-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDrosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
Alzheimer’s disease is the most common cause of dementia in the aging population. It is a progressive neurodegenerative disorder that attacks the brain neurons, resulting in loss of memory, thinking and behavioral changes. One pathological hallmark is aggregation of the microtubule-associated protein Tau. A growing body of evidence highlights the importance of caspase-dependent Tau truncation in initiation and potentiation of Tau aggregation. Here we use the fruit fly Drosophila to examine the links between circadian rhythms, aging, apoptosis and Alzheimer’s Disease. We identified a regulator (spag) of the circadian kinase Dbt that functions to stabilize Dbt during the middle of the day. In addition, the caspase Dronc is regulated by Dbt and Spag and, when activated by reduction of either, targets Tau for cleavage, leading to behavioral deficits and shortened lifespans. The expression of activated caspase occurs in several parts of the brain in a manner requiring signaling from a neuropeptide produced by circadian cells. Wild type flies with no genetic modifications eventually exhibit modified Dbt and expression of activated caspase at specific times of day, further demonstrating the links between the circadian clock, light and apoptosis.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005171
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005171Summary
Alzheimer’s disease is the most common cause of dementia in the aging population. It is a progressive neurodegenerative disorder that attacks the brain neurons, resulting in loss of memory, thinking and behavioral changes. One pathological hallmark is aggregation of the microtubule-associated protein Tau. A growing body of evidence highlights the importance of caspase-dependent Tau truncation in initiation and potentiation of Tau aggregation. Here we use the fruit fly Drosophila to examine the links between circadian rhythms, aging, apoptosis and Alzheimer’s Disease. We identified a regulator (spag) of the circadian kinase Dbt that functions to stabilize Dbt during the middle of the day. In addition, the caspase Dronc is regulated by Dbt and Spag and, when activated by reduction of either, targets Tau for cleavage, leading to behavioral deficits and shortened lifespans. The expression of activated caspase occurs in several parts of the brain in a manner requiring signaling from a neuropeptide produced by circadian cells. Wild type flies with no genetic modifications eventually exhibit modified Dbt and expression of activated caspase at specific times of day, further demonstrating the links between the circadian clock, light and apoptosis.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that involves neuronal cell loss, extracellular amyloid plaques, and intracellular neurofibrillary tangles. During AD and other neurodegenerative diseases, neurons induce a series of proteases, including caspases, and a number of key proteins are cleaved by caspases including APP, Presenilin (PS1, PS2), Tau and Huntingtin [1–5]. This has led to the suggestion that the extensive neuronal loss observed in AD may result from the activation of apoptotic related pathways [6]. Caspases can be activated within a cell without immediately causing classical apoptosis, and there is evidence for prolonged caspase activation without neuronal death [7]. For instance, in AD chronic caspase activation may lead to cleavage of Tau and other essential cellular proteins and contribute to neuronal pathology prior to cell death [3]. Caspase-cleaved Tau is present in AD, but not control brain [8,9]. Additionally, caspase-cleaved Tau is more fibrillogenic in vitro than full-length Tau [9].
Drosophila melanogaster has been used as a model system for the analysis of AD [10]. The Drosophila Tau protein is similar to the mammalian one and accumulates in axonal processes [11]. When it is overexpressed in neurons or eyes, fly Tau induces apoptotic neuronal cell death [12]. In addition, synaptic dysfunctions induced by human or fly Tau have been produced and analyzed in Drosophila larval motor neurons and neuromuscular junctions. Expression of Tau within motor neurons generates altered morphology in the presynaptic terminals and defective synaptic transmission and microtubule-based axonal transport [13,14]. The fly mushroom body is the key locus for olfactory learning and memory in Drosophila, where human or fly Tau expression causes an impairment of associative learning and memory followed by neurodegeneration [15]. Finally, when expressed in the fly eye or brain, human Tau produces aspects of human tauopathies, including neurodegeneration [16,17].
