-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
Juvenile hormones (JHs) play critical roles in the development of arthropods, comprising half the animal biomass of the oceans and over a million insect species, which have an enormous impact on ecosystems, agriculture (pollinators and pests) and health of mankind (disease vectors). Despite decades of research, a receptor for these unique sesquiterpenoid hormones has remained elusive. Here, we provide definitive genetic evidence establishing that the essential biological function of the Gce/Met protein during insect development is critically dependent on its ability to bind JH, in effect functionally defining a JH receptor. Unequivocal identification of a JH receptor has profound implications for our understanding of arthropod biology. It also defines a molecular target for development of environmentally friendly, safer insecticides.
Published in the journal: . PLoS Genet 11(7): e32767. doi:10.1371/journal.pgen.1005394
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005394Summary
Juvenile hormones (JHs) play critical roles in the development of arthropods, comprising half the animal biomass of the oceans and over a million insect species, which have an enormous impact on ecosystems, agriculture (pollinators and pests) and health of mankind (disease vectors). Despite decades of research, a receptor for these unique sesquiterpenoid hormones has remained elusive. Here, we provide definitive genetic evidence establishing that the essential biological function of the Gce/Met protein during insect development is critically dependent on its ability to bind JH, in effect functionally defining a JH receptor. Unequivocal identification of a JH receptor has profound implications for our understanding of arthropod biology. It also defines a molecular target for development of environmentally friendly, safer insecticides.
Introduction
Arthropods possess unique sesquiterpenoid hormones, represented by the juvenile hormones (JHs) of insects [1] and their non-epoxidized precursor, methyl farnesoate (MF) in crustaceans [2,3]. JHs regulate insect metamorphosis, polymorphism and social caste determination, and adult reproductive physiology [1,4–6]. Although the sesquiterpenoid structure of JH was determined nearly five decades ago [7], a receptor for these important hormones has been notoriously difficult to identify. Non-peptide lipophilic hormones usually exert genomic effects by activating nuclear receptor proteins [8–10]. One insect member of the nuclear receptor family, Ultraspiracle (USP), has been proposed as a mediator of sesquiterpenoid action, initially of JH itself [11] and currently of MF [6,12–14]. USP is an appealing JH receptor candidate given its homology to the vertebrate retinoid X receptor (RXR) and an apparent level of similarity between JH and the RXR ligand, 9-cis-retinoic acid [15]. Moreover, USP is a subunit of the insect ecdysone receptor complex [10,16,17], thus providing a potential point where the steroid and JH signaling pathways might converge. Whether or not the putative hormone-binding pocket of USP is capable of biologically significant ligand binding is still debated [13,14,18,19].
Discovery of the Methoprene-tolerant (Met) gene that confers resistance to the JH analog insecticide methoprene in the fruit fly, Drosophila melanogaster, has provided an alternative JH receptor candidate [20]. Nonetheless, absence of obvious effects of Met mutations on D. melanogaster development argued against the JH receptor function of Met until knockdown of Met in the flour beetle, Tribolium castaneum, produced precocious metamorphosis phenotypes consistent with disrupted JH signaling [21]. Later, it was shown in D. melanogaster that simultaneous mutation of Met and deletion of its paralog, the germ cell-expressed (gce) gene, resulted in non-conditional lethality during the larva-pupa transition [22], corresponding to the lethal phase associated with deficiency of JH [22,23]. The Met and gce paralogs in D. melanogaster arose via gene duplication during "higher fly" evolution, whereas mosquitoes or beetles possess only a single gene [24]. Based mainly on evidence related to the position of introns, gce is ancestral to Met and, in spite of the nomenclature, D. melanogaster gce is more similar to the single Met genes found in other insects [24].
Met and Gce belong to the basic helix-loop-helix (bHLH)/Per-Arnt-Sim (PAS) family of transcription factors [25] that are distinctly different from nuclear receptor proteins. Although no bHLH-PAS protein has previously been proven to be a receptor for an authentic hormone, the vertebrate aryl hydrocarbon receptor (AhR) is a transcription factor activated by xenobiotics (e.g., dioxin), or by endogenous ligands such as tryptophan metabolites, binding to its PAS-B domain [26,27].
Like JH, Gce/Met is unique to arthropods, and thus may have evolved to mediate JH signaling in insects, crustaceans, and other related taxa. In vitro, the Met proteins from D. melanogaster [28,29], T. castaneum [29], and the Aedes aegypti mosquito [30] bind native JH (JH III) with nanomolar affinities. Specific mutations within the PAS-B domain of T. castaneum or A. aegypti Met preclude this JH binding [29,30]. JH induces Met to bind to another bHLH-PAS protein Taiman (Tai), also known as FISC or SRC [29,31–33]. The resulting complex binds JH-response DNA motifs and activates target gene transcription [30–35]. Similarly to Met, Tai has also been shown to mediate effects of JH on metamorphosis [36] and reproduction [37,38] in some insects. The D. melanogaster Met and Gce proteins interact with a chaperone Hsp83, which facilitates nuclear import of Met and expression of JH-induced genes such as Krüppel homolog 1 (Kr-h1) [34]. Most recently, Met and Gce were shown to mediate the effect of the JH precursor MF, which has been established as a circulating hormone in D. melanogaster [39].
Taken together, the above results favor Gce/Met as a JH receptor candidate. However, to establish conclusively that Gce/Met is a JH receptor, it must also be demonstrated that binding of the hormone is a necessary condition for functioning of the candidate receptor in vivo, during normal insect development. This study employs the power of Drosophila genetics to provide this critical missing evidence. It shows that transgenic Gce or Met proteins restore the natural sensitivity to JH mimics in the Methoprene-tolerant mutants and rescue the non-conditionally lethal Met gce double-mutant flies as long as their JH-binding pocket is intact.
Results and Discussion
Gce and Tai activate transcription in response to JH III, synthetic JH mimics, and MF
The D. melanogaster S2 cell line expresses endogenous mRNAs encoding both Met and Gce paralogs and their single partner protein Tai (S1 Fig). We initially tested whether Met, Gce and Tai mediated ligand-dependent transcriptional activation in the S2 cells. A luciferase reporter JHRE-luc was constructed using eight tandem copies of a JH-response element (JHRE) from the A. aegypti early trypsin gene [30,31] (Fig 1A). JHRE-luc was activated by a native JH (JH III), the JH mimic methoprene, and by MF in a dose-dependent manner (Fig 1B). Mutation of the JHRE inhibited the response to JH III (Fig 1B). RNAi-mediated knockdown of either tai or gce but not of Met prevented JH III or MF from inducing JHRE-luc (Fig 1C). Expression of additional Tai enhanced this hormone-dependent activation, again in a manner dependent primarily on gce and tai (Fig 1D). Similar results were obtained utilizing pyriproxyfen, a potent JH mimic of distinct, pyridine-based chemical structure [40] (S2 Fig).
