-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNovel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
Class I PI3Ks are important signalling enzymes and drug targets in cancer and inflammation. We report that p110α and p110β, the two ubiquitously expressed class I PI3K isoforms, control fertility, with no evidence for such a role for p110δ, a PI3K highly expressed in leukocytes. Infertility is therefore a possible but reversible side-effect of PI3K-targeted therapies. Using a new mouse model of systemic p110β inactivation, we found that p110β is critical for ensuring the quality of eggs in females and for sperm formation in males. p110β inactivation leads to a specific blockade in sperm development, without affecting the spermatogenic stem cell pool. This, together with the observation that p110β inactivation has no detectable organismal side effects in the adult stage, makes this kinase a potential drug target for a male contraceptive. Besides its previously reported role in the spermatogenic cells themselves, we now report that p110β also regulates the action of androgens, the male sex hormones, specifically in the Sertoli cells that surround the developing sperm, without affecting androgen action in other tissues. In cancer, however, p110β may acquire the capacity to regulate androgen action in tissues other than Sertoli cells, as was previously documented in prostate cancer.
Published in the journal: . PLoS Genet 11(7): e32767. doi:10.1371/journal.pgen.1005304
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005304Summary
Class I PI3Ks are important signalling enzymes and drug targets in cancer and inflammation. We report that p110α and p110β, the two ubiquitously expressed class I PI3K isoforms, control fertility, with no evidence for such a role for p110δ, a PI3K highly expressed in leukocytes. Infertility is therefore a possible but reversible side-effect of PI3K-targeted therapies. Using a new mouse model of systemic p110β inactivation, we found that p110β is critical for ensuring the quality of eggs in females and for sperm formation in males. p110β inactivation leads to a specific blockade in sperm development, without affecting the spermatogenic stem cell pool. This, together with the observation that p110β inactivation has no detectable organismal side effects in the adult stage, makes this kinase a potential drug target for a male contraceptive. Besides its previously reported role in the spermatogenic cells themselves, we now report that p110β also regulates the action of androgens, the male sex hormones, specifically in the Sertoli cells that surround the developing sperm, without affecting androgen action in other tissues. In cancer, however, p110β may acquire the capacity to regulate androgen action in tissues other than Sertoli cells, as was previously documented in prostate cancer.
Introduction
Upon stimulation of cells with extracellular ligands, class I phosphoinositide 3-kinases (PI3Ks) generate lipids that modulate the function of a range of signalling proteins, including protein kinases (such as Akt/PKB), regulators of small GTPases and adaptor proteins. These PI3K effectors regulate an array of cellular outputs, including cell cycle progression, cell survival, metabolism, translation, transcription and cell motility. Class I PI3Ks have been implicated in cancer, immunity and metabolism, and are the subject of active drug development efforts [1–4].
Mammals have four class I PI3K catalytic isoforms (called p110s) that occur in a heterodimeric complex with a regulatory subunit. Class IA catalytic subunits (p110α, β and δ) are bound to an SH2 domain-containing p85 regulatory subunit, that binds to Tyr phosphorylated membrane-associated proteins, whereas the p84 and p101 regulatory subunits lack SH2 domains and link the single class IB PI3K, p110γ, to G protein-coupled receptors (GPCRs). Tyrosine kinases activate p110α, β and δ, whereas GPCRs regulate p110β and γ. While p110α and β are ubiquitously expressed, p110γ and δ are mainly found in leukocytes but can also be expressed at lower levels in other cell types [5].
Studies using PI3K mutant mice and pharmacological PI3K inhibitors have largely focused on p110α, γ and δ and revealed isoform-selective signalling functions for the class I PI3Ks [1,6,7]. Comparatively less is known about p110β. Several genetic mouse models of p110β inactivation have been created, including mice with full [8] or partial [9,10] deletion of the p110β gene, and mice that produce a hybrid mouse/human inactive p110β protein [11,12]. Conflicting data have been obtained using these different mouse models: whereas one p110β gene deletion model [8] displays a fully penetrant, very early embryonic lethality (at embryonic day E3.5), p110β gene deletion using another strategy [9,10], or its replacement with a cDNA encoding for an inactive p110β enzyme [11,12], show only partial embryonic lethality that occurs at later stages of development. Mice that survived full p110β inactivation were apparently normal [11] but showed a mild growth retardation that normalized after 6 months of age, at which stage these mice became mildly insulin-resistant, with increased blood glucose levels [11]. Homozygous inactivation of p110β with this strategy had no impact on female fertility but led to male sterility [12].
The PI3K signalling pathway has previously been implicated in germ cell-intrinsic regulation of fertility. This was documented through conditional inactivation of PTEN in the female and male germlines [13–15] or by inactivation of PDK1 in the male germline [15]. Organismal inactivation of Akt1 or Akt2 has also been reported to lead to reduced testis size, reduced male fertility and increased apoptosis in male germ cells [16–18]. Mice in which the endogenous c-kit tyrosine kinase receptor no longer binds class IA PI3Ks further revealed a role for this group of PI3Ks in male [19,20] and female fertility [19]. Subsequent studies using mice with systemic inactivation of p110β [11] suggested that this kinase provides PI3K activity downstream of c-kit in male germ cells [12], although a germ-cell-intrinsic role of p110β remains to be formally proven.
In this study, we have investigated the organismal role of p110β by inactivating it in mice using a gene targeting strategy that we previously applied to p110α and p110δ [21–24]. We have created a knockin mouse line in which a point mutation in the kinase domain renders the endogenous p110β inactive but preserves its expression levels, thus mimicking the action of a kinase inhibitor. Such systemic inactivation of p110β in mice resulted in a substantial, although not fully penetrant, embryonic lethality, with the surviving mice showing defects in fertility, especially in males. In addition to the previously suggested role for p110β in the germ cell compartment [12], we find that p110β also has a germ cell-extrinsic role in the regulation of fertility, namely by regulating androgen receptor (AR) gene expression in SCs, which is known to be critical for the proper development of the male germ cells. We also present evidence of a role for p110α, the other ubiquitously expressed class I PI3K, in male and female fertility.
Results
Generation of a mouse line with inactive p110β
Using a strategy previously applied to p110α and p110δ [22,24], we created a mouse line in which endogenous p110β is converted to a kinase-dead protein. This was achieved by introducing a germline point mutation in Pik3cb, the gene encoding p110β, which converts the critical ATP-binding DFG motif in the p110β kinase domain to AFG (S1 Fig and S1 Text). Experiments using mice homozygous for this mutant p110β (referred to as p110βD931A/D931A mice) showed that the p110βD931A protein lacked catalytic activity (S2A Fig) but was expressed at the same level as wild-type (WT) p110β (S2B Fig). In addition, it did not affect expression of p85 (S2B Fig) nor the expression (S2B Fig) or activity (S2A Fig) of p110α. In heterozygous p110βD931A/WT mice, p110β lipid kinase activity was reduced by approximately 50%, with the remaining activity being significantly sensitive to the p110β-selective inhibitor TGX-221 (S2C Fig). Loss of p110β activity did not decrease total PI3K activity present in phosphoTyr-peptide precipitates (which pull down all p85 subunits; S2A Fig) nor the basal phosphorylation of Akt/PKB on S473 in the lungs and testes (S2B Fig). Taken together, these data show that germline conversion of p110β to the p110βD931A form inactivates this kinase without affecting the expression or activity of other, non-targeted, PI3K isoforms.
p110β inactivation leads to substantial loss of embryonic viability
Intercrosses of p110βD931A/WT mice yielded a significantly lower than expected fraction of homozygous p110βD931A/D931A mice born, based on a normal Mendelian distribution, both on a mixed C57BL/6 x 129S2/Sv or on a pure C57BL/6 background (10% and 1% versus 25% expected, respectively) (S3A Fig). The reason for the lethality of p110βD931A/D931A embryos is unknown at the moment. Indeed, it was not possible to identify a specific time point of embryonic lethality, as embryos were found to die at different stages of embryonic development (S3A Fig). This is in stark contrast to the fully penetrant embryonic lethality of homozygous p110α kinase-dead mice that all die at E10.5 [22].
Homozygous p110β kinase-dead males are infertile
p110βD931A/D931A embryos (S3B Fig) and 4-week-old male mice (S3C Fig) showed a mild growth delay. However, no weight differences were seen in male or female adult mice (S3D Fig). Necropsy and comprehensive histological analysis (see S1 Table for a list of organs analyzed) of ~6-month-old p110βD931A/D931A mice did not reveal any detectable alterations or pathology, apart from reduced size (S4 Fig) and altered histology (see below) of the testes (Fig 1A shows the organ weights of 12-week-old mice). p110βD931A/D931A males, on both pure and mixed genetic backgrounds, were found to be sterile upon mating with WT females (Fig 1B), suggesting oligo - or azoospermia. p110βD931A/WT males, when mated with WT females, also showed a 20% reduction in litter frequency compared to WT males (Fig 1B), although the litter size was unaltered (Fig 1C).
