-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBlimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
The transcriptional repressor Blimp1/Prdm1 plays a pivotal role in the metabolic switch that occurs in the small intestine during the suckling to weaning transition. Notably, expression profiling of perinatal Blimp1-deficient small intestine revealed premature activation of metabolic genes normally restricted to post-weaning enterocytes. To further elucidate the function of Blimp1 in intestinal development, we engineered a novel Blimp1-eGFP-fusion knock-in mouse strain to perform ChIP-seq analysis. In addition to identifying which metabolic genes are direct Blimp1 targets, ChIP-seq analysis revealed a highly conserved Blimp1/Irf-1 overlapping sites that function to control MHC class I antigen processing during acquisition of neonatal tolerance in the first weeks after birth during early colonization of the intestinal tract by commensal microorganisms. Moreover, immunohistochemical analysis of human fetal intestine suggests that a BLIMP1/IRF-1 axis may also function in human intestinal epithelium development.
Published in the journal: . PLoS Genet 11(7): e32767. doi:10.1371/journal.pgen.1005375
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005375Summary
The transcriptional repressor Blimp1/Prdm1 plays a pivotal role in the metabolic switch that occurs in the small intestine during the suckling to weaning transition. Notably, expression profiling of perinatal Blimp1-deficient small intestine revealed premature activation of metabolic genes normally restricted to post-weaning enterocytes. To further elucidate the function of Blimp1 in intestinal development, we engineered a novel Blimp1-eGFP-fusion knock-in mouse strain to perform ChIP-seq analysis. In addition to identifying which metabolic genes are direct Blimp1 targets, ChIP-seq analysis revealed a highly conserved Blimp1/Irf-1 overlapping sites that function to control MHC class I antigen processing during acquisition of neonatal tolerance in the first weeks after birth during early colonization of the intestinal tract by commensal microorganisms. Moreover, immunohistochemical analysis of human fetal intestine suggests that a BLIMP1/IRF-1 axis may also function in human intestinal epithelium development.
Introduction
The zinc finger transcriptional repressor Blimp1 originally cloned as a negative regulator of beta-interferon gene expression [1] is known to control cell fate decisions in the developing embryo and adult organism [2]. Blimp1 acting as a master regulator of plasma cell differentiation directly represses expression of key transcription factor genes such as c-Myc, Id3, CIITA and PAX5 required for B-lymphocyte function and proliferation [3,4]. In the early embryo, Blimp1 silences the default somatic pathway and instructs a discrete subset of epiblast cells exposed to the highest levels of BMP4 signaling at the base of the allantois to become primordial germ cells (PGC) [5,6]. At later stages, Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, secondary heart field, and sensory vibrissae [7]. Blimp1 also plays an essential role in placental morphogenesis, governing terminal differentiation of the invasive spiral artery-associated trophoblast giant cells [8].
Considerable data strongly suggests that Blimp1 transcriptional targets are cell type-specific. For example Blimp1 directly represses c-Myc expression to block proliferation in B cells, macrophages, and sebaceous gland progenitors [4,9,10]. In contrast however c-Myc is not a key target in activated effector T cells. Rather Blimp1 blocks IL-2 production required for T cell proliferation [11,12]. Within the CD4+ T-cell lineage Blimp1 selectively attenuates Th1 subset development by extinguishing expression of IFNγ, Tbx21 and Bcl6 [13]. Nfat5, Fos, Dusp16 and Prdm1 itself are direct targets in the skin epidermis [14]. Recent ChIP-seq experiments analyzing transfected P19 embryonic carcinoma (EC) cells demonstrate that Blimp1 directly represses numerous developmental and somatic regulators [15]. Cooperative binding with AP2γ and Prdm14 dramatically shifts gene expression profiles and initiates the transcriptional programme required for PGC specification [15].
Blimp1 is strongly expressed in the intestinal epithelium throughout fetal development but beginning at birth becomes dramatically down-regulated in the crypt progenitors, corresponding to the adult intestinal stem cell compartment [7,16,17]. Conditional deletion experiments revealed an essential role in governing postnatal reprogramming of intestinal enterocytes during the suckling to weaning transition [16,18]. Transcriptional profiling experiments demonstrate Blimp1 functional loss results in global changes in gene expression patterns. Thus numerous immature enterocyte markers including digestive enzymes required for processing maternal milk were markedly reduced, whereas in contrast several key components of the adult biochemical signature were substantially and prematurely activated [16].
To further investigate Blimp1 functional contributions during this developmental transition and identify its direct targets in immature intestinal enterocytes, we created a knock-in allele engineered to express an enhanced green fluorescent protein (eGFP)-tagged fusion protein reactive with the well-characterized ChIP quality anti-GFP monoclonal antibody. Here we demonstrate that the fusion protein faithfully reconstitutes Blimp1-dependent functional activities. Thus homozygous embryos exclusively expressing the eGFP-tagged Blimp1-fusion protein develop normally, and healthy homozygous adults recovered at the predicted Mendelian ratios were indistinguishable from wild type littermates. Moreover, the knock-in allele efficiently rescues Blimp1-dependent plasma cell differentiation. EGFP-tagged Blimp1 was strongly expressed in the developing intestine allowing us to undertake unbiased ChIP-seq analysis. Several candidate target genes strongly up-regulated in conditional loss mutants were identified as direct targets including Cyp4v3, Slc16a5 and Myo18b. Interestingly, comparisons of our ChIP-seq peaks with those reported for P19 EC cell and human HeLa cell datasets revealed several highly conserved genomic targets, including key components of the MHC class I peptide-loading pathway [19].
SELEX experiments revealed that the Blimp1 consensus-binding motif closely resembles the IRF-E sequence [20] recognized by IRF1, an activator of β-interferon gene expression [21–23]. Recent studies suggest that competitive BLIMP1 and IRF1 binding regulates expression of IFN-inducible components of the MHC class I peptide loading machinery [24]. Here we performed genome-wide ChIP-seq analysis to define the extent of overlap between Blimp1 and Irf1 occupancy in vivo under physiological conditions in the intestinal epithelium. We found overlapping Blimp1/Irf1 binding sites proximal to the promoters of 24% (24 of 99) of human/mouse conserved Blimp1 target genes including Psmb8, Psmb10, Psme1, Tapbp and Erap1. These findings strengthen the idea that Blimp1 occupancy directly antagonizes Irf1-dependent activation of MHC class I antigen presentation. Consistent with this suggestion we demonstrate that Irf1 is constitutively expressed in the fetal intestine and throughout postnatal stages. The onset of MHC class I expression in the developing intestine precisely coincides with down-regulated Blimp1 expression and the appearance of crypt-derived Blimp1-negative adult enterocytes during the suckling to weaning transition. Besides its role in governing the switch to adult metabolic pathways, Blimp1 co-occupancy at these Irf1-target genes promotes neonatal tolerance in the first weeks after birth during early colonization of the intestinal tract by commensal microorganisms.
Results
The eGFP-tagged Blimp1 knock-in allele rescues plasma cell differentiation and is efficiently expressed in the developing placenta and embryonic small intestine
To enable identification of Blimp1 target genes in diverse embryonic and adult tissues in vivo under physiological conditions, we engineered a novel eGFP-tagged Blimp1 knock-in allele Prdm1BEG by introducing the cDNA expression cassette into the first coding exon (Fig 1A). This strategy, used successfully for construction of a Prdm1 cre LacZ reporter allele, preserves all known regulatory and structural features of the endogenous locus [8]. Consistent with this homozygous embryos exclusively expressing the Blimp1-eGFP fusion protein develop normally. Additionally, healthy weanlings recovered from intercross matings at the predicted Mendelian ratios, were indistinguishable from wild type littermates (Fig 1B) and display no signs of disease when housed in a specific pathogen free environment.
