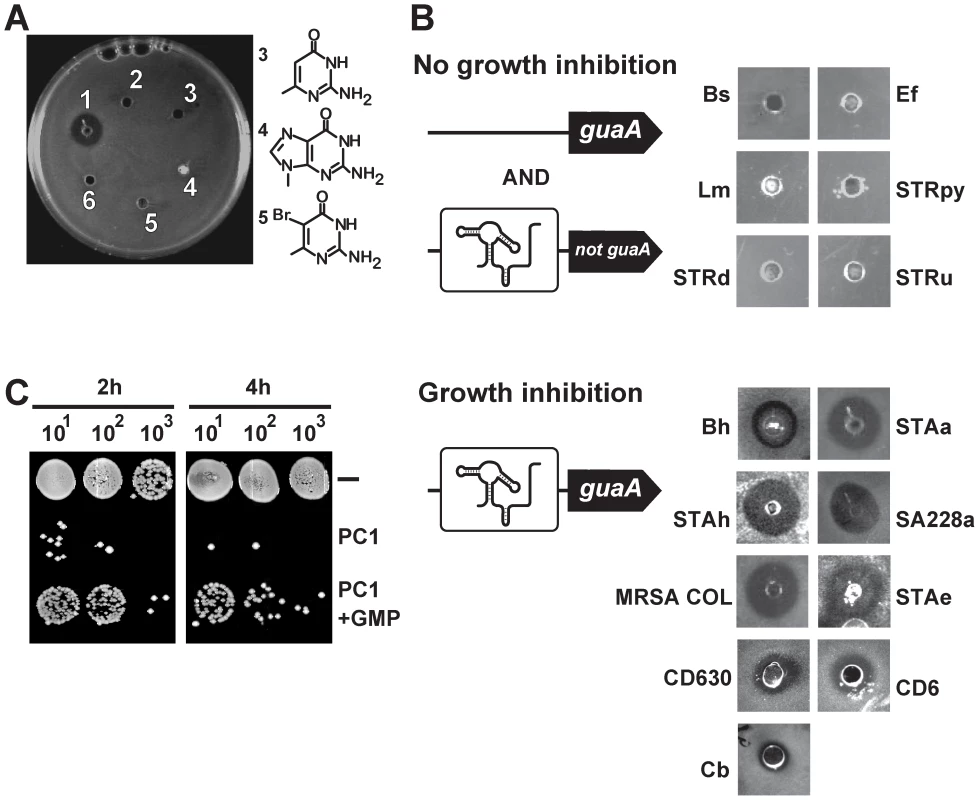
(A) Antibiograms performed on the S. aureus ATCC 29213 strain using 75 µg of PC1 (1), PC2 (2), 2-amino-4-hydroxy-6-methylpyrimidine (3), 9-methyl guanine (4), 5-bromo-6-methyl pyrimidine (5) and PBS as control (6). Chemical structures of PC1 and PC2 are shown in Figure 2B. (B) PC1 antibiograms using bacterial strains with guanine riboswitches. While PC1-insensitive strains do not have guaA under a riboswitch control (Bs: Bacillus subtilis, Ef: Enterococcus feacium, Lm: Listeria monocytogenes, STRpy: Streptococcus pyogenes, STRd: Streptococcus dysgalactiae and STRu: Streptococcus uberis), PC1-sensitive strains control the guaA gene expression via a riboswitch mechanism (Bh: Bacillus halodurans, STAa: S. aureus ATCC 29213, STAh: S. haemolyticus, SA228a: S. aureus resistant to beta-lactam, erythromycin, ciprofloxacin, gentamicin and tetracycline but susceptible to vancomycin, MRSA COL: methicilin resistant S. aureus COL, STAe: S. epidermidis, Cb: Clostridium botulinum, CD630: C. difficile strain 630, CD6: C. difficile representing the hypervirulent NAP1/027 strain). (C) Influence of GMP on PC1 bacterial growth inhibition. Spots from serial dilutions of S. aureus cultures in cation-adjusted Muller-Hinton broth (CAMHB) in absence or presence of 600 µg/mL PC1 or 600 µg/mL PC1 supplemented with 100 µM GMP. To explore the antibacterial activity spectrum of PC1, we used several Gram-positive bacterial species which are potential human pathogens containing guanine riboswitches. Of the 15 species tested, 9 showed marked cellular growth inhibition, including MDR strains and the C. difficile CD6 isolate representing the hypervirulent NAP1/027 strain (Figure 3B). Interestingly, when analyzing guanine riboswitch-regulated genes, we observed that all PC1-responsive strains had guaA under riboswitch control whereas the PC1-unresponsive ones did not employ riboswitch regulation to control guaA. The best example of this correlation is that while 16S rDNA sequence analysis indicates that B. subtilis and Bacillus halodurans are very closely related species [29], B. halodurans has a guaA-controlled riboswitch and is sensitive to PC1 whereas B. subtilis lacks a guaA-controlled riboswitch and is resistant to PC1. Antibiogram results also showed that strains exhibiting pronounced MDR phenotypes are sensitive to PC1 suggesting that the antimicrobial activity does not involve action mechanisms common to other known antibiotics.
