-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNatural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
The increase in average temperatures across the globe has been predicted to have negative impacts on agricultural productivity. Therefore, there is a need to understand the molecular mechanisms that underlie plant growth responses to varying temperature regimes. At present, very little is known about the genes and pathways that modulate thermo-sensory growth responses in plants. In this article, the authors exploit natural variation in the commonly occurring weed thale cress (Arabidopsis thaliana) and identify a gene referred to as ICARUS1 to be required for plant growth at higher ambient temperatures. Plants carrying lesions in this gene stop growing at high temperatures and revert to growth when temperatures reduce. Using a combination of computational, molecular and cell biological approaches, the authors demonstrate that allelic variation at ICARUS1, which encodes an enzyme required for the fundamental biochemical process of tRNAHis maturation, underlies variation in thermo-sensory growth responses of A. thaliana. Furthermore, the authors discover that the deleterious impact of a natural mutation in ICARUS1 is suppressed through alternative splicing, thus suggesting the potential for alternative splicing to buffer the impacts of some natural mutations. These results support that modulation of fundamental processes, in addition to transcriptional regulation, mediate thermo-sensory growth responses in plants.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005085
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005085Summary
The increase in average temperatures across the globe has been predicted to have negative impacts on agricultural productivity. Therefore, there is a need to understand the molecular mechanisms that underlie plant growth responses to varying temperature regimes. At present, very little is known about the genes and pathways that modulate thermo-sensory growth responses in plants. In this article, the authors exploit natural variation in the commonly occurring weed thale cress (Arabidopsis thaliana) and identify a gene referred to as ICARUS1 to be required for plant growth at higher ambient temperatures. Plants carrying lesions in this gene stop growing at high temperatures and revert to growth when temperatures reduce. Using a combination of computational, molecular and cell biological approaches, the authors demonstrate that allelic variation at ICARUS1, which encodes an enzyme required for the fundamental biochemical process of tRNAHis maturation, underlies variation in thermo-sensory growth responses of A. thaliana. Furthermore, the authors discover that the deleterious impact of a natural mutation in ICARUS1 is suppressed through alternative splicing, thus suggesting the potential for alternative splicing to buffer the impacts of some natural mutations. These results support that modulation of fundamental processes, in addition to transcriptional regulation, mediate thermo-sensory growth responses in plants.
Introduction
Environmental perturbations can often reveal cryptic phenotypes, which in turn can uncover mechanisms associated with environmental regulation of growth and development [1–5]. Light and temperature are the two key environmental factors that have major impacts on plant development. The molecular mechanisms associated with light signaling and its regulation of plant development is very well studied [6–9]. In contrast, temperature response has been studied traditionally at extreme conditions characterized by heat shock response or cold stress response [10–13]. However, even small differences in ambient growth temperature can have profound effects on plant growth and development [12, 14, 15].
Vernalization, the acceleration of flowering in response to exposure to winter-like temperatures, is one of the developmental processes well studied at the molecular level [16, 17]. In contrast to this response to extreme temperatures, very little is known about the molecular mechanisms underlying thermo-sensory responses within moderate growth temperature ranges [14]. Plants grown at higher ambient temperatures display elongated hypocotyls and petioles, increased leaf serration, as well as early flowering [18–21]. Thermo-sensory responses have been suggested to involve chromatin remodeling involving histone dynamics [22–24]. For example, the incorporation and eviction of histone H2A.Z onto the nucleosomes modulated through the SWR1 complex has been suggested to underlie transcriptional regulation of thermal response in plants [23]. In fact, a direct measurement of transcriptional rates suggested that there exists a global transcriptional process modulating mRNA abundance by temperature [25]. However, the presence of H2A.Z in the gene body accounted for only part of this, suggesting that other factors contribute to the modulation of plant growth responses to ambient temperature variation.
In this thermo-sensory transcriptional network, the PHYTOCHROME INTERACTING FACTOR 4 (PIF4) has been suggested to be a central hub [18,20,26]. It has been shown that elevated ambient temperature leads to an increase in auxin levels, which in itself is under the control of PIF4 [18,20,26]. Higher temperatures induce flowering and this process has been suggested to be mediated through PIF4, in addition to other known flowering time related genes [21,27,28]. Finally, altered regulation of the circadian clock has also been suggested to play a role in governing the plant growth in different temperatures (thermo-sensory growth response) [29]. The evening complex night-time repressor comprised of EARLY FLOWERING 3 (ELF3), ELF4 and LUX ARRYTHMO1 (LUX) is inhibited by higher temperatures, which in turn can regulate the PIF4 gene, modulating thermal response [29]. Thus an overarching theme that appears to emerge from these studies is that the thermal response in plants mostly occurs at a transcriptional level.
Furthermore, natural populations of Arabidopsis thaliana (A. thaliana) exhibit extensive variation in diverse traits including thermo-sensory growth and developmental responses [30]. The analysis of such natural variation has been very useful in identifying new mechanisms involved in the regulation of development by temperature, as illustrated with our current understanding of the vernalization process [17]. The first analyses of natural variation for growth processes in relation to high ambient temperature have already identified novel factors such as the ISOPROPYL MALATE ISOMERASE LARGE SUB UNIT1 (IIL1) and the ERECTA genes [3,31]. In addition, natural variation in thermal response for flowering time has identified FLOWERING LOCUS M (FLM) as a regulatory factor, whose further analysis has suggested a role for FLM alternative splicing in the modulation of flowering by ambient temperature [21,32,33]. Thus our understanding of the molecular mechanisms and pathways that govern natural variation in thermo-sensory growth responses in plants is just beginning to emerge.
In this study, we have undertaken a natural variation approach and discovered that the uncharacterized and universally present gene, ICARUS1 (ICA1), is required for plant growth specifically at high growth temperatures. Plants carrying loss-of-function alleles of ICA1 are severely reduced in growth at high temperatures, but resume growth when reverted to lower thermal regimes. ICA1 encodes a member of the tRNAHis guanylyl transferase (Thg1) superfamily [34]. The Thg1 superfamily has been of biochemical interest as its members share a striking structural similarity to nucleic acid polymerases and catalyze the addition of a guanosine residue to the 5’ end of the tRNAHis, in an unusual 3’-5’ phosphodiester bond formation, which is required for tRNAHis maturation [34, 35]. However, their biological impact at the organismal level remains to be described. We have characterized the first plant member of this important class of proteins, demonstrating its biological role at cellular and organismal levels. In addition, we reveal substantial alternative splicing at the ICA1 locus, which is associated with phenotypic suppression, indicating that alternative splicing can buffer the potential negative effects of some natural mutations. Together, our results show that allelic variation of a Thg1 superfamily gene contributes to the natural variation in the thermo-sensory growth response of plants.
Results
Sij-4 and Don-0 accessions display conditional and reversible growth phenotypes depending on ambient temperature
To identify factors required for plant growth at high ambient temperatures, we grew a worldwide collection of A. thaliana accessions at standard (21–23°C) and high temperatures (27–28°C) and screened for accessions with severe growth defects at higher temperatures. We identified the Sij-4 and the Don-0 strains to be highly temperature-sensitive (Fig 1A). At high temperatures, they both had altered leaf morphology and reduced expansion of leaf blades (Fig 1A). The outgrowth of the first true leaf primordium was delayed and the cell morphology was also affected (Fig 1B and 1C). The high temperature-induced hypocotyl elongation was not observed in Sij-4 indicating a general impairment of growth at high temperatures (Fig 1D). Plants grown at intermediate temperatures displayed milder phenotypes indicating a quantitative nature of the growth defects (S1A Fig). In addition, these phenotypes were reversible on thermal shifts, although reversion was restricted mainly to the newly developed leaves, which initiated or stop growth after shifting to 21 or 28°C, respectively (Fig 1E). However, when Don-0 plants were transferred from 28 to 21°C, the oldest existing leaves did not grow, whereas younger existing leaves grew abnormally, leading to deformed leaves. Similar phenotypes were displayed by the two accessions throughout development with adult plants showing very reduced growth, smaller, pale and serrated leaves with reduced expansion of the leaf blade, altered phyllotaxy, plant architecture and severely impaired seed production (S1A–S1C Fig). Analyses of F1 and F2 populations derived from inter-crossing Sij-4 and Col-0 indicated that the growth defects were recessive and monogenic (280/1080 plants with growth defects, Chi-square p = 0.482; Figs 1D and S1E and S2). Further analysis of hypocotyl elongation in the F2 progeny revealed a highly significant genetic correlation between the leaf and hypocotyl phenotypes (rG2 = 0.88, p<0.0001) suggesting a shared genetic basis (S2B Fig).
