-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTurning into a Frataxin-Dependent Organism
Iron sulfur (Fe-S) clusters are ubiquitous cofactors found in proteins which function in very diverse pathways ranging from respiration to DNA repair. The mitochondrial Fe-S biogenesis machinery ISC was inherited from the bacterial ancestor of mitochondria. In both prokaryotes and eukaryotes, deficiency of core ISC components is associated with drastic decrease in Fe-S proteins activities and causes severe phenotypes. In this context, the case of frataxin, an ISC associated component, is surprising since the lack of frataxin in prokaryotes leads to very mild phenotypes in comparison to eukaryotes. Here, we showed that in an E. coli strain, a single mutation in a key component of the Fe-S cluster biogenesis pathway, namely the scaffold protein, was sufficient to impose a strict frataxin dependency. Remarkably, this mutation substituted an Ile residue that is conserved in prokaryotic scaffolds, for one Met residue that is conserved in eukaryotic scaffolds. These results provide a lead towards understanding the differences between otherwise highly related prokaryotic and eukaryotic ISC Fe-S cluster biogenesis machineries, and provide a new entry point into deciphering the molecular role of frataxin.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005134
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005134Summary
Iron sulfur (Fe-S) clusters are ubiquitous cofactors found in proteins which function in very diverse pathways ranging from respiration to DNA repair. The mitochondrial Fe-S biogenesis machinery ISC was inherited from the bacterial ancestor of mitochondria. In both prokaryotes and eukaryotes, deficiency of core ISC components is associated with drastic decrease in Fe-S proteins activities and causes severe phenotypes. In this context, the case of frataxin, an ISC associated component, is surprising since the lack of frataxin in prokaryotes leads to very mild phenotypes in comparison to eukaryotes. Here, we showed that in an E. coli strain, a single mutation in a key component of the Fe-S cluster biogenesis pathway, namely the scaffold protein, was sufficient to impose a strict frataxin dependency. Remarkably, this mutation substituted an Ile residue that is conserved in prokaryotic scaffolds, for one Met residue that is conserved in eukaryotic scaffolds. These results provide a lead towards understanding the differences between otherwise highly related prokaryotic and eukaryotic ISC Fe-S cluster biogenesis machineries, and provide a new entry point into deciphering the molecular role of frataxin.
Introduction
Fe-S bound proteins are ubiquitous and involved in a wide variety of cellular processes such as respiration, regulation of gene expression and central metabolism [1,2]. Maturation of Fe-S proteins is an essential cellular process for both eukaryotic and prokaryotic organisms. The mitochondrial ISC Fe-S biogenesis machinery has been proposed to be inherited from a bacterial ancestor, and they function in a similar way by utilizing two major steps: (i) an assembly step in which the cluster forms transiently on a scaffold protein, and (ii) a delivery step in which the cluster is transferred to apotargets via dedicated carriers [3–5]. The ISC scaffold (Isu for eukaryotes / IscU for prokaryotes) contains three conserved cysteine residues that are essential for Fe-S cluster binding and a conserved motif that is specifically recognized by DnaKJ related chaperones/co-chaperones to facilitate cluster release [6–10]. Sulfur is produced from L-cysteine by the cysteine desulfurase, (Nfs1 for eukaryotes / IscS for prokaryotes) a pyridoxal-5’-phosphate (PLP)-dependent enzyme [11–15]. The sulfur is bound in the form of a persulfide to an active-site cysteine residue of the cysteine desulfurase and is subsequently transferred to the scaffold [15–18]. Frataxin (FXN in human, Yfh1 in yeast and CyaY in bacteria) is a protein present in mammals, plants and bacteria [19]. FXN interacts with the cysteine desulfurase/scaffold complex [20–26]. In both prokaryotes and eukaryotes, deficiency of core ISC components including the ISC scaffold or cysteine desulfurase is associated with severely defective Fe-S cluster biogenesis that translates into drastic phenotypes [12–14,26–29]. In contrast, the consequences resulting from deficiency in FXN differ in eukaryotes or prokaryotes. In yeast, deficiency in frataxin (Yfh1) results in defective growth, mitochondrial iron accumulation, decreased heme synthesis, loss of Fe-S cluster protein activity and hypersensitivity to oxidants [30–34]. In humans, altered levels of FXN lead to a drastic decrease in Fe-S protein activities and cause the neurodegenerative disease Friedreich’s ataxia [35–39]. We and others recently established the participation of E. coli frataxin (CyaY) in ISC-assisted biogenesis of Fe-S clusters. Accordingly, ΔcyaY mutants exhibit pleiotropic but mild phenotypes [40–44]. Both the physiological advantage and the molecular reasons underlying this apparent loss in importance of CyaY in prokaryotes remain obscure.
Recently, in their analysis in Saccharomyces cerevisiae, the Dancis lab reported that a point mutation in the scaffold protein Isu1 could bypass a Yfh1 deletion [45]. This demonstrated that a single mutation could make survival of a eukaryote independent of FXN. In the present study, we investigated whether the reverse was true, i.e. could E. coli be turned into a CyaY(FXN)-dependent organism. This proved to be possible and required a single amino acid change in the IscU scaffold as well. Genetic, physiological, biochemical, bioinformatic and phylogenomic approaches were carried out to characterize this E. coli variant. The results of these studies led us to propose an evolutionary scenario according to which frataxin is an ISC-associated factor that appeared in Proteobacteria. It was then acquired by eukaryotes via endosymbiotic mitochondrial event where it became essential. Meanwhile its importance diminished in bacteria possibly because these later contained other Fe-S cluster biogenesis systems, such as SUF in many instances.
Results
CyaY is essential in a “eukaryotized” E. coli
The studies by the Dancis group revealed that the contribution of frataxin to Fe-S cluster biogenesis might depend on the identity of the residue present at position 108 in IscU [45]. To test this hypothesis, we exchanged the 108th ATT Ile codon with an ATG Met codon in the iscU sequence, and the cognate iscUI108M allele was introduced into the E. coli chromosome, giving rise to the BR755 (iscUIM) strain. Moreover, in order to make this strain more eukaryotic-like, we deleted the suf operon encoding the second E. coli Fe-S cluster biogenesis system, giving rise to the BR763 (iscUIM Δsuf) strain. Growth of the BR763 strain in LB or in minimal M9 medium was similar to the reference strain DV901 (Fig 1A and 1B). In contrast, introduction of the cyaY deletion in the iscUIM Δsuf strain had a drastic negative impact on growth in glucose M9 minimal medium (Fig 1B). To test whether the growth defect of the iscUIM Δsuf ΔcyaY strain in minimal medium was related to defects in Fe-S proteins, we tested whether it was auxotrophic for the amino acids Ile, Leu, and Val whose synthesis depends on Fe-S enzymes. Addition of all of the 20 amino acids restored growth, whereas omitting Ile, Leu, and Val failed to rescue growth (Fig 1C). However, adding only Ile, Leu, and Val failed to restore growth showing that Ile, Leu, and Val were necessary but not sufficient. Adding Cys and Met in addition to Ile, Leu, and Val, did not rescue growth of the iscUIM Δsuf ΔcyaY indicating that other processes must also be impaired in this strain. Addition of vitamins improved marginally growth of the iscUIM Δsuf ΔcyaY strain (S1 Fig). In rich medium, the growth defect of the iscUIM Δsuf ΔcyaY strain indicated that, in addition to nutritional requirements, this strain was also impaired in other processes (Fig 1A).