Because our data suggest that PC1 acts by repressing the GMP synthetase guaA, we reasoned that the PC1 inhibitory activity should be reduced by GMP supplementation. S. aureus cells were thus grown with or without supplemented GMP, and colony forming units (CFU) were determined following serial microdilutions (Figure 3C). As predicted, bacterial growth inhibition was relieved when GMP was provided to cells grown in presence of PC1 for 2 h or 4 h, supporting the hypothesis that bacterial growth inhibition is caused by the riboswitch-mediated guaA gene repression that results in GMP cellular depletion.
The PC1 specificity was also confirmed using the Gram-negative bacterium Escherichia coli ATCC 35695, a strain that does not contain guanine riboswitches. As expected, E. coli showed no growth inhibition in presence of PC1 even when using strains deficient for the AcrAB efflux system or having increased membrane permeability (Figure S4). These results suggest that the inability of PC1 to inhibit E. coli most probably results from guaA not being under the control of a guanine riboswitch in E. coli.
Bactericidal activity and specificity of PC1
To further characterize the riboswitch inhibitory action mechanism of PC1, S. aureus cells were grown in CAMHB in presence of various ligand concentrations. We obtained a PC1 dose-dependent growth inhibition response characterized by a MIC of 0.625 mg/mL (Figure 4A). PC2 was also used and its antibiotic activity was found to be less efficient than PC1, as observed in B. subtilis (Figure S2). When compared to known antibiotics, PC1 was found to have an extremely rapid bactericidal activity similar to ciprofloxacin, one of the most bactericidal antibiotics (Figure 4B). For instance, a 4 h treatment with PC1 led to 6.67±0.58 and 5.42±1.02 log reductions in CFU/mL compared to the untreated control for cultures of S. aureus ATCC 29213 and C. difficile CD6, respectively. When the same experiment was repeated by adding either GMP or AMP to the culture for 8 hours, bacterial growth was restored by a factor of 103 only in presence of GMP (Figure 4C), suggesting that PC1 growth inhibition activity is specific to guanine metabolism.
Fig. 4. PC1 shows bactericidal activity through cellular GMP depletion. 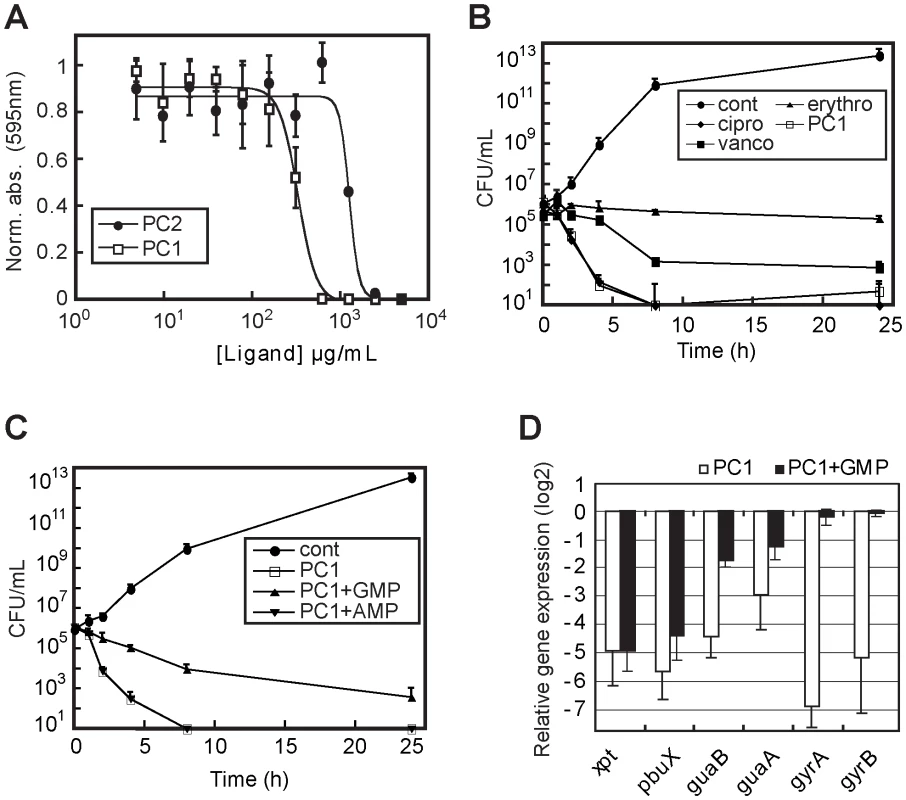
(A) Minimal inhibitory concentrations (MIC) determination of PC1 and PC2 on S. aureus strain ATCC 29213 in CAMHB. MIC values of 600 µg/mL and 5000 µg/mL were obtained for PC1 and PC2, respectively. (B) Bactericidal activity of PC1 and other known antibiotics against S. aureus as a function of time. For determination of the bactericidal effect of PC1, bacteria were inoculated at 105 CFU/mL in absence (cont) or presence of 0.5 µg/mL ciprofloxacin (cipro), 0.5 µg/mL erythromycin (erythro), 1 µg/mL vancomycin (vanco) and 600 µg/mL PC1. The concentration of each antibiotic corresponds to their MIC. (C) Bactericidal activity of PC1 against S. aureus as a function of time in absence (cont) or presence of 600 µg/mL PC1, and in presence of PC1 with 100 µM GMP or AMP. (D) Relative expression of S. aureus genes under the control of a guanine riboswitch when grown in presence of PC1 or PC1 with GMP. Results obtained in presence of PC1 are normalized using xpt gene expression. Bacteria were inoculated at 108 CFU/mL in CAMHB in absence or presence of 600 µg/mL PC1 with or without 100 µM GMP. Each experiment was performed three times and the average as well as standard deviations are shown. To analyze the PC1-mediated riboswitch inhibition on S. aureus gene expression, we performed a transcriptomic microarray analysis containing a selection of