-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLarge-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
Receptor-like kinases (RLKs) are important regulators in signal transduction in plants. However, the large number of RLKs and their high sequence similarity has hampered the analysis of RLKs. One of the largest subgroups of RLKs, the cysteine-rich receptor-like kinases (CRKs), has been suggested to be involved in mediating the effects of reactive oxygen species (ROS). While ROS are recognized as important signalling elements with a large variety of roles in plants, their ligands and achievement of signalling specificity remain unknown. Using insertion mutants we analysed the roles of CRKs in plant development and stress responses and show that CRKs have important roles as mediators of signalling specificity during regulation of stomatal aperture. Our study shows that, despite their large number and high sequence conservation, individual CRKs have intriguingly distinct functions in different aspects of plant life. This makes the CRKs promising candidates for future studies of their biochemical function.
Published in the journal: . PLoS Genet 11(7): e32767. doi:10.1371/journal.pgen.1005373
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005373Summary
Receptor-like kinases (RLKs) are important regulators in signal transduction in plants. However, the large number of RLKs and their high sequence similarity has hampered the analysis of RLKs. One of the largest subgroups of RLKs, the cysteine-rich receptor-like kinases (CRKs), has been suggested to be involved in mediating the effects of reactive oxygen species (ROS). While ROS are recognized as important signalling elements with a large variety of roles in plants, their ligands and achievement of signalling specificity remain unknown. Using insertion mutants we analysed the roles of CRKs in plant development and stress responses and show that CRKs have important roles as mediators of signalling specificity during regulation of stomatal aperture. Our study shows that, despite their large number and high sequence conservation, individual CRKs have intriguingly distinct functions in different aspects of plant life. This makes the CRKs promising candidates for future studies of their biochemical function.
Introduction
Receptor protein kinases play key roles in mediating perception of extracellular signals. These signals trigger intracellular signalling cascades allowing cells to respond and adapt to internal and external stimuli. Receptor kinases contain an extracellular signal-sensing domain connected by a single transmembrane domain to an intracellular protein kinase domain [1]. During evolution, different systems for the same function have emerged in animals and plants: animals deploy receptor-tyrosine kinases whereas plants utilize receptor-like kinases (RLKs), which are dual specificity serine/threonine and tyrosine kinases [2–4]. In contrast to mammals, all sequenced plant genomes contain a large number of RLKs, as illustrated by Arabidopsis and rice which encode more than 600 and 1100 RLKs in their genomes [1], respectively. The highly diverse extracellular regions of RLKs typically contain one or more protein domains or combinations of different domains. These domains have been used to divide RLKs into different sub-groups [1]. To date only a few RLKs have been functionally characterised but expression analyses have linked a large number of RLKs to many different physiological processes and signalling networks in plant development, pathogen defence, and abiotic stress response [5–8].
The cysteine-rich receptor-like kinases (CRKs, originally referred to as domain of unknown function 26 [DUF26] RLKs [9]) represent one of the largest groups of RLKs with 44 members in Arabidopsis thaliana [7]. Most CRKs have a typical RLK domain architecture, but three CRKs (CRK43, CRK44 and CRK45) consist only of the cytoplasmic domain reminiscent of receptor-like cytoplasmic kinases (RLCKs) [1]. The extracellular domain of CRKs encompasses two copies of the DUF26 domain (PF01657; http://pfam.sanger.ac.uk/family/PF01657; stress-antifung domain), which contains three cysteine residues in a conserved configuration (C-X8-C-X2-C) and is a predicted target for redox modifications. The DUF26 domain is also present in eight Arabidopsis PLASMODESMATA-LOCATED PROTEINs (PDLPs) [10]. The domain structure of these PDLPs is similar to CRKs but lacks the intracellular protein kinase, analogous to the leucine-rich repeat (LRR) receptor-like proteins (RLPs). Experimental evidence suggests that PDLPs are involved in the regulation of cell-to-cell communication and are important for pathogen defence [11,12]. Furthermore, more than 50 secreted proteins in Arabidopsis contain DUF26 domains but their roles have so far not been elucidated.
Several CRKs show elevated transcript levels in response to salicylic acid (SA) and pathogens [13–17] as well as ozone (O3) and drought [7,18]. Altered transcript abundance due to conditions affecting cellular redox and reactive oxygen species (ROS) balance [7,19], and the presence and spacing of the conserved cysteines in the DUF26 domain suggest that CRKs might be connected to ROS and redox signalling [7,14,20]. However, the functional role of the DUF26 domain is still unclear.
Previous studies have suggested the involvement of some CRK family members in pathogen defence and osmotic stress. Overexpression of CRK4, CRK5, CRK19, and CRK20 induced hypersensitive response-like (HR-like) cell death [13,14] and overexpression of CRK4, CRK6, CRK13, and CRK36 resulted in enhanced tolerance to the bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (Pto DC3000) [21,22]. Also a loss-of-function mutant crk20 showed a slight reduction in Pto DC3000 growth [23]. A Medicago truncatula CRK, SymCRK, was found to be involved in preventing early senescence and defence responses during symbiotic interactions [24]. Knock-down of CRK36 resulted in increased sensitivity to abscisic acid (ABA) and osmotic stress [25], and altered seed germination 6 (asg6; crk2), a mutant deficient in CRK2 function, has been associated with changes in seed germination in response to ABA [26]. A mutation in crk7 led to slightly increased sensitivity to extracellular ROS [27], and a mutation in crk5 resulted in impaired stomatal conductance, accelerated senescence as well as enhanced cell death in response to ultraviolet radiation [28]. Given the large number of CRKs and several transcript profiling experiments which suggest that CRKs are involved in a variety of environmental responses [6–8,18,19], it is surprising how little is known about the physiological and biochemical functions of this RLK family. However, based on transcriptional analysis CRKs might have far more complex functions, for example also in signalling in response to N-acetylglucosamine (GlcNAc) oligomers in the plant cell wall [29].
In this study we describe a comprehensive phenotypic analysis of a T-DNA insertion collection for the entire CRK gene family. This phenomics approach revealed novel roles for CRKs in control of plant development and biotic and abiotic stress adaptation. In spite of high amino acid sequence similarity, we observed that many CRKs mediate specific functions, with CRK2 and CRK5 playing predominant roles in growth regulation and stress adaptation, respectively. Our results imply a model for CRK function, placing CRKs as putative elements between ROS production and downstream signalling leading to pathogen - and abiotic stress-induced stomatal closure. This provides a framework for future detailed analysis of the molecular mechanisms underlying CRK signalling.
Results
Compilation and curation of a T-DNA insertion collection for the CRK group of RLKs
Transcriptional analyses have shown that CRK genes are responsive to several external stimuli. However, only limited information is available on their physiological roles. Based on the amino acid sequences of the coding region, the 44 Arabidopsis thaliana CRKs (plus the putative pseudogene CRK35 At4g11500 and the truncated CRK9 At4g23170) form five distinct groups (Fig 1A). Similar groups can be identified in phylogenetic trees based on the intracellular kinase domain (S1A Fig) as well as on the extracellular region (S1B Fig). This suggests co-evolution of the extra - and intracellular domains of Arabidopsis CRKs. A group of six CRKs, group I, constitutes a basal clade which forms a sister group distinct from groups II-V. The position of CRK45, At4g11890, which lacks the extracellular and transmembrane region, is ambiguous as it clusters with low bootstrap support with the basal group in the phylogenetic tree based on the entire coding region (Fig 1A) but as a sister to groups II and III in the tree based on the kinase domain (S1A Fig). Thus, CRK45 is not assigned to any CRK subgroup. Group I CRKs are distributed across chromosomes 1, 4 and 5 and only CRK2 and CRK3 are located next to each other, whereas genes encoding CRKs in groups II-V are, with the exception of CRK4 on chromosome 3, located on chromosome 4 and organized in repeats forming clusters of CRK genes (S2 Fig).
Fig. 1. Phylogenetic clustering of the Arabidopsis thaliana CRK group of RLKs and summary of the crk T-DNA insertion collection. 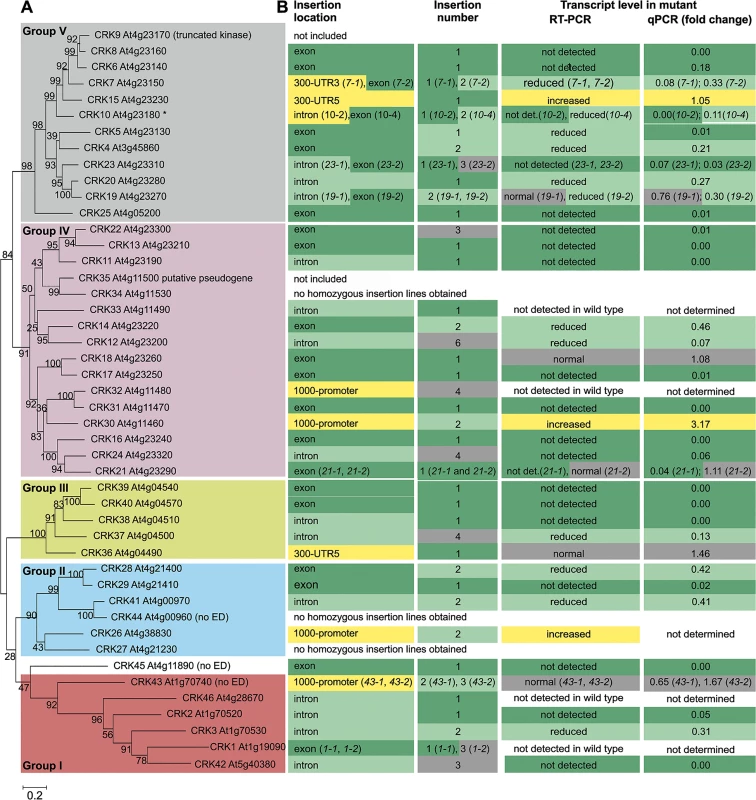
(A) The coding region of the CRKs of Arabidopsis thaliana (including the truncated CRK9 At4g23170 and the putative pseudogene CRK35 At4g11500) was aligned using Muscle. The maximum-likelihood phylogenetic tree was estimated in MEGA6 using all sites (no gap penalty). The initial guide tree was constructed using maximum parsimony. Values at branch nodes represent bootstrap values (1000 replicates). CRK43 (At1g70740), CRK44 (At4g00960) and CRK45 (At4g11890) lack signal peptide, CRK ectodomain (ED) and transmembrane domain. (B) Information on T-DNA insertion lines for corresponding crk mutants is summarized: location of the T-DNA insertion in the gene (detailed information in S3 Fig), number of T-DNA insertions per line (determined by quantitative PCR; S1 Table) and transcript level of the corresponding crk mutant (according to semi-quantitative RT-PCR and qPCR; detailed information in S1 Table). For two additional crk10 alleles (crk10-1 and crk10-3) information can be found in S1 Table. In order to investigate the function of individual CRK family members, a collection of 82 T-DNA insertion mutants was compiled from the Nottingham Arabidopsis Stock Centre (NASC; Figs 1B and S3 and S4 and S1 Table). CRK35 (putative pseudogene) and CRK9 (truncated) were excluded and, under our conditions, no homozygous T-DNA insertion lines could be obtained for crk27, crk34, and crk44 in the Col-0 background (Figs 1B and S3 and S1 Table). A total of 50 homozygous T-DNA insertion lines representing 41 crk mutants were isolated. In 21 crk lines the corresponding wild-type CRK transcript was not detected while levels were reduced in thirteen lines (Figs 1B and S4 and S1 Table). Of those thirteen lines seven have an insertion in an exon suggesting that they would produce a truncated protein while five lines carried insertions in introns. Only one line, crk7-1, with reduced transcript level of a CRK carried an insertion in the 5’ untranslated region (UTR). The status of the crk1-1, crk1-2, crk32, crk33, and crk46 mutants is unclear (Figs 1B and S3 and S1 Table) since transcripts for those four CRKs (CRK1, CRK32, CRK33, CRK46) were not detectable in the Col-0 wild type. In three crk lines where insertions were located in the 5’ UTR or the upstream promoter region, the corresponding CRK transcript abundance was increased compared to wild type. In eight lines no differences in corresponding CRK transcript levels were detected between the T-DNA insertion line and wild type (Figs 1B and S4 and S1 Table). Of those lines, two carried the insertions in the exonic region, thus possibly resulting in a truncated protein; one line carried the insertion in an intron, while the remaining five lines carried insertions in the regions upstream of the start codon (S3 Fig). The eleven lines in which corresponding CRK transcript levels were increased or not altered compared to Col-0 wild type were excluded from subsequent analyses (Fig 2). Thus a total of 39 lines were used (Fig 2). Based on quantitative PCR (qPCR) analysis 27 of the T-DNA insertion lines contained a single insertion, twelve contained two insertions while the rest contained more than two insertions in their genomes (Fig 1B and S1 Table). An age-matched seed collection was generated and used for all subsequent phenotypic analyses where we investigated the role of CRKs in aspects of growth/development, abiotic stress responses, biotic stress responses, photosynthesis and stomatal regulation (Fig 2 and S2 Table).