Like many organisms, Drosophila operates on a 24-hour cycle that is maintained by environmental input to an internal body clock [18]. The clock depends on oscillations in the activation of specific genes at certain times of the day. The key feature of these oscillations is a negative feedback loop, in which transcriptional regulators like Period (Per) repress transcription of their mRNAs. The Drosophila Doubletime (Dbt) protein is homologous to mammalian Casein Kinase 1 and phosphorylates Per monomers, resulting in Per degradation [19,20]. In addition to its role in regulating circadian rhythms, Dbt has also been shown to be involved in regulating cell death pathways. For example, overexpression of Dbt in the fly eye has been shown to rescue the eye morphology defect caused by expression of proapoptotic proteins such as Reaper and Hid [21].
Circadian rhythm disturbances affect as many as 25% of AD patients during some stage of their disease [22]. As a consequence, sleep, the biological clock, and core body temperature are affected. Some common symptoms of AD that are related to disturbances in the circadian clock are insomnia, nocturnal behavioral changes, and excessive daytime sleepiness. A postmortem study found significant differences in the expression pattern of circadian genes between Alzheimer patients and controls [23]. In addition, the 3xTg (triple transgenic) mouse models of AD, which exhibit Tau neuropathology, showed deteriorated circadian organization of locomotor behavior [24]. However, it is not known if circadian components are directly linked to disease onset or if circadian dysfunction is just a consequence of AD.
Our search for proteins that interact with the Drosophila circadian kinase Dbt has led to results demonstrating that Dbt and one of these interactors connect the circadian, cell death and neurodegenerative pathways. Initially, a screen was performed for effectors of Dbt’s circadian function; we screened a list of candidate genes (mostly phosphatase catalytic subunits) with dsRNAi lines crossed to the timGAL4 driver for those that would alter circadian periods and lead to changes in Dbt electrophoretic mobility, potentially indicative of those that would lead to autophosphorylation of Dbt. This screen identified spaghetti (CG13570, or spag). spag encodes a tetratricopeptide repeat (TPR)-containing protein initially identified in a screen for modifiers of protein aggregation in Huntington disease and was reported to interact both with Huntingtin protein and the chaperone protein HSP90 [25]. Here, we show that spag knock-down or reduced Dbt activity in circadian cells leads to longer circadian periods and expression of activated initiator caspase only during the middle of the day. Links to the circadian clock are shown by the effects of light, circadian mutants and circadian cells, while links to AD are shown by the resulting cleavage of Tau, enhancement of neurodegeneration, reduced healthspan and effects of aging.
Results
Spaghetti knockdown leads to circadian rhythm disruption, reductions in Dbt, and Dbt-dependent caspase activation after extended light treatment
We targeted the clock cells in flies with spag RNAi knock-down (timGAL4>UAS-dcr; UAS-spag RNAi) and observed altered locomotor activity rhythms. spag knockdown flies exhibited long periods (25.5–26.5 h) or arrhythmic locomotor activity (S1 Fig and Table 1), depending on the specific RNAi transgene, the inclusion of dcr in the genotype and the duration of transgene expression after eclosion. Null mutations of spag and ubiquitous knock-down of spag were lethal, so all of our analysis has employed knock-downs of spag in circadian cells with the timGAL4 or pdfGAL4 drivers.
