-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIncreased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
Cells of the myeloid lineage are significant targets for human immunodeficiency virus (HIV) in humans and simian immunodeficiency virus (SIV) in monkeys. Monocytes play critical roles in innate and adaptive immunity during inflammation. We hypothesize that specific subsets of monocytes expand with AIDS and drive central nervous system (CNS) disease. Additionally, there may be expansion of cells from the bone marrow through blood with subsequent macrophage accumulation in tissues driving pathogenesis. To identify monocytes that recently emigrated from bone marrow, we used 5-bromo-2′-deoxyuridine (BrdU) labeling in a longitudinal study of SIV-infected CD8+ T lymphocyte depleted macaques. Monocyte expansion and kinetics in blood was assessed and newly migrated monocyte/macrophages were identified within the CNS. Five animals developed rapid AIDS with differing severity of SIVE. The percentages of BrdU+ monocytes in these animals increased dramatically, early after infection, peaking at necropsy where the percentage of BrdU+ monocytes correlated with the severity of SIVE. Early analysis revealed changes in the percentages of BrdU+ monocytes between slow and rapid progressors as early as 8 days and consistently by 27 days post infection. Soluble CD163 (sCD163) in plasma correlated with the percentage of BrdU+ monocytes in blood, demonstrating a relationship between monocyte activation and expansion with disease. BrdU+ monocytes/macrophages were found within perivascular spaces and SIVE lesions. The majority (80–90%) of the BrdU+ cells were Mac387+ that were not productively infected. There was a minor population of CD68+BrdU+ cells (<10%), very few of which were infected (<1% of total BrdU+ cells). Our results suggest that an increased rate of monocyte recruitment from bone marrow into the blood correlates with rapid progression to AIDS, and the magnitude of BrdU+ monocytes correlates with the severity of SIVE.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000842
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000842Summary
Cells of the myeloid lineage are significant targets for human immunodeficiency virus (HIV) in humans and simian immunodeficiency virus (SIV) in monkeys. Monocytes play critical roles in innate and adaptive immunity during inflammation. We hypothesize that specific subsets of monocytes expand with AIDS and drive central nervous system (CNS) disease. Additionally, there may be expansion of cells from the bone marrow through blood with subsequent macrophage accumulation in tissues driving pathogenesis. To identify monocytes that recently emigrated from bone marrow, we used 5-bromo-2′-deoxyuridine (BrdU) labeling in a longitudinal study of SIV-infected CD8+ T lymphocyte depleted macaques. Monocyte expansion and kinetics in blood was assessed and newly migrated monocyte/macrophages were identified within the CNS. Five animals developed rapid AIDS with differing severity of SIVE. The percentages of BrdU+ monocytes in these animals increased dramatically, early after infection, peaking at necropsy where the percentage of BrdU+ monocytes correlated with the severity of SIVE. Early analysis revealed changes in the percentages of BrdU+ monocytes between slow and rapid progressors as early as 8 days and consistently by 27 days post infection. Soluble CD163 (sCD163) in plasma correlated with the percentage of BrdU+ monocytes in blood, demonstrating a relationship between monocyte activation and expansion with disease. BrdU+ monocytes/macrophages were found within perivascular spaces and SIVE lesions. The majority (80–90%) of the BrdU+ cells were Mac387+ that were not productively infected. There was a minor population of CD68+BrdU+ cells (<10%), very few of which were infected (<1% of total BrdU+ cells). Our results suggest that an increased rate of monocyte recruitment from bone marrow into the blood correlates with rapid progression to AIDS, and the magnitude of BrdU+ monocytes correlates with the severity of SIVE.
Introduction
Monocytes of bone marrow origin are circulating precursors that give rise to and replenish macrophage populations in tissues, including the brain [1]. Monocytes that originate from hematopoietic stem cells in bone marrow undergo three stages of differentiation from monoblasts to promonocytes and then monocytes where they are released into the circulation [2]–[4]. Current dogma defines that human and non-human primate monocytes do not divide out of the bone marrow [5]. Blood monocytes are thought to circulate in the vasculature for approximately 24–72 hours before differentiation into macrophages in tissues [2], [6]. Continuous extravasation and differentiation of circulating monocytic precursors has long been considered the sole source of tissue macrophages [7]. Other mechanisms to maintain tissue macrophage homeostasis have been identified and described in rodents including: 1) self-renewal of differentiated resident cells and 2) homing and limited proliferation of bone marrow derived precursors in tissues [5], [8]–[11]. Such mechanisms are not thought to function in humans. Nevertheless, in both rodents and primates in acute inflammation, monocytes are recruited to tissue compartments [2], [12]–[14]. With acute inflammation, the half-life of circulating monocytes is decreased coincident with an accumulation of macrophages at the inflamed site [15], [16]. The half-life of circulating monocytes in chronic inflammation is undefined. Prior studies of monocyte kinetics used autoradiographic analysis and radiolabeled thymidine or indium chloride incorporation, which was powerful but of limited utility due to the toxicity of radiolabel [6], [15]–[18]. More recently, we and others used the thymidine analog 5′-bromo-2′-deoxyuridine (BrdU) to quantify the turnover and release of monocytes from bone marrow [19]–[24]. BrdU is incorporated into cellular DNA during replication, at the S-phase of the cell cycle. Monocytes are released from the bone marrow into the circulation shortly after the completion of S phase, thus BrdU is a reliable marker for monocytes newly released into blood [20], [21]. We have shown that increased BrdU incorporation in monocytes with SIV infection is associated with macrophage cell death in lymph nodes [20]. Increased percentage of BrdU+ monocytes correlated with AIDS more so than CD4+ T lymphocyte loss or viral load [20]. In this report, we confirm and extend these observations in a serial pathogenesis study of monocyte expansion with emphasis on the severity of SIVE and identification of a plasma marker of monocyte expansion.
Monocytes have been shown to play a critical role in HIV and SIV disease pathogenesis [25]–[29]. The expansion of the number and/or relative percentage of CD14+CD16+ monocytes correlates with the incidence of HIV encephalitis (HIVE) and accumulation of monocyte/macrophages in HIVE lesions correlates with dementia [25], [30], [31]. Similarly, our laboratory has shown that SIV-infected CD8+ T lymphocyte depleted rhesus macaques have a biphasic increase in the percentage and absolute numbers of CD14+CD16+ monocytes with viremia and later with the development of AIDS [32]–[35]. Whether this is from increased recruitment of monocytes from bone marrow or recirculation from tissue sources is not defined.
We hypothesized that increased monocyte production from bone marrow and traffic into the brain during SIV infection correlates with rapid development of AIDS and severity of SIVE. To test this, we examined BrdU+ incorporation of monocytes in a longitudinal serial-sample pathogenesis study using SIV-infected CD8+ T lymphocyte depleted rhesus monkeys [36]–[38]. We show BrdU+ monocytes are negative for the proliferation marker Ki-67, consistent with monocytes labeled with BrdU in the marrow that do not proliferate in the blood. We demonstrate a significant correlation between the increased percentage of BrdU+ monocytes in blood at necropsy and the severity of SIV disease. Moreover, we find within CD8+ T lymphocyte depleted animals the magnitude of BrdU+ monocytes is equal with the rate of disease progression. We have identified BrdU+ monocyte/macrophages accumulating in the CNS perivascular space and SIVE lesions. About 80–90% of BrdU+ cells are Mac387+ that are not productively infected and likely representative of recently recruited monocyte/macrophages. A rare population of CD68+ macrophages are BrdU+ and also productively infected. These results suggest that an increased number of monocytes emigrating from the bone marrow occurs with rapid progression to AIDS and correlates with the severity of SIVE at necropsy. These data further point to the traffic of BrdU+ cells into the CNS. We did not find a correlation between BrdU+ monocytes in blood and plasma LPS levels, but found a correlation with soluble CD163 (sCD163) levels in plasma, consistent with monocyte activation and stimulation of innate immunity [39]–[45]. Overall these data suggest that increased monocyte production from bone marrow, traffic to the brain, and overall monocyte activation play major roles in HIV and SIV neuropathogenesis.
Results
In this pathogenesis study, we utilized seven SIVmac251-infected rhesus macaques that were depleted of CD8+ T lymphocytes by three injections of a CD8-specific mouse-human chimeric antibody (cM-T807) at 6, 8 and 12 days post infection. Of these, one animal was CD8+ T lymphocyte depleted for 21 days (“transiently” depleted) and six for greater than 28 days (“persistently” depleted) (Table 1). Animals were sacrificed upon the development of AIDS (criteria described in Materials and Methods). This cohort could be subdivided into slow and rapid progressors. Of the rapid progressors, five animals were sacrificed with AIDS (56, 75, 77, 89 and 92 days post infection) and four had SIV encephalitis (SIVE) (criteria described in Materials and Methods). Of the two slow progressors, one was sacrificed at 131 days post infection and the other did not develop AIDS and is still alive (Table 1).