Fig. 1. p110β kinase activity positively regulates female and male fertility. 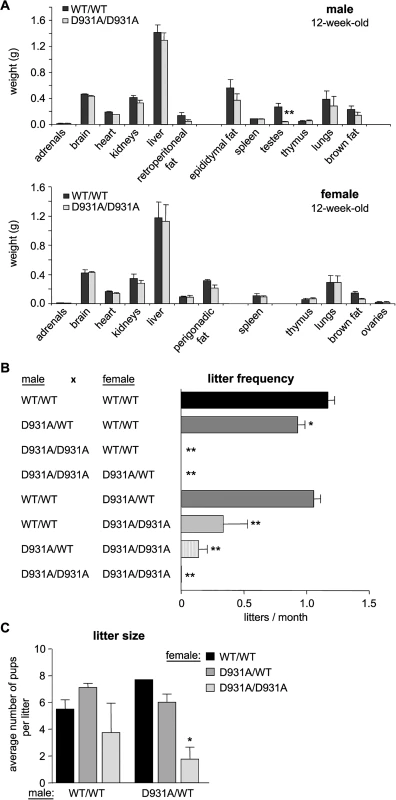
A) Weight of organs in 12-week-old mice (n = 4). B) Mice with the indicated genotype were bred for a 6-month period (cages of 2 females with 1 male; > 3 couples) and the average number of litters per month was assessed. Mann-Whitney: **, p<0.01. C) Average size of litters obtained from breeding pairs (2 females with one male for ≥4 months). Unpaired t-test: *, p<0.05; **, p<0.01. Maternal p110β activity contributes to effective transitioning of the 2-cell embryo to the morula/blastocyst stage
Female p110βD931A/D931A mice also showed a substantial reduction in fertility. Indeed, p110βD931A/D931A females, when crossed with WT males, had a reduction of 70% in their capacity to have recurrent litters (0.34 litters born per month versus 1.20 in intercrosses of WT mice; Fig 1B), a reduced litter size when crossed with p110βD931A/WT males (Fig 1C) and a 24%-reduction in the percentage and absolute number of ovulated oocytes that made it to E13.5 embryos (Fig 2A).
Fig. 2. Maternal and embryonic p110β kinase activity regulate preimplantation embryogenesis. 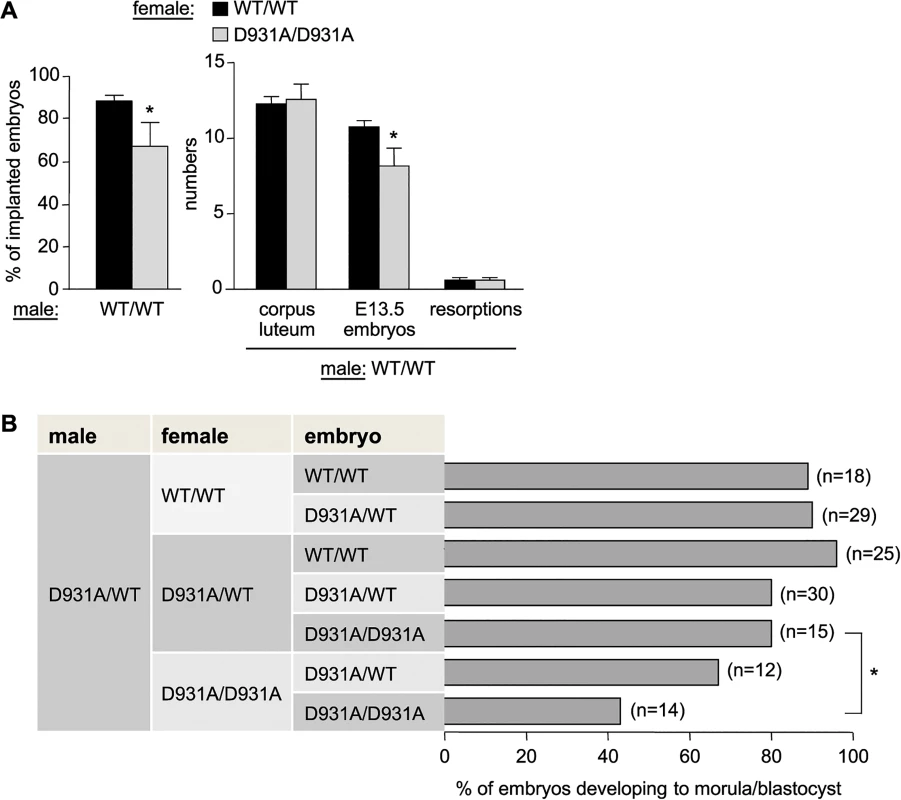
A) Females of the indicated genotype were crossed with WT males (n = 5 females crossed with 2 different males). The percentage of ovulations which became implanted embryos (left panel) was calculated as follows: [numbers of implanted E13.5 embryos + number of resorptions]/corpus luteum numbers in the ovaries (indicative of the number of ovulated oocytes)] x 100 (right panel). Mann-Whitney: *, p<0.05. B) Females of the indicated genotype were superovulated and mated with a p110βD931A/WT male. Two-cell embryos were recovered from the oviducts and cultured in vitro for 4 days, at which time embryos were scored for development to the morula/blastocyst stage or any earlier developmental stage, and genotyped. Mann-Whitney: *, p<0.05. p110βD931A/D931A females showed normal follicle maturation (S5A Fig) and oestrus cycles (S5B Fig) and generated the same number of 2-cell embryos upon superovulation and mating with WT males (S5C Fig), suggesting normal ovulation in these mice. However, 2-cell p110βD931A/D931A embryos recovered from p110βD931A/D931A females had a decreased in vitro ability to develop into morula and blastocysts and to survive ex vivo, compared to p110βD931A/D931A embryos generated by p110βD931A/WT females (Fig 2B; representative ex vivo cultures and genotyping results are shown in S5D and S5E Fig). Taken together, these data indicate that the lack of embryonic p110β activity is not, per se, detrimental to preimplantation development to blastocyst if the female is heterozygous for p110β inactivation. Thus, a maternal pool of p110β, provided by the oocyte cytoplasm and/or the host environment, likely participates in healthy embryonic development to blastocyst.
p110β activity is essential for the first round of spermatogenic meiosis after birth
We next analyzed the p110βD931A/D931A male sterility phenotype in more detail. At 12 weeks of age, the testes were the only organs reduced in weight (by more than 70% compared to WT testes) in the p110βD931A/D931A males (Figs 1A and 3A). Testes of p110βD931A/D931A males had descended normally into the scrotum (S6 Fig), indicating that the first intrauterine/early postnatal peaks of testosterone production had led to correct perinatal testicular development [25]. Serum levels of testosterone and luteinizing hormone (LH) were not significantly altered (S7 Fig), in accordance with the unaffected organ weight of other androgen target tissues, including prostate, seminal vesicles and epididymal and retroperitoneal fat (Figs 1A and 3A). However, compared to WT controls, p110βD931A/D931A males had a 37% increase in the serum levels of follicle-stimulating hormone (FSH; S7 Fig), a phenomenon known to occur upon spermatogenic arrest [25].
Fig. 3. Systemic organismal inactivation of p110β reveals a role for p110β in SCs. 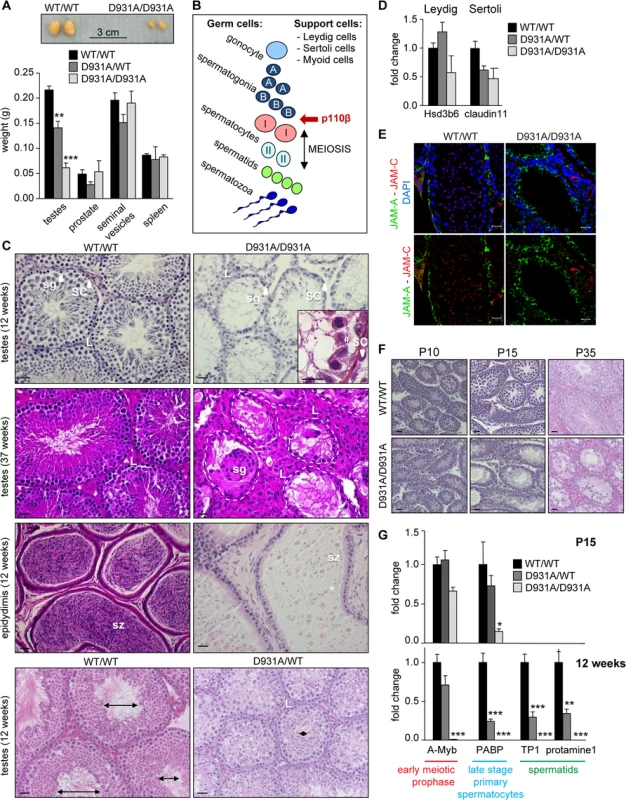
A) Weight of reproductive organs in 12-week-old mice (n>6). Mann-Whitney: **, p<0.01; ***, p<0.001. B) Schematic of the spermatogenic lineage, testis support cells and the earliest stage of interference by p110β. C) H&E-stained sections of 12-week-old and 37-week-old testis and 12-week-old epididymis. sg, spermatogonia; sz, spermatozoa; SC, Sertoli cell; L, Leydig cell; #, presence of germ cells in p110βD931A/D931A seminiferous tubules; *, abnormally detached undifferentiated spermatids. Lumen size is indicated by double ended arrows. Scale: 20 μm. D) mRNA expression levels of the indicated genes at 12-week-old as determined by RT-qPCR normalized with 18S expression and corrected for total testis weight (n = 4). Student's t-test: non-significant. E) Immunofluorescence staining for JAM-A (Sertoli cell-specific protein localized in tight junctions) and JAM-C (spermatid-specific protein) counterstained with DAPI on cryosections of testes of 12-week-old mice (n = 3) [64]. * indicate cell membranes of Sertoli cells; white arrow are JAM-C positive spermatids. Scale: 20 μm. F) H&E-stained sections of testes during post-natal development (P10: n = 4; P15: n = 2; P35: n = 3). Scale: 20 μm. G) mRNA expression levels of the indicated genes at P15 (top panel) and 12 weeks (bottom panel) as determined by RT-qPCR normalized with 18S expression and corrected for total testis weight (n = 4). Student's t-test: *, p<0.05; **, p<0.01; ***, p<0.001. The appearance and size of the interstitial tissue, that surrounds the germ cell-containing seminiferous tubules (Fig 3B) and is composed of the testosterone-producing Leydig cells (labelled L in Fig 3C and S8 Fig), were analysed in WT and p110βD931A/D931A males. p110β inactivation did not lead to significant changes in the mRNA expression levels of the Leydig cell marker Hsd3b6 (3-β-hydroxysteroid dehydrogenase 6), the localisation of the HSD3B protein (Fig 3D and S8 Fig) or the number of HSD3B-positive cells (S8E Fig). However, a tendency for an increase in Leydig cell numbers was observed in aged p110βD931A/D931A mice.
