-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInteractions with Bacteria in the Context of Human Health and Disease
article has not abstract
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000886
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1000886Summary
article has not abstract
Humans are colonized by diverse populations of bacteria and fungi when in a healthy state and in the settings of disease, and the interactions between these microbial populations can be beneficial or detrimental to the host [1]. Among these microbial populations, Candida albicans is the fungus most commonly detected in association with humans [2], and numerous studies have described C. albicans interactions with its bacterial neighbors [1]. Here, with a focus on C. albicans, we provide examples of how bacterial-fungal interactions can influence human health. In addition, we highlight studies that give insight into the molecular mechanisms that govern the physical associations, interspecies communication, and changes in microbial behavior and survival that occur when bacteria and fungi occupy the same sites.
Bacterial−C. albicans Interactions Can Promote or Prevent Disease
Bacterial and fungal co-infections have been implicated in enhanced host colonization and virulence. For instance, C. albicans and Escherichia coli exhibit a cooperative interaction wherein E. coli enhances adhesion of C. albicans to bladder mucosa and increases the likelihood of fungal urinary tract infections [3]. Likewise, the risk of ventilator-associated pneumonia due to infection by Pseudomonas aeruginosa is markedly greater in patients colonized by C. albicans [4], and accordingly, antifungal treatments can reduce the likelihood of developing this systemic disease [5]. Moreover, denture stomatitis, an inflammation of the oral mucosa in denture wearers, is influenced by the presence of C. albicans and other oral microorganisms [6]. In fact, several studies demonstrate an association between C. albicans and oral bacteria such as Streptococcus (Figure 1A), Actinomyces, and Fusobacterium species [1], [7], and these physical interactions likely contribute to denture colonization and oral candidiasis.
Fig. 1. An overview of how interactions between C. albicans and Gram-positive members of the human flora may influence disease. 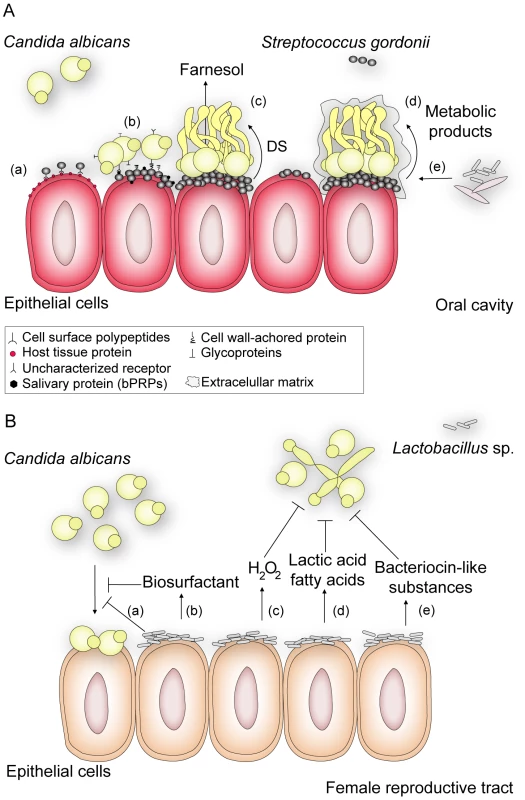
(A) S. gordonii, a normal colonizer of the oral cavity, enhances C. albicans survival and persistence, thus contributing to the development of C. albicans infections. In this environment, S. gordonii adhere to cells by expressing a complex repertoire of cell surface polypeptides (AgI/II) (a) that recognize a range of host tissue proteins and cellular receptors [7]. After bacterial attachment, C. albicans can selectively attach to surface-bound bacteria by means of protein-protein interactions [10], [12] or by direct recognition of salivary proteins (basic proline-rich proteins, bPRPs) previously adsorbed by S. gordonii cells [11] (b). Coaggregation of the bacterium and C. albicans contributes to biofilm formation and results in closer proximity for cell-cell communication. Through a diffusible signal (DS) molecule (c), S. gordonii suppresses farnesol-mediated inhibition of hypha formation, thereby enhancing the potential for C. albicans to form biofilms and thus its ability to invade tissue [7]. In addition, Streptococcus promotes (d) fungal growth by secreting metabolic products that can be used as a carbon source by C. albicans [1]. Likewise, C. albicans enhances the survival and colonization of S. gordonii by reducing the oxygen tension to levels preferred by streptococci and by providing bacterial growth stimulatory factors as a result of nutrient metabolism [1], [11]. These favorable conditions promote the formation of mature fungal-bacterial biofilms surrounded by an extracellular matrix, to which (e) other bacterial or fungal species can bind. These interactions may make oral infections more persistent and harder to treat. (B) Lactobacillus sp., which normally inhabits the female reproductive tract, defends the host against colonization of pathogens such as C. albicans. Evidence suggests that the bacterium reduces the adhesion of C. albicans to epithelial cells either by (a) outcompeting fungal cells for adhesion sites, such as cellular receptors to which Lactobacillus has higher affinity, or (b) by secreting biosurfactants such as surlactin that physically decrease fungal binding. Most Lactobacillus strains release (c) hydrogen peroxide (H2O2) and (d) lactic acid or other fatty acids that inhibit C. albicans proliferation and invasive hypha formation. Bacteriocin-like substances (e) produced by Lactobacillus suppress the fungal growth to directly decrease its load [8]. In contrast, lactic acid bacteria (Figure 1B), which normally inhabit the intestinal and female reproductive tracts, compete with C. albicans for adhesion sites and secrete substances that inhibit fungal attachment to control C. albicans invasion and disease [8]. Interestingly, imbalance in the normal bacterial flora caused by treatment with broad-spectrum antibiotics is a predisposing factor associated with C. albicans colonization of immunocompromised patients, probably due to decreased numbers of bacterial competitors [9]. Thus, antibiotic therapies that specifically target pathogens, in contrast to broad spectrum antibiotics, may help prevent secondary problems that arise upon perturbation of beneficial bacterial-fungal interactions.