Fig. 1. Transcriptional activation by JH III, methyl farnesoate (MF), and methoprene through Gce in Drosophila S2 cells. 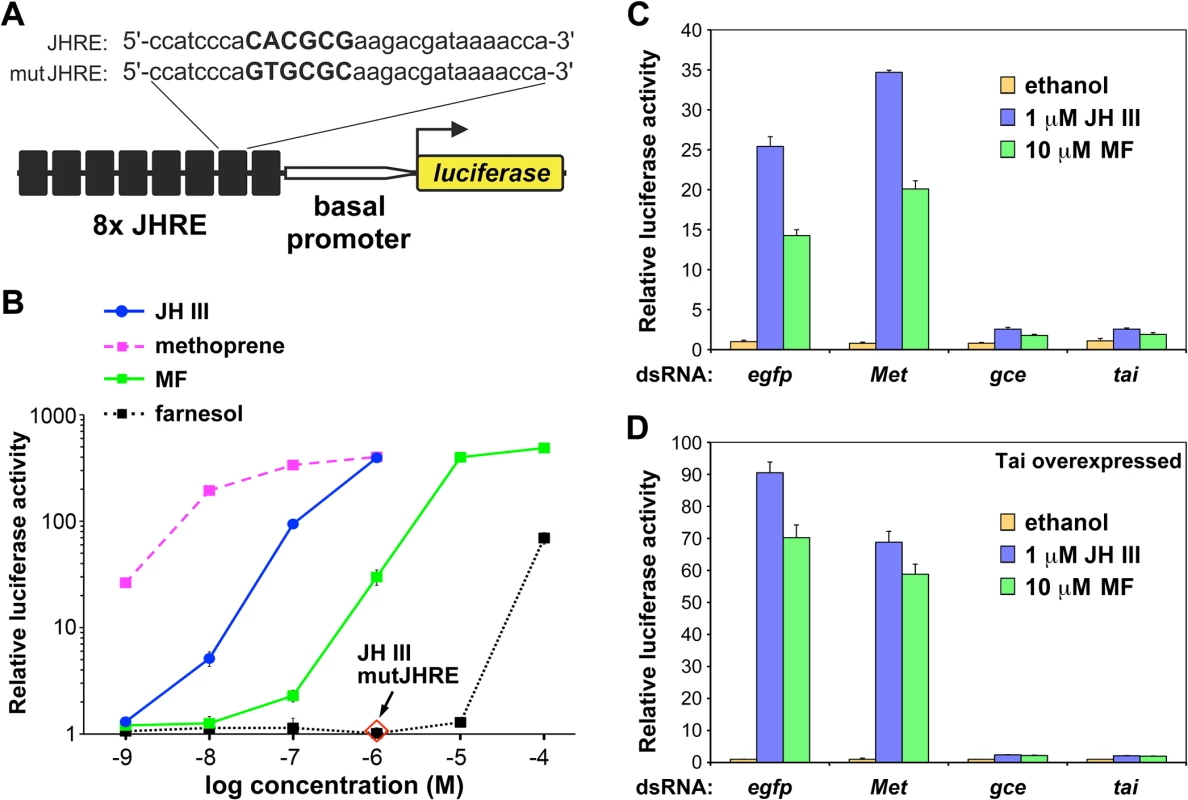
(A) A luciferase reporter construct carrying 8 copies of a JH response element (JHRE). The JHRE and the basal promoter derive from the Aedes aegypti early trypsin gene [30,31]. The core (capital letters) of the JHRE was mutated to produce mutJHRE. (B) S2 cells responded to the indicated compounds by activating the JHRE-luc reporter, but not mutJHRE-luc (1 μM JH III). Farnesol is a biologically inactive control. (C-D) Activation of JHRE-luc by 1 μM JH III or 10 μM MF relative to basal activity (ethanol) required both Gce and Tai as revealed by dsRNA-mediated knockdown of either protein; egfp dsRNA served as a control. Co-transfection with a Tai-expressing plasmid enhanced the Gce-dependent activation by JH III and MF (D). Data were normalized to Renilla luciferase activity and plotted as mean ± SD (n = 3). The observation that Gce and Tai were required for activation of JHRE-luc by MF (Fig 1C and 1D), is consistent with a previous finding that MF activated transcription through an ortholog of Gce/Met from the silkworm, Bombyx mori [29,31–33] and the recent finding that this natural JH precursor is a circulating hormone in D. melanogaster [39].
Gce binds JH and its agonists including MF
As the effect of JH in the S2 cell-based assay was essentially mediated by Tai and Gce, we examined the ability of the Gce protein in vitro to bind the activating ligands. [3H]JH III bound to Gce with a Kd of 19.3 ± 4.5 nM (Fig 2A), an affinity within the physiological hormone range [13]. Following on from the reporter gene activation data (Fig 1B), methoprene, pyriproxyfen, and MF all effectively competed with [3H]JH III for binding to Gce (Fig 2B), consistent with both JH mimics and MF acting as JH receptor agonists. Similarly to binding affinities previously determined for the PAS-B domain of T. castaneum Met [29], pyriproxyfen was the strongest competitor for binding to Gce, followed by methoprene and MF (Fig 2B). The higher potency of methoprene to activate JHRE-luc, relative to JH III (Fig 1B) may be explained by the fact that the synthetic insecticide is chemically and biologically more stable than JH III. Due to marginal levels of total [3H]JH III bound to the in-vitro translated D. melanogaster Met protein [29], we were unable to determine the ligand-binding affinities for Met.
Fig. 2. Binding of Gce by JH III, methyl farnesoate (MF), pyriproxyfen, and methoprene. 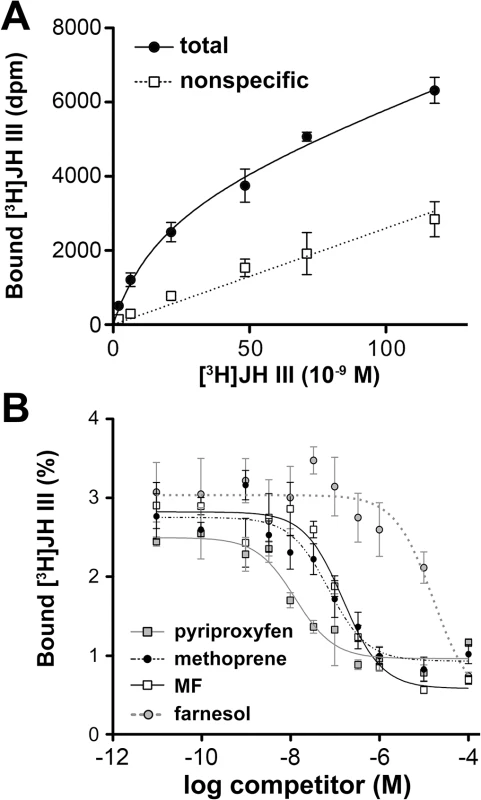
(A) Gce translated in vitro was incubated with [3H]JH III in the absence (total binding) or presence (nonspecific binding) of a 100-fold molar excess of unlabeled JH III. Data are mean ± SD (n = 3); the calculated Kd is 19.3 ± 4.5 nM. (B) Competition binding assay: Gce was incubated with 2 pmol of [3H]JH III and increasing concentrations of the indicated compounds. The data (mean ± SEM from 3–5 experiments) indicated Ki values of 5.7 ± 2.8 nM for pyriproxyfen, 45.8 ± 28.9 nM for methoprene, 87.9 ± 22.2 nM for MF, and 8.1 ± 2.4 μM for farnesol (a biologically inactive control). The activation and binding of Gce by MF is significant, as this circulating JH precursor prevails over JH III in D. melanogaster larvae [13,39] and exerts its own hormonal function [39]. Interestingly, MF has been reported to bind D. melanogaster USP with a high affinity (Kd = 40 nM) [12], comparable to the Ki of 87.9 nM we observed for MF binding to Gce (Fig 2B). USP has therefore been proposed as an intracellular MF receptor [6,13,14]. However, in agreement with genetic evidence [39], our RNAi data (Fig 1C and 1D) clearly show that Gce and Tai are essential for MF to induce expression of the JHRE-dependent reporter and thus act as a MF receptor.