Homozygous inactivation of p110β did also not affect the presence of SCs in the testes (Fig 3C). In addition, no differences were seen in the expression of the SC-specific mRNA claudin 11 in 12-week-old mice (Fig 3D), or in the number of SC-marker SOX9-positive cells during testes development and aging (S8E Fig). In addition, the localization of the adhesion molecule JAM-A was not affected in p110βD931A/D931A SCs (Fig 3E), indicative of an intact blood-testis barrier of the seminiferous tubules.
During spermatogenesis, specialized stem cells, called spermatogonia, undergo meiosis and cell differentiation into, sequentially, spermatocytes, spermatids and spermatozoa (Fig 3B) [26]. Spermatogonia reside on the basement membrane of the seminiferous tubules and are surrounded by supporting SCs. To understand the spermatogenic defect caused by p110β inactivation in more detail, we analysed the differentiation of spermatogonia. The first round of meiosis occurs between post-natal stage P10 to P15. The spermatogenic cell pool was altered in p110βD931A/D931A males at P10, the known time point of onset of meiosis, as evidenced by staining of the germ cell marker DDX4 (S8A Fig). At P15, the H&E staining and DDX4 staining of p110βD931A/D931A testes revealed a clear depletion of germ cells, coinciding with the known time point of primary spermatocyte appearance, and further observed at P35 (Fig 3F and S8B Fig). At 12 weeks of age, the seminiferous tubules of p110βD931A/D931A testes contained only a few spermatogenic cells, many of which were detached (Fig 3C and S8 Fig, DDX4 marker), and no JAM-C positive cells (Fig 3E), indicative of a massive loss of spermatids. However, a low concentration of isolated round and elongating spermatids and few mature spermatozoa were present in the cauda epididymis of testes of 12-week-old p110βD931A/D931A mice (Fig 3C). At 37 weeks of life, most of the tubular cross-sections of p110βD931A/D931A testes displayed a ‘SC only’ appearance (Fig 3C).
We next analysed the gene expression of selected differentiation markers (A-Myb, PABP, TP1 and protamine1) in the testes. At P15, the expression of A-Myb, a marker of the early meiotic prophase, was not significantly altered in p110βD931A/D931A testes (Fig 3G) whereas expression of PABP, a marker for late stage spermatocytes, was severely reduced (Fig 3G), suggesting an altered progression in germ cell differentiation. The expression of all these markers was undetectable at 12 weeks of age (Fig 3G).
In heterozygous p110βD931A/WT males, the expression of both A-Myb and PABP was similar to that in WT mice at P15 (Fig 3G). In adult heterozygous p110βD931A/WT males that have a modestly reduced fertility (Fig 1B) and a 35% reduction in testis size compared to WT mice (Fig 3A), the diameter and lumen size of the seminiferous tubules were severely reduced at 12 weeks of age (Fig 3C, bottom panel). Accordingly, the expression of markers of late primary spermatocyte (PABP) and spermatid (TP1 and protamine1) stages was strongly reduced in these mice, while the expression of A-Myb was unaffected (Fig 3G), indicative of unaffected spermatogenesis in young p110βD931A/WT males but a progressive depletion of spermatogenic germ cells upon aging.
In summary, our data reveal an essential role for p110β in early meiosis (Fig 3B). The presence of small numbers of post-meiotic germ cells in the epididymis of 12-week-old p110βD931A/D931A males suggests that meiosis is possible in the absence of p110β activity but that it occurs with a possible altered efficiency, loss-of-contact and sloughing off of the germinal lineage from SCs before timely spermiogenesis, resulting in severely impaired sperm production.
Selective inactivation of p110β in SCs leads to male sterility but does not affect germ cell composition
The detachment of the germinal cell lineage and inefficient primary spermatocyte formation, observed in p110βD931A/D931A testes at P15, are both phenomena that are known to be controlled by SCs [25,27]. This suggests that p110β activity could be important in SCs, despite the lack of obvious histological differences in p110βD931A/D931A SCs (Fig 3C) and the unaltered staining for the SC-specific marker SOX9 (S8 Fig).
To assess the possible role of p110β expressed in SCs (Fig 4A), we crossed mice with a conditional inactivating allele of p110β (p110βflox [9]) with mice expressing the Cre recombinase under the control of the SC-specific AMH promoter (AMH-Cre mice [28]). 12-week-old mice with recombined Pik3cb loci in AMH-Cre-expressing SCs (referred to as SCβ-DEL; Fig 4B) had a decrease in the weight of the testes (48%) and epididymis (25%) with no alterations in the weight of the prostate, seminal vesicles or spleen (Fig 4C). The diameter of the seminiferous tubules was also reduced in SCβ-DEL testes (Fig 4D), but, in contrast to p110βD931A/D931A, the germ cell composition of the testes was unaltered (Fig 4D). In line with this, the expression of markers of each germ cell stage was unchanged in SCβ-DEL testes at 12 weeks of age, with the exception of the primordial germ cell marker Trap1a (Fig 4E). Importantly, however, none of the SCβ-DEL mice gave rise to offspring when crossed with WT females (Fig 4F). These data show that, like systemic organismal inactivation, SC-specific inactivation of p110β leads to male sterility but likely through a different mechanism than primary spermatocyte formation. These data suggest that, in addition to its key role in SCs, p110β may also regulate spermatogenesis in a germ cell-intrinsic manner.
Fig. 4. Tissue-specific inactivation of p110β in SCs causes male sterility. 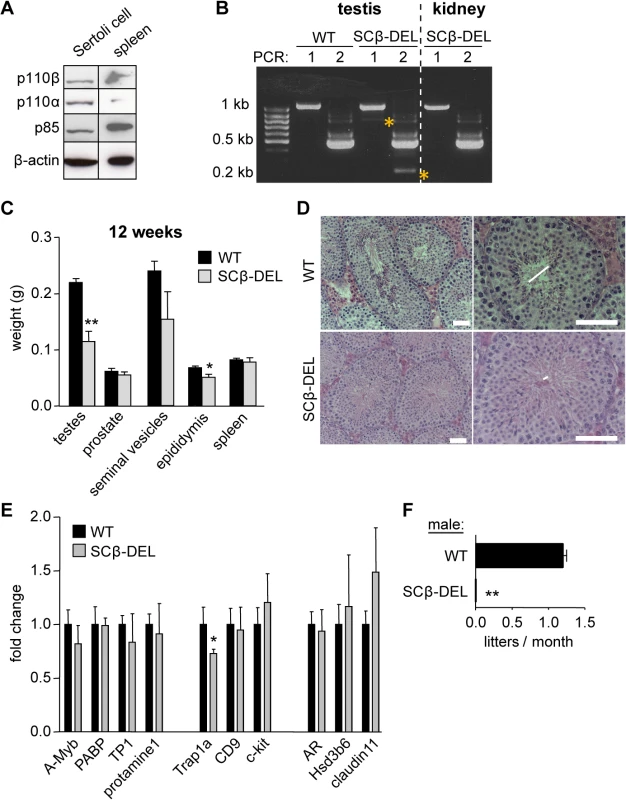
A) p110 protein expression in primary rat SC culture. B) Following genotyping, AMH-Cre+ testes were checked for Pik3cb recombination levels by a RT-nested PCR. No recombination was detected in Cre- testis (not shown) or in other organs. PCR 1 for Pik3cb exons 16–24: WT 1067 bp; β-DEL 797 bp; PCR 2 for Pik3cb exons 19–23: WT 474 bp; β-DEL 204 bp. β-DEL amplicons in both PCRs are indicated with a yellow star, and were only detected after the second PCR, consistent with the fact that SCs are less abundant in testis than the spermatogenic lineage. C) Weight of the indicated organs in recombined AMH-Cre+p110βflox/flox mice, referred to as SCβ-DEL (n = 5). Mann-Whitney: *<p0.05, **, p<0.01. D) Histology of 12-week-old SCβ-DEL testes (n = 3). Lumen size is indicated by horizontal lines. Scale 50 μm. E) mRNA expression levels of the indicated genes in testes of 12-week-old mice (n = 4) mice as determined by RT-qPCR normalized with 18S expression and corrected for total testis weight (n = 4). Student's t-test: *, p<0.05. F) Breeding efficiency of WT and SCβ-DEL males crossed with WT C57BL/6 females (n = 5). Despite some variability in the histological phenotype, all mice with a recombined Pik3cb were found to be sterile. Unpaired t-test: **, p<0.01. The male fertility phenotype upon p110β inactivation shares similarities with SC-specific deletion of the androgen receptor (AR)
We next searched for the mechanism by which p110β activity regulates SC function. The AR has a key role in controlling spermatogenesis. As germ cells do not express the AR, androgen regulates fertility indirectly through regulating gene expression in SCs, which influences germ cell maturation. Testosterone binding to the AR in SCs is a key signal in the regulation of the first round of meiosis in male gametogenesis during early postnatal development [25,29]. Other roles of testosterone in SCs include the attachment of developing spermatids to the SCs [30,31] and lumen formation of seminiferous tubules [32], both of which were found to be affected by full inactivation of p110β. Indeed, the phenotype of p110βD931A/D931A testes was reminiscent of some aspects of SC-specific AR knockout (SCARKO) mice [30]. Compared to p110βD931A/D931A mice, in SCARKO mice the reduction in the diameter of the seminiferous tubules is milder, and sperm cell differentiation is blocked at a later (round spermatid) stage (Fig 3B). SCs start to express the AR at P4 [33], with increased expression at P15 [32], coinciding with the stage at which the p110βD931A/D931A testis phenotype becomes apparent (Fig 3F and 3G and S8 Fig). We therefore hypothesized that p110β that is expressed in SCs could regulate some aspects of AR activity.