Fig. 1. ICA1 growth phenotypes in natural accessions of Arabidopsis depend on temperature. 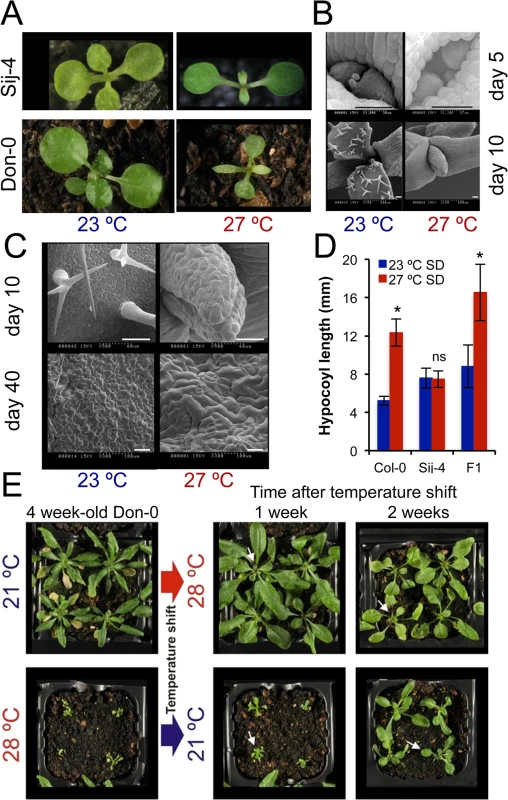
(A) 2 week-old Sij-4 and Don-0 plants grown at 23°C and 27°C. (B & C) Scanning electron microscopy of Sij-4 grown at 23°C and 27°C. Differences in cell morphology are visible upon high magnification (C) from day 10. Scale bar = 50μM. (D) Hypocotyl elongation at 23°C and 27°C under short days (SD) in Col-0, Sij-4 and F1 plants derived from a cross between these two strains. 15–30 plants per genotype were analyzed for hypocotyl length measurement. *: p<0.0001; ns: not significant in Student t-tests comparing growth at the two temperatures. (E) Reversibility of the growth phenotype of Don-0 adult plants (4 week-old) in temperature shift experiments between 21 and 28°C. White arrows indicate new leaves developed after temperature shift. Positional cloning of ICARUS1 identifies a universal protein required for plant growth at high temperatures
To identify the underlying genetic loci, we first analyzed F1 plants derived from a cross between the two sensitive accessions, Don-0 and Sij-4, which failed to complement each other indicating a common causal locus (S1D Fig). Consistent with this, genetic mapping using Don-0 x Ler and Sij-4 x Col-0 F2 populations identified an overlapping region located in the middle of chromosome 2 associated with the phenotype (Fig 2A and 2B). To reflect temperature sensitivity, we named the presumed causal locus within this region ICARUS1 (ICA1, after the Greek mythological character, who flew too close to the sun with wings attached with wax that melted at high temperature). Additional fine mapping using F2 populations derived from Sij-4 x Col-0 and Sij-4 x Ler crosses, located ICA1 in a 37 or 393 kb genomic region, respectively (Fig 2B). The two mapping intervals overlap in a 5.9 kb region that contains a single annotated gene, At2g31580. Two T-DNA insertion lines in At2g31580 (designated as ica1-1 and ica1-2) in Col-0 background also displayed pleiotropic growth defects depending on temperature similar to Sij-4 and Don-0, although the exonic insertion line ica1-2 showed stronger phenotypes (Figs 2C and S3A–S3G). Crosses between ica1-1 and Sij-4 failed to complement the pleiotropic growth phenotypes, indicating that At2g31580 is ICA1 (S3E Fig). This was further supported by transgenic lines carrying an artificial microRNA against At2g31580 in the Col-0 background, which displayed the ica1 phenotype only at high temperatures (Figs 2D and S1F–S1H). Finally, the ica1 phenotype was complemented in transgenic lines carrying the genomic sequence encompassing the ICA1 locus from either Ler (ICA1gDNA-Ler) or Col-0 (ICA1gDNA-Col) driven by either the endogenous promoter (in Don-0 or ica1-2 background) or the 35S CaMV promoter (in Sij-4 background) confirming that At2g31580 is the ICA1 gene (Figs 2E and 2F and S3H).
Fig. 2. Positional cloning of ICA1. 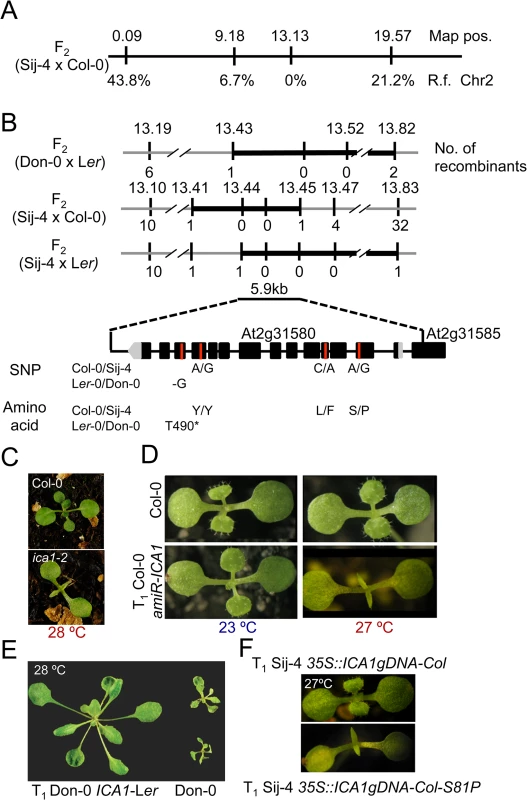
(A) Whole genome scanning of F2 (Sij-4 x Col-0) along with marker positions and recombination frequencies (R.f.) with ICA1. Map positions are given in Mb. (B) Fine mapping of ICA1 in F2 (Don-0 x Ler-0), F2 (Sij-4 x Col-0) and F2 (Sij-4 x Ler-0) segregating populations. Map positions and the number of recombinants are indicated above and below the line respectively. An overlapping interval of 5.9 kb contains a single gene. The single nucleotide deletion of Don-0 and the missense mutations between Col-0 and Sij-4 along with the corresponding amino acid changes are given below. (C) Phenotype of the Col-0 and ica1-2 (Col-0 background) grown at 27°C. (D) Phenotype of 35S::amiR-ICA1 in Col-0 background at 23°C and 27°C. (E) Phenotypic complementation of Don-0 with an ICA1-Ler transgene at 28°C (4 week-old plants). (F) Transgenic suppression of ICA1-Sij-4 phenotype at 27°C by 35S::ICA1gDNA-Col and lack of phenotypic suppression with the S81P mutation. ICA1 encodes a universal protein belonging to the tRNAHis guanylyl transferase (Thg1) super-family [34,36]. In contrast to human/yeast orthologs, ICA1 encodes two tandemly repeated units of Thg1 within the protein and is known to be nuclear localized [37] (S4A Fig). To find out whether structural or regulatory polymorphisms result in Don-0 and Sij-4 loss-of-function alleles, we analyzed their ICA1 sequence and expression levels. We did not find any obvious differences in ICA1 expression levels across the accessions at lower temperatures, although there was a slight increase in ICA1 expression of Sij-4 at higher temperatures (S5A and S5B Fig). Moreover, sequence analysis revealed a single 1 bp deletion in exon-12 of Don-0 ICA1, which is predicted to truncate the C-terminal half (position T490*, Fig 2B). Also, the Sij-4 strain harbors two nonsynonymous substitutions, of which the serine to proline (S81P) substitution corresponds to the nucleotide-binding site in the yeast homologue (equivalent to H34)[38] (Figs 2B and S4B). To test if this substitution affects ICA1 function, we generated Sij-4 transgenic lines carrying a 35S::ICA1gDNA-Col construct with an S81P substitution. Contrary to 35S::ICA1gDNA-Col original construct, this transgene was unable to complement Sij-4 growth defects (Fig 2F), indicating that S81P is the causal polymorphism. The locations of both structural mutations in Don-0 and Sij-4 further suggest that the two ICA1 halves are required for its function and the structural perturbations might affect either the activity or the stability of ICA1 protein.
