-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaKeratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
Experimental leishmaniasis is an excellent model system for analyzing Th1/Th2 differentiation. Resistance to Leishmania (L.) major depends on the development of a L. major specific Th1 response, while Th2 differentiation results in susceptibility. There is growing evidence that the microenvironment of the early affected tissue delivers the initial triggers for Th-cell differentiation. To analyze this we studied differential gene expression in infected skin of resistant and susceptible mice 16h after parasite inoculation. Employing microarray technology, bioinformatics, laser-microdissection and in-situ-hybridization we found that the epidermis was the major source of immunomodulatory mediators. This epidermal gene induction was significantly stronger in resistant mice especially for several genes known to promote Th1 differentiation (IL-12, IL-1β, osteopontin, IL-4) and for IL-6. Expression of these cytokines was temporally restricted to the crucial time of Th1/2 differentiation. Moreover, we revealed a stronger epidermal up-regulation of IL-6 in the epidermis of resistant mice. Accordingly, early local neutralization of IL-4 in resistant mice resulted in a Th2 switch and mice with a selective IL-6 deficiency in non-hematopoietic cells showed a Th2 switch and dramatic deterioration of disease. Thus, our data indicate for the first time that epidermal cytokine expression is a decisive factor in the generation of protective Th1 immunity and contributes to the outcome of infection with this important human pathogen.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000871
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000871Summary
Experimental leishmaniasis is an excellent model system for analyzing Th1/Th2 differentiation. Resistance to Leishmania (L.) major depends on the development of a L. major specific Th1 response, while Th2 differentiation results in susceptibility. There is growing evidence that the microenvironment of the early affected tissue delivers the initial triggers for Th-cell differentiation. To analyze this we studied differential gene expression in infected skin of resistant and susceptible mice 16h after parasite inoculation. Employing microarray technology, bioinformatics, laser-microdissection and in-situ-hybridization we found that the epidermis was the major source of immunomodulatory mediators. This epidermal gene induction was significantly stronger in resistant mice especially for several genes known to promote Th1 differentiation (IL-12, IL-1β, osteopontin, IL-4) and for IL-6. Expression of these cytokines was temporally restricted to the crucial time of Th1/2 differentiation. Moreover, we revealed a stronger epidermal up-regulation of IL-6 in the epidermis of resistant mice. Accordingly, early local neutralization of IL-4 in resistant mice resulted in a Th2 switch and mice with a selective IL-6 deficiency in non-hematopoietic cells showed a Th2 switch and dramatic deterioration of disease. Thus, our data indicate for the first time that epidermal cytokine expression is a decisive factor in the generation of protective Th1 immunity and contributes to the outcome of infection with this important human pathogen.
Introduction
Experimental leishmaniasis has been the first model to directly demonstrate the relevance of the T helper 1/T helper 2 (Th1/Th2) dichotomy for the outcome of an infection or disease in vivo. Upon cutaneous infection with L. major, C57BL/6 mice generate a Th1 response and subsequently control infection, mainly due to activation of macrophages by IFN-γ. BALB/c mice develop a Th2 response and succumb to progressive disease (for a review see [1]).
The decisive events for the development of a Th1 or Th2 response take place early after infection - most likely during the first two days - since an influence on Th1/2 differentiation can only be achieved by pharmacological manipulation in this critical time frame [1]. The regulatory mechanisms responsible for this differentiation have long been supposed to primarily occur in the lymph node [1]. However, the molecular and cellular mechanisms preceding the immunological mechanism in the lymph nodes are not identified so far.
During the last years it became increasingly clear that the skin as the site of primary infection also influences the direction of the immune response. We and others have demonstrated that already 2 days after infection there is a higher percentage of granulocytes in the infiltrate of BALB/c compared to C57BL/6 mice and that antibody-mediated elimination of these cells in susceptible mice results in a Th1 response and restoration of resistance [2], [3].
These differences in granulocyte infiltration point to important differences in the microenvironment of the infected tissue within the first hours of infection, which can be decisive for the direction of the T cell response. Accordingly, a change in the route or site of infection, e.g. via blood stream or nasal mucosa instead of skin, results in a Th2 response and non-healing disease in originally resistant mice [4], [5] confirming a relevant influence of the local environment of the skin on T-cell priming in experimental leishmaniasis.
The infected tissue has been suggested to generate signals within the first hours after inoculation of parasites which are then integrated and transferred via dendritic cells (DCs) to T-cells in draining lymph nodes where they induce Th1 or Th2 differentiation. These signals have been termed “tissue” or “danger” signals [6]–[8]. The types of such signals at the site of infection are as manifold as their potential cellular sources since they could derive from either resident or infiltrating cells.
Therefore our aim was to perform a global search for such early tissue signals at the site of infection which have the potential to influence the specific immune response.
Using microarray technology and bioinformatics we confirmed the existence of tissue signals which appear within the first hours at the site of infection and identified for the first time the epidermis as an important source for these signals. Among these signals we identified chemokines for macrophage recruitment as well as several cytokines with the potential to induce Th1 response (e.g. via influencing DC) which showed a stronger expression in the epidermis of resistant mice. Such cytokines included IL-4 and IL-6. While early neutralization of IL-4 resulted in a Th2 switch in originally resistant mice, also early production of IL-6 in the skin was revealed as a novel decisive factor in the generation of protective Th1 immunity. Thus, we present strong evidence that early activation of epidermal cells influences the resulting T-cell response against L. major.
Results
Screening by gene array and PCR for genes regulated in the early phase of experimental leishmaniasis
To test our hypothesis that immunomodulatory mediators relevant for defense against infection with L. major and the generation of a Th1/Th2 response are expressed early at the site of initial infection, we screened for gene expression patterns using both global microarray analysis as well as the more sensitive real time PCR technique.
Employing microarray technology we detected significant up-regulation of 189 genes and down-regulation of 16 genes in both mouse strains 16 hours after infection (Table S1, S2). The total number of regulated genes was greater in resistant than in susceptible mice (205 vs. 146 genes). While only 4 genes were regulated significantly stronger in susceptible BALB/c mice, 59 genes were regulated significantly stronger in resistant C57BL/6 mice (Table 1 and Table S3, S4) and encompassed genes with a well known function in Th1/Th2 differentiation (such as IL-1β and osteopontin (opn)) [9], [10].
Tab. 1. Genes with differential regulation in C57BL/6 or BALB/c mice upon <i>L. major</i> infection. 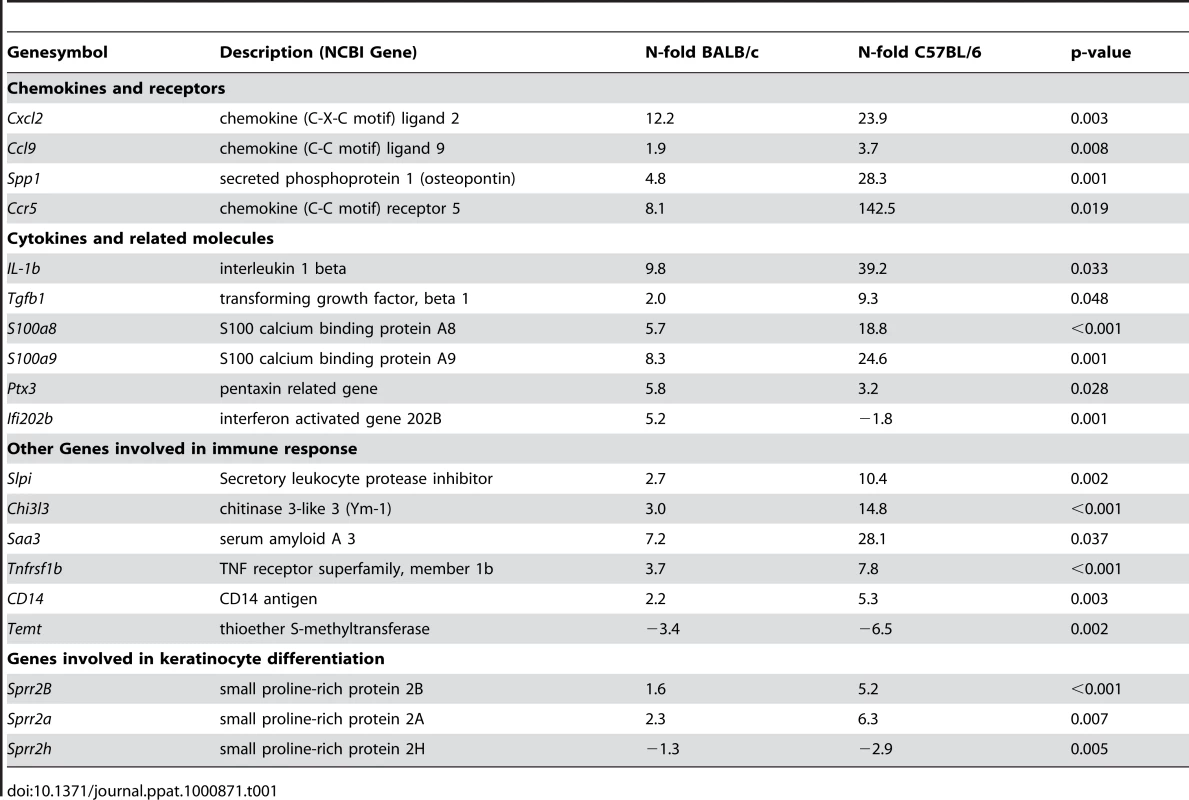
Considering the fact that whole tissue samples were analyzed one has to take into account that some genes could be highly regulated in a fraction of cells, while their absolute expression levels could still remain under the detection limit of the microarray analysis. Thus, the much more sensitive real-time PCR was applied i) to confirm the microarray data for selected genes ii) to extend the gene expression analysis especially to immunomodulatory mediators with known or suspected influence on Th1/2 differentiation whose expression was not detected by the less sensitive microarray analysis (Figure 1A and Table S5). PCR not only confirmed the induction of genes revealed by microarray analysis, but due to the higher sensitivity of the PCR analysis an up regulation of several chemokines and of the cytokines IL-12, TNFα, IL-4, and IL-6 could also be detected. All these genes were more strongly induced in C57BL/6 than in BALB/c.