Fig. 1. The iscUIM Δsuf ΔcyaY strain exhibits growth defect. 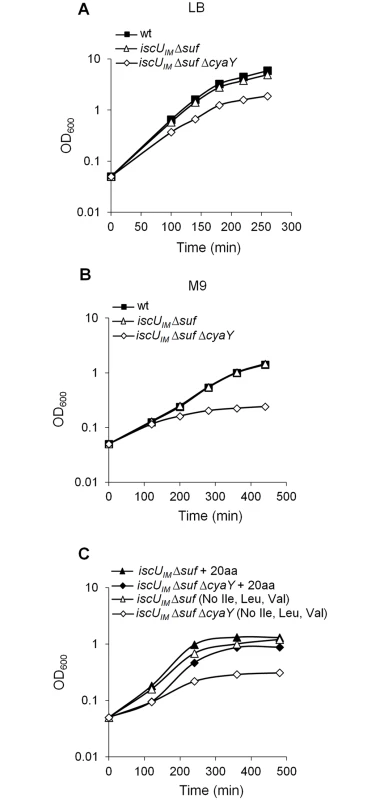
Growth of wt (DV901), iscUIM Δsuf (BR763) and iscUIM Δsuf ΔcyaY (BR767) strains in LB (A). The wt (DV901), iscUIM Δsuf (BR763) and iscUIM Δsuf ΔcyaY (BR767) strains were grown overnight in glucose M9 minimal medium supplemented with all 20 amino acids. Cultures were then diluted into fresh glucose M9 minimal medium (B). Strains iscUIM Δsuf (BR763) and iscUIM Δsuf ΔcyaY (BR767) were grown overnight in glucose M9 minimal medium supplemented with all 20 amino acids. Cultures were then diluted into a fresh glucose M9 minimal medium supplemented with all amino acids or with all except Ile, Leu and Val (C). Growth was monitored at 600 nm. The experiment was repeated at least three times. One representative experiment is shown. A second assay measuring killing efficiency by aminoglycosides (gentamicin, Gm, and kanamycin, Kan) was used. This assay is an indirect read-out of ISC-mediated Fe-S cluster biogenesis efficiency but is independent of SUF functioning. Indeed, uptake of aminoglycosides is dependent upon proton motive force (p.m.f) at the cytoplasmic membrane, which depends upon the activity of Nuo (also called Complex I), a multi-protein complex containing 9 Fe-S clusters, whose maturation depends predominantly on the ISC system and only marginally on the SUF system [46]. The iscUIM strain was found to exhibit wild-type sensitivity to Gm and Kan, whereas the ΔcyaY derivative iscUIM ΔcyaY showed enhanced resistance, again suggesting that ISC dependent Fe-S cluster biogenesis was compromised in the absence of CyaY in this background (Fig 2). As a matter of fact, the iscUIM ΔcyaY strain exhibited a level of resistance similar to that of a ΔiscU strain, illustrating the important contribution of CyaY in a background using a eukaryotic-like IscUIM scaffold. In contrast, the wt strain remained sensitive to Gm and Kan whether or not CyaY was present (Fig 2).
Fig. 2. The iscUIM ΔcyaY strain is resistant to aminoglycosides. 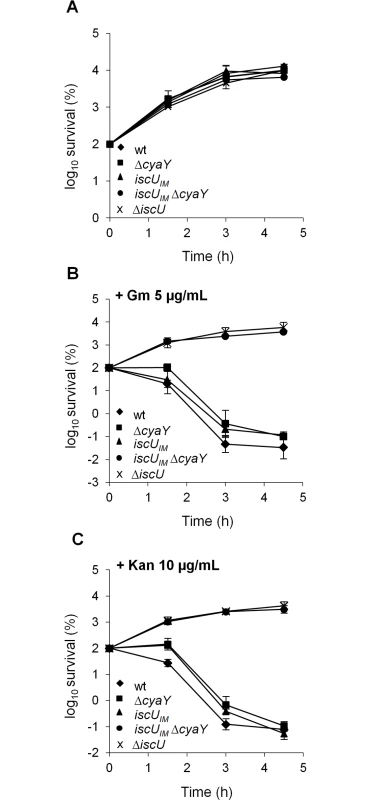
Survival of wt (DV901), iscUIM (BR755) and their ΔcyaY derivatives (DV925 and BR756) without antibiotic (A) and after (B) Gentamicin (Gm) (5 μg/mL) and Kanamycin (Kan) (10 μg/mL) (C) treatment. Survival, measured by colony-forming units (CFU) per mL, was normalized relative to time zero at which the antibiotic was added (midexponential phase cells; ~5 ×107 CFU/mL) and was plotted as log10 of % survival. Error bars represent the standard error from three independent experiments. Because frataxin deficiency, in yeast, led to hypersensitivity to oxidants, we also tested the importance of CyaY in the “eukaryotized” background. Fig 3 shows that introduction of the cyaY deletion in the iscUIM Δsuf strain led to hypersensitivity to hydrogen peroxide and to paraquat, a superoxide generator.
Fig. 3. The iscUIM Δsuf ΔcyaY strain is hypersensitive to oxidative stress. 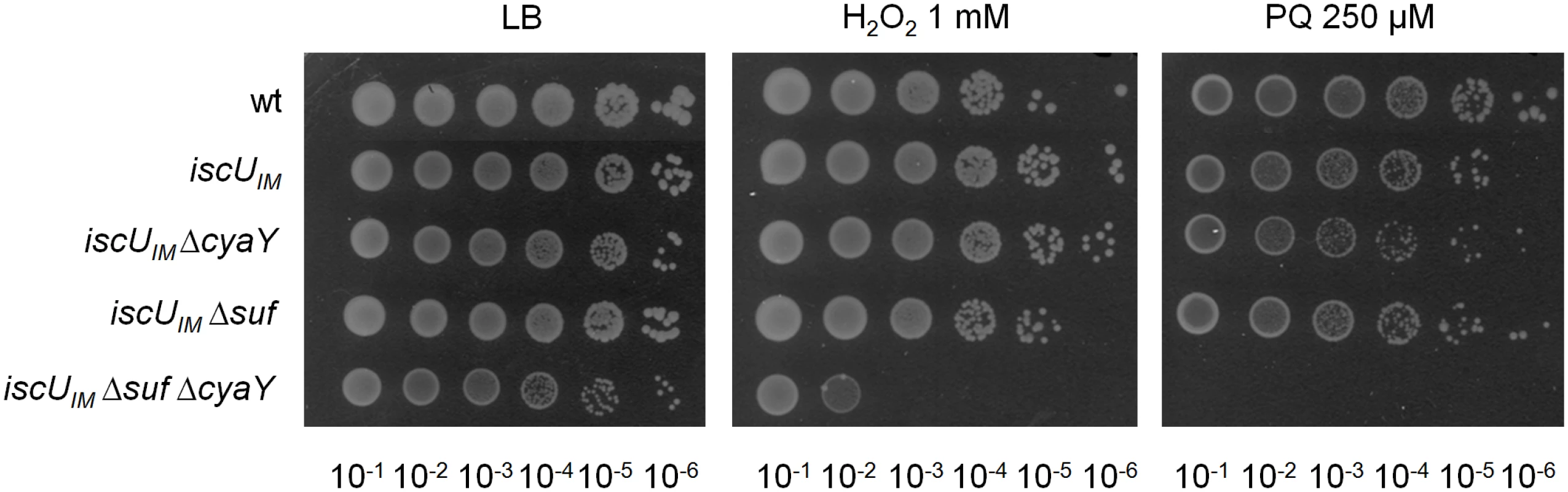
The wt (DV901), iscUIM (BR755), iscUIM ΔcyaY (BR756), iscUIM Δsuf (BR763) and iscUIM Δsuf ΔcyaY (BR767) strains were grown overnight at 37°C in LB medium. Cultures were diluted in sterile PBS, and 5 μL were directly spotted onto LB medium plates containing either 1 mM H2O2 or 250 μM paraquat. Growth was analysed after overnight incubation at 37°C. Each spot represents a 10-fold serial dilution. Altogether, these results indicate that an E. coli lacking SUF can be turned into a frataxin-dependent organism simply by changing a single residue in the IscU scaffold.
The iscUIM-associated defects are due to decreased Fe-S biogenesis
In order to ascertain that the drastic defects observed in the iscUIM ΔcyaY strain were directly due to a dysfunction of Fe-S cluster biogenesis, we tested the activity of several Fe-S cluster-containing proteins. These latter were IscR, a [2Fe-2S] transcriptional regulator, Nuo and Sdh, two multi-protein complexes containing 9 and 3 Fe-S clusters, respectively. In full agreement with the phenotypic tests reported above, introduction of a ΔcyaY mutation in a strain synthesizing the eukaryote-like IscUIM scaffold essentially recapitulated the effect of deleting the scaffold-encoding gene iscU (Fig 4A, 4B and 4C). As a point of comparison, in the iscUIM strain, the IscR, Nuo and Sdh activities were decreased by 1.5–2 fold when compared to the wt strain (Fig 4A, 4B and 4C). Immunoblot analysis of the IscR, Nuo and IscUIM proteins ruled out that the decreased activities were due to reduced amounts of target or scaffold proteins (Figs 4D and S2). Altogether, these results indicate that even though it is a conservative change, a single Ile-to-Met substitution in the IscU scaffold alters Fe-S biogenesis efficiency.