MF is a "juvenile hormone" of crustaceans, where it promotes reproductive maturation and specific developmental events [41,42]. Interestingly, similar to JH in insects [29,31–33], MF has been shown to stimulate interaction between Met and Tai/SRC orthologs from the cladoceran crustaceans, Daphnia pulex and D. magna [43]. Moreover, when a threonine residue in the PAS-B domain of Daphnia Met was replaced with valine that occurs in the corresponding position critical for JH III binding in insects, namely V315 in D. melanogaster Gce (Fig 3A and 3B) or V297 in T. castaneum Met [29], the Daphnia Met protein became more responsive to JH III, without losing its responsiveness to MF [43]. Together with our current findings, this recent evidence suggests that Gce/Met has evolved as a receptor for sesquiterpenoid hormones in a common ancestor of crustaceans and insects.
Fig. 3. Gce and Met mutated in their JH-binding domains are incapable of activating transcription. 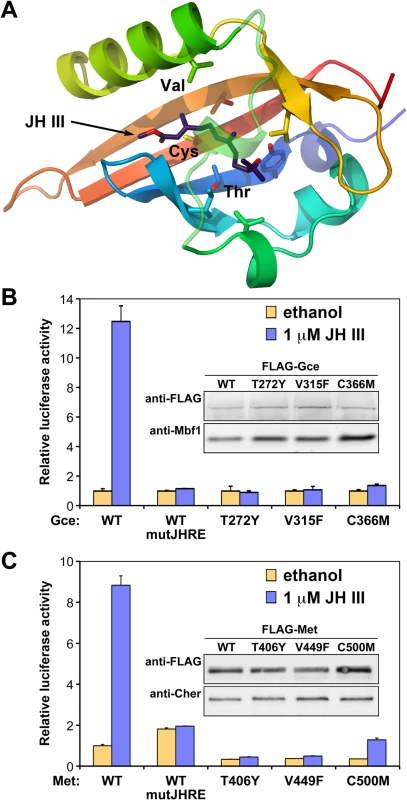
(A) Positions of three conserved amino acids important for binding of JH III based on our model of the T. castaneum Met PAS-B domain [29]. (B) Only wild-type Gce (WT) capable of binding JH III (S3 Fig) activated the JHRE-luc reporter in S2 cells. (C) Similar results were obtained for Met, which also lost its ability to activate JHRE-luc in response to JH III when its PAS-B domain was mutated at the corresponding conserved residues. In both experiments (B and C), the endogenous gce and Met were suppressed by RNAi. Data were normalized to Renilla luciferase activity and plotted as mean ± SD (n = 3). The WT and mutated Gce and Met variants were all stable as detected on immunoblots (insets) using their FLAG tags; antibody against the Mbf1 or Cheerio proteins served as controls. Gce/Met requires ligand binding to induce transcription in response to JH
To determine whether Gce required direct binding of JH for its function, we individually mutated three amino acids (T272Y, V315F, and C366M) in the ligand-binding pocket of Gce PAS-B (Fig 3A). The same substitutions of the corresponding residues have been shown to abolish binding to JH III in the Met proteins from T. castaneum [29] and A. aegypti [30]. As expected, all three mutated Gce proteins lost the ability to bind [3H]JH III in vitro (S3 Fig), indicating that these conserved T, V and C residues are critical for hormone binding by D. melanogaster Gce.
To test whether binding of JH was necessary for Gce to activate transcription, FLAG epitope-tagged wild-type (FLAG-GceWT) or mutated (FLAG-GceT272Y,-GceV315F, and-GceC366M) proteins were expressed in S2 cells, in which the endogenous Gce and Met were suppressed by RNAi. Clearly, only FLAG-GceWT responded to JH III to activate the JHRE-luc reporter, whereas the three Gce variants incapacitated for hormone binding did not (Fig 3B). The wild-type and mutated Gce proteins all appeared to be stable in the S2 cells (Fig 3B, inset).
Although the endogenous Met protein did not appear to play a major role in the JH-dependent activation of JHRE-luc in the S2 cell line (Fig 1C and 1D), D. melanogaster Met could in fact substitute for Gce in this reporter assay when transfected to the cells (Fig 3C). The S2 cells were again subjected to RNAi-mediated depletion of the endogenous Met and Gce proteins but not of the added Met protein that was expressed from a synthetic DNA construct. Like Gce, Met but not its mutated versions, mediated induction of JHRE-luc by JH III (Fig 3C). Importantly, the functional JHRE-luc reporter was not activated by Met that had been mutated in its PAS-B domain with individual substitutions T406Y, V449F, and C500M that correspond to the T272Y, V315F, and C366M mutations in Gce (Fig 3C). These mutations did not lead to degradation of Met (Fig 3C, inset). Although we have been unable to directly confirm the effect of these mutations on the ligand-binding activity of D. melanogaster Met, it is most likely that they prevent JH III binding just as equivalent substitutions of these highly conserved residues do in Gce (S3 Fig) and in the Met proteins from T. castaneum [29] and A. aegypti [30]. These results strongly suggest that the JH-binding capacity is required for the normal function of D. melanogaster Met.
Expression of an endogenous JH-response gene relies on the JH-binding activity of Gce
In D. melanogaster the Met and gce genes reside on the X chromosome and their simultaneous loss in females that are homozygous or males that are hemizygous for the Met27 and gce2.5k null alleles is lethal at the onset of pupation [22]. The Met27 gce2.5k double mutants are known to express reduced mRNA levels of the Kr-h1 gene, which is a direct target of Gce/Met [22,34,39]. In order to demonstrate that the JH-binding capacity of Gce is important in vivo for transcription of this relevant JH-response gene, we expressed the wild-type and mutated forms of Gce using the ubiquitous armadillo-Gal4 driver (arm-Gal4) in the Met27 gce2.5k background. To avoid the lethal phase in this strain, we examined Kr-h1 expression in Met27 gce2.5k/Y male larvae that were selected and genotyped during mid-third instar. The equal performance of the Gce variants was ensured by inserting all transgenic UAS-gce constructs into the same genetic locus [44].