Fig. 2. Phenotypic analysis of the Arabidopsis thaliana CRK protein family. 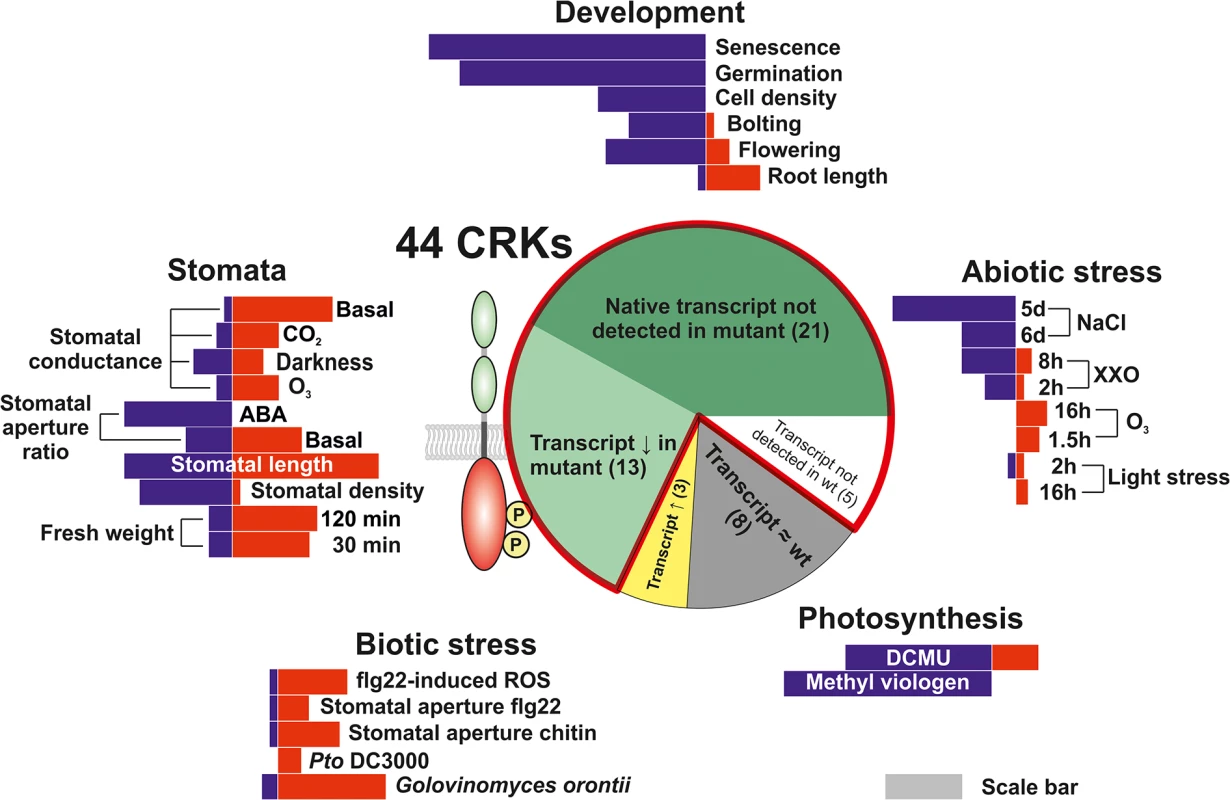
A T-DNA insertion collection for the CRK family was compiled and subjected to phenotyping addressing aspects of plant development, biotic and abiotic stress responses, photosynthesis as well as stomatal regulation. Length of red and blue bars in the five phenotyping sections is representative of the number of crk lines found to have phenotypes in the thematic area. Information about the sections in the pie chart is displayed in Figs 1 and S3 and S4 and S1 Table. The red outline in the pie chart highlights the lines included in the analyses and figures throughout the manuscript. The gray scale bar serves as a reference for comparison. The length of the scale bar corresponds to ten lines. Development is altered in crk mutants
Most crk mutants displayed normal morphology similar to the Col-0 wild type (S5 Fig). By contrast, crk2 displayed a clear dwarf phenotype (Fig 3A). Complementation of the crk2 mutation restored morphology similar to the Col-0 wild type (Fig 3A). The crk5 mutant was slightly smaller compared to Col-0 wild type, in particular after five weeks of growth [28], and overexpression of CRK5 in the mutant background led to slightly larger rosettes. Under long day conditions (16h light/8h darkness) 34 crk mutants displayed early senescence (Figs 3B and S6A). Under the same conditions, four crk mutants flowered earlier than Col-0 wild type (Figs 3C and S6B). The only mutant that flowered later compared to Col-0 wild type was the dwarf crk2 (Fig 3C). Germination was delayed in 32 crk mutants as shown by analysis of endosperm rupture (Fig 3D and S3 Table). Pavement cell density was reduced in eleven crks compared to Col-0 wild type (Figs 3E and S6C). Germination and pavement cell density were not altered in the dwarf crk2 compared to Col-0 wild type (Fig 3D and 3E). In addition, crk28, crk29, and crk42 showed slightly longer roots compared to Col-0 wild type seedlings (Fig 3F).
Fig. 3. Plant development is affected in several crk mutants. 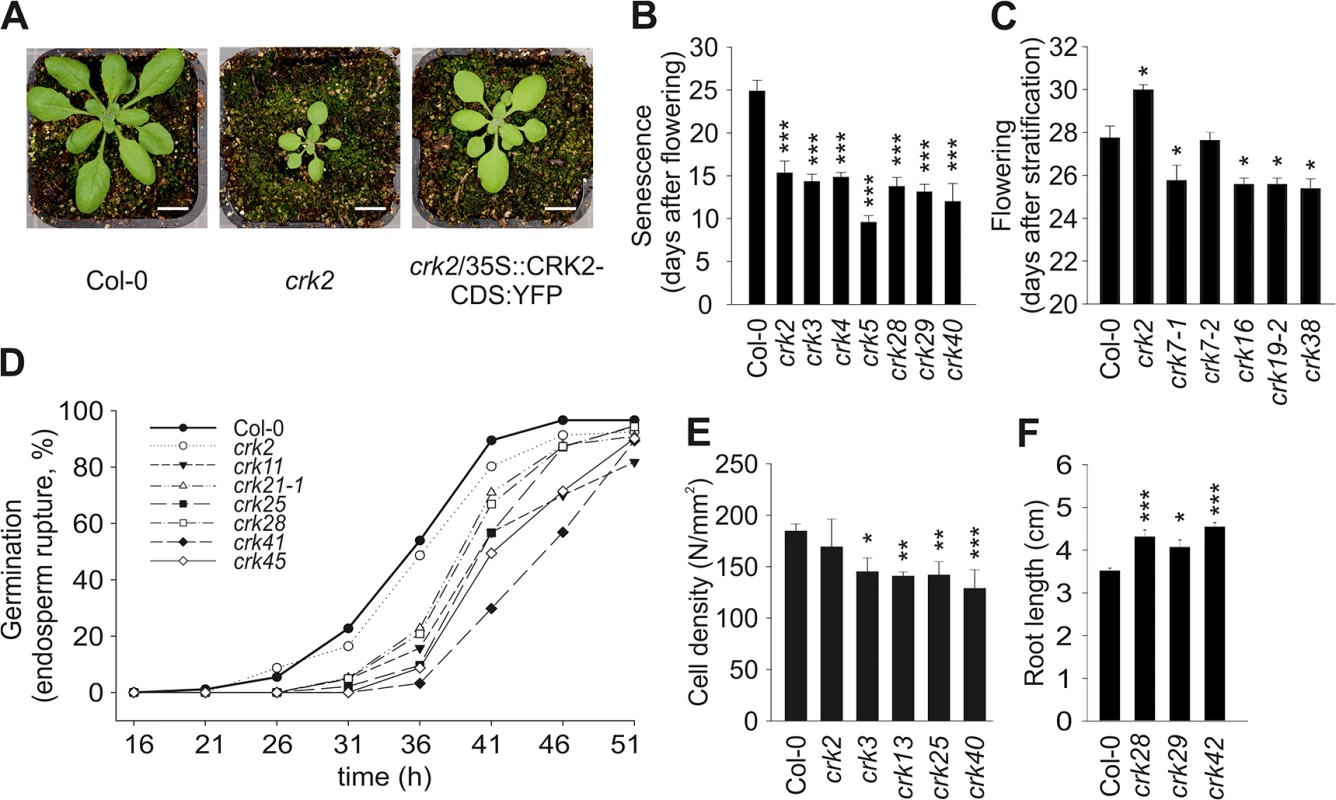
(A) Representative pictures of 17-day old seedlings of Col-0 wild type and crk2. Complementation of crk2 with 35S::CRK2-CDS:YFP rescued the growth defect of the mutant. Plants were grown under the following conditions: 250 μmol m-2 s-1 light intensity under 12 h-day length (day: 23°C, 70% relative humidity; night: 18°C, 90% relative humidity). Bar = 1 cm. Pictures are representative of three independent experiments. (B) A selection of crk mutant lines showing earlier senescence compared to Col-0 wild type. Results are means ± SE (n = 8). (C) Several crk mutants flowered earlier compared to wild type while crk2 flowered later. Results are means ± SE (n = 8). (D) Time course analysis of endosperm rupture showed delayed germination in several crk mutants compared to wild type. Results represent means from three independent biological experiments (n = 30). Testa and endosperm rupture were assessed every 5 hours up to 51 hours of imbibition. A seed was considered as germinated when the radicle protruded through both envelopes. (E) Several crk mutants exhibit a lower pavement cell density (number of pavement cells / mm2) in cotyledons. Results are means ± SE (n = 15). (F) Three crks showed slightly longer roots compared to wild type (measured eight days after stratification). Results are means ± SE (n = 16). (B-F) Differences between mutants and Col-0 wild type were compared and analysed using one-way-ANOVA (post hoc Dunnett, asterisks indicate statistical significance at *P<0.05, **P<0.01 and ***P<0.001) for (B, C, E) and linear model with single step p-value adjustment (F). All experiments were repeated three times with similar results. In summary, our results suggest that the CRKs are not exclusively involved in stress and pathogen responses, as previously suggested, but in addition contribute to the regulation of specific developmental processes. The only mutant displaying a clear dwarf phenotype was crk2 which supports the earlier predictions that CRK2 might be involved in growth regulation in response to ABA [26].
Responses of crk mutants to abiotic stresses
Elevated production of ROS is part of the response to many abiotic stresses [20]. CRK transcript levels were strongly regulated in response to abiotic stresses (S7 Fig) [7,19] including ozone (O3) and ultraviolet radiation (UV). O3-induced ROS formation in the extracellular space rapidly induced transcript accumulation for several CRKs, while high light-induced ROS formation in the chloroplasts showed no effect [7]. In line with this, high light stress did not induce extensive damage in the crk mutants (S8 Fig). Only crk2 and crk45 showed significant light stress-induced electrolyte leakage that differed from the response in wild type plants after nine or 24 hours, respectively.
Elevated ROS production in chloroplasts can also be induced by methyl viologen (MV, also known as Paraquat), which leads to increased superoxide production in the reducing end of the PSII. Moreover, chloroplastic ROS formation can also be induced by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), which causes increased production of singlet oxygen by affecting the redox status of the plastoquinone pool. The use of MV or DCMU allowed the assessment of the crk responses to increased chloroplastic ROS production by measurement of photosynthetic energy transfer, which is a more sensitive measurement compared to electrolyte leakage. Treatment of plants with MV or DCMU resulted in stronger photoinhibition in several lines (e.g. crk2, crk5, crk8, crk17, crk20, crk40, crk45) compared to wild type, depending on the ROS inducer that was used (Figs 4A–4C and S9). Photosynthetic impairment of the crk5 mutant was rescued by overexpression of CRK5 [28].