Systemic inactivation of p110β in mice did not affect AR mRNA expression in the testes (Fig 5A, left panel). In contrast, the mRNA expression of the SC-specific AR-responsive homeobox-gene Rhox5 was reduced in both p110βD931A/D931A and p110βD931A/WT 12-week-old testes (Fig 5A, right panel). The expression of Rhox5 is critical for the full efficiency of meiosis [29,32,34]. p110β inactivation also led to reduced expression of other SC-specific AR targets in adult testes, including TJP1 (Tight Junction Protein 1) and claudin11 (a transmembrane protein important for tight junctions), although the reduction in the expression of claudin11 did not reach statistical significance (Fig 5A, right panel). In contrast, systemic p110β inactivation did not affect the expression of Leydig cell-specific AR target genes such as Hsd3b6 [35] (Fig 3D), in line with the lack of obvious defects in this cell population upon p110β inactivation (Fig 3C and 3D and S8 Fig).
Fig. 5. p110β activity regulates AR mRNA targets specifically in SCs. 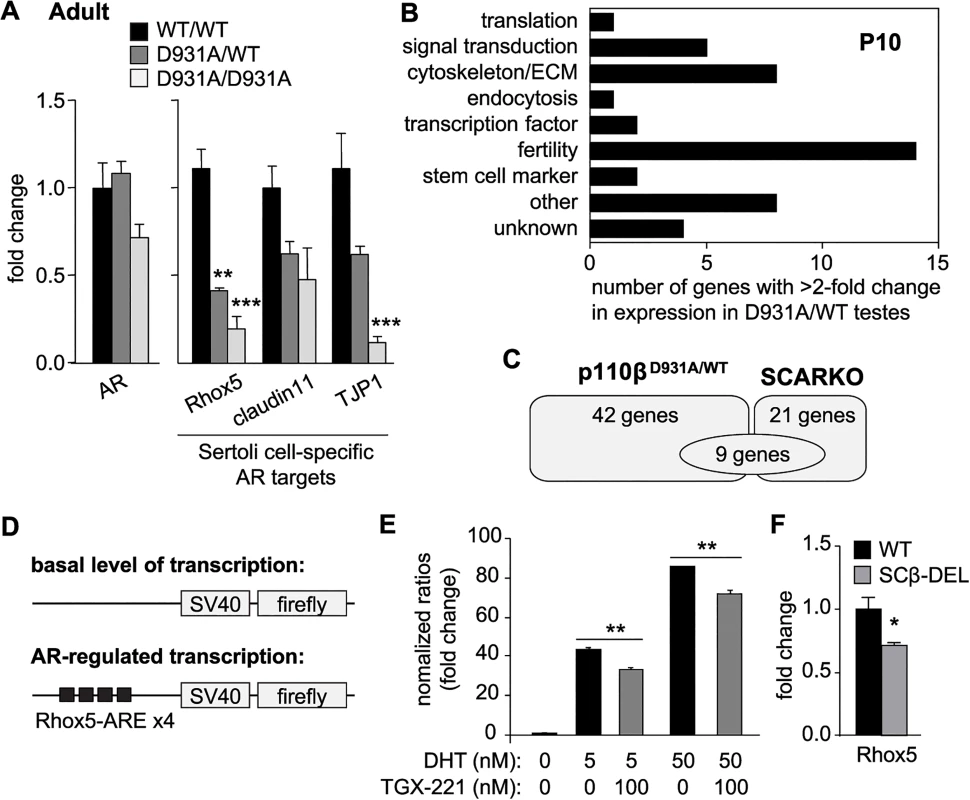
A) mRNA expression of selected markers in adult testes of mice with the indicated genotypes as determined by RT-qPCR and normalized with 18S expression and corrected for total testis weight (n>4). Student's t-test: **, p<0.01; ***, p<0.001. B) Functional classification of genes with >2-fold altered expression in testes of p110βD931A/WT males, compared to WT mice (for details see S3 Table). C) Gene expression pattern in p110βD931A/WT testis compared to SCARKO testis [38] (for details see S4 Table). D) Schematic representation of plasmids transiently transfected in MSC-1 cells. E) Transient co-transfection of Rhox5-AREs luciferase reporter and Flag-AR of MSC-1 cells. 5α-DHT (5 and 50 nM) was used to activate AR transactivation activity. TGX-221 was used at 100 nM. Results are presented as induction factors (averages ± SEM of ≥3 independent experiments performed in triplicate). Student's t-test: **, p<0.01. F) mRNA expression of Rhox5 in testes of mice of the indicated genotypes (n>4) as determined by RT-qPCR and normalized with 18S expression and corrected for total testis weight. Student's t-test: *, p<0.05. The AR also has a pivotal role in the regulation of extragonadal reproductive glands, muscle mass, fat deposition and bone or brain function [36,37], none of which were notably affected in p110βD931A/D931A mice (Fig 3A). Taken together, these data indicate that p110β regulates a subset of AR target genes, specifically in SCs.
Gene network analysis in testes identifies AR-regulated genes in SCs as p110β targets
In order to gain further insight into the functional link between p110β and AR, we performed an unbiased global gene expression analysis in WT and p110βD931A/WT testes at P10. This early time point was selected in order to investigate the events associated with the initiation of the p110β-associated fertility phenotype. The use of p110βD931A/WT testes is also expected to reveal the primary transcriptional targets of AR regulated by this PI3K, as homozygous inactivation of p110β likely results in ‘knock-on’ effects on spermatogenesis regulation. One such effect is the induction of the FSH/LH feedback loop that arises as a consequence of impaired production of spermatozoa. Indeed, the plasma levels of FSH were significantly increased in p110βD931A/D931A but not p110βD931A/WT males (S7 Fig). However, a drawback of using p110βD931A/WT mice is the potentially low magnitude of change in the gene expression as compared to WT mice. For this reason, we considered a 2-fold difference in gene expression significant in this setting.
The expression of 42 genes was found to be altered ≥2-fold between WT and p110βD931A/WT testes (17 genes downregulated, p-value 0.0052–0.00013; 25 upregulated, p-value 0.015–0.00013; S2 Table). The functions of these genes span various biological contexts, with genes known to regulate fertility forming the main group (Fig 5B and S3 Table). A comparison of the gene expression profiles of p110βD931A/WT and SCARKO P10 testes [38] (Fig 5C and S4 Table) showed that, of the 21 genes significantly modified in SCARKO, 9 also showed an altered expression between WT and p110βD931A/WT testes. p110β activity thus regulates the expression of a fraction of known SC-specific AR-regulated genes, while other genes regulated by p110β appear not to be dependent on Sertoli-cell specific AR activity. This is indicative of the AR in SCs having p110β-independent functions but also of p110β having 1) AR-independent functions in SCs and 2) SC-independent functions in the testes, such as the regulation of germ cell survival and proliferation.
p110β activity modulates AR-regulated gene transcription
The AR resides in the cytoplasm and upon binding to testosterone translocates to the nucleus where it binds to its DNA-response elements in the promoter or enhancer regions of androgen target genes. To demonstrate that p110β activity has the ability to regulate the genomic functions of AR, we transiently transfected the mouse SC line MSC-1 [39] with SV40 promoter-containing luciferase reporter constructs with hormone-responsive elements, including elements responsive to AR only (Rhox5 AR elements (AREs) and Eppin-AREs) or to both AR and Glucocorticoid Receptor (GR) (Tat-GRE, a known binding element for both AR and GR [40]) (Fig 5D and S9A Fig). Stimulation of MSC-1 cells transfected with the Rhox5-ARE reporter with the androgen 5α-dihydrotestosterone (5α-DHT), which activates the endogenous AR, induced a significant increase in luciferase activity (S9B Fig), with the concomitant transfection of AR strongly enhancing Rhox5-ARE reporter activity (S9C Fig). Importantly, pre-treatment of cells overexpressing AR with the p110β inhibitor TGX-221 decreased 5α-DHT-induced luciferase expression driven by Rhox5-AREs (Fig 5E), Eppin-AREs (S9D Fig) and Tat-GREs (S9E Fig). While the observed decrease in AR-dependent transcriptional activation upon p110β inhibition was modest in cell culture, a strong impact on the expression of Rhox5 was seen in vivo, with a 29%, 59% and 81% decrease in gene expression in adult SCβ-DEL, p110βD931A/WT and p110βD931A/D931A males, respectively, compared to WT mice (Fig 5F and 5A, right panel). Taken together, these data show that p110β activity regulates AR transcriptional activity, contributing to the expression of the SC-specific AR target Rhox5 in vivo.