Fig. 1. The eGFP-tagged Blimp1 knock-in allele efficiently rescues plasma cell differentiation. 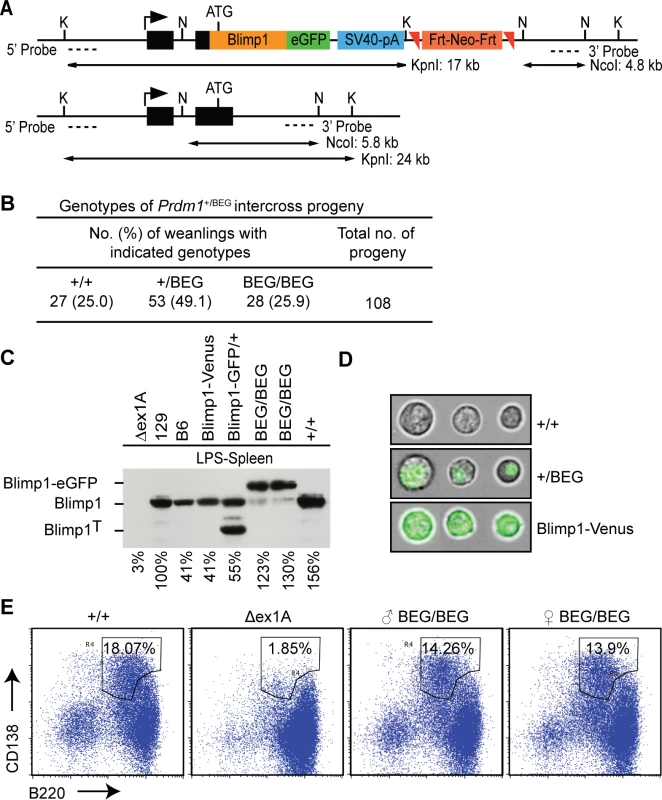
(A) Schematic representation of the targeting vector, wild type locus, and Southern blot screening strategy. (B) Intercross matings of heterozygous BEG animals generate Mendelian ratios of wild type, heterozygous and homozygous mutant progeny. (C) Western blot analysis demonstrates the eGFP-Blimp1 fusion protein is robustly induced in LPS-treated splenocytes. Levels of Blimp1 protein were quantified relative to the 129/SvEv wild type sample. Additional positive controls were wild type C57BL/6 and a BEG intercross littermate (+/+), heterozygous Blimp1-Venus BAC transgene (Blimp1-Venus) [25] or Blimp1-IRES-GFP (Blimp1-GFP/+) [26] reporter strains. Prdm1ΔEx1A splenocytes lacking the ability to generate plasma cells were included as a negative control [27]. (D) Imagestream analysis of LPS-treated splenocytes reveals nuclear localization of the Blimp1-eGFP fusion protein in contrast to the cytoplasmic/plasma membrane localization of Venus expressed under the control of the Blimp1 BAC-transgene. (E) Homozygous mutant B cells that exclusively express the eGFP-tagged Blimp1-fusion protein efficiently undergo plasma cell (CD138+B220+) terminal differentiation. Western blot experiments demonstrate the eGFP-tagged fusion protein is strongly expressed in LPS-stimulated splenocytes (Fig 1C). The eGFP-fusion protein efficiently translocates to the nucleus (Fig 1D) and rescues plasma cell differentiation (Fig 1E). Robust expression in the spongiotrophoblast and invading spiral artery trophoblast cells in E9.5 Prdm1BEG/BEG placenta faithfully reconstitutes Blimp1 functional requirements (Fig 2A)[8]. Western blot analysis demonstrates robust expression in the embryonic small intestine (Fig 2B). Immunohistochemical staining confirmed intestinal expression is restricted to the villus epithelium (Fig 2C). Small intestine tissue architecture and body weights were indistinguishable from wild type littermates at all stages examined.
Fig. 2. The eGFP-tagged Blimp1-fusion protein is efficiently expressed in the developing placenta and embryonic small intestine, but fails to rescue germ cell defects. 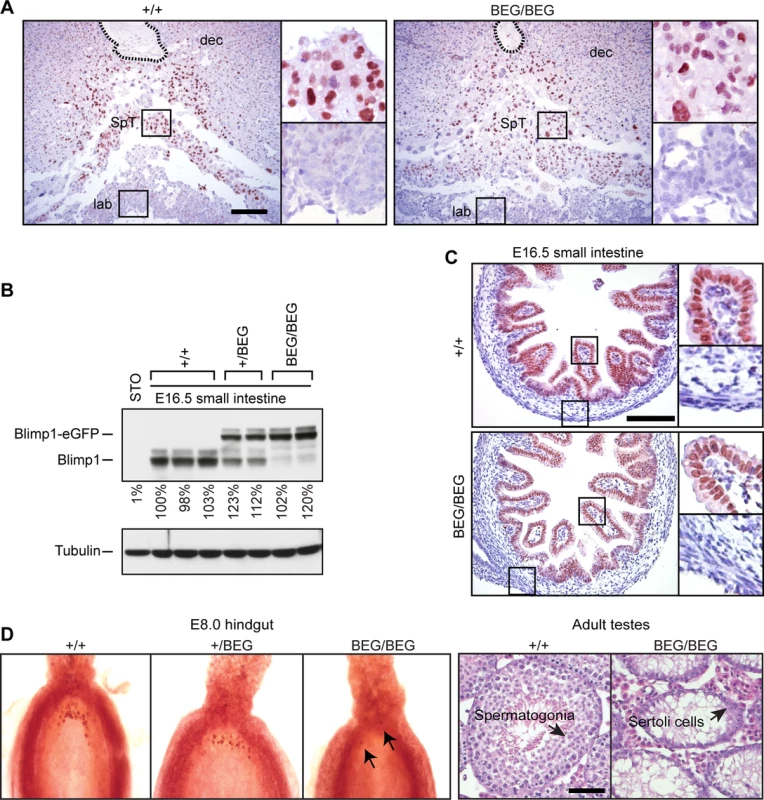
(A) Immunohistochemical staining of E9.5 placentae demonstrates the eGFP-tagged Blimp1-fusion protein is correctly expressed in the spongiotrophoblast layer. The position of the central maternal artery is outlined. Bar, 200 μm. SpT, spongiotrophoblast; dec, maternal decidua; lab, labyrinth trophoblast. (B) Western blot analysis demonstrates robust expression in E16.5 small intestine. RIPA lysates of STO fibroblasts are included as a negative control. Levels of Blimp1 expression were quantified relative to the first wild type littermate. (C) Immunohistochemical staining demonstrates nuclear expression of both endogenous and eGFP-tagged Blimp1 protein in E16.5 villus epithelium. Bar, 200 μm. (D) The eGFP-tagged Blimp1 knock-in allele fails to reconstitute germ cell formation. Fast red alkaline phosphatase staining at E7.5 demonstrates markedly reduced numbers of PGCs (black arrows). Hematoxylin- and eosin-stained sections confirm that adult homozygous BEG/BEG testes lack spermatocytes. Bar, 200 μm. Strikingly however, in contrast to mice carrying the exon 1A deletion with modestly reduced expression levels [27], here we found that healthy adult homozygous animals were sterile. Furthermore, as judged by fast red alkaline phosphatase staining, embryos exclusively expressing the knock-in allele have greatly diminished numbers of PGCs and adult testes lack spermatocytes (Fig 2D). These results demonstrate that the knock-in allele lacks the ability to induce the germ cell transcriptional programme. It is of course possible that functionality of the eGFP-tagged fusion protein is selectively compromised in PGCs due to its inability to recruit the entire cohort of epigenetic partners necessary for silencing of the default somatic pathway. On the other hand, Blimp1 functional requirements in the germ cell lineage are known to be exquisitely dose-dependent [5,6]. In all likelihood the failure to rescue germ cell defects simply reflects inadequate expression levels.
To identify Blimp1 targets in the developing intestine a well-characterized anti-GFP mouse monoclonal antibody [15,28] was exploited for ChIP-seq analysis. We identified 2689 Blimp1 binding events in embryonic (E18.5) small intestine (S1 Dataset). Blimp1 binding proximal to selected target genes was validated by ChIP-qPCR (S1 Fig). Analysis of peak locations relative to gene annotations revealed a broad distribution of Blimp1 binding throughout the genome with a bias towards regions proximal to TSSs (Fig 3A and 3B). A comparable number of ChIP-seq peaks (n = 3018) were recently documented in transfected P19 embryonic carcinoma cells over-expressing the identical eGFP-tagged Blimp1 fusion protein [15]. De novo motif analysis of sequences underlying all 2689 Blimp1 peaks revealed a highly significant consensus DNA binding motif (Fig 3C) that closely resembles the canonical Blimp1 binding motif originally identified in SELEX experiments [20], as well as that identified via ChIP-on-Chip analysis of a human myeloma cell line [29].
Fig. 3. ChIP-seq analysis of genome-wide Blimp1 binding sites in E18.5 small intestine. 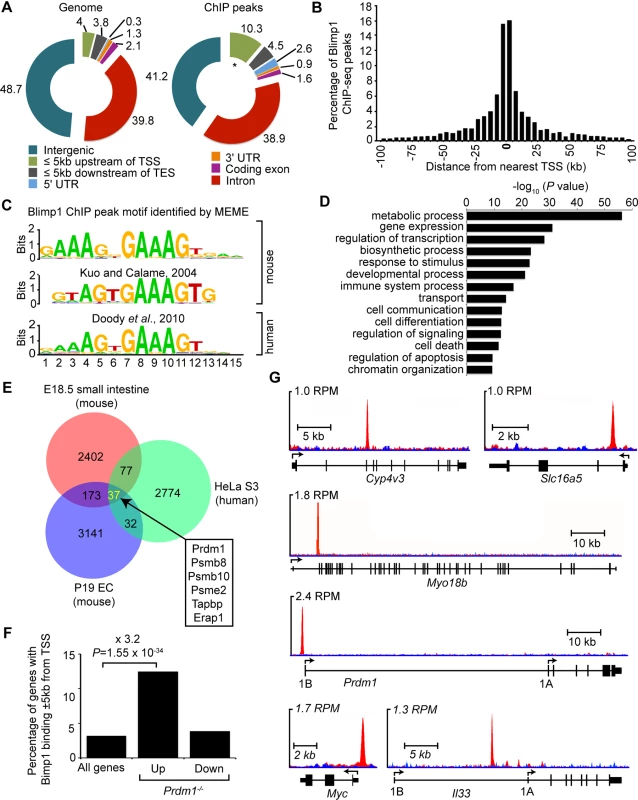
(A) Distribution of all Blimp1 peak locations in comparison to whole genome at defined genomic regions. * P = 2.5 x 10−45 in comparison to whole genome for the same region. (B) The distance of each Blimp1 ChIP-seq peak from the nearest TSS binned at 5 kb intervals. (C) De novo motif analysis (MEME) identifies significant enrichment of a consensus Blimp1 binding motif underlying Blimp1 peaks (n = 2689, MEME E value = 4.7 x 10−712) that closely resembles previously reported mouse (STAMP E value = 3.4 x 10−7) and human BLIMP1 motifs (TOMTOM P value = 2.8 x 10−9). (D) Functional annotation analysis using GREAT reveals Blimp1 preferentially binds to promoter regions upstream of genes associated with metabolism, transcription, developmental and immune processes. (E) A subset of overlapping Blimp1 peaks shared with previously reported mouse and human ChIP-seq datasets. (F) Up-regulated genes in conditional mutants are significantly (P = 1.1 x 10−22) enriched for Blimp1 binding (+/- 5 kb of TSS) relative to all genes on the array. (G) UCSC track view of ChIP (red) and input (blue) wiggle plot overlays showing enrichment of GFP ChIP-seq density in Prdm1BEG/BEG embryonic small intestine proximal to the promoters of Cyp4v3, Slc16a5, Myo18b, Prdm1, Myc and Il33. Blimp1 occupies the proximal promoters of a subset of highly conserved core genomic targets
Functional annotation of genes with proximal Blimp1 binding revealed significant enrichment of GO terms associated with metabolic process and transcriptional regulation (Fig 3D). Comparisons with ChIP-seq peaks recently identified in transfected embryonic carcinoma cells [15] revealed a small subset of overlapping peaks corresponding to roughly 7–8% of each dataset (Fig 3E). The majority (171 out of 210) fall within 5 kb of annotated gene TSSs. Additionally, comparisons with peaks identified in human HeLa S3 cells [30] revealed 114 highly conserved core Blimp1 genomic targets, including 35 genes (37 peaks) universally present within all three datasets (Fig 3E and S2 Dataset). Interestingly, IFN-inducibility is a common feature shared by several of these components of the MHC class 1 antigen processing machinery i.e. Psmb8, Psmb10, Psme2, Tapbp and Erap1 [19,31].