Transcriptional and computational analyses suggest impairment of cell cycle in Sij-4
In order to understand the developmental mechanisms that lead to the growth defects observed in Sij-4, we analyzed genes that are differentially expressed between Col-0 and Sij-4 at different temperatures using RNA-seq analysis. In agreement with the temperature-dependence of the Sij-4 phenotype, more genes were differentially expressed between Col-0 and Sij-4 at 27°C than at 23°C (5449 versus 1661; S1 and S2 Tables). To assess whether the gene expression is specifically affected at higher temperatures or the differences in expression levels are more pronounced, we compared the fold changes in gene expression between Col-0 and Sij-4 at 23°C with the same genes at 27°C. There was a significant correlation between the fold changes, suggesting that the differences in expression levels were exacerbated at 27°C consistent with the quantitative nature of the observed phenotypes (S6 Fig). Of the 4236 genes differentially expressed only at 27°C (S3 Table), 2723 (65%) were down regulated in Sij-4 compared to Col-0. Gene ontology analysis [39,40] detected a highly significant enrichment of genes associated with cell proliferation, cytokinetic processes and DNA replication suggesting alterations of cell cycle regulation (S4 and S5 Tables). A comparison with a previously published analysis [41] revealed that the down regulation of gene expression in Sij-4 is more evident in genes expressed in the S and M phases of the cell cycle (S6 Table).
Since mutations in tRNAHis guanylyl transferase will possibly affect the availability of amino acyl histidine tRNA to be incorporated into proteins, we reasoned that proteins with higher number of histidine residues are more likely to be affected by mutations in ICA1. Accordingly, we undertook a computational analysis of the proteome of A. thaliana to identify processes enriched among proteins with relatively high histidine content, which revealed a significant enrichment for genes associated with cell cycle processes (S7–S9 Tables). Therefore, we hypothesized that a translational disruption of these proteins and the transcriptional down-regulation of cell cycle genes could confer the growth defects caused by the ICA1 loss-of-function alleles.
ICA1-Sij-4 loss-of-function alleles lead to a block in G2/M transition at high ambient temperatures
To further evaluate the potential impairment of the cell cycle, we tested if the Sij-4 growth phenotypes result from defects in cell proliferation using specific cell cycle markers. To this end, we used a marker line expressing the CyclinB1,1::GFP fusion protein driven by CycB1,1 promoter, which is known to be expressed only during the G2/M transition checkpoint and degraded at the end of the M phase [42–44]. Analysis of a segregating F2 population derived between pCycB1;1::CycB1;1-GFP line in Col-0 background and Sij-4, showed that CyclinB1,1-GFP expression was stronger in plants exhibiting the loss-of function mutant phenotype (henceforth referred to as ICA1-Sij-4) compared to normal appearing plants (henceforth referred to as ICA1-Col-0) (Fig 3A), which suggests that a fraction of ICA1-Sij-4 cells may be arrested in the G2/M transition phase. In agreement with this, RNA-seq analysis showed that 82% (67 out of 82) of the genes with expression that peaked at the G2/M boundary [45] were significantly down regulated in Sij-4 compared to Col-0 at high temperature (S10 Table). Cells arrested in the G2/M transition often undergo endocycling, and the timing of this arrest correlates with changes in cell size [46]. Consistent with this, we observed enlarged cells in Sij-4 plants using the pATML1::mCitrine-RCI2A plasma membrane marker [46] (Fig 3B). In addition, we used a histone H2B marker [46] (pATML1::H2B-mYFP) to visualize ICA1-Col-0 and ICA1-Sij-4 nuclei in abaxial epidermal cells, revealing larger elongated nuclei suggestive of increased DNA content (Fig 3C and 3D). This was verified by flow cytometric analyses, where Col-0 plants showed more than 90% of the cell nuclei with either 2C or 4C DNA content (Figs 3E and S7). By contrast, Sij-4 plants grown at high temperatures also contained nuclei with 32C and 64C at the expense of the 2C and 4C cells, which indicates that a significant proportion of the cells have gone through additional DNA replications (Figs 3E and S7). Introduction of the ICA1-Col-0 allele driven by the 35SCaMV promoter (35S::ICA1gDNA-Col) in Sij-4 suppressed this endoreduplication, hence indicating that it is caused by ICA1 allelic variation (Figs 3E and S7). Thus, the growth defects observed in loss-of-function alleles of ICA1 at high temperature are associated with disruptions in cell cycle regulation.
Fig. 3. ICA1 affects cell cycle and endoreduplication. 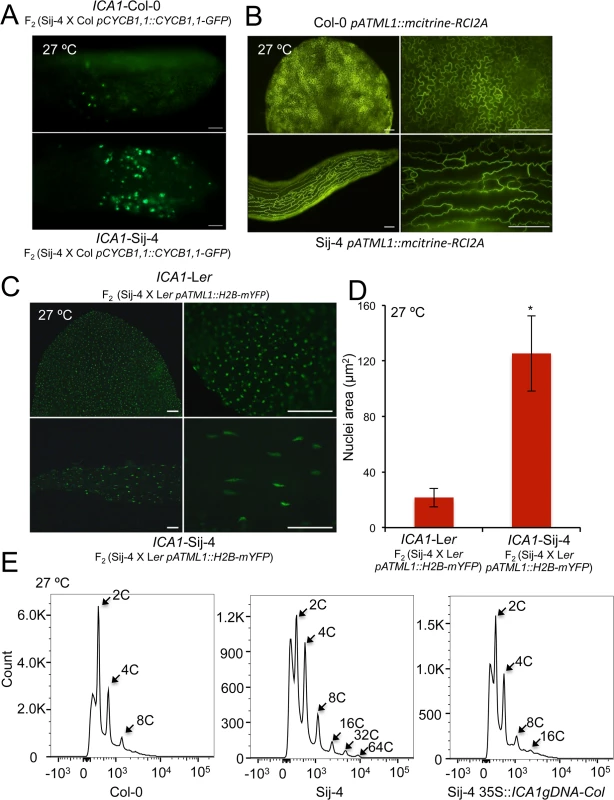
(A) Expression pattern of Cyclin B1;1 in leaves of ICA1-Sij-4 and ICA1-Col plants segregating in an F2 (Sij-4 x Col-0) population grown at 27°C and analyzed using a pCycB1;1::CycB1;1-GFP marker. (B) Shape of abaxial epidermal cells of the first leaf from Col-0 and Sij-4 plants grown at 27°C and visualized using the plasma membrane marker (pATML1::mCitrine-RCI2A). Magnification: left 10X, right 40X. (C) Epidermal cell nuclei of the first leaf of ICA1-Sij-4 and ICA1-Ler plants selected in an F2 (Sij-4 x Ler) family grown at 27°C and visualized with the histone H2B marker (pATML1::H2B-mYFP). Magnification: left 10X, right 40X. (D) Quantification of nuclei sizes measured as mean (± standard deviation) nuclei area in ICA1-Sij-4 and ICA1-Ler-0 plants selected in an F2 (Sij-4 x Ler-0) family grown at 27°C. *: p<0.0001 in Student t-test comparing nuclei sizes of both genotypic classes. (E) Flow cytometry analysis of Sij-4 plants compared with Col-0 and 35S::ICA1gDNA-Col in Sij-4 background at 27°C. Scale bars in A, B and C are 100μM. ICA1-Sij-4 cells are hypersensitive to DNA damage
The G2/M cell cycle checkpoint allows the repair of DNA after DNA synthesis and it has been shown that DNA damage can induce endoreduplication in Arabidopsis [47,48]. Since at 27°C, a fraction of ICA1-Sij-4 cells were arrested at the G2/M transition coupled with endoreduplication, we tested if ICA1 loss-of-function alleles affect the capacity of the plants to respond to DNA damage. To this end, we used the first leaf assay, which is based on the growth arrest of the first true leaves when subjected to DNA damage during early plant development [49,50]. Seedlings of Sij-4, Col-0, 35S::ICA1gDNA-Col lines in Sij-4 background and 35S::amiR-ICA1 in Col-0 background were subjected to DNA damage by Bleomycin treatment and evaluated for their subsequent leaf development at 23°C (Fig 4A). Sij-4 seedlings were hypersensitive to Bleomycin with more than 80% of the plants lacking the first leaves compared to 15% in Col-0. The Sij-4 hypersensitivity was reduced in the 35S::ICA1gDNA-Col lines in Sij-4 background, while 35S::amiR-ICA1 lines in Col-0 background displayed enhanced sensitivity to Bleomycin (Fig 4B). These results suggest that ICA1 is also affecting the capacity to repair DNA damage, even under standard growth temperatures.