Tab. 1. SIV CD8+ T lymphocyte depleted animals used in the study. 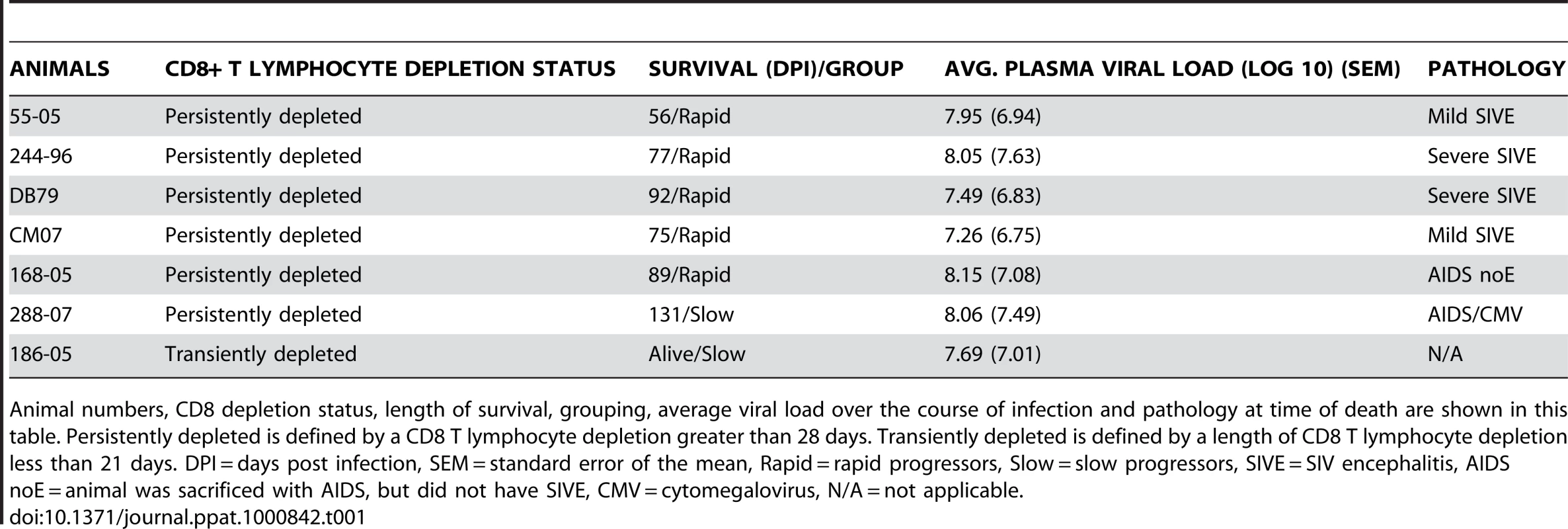
Animal numbers, CD8 depletion status, length of survival, grouping, average viral load over the course of infection and pathology at time of death are shown in this table. Persistently depleted is defined by a CD8 T lymphocyte depletion greater than 28 days. Transiently depleted is defined by a length of CD8 T lymphocyte depletion less than 21 days. DPI = days post infection, SEM = standard error of the mean, Rapid = rapid progressors, Slow = slow progressors, SIVE = SIV encephalitis, AIDS noE = animal was sacrificed with AIDS, but did not have SIVE, CMV = cytomegalovirus, N/A = not applicable. To examine if the rate of monocyte turnover and traffic into the brain during SIV infection correlates with disease progression, we performed serial sample of monocytes 24hrs after BrdU injection at multiple time points. Flow cytometry on whole blood was used to identify BrdU+ monocytes with the gating strategy defined in Figure 1. Monocytes were first identified by FSC versus SSC profiles, and selection of HLA−DR+ cells with exclusion of CD3+, CD8+, and CD20+ cells. The percentage of BrdU+ monocytes was calculated from total monocytes: CD14+CD16−, CD14+CD16+ and CD14−CD16+ (excluding CD14−CD16 − cells) (Figure 1). We have previously shown that monocytes in the bone marrow were BrdU+Ki-67+ [20]. However, in blood we found that CD14+ monocytes are BrdU+Ki-67 − (Figure 1). These results are consistent with non-proliferating blood monocytes labeled with BrdU in bone marrow that lose Ki-67 post-proliferation (within 24–48 hours), when cells are in the blood [3], [46]–[49]. The CD14−CD16 − cells, likely comprised of CD34+ hematopoietic stem cells and dendritic cells (DCs), are BrdU+ and Ki-67+ (Figure 1). These data are consistent with recent reports [20], [21].
Fig. 1. Gating strategy for identifying BrdU+ monocytes. 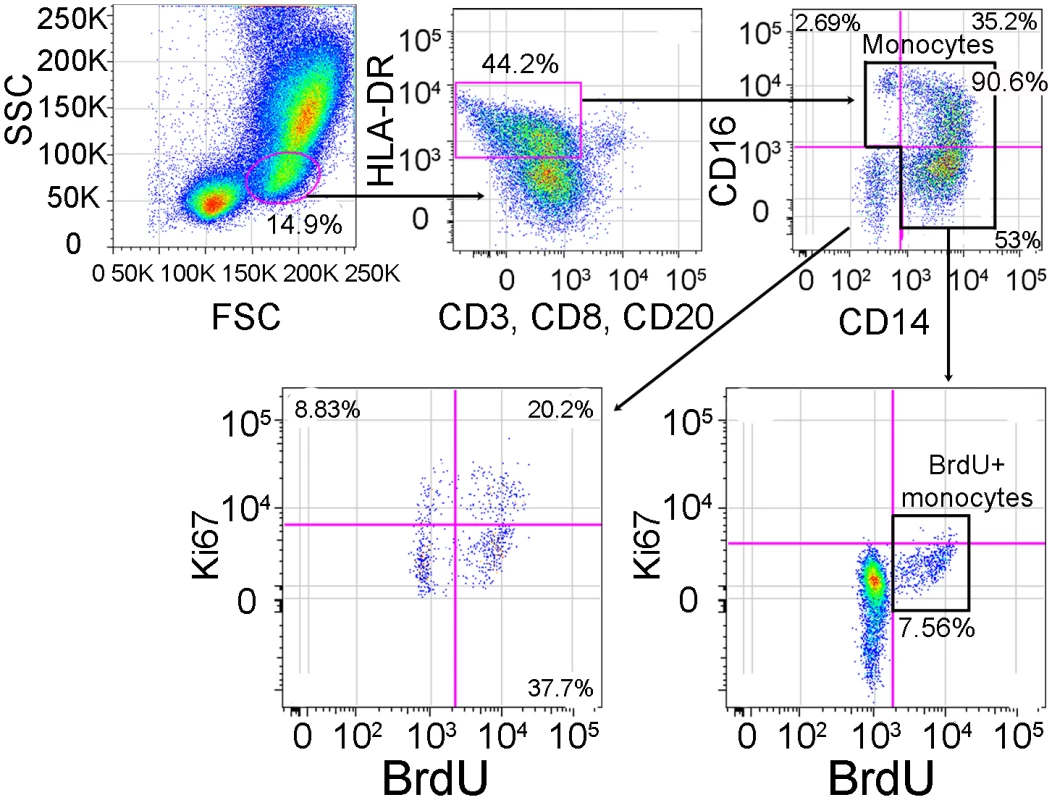
In order to identify BrdU+ monocytes after in vivo BrdU injection, we used flow cytometry on whole blood. We first gated on the monocytes based on their forward (FSC) vs. side scatter (SSC) properties (far left panel), then excluded HLA-DR negative cells as well as T-lymphocytes using anti-CD3, NK cells using anti-CD8 and B-lymphocytes using anti-CD20 (second panel from left). Next, we examined monocytes based on their expression of CD14 and CD16 (right top panel). We examined the expression of Ki-67 and BrdU on monocytes (including CD14+CD16−, CD14+CD16+ and CD14−CD16+) (bottom right panel). For the purpose of this study, CD14−CD16− cells were excluded from all further analyses since this population contains CD34+ hematopoietic stem cells and dendritic cells (bottom left panel). This gating and data are representative of an infected rapid progressor at day 27 post-infection out of all animals studied. BrdU incorporation in monocytes 24hrs post-BrdU injection in control, uninfected CD8+ T lymphocyte depleted animals showed that CD8+ T lymphocyte depletion alone did not affect monocyte turnover (Figure 2A; n = 4). The percentage of BrdU+ monocytes after CD8+ T lymphocyte depletion was the same as pre-depletion (approximately 2%). There was no correlation between the percentage of BrdU+ monocytes and days post CD8+ T lymphocyte depletion in uninfected animals (r = 0.02427, P = 0.9289). There was an increase in BrdU+ monocytes in CD8+ T lymphocyte-depleted infected (Figure 2B–C) versus uninfected control animals (Figure 2A) and a higher percentage of BrdU+ monocytes in rapid (Figure 2C) versus slow progressors (Figure 2B). Differences in the percentage of BrdU+ monocytes between animals with rapid versus slow progression were evident as early as 8 and consistent at 27 days post infection (Figure 2B–C). No correlation was detected between the percentage of BrdU+ monocytes and days post infection in slow progressors (Figure 2B; r = −0.1464, P = 0.7294). In fact, the percentage of BrdU+ monocytes in these animals was similar to non-infected controls. There was a dramatic increase in the percentage of BrdU+ monocytes in the rapid progressors and a significant correlation between the percentage of BrdU+ monocytes and days post infection (Figure 2C; r = 0.7651, P = 0.0006). At end stage disease, the percentage of BrdU+ monocytes correlated with the severity of SIVE (Figure 2C). In rapid progressors, the percentage of BrdU+ cells prior to infection ranged from 0.85%, to 2.77%. This percentage increased with infection and at necropsy was 6.27% in an animal with AIDS but no SIVE (AIDS noE), 11.0% in an animal with mild SIVE, and 23.4% and 31.5% in animals with severe SIVE (Figure 2C). Thus, the percentage of BrdU+ monocytes correlated with time after infection in rapid progressors and was increased with severity of SIVE at necropsy (Figure 2C).