Tab. 1. Locomotor activity rhythms of flies with spag RNAi knockdown. 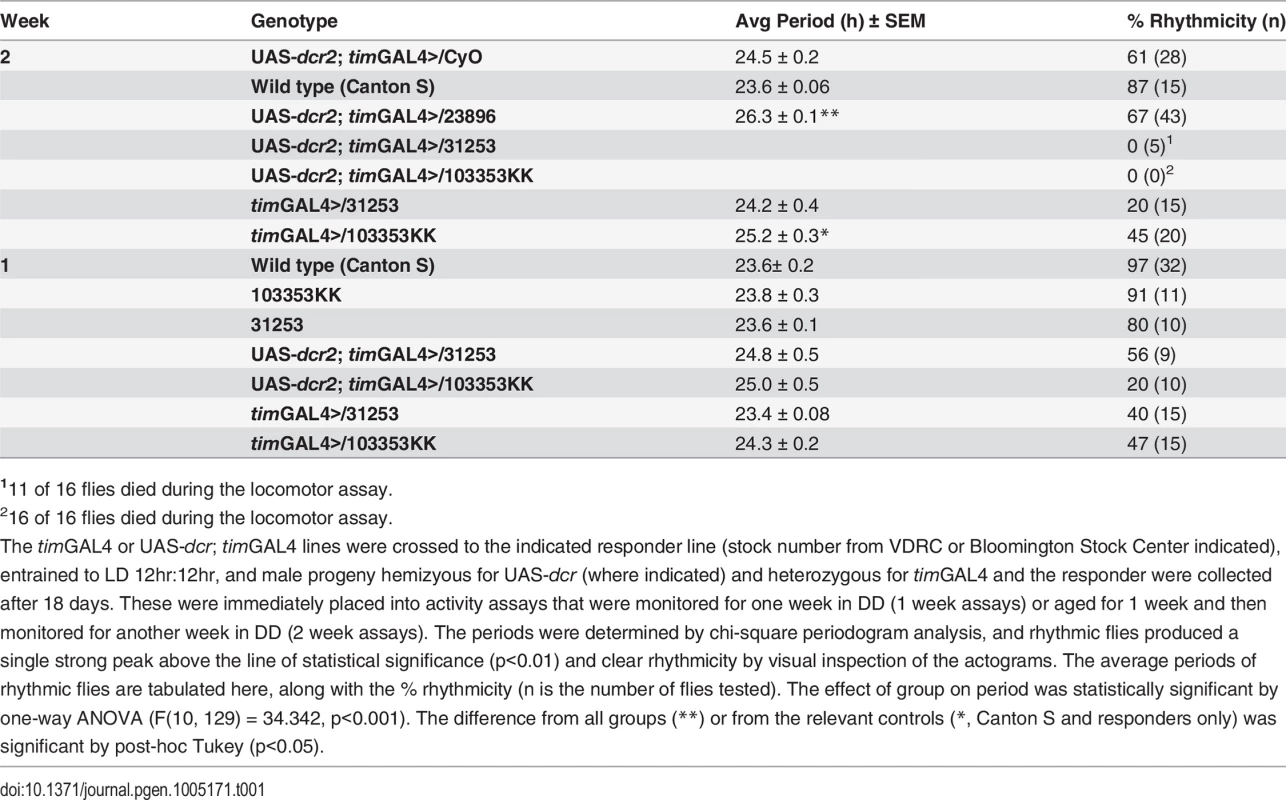
111 of 16 flies died during the locomotor assay. In addition to locomotor defects, knockdown of spag led to a decrease in Dbt protein levels and an increase in the levels of activated initiator caspase Dronc, detected with an antibody that only detects the cleaved (and thereby activated) form of Dronc [26] (Figs 1A and S2A; See Materials and Methods). Interestingly, the decrease in Dbt and the accumulation of activated Dronc occurred mostly during the daytime close to ZT7 (Lights are on from ZT0-12 and off from ZT12-24). To determine the time course more precisely, head extracts were collected at one hour intervals from ZT1-7, and Dbt disappearance and activated caspase were detected by ZT6 (Fig 1B). In addition, the levels of activated Dronc were reduced after ZT9 and gone by ZT11 (Fig 1B). These effects of spag knock-down were further confirmed in Drosophila S2 cells, which lack a circadian rhythm, and knockdown of spag with two different non-overlapping dsRNAi’s led to a decrease in Dbt levels, followed by accumulation of activated Dronc (Figs 1C and S3A). The loss of Dbt from Drosophila S2 cells or fly heads was preceded by a post-translational modification of Dbt that produced a mobility shift with SDS-PAGE (S2B–S2E Fig), and in fly heads the loss was not always complete but was enhanced at higher temperatures, longer times post-eclosion and by light rather than darkness (S2C, S2D, and S2E Fig). Light is a strong trigger, as Dronc was activated 7 hrs after light periods starting at ZT13 in a number of lines (including spag RNAi) that produced activated Dronc during the day (S2F Fig). A variable amount of Dbt reduction was also observed in the middle of the night (ZT19; Figs 1A and S3B), although activated Dronc was not detected. In summary, reduced spag levels in circadian cells triggers post-translational modification of Dbt, reduced Dbt levels and accumulation of activated caspase at specific times of day.