Fig. 4. Abiotic stress responses are affected in crk mutants. 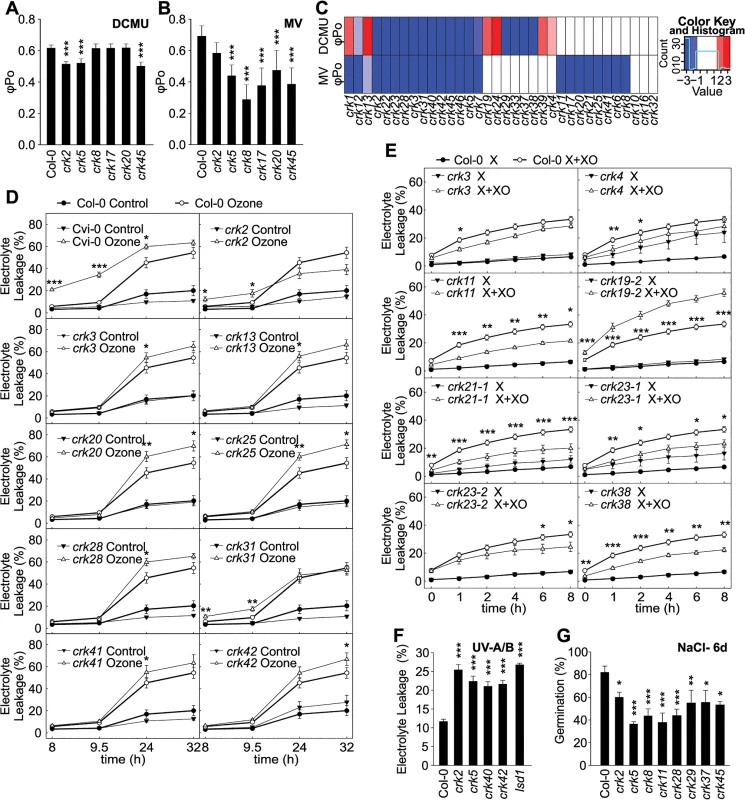
(A and B) Treatment with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) for two hours (A) or methyl viologen (MV) for 48 hours (B) resulted in stronger impairment of photosynthesis in a subset of crk mutants compared to Col-0 wild type as shown by changes in the maximum quantum yield of primary photochemistry (φPo). Significant differences in relation to wild type are indicated (n = 12, bars represent SD; one-way ANOVA with post hoc Tukey HSD) *** P<0.001. The experiment was repeated three times with similar results. (C) Clustering of DCMU and MV experiments. Red and blue indicate increased or decreased response with respect to Col-0, respectively (average of alleles). Color intensity is proportional to a Benjamini-Hochberg false discovery rate (FDR) adjusted Z statistic, which takes the estimated means and their variation into account. Roughly, |Z|>2 corresponds to an FDR<5%, and |Z|<2.6 to an FDR<1%. Results were clustered using a complete linkage algorithm with 1-Pearson correlation as distance. (D) In several crk mutant lines exposure to O3 resulted in elevated electrolyte leakage. Electrolyte leakage was measured at indicated time points after start of the O3 exposure that lasted 6 h (350 ppb). Mean values ± SD from two independent experiments are presented (n = 12, one-way ANOVA with post hoc Tukey HSD). (E) Xanthine-Xanthine Oxidase (X+XO) infiltration induced different levels of electrolyte leakage in crk mutants compared to wild type (0.1 U ml-1, 4 h). Mean values ± SD from three independent experiments are presented (n = 16, linear model with single-step p-value adjustment for multiple testing). (F) Treatment with ultraviolet-A (UV-A) and–B (UV-B) radiation led to increased electrolyte leakage in crks compared to wild type. Mean values ± SD from three independent experiments are presented (n = 12, one-way ANOVA with post hoc Tukey HSD). (G) Effect on NaCl on the germination crk seedlings at 6 days after stratification. Values represent mean of the ratio (germination percentage on 120 mM NaCl / percentage on control medium) for each line (n = 15, linear model, single-step p-value adjustment). Experiments were performed three times. Asterisks indicate statistically significant differences between crks and Col-0 (*P<0.05, **P<0.01 and ***P<0.001) (D-G). Error bars indicate ± SE (D, E and F). Relative electrolyte leakage was calculated as a ratio of the value measured at the indicated time and the total electrolyte leakage after freezing (D and E) or autoclaving the samples (F). In summary, these results suggest that some CRKs are involved in sensing or adaptation to changes in ROS or redox balance in the chloroplast and could be involved in signal transduction processes that culminate in regulation of photosynthetic electron transport. Like most RLKs, CRKs are predicted to localize to the plasma membrane and no CRKs have been identified in studies exploring the chloroplast proteome [30,31]. Thus, at the moment it is not clear how the CRKs communicate with the chloroplast but they might participate in communication between apoplast and chloroplast similarly to what has been described during PAMP-triggered immunity [32].
Even though chloroplasts and peroxisomes are the main sources of intracellular ROS in plants, extracellular ROS production is also important in the response abiotic stimuli [20]. Extracellular ROS production can be specifically induced by exposure to the air pollutant O3 or infiltration with the enzymatic system Xanthine-Xanthine Oxidase (X+XO) [33]. Several crk mutants displayed differential responses to extracellular ROS in comparison to Col-0 wild type plants. Responses to O3 were generally subtle (Figs 4D and S10). At 9.5 hours after the onset of O3 treatment crk2 and crk31 showed increased electrolyte leakage while at later timepoints crk3, crk13, crk20, crk25, crk28, crk41, and crk42, showed elevated electrolyte leakage (Fig 4D). Enhanced O3-induced cell death, visible as lesion formation, was observed for crk2, crk3, crk5, crk7-1, crk7-2, crk11, crk13, crk19-2, crk20, crk22, crk23-2, crk24, crk28, crk31, crk37, crk38, and crk46 (S11 Fig). In response to treatment with X+XO, crk19-2 showed increased electrolyte leakage while crk4, crk11, crk21-1, crk23-1, crk23-2, crk29, crk37, crk38 and crk46 showed reduced electrolyte leakage compared to Col-0 wild type (Figs 4E and S12).
In response to UV-A and-B, crk2, crk5, crk40, and crk42 displayed significantly elevated electrolyte leakage compared to Col-0 wild type indicating more damage similar to lesion simulating disease 1 (lsd1), which was used as positive control (Figs 4F and S13A). Complementation of crk5 rescued the hypersensitivity to UV-A and–B radiation [26]. Exposure to salt (NaCl) is another environmentally relevant abiotic stress. Overall, twenty one crk mutants, including crk2, crk5, crk8, crk11, crk28 crk29 crk37, and crk45, showed delayed germination compared to the Col-0 wild type on medium containing 120 mM NaCl (Figs 4G and S13B and S1C). After six days of growth on medium supplemented with 120 mM NaCl 13 crk mutants still maintained the delayed germination (S13C Fig).
While previous studies have emphasized the roles of CRKs in the response to pathogens and cell death regulation [13,14,21–23], our observations suggest that CRKs are also important regulators of the response to abiotic stresses, such as UV-A and–B, salt, and O3, possibly through extracellular ROS. In addition, our results suggest that the CRKs could be involved in controlling processes that indirectly regulate photosynthetic electron transport in the chloroplast.
Stomatal development and regulation is altered in the crk mutants
Stomata are key structures in the control of plant responses to drought stress, pathogen infection, and other stimuli. Control of the stomatal aperture is a complex process involving plant hormones, most prominently ABA, ROS, and calcium (Ca2+) signalling to mediate and integrate plant-derived and environmental signals [34]. Transcriptional analysis suggested that several CRKs are involved in the control of drought responses (S7 Fig) [18]. Most CRKs displayed lower transcript abundance in guard cells compared to total leaf based on microarray meta-analysis (S14A Fig) while according to qPCR several CRKs displayed higher transcript levels in guard cells (S14B Fig). The difference between different methods to analyze gene expression could be due to the different preparation methods for guard cells. For the qPCR experiments enzymatic digestion was used for the isolation of guard cell protoplasts which might cause an additional pathogen treatment. As several CRKs have been described to show elevated transcript levels in response to pathogen or elicitor treatments, this could likely be the source of this difference. Water loss from detached leaves or rosettes can be used as a measure of initial stomatal aperture and the rate of stomatal closure. Water loss was enhanced in crk2, crk5, and crk31, as indicated by rapid decrease of rosette weight due to impaired stomatal regulation (Fig 5A and S4 Table). Complementation of the crk2 and crk5 mutations rescued water loss phenotypes of these mutants (Fig 5B and 5C). Several other crks, notably crk45, lost less water after detachment compared to Col-0 wild type (Fig 5A). Complementation of the crk45 mutation using an overexpression construct (see materials and methods) rescued the phenotype and led to increased water loss compared to the mutant and Col-0 wild type (Fig 5D).
Fig. 5. Stomatal development and responses are impaired in specific crks. 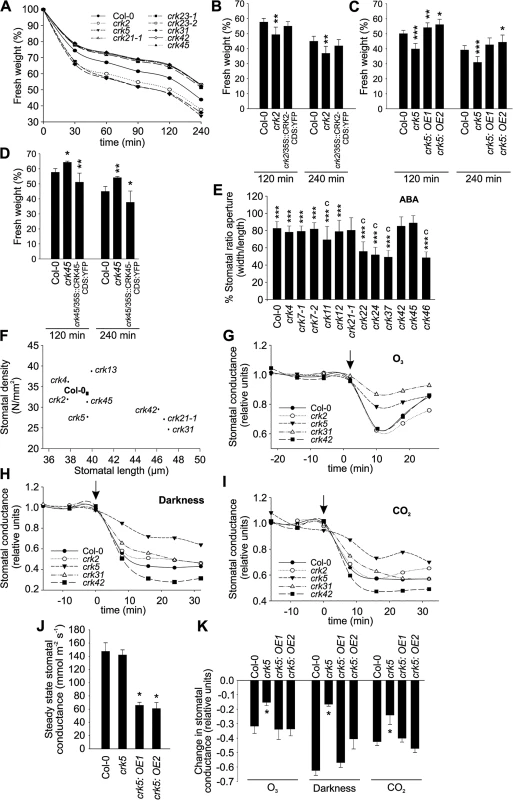
(A) A subset of the crk mutants showed altered water loss (shown as decrease of fresh weight) compared to Col-0 wild type plants after detachment of shoots from roots as evaluated from rosette weight. Complementation of the crk2 (B), crk5 (C) or crk45 (D) mutants restored a wild type-like water loss phenotype as interpreted from decrease of fresh weight of excised rosettes. Asterisks indicate differences between crk mutants or complementation lines and Col-0 with statistical significance at *P<0.05, **P<0.01 and ***P<0.001 according to one-way ANOVA with post hoc Tukey HSD. The experiment was repeated three times with similar results. (E) Stomatal apertures were measured 2 h after abscisic acid (ABA) treatment. Some crk mutants are impaired in stomatal closure 2 h after treatment with 10 μM ABA. Results are means of % stomatal aperture ratio (width/length) ± SE (average number of stomata measured = 250). Asterisks indicate statistical significance between control and ABA treatment at *P<0.05, **P<0.01 and ***P<0.001 (linear model, single-step p-value adjustment). Lowercase letters indicate statistical significance between wild type Col-0 and crk mutant at P<0.05 (a), P<0.01 (b) and P<0.001 (c) according to one-way ANOVA with post hoc Dunnett’s test. (F) Stomatal density (number of stomata/mm2) is correlated with stomatal length (μm). Most of the crks exhibit a smaller stomata density which correlates with longer stomata (Pearson correlation -0.69, p-value = 0.04). Results are means (average number of stomata measured = 500). (G-I) Time courses of stomatal conductance (relative units) in response to a 3 min pulse of 500–600 ppb of O3 (G), darkness (H) and elevation of CO2 from 400 ppm to 800 ppm (I) in a subset of crk mutants and Col-0. Stimuli were applied at 0 time point, which is indicated by an arrow; pre-treatment stomatal conductance was used for normalization. Graph shows the mean of two experiments (n = 6). (J) Overexpression of CRK5 led to lower stomatal conductance compared to Col-0 wild type. (K) Complementation of the crk5 mutant restored wild type-like phenotype in the response to a 3-min pulse of 500–600 ppb of O3, darkness, and elevating CO2 from 400 to 800 ppm. Asterisks indicate differences between crk mutants or complementation lines and Col-0 with statistical significance at *P<0.05 according to one-way ANOVA with post hoc Tukey HSD. The experiment was repeated three times with similar results. Stomatal openness (measured as the ratio of width to length) in response to the plant hormone ABA was altered in several crk mutants (Figs 5E and S15A), suggesting that CRKs may also participate in ABA-dependent control of the stomatal aperture. The crk22, crk24, crk37, and crk46 mutants showed stronger ABA-induced stomatal closure compared to Col-0 wild type (Figs 5E and S15A). Similar to other mutants [35], we found a negative correlation between stomatal length and density for crks (Figs 5F and S15B). Furthermore, the analysis showed a cluster of crks that had reduced stomatal density and increased stomatal length as compared to Col-0 (S15B Fig).
To compare microscopic measurements of the stomatal aperture with stomatal function we measured stomatal conductance in gas exchange experiments using intact, soil-grown rosettes [36]. Thirteen crks displayed slightly increased basal steady-state stomatal conductance under control conditions (S16A Fig). Under conditions that induce rapid stomatal closure [36] (elevated CO2, darkness, pulse of O3) the rapid decrease in stomatal conductance was less pronounced in crk5 and crk31 compared to wild type plants (Figs 5G–5I, S16B–S16D, S17, S18, S19 and S20). These two mutants also exhibited increased water loss (Fig 5A). Complementation of the crk5 mutant restored wild type-like stomatal responses in the mutant in response to O3, darkness and CO2 (Fig 5J and 5K). Some crk mutants showed slightly increased stomatal closure compared to Col-0 wild type in response to the stimuli tested (S16, S17, S18, S19 and S20 Figs). In response to CO2, stomatal closure of crk31 was also somewhat (but not statistically significantly) reduced (Figs 5I and S19). In addition to the major regulators, many additional CRKs may be involved in stomatal closure but are compensated by the redundancy within the gene family. This is suggested by the fact that the overall responses to O3, CO2, and darkness correlate significantly even when including the non-significant responses (Fig 6A–6C). This could result from compensation that is not perfect, as would be expected with genes which have slightly different structures (Fig 6A–6C).