Fig. 2. Increased percentage of BrdU+ monocytes is predictive rapid progression to AIDS and severity of SIVE. 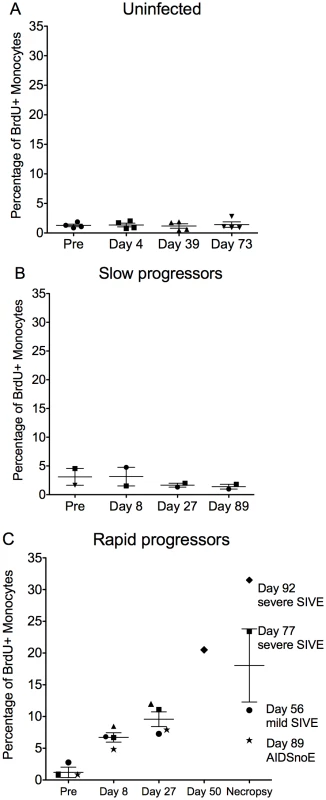
(A–C): BrdU was injected prior to (n = 1) and after SIVmac251 infection (n = 3) and the percentages of BrdU+ monocytes 24hrs after BrdU injection was determined by flow cytometric analysis. A. In four uninfected CD8+ T lymphocyte depleted animals, the percentage of BrdU+ monocytes remained approximately 2% of total monocytes in all time points. Thus, CD8+ T lymphocyte depletion alone without SIV infection does not alter monocyte turnover (n = 4). CD8+ T lymphocyte depleted SIV infected animals were divided into two groups: slow progressors (B) and rapid progressors (C). B. Monocyte turnover is unchanged at all points examined after infection in slow progressors (n = 2). C. The percentages of BrdU+ monocytes increased dramatically with rapid disease in animals that succumbed to AIDS (n = 5). The magnitude of BrdU+ incorporation of monocytes at necropsy can differentiate mild and severe SIVE. Each animal is represented by a different symbol. Error bars are standard error of the mean. To further study if increased monocyte turnover can predict rapid progression to AIDS, we examined other parameters previously linked to disease progression including CD4+ T lymphocyte numbers, CD4+ T lymphocyte turnover, and plasma viral load (Figure 3A–D). Using our model of CD8+ T lymphocyte depletion and SIV infection, plasma viral loads peaked by day 8 and remained high throughout the course of disease, consistent with persistently depleted animals (Table 1) [32], [36]. No correlation was found between percentage of BrdU+ monocytes and percentage of BrdU+CD4+ T lymphocytes (P = 0.2845) or numbers of CD4+ T lymphocytes (P = 0.6641) (Figure 3A and B, respectively). In addition, there was no correlation between plasma virus and the percentage of BrdU+ monocytes (P = 0.7880) or percentage of BrdU+CD4+ T lymphocytes (P = 0.3701) (Figure 3C and D, respectively). Because the number of CD4+ T cells increase with CD8+ lymphocyte depletion, the CD4+ numbers are not comparable to non-CD8 depleted animals [38].
Fig. 3. Percentage of BrdU+ monocytes does not correlate with plasma virus or CD4+ T lymphocyte turnover or numbers. 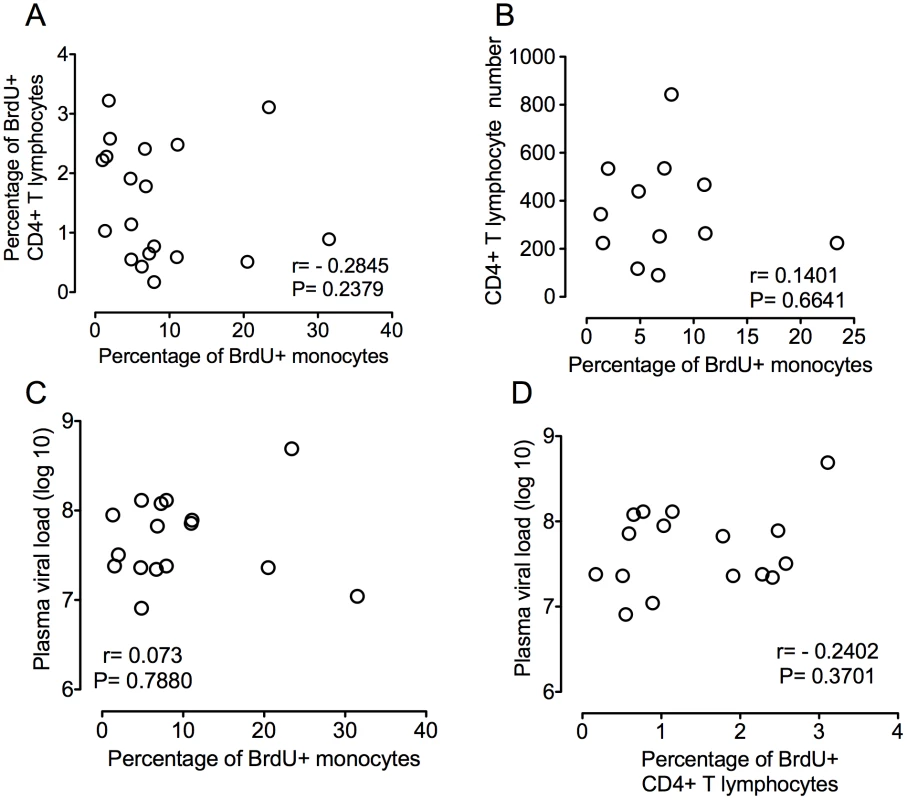
A. Paired XY values for percentage of BrdU incorporation in CD4+ T lymphocytes and monocytes in all infected animals are plotted. No correlation is found between the percentage of BrdU+ monocytes and the percentage of BrdU+ CD4+ T lymphocytes (P = 0.2379). B. Paired XY values for percentage of BrdU incorporation monocytes and CD4+ T lymphocyte counts in all seven infected animals are plotted. No correlation is found between the percentage of BrdU+ monocytes and CD4+ T cell counts (P = 6641). C. Paired XY values for percentage of monocyte BrdU incorporation and plasma viral load in all infected animals are plotted. No correlation is found between the percentage of BrdU+ monocytes and plasma viral loads P = 7880. D. Paired XY values for percentage of BrdU+ CD4+ T cells and plasma virus in all infected animals are plotted. No correlation is found between the percentage of BrdU+ CD4+ T cells and plasma viral loads P = 0.3701. A Spearman rank test is used for statistics. We examined the kinetics of BrdU+ monocyte subsets (CD14+CD16 − and CD14+CD16+) entering and exiting blood at four time points: pre-infection (9 days before infection), peak infection (7 days post infection), 26 days post infection, and either 88 days post infection (slow progressors) or 24hrs prior to necropsy (rapid progressors) (Figure 4A–B and 4C–D). The percentage of BrdU incorporation was measured at 24hrs and 48hrs and either 96 or 120hrs after a single BrdU injection. First, BrdU incorporation was examined before SIV infection. In all animals, the percentage of “classical” CD14+CD16 − monocytes that were BrdU+ peaked in blood 48hrs post BrdU (Figure 4A and C; red lines), whereas the “inflammatory” CD14+CD16+ cells that were BrdU+ peaked at 96hrs post BrdU (Figure 4B and D; red lines). This suggests that CD14+CD16+ cells might arise from CD14+CD16 − monocytes in the blood after leaving the bone marrow or that the majority of the CD14+CD16+ cells leave the bone marrow later than the CD14+CD16 − cells. Second, the effect of SIV infection on kinetics of BrdU+ monocyte subsets was examined. In contrast to slow progressors, a peak in the percentage of BrdU+CD14+CD16 − monocytes in rapid progressors appeared at 24hrs post BrdU after infection (Figure 4C; blue, green, black lines). The percentage of BrdU+CD14+CD16+ monocytes was increased after infection at 48hrs post BrdU in both slow and rapid progressors (Figures 4B and D; blue, green, black lines), with a greater increase in the rapid progressors (Figure 4D). There was no change in any of the monocyte subsets in the uninfected CD8+ T lymphocyte depleted animals (data not shown).
