-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
Bacterial translocation from the gut and subsequent immune activation are hallmarks of HIV infection and are thought to determine disease progression. Intestinal barrier integrity is impaired early in acute retroviral infection, but levels of plasma lipopolysaccharide (LPS), a marker of bacterial translocation, increase only later. We examined humanized mice infected with HIV to determine if disruption of the intestinal barrier alone is responsible for elevated levels of LPS and if bacterial translocation increases immune activation. Treating uninfected mice with dextran sodium sulfate (DSS) induced bacterial translocation, but did not result in elevated plasma LPS levels. DSS-induced translocation provoked LPS elevation only when phagocytic cells were depleted with clodronate liposomes (clodrolip). Macrophages of DSS-treated, HIV-negative mice phagocytosed more LPS ex vivo than those of control mice. In HIV-infected mice, however, LPS phagocytosis was insufficient to clear the translocated LPS. These conditions allowed higher levels of plasma LPS and CD8+ cell activation, which were associated with lower CD4+/CD8+ cell ratios and higher viral loads. LPS levels reflect both intestinal barrier and LPS clearance. Macrophages are essential in controlling systemic bacterial translocation, and this function might be hindered in chronic HIV infection.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000867
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000867Summary
Bacterial translocation from the gut and subsequent immune activation are hallmarks of HIV infection and are thought to determine disease progression. Intestinal barrier integrity is impaired early in acute retroviral infection, but levels of plasma lipopolysaccharide (LPS), a marker of bacterial translocation, increase only later. We examined humanized mice infected with HIV to determine if disruption of the intestinal barrier alone is responsible for elevated levels of LPS and if bacterial translocation increases immune activation. Treating uninfected mice with dextran sodium sulfate (DSS) induced bacterial translocation, but did not result in elevated plasma LPS levels. DSS-induced translocation provoked LPS elevation only when phagocytic cells were depleted with clodronate liposomes (clodrolip). Macrophages of DSS-treated, HIV-negative mice phagocytosed more LPS ex vivo than those of control mice. In HIV-infected mice, however, LPS phagocytosis was insufficient to clear the translocated LPS. These conditions allowed higher levels of plasma LPS and CD8+ cell activation, which were associated with lower CD4+/CD8+ cell ratios and higher viral loads. LPS levels reflect both intestinal barrier and LPS clearance. Macrophages are essential in controlling systemic bacterial translocation, and this function might be hindered in chronic HIV infection.
Introduction
The clinical course of HIV infection varies considerably among patients, and the variability is even greater in simian models. Asian monkeys infected with simian immunodeficiency virus (SIV) rapidly progress to AIDS, but African monkeys do not get sick [1]. In general, a pathogenic course of retroviral infection is characterized by high levels of immune activation [2], and bacterial translocation from the intestinal tract has been implicated as an underlying activating mechanism [3]–[8]. The integrity of the intestinal barrier is impaired early in acute retroviral infections [9], and a substantial fraction of the intestinal CD4+ T cells are lost within days after infection [10], [11].
However, bacterial translocation manifests itself only later. Low T-cell numbers in the gut are an important characteristic of HIV or SIV pathogenesis [12], [13], but intestinal CD4+ T-cell depletion does not predict the outcome of SIV infection in monkeys [14]. In SIV-infected African monkeys, for example, bacterial translocation is prevented despite low numbers of intestinal CD4+ T cells [15]. Thus, intestinal CD4+ T-cell depletion alone cannot explain bacterial translocation and the subsequent rise in plasma lipopolysaccharide (LPS) levels in chronic HIV infection [8]. Preferential depletion of Th17 cells is associated with disruption of the intestinal barrier in pathogenic retroviral infections [16], [17], but overall, the mechanism linking bacterial translocation and HIV pathogenesis is not fully understood.
Seeking evidence for this mechanism, we examined relationships among intestinal barrier integrity, microbial translocation, immune activation, and HIV replication in a mouse-model of HIV infection. In this model, RAG2−/−gammac−/− mice are transplanted with human cord-blood hematopoietic stem cells. A human lymphoid system develops [18], and the “humanized” mice can be infected with HIV [19]–[21]. The humanized mice combine the advantages of studying HIV in human cells and in a small-animal model that facilitates experimental manipulations. Furthermore, these mice have low intestinal lymphocyte numbers [22]. In HIV infection, low numbers result mostly from virus-mediated depletion. In the humanized mice, however, they result from a low local level of engraftment by human cells. Thus, in the humanized mice, low numbers of intestinal T cells are uncoupled from the effects of viral replication. Moreover, the intestinal barrier can be disrupted by adding dextran sodium sulfate (DSS) to the drinking water of mice [23]. DSS treatment leads to apoptosis [24] and reduced proliferation of intestinal epithelial cells [25], mimicking the enterocyte apoptosis seen in SIV infection [26].
In the current study, we dissected the effects of bacterial translocation alone or in the context of HIV infection by combining DSS and HIV in humanized mice. We defined the consequences of HIV infection and bacterial translocation on plasma LPS levels, LPS clearance by macrophages, immune activation, and T-cell loss.
Results
DSS treatment or HIV infection induce a similar amount of bacterial translocation
In a DSS dose-response experiment, we established a treatment protocol that increases bacterial translocation without inducing colitis (Fig. S1). Briefly, we quantified bacterial translocation in groups of HIV-uninfected and -infected mice with mock or DSS treatment (i.e., HIV−/DSS−, HIV−/DSS+, HIV+/DSS−, or HIV+/DSS+ mice). We infected humanized mice with the CCR5-tropic HIV strain YU-2 and verified the infection by RT-PCR of plasma HIV RNA 4–6 weeks after inoculation. Thereafter, infected and uninfected control mice were treated with 0 or 0.75% DSS for 2 weeks. We cultured organ suspensions of mesenteric lymph nodes (MLN) and spleens, quantified bacterial colonies (Fig. 1A), and calculated a translocation index, based on quantity, diversity, and location of recovered bacteria (Fig. 1B). HIV−/DSS − control mice showed some baseline translocation with roughly a third of the animals containing bacteria in the MLN suspensions. Only few animals showed systemic dissemination to the spleen. DSS treatment increased bacterial translocation; in HIV−/DSS+ mice percentages of cultures containing bacteria doubled, and particularly in the spleen bacterial loads were higher. Organ cultures yielded mainly Lactobacilli, Staphylococcus xylosus, a typical mouse commensal, and Enterococci, whereas stool cultures yielded a multitude of aerobic and anaerobic bacteria. Only some bacterial species translocated to the organs in sufficient numbers to be cultured. HIV+ mice had similar microbiology results to HIV−/DSS+ mice. Overall, the range and amount of translocation were comparable between HIV−/DSS+, HIV+/DSS−, and HIV+/DSS+ mice. HIV infection alone seemed to facilitate bacterial invasion from the gut, and DSS treatment did not further increase the bacterial translocation in HIV+ humanized mice.