Fig. 6. Scatter plots of stomatal regulation in crk mutants. 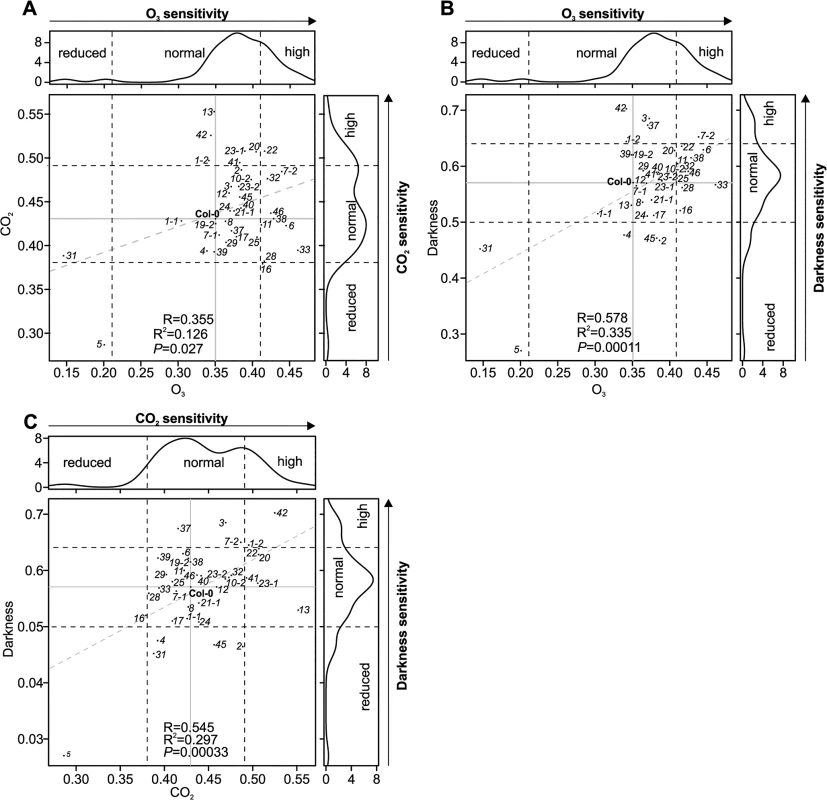
The crk5 was insensitive to all studied stimuli, whereas crk31 was particularly insensitive to O3. The lines crk19-1 and crk22 were more sensitive to the analysed stimuli. (A) Scatter plot of stomatal responses of crk mutants to O3 (x-axis) and CO2 (y-axis). (B) Scatter plot of stomatal responses of crk mutants to O3 (x-axis) and darkness (y-axis) (C) Scatter plot of stomatal responses of crk mutants to CO2 (x-axis) and darkness (y-axis). Dashed lines indicate the cut-off for reduced, normal, and high response with respect to Col-0. Grey dashed lines show regression fit with correlation (R), coefficient of determination (R2) and significance reported in lower right corner in each plot. Reduced or increased responses were statistically significant in the majority of mutants (see respective barplots in S17, S18 and S19 Figs). CRKs have not been implicated in the regulation of stomatal openness and closure previously. Our findings suggest that specific CRKs are involved in controlling basal stomatal aperture and stomatal responses to environmental stimuli, which are critical to plant survival. In addition, CRKs also participate in the regulation of stomatal numbers.
CRKs are integral components of plant defence
Apoplastic ROS production also plays an important role in pathogen defence [37]. It can be triggered by treatments with pathogen-, microbe-, or damage-associated molecular patterns (PAMPs; MAMPs; DAMPs, respectively), for example flg22, a peptide derived from flagellin, an integral component of the bacterial flagellum [38,39]. Basal ROS production was slightly reduced in thirteen and elevated in two crk lines (S21 Fig). In response to elicitation with flg22 eleven crk lines displayed significantly increased ROS production while ROS production was decreased in crk2, crk3, crk13, and crk31 (Figs 7A and S22A). This suggests that CRKs might be involved in control of ROS production, though the mechanism is not clear.
Fig. 7. Immunity to bacterial pathogens is impaired in crk mutants. 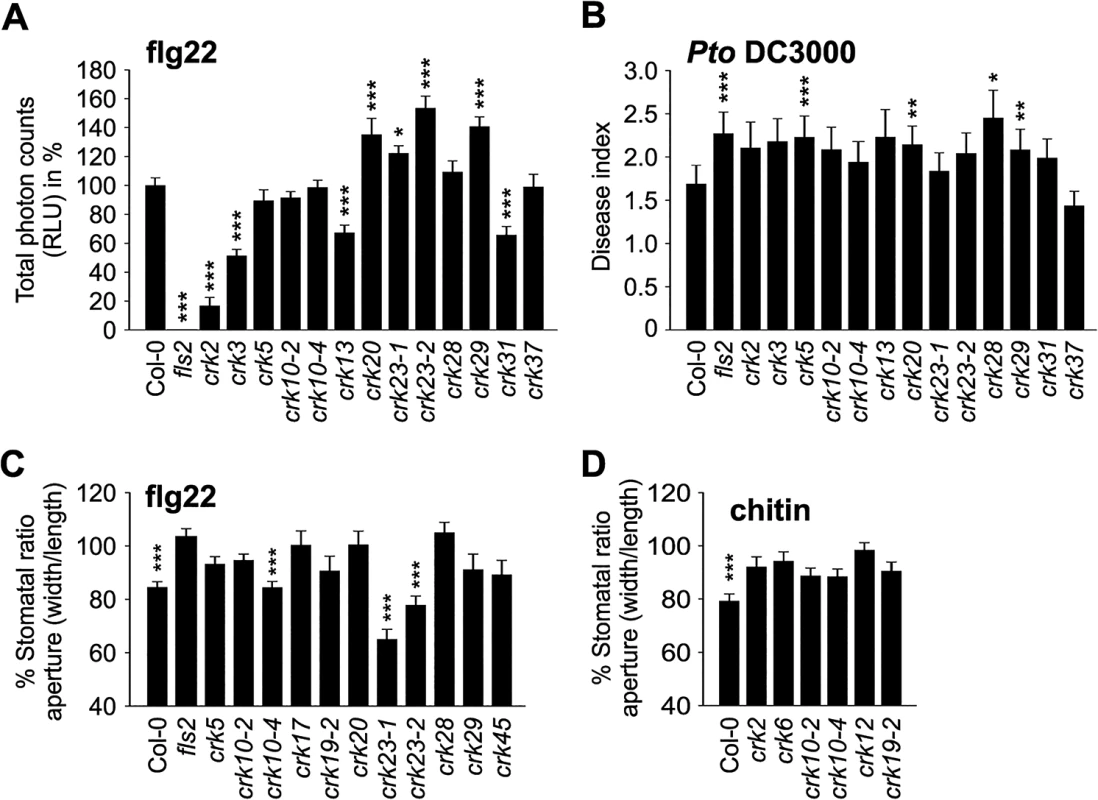
(A) ROS production was enhanced in several crks compared to Col-0 wild type after elicitation with 100 nM flagellin (flg22) in 4 week-old leaves. Data show the percentage of the mean of the total RLU (relative light units) to Col-0 ± SE (n = 24). Asterisks indicate differences between crks and Col-0, statistical significance *P<0.05, **P<0.01 and ***P<0.001 (one-way ANOVA post hoc Dunnett). (B) A subset of crks was more susceptible to Pto DC3000 (spray infection of 2-week old seedlings at 108 cfu ml-1) compared to Col-0. Disease symptoms were scored 3 days post inoculation: 0, no symptom; 1, one symptomatic cotyledon; 2, two symptomatic cotyledons; 3, dead seedling. Results are means ± SE (n = 48). Asterisks indicate differences between crks and Col-0, statistical significance *P<0.05, **P<0.01 and ***P<0.001 (Mann-Whitney test, Benjamini-Hochberg correction for multiple comparisons). (C) Some crk mutants are impaired in stomatal closure after 2 h treatment with 10 mM flg22. Results are presented as mean of stomatal aperture ratio (width/length) after treatment compared to pre-treatment values in percentages ± SE (average number of stomata measured = 250). Asterisks indicate statistical significance between control treatment at *P<0.05, **P<0.01 and ***P<0.001 (linear model, single-step p-value adjustment). (D) Stomatal apertures were measured 2 h after chitin treatment. Stomatal closure is impaired in several crk mutants after treatment with chitin (1 g l-1) for two hours (five selected mutants are shown). Results are means of % stomatal aperture ratio (width/length) after treatment ± SE (average number of stomata measured = 250). Asterisks indicate statistical significance between control treatment at *P<0.05, **P<0.01 and ***P<0.001 (linear model, single-step p-value adjustment). All experiments were repeated three times with similar results. Several crk lines were more susceptible than the wild type to surface infection with the hemi-biotrophic bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (Pto DC3000; Figs 7B and S22B). Notably, elevated flg22-induced ROS production did not fully match the responses to Pto DC3000 infection (Figs 7A and 7B and S22). The crk5 and crk28 mutants exhibited normal ROS production (Fig 7A) but showed increased disease symptoms (Fig 7B), while crk23 showed elevated flg22-induced ROS production but did not differ from Col-0 wild type in its susceptibility to Pto DC3000. The crk20 and crk29 mutants were also more susceptible to infection by Pto DC3000 in spite of the elevated ROS production.
The different level of ROS production in the crk mutants compared to Col-0 wild type after elicitation with flg22 suggests that at least some CRKs might act through ROS signalling pathways rather than direct pathogen perception. To test this hypothesis, we measured PAMP-induced stomatal closure in Col-0 wild type and crk plants. PAMP perception through RLKs leads to NADPH oxidase activation and extracellular ROS production and induces stomatal closure [40–42]. While ROS production was normal or even elevated in most crks, stomatal closure triggered by flg22 was impaired in several mutants including crk5, crk17, crk20, and crk28 (Figs 7C and S23A) and corresponds to their increased susceptibility to Pto DC3000 (Figs 7B and S22B). In response to the PAMP chitin, a fungal cell wall component, stomatal closure was reduced in several crk mutants including crk2, crk6, crk10-2, crk10-4, crk12, and crk19-2 (Figs 7D and S23B). Significant ABA-, flg22-, and chitin-induced stomatal responses were mostly mediated by different CRKs (Fig 8A and 8B); only a few CRKs participated in more than one process. Responses to the PAMPs flg22 and chitin might be mediated mostly by different CRKs (Fig 8C). Again, in addition to the major regulators, the responses overall show a significant correlation even when including also the non-significant crk mutants (Fig 8A–8C). This may be due to the redundancy within the gene family, similar to the result observed in stomatal conductance (Fig 6A–6C). Most crk mutants that were affected in stomatal immunity displayed higher transcript abundance in guard cell protoplasts compared to whole, untreated leaves (S14B Fig).
Fig. 8. Scatter plots for stomatal regulation in crk mutants. 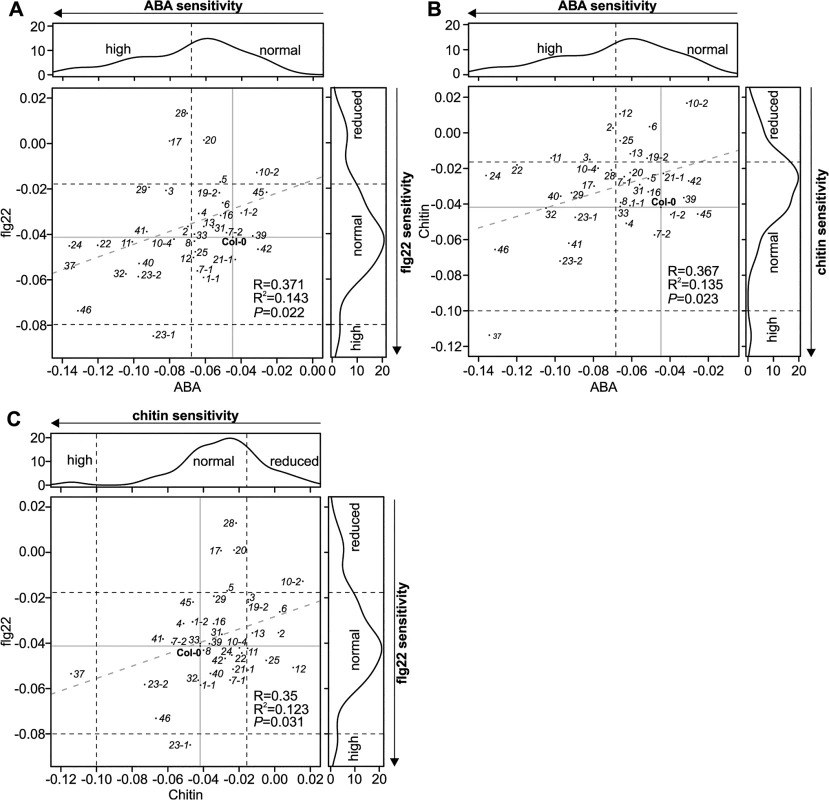
(A) Scatter plot of stomatal responses of crk mutants to ABA (x-axis) and flagellin (flg22; y-axis). (B) Scatter plot of stomatal responses of crk mutants to ABA (x-axis) and chitin (y-axis). (C) Scatter plot of stomatal responses of crk mutants to chitin (x-axis) and flg22 (y-axis). Black dashed lines indicate the cut-off for reduced, normal, and high response with respect to Col-0. Grey dashed lines show regression fit with correlation (R), coefficient of determination (R2) and significance reported in lower right corner in each plot. Reduced or increased responses were statistically significant in the majority of mutants (see respective barplots in S15A and S23 Figs). While Pto DC3000 can infect plant leaves through stomata [43–45], powdery mildews follow a different strategy by penetrating and colonizing epidermal cells [46–48]. Infection of wild type Col-0 with the biotrophic virulent powdery mildew fungus, Golovinomyces orontii (Go), or the non-host powdery mildew fungus Blumeria graminis f. sp. hordei (Bgh) resulted in altered expression of several CRK genes (S24A Fig) and susceptibility towards the pathogens was also affected in crks. Several crks showed increased susceptibility to Go, whereas responses to Bgh were more subtle. Specifically, crk2 and crk5 displayed less visible mildew symptoms in response to Go infection (Fig 9A and 9B), whereas crk17, crk20, crk23-1, crk23-2, crk25, crk28, crk32, and crk38 were more susceptible (Figs 9A and 9B and S24B and S24C). Furthermore, crk20 and both alleles of crk23 (crk23-1 and crk23-2) showed consistently enhanced pigmentation of the leaves following Go infection (Fig 9B). Additionally, crk1-1, crk17, crk25, and crk32 showed this response also to the non-pathogenic Bgh (Fig 9C).