Fig. 4. Monocyte subsets leave the bone marrow at different rates that are accelerated in animals that develop SIVE. 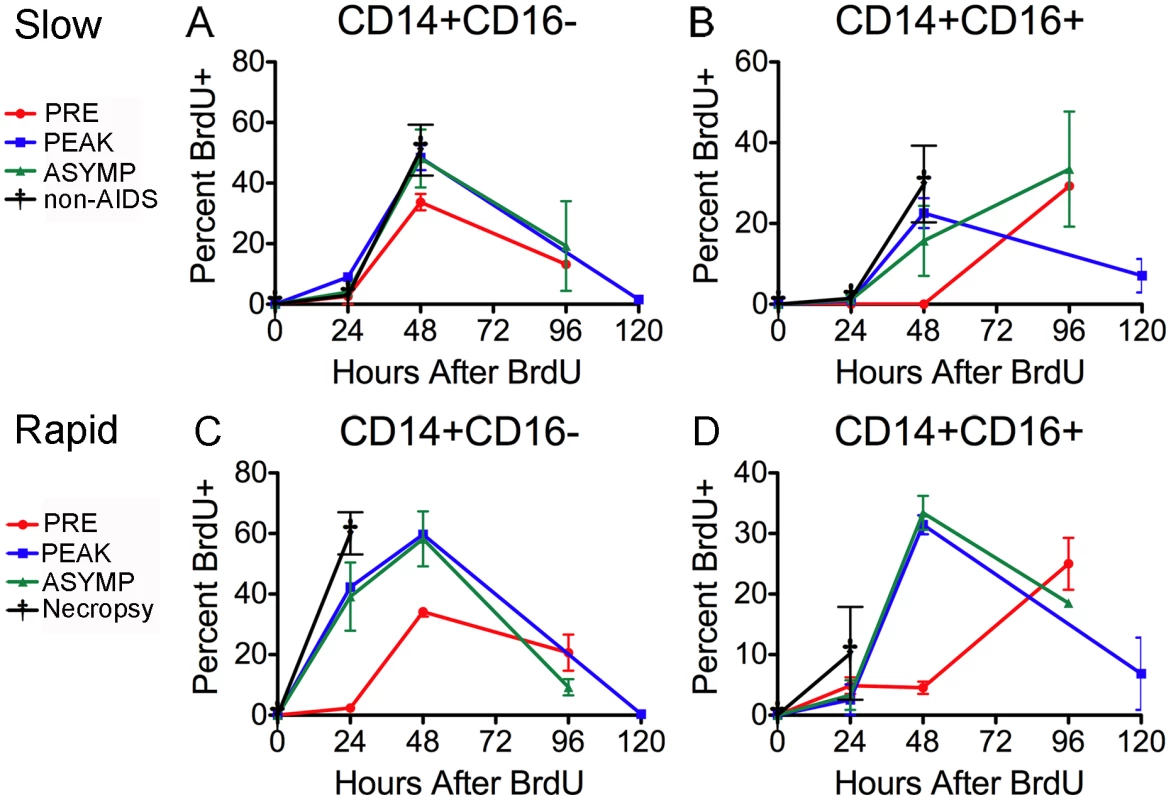
BrdU was injected four times over the course of the study. Red = pre-infection (days −10 days post infection (dpi)), Blue = peak infection (7 dpi), Green = “asymptomatic” period (26 dpi) and Black = 88 dpi (slow progressors = A–B) or necropsy (rapid progressors = C–D). BrdU injections were given at 24hrs before necropsy. The percentage of BrdU+ monocytes in each subset was determined 24hr and 48hr and either 96 or 120 hrs after BrdU injection (Time: 0 hr). (A–B) Dots represent the averages of the percentage of BrdU+ cells in the subsets of slow progressors that remained asymptomatic throughout the period examined. A. There is no change in the percentage of BrdU+ CD14+CD16− cells between pre- and post-infection time points. B. There is a slight increase in the percentage of BrdU+ CD14+CD16+cells after infection that is apparent at 48hrs. (C–D) Dots are averages from two rapid progressors (244-96 and 55-05). The percentage of BrdU+ monocytes was only examined 24hrs after BrdU pulse for animals, DB79 and CM07. C. The difference in the percentage of BrdU+CD14+CD16− cells between pre- and post-infection is apparent at 24hrs. D. The difference in the percentage of BrdU+ CD14+CD16+cells between pre- and post-infection occurs at 48hrs. The error in all graphs is the standard error of the mean. Consistent with previous literature describing an increase of CD14+CD16+ cell numbers in animals with SIVE [25]–[27], [30], [50], [51], the absolute number of CD14+CD16+ monocytes was elevated in the rapid progressors (data not shown). There was no change in the absolute number of CD14+CD16+ monocytes in the slow progressors throughout infection (data not shown).
Recent studies have shown an association of high plasma LPS with increased sCD14 thus implicating monocyte activation in HIV infection [52], [53]. Exact correlates between HIV dementia and plasma LPS were not found [53]. We therefore examined plasma LPS as a potential stimulus for emigration of monocytes from the bone marrow. There were no significant differences in plasma LPS levels between rapid and slow progressors (Figure 5A; rapid progressors = solid lines, slow progressors = dotted lines) and no significant correlation between the percentage of BrdU+ monocytes and plasma LPS levels (Figure 5B; r = −0.2040, P = 0.5038).
Fig. 5. sCD163 levels in plasma, but not LPS or CCL2, correlates with percentage of BrdU+ in total monocytes. 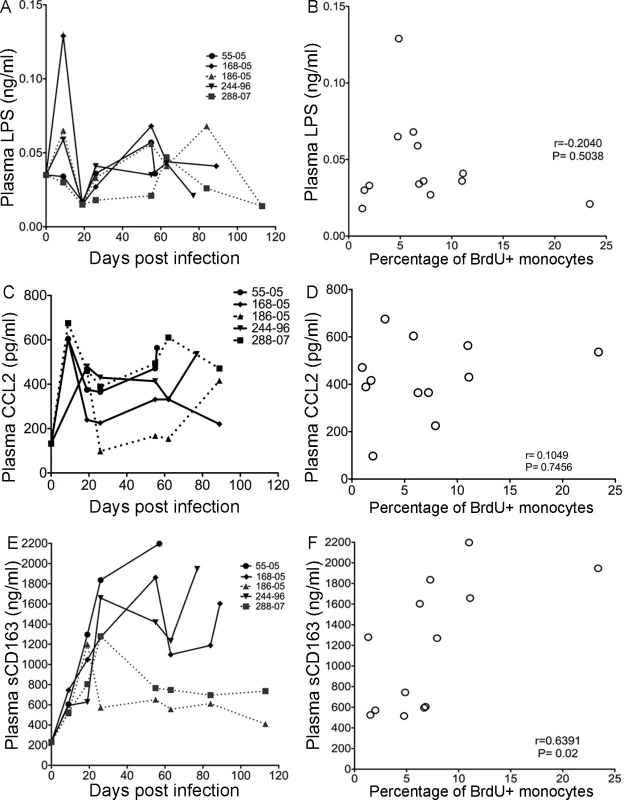
A. LPS plasma levels in the individual SIV infected animals after infection are shown. Solid lines are plasma LPS levels in rapid progressors. Dashed lines are plasma LPS levels in slow progressors. B. There is no correlation between plasma LPS levels with the percentage of BrdU+ monocytes. C. CCL2/MCP-1 plasma levels in individual SIV infected animals after infection are shown. The solid lines represent plasma CCL2/MCP-1 levels in rapid progressors. The dashed lines represent plasma CCL2/MCP-1 levels in slow progressors. D. There is no correlation between plasma CCL2/MCP-1 and the percentage of BrdU+ monocytes. E. sCD163 plasma levels in individual SIV infected animals after infection are shown. Solid lines are sCD163 plasma in rapid progressors. Dashed lines are sCD163 plasma in slow progressors. F. There is a significant correlation between sCD163 plasma levels and percentage of BrdU+ monocytes. The Spearman rank test is used. To examine whether increased circulating CCL2/monocyte chemoattractant protein 1 (MCP-1) can result in enhanced monocyte emigration from bone marrow, plasma CCL2 levels in SIV-infected animals were examined (Figure 5C). Plasma CCL2 levels increased at 9 days post infection and then moderately decreased in all animals, except one slow progressor whose CCL2 concentration returned to pre-infection levels (Figure 5C; rapid progressors = solid lines, slow progressors = dotted lines). There was no correlation between the percentages of BrdU+ monocytes and plasma CCL2 (Figure 5D; r = 0.1049, P = 0.7456). Interestingly, sCD163 did correlate with BrdU incorporation, consistent with activation of monocyte/macrophages and increased monocyte traffic from the bone marrow (Figure 5E and F) [35], [39]–[42], [45], [54], [55]. In all animals examined, plasma sCD163 levels increased by 9 days post infection, the time point when slow and rapid progressors can be distinguished by differences in the percentage of BrdU+ monocytes (Figure 5E). At day 20, in slow progressors sCD163 plasma levels decreased (Figure 5E, dotted lines), but in rapid progressors sCD163 levels continued to increase (Figure 5E, solid lines). There was a significant correlation between the percentage of BrdU+ monocytes and plasma sCD163 levels (Figure 5F; r = 0.6391, P = 0.02). Thus, sCD163 levels, but not plasma LPS or CCL2, correlated with the increased percentage of BrdU+ monocytes in the blood of CD8+ T lymphocyte depleted SIV-infected macaques.
In a recent study, we showed that higher levels of BrdU+ monocytes in blood were associated with macrophage apoptosis in lymph nodes [20]. By flow cytometry, we did not find Annexin V+ BrdU+ monocytes suggesting that monocytes were not undergoing apoptosis in blood (data not shown). Herein, we examined BrdU labeled cells in the CNS investigating their distribution in the brains of three animals, two with severe SIVE and one with mild SIVE (Figure 6). The majority of the BrdU+ cells in all brains were located within SIVE lesions and in the vasculature (Figure 6A and B; BrdU: DAB). BrdU+ cells comprised 15.6 and 17.5% of total cells within SIVE lesions in two rapid progressors with severe SIVE (Table 2, Figure 6A). In the animal with mild SIVE, similar percentages of BrdU+ cells were found in lesions, but there were fewer overall lesions. To determine the identity of the BrdU+ cells, double immunohistochemistry was performed using antibodies against CD3 for T cells, GFAP for astrocytes, CD68 for resident macrophages and Mac387 for recently infiltrated monocyte/macrophages [56], [57]. There was little to no CD3+ T lymphocytes found in any sections examined (Figure 6C; BrdU: DAB and CD3: Vector Blue). BrdU+ cells were found in close proximity to GFAP+ astrocytes, but very few scattered double positive astrocytes (GFAP+BrdU+) were found (Figure 6D and E; BrdU: DAB and GFAP: Vector Blue). Approximately 10% of total BrdU+ cells in SIVE lesions were CD68+ and these BrdU+ cells comprised between 1.8 and 4.6% of all CD68+ macrophages in lesions (Figure 6F and G; BrdU: DAB and CD68: Vector Blue and Table 2). Between 81 to 92% of all BrdU+ cells in SIVE lesions were Mac387+, representing approximately a third of the total Mac387+ monocyte/macrophages in lesions (Figure 6H and I; BrdU: Vector Blue and Mac387: DAB and Table 2). The animal with mild SIVE had similar percentages of BrdU+Mac387+ and BrdU+CD68+ cells in lesions as the animals with severe SIVE. In the brains of control non-infected CD8+ T lymphocyte depleted animals, few scattered BrdU+ macrophages were detected, representing normal monocyte traffic (data not shown).