Fig. 1. Knock-down of spaghetti reduces Dbt and causes accumulation of activated initiator caspase at ZT7. 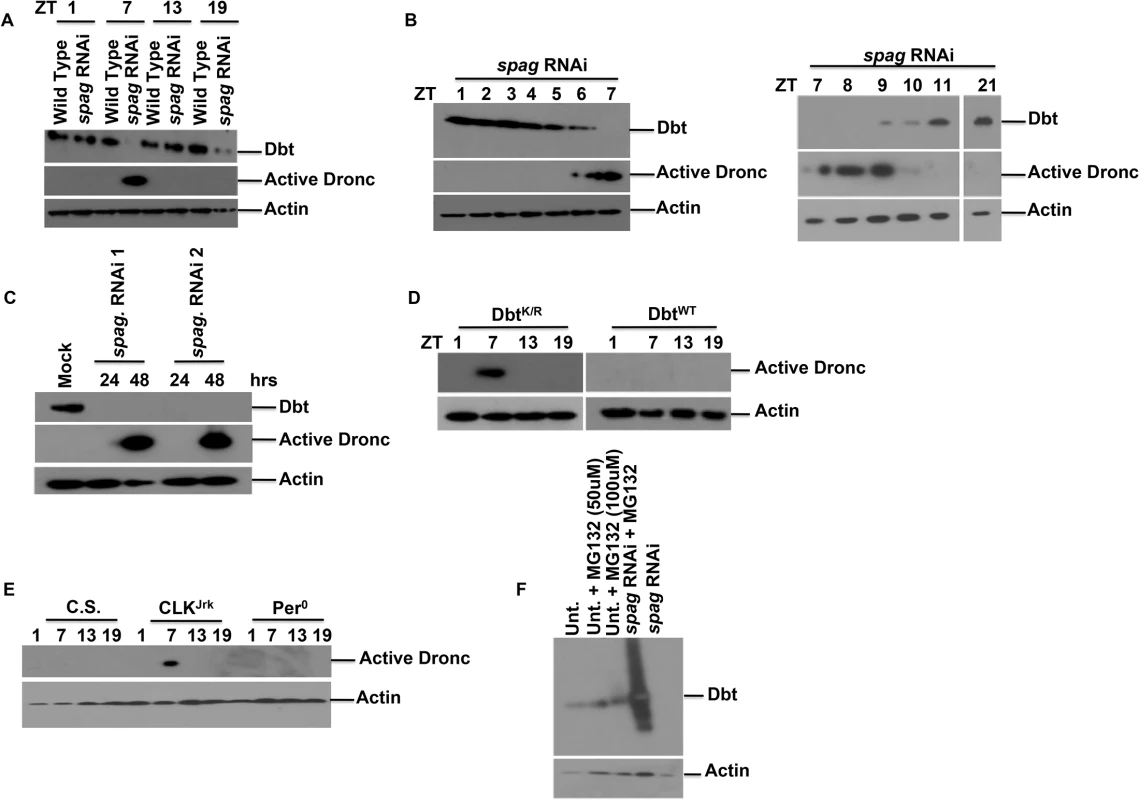
(A) Wild type Canton S or timGAL4>UAS-spag RNAi fly heads were harvested at various times as indicated (ZT: hours after lights on in a 12hr:12hr light:dark cycle) and immunoblotted for Dbt, active Dronc and actin as a loading control. (B) timGAL4>spag RNAi fly heads were harvested at the indicated times in LD (ZT) and immunoblotted for Dbt or active Dronc. (C) S2 cells were harvested 24 and 48 hours after spag dsRNA addition (two different nonoverlapping dsRNAis were used) and immunoblotted for Dbt, active Dronc and actin. (D) timGAL4>UAS-dbtK/R and-dbtWT fly heads were harvested at the indicated times and immunoblotted for active Dronc and actin. (E) ClkJrk flies express activated Dronc at ZT 7. Fly heads from various clock mutants or wild type flies were collected at the indicated times and analyzed for activated caspase expression. (F) Reduced Dbt levels caused by RNAi knock-down of spag are caused by the proteasome. S2 cells were untreated or treated with spag dsRNA +/- MG132 (a proteasome inhibitor) and immunoblotted for Dbt and actin. To address whether the caspase activation was a consequence of loss of Dbt function we expressed the kinase-dead (DbtK/R) form of Dbt in circadian cells with a timGAL4 driver. The DbtK/R protein acts as a dominant negative to antagonize endogenous Dbt [27]. DbtK/R flies also showed elevated caspase activity at ZT7, while flies expressing DbtWT did not (Fig 1D). The results suggest that the kinase activity of Dbt negatively regulates expression of activated Dronc, and that reductions in this activity can cause expression of activated Dronc.