Numerous up-regulated genes previous identified in our transcriptional profiling experiments contain predicted Blimp1 binding sites proximal to their promoter regions [16]. To evaluate whether these candidates are direct Blimp1 target genes, we compared the list of significantly altered genes (P<0.05 with Benjamini and Hochberg multiple testing correction) with the present ChIP-seq Blimp1 binding events. We observed significant enrichment of Blimp1 binding sites proximal to up-regulated genes (Fig 3F). In contrast, there was no significant overlap between genes with proximal Blimp1 binding and those having decreased expression in conditional mutants. Genes with markedly increased expression that show proximal Blimp1 binding (S3 Dataset) include Cyp4v2, Slc15a5, Myo18b and Il33 (increased 27.7, 16.1 16.5 and 4.6 fold in mutants, respectively, Fig 3G). Notably, as judged by these criteria, Il33 expressed at high levels in epithelial barrier tissues and believed to play a key role in amplifying innate immunity [32,33], is a direct Blimp1 target. Surprisingly Sucrase isomaltase (Sis) (365.2 fold increased in mutants) predicted in silico to contain multiple Blimp1 binding sites proximal to its promoter [16] lacks occupancy. Similarly, Arginase type 2 (Arg2) (174.5 fold increased in mutants) gave no detectable ChIP-seq peak. In vivo in the context of the E18.5 embryonic intestine, compacted chromatin near genomic regions surrounding these potential target sites appears to be inaccessible. We observed robust Blimp1 binding proximal to the c-Myc P1 promoter (Fig 3G). However, as previously reported expression is not significantly altered in Prdm1-/- small intestine [16,18]. Indeed, the vast majority of binding events detectable in the present ChIP-seq experiments play no obvious role in Blimp1-dependent transcriptional regulation, and rather seem to reflect so-called neutral occupancy [34].
Blimp1 transcripts are significantly up-regulated in conditional null Prdm1-/- small intestine [16]. Similarly in mouse keratinocytes an intronic Blimp1 binding site downstream of exon 3 mediates a negative feedback loop [14]. However here we failed to detect occupancy at the intronic site described by Magnusdottir et al. [14]. Rather we observe robust binding at the alternative distal 1B promoter region (Fig 3G)[27] that drives expression in the yolk sac endoderm [27] and embryonic intestine (S2 Fig). This highly conserved ChIP-seq peak was universally present in both mouse and human datasets [15,30]. Interestingly the sea urchin Blimp1 orthologue efficiently binds to the conserved Blimp1 consensus motif [24] and a negative autoregulatory loop upstream of its alternative promoter has been implicated in the specification of endomesodermal territories [35]. Collectively these observations suggest that Blimp1 itself is a direct target of repression but mechanistic details may be cell-type specific.
Irf1 is constitutively expressed in the developing small intestine
It has been known for many years that the Blimp1 consensus binding motif shares a high degree of sequence overlap with IRF-E core sequence containing GAAAGT/C or GAAACT/C [20,36]. We also observed here that the IRF1 DNA binding motif was the second most significant match (P = 2.8 x 10−8) after Blimp1 to the de novo identified consensus DNA binding motif underlying all Blimp1 ChIP-seq peaks (S3 Fig). Irf1 expression has been previously documented in purified PGCs [37]. Similarly here, as shown in Fig 4A, we observe weak Irf1 staining in migrating PGCs and the pseudo-stratified epithelium of the primitive gut tube as early as E10.5. Western blot analysis demonstrates Irf1 abundantly expressed in the developing intestine at E16.5 (Fig 4B) is maintained during the time frame when Blimp1 becomes down regulated between postnatal day 7 and 28 [16]. Strong nuclear staining is readily detectable in both the villus epithelium and the crypts at postnatal stages (Fig 4C). Data from the online EurExpress RNA in situ hybridization (ISH) database [38] indicates that Irf1 transcripts are present in the developing gut tube as early as E14.5 prior to villus formation (Fig 4D). Irf1 is also constitutively expressed in the developing thymus. Transcriptional profiling data (Small intestine postnatal development, GEO accession number GDS2989) [39] demonstrates high levels of Irf1 and Prdm1, but importantly the closely related family member Irf2, known to bind the same IRF-E consensus sequence as Irf1, is only minimally co-expressed in the intestine (Fig 4E)[20,36,40,41].
Fig. 4. Irf1 is constitutively expressed in the developing thymus and small intestine. 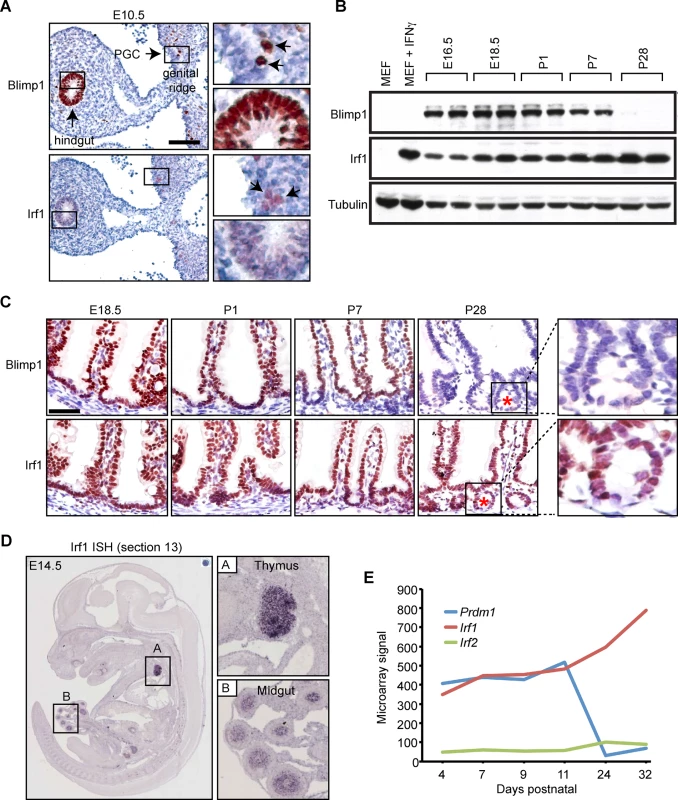
(A) Immunohistochemical staining reveals weak Irf1 expression in the pseudo-stratified epithelium of the developing gut tube and PGCs (black arrows) closely overlaps with robust Blimp1 expression. Bar, 100 μm. (B) Western blot analysis demonstrates Irf1 constitutively expressed in E16.5 intestine and throughout post-natal stages. In contrast, Blimp1 expression becomes down-regulated during the suckling to weaning transition. (C) Irf1 and Blimp1 nuclear staining in the villus epithelium from E18.5 to early postnatal stages (P7). Irf1 expression maintained in villus epithelium at post-weaning stages is readily detectable in the developing crypts of Lieberkuhn (indicated by red asterisk). Bar, 50 μm. (D) ISH analysis reveals Irf1 robustly expressed in the developing thymus and midgut at E14.5 (data from EurExpress) [38]. (E) Microarray expression data (GEO accession number GDS2989) reveals abundant levels of Irf1, but not Irf2 transcripts in the early postnatal small intestine are up-regulated at later stages when Blimp1 expression becomes extinguished. Binding of Irf1 and Blimp1 overlaps at the proximal promoters of antigen processing genes
Next to directly identify Irf1 targets in the E18.5 small intestine we performed ChIP-seq analysis. We identified 1996 binding events (S4 Dataset) broadly distributed throughout the genome displaying a bias towards regions proximal to TSSs (S4A and S4B Fig) [42]. Functional annotation revealed significant enrichment for GO terms associated with immune function (Fig 5A) and antigen recognition (Fig 5E). Comparison of our Irf1 ChIP seq peak dataset with genomic coordinates previously reported for mouse LPS-treated BMDC [42] revealed a high degree of conservation (Fig 5B). Nearly half (48%) of our Irf1 peaks display a corresponding peak in the previously reported dataset (31% overlap). The de novo Irf1 DNA binding motif identified here and that described by Garber et al. [42] were indistinguishable (Fig 5C) and closely matches those described for IRF1 in primary human monocytes (P = 3.3 x 10−11) [43], Blimp1 (P = 3.4 x 10−6) as well as IRF2 (P = 5.1 x 10−6). Roughly 33% IRF1/BLIMP1 overlap was predicted in human myeloma cells [29], whereas here Irf1 and Blimp1 ChIP-seq peaks show approximately 12% (n = 331, S5 Dataset) overlap.