Bacteria and Fungi Promote Coaggregation and Formation of Mixed-Species Biofilms
Both singly and together, bacteria and fungi form highly structured, often surface-associated, communities termed biofilms. A significant proportion of human microbial infections are biofilm-associated, wherein the formation of mixed-species biofilms could create a protected environment that allows for survival to external assaults and facilitates different bacterial-fungal interactions [1], [2]. The known relationships between C. albicans and oral streptococci illustrate the various ways by which bacteria and fungi can attach to one another or coaggregate using specific cell surface factors, leading to mixed-species biofilms [2], [7]. These adhesive interactions between C. albicans and other indigenous oral microbes can be mediated by protein-protein and lectin-carbohydrate interactions, and hydrophobic and electrostatic interactions may contribute as well. C. albicans molecules such as agglutinin-like sequences (Als) and specific cell surface glycoproteins have been identified as being important for coadhesion to mixed microbial communities in biofilms [1], [10]. O'Sullivan and colleagues [11] demonstrated that the oral bacterium Streptococcus gordonii adsorbs salivary proline-rich proteins that are recognized by C. albicans and act as receptors for the fungus (Figure 1A). Importantly, S. gordonii cell surface polypeptides also contribute to coadherance with the fungi [12]. Underscoring the complexity of these interactions, adherence and coaggregation of C. albicans with oral bacteria is species specific, and is mediated by bacterial receptors that might be expressed only under particular environments.
Interdomain Signaling via Quorum-Sensing Molecules and Other Microbial Products Modulates Fungal and Bacterial Behavior
Single species bacterial and fungal populations modulate their collective behavior using extracellular signals known as quorum-sensing molecules [13], [14]. Since this regulation generally occurs in response to cell density, processes like co-aggregation and biofilm formation promote the synthesis and secretion of quorum-sensing molecules, increasing the likelihood that neighboring cells will experience the signals at levels sufficient to induce a response. Thus, the study of bacterial-fungal interactions in association with mixed-species biofilms reveals that cross-kingdom communication between bacteria and fungi is a common process, and that quorum-sensing signals, known for their roles in intraspecies communication, can also mediate crosstalk between bacteria and fungi. C. albicans, as an illustration, induces its switch from hyphal growth to yeast growth using a secreted quorum-sensing molecule called farnesol [14], which inhibits the Ras1-controlled pathway involved in hyphal growth [15]. Strikingly, this small molecule can also modulate bacterial behavior and virulence by altering the production of toxic phenazines, such as pyocyanin in P. aeruginosa [13]. Moreover, farnesol can also induce the generation of reactive oxygen species in a number of microorganisms, likely through effects on electron transport chain components [16], and this process may play an important role in competition with bacteria.
Notably, a number of Gram-negative bacteria secrete molecules with farnesol-like activities in that they induce a shift to yeast form growth by the fungus [17]. For instance, P. aeruginosa–produced 3-oxo-C12-homoserine lactone reaches concentrations in mixed-species biofilms that repress C. albicans filamentation [17]. By responding to these signaling molecules, the fungus may disperse from sites where other co-inhabitants such as antifungal-producing bacteria are present, conferring a potential selective advantage. Conversely, in mixed biofilms of S. gordonii and C. albicans (Figure 1A), a bacterially secreted diffusible signal enhances hyphal development by relieving the effects of farnesol on the fungus [7]. Moreover, cell wall–derived molecules such as bacterial muramyl dipeptides induce C. albicans hyphal growth, which may also promote fungal invasion of host tissues and virulence [18]. These findings support the concept that both eukaryotic and prokaryotic microorganisms sense and respond to the diverse diffusible signaling molecules produced in the niches where they coexist. Furthermore, we may find that the chemical warfare between bacteria and fungi leads to increased toxin production and increased host damage and inflammation.