Fig. 1. Gastrointestinal barrier dysfunction did not completely explain HIV-associated plasma LPS elevation in humanized mice. 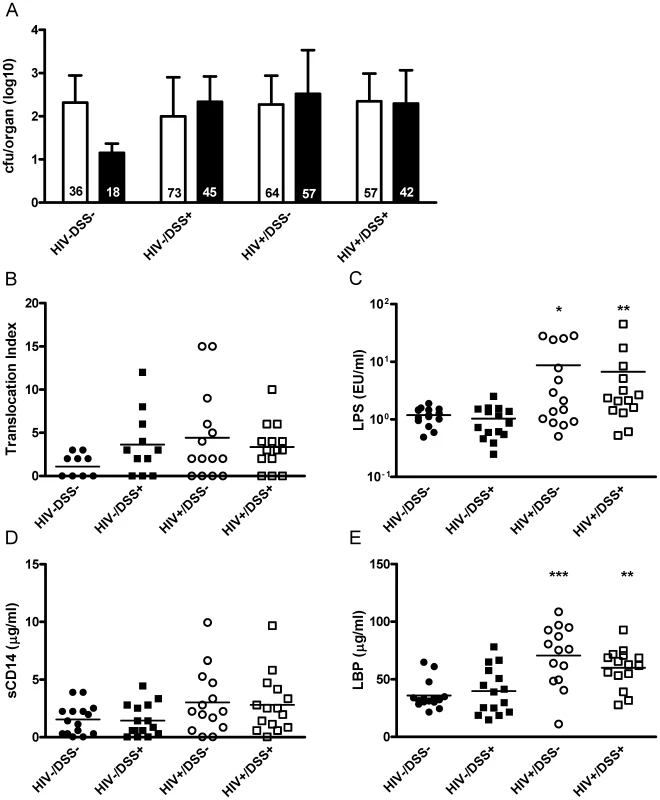
4 to 6 weeks after HIV or mock infection, humanized mice received DSS 0.75% or normal drinking water for 2 weeks. (A) In cultures of organ suspensions, bacterial colony forming units from MLN (white bar) and spleens (black bar, mean, SD) were quantified; percentages of positive organs in the different groups are indicated at the bottom of the respective bars (n = 50, pooled data from two independent experiments). (B) The mice showed a trend towards higher levels of bacterial translocation (assessed by an index that includes number, species, and location of bacteria detected, P = 0.1, 0.08 and 0.13, respectively) after HIV infection or DSS treatment. (C) DSS+/HIV− mice (black square) had plasma LPS levels similar to those of control mice (black circle). Only HIV+ mice without (white circle, *, P = 0.015) or with DSS treatment (white square, **, P = 0.005) showed significant increases of plasma LPS (n = 59, pooled data from two independent experiments). (D and E) Both groups of HIV+ mice showed a trend towards higher plasma sC14 (P = 0.06 for HIV+/DSS− and P = 0.1 for HIV+/DSS+ mice) and had significantly higher LBP values (***, P = 0.0006 for HIV+/DSS− and **, P = 0.008 for HIV+/DSS+ mice). Bacterial translocation does not always induce LPS elevation
In humans bacterial translocation is quantified by measuring surrogate markers, such as plasma LPS, soluble CD14 (sCD14), or LPS binding protein (LBP). We compared these markers to the direct measurement of intestinal barrier function in humanized mice.
Plasma LPS measurements showed a contrasting picture to microbiology results. Only HIV+ mice exhibited elevated levels of LPS in the systemic circulation. HIV−/DSS+ mice, which had increased intestinal permeability according to the organ culture results, controlled plasma LPS levels (Fig. 1C). In accordance with the elevated LPS levels, HIV+ mice also exhibited higher plasma sCD14 and LBP levels (Fig. 1D and E). We did not measure endotoxin core antibodies (EndoCAb) since humanized RAG2−/−gammac−/− mice in general have very poor antibody responses, serum immunoglobulin concentrations are several log lower than in humans [18], and mostly IgM is produced with IgG appearing only several months after humanization [20].
The HIV+ mice might have had a greater influx of smaller bacterial products and as a consequence higher plasma LPS levels. To eliminate this possibility, we measured the integrity of the intestinal barrier by gavaging mice with fluorescein isothiocyanate (FITC)-dextran with a molecular weight similar to that of LPS (Fig. S2B). Furthermore, we included wild-type and non-humanized mice to determine the consequences of irradiation and transplantation. After 4 hours, FITC-dextran was detected at similar concentrations in the plasma of HIV−/DSS − control mice, wild-type BALB/c, and non-humanized RAG2−/−gammac−/− mice. Thus, independent of humanization, RAG2−/−gammac−/− and wild-type mice had equal intestinal permeabilities, and the absence of intestinal lymphocytes had little effect on permeability in this model. In DSS-treated or HIV-infected mice there was a trend towards higher FITC-dextran values. In histological sections of the intestines, we found no evidence for exacerbated damage in HIV+ mice, and DSS-treated mice showed moderate changes (Fig. S2A).
Defects in intestinal barrier integrity influenced translocation, but alone they were not sufficient to induce LPS elevation. In HIV-infected mice, some additional factors contributed to higher plasma LPS levels.
Bacterial translocation can be compensated for by increased macrophage phagocytosis
We hypothesized that plasma LPS levels are a marker for bacterial translocation and for the clearance of bacterial products from the systemic circulation. One of the main LPS clearance mechanisms is phagocytosis by liver macrophages [27], [28]. We tested the influence of LPS clearance on plasma LPS levels by depleting macrophages in the humanized mice. Clodronate liposomes induce apoptosis of phagocytic cells, thus humanized mice injected with clodronate liposomes exhibited a strong reduction of macrophage numbers (Fig. 2A). When we simultaneously treated the mice with 0.75% DSS for 1 week, plasma LPS levels increased (Fig. 2B). Disturbing the intestinal barrier or reducing the number of macrophages alone caused no change in plasma LPS.