Fig. 5. ChIP-seq analysis of genome-wide Irf1 binding sites in E18.5 small intestine. 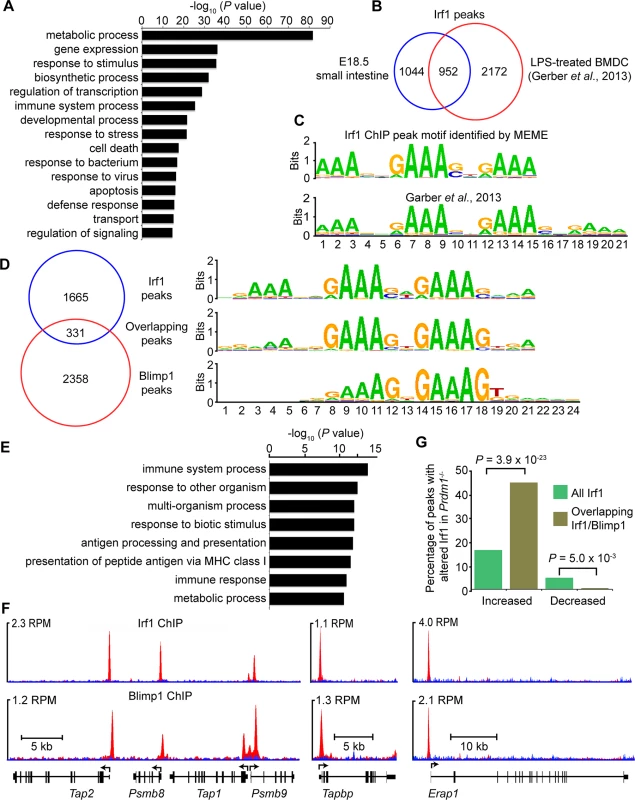
(A) Functional annotation analysis of genes bound by Irf1. (B) Overlapping Irf1 peaks between those identified in the present study and a mouse LPS-BMDC ChIP-seq dataset. (C) De novo motif analysis identifies significant enrichment of the consensus IRF1 binding motif underlying Irf1 peaks (n = 1996, MEME E value 1.9 x 10−802) that closely resembles the motif identified by Garber et al. [42] (STAMP E value = 0). (D) Comparison of our Irf1 and Blimp1 ChIP-seq datasets identifies a subset of overlapping peaks that selectively display the IRF1 motif. (E) Functional annotation analysis of genes bound by both Irf1 and Blimp1 demonstrates enrichment of immune response and antigen processing genes. (F) UCSC track view of ChIP (red) and input (blue) Wiggle plots. Both Irf1 and Blimp1 ChIP-seq peaks were present proximal to the promoters of Psmb8, Psmb9, Tap1, Tap2, Tapbp and Erap1. Positions of the TSS and direction of transcription are indicated by the arrows. (G) Overlapping Irf1 and Blimp1 peaks near a subset of Irf1 target genes display increased Irf1 occupancy in the absence of Blimp1 competition. Functional annotation of the 331 overlapping Irf1/Blimp1 ChIP-seq peaks using GREAT revealed significant enrichment of GO terms associated with MHC class I antigen processing (Fig 5E). To evaluate competitive Blimp1 and Irf1 binding we compared the number of reads underlying Irf1 peaks in Blimp1 mutant versus wild type small intestines. Mutants displayed a significant preference for increased Irf1 occupancy at overlapping Blimp1/Irf1 sites (Fig 5G and S5 Dataset). Conversely, overlapping regions were significantly less likely to show reduced Irf1 occupancy in Blimp1 mutants.
The onset of MHC class I expression coincides with down-regulated Blimp1 expression in the developing intestine
To directly examine MHC class I expression in the developing small intestine, we performed Western blot experiments. Irf1 is strongly expressed at E16.5 and throughout postnatal intestinal development (Fig 6A). In contrast Blimp1 expression is completely lost by P28, co-incident with the onset of MHC class I expression. Immunohistochemistry similarly demonstrate MHC class I expression, readily detectable in embryonic Peyer’s patches at E18.5, increases dramatically by P28 (Fig 6B and 6C). These findings strongly suggest that Blimp1 repression of IFN-dependent components of the MHC class I peptide-loading pathway plays a key role in restraining premature activation of host immune responses in utero and early postnatal stages.
Fig. 6. The onset of MHC class I expression coincides with down-regulated Blimp1 expression during the suckling to weaning transition. 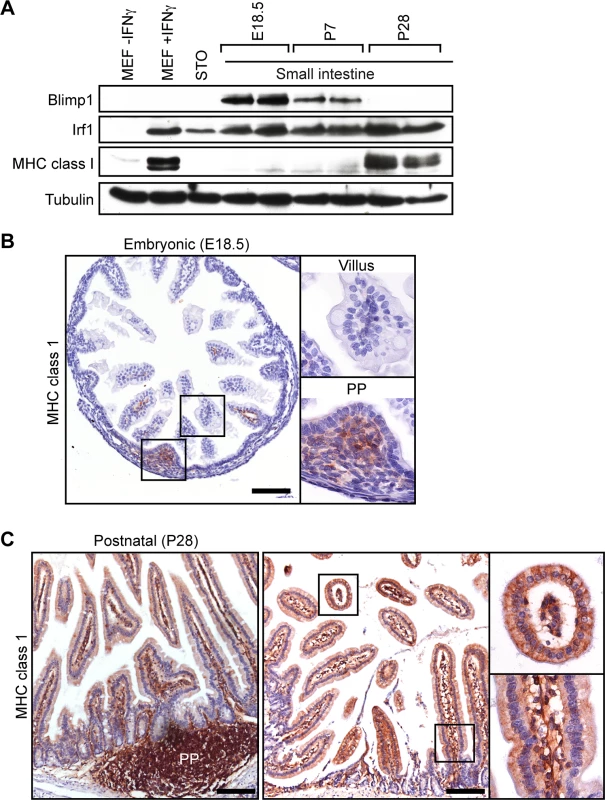
(A) Western Blot analysis reveals down-regulated Blimp1 and slightly increased Irf1 co-incident with the onset MHC class I expression detectable at post weaning stages. As a positive control Irf1 and MHC class I were strongly induced in IFNγ-treated MEFs. (B) Immunohistochemical analysis demonstrates MHC class I expression initially appears in immature Peyer’s patches (PP). Bar, 200 μm. (C) MHC class I is robustly expressed in post-natal villus epithelium (P28), as well as mature Peyer’s patches and villus mesenchyme. Bar, 200 μm. The ability of Blimp1 to mediate gene silencing and re-organize the chromatin landscape at its target genes depends on recruitment of histone-modifying enzymes [2]. Recent work suggests that postnatal maturation of the intestinal epithelium during the suckling to weaning transition is accompanied by global changes in the epigenetic machinery [44]. Decreased Hdac1 and Hdac2 expression was associated with a modest reduction in histone acetylation. However, protein stability may be compromised due to increased levels of digestive enzymatic activities during intestinal maturation. In snap frozen samples directly lysed in SDS sample buffer we observed consistently high levels of Blimp1 epigenetic partners including Lsd-1, G9a, Hdac1 and Hdac2 throughout the postnatal period (S5 Fig). Thus dramatic changes in transcriptional profiles during postnatal maturation of the intestinal epithelium cannot simply be explained due to developmentally regulated shifts in the composition of co-repressor complexes.
Co-expression of BLIMP1 and IRF1 in human fetal intestine reveals a potential conserved functional role
Human placenta architecture, the duration of pregnancy, suckling behaviors and time frames of intestinal maturation have been adapted to fit the lifestyles of different mammalian species. In mice, crypt progenitors emerge from the inter-villous epithelium only after birth. Migration into the underlying mesenchyme leads to the formation of mature crypts and crypt-derived Blimp1-negative adult enterocytes gradually repopulate the intestinal epithelium during the suckling to weaning transition [16]. By contrast in humans overt cryptogenesis begins much earlier. Crypt-like structures initially appear in the small intestine in utero at approximately 12 weeks [45]. Considerable evidence suggests the gut epithelium retains its immature status throughout fetal development. Consistent with this human fetal enterocytes lack ARG2 expression [46]. Additionally a human H4 fetal intestinal enterocyte cell line co-expresses PRDM1 and very low levels of ARG2 and SIS [47].
As shown in Fig 7A, immunohistochemistry demonstrate that BLIMP1 is constitutively expressed throughout the early epithelium at the pseudostratified stage and persists in the forming villous structures at 15 weeks of gestation (Fig 7B). In contrast IRF1 expression is undetectable in the gut epithelium prior to villus formation (Fig 7C). Slightly later at 15 weeks we detect IRF1 co-expressed together with BLIMP1 (Fig 7D). In humans BLIMP1 expression in crypt-like structures at late gestation pregnancy is probably required for continued production of immature enterocytes.