Fig. 4. Activities of Fe-S proteins in iscUIM and ΔcyaY strains. 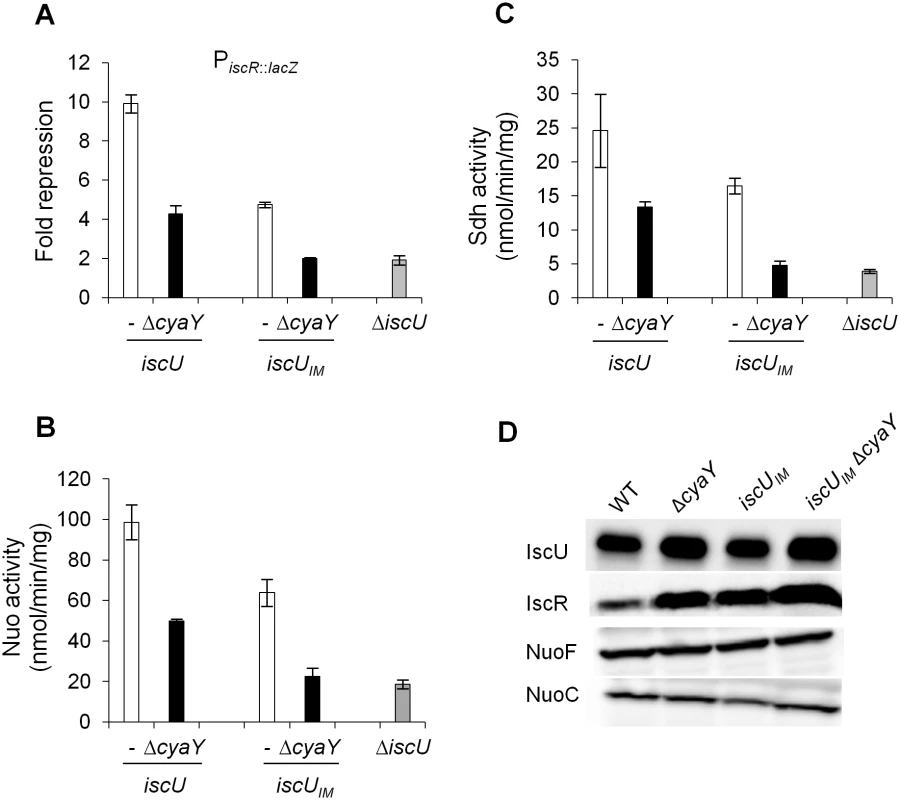
Repression of the IscR-regulated gene (iscR::lacZ) (A), Nuo (B) and Sdh (C) activities in the wt (DV901) (white bars), iscUIM (BR755) (white bars), their ΔcyaY derivatives (DV925, BR756) (black bars), and ΔiscU (BR667) (grey bars) strains. The amount of IscR-dependent repression (fold repression) was determined by dividing the β-galactosidase activity present in the strain lacking IscR (DV915) by the β-galactosidase activity measured for each strain. Error bars represent the standard error from three independent experiments. (D) Cell extracts of indicated strains were subjected to immunoblot analysis using antibodies raised against IscU, IscR, NuoF and NuoC. The IscUIM forms Fe-S cluster at a slower rate
In order to understand the molecular basis for the effect caused by the mutation, the IscUIM protein was submitted to a thorough in vitro analysis. A plasmid encoding a His-tagged IscUIM was constructed, and the tagged protein was purified in large quantities. The CD spectra of IscUIM and IscUWT were similar, indicating that the mutation did not affect the secondary structure of the protein (Fig 5A). Also gel filtration experiments indicated that the IscUIM formed dimers like the IscUWT (S3 Fig).
Fig. 5. Analysis of IscUIM in vitro. 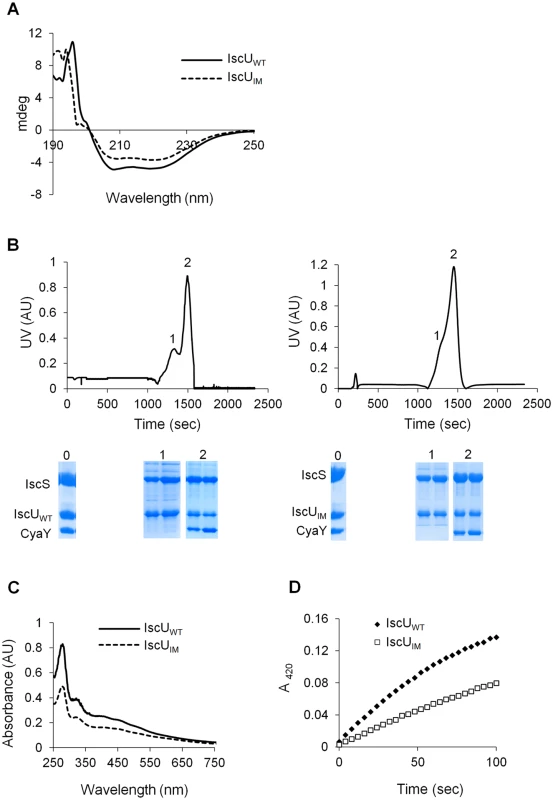
(A) Comparison of the CD spectra (expressed in mdeg) recorded in the region 190–250 nm between IscUWT (filled line) and IscUIM (dotted line). (B) Purified IscS, CyaY and IscUWT (left panel) or IscUIM (right panel) were mixed in 1:1:1 ratio (144 μM of each protein) in the presence of 4-fold excess of Fe(SO4)2(NH4)2, 10-fold excess of L-cysteine and 5 mM DTT and incubated for 40 minutes. The mixture was then loaded onto a QFF column equilibrated with 50 mM Tris pH 8. Proteins were eluted with 50 mM Tris pH 8 containing 1M NaCl. SDS-PAGE analyses have been performed on samples from the column on-put (0) and the peaks 1 and 2 for each mixture. (C) Reconstitution of [2Fe-2S] IscUWT (filled line) and IscUIM (dotted line) followed by UV-visible absorption spectroscopy. Apo-IscUWT or apo-IscUIM (144 μM) were incubated with 5 mM DTT, 1.44 μM IscS, 2 mM L-cysteine and 0.43 mM Fe(SO4)2(NH4)2 in 50 mM Tris-HCl pH 8. (D) Comparison of the kinetics of enzymatic Fe-S cluster formation on IscUWT (black diamonds) and IscUIM (white squares). Experiment was carried out using 25 μM IscUWT or IscUIM, 25 μM IscS, 100 μM Fe(SO4)2(NH4)2, 250 μM L-cysteine, 2 mM DTT. Fe-S cluster formation was followed by absorbance at 420 nm. The experiment was repeated at least three times. One representative experiment is shown. The IscUWT was previously shown to be isolated from complexes together with IscS and IscS-CyaY [23,24]. Therefore, we investigated whether the IscUIM had similar behavior. To this purpose an anion exchange chromatography approach was used. Purified reconstituted IscUWT or IscUIM was mixed anaerobically with molar stoichiometric amount of IscS and CyaY proteins. The mixtures were loaded onto an anion exchange column (QFF), and the collected fractions were analysed by SDS-PAGE. Using IscUWT, a first peak (peak 1), containing IscU and IscS, eluted at 640 mM NaCl while a second major peak (peak 2), which eluted at 780 mM NaCl contained the IscS, IscU and CyaY proteins (Fig 5B left panel). The proteins recovered in peak 1 and peak 2 were part of a complex, since each individual protein, IscUWT, IscS and CyaY eluted from the column at 400, 430 and 530 mM NaCl, respectively (S4 Fig). A similar result was obtained when using IscUIM instead of IscUWT (Figs 5B right panel and S4). Thus, these data show that the ability of IscU to associate with IscS and CyaY was not altered by the Ile-to-Met mutation.
Lastly, we investigated whether IscUIM could assemble a [2Fe-2S] cluster. Fig 5C shows that after anaerobic Fe-S cluster reconstitution, IscUWT and IscUIM displayed similar UV-vis. spectra characteristic of [2Fe-2S] clusters, with absorption maxima at 320, 410 and 456 nm (Fig 5C) [47–49]. However, the rate of Fe-S cluster formation differed between the two. Indeed the rate of Fe-S cluster formation was slowed down by approximately 2-fold when using the IscUIM mutant (Fig 5D). Altogether, these biochemical investigations revealed that the Ile-to-Met mutation specifically altered the efficiency of Fe-S cluster formation on IscUIM, with no major effect on the structure of IscUIM or its capacity to interact with its partners IscS and CyaY.
Evolution of CyaY and IscU in prokaryotes
CyaY contains a single domain of ~100 residues referred to as PF01491 in the Pfam database. By using this domain as a query, we detected 598 homologous proteins within 2742 complete prokaryotic genomes available in the local bank of complete genomes (2 March, 2014) (S1 Table).
Homologs of CyaY were found in Alpha-, Beta-, Gammaproteobacteria, Acidobacteria and Deltaproteobacteria species, and in one representative of Chlorobi phylum (Chloroherpeton thalassium ATCC 35110) (Fig 6). These data indicate that CyaY is not widely distributed among prokaryotes. The absence of a CyaY encoding gene in the ancestor of most bacterial phyla suggests that a CyaY encoding gene was absent in LBCA. The phylogenetic analysis of CyaY also showed that the representatives of Chlorobi and Acidobacteria phylum, which emerge within the Gammaproteobacteria, have probably acquired cyaY gene by HGT (dotted black arrows) (Figs 6 and S5). Altogether, these results suggest that the CyaY protein originated in the bacterial domain, likely in the common ancestor of the Proteobacteria with massive loss in Delta/Epsilonproteobacteria subdivision.