Fig. 2. The combination of bacterial translocation and disturbed LPS clearance induced plasma LPS elevation. 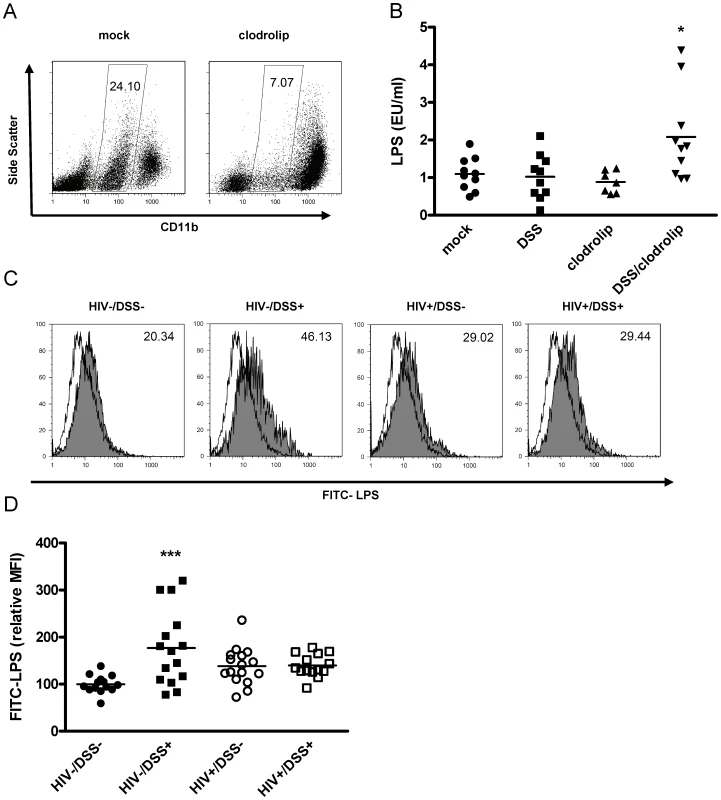
(A) Humanized mice were injected intraperitoneally with clodrolip (1 mg/20 g body weight) to deplete phagocytic CD11b intermediate cells (spleens of representative mock PBS or clodrolip treated mice 48 h after injection). (B) After 1 week of DSS 0.75% (square, block arrow down) treatment and a second injection of clodrolip (0.5 mg/20 g body weight) (block arrow up, block arrow down), plasma LPS was only increased in mice that received both treatments (*, P = 0.006, n = 37, pooled data from two independent experiments). (C) Liver macrophages, isolated from HIV− or HIV+ mice that received either normal drinking water or 0.75% DSS for 2 weeks, were incubated ex vivo with FITC-LPS at 37°C or 4°C (shaded or open histogram), and mean fluorescence intensity of phagocytic cells (values upper right corner) was measured. (D) Values were normalized to the mean FITC-LPS signal of cells from HIV−/DSS− mice (black circle). DSS-induced bacterial translocation increased FITC-LPS phagocytosis (black square, ***, P<0.0001), but HIV infection abrogated this effect (white circle, P = 0.2), independent of DSS treatment (white square, P = 0.19) (n = 59, pooled data from two independent experiments). To assess macrophage function during HIV infection, we isolated liver macrophages from all four groups of mice (i.e., HIV−/DSS−, HIV−/DSS+, HIV+/DSS−, and HIV+/DSS+ mice) and incubated the cells ex vivo with FITC-LPS (Fig. 2C). All macrophages took up some LPS, but cells isolated from HIV−/DSS+ mice up-regulated their phagocytic capacity significantly compared to cells from control animals. Cells from HIV+ mice showed a slight, statistically insignificant increase of LPS phagocytosis (Fig. 2D). Further evidence of altered macrophage function in HIV+ mice was an increase of pro-inflammatory cytokines, such as IL-12 and TNF-alpha in the plasma (Fig. S3). Macrophage numbers in liver and intestines were similar in all groups (data not shown). The results imply that bacterial translocation and LPS influx can be compensated for by increased LPS phagocytosis and that this function was disturbed in HIV-infected mice.
Bacterial translocation activates T cells
Since bacterial translocation has been implicated in immune activation during chronic HIV infection, we examined the effects of bacterial translocation alone or in the context of HIV infection on the expression of cell-surface markers of T-cell activation. We determined T-cell activation levels in the spleens of humanized mice by measuring percentages of CD38 HLA-DR double positive cells in human CD4+ or CD8+ cells (Fig. 3A). CD4+ cells from HIV−/DSS+ animals showed a slight increase of activation, but activation of CD8+ cells was more apparent (Fig. 3B). Both groups of HIV-infected mice had even higher CD8+ cell activation levels, with no difference between DSS − and DSS+ groups. There was a trend towards slightly higher CD4+ cell activation in HIV+ mice.