Fig. 1. Analysis of gene and protein expression in L. major infected skin. 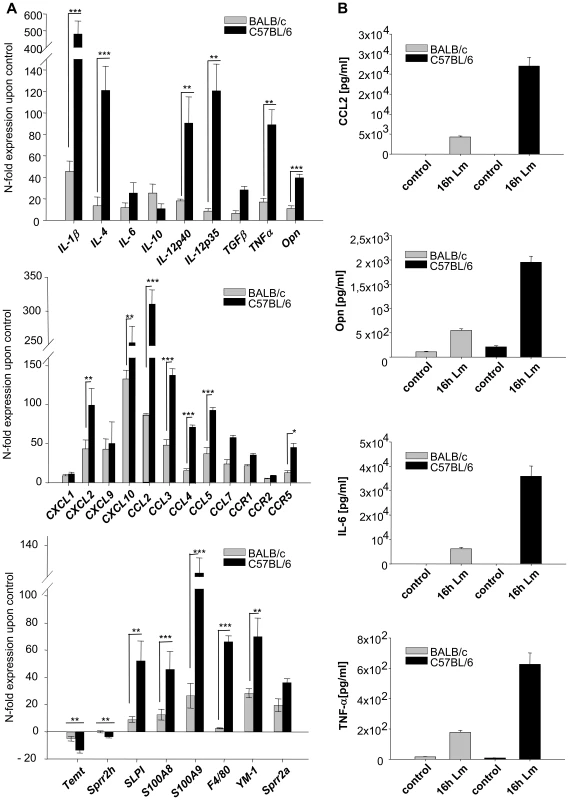
A) RT-PCR Analysis of gene expression in 16 h L. major infected skin. The PCR data were normalized to GAPDH expression and mean N-fold regulation and SEM in comparison to PBS injected controls was calculated (n = 3). Grey bars: Regulation in BALB/c mice; Black bars: Regulation in C57BL/6 mice. * = P<0.05, ** = P<0.01, *** = P<0,001 for differences between C57BL/6 and BALB/c mice, Student's t-test. B) Protein secretion in L. major infected skin. Soluble CCL2, Opn, TNFα (16h after infection) and IL-6 (8h after infection) protein was measured in infected foots as described in Materials and Methods. Striped grey bars: Mean and SE in BALB/c mice; Black bars: Mean and SE C57BL/6 mice. The experiment was performed three times with similar results. For selected genes, among them those with suspected influence on Th1/2 differentiation (such as opn, TNFα, IL-6), we confirmed both expression on protein level and higher up-regulation in C57BL/6 mice by immunoprecipitation as well as cytometric bead assay (Figure 1B).
Functional clustering
Besides cytokines many other genes were regulated in the skin in response to L. major. To get some functional insight into the pattern of regulated genes we applied an automated unbiased functional clustering using GENMAPP software [11], [12] and gene ontology annotations to determined which functional clusters among the regulated genes were statistically overrepresented. Accordingly, we found a statistically significant overrepresentation of genes involved in “inflammatory response”, “chemotaxis” and “cytokines” in the group of genes up-regulated in both mouse strains (Table S6, S7).
In agreement with the PCR-data we detected a significant over-representation of genes involved in “chemotaxis” and in “cell-mediated immune response” among those genes which were significantly stronger regulated in resistant mice. Importantly, genes involved in keratinocyte differentiation were also overrepresented in this group of genes (Table 2). These data indicate that gene expression of keratinocytes is markedly influenced early after infection with L. major.
Tab. 2. Functional gene clusters overrepresented. 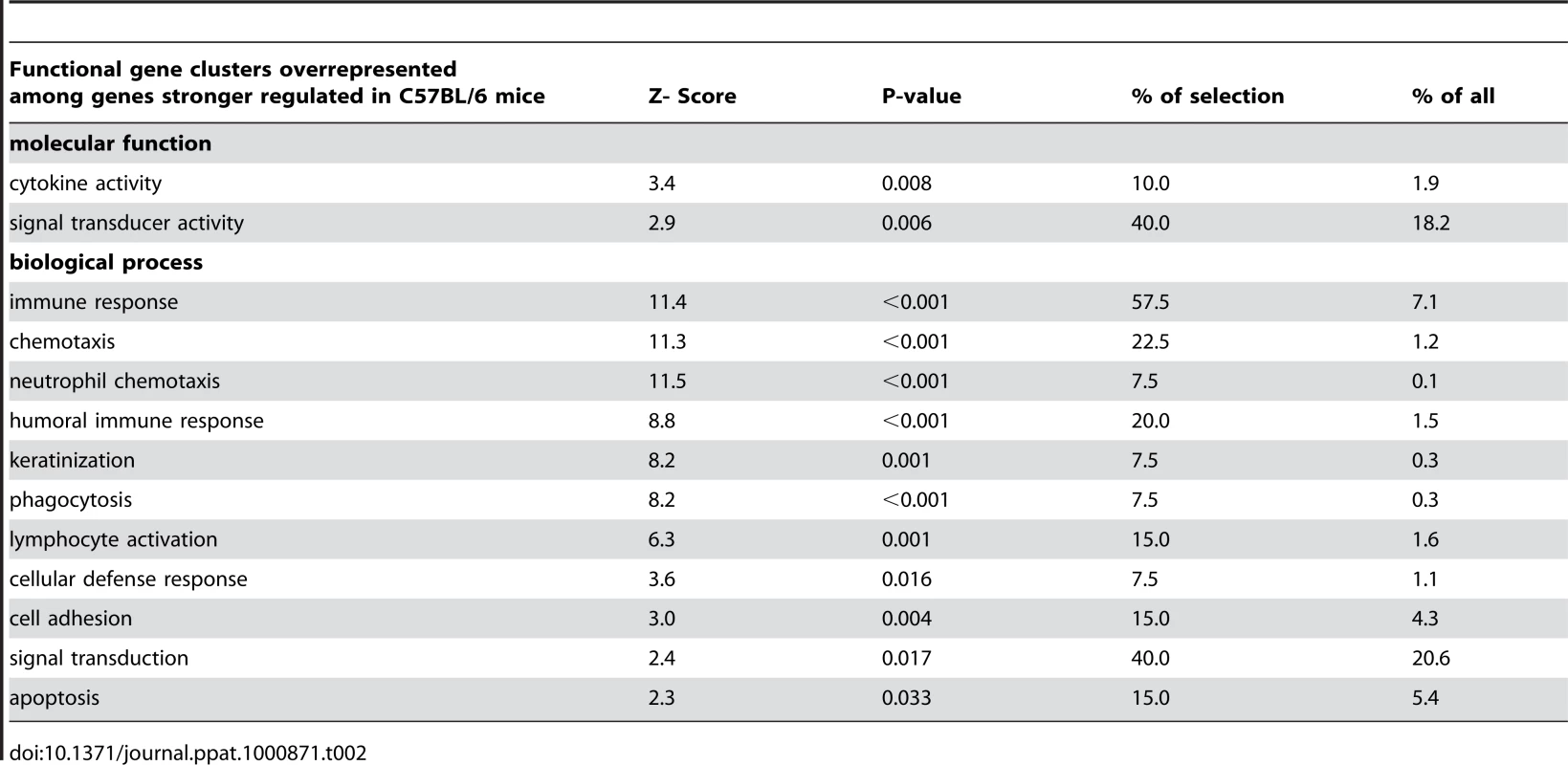
Microdissection and RNA in-situ-hybridization (RISH) revealed keratinocytes as a source of early cutaneous gene expression
Functional clustering and detection of overrepresented transcription factor binding sites both indicated involvement of keratinocytes in the early immune response. This prompted us to analyze more closely the role of the epidermis in early cutaneous gene expression during L. major infection in vivo by laser microdissection of epidermal keratinocytes from skin 16 hours after infection. For further verification, we applied in-situ-hybridization as it additionally allows spatial allocation of gene induction also in other cells outside the epidermis (e.g. infiltrating leukocytes).