Fig. 6. Model for CyaY protein evolution. 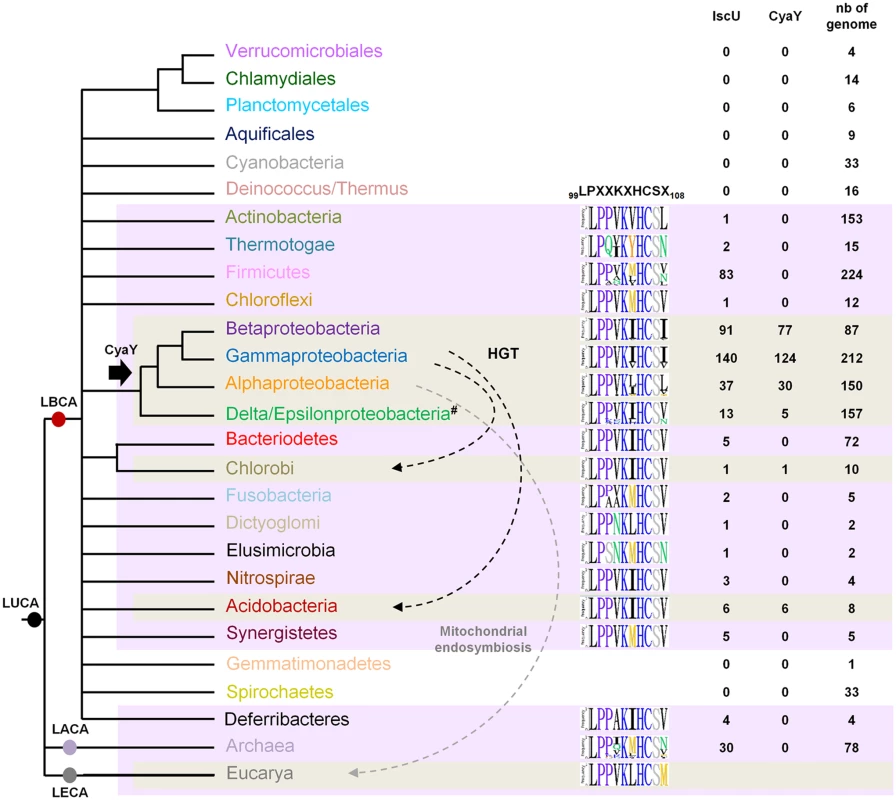
Schematic representation of the universal tree of life, for which complete genome sequences are available. LUCA (Last Universal Common Ancestor), LECA (Last Eukaryotic Common Ancestor), LACA (Last Archaeal Common Ancestor) and LBCA (Last Bacterial Common Ancestor). For each prokaryotic phylum (whose color code is the same as the one used in S5 Fig), the number of genomes encoding a CyaY and a IscU homolog with respect to the number of complete available genomes is given. The black arrow indicates the presence of a CyaY encoding gene in the ancestor of a given lineage. The evolutionary event at the origin of the cyaY gene in the Delta/Epsilon subgroup cannot be definitively inferred. One hypothesis is that the cyaY gene is originated in the common ancestor of the Proteobacteria which together with a probable massive loss of cyaY (#) in Delta/Epsilonproteobacteria subgroup explains the presence of CyaY in the species of the Delta/Epsilonproteobacteria subgroup. Dotted arrows indicate horizontal gene transfer events (HGT) (black) and the mitochondrial endosymbiosis (grey). Sequence-logo of the region 99–108 in IscU homologs is also represented using Phylo-mLogo. This region contains the LPPVK motif and amino acid residues at position 108. IscU homologs were retrieved using the PF01592 domain and were aligned using the multiple alignment program MAFFT v7.045b (S1 Table). We imposed some additional criteria in order for a protein to be considered as an IscU homolog: (i) the presence of the three conserved cysteine residues that are required for the scaffold activity of IscU, (ii) the presence of the sequence that is recognized by the chaperone/co-chaperone system of the ISC system (LPPVK in E. coli IscU) (iii) no other additional domain such as those that could be found in NifU, and (iv) at least one other isc-related gene as a neighbor gene. Using these criteria, well studied U-like proteins such as the SufU protein of Bacillus subtilis and the NifU protein of Azotobacter vinelandii and their close homologs were eliminated. We then showed that all the prokaryotic species that possessed CyaY also contained an IscU homolog. However, the reverse situation was not true, since numerous prokaryotic species possessing IscU did not contain a CyaY encoding gene (Fig 6).
Sequence alignment of the 429 prokaryotic IscU homologs showed that amino acids at position 108 were mostly (369/429) Ile, Leu or Val (Figs 6 and S6). A few IscU examples exhibited a Met or Asn amino acid. Interestingly, an IscUM protein was found in 3 out of 28 species of the Rickettsiales order (Alphaproteobacteria) (Orientia tsutsugamushi str. Boryong, YP_001248706; Neorickettsia risticii str. Illinois, YP_003081518 and Neorickettsia sennetsu str. Miyayama, YP_506192). An IscUM protein was also found in two Archaea species (2 out of 30 species) Methanosarcina barkeri str. Fusaro (YP_305925), and Methanosarcina acetivorans C2A (NP_617616).
Discussion
ISC machineries from both eukaryotes and prokaryotes are considered to be homologous. They share many components including cysteine desulfurases, scaffolds, dedicated-chaperone proteins and A-type carriers. A defect in any of these conserved components provokes a drastic drop in Fe-S cluster biogenesis in either eukaryotes or prokaryotes [12–14,26–29,50,51]. The case of frataxin is different, however, as a lack of FXN in eukaryotes, humans or yeast, is markedly more detrimental than a lack of CyaY in prokaryotes such as E. coli or Salmonella [30–44]. A possible explanation for the difference is that variation within the Fe-S cluster assembly machineries provides different contexts, which in turn make the contribution of FXN of greater importance than that of CyaY. In this regard, it is important to recall that the core eukaryotic ISC system includes a component, Isd11, which interacts with Nfs1 [52–54]. A model was recently proposed according to which the eukaryotic Nfs1 cysteine desulfurase remains in an OFF state unless it interacts with FXN and Isd11 [55–58]. However, no Isd11-like proteins are present in E. coli and this regulation of IscS activity does not apply [52,53].
Recent genetic analysis by the Dancis group showed that modifying part of the ISC machinery could render it independent of FXN. Indeed, in a search for suppressing mutation that could bypass the lack of FXN, these authors identified a mutation in the scaffold-encoding gene ISU1 [45]. The suppressing mutation allowed Isu1 to activate Nfs1, thereby mimicking FXN [55,59]. Remarkably, this mutation changed a Met residue, conserved in eukaryotes, to an Ile residue, conserved into prokaryote IscU proteins. Although largely speculative, this result may open the way to deciphering the contribution of frataxin in the functioning of ISC machineries, and possibly provide a lead towards understanding the differences between prokaryotes and eukaryotes.
In the present work, we carried out a bioinformatic analysis of IscU sequences in prokaryotes. This allowed us to confirm that position 108 was mostly occupied by Ile, as in E. coli, Leu or Val. By contrast, position 108 in prokaryotes was almost never occupied by Met (see below for an exception), which is the situation most frequently encountered in eukaryotes. In an effort to address the importance of this residue experimentally, the Ile residue was changed to Met at position 108 of IscU and expressed into E. coli lacking SUF. The results confirmed the influential role of that position. First, the E. coli strain containing a eukaryotic-like IscUIM became fully dependent on CyaY. Thus, this strain was unable to mature a series of Fe-S cluster containing proteins such the transcriptional regulator IscR, a [2Fe-2S] protein, or Nuo and Sdh, multi-cluster containing enzymes of the electron transport chain. Moreover, such a strain became auxotrophic for various amino acids, including Ile, Leu and Val, the branched amino acids whose synthesis depends on the Fe-S cluster containing proteins, dihydroxy-acid dehydratase (IlvD) and isopropylmalate dehydratase (LeuD). In addition, the strain showed hypersensitivity to oxidative stress, a phenotype linked to FXN deficiency in eukaryotes [39,60,61]. Therefore, a single Ile-to-Met substitution was sufficient to turn E. coli into a frataxin-dependent organism for Fe-S cluster biogenesis.