Fig. 7. BLIMP1 and IRF1 are co-expressed in fetal human intestine. 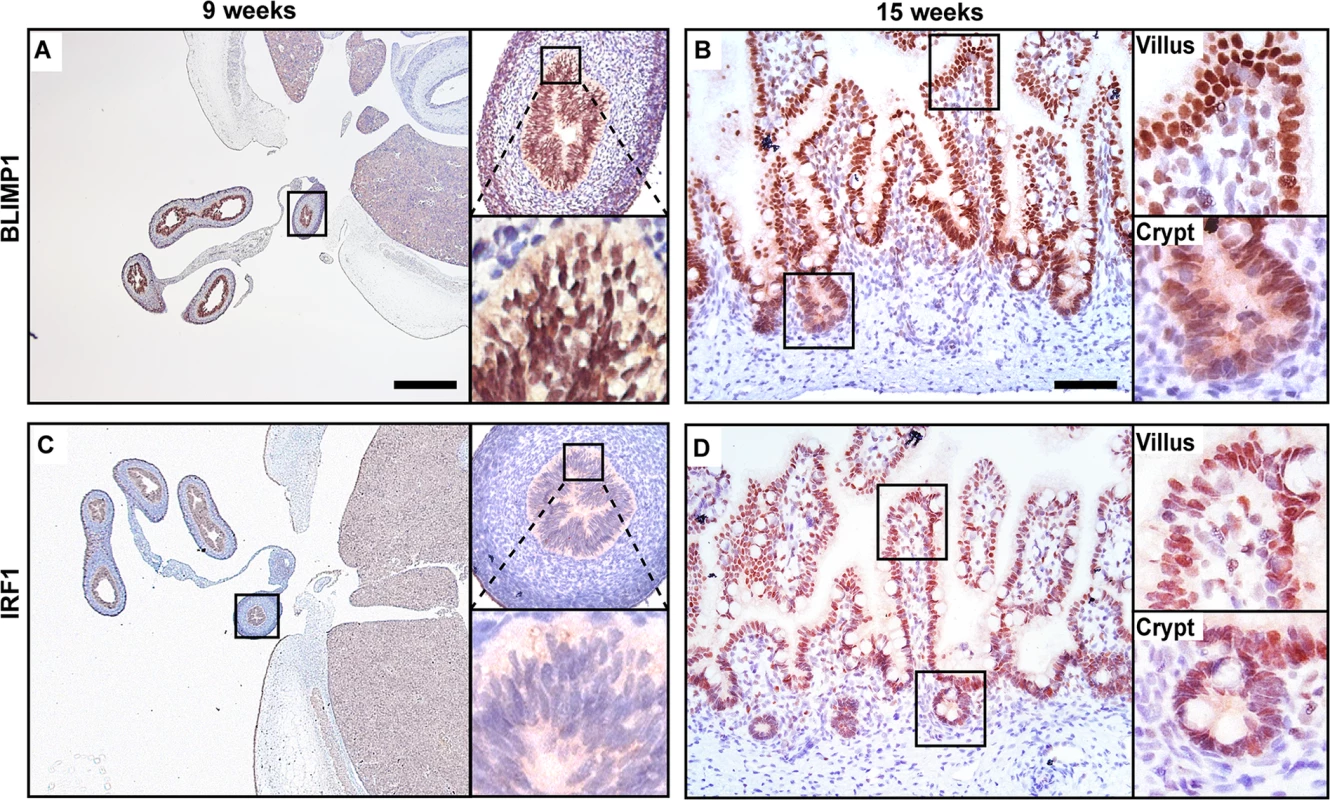
Nuclear BLIMP1 staining detected in the pseudostratified epithelium of the developing gut tube at (A) 9 weeks of gestation persists in (B) the villus epithelium including the immature crypts of Lieberkuhn at 15 weeks of gestation. (C) Although not detected in the gut tube prior to villus formation nuclear IRF1 staining is present in the villus epithelium at (D) 15 weeks of gestation. (A, C) Bar, 800 μm, (B, D) Bar 200 μm. Discussion
Target gene expression controlled by the zinc finger transcriptional repressor Blimp1/Prdm1 has been extensively characterized in the context of B-cell terminal differentiation to become antibody secreting plasma cells, the development of diverse CD4+ T lymphocyte subsets [4,48,49] as well as cell fate decisions governing effector versus regulatory CD8+ T cell homeostasis [12,50–53]. In guiding responses towards diverse antigenic stimuli, Blimp1 selectively shifts gene expression profiles in these lineage-committed precursors to directly influence developmental choices and ensure maximally effective protective immunity.
It has been known for many years that the Blimp1 consensus motif closely overlaps with the IRF-E sequence recognized by IRF1/IRF2 [20,29]. The structure of IRF1 bound to DNA reveals contacts mediated by a conserved cluster of tryptophan repeats [54]. In contrast the first two C2H2 zinc fingers of Blimp1 are required for recognition of its consensus motif [55]. Nonetheless, remarkably these structurally diverse proteins have the ability to interact with the same DNA sequence upstream of important target gene promoters.
The present experiments reveal considerable overlap between Blimp1 and Irf1 ChIP-Seq peaks. When further analyzed using Weeder, we identified an IRF-E DNA binding motif GAAAGTGAAA [20] underlying a subset of the 331 overlapping Blimp1/Irf1 ChIP-seq peaks. Of these 7% (n = 22) failed to match the motif, 23% (n = 75) contained a single match, and 70% (n = 234) contained 2 or more IRF-E sequences. For example, the overlapping peak proximal to the Psmb10 promoter contained 8 motif matches. The Tap1 and Psmb8 bi-directional promoter also displays overlapping occupancy [56,57]. Closer inspection of the peak profiles reveals two closely adjacent Blimp1 peaks but only one overlaps with Irf1. The close arrangement of multiple adjacent consensus binding sites potentially allows co-occupancy of Blimp1 and Irf1 at these promoter regions. It is tempting to speculate that simultaneous binding of both repressor (Blimp1) and activator (Irf1) provides a rapid transcriptional switch mechanism.
Previous experiments demonstrate that MHC class I surface expression, absent at early embryonic stages of development is interferon-inducible [58,59]. Similarly temporal and spatially restricted Irf1 expression is tightly regulated in the early embryo [60]. Irf1 constitutively expressed in the developing thymus plays an essential role in positive and negative selection of the CD8+ T cell repertoire [57,61]. MHC class I-restricted CD8+ T lymphocytes are directed against peptides derived from cytosolic proteins degraded by the proteasome that are translocated across the ER membrane by the TAP1/TAP2 transporter, stabilized by interactions with the dedicated class I chaperone Tapasin, and edited by ER aminopeptidases [19]. T cell receptor (TCR) repertoire selection is thought to depend on affinity differences as developing T cells interact with self-peptide MHC complexes displayed by thymic antigen-presenting cells in the cortical and medullary compartments [62]. During inflammatory and anti-viral responses the MHC class I peptide loading pathway becomes dramatically up-regulated to activate host defenses [31]. Interestingly both the adult thymus and small intestine constitutively express so-called immunoproteasome subunits [63]. We demonstrate here that numerous IFN-inducible components of the MHC class I antigen presenting pathway are direct Blimp1 targets in the embryonic intestinal epithelium. Irf1 constitutively expressed throughout intestinal development is poised to activate the MHC class I pathway in mature enterocytes coincident with down-regulated Blimp1 expression. In the neonatal intestine however, Blimp1 antagonizes Irf1 target gene expression to prevent premature activation of the MHC class I peptide-loading machinery.
Besides its essential role in absorption of nutrients, the intestinal epithelium also function as a protective barrier to prevent infection. The formation of gut-associated lymphoid tissues including the Peyer’s patches is initiated in utero but maturation of the mucosal immune system is incomplete at birth. Neonatal immune tolerance during postnatal colonization by commensal bacteria is essential for the establishment of a well-balanced host-commensal relationship. It is widely accepted that maternal tolerance of the allogenic fetus is largely dependent on the absence of embryonic MHC class I expression [58,59]. The present experiments demonstrate that another key feature of mammalian development is competitive binding by Blimp1 and Irf1 at the promoters of IFN-inducible components of the MHC class I machinery in fetal intestine to guarantee immune tolerance during postnatal intestinal maturation. We also identify BLIMP1 expression in human fetal enterocytes, implicating a potentially conserved functional role in guiding neonatal intestinal maturation and metabolic adaption. Although obtaining healthy preterm and neonatal material presents a considerable obstacle, nonetheless our future studies will aim to learn more about how BLIMP1 functions in humans to coordinate neonatal immune tolerance and the developmental switch from immature to mature enterocyte transcriptional programmes.
Note added in proof. While this manuscript was under review, another genome wide Blimp-1 ChIP data set was published by Saitou and colleagues (Kurimoto, K., Yabuta. Y., Hayashi, K. Ohta, H., Kiyonari, H., Mitani, T, Moritoki, Y., Kohri, K., Kimura, H., Yamamoto, T., Katou, Y, Shirahige, K., and M. Saitou (2015) Quantitative dynamics of chromatin remodelling during germ cell specification from mouse embryonic stem cells. Cell Stem Cell)[64]. The authors similarly used a Blimp-1-eGFP fusion knock-in strategy, but in contrast to our study, the eGFP cassette was inserted at the N-terminus and the resulting homozygous mice are fertile. They focused exclusively on expression during in vitro PGC specification, so it remains unclear whether germ cell defects in our BEG allele may reflect compromised recruitment of epigenetic partners and/or marginally reduced expression levels. Remarkably despite cell type differences, we see that there is roughly 48% overlap between the Blimp-1 ChIP seq peaks identified here in small intestine and those identified in PGCs by Kurimoto et al. Importantly overlapping targets include Prdm1, Il33, Psmb9, Psmb10, Psmg4, Psme2, Tap1, Tap2, Tapbp, and Erap1.