To determine if other clock mutants showed activated caspase expression we collected heads of per0 and ClkJrk mutant flies and analyzed for activated caspase expression. ClkJrk flies showed activated caspase expression at ZT7 like flies expressing DbtK/R, but per0 did not (Fig 1E), and more extensive analysis of ClkJrk flies showed activated caspase only at ZT7-9 in ClkJrk flies (S4 Fig). timGAL4>UAS-dbtK/R flies express high levels of PER [27], which should repress the CLK/CYC transcription factor and thereby produce a condition like that found in ClkJrk flies, which lack CLK-dependent transcription. Therefore, activated caspase is produced in two different circadian mutants with similar effects on the circadian transcription cycle. In order to determine if the time of the circadian clock (e.g., ZT7) is needed for production of activated Dronc, we examined the timing of Dronc induction in perS (or perL); timGAL4>UAS-dbtK/R flies, along with its activation in ClkJrk flies and wild type flies, and in all mutant flies activation was detected from ZT7-9 (S4 Fig), despite the fact that the perS mutation significantly shortened the period of locomotor activity for the timGAL4>UAS-dbtK/R genotype (31.0 ± 0.4, n = 14 vs 33.7 ± 0.9, n = 15 for the perS and per+; dbtK/R genotypes respectively; all perL; dbtK/R flies, n = 16, were arrhythmic). Taken together with the ZT7 time of Dronc activation for the largely arrhythmic timGAL4>UAS-dbtK/R and ClkJrk genotypes, the absence of Dbt reductions in DD (S2E Fig), and the production of activated Dronc in light-pulsed flies at night (S2F Fig), these results suggest that the production of activated caspase is a transient response to prolonged light exposure that also involves reductions in several circadian genes (e.g., spag, dbt and clk).
Dbt is targeted for degradation via the proteasome
S2 cells were treated with spag dsRNA with and without the proteasome inhibitor MG132 and immunoblotted for Dbt. Endogenous Dbt levels were stabilized in the presence of the proteasome inhibitor in the presence of spag RNAi, which led to complete absence of Dbt in the absence of proteasome inhibitor, but higher Dbt levels in the presence of proteasome inhibitor and spag RNAi were obtained than with proteasome inhibitor only (Fig 1F). The higher levels of Dbt in the presence of spag RNAi and proteasome inhibitor than with proteasome inhibitor only, coupled with the lower levels of Dbt with spag RNAi only, suggest that Spag may have both proteasome-dependent positive and proteasome-independent negative effects on Dbt levels. This result also demonstrates that Dbt is degraded by the proteasome in response to spag knock-down. The transient accumulation of forms of Dbt with slow electrophoretic mobility (S2B–S2E Fig) suggests that phosphorylation and ubiquitination of Dbt is the initial consequence of the spag knock-down (See [28,29] for evidence of phosphorylation.), with proteasomal degradation of Dbt a subsequent consequence.
DbtWT can rescue spag RNAi-induced caspase activation
When DbtWT is expressed in fly head circadian cells in a spag RNAi background accumulation of activated Dronc is blocked (Fig 2A), suggesting that the effects of spag knock-down are mediated by reductions in Dbt. In addition, when Dbt is overexpressed in S2 cells it is able to block the accumulation of activated caspase associated with spag or dbt RNAi (Fig 2B). However, Spag overexpression was not able to block activated caspase accumulation brought on by dbt RNAi but did block activated caspase accumulation by spag RNAi, suggesting that Spag is upstream of Dbt and confirming the specificity of the dsRNAi knock-down for spag (Fig 2B).