The other ubiquitous class I isoform p110α also regulates fertility
The testis phenotype upon global p110β inactivation is stronger than that observed in SCARKO mice (see above). This is possibly due to an additional role that p110β has directly in the spermatogenic germ cell lineage, in addition to its ability to regulate AR signalling in SCs, for example its previously reported involvement in c-kit receptor-positive male germ cells [12].
An important question is also whether other class IA PI3K isoforms than p110β are involved in the regulation of fertility. The male fertility phenotype of the p110βD931A/D931A mice appears to be less pronounced compared to that of c-kit-p85 null mice (knockin mice in which c-kit can no longer interact with the p85 regulatory subunit of class IA PI3Ks; [19,20]). In the c-kit-p85 null mice, c-kit expression is drastically reduced in mutant seminiferous tubules already at P8 [19] and the spermatogenic germ cell pool is fully depleted at P21 [20]. In contrast, despite a strong reduction in the mRNA expression of the stem cell marker Trap1a (Fig 6A), the testes of p110βD931A/D931A adult males still showed mRNA expression of the CD9 and c-kit stem cell markers (Fig 6A) and protein expression of c-kit (Fig 6B), demonstrating that they contained germ cells. In addition, some c-kit-positive spermatogonial cells, surrounded by c-kit-positive Leydig cells (respectively indicated by * and # in Fig 6C), were present in the seminiferous tubules of p110βD931A/D931A males. These findings suggest that another class IA PI3K isoform than p110β could be involved in spermatogonial signalling, possibly downstream c-kit.
Fig. 6. p110α couples to c-kit in testes and regulates male fertility. 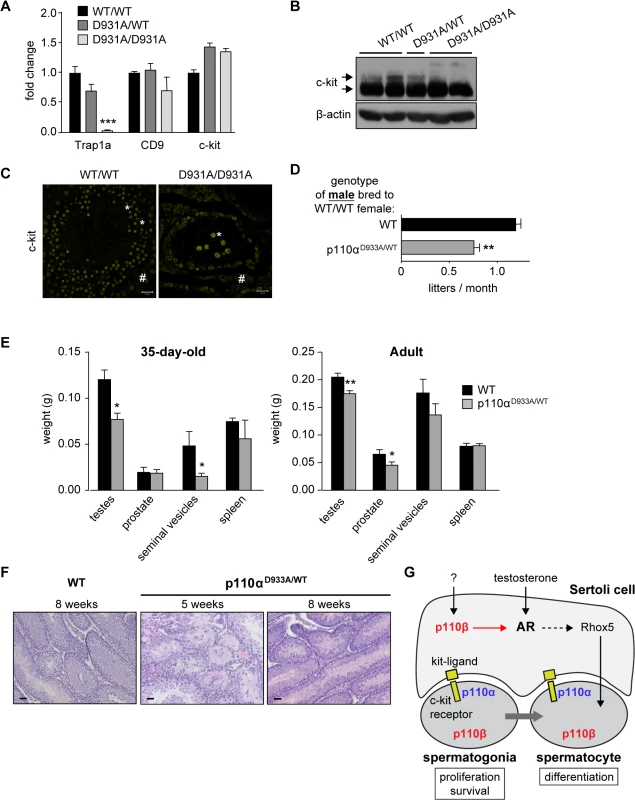
A) mRNA expression of stem cell markers in testes of adult mice of the indicated genotypes as determined by RT-qPCR and normalized with 18S expression and corrected for total testis weight (n = 5). Student's t-test: ***, p<0.001. B) Western blot analysis of c-kit expression in adult testes of mice with the indicated genotypes (n = 4). Arrows point to the 100 kDa and 150 kDa forms of c-kit. C) Immunofluorescence using anti-c-kit TRITC-labeled antibodies in adult WT and p110βD931A/D931A testes (n = 3). Scale: 20 μm. * c-kit-positive germ cells; # c-kit-positive Leydig cells. D) Breeding efficiency of p110αD933A/WT males crossed with 2 WT females over a 4 month period (C57BL/6 background). Unpaired t-test: **, p<0.01. E) Weight of reproductive organs in P35 (n>3) and adult 12-week-old WT and p110αD933A/WT mice (n>6). Mann-Whitney: *, p<0.05. F) H&E staining of testes of D35 and adult WT and p110αD933A/WT mice (n>3). Scale: 20 μm. G) A schematic representation of the roles of p110α and p110β in the regulation of male fertility. We therefore assessed the possible contribution of p110α to male fertility, using mice with a kinase-dead knockin allele of p110α [22,23]. Homozygous p110αD933A/D933A mice are embryonic lethal [22] but heterozygous p110αD933A/WT males were found to be subfertile (Fig 6D) with a significant decrease in testis size at 35 days after birth and in the adult stage (Fig 6E). An incompletely penetrant (2 mice out of 5) mixed atrophy of seminiferous tubules of p110αD933A/WT was observed at 5 weeks of age. This atrophy was found to be reversed at 8 weeks of age (Fig 6F). In addition, p110αD933A/WT females, when crossed with WT males, had a 35% reduced average litter frequency, compared to WT mice (S10 Fig). In contrast, analysis of homozygous p110δ kinase-dead testes showed no significant defects in ageing mice (S11 Fig).
Given that all class IA PI3K isoforms bind p85 [41], the association to the c-kit receptor of all p85-bound p110 isoforms is expected to be impaired in c-kit-p85 null mice. We found that in unstimulated 12-week-old testes, only p110α, but not p110β or δ, co-immunoprecipitated with c-kit (S12 Fig), whereas p110α and δ, but not p110β, co-immunoprecipitated with c-kit in spleen (S12 Fig), a tissue that is enriched in leukocytes and in which p110δ is known to transmit c-kit signalling [21,41,42]. These data suggest that only p110α contributes to c-kit signalling in adult testes, while p110β is not significantly recruited to c-kit receptor at this developmental stage.
These data show that both p110α and p110β, but not p110δ, contribute to male and female fertility in mice, with p110α, as a tyrosine kinase-linked class I PI3K, most likely executing this biological function through c-kit. The role of p110α in Sertoli cells is unknown.
Discussion
Using a mouse model of constitutive inactivation of the ubiquitously expressed p110β isoform of PI3K, we document that only few mice with inactive p110β survive into adulthood, for reasons that are unclear at the moment. Interestingly, the only apparent phenotypes in the p110β kinase-dead mice that are born are subfertility in females and complete infertility in males. The importance of PI3K signalling in fertility was initially uncovered using mice in which the c-kit tyrosine kinase, an essential regulator of fertility in germ cells, was engineered to no longer interact with all class IA PI3Ks [19,20].
Our data reveal that both of the ubiquitously expressed class IA isoforms, p110α and p110β, regulate fertility in male germ cells (Fig 6G), with no fertility phenotypes observed upon full inactivation of p110δ, a leukocyte-restricted class I PI3K isoform.
A recent study demonstrated that the p110β isoform signals downstream of c-kit [12], uncovering a potential germ-cell intrinsic function of p110β in mice. However, the male fertility phenotype of p110β kinase-dead mice differs from that of c-kit/PI3K mutant mice, pointing to an additional, germ cell-extrinsic, function of p110β in the regulation of male fertility. Indeed, the testicular phenotype of mice with inactive p110β is reminiscent of that of mice with defective SCs, which are known to control the formation of the lumen of seminiferous tubules, attachment of the germinal cell lineage and efficient sperm formation and maturation [25,27,32]. Little is known about PI3K function in male germ cell support cells, with some evidence for a role of PI3K signalling in primary culture of SCs [43]. Importantly, SC-specific inactivation of p110β also led to male sterility, highlighting its important role in these support cells. Indeed, a decrease in the mRNA expression of the homeobox gene Rhox5, critical for the full efficiency of meiosis [29,32,34], was observed upon SC-specific genetic deletion of p110β as well as upon global genetic inactivation of p110β, suggesting that the catalytic activity of p110β was also important for SC function. Of note, the progenitor germ cell marker Trap1a was also found to be decreased in both mouse models (Fig 4E and Fig 6A), although the in vivo implication of this is currently unknown.
The male fertility phenotype of SC-selective p110β inactivation was less prominent than upon systemic p110β inactivation, in that it did not affect the germ cell composition of the mice, further suggesting potential germ cell-intrinsic roles for p110β, such as in c-kit-positive sperm cells. Of note, we cannot rule out that p110α also plays a role in SCs, as it is expressed in this cell type (Fig 4A). Taken together, our data show that the previously reported male infertility phenotype upon p110β inactivation [12] is not limited to a potential germ cell-intrinsic role of p110β in c-kit signalling, but is also related to an important role for p110β in the SC support cells.