Fig. 6. Histopathologic studies showed evidence of BrdU+ cells, the majority of which are Mac387+, in the brains of macaques with SIVE. 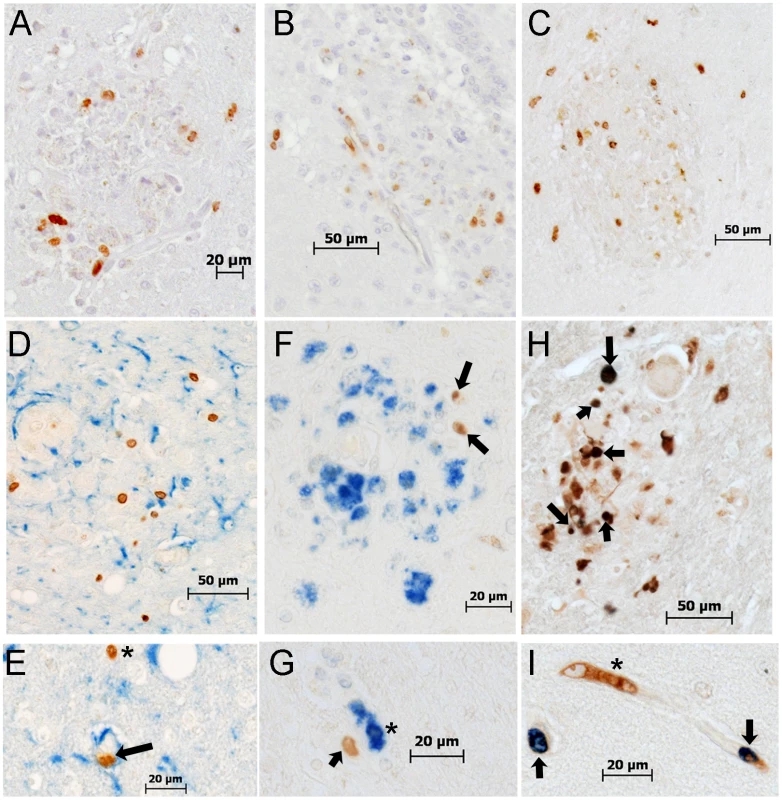
A–B: Immunohistochemistry with an antibody against BrdU is utilized in brain sections of rapid progressors. A: BrdU+ cells are present in and around SIVE lesions (BrdU: DAB, brown) B: BrdU+ cells are seen in and around the vasculature (BrdU: DAB, brown) C: Immunohistochemistry with antibodies against CD3 (Vector Blue, blue) and BrdU (DAB, brown) is utilized in brain sections of rapid progressors to examine if BrdU+ cells are CD4+ T lymphocytes. No double label BrdU+ and CD3+ cells are found in the brains of SIVE+ macaques. D and E: Immunohistochemistry with antibodies against GFAP (Vector Blue, blue) and BrdU (DAB, brown) is utilized in brain sections of rapid progressors to examine if BrdU+ cells are astrocytes. There are very few BrdU+ astrocytes seen in all sections examined. The arrow points to a BrdU+ in a vessel surrounded by astrocyte foot processes. The asterisk points out a BrdU+ cells in close proximity to GFAP+ astrocytes. F and G: Immunohistochemistry with antibodies against CD68 (DAB, brown) and BrdU (Vector Blue, blue) is utilized in brain sections of rapid progressors to examine if BrdU+ cells were CD68+ mature macrophages. Few BrdU+CD68+ cells are seen. H and I: Double staining with antibodies against BrdU (Vector Blue, blue) and Mac387 (DAB, brown) is utilized to determine if BrdU+ cells in the brain are early monocyte/macrophage infiltrates. BrdU and Mac387 did co-localization in the brain of SIVE animals. The arrows indicate BrdU+Mac387+ monocyte/macrophages, in a lesion (H) or perivascular region (I). The asterisk is a Mac387 BrdU- cell in the vasculature. Brain sections are representative of three SIVE+ animals examined for all stains. Tab. 2. Percentages of BrdU+, Mac387+, CD68+ and SIVp28+ cells in SIVE lesions. 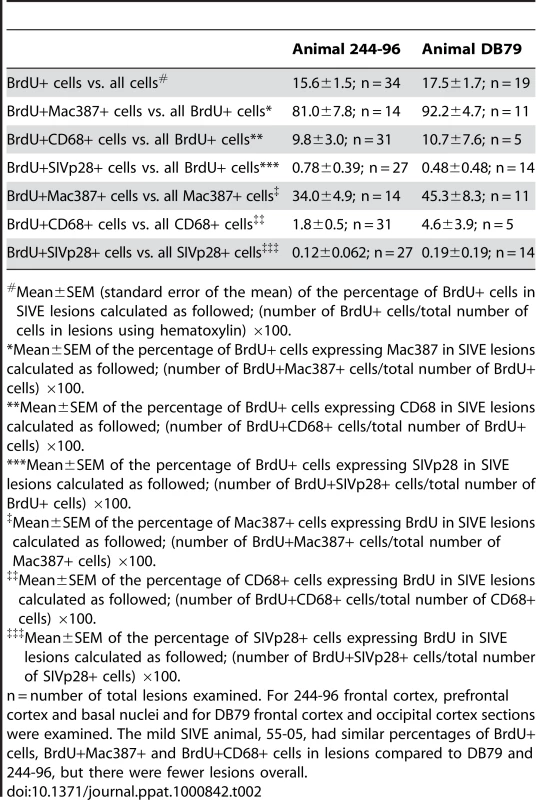
Mean±SEM (standard error of the mean) of the percentage of BrdU+ cells in SIVE lesions calculated as followed; (number of BrdU+ cells/total number of cells in lesions using hematoxylin) ×100. Less than 1% of all BrdU+ cells in SIVE lesions were SIVp28+ and less than 0.2% of all SIVp28+ cells were also BrdU+ (Table 2). Double positive BrdU and SIVp28 cells are very rare events, thus, resulting in few productively infected BrdU monocyte/macrophages. Immunofluorescence confirmed the presence of numerous BrdU+ cells in blood vessels (Figure 7A), perivascular cuffs (Figure 7A), and SIVE lesions (Figure 7B–D) and showed that the majority of the BrdU+ cells were Mac387+ cells (Figure 7C; BrdU: Vector Blue and Mac387: DAB) that were not productively infected. Immunofluorescence was used to identify that the productively SIV infected BrdU+ cells were CD68+ macrophages (Figure 7A–B and 7D; white arrow). Thus, the majority of the BrdU+ cells in the brain were Mac387+ that were not productively infected, representing monocyte/macrophages that were labeled with BrdU in the bone marrow and had recently trafficked to the brain (likely from the last two BrdU pulses). These data are consistent with Mac387 as one of the earliest differentiation markers expressed on monocyte/macrophages as they enter tissues [56], [57].
Fig. 7. The majority of BrdU+ monocyte/macrophages in the brain are not productively infected. 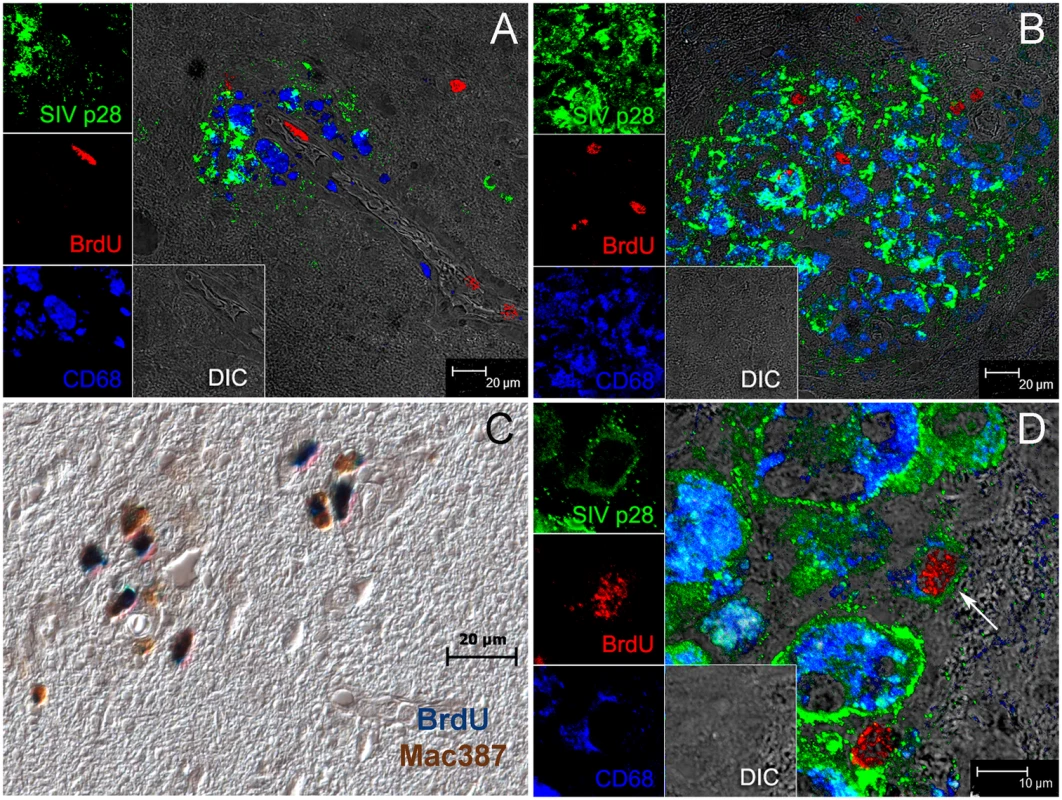
Triple label with antibodies against BrdU (red), SIV p28 (green), CD68 (blue) is used to determine if BrdU+ cells are productively infected. Side panels are single-color images of SIVp28 (green), BrdU (red), CD68 (blue) and differential interference contrast (DIC). A: BrdU+ (red) cells in blood vessels and a BrdU+ cell infiltrated in the brain B–D: Multiple BrdU+ cells are detected in and around SIVE lesion. B: BrdU+ cells are seen in and around this SIVE lesion; the majority BrdU+ cells are SIV p28− and thus not productively infected. C: The majority of the BrdU+ cells in lesions are Mac387+. Double label with antibodies against BrdU (blue) and Mac387 (brown) in an SIVE lesion is shown. D: An SIVE lesion in the brain of a rapid progressor showing a rare triple positive cell: SIV-infected CD68+BrdU+ macrophage (white arrow). Data presented here are representative of n = 3 macaques with SIVE. Multiple tissue sections from different brain regions were examined. Discussion
Here we have presented data showing that increased monocyte turnover is predictive of rapid development of AIDS. We have demonstrated that an increased percentage of BrdU+ monocytes in blood correlated with the severity of SIVE at necropsy. Interestingly, the differences in the percentage of BrdU+ monocytes were apparent by 8 days post infection and differentiated between slow and rapid progression by 27 days post infection. This data lends support to the importance of early monocyte activation and deregulation in AIDS pathogenesis. Furthermore, sCD163 levels, but not plasma LPS or CCL2, correlated with increased percentages of BrdU+ monocytes in the blood of SIV-infected macaques. In addition, we showed a differential rate of turnover in two major monocyte populations (CD14+CD16 − and CD14+CD16+) and the acceleration of their turnover in rapid progressors. BrdU+ cells were detected in the brains of animals with SIVE; the majority of these cells were Mac387+ that were SIV p28 − and a minor population of CD68+ macrophages, few of which were productively infected. The data presented here underscore the importance of increased monocyte turnover and traffic to the brain during SIVE, and emphasize in this model that CNS lesion formation is an active process requiring monocyte/macrophage recruitment, likely a result of enhanced innate immune responses [27], [35].