How could a single conservative Ile-to-Met change have such a crucial impact on Fe-S cluster biogenesis? A hypothesis is that the Ile-to-Met mutation alters the IscU protein, diminishing its efficiency in contributing to the overall Fe-S cluster biogenesis process and that in this context, the contribution of CyaY becomes essential. To test this hypothesis, we carried out a thorough biochemical characterization of the IscUIM variant and could rule out structural or stability defects. This fits with the in vivo observation that the IscUIM protein was as abundant as the wt protein and failed to exhibit instability as assessed by immunoblot analysis. Moreover, we observed that the IscUIM protein interacted with its natural partners, IscS and CyaY, in a mode indistinguishable from the wild type. In contrast, in vitro, IscUIM was found to assemble Fe-S clusters at a rate 2-fold slower than the wild type. Interestingly, these data are consistent with the in vivo observation that E. coli containing a chromosomal copy of the mutated iscU allele was 2-fold less efficient in maturing IscR than the wt strain. Hence, altogether these results support the notion that the Ile-to-Met mutation altered the kinetic formation of Fe-S clusters on IscU. A possible structural explanation for the effect might be that the mutation modifies the accessibility of sulfur or iron for Fe-S cluster intermediate formation, as the 108th position is in close vicinity to the Cys106, one of the three Cys residues acting as ligands. Regardless of the structural basis for this effect, the fact is that this analysis revealed that a eukaryotic-like IscU is slightly less efficient than the E. coli one in assembling a cluster and as a consequence, the contribution of frataxin becomes more significant for helping the process to go on. As previously shown, we observed that via its interaction with IscS, CyaY slowed down the kinetic of Fe-S cluster formation on IscU (S7 Fig) [22,62]. At first this contradicts the view of CyaY acting as a positive effector for Fe-S cluster formation and this has already been discussed at length in the literature [22,62,63]. But what matters here is that CyaY also inhibited, and to the same extent, Fe-S cluster assembly by the IscUIM variant (S7 Fig). This indicated that the CyaY action is not strictly connected to the nature of the residue at position 108. Thus one possibility is that CyaY and IscUIM influence the overall Fe-S cluster biogenesis process at different steps. The fact that the CyaY dependency is not bypassed by increasing amount of the IscUIM scaffold, as indicated in vivo by the CyaY-dependent maturation of IscR when IscUIM was overproduced (S8 Fig), is consistent with this hypothesis. Further biochemical analyses are needed to investigate the possible sites of action in the Fe-S cluster assembly process, such as iron donation, control of sulfur flux, Fe-S cluster transfer to downstream recipients, or HscBA-associated steps, for the CyaY and IscUIM effect.
The involvement of CyaY in Fe-S cluster biogenesis was proposed in the early 2000’s on the basis of co-occurrence of cyaY and hscBA genes [64]. This led to the belief that CyaY would be as conserved as the other ISC components. The reason why the cognate structural gene was not part of the isc operon in bacteria remained unclear. Here, exploiting the larger number of genomes now available for analysis, we reinvestigated the distribution of CyaY and its co-occurrence with the ISC system. Surprisingly, CyaY was found to be much less conserved in eubacteria than previously thought, as its presence was mostly restricted to Alpha-, Beta-, and Gammaproteobacteria. Interestingly, in these bacteria, none of the genes encoding components related to Fe-S cluster biogenesis were to be found in the vicinity of cyaY. Phylogenic analysis revealed that CyaY originated in the last common ancestor of Proteobacteria. This contrasts with the story for A-type Fe-S cluster carriers, which we previously found to be present in the last bacterial common ancestor [65,66]. Even more surprising was the fact that many genomes contained iscU but not cyaY, suggesting that these bacteria learned how to make Fe-S clusters in an ISC-dependent and CyaY-independent way. In contrast, all genomes containing cyaY also contained iscU. Hence overall this leads to picture CyaY as a Fe-S cluster biogenesis factor associated with the ISC machinery in most eukaryotes and in a restricted number of prokaryotes. Interestingly, not only some lineages such as Deltaproteobacteria, but also some species within the Alpha - and Betaproteobacteria have lost CyaY, indicating that there might have been some evolutionary drift favoring organisms that evolve without it. Amino acids encoded by the codon at position 108 of IscU are essentially Ile, Leu or Val. Methionine appears in only two cases, in Methanobacteria and some Rickettsiae species that also have a cyaY gene. Rickettsiae are thought to have given rise to mitochondria via the first endosymbiosis event. Hence, it is tempting to speculate that the current mitochondrial Isu protein originated from the IscUM version that was already present in the ancestor of Rickettsiae.
Based upon the above considerations, one can envision the following scenario: i) Frataxin appeared in the ancestor of the Proteobacteria, and joined the ISC system for Fe-S cluster biogenesis, ii) Mitochondria developed from Proteobacteria by endosymbiosis, in particular from Rickettsiae, acquiring components what would give rise to the actual IsuM and FXN, iii) Proteobacteria acquired SUF, which released the pressure on ISC, and in parallel they explored variation in the ISC scaffold at position 108. In particular, the Met-to Ile, Leu, Val changes happened to improve Fe-S cluster assembly, iv) Frataxin dependency was loosened in Proteobacteria that have a more efficient ISC scaffold and other Fe-S back up system.
Materials and Methods
Bacterial strains and growth conditions
The E. coli K-12 strain MG1655 and its derivatives used in this study are listed in Table 1. Deletion mutations from the KEIO collection were introduced by P1 transduction [67]. Transductants were verified by PCR, using primer pairs hybridizing upstream and downstream of the deleted gene. Strain BR755 producing the IscUI108M variant from a chromosomal copy was constructed as follows: a DNA fragment carrying the iscUI108M allele was obtained after a mutagenesis procedure by overlap extension PCR reactions using the following primer pairs: IscU-UPBamH1/IscUI108M-DO, IscUI108M-UP/IscU-DOXbaI, IscU-UPBamH1/IscU-DOXbaI (S2 Table). This DNA fragment was introduced in a strain in which the iscU gene had been replaced by a cat-sacB cassette as previously described [68]. The Suc-resistant clones were checked for Cm sensitivity, and the appropriate region was sequenced. The iscUI108M allele was transduced into desired strains by using a KanR-linked marker in the yphD gene, which is located close to the iscU gene. The Δsuf mutation was introduced in the iscUIM background strains that contained the eukaryotic Fe-S cluster independent mevalonate pathway for IPP biosynthesis (MVA), in case the combination of iscUIM ΔcyaY would have been lethal [50,51]. This precaution proved to be unnecessary since the iscUIM Δsuf and iscUIM Δsuf ΔcyaY strains could be obtained without the addition of arabinose and mevalonate. Addition of arabinose and mevalonate did not improve growth of the iscUIM Δsuf and iscUIM Δsuf ΔcyaY strains; therefore, all the experiments have been done without. However, the iscUIM Δsuf and iscUIM Δsuf ΔcyaY strains are auxotroph for tryptophan since the MVA synthetic operon was inserted in the trp operon, therefore when grown in M9 glucose minimal medium tryptophan was added [50].
Tab. 1. Bacterial strains and plasmids used in this study. 
Oligonucleotides used in this study are listed in S2 Table. Supplementary strains are listed in S3 Table.
E. coli strains were grown at 37°C in Luria—Bertani (LB) rich medium or in minimal medium (M9) supplemented with glucose (0.4%) and MgSO4 (1 mM). Arabinose (0.2%), amino acids (0.5 mM), sucrose (5%), thiamine (0.2 μg/mL) and nicotinic acid (12.5 μg/mL) were added as required. Solid media contained 1.5% agar. Antibiotics were used at the following concentrations: chloramphenicol 25 μg/mL, kanamycin 30 μg/mL, tetracycline 25 μg/mL, gentamicin 5 μg/mL and ampicillin 50 μg/mL.
Plasmid construction
Plasmid pIscU was constructed by PCR amplification of the coding region of iscU from E. coli MG1655 chromosomal DNA using the following primer pair: NcoI-IscU/HindIII-IscU (S2 Table). The PCR product was then digested by NcoI and HindIII and cloned into the NcoI/HindIII linearized pBAD24 vector.
Production of the IscUIM variant exhibiting a single amino acid substitution isoleucine to methionine at position 108 was obtained by site-directed mutagenesis in the pIscU plasmid to generate pIscUIM using the following primer pair: IscUI108M_for/IscUI108M_rev (S2 Table).
Plasmids pETIscUWT and pETIscUIM were constructed by PCR amplification of the coding region of iscU from E. coli MG1655 chromosomal DNA and from the pIscUIM vector, respectively, using the following primer pair: NdeI-IscU/HindIII-IscU (S2 Table). The PCR products were then digested by NdeI and HindIII and cloned into the NdeI/HindIII linearized pET21a+ vector.
Plasmids pET22b-CyaY and pQE-IscS for production of recombinant E. coli CyaY and IscS, were described previously [69].
Generation of survival curves
Overnight cultures were diluted and grown aerobically in LB at 37°C to an OD600 of 0.2. At this point, antibiotics were added to the cells (Gm at 5 μg/mL and Kan at 10 μg/mL). At different incubation times, 100 μL of cells were diluted in PBS buffer, spotted on LB agar and then incubated at 37°C overnight. Cell survival was determined by counting colony-forming units per mL (CFU/mL). The absolute CFU at time-point 0 (used as the 100%) was ≈ 5x107 CFU/mL.
Paraquat and hydrogen peroxide sensitivity test
Overnight cultures were diluted in sterile PBS and 5 μL were directly spotted onto LB plates containing either paraquat (250 μM) or H202 (1 mM). The plates were incubated overnight at 37°C before growth was scored.