Fig. 2. Overexpression of DbtWT can rescue the caspase activation associated with spag RNAi. 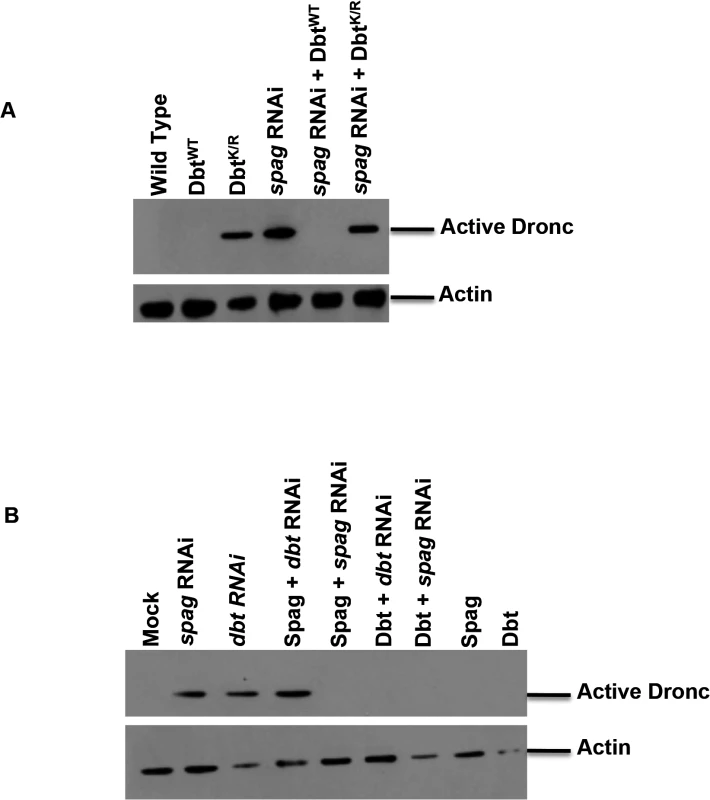
(A) Fly heads expressing DbtWT, DbtK/R, spag RNAi (31253), spag RNAi+DbtK/R or spag RNAi+DbtWT with the timGAL4 driver were collected at ZT7 and analyzed for active Dronc or actin. (B) S2 cells stably overexpressing Spag or DbtWT were treated with spag or dbt dsRNA and immunoblotted for active Dronc or actin. Lowered Spag and Dbt activity in circadian cells lead to accumulation of activated Dronc in the optic lobes via PDF signaling
Fly brains from timGAL4>UAS-spag RNAi flies collected at ZT7 showed elevated levels of active caspase not found in control brains, but not at ZT19 (Fig 3A and 3B). This was also observed using the pdfGAL4 driver, which is expressed in the PDF-secreting brain neurons that drive circadian rhythms of locomotor activity in constant darkness, and with expression of the dominant negative DbtK/R with both drivers (Figs 3D and 4A for ZT7 and S5A for ZT19). tim-GAL4 clock cells expressing DbtK/R expressed both activated caspase and Dbt-MYC signal, but activated caspase was expressed in other cells as well (Fig 3D). Taken together, these results suggest both autonomous and non-autonomous effects of the transgene expression.