S. aureus genes involved in different cellular processes such as virulence, secretion, general stress responses, sensory/regulatory systems, antibiotic resistance, iron transport and general biosynthesis [30] (Figure 4D). Among the 468 genes analyzed, 72% were repressed by at least two folds when S. aureus was treated with PC1 where the 16S rRNA gene was the most repressed (Table S1). This result is consistent with a riboswitch-mediated guaA gene expression inhibition leading to GMP cellular depletion and RNA synthesis inhibition. This is supported by the low expression of the guanine riboswitch operon (xpt, pbuX, guaB and guaA) as well as the two DNA gyrase subunits (gyrA and gyrB), which were used as housekeeping gene controls (Figure 4D). Of all the monitored genes in the microarray analysis, only ahpF and ahpC, two genes involved in stress response mechanisms, were activated by the PC1 treatment. However, when S. aureus was treated with PC1 and GMP, the microarray data showed an expression profile in which only 21% of the genes surveyed were repressed. Whereas the housekeeping gyrase genes were no longer repressed, the expression of the guanine riboswitch operon was still reduced, consistent with PC1 binding the riboswitch operon and inhibiting gene expression. The other repressed genes mainly comprised those involved in virulence and cell wall synthesis suggesting that the GMP supplemented cells were still under stress [31], which is in agreement with the partial growth rescue observed in Figure 4C. GMP is able to rescue PC1-treated cells in a dose-dependent manner (data not shown) but its low solubility prevents full recovery at higher doses. It is also probable that GMP-related feedback inhibitory mechanisms were responsible for some of the gene repression observed (as in the case of the guanylate kinase gmk). Taken together, these results are consistent with PC1 mainly acting through a riboswitch inhibition mechanism that ultimately results in GMP cellular deprivation and S. aureus growth inhibition.
PC1 inhibits S. aureus growth in a murine model
Because our data showed that the growth repression activity of PC1 is influenced by the presence of GMP, we decided to assess the bactericidal activity of PC1 in a murine mastitis model of S. aureus infection, which adequately represents the clinical context. Indeed, in addition to morbid nosocomial infections caused by S. aureus, this bacterium is one of the major pathogen leading to bovine mastitis, which is the most frequent and costly disease for dairy producers with current antibiotic therapies usually failing to eliminate infections from dairy herds [32]. The antimicrobial activity of PC1 was therefore first tested on several S. aureus isolates from mastitic cows, some of which having persisting chronic infections (Figure 5A). A bactericidal effect of at least 4 orders of magnitude was observed after a 4 h treatment with PC1. Next, to ascertain that guaA was expressed in vivo and that this gene may be important during infection, we monitored the expression level of guaA by real-time PCR. When strain 1290 was grown either in broth culture in vitro or when it was directly isolated from the mastitic milk of infected cows (M. Allard and F. Malouin, in preparation), very similar expression levels were found for guaA and the essential gene gyrB in both environments. This suggests that PC1 could have an impact on guaA expression in vivo and thus be used to treat S. aureus infections.
Fig. 5. PC1 inhibits S. aureus clinical isolates in vitro and in vivo. 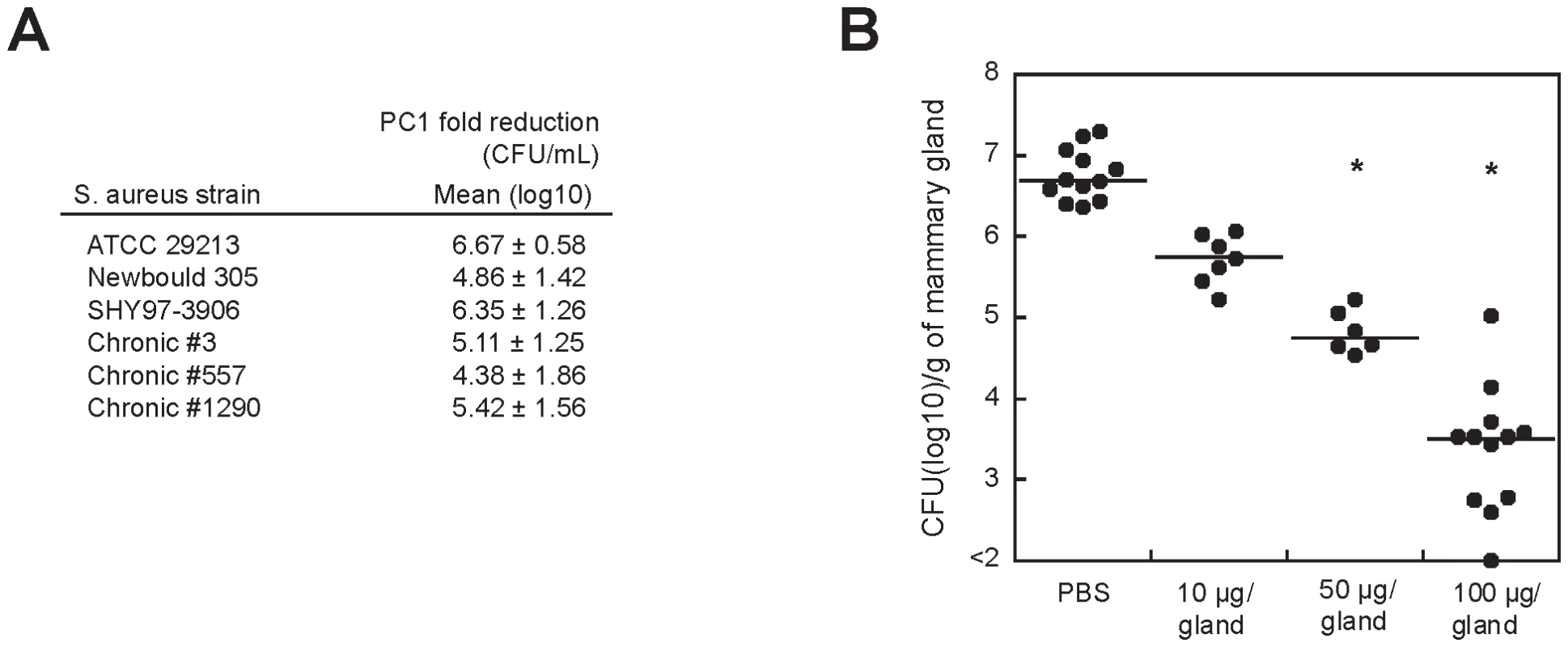