Fig. 3. HIV-infected humanized mice had high levels of CD8+ T-cell activation. 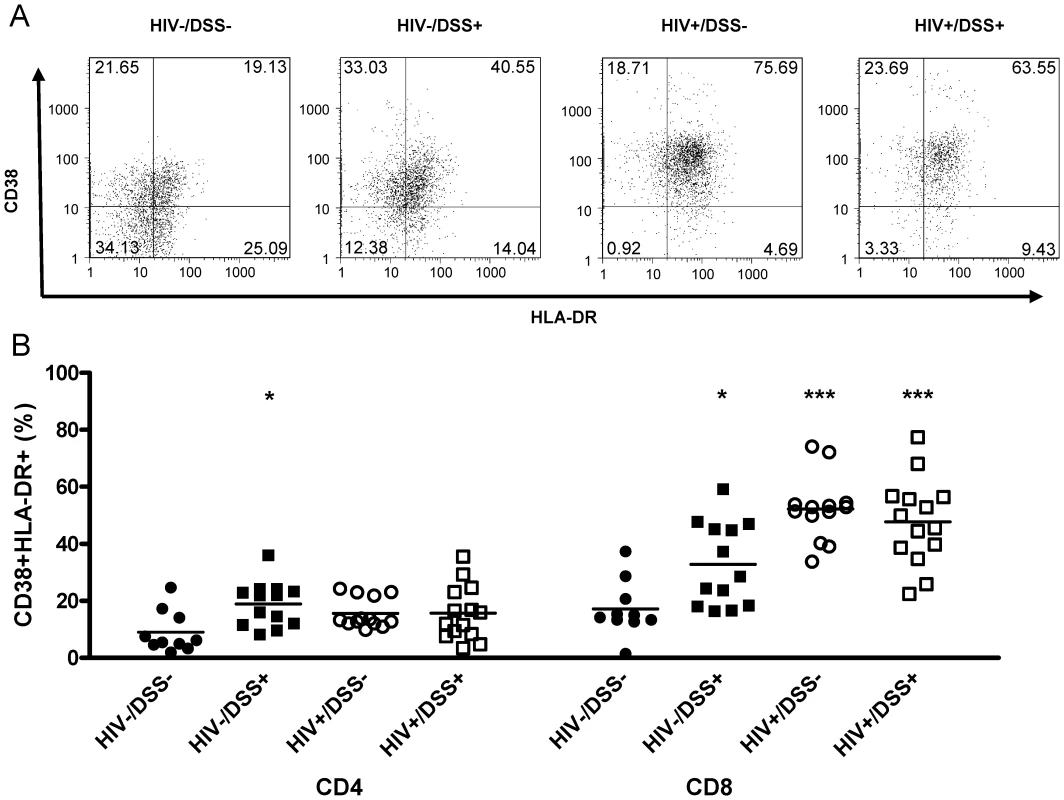
Control (black symbols) or HIV-infected mice (white symbols) received normal drinking water (circles) or 0.75% DSS (squares) for 2 weeks. (A) Activation levels in the spleen were determined by flow cytometry of human HLA-DR and CD38 staining of CD45+CD8+ (one representative animal per group) and CD45+CD4+ splenocytes. (B) DSS treatment alone slightly increased activation levels of CD4+ (*, P = 0.004) and CD8+ cells (*, P = 0.007), over those in uninfected, untreated control mice. HIV infection drastically increased CD8+ cell activation in animals that received normal (***, P<0.0001) or DSS (***, P<0.0001) water (n = 50, pooled data from two independent experiments). Thus, bacterial translocation, even if no detectable plasma LPS elevation occurred, activated CD4+ and CD8+ cells in HIV - mice. In HIV+ mice, where levels of plasma LPS were increased, CD8+ cell activation was even stronger.
T-cell activation promotes viral replication and CD4+ T-cell loss
In HIV - mice, levels of CD4+ and CD8+ cell activation were tightly correlated (Fig. 4A). If that relationship of CD4+ and CD8+ cell activation was the same in HIV+ mice, then CD4+ cell activation levels should have been even higher in HIV+ mice than in HIV−/DSS+ mice. The correlation between activation levels of CD4+ and CD8+ cells in HIV+ mice, however, was partially lost (Fig. 4B), and the HIV+ mice had a relative deficit of activated CD4+ cells.
Fig. 4. Activation of CD8+ cells was associated with lower CD4+/CD8+ cell ratios and higher viral loads. 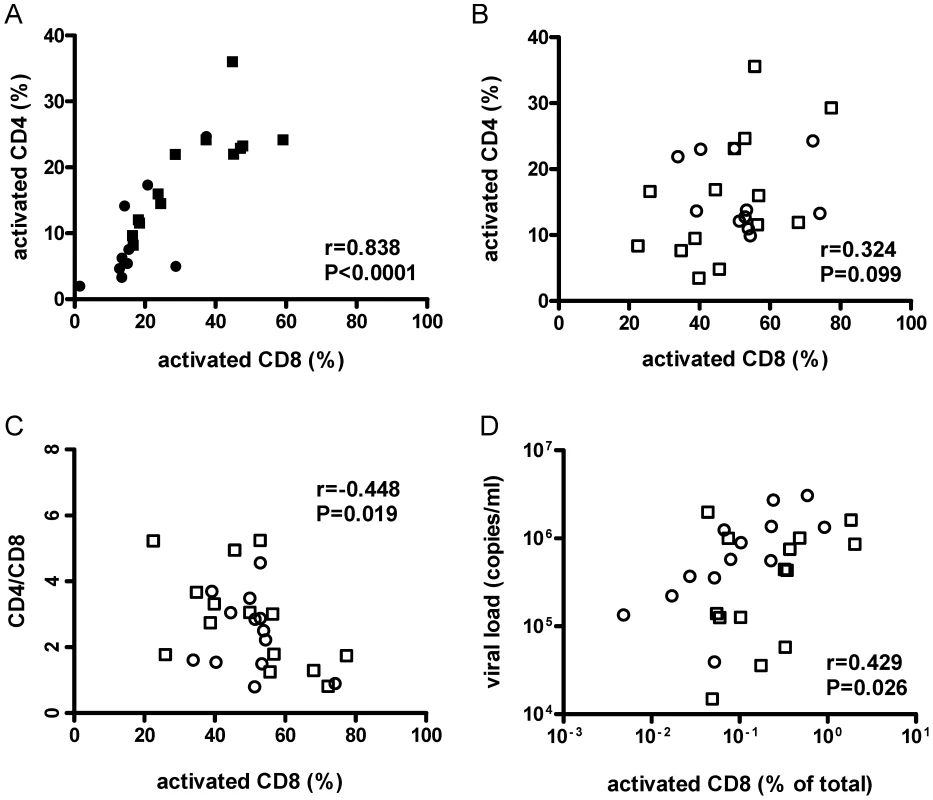
Human CD8+ splenocyte activation levels defined by HLA−DR+ and CD38+ co-staining from HIV−/DSS− (black circle), HIV−/DSS+ (black square), HIV+/DSS− (white circle), and HIV+/DSS+ animals (white square) were correlated with CD4+ cell-activation levels, CD4+/CD8+ cell ratio, and viral load. (A) In uninfected mice, activation levels of CD8+ and CD4+ cells were tightly correlated (n = 23, pooled data from two independent experiments). (B) But in HIV+ mice, this relationship was not as clear (n = 27, pooled data from two independent experiments). (C) In HIV+ mice, higher percentages of activated CD8+ cells correlated with lower CD4+/CD8+ cell ratios. (D) When activation levels were adjusted for overall engraftment by calculating percentages of HLA-DR+CD38+CD8+ cells of total cells, including murine cells, CD8+ cell activation correlated also with higher viral loads. In humanized mice, absolute CD4+ T-cell numbers differ, because the efficiency of human engraftment is variable. However, CD4+/CD8+ cell ratios are independent of engraftment and are, thus, reasonably reliable estimates of CD4+ cell depletion. The ratios were similar for all four groups (Fig. S4A). Individual variations of CD4+/CD8+ cell ratios between mice were probably too large to detect small changes between groups in the relatively short time of the experiment. Furthermore, no difference in HIV replication was observed between HIV+/DSS − and HIV+/DSS+ mice (Fig. S4B). Nevertheless, in HIV+ mice, percentages of activated CD8+ cells correlated with lower CD4+/CD8+ cell ratios (Fig. 4C). Hence, HIV+ mice with high levels of CD8+ cell activation lost more CD4+ cells. No correlation between CD4+/CD8+ cell ratios and CD8+ cell activation was seen in HIV − mice (data not shown, P = 0.423, r = 0.190). In the absence of HIV infection, no loss of CD4+ cells occurred, indicating that HIV causes the preferential loss of activated CD4+ cells. Indeed, higher CD8+ cell activation levels—this time calculated in relation to total splenocytes to take engraftment level also into account—correlated with higher viral loads (Fig. 4D). In the HIV+ humanized mice, activation of CD4+ cells seemed to be masked by HIV infection and rapid loss of these cells, maybe even before full expression of activation markers.