Materials and Methods
Generation of the Blimp1-eGFP knock-in allele
For the 5’ homology region, a StuI-XbaI fragment was excised from a genomic subclone [6] and introduced into a modified pBluescriptII plasmid (Stratagene). To complete the 5’ homology region PCR (Platinum Pfx polymerase, Invitrogen) was performed using the primers Prdm1-KI-F: AGAAACCAGCGCTTCTGTTTTAGTACGCGGAGC and Prdm1-KI-R: GAGAGGCGCGCCGAGAACTAGTCTCTGCCAGTCCTTGAAACTTCACGGAGCC with the bacterial artificial chromosome bMQ-375h16 as template. The PCR product was digested (XbaI, AscI) and cloned to introduce SpeI and AscI (underlined) cloning sites into Prdm1 exon 3. The Blimp1-eGFP fusion construct plus SV40 polyadenylation signal [65] excised using XhoI-NotI was subcloned into a pBluscriptII based shuttle vector containing an upstream NheI site and downstream AscI site. This Blimp1-eGFP cDNA expression cassette was then ligated into SpeI and AscI sites in Prdm1 exon 3. Finally, a fragment containing a Frt flanked Neomycin resistance cassette [66], the MfeI-SphI 3’ homology region [6] and Hsv-TK cassette were inserted using AscI and PmeI restriction sites. The NotI linearized targeting vector was introduced into CCE embryonic stem (ES) cells by electroporation.
Southern blot screening was performed as described using the restriction enzyme and probe combinations shown in Fig 1A [27]. Southern probes were amplified by PCR using the following primers: 5'-Forward: GATAGGATCCTTTCCAGCTGTTACTATGTAGG, 5'-Reverse: GATACTCGAGCTTATGCTTCATAGTTAATTTGG, 3'-Forward: GATAGAATTCAATGCCATTTGTCAGGGAGC, 3'-Reverse: GATACTCGAGCTTTTGGCCACAGGACAATG. For excision of the Frt-Neo cassette, correctly targeted ES cell clones were transiently transfected with pCAGGS-FlpO (generously provided by Bill Skarnes) and subsequently screened using the 3' probe together with KpnI genomic digest.
Animals
Genotyping of mice carrying the novel eGFP knock-in (BEG) allele was performed with Forward primers BEG Mut (GTTATTGGCGTGGTAAGTAAGG) and WT (AGGCATCCTTACCAAGGAAC) and Reverse primers BEG Mut (ATTTATCACTGTGAGCTCTCCAG) and WT (GCTGAAGGGAGGAAGAAATG). The cycling conditions were 94°C for 20 s, 58°C for 30 s, and 72°C for 45 s for 35 cycles. Conditional mutants selectively lacking Blimp1 function in the developing intestine, generated by crossing Prdm1CA/CA animals to the Villin cre transgenic strain were genotyped as described [16]. The targeted deletion that selectively eliminates exon 1A promoter usage and disrupts Blimp1 dependent plasma cell differentiation as well as the BAC transgenic reporter strain expressing membrane targeted Venus under the control of the Blimp1 regulatory elements have been described [25,27]. All animal experiments were performed in accordance with Home Office (UK) regulations and were approved by the University of Oxford Local Ethical Committee.
Western blot analysis
Cell cultures to induce plasma cell differentiation and Western blot analysis of LPS-stimulated splenocytes were performed as described previously [65]. To insure quantitative protein recoveries, embryonic and postnatal intestinal tissues were flushed with PBS, snap frozen and immediately dissociated directly in SDS sample buffer. The primary antibodies were: rat monoclonal anti-Blimp1 (clone 5E7, SC-130917; Santa Cruz), rabbit polyclonal anti-Irf1 rabbit (M-20, Santa Cruz, SC-640), rat monoclonal anti-MHC class I (ER-HR52, SC-59199; Santa Cruz), hamster monoclonal anti-G9a (clone 14–1, D141-3; MBL), rabbit polyclonal anti-HDAC1 (AB7028; Abcam), rabbit polyclonal anti-HDAC2 (AB7029; Abcam), rabbit monoclonal anti-LSD1 (clone EPR6825, AB129195; Abcam), rabbit polyclonal anti-CoREST (07–455, Merck Millipore) and rabbit polyclonal anti-β-tubulin (SC-9104; Santa Cruz). Secondary antibodies were anti-mouse immunoglobulin (Ig)–horseradish peroxidase (HRP) (NA931V; GE Healthcare), anti-rat Ig—HRP (NA935V; GE Healthcare), anti-rabbit Ig—HRP (NA934V; GE Healthcare), or anti-Armenian hamster Ig—HRP (SC-2904; Santa Cruz). Levels of Blimp1-eGFP fusion protein relative to levels of endogenous Blimp1 protein were quantified on Western blots using a ChemiDoc XRS+ system and Image Lab software (BIO-RAD).
Immunofluorescence analysis
Cell staining experiments were performed as described [65] using a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (Tree Star). High-resolution images were captured using an ImageStreamX Mk II imaging flow cytometer (AMNIS) and analyzed using IDEAS software (AMNIS).
Histology and immunohistochemistry
Placental and intestinal tissue samples were fixed overnight in 4% paraformaldehyde (PFA) in PBS, dehydrated in ethanol, embedded in paraffin and sectioned (6 μm) Immunohistochemistry was performed as described [8]. Primary antibodies were rat monoclonal anti-Blimp1 (clone 5E7, SC-130917; Santa Cruz), or for human tissue rat monoclonal anti-Blimp1 (clone 6D3, 14-5963-82; eBioscience), rabbit monoclonal anti-Irf1 (8478; Cell signaling) or rat monoclonal anti-MHC class I (ER-HR52, SC-59199; Santa Cruz). Visualization of primordial germ cells, by staining for alkaline phosphatase was performed as described [67]. Testes samples were fixed overnight in Bouin’s fixative, dehydrated in ethanol, embedded in paraffin, sectioned (6 μm) and stained with hematoxylin and eosin.
ChIP-seq analysis
Small intestines were dissected, flushed with PBS, cut into small pieces and cross-linked with 1% formaldehyde in PBS for 15 min at 4°C followed by 35 min at 25°C [68] and subsequently processed for ChIP using either 10 μg of mouse anti-GFP IgG2a (clone 3E6, A11120; Invitrogen), polyclonal rabbit anti-Irf1 antibody (M-20, Santa Cruz, SC-640) or as a control, normal rabbit IgG (Santa Cruz, SC-2027) as described previously [28]. The DNA samples were multiplexed and sequenced using two lanes on an Illumina HiSeq 2000 sequencer. Duplicate test (GFP ChIP of Prdm1BEG/BEG, or Irf1 ChIP of WT and Villin-cre conditional Blimp1 mutants, [16]) samples or individual negative control (GFP ChIP of wild type or, normal rabbit IgG ChIP) and input samples were analyzed.
Sequence reads were mapped to the mm9 mouse genome release with Stampy using default parameters [69]. Peak calling was performed with MACS1.4.2 [70,71], using default parameters to call areas of enrichment in ChIP samples over input. Regions of enrichment detected in negative controls samples were removed from subsequent analysis. GFP ChIP peaks called in wild type samples were subtracted from GFP peaks called in BEG/BEG samples. Similarly, normal rabbit IgG ChIP peaks were subtracted from Irf1 peaks. The overlapping peaks in duplicate ChIP samples were then identified and the core region of overlap was further analyzed. The genomic distribution of ChIP-seq peaks compared to gene annotations was determined using CEAS [72]. Genes of Ensembl release 67 with proximal Blimp1 or Irf1 binding were identified using custom Perl scripts. De novo identification of motifs within ChIP-seq peaks was performed using MEME suite tools [73]. Functional annotation of ChIP-seq peaks was performed with GREAT version 2.0.2 using the basal plus extension rule, annotating genes within 5 kb of transcription start sites initially or within 25 kb when no proximal gene in known to exist [74]. Regions of overlap between the ChIP-seq peaks identified in present study with other published datasets were compared using custom Perl scripts. Peak regions in human datasets were first converted to mm9 using the UCSC liftOver function. The association between Blimp1 binding (± 5kb of TSS) and genes differentially expressed in embryonic Prdm1-/- small intestine [16] (Gene Expression Omnibus database, www.ncbi.nlm.nih.gov/geo, accession no. GSE29658) was calculated by chi-square test.