Consistent with previous reports [22,34,39], we observed reduced Kr-h1 levels in Met27 gce2.5k mutants (Fig 4) albeit the difference was less dramatic, likely due to the earlier stage of our animals at which Kr-h1 expression is lower and less dependent on JH [45]. Addition of the transgenic GceWT protein to the Met27 gce2.5k background significantly augmented Kr-h1 expression near levels occurring in Met+ gce+ sibling male larvae (Fig 4). In contrast, the Kr-h1 transcript remained low when any of the three mutated forms of Gce were expressed (Fig 4). Therefore, only when capable of binding its hormonal ligand (S3 Fig), Gce could compensate for the missing endogenous receptor proteins in restoring the normal expression of their target gene. As the function of Kr-h1 is essential for D. melanogaster to complete the prepupal stage [45], compromised Kr-h1 expression may be contributing to the lethality resulting from the absence of Gce and Met.
Fig. 4. The ligand-binding capacity of Gce is required for normal expression of the direct JH-response gene Kr-h1 in vivo. 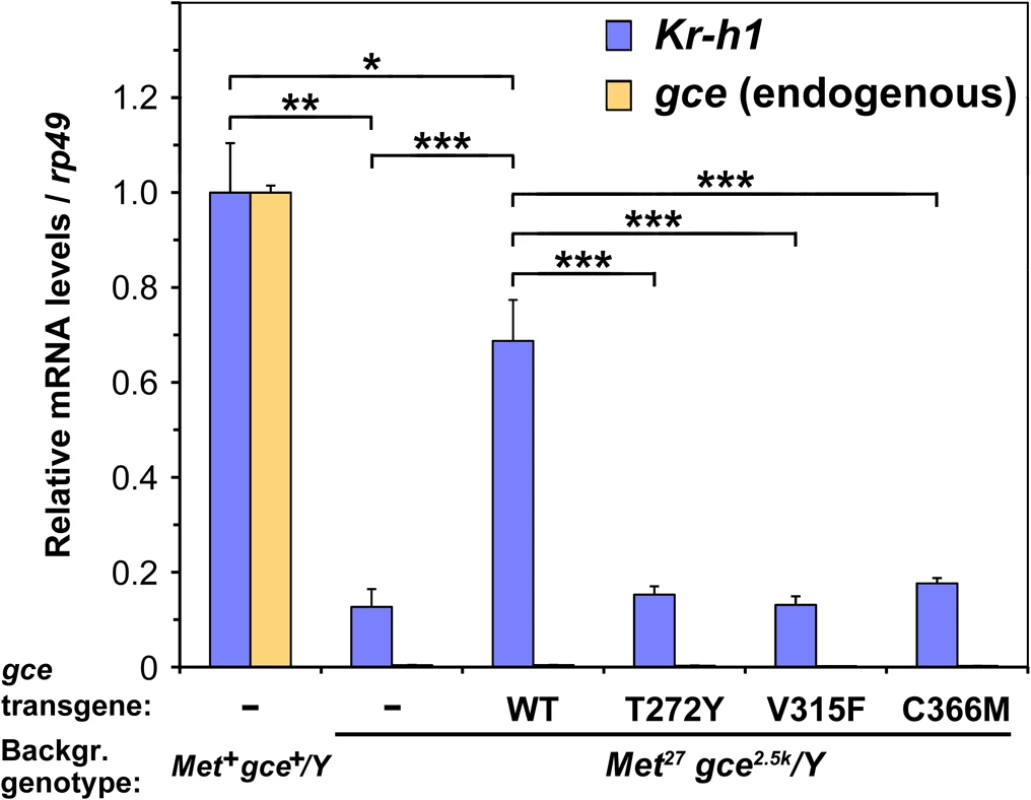
Reduction in Kr-h1 mRNA levels in Met27 gce2.5k mutant larvae can be compensated by transgenic expression of the functional Gce protein but not by any of its mutated forms, incapable of binding JH III (S3 Fig). Balanced Met27 gce2.5k/FM7c; arm-Gal4 females were crossed with males bearing the UAS-gce transgenes. The male progeny were selected as mid-third instar larvae and genotyped by PCR detecting the gce2.5k deletion [22] to distinguish Met27 gce2.5k/Y from Met+ gce+/Y siblings carrying the FM7c balancer chromosome. Groups of four larvae were subjected to qRT-PCR with primers detecting the endogenous Kr-h1α and gce transcripts (S2 Table). Values relative to mRNA levels in Met+ gce+/Y controls (set to 1) are mean ± SD from three biological replicates for each genotype, five replicates for GceWT. The significance levels of differences determined by t-test were P < 0.02 (*), P = 0.0002 (**), and P < 0.0002 (***). The ligand-binding capacity of Gce/Met is necessary for the normal response of Drosophila to JH mimics
To further investigate the receptor function of Gce in vivo, we tested the relationship of JH binding to the phenomenon of "methoprene tolerance"–the insecticide resistance phenotype for which the D. melanogaster Met mutants were originally isolated and named [20]. Strains singly mutant either for Met or, to a lesser extent gce, resist doses of JH mimics that kill flies possessing both wild-type genes [20,22,46,47]. It has been shown that ubiquitous expression of a gce+ transgene using the Gal4/UAS system is sufficient to reinstate sensitivity to methoprene in the Met27 null mutants [46].
We took this approach with our GceWT, GceT272Y, GceV315F, and GceC366M transgenic constructs. When expressed under the arm-Gal4 driver, only GceWT restored sensitivity to dietary methoprene in Met27 homozygotes (Fig 5A). In fact, these Met27 animals expressing GceWT became more sensitive to methoprene than Met+ controls, reflecting a dominant effect of the additional GceWT protein. In contrast, Met27 males and females expressing any of the three mutated Gce variants remained resistant and emerged as adults after feeding on methoprene (Fig 5A). Similar results were obtained with pyriproxyfen (S4 Fig). Thus, the lethal action of the insecticidal JH mimics relies on the ligand-binding capacity of the transgenic Gce protein.