Discussion
Dysfunction of the intestinal barrier has severe consequences for the whole organism. It leads to translocation of bacteria from the gut and mediates inflammation. In chronic HIV infection, for instance, elevated levels of circulating bacterial products are associated with T-cell activation and disease progression [8]. We established a humanized mouse model of intestinal barrier dysfunction to determine the systemic effects of bacterial translocation. In our model, permeability corresponded well with translocation. But plasma LPS levels, the classical marker of bacterial translocation, depended only partially on barrier dysfunction. Macrophages compensated for the increased bacterial translocation by up-regulating their phagocytic capacity and thereby kept plasma LPS levels in a normal range. In HIV-infected mice, however, LPS clearance was inadequate leading to increased plasma LPS levels, high T-cell activation, and vigorous HIV replication.
A multilayered barrier protects the body from invading intestinal bacteria. The first line of defense is a tight, mucus-coated sheath of intestinal epithelial cells. Moreover, leukocytes in the underlying lamina propria contribute to the protection against bacteria. The humanized mice we used have low intestinal lymphocytes numbers [22]. Therefore, changes of the epithelial integrity probably have a relatively big impact on translocation in humanized mice, even without obvious histopathological changes. While there was no obvious difference in FITC-dextran translocation between humanized and wild-type mice, intestinal permeability tended to increase after HIV infection or low dose DSS treatment (Fig. S2B). Notably, HIV+ mice had high plasma TNF-alpha levels (Fig. S3). TNF-alpha disrupts tight junctions and induces apoptosis of intestinal epithelial cells [29], thereby mediating barrier dysfunction. Baseline translocation in HIV−/DSS − mice was quite high with one third of the MLN cultures containing bacteria (Fig. 1A and B). HIV+ or DSS+ mice more frequently had bacteria translocating to MLN and spleen—in accordance with the increased FITC-dextran permeability. From our data, it is not possible to infer the exact role disturbance of epithelial permeability and intestinal lymphocyte depletion plays in human HIV infection. But in general, the amount of bacterial translocation depends on barrier integrity.
Surprisingly, plasma LPS levels showed a different pattern (Fig. 1C). They did not depend strictly on permeability and translocation. In our experiments, HIV+ mice had elevated plasma LPS levels. HIV−/DSS+ mice controlled the increased influx of bacteria from the gut by raising their ability to clear LPS. LPS elevation only occurred in animals that had disturbance of the intestinal barrier and at the same time LPS clearance defects, either because of macrophage depletion or HIV infection (Fig. 2). Thus, macrophage phagocytosis seems to be critical in protecting against systemic translocation and failed clearance leads to systemic elevation of bacterial products. This hypothesis is supported by studies of inflammatory bowel disease. Plasma LPS levels are elevated in active inflammatory bowel disease, when the intestinal barrier is disrupted [4]. Furthermore, patients with Crohn's disease clear subcutaneously injected bacteria very slowly, their macrophages secrete few pro-inflammatory cytokines in response to bacteria or TLR ligands, and the transcription profiles of these macrophages show defects in vesicle trafficking and cytoskeletal organization [30]. This indicates that defects similar to the dysfunctional phagocytosis seen in our model might be important in inflammatory bowel diseases.
The mechanism leading to macrophage dysfunction in HIV+ mice is not clear. It is tempting to postulate induction of an “endotoxin-tolerant” macrophage phenotype in HIV−/DSS+ mice, and loss of tolerance induction in HIV+ mice. Endotoxin tolerance is characterized by programming of macrophages towards phagocytosis instead of production of pro-inflammatory cytokines upon LPS re-exposure [31]. Indeed, plasma IL-12 and TNF-alpha levels were normal in HIV−/DSS+ mice despite increased bacterial translocation. In contrast, HIV+ mice had high levels of pro-inflammatory cytokines (Fig. S3). At the moment, the factors inhibiting tolerance induction are unknown. Duration of translocation might play a role: HIV−/DSS+ mice had barrier dysfunction for the relatively short period of two weeks. Control of longer lasting translocation might not be as easy. Otherwise, the pro-inflammatory state generated by HIV infection might influence macrophage function. Macrophages integrate a broad range of environmental information. Some cytokines [32] and bacterial products [33], [34] sensitize cells to LPS stimulation. Furthermore, viral products and/or cytokines produced due to virus infection interfere with endotoxin tolerance induction in chronically HCV - [35] or HIV - [36] infected patients. Instead monocytes from these patients produce more TNF-alpha upon LPS re-stimulation.