β-Galactosidase assay
Strains were grown at 37°C in LB rich medium, to an OD600 of ~1.5. β-galactosidase assays were carried out as previously described [70].
Enzymatic assays
NADH dehydrogenase activity
NADH dehydrogenase activity was assayed as previously described [71]. Briefly, cells were grown to an OD600 of 0.6–0.8, harvested by centrifugation, resuspended in MES-10% glycerol buffer pH 6.5, and disrupted in a French press. Aliquots of the whole-cell extract were immediately frozen in liquid nitrogen and stored at -80°C until used. Enzymatic activity was measured spectrophotometrically at 30°C by following absorbance at 340 nm in a reaction mixture containing 50 mM MES, pH 6.5, 10% glycerol and 200 μM D-NADH, as a specific substrate. Protein concentration was determined using the protein A280 method on NanoDrop2000 spectrophotometer.
Succinate dehydrogenase activity
Succinate dehydrogenase activity (Sdh) was assayed as described previously [71]. Briefly, cells were grown to an OD600 of 0.6–0.8, harvested by centrifugation, resuspended in MES-10% glycerol buffer pH 6.5, and disrupted in a French press. Following centrifugation (11 000 rpm for 15 min at 4°C), the supernatant was submitted to ultracentrifugation (45 000 rpm for 2 h at 4°C) to obtain the membrane fraction. Sdh activity was assayed for the pellet fraction resuspended in MES-10% glycerol buffer pH 6.5. Because Sdh is partially inhibited by oxaloacetate, the enzyme was first activated by incubation in 50 mM Tris-HCl pH 7.5, 4 mM succinate and 1 mM KCN for 30 min at 30°C [72,73]. Sdh activity was then measured spectrophotometrically at 30°C by following the phenazine ethosulfate (PES)-coupled reduction of DCPIP at 600 nm, in a reaction mixture containing 50 mM Tris-Hcl pH 7.5, 4 mM succinate, 1 mM KCN, 400 μM PES and 50 μM DCPIP. Protein concentration was determined using a NanoDrop2000 spectrophotometer to determine the protein A280.
Western blot analysis
Equal quantities of protein were applied to SDS-PAGE and transferred onto nitrocellulose membranes. The membrane filters were incubated with appropriate antibodies (1/200, 1/2000, 1/2000, 1/150 dilutions of the anti-IscU, anti-NuoF, anti-NuoC and anti-IscR serums, respectively). Immunoblots were developed by using horseradish peroxidase-conjugated goat anti-rabbit antibody, followed by chemiluminescence detection.
Expression and purification of proteins
Recombinant CyaY, IscUWT and IscUIM proteins containing a C-terminal His6 tag were expressed in E. coli and purified as follows: E. coli BL21 (DE3)/pETcyaY was grown in LB medium containing 50 μg/mL ampicillin at 37°C. Protein expression was induced for 4 h by the addition of 0.5 mM isopropyl β-D-thiogalactoside (IPTG) at an OD600 ≈ 0.5. The bacterial pellet was resuspended in buffer A (0.1 M Tris-HCl, pH 8, 500 mM NaCl, 20 mM imidazole) and disrupted in a French press. After centrifugation (15 min, 11 000 rpm, 4°C), the supernatant was loaded onto a 1-mL HisTrap affinity column (GE Healthcare) equilibrated with buffer A. Proteins were eluted with a gradient of buffer A containing 500 mM imidazole. Protein-containing fractions were desalted with a Nap-25 column (Amersham Biosciences) and then concentrated. A similar procedure was used to purify IscUWT and IscUIM proteins except that protein expression was induced by the addition of 1 mM IPTG. Recombinant E. coli IscS containing an N-terminal His6 tag was expressed and purified as previously described [74]. The protein concentration was estimated by measuring the absorbance at 280 nm with the NanoDrop2000 spectrophotometer and using the calculated molar extinction coefficient.
Circular dichroism experiments
CD spectra were recorded on a Jasco J-815 spectropolarimeter by using Hellma 110-QS cuvettes of 1 mm path length. CD measurements were performed in 50 mM Tris-HCl pH 8, 50 mM NaCl using protein concentrations of 2 μM. 20 scans were averaged and the buffer baseline was subtracted.
In vitro Fe-S reconstitution
The purified His-tagged IscUWT and IscUIM proteins were obtained in the apo-form. The purified proteins were reconstituted anaerobically in a glove box as described previously [48]. Briefly, 144 μM protein was mixed with 5 mM DTT, 1.44 μM IscS, 2 mM L-cysteine and 0.43 mM Fe(SO4)2(NH4)2 in a total volume of 500 μL of buffer A (50 mM Tris-HCl pH 8). Formation of Fe-S clusters on IscU was followed by UV-visible absorption spectroscopy using a Cary 1 Bio spectrophotometer. After 3 h incubation, samples were loaded onto a 1-mL anion exchange column (QFF) (GE Healthcare) equilibrated with buffer A and eluted with a gradient of buffer A containing 1 M NaCl. Protein fractions were concentrated on a Microcon concentrator (Amicon) and each concentrate was analysed for its Fe content, and for its UV-visible spectrum.
Ion exchange chromatography
Purified His-tagged IscUWT or IscUIM, IscS and CyaY proteins were mixed anaerobically in a 1 : 1:1 ratio (144 μM of each protein) for 40 minutes with 4-fold excess of Fe(SO4)2(NH4)2, 10-fold excess of L-cysteine and 5 mM DTT in a total volume of 500 μL of buffer A (50 mM Tris-HCl pH 8). The mixture was loaded onto a 1-mL QFF column (GE Healthcare) equilibrated with buffer A and eluted with a gradient of buffer A containing 1 M NaCl. Proteins elution was visualized by SDS-PAGE.
Kinetics of Fe-S formation
To assess kinetics of cluster formation on IscUWT or IscUIM, absorbance at 420 nm was measured as a function of time. 25 μM IscUWT or IscUIM was incubated anaerobically with 100 μM Fe(SO4)2(NH4)2, 2 mM DTT in 50 mM Tris-HCl pH 8. Subsequently, 25 μM IscS and 250 μM L-cysteine were added to start the reaction.
Bioinformatic and phylogenomic analyses
The 2742 complete prokaryotic proteome (2591 bacterial and 151 archaeal) available at the NCBI in March 03, 2014 were downloaded (ftp://ftp.ncbi.nlm.nih.gov/genomes/). The HMMER package v3.0b2 and self-written scripts were then used to search for CyaY homologs in these complete genomes, requiring the presence of Frataxin-like domain (PFAM accession number PF01491) [75]. Alignments E-value with the 599 profile less than 0.1 were considered as significant. To retrieve CyaY sequence we imposed homology with the entire CyaY sequence and an E-value with 1.7e-7 as threshold. In addition, alignments have been visually inspected. Proteins of the YjbR family, such as YdhG from Bacillus subtilis have not been detected since despite their structural similarity with CyaY they lack similarity at the sequence level [76,77]. The corresponding sequences were subsequently analysed with the same software in order to determine the presence of additional known functional domains. Additional BLASTP/tBLASTN searches were performed in complete genomes to ensure that the CyaY family was exhaustively sampled and in the nr database at the NCBI to retrieve eukaryotic sequences [78]. For each homolog, the gene context, defined as the 5 neighboring genes located upstream and downstream, was investigated using MGcV (Microbial Genomic context Viewer) [79].
The retrieved homologous sequences were aligned using MAFFT v7.045b [80]. The best resulting alignment was then visually inspected and manually refined using ED program from the MUST package [81]. The regions in a multiple sequence alignment that were suited for phylogenetic inference were selected by using BMGE (BLOSUM30 similarity matrix) [82].
The phylogeny of all the prokaryotic CyaY was constructed using both maximum likelihood (ML) and Bayesien methods. ML analyses were run using PHYML version 3.1 with the Le and Gascuel (LG) model (amino acid frequencies estimated from the dataset) and a gamma distribution (4 discrete categories of sites and an estimated alpha parameter) to take into account evolutionary rate variations across sites [80]. The robustness of each branch was estimated by the non-parametric bootstrap procedure implemented in PhyML (100 replicates of the original dataset with the same parameters). Bayesian analyses were performed using MrBayes version 3.2.2 with a mixed model of amino acid substitution including a gamma distribution (4 discrete categories) and an estimated proportion of invariant sites [83]. MrBayes was run with four chains for 1 million generations and trees were sampled every 100 generations. To construct the consensus tree, the first 1500 trees were discarded as ‘‘burnin”.
For the dataset construction IscU homologs was retrieved from complete proteome available in the local databank (see above) using BLASTP. The distinction between homologous and non-homologous sequences was assessed by visual inspection of each BLASTP outputs (no arbitrary cut-off on the E-value or score). We imposed some additional criterion in order for a protein to be considered as an IscU homologs: the presence of the three conserved cysteine residues that are required for the scaffold activity of IscU, no other additional domain such as those that could be found in NifU, and at least one other isc-related gene as a neighbor gene. The IscU homologs were gathered in a dataset and the corresponding sequences were aligned using MAFFT v7.045b [80].