Fig. 4. Sij-4 plants are hypersensitive to DNA damage. 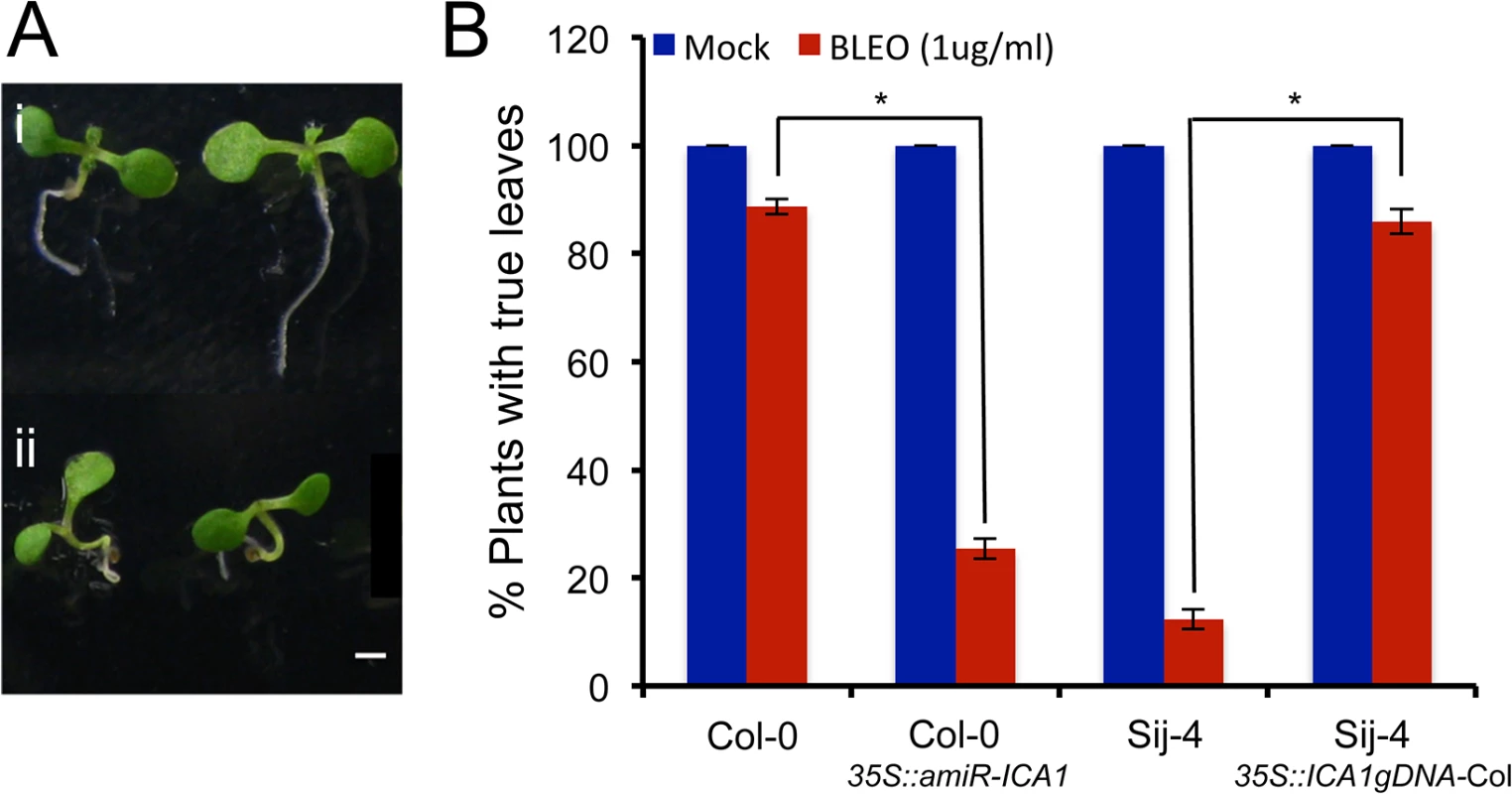
The sensitivity to DNA damage was assessed by the emergence of first leaves after Bleomycin treatment, which introduces double strand DNA breaks. (A) First leaf development in 10-day-old Col-0 seedlings grown at 23°C under long days. Examples for Sij-4 plants with (i) and without (ii) true first leaves are shown. (B) Percentage of plants developing true leaves when treated with Bleomycin (BLEO) or mock treated. Results from three independent experiments are shown for plants of Col-0, Sij-4, 35S::ICA1gDNA-Col in Sij-4 background and 35S::amiR-ICA1 in Col-0 background. Error bars indicate standard deviation from three biological replicates with 50 to 100 seedlings each. *: p<0.0001 in Student t-tests comparing pairs of genotypes. Natural ICA1 loss-of-function alleles are rare at global and regional scales but occur at high frequency in some local populations
To assess the population frequency of ICA1-mediated growth alterations in response to temperature, we carried out additional phenotypic screening of a well-characterized regional collection of wild accessions from the Iberian Peninsula, where the accession Don-0 was originally isolated [51] (S8 Fig). Thus, we identified the Frc-0 strain displaying the temperature-dependent growth defects of Don-0 and Sij-4 (Fig 5A). F1 plants, derived from an Frc-0 x Don-0 cross, showed similar phenotypes to parental lines, indicating that Frc-0 carries an ICA1 loss-of-function allele (Fig 5A). Sequence analysis identified a different SNP in the ICA1-Frc-0 allele, which causes a serine to proline substitution at position 84 (S84P). Since this serine is highly conserved in most plants and animals and is located close to the similar S81P polymorphism of Sij-4, it is likely that this structural mutation causes ICA1 loss-of-function (Fig 5C). Therefore, we found a total of three different natural ICA1 loss-of-function alleles from geographically distant locations (S8 Fig). To evaluate if these natural ICA1 mutations might be tolerated at local population level, we also analyzed allele frequencies in Don-0 and Frc-0 populations from the Iberian Peninsula. Phenotypic and genotypic analyses of several individuals per population showed that Don and Frc locations differed in the amount genome-wide genetic diversity per population (gene diversity of 0 and 0.05 respectively) and were highly differentiated (average number of allelic differences of 0.33±0.02). However, the ICA1-Frc-0 allele is nearly fixed in Frc location (6 homozygous ICA1-Frc-0 out of 6 individuals) and the ICA1-Don-0 allele was maintained at high frequency over time, since 7 out of 9 individuals were homozygous for ICA1-Don-0 allele six years after the initial Don collection [52].
Fig. 5. ICA1 allelic variation and intragenic suppression by alternative splicing. 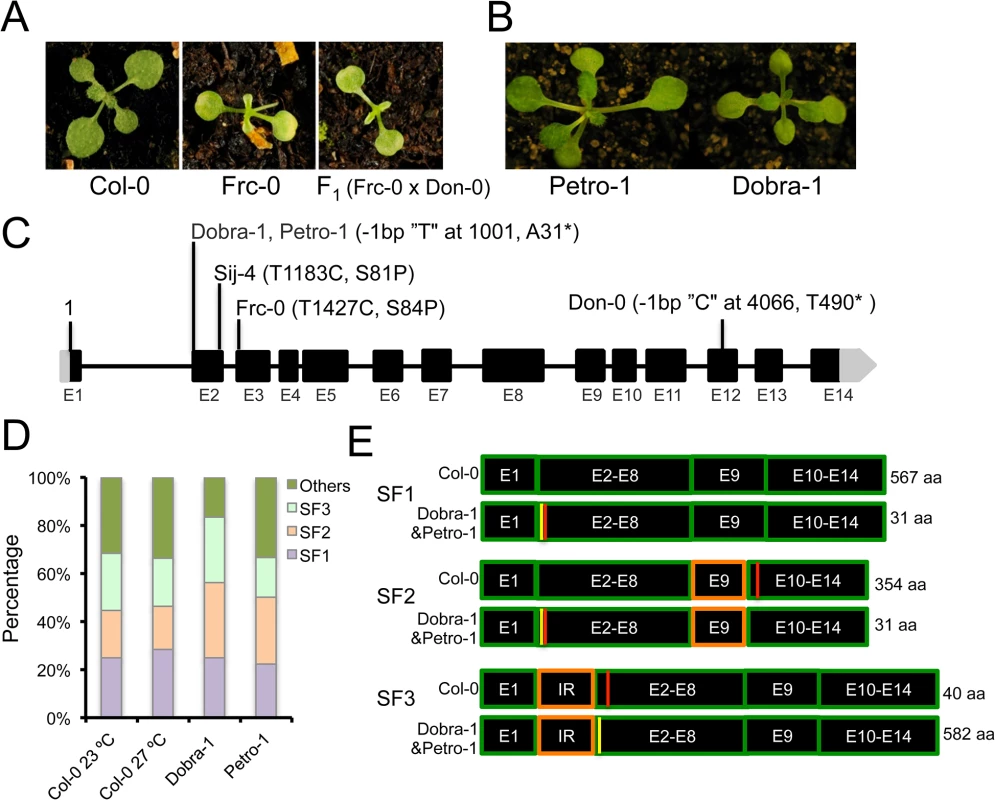
(A) Frc-0 displays the growth defect at 27°C and the phenotype of F1 (Frc-0 x Don-0) plants demonstrates that ICA1-Frc-0 is another ICA1 loss-of-function allele. (B) Absence of the growth defects in Petro-1 and Dobra-1 accessions grown at 27°C. (C) Natural polymorphisms of major effect observed in ICA1 and their genomic positions. (D) Proportions of alternatively spliced transcripts of ICA1 at 23°C and 27°C in Col-0 and at 27°C in Dobra-1 and Petro-1. (E) Schematic representation of the three major splice forms (SF1, SF2 and SF3), along with the predicted stop codons (shown as red lines) in Col-0 and Dobra-1/Petro-1. The single nucleotide deletion of Dobra-1/Petro-1 is shown as a yellow line, whereas the region affected by alternative splicing is marked in orange color. The predicted protein lengths are indicated in the right side of panel. SF2 is due to an alternative splice acceptor site for I8 in E9 resulting in a shorter E9 exon; SF3 is due to an alternative splice acceptor site in the first intron resulting in a partial intron retention (IR). Natural intragenic suppression of mutations through alternative splicing
As a complementary approach to find additional natural ICA1 loss-of-function alleles, we analyzed ICA1 sequences from the Arabidopsis 1001genome project, which recovered the S81P and T490* mutations in Sij-4 and Don-0 respectively. In addition, an identical single bp deletion was found in the Petro-1 and Dobra-1 accessions. This mutation is predicted to shift the reading frame and generate a short truncated protein of 31 amino acids (Fig 5C). While we confirmed this deletion through Sanger sequencing, Petro-1 and Dobra-1 failed to display the described phenotypes associated with ICA1 loss-of-function, suggesting the presence of modifiers (Fig 5B). To assess whether intragenic second-site suppression could account for the lack of disrupted phenotypes in Petro-1 and Dobra-1, we compared ICA1 cDNAs from these strains with those of Col-0. We first cloned and sequenced Col-0 cDNAs isolated from 23°C (76 clones) or 27°C (95 clones), which identified a total of 22 different transcripts that uncovered extensive alternative splicing at ICA1 (S11 Table). Three splice forms, referred to as SF1, SF2 and SF3, accounted for 70% of transcripts (Fig 5D and S11 Table) and no significant difference was found in the frequency of ICA1 transcripts between the two temperatures (S11 Table and Fig 5D). Only the SF1 transcript encodes the full length ICA1 protein of 567 amino acids, whereas transcripts SF2 and SF3 are predicted to generate truncated proteins of 354 or 40 amino acids respectively (Fig 5E). Since the truncated proteins are smaller than that encoded by Don-0 strain, these are likely to be nonfunctional. SF3 results from the use of an alternative splice acceptor site in intron 1 leading to the retention of 46 bp, which is predicted to shift the reading frame and to generate a premature stop codon in exon 2 (Fig 5E). Sequencing of cDNAs from Dobra-1 (48 clones) and Petro-1 (18 clones) revealed a splicing pattern similar to Col-0 (S11 Table). The single nucleotide deletion of Dobra-1/Petro-1 leads to a premature stop codon that would result in a truncated protein of 31 amino acids in transcripts encoded by SF1 and SF2. However, this deletion restores the SF3 open reading frame, resulting in a protein similar to that encoded by Col-0 SF1 with the addition of 15 amino acids in the N-terminal extension of ICA1 (S9 Fig and S11 Table). Thus, alternative splicing enables the natural intragenic suppression of an otherwise ICA1 loss-of-function allele.