Fig. 9. Immunity to powdery mildews is impaired in crk mutants. 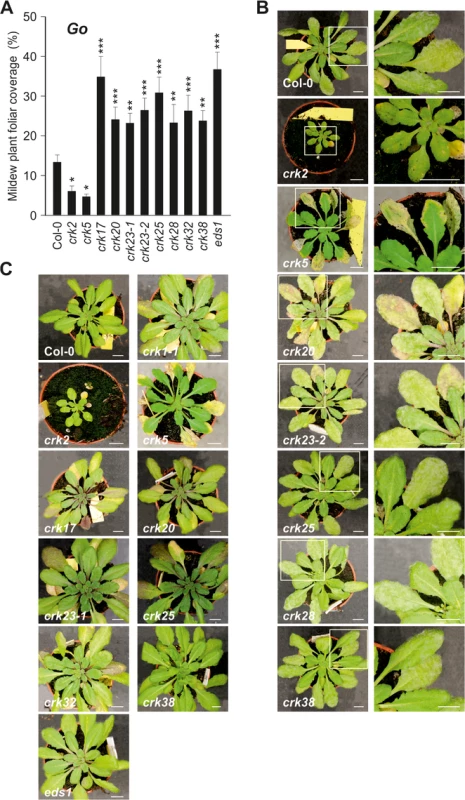
(A) Relative amount of plant foliar mildew coverage (in percent) caused by the virulent biotrophic powdery mildew Golovinomyces orontii (Go) on crks compared to Col-0 wild type and Go-super-susceptible eds1. Results are mean ± SE (n = 15). The experiment was conducted three times and the amount of disease was normalised between experiments by setting the infection cover of Col-0 to one. Asterisks indicate differences between crks and Col-0, statistical significance *P<0.05, **P<0.01 and ***P<0.001 (linear mixed model with Benjamini-Hochberg false discovery rate correction). (B) Pictures of Go infected crks and close-up of infected leaves. In some cases, infected leaves displayed increased pigmentation and crk5 showed accelerated death of the infected leaves. Bar = 1 cm. (C) No infection was observed with the non-host powdery mildew Blumeria graminis f. sp. hordei (Bgh) but several crks displayed increased pigmentation. Bar = 1 cm. All experiments were repeated three times with similar results. While previous reports have already suggested that CRKs participate in defence against bacterial pathogens, our results show that CRKs are also involved in the defence against fungal pathogens. CRKs participate in the control of pathogen-induced ROS signalling, stomatal responses and pre-invasive immunity.
Discussion
This study addresses the physiological and cellular roles of the CRK protein family—with 44 members, one of the largest subgroups of RLKs in Arabidopsis (Fig 2). As a result of the large-scale phenotyping of a crk T-DNA insertion mutant collection we were able to identify clear and specific phenotypes for several crk mutants. Clustering of all phenotypic differences of crk mutants compared to Col-0 wild type allowed the generation of a genetic and phenotypic framework (Figs 10 and S25). Previous reports have linked ectopic overexpression of individual CRKs with pathogen defence and regulation of cell death [12,13,20] and CRK45, one of the few CRKs lacking ecto - and transmembrane domains, was found to interact with pathogen effectors in a large-scale screen [49]. However, it is unclear whether other CRKs could be direct targets for pathogen effectors. Meta-analysis of microarray data (S7 Fig) suggested the involvement of CRKs in response to a variety of additional stimuli beyond pathogen defence, including O3, UV, light, salt, and drought stress. Several crks displayed phenotypes in response to those stimuli (Figs 10 and S25). In addition, growth and development were altered in several crks (Figs 10 and S25).
Fig. 10. Integrated cluster analysis of crk mutant phenotypes. 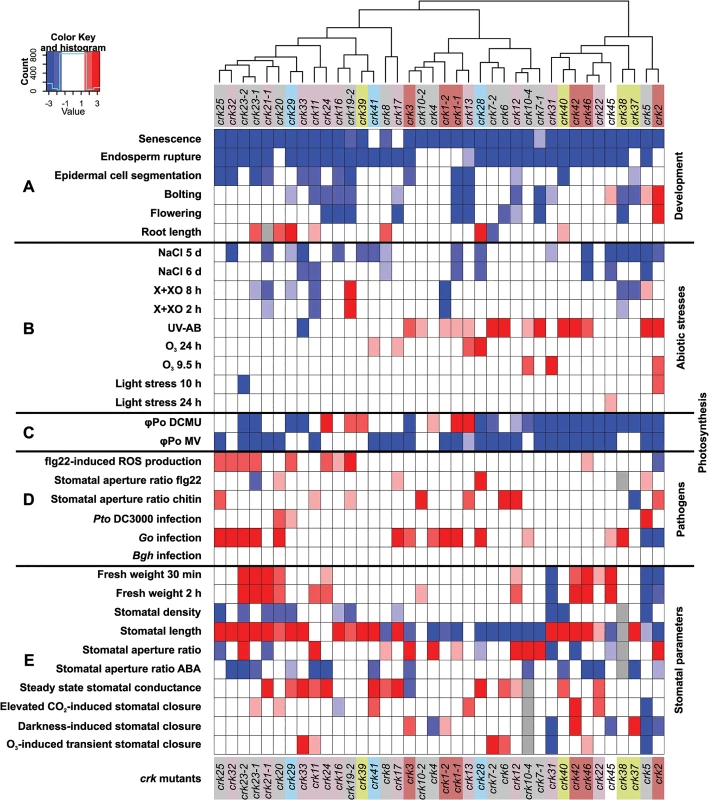
An age-matched collection of T-DNA insertion lines in CRK genes was analyzed for developmental and stress-related phenotypes. (A) Analysis of developmental phenotypes of crk mutant lines: senescence, germination (endosperm rupture), epidermal cell segmentation, bolting, flowering, and root length. (B) Analysis of abiotic stresses phenotypes of crk lines: germination of crk lines on medium containing NaCl, cell death (measured by electrolyte leakage) in response to Xanthine-Xanthine Oxidase (X+XO), ultraviolet light (UV-AB), ozone (O3), or light stress. (C) Analysis of photosynthesis responses upon treatment with DCMU or methyl viologen (MV). (D) Pathogen phenotypes. ROS production in response to treatment with the bacterial elicitor flagellin (flg22). Stomatal aperture ratio in response to flg22 and chitin treatments and crk susceptibility to the hemibiotrophic bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (Pto infection) or the biotrophic fungal pathogens Golovinomyces orontii (Go) (virulent on Arabidopsis) or Blumeria graminis f.sp. hordei (Bgh, a barley pathogen, non-pathogenic on Arabidopsis). (E) Analysis of stomatal parameters: fresh weight (for determination of water loss), density, length, aperture, stomatal aperture in response to ABA treatment, steady state stomatal conductance, stomatal closure in response to elevated CO2, O3, and darkness. Experiments were made comparable by bootstrap sampling to n = 15 followed by averaging over bootstrap estimates. Red and blue indicate statistically significant increase or decrease in response compared to Col-0 wild type, respectively, while white indicates a response that is similar to wild type Col-0. The intensity of color is proportional to the Benjamini-Hochberg false discovery rate (FDR) adjusted Z statistic which takes the estimated means and their variation into account. As a rough guideline, |Z|>1.67 corresponds to a FDR<10% (shown with light hue), and |Z|<2.6 to a strong FDR<1% (intense color). White: non-significant response; grey: not measured. A corresponding plot displaying the adjusted Z statistics without thresholding is shown in S25 Fig. Roles for ROS/redox signalling in biotic or abiotic stress response have been shown repeatedly over the last decade [20,50]. Similarly, plant development [51], root growth [52,53], senescence [54], germination [55], cell expansion [56], flowering [57–59], and cell cycle control [60] are tightly integrated with ROS and redox-dependent processes. This might suggest that CRKs could be connected to ROS/redox signalling in both stress and developmental processes. Three CRKs (CRK27, CRK34, and CRK44) might fulfil critical roles for plant survival. Their expression in different plant tissues and organs did not reveal any striking patterns (S5 Table) and no assumption towards their function. In the Col-0 genetic background no T-DNA insertion lines were obtained for CRK27, CRK34, and CRK44. Their roles will however need to be verified in the future.
Overall, some of the most striking phenotypes were found for crk2 and crk5, members of the basal group I and group V, respectively (Fig 10). Some phenotypes of crk2 might be caused by its dwarf morphology. However, this, together with the underlying cause of the dwarfism in crk2, will require more detailed analysis in the future.
Earlier studies have suggested that the high degree of amino acid sequence similarity between CRK family members would be the main reason for redundancy and lack of loss-of-function mutant phenotypes [13,14,21,22]. Our findings suggest that CRKs do not function in an exclusively redundant fashion. Specific CRKs, for example CRK2 of the basal phylogenetic group I, could function as primary regulators while others might provide calibration for more fine-tuned responses. This would offer an explanation for the intriguingly large number of CRKs in Arabidopsis thaliana, and also in other species.
Tissue and cell specificity of various CRKs might also be a reason for the large number of different genes, as they would be under different transcriptional regulation. However, according to eFP browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) [61] most CRKs seem to be present in low levels in most tissues (S5 Table). Only CRK2 and CRK3 show strikingly higher transcript abundance in guard cells, hypocotyl and in vascular tissues (S5 Table). Expression of CRKs might however also be regulated in response to external stimuli. This is the case for CRK7 [27] and CRK5 [28].
Regulation of the stomatal aperture is an important factor in the response to a wide range of stimuli [45,62,63] and has been shown to involve ROS signalling [64]. Several crks showed differences in ABA - or stress-induced stomatal closure (Fig 11A). Thus, it is tempting to link the crk phenotypes defective in ABA signalling with altered ROS signalling. Two crks (crk5 and crk31) showed defective responses to stomatal closure induced by abiotic factors. Expression of CRK5 is not restricted to guard cells (S14B Fig), underlining the importance of whole-leaf processes mediated by CRK5 in stomatal movements. CRKs might also be involved in determining basal openness of stomata. Interestingly, different CRKs control basal steady-state stomatal openness and stomatal responsiveness to stimuli.