(A) Fold reduction in viable counts (log10 CFU/mL) for the reference S. aureus strain ATCC 29213 and for selected bovine isolates after a 4 h exposure to PC1 as compared to the untreated culture. Newbould 305 (ATCC 29740) and SHY97-3906 are bovine isolates from typical mastitis cases and isolates 3, 557 and 1290 were from cows with persisting intra-mammary infections and chronic mastitis. (B) Bacterial counts (CFU) obtained from mice mammary glands 10 h post-infection with S. aureus. Mice mammary glands were treated (intra-mammary administration) 4 h after infection with PBS with or without PC1 at 10, 50 or 100 µg/gland. Each dot represents the CFU of each individual gland (n = 6–12) and the median value for each group is indicated by the bar. Statistical differences (P<0.05) between CFU recovered from treated and untreated animals are shown by asterisks (non-parametric Kruskal-Wallis ANOVA with Dunn's post test). The proof of concept for the therapeutic efficacy of PC1 was established in our murine model of S. aureus-induced mastitis [33]. At 4 h post-infection, different concentrations of PC1 were administered to infected mice that were sacrificed 6 h later (Figure 5B). When compared to mice that were not treated with PC1, viable bacterial counts in the mammary gland were drastically reduced in a dose-dependent manner. This strong therapeutic effect was highly comparable to what we observed with known antibiotics. For example, amoxicillin decreased the bacterial load in the mammary gland to a log10 median value of 3.97 CFU/g of gland at a dose of 50 µg/gland. Noteworthy, a dose of 50 µg of amoxicillin would represent 100xMIC/g of gland, whereas a similar dose would only represent a twelfth of the MIC/g of gland for PC1. This result is consistent with the idea that PC1 is most efficient in the mammary gland environment suggesting that the microaerobic condition (i.e., low oxidative environment) of the mastitic milk[34] helps PC1 therapeutic efficacy. Consistent with these results, we found that the potency of PC1 was significantly increased by preventing its oxidation using a reductive agent such as DTT in susceptibility tests in vitro (Figure S5).
Discussion
Despite previous large scale screen data suggesting that guaA is not essential for S. aureus growth [23], [35] in relatively rich media, we show here that blocking guaA expression can lead to bactericidal activity in various bacterial species. In support of guaA for cell viability, it has been recently reported that mutations occurring in guaA prevent Streptococcus suis [36] and Salmonella thyphimurium [37] from properly infecting porcine and murine models, respectively, suggesting that GMP bioavailability may be reduced during host infection and that guaA is likely to be crucial for bacterial infection in mammals. Thus, together with studies showing the importance of guaA for bacterial growth in urine or blood [38], [39], our data suggest that mammalian infection sites may significantly differ in their nutrient compositions from those used in large scale screens [23], [35], and that care should be taken when assessing the “essentiality” of a gene. Furthermore, when assessing whether S. aureus could develop resistance to PC1, no resistant bacteria were obtained after more than 30 passages suggesting that maintaining a functional guaA-regulated riboswitch is a vital process (Figure S6). Taken together, the demonstration that guaA expression is normally maintained in S. aureus grown in vivo and the strong therapeutic effect resulting from PC1 treatment indicate that guaA is an important contributor to the survival of S. aureus during infection and that it can be used as an antibiotic target.