For comparative Irf1 ChIP-seq analysis reads mapping within identified peaks were counted using HTSeq-count [75]. TMM normalization factors and tagwise dispersions were then computed, and differential occupancy between sites in mutant and wild type determined by exact test using edgeR [76,77]. False discovery rates were calculated using the Benjamini and Hochberg method. Sites differentially bound with an FDR ≤ 0.05 were considered significant.
qPCR validation
QPCR analysis of triplicate GFP or control IgG ChIP, and input samples of Prdm1BEG/BEG and WT E18.5 intestine was performed using QuantiTech SYBR Green master mix (Q2040143; Qiagen) on a Rotor-Gene Q (Qiagen). Primers were designed to amplify 100–200 bp regions central to ChIP-seq peak genomic coordinates. Selected genes included c-Myc, Psmb8, Psmb9, Tap1, Tap2 and Tapbp [20,24] and several candidates with increased transcript levels in Blimp1 mutants i.e. Prdm1 (45.3 fold), Slc16a5 (16.1 fold), Gsta1 (16.8 fold), 2210407C18Rik (15.7 fold), Trib3 (2.9 fold) and Cyp4v3 (68.9 fold) [16]. A non-enriched ChIP-seq region in the 3’UTR of the Prdm1 gene was used as a negative control. Primer sequences are shown in S6 Dataset. Fold enrichment of ChIP over input was determined relative to a standard curve generated from log diluted sheared genomic DNA.
RT-PCR
Total RNA from yolk sac and embryonic small intestinal tissues was isolated using an RNeasy Mini kit (Qiagen), and reverse transcription-PCR (RT-PCR) was performed using the OneStep RT-PCR kit (Qiagen) as previously described [27]. Primers Ex1AFor and Ex1BFor in combination with Ex3Rev distinguish Prdm1 exon 1A and Prdm1 exon 1B transcripts whereas total Prdm1 transcripts were detected with primers Ex4For and Ex5Rev [27].
Accession numbers
The ChIP-seq data have been deposited in NCBI GEO with the accession number GSE66069.
Supporting Information
Zdroje
1. Keller AD, Maniatis T (1991) Identification and characterization of a novel repressor of beta-interferon gene expression. Genes Dev 5 : 868–879. 1851123
2. Bikoff EK, Morgan MA, Robertson EJ (2009) An expanding job description for Blimp-1/PRDM1. Curr Opin Genet Dev 19 : 379–385. doi: 10.1016/j.gde.2009.05.005 19592232
3. Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, et al. (2002) Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17 : 51–62. 12150891
4. Martins G, Calame K (2008) Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol 26 : 133–169. doi: 10.1146/annurev.immunol.26.021607.090241 18370921
5. Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, et al. (2005) Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436 : 207–213. 15937476
6. Vincent SD, Dunn NR, Sciammas R, Shapiro-Shalef M, Davis MM, et al. (2005) The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development 132 : 1315–1325. 15750184
7. Robertson EJ, Charatsi I, Joyner CJ, Koonce CH, Morgan M, et al. (2007) Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development 134 : 4335–4345. 18039967
8. Mould A, Morgan MA, Li L, Bikoff EK, Robertson EJ (2012) Blimp1/Prdm1 governs terminal differentiation of endovascular trophoblast giant cells and defines multipotent progenitors in the developing placenta. Genes Dev 26 : 2063–2074. doi: 10.1101/gad.199828.112 22987638
9. Lin Y, Wong K, Calame K (1997) Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science 276 : 596–599. 9110979
10. Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, et al. (2006) Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126 : 597–609. 16901790
11. Gong D, Malek TR (2007) Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol 178 : 242–252. 17182561
12. Martins GA, Cimmino L, Liao J, Magnusdottir E, Calame K (2008) Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J Exp Med 205 : 1959–1965. doi: 10.1084/jem.20080526 18725523
13. Cimmino L, Martins GA, Liao J, Magnusdottir E, Grunig G, et al. (2008) Blimp-1 attenuates Th1 differentiation by repression of ifng, tbx21, and bcl6 gene expression. J Immunol 181 : 2338–2347. 18684923
14. Magnusdottir E, Kalachikov S, Mizukoshi K, Savitsky D, Ishida-Yamamoto A, et al. (2007) Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc Natl Acad Sci U S A 104 : 14988–14993. 17846422
15. Magnusdottir E, Dietmann S, Murakami K, Gunesdogan U, Tang F, et al. (2013) A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat Cell Biol 15 : 905–915. doi: 10.1038/ncb2798 23851488
16. Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ (2011) The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A 108 : 10585–10590. doi: 10.1073/pnas.1105852108 21670299
17. Chang DH, Cattoretti G, Calame KL (2002) The dynamic expression pattern of B lymphocyte induced maturation protein-1 (Blimp-1) during mouse embryonic development. Mech Dev 117 : 305–309. 12204275
18. Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, et al. (2011) Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun 2 : 452. doi: 10.1038/ncomms1463 21878906
19. Blum JS, Wearsch PA, Cresswell P (2013) Pathways of antigen processing. Annu Rev Immunol 31 : 443–473. doi: 10.1146/annurev-immunol-032712-095910 23298205
20. Kuo TC, Calame KL (2004) B lymphocyte-induced maturation protein (Blimp)-1, IFN regulatory factor (IRF)-1, and IRF-2 can bind to the same regulatory sites. J Immunol 173 : 5556–5563. 15494505
21. Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, et al. (1988) Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell 54 : 903–913. 3409321
22. Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19 : 623–655. 11244049
23. Pine R, Decker T, Kessler DS, Levy DE, Darnell JE, Jr. (1990) Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon - and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol 10 : 2448–2457. 2342456
24. Doody GM, Stephenson S, McManamy C, Tooze RM (2007) PRDM1/BLIMP-1 modulates IFN-gamma-dependent control of the MHC class I antigen-processing and peptide-loading pathway. J Immunol 179 : 7614–7623. 18025207
25. Ohinata Y, Sano M, Shigeta M, Yamanaka K, Saitou M (2008) A comprehensive, non-invasive visualization of primordial germ cell development in mice by the Prdm1-mVenus and Dppa3-ECFP double transgenic reporter. Reproduction 136 : 503–514. doi: 10.1530/REP-08-0053 18583473
26. Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, et al. (2004) Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med 200 : 967–977. 15492122
27. Morgan MA, Magnusdottir E, Kuo TC, Tunyaplin C, Harper J, et al. (2009) Blimp-1/Prdm1 alternative promoter usage during mouse development and plasma cell differentiation. Mol Cell Biol 29 : 5813–5827. doi: 10.1128/MCB.00670-09 19737919
28. Bogani D, Morgan MA, Nelson AC, Costello I, McGouran JF, et al. (2013) The PR/SET domain zinc finger protein Prdm4 regulates gene expression in embryonic stem cells but plays a nonessential role in the developing mouse embryo. Mol Cell Biol 33 : 3936–3950. doi: 10.1128/MCB.00498-13 23918801
29. Doody GM, Care MA, Burgoyne NJ, Bradford JR, Bota M, et al. (2010) An extended set of PRDM1/BLIMP1 target genes links binding motif type to dynamic repression. Nucleic Acids Res 38 : 5336–5350. doi: 10.1093/nar/gkq268 20421211
30. Wang J, Zhuang J, Iyer S, Lin X, Whitfield TW, et al. (2012) Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res 22 : 1798–1812. doi: 10.1101/gr.139105.112 22955990
31. Strehl B, Seifert U, Kruger E, Heink S, Kuckelkorn U, et al. (2005) Interferon-gamma, the functional plasticity of the ubiquitin-proteasome system, and MHC class I antigen processing. Immunol Rev 207 : 19–30. 16181324
32. Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, et al. (2012) Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol 188 : 3488–3495. doi: 10.4049/jimmunol.1101977 22371395
33. Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, et al. (2010) IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A 107 : 18581–18586. doi: 10.1073/pnas.1003059107 20937871
34. Spivakov M (2014) Spurious transcription factor binding: non-functional or genetically redundant? Bioessays 36 : 798–806. doi: 10.1002/bies.201400036 24888900
35. Livi CB, Davidson EH (2006) Expression and function of blimp1/krox, an alternatively transcribed regulatory gene of the sea urchin endomesoderm network. Dev Biol 293 : 513–525. 16581059
36. Honda K, Takaoka A, Taniguchi T (2006) Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25 : 349–360. 16979567
37. Okamura D, Maeda I, Taniguchi H, Tokitake Y, Ikeda M, et al. (2012) Cell cycle gene-specific control of transcription has a critical role in proliferation of primordial germ cells. Genes Dev 26 : 2477–2482. doi: 10.1101/gad.202242.112 23154982
38. Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, et al. (2011) A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol 9: e1000582. doi: 10.1371/journal.pbio.1000582 21267068
39. Schjoldager KT, Maltesen HR, Balmer S, Lund LR, Claesson MH, et al. (2008) Cellular cross talk in the small intestinal mucosa: postnatal lymphocytic immigration elicits a specific epithelial transcriptional response. Am J Physiol Gastrointest Liver Physiol 294: G1335–1343. doi: 10.1152/ajpgi.00265.2007 18388184
40. Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, et al. (1989) Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 58 : 729–739. 2475256
41. Tanaka N, Kawakami T, Taniguchi T (1993) Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol Cell Biol 13 : 4531–4538. 7687740
42. Garber M, Yosef N, Goren A, Raychowdhury R, Thielke A, et al. (2012) A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol Cell 47 : 810–822. doi: 10.1016/j.molcel.2012.07.030 22940246
43. Shi L, Perin JC, Leipzig J, Zhang Z, Sullivan KE (2011) Genome-wide analysis of interferon regulatory factor I binding in primary human monocytes. Gene 487 : 21–28. doi: 10.1016/j.gene.2011.07.004 21803131
44. Tou L, Liu Q, Shivdasani RA (2004) Regulation of mammalian epithelial differentiation and intestine development by class I histone deacetylases. Mol Cell Biol 24 : 3132–3139. 15060137
45. Montgomery RK, Mulberg AE, Grand RJ (1999) Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 116 : 702–731. 10029630
46. Kohler ES, Sankaranarayanan S, van Ginneken CJ, van Dijk P, Vermeulen JL, et al. (2008) The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and - catabolizing enzymes. BMC Dev Biol 8 : 107. doi: 10.1186/1471-213X-8-107 19000307