Discussion
Modulation of fundamental processes related to protein biosynthesis might regulate thermo-sensory growth responses in plants
Temperature regulation of plant growth and development has been described until now mostly in the context of transcriptional regulation [14,23]. However, in this study we have demonstrated that ICA1, which encodes a member of the universally present Thg1 superfamily that is known to be involved in the tRNAHis maturation [34–36], is required for normal plant growth specifically at high ambient temperatures. Consequently, ICA1 appears to be an essential factor that is necessary for the regulation of thermo-sensory growth responses in A. thaliana. Both, natural and induced, ICA1 loss-of-function alleles show strong temperature-sensitive pleiotropic effects on plant growth throughout vegetative and reproductive development. These pleiotropic phenotypes are consistent with a function for ICA1 in a basic molecular process like the tRNAHis maturation mediated by Thg1 proteins. In agreement with this ICA1 function, our results suggest that most growth defects caused by ICA1 loss-of-function alleles could be due to indirect ICA1 effects on proteins involved in cell cycle processes and containing high histidine. In addition, the quantitative and reversible nature of the growth defects caused by ICA1 loss-of-function alleles further suggest the precise modulation of this fundamental biological process depending on temperature. Interestingly, a similarly strong temperature-sensitive growth phenotype has been previously shown in the Bur-0 strain to be caused by a mutation in the IIL1 locus that encodes an enzyme involved in leucine biosynthesis [3]. Therefore, thermal responses in Arabidopsis growth are likely regulated not only by direct transcriptional regulation, but also by modulation of other fundamental biological processes related to the general regulation of protein biosynthesis.
Thg1 family members share a conserved role in cell cycle regulation
We have characterized at the cellular and organismal level the first plant member of the highly conserved Thg1 superfamily, ICA1, showing its crucial developmental effects in A. thaliana. The strong ICA1 pleiotropic effects found at the organism level appear determined by its effect at the cellular level, since ICA1 is required for plant cell cycle progression and cell division. Consistent with a role in growth rather than cellular differentiation, the cell division disruption caused by ICA1 did not affect the general pattern of vegetative and reproductive organs, but mostly alters their sizes. The yeast homologue of ICA1 (Thg1p) was identified as a protein interacting with the replication origin recognition complex, and defective alleles led to defects in cell division similar to those that we have found in Arabidopsis [53]. In addition, the human homologue, referred to as ICF45, was isolated in a cDNA library screen as a factor expressed in a cell-division dependent manner [54]. Together these findings indicate that Thg1 proteins display a conserved function in cell division in most eukaryotes, from uni - to multicellular organisms.
Despite the primary molecular function described for Thg1 proteins are to provide mature tRNAHis [36], several observations have suggested additional potential functions. First, the Thg1 proteins are also present in organisms in which the 5’ G is already encoded in their genome, suggesting that this enzyme may have another ancestral function [34]. Second, the Thg1 proteins share a striking structural similarity with nucleic acid polymerases [38,55,56]. Third, they are unique in their ability to use both NTPs and dNTPs as substrates in the 3’-5’ polymerization reaction that they catalyze [57]. Fourth, Thg1 proteins have been shown to interact with the origin recognition complex, which has a primary role in DNA replication [53]. Based on these observations, it has been suggested that Thg1 proteins may also have a function in DNA/RNA repair [34,58]. In agreement with this hypothesis, ICA1 loss-of-function alleles display hypersensitivity to DNA damage. Hence, further investigations on the potential role of Thg1 superfamily in DNA/RNA repair and its link to cell cycle regulation are warranted.
Allelic variation at ICA1 accounts for natural variation in thermo-sensory growth responses of Arabidopsis thaliana
The characterization of Arabidopsis wild accessions revealed several instances of temperature-sensitive growth arrest, which is largely determined by ICA1 allelic variation. Natural loss-of-function alleles of ICA1 have arisen multiple times independently, since we found three different ICA1 natural loss-of-function mutations in global and regional collections from distinct geographic locations. Overall, the low frequency of such alleles (3 out of more than 300 analyzed strains) and their restriction to individual populations, suggest that ICA1 loss-of-function is deleterious in most natural environments and locations. This is further supported by the buffering of a potentially deleterious mutation through alternative splicing found in Petro-1 and Dobra-1 accessions. However, ICA1 loss-of-function alleles show high frequency at the local population level, thus suggesting that they are neutral in these populations. Therefore, this cryptic genetic variation at ICA1 is, most likely, conditionally neutral under natural conditions. Nevertheless, the quantitative and reversible nature of the temperature dependency, both at the organism phenotypic level and at the level of gene expression, as well as the presence of splice variants that encode potentially non-functional proteins in significant proportions (~70%, S11 Table), also suggest that ICA1 effects might be regulated in a quantitative manner to modulate plant growth in relation to temperature. Accordingly, we speculate that the fine tuning of plant growth by the reversible growth arrest caused by natural ICA1 loss-of-function alleles might reflect an alternative mechanism to respond to abiotic stress, which could be locally advantageous under certain unfavorable high temperatures.