The major limitation to validate an antibiotic that targets riboswitches is to evaluate the antibiotic specificity of action. In this particular case, PC1 is not a broad-spectrum antibacterial drug given that it does not target all bacteria containing guanine riboswitches, but only those in which guaA is under the control of a riboswitch. It is not excluded that other riboswitch-controlled genes may participate in the PC1-dependent bacterial growth inhibition (e.g., guaB), or that PC1 may bind other cellular targets, which alone or in combination with the riboswitch-controlled gene repression, would repress bacterial growth. Nevertheless, the restricted nature of growth inhibition likely indicates that PC1 inhibits bacterial growth through riboswitch binding and not via an alternative mechanism such as DNA incorporation (Figure 6). For instance, when performing antibiograms using the guanine analog 6-thioguanine, a general growth inhibition was observed in E. coli and S. aureus (Figure S7), consistent with its incorporation into DNA that perturbs the epigenetic pathway of gene regulation [40]. The selective antibacterial activity of PC1 toward S. aureus was also supported by the lack of apparent toxicity for mice treated with the experimental compound at concentrations as high as 100 µg/gland with no sign of discomfort including vocalizations, curved back, piloerection and hypothermia. There was also no apparent cytotoxicity upon histological observations of mammary tissues in PC1-treated mice compared to PBS-treated glands (Figure S8).
Fig. 6. Scheme representing the action mechanism of PC1 on S. aureus guanine pathway. 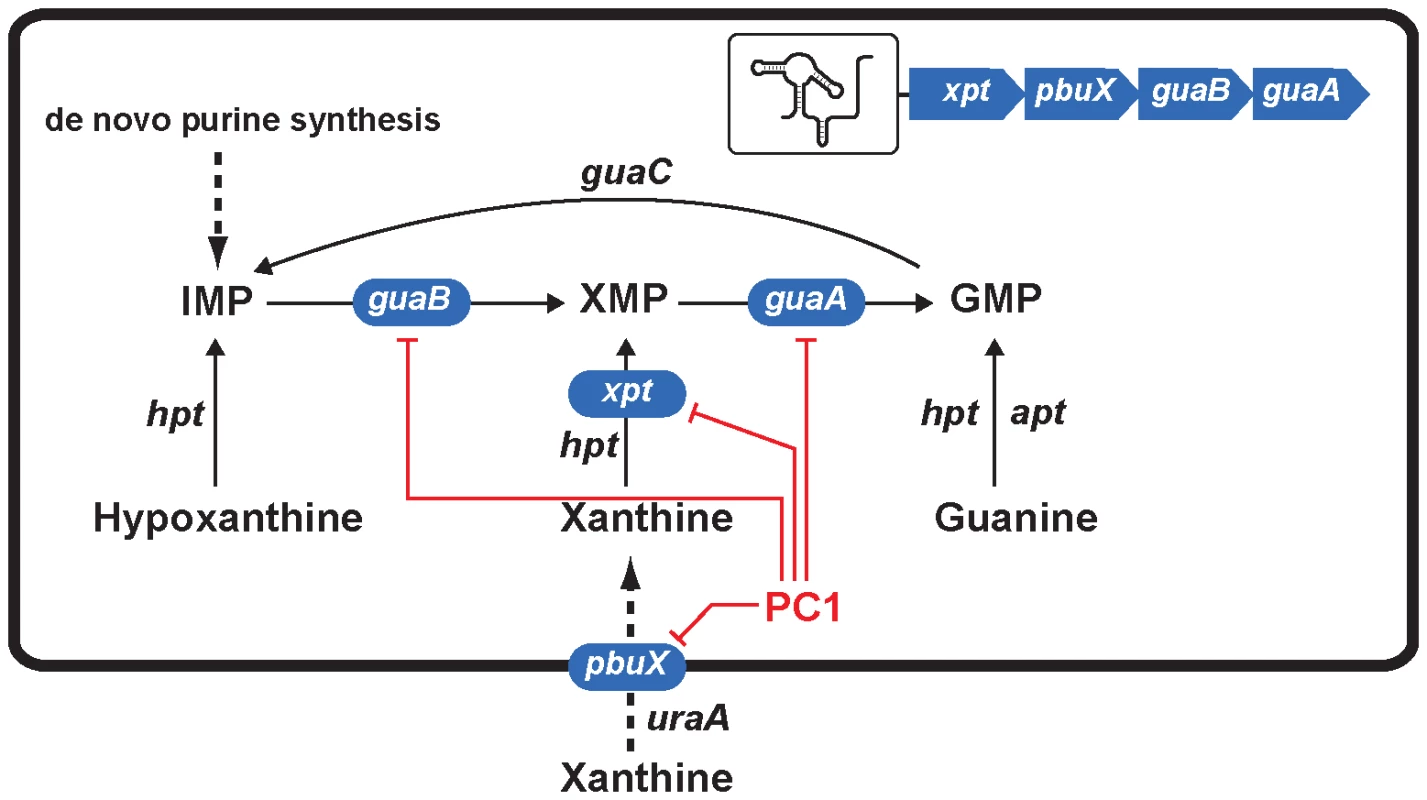
Genes highlighted in blue correspond to those of the operon xpt-pbuX-guaB-guaA that is controlled by the guanine riboswitch. Red lines indicate genes that are inhibited by PC1 via its binding to the guanine riboswitch. Of these four inhibited genes, guaA and guaB are known to be critical for guanine nucleotide biosynthesis [22], [23], [36], [37], [38], [39] and their inhibition very likely lead to the repression of GMP synthesis and most probably of RNA and DNA production. Recently, the Breaker group used purine derivatives modified in position 2 or 4 to target guanine riboswitches and found three molecules that inhibited bacterial growth [15]. Among these molecules, only one was able to repress the expression of a guanine riboswitch-controlled reporter gene suggesting that at least two molecules inhibited cell growth through a different mechanism of action. It is possible that the mode of action of these two molecules involves nucleic acids incorporation following ribosylation at position 9 of the purine analog, as demonstrated for 6-thioguanine. It is also interesting to mention that the antibiotic activity was only observed using B. subtilis strains cultivated in minimal medium whereas no growth inhibition was detected in rich media. In our study, we selected guanine riboswitch ligands that cannot be ribosylated to prevent alternative modes of action, and favored a pyrimidine compound (PC1) that retained most of the key functional groups. By testing various bacterial species, we observed that the bactericidal activity of PC1 was only seen against bacteria using a guanine riboswitch to control guaA expression, and this even when bacteria were grown in a rich medium. This demonstrates that analog binding to riboswitch aptamers is not the only determinant to achieve selective and efficient bactericidal effects.