After laser capturing of microdissected keratinocytes (Figure 2, left panel), we found induction of many immune mediators by RT-PCR, among them IL-4, opn, TNFα, IL-1β, IL-12 and IL-6 (Figure 2, right panel, Table S8). This way we showed that the stronger induction of cytokines applied not only for the whole skin, but in particular also for keratinocytes of resistant C57BL/6 mice.
Fig. 2. Analysis of gene in keratinocytes isolated from L. major infected skin. 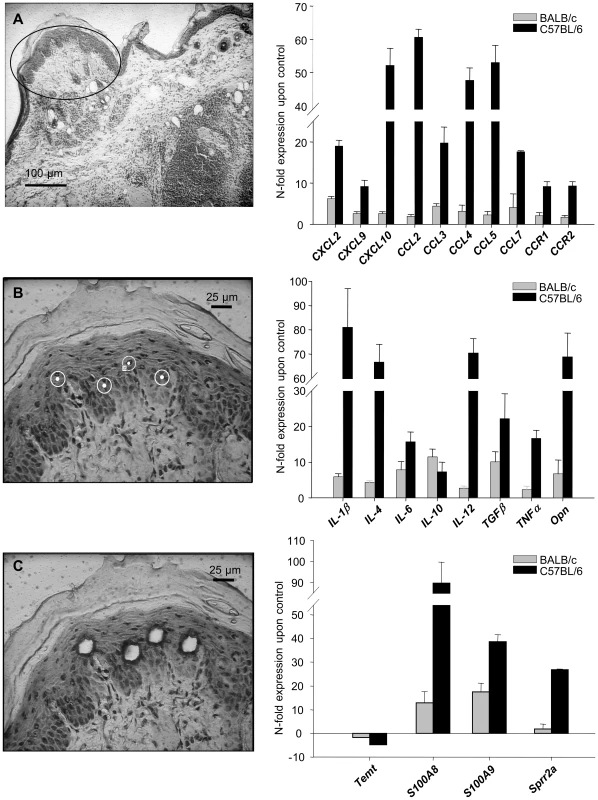
Laser capture microdissection (left panel). Single keratinocytes were dissected from cryosections of infected and control skin as described in Materials and methods. A) Overview, cryosection (BALB/c mice 16 h after infection). B,C) Single keratinocytes of the stratum spinosum were selected, laser microdissected and pressure catapulted. RT-PCR Analysis of gene expression in microdissected keratinocytes from 16h L. major infected skin (right panel). The PCR data were normalized to GAPDH expression and N-fold regulation and SEM in comparison to PBS injected controls was calculated (n = 2). Grey bars: Regulation in BALB/c mice; Black bars: Regulation in C57BL/6 mice. Similarly, when we analyzed the cellular cutaneous expression pattern by RISH (Figure 3A) or by immunohistochemistry for opn (Figure 3B) in C57BL/6 mice we found major expression of opn, CXCL10, CXCL2 and TNFα by the epidermis.
Fig. 3. In-situ-hybridization and immunohistochemistry. 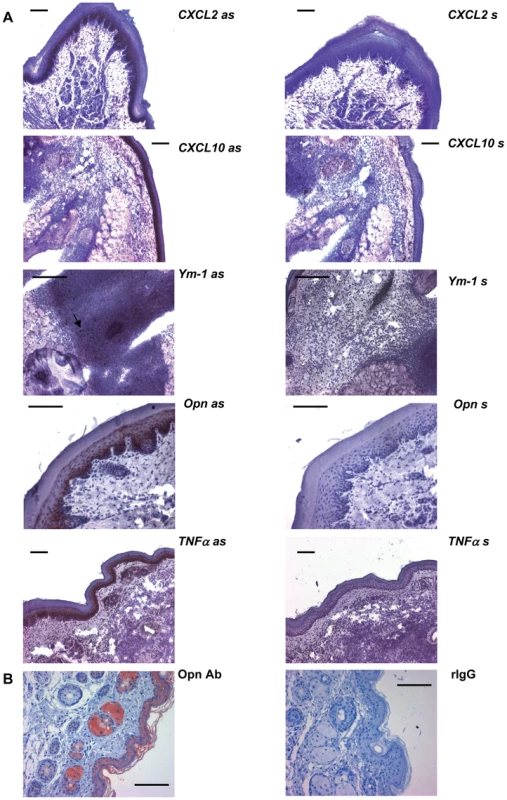
The cellular expression pattern of the indicated genes was analyzed in footpads of C57BL/6 mice infected 16 h with L. major by in-situ-hybridization (A) and immunohistochemistry (B). The arrow indicates Ym-1 positive cells in the infiltrate. Bars represent 50 µm; as = antisense RNA probe; s = sense RNA probe; Opn Ab = rat anti mouse monoclonal antibody against opn; rIgG = rat IgG. These results do not exclude that other cells present in the infected dermis also contribute to the observed induction of gene expression. However, they do reveal that keratinocytes are a hitherto unknown, yet important source of the early and distinctly expressed genes in infected footpads.
More prominent expression of macrophage chemokines preceded more prominent macrophage infiltration in resistant C57BL/6 mice
Functional clustering had revealed an over-representation of chemotactic factors among the more strongly induced genes in resistant mice. Opn was the chemokine with the most prominent differential expression. It is known as a cytokine able to induce a Th1 response and as a chemoattractant for macrophages, but has not been linked to leishmaniasis yet.
We therefore investigated, whether epidermal expression of macrophage chemokines (opn, CCL5, CCL2) correlated with a more rapid infiltration of mature macrophages in resistant mice [2]. Time-course experiments with real-time PCR revealed that the stronger peak in expression of macrophage chemokines after 8–24 h of infection in C57BL/6 mice was indeed followed by a stronger expression of Emr1, the gene encoding F4/80, a well established marker for mature macrophages (Figure 4). Thus, our global search for early tissue signals at the site of infection revealed the epidermal expression of chemokines which help to explain the known differences in leukocyte recruitment during experimental leishmaniasis.