The fertility phenotype of mice with inactive p110β strongly resembles that of mice with SC-selective deletion of the AR (SCARKO mice; [29]). We found that p110β activity regulates the expression of SC-specific genes that are essential for the differentiation of germ cell lineage and known to be regulated by AR [40]. Previous work has also implicated p110β as a positive regulator of AR transactivation in prostate cancer cell lines [44] and PI3K/mTOR signalling has been shown to either positively or negatively modulate AR transactivation both in prostate cancer cell lines and genetic mouse models of prostate cancer [45,46].
At present, the upstream signals that activate p110β in SCs are unknown. Between P10 and P15, the later time point being the one at which the p110β-linked phenotype becomes largely apparent in the testes, SCs regulate the induction of germ cell differentiation through the combined action of the AR and FSH, a ligand that signals through the FSH receptor (FSHR), a GPCR only expressed SCs [47,48]. FSHR deletion in mice mildly perturbs SC function and the progression of germ cells through spermatogenesis but when combined with AR deletion in SCs severely blocks this process [48]. As p110β mainly signals downstream of GPCRs [9–11,49,50], it is conceivable that p110β could mediate some of the action of FSH, and in particular the potential synergistic activity of FSH signalling on AR function. This remains to be investigated further.
We find that p110β activity modulates the transcriptional activity of the AR on DNA response elements from Rhox5 or Eppin promoters. However, the exact way in which the lipid kinase activity of p110β signals to the AR is currently unclear. Although p110β is expected to act mainly in the cytosol, recent reports suggest that it could also act inside the nucleus where it has been found to regulate DNA repair and replication [51,52] and to directly interact with the AR in ChIP assays [44].
Female mice with full inactivation of p110β had a significant reduction in litter size and frequency. This might be explained by our finding that p110β activity (either in the maternal environment and/or intrinsically in the developing egg) contributes to the transition of explanted 2-cell embryos to the morula/blastocyst stage in vitro. These data are in line with previously published evidence, using PDK1 or PTEN inactivation in oocytes, which show that maternal PI3K signalling is crucial for embryonic genome activation and preimplantation embryogenesis in mice [53]. Preimplantation embryos may generate intrinsic signals that promote their survival and development, with paracrine/autocrine factors activating intracellular signalling events needed for early embryonic development [54,55]. Class I PI3K activity is known to contribute to the constitutive PtdIns(3,4,5)P3 lipid synthesis observed in mouse preimplantation embryos [56]. Moreover, granulosa cells surrounding the oocyte were shown to act via the PI3K/Akt/mTOR pathway to promote the translation of maternal oocyte mRNAs that are critical for preimplantation embryo development [57]. It is possible that p110β signalling downstream of the GPCR agonist LPA [9], known to be important in preimplantation embryos [58], contributes to the embryonic lethality upon p110β inactivation.
In cancer, p110β is often, but not always, the key PI3K isoform in cells with inactive PTEN [50,59–61]. p110β-selective drugs (such as GSK2636771 [62], clinicaltrials.gov identifier NCT01458067) are currently being tested in cancers with inactive PTEN, including prostate cancer. Our data suggest that such compounds, but also the broader spectrum class I PI3K inhibitors that hit both p110α and p110β, might have side-effects on human fertility.
Our data also provide a new lead for the development of male contraceptives. Indeed, in an organismal developmental context, p110β regulates AR targets only in SCs but not in other AR-responsive tissues, including prostate, seminal vesicles and epididymal/retroperitoneal fat, which would ensure minimal off-target effects of a p110β inhibitor. A male contraceptive should be free from side effects with a reversible action on sperm once the "male pill" is no longer taken. Our data suggest that p110β inhibitors could meet these requirements, with no overall phenotypes in p110β-deficient males other than sterility, due to a highly specific blockade of sperm maturation from spermatogonia to the primary spermatocyte stage (Fig 3B), while retaining most of the spermatogonial pool of cells that are at the origin of sperm development. Disorders of male and female fertility are on the increase. Our findings have additional potential clinical implications for unraveling mechanisms of idiopathic male and female infertility. Idiopathic non-obstructive azoo/oligozoospermia is a major health problem, accounting for about 30% of all male infertility cases. It is likely, and widely speculated, that novel mutations in genes regulating spermatogenesis will be discovered as causes of such situations. It is tempting to speculate that Pik3cb could be one of such candidate genes.
Materials and Methods
Reagents
Small molecule inhibitors were dissolved in DMSO, with final concentration of DMSO in the assays maximally 0.2%. TGX-221 was from Cayman. Some antibodies to class IA PI3Ks (p110α and p110β) were generated in-house (for details, see Ref. [9]), p110β antibodies for immunoblotting was from Santa Cruz Biotechnology (sc-602). Additional antibodies were from Upstate (p85-pan; 06–195), BD Biosciences (p110α; 94520–150); Alexis (p110γ; clone H1); Cell Signaling Technology (pS473-Akt, pT308-Akt, Akt, pS176-IKKα, p-S240/244-S6 or S6); Santa-Cruz Biotechnology (pT202-pY204-p44/42, c-kit (C19 and M14)) and Sigma (α-tubulin, β-actin). Cell culture reagents were from Invitrogen. Dihydroxytestosterone 5α-DHT was from Sigma.
Ethics statement
Mice were kept in individually-ventilated cages. All procedures and animal care were conducted under the UK Licence PPL 70/7447, in accordance with the UK Animals (Scientific Procedures) Act 1986, with local ethics approval at University College London.
Mice
Embryos or pups from timed pregnant mice were dissected at different time points, and those from E13.5 pregnant mice from mixed C57BL/6 x 129S2/Sv or C57BL/6 background were used to prepare MEFs as described [9]. Breeding efficiency was analysed in cages with 1 male and 2 females.
Histology
Necropsy was performed after perfusion of mice with 4% formalin. Organs were fixed for 24 h in 4% PFA, washed twice in water and stored in 70% ethanol until embedding in paraffin. For analysis of testes, fixation was in Bouin’s solution (overnight) rather than PFA. H&E staining was performed on 2 μM sections. The diameter of the seminiferous cords/tubules was measured at 400× magnification using an ocular micrometer calibrated with a stage micrometer (Hamamatsu). Between 100 tubules that were either round or nearly round were chosen randomly and measured for each animal. For IHC, cryosections were stained with antibodies to JAM-A or JAM-C (kind gift from Sussan Nourshargh, Queen Mary University London) or anti-goat c-kit (Santa Cruz; 1/400).
Cell lysis and immunoprecipitation
Mouse tissue and cultured cells were lysed in 1% w/v Triton X-100 in 50 mM Tris.HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, supplemented with protease and phosphatase inhibitor cocktails. Protein concentration was quantified by the BCA method for tissue or Bradford assay for cell lysates. IP of c-kit was performed after preclearing lysates with Sepharose protein A/G. Proteins were resolved on 8% SDS/PAGE gels and immunoblotted as described [9].
Cell transfection and luciferase reporter assay
The murine MSC-1 SC line was transiently transfected with pcDNA3 plasmids with or without the DNA sequence encoding Flag-tagged human AR, together with a Firefly luciferase expression plasmid driven by AR - and/or GR-responsive elements, as shown in S9 Fig. Two controls were applied: 1) Firefly luciferase expression was normalised to expression of a co-transfected plasmid in which Renilla luciferase is driven by the SV40 promoter and 2) the luciferase values were normalised to values from wells transfected only with the plasmid in which Firefly luciferase is driven by the SV40 promoter, to account for non-specific induction of gene expression. Data are expressed as fold-increase of normalized Firefly/Renilla ratios. All transfections were performed in triplicate in 6-well plates. Indicated are the mean induction factors ± SEM after stimulation with 5–50 nM of 5α-DHT for 24 h. The DNA elements used and their nucleotide sequence were as follows: Rhox5-ARE-1 (5'-AGATCTCATTCTGTTCC-3'), Eppin-ARE (5'-AGAACTTGGTGTTCC-3) and TAT-GRE2 (5'-TGTACAGGATGTTCT-3') and were described in detail in [40,63].
mRNA expression analysis
Tissue samples of (P10, P15 and P35 and week 8 and 12) testes were collected and snap-frozen in liquid nitrogen. All samples heterozygous for p110β were from mice on the C57BL/6 background. cDNA was synthesized from DNaseI-treated total RNA (RNeasy kit, Qiagen, Chatsworth, CA) using Superscript II RNaseH– reverse transcriptase and random hexamer primers (Invitrogen). Primer pairs spanning an intron were designed by Applied Biosystems or previously published in [29] (for details see supplemental data). For quantification of gene expression, the ABI Prism 7700 sequence detector PCR detection system (Applied Biosystems) was used with a two-step RT-quantitative-PCR protocol. Gene expression was corrected for well-to-well loading variation by expressing data as a ratio to 18S rRNA. All samples and standard curves were run in triplicate. Data are analyzed using relative standard curves to allow comparison between all samples. Normalization of data to the total weights of the testes was performed to take into account the differential composition due to differential development of spermatogenesis. For Illumina array, testes from WT and heterozygous mutant P10 pups (on the C57BL/6 background) were harvested and snap-frozen. Purified mRNA was subjected to a quality check (Experion) and subjected to Illumina array analysis (Mouse Ref8v2 arrays). Five samples from each genotype in duplicate were subjected to the analysis. Quality Control and normalization were performed using BeadStudio (Illumina). Statistical analyses were performed using Bioconductor (www.bioconductor.org) packages within the open source R statistical environment (www.r-project.org). After filtering, the Limma package for differential expression analysis was used. Significant changes in gene expression were detected using a False Discovery Rate (FDR) < = 0.05. Data are represented as fold modification in log 2.
