-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaYeast Killer Elements Hold Their Hosts Hostage
article has not abstract
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005139
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1005139Summary
article has not abstract
Cytoplasmic linear double-stranded DNA plasmids (or virus-like elements [VLEs]) encoding a secreted toxin, immunity to the toxin, and their own replication and transcription systems were first discovered in the yeast Kluyveromyces lactis by Norio Gunge [1]. The K. lactis toxin, like similar protein toxins produced by similar cytoplasmic linear DNA plasmids in Pichia acacia and Debaryomyces robertsiae [2–4], kills cells by its highly specific tRNA-cleaving activity, cutting next to the anticodon of tRNAGln or tRNAGlu [5].
The immunity protein makes cells insensitive to the corresponding toxin when expressed from one of the cytoplasmic DNA VLEs used as a vector [6] (Fig 1A). However, these immunity proteins are not expressed when their genes are introduced into nuclear circular DNA plasmids under a chromosomal promoter [6]. Now, Meinhardt et al. show that these immunity genes are not expressed because the mRNA is cleaved into multiple small pieces, each polyadenylated [7]. The cytoplasmic DNA VLEs are very A/T rich, and the authors found that the nuclear transcription apparatus recognized several sequences within the immunity gene open reading frame as polyadenylation sites, cleaved the RNA, and appended a polyA 3' tag. To confirm this notion, they recoded the gene, substituting G/C-rich codons for A/T-rich codons, raising the G/C content dramatically. They found that this recoded gene was now well expressed.
Fig. 1. Biology of killer plasmids and viruses of yeasts. 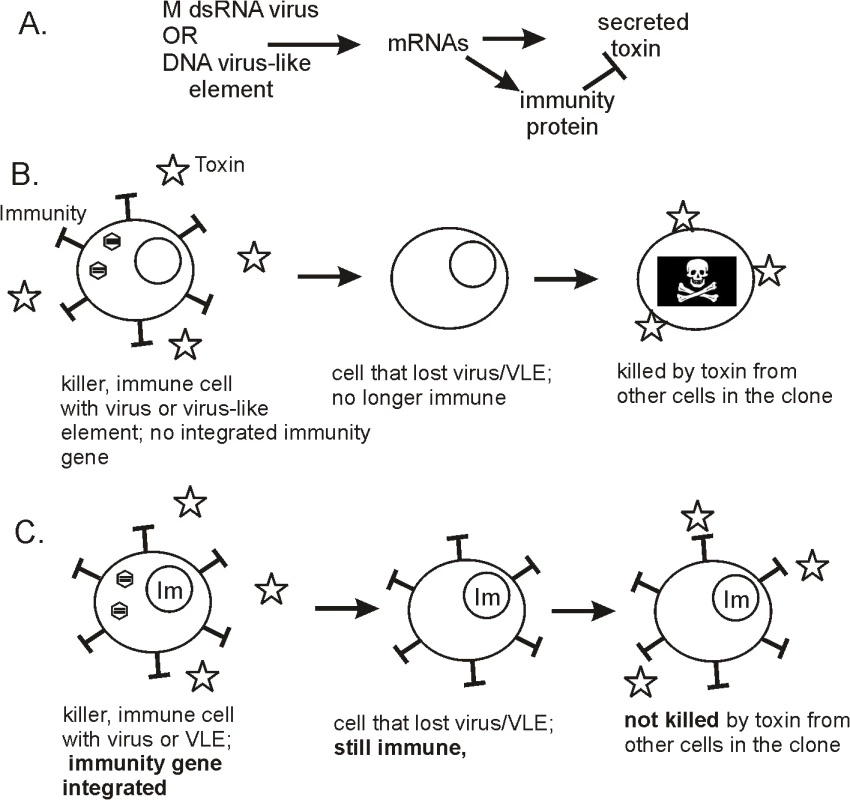
(A) Killer elements, whether dsRNA or DNA based, encode a secreted protein toxin and immunity to the toxin. (B) In a clone of killer cells, if one cell loses the virus or virus-like element (VLE), it no longer makes the immunity protein and becomes sensitive to the killer toxin secreted by other cells in the colony. This mechanism enforces maintenance of the killer dsRNA or DNA. (C) If the gene for the immunity protein is integrated into the cell's genome, then loss of the dsRNA virus or DNA plasmid does not result in sensitivity to the toxin, and maintenance of the virus or VLE is no longer enforced. The authors interpret their results as a demonstration of a virus-like element mechanism to prevent the cells from incorporating the immunity gene into the nucleus [7]. Such a transfer would make cells able to survive loss of the cytoplasmic VLE because even though the immunity gene on the VLE is lost, the cells are still immune because of the chromosomal copy (Fig 1B and 1C). They state, "From an evolutionary point of view, toxin and immunity functions implemented in VLEs have to be considered as players of an autoselection system rather than providing advantages for the respective host, although the latter clearly benefits from the conferred killer phenotype." This view is particularly interesting and could be tested by determining whether the DNA killer plasmids/VLEs are widespread in wild strains or not. If the killer toxin encoding elements are a net advantage to their hosts, their ability to ignore the rules of meiosis, combined with their weaponizing their host, would make them very common. The mitochondria are a familiar, advantageous non-chromosomal genetic element, which is found in all wild yeasts. If the DNA killer VLEs encoding killer toxins are scarce, it would indicate that they have detrimental effects on the host more than balancing the obvious benefit.