Fig. 11. Models of CRK function and how they could provide specificity of stomatal aperture regulation. 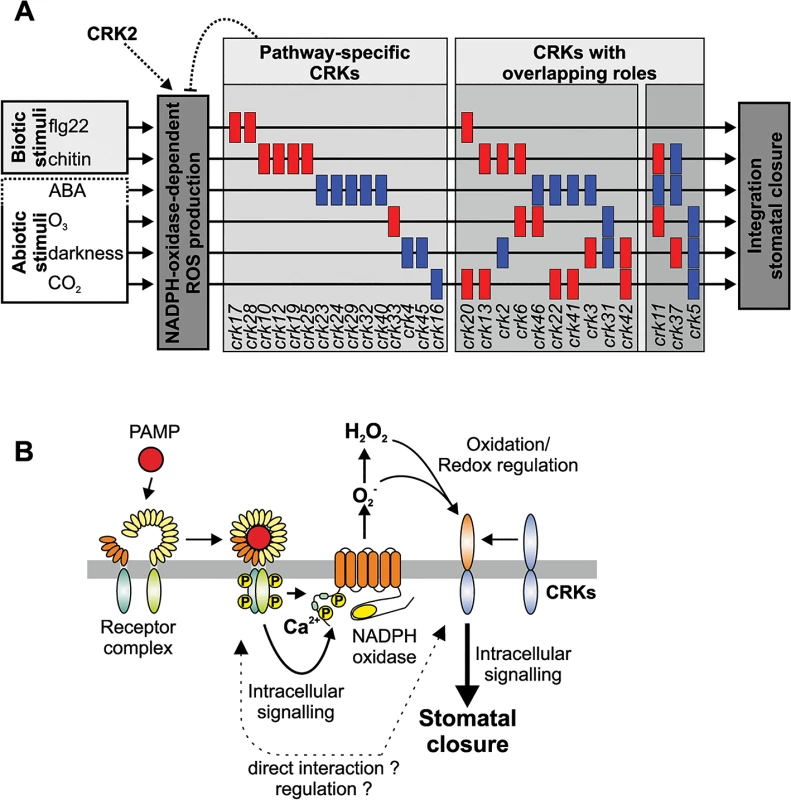
(A) CRKs might act as pathway-specific or multi-pathway regulators of stomatal aperture in response to the PAMPs flg22 and chitin but also the stress hormone ABA and the abiotic stimuli O3, darkness and CO2. The figure has been created from data presented in Fig 10. (B) CRKs are involved in the response to pathogens downstream of extracellular ROS production. PAMPs are recognized by pattern recognition receptor complexes. Subsequently, intracellular signalling leads to activation of extracellular superoxide production by NADPH oxidases. ROS perception subsequently leads to intracellular signalling and ultimately stomatal closure. CRKs are implicated in linking extracellular ROS production to intracellular signalling and might regulate and/or interact directly with the recognition receptor complexes. Stomatal closure can also be triggered by application of PAMPs, e.g. flg22 or chitin. Signal transduction from receptor-mediated PAMP perception by intracellular signalling and ROS production down to stomatal closure is becoming increasingly better understood [45,65]. There are convergence points in the signalling pathways of ABA - and PAMP-induced stomatal closure, for example ROS production by the NADPH oxidase RBOHD [34,41,66]. Consistent with enhanced disease symptoms upon Pto DC3000 infection of several crks, mutations in several CRKs impaired flg22-triggered stomatal immunity (Fig 11A). The results suggest that PAMP perception and the earliest signalling events upstream of ROS production are likely not affected in crk mutants. Chitin-induced stomatal closure was also compromised in crks, but different crks were affected in chitin-induced stomatal closure compared to flg22 (Fig 11A) suggesting that CRKs could provide signalling specificity.
Even though powdery mildew fungi do not use stomata as an infection route but directly penetrate epidermal cells, several crks impaired in chitin-induced stomatal immunity also displayed enhanced susceptibility to Go. This indicates that CRKs are also signalling components in guard cell function-independent plant defence responses. Interestingly, crk mutants that were impaired in stomatal immunity were not altered in ABA-induced stomatal closure (Fig 11A). Together, this indicates that many CRKs might fulfil independent functions in PAMP - or ABA-triggered processes in guard cells (Fig 11A), while a few CRKs might control common, and presumably basal, aspects of guard cell function (Fig 11B). While CRKs in Arabidopsis thaliana are involved in immune signalling evidence from legumes suggests that CRKs might also participate in the control of symbiosis or even in distinguishing between pathogenic or beneficial microbes [22]. This suggests that there might be additional functions for CRKs which cannot be addressed in Arabidopsis.
The most prominent feature of the CRK protein family is the presence of two cysteine-rich DUF26 domains (with C-X8-C-X2-C-motifs) in the extracellular region. Structural analysis of the DUF26 domain of ginkbilobin-2 (Gnk2), a Ginkgo biloba protein containing a single DUF26 domain with a proposed function as an antimicrobial protein, suggests that the cysteines form disulphide bonds [67,68]. The role of the CRK ectodomain is still unknown. It could either bind a ligand (peptide or other) or be crucial for the formation of complexes with other receptors. Recently, it has been suggested that the DUF26 domain in Gnk2 might be involved in mannose binding [69]. The residues required for mannose binding in Gnk2 are, however, not conserved in the DUF26 domains of the CRKs. It has also been suggested that the cysteines in the DUF26 domain could be a target for redox modification which might lead to a conformational change, for example through opening of disulphide bridges. However, redox regulation of CRK ectodomain structure and ligand binding might not be mutually exclusive.
A connection between CRK function and redox or ROS-related processes is also suggested by the strict and specific regulation of genes encoding CRKs under ROS-producing conditions [7,19]. Through this CRKs might participate in feedback regulation of ROS production where they might sense extracellular ROS and be part of a “ROS amplification loop”. This would place the CRKs in the “ROS wave” [70] by perceiving ROS from neighbouring cells and transducing the signal into the cytosol, subsequently regulating NADPH oxidase activity and signal propagation (Fig 11B). This is particularly interesting since the precise control and adjustment of ROS production in response to different stimuli is still unresolved even though activation and regulation of NADPH oxidases and other ROS-producing enzymes through protein phosphorylation and RLKs is becoming better understood. Proteins with DUF26 domains are restricted to plants but other cysteine-rich domains could fulfil analogous functions to CRKs with respect to sensing of extracellular ROS in other organisms. The data shown here suggests that one of the main functions of CRKs could be to provide signalling specificity downstream of extracellular ROS production. However, it is unclear how this regulation might work exactly. Recent evidence suggests that CRKs might be able to interact with pattern recognition receptors [22] but the specificity and the precise role of this interaction will require further investigation. It suggests however, that CRKs might act in concert with other receptors and RLKs, possibly also during the regulation of plant development and during abiotic stress responses.
From the phenotypic framework (Figs 2 and 10) it will now be possible to dissect the molecular mechanisms through which the CRKs function. Conceptually, perception of cell-to-cell or environmental signals through CRKs could follow different modes of action as positive or negative regulators. However, it is likely that CRKs have multiple rather than single downstream targets. How CRK signalling is integrated in synergistic or antagonistic fashion might be highly process specific. The genetic and phenotypic framework and the proposed models for modes of CRK action will allow targeted and detailed mechanistic analysis of CRK function in the future. Ultimately, this will allow improvement of plant growth and tolerance to complex environmental challenges. Our results demonstrate that discovery of subtle phenotypic responses and aspects, which might otherwise be missed, can be facilitated with thorough phenotypic analysis of comprehensive mutant collections for large gene families, instead of studying individual family members.
Materials and Methods
Plant materials
All T-DNA insertion crk lines were obtained from the Nottingham Arabidopsis Stock Centre (NASC, http://nasc.life.nott.ac.uk/) and were confirmed by PCR (primers are listed in S1 Table). An age-matched seed collection was generated and used for all experiments. The seed collection has been donated to the European Arabidopsis Stock Centre in Nottingham (http://www.arabidopsis.info) and can be obtained from there.
Growth conditions
Siliques were harvested at maturity and dried at room temperature for 10 days prior to the collection of seeds. Freshly harvested seeds were after-ripened for 3 months at 20°C (approximately 30% relative humidity) in darkness and further used in germination tests.
After stratification for 3 days at 4°C, seeds were grown in a mixture of soil and perlite (3 : 1) or on Jiffy Peat Pellets in the growing room under the following conditions: 8/16 h photoperiod, temperature 22/18°C (day/night, respectively), relative humidity of 70 ± 5%, and PAR (100–150 μmol m-2 s-1). Experiments were performed on 4 week-old plants, unless otherwise stated. For pathogen assays and stomatal analysis Arabidopsis thaliana plants were grown on general soil (Arabidopsis mix, John Innes Centre, Norwich), or for infection assays on Jiffy pellets (Jiffy Products, Norway) under 10 h or 16 h of light at 20–22°C and 65% humidity. Mutant fls2 lines have been described previously [71].
O3 and light stress experiments
O3 exposure and high-light treatments started at 9 am and were continued for 6 h. 18-day-old plants were used for O3 experiments. Leaf rosettes were harvested 7 h after the start of the treatment then washed with ultra-pure water and transferred into 15 ml ultra-pure water for electrolyte leakage measurements (n = 4). Experiments were repeated twice.
Ozone (350 ppb) and high-light (1430 μmol m-2s-1 photosynthetically available radiation [PAR; 400–700 nm], 11 mW m-2 UV-B radiation [280–315 nm], 25.4 W m-2 UV-A radiation [315–400 nm]) treatments were performed at the Research Unit Environmental Simulation of the Helmholtz Zentrum München (Germany) in the walk-in-size chambers and in a small sun simulator respectively. Spectral measurements were performed using a double monochromator system TDM300 (Bentham, Reading, England). Arabidopsis plants were cultivated on multiplication substrate (Floradur) mixed with quartz sand (Dorfner) in the respective ratio 5 : 1. After a 2-day pretreatment at 4°C, pots were cultivated under the following conditions: 250 μmol m-2 s-1 PAR under the exclusion of UV radiation (<400 nm), under 12h-day length (day: 23°C, 70% relative humidity; night: 18°C, 90% relative humidity).
Xanthine-xanthine oxidase experiments
Extracellular superoxide was generated by vacuum infiltration of 1 mM xanthine (X) and 0.1 U ml-1 xanthine oxidase (XO, Sigma-Aldrich) into the leaf discs from 4-week-old plants as previously described [33,72]. Cell death was monitored by electrolyte leakage measurements with a conductivity meter (Mettler Toledo) at the indicated times after the end of the treatment.
Confocal microscopy and image analysis
High-throughput confocal imaging was performed using the Opera microscope (PerkinElmer, Germany) as published [73]. For quantification of cell numbers, cotyledons were stained with propidium iodide according to Lucas et al. [74] and measured using PDQUANT as described previously [73].
Pathogen inoculation
Bacterial inoculation assays were performed as described previously [75]. Briefly, Pto DC3000 was sprayed onto leaf surface at 108 CFU ml-1 and disease symptoms were scored 3 days post inoculation.
For mildew infection, plants were grown under 8/16 h photoperiod (200 μmol m-2 s-1) at 22°C ± 1°C. Three to four week old plants were inoculated in a settling tower with about 1 spore per mm2 of a virulent Golovinomyces orontii (Go) powdery mildew isolate or 10 spores per mm2 of a non-host Blumeria graminis f. sp. hordei (Bgh) powdery mildew isolate. Symptoms and mildew coverage was assessed after 6 days. Mildew coverage in percent per plant was scored from digital images using the image processing software ImageJ (http://imagej.nih.gov/ij/). The experiment was conducted three times with 5 replicate plants.
Quantitative real-time reverse transcription polymerase chain reaction (qPCR) analysis
Plants for qPCR were grown as above, samples were taken at 6, 16, and 24 h after inoculation with Go or Bgh. Five plants were pooled into one sample and the experiment was conducted three times. RNA extraction and cDNA synthesis was as described earlier [46] (primer sequences for CRK transcripts [7]). Relative CRK gene expression was analyzed by the comparative CT method. CT values were normalized to 18S rRNA and expression between uninfected control and Go/Bgh powdery mildew infected plants were compared using the 2-ΔΔCt method. Significant differences were determined according to student’s t test.
Guard cells were isolated as described [76] and cDNA was generated from RNA isolated from guard cell protoplasts. Expression of CRKs was compared with cDNA from total RNA isolated from untreated Arabidopsis leaves. Actin-2 (At3g18780), YLS8 (At5g08290), PP2A (At1g13320), TIP41 (At4g34270), and At4g35510 were used as normalization genes for the analysis using the 2-ΔΔCt method.
Bioassays for PAMP-induced responses
ROS assays were performed as described previously [41]. Briefly, 16 leaf discs were excised per genotype of four weeks-old plants and treated with 1 μM flg22. ROS was measured with a Varioskan multiplate reader (Thermo Fisher Scientific, USA) for 35 min.
Electrolyte leakage measurement after UV-A and-B treatment
For ultraviolet light source, UVC 500 Crosslinker (Hoefer Pharmacia Biotech, San Francisco, CA, USA) equipped with three UV-B lamps (type G8T5E, Sankyo Denki, peak wavelength 306 nm) and two UV-A lamps (type TL8WBLB, Philips, peak wavelength 365 nm) were used. Plants were exposed to single radiation episode until a cumulative dose of 1500 mJ cm-2 was reached (roughly 10 minutes). After 4 days leaves were excised, fresh weight measured (g) and transferred into 50 ml falcon tubes containing 35 ml MilliQ water. The relative electrolyte leakage was measured with a conductance meter (WTW, INOLAB Cond Level 1) and calculated as a ratio between the value obtained after 1 h incubation and the total electrolyte leakage evaluated after autoclaving the samples.
Analysis of the fluorescence transients (O-J-I-P test) upon MV and DCMU treatment
The O-J-I-P test was performed as described [77] using FluorCam and the associated software (Photon System Instruments, Czech Republic). Plants were dark-adapted for 30 min prior to measurement. The maximum quantum yield of primary photochemistry (φPo), the size of the plastoquinone pool (qPQ) and the total dissipation of untrapped excitation energy from photosystem II (PSII) reaction center (DLo/RC) were calculated. φPo represents the probability that an absorbed photon is trapped by the reaction center and used for primary photochemistry, reducing QA to QA-. Analysis of the fluorescence transients was made on whole rosettes and included two sets of plants: one set sprayed with 32 μM MV and kept in 8/16 photoperiod for two days and a second set sprayed with 20 μM DCMU (dissolved in 75% ethanol) and kept in darkness for two hours.
Endosperm rupture assays
To assess germination of crk mutants, endosperm rupture assays were performed by placing after-ripened seeds in 9 cm Petri dishes (30 seeds per dish, three independent biological replicates) on 1% agar with addition of 0.01% PPM (Plant Preservative Mixture, Plant Cell Technology, USA). All assays were performed at 20°C under PAR of 200 μmol m-2 s-1. Testa and endosperm rupture were assessed every 5 hours up to 51 hours of imbibition. A seed was considered as germinated when the radicle protruded through both envelopes.