Gene duplication may account for the temperature conditionality of ICA1 phenotypes
Even though ICA1 is required for growth at high ambient temperature but not at standard temperature, the precise mechanism accounting for the temperature sensitivity of the phenotypes caused by ICA1 loss-of-function alleles remains unknown. It is possible that this conditionality is an indirect effect derived from the temperature regulation of any downstream molecular component that is necessary for ICA1 effects on cell division. Alternatively, the temperature sensitivity of ICA1 effects might be the result of functional divergence of Thg1 proteins, because A. thaliana carries two genes encoding members of the Thg1 superfamily that may act redundantly [37]. Most likely, this duplication accounts for the viability of ICA1 loss-of-function genotypes, in comparison with the lethality of null alleles in the single copy Thg1 gene of yeast [36]. However, the strong phenotypes of ICA1 loss-of-function alleles at high temperature indicate that the two close paralogs are not fully redundant but show certain functional diversification. Future studies will further elucidate the relative contribution of functional divergence of plant Thg1 encoding genes to the temperature sensitivity of ICA1 growth phenotypes.
Materials and Methods
Plant material and growth conditions
A. thaliana accessions Col-0, Sij-4, Petro-1 and Dobra-1 and the T-DNA insertions lines for At2g31580 (SALK035242 and Wisc DsLox Hs 036_12H, in intron 1 and exon 8, and referred to as ica1-1 and ica1-2, respectively) were obtained from Arabidopsis Biological Resource Center. The Iberian collection used for the phenotypic screen has been previously described [51]. Frc-0 and Don-0 local populations were sampled in this study by collecting six and nine individuals respectively. Genetic diversity of the two populations was analyzed by genotyping a genome-wide set of 249 SNPs as previously described. [59].
Plants for phenotyping, crossing and propagation were grown on soil, at 23°C, in growth rooms under long days (LD, 16-h-light/8-h-dark cycles). Temperature dependent phenotypic analyses were done under short days (SD, 8-hr-light/16-hr-dark cycles) at 23°C and 27°C (for most of the experiments with Sij-4) or in long days at 21°C or 28°C (for most of the experiments with Don-0). For DNA damage assays and transcriptome analyses, sterilized seeds were plated on 0.5x MS media supplemented with 1% sucrose, and plates were placed in growth chambers (Percival Scientific, Canada) in SD at the required temperatures. For transformation, to accelerate flowering, Sij-4 was vernalized by imbibing seeds in water and placing them at 4°C in the dark for at least 4 weeks before planting.
For measurements of hypocotyl elongation in response to high temperature, F2 (Sij-4 x Col-0) seeds were grown at 23°C and 27°C in SD, after 2 days of stratification at 4°C in darkness. Two week-old F2 seedlings were then collected and photographed. Early leaf development phenotype was scored based on ICA1 loss-of-function leaf phenotype and the hypocotyl length was measured with ImageJ64 for Mac.
Positional cloning
F1 plants derived from reciprocal crosses between Sij-4 and Col-0 were tested at 27°C SD to evaluate ICA1 phenotype. The initial mapping was performed using 96 plants with ICA1-Sij-4 phenotype collected from a F2 (Sij-4 x Col-0) population and 300 ICA1-Don-0 phenotype plants derived from a F2 (Don-0 x Ler) family. For fine mapping we used 2500 and 1500 mutant plants respectively from F2 (Sij-4 x Col-0) and F2 (Sij-4 x Ler) populations. Genetic markers used for fine mapping are given in S11 Table. Analyses of available sequences from Sij-4, Bur-0, C24, Col-0 and Ler-0 (1001 genome project; http://1001genomes.org/) were performed to identify nucleotide polymorphisms in mapping intervals. The final mapping interval (5.9 Kb) was then fully sequenced in Sij-4 and a 5.3 Kb region containing the ICA1 gene was sequenced in Don-0 to identify sequence variants.
Generation of constructs and transgenic lines
For ICA1 complementation in Sij-4, a construct containing 4.7 Kb genomic DNA encompassing the entire coding region of ICA1 from Col-0 (35S::ICA1gDNA-Col) driven by 35S CaMV promoter was cloned into the Gateway entry vector pDONR207 and moved into pFK210 through LR reaction. Constructs in pFK210 were then electroporated into Agrobacterium tumefaciens GV3001 and transformed into Sij-4 plants by floral dipping [60]. Similarly, complementation of Don-0 and the ica1-2 T-DNA mutant was done using a 5.3 Kb Ler genomic fragment containing the complete ICA1 coding region and the intergenic adjacent sequences cloned in the binary vector pCAMBIA3300.
To generate a construct containing the S81P mutation (35S::ICA1gDNA-Col-S81P), the 35S:: ICA1gDNA-Col construct was used as template to introduce a T to C conversion (corresponding to TAIR10 position 13444655, Chr 2) by site-directed mutagenesis, according to QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies). The 35S::amiR-ICA1 construct was designed using primers listed in S12 Table according to Schwab et al [61] and cloned into pFK210 for transformation.
Microscopy
To investigate the cell cycle of plants carrying ICA1 loss-of-function alleles, a pCyclinB1;1::CyclinB1;1-GFP stable transgenic line in Col-0 background (kindly donated by Peter Doerner, University of Edinburgh, UK) was crossed with Sij4. Two week-old F2 young seedlings derived from that cross were dissected to obtain shoot apices and early leaves for fluorescence microscopy analysis. The pCyclinB1;1::CyclinB1;1-GFP signal was then observed by fluorescence microscopy and compared between plants showing ICA1-Sij-4 and ICA1-Col phenotypes. Nucleus and cell sizes of abaxial epidermal cells from the first pair of true leaves were measured using the nucleus marker pAR98 (pATML1::H2B-mYFP) and the plasma membrane marker pAR169 (pATML1::mCitrine-RCI2A)[46] respectively. Plasma membrane marker pAR169 was introduced by plant transformation in Sij-4 and Col-0 plants to compare between ICA1-Sij-4 and ICA1-Col cells. For nuclear marker pAR98, a stable transgenic line in Ler background was crossed with Sij-4, and the F2 plants were used to compare between ICA1-Sij-4 and ICA1-Ler cells. The nuclear area was measured manually using more than 15 nuclei per genotype using ImageJ. The surface of 5,10 or 40 day-old first true leaves was visualized using low-temperature SEM according to Feiler et al [62].
Flow cytometry
For measurements of DNA content, 3 week-old plants of Col, Sij-4 and 35S::ICA1gDNA-Col in Sij-4 background grown at 27°C SD, were chopped with a sharp razor, and the nuclei stained with propidium iodide according to the manufacturer’s protocol (CyStain UV Precise P; PARTEC). The distribution of DNA content in cell populations was then measured with a flow cytometry analyzer LRS IIb (BD Biosciences). The DNA contents of Sij-4 and 35S::ICA1gDNA-Col in Sij-4 plants were compared against Col-0 and the significance was tested using a Chi-square test.
DNA damage assays
The first leaf assay was performed to evaluate DNA damage response with Col-0, Sij-4, 35S::ICA1gDNA-Col in Sij-4 background and 35S::amiR-ICA1 in Col-0 background grown under 23°C SD as described previously [50]. Briefly, four-day-old seedlings were treated with a DNA damage reagent, the radiomimetic drug Bleomycin (BLEO, EMD Millipore) and the development of the first two leaves was analyzed at early stage. Seedlings were transferred to liquid 0.5 MS medium at day 4, either with or without 1μg/ml or 2μg/ml of BLEO. At day 9, BLEO-treated seedlings were washed with plain liquid 0.5 MS media and transferred back to 0.5 MS plates. The phenotype of first true leaves was then scored 24 hours later at day 10.
Analysis of ICA1 expression and alternative splicing
Leaf tissue from 4 week-old plants grown at 23°C and 27°C under SD was collected and RNA was isolated with Trizol (Invitrogen). RNA was reverse transcribed using the Roche first strand cDNA synthesis kit (Roche). For ICA1 expression, RNA was isolated from three week-old plants grown at 23°C and 27°C under long days analyzed by quantitative PCR using primers described in S12 Table. To analyze alternative splicing, cDNAs were amplified from Col-0, Petro-1 and Dobra-1 grown at 23°C or 27°C using full length ICA1 coding region primers described in S12 Table, and cloned in to pGEM-T vector (Promega). Clones were sequenced with pUC/M13F and pUC/M13R primers (Macrogen, South Korea). After quality control, sequences were obtained for 48–96 clones as shown in S11 Table (76 and 96 colonies from Col-0 23°C and 27°C respectively; 48 colonies from Dobra-1 and 18 colonies from Petro-1 in 27°C short day). Sequences were aligned with Seqman (DNAStar Lasergene) to identify alternative splicing at 23°C and 27°C.