These data confirm and extend the observations recently reported by Hasegawa and colleagues, who examined BrdU incorporation in animals at different stages of SIV infection and found an increase in the percentage of BrdU+ monocytes with acute and chronic infection and a greater expansion with AIDS [20]. Our results are consistent with that finding, but add the observation that increased BrdU incorporation in monocytes correlates with the severity of SIVE and can distinguish between slow and rapid progressors.
We have presented evidence that increased monocyte traffic from bone marrow into the circulation correlates with the rapid progression to AIDS and severity of SIVE. BrdU+ monocytes traffic from bone marrow through the circulation into the brain, fostering the development of AIDS and SIVE. An increase in monocyte traffic in uninfected animals or slow progressors was not found. These data underscore the important role of monocyte activation and augmented traffic from the bone marrow to the brain in SIV neuropathogenesis. Bone marrow diffusion has been reported to correlate with the incidence of HIV dementia [58]. Additionally, anemia before the onset of AIDS is predictive of HIV neuropathogenesis [35], [58]. It has been demonstrated in SIV infected monkeys that rapid disease progression is the best correlate with the development of SIVE [59]. Our data support these observations and extend these to include that the rate of monocyte turnover, demonstrated by the percentage of BrdU+ monocytes, correlates with the rapid development of AIDS and the severity of SIVE.
The CD8 depletion, SIV infection model produces rapid AIDS with a high incidence of SIVE by depleting cytotoxic T lymphocytes. Using this model, there were both slow and rapid disease progressing animals with varying degrees of severity of SIVE that correlated with the percentage of BrdU+ monocytes. However, CD4+ T lymphocytes, which are linked to CD8+ T lymphocytes, are not regulated in a normal fashion due to depletion of CD8+ T lymphocytes [38], a possible reason for the lack of a correlation between CD4+ T lymphocytes with BrdU+ monocytes in our study. However, in a previous report using non-CD8 depleted SIV infected monkeys, high monocyte turnover also did not correlate with CD4+T cell number or plasma viral load [20].
We found that the percentage of BrdU+ monocytes does not correlate with LPS or CCL2 levels in plasma, but interestingly sCD163 did; this is consistent with the notion that activation of monocyte/macrophages and the increase of monocyte traffic from the bone marrow drive CNS pathogenesis [39], [40]. Previous in vivo and in vitro studies have shown that CD163 expression on monocytes inversely correlated with sCD163 in plasma or tissue culture media directly linking sCD163 to monocyte activation [39], [45], [55]. In vitro studies show picogram levels of LPS results in sCD163 release from monocytes, underscoring the role of innate immune responses in AIDS pathogenesis [45]. In addition, the level of sCD163 has been shown to increase in association with macrophage mediated diseases, including sepsis [40] and Gaucher's disease [60], characterized by macrophage accumulation in the liver and spleen [60]. Thus, increased traffic of monocytes from the bone marrow (increased BrdU+ monocytes in the circulation) and increased levels of sCD163 in the plasma are consistent with an inflammatory environment resulting from stimulation of innate immunity that may play important roles in the development of SIVE.
Release of monocytes from the bone marrow could be triggered by increased apoptosis of monocytes in the blood. Although CD3+CD4+ T cells from HIV-1 subjects have been shown to undergo constitutive and induced apoptosis [61], HIV-1+ monocytes are resistant [61]. Recently, it was demonstrated that peripheral blood monocytes from chronically HIV-1 infected individuals have a stable anti-apoptotic gene signature suggesting a greater resistance to apoptosis in circulating monocytes during HIV infection [61]. In this study, pro-apoptotic genes were down-regulated and anti-apoptotic genes were up-regulated in infected monocytes [61]. When we examined whole blood by flow cytometry for the presence of apoptotic monocytes, we did not detect monocytes that were Annexin V and propidium iodide (PI) positive 24hrs after BrdU injection (data not shown). Thus, we could rule out apoptosis of peripheral blood monocytes as a trigger for the increased release of monocytes from the bone marrow. Apoptosis of tissue macrophages in lymph nodes has already been demonstrated to correlate with the percentage of BrdU monocyte label in blood [20].
A report by Serbina and Pamer suggested that monocyte emigration from bone marrow during bacterial infection requires signals mediated by the chemokine receptor CCR2 [62]. Using a Ccr2−/− mouse model, it was found that CCR2 is important for release of Ly6Chi monocytes (equivalent to the CD14+CD16 − monocytes in humans) from bone marrow [62]. A more recent paper concluded that not only CCL2, but also CCL7 (MCP-3) was critical in monocyte mobilization from the bone marrow [63]. We did not see a correlation between CCL2 plasma levels and the percentage of BrdU+ monocytes, but that does not rule out the involvement of CCR2 and additional ligands. In addition, the chemokine CXCL12 (SDF-1) and its receptor CXCR4 are known to be involved in the retention of hematopoietic stem cells in the bone marrow [64], [65]. The roles of these additional factors are worthy of exploration in the SIV model of disease and may lead to further insight into the increased release of monocytes from the bone marrow during AIDS and SIVE progression.
Not only did we find differences in the percent of BrdU+ total monocytes between slow and rapid progressors, but we found kinetic differences in BrdU incorporation of two monocyte subsets, CD14+CD16 − and CD14+CD16+ entering the blood from the bone marrow. In the rapid progressors, the percentage of BrdU incorporation was increased dramatically with infection in both populations. The CD14+CD16 − (CCR2+) monocyte population may have accelerated release due to increased levels of MCP-3, as discussed above. The CD14+CD16+ monocyte blood population has been shown to be phenotypically similar to perivascular macrophages in the brain, which supports the concept that the CD14+CD16+ monocytes transmigrate into the brain to differentiate further into perivascular macrophages [25], [33]. Our data suggests that although CD14+CD16+ cells leave the bone marrow later than CD14+CD16 − cells, the CD14+CD16+ cells spend less time in the circulation, possibly a result of traffic to tissues. This likelihood is supported by the finding that CD14+CD16+ monocytes are inflammatory mediators expressing high levels of pro-inflammatory cytokines and are potent antigen presenting cells. We have not ruled out the possibility that CD14+CD16 − monocytes convert to CD14+CD16+ monocytes in blood [66].
The overall number of brain macrophages in HIVE and SIVE is increased despite evidence of proliferation in situ, supported by the notion that the majority of these macrophages are derived from the periphery [25], [34]. Here, we present evidence that newly infiltrated BrdU+ monocyte/macrophages are a significant population of monocyte/macrophages in perivascular cuffs and lesions in brains of SIVE+ animals. Recruited monocytes may increase the number of viral target cells in the brain. Most of the BrdU+ cells were Mac387+ cells representing newly recruited and infiltrated monocytes/macrophages [56], [57]. It is not known how long the BrdU+Mac387+ cells stay in the CNS with lesion formation nor at which BrdU pulse these cells were labeled in the periphery. This might, in part, explain why CD68+BrdU+ cells are found in the CNS; they might have trafficked earlier as less mature cells and undergone maturation within tissues. Additionally, the CD68+ BrdU+ cells could have migrated directly from the blood, thus bringing HIV into the CNS. Lastly, they may represent macrophages that divided in the CNS, although we believe this in unlikely since we found little evidence of BrdU+ microglia, astrocytes or epithelial cells in our studies. We have previously shown using specific markers for cell proliferation, including Ki-67 and topoisomerase II alpha, that macrophages within SIVE lesions were not undergoing significant active proliferation in the time frame studied [34].
Increased monocyte turnover correlated with high levels of sCD163 in plasma. These findings along with potential anti-inflammatory properties of sCD163 [44], which is directly shed from the M2 anti-inflammatory alternative activated macrophages [39], [67], suggest that BrdU+ cells recruited to the CNS may aid in lesion resolution. Monocyte/macrophages may traffic to the brain as inflammatory cells in response to or causing neuronal injury or as anti-inflammatory vehicles to minimize injury to the brain during SIV infection.