Root length, bolting, flowering and senescence assays
Seeds were sterilized, germinated and grown as described [78]. Root length measurements were performed 8 days after stratification in two independent assays with 6 plants each. Interesting lines were additionally screened twice.
To measure bolting, flowering and senescence germinated seeds were transferred to soil and grown with 16 h-light/8 h-dark photoperiods at 22°C. Plants were considered bolting at the first appearance of the inflorescence, flowering at the opening of the flower petals and senescing at the first yellowing of the rosette leaves.
Salt stress
Salt treatment assays were performed by placing seeds in Petri dishes (15 seeds per lines, three independent biological replicates) on MS medium containing 1% sucrose, 1% agar, buffered to pH 5.7 with 2.8 mM MES, with or without 120 mM NaCl; under 12h-day length (100–150 μmol m-2 s-1, day: 22°C—night: 19°C, 70% relative humidity). A seed was considered as germinated when the 2 cotyledons were visible. For each line, germination rate was expressed as the ratio (germination percentage on NaCl plates /germination percentage on control plates), at 5 and 6 days after stratification.
Fresh weight (for water loss analysis)
Plants were grown as described in the plant materials section. Weight of detached whole rosettes was followed until 4 h at room temperature (five 3-week old plants per lines, for three independent biological replicates).
Whole plant gas exchange experiments
To analyse steady-state stomatal conductance and stomatal responses to darkness, elevated CO2 and O3, 21–26 days old plants and a custom made gas exchange device were used. As crk2 had reduced growth rate, older plants (26 to 32 days old) were analysed. The device and plant growth conditions have been described previously [36,79]. First, plants were inserted into the device and kept at 150 μmol m-2 s-1 light, 65% air humidity and ambient CO2 concentration (400 ppm) until stomatal conductance had stabilized. To address stomatal response to darkness, light was switched off, to address elevated CO2-induced stomatal closure, CO2 concentration was increased to 800 ppm and in order to address stomatal closure induced by O3, a three min pulse of 500–600 ppb of O3 was applied. In all experiments, stomatal conductance was followed for 32 min from application of closure-inducing stimuli.
Complementation lines
CRK2 and CRK45 coding regions were cloned into pDONRzeo (Invitrogen) via Gateway site-specific recombination. Coding regions were then assembled together with CaMV 35S-promoter and Venus YFP [80] C-terminal tag into the pBm43GW [81] expression vector, using MultiSite Gateway technology (Invitrogen), creating a translational fusion protein. Primers are listed in S1 Table. Constructs were transformed into the corresponding T-DNA insertion plants by GV3101 Agrobacterium-mediated floral dipping [82]. For selection of successful transformants, seeds were plated on ½ MS media supplemented with 1% sucrose and 20 μg/mL Basta. Plants were grown for one week in vitro before being transferred to soil. T1 plants were used for experiments with each plant constituting an individual insertion event.
CRK5 complementation/over-expression lines have been described in Burdiak et al. [28]. Two individual homozygous T3 lines with a single insert were used.
Data analysis
All data analysis was carried out using R, version 2.15.1.
Estimation of Z scores
All experiments were made commeasurable by computing the Z score statistic of the comparison of each of the alleles versus the Col-0 wild type reference using appropriate statistical test for the given data set. Sample sizes across different experiments were normalised by bootstrap sampling such that in each bootstrap data set the number of samples for a given condition was set to n = 15. The Z score for each experiment was computed as the mean of the Z score estimates from bootstrap data sets. In cases where the null distribution of the statistical test did not follow Z distribution (e.g. with Mann-Whitney test), it was approximated with the Z-distribution. To reduce the number of false positives produced by the analysis, the Z-scores in each experiment were transformed to P-values and corrected using Benjamini-Hochberg false discovery rate adjustment. The adjusted P-values were then transformed back to obtain adjusted Z-scores. Adjusted Z-score values >2 or < -2 (corresponding to a false discovery rate < 5%) were considered to be statistically significant. In experiments involving a time course and many measurements, each time point was analysed separately to have statistical power comparable to non-time course experiments.
The list of specific statistical models and comparisons that were used to get Z scores and subsequently used for construction of heat maps are displayed in S6 Table. Unless stated otherwise, the Z score statistic from the statistical model was obtained comparing each crk genotype to Col-0 wild type.
Comparisons applying linear models were carried out with multcomp R package (http://cran.r-project.org/web/packages/multcomp/index.html). Heatmap of adjusted Z scores was constructed using hierarchical clustering with Ward’s method and applying Euclidean distance metric.
Estimation of phylogenetic trees
Phylogenetic trees were estimated using MEGA6 [83]. Alignments were carried out using Muscle [84,85]. Trees for the entire coding region were estimated using all positions while trees for the kinase domains and extracellular regions were estimated using complete deletion for gaps. The initial guide tree was estimated using maximum parsimony. 1000 bootstrap replicates were used for all trees.
Estimation of statistical significances
Statistical significances were estimated by constructing separate linear mixed model for each of the crk genotypes including Col-0 wild type as the reference data, modelling different time points as fixed effects. Comparisons were carried out using multcomp package for R (http://cran.r-project.org/web/packages/multcomp/index.html) and applying single step p-value adjustment for multiple comparisons. In light stress and fresh weight experiments, the model consisted of genotype, time and their interaction as fixed effects, and experiment replicate as random effect. Each crk genotype was compared to Col-0 in all time points. In O3, X+XO, salt experiments, the linear mixed model consisted of genotype, time and their interaction as fixed effects, and experiment replicate as random effect. Each crk genotype was compared to Col-0 under treatment in all time points.
In Go scoring, the leaf coverage percentage was modelled with genotype as fixed effect and experiment replicate as random effect. In germination assay analysis, the linear mixed model consisted of genotype, time and their interaction as fixed effects. In both experiments, each crk genotype was compared to Col-0.
Gene expression data
Pre-processing of the gene expression data and the accession numbers in databases has been previously described [86]. In brief, the data was downloaded from NASCArrays (http://affymetrix.arabidopsis.info/narrays/experimentbrowse.pl), ArrayExpress (http://www.ebi.ac.uk/microarrayas/ae/), Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), and The Integrated Microarray Database System (http://ausubellab.mgh.harvard.edu/imds). Arrays were normalised with Robust Multi-array Average (RMA) [87], and log2 ratio of the mean of treatment and control expressions across biological replicates was computed, resulting in 141 differential expression profiles. Bayesian Hierarchical Clustering of CRKs present on the arrays was carried out using R package BHC [88] using log2 fold change ±1 as discretization threshold.
Supporting Information
Zdroje
1. Shiu SH, Bleecker AB (2003) Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132 : 530–543. 12805585
2. Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, et al. (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16 : 1220–1234. 15105442
3. Bojar D, Martinez J, Santiago J, Rybin V, Bayliss R, et al. (2014) Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. Plant J 78 : 31–43. doi: 10.1111/tpj.12445 24461462
4. Oh MH, Wang X, Kota U, Goshe MB, Clouse SD, et al. (2009) Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc Natl Acad Sci USA 106 : 658–663. doi: 10.1073/pnas.0810249106 19124768
5. Lehti-Shiu MD, Shiu SH (2012) Diversity, classification and function of the plant protein kinase superfamily. Philos Trans R Soc Lond B Biol Sci 367 : 2619–2639. doi: 10.1098/rstb.2012.0003 22889912
6. Munné-Bosch S, Queval G, Foyer CH (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 161 : 5–19. doi: 10.1104/pp.112.205690 23151347
7. Wrzaczek M, Brosché M, Salojärvi J, Kangasjärvi S, Idänheimo N, et al. (2010) Transcriptional regulation of the CRK/DUF26 group of receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biol 10 : 95. doi: 10.1186/1471-2229-10-95 20500828
8. Chae L, Sudat S, Dudoit S, Zhu T, Luan S (2009) Diverse transcriptional programs associated with environmental stress and hormones in the Arabidopsis receptor-like kinase gene family. Mol Plant 2 : 84–107. doi: 10.1093/mp/ssn083 19529822
9. Chen Z (2001) A superfamily of proteins with novel cysteine-rich repeats. Plant Physiol 126 : 473–476. 11402176
10. Amari K, Boutant E, Hofmann C, Schmitt-Keichinger C, Fernandez-Calvino L, et al. (2010) A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Pathog 6: e1001119. doi: 10.1371/journal.ppat.1001119 20886105
11. Lee JY, Wang X, Cui W, Sager R, Modla S, et al. (2011) A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 23 : 3353–3373. doi: 10.1105/tpc.111.087742 21934146
12. Caillaud MC, Wirthmueller L, Sklenar J, Findlay K, Piquerez SJ, et al. (2014) The plasmodesmal protein PDLP1 localises to haustoria-associated membranes during downy mildew infection and regulates callose deposition. PLoS Pathog 10: e1004496. doi: 10.1371/journal.ppat.1004496 25393742
13. Chen K, Du L, Chen Z (2003) Sensitization of defense responses and activation of programmed cell death by a pathogen-induced receptor-like protein kinase in Arabidopsis. Plant Mol Biol 53 : 61–74. 14756307
14. Chen K, Fan B, Du L, Chen Z (2004) Activation of hypersensitive cell death by pathogen-induced receptor-like protein kinases from Arabidopsis. Plant Mol Biol 56 : 271–283. 15604743
15. Ohtake Y, Takahashi T, Komeda Y (2000) Salicylic acid induces the expression of a number of receptor-like kinase genes in Arabidopsis thaliana. Plant Cell Physiol 41 : 1038–1044. 11100776
16. Czernic P, Visser B, Sun W, Savoure A, Deslandes L, et al. (1999) Characterization of an Arabidopsis thaliana receptor-like protein kinase gene activated by oxidative stress and pathogen attack. Plant J 18 : 321–327. 10377997
17. Du L, Chen Z (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen - and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J 24 : 837–847. 11135117
18. Marshall A, Aalen RB, Audenaert D, Beeckman T, Broadley MR, et al. (2012) Tackling drought stress: receptor-like kinases present new approaches. Plant Cell 24 : 2262–2278. doi: 10.1105/tpc.112.096677 22693282
19. Lehti-Shiu MD, Zou C, Hanada K, Shiu SH (2009) Evolutionary history and stress regulation of plant receptor-like kinase/pelle genes. Plant Physiol 150 : 12–26. doi: 10.1104/pp.108.134353 19321712
20. Wrzaczek M, Brosché M, Kangasjärvi J (2013) ROS signaling loops—production, perception, regulation. Curr Opin Plant Biol 16 : 575–582. doi: 10.1016/j.pbi.2013.07.002 23876676
21. Acharya BR, Raina S, Maqbool SB, Jagadeeswaran G, Mosher SL, et al. (2007) Overexpression of CRK13, an Arabidopsis cysteine-rich receptor-like kinase, results in enhanced resistance to Pseudomonas syringae. Plant J 50 : 488–499. 17419849
22. Yeh Y-H, Chang Y-H, Huang P-Y, Huang J-B, Zimmerli L (2015) Enhanced Arabidopsis pattern-triggered immunity by overexpression of cysteine-rich receptor-like kinases. Frontiers in Plant Science 6 : 322. doi: 10.3389/fpls.2015.00322 26029224
23. Ederli L, Madeo L, Calderini O, Gehring C, Moretti C, et al. (2011) The Arabidopsis thaliana cysteine-rich receptor-like kinase CRK20 modulates host responses to Pseudomonas syringae pv. tomato DC3000 infection. J Plant Physiol 168 : 1784–1794. doi: 10.1016/j.jplph.2011.05.018 21742407
24. Berrabah F, Bourcy M, Eschstruth A, Cayrel A, Guefrachi I, et al. (2014) A nonRD receptor-like kinase prevents nodule early senescence and defense-like reactions during symbiosis. New Phytol 203 : 1305–1314. doi: 10.1111/nph.12881 24916161
25. Tanaka H, Osakabe Y, Katsura S, Mizuno S, Maruyama K, et al. (2012) Abiotic stress-inducible receptor-like kinases negatively control ABA signaling in Arabidopsis. Plant J 70 : 599–613. doi: 10.1111/j.1365-313X.2012.04901.x 22225700
26. Bassel GW, Glaab E, Marquez J, Holdsworth MJ, Bacardit J (2011) Functional network construction in Arabidopsis using rule-based machine learning on large-scale data sets. Plant Cell 23 : 3101–3116. doi: 10.1105/tpc.111.088153 21896882
27. Idänheimo N, Gauthier A, Salojärvi J, Siligato R, Brosché M, et al. (2014) The Arabidopsis thaliana cysteine-rich receptor-like kinases CRK6 and CRK7 protect against apoplastic oxidative stress. Biochem Biophys Res Commun 445 : 457–462. doi: 10.1016/j.bbrc.2014.02.013 24530916
28. Burdiak P, Rusaczonek A, Witon D, Glow D, Karpinski S (2015) Cysteine-rich receptor-like kinase CRK5 as a regulator of growth, development, and ultraviolet radiation responses in Arabidopsis thaliana. J Exp Bot in press.