RNA-seq transcript analysis
For transcriptome analysis, about one hundred 6-day-old seedlings of Col-0 or Sij-4 grown at 23°C or 27°C in growth chambers (GR-36, Percival Scientific, Canada) in SD were harvested around 1.00 PM (5 hours after the beginning of the light regime). The RNA was extracted using Isolate II RNA plant kit (Bioline Pty Ltd, Australia) and RNASeq was done with Illumina HiSeq2000 platform by BGI (BGI, China). Three and two biological replicates were used for Col-0 and the Sij-4 samples respectively. Analysis of differential expression between samples was conducted using the edgeR Bioconductor package [63]. Paired-end Illumina RNA-Seq reads of 90bp length were aligned to the TAIR10 reference Arabidopsis genome, using the Subread pipeline's subread-align program with its default parameters [64]. Raw abundance counts of each gene were subsequently produced by running the Subread pipeline’s featureCounts program on the SAM file produced in the previous step, using the TAIR10 Arabidopsis genome annotation file (downloaded from TAIR) and the-p and-R parameters to convert mapped reads to mapped RNA fragments (a pair of forward and reverse reads) and to output read counting results for each fragment, respectively. The resulting list of abundance counts for each gene was used as input data to the edgeR differential expression software. Differential expression was analyzed by the edgeR BioConductor package using the GLM (generalized linear model) approach, with replicate number added as a factor to the generalized linear model to mitigate for a batch effect. In case of Col-0, where three replicates were present, 'replicate 1' and 'replicate 2 or 3' were used as factors, since replicates 2 and 3 were produced at the same time and were not subject to a batch effect between the two. As per edgeR defaults, p-values for differential expression were adjusted for multiple hypotheses testing by Benjamini-Hochberg p-value correction. To analyze the correlation between changes in expression, log fold changes from the edgeR output of the differential expression between Col-0 and Sij-4 at 23°C and 27°C was plotted against each other and the Pearson correlation was calculated. There was no difference in Pearson correlation between genes that were down regulated or up regulated in Sij-4 suggesting that the observed differences are not associated with the polymorphisms between Sij-4 and Col-0 affecting the alignment of reads.
GO enrichment analysis
The gene lists generated through the analysis of differential expression were used in the online program GOrilla to identify enriched GO terms [39] and the GOrilla output was summarized and visualized through REViGO [40]. In order to identify the Histidine rich proteins, we used three different approaches. First, we used a single list of the Arabidopsis proteins ranked by amino acid content (numbers) and analyzed through GOrilla and REViGO as described above. Second, we took the top 5% of the proteins with high amino acid content and compared this list using the whole proteome as the background through GOrilla and REViGO. Third, we removed the tail ends of the distribution taking only proteins that fell in between the 10th and 90th percentile in terms of the protein length and analyzed the top 5% of these through a similar analysis. The analysis was done with all amino acids, with all proteins ranked by length or with the top 5% of the largest proteins with the entire proteome as controls.
Databases
The sequences of the ICA1 from various strains and the sequences of different splice forms are available through Genbank accession numbers KP759903-KP759939. The transcriptome data has been submitted to the NCBI Sequence Read Archive and is available under the accession number SRP053394.
Supporting Information
Zdroje
1. Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417(6889):618–24. Epub 2002/06/07. 12050657
2. Gibson G, Dworkin I. Uncovering cryptic genetic variation. Nature Reviews Genetics. 2004;5(9):681–90. Epub 2004/09/17. 15372091
3. Sureshkumar S, Todesco M, Schneeberger K, Harilal R, Balasubramanian S, Weigel D. A genetic defect caused by a triplet repeat expansion in Arabidopsis thaliana. Science. 2009;323(5917):1060–3. Epub 2009/01/20. doi: 10.1126/science.1164014 19150812
4. Ledon-Rettig CC, Pfennig DW, Chunco AJ, Dworkin I. Cryptic Genetic Variation in Natural Populations: A Predictive Framework. Integrative and comparative biology. 2014;54(5):783–93. Epub 2014/06/20. doi: 10.1093/icb/icu077 24944116
5. Paaby AB, Rockman MV. Cryptic genetic variation: evolution's hidden substrate. Nature Reviews Genetics. 2014;15(4):247–58. Epub 2014/03/13. doi: 10.1038/nrg3688 24614309
6. Casal JJ. Photoreceptor signaling networks in plant responses to shade. Annual review of plant biology. 2013;64 : 403–27. Epub 2013/02/05. doi: 10.1146/annurev-arplant-050312-120221 23373700
7. Chen M, Chory J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011;21(11):664–71. Epub 2011/08/20. doi: 10.1016/j.tcb.2011.07.002 21852137
8. Kami C, Lorrain S, Hornitschek P, Fankhauser C. Light-regulated plant growth and development. Curr Top Dev Biol. 2010;91 : 29–66. Epub 2010/08/14. doi: 10.1016/S0070-2153(10)91002-8 20705178
9. Fankhauser C, Chory J. Light control of plant development. Annual review of cell and developmental biology. 1997;13 : 203–29. Epub 1997/01/01. 9442873
10. Knight MR, Knight H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. The New phytologist. 2012;195(4):737–51. Epub 2012/07/24. doi: 10.1111/j.1469-8137.2012.04239.x 22816520
11. Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD. Complexity of the heat stress response in plants. Current opinion in plant biology. 2007;10(3):310–6. Epub 2007/05/08. 17482504
12. Penfield S. Temperature perception and signal transduction in plants. The New phytologist. 2008;179(3):615–28. Epub 2008/05/10. doi: 10.1111/j.1469-8137.2008.02478.x 18466219
13. Fitter AH, Fitter RS. Rapid changes in flowering time in British plants. Science. 2002;296(5573):1689–91. 12040195
14. Wigge PA. Ambient temperature signalling in plants. Current opinion in plant biology. 2013;16(5):661–6. Epub 2013/09/12. doi: 10.1016/j.pbi.2013.08.004 24021869
15. Samach A, Wigge PA. Ambient temperature perception in plants. Current opinion in plant biology. 2005;8(5):483–6. 16054430
16. Wang ZW, Wu Z, Raitskin O, Sun Q, Dean C. Antisense-mediated FLC transcriptional repression requires the P-TEFb transcription elongation factor. Proc Natl Acad Sci U S A. 2014;111(20):7468–73. Epub 2014/05/07. doi: 10.1073/pnas.1406635111 24799695
17. Baulcombe DC, Dean C. Epigenetic regulation in plant responses to the environment. Cold Spring Harbor perspectives in biology. 2014;6(9):a019471. Epub 2014/09/04. doi: 10.1101/cshperspect.a019471 25183832
18. Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci U S A. 1998;95(12):7197–202. Epub 1998/06/17. 9618562
19. Todesco M, Balasubramanian S, Cao J, Ott F, Sureshkumar S, Schneeberger K, et al. Natural variation in biogenesis efficiency of individual Arabidopsis thaliana microRNAs. Curr Biol. 2012;22(2):166–70. Epub 2011/12/31. doi: 10.1016/j.cub.2011.11.060 22206705
20. Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, et al. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19(5):408–13. Epub 2009/03/03. doi: 10.1016/j.cub.2009.01.046 19249207
21. Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS genetics. 2006;2(7):e106. Epub 2006/07/15. 16839183
22. Boden SA, Kavanova M, Finnegan EJ, Wigge PA. Thermal stress effects on grain yield in Brachypodium distachyon occur via H2A.Z-nucleosomes. Genome biology. 2013;14(6):R65. Epub 2013/06/27.
23. Kumar SV, Wigge PA. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140(1):136–47. Epub 2010/01/19. doi: 10.1016/j.cell.2009.11.006 20079334
24. Franklin KA. Plant chromatin feels the heat. Cell. 2010;140(1):26–8. Epub 2010/01/21. doi: 10.1016/j.cell.2009.12.035 20085701
25. Sidaway-Lee K, Costa MJ, Rand DA, Finkenstadt B, Penfield S. Direct measurement of transcription rates reveals multiple mechanisms for configuration of the Arabidopsis ambient temperature response. Genome biology. 2014;15(3):R45. Epub 2014/03/04. doi: 10.1186/gb-2014-15-3-r45 24580780
26. Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci U S A. 2011;108(50):20231–5. Epub 2011/11/30. doi: 10.1073/pnas.1110682108 22123947
27. Kumar SV, Lucyshyn D, Jaeger KE, Alos E, Alvey E, Harberd NP, et al. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature. 2012;484(7393):242–5. Epub 2012/03/23. doi: 10.1038/nature10928 22437497
28. Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes & development. 2007;21(4):397–402.
29. Mizuno T, Nomoto Y, Oka H, Kitayama M, Takeuchi A, Tsubouchi M, et al. Ambient temperature signal feeds into the circadian clock transcriptional circuitry through the EC night-time repressor in Arabidopsis thaliana. Plant & cell physiology. 2014;55(5):958–76. Epub 2014/02/07.