Fig. 5. The capacity to bind JH is essential for Gce/Met function in vivo. 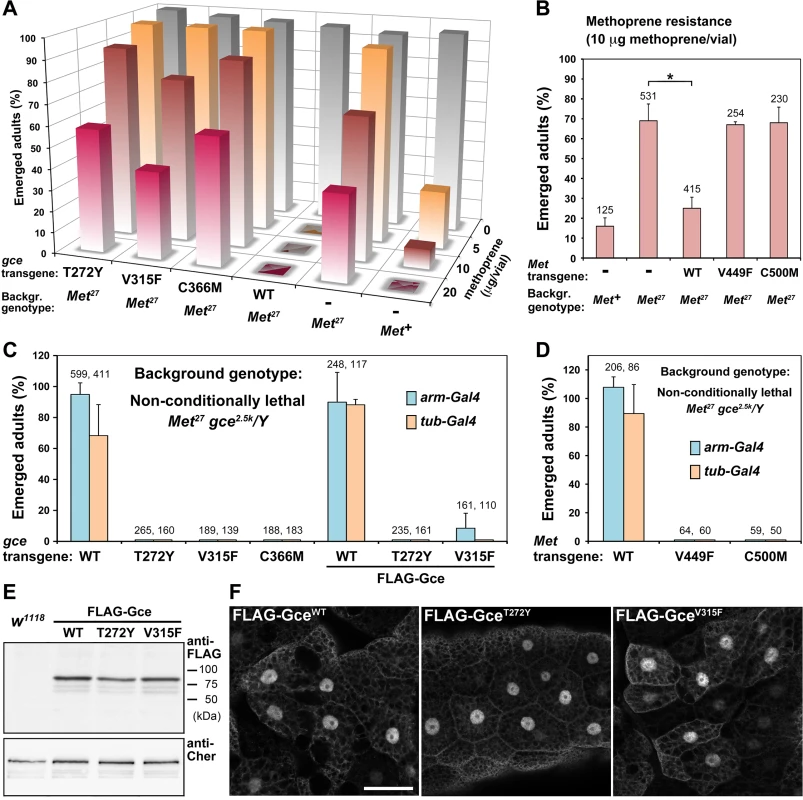
(A-B) Met27 mutants tolerate methoprene better than Met+ control flies [47] or Met27 flies expressing transgenic GceWT or MetWT proteins. Met27/Y males carrying the indicated UAS-gce or UAS-Met transgenes were mated to Met27; arm-Gal4 females, and the F1 progeny was fed methoprene. In the presence of methoprene, GceWT totally blocked adult development and MetWT significantly (*; P < 0.0003) reduced survival relative to the Met27 strain, whereas mutated Gce or Met did not have this effect. Values are per cent average numbers of emerged adults relative to total numbers of pupated animals. Each column represents 200–430 animals (or the indicated numbers in B) counted in 2–3 independent trials. (C-D) Balanced Met27 gce2.5k/FM7c; arm-Gal4 (or tub-Gal4) females were crossed with males bearing the UAS-gce or UAS-Met transgenes, and emerged Met27 gce2.5k/Y adult males were scored relative to their FM7c/Y (Met+ gce+) siblings (1:1 ratio was considered 100% rescue). Non-conditionally lethal Met27 gce2.5k/Y males were rescued to adulthood by transgenic GceWT, FLAG-GceWT, and MetWT proteins but not by their mutated versions except for a few flies rescued by one randomly inserted FLAG-GceV315F construct expressed under arm-Gal4 (C). Data are mean ± SD; numbers of all scored F1 males are given above columns. (E) FLAG-Gce WT and mutated proteins were stable when expressed in vivo as shown on an immunoblot (anti-FLAG antibody) from adult flies. The Cheerio (Cher) protein served as a control. (F) FLAG-Gce WT and mutated proteins were detected with the anti-FLAG antibody in the nuclei of larval fat body cells. Bars, 50 μm. To obtain similar information for Met, we repeated this experiment with fly strains expressing the D. melanogaster wild-type Met protein or its mutated versions MetV449F and MetC500M (our initial attempt to transform flies with UAS-MetT406Y failed). Although the flies expressing MetWT under the arm-Gal4 driver did not become more sensitive than control Met+ flies, their response to dietary methoprene significantly increased relative to the original Met27 mutants or the same mutants carrying the MetV449F and MetC500M transgenes (Fig 5B). Our data thus demonstrate that Gce and Met are mutual substitutes in rendering flies sensitive to exogenous JH mimics as long their ligand-binding pockets are unaffected by specific mutations.
The capacity of Gce/Met to prevent lethality in Met gce double-mutant flies depends on hormone binding
The non-conditional lethality of the Met27 gce2.5k double-mutants can be rescued with transgenic constructs providing either Met+ or gce+ function, thus reflecting partial redundancy between Met and Gce [22]. This genetic rescue offers an ideal system to answer the ultimate question as to whether Gce/Met requires its JH-binding capacity to sustain normal development of the animal. Using two ubiquitous drivers, arm-Gal4 and α-tubulin (tub-Gal4), and transgenic UAS-gce and UAS-Met constructs uniformly inserted to the attP2 chromosomal site [44], we expressed the functional or mutated proteins in the Met27 gce2.5k background. Indeed, expression of GceWT or MetWT under both drivers rescued a major proportion of Met27 gce2.5k/Y hemizygous males to adulthood (Fig 5C and 5D). In striking contrast, the mutated GceT272Y, GceV315F, GceC366M, MetV449F or MetC500M proteins did not allow any Met27 gce2.5k/Y adults to emerge (Fig 5C and 5D). Therefore, only Gce/Met with intact JH-binding function can substitute for the absence of both genes during normal development.
To examine whether Gce incapacitated for JH binding was stable in vivo, we expressed FLAG-tagged versions of GceWT, GceT272Y, and GceV315F in transgenic D. melanogaster. Again, only the functional but not the JH binding-deficient tagged protein provided a clear rescue of the Met27 gce2.5k mutants (Fig 5C). Interestingly, marginal rescue of 7.5% of emerging adults was observed, albeit only with the arm-Gal4 driver, with FLAG-GceV315F (Fig 5C), suggesting that this mutated protein might retain some residual functionality. The discrepancy between this weak effect and the total absence of rescue by untagged GceV315F (Fig 5C) might result from variable expression level of the FLAG-tagged construct that, unlike the untagged constructs, had been integrated to random loci rather than to the specific attP2 site. Indeed, from three independent FLAG-GceV315F transgenic lines, only one showed the partial genetic rescue.
Importantly, all three FLAG-tagged Gce variants were detected on immunoblots from whole transgenic flies (Fig 5E), and all were observed primarily in the nuclei of larval fat body cells (Fig 5F), regardless of whether or not Gce was mutated to prevent binding of JH. Thus, the failure of mutated Gce to compensate for the loss of the endogenous Met and Gce proteins was more likely caused by inability to bind JH rather than by degradation or mislocalization of the mutated protein.
In conclusion, our study shows that the capacity of Gce, and most likely also of Met, to promote gene expression and sustain normal development requires direct hormone binding to the protein in vivo. The case that Gce/Met acts as a JH receptor in insects is now unequivocal. Establishment of the nature of this receptor resolves a central problem in arthropod endocrinology. The ability of Gce to respond to methyl farnesoate, the crustacean JH, suggests that the role of Gce/Met in sesquiterpenoid signaling predates the evolutionary separation of the hexapod and crustacean lineages. Furthermore, it is of interest that Gce/Met provides the first clear example of a bHLH-PAS protein acting as a receptor for a genuine animal hormone.
Materials and Methods
Vectors for Gce and Met protein expression
DNA sequences corresponding to the D. melanogaster Gce (amino acids 1–689; NCBI Reference Sequence NP_511160.1) and Met (amino acids 1–716; NCBI Reference Sequence NP_511126.2) were synthesized for optimal D. melanogaster codon usage to encode the Gce and Met wild-type (WT) and mutated (T272Y, V315F, C366M, T406Y, V449F, C500M) variants. For transcription/translation in vitro, these DNA fragments were cloned using the Eco RI and Kpn I restriction sites behind the T7 promoter in the pK-Myc-C2 plasmid [48]. The same gce and Met DNA sequences were inserted under the UAST promoter in two different vectors for D. melanogaster transformation: pTFW (Drosophila Genomics Resource Center), in which Gce was N-terminally tagged with a FLAG epitope, and pUASTattB [49] that permitted integration of the gce/Met transgenes into the specific attP2 chromosomal landing site [44].