SC-specific deletion of p110β
p110βflox/flox mice (C57BL/6 background) were crossed with SC-specific Cre expressing mouse line AMH-Cre (C57BL/6 background) [28]. For detection of Cre-mediated excision of exons 21 and 22 of the p110β catalytic domain, mRNA was extracted from the testis and transcribed into cDNA and used as a template for a nested PCR to amplify exon 16–24 of Pik3cb using primers located in exon 16 (5’-CACTCCTGCTGTGTCCGTACA-3’) and 24 (5’-TCAGTGCTTCCTCCTCGCTCT-3’) followed by amplification of exons 19–23 using primers located in exon 19 (5’-TTGGACCTGCGGATGCTCCCCTAT-3’) or exon 23 (5’-CGCATCTTCACAGCACTGGCGGA-3’). The generation of a 204 bp (base pair) PCR fragment in testis samples from AMH-Cre+p110βflox/flox (SCβ-DEL) mice indicated successful splicing of exon 20 onto exon 23, resulting in the generation of a mRNA encoding an internally truncated p110β protein [9].
In vitro culture of 2-cell embryos
6 - to 8-week-old female mice of the indicated genotypes on a C57BL/6 x 129 mixed background were superovulated by intraperitoneal injection of 7.5 IU pregnant mare's serum gonadotrophin (PMSG, Intervet) followed 48 h later by injection of 5 IU human chorionic gonadotrophin (hCG, Intervet). Female mice were mated with males of the indicated genotype (mixed background) at the time of hCG administration, and two-cell embryos were collected from the oviducts 1.5 days later (E1.5) in HEPES-buffered KSOM (Specialty Media) supplemented with amino acids. The numbers of 2-cell embryos recovered from WT and p110βD931A/D931A females were similar, suggesting normal ovulation upon p110β inactivation. Embryos were cultured for 4 days in a 5% CO2 incubator in KSOM supplemented with amino acids. In order to reach high-density culture, embryos were placed into small drops of KSOM under mineral oil, at a density of one embryo per μl (typically, 15–20 embryos in 15–20 μl drops), as previously described [56]. After microscopic scoring of the stage of development, each embryo was digested for 2 h at 55°C in 5 μl of tail digestion buffer (100 mM NaCl, 10 mM Tris pH 8, 25 mM EDTA, 0.5% SDS) with proteinase K and pronase E (0.4 μg/ml); the reaction was stopped with 45 μl of TE (Tris.EDTA pH 8.0) and genotyping PCR performed on 2 μl of the reaction as described above. Embryos with failed genotyping (14% of all embryos cultured) were not taken into account: 6% were blastocysts or morulas and 8% were developmentally arrested embryos.
Statistical analysis
In vitro and in vivo parameters were compared between two groups using the non-parametric Mann–Whitney U-test or unpaired t-test; quantifications and in vitro parameters using Student's t-test.
Supporting Information
Zdroje
1. Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11 : 329–341. doi: 10.1038/nrm2882 20379207
2. Wong KK, Engelman JA, Cantley LC (2010) Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 20 : 87–90. doi: 10.1016/j.gde.2009.11.002 20006486
3. Braccini L, Ciraolo E, Martini M, Pirali T, Germena G, et al. (2012) PI3K keeps the balance between metabolism and cancer. Adv Biol Regul 52 : 389–405. doi: 10.1016/j.jbior.2012.04.002 22884032
4. Rodon J, Dienstmann R, Serra V, Tabernero J (2013) Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 10 : 143–153. doi: 10.1038/nrclinonc.2013.10 23400000
5. Whitehead MA, Bombardieri M, Pitzalis C, Vanhaesebroeck B (2012) Isoform-selective induction of human p110delta PI3K expression by TNFalpha: identification of a new and inducible PIK3CD promoter. Biochem J 443 : 857–867. doi: 10.1042/BJ20112214 22375552
6. Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC (2005) Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 30 : 194–204. 15817396
7. Hirsch E, Braccini L, Ciraolo E, Morello F, Perino A (2009) Twice upon a time: PI3K's secret double life exposed. Trends Biochem Sci 34 : 244–248. doi: 10.1016/j.tibs.2009.02.003 19376709
8. Bi L, Okabe I, Bernard DJ, Nussbaum RL (2002) Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome 13 : 169–172. 11919689
9. Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, et al. (2008) The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci U S A 105 : 8292–8297. doi: 10.1073/pnas.0707761105 18544649
10. Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Damoulakis G, et al. (2011) PI3Kbeta plays a critical role in neutrophil activation by immune complexes. Sci Signal 4: ra23.
11. Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, et al. (2008) Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal 1: ra3.
12. Ciraolo E, Morello F, Hobbs RM, Wolf F, Marone R, et al. (2010) Essential role of the p110beta subunit of phosphoinositide 3-OH kinase in male fertility. Mol Biol Cell 21 : 704–711. doi: 10.1091/mbc.E09-08-0744 20053680
13. Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, et al. (2003) Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 130 : 1691–1700. 12620992
14. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, et al. (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319 : 611–613. doi: 10.1126/science.1152257 18239123
15. Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH (2011) Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest 121 : 3456–3466. doi: 10.1172/JCI57984 21865646
16. Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, et al. (2001) Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 15 : 2203–2208. 11544177
17. Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, et al. (2006) Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol 26 : 8042–8051. 16923958
18. Li Q, He H, Zhang YL, Li XM, Guo X, et al. (2013) Phosphoinositide 3-Kinase p110delta Mediates Estrogen - and FSH-Stimulated Ovarian Follicle Growth. Mol Endocrinol 27 : 1468–1482. doi: 10.1210/me.2013-1082 23820902
19. Blume-Jensen P, Jiang G, Hyman R, Lee KF, O'Gorman S, et al. (2000) Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3'-kinase is essential for male fertility. Nat Genet 24 : 157–162. 10655061
20. Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, et al. (2000) Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J 19 : 1312–1326. 10716931
21. Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, et al. (2004) Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature 431 : 1007–1011. 15496927
22. Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, et al. (2006) Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441 : 366–370. 16625210
23. Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, et al. (2008) Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 453 : 662–666. doi: 10.1038/nature06892 18449193
24. Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, et al. (2002) Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 297 : 1031–1034. 12130661
25. O'Shaughnessy PJ, Morris ID, Huhtaniemi I, Baker PJ, Abel MH (2009) Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: data from mutant and genetically modified mice. Mol Cell Endocrinol 306 : 2–8. doi: 10.1016/j.mce.2008.11.005 19059463
26. Borg CL, Wolski KM, Gibbs GM, O'Bryan MK (2010) Phenotyping male infertility in the mouse: how to get the most out of a 'non-performer'. Hum Reprod Update 16 : 205–224. doi: 10.1093/humupd/dmp032 19758979
27. De Gendt K, Atanassova N, Tan KA, de Franca LR, Parreira GG, et al. (2005) Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology 146 : 4117–4126. 15919750
28. Lecureuil C, Fontaine I, Crepieux P, Guillou F (2002) Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 33 : 114–118. 12124943
29. De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, et al. (2004) A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A 101 : 1327–1332. 14745012
30. Wong CH, Xia W, Lee NP, Mruk DD, Lee WM, et al. (2005) Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology 146 : 1192–1204. 15591141
31. Shupe J, Cheng J, Puri P, Kostereva N, Walker WH (2011) Regulation of Sertoli-germ cell adhesion and sperm release by FSH and nonclassical testosterone signaling. Mol Endocrinol 25 : 238–252. doi: 10.1210/me.2010-0030 21177760
32. Hazra R, Corcoran L, Robson M, McTavish KJ, Upton D, et al. (2013) Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol Endocrinol 27 : 12–24. doi: 10.1210/me.2012-1219 23160479
33. Willems A, De Gendt K, Allemeersch J, Smith LB, Welsh M, et al. (2010) Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int J Androl 33 : 507–517. doi: 10.1111/j.1365-2605.2009.00964.x 19392831
34. Maclean JA 2nd, Chen MA, Wayne CM, Bruce SR, Rao M, et al. (2005) Rhox: a new homeobox gene cluster. Cell 120 : 369–382. 15707895
35. O'Hara L, McInnes K, Simitsidellis I, Morgan S, Atanassova N, et al. (2014) Autocrine androgen action is essential for Leydig cell maturation and function, and protects against late-onset Leydig cell apoptosis in both mice and men. FASEB J ahead of print.