Although we detected very few SIVp28+ BrdU+ cells in SIVE brains, this does not rule out the possibility that the BrdU+ cells are not latently infected. In fact, our data suggests BrdU+ cells may act as viral reservoirs that may with maturation actively replicate virus. Our finding that only the BrdU+CD68+ monocytes are productively infected underscores the importance of monocyte/macrophage maturation (possibly Mac387 to CD68 expression) for active viral replication. Invasion of the CNS by BrdU+Mac387+ perivascular macrophages with concomitant viral infection of the CNS compartment upon maturation is a likely scenario for this. Overall, our data examining BrdU+ monocytes in blood and BrdU+ monocyte/macrophages with the brain, underscore the role of monocyte/macrophages derived from bone marrow in AIDS pathogenesis and CNS disease.
Materials and Methods
Ethics statement
All animals were handled in strict accordance with good animal practice as defined by the Harvard University's Institutional Animal Care and Use Committee, and all animal work was approved by this committee.
Animals, viral infection and CD8+ T lymphocyte depletion
Eleven rhesus macaques were utilized in this study. Seven animals were infected with SIVmac251 (20 ng of SIV p27) by intravenous injection, kindly provided by Ronald Desrosiers. In order to achieve rapid disease progression with high incidence of SIVE, animals were CD8+ T lymphocyte depleted by treatment with a human anti-CD8 antibody cM-T807 administered s.c. (10mg/kg) at 6 days post infection and i.v. (5mg/kg) at 8 and 12 days post infection (previously described [32]). Four uninfected animals were used as controls: two were CD8+ T lymphocyte depleted by treatment with a human anti-CD8 antibody cM-T807 administered once (50mg/kg) i.v. and two were CD8+ T lymphocyte depleted by treatment with a rhesus anti-CD8 antibody cM-T807 administered once (50mg/kg) i.v. These antibodies were provided by the NIH Non-human Primate Reagent Resource (RR016001, AI040101). CD8+ T lymphocyte depletion was monitored by flow cytometry prior to antibody treatment and weekly thereafter during infection (as previously described [32]). All animals were housed at Harvard University's New England Regional Primate Research Center in accordance with standards of the American Association for Accreditation of Laboratory Animal Care. Animals were anesthetized with ketamine-HCl and euthanized by an intravenous pentobarbital overdose and exsanguinated. Animals were sacrificed with any of the following 5 criteria: 1. weight loss >15% body weight in 2 weeks or >30% body weight in 2 months, 2. documented opportunistic infection, 3. persistent anorexia >3 days without explicable cause, 4. severe intractable diarrhea, progressive neurological signs or 5. significant cardiac and/or pulmonary signs. The diagnosis of AIDS was determined by the presence of AIDS defining lesions: Pneumocystis pneumonia, Mycobacterium avium infection (most commonly small intestine, liver and mesenteric lymph node), intestinal adenovirus infection (most common in small intestine). Other, less common lesions include SIV giant cell disease in the lung, gut, and lymph nodes and SIV associated arteriopathy. SIV encephalitis (SIVE) was defined by the presence of multinucleated giant cells (MNGC) and accumulation of macrophages, some of which are infected [59], [68]–[70]. No animals with opportunistic infections in the CNS were used in this study.
Viral load determination
Plasma SIV RNA was quantified using real-time PCR as previously described [71]. SIV virions were pelleted from 0.5ml EDTA plasma by centrifugation at 20,000 g for 1 hour. The fluorescently labeled, real-time PCR probe employed contained a non-fluorescent quencher, BHQ-1, at its 3′ end. The threshold sensitivity was 100 copy Eq/ml, with an average interassay coefficient of variation of less than 25%.
BrdU administration
A 30 mg/ml stock of solution was prepared by adding 5-bromo-2′-deoxyuridine (BrdU) (Sigma) to 1× PBS (without Ca2+ and Mg2+), U.S.P. grade (Aestus Pharmaceuticals) and heated to 60°C in water bath (as previously described [20], [24]). BrdU was administered as a slow bolus i.v. injection at a dose of 60 mg BrdU/kg body weight. In the uninfected animals, BrdU was given four times throughout the study (days −11, 3, 38 and 72 days post-CD8 antibody administration). In the infected animals, BrdU was administered pre-infection (day −9), peak infection (day 7), and days 26, day 50 (n = 1) and 88 (n = 2) post infection and 24 hours prior to necropsy (n = 4; days 55, 76, 88 and 91). CM07 did not receive BrdU prior to necropsy.
Flow cytometry
Flow cytometric analyses were performed with 100 µl aliquots of EDTA-coagulated whole blood. Erythrocytes were lysed using ImmunoPrep Reagent System (Beckman Coulter), washed twice with PBS containing 2% FBS, then incubated for 15 minutes at room temperature with fluorochrome-conjugated surface antibodies including anti-HLA-DR-PerCp-Cy5.5 (clone L243), anti-CD16-PE-Cy7 (clone 3G8; BD Biosciences), anti-CD3-APC (clone SP34-2), anti-CD8-APC (clone RPA-T8;), anti-CD20-APC (clone 2H7) and anti-CD14-Pacific blue (clone M5E2) (BD Biosciences). For intracellular staining, cells were fixed and permeabilized with BD Cytofix/Cytoperm™ buffer (BD Biosciences) for 30 mins at room temperature. Cells were again washed and incubated with BD Cytoperm Plus™ buffer for 10 mins on ice, then washed and incubated with DNase (30mg) for 1hr at 37°C, washed and then stained for intracellular antigen with anti-BrdU-FITC (clone 3D4; BD Biosciences) and anti-Ki-67-PE (clone B56; BD Biosciences) for 20 mins at room temperature. For controls, BrdU naïve animals and isotype controls were used. To test for apoptotic monocytes anti-Annexin V (Invitrogen) and propidium iodine (PI; BD Biosciences) were used. Samples were acquired on a BD FACS Aria (BD Biosciences) and analyzed with Tree Star Flow Jo version 8.7.
sCD163 and CCL2 ELISAs and LAL assay
Soluble CD163 (sCD163) and CCL2 plasma levels were quantified by ELISA according to manufacturer's protocol (Trillium Diagnostics and R&D Systems, respectively). The Diazo-coupled Limulus amebocyte lysate (LAL) assay (Associates of Cape Cod Inc.) was used to quantify endotoxin/liposaccharide (LPS) levels in plasma from SIV-infected animals, according to the manufacturer's protocol. Briefly, samples diluted 1/5 were inactivated for 30 min at 65°C and incubated with LAL for 30 min at 37°C. Addition of reagents led to formation of a magenta derivative that absorbs light at 570 nm. For LAL assay, samples were handled with non-pyrogenic plastic or glassware to avoid LPS contamination.
Immunohistochemistry
Formalin-fixed, paraffin-embedded brain tissues were deparaffinized and assessed by immunohistochemistry for BrdU (Mouse IgG1; Dako, 1∶50, 1hr room temperature. Before primary antibody incubation, non-serum protein block was applied. The EnVision+ System - horseradish peroxidase (HRP) (EnVision+ Kit; DAKO) was used according to the manufacturers' instructions. The color reaction product was developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB; DAKO) as the chromogenic substrate for HRP. The sections were counterstained with hematoxylin and then dehydrated and mounted. Controls consisted of the addition of isotype-matched immunoglobulin. To detect BrdU+ cells and CD3+ T lymphocytes, GFAP+ astrocytes, SIVp28+ infected cells, CD68+ macrophages and Mac387+ monocyte/macrophages in monkey brains, double-label immunohistochemistry was performed using the DAKO Double Stain System, according to the manufacturer's instructions. The color reaction product was developed using DAB and Vector Blue (Vector Laboratories). Sections were visualized under a Zeiss Axio Imager M1 microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) using Plan-Apochromat ×20/0.8 and ×40/0.95 Korr objectives.
Confocal microscopy
Tissues were collected in 10% neutral buffered formalin, and embedded in paraffin. Tissues were sectioned at 6 µm and deparaffinized with xylene and hydrated in graded alcohols. Immunohistochemical staining followed a basic protocol using a citrate antigen retrieval method. For immunofluorescence, sections were blocked with 10% normal goat serum (NGS) in PBS with 0.2% Fish Skin Gelatin (FSG) (Sigma) for 40 min. Tissues were incubated with rat anti-BrdU (IgG2a; BU1/75, Novus Biologicals, 1∶50, 1hr RT) followed by AlexaFluor 568 conjugated goat anti-rat IgG (Molecular Probes; 1∶500), then with mouse anti-SIV p28 (IgG1; Microbix Biosystems, 1∶500) followed by AlexaFluor 488 conjugated goat anti-mouse IgG1 (Molecular Probes; 1∶500) and then CD68-biotin conjugated (1∶20, overnight at 4°C) followed by streptavidin conjugated AlexaFluor 568 (Molecular Probes; 1∶500). After immunofluorescence labeling and washing, sections were treated with 50 mmol/L CuSO4 ammonium buffer for 45 minutes to quench auto-fluorescence. Confocal microscopy was performed using a Leica TCS SP2 laser-scanning microscope equipped with 3 lasers (Leica Microsystems, Exton, PA). Individual optical slices represent 0.2 µm. Optical slices were collected at 512×512 pixel resolution. The fluorescence of individual fluorochromes was captured separately in a sequential mode, after optimization to reduce bleed-through between channels (photomultiplier tubes) using Leica software. NIH Image v1.62 and Adobe Photoshop v7 software were used to assign correct colors of up to four channels collected (3 fluorochromes: Alexa 488 (green), Cy3, Alexa 568 (red), and Alexa 647 (far red), and the differential interference contrast image (gray scale).
Statistical analysis
For statistical analyses we used the Prism version 5.0a (GraphPad Software, Inc., San Diego, CA) software. Spearman rank test was used for all correlations.