Fig. 4. RT-PCR Time course analysis of gene expression in 1h to 72h L. major infected skin. 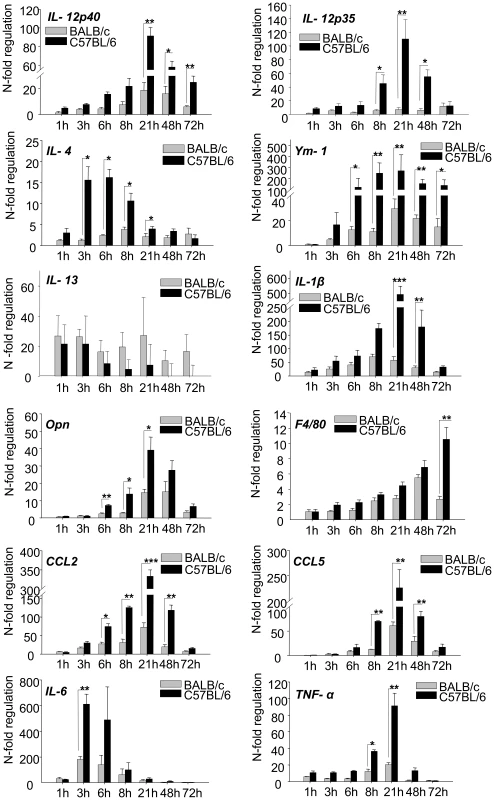
The PCR data were normalized to GAPDH expression and mean N-fold regulation and SEM in comparison to PBS injected controls was calculated (n = 3) Grey bars: Regulation in BALB/c mice; Black bars: Regulation in C57BL/6 mice. * = P<0.05, ** = P<0.01, *** = P<0.001 for differences between C57BL/6 and BALB/c mice, Student's t-test. Time course analysis of cytokine expression reveals their relevance for differentiation of Th1 cells
Our experiments had revealed that 16 hours after infection, the epidermis in resistant C57BL/6 mice is a major and stronger source not only for chemokines, but also for cytokines known to have the potential to induce Th1 cells (IL-12, IL-1β, TNFα and opn). Interestingly, keratinocytes in resistant C57BL/6 mice had also shown a higher induction of IL-4 and thus of a cytokine characteristically released by Th2 cells and counteracting Th1 cytokines. However, IL-4 has previously been reported to be necessary for Th1 differentiation and resistance to L. major in vivo, but only when administered exclusively during the first 8 hours of infection [13].
This accentuates the relevance of the early crucial time frame for Th1/Th2 differentiation and demonstrates that not only expression, but also duration of expression can be crucial for the effect of cytokines.
When we performed time course analyses for several cytokines, we detected an early peak of cytokine gene expression for IL-4 and IL-6 at 3-6 hours followed by a peak of IL1-β and IL-12 expression at 21 hours which was stronger in resistant mice and declined to baseline levels within one or two days (Figure 4). This temporal expression pattern thus correlated perfectly with the described crucial time frame for Th1/Th2 differentiation. For IL-4 it was particularly consistent with its previously shown pharmacological effects on Th1, but not Th2 cell differentiation. Our results would thus unravel the so far undetected source for IL-4 as a Th1-inducing cytokine in vivo. Of note, there were no strain specific differences in the expression of other Th2 cytokines such as IL-13.
We did not succeed in the - notoriously difficult - demonstration of IL-4 protein (due to the often low but yet efficacious concentrations in the tissue). However, as an indirect sign for the biological activity of IL-4 we demonstrated that Ym-1 (Figure 4), a gene specifically regulated by IL-4 and IL-13 in macrophages, was expressed in infiltrating leucocytes (by RISH, Figure 3A) and was more strongly induced in resistant mice following the induction of IL-4 (Figure 4).
There was a striking resemblance between IL-4 and IL-6 with regard to their epidermal and temporal expression pattern (Figure 4). Expression of IL-6 peaked even more rapidly.
Local deficiency of IL-4 and IL-6 resulted in increased susceptibility towards L. major infection
While it had been demonstrated that early treatment of susceptible mice with IL-4 resulted in a Th1 shift [13], our data now pointed to a corresponding relevance of endogenous IL-4 in resistant mice for induction of the Th1 immune response. To prove this hypothesis we injected 1µg of neutralizing anti-IL-4 antibody in the infected footpads of resistant mice at the time of parasite inoculation and 4h later. When we measured L. major specific cytokine secretion by CD4+-T-cells one week later we detected increased levels of IL-4 and IL-13 and simultaneously decreased levels of IFNγ (Figure 5). Thus, neutralization of endogenous IL-4 in infected footpads at the time of infection resulted in a clear Th2 switch.
Fig. 5. Early anti-IL-4 treatment induces a Th2 switch. 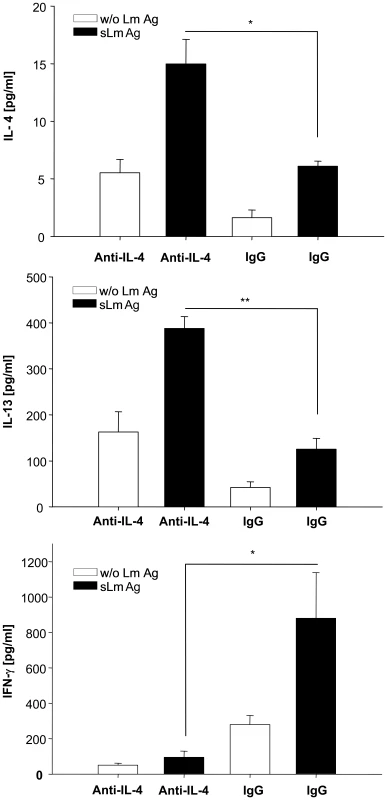
IL-4, IL-13 and IFNγ secretion of CD4+ cells isolated one week after infection from draining lymph nodes of C57BL/6 mice which had been injected with 1 µg of neutralizing anti-IL-4 or irrelevant antibody into the infected footpad at the time of parasite inoculation and 4h later. Cells were incubated for 48 h with syngenic DC stimulated for 48h with soluble Leishmania antigen (sLmAg) (black bars) or with unstimulated syngenic DC (open bars). Cytokines in culture supernatants were assayed by cytometric bead assay (mean ± SE, n = 3) * = p<0.05, ** = p<0.01, Student's t-test. Due to this time course and its likewise dual role in Th1/Th2-differentiation [14]–[18], we hypothesized that IL-6 could act in a similar way as IL-4 and support Th1-immunity during a narrow, early time frame of L. major infection. To analyze whether locally produced IL-6 affects adaptive immunity and resistance in L. major infection we generated mice with a selective IL-6 deficiency in non-hematopoietic cells (e.g. keratinocytes). To this end we reconstituted lethally irradiated, IL-6-deficient mice with IL-6 competent wild type bone marrow (wt→IL-6−/− chimeric mice). As control group, irradiated C57BL/6 wild type mice were reconstituted with wild type bone marrow (wt→wt mice) and irradiated IL-6−/− mice were reconstituted with wild type bone marrow from IL-6−/− mice (IL-6−/−→IL-6−/− chimeric mice).
After infection with L. major, the deficiency of IL-6 in non-hematopoietic cells and keratinocytes (wt→IL-6−/− chimeric mice) resulted in a marked deterioration of disease (Figure 6A), shown by increased footpad swelling as well as by significantly increased numbers of living parasites in footpads (Figure 6B) and lymph-nodes (data not shown) compared to the control groups of mice. This aggravation correlated with a significant reduction in secretion of IFNγ by L. major –specific CD4+ T-cells (restimulated in vitro with L. major antigen presented by DC) and a corresponding increase in the antigen-specific secretion of IL13 by L. major-specific CD4+ T-cells (Figure 6C).