30. Alonso-Blanco C, Mendez-Vigo B. Genetic architecture of naturally occurring quantitative traits in plants: an updated synthesis. Current opinion in plant biology. 2014;18 : 37–43. Epub 2014/02/26. doi: 10.1016/j.pbi.2014.01.002 24565952
31. Patel D, Basu M, Hayes S, Majlath I, Hetherington FM, Tschaplinski TJ, et al. Temperature-dependent shade avoidance involves the receptor-like kinase ERECTA. Plant J. 2013;73(6):980–92. Epub 2012/12/04. doi: 10.1111/tpj.12088 23199031
32. Balasubramanian S, Weigel D. Temperature Induced Flowering in Arabidopsis thaliana. Plant Signal Behav. 2006;1(5):227–8. Epub 2006/09/01. 19704664
33. Pose D, Verhage L, Ott F, Yant L, Mathieu J, Angenent G, et al. Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature. 2013. 503(7476): 414–417. doi: 10.1038/nature12633 24067612
34. Jackman JE, Gott JM, Gray MW. Doing it in reverse: 3'-to-5' polymerization by the Thg1 superfamily. RNA (New York, NY. 2012;18(5):886–99. Epub 2012/03/30. doi: 10.1261/rna.032300.112 22456265
35. Heinemann IU, Nakamura A, O'Donoghue P, Eiler D, Soll D. tRNAHis-guanylyltransferase establishes tRNAHis identity. Nucleic acids research. 2012;40(1):333–44. Epub 2011/09/06. doi: 10.1093/nar/gkr696 21890903
36. Gu W, Jackman JE, Lohan AJ, Gray MW, Phizicky EM. tRNAHis maturation: an essential yeast protein catalyzes addition of a guanine nucleotide to the 5' end of tRNAHis. Genes & development. 2003;17(23):2889–901. Epub 2003/11/25.
37. Placido A, Sieber F, Gobert A, Gallerani R, Giege P, Marechal-Drouard L. Plant mitochondria use two pathways for the biogenesis of tRNAHis. Nucleic acids research. 2010;38(21):7711–7. Epub 2010/07/28. doi: 10.1093/nar/gkq646 20660484
38. Hyde SJ, Eckenroth BE, Smith BA, Eberley WA, Heintz NH, Jackman JE, et al. tRNA(His) guanylyltransferase (THG1), a unique 3'-5' nucleotidyl transferase, shares unexpected structural homology with canonical 5'-3' DNA polymerases. Proc Natl Acad Sci U S A. 2010;107(47):20305–10. Epub 2010/11/10. doi: 10.1073/pnas.1010436107 21059936
39. Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC bioinformatics. 2009;10 : 48. Epub 2009/02/05. doi: 10.1186/1471-2105-10-48 19192299
40. Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6(7):e21800. Epub 2011/07/27. doi: 10.1371/journal.pone.0021800 21789182
41. Menges M, Hennig L, Gruissem W, Murray JA. Genome-wide gene expression in an Arabidopsis cell suspension. Plant molecular biology. 2003;53(4):423–42. Epub 2004/03/11. 15010610
42. Ferreira PC, Hemerly AS, Engler JD, van Montagu M, Engler G, Inze D. Developmental expression of the arabidopsis cyclin gene cyc1At. Plant Cell. 1994;6(12):1763–74. Epub 1994/12/01. 7866022
43. Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P. Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999;20(4):503–8. Epub 1999/12/22. 10607302
44. Jacqmard A, De Veylder L, Segers G, de Almeida Engler J, Bernier G, Van Montagu M, et al. Expression of CKS1At in Arabidopsis thaliana indicates a role for the protein in both the mitotic and the endoreduplication cycle. Planta. 1999;207(4):496–504. Epub 1999/03/27. 10093894
45. Menges M, de Jager SM, Gruissem W, Murray JA. Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J. 2005;41(4):546–66. Epub 2005/02/03. 15686519
46. Roeder AH, Chickarmane V, Cunha A, Obara B, Manjunath BS, Meyerowitz EM. Variability in the control of cell division underlies sepal epidermal patterning in Arabidopsis thaliana. PLoS biology. 2010;8(5):e1000367. Epub 2010/05/21. doi: 10.1371/journal.pbio.1000367 20485493
47. Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nature reviews Cancer. 2007;7(11):861–9. Epub 2007/10/19. 17943134
48. Adachi S, Minamisawa K, Okushima Y, Inagaki S, Yoshiyama K, Kondou Y, et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(24):10004–9. Epub 2011/05/27. doi: 10.1073/pnas.1103584108 21613568
49. Kandasamy MK, McKinney EC, Deal RB, Smith AP, Meagher RB. Arabidopsis actin-related protein ARP5 in multicellular development and DNA repair. Developmental biology. 2009;335(1):22–32. Epub 2009/08/15. doi: 10.1016/j.ydbio.2009.08.006 19679120
50. Rosa M, Von Harder M, Cigliano RA, Schlogelhofer P, Mittelsten Scheid O. The Arabidopsis SWR1 chromatin-remodeling complex is important for DNA repair, somatic recombination, and meiosis. Plant Cell. 2013;25(6):1990–2001. Epub 2013/06/20. doi: 10.1105/tpc.112.104067 23780875
51. Mendez-Vigo B, Pico FX, Ramiro M, Martinez-Zapater JM, Alonso-Blanco C. Altitudinal and climatic adaptation is mediated by flowering traits and FRI, FLC, and PHYC genes in Arabidopsis. Plant Physiol. 2011;157(4):1942–55. Epub 2011/10/13. doi: 10.1104/pp.111.183426 21988878
52. Pico FX, Mendez-Vigo B, Martinez-Zapater JM, Alonso-Blanco C. Natural genetic variation of Arabidopsis thaliana is geographically structured in the Iberian peninsula. Genetics. 2008;180(2):1009–21. Epub 2008/08/22. doi: 10.1534/genetics.108.089581 18716334
53. Rice TS, Ding M, Pederson DS, Heintz NH. The highly conserved tRNAHis guanylyltransferase Thg1p interacts with the origin recognition complex and is required for the G2/M phase transition in the yeast Saccharomyces cerevisiae. Eukaryotic cell. 2005;4(4):832–5. Epub 2005/04/12. 15821142
54. Guo D, Hu K, Lei Y, Wang Y, Ma T, He D. Identification and characterization of a novel cytoplasm protein ICF45 that is involved in cell cycle regulation. The Journal of biological chemistry. 2004;279(51):53498–505. Epub 2004/10/02. 15459185
55. Hyde SJ, Rao BS, Eckenroth BE, Jackman JE, Doublie S. Structural studies of a bacterial tRNA(HIS) guanylyltransferase (Thg1)-like protein, with nucleotide in the activation and nucleotidyl transfer sites. PLoS ONE. 2013;8(7):e67465. Epub 2013/07/12. doi: 10.1371/journal.pone.0067465 23844012
56. Nakamura A, Nemoto T, Heinemann IU, Yamashita K, Sonoda T, Komoda K, et al. Structural basis of reverse nucleotide polymerization. Proc Natl Acad Sci U S A. 2013;110(52):20970–5. Epub 2013/12/11. doi: 10.1073/pnas.1321312111 24324136
57. Jackman JE, Phizicky EM. tRNAHis guanylyltransferase catalyzes a 3'-5' polymerization reaction that is distinct from G-1 addition. Proc Natl Acad Sci U S A. 2006;103(23):8640–5. Epub 2006/05/30. 16731615
58. Rao BS, Maris EL, Jackman JE. tRNA 5'-end repair activities of tRNAHis guanylyltransferase (Thg1)-like proteins from Bacteria and Archaea. Nucleic acids research. 2011;39(5):1833–42. Epub 2010/11/06. doi: 10.1093/nar/gkq976 21051361
59. Brennan AC, Mendez-Vigo B, Haddioui A, Martinez-Zapater JM, Pico FX, Alonso-Blanco C. The genetic structure of Arabidopsis thaliana in the south-western Mediterranean range reveals a shared history between North Africa and southern Europe. BMC plant biology. 2014;14 : 17. Epub 2014/01/15. doi: 10.1186/1471-2229-14-17 24411008
60. Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43. Epub 1999/03/09. 10069079
61. Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18(5):1121–33. 16531494
62. Feiler HS, Desprez T, Santoni V, Kronenberger J, Caboche M, Traas J. The higher plant Arabidopsis thaliana encodes a functional CDC48 homologue which is highly expressed in dividing and expanding cells. The EMBO journal. 1995;14(22):5626–37. Epub 1995/11/15. 8521820
63. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England). 2010;26(1):139–40. Epub 2009/11/17. doi: 10.1093/bioinformatics/btp616 19910308
64. Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic acids research. 2013;41(10):e108. Epub 2013/04/06. doi: 10.1093/nar/gkt214 23558742
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