47. Lu L, Li T, Williams G, Petit E, Borowsky M, et al. (2011) Hydrocortisone induces changes in gene expression and differentiation in immature human enterocytes. Am J Physiol Gastrointest Liver Physiol 300: G425–432. doi: 10.1152/ajpgi.00011.2010 21148402
48. Nutt SL, Fairfax KA, Kallies A (2007) BLIMP1 guides the fate of effector B and T cells. Nat Rev Immunol 7 : 923–927. 17965637
49. Crotty S, Johnston RJ, Schoenberger SP (2010) Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol 11 : 114–120. doi: 10.1038/ni.1837 20084069
50. Shin HM, Kapoor VN, Guan T, Kaech SM, Welsh RM, et al. (2013) Epigenetic modifications induced by Blimp-1 Regulate CD8(+) T cell memory progression during acute virus infection. Immunity 39 : 661–675. doi: 10.1016/j.immuni.2013.08.032 24120360
51. Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, et al. (2009) Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31 : 296–308. doi: 10.1016/j.immuni.2009.05.014 19664941
52. Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, et al. (2009) A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity 31 : 309–320. doi: 10.1016/j.immuni.2009.06.019 19664943
53. Kallies A, Xin A, Belz GT, Nutt SL (2009) Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity 31 : 283–295. doi: 10.1016/j.immuni.2009.06.021 19664942
54. Escalante CR, Yie J, Thanos D, Aggarwal AK (1998) Structure of IRF-1 with bound DNA reveals determinants of interferon regulation. Nature 391 : 103–106. 9422515
55. Keller AD, Maniatis T (1992) Only two of the five zinc fingers of the eukaryotic transcriptional repressor PRDI-BF1 are required for sequence-specific DNA binding. Mol Cell Biol 12 : 1940–1949. 1569931
56. Wright KL, White LC, Kelly A, Beck S, Trowsdale J, et al. (1995) Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J Exp Med 181 : 1459–1471. 7699330
57. White LC, Wright KL, Felix NJ, Ruffner H, Reis LF, et al. (1996) Regulation of LMP2 and TAP1 genes by IRF-1 explains the paucity of CD8+ T cells in IRF-1-/ - mice. Immunity 5 : 365–376. 8885869
58. Ozato K, Wan YJ, Orrison BM (1985) Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A 82 : 2427–2431. 2581247
59. Bikoff EK, Jaffe L, Ribaudo RK, Otten GR, Germain RN, et al. (1991) MHC class I surface expression in embryo-derived cell lines inducible with peptide or interferon. Nature 354 : 235–238. 1720508
60. Harada H, Willison K, Sakakibara J, Miyamoto M, Fujita T, et al. (1990) Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell 63 : 303–312. 2208287
61. Penninger JM, Sirard C, Mittrucker HW, Chidgey A, Kozieradzki I, et al. (1997) The interferon regulatory transcription factor IRF-1 controls positive and negative selection of CD8+ thymocytes. Immunity 7 : 243–254. 9285409
62. Klein L, Kyewski B, Allen PM, Hogquist KA (2014) Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol 14 : 377–391. doi: 10.1038/nri3667 24830344
63. Kuckelkorn U, Ruppert T, Strehl B, Jungblut PR, Zimny-Arndt U, et al. (2002) Link between organ-specific antigen processing by 20S proteasomes and CD8(+) T cell-mediated autoimmunity. J Exp Med 195 : 983–990. 11956289
64. Kurimoto K, Yabuta Y, Hayashi K, Ohta H, Kiyonari H, et al. (2015) Quantitative Dynamics of Chromatin Remodeling during Germ Cell Specification from Mouse Embryonic Stem Cells. Cell Stem Cell 16 : 517–532. doi: 10.1016/j.stem.2015.03.002 25800778
65. Morgan MA, Mould AW, Li L, Robertson EJ, Bikoff EK (2012) Alternative splicing regulates Prdm1/Blimp-1 DNA binding activities and corepressor interactions. Mol Cell Biol 32 : 3403–3413. doi: 10.1128/MCB.00174-12 22733990
66. Hoch RV, Soriano P (2006) Context-specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development 133 : 663–673. 16421190
67. Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, et al. (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13 : 424–436. 10049358
68. Verzi MP, Shin H, Ho LL, Liu XS, Shivdasani RA (2011) Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Mol Cell Biol 31 : 2026–2039. doi: 10.1128/MCB.01250-10 21402776
69. Lunter G, Goodson M (2011) Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res 21 : 936–939. doi: 10.1101/gr.111120.110 20980556
70. Feng J, Liu T, Qin B, Zhang Y, Liu XS (2012) Identifying ChIP-seq enrichment using MACS. Nat Protoc 7 : 1728–1740. doi: 10.1038/nprot.2012.101 22936215
71. Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137. doi: 10.1186/gb-2008-9-9-r137 18798982
72. Shin H, Liu T, Manrai AK, Liu XS (2009) CEAS: cis-regulatory element annotation system. Bioinformatics 25 : 2605–2606. doi: 10.1093/bioinformatics/btp479 19689956
73. Bailey TL, Boden M, Buske FA, Frith M, Grant CE, et al. (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37: W202–208. doi: 10.1093/nar/gkp335 19458158
74. McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, et al. (2010) GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28 : 495–501. doi: 10.1038/nbt.1630 20436461
75. Anders S, Pyl PT, Huber W (2015) HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 31 : 166–169. doi: 10.1093/bioinformatics/btu638 25260700
76. Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 : 139–140. doi: 10.1093/bioinformatics/btp616 19910308
77. Nikolayeva O, Robinson MD (2014) edgeR for differential RNA-seq and ChIP-seq analysis: an application to stem cell biology. Methods Mol Biol 1150 : 45–79. doi: 10.1007/978-1-4939-0512-6_3 24743990
Štítky
Genetika Reprodukční medicína
Článek Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density ImputationČlánek AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct MechanismsČlánek A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seqČlánek TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive HematopoiesisČlánek Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 7
-
Všechny články tohoto čísla
- LINE-1 Retroelements Get ZAPped!
- /p23: A Small Protein Heating Up Lifespan Regulation
- Hairless Streaks in Cattle Implicate TSR2 in Early Hair Follicle Formation
- Ribosomal Protein Mutations Result in Constitutive p53 Protein Degradation through Impairment of the AKT Pathway
- Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in
- BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma
- Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
- EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in
- Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins
- Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
- A Year of Infection in the Intensive Care Unit: Prospective Whole Genome Sequencing of Bacterial Clinical Isolates Reveals Cryptic Transmissions and Novel Microbiota
- Inference of Low and High-Grade Glioma Gene Regulatory Networks Delineates the Role of Rnd3 in Establishing Multiple Hallmarks of Cancer
- Novel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
- A Novel Locus Harbouring a Functional Nonsense Mutation Identified in a Large Danish Family with Nonsyndromic Hearing Impairment
- Checkpoint Activation of an Unconventional DNA Replication Program in
- A Genetic Incompatibility Accelerates Adaptation in Yeast
- The SMC Loader Scc2 Promotes ncRNA Biogenesis and Translational Fidelity
- Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
- Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation
- JAK/STAT and Hox Dynamic Interactions in an Organogenetic Gene Cascade
- Emergence, Retention and Selection: A Trilogy of Origination for Functional Proteins from Ancestral LncRNAs in Primates
- MoSET1 (Histone H3K4 Methyltransferase in ) Regulates Global Gene Expression during Infection-Related Morphogenesis
- Arabidopsis PCH2 Mediates Meiotic Chromosome Remodeling and Maturation of Crossovers
- AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
- A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seq
- Tempo and Mode of Transposable Element Activity in Drosophila
- The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres
- SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation
- A Genome Scan for Genes Underlying Microgeographic-Scale Local Adaptation in a Wild Species
- TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis
- Analysis of the Relationships between DNA Double-Strand Breaks, Synaptonemal Complex and Crossovers Using the Mutant
- Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools
- Allelic Spectra of Risk SNPs Are Different for Environment/Lifestyle Dependent versus Independent Diseases
- CSB-PGBD3 Mutations Cause Premature Ovarian Failure
- Irrepressible: An Interview with Mark Ptashne
- Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
- Inactivation of Retinoblastoma Protein (Rb1) in the Oocyte: Evidence That Dysregulated Follicle Growth Drives Ovarian Teratoma Formation in Mice
- Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
- Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA
- Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage
- RFX2 Is a Major Transcriptional Regulator of Spermiogenesis
- A Role for Macro-ER-Phagy in ER Quality Control
- Corp Regulates P53 in via a Negative Feedback Loop
- Common Cell Shape Evolution of Two Nasopharyngeal Pathogens
- Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in
- Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression
- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- mTOR Directs Breast Morphogenesis through the PKC-alpha-Rac1 Signaling Axis
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
- Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast
- Encodes a Highly Conserved Protein Important to Neurological Function in Mice and Flies
- Identification of a Novel Regulatory Mechanism of Nutrient Transport Controlled by TORC1-Npr1-Amu1/Par32
- Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly
- Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
- TFIIS-Dependent Non-coding Transcription Regulates Developmental Genome Rearrangements
- Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen
- Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- The Catalytic and Non-catalytic Functions of the Chromatin-Remodeling Protein Collaborate to Fine-Tune Circadian Transcription in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