Fig. 6. Experimental leishmaniasis in wt→IL-6 −/− chimeric mice. 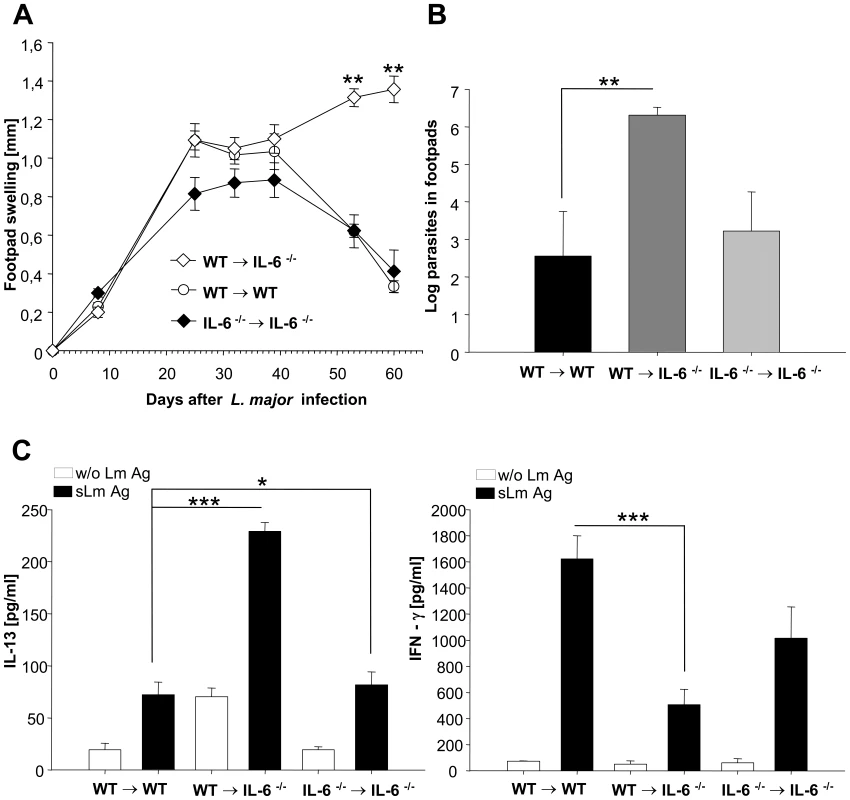
A) Footpad swelling (compared to the not infected contra lateral footpad) (mm) of infected wt→IL-6 −/− chimeric mice, control wt→wt mice and control IL-6 −/− →IL-6 −/− chimeric mice ** = p<0.01, Student's t-test. B) Limiting dilution assay (LDA) from footpads of wt→IL-6 −/− chimeric mice, control wt→wt mice and control IL-6 −/− →IL-6 −/− chimeric mice 9 weeks after infection (mean */− SE of log parasites) ** = p<0.01, Student's t-test. C) IFNγ and IL-13 secretion of CD4+ cells isolated from draining lymph nodes of wt→IL-6 −/− chimeric mice, control wt→wt mice and control IL-6 −/− →IL-6 −/− chimeric mice 9 weeks after infection. Cells were incubated for 48 h with syngenic dendritic cells stimulated for 48h with soluble Leishmania antigen (s LmAg) (black bars) or with unstimulated syngenic dendritic cells (without (w/o) Lm Ag, open bars). Cytokines in culture supernatants were assayed by cytometric bead assay (mean ± SE, n = 3) * = p<0.05, *** = p<0.001, Student's t-test. These data indicate that there is a switch from a Th-1 response towards a Th-2 response in wt→IL-6−/− chimeric mice. The switched Th-cell cytokine pattern proofed to be biologically highly relevant as it directly correlated with the course of disease in wt→IL-6−/− chimeric mice, i.e. a highly significant increase in footpad swelling and a more than 1000 fold higher local parasite titer.
Discussion
Resistance in experimental leishmaniasis depends on the development of a L. major specific Th1 response, while Th2 differentiation in BALB/c mice results in susceptibility. The decisive events for the development of a Th1 or Th2 response take place during the first 3 days of infection [9], [19]–[22]. There is growing evidence that the microenvironment of the infected tissue delivers the initial triggers that affect Th-cell differentiation. The nature or source of such triggers, however, is still enigmatic.
Using a combination of gene array analysis, functional clustering, microdissection, RISH and Real-time PCR, we were able to confirm the hypothesis that molecules relevant for defense against infection with L. major and for the generation of a Th1/Th2 response not only are early induced at the site of infection, but also at great part by the cell-rich epidermis and with a significantly stronger expression in resistant mice. While the relevance of opn, IL-12 IL-1β, and TNFα for the crucial recruitment of macrophages and the induction of a Th1 response are well known [1], [9], [10], [23], the crucial relevance of IL-4 and IL-6 for resistance and Th1 response had to be especially elaborated.
Although an early expression of some chemokines and cytokines in infiltrating leukocytes and in the skin has been found previously [24]–[28], such a pronounced and differential expression of immune-modulatory mediators in the skin within the first hours of L. major infection was hitherto not described. Strain specific differences were so far only reported for CXCL10 [24]. Moreover, the major source for cytokine production in the first hours of infection was not known.
The results of functional clustering pointed to a relevant, so far unrevealed involvement of keratinocytes in the early immune response. Our spatial analysis of gene expression by microdissection, in-situ hybridization and immunohistochemistry confirmed that subcutaneous injection of L. major induces several genes and especially cytokines in keratinocytes during the first hours of infection.
Moreover, detection of proteins in supernatants of minced footpad tissue strongly suggests that induction of genes results in expression and efficient secretion of epidermally produced immune-mediators, which affect the parasite-induced immune response.
The mechanism by which L. major induces gene expression in keratinocytes early after infection remains enigmatic. A direct interaction between L. major and keratinocytes seems unlikely because of the subcutaneous infection and because keratinocytes do not take up L. major in vitro [29]–[32]. It is possible that epidermal gene induction is caused by cytokines (e.g. CCL4, IL-8 and CCL2) released from resident macrophages and early infiltrating granulocytes [2], [24], [33]–[38], after their initial contact with the parasites. By such a crosstalk, epidermal keratinocytes would be involved in an amplification of the L. major-related inflammatory tissue signal, since they are capable of synthesizing and secreting large amounts of cytokines [39]. Such an amplification due to high numbers of cells could be essential since only few resident macrophages are present early after infection and infiltrating granulocytes are not equipped for sustained transcription of inflammatory mediators.
Most importantly, the induction of gene expression in keratinocytes was significantly different between susceptible and resistant mice, indicating an influence of epidermal cells on the direction of the ensuing immune response.
One mechanism by which this influence is executed could be the differential recruitment of leukocytes. A higher percentage of granulocytes in BALB/c mice is relevant for susceptibility, while a higher percentage of mature macrophages in C57BL/6 mice is associated with resistance towards L. major [2], [3], [40]. Here we demonstrate the temporal correlation between a significantly stronger and transient expression of macrophage chemoattractants (CCL5, CCL2 and opn) with a peak after 8h post infection and an ensuing significantly more pronounced macrophage infiltration 3 days after infection.
Opn was also described as an early Th1-inducing cytokine acting on DC [10], [41]. Together with the significantly stronger expression of the Th1-inducing cytokines IL-12, TNFα and IL-1β in the epidermis of resistant mice, this points to a direct effect of epidermal gene expression on Th1/2 differentiation. IL-12 is the best characterized Th1-inducing cytokine so far [19]–[21], although a stronger early expression of IL-12 in vivo in resistant mice has so far never been demonstrated, neither for the skin nor for the lymph nodes. Similarly, IL-1β has so far not been shown to be differentially expressed in skin, whereas we had previously described a more prominent expression of IL-1α in lymph nodes of resistant mice [9].
The time course of transient IL-12 and IL-1β expression from 8 h to 72 h after infection correlates perfectly with the critical time frame for Th1/Th2 differentiation [9], [19]–[21]. In confirmation of this relevance, we and others previously showed that application of either IL-12 or IL-1β at the site of infection during the first three days after injection with L. major was able to promote Th1 differentiation and to inhibit disease progression in BALB/c mice [9], [42]. This study now shows that keratinocytes are an essential natural source to provide early significant amounts of IL-12 or IL-1β in C57BL/6 mice.
In addition, our finding of a transient IL-4 expression in the skin of resistant mice may explain a paradoxon in experimental leishmaniasis: IL-4 on one hand is the best characterized Th2 cytokine; correspondingly, IL-4 production in the draining lymph nodes of BALB/c has been associated with induction of a Th2 response [43]–[45]. On the other hand, IL-4 is able to induce production of IL-12p70 in murine and human DC in vitro [46], [47] and to promote a protective Th1 immune response in susceptible BALB/c mice but only when administered during the first 8 h of infection, and not if given for a prolonged period of time [13]. Thus, IL-4 induces a Th1 response earlier in infection, most likely via increasing IL-12 secretion from DCs in a defined early time frame. However, it remained unclear whether endogenous IL-4 is involved in Th1 immunity.
We now demonstrated that the epidermis could provide IL-4 whose expression is temporally restricted to exactly the crucial early time frame. While it is technically problematic to detect the low local levels of IL-4 protein, we found that expression of Ym-1, a gene specifically regulated by IL-4 in macrophages (and IL-13 which did not shown differential expression), exactly followed the differential IL-4 expression with a stronger peak in C57BL/6 mice. This could indicate a temporally defined and quantitatively distinct local presence of biological active IL-4. More important, we could clearly demonstrate that neutralizing early endogenous IL-4 in resistant mice locally by anti-IL-4 antibody resulted in a switch to a Th2 cytokine secretion pattern of CD4+ T-cells from draining lymph nodes one week later (Figure 5). This clearly indicates that early endogenous IL-4, most likely produced by keratinocytes, is mandatory for the induction of a Th1 response against L. major.