This study shows for the first time that antibiotics targeting riboswitches may be efficient to kill bacterial pathogens in vitro as well as in mammalian infection models. We found here that the selective bacterial killing of PC1 is only achieved when guaA, a GMP synthetase, is under the control of the riboswitch and when the antimicrobial agent cannot be ribosylated. The narrow spectrum of activity we demonstrated here for PC1 is very interesting since two of the target bacteria, S. aureus and C. difficile, are among the most problematic nosocomial pathogens. The spread of MDR in those bacterial species also stresses the importance to develop new antibiotics that avoid current mechanisms of resistance. The use of narrow spectrum drugs should be encouraged whenever possible to reduce any selective pressure for resistance in non-targeted bacteria. Here, we also showed that the development of resistance toward PC1 is likely to be infrequent.
Methods
Ethics statement
The institutional ethics committee on animal experimentation of the Faculté des sciences of the Université de Sherbrooke (QC, Canada) approved these experiments and the guidelines of the Canadian Council on Animal Care were respected during all the procedures.
Reagents
4-hydroxy-2,5,6-triaminopyrimidine (PC1), 2,4-diamino-6-hydroxypirimidine (PC2) and 9-methylguanine were purchased from Fluka. 2-amino-5-bromo-6-methyl-4-pyrimidol and 2-amino-4-hydroxy-6-methylpyrimidine were purchased from Aldrich.
Strategy of ligand selection
Our ligand selection took into account guanine binding requirements [25] and crystal structure interactions [21], [26], [27]. Planar molecules were selected to preserve stacking interactions with adenines 21 and 52 in the guanine aptamer binding site. One of our important selection criteria was to avoid the presence of functional groups that could serve as a ribosylation site in purine or pyrimidine analogs that would allow subsequent nucleic acid incorporation and non-specific antibiotic effect. Successfully identified molecules were drawn using Chem3D Pro (CambridgeSoft) and docked onto the guanine aptamer crystal structure (PDB 1U8D). The in silico procedure was important to validate aptamer-ligand interactions and to avoid sterical obstructions that would perturb ligand binding.
Transcription of RNA
For the production of guanine riboswitch aptamers, DNA templates were prepared from partial duplexes and transcribed using T7 RNA polymerase as previously described [28]. The aptamer sequences used in this study are based on the genomic sequence to which a GCG sequence is added to the 5′ side to allow high transcription yield and to minimize the 5′ heterogeneity [28].
In-line probing assays
[5′-32P] RNA molecules were incubated for 96 h at 25°C in 50 mM Tris-HCl buffer, pH 8.5, 20 mM MgCl2 and 100 mM KCl in absence or in presence of indicated ligand concentrations. The reactions were stopped with a 97% formamide solution containing 10 mM EDTA and samples were purified by electrophoresis in 10% polyacrylamide gels (acrylamide:bisacrylamide; 19 : 1) containing 8 M urea. Gel were dried and exposed to Phosphor Imager screens.
βeta-galactosidase assays
Regulation of the beta-galactosidase reporter gene expression in presence of PC1 or PC2 was determined using an xpt-lacZ transcriptional fusion construct integrated in the genome of B. subtilis by recombination. The beta-galactosidase activity was measured after 4 h of growth at 37°C in minimal medium in absence or presence of the indicated ligand concentrations [41].
Antibiogram assays
Bacteria were inoculated at 105 CFU/mL in melted Muller-Hinton agar. After agar medium was solidified, six wells of 4 mm in diameter were made and filled with 10 µL of the tested molecules (5 mg/mL). Plates were incubated for 16 h at 37°C.
Antibiotic minimal inhibitory concentrations
The minimal inhibitory concentration (MIC) of PC1 and PC2 against S. aureus strain ATCC 29213 was determined using a microdilution method in 96-well plates [30]. Bacteria were inoculated at 105 CFU/mL and incubated at 37°C for 24 h in cation-adjusted Muller-Hinton broth (CAMHB). Bacterial growth was detected by measuring the OD at 595 nm on a microplate reader.