Chemical Interactions between Fungi and Bacteria
In addition to providing attachment sites for different species, bacterial-fungal communities create environmental conditions that promote or control the growth of other microbes. Actively respiring C. albicans reduces oxygen tension levels and provides stimulatory factors for streptococci in the oral environment, while the latter provides nutrients that promote fungal growth [1]. In contrast, commensal bacteria that inhabit the female reproductive tract, such as Lactobacillus spp. (Figure 1B), inhibit the growth and virulence of C. albicans potentially through secretion of organic acids and production of hydrogen peroxide (H2O2). Supporting these in vitro findings, it has been shown that 96% of healthy women have H2O2-generating Lactobacillus species as part of their microflora, while these bacterial populations are lower in women suffering from vaginosis [8].
Bacterial-Fungal Interactions Influence Antibiotic Resistance and Host Response to Infection
Mixed bacterial-fungal infections can correlate with increased frequency or severity of disease. In fact, while C. albicans is the fourth leading cause of mortality due to systemic infections [1], [19], the risk of mortality may increase upon bacterial and fungal co-infection. C. albicans and Staphylococcus aureus, for instance, have synergistic effects where mice inoculated with only S. aureus show low mortality, whereas co-inoculation with C. albicans leads to mortality increases [1], [20]. It is not yet known how the host immune response is perturbed when bacterial and fungal pathogens are both present, but current research seeks to address this important question. The cooperative effects observed in mixed fungal-bacterial infections in vivo could be due to formation of biofilms, since this form of growth can promote resistance to both host clearance pathways and antimicrobial agents. Harriott and Noverr demonstrated that the human pathogen S. aureus forms larger biofilms with increased resistance to vancomycin when it is co-cultured with C. albicans [19]. Biofilms of C. albicans and oral streptococci are similarly more resistant to antibiotics than their single species counterparts [1], [2]. Matrix polymers produced by both organisms might result in a more viscous matrix that is more effective at restricting the penetration of drugs [2], [19]. Understanding the physiology of bacteria and fungi coexisting within mixed microbial communities will greatly aid our ability to effectively treat opportunistic polymicrobial infections and to modulate the behavior of potentially pathogenic bacteria and fungi in beneficial ways.
Zdroje
1. ShirtliffME
PetersBM
Jabra-RizkMA
2009 Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett 299 1 8
2. DouglasLJ
2003 Candida biofilms and their role in infection. Trends Microbiol 11 30 36
3. LevisonME
PitsakisPG
1987 Susceptibility to experimental Candida albicans urinary tract infection in the rat. J Infect Dis 155 841 846
4. AzoulayE
TimsitJF
TaffletM
de LassenceA
DarmonM
2006 Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 129 110 117
5. NseirS
JozefowiczE
CavestriB
SendidB
Di PompeoC
2007 Impact of antifungal treatment on Candida-Pseudomonas interaction: a preliminary retrospective case-control study. Intensive Care Med 33 137 142
6. Baena-MonroyT
Moreno-MaldonadoV
Franco-MartinezF
Aldape-BarriosB
QuindosG
2005 Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal 10 Suppl 1 E27 E39
7. BamfordCV
d'MelloA
NobbsAH
DuttonLC
VickermanMM
2009 Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun 77 3696 3704
8. BorisS
BarbesC
2000 Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2 543 546
9. HogenauerC
HammerHF
KrejsGJ
ReisingerEC
1998 Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis 27 702 710
10. KlotzSA
GaurNK
De ArmondR
SheppardD
KhardoriN
2007 Candida albicans Als proteins mediate aggregation with bacteria and yeasts. Med Mycol 45 363 370
11. O'SullivanJM
JenkinsonHF
CannonRD
2000 Adhesion of Candida albicans to oral streptococci is promoted by selective adsorption of salivary proteins to the streptococcal cell surface. Microbiology 146(Pt 1) 41 48
12. HolmesAR
McNabR
JenkinsonHF
1996 Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect Immun 64 4680 4685
13. CuginiC
CalfeeMW
FarrowJM3rd
MoralesDK
PesciEC
2007 Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol Microbiol 65 896 906
14. HornbyJM
JensenEC
LisecAD
TastoJJ
JahnkeB
2001 Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67 2982 2992
15. Davis-HannaA
PiispanenAE
StatevaLI
HoganDA
2008 Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol 67 47 62
16. MachidaK
TanakaT
1999 Farnesol-induced generation of reactive oxygen species dependent on mitochondrial transmembrane potential hyperpolarization mediated by F(0)F(1)-ATPase in yeast. FEBS Lett 462 108 112
17. HoganDA
VikA
KolterR
2004 A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol 54 1212 1223
18. XuXL
LeeRT
FangHM
WangYM
LiR
2008 Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 4 28 39
19. HarriottMM
NoverrMC
2009 Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother 53 3914 3922
20. CarlsonE
1983 Effect of strain of Staphylococcus aureus on synergism with Candida albicans resulting in mouse mortality and morbidity. Infect Immun 42 285 292
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