36. Wang RS, Yeh S, Tzeng CR, Chang C (2009) Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 30 : 119–132. doi: 10.1210/er.2008-0025 19176467
37. De Gendt K, Verhoeven G (2012) Tissue - and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol 352 : 13–25. doi: 10.1016/j.mce.2011.08.008 21871526
38. Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, et al. (2006) The effect of a sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol 20 : 321–334. 16166195
39. Eskola V, Ryhanen P, Savisalo M, Rannikko A, Kananen K, et al. (1998) Stable transfection of the rat follicle-stimulating hormone receptor complementary DNA into an immortalized murine Sertoli cell line. Mol Cell Endocrinol 139 : 143–152. 9705082
40. Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, et al. (2007) Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci U S A 104 : 4961–4966. 17360365
41. Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, et al. (1997) P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci U S A 94 : 4330–4335. 9113989
42. Sun J, Pedersen M, Ronnstrand L (2008) Gab2 is involved in differential phosphoinositide 3-kinase signaling by two splice forms of c-Kit. J Biol Chem 283 : 27444–27451. doi: 10.1074/jbc.M709703200 18697750
43. Dupont J, Musnier A, Decourtye J, Boulo T, Lecureuil C, et al. (2010) FSH-stimulated PTEN activity accounts for the lack of FSH mitogenic effect in prepubertal rat Sertoli cells. Mol Cell Endocrinol 315 : 271–276. doi: 10.1016/j.mce.2009.09.016 19778579
44. Zhu Q, Youn H, Tang J, Tawfik O, Dennis K, et al. (2008) Phosphoinositide 3-OH kinase p85alpha and p110beta are essential for androgen receptor transactivation and tumor progression in prostate cancers. Oncogene 27 : 4569–4579. doi: 10.1038/onc.2008.91 18372911
45. Lin HK, Hu YC, Lee DK, Chang C (2004) Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol 18 : 2409–2423. 15205473
46. Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, et al. (2011) Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19 : 575–586. doi: 10.1016/j.ccr.2011.04.008 21575859
47. Ketelslegers JM, Catt KJ (1974) Receptor binding properties of 125I-hFSH prepared by enzymatic iodination. J Clin Endocrinol Metab 39 : 1159–1162. 4372248
48. Abel MH, Baker PJ, Charlton HM, Monteiro A, Verhoeven G, et al. (2008) Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology 149 : 3279–3285. doi: 10.1210/en.2008-0086 18403489
49. Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, et al. (2012) G protein-coupled receptor-mediated activation of p110beta by Gbetagamma is required for cellular transformation and invasiveness. Sci Signal 5: ra89.
50. Jia S, Liu Z, Zhang S, Liu P, Zhang L, et al. (2008) Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454 : 776–779. doi: 10.1038/nature07091 18594509
51. Kumar A, Fernandez-Capetillo O, Carrera AC (2010) Nuclear phosphoinositide 3-kinase beta controls double-strand break DNA repair. Proc Natl Acad Sci U S A 107 : 7491–7496. doi: 10.1073/pnas.0914242107 20368419
52. Kumar A, Redondo-Munoz J, Perez-Garcia V, Cortes I, Chagoyen M, et al. (2011) Nuclear but not cytosolic phosphoinositide 3-kinase beta has an essential function in cell survival. Mol Cell Biol 31 : 2122–2133. doi: 10.1128/MCB.01313-10 21383062
53. Zheng W, Gorre N, Shen Y, Noda T, Ogawa W, et al. (2010) Maternal phosphatidylinositol 3-kinase signalling is crucial for embryonic genome activation and preimplantation embryogenesis. EMBO Rep 11 : 890–895. doi: 10.1038/embor.2010.144 20930845
54. Kane MT, Morgan PM, Coonan C (1997) Peptide growth factors and preimplantation development. Hum Reprod Update 3 : 137–157. 9286738
55. O'Neill C (2008) The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update 14 : 275–288. doi: 10.1093/humupd/dmn002 18281694
56. Halet G, Viard P, Carroll J (2008) Constitutive PtdIns(3,4,5)P3 synthesis promotes the development and survival of early mammalian embryos. Development 135 : 425–429. 18094023
57. Chen J, Torcia S, Xie F, Lin CJ, Cakmak H, et al. (2013) Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol 15 : 1415–1423. doi: 10.1038/ncb2873 24270888
58. Kobayashi T, Yamano S, Murayama S, Ishikawa H, Tokumura A, et al. (1994) Effect of lysophosphatidic acid on the preimplantation development of mouse embryos. FEBS Lett 351 : 38–40. 8076690
59. Berenjeno IM, Guillermet-Guibert J, Pearce W, Gray A, Fleming S, et al. (2012) Both p110alpha and p110beta isoforms of PI3K can modulate the impact of loss-of-function of the PTEN tumour suppressor. Biochem J 442 : 151–159. doi: 10.1042/BJ20111741 22150431
60. Torbett NE, Luna-Moran A, Knight ZA, Houk A, Moasser M, et al. (2008) A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. Biochem J 415 : 97–110. doi: 10.1042/BJ20080639 18498248
61. Wee S, Wiederschain D, Maira SM, Loo A, Miller C, et al. (2008) PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci U S A 105 : 13057–13062. doi: 10.1073/pnas.0802655105 18755892
62. Arkenau HT MJ, Rose Lemech R, Infante JR, Burris HA, Bang YJ, Eder JP, Herbst RS, Sharma S, Chung HC, Decordova S, Swales KE, Garrett MD, Loftiss JI, Durante M, Russo MW, Suttle BB, Motwani M, Kumar R, De Bono JS; Cannon S (2014) A phase I/II, first-in-human dose-escalation study of GSK2636771 in patients (pts) with PTEN-deficient advanced tumors. J Clin Oncol 32 : 5s, 2014 (suppl; abstr 2514^).
63. Denolet E, Gendt KD, Swinnen JV, Verrijdt G, Deboel L, et al. (2006) Transfection with steroid-responsive reporter constructs shows glucocorticoid rather than androgen responsiveness in cultured Sertoli cells. J Steroid Biochem Mol Biol 98 : 164–173. 16388947
64. Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH (2004) Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature 431 : 320–324. 15372036
65. Schwenk F, Baron U, Rajewsky K (1995) A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23 : 5080–5081. 8559668
Štítky
Genetika Reprodukční medicína
Článek Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density ImputationČlánek AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct MechanismsČlánek A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seqČlánek TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive HematopoiesisČlánek Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 7
-
Všechny články tohoto čísla
- LINE-1 Retroelements Get ZAPped!
- /p23: A Small Protein Heating Up Lifespan Regulation
- Hairless Streaks in Cattle Implicate TSR2 in Early Hair Follicle Formation
- Ribosomal Protein Mutations Result in Constitutive p53 Protein Degradation through Impairment of the AKT Pathway
- Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in
- BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma
- Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
- EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in
- Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins
- Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
- A Year of Infection in the Intensive Care Unit: Prospective Whole Genome Sequencing of Bacterial Clinical Isolates Reveals Cryptic Transmissions and Novel Microbiota
- Inference of Low and High-Grade Glioma Gene Regulatory Networks Delineates the Role of Rnd3 in Establishing Multiple Hallmarks of Cancer
- Novel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
- A Novel Locus Harbouring a Functional Nonsense Mutation Identified in a Large Danish Family with Nonsyndromic Hearing Impairment
- Checkpoint Activation of an Unconventional DNA Replication Program in
- A Genetic Incompatibility Accelerates Adaptation in Yeast
- The SMC Loader Scc2 Promotes ncRNA Biogenesis and Translational Fidelity
- Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
- Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation
- JAK/STAT and Hox Dynamic Interactions in an Organogenetic Gene Cascade
- Emergence, Retention and Selection: A Trilogy of Origination for Functional Proteins from Ancestral LncRNAs in Primates
- MoSET1 (Histone H3K4 Methyltransferase in ) Regulates Global Gene Expression during Infection-Related Morphogenesis
- Arabidopsis PCH2 Mediates Meiotic Chromosome Remodeling and Maturation of Crossovers
- AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
- A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seq
- Tempo and Mode of Transposable Element Activity in Drosophila
- The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres
- SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation
- A Genome Scan for Genes Underlying Microgeographic-Scale Local Adaptation in a Wild Species
- TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis
- Analysis of the Relationships between DNA Double-Strand Breaks, Synaptonemal Complex and Crossovers Using the Mutant
- Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools
- Allelic Spectra of Risk SNPs Are Different for Environment/Lifestyle Dependent versus Independent Diseases
- CSB-PGBD3 Mutations Cause Premature Ovarian Failure
- Irrepressible: An Interview with Mark Ptashne
- Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
- Inactivation of Retinoblastoma Protein (Rb1) in the Oocyte: Evidence That Dysregulated Follicle Growth Drives Ovarian Teratoma Formation in Mice
- Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
- Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA
- Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage
- RFX2 Is a Major Transcriptional Regulator of Spermiogenesis
- A Role for Macro-ER-Phagy in ER Quality Control
- Corp Regulates P53 in via a Negative Feedback Loop
- Common Cell Shape Evolution of Two Nasopharyngeal Pathogens
- Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in
- Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression
- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- mTOR Directs Breast Morphogenesis through the PKC-alpha-Rac1 Signaling Axis
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
- Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast
- Encodes a Highly Conserved Protein Important to Neurological Function in Mice and Flies
- Identification of a Novel Regulatory Mechanism of Nutrient Transport Controlled by TORC1-Npr1-Amu1/Par32
- Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly
- Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
- TFIIS-Dependent Non-coding Transcription Regulates Developmental Genome Rearrangements
- Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen
- Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- The Catalytic and Non-catalytic Functions of the Chromatin-Remodeling Protein Collaborate to Fine-Tune Circadian Transcription in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