Antibiotic bactericidal activity
Time-kill experiments were performed for the determination of the bactericidal effect of test antibiotics. Bacteria were inoculated at 105 CFU/mL in CAMHB in absence or presence of the antibiotic at its MIC with or without 100 µM GMP. Bacterial permeability to GMP was increased by adding 0.002% Triton X-100. At several time points, bacteria were sampled and serially diluted before spreading on tryptic soya agar (TSA) plates for CFU determinations. Plates were incubated for 24 h at 37°C.
Transcriptomic microarray
Bacteria were inoculated at 108 CFU/mL in CAMHB in absence or presence of 600 µg/mL PC1 or 600 µg/mL PC1 supplemented with 100 µM GMP. After 30 min of growth, RNA was extracted and 2.5 µg of RNA were submitted to reverse transcription to generate fluorescent probes through an aminoallyl cDNA labeling procedure before being hybridized on the microarray [30].
Murine mastitis model
Experimental conditions used here for the mastitis model were previously optimized for S. aureus Newbould and antibiotic treatment [42]. CD-1 lactating mice (Charles River, St. Constant, Canada) were used 12 to 14 days after offspring birth and typically weighed 35 to 40 g. Pups were removed 1 h before bacterial inoculation of mammary glands and a mixture of ketamine/xylazine at 87 and 13 mg/kg of weight, respectively, was used for anesthesia of lactating mice. A 100 µl syringe with a 33-gauge blunt needle was used to inoculate both L4 (on the left) and R4 (on the right) abdominal mammary glands. These large glands constitute the fourth pair found from head to tail. Each udder canal was exposed by a small cut at the near end of the teat under a binocular and 100 µL of bacterial suspension (1 CFU/µL) was injected through the orifice. Mice mammary glands were treated 4 h after infection with PBS or PBS with 10, 50 and 100 µg/gland of PC1 and mice were sacrified 6 h later for mammary gland sampling and homogenization. The tissues used for CFU counts were homogenized in 2 mL of PBS and the bacterial content was evaluated by serial logarithmic dilutions on agar. The detection limit was 100 CFU/g of gland.
Supporting Information
Zdroje
1. BlountKF
BreakerRR
2006 Riboswitches as antibacterial drug targets. Nat Biotechnol 24 1558 1564
2. TalbotGH
BradleyJ
EdwardsJEJr
GilbertD
ScheldM
2006 Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42 657 668
3. PopovichKJ
HotaB
WeinsteinRA
2008 Treatment of community-associated methicillin-resistant staphylococcus aureus. Curr Infect Dis Rep 10 411 420
4. ValentiniP
ParisiG
MonacoM
CreaF
SpanuT
2008 An uncommon presentation for a severe invasive infection due to methicillin-resistant Staphylococcus aureus clone USA300 in Italy: a case report. Ann Clin Microbiol Antimicrob 7 11
5. PofelskiJ
PaveseP
BrionJP
MarrakchiC
GayE
2003 Staphylococcus aureus meningitis with intermediate sensitivity to glycopeptides. Therapeutic indications. Presse Med 32 217 220
6. LooVG
PoirierL
MillerMA
OughtonM
LibmanMD
2005 A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 353 2442 2449
7. KuijperEJ
CoignardB
TullP
2006 Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect 12 Suppl 6 2 18
8. KnowlesDJ
FoloppeN
MatassovaNB
MurchieAI
2002 The bacterial ribosome, a promising focus for structure-based drug design. Curr Opin Pharmacol 2 501 506
9. MonaghanRL
BarrettJF
2006 Antibacterial drug discovery–then, now and the genomics future. Biochem Pharmacol 71 901 909
10. SteitzTA
2005 On the structural basis of peptide-bond formation and antibiotic resistance from atomic structures of the large ribosomal subunit. FEBS Lett 579 955 958
11. BlountKF
WangJX
LimJ
SudarsanN
BreakerRR
2007 Antibacterial lysine analogs that target lysine riboswitches. Nat Chem Biol 3 44 49
12. SudarsanN
Cohen-ChalamishS
NakamuraS
EmilssonGM
BreakerRR
2005 Thiamine pyrophosphate riboswitches are targets for the antimicrobial compound pyrithiamine. Chem Biol 12 1325 1335
13. LeeER
BlountKF
BreakerRR
2009 Roseoflavin is a natural antibacterial compound that binds to FMN riboswitches and regulates gene expression. RNA Biol 6
14. OttE
StolzJ
LehmannM
MackM
2009 The RFN riboswitch of Bacillus subtilis is a target for the antibiotic roseoflavin produced by Streptomyces davawensis. RNA Biol 6 276 280
15. KimJN
BlountKF
LimJ
LinkKH
BreakerR
2009 Design and antimicrobial action of purine analogs that bind guanine riboswitches. ACS Chem Biol 4 915 927
16. AtaideSF
WilsonSN
DangS
RogersTE
RoyB
2007 Mechanisms of resistance to an amino acid antibiotic that targets translation. ACS Chem Biol 2 819 827
17. WittorfJH
GublerCJ
1971 Coenzyme binding in yeast pyruvate decarboxylase. Kinetic studies with thiamine diphosphate analogues. Eur J Biochem 22 544 550