Ligand-binding assays
Racemic (RS) tritiated JH III (10–20 Ci mMol-1) was purchased from Perkin Elmer. Racemic JH III, pyriproxyfen, trans,trans-farnesol and methoprene were from Sigma-Aldrich, and (E,E)-methyl farnesoate (MF) from Echelon Biosciences. The WT, T272Y, V315F, and C366M variants of Gce were produced with the rabbit reticulocyte lysate TnT Quick Coupled transcription/translation system (Promega) using 400 ng of template plasmid per 50-μl reaction. Each reaction was divided into 15-μl aliquots that were assessed for binding of [3H]JH III using the dextran-coated charcoal (DCC) method as described previously [29]. The dissociation constant (Kd) was determined for GceWT binding to [3H]JH III, and the Ki values for methoprene, pyriproxyfen, MF and farnesol were calculated from competition assays with the unlabeled compounds using GraphPad Prism 5.00 (GraphPad Software) as described [29].
Luciferase reporter assays
A JH-responsive luciferase reporter (JHRE-luc) was generated using a JH response element (JHRE) (5' - CCATCCCACACGCGAAGACGATAAAACCA - 3') identified upstream of the Aedes aegypti early trypsin (AaET) gene [31]. A mutated version of this element (5' - CCATCCCAGTGCGCAAGACGATAAAACCA -3') was used to generate a negative-control mutJHRE-luc. DNA sequences were synthesized to include eight copies of either JHRE or mutJHRE, followed by a 140-bp minimal promoter of the AaET gene (nucleotides -77 to +63). These sequences were cloned to the pGL4.17 vector containing the firefly (Photinus pyralis) luc2 gene (Promega). D. melanogaster Schneider 2 (S2) cells were cultured in Shields and Sang M3 Insect Medium (Sigma-Aldrich) containing 8% of heat-inactivated fetal bovine serum (Life Technologies) at 25°C. For luciferase reporter assays, S2 cells were seeded in a 12-well plate containing 900 μl of medium per well, and cultured for 24 h. The JHRE-luc (or mutJHRE-luc) reporter plasmid (0.25 μg per well) was co-transfected with a pCopia plasmid (0.1 μg per well) encoding Renilla luciferase using the X-tremeGENE HP DNA Transfection Reagent (Roche). Where appropriate, the D. melanogaster Tai protein was expressed from a pCMA plasmid (0.25 μg per well) containing tai cDNA [31,50]. Expression of either the wild-type or mutated FLAG-tagged Gce and Met variants was achieved by co-transfecting 0.25 μg of a pTFW vector carrying the respective gce or Met DNA sequence under the UAST promoter with 0.1 μg of a plasmid expressing the Gal4 transcription factor under a D. melanogaster actin promoter. The total DNA load per well was kept constant at 1 μg by inclusion of non-specific plasmid DNA. Following transfection, cells were incubated for 48 h and treated for another 12 h with JH III, methoprene, pyriproxyfen, MF or farnesol (all dissolved in ethanol). The cells were then processed with the Dual-Luciferase reporter assay system (Promega). Relative luciferase activity was measured using the Orion II microplate luminometer (Berthold Detection Systems) and data were normalized against Renilla luciferase activity.
RNAi in S2 cells
Met, gce and tai cDNAs were obtained by reverse transcription of total D. melanogaster embryonic RNA, followed by PCR amplification with specific primer sets (S1 Table). The cDNA fragments were flanked with T7 promoter sequences to enable synthesis of double-stranded RNA (dsRNA) using T7 RNA polymerase (MEGAscript, Ambion). A 720-bp dsRNA derived from the egfp gene served as a control. To knock down Met, gce, and tai genes in S2 cells, 3 μg of dsRNA per well of a 12-well plate were added together with plasmid DNA in the transfection mixture. The dsRNA sequences targeting endogenous gce and Met did not interfere with expression of the Gce and Met (WT or mutated) proteins transfected with the pTFW vector, as those were encoded by synthetic DNA divergent from the endogenous DNA sequences. Moreover, gce dsRNA targeted an upstream region of the native gce transcript that did not overlap with the synthetic sequence included in the pTFW-gce constructs.
mRNA quantification
Total RNA isolated from whole mid-third instar D. melanogaster larvae or S2 cells using the Trizol reagent (Life Technologies) was treated with TURBO DNase (Ambion), and 1.5 μg of RNA was reverse transcribed to cDNA (Superscript II, Life Technologies). Relative transcript levels were measured in a C1000 Thermal Cycler (Bio-Rad) using the iQ SYBR Green Supermix kit (Bio-Rad) using specific primer sets (S2 Table) and normalized against levels of the ribosomal protein 49 (rp49) mRNA.
Drosophila transgenesis
Targeted insertion of gce transgenes into the attP2 landing site on the third chromosome (cytological position 68A4) was achieved using the bacteriophage ϕC31 integrase method [44]. The pUASTattB constructs containing the WT and mutated gce or Met sequences were injected into embryos of the y w P{nos-ϕC31\int.NLS}X; P{CaryP}attP2 host strain (Genetic Services, Inc. or BestGene, Inc.). Several independent transgenic lines for expression of the FLAG-tagged GceWT, GceT272Y, and GceV315F proteins were generated through conventional P-element mediated transformation by injecting embryos of the w1118 host strain with the pTFW-based vectors (Genetic Services, Inc.). In all cases, expression of the transgenic proteins was induced using the Gal4/UAS system [51] with the ubiquitous armadillo (arm-Gal4) or α-tubulin (tub-Gal4) drivers (Bloomington Drosophila Stock Center, Indiana).
Genetic rescue experiments
D. melanogaster with unaffected Met+ function are sensitive to exogenous JH or its mimics as early prepupae [52], whereas flies deficient for Met tolerate exposure to these compounds [20,47]. To test for restoration of methoprene sensitivity to Met27 mutants, homozygous Met27; arm-Gal4 females were mated with Met27/Y; UAS-gce or Met27/Y; UAS-Met males carrying the wild-type or mutated transgenes, all inserted into the same attP2 landing site [44]. The uniform Met27; arm-Gal4/+; UAS-gce (or UAS-Met)/+ F1 progeny was exposed to methoprene, pyriproxyfen or ethanol alone from the outset of larval feeding, and numbers of emerged adults were scored relative to all animals forming pupae.
To test for rescue of viability in the non-conditionally lethal Met27 gce2.5k double mutants, balanced Met27 gce2.5k/FM7c; arm-Gal4 or Met27 gce2.5k/FM7c; tub-Gal4 females were crossed with w1118/Y; UAS-gce or w1118/Y; UAS-Met males harboring the wild-type or mutated gce or Met transgenes in the attP2 landing site. To detect the transgenic Gce proteins, we used males with UAS-FLAG-gce transgenes carried on the pTFW vector and inserted into random genomic loci.