This reasoning may apply as well to the yeast killer systems based on double-stranded RNA in virus particles. There are many such dsRNA-based killer systems, but the most studied system is the L-A virus and its toxin - and immunity-encoding satellite RNA, M dsRNA, in Saccharomyces cerevisiae. M dsRNA is encapsidated in and replicated by the Gag major coat protein and Gag-Pol fusion protein, both encoded by L-A dsRNA (reviewed in [8]). The yeast killer toxins are easily detected on plates, but killing is only noticeable when the killer cells far outnumber the sensitive cells. Typically, on plates carefully buffered at the pH optimum for the toxin, about 5 x 106 cells of the toxin-sensitive strain are spread over the entire plate, and a small patch of the killer strain, initially containing perhaps 2 x 107 cells all in one spot, is applied on the lawn. After two days of incubation at 20°C (the optimum temperature for the toxin), the sensitive strain will have formed a lawn except for an area of about 2 mm around the killer strain patch. A killer strain in a small minority among non-killers will not be able to kill other cells.
While the dsRNA-encoded killer toxins provide the host with a weapon, this must not be a very effective weapon. Surveys of wild strains by several groups find only a small minority producing killer toxins. For example, Philliskirk and Young found only 11 killer strains of 592 wild strains of S. cerevisiae examined [9], while Nakayashiki et al. found no killers among 70 wild strains [10].
Meinhardt et al. have found a mechanism by which the DNA VLEs attempt to enforce their maintenance on the host, killing host cells that have the temerity to withdraw the welcome mat [7]. Most cells in a colony will carry the VLEs, and the few that lose it will be subjected to the action of the toxin (Fig 1B). The implication is that the VLEs were selected to have their very high A/T content (>75%) in a host with a much lower A/T (59%) because that would prevent the host from incorporating the immunity gene into a chromosomal locus, an event that would allow escape from the killer toxin action [7] (Fig 1C).
Drinnenberg et al. found that yeast species that have no RNAi system are just those that have dsRNA virus-based killer systems, and they propose that the advantage of having such armament drove the loss of the RNAi system, which would otherwise eliminate the toxin-encoding dsRNA segment [11]. If the dsRNA toxin-immunity systems have the same autoselection role suggested by Meinhardt et al. for the DNA killer systems then they would not be advantageous on the net, and could not select for loss of the RNAi system. If the killer systems were a net benefit to the host, they would be more widespread. The potential of the toxin-immunity-encoding M segments to damage their hosts is suggested by their conditional or absolute lethality to cells defective in the yeast "innate immunity" system comprising the SKI genes [12]. The SKI genes block expression of non-polyA mRNAs, such as these viral mRNAs [13]. The mechanism of lethality in this case is completely unknown, and may be as trivial as ineffective immunity to the overproduced toxin. Perhaps the parasitic dsRNA viruses have selected hosts that, for another reason, lack the RNAi system.
In summary, Meinhardt et al. [7] have presented a clear demonstration of a mechanism for a parasite forcing its host to welcome it. Their insightful interpretation of the evolutionary significance of their results may have application beyond the cytoplasmic DNA systems that they studied.
Zdroje
1. Gunge N, Tamaru A, Ozawa F, Sakaguchi K (1981) Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bateriol 145 : 382–390.
2. Worsham PL, Bolen PL (1990) Killer toxin production in Pichia acaciae is associated with linear DNA plasmids. Curr Genet 18 : 77–80. 2245477
3. Cong YS, Yarrow D, Li YY, Fukuhara H (1994) Linear DNA plasmids from Pichia etchellsii, Debaryomyces hansenii and Wingea robertsiae. Microbiology 140 : 1327–1335. 8081497
4. Klassen R, Meinhardt F (2002) Linear plasmids pWR1A and pWR1B of the yeast Wingea robertsiae are associated with a killer phenotype. Plasmid 48 : 142–148. 12383731
5. Lu J, Huang B, Esberg A, Johansson MJ, Bystrom AS (2005) The Kluyveromyces lactis γ-toxin targets tRNA anticodons. RNA 11 : 1648–1654. 16244131
6. Paluszynski JP, Klassen R, Meinhardt F (2007) Pichia acaciae killer system: genetic analysis of toxin immunity. Appl Environ Microbiol 73 : 4373–4378. 17483256
7. Meinhardt F, Kast A, Voges R, Schroth M, Schaffrath R, Klassen R. (2015) Autoselection of cytoplasmic yeast virus like elements encoding toxin/antitoxin systems involves a nuclear barrier for immunity gene expression. PlOS Genet in press.
8. Wickner RB, Fujimura T, Esteban R (2013) Viruses and prions of Saccharomyces cerevisiae. Adv Virus Res 86 : 1–36. doi: 10.1016/B978-0-12-394315-6.00001-5 23498901
9. Philliskirk G, Young TW (1975) The occurence of killer character in yeasts of various genera. Antonie van Leeuwenhoik J Microbiol Serol 41 : 147–151. 239627
10. Nakayashiki T, Kurtzman CP, Edskes HK, Wickner RB (2005) Yeast prions [URE3] and [PSI+] are diseases. Proc Natl Acad Sci U S A 102 : 10575–10580. 16024723
11. Drinnenberg IA, Fink GR, Bartel DP (2011) Compatibility with killer explains the rise of RNAi-deficient fungi. Science 333 : 1592. doi: 10.1126/science.1209575 21921191
12. Ridley SP, Sommer SS, Wickner RB (1984) Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol Cell Biol 4(4): 761–770. 6371496
13. Masison DC, Blanc A, Ribas JC, Carroll K, Sonenberg N, Wickner RB. (1995) Decoying the cap - mRNA degradation system by a dsRNA virus and poly(A) - mRNA surveillance by a yeast antiviral system. Mol Cell Biol 15 : 2763–2771. 7739557
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