Since IL-4 is induced in the epidermis - and thus in direct vicinity to DCs -, we suggest that under physiological conditions epidermal IL-4 could act in a paracrine way on DCs so that they are prepared to release IL-12 when migrating to the lymph node.
Similar to IL-4 we found a very rapid, but also temporally restricted induction of IL-6 (mRNA as well as protein) in the skin which likewise was significantly stronger in resistant mice. Microdissection revealed that keratinocytes are an important source of local IL-6 expression. Like IL-4, IL-6 has been demonstrated to induce Th2 differentiation via direct action on Th cells [14], [15], while it was also required for the development of Th1 immunity in murine tuberculosis, colitis and experimental autoimmune encephalomyelitis [16]–[18]. Besides, IL-6 appears to be involved in differentiation of Th17 cells and in inhibition of regulatory T-cells [48] while it also inhibited differentiation, maturation and activation of DC [49]–[52]. In the light of these results it is reasonable to conclude that the effect of IL-6 on either Th1 or Th2 differentiation in an emerging immune response crucially depends on timing as well as site of action. In case of its complete genetic deletion, these effects may set each other off, which may explain why it resulted in only minimal net effects on the course of experimental leishmaniasis [53], [54].
In order to assess whether lack of cutaneous IL-6 production would impair development of Th1 cells, we demonstrated that IL-6 −/ − mice with IL-6 competent bone marrow and thus with a constitutional lack of IL-6 at the site of infection, became markedly more susceptible to L. major (more than 1000 fold more parasites compared to control animals) and showed a shift from a Th1 towards a Th2 response. Thus, IL-6 is a new tissue signal whose early local presence promotes Th1 cells.
The mechanism by which IL-6 affects the Th1 response is currently unclear. It may not primarily instruct DC for Th1 priming [49]–[52], but could inhibit conversion of naïve T-cells into Foxp3+ regulatory T-cells (Treg) [48] so that its local absence leads to increased influence of Treg on Th1 cells. However, the function of Treg in experimental leishmaniasis is not completely clear and both inhibitory as well as exaggerating effects of Treg on the course of L major infection have been described [55]–[59]. Thus, it is also tempting to speculate that the immediate induction of IL-6 after contact with the parasites may prompt resident and early infiltrating cells to generate a Th1 promoting local micromilieu, characterized e.g. by ensuing expression of IL-4, IL1β, IL-12 and opn.
In summary, our approach directed at a global view of gene expression and thus reflecting the biology of systems revealed a stronger expression of immunomodulatory mediators in the infected skin and epidermis of resistant compared to susceptible mice. Our approach for the first time defines the global pattern of the early “tissue signal” and moreover identifies keratinocytes as a critical modulator of the microenvironment in L. major infected skin. Furthermore, our data indicate for the first time that epidermal cytokine expression, e. g. of IL-4 and IL-6 is a decisive factor in the generation of protective Th1 immunity and contributes actively to the outcome of inflammatory reactions.
Materials and Methods
Ethics statement
All experiments with mice were performed with the approval of the State Review Board of Nordrhein-Westfalen (Germany) according to the German law for animal welfare (Tierschutzgesetz) § 8; reference number 8.87–50.10.36.08.009.
Mice
Specific pathogen-free, female C57BL/6 and BALB/c mice were purchased from Charles River, Germany, and were housed in microisolator cages and given mouse chow and water ad libitum. Mice were 8–12 wk of age when used in experiments. All experiments with mice were performed with the approval of the State Review Board of Munster (Germany).
Parasites and experimental infection
L. major (MHOM/IL/81/FE/BNI) parasites were cultivated in Schneider's Drosophila Medium supplemented with 10% FCS, 2% human urine, 2% glutamine, and 1% Penicillin/Streptomycin. Soluble Leishmania antigen (SLA) was prepared by 5 repeated freeze and thaw cycles in phosphate buffered saline (PBS).
Mice were infected subcutaneously by application of 2×107 promastigotes (stationary phase) of L. major in 50 µl PBS into the left hind footpad. The right footpad was injected with 50 µl PBS alone and served as internal control to ensure that gene expression was not caused by the injection stimulus. After sacrifice, footpads from mice were harvested 1 to 72 hours after infection for gene expression analysis. In the experiments neutralizing local IL-4 1 µg of neutralizing rat anti-mouse IL-4-antibody or irrelevant rat IgG (Biolegend, Uithoorn, Netherlands) was used. Mice were injected at the time of parasite inoculation and 4h later.
DNA microarray and statistical data analysis
In three independent experiments, total RNA from 16 h L. major infected C57BL/6 or BALB/c mice and PBS injected control animals was isolated and subsequently processed for microarray hybridization using Affymetrix Murine Genome MG_U74Av2 arrays according to the manufacturer's instructions (Affymetrix). Microarray data were analyzed using MicroArray Suite Software 5.0 (Affymetrix) using data from corresponding control samples as baseline and further studied applying the Expressionist Suite software package (GeneData), which allows identification of genes that are significantly regulated in multiple independent experiments as described previously [60].
We retained only genes which were significantly regulated in every single experiment (change p-value <0.05, fold-change ≥2, expression over background) as well as in the complete set of experiments (fold-change of ≥3.0, p-value of <0.05, paired t-test).
To compare L. major induced alterations of cutaneous expression patterns between resistant and susceptible mouse strains, signal log ratios of infected versus uninfected control samples in both mice strains were evaluate by paired t-test. We retained only genes with a p-value <0.05 and a differential fold-change regulation of ≥1.75.
Principal component analysis (PCA) was applied to mathematically reduce the dimensionality of the entire spectrum of gene expression values of a microarray experiment to three components as described previously [61] and revealed that individual experimental groups were well reproducible and clearly separated.
Functional clustering
To analyze the microarray data in the context of biological functions, we used information available from the Gene Ontology (GO) consortium (http://www.geneontology.org) [11], [12]. The GO terms represent a defined vocabulary describing the biological process, cellular components, and molecular functions of genes in a hierarchical directed acyclic graph structure. Statistical analysis was performed for groups of >10 genes using GenMAPP software [11], [12]. For each of the existing GO terms, the cumulative number of genes meeting our criteria (e.g. up - or down-regulated) and of all genes represented on the microarray was calculated. The Z score is calculated for every GO term by subtracting the expected number of genes meeting the criterion from the actual number, and division of this value by the standard deviation of the actual number of genes:with N as the total number of genes measured, R as the total number of genes meeting the criterion, n as the total number of genes in the specific GO term, and r as the number of genes meeting the criterion in the specific GO term. A positive Z score indicates that there are more genes meeting the criterion in the specific GO term than expected by chance. The Z-score is transferred to p-values under the assumption of a hypergeometric distribution.
RNA in situ hybridization (RISH) and immunohistochemistry
For RISH, the complete coding regions of the genes analyzed were amplified by PCR and cloned into pBluecript (Stratagene, La Jolla, CA) (Table S9). For antisense and sense probes, the plasmid was linearized by appropriate restriction enzymes and T7 or T3 RNA polymerase was used to synthesize digoxigenin labeled probes. The RISH was performed on 4 µm frozen sections according to an protocol described earlier [62].
Immunohistochemical staining was performed [2] using a rabbit anti-mouse opn antibody (Assay Designs, Ann Arbor, MI, USA).
Preparation and staining of slides for laser microdissection and pressure catapulting
Tissue-Tec O.C.T. (Sakura, Germany) -embedded hind feet were cut in 12 µm sections on a microtome, transferred onto PEN-covered glass slides (PALM, Germany) and immediately stored at −80°C. Sections were stained with cresyl violet (1% in aqua bidest) followed by rehydration in 100, 96, 70% ethanol and dried at 37°C for half an hour.
Laser microbeam microdissection & pressure catapulting (LMPC)
Collection of keratinocytes by LMPC was performed as described [63], utilizing the PALM Laser Microbeam Microdissection System with Laser Pressure Catapulting and Robocut Software (PALM, Germany). A minimum of 200 cell equivalents (about 1000 visible cells) were isolated and collected into a reaction tube cap, which was filled with TRIzol Reagent (Invitrogen, Germany).