Immunoblotting and antibody tissue staining
Immunoblots were prepared from total D. melanogaster S2 cell lysates or from entire adult transgenic flies and processed with an anti-FLAG antibody (Sigma-Aldrich; 1 : 4000) and with anti-Mbf1 or anti-Cheerio antibodies as previously described [53]. Clones overexpressing WT or mutant Gce proteins were induced using the heat-shock-FLPout technique [54], whereby y w hs-flp; act>y+>Gal4, UAS-GFP females were mated to UAS-FLAG-gce transgenic males. Fat bodies dissected from larval progeny one day after heat shock (37°C, 30 min) were stained with anti-FLAG (Sigma-Aldrich; 1 : 1000) and Cy3-conjugated (Cell Signaling) antibodies, and images were captured with the Olympus FV1000 confocal microscope.
Supporting Information
Zdroje
1. Nijhout H (1994) Insect hormones. Princeton: Princeton University Press. 267 p.
2. Cusson M, Yagi KJ, Ding Q, Duve H, Thorpe A, et al. (1991) Biosynthesis and release of juvenile hormone and its precursors in insects and crustaceans: the search for a unifying arthropod endocrinology. Insect Biochem 21 : 1–6.
3. Laufer H, Biggers WJ (2001) Unifying concepts learned from methyl farnesoate for invertebrate reproduction and post-embryonic development. Am Zool 41 : 442–457.
4. Raikhel AS, Brown MR, Bellés X (2005) Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science. Amsterdam: Elsevier/Pergamon. pp. 433–491.
5. Jindra M, Palli SR, Riddiford LM (2013) The juvenile hormone signaling pathway in insect development. Annu Rev Entomol 58 : 181–204. doi: 10.1146/annurev-ento-120811-153700 22994547
6. Dubrovsky EB, Bernardo TJ (2014) The juvenile hormone receptor and molecular mechanisms of juvenile hormone action. Adv Insect Physiol 46 : 305–388.
7. Röller H, Dahm KH, Sweeley CC, Trost BM (1967) Die Struktur des Juvenilhormones. Angew Chemie 4 : 190–191.
8. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, et al. (1995) The nuclear receptor superfamily: the second decade. Cell 83 : 835–839. 8521507
9. King-Jones K, Thummel CS (2005) Nuclear receptors–a perspective from Drosophila. Nat Rev Genet 6 : 311–323. 15803199
10. Hill RJ, Billas IML, Bonneton F, Graham LD, Lawrence MC (2013) Ecdysone receptors: from the Ashburner model to structural biology. Annu Rev Entomol 58 : 251–271. doi: 10.1146/annurev-ento-120811-153610 23072463
11. Jones G, Sharp PA (1997) Ultraspiracle: an invertebrate nuclear receptor for juvenile hormones. Proc Natl Acad Sci USA 94 : 13499–13503. 9391054
12. Jones G, Jones D, Teal P, Sapa A, Wozniak M (2006) The retinoid-X receptor ortholog, ultraspiracle, binds with nanomolar affinity to an endogenous morphogenetic ligand. FEBS J 273 : 4983–4996. 17064257
13. Jones G, Teal P, Henrich VC, Krzywonos A, Sapa A, et al. (2013) Ligand binding pocket function of Drosophila USP is necessary for metamorphosis. Gen Comp Endocrinol 182 : 73–82. doi: 10.1016/j.ygcen.2012.11.009 23211750
14. Jones D, Jones G, Teal P (2013) Sesquiterpene action, and morphogenetic signaling through the ortholog of retinoid X receptor, in higher Diptera. Gen Comp Endocrinol 194 : 326–335. doi: 10.1016/j.ygcen.2013.09.021 24120505
15. Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, et al. (1992) 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68 : 397–406. 1310260
16. Yao TP, Segraves WA, Oro AE, McKeown M, Evans RM (1992) Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell 71 : 63–72. 1327536
17. Thomas HE, Stunnenberg HG, Stewart AF (1993) Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature 362 : 471–475. 8385270
18. Nowickyj SM, Chithalen JV, Cameron D, Tyshenko MG, Petkovich M, et al. (2008) Locust retinoid X receptors: 9-cis-retinoic acid in embryos from a primitive insect. Proc Natl Acad Sci USA 105 : 9540–9545. doi: 10.1073/pnas.0712132105 18606996
19. Ren B, Peat TS, Streltsov VA, Pollard M, Fernley R, et al. (2014) Unprecedented conformational flexibility revealed in the ligand-binding domains of the Bovicola ovis ecdysone receptor (EcR) and ultraspiracle (USP) subunits. Acta Crystallogr D Biol Crystallogr 70 : 1954–1964. doi: 10.1107/S1399004714009626 25004972
20. Wilson TG, Fabian J (1986) A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev Biol 118 : 190–201. 3095161
21. Konopova B, Jindra M (2007) Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci USA 104 : 10488–10493. 17537916
22. Abdou MA, He Q, Wen D, Zyaan O, Wang J, et al. (2011) Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem Mol Biol 41 : 938–945. doi: 10.1016/j.ibmb.2011.09.003 21968404
23. Riddiford LM, Truman JW, Mirth CK, Shen YC (2010) A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137 : 1117–1126. doi: 10.1242/dev.037218 20181742
24. Baumann A, Fujiwara Y, Wilson TG (2010) Evolutionary divergence of the paralogs Methoprene tolerant (Met) and germ cell expressed (gce) within the genus Drosophila. J Insect Physiol 56 : 1445–1455. doi: 10.1016/j.jinsphys.2010.05.001 20457161
25. Ashok M, Turner C, Wilson TG (1998) Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA 95 : 2761–2766. 9501163
26. Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B (2011) Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci 124 : 1–22. doi: 10.1093/toxsci/kfr218 21908767
27. Stockinger B, Di Meglio P, Gialitakis M, Duarte JH (2014) The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 32 : 403–432. doi: 10.1146/annurev-immunol-032713-120245 24655296
28. Miura K, Oda M, Makita S, Chinzei Y (2005) Characterization of the Drosophila Methoprene-tolerant gene product. FEBS J 272 : 1169–1178. 15720391
29. Charles J-P, Iwema T, Epa VC, Takaki K, Rynes J, et al. (2011) Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA 108 : 21128–21133. doi: 10.1073/pnas.1116123109 22167806
30. Li M, Liu P, Wiley JD, Ojani R, Bevan DR, et al. (2014) A steroid receptor coactivator acts as the DNA-binding partner of the methoprene-tolerant protein in regulating juvenile hormone response genes. Mol Cell Endocrinol 394 : 47–58. doi: 10.1016/j.mce.2014.06.021 25004255
31. Li M, Mead EA, Zhu J (2011) Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA 108 : 638–643. doi: 10.1073/pnas.1013914108 21187375
32. Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR (2011) Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem 286 : 8437–8447. doi: 10.1074/jbc.M110.191684 21190938
33. Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, et al. (2012) Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA 109 : 11729–11734. doi: 10.1073/pnas.1204951109 22753472
34. He Q, Wen D, Jia Q, Cui C, Wang J, et al. (2014) Heat shock protein 83 (Hsp83) facilitates Methoprene-tolerant (Met) nuclear import to modulate juvenile hormone signaling. J Biol Chem 289 : 27874–27885. doi: 10.1074/jbc.M114.582825 25122763
35. Zou Z, Saha TT, Roy S, Shin SW, Backman TWH, et al. (2013) Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci USA 110: E2173–E2181. doi: 10.1073/pnas.1305293110 23633570
36. Lozano J, Kayukawa T, Shinoda T, Bellés X (2014) A role for taiman in insect metamorphosis. PLoS Genet 10: e1004769. doi: 10.1371/journal.pgen.1004769 25356827
37. Smykal V, Bajgar A, Provaznik J, Fexova S, Buricova M, et al. (2014) Juvenile hormone signaling during reproduction and development of the linden bug, Pyrrhocoris apterus. Insect Biochem Mol Biol 45 : 69–76. doi: 10.1016/j.ibmb.2013.12.003 24361539
38. Guo W, Wu Z, Song J, Jiang F, Wang Z, et al. (2014) Juvenile hormone-receptor complex acts on mcm4 and mcm7 to promote polyploidy and vitellogenesis in the migratory locust. PLoS Genet 10: e1004702. doi: 10.1371/journal.pgen.1004702 25340846
39. Wen D, Rivera-Perez C, Abdou M, Jia Q, He Q, et al. (2015) Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLoS Genet 11: e1005038. doi: 10.1371/journal.pgen.1005038 25774983
40. Ishaaya I, Horowitz AR (1995) Pyriproxyfen, a novel insect growth regulator for controlling whiteflies: mechanisms and resistance management. Pestic Sci 43 : 227–232.