Zdroje
1. SoulasC
DonahueRE
DunbarCE
PersonsDA
AlvarezX
2009 Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am J Pathol 174 1808 1817
2. Gonzalez-MejiaME
DoseffAI
2009 Regulation of monocytes and macrophages cell fate. Front Biosci 14 2413 2431
3. van FurthR
1989 Origin and turnover of monocytes and macrophages. Curr Top Pathol 79 125 150
4. TushinskiRJ
OliverIT
GuilbertLJ
TynanPW
WarnerJR
1982 Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28 71 81
5. LandsmanL
VarolC
JungS
2007 Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol 178 2000 2007
6. WhitelawDM
1972 Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet 5 311 317
7. VarolC
YonaS
JungS
2009 Origins and tissue-context-dependent fates of blood monocytes. Immunol Cell Biol 87 30 38
8. SawyerRT
StrausbauchPH
VolkmanA
1982 Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest 46 165 170
9. TarlingJD
LinHS
HsuS
1987 Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. J Leukoc Biol 42 443 446
10. WijffelsJF
de RoverZ
BeelenRH
KraalG
van RooijenN
1994 Macrophage subpopulations in the mouse spleen renewed by local proliferation. Immunobiology 191 52 64
11. SwirskiFK
NahrendorfM
EtzrodtM
WildgruberM
Cortez-RetamozoV
2009 Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325 612 616
12. van FurthR
Diesselhoff-den DulkMM
1984 Dual origin of mouse spleen macrophages. J Exp Med 160 1273 1283
13. NahrendorfM
SwirskiFK
AikawaE
StangenbergL
WurdingerT
2007 The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204 3037 3047
14. ArnoldL
HenryA
PoronF
Baba-AmerY
van RooijenN
2007 Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204 1057 1069
15. Van FurthR
Diesselhoff-den DulkMC
MattieH
1973 Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med 138 1314 1330
16. VolkmanA
CollinsFM
1974 The cytokinetics of monocytosis in acute salmonella infection in the rat. J Exp Med 139 264 277
17. OhgamiM
DoerschukCM
GieRP
EnglishD
HoggJC
1991 Monocyte kinetics in rabbits. J Appl Physiol 70 152 157
18. MeuretG
BammertJ
HoffmannG
1974 Kinetics of human monocytopoiesis. Blood 44 801 816
19. GotoY
HoggJC
SuwaT
QuinlanKB
van EedenSF
2003 A novel method to quantify the turnover and release of monocytes from the bone marrow using the thymidine analog 5′-bromo-2′-deoxyuridine. Am J Physiol Cell Physiol 285 C253 259
20. HasegawaA
LiuH
LingB
BordaJT
AlvarezX
2009 The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114 2917 2925
21. BrownKN
WijewardanaV
LiuX
Barratt-BoyesSM
2009 Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog 5 e1000413 doi:10.1371/journal.ppat.1000413
22. ShihCH
van EedenSF
GotoY
HoggJC
2005 CCL23/myeloid progenitor inhibitory factor-1 inhibits production and release of polymorphonuclear leukocytes and monocytes from the bone marrow. Exp Hematol 33 1101 1108
23. GotoY
HoggJC
WhalenB
ShihCH
IshiiH
2004 Monocyte recruitment into the lungs in pneumococcal pneumonia. Am J Respir Cell Mol Biol 30 620 626
24. WangX
DasA
LacknerAA
VeazeyRS
PaharB
2008 Intestinal double-positive CD4+CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood 112 4981 4990
25. Fischer-SmithT
CroulS
SverstiukAE
CapiniC
L'HeureuxD
2001 CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 7 528 541
26. KimWK
AvarezX
WilliamsK
2005 The role of monocytes and perivascular macrophages in HIV and SIV neuropathogenesis: information from non-human primate models. Neurotox Res 8 107 115
27. KimWK
CoreyS
AlvarezX
WilliamsK
2003 Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 74 650 656
28. WilliamsKC
HickeyWF
1995 Traffic of hematogenous cells through the central nervous system. Curr Top Microbiol Immunol 202 221 245
29. KedzierskaK
CroweSM
2002 The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem 9 1893 1903
30. PulliamL
GasconR
StubblebineM
McGuireD
McGrathMS
1997 Unique monocyte subset in patients with AIDS dementia. Lancet 349 692 695
31. ThieblemontN
WeissL
SadeghiHM
EstcourtC
Haeffner-CavaillonN
1995 CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol 25 3418 3424
32. WilliamsK
WestmorelandS
GrecoJ
RataiE
LentzM
2005 Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest 115 2534 2545
33. WilliamsKC
CoreyS
WestmorelandSV
PauleyD
KnightH
2001 Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193 905 915
34. WilliamsK
SchwartzA
CoreyS
OrandleM
KennedyW
2002 Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol 161 575 585
35. WilliamsKC
HickeyWF
2002 Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci 25 537 562
36. SchmitzJE
SimonMA
KurodaMJ
LiftonMA
OllertMW
1999 A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol 154 1923 1932
37. SchmitzJE
JohnsonRP
McClureHM
MansonKH
WyandMS
2005 Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J Virol 79 8131 8141
38. SchmitzJE
KurodaMJ
SantraS
SassevilleVG
SimonMA
1999 Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283 857 860
39. DavisBH
ZarevPV
2005 Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom 63 16 22
40. MollerHJ
PeterslundNA
GraversenJH
MoestrupSK
2002 Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 99 378 380
41. BuechlerC
RitterM
OrsoE
LangmannT
KluckenJ
2000 Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro - and antiinflammatory stimuli. J Leukoc Biol 67 97 103
42. FabriekBO
DijkstraCD
van den BergTK
2005 The macrophage scavenger receptor CD163. Immunobiology 210 153 160
43. KimWK
AlvarezX
FisherJ
BronfinB
WestmorelandS
2006 CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol 168 822 834
44. MoestrupSK
MollerHJ
2004 CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med 36 347 354
45. WeaverLK
Hintz-GoldsteinKA
PioliPA
WardwellK
QureshiN
2006 Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol 80 26 35
46. CordoneI
MatutesE
CatovskyD
1992 Characterisation of normal peripheral blood cells in cycle identified by monoclonal antibody Ki-67. J Clin Pathol 45 201 205
47. GoudTJ
van FurthR
1975 Proliferative characteristics of monoblasts grown in vitro. J Exp Med 142 1200 1217
48. van FurthR
RaeburnJA
van ZwetTL
1979 Characteristics of human mononuclear phagocytes. Blood 54 485 500
49. GerdesJ
LemkeH
BaischH
WackerHH
SchwabU
1984 Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133 1710 1715
50. PasslickB
FliegerD
Ziegler-HeitbrockHW
1989 Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74 2527 2534
51. MarcondesMC
LaniganCM
BurdoTH
WatryDD
FoxHS
2008 Increased expression of monocyte CD44v6 correlates with the deveopment of encephalitis in rhesus macaques infected with simian immunodeficiency virus. J Infect Dis 197 1567 1576
52. BrenchleyJM
PriceDA
SchackerTW
AsherTE
SilvestriG
2006 Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12 1365 1371
53. AncutaP
KamatA
KunstmanKJ
KimEY
AutissierP
2008 Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE 3 e2516 doi:10.1371/journal.pone.0002516
54. FabriekBO
Van HaastertES
GaleaI
PolflietMM
DoppED
2005 CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia 51 297 305
55. WeaverLK
PioliPA
WardwellK
VogelSN
GuyrePM
2007 Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol 81 663 671
56. BruckW
PoradaP
PoserS
RieckmannP
HanefeldF
1995 Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 38 788 796
57. OtaniI
MoriK
SataT
TeraoK
DoiK
1999 Accumulation of MAC387+ macrophages in paracortical areas of lymph nodes in rhesus monkeys acutely infected with simian immunodeficiency virus. Microbes Infect 1 977 985
58. RaginAB
WuY
StoreyP
CohenBA
EdelmanRR
2006 Bone marrow diffusion measures correlate with dementia severity in HIV patients. AJNR Am J Neuroradiol 27 589 592
59. WestmorelandSV
HalpernE
LacknerAA
1998 Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol 4 260 268
60. MollerHJ
de FostM
AertsH
HollakC
MoestrupSK
2004 Plasma level of the macrophage-derived soluble CD163 is increased and positively correlates with severity in Gaucher's disease. Eur J Haematol 72 135 139
61. GiriMS
NebozyhnM
RaymondA
GekongeB
HancockA
2009 Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus - and host-mediated apoptosis resistance. J Immunol 182 4459 4470
62. SerbinaNV
PamerEG
2006 Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7 311 317
63. TsouCL
PetersW
SiY
SlaymakerS
AslanianAM
2007 Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 117 902 909
64. LapidotT
PetitI
2002 Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 30 973 981
65. PeledA
PetitI
KolletO
MagidM
PonomaryovT
1999 Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283 845 848
66. AncutaP
LiuKY
MisraV
WaclecheVS
GosselinA
2009 Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16 - monocyte subsets. BMC Genomics 10 403
67. KomoharaY
HiraharaJ
HorikawaT
KawamuraK
KiyotaE
2006 AM-3K, an anti-macrophage antibody, recognizes CD163, a molecule associated with an anti-inflammatory macrophage phenotype. J Histochem Cytochem 54 763 771
68. SassevilleVG
LacknerAA
1997 Neuropathogenesis of simian immunodeficiency virus infection in macaque monkeys. J Neurovirol 3 1 9
69. NathA
1999 Pathobiology of human immunodeficiency virus dementia. Semin Neurol 19 113 127
70. BellJE
1998 The neuropathology of adult HIV infection. Rev Neurol (Paris) 154 816 829
71. LifsonJD
RossioJL
PiatakMJr
ParksT
LiL
2001 Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol 75 10187 10199
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