RNA Isolation, reverse transcription and random based PCR amplification
For total skin RNA extraction, skin from the hind footpad was excised and pulverized in liquid nitrogen and Potter homogenized in TRIzol Reagent. Subsequent RNA isolation was performed using TRIzol Reagent (Invitrogen, Germany) and subsequently RNeasy Minikit (Qiagen, Germany) cleanup preparation including DNAse digestion. The RNA from laser microdissected keratinocytes was TRIzol extracted, following the manufacturer's protocol for small quantities of cells. The quality and approximate quantity of the resulting RNA was determined using the microfluidics system (Agilent 2100 Bioanalyzer, Agilent Technologies). Whole skin material was transcribed into cDNA utilizing H-minus reverse transcriptase (MBI Fermentas, Germany). cDNA synthesis and amplification of laser microdissected samples were performed with the Microarray Target Amplification Kit (Roche Diagnostics, Germany), following the manufacturer's instructions. Total RNA extracted from 1000 keratinocytes was used as template for first-strand synthesis.
Quantitative RT-PCR
Expression of selected genes was confirmed by real-time RT-PCR as described previously [60], see Table S10 for primer sequence information.
Protein measurements
To determine secreted proteins in infected footpad tissue, dissected feet were flushed repeatedly with a total of 500 µl PBS and adjusted to equal protein concentrations. Cytokine concentrations were determined using the cytometric bead assay (BD Bioscience, San Jose, CA, USA).
Osteopontin was immunoprecipitated using monoclonal goat anti-opn antibody (RD Systems, Minneapolis, MN, USA), following Protein A sepharose, elution of protein and subsequent analysis using polyacrylamid gel electrophoresis and western blotting.
Samples were adjusted to equal protein concentrations and separated by SDS/PAGE (12% acrylamide). Subsequently, proteins were transferred by blotting onto membrane (Pall GmbH, Dreieich, Germany), incubated for 1 h in NaCl/Tris/0.1% (v/v) Tween 20/5% (w/v) nonfat dried milk, and then for 1 h in NaCl/Tris/5% (w/v) nonfat dried milk containing goat anti-opn antibody. The membrane was washed three times in NaCl/Tris/0.1% Tween-20 and incubated with a phosphatase-conjugated secondary anti-goat-IgG to visualize opn using the standard phosphatase reaction. The bands were scanned densitometrically.
Generation of chimeric mice
C57BL/6 mice or IL-6 gene deficient mice were lethally irradiated (5.0 Gy on 2 days resulting in a cumulative dose of 10 Gy) and reconstituted with bone marrow cells (107 cells/mouse) obtained from wt C57BL/6 or from IL6−/ − mice. For experimental leishmaniasis chimeric mice were used 6 weeks after bone marrow reconstitution.
Experimental leishmaniasis
Cutaneous leishmaniasis was initiated by subcutaneous application of 2×107 promastigotes (stationary phase) of L. major in 50 µl PBS into the left hind footpad. Footpad thickness was assessed weekly using a metric caliper. Specific swelling of the infected footpad was assessed by subtracting the diameter of the infected footpad from that of the non-infected footpad. Footpads, lymph nodes and spleen from each mice were harvested for limiting dilution assay (LDA) and determination of cytokine profile. The experiments were repeated 2–3 times.
Limiting dilution assay
Parasite numbers in bone marrow and liver as a parameter for systemic spread were determined 8–12 weeks after infection by a limiting dilution assay (LDA) modified by using leishmania medium as specified above instead of slant blood agar [64].
Cytokine and proliferation assay
For cytokine assay mice were euthanized and draining lymph nodes were aseptically removed. A single cell suspension was prepared and CD4+ T cells were collected using biomagnetic enrichment procedures (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's recommendations.
Bone marrow derived dendritic cells (DC) were generated as previously described [65]. Shortly, the femur bone was aseptically removed from euthanized C57BL/6 mice and the bone marrow was flushed out. Bone marrow DCs were expanded with IL-4 and GM-CSF for 6 days. DCs (1×106 cells/ml) were incubated with SLA equivalent to 5×106 L. major for 48 h. For assessment of cytokine secretion DC and CD4+ T cells (5×104/100 µl) were mixed in a ratio 1∶5 and cultured in RPMI1640 plus 2 mM glutamine, 50 µM mercaptoethanol and 10% FCS for 48 hours. Culture supernatants were assayed by cytometric bead assay BD Bioscience, San Jose, CA, USA) according to the manufacturer's instructions.
Statistical analysis
To determine whether differences were statistically significant, Student's t test was performed, using a two-tailed distribution. Indication of p-values are as follows * <0.05; ** <0.01; *** <0.001.
Annotation of genes and proteins mentioned in the manuscript (database NCBI Gene)
CCL2 GeneID: 20296, CCL3 GeneID: 20302, CCL4 GeneID: 20303, CCL5 GeneID: 20304, CCL7 GeneID: 20306, CCL9 GeneID: 20308, CCR1 GeneID: 12768, CCR2 GeneID: 12772, CCR5 GeneID: 12774, CD14 GeneID: 12475, Chi3l3 GeneID: 12655, CXCL10 GeneID: 15945, CXCL1 GeneID: 14825, CXCL2 GeneID: 20310, CXCL9 GeneID: 17329, Emr1 GeneID: 13733, Ifi202b GeneID: 26388, IFN-g GeneID: 15978, IL-10 GeneID: 16153, IL-12p35 GeneID: 16159, IL-12p40 GeneID: 16160, IL-13 GeneID: 16163, IL-1a GeneID: 16175, IL-1b GeneID: 16176, IL-4 GeneID: 16189, IL-6 GeneID: 16193, Ost GeneID: 20750, Ptx3 GeneID: 19288, S100a8 GeneID: 20201, S100a9 GeneID: 20202, Saa3 GeneID: 20210, Slpi GeneID: 20568, Sprr2a GeneID: 20755, Sprr2B GeneID: 20756, Sprr2h GeneID: 20756, Temt GeneID: 21743, Tgfb1 GeneID: 21803, TNFa GeneID: 21926, Tnfrsf1b GeneID: 21938.