Sequence-logo of IscU alignment was generated using Phylo-mLogo visualization tool in order to highlight the LPPVK motif and residues in position 108 [84].
Additional materials and methods are mentioned in S1 Text.
Supporting Information
Zdroje
1. Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8 : 436–446. doi: 10.1038/nrmicro2356 20467446
2. Crack JC, Green J, Thomson AJ, Le Brun NE. Iron-sulfur cluster sensor-regulators. Curr Opin Chem Biol. 2012;16 : 35–44. doi: 10.1016/j.cbpa.2012.02.009 22387135
3. Balk J, Schaedler TA. Iron cofactor assembly in plants. Annu Rev Plant Biol. 2014;65 : 125–53. doi: 10.1146/annurev-arplant-050213-035759 24498975
4. Roche B, Aussel L, Ezraty B, Mandin P, Py B, Barras F. Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim Biophys Acta. 2013;1827 : 923–937. doi: 10.1016/j.bbabio.2013.05.001 23660107
5. Stehling O, Lill R. The role of mitochondria in cellular iron-sulfur protein biogenesis: mechanisms, connected processes, and diseases. Cold Spring Harb Perspect Med. 2013;3 : 1–17. 23986915
6. Schilke B, Williams B, Knieszner H, Pukszta S, D’Silva P, Craig EA, et al. Evolution of mitochondrial chaperones utilized in Fe-S cluster biogenesis. Curr Biol. 2006;16 : 1660–1665. 16920629
7. Vickery LE, Cupp-Vickery JR. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit Rev Biochem Mol Biol. 2007;42 : 95–111. 17453917
8. Bandyopadhyay S, Chandramouli K, Johnson MK. Iron-sulfur cluster biosynthesis. Biochem Soc Trans. 2008;36 : 1112–1119. doi: 10.1042/BST0361112 19021507
9. Bonomi F, Iametti S, Morleo A, Ta D, Vickery LE. Facilitated transfer of IscU-[2Fe-2S] clusters by chaperone-mediated ligand exchange. Biochem. 2011;50 : 9641–9650.
10. Marinoni EN, de Oliveira JS, Nicolet Y, Raulfs EC, Amara P, Dean DR, et al. (IscS-IscU)2 complex structures provide insights into Fe2S2 biogenesis and transfer. Angew Chem Int Ed. 2012;51 : 5439–5442. doi: 10.1002/anie.201201708 22511353
11. Zheng L, White RH, Cash VL, Jack RF, Dean DR. Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc Natl Acad Sci USA. 1993;90 : 2754–2758. 8464885
12. Kispal G, Csere P, Prohl C, Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999;18 : 3981–3989. 10406803
13. Li J, Kogan M, Knight SA, Pain D, Dancis A. Yeast mitochondrial protein, Nfs1p, coordinately regulated iron-sulfur cluster proteins, cellular iron uptake, and iron distribution. J Biol Chem. 1999;274 : 33025–33034. 10551871
14. Schwartz CJ, Djaman O, Imaly JA, Kiley PJ. The cysteine desulfurase, IscS, has a major role in vivo Fe-S cluster formation in Escherichia coli. Proc Natl Acad Sci USA. 2000;97 : 9009–9014. 10908675
15. Hidese R, Mihara H, Esaki N. Bacterial cysteine desulfurases: versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl Microbiol Biotechnol. 2011;91 : 47–61. doi: 10.1007/s00253-011-3336-x 21603932
16. Smith AD, Agar JN, Johnson KA, Frazzon J, Amster IJ, Dean DR, et al. Sulfur transfer from IscS to IscU: the first step in iron-sulfur cluster biosynthesis. J Am Chem Soc. 2001;123 : 11103–11104. 11686732
17. Smith AD, Frazzon J, Dean DR, Johnson MK. Role of conserved cysteines in mediating sulfur transfer from IscS to IscU. FEBS Lett. 2005;579 : 5236–5240. 16165131
18. Urbina HD, Silberg JJ, Hoff KG, Vickery LE. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J Biol Chem. 2001;276 : 44521–44526. 11577100
19. Gibson TJ, Koonin EV, Musco G, Pastore A, Bork P. Friedreich’s ataxia protein: phylogenetic evidence for mitochondrial dysfunction. Trends Neurosci. 1996;19 : 465–468. 8931268
20. Gerber J, Mühlenhoff U, Lill R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003;4 : 906–911. 12947415
21. Wang T, Craig EA. Binding of yeast frataxin to the scaffold for Fe-S cluster biogenesis, Isu. J Biol Chem. 2008;283 : 12674–12679. doi: 10.1074/jbc.M800399200 18319250
22. Adinolfi S, Iannuzzi C, Prischi F, Pastore C, Iametti S, Martin SR, et al. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat Struct Mol Biol. 2009;16 : 390–396. doi: 10.1038/nsmb.1579 19305405
23. Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, Trempe JF, et al. Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 2010;8: e1000354. doi: 10.1371/journal.pbio.1000354 20404999
24. Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, Martin SR, et al. Structural bases for the interaction of frataxin with the central components of iron-sulfur cluster assembly. Nat Commun. 2010;1 : 95. doi: 10.1038/ncomms1097 20981023
25. Tsai CL, Barondeau DP. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry. 2010;49 : 9132–9139. doi: 10.1021/bi1013062 20873749
26. Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donzé M, Reutenauer L, et al. Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS One. 2011;6: e16199. doi: 10.1371/journal.pone.0016199 21298097
27. Tokumoto U, Takahashi Y. Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron-sulfur proteins. J Biochem. 2001;130 : 63–71. 11432781
28. Li K, Tong WH, Hughes RM, Rouault TA. Roles of the mammalian cytosolic cysteine desulfurase, ISCS, and scaffold protein, ISCU, in iron-sulfur cluster assembly. J Biol Chem. 2006;281 : 12344–12351. 16527810
29. Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis Model Mech. 2012;5 : 155–164. doi: 10.1242/dmm.009019 22382365
30. Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, et al. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276 : 1709–1712. 9180083
31. Foury F, Cazzalini O. Deletion of the yeast homologue of the human gene associated with Friedreich’s ataxia elicits iron accumulation in mitochondria. FEBS Lett. 1997;11 : 373–377.
32. Foury F. Low iron concentration and aconitase deficiency in a yeast frataxin homologue deficient strain. FEBS Lett. 1999;456 : 281–284. 10456324
33. Lesuisse E, Santos R, Matzanke BF, Knight SA, Camadro JM, Dancis A. Iron use for haeme synthesis is under control of the yeast frataxin homologue (Yfh1). Hum Mol Genet. 2003;12 : 879–889. 12668611