So far, it is unknown if LPS clearance dysfunction also exists in HIV-positive humans, although some evidence supports the existence of macrophage defects in chronic HIV infection. Plasma LPS levels in acutely HIV-infected patients are not elevated [8], despite early depletion of gut lymphocytes. During treatment interruption, LPS levels rise only after a few weeks of viral replication [7]. Early after antiretroviral therapy is stopped, falling EndoCAb levels indicate functional LPS clearance. Thereafter, elevation of LPS levels suggests the onset of clearance defects. In fact, HIV inhibits macrophage maturation [37] and phagocytosis [38], [39]. Monocytes from HIV-infected individuals show impaired Mycobacterium phagocytosis [40]; Saccharomyces up-take is also decreased, and LPS-mediated enhancement of phagocytosis is less than the enhancement in monocytes from healthy donors [41]. Moreover, monocytes from HIV-infected patients show an attenuated pro-inflammatory cytokine response to LPS stimulation ex vivo [8], [42], maybe due to in vivo pre-stimulation. Serum IL-12 and TNF-alpha levels, which reflect in vivo cytokine production, are higher [43]–[46] - similar to the increased cytokine levels we observed in HIV+ mice. Overall, our findings in HIV-infected humanized mice resemble the results from HIV-infected humans.
Certainly, not all aspects of human HIV infection can be modeled accurately in humanized mice. For example, direct HIV infection of macrophages is rare in the mice. Engraftment of human monocytes was low; less than 2% of all monocytes were of human origin (data not shown). Most macrophages are of murine origin and therefore resistant to HIV infection. In humans, macrophage permissiveness to HIV infection varies from tissue to tissue. For example, intestinal macrophages are quite resistant to infection, but vaginal macrophages are readily infected [47]. However, productive infection of macrophages is, in general, infrequent in vivo [48], except for late stages of disease when opportunistic infections occur [49]. To definitely determine the importance of direct viral infection of macrophages, other models with bigger populations of human myeloid cells would be useful.
Lymphoid engraftment, however, is quite good in humanized mice. This allowed the investigation of T-cell activation in DSS-treated or HIV-infected mice. Bacterial translocation induced CD4+ and CD8+ cell activation (Fig. 4). Other activating factors (i.e., stimulation of TLR7/8 by HIV RNA [50]–[52]) might also have played a role. However, in HIV−/DSS+ mice, such other activating factors were not present, and bacterial translocation alone activated T cells. Since plasma LPS levels were not elevated in these mice, immune activation might have been mediated by other bacterial components, such as peptioglycan, flagellin, or bacterial DNA. Moreover, LPS flux—input from the gut and subsequent clearance—was greater in these mice. A greater LPS flux might activate the immune system. At least, it induced and sustained stimulation of macrophage phagocytosis. Uncontrolled bacterial translocation, as seen in HIV+ mice, might reasonably caused higher levels of lymphocyte activation. Not surprisingly, DSS treatment had no effect on T-cell activation in HIV+ mice. The bacterial translocation indices and LPS phagocytosis capacities were almost identical in HIV+/DSS − and HIV+/DSS+ groups, leading to similar LPS, LBP and sC14 levels and, therefore, to similar activation levels.
Our results might explain why levels of CD8+ T-cell activation are especially good markers of disease outcome [53], even though CD8+ T cells are not direct viral targets. In HIV-uninfected mice CD8+ and CD4+ cell activation levels were tightly correlated. Thus, CD8+ T-cell activation levels seem to predict the amount of activated and HIV-permissive CD4+ T cells. These cells have a very short lifespan; the half-life of an infected CD4+ T cell is estimated at 1.6 days [54]. Additionally, measurements of lymphocyte telomere lengths indicate that, in HIV-infected individuals, only CD8+ T-cell turnover is increased, while telomeres in CD4+ T cells show no excessive turnover [55]. CD4+ T cells might be lost due to HIV infection before cell division is accomplished. This could also explain the relative deficit of activated CD4+ cells we observed in HIV+ animals. HIV preferably infects activated CD4+ cells [56]. Indeed, those mice with the highest percentages of activated CD8+ cells had the lowest CD4+/CD8+ cell ratios and the highest HIV plasma loads.
In conclusion, we identified a critical role of macrophages in protection from systemic bacterial translocation. In humanized mice, failure of LPS clearance was associated with high levels of T-cell activation and HIV replication. Macrophage dysfunction might be an underestimated mechanism in HIV-induced immunodeficiency and certainly warrants further investigation.
Materials and Methods
Ethics statement
All experiments were approved by ethical committees of the University Zurich and the Federal Veterinary Department and were conducted according to local guidelines (TschV, Zurich) and the Swiss animal protection law (TschG). Cord blood was collected with written consent of the parents.
Generation and HIV infection of humanized mice
Mice were reconstituted and infected as described [19]. Briefly, newborn RAG2−/−gammac−/− mice were irradiated with 2×2 Gy. CD34+ cells were isolated from human cord blood with immunomagnetic beads (Miltenyi Biotec), and 2.75±0.5×105 cells were transplanted into each mouse. After 10 to 16 weeks, the degree of blood engraftment was determined by flow cytometry of peripheral blood mononuclear cells stained for the panhuman marker CD45 in all mice (mean human cells/live cells 5.6±5.4% SD). Mice were infected intraperitoneally with HIV YU-2, 2×105 of the tissue-culture infectious dose50 per mouse. Plasma viral loads were measured by RT-PCR (Amplicor, Roche) 4–6 weeks after inoculation and at the end of each experiment. The detection limit was 1,600 HIV RNA copies/ml. Activation levels of T cells were measured by flow cytometry of splenocytes stained for human CD45, CD4, CD8, HLA-DR, CD38, and appropriate isotype controls (all from BD Biosciences). In all experiments, mouse litters and cord blood donors were evenly distributed to all experimental groups.
Induction and measurement of bacterial translocation
Bacterial translocation was induced by adding 0.75% (w/v) DSS (molecular weight 40,000, MP Biomedicals) to the drinking water for 2 weeks. Spleen and MLN were removed aseptically, mashed with a pestle in PBS, and plated on sheep blood and MacConkey agar plates. Plates were only incubated aerobically, since in a pilot experiment, no anaerobic bacteria could be detected in organ cultures. As a control, diluted stool samples were cultured both in aerobic and anaerobic conditions. A descriptive bacterial translocation index was calculated from organ culture results (no bacterial growth = 0 points, 10 to 99 cfu/organ = 1 point, 100 to 999 cfu/organ = 2 points, >1000 cfu/organ = 3 points, for all organs points were multiplied by the number of bacterial species detected. For spleen cultures, points were doubled, and finally, all points from one mouse were added up). LPS was quantified by endpoint chromogenic limulus amoebocyte lysate assay (Lonza). Plasma samples were diluted 1∶10 with endotoxin free water, incubated at 85°C for 12 min, and assayed according to the manufacturer's instructions. Standard endotoxin (Lonza) was diluted to cover plasma LPS values within a range of 0.5 to 20 EU/ml. Plasma mouse sCD14 and LBP were measured by ELISA (both from CellSciences), according to the manufacturer's instructions. For sCD14 measurement, samples were diluted 1∶150, and for LBP measurement, 1∶800.