Supporting Information
Zdroje
1. SacksD
Noben-TrauthN
2002 The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2 845 858
2. SunderkotterC
KunzM
SteinbrinkK
Meinardus-HagerG
GoebelerM
1993 Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol 151 4891 4901
3. Tacchini-CottierF
ZweifelC
BelkaidY
MukankundiyeC
VaseiM
2000 An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol 165 2628 2636
4. NaborsGS
NolanT
CroopW
LiJ
FarrellJP
1995 The influence of the site of parasite inoculation on the development of Th1 and Th2 type immune responses in (BALB/c×C57BL/6) F1 mice infected with Leishmania major. Parasite Immunol 17 569 579
5. ConstantSL
BrogdonJL
PiggottDA
HerrickCA
VisintinI
2002 Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest 110 1441 1448
6. KapsenbergML
2003 Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3 984 993
7. MatzingerP
2002 An innate sense of danger. Ann N Y Acad Sci 961 341 342
8. MatzingerP
2007 Friendly and dangerous signals: is the tissue in control? Nat Immunol 8 11 13
9. Von StebutE
EhrchenJM
BelkaidY
KostkaSL
MolleK
2003 Interleukin 1alpha promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med 198 191 199
10. AshkarS
WeberGF
PanoutsakopoulouV
SanchiricoME
JanssonM
2000 Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287 860 864
11. DahlquistKD
SalomonisN
VranizanK
LawlorSC
ConklinBR
2002 GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet 31 19 20
12. DonigerSW
SalomonisN
DahlquistKD
VranizanK
LawlorSC
2003 MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol 4 R7
13. BiedermannT
ZimmermannS
HimmelrichH
GumyA
EgeterO
2001 IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol 2 1054 1060
14. DiehlS
AnguitaJ
HoffmeyerA
ZaptonT
IhleJN
2000 Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13 805 815
15. RinconM
AnguitaJ
NakamuraT
FikrigE
FlavellRA
1997 Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med 185 461 469
16. LadelCH
BlumC
DreherA
ReifenbergK
KopfM
1997 Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun 65 4843 4849
17. YamamotoM
YoshizakiK
KishimotoT
ItoH
2000 IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol 164 4878 4882
18. SeradaS
FujimotoM
MiharaM
KoikeN
OhsugiY
NomuraS
YoshidaH
NishikawaT
TerabeF
OhkawaraT
TakahashiT
RipleyB
KimuraA
KishimotoT
NakaT
2008 IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 105 9041 9046
19. SypekJP
ChungCL
MayorSE
SubramanyamJM
GoldmanSJ
SieburthDS
WolfSF
SchaubRG
1993 Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med 177 1797 1802
20. HeinzelFP
SchoenhautDS
RerkoRM
RosserLE
GatelyMK
1993 Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med 177 1505 1509
21. HeinzelFP
RerkoRM
AhmedF
PearlmanE
1995 Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol 155 730 739
22. von StebutE
2007 Cutaneous Leishmania infection: progress in pathogenesis research and experimental therapy. Exp Dermatol 16 340 346
23. von StebutE
MetzM
MilonG
KnopJ
MaurerM
2003 Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1alpha/beta released from neutrophils recruited by mast cell-derived TNFalpha. Blood 101 210 215
24. MullerK
vanZG
HansenB
LaufsH
JahnkeN
SolbachW
LaskayT
2001 Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med Microbiol Immunol 190 73 76
25. VesterB
MullerK
SolbachW
LaskayT
1999 Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infect Immun 67 3155 3159
26. MatteC
OlivierM
2002 Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. J Infect Dis 185 673 681
27. AntoniaziS
PriceHP
KropfP
FreudenbergMA
GalanosC
SmithDF
MullerI
2004 Chemokine gene expression in toll-like receptor-competent and -deficient mice infected with Leishmania major. Infect Immun 72 5168 5174
28. KatzmanSD
FowellDJ
2008 Pathogen-imposed skewing of mouse chemokine and cytokine expression at the infected tissue site. J Clin Invest 118 801 811
29. MbowML
DeKreyGK
TitusRG
2001 Leishmania major induces differential expression of costimulatory molecules on mouse epidermal cells. Eur J Immunol 31 1400 1409
30. BlankC
FuchsH
RappersbergerK
RollinghoffM
MollH
1993 Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J Infect Dis 167 418 425
31. MollH
FloheS
RollinghoffM
1995 Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol 25 693 699
32. WillA
BlankC
RollinghoffM
MollH
1992 Murine epidermal Langerhans cells are potent stimulators of an antigen-specific T cell response to Leishmania major, the cause of cutaneous leishmaniasis. Eur J Immunol 22 1341 1347
33. ChaussabelD
SemnaniRT
McDowellMA
SacksD
SherA
NutmanTB
2003 Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102 672 681
34. RacoosinEL
BeverleySM
1997 Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp Parasitol 85 283 295
35. BadolatoR
SacksDL
SavoiaD
MussoT
1996 Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Exp Parasitol 82 21 26
36. CillariE
DieliM
MalteseE
MilanoS
SalernoA
LiewFY
1989 Enhancement of macrophage IL-1 production by Leishmania major infection in vitro and its inhibition by IFN-gamma. J Immunol 143 2001 2005
37. van ZandbergenG
KlingerM
MuellerA
DannenbergS
GebertA
SolbachW
LaskayT
2004 Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol 173 6521 6525
38. van ZandbergenG
HermannN
LaufsH
SolbachW
LaskayT
2002 Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect Immun 70 4177 4184
39. AlbanesiC
ScarponiC
GiustizieriML
GirolomoniG
2005 Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy 4 329 334
40. LopezKS
DingesS
GriewankK
IwakuraY
UdeyMC
von StebutE
2009 IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol 182 3039 3046
41. RenklAC
WusslerJ
AhrensT
ThomaK
KonS
UedeT
MartinSF
SimonJC
WeissJM
2005 Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood 106 946 955
42. FilippiC
HuguesS
CazarethJ
JuliaV
GlaichenhausN
UgoliniS
2003 CD4+ T cell polarization in mice is modulated by strain-specific major histocompatibility complex-independent differences within dendritic cells. J Exp Med 198 201 209
43. LaunoisP
OhtekiT
SwihartK
MacDonaldHR
LouisJA
1995 In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1-. Eur J Immunol 25 3298 3307
44. LaunoisP
MaillardI
PingelS
SwihartKG
XenariosI
Acha-OrbeaH
DiggelmannH
LocksleyRM
MacDonaldHR
LouisJA
1997 IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6 541 549
45. HimmelrichH
LaunoisP
MaillardI
BiedermannT
Tacchini-CottierF
LocksleyRM
RockenM
LouisJA
2000 In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J Immunol 164 4819 4825
46. HochreinH
O'KeeffeM
LuftT
VandenabeeleS
GrumontRJ
MaraskovskyE
ShortmanK
2000 Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med 192 823 833
47. NiggAP
ZahnS
RuckerlD
HolscherC
YoshimotoT
EhrchenJM
WolbingF
UdeyMC
von StebutE
2007 Dendritic cell-derived IL-12p40 homodimer contributes to susceptibility in cutaneous leishmaniasis in BALB/c mice. J Immunol 178 7251 7258
48. KornT
MitsdoerfferM
CroxfordAL
AwasthiA
DardalhonVA
GalileosG
VollmarP
StriteskyGL
KaplanMH
WaismanA
KuchrooVK
OukkaM
2008 IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 105 18460 18465
49. LiangS
RistichV
AraseH
DaussetJ
CarosellaED
HoruzskoA
2008 Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Natl Acad Sci U S A 105 8357 8362
50. KitamuraH
KamonH
SawaS
ParkSJ
KatunumaN
IshiharaK
MurakamiM
HiranoT
2005 IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity 23 491 502
51. ChomaratP
BanchereauJ
DavoustJ
PaluckaAK
2000 IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 1 510 514
52. TakenakaH
MaruoS
YamamotoN
WysockaM
OnoS
KobayashiM
YagitaH
OkumuraK
HamaokaT
TrinchieriG
FujiwaraH
1997 Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J Leukoc Biol 61 80 87
53. MoskowitzNH
BrownDR
ReinerSL
1997 Efficient immunity against Leishmania major in the absence of interleukin-6. Infect Immun 65 2448 2450
54. TitusRG
DeKreyGK
MorrisRV
SoaresMB
2001 Interleukin-6 deficiency influences cytokine expression in susceptible BALB mice infected with Leishmania major but does not alter the outcome of disease. Infect Immun 69 5189 5192
55. AndersonCF
OukkaM
KuchrooVJ
SacksD
2007 CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med %19;204 285 297
56. SuffiaIJ
RecklingSK
PiccirilloCA
GoldszmidRS
BelkaidY
2006 Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med %20;203 777 788
57. BelkaidY
2003 The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert Opin Biol Ther 3 875 885
58. BelkaidY
PiccirilloCA
MendezS
ShevachEM
SacksDL
2002 CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420 502 507
59. AseffaA
GumyA
LaunoisP
MacDonaldHR
LouisJA
Tacchini-CottierF
2002 The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol 169 3232 3241
60. EhrchenJ
SteinmullerL
BarczykK
TenbrockK
NackenW
EisenacherM
NordhuesU
SorgC
SunderkotterC
RothJ
2007 Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 109 1265 1274
61. ViemannD
SchmidtM
TenbrockK
SchmidS
MullerV
KlimmekK
LudwigS
RothJ
GoebelerM
2007 The contact allergen nickel triggers a unique inflammatory and proangiogenic gene expression pattern via activation of NF-kappaB and hypoxia-inducible factor-1alpha. J Immunol 178 3198 3207
62. EhrchenJ
HeuerH
SigmundR
SchaferMK
BauerK
2001 Expression and regulation of osteopontin and connective tissue growth factor transcripts in rat anterior pituitary. J Endocrinol 169 87 96
63. WestphalG
BurgemeisterR
FriedemannG
WellmannA
WernertN
WollscheidV
BeckerB
VogtT
KnuchelR
StolzW
SchutzeK
2002 Noncontact laser catapulting: a basic procedure for functional genomics and proteomics. Methods Enzymol 356 80 99
64. EhrchenJ
HelmingL
VargaG
PascheB
LoserK
GunzerM
SunderkotterC
SorgC
RothJ
LengelingA
2007 Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J 21 3208 3218
65. EhrchenJM
RothJ
RoebrockK
VargaG
DomschkeW
NewberryR
SorgC
Muller-TidowC
SunderkotterC
KucharzikT
SpahnTW
2008 The absence of cutaneous lymph nodes results in a Th2 response and increased susceptibility to Leishmania major infection in mice. Infect Immun 76 4241 4250
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