41. Nagaraju GPC (2011) Reproductive regulators in decapod crustaceans: an overview. J Exp Biol 214 : 3–16. doi: 10.1242/jeb.047183 21147963
42. Miyakawa H, Toyota K, Sumiya E, Iguchi T (2014) Comparison of JH signaling in insects and crustaceans. Curr Opin Insect Sci 1 : 81–87.
43. Miyakawa H, Toyota K, Hirakawa I, Ogino Y, Miyagawa S, et al. (2013) A mutation in the receptor Methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nat Commun 4 : 1856. doi: 10.1038/ncomms2868 23673641
44. Groth AC, Fish M, Nusse R, Calos MP (2004) Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166 : 1775–1782. 15126397
45. Pecasse F, Beck Y, Ruiz C, Richards G (2000) Krüppel-homolog, a stage-specific modulator of the prepupal ecdysone response, is essential for Drosophila metamorphosis. Dev Biol 221 : 53–67. 10772791
46. Baumann A, Barry J, Wang S, Fujiwara Y, Wilson TG (2010) Paralogous genes involved in juvenile hormone action in Drosophila melanogaster. Genetics 185 : 1327–1336. doi: 10.1534/genetics.110.116962 20498297
47. Wilson TG, Ashok M (1998) Insecticide resistance resulting from an absence of target-site gene product. Proc Natl Acad Sci USA 95 : 14040–14044. 9826649
48. Valenta T, Lukas J, Korinek V (2003) HMG box transcription factor TCF-4's interaction with CtBP1 controls the expression of the Wnt target Axin2/Conductin in human embryonic kidney cells. Nucleic Acids Res 31 : 2369–2380. 12711682
49. Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA 104 : 3312–3317. 17360644
50. Bai J, Uehara Y, Montell DJ (2000) Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell 103 : 1047–1058. 11163181
51. Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 : 401–415. 8223268
52. Riddiford LM, Ashburner M (1991) Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol 82 : 172–183. 1906823
53. Külshammer E, Uhlirova M (2013) The actin cross-linker Filamin/Cheerio mediates tumor malignancy downstream of JNK signaling. J Cell Sci 126 : 927–938. doi: 10.1242/jcs.114462 23239028
54. Struhl G, Basler K (1993) Organizing activity of wingless protein in Drosophila. Cell 72 : 527–540. 8440019
Štítky
Genetika Reprodukční medicína
Článek Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density ImputationČlánek AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct MechanismsČlánek A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seqČlánek TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive HematopoiesisČlánek Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 7
-
Všechny články tohoto čísla
- LINE-1 Retroelements Get ZAPped!
- /p23: A Small Protein Heating Up Lifespan Regulation
- Hairless Streaks in Cattle Implicate TSR2 in Early Hair Follicle Formation
- Ribosomal Protein Mutations Result in Constitutive p53 Protein Degradation through Impairment of the AKT Pathway
- Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in
- BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma
- Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
- EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in
- Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins
- Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
- A Year of Infection in the Intensive Care Unit: Prospective Whole Genome Sequencing of Bacterial Clinical Isolates Reveals Cryptic Transmissions and Novel Microbiota
- Inference of Low and High-Grade Glioma Gene Regulatory Networks Delineates the Role of Rnd3 in Establishing Multiple Hallmarks of Cancer
- Novel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
- A Novel Locus Harbouring a Functional Nonsense Mutation Identified in a Large Danish Family with Nonsyndromic Hearing Impairment
- Checkpoint Activation of an Unconventional DNA Replication Program in
- A Genetic Incompatibility Accelerates Adaptation in Yeast
- The SMC Loader Scc2 Promotes ncRNA Biogenesis and Translational Fidelity
- Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
- Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation
- JAK/STAT and Hox Dynamic Interactions in an Organogenetic Gene Cascade
- Emergence, Retention and Selection: A Trilogy of Origination for Functional Proteins from Ancestral LncRNAs in Primates
- MoSET1 (Histone H3K4 Methyltransferase in ) Regulates Global Gene Expression during Infection-Related Morphogenesis
- Arabidopsis PCH2 Mediates Meiotic Chromosome Remodeling and Maturation of Crossovers
- AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
- A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seq
- Tempo and Mode of Transposable Element Activity in Drosophila
- The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres
- SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation
- A Genome Scan for Genes Underlying Microgeographic-Scale Local Adaptation in a Wild Species
- TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis
- Analysis of the Relationships between DNA Double-Strand Breaks, Synaptonemal Complex and Crossovers Using the Mutant
- Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools
- Allelic Spectra of Risk SNPs Are Different for Environment/Lifestyle Dependent versus Independent Diseases
- CSB-PGBD3 Mutations Cause Premature Ovarian Failure
- Irrepressible: An Interview with Mark Ptashne
- Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
- Inactivation of Retinoblastoma Protein (Rb1) in the Oocyte: Evidence That Dysregulated Follicle Growth Drives Ovarian Teratoma Formation in Mice
- Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
- Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA
- Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage
- RFX2 Is a Major Transcriptional Regulator of Spermiogenesis
- A Role for Macro-ER-Phagy in ER Quality Control
- Corp Regulates P53 in via a Negative Feedback Loop
- Common Cell Shape Evolution of Two Nasopharyngeal Pathogens
- Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in
- Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression
- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- mTOR Directs Breast Morphogenesis through the PKC-alpha-Rac1 Signaling Axis
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
- Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast
- Encodes a Highly Conserved Protein Important to Neurological Function in Mice and Flies
- Identification of a Novel Regulatory Mechanism of Nutrient Transport Controlled by TORC1-Npr1-Amu1/Par32
- Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly
- Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
- TFIIS-Dependent Non-coding Transcription Regulates Developmental Genome Rearrangements
- Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen
- Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- The Catalytic and Non-catalytic Functions of the Chromatin-Remodeling Protein Collaborate to Fine-Tune Circadian Transcription in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