Ex vivo LPS phagocytosis
Liver mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation. To further purify macrophages, cells were incubated for 3 h in RPMI, 10% FCS, penicillin/streptomycin, and L-glutamine at 37°C, 5% CO2, and then washed two times with room temperature PBS to remove non-adherent cells. This procedure yielded over 90% murine CD11b+ cells. Cells were then incubated with 0.1 mg/ml FITC-LPS (Sigma) in RPMI, 10% FCS, penicillin/streptomycin, and L-glutamine at 37°C, 5% CO2 for 1 h. As a control, cells were also incubated with FITC-LPS at 4°C. Cells were then washed two times with cold PBS, detached with trypsin, washed once again, and analyzed by flow cytometry. FITC-LPS mean fluorescent intensity was normalized to the fluorescence of samples from HIV−/DSS − mice.
Macrophage depletion
Mice were injected intraperitoneally with clodrolip (1 mg/20 g body weight) or with PBS [57]. After 48 h, depletion was verified in four mice by staining of spleen and liver cells for CD11b. To maintain depletion, mice were treated a second time with clodrolip 0.5 mg/20 g body weight or with PBS intraperitoneally 4 days after the first injection. Concurrently, half of the mice received DSS 0.75% (w/v) in their drinking water. After 1 week, the mice were sacrificed, and their plasma LPS levels were measured.
Statistical analysis
GraphPad Prism 5.02 was used for statistical analysis. Data were analyzed by parametric one-way analysis of variance, followed by Bonferroni post-test. All p-values shown are adjusted for multiple comparisons. In the figures, p-values are presented for comparisons between treatment groups and controls and are denoted by asterisks. Values for HIV/DSS experiments for plasma LPS, viral load, FITC-dextran fluorescence, and percentages of activated CD8+ cells (of total cells) were log transformed before analysis to reduce right-skewing of the data. For all correlations, Pearson's correlation coefficient was calculated. In all figures, points represent values of individual mice, and lines depict mean values.
Supporting Information
Zdroje
1. PandreaI
SodoraDL
SilvestriG
ApetreiC
2008 Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends in Immunology 29 419 428
2. DouekDC
RoedererM
KoupRA
2009 Emerging Concepts in the Immunopathogenesis of AIDS. Annual Review of Medicine 60
3. AncutaP
KamatA
KunstmanKJ
KimE-Y
AutissierP
2008 Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE 3 e2516 doi:10.1371/journal.pone.0002516
4. GregsonJN
SteelA
BowerM
GazzardBG
GotchFM
2009 Elevated plasma lipopolysaccharide is not sufficient to drive natural killer cell activation in HIV-1-infected individuals. AIDS 23 29 34
5. HuntPW
BrenchleyJ
SinclairE
McCuneJM
RolandM
2008 Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 197 126 133
6. MarchettiG
BellistriGM
BorghiE
TincatiC
FerramoscaS
2008 Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 22 2035 2038
7. PapasavvasE
PistilliM
ReynoldsG
BuckiR
AzzoniL
2009 Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS 23 369 375
8. BrenchleyJM
PriceDA
SchackerTW
AsherTE
SilvestriG
2006 Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12 1365 1371
9. HeiseC
MillerCJ
LacknerA
DandekarS
1994 Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis 169 1116 1120
10. VeazeyRS
DeMariaM
ChalifouxLV
ShvetzDE
PauleyDR
1998 Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280 427 431
11. MehandruS
PolesMA
Tenner-RaczK
HorowitzA
HurleyA
2004 Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 200 761 770
12. HoferU
SpeckRF
2009 Disturbance of the gut-associated lymphoid tissue is associated with disease progression in chronic HIV infection. Semin Immunopathol 31 257 266
13. PaiardiniM
FrankI
PandreaI
ApetreiC
SilvestriG
2008 Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev 10 36 46
14. GordonSN
KlattNR
BosingerSE
BrenchleyJM
MilushJM
2007 Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol 179 3026 3034
15. PandreaIV
GautamR
RibeiroRM
BrenchleyJM
ButlerIF
2007 Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol 179 3035 3046
16. BrenchleyJM
PaiardiniM
KnoxKS
AsherAI
CervasiB
2008 Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112 2826 2835
17. RaffatelluM
SantosRL
VerhoevenDE
GeorgeMD
WilsonRP
2008 Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14 421 428
18. TraggiaiE
ChichaL
MazzucchelliL
BronzL
PiffarettiJ-C
2004 Development of a Human Adaptive Immune System in Cord Blood Cell-Transplanted Mice. Science 304 104 107
19. BaenzigerS
TussiwandR
SchlaepferE
MazzucchelliL
HeikenwalderM
2006 Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−gamma c−/ − mice. Proc Natl Acad Sci U S A 103 15951 15956
20. GorantlaS
SnellerH
WaltersL
SharpJG
PirruccelloSJ
2007 Human Immunodeficiency Virus Type 1 Pathobiology Studied in Humanized BALB/c-Rag2−/−{gamma}c−/ − Mice. J Virol 81 2700 2712
21. BergesBK
WheatWH
PalmerBE
ConnickE
AkkinaR
2006 HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/ − (RAG-hu) mouse model. Retrovirology 3 76
22. HoferU
BaenzigerS
HeikenwalderM
SchlaepferE
GehreN
2008 RAG2−/ − gamma(c)−/ − mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. J Virol 82 12145 12153
23. WirtzS
NeufertC
WeigmannB
NeurathMF
2007 Chemically induced mouse models of intestinal inflammation. Nat Protocols 2 541
24. VetuschiA
LatellaG
SferraR
CaprilliR
GaudioE
2002 Increased Proliferation and Apoptosis of Colonic Epithelial Cells in Dextran Sulfate Sodium-Induced Colitis in Rats. Digestive Diseases and Sciences 47 1447 1457