29. Vanholme B, Vanholme R, Turumtay H, Goeminne G, Cesarino I, et al. (2014) Accumulation of N-acetylglucosamine oligomers in the plant cell wall affects plant architecture in a dose-dependent and conditional manner. Plant Physiol 165 : 290–308. doi: 10.1104/pp.113.233742 24664205
30. Ferro M, Brugiere S, Salvi D, Seigneurin-Berny D, Court M, et al. (2010) AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol Cell Proteomics 9 : 1063–1084. doi: 10.1074/mcp.M900325-MCP200 20061580
31. Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjolander K, et al. (2004) The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol 14 : 354–362. 15028209
32. Göhre V, Jones AM, Sklenar J, Robatzek S, Weber AP (2012) Molecular crosstalk between PAMP-triggered immunity and photosynthesis. Mol Plant Microbe Interact 25 : 1083–1092. doi: 10.1094/MPMI-11-11-0301 22550958
33. Wrzaczek M, Brosché M, Kollist H, Kangasjärvi J (2009) Arabidopsis GRI is involved in the regulation of cell death induced by extracellular ROS. Proc Natl Acad Sci USA 106 : 5412–5417. doi: 10.1073/pnas.0808980106 19279211
34. Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, et al. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22 : 2623–2633. 12773379
35. Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE (2012) Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos Trans R Soc Lond B Biol Sci 367 : 547–555. doi: 10.1098/rstb.2011.0272 22232766
36. Kollist T, Moldau H, Rasulov B, Oja V, Ramma H, et al. (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol Plant 129 : 796–803.
37. Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141 : 373–378. 16760490
38. Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18 : 465–476. 16377758
39. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 : 265–276. 10377992
40. Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, et al. (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 54 : 43–55. doi: 10.1016/j.molcel.2014.02.021 24630626
41. Mersmann S, Bourdais G, Rietz S, Robatzek S (2010) Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol 154 : 391–400. doi: 10.1104/pp.110.154567 20592040
42. Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11: e1001513. doi: 10.1371/journal.pbio.1001513 23526882
43. Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, et al. (2009) RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol 7: e1000139. doi: 10.1371/journal.pbio.1000139 19564897
44. Lozano-Durán R, Bourdais G, He SY, Robatzek S (2014) The bacterial effector HopM1 suppresses PAMP-triggered oxidative burst and stomatal immunity. New Phytol 202 : 259–269. doi: 10.1111/nph.12651 24372399
45. Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126 : 969–980. 16959575
46. Rayapuram C, Jensen MK, Maiser F, Shanir JV, Hornshoj H, et al. (2012) Regulation of basal resistance by a powdery mildew-induced cysteine-rich receptor-like protein kinase in barley. Mol Plant Pathol 13 : 135–147. doi: 10.1111/j.1364-3703.2011.00736.x 21819533
47. Micali C, Göllner K, Humphry M, Consonni C, Panstruga R (2008) The powdery mildew disease of Arabidopsis: A paradigm for the interaction between plants and biotrophic fungi. Arabidopsis Book 6: e0115. doi: 10.1199/tab.0115 22303240
48. Jensen MK, Hagedorn PH, de Torres-Zabala M, Grant MR, Rung JH, et al. (2008) Transcriptional regulation by an NAC (NAM-ATAF1,2-CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis. Plant J 56 : 867–880. doi: 10.1111/j.1365-313X.2008.03646.x 18694460
49. Mukhtar MS, Carvunis AR, Dreze M, Epple P, Steinbrenner J, et al. (2011) Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science 333 : 596–601. doi: 10.1126/science.1203659 21798943
50. Karpinski S, Szechynska-Hebda M, Wituszynska W, Burdiak P (2013) Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ 36 : 736–744. doi: 10.1111/pce.12018 23046215
51. Rodriguez AA, Grunberg KA, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129 : 1627–1632. 12177475
52. Manzano C, Pallero-Baena M, Casimiro I, De Rybel B, Orman-Ligeza B, et al. (2014) The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol 165 : 1105–1119. 24879433
53. Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143 : 606–616. doi: 10.1016/j.cell.2010.10.020 21074051
54. Lee S, Seo PJ, Lee HJ, Park CM (2012) A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J 70 : 831–844. doi: 10.1111/j.1365-313X.2012.04932.x 22313226
55. Kranner I, Roach T, Beckett RP, Whitaker C, Minibayeva FV (2010) Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J Plant Physiol 167 : 805–811. doi: 10.1016/j.jplph.2010.01.019 20303611
56. Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422 : 442–446. 12660786
57. Barth C, De Tullio M, Conklin PL (2006) The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot 57 : 1657–1665. 16698812
58. Lai AG, Doherty CJ, Mueller-Roeber B, Kay SA, Schippers JH, et al. (2012) CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci USA 109 : 17129–17134. doi: 10.1073/pnas.1209148109 23027948
59. McInnis SM, Desikan R, Hancock JT, Hiscock SJ (2006) Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytol 172 : 221–228. 16995910
60. Foyer CH, Pellny TK, Locato V, De GL (2008) Analysis of redox relationships in the plant cell cycle: determinations of ascorbate, glutathione and poly (ADPribose)polymerase (PARP) in plant cell cultures. Methods Mol Biol 476 : 199–215. 19157018
61. Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, et al. (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37 : 501–506. 15806101
62. Mühlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mateo A, et al. (2008) Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20 : 2339–2356. doi: 10.1105/tpc.108.059618 18790826
63. Mateo A, Mühlenbock P, Rusterucci C, Chang CC, Miszalski Z, et al. (2004) LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol 136 : 2818–2830. 15347794
64. Miao Y, Lv D, Wang P, Wang XC, Chen J, et al. (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18 : 2749–2766. 16998070
65. Sawinski K, Mersmann S, Robatzek S, Bohmer M (2013) Guarding the green: pathways to stomatal immunity. Mol Plant Microbe Interact 26 : 626–632. doi: 10.1094/MPMI-12-12-0288-CR 23441577
66. Dubiella U, Seybold H, Durian G, Komander E, Lassig R, et al. (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110 : 8744–8749. doi: 10.1073/pnas.1221294110 23650383
67. Miyakawa T, Miyazono K, Sawano Y, Hatano K, Tanokura M (2009) Crystal structure of ginkbilobin-2 with homology to the extracellular domain of plant cysteine-rich receptor-like kinases. Proteins 166 : 247–251.
68. Sawano Y, Miyakawa T, Yamazaki H, Tanokura M, Hatano K (2007) Purification, characterization, and molecular gene cloning of an antifungal protein from Ginkgo biloba seeds. Biol Chem 388 : 273–280. 17338634
69. Miyakawa T, Hatano K, Miyauchi Y, Suwa Y, Sawano Y, et al. (2014) A secreted protein with plant-specific cysteine-rich motif functions as a mannose-binding lectin that exhibits antifungal activity. Plant Physiol 166 : 766–778. doi: 10.1104/pp.114.242636 25139159
70. Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, et al. (2011) ROS signaling: the new wave? Trends Plant Sci 16 : 300–309. doi: 10.1016/j.tplants.2011.03.007 21482172
71. Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, et al. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428 : 764–767. 15085136
72. Wrzaczek M, Vainonen JP, Stael S, Tsiatsiani L, Help-Rinta-Rahko H, et al. (2015) GRIM REAPER peptide binds to receptor kinase PRK5 to trigger cell death in Arabidopsis. EMBO J 34 : 55–66. doi: 10.15252/embj.201488582 25398910
73. Fitzgibbon J, Beck M, Zhou J, Faulkner C, Robatzek S, et al. (2013) A developmental framework for complex plasmodesmata formation revealed by large-scale imaging of the Arabidopsis leaf epidermis. Plant Cell 25 : 57–70. doi: 10.1105/tpc.112.105890 23371949
74. Lucas JR, Nadeau JA, Sack FD (2006) Microtubule arrays and Arabidopsis stomatal development. J Exp Bot 57 : 71–79. 16303827
75. Göhre V, Spallek T, Haweker H, Mersmann S, Mentzel T, et al. (2008) Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol 18 : 1824–1832. doi: 10.1016/j.cub.2008.10.063 19062288
76. Pandey S, X-Q W, Coursol SA, Assmann SA (2014) Preparation and applications of Arabidopsis thaliana guard cell protoplasts. New Phytol 153 : 517–526.
77. Van Heerden PD, Tsimilli-Michael M, Kruger GH, Strasser RJ (2003) Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiol Plant 117 : 476–491. 12675738
78. van Esse GW, van MS, Stigter H, ten Hove CA, Molenaar J, et al. (2012) A mathematical model for BRASSINOSTEROID INSENSITIVE1-mediated signaling in root growth and hypocotyl elongation. Plant Physiol 160 : 523–532. doi: 10.1104/pp.112.200105 22802611
79. Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, et al. (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol 162 : 1652–1668. doi: 10.1104/pp.113.220608 23703845
80. Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, et al. (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20 : 87–90. 11753368
81. Karimi M, De Meyer B, Hilson P (2005) Modular cloning in plant cells. Trends Plant Sci 10 : 103–105. 15749466
82. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 : 735–743. 10069079
83. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30 : 2725–2729. doi: 10.1093/molbev/mst197 24132122
84. Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5 : 113. 15318951
85. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32 : 1792–1797. 15034147
86. Georgii E, Salojärvi J, Brosché M, Kangasjärvi J, Kaski S (2012) Targeted retrieval of gene expression measurements using regulatory models. Bioinformatics 28 : 2349–2356. 22743225
87. Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20 : 307–315. 14960456
88. Savage R, Cooke E, Darkins R, Xu Y (2011) BHC: Bayesian Hierarchical Clustering. R package version 1.8.0.
Štítky
Genetika Reprodukční medicína
Článek Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density ImputationČlánek AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct MechanismsČlánek A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seqČlánek TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive HematopoiesisČlánek Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 7
-
Všechny články tohoto čísla
- LINE-1 Retroelements Get ZAPped!
- /p23: A Small Protein Heating Up Lifespan Regulation
- Hairless Streaks in Cattle Implicate TSR2 in Early Hair Follicle Formation
- Ribosomal Protein Mutations Result in Constitutive p53 Protein Degradation through Impairment of the AKT Pathway
- Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in
- BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma
- Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
- EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in
- Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins
- Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
- A Year of Infection in the Intensive Care Unit: Prospective Whole Genome Sequencing of Bacterial Clinical Isolates Reveals Cryptic Transmissions and Novel Microbiota
- Inference of Low and High-Grade Glioma Gene Regulatory Networks Delineates the Role of Rnd3 in Establishing Multiple Hallmarks of Cancer
- Novel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
- A Novel Locus Harbouring a Functional Nonsense Mutation Identified in a Large Danish Family with Nonsyndromic Hearing Impairment
- Checkpoint Activation of an Unconventional DNA Replication Program in
- A Genetic Incompatibility Accelerates Adaptation in Yeast
- The SMC Loader Scc2 Promotes ncRNA Biogenesis and Translational Fidelity
- Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
- Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation
- JAK/STAT and Hox Dynamic Interactions in an Organogenetic Gene Cascade
- Emergence, Retention and Selection: A Trilogy of Origination for Functional Proteins from Ancestral LncRNAs in Primates
- MoSET1 (Histone H3K4 Methyltransferase in ) Regulates Global Gene Expression during Infection-Related Morphogenesis
- Arabidopsis PCH2 Mediates Meiotic Chromosome Remodeling and Maturation of Crossovers
- AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
- A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seq
- Tempo and Mode of Transposable Element Activity in Drosophila
- The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres
- SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation
- A Genome Scan for Genes Underlying Microgeographic-Scale Local Adaptation in a Wild Species
- TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis
- Analysis of the Relationships between DNA Double-Strand Breaks, Synaptonemal Complex and Crossovers Using the Mutant
- Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools
- Allelic Spectra of Risk SNPs Are Different for Environment/Lifestyle Dependent versus Independent Diseases
- CSB-PGBD3 Mutations Cause Premature Ovarian Failure
- Irrepressible: An Interview with Mark Ptashne
- Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
- Inactivation of Retinoblastoma Protein (Rb1) in the Oocyte: Evidence That Dysregulated Follicle Growth Drives Ovarian Teratoma Formation in Mice
- Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
- Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA
- Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage
- RFX2 Is a Major Transcriptional Regulator of Spermiogenesis
- A Role for Macro-ER-Phagy in ER Quality Control
- Corp Regulates P53 in via a Negative Feedback Loop
- Common Cell Shape Evolution of Two Nasopharyngeal Pathogens
- Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in
- Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression
- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- mTOR Directs Breast Morphogenesis through the PKC-alpha-Rac1 Signaling Axis
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
- Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast
- Encodes a Highly Conserved Protein Important to Neurological Function in Mice and Flies
- Identification of a Novel Regulatory Mechanism of Nutrient Transport Controlled by TORC1-Npr1-Amu1/Par32
- Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly
- Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
- TFIIS-Dependent Non-coding Transcription Regulates Developmental Genome Rearrangements
- Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen
- Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- The Catalytic and Non-catalytic Functions of the Chromatin-Remodeling Protein Collaborate to Fine-Tune Circadian Transcription in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