18. HeinrichPC
SteffenH
JanserP
WissO
1972 Studies on the reconstitution of apotransketolase with thiamine pyrophosphate and analogs of the coenzyme. Eur J Biochem 30 533 541
19. WoolleyDW
1951 An enzymatic study of the mode of action of pyrithiamine (neopyrithiamine). J Biol Chem 191 43 54
20. KozminSG
SchaaperRM
ShcherbakovaPV
KulikovVN
NoskovVN
1998 Multiple antimutagenesis mechanisms affect mutagenic activity and specificity of the base analog 6-N-hydroxylaminopurine in bacteria and yeast. Mutat Res 402 41 50
21. GilbertSD
MediatoreSJ
BateyRT
2006 Modified pyrimidines specifically bind the purine riboswitch. J Am Chem Soc 128 14214 14215
22. ShimaokaM
TakenakaY
MiharaY
KurahashiO
KawasakiH
2006 Effects of xapA and guaA disruption on inosine accumulation in Escherichia coli. Biosci Biotechnol Biochem 70 3069 3072
23. KobayashiK
EhrlichSD
AlbertiniA
AmatiG
AndersenKK
2003 Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A 100 4678 4683
24. KimJN
BreakerRR
2008 Purine sensing by riboswitches. Biol Cell 100 1 11
25. MandalM
BoeseB
BarrickJE
WinklerWC
BreakerRR
2003 Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113 577 586
26. SerganovA
YuanYR
PikovskayaO
PolonskaiaA
MalininaL
2004 Structural basis for discriminative regulation of gene expression by adenine - and guanine-sensing mRNAs. Chem Biol 11 1729 1741
27. BateyRT
GilbertSD
MontangeRK
2004 Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432 411 415
28. MulhbacherJ
LafontaineDA
2007 Ligand recognition determinants of guanine riboswitches Nucleic Acids Res 35 5568 5580
29. PorwalS
LalS
CheemaS
KaliaVC
2009 Phylogeny in aid of the present and novel microbial lineages: diversity in Bacillus. PLoS ONE 4 e4438 doi:10.1371/journal.pone.0004438
30. MoisanH
BrouilletteE
JacobCL
Langlois-BeginP
MichaudS
2006 Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J Bacteriol 188 64 76
31. BischoffM
DunmanP
KormanecJ
MacapagalD
MurphyE
2004 Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. J Bacteriol 186 4085 4099
32. SearsPM
McCarthyKK
2003 Management and treatment of staphylococcal mastitis. Vet Clin North Am Food Anim Pract 19 171 185
33. BrouilletteE
MalouinF
2005 The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect 7 560 568
34. MayerSJ
WatermanAE
KeenPM
CravenN
BourneFJ
1988 Oxygen concentration in milk of healthy and mastitic cows and implications of low oxygen tension for the killing of Staphylococcus aureus by bovine neutrophils. J Dairy Res 55 513 519
35. JiY
ZhangB
VanSF
Horn
WarrenP
2001 Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science 293 2266 2269
36. WilsonTL
JeffersJ
Rapp-GabrielsonVJ
MartinS
KleinLK
2007 A novel signature-tagged mutagenesis system for Streptococcus suis serotype 2. Vet Microbiol 122 135 145
37. McFarlandWC
StockerBA
1987 Effect of different purine auxotrophic mutations on mouse-virulence of a Vi-positive strain of Salmonella dublin and of two strains of Salmonella typhimurium. Microb Pathog 3 129 141
38. RussoTA
JodushST
BrownJJ
JohnsonJR
1996 Identification of two previously unrecognized genes (guaA and argC) important for uropathogenesis. Mol Microbiol 22 217 229
39. SamantS
LeeH
GhassemiM
ChenJ
CookJL
2008 Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS Pathog 4 e37 doi:10.1371/journal.ppat.0040037
40. SwannPF
WatersTR
MoultonDC
XuYZ
ZhengQ
1996 Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 273 1109 1111
41. LemayJF
PenedoJC
TremblayR
LilleyDM
LafontaineDA
2006 Folding of the adenine riboswitch. Chem Biol 13 857 868
42. BrouilletteE
GrondinG
TalbotBG
MalouinF
2005 Inflammatory cell infiltration as an indicator of Staphylococcus aureus infection and therapeutic efficacy in experimental mouse mastitis. Vet Immunol Immunopathol 104 163 169
43. MazzariolA
CornagliaG
NikaidoH
2000 Contributions of the AmpC beta-lactamase and the AcrAB multidrug efflux system in intrinsic resistance of Escherichia coli K-12 to beta-lactams. Antimicrob Agents Chemother 44 1387 1390
44. SampsonBA
MisraR
BensonSA
1989 Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 122 491 501
45. TaylorEC
LouxHM
FalcoEA
HitchingsGH
1955 Pyrimidopteridines by oxidative self-condensation of aminopyrimidines. J Am Chem Soc 77 2243 2248
46. JonesME
BoeninkNM
VerhoefJ
KohrerK
SchmitzFJ
2000 Multiple mutations conferring ciprofloxacin resistance in Staphylococcus aureus demonstrate long-term stability in an antibiotic-free environment. J Antimicrob Chemother 45 353 356
47. WichelhausT
SchaferV
BradeV
BoddinghausB
2001 Differential effect of rpoB mutations on antibacterial activities of rifampicin and KRM-1648 against Staphylococcus aureus. J Antimicrob Chemother 47 153 156
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