25. TessnerTG
CohnSM
SchloemannS
StensonWF
1998 Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology 115 874 882
26. LiQ
EstesJD
DuanL
JessurunJ
PambuccianS
2008 Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J Infect Dis 197 420 429
27. HopfU
RamadoriG
MollerB
GalanosC
1984 Hepatocellular clearance function of bacterial lipopolysaccharides and free lipid A in mice with endotoxic shock. Am J Emerg Med 2 13 19
28. FreudenbergMA
FreudenbergN
GalanosC
1982 Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol 63 56 65
29. GitterA
BendfeldtK
SchulzkeJ
FrommM
2000 Leaks in the epithelial barrier caused by spontaneous and TNF-{alpha}-induced single-cell apoptosis. FASEB J 14 1749 1753
30. SmithAM
RahmanFZ
HayeeBH
GrahamSJ
MarksDJB
2009 Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med 206 1883 1897
31. BiswasSK
Lopez-CollazoE
2009 Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends in Immunology 30 475 487
32. Adib-ConquyM
CavaillonJ-M
2002 Gamma Interferon and Granulocyte/Monocyte Colony-stimulating Factor Prevent Endotoxin Tolerance in Human Monocytes by Promoting Interleukin-1 Receptor-associated Kinase Expression and Its Association to MyD88 and Not by Modulating TLR4 Expression. Journal of Biological Chemistry 277 27927 27934
33. SchuchmannM
HermannF
HerkelJ
van der ZeeR
GallePR
2004 HSP60 and CpG-DNA-oligonucleotides differentially regulate LPS-tolerance of hepatic Kupffer cells. Immunology Letters 93 199 204
34. DalpkeAH
LehnerMD
HartungT
HeegK
2005 Differential effects of CpG-DNA in Toll-like receptor-2/-4/-9 tolerance and cross-tolerance. Immunology 116 203 212
35. DolganiucA
NorkinaO
KodysK
CatalanoD
BakisG
2007 Viral and Host Factors Induce Macrophage Activation and Loss of Toll-Like Receptor Tolerance in Chronic HCV Infection. Gastroenterology 133 1627 1636
36. LesterRT
YaoX-D
BallTB
McKinnonLR
OmangeWR
2009 HIV-1 RNA Dysregulates the Natural TLR Response to Subclinical Endotoxemia in Kenyan Female Sex-Workers. PLoS ONE 4 e5644 doi:10.1371/journal.pone.0005644
37. MuthumaniK
HwangDS
ChooAY
MayilvahananS
DayesNS
2005 HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int Immunol 17 103 116
38. BiggsBA
HewishM
KentS
HayesK
CroweSM
1995 HIV-1 infection of human macrophages impairs phagocytosis and killing of Toxoplasma gondii. J Immunol 154 6132 6139
39. KedzierskaK
AzzamR
ElleryP
MakJ
JaworowskiA
2003 Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy. Journal of Clinical Virology 26 247 263
40. KafKedzierska
JagMak
AaeJaworowski
AbgGreenway
AaViolo
2001 nef-deleted HIV-1 inhibits phagocytosis by monocyte-derived macrophages in vitro but not by peripheral blood monocytes in vivo. AIDS 15 945 955
41. BaquiAA
MeillerTF
ZhangM
FalklerWAJr
1999 The effects of HIV viral load on the phagocytic activity of monocytes activated with lipopolysaccharide from oral microorganisms. Immunopharmacol Immunotoxicol 21 421 438
42. MarshallJD
ChehimiJ
GriG
KostmanJR
MontanerLJ
1999 The Interleukin-12-Mediated Pathway of Immune Events Is Dysfunctional in Human Immunodeficiency Virus-Infected Individuals. Blood 94 1003 1011
43. RockstrohJK
KreuzerKA
SauerbruchT
SpenglerU
1998 Protein levels of interleukin-12 p70 Holomer, its p40 chain and interferon-gamma during advancing HIV infection. Journal of Infection 37 282 286
44. ByrnesAA
HarrisDM
AtabaniSF
SabundayoBP
LanganSJ
2008 Immune activation and IL-12 production during acute/early HIV infection in the absence and presence of highly active, antiretroviral therapy. J Leukoc Biol 84 1447 1453
45. AyehunieS
SonnerborgA
Yemane-BerhanT
ZewdieDW
BrittonS
1993 Raised levels of tumour necrosis factor-alpha and neopterin, but not interferon-alpha, in serum of HIV-1-infected patients from Ethiopia. Clin Exp Immunol 91 37 42
46. von SydowM
SonnerborgA
GainesH
StrannegardO
1991 Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses 7 375 380
47. ShenR
RichterHE
ClementsRH
NovakL
HuffK
2009 Macrophages in Vaginal but Not Intestinal Mucosa Are Monocyte-Like and Permissive to Human Immunodeficiency Virus Type 1 Infection. J Virol 83 3258 3267
48. EmbretsonJ
ZupancicM
RibasJL
BurkeA
RaczP
1993 Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362 359 362
49. OrensteinJM
FoxC
WahlSM
1997 Macrophages as a Source of HIV During Opportunistic Infections. Science 276 1857 1861
50. BaenzigerS
HeikenwalderM
JohansenP
SchlaepferE
HoferU
2009 Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood 113 377 388
51. MandlJN
BarryAP
VanderfordTH
KozyrN
ChavanR
2008 Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med 14 1077 1087
52. HeilF
HemmiH
HochreinH
AmpenbergerF
KirschningC
2004 Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science 303 1526 1529
53. LiuZ
CumberlandWG
HultinLE
PrinceHE
DetelsR
1997 Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol 16 83 92
54. PerelsonAS
NeumannAU
MarkowitzM
LeonardJM
HoDD
1996 HIV-1 Dynamics in Vivo: Virion Clearance Rate, Infected Cell Life-Span, and Viral Generation Time. Science 271 1582 1586
55. WolthersKC
WismanGBA
OttoSA
de Roda HusmanA-M
SchaftN
1996 T Cell Telomere Length in HIV-1 Infection: No Evidence for Increased CD4+ T Cell Turnover. Science 274 1543 1547
56. CullenBR
GreeneWC
1989 Regulatory pathways governing HIV-1 replication. Cell 58 423 426
57. ZeisbergerSM
OdermattB
MartyC
Zehnder-FjallmanAH
Ballmer-HoferK
2006 Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer 95 272 281
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