34. Pastore A, Puccio H. Frataxin: a protein in search for a function. J Neurochem. 2013;1 : 43–52.
35. Campuzano V, Montermini L, Molto MD, Pianese L, Cossée M, Cavalcanti F, et al. Freidreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271 : 1423–1427. 8596916
36. Rötig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17 : 215–217. 9326946
37. Stehling O, Elsässer HP, Brückel B, Mühlenhoff U, Lill R. Iron-sulfur protein maturation in human cells: evidence for a function of frataxine. Hum Mol Genet. 2004;13 : 3007–3015. 15509595
38. Pandolfo M, Pastore A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J Neurol. 2009;256 : 9–17. doi: 10.1007/s00415-009-1003-2 19283345
39. Santos R, Lefevre S, Sliwa D, Seguin A, Camadro JM, Lesuisse E. Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal. 2010;13 : 651–690. doi: 10.1089/ars.2009.3015 20156111
40. Li DS, Ohshima K, Jiralerspong S, Bojanowski MW, Pandolfo M. Knock-out of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS. 1999;456 : 13–16. 10452520
41. Vivas E, Skovran E, Downs DM. Salmonella enterica strains lacking the frataxin homolog CyaY show defects in Fe-S cluster metabolism in vivo. J Bacteriol. 2006;188 : 1175–1179. 16428423
42. Pohl T, Walter J, Stolpe S, Soufo JH, Grauman PL, Friedrich T. Effects of the deletion of the Escherichia coli frataxin homologue CyaY on the respiratory NADH: ubiquinone oxidoreductase. BMC Biochem. 2007;8 : 13. doi: 10.1186/1471-2091-8-13 17650323
43. Velayudhan J, Karlinsev JE, Frawley ER, Becker LA, Nartea M, Fang FC. Distinct roles of the Salmonella enterica serovar Typhimurium CyaY and YggX proteins in the biosynthesis and repair of iron-sulfur clusters. Infect Immun. 2014;82 : 1390–1401. doi: 10.1128/IAI.01022-13 24421039
44. Roche B, Huguenot A, Barras F, Py B. The iron-binding CyaY and IscX proteins assist the ISC-catalyzed Fe-S biogenesis in Escherichia coli. Mol Microbiol. 2015;4 : 605–623. doi: 10.1111/mmi.12888 25430730
45. Yoon H, Golla R, Lesuisse E, Pain J, Donald JE, Lyver ER, et al. Mutation in the Fe-S scaffold protein Isu bypasses frataxin deletion. Biochem J. 2012;441 : 473–480. doi: 10.1042/BJ20111637 21936771
46. Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, Brochado AR, et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science. 2013;340 : 1583–1587. doi: 10.1126/science.1238328 23812717
47. Dailey HA, Finnegan MG, Johnson MK. Human ferrochelatase is an iron-sulfur protein. Biochemistry. 1994;33 : 403–407. 8286370
48. Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39 : 7856–7862. 10891064
49. Adinolfi S, Rizzo F, Masino L, Nair M, Martin SR, Pastore A, et al. Bacterial IscU is a well folded and functional single domain protein. Eur J Biochem. 2004;271 : 2093–2100. 15153099
50. Loiseau L, Gerez C, Bekker M, Ollagnier-de Choudens S, Py B, Sanakis Y, et al. ErpA, an iron sulfur (Fe-S) protein of the A-type essential for respiratory metabolism in Escherichia coli. Proc Natl Acad Sci USA. 2007;104 : 13626–13631. 17698959
51. Vinella D, Loiseau L, Ollagnier-de-Choudens S, Fontecave M, Barras F. In vivo [Fe-S] cluster acquisition by IscR and NsrR, two stress regulators in Escherichia coli. Mol Microbiol. 2013;3 : 493–508. doi: 10.1111/mmi.12135 23320508
52. Adam AC, Bornhövd C, Prokisch H, Neupert W, Hell K. The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. 2006;25 : 174–183. 16341090
53. Wiedemann N, Urzica E, Guiard B, Müller H, Lohaus C, Meyer HE, et al. Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins. EMBO J. 2006;25 : 184–195. 16341089
54. Richards TA, van der Giezen M. Evolution of the Isd11-IscS complex reveals a single alpha-proteobacterial endosymbiosis for all eukaryotes. Mol Biol Evol. 2006;23 : 1341–1344. 16648156
55. Pandey A, Golla R, Yoon H, Dancis A, Pain D. Persulfide formation on mitochondrial cysteine desulfurase: enzyme activation by a eukaryote-specific interacting protein and Fe-S cluster synthesis. Biochem J. 2012;448 : 171–187. doi: 10.1042/BJ20120951 22928949
56. Lim SC, Friemel M, Marum JE, Tucker EJ, Bruno DL, Riley LG, et al. Mutations in LYRM4, encoding iron-sulfur cluster biogenesis factor ISD11, cause deficiency of multiple respiratory chain complexes. Hum Mol Genet. 2013;22 : 4460–4473. doi: 10.1093/hmg/ddt295 23814038
57. Pandey A, Gordon DM, Pain J, Stemmler TL, Dancis A, Pain D. Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly. J Biol Chem. 2013;288 : 36773–36786. doi: 10.1074/jbc.M113.525857 24217246
58. Parent A, Elduque X, Cornu D, Belot L, Le Caer JP, Grandas A, et al. Mammalian frataxin directly enhances sulfur transfer of NFS1 persulfide to both ISCU and free thiols. Nat Commun. 2015;6 : 5686. doi: 10.1038/ncomms6686 25597503
59. Yoon H, Knight SA, Pandey A, Pain J, Zhang Y, Pain D, et al. Frataxin-bypassing Isu1: characterization of the bypass activity in cells and mitochondria. Biochem J. 2014;459 : 71–81. doi: 10.1042/BJ20131273 24433162
60. Seguin A, Bayot A, Dancis A, Rogowska-Wrzesinska A, Auchère F, Camadro JM, et al. Overexpression of the yeast frataxin homolog (Yfh1): contrasting effects on iron-sulfur cluster assembly, heme synthesis and resistance to oxidative stress. Mitochondrion. 2009;9 : 130–138. doi: 10.1016/j.mito.2009.01.007 19460301
61. Lefevre S, Sliwa D, Rustin P, Camadro JM, Santos R. Oxidative stress induces mitochondrial fragmentation in frataxin-deficient cells. Biochem Biophys Res Commun. 2012;418 : 336–341. doi: 10.1016/j.bbrc.2012.01.022 22274609
62. Iannuzzi C, Adinolfi S, Howes BD, Garcia-Serres R, Cemancey M, Latour JM, et al. The role of CyaY in iron-sulfur cluster assembly on the E. coli IscU scaffold protein. PLoS One. 2011;6: e21992. doi: 10.1371/journal.pone.0021992 21799759
63. Bridwell-Rabb J, Iannuzzi C, Pastore A, Barondeau DP. Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis. Biochemistry. 2012;51 : 2506–2514. doi: 10.1021/bi201628j 22352884
64. Huynen MA, Snel B, Bork P, Gibson TJ. The phylogenetic distribution of frataxin indicates a role in iron-sulfur cluster protein assembly. Hum Mol Genet. 2001;10 : 2463–2468. 11689493
65. Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers. PLoS Genet. 2009;5: e1000497. doi: 10.1371/journal.pgen.1000497 19478995
66. Py B, Gerez C, Angelini S, Planel R, Vinella D, Loiseau L, et al. Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol Microbiol. 2012;86 : 155–171. doi: 10.1111/j.1365-2958.2012.08181.x 22966982
67. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the KEIO collection. Mol Syst Biol. 2006;2 : 2006.0008. 16738554
68. Mandin P, Gottesman S. A genetic approach for finding small RNAs regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol Microbiol. 2009;72 : 551–565. doi: 10.1111/j.1365-2958.2009.06665.x 19426207
69. Layer G, Ollagnier-de-Choudens S, Sanakis Y, Fontecave M. Iron-sulfur cluster biosynthesis. J Biol Chem. 2006;281 : 16256–16263. 16603772
70. Miller J.H. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press. Cold Sppring Harbor, NY;1972.
71. Seaver LC, Imlay JA. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J Biol Chem. 2004;279 : 48742–48750. 15361522
72. Kolaj-Robin O, O’Kane SR, Nitschke W, Léger C, Baymann F, Soulimane T. Biochemical and biophysical characterization of succinate: quinone reductase from Thermus thermophilus. Biochim Biophys Acta. 2011;1807 : 68–79. doi: 10.1016/j.bbabio.2010.10.009 20951673
73. Zhao Z, Rothery RA, Weiner JH. Effects of site-directed mutations on heme reduction in Escherichia coli nitrate reductase A by menaquinol: a stopped-flow study. Biochemistry. 2003;42 : 14225–14233. 14640690
74. Tokumoto U, Nomura S, Minami Y, Mihara H, Kato S, Kurihara T, et al. Network of protein-protein interactions among iron-sulfur assembly proteins in Escherichia coli. J Biochem. 2002;131 : 713–719. 11983079
75. Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39 : 29–37.
76. Qi W, Cowan JA. A structural and functional homolog supports a general role for frataxin in cellular iron chemistry. Chem Commun (Camb). 2010;46 : 719–721. doi: 10.1039/b911975b 20087498
77. Albrecht AG, Landmann H, Nette D, Burghaus O, Peuckert F, Seubert A, et al. The frataxin homologue Fra plays a key role in intracellular iron channeling in Bacillus subtilis. Chembiochem. 2011;12 : 2052–2061. doi: 10.1002/cbic.201100190 21744456
78. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25 : 3389–3402. 9254694
79. Overmars L, Kerkhoven R, Siezen RJ, Francke C. MGcV: the microbial genomic context viewer for comparative genome analysis. BMC Genomics. 2013;14 : 209. doi: 10.1186/1471-2164-14-209 23547764
80. Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30 : 772–780. doi: 10.1093/molbev/mst010 23329690
81. Philippe H. MUST, a computer package of Management Utilities for Sequences and Trees. Nucleic Acids Res. 1993;21 : 5264–5272. 8255784
82. Criscuolo A Gribaldo S. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol. 2010;10 : 210. doi: 10.1186/1471-2148-10-210 20626897
83. Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17 : 754–755. 11524383
84. Shih AC, Lee DT, Peng CL, Wu YW. Phylo-mLogo: an interactive and hierarchical multiple-logo visualization tool for alignment of many sequences. BMC Bioinformatics. 2007;8 : 63. doi: 10.1186/1471-2105-8-63 17319966
85. Cho SJ, Lee MG, Yang JK, Lee JY, Song HK, Suh SW. Crystal structure of Escherichia coli CyaY protein reveals a previously unidentified fold for the evolutionarily conserved frataxin family. Proc Natl Acad Sci USA. 2000;97 : 8932–8937. 10908679
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