Fig. 3. Spag knock-down or reduction in Dbt activity in clock cells induces activated caspase expression. 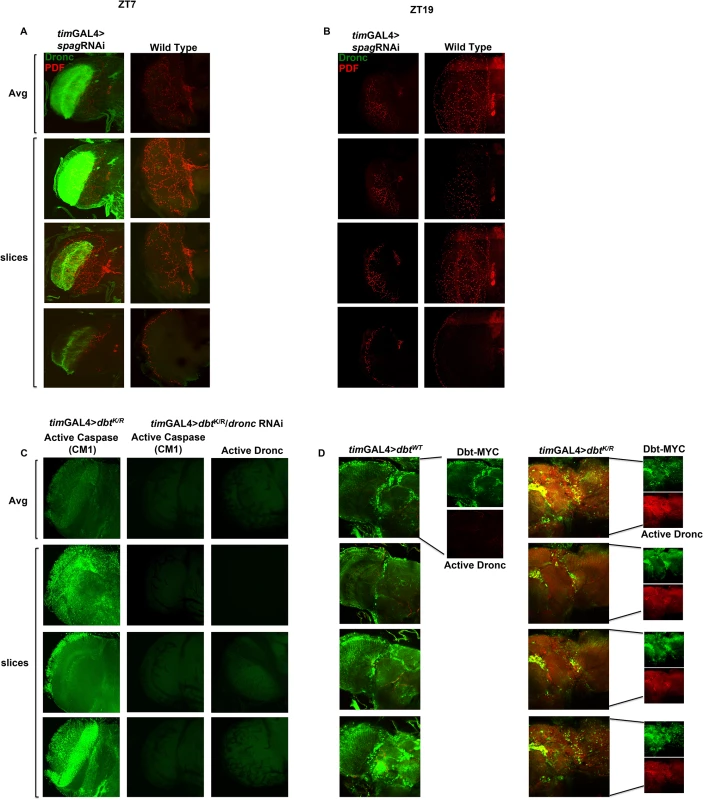
(A) Knockdown of spag using the timGAL4 driver induces accumulation of activated caspase. Whole brains were collected at ZT7 and active caspase (green) and PDF (red) were detected. The first image of each column shows the average intensity of the Z-stack image, followed by individual slices from the given Z-stack. Wild type controls showed no activated Dronc. (B) Whole brains were collected at ZT19 and PDF (red) but no active caspase (green) were detected in either genotype. (C) The caspase signal detected in fly brains is Dronc specific. Active caspase detected using anti-CM1 or anti-active Dronc at ZT7 in DbtK/R or DbtK/R and Dronc RNAi fly brains. (D) Caspase activation is detected in clock cells and non-clock cells. Whole brains were collected at ZT7 and imaged for Dbt-MYC (green) and active caspase (red), which was detected only in tim-GAL4>UAS-dbtK/R brains in both cells expressing Dbt-MYC and surrounding cells. Fig. 4. Pdf signaling from circadian neurons is required for accumulation of activated caspase in optic lobes. 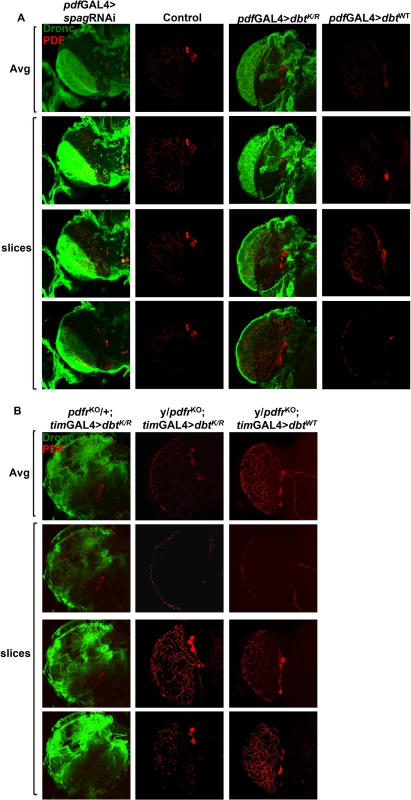
(A) Whole brains were collected at ZT7 from pdfGAL4>UAS-spagRNAi (or-dbtK/R, -dbtWT) flies and wild type control (pdfTm3; progeny inheriting a balancer chromosome rather than the UAS responder) and processed for detection of PDF (red) and active caspase (green). Knock-down of spag or reduction of Dbt activity both produced activated caspase expression in the optic lobes in a region innervated by the PDF+ axons. (B) Whole brains were collected at ZT7 from timGAL4>UAS-dbtK/R (or dbtWT) flies and processed for detection of PDF (red) and active caspase (green). Flies that were heterozygous for the pdf receptor deletion (pdfrKO/+) expressed activated Dronc with DbtK/R, while those that were hemizgous for the mutation (pdfrKO males) did not. The first image of each column is the average intensity of the Z-stack image, followed by individual slices from the Z-stack. See S5 Fig for ZT19 analysis, which showed no expression of activated caspase under any condition. The elevated activated Dronc was particularly prominent in the optic lobe where the PDF+ axons terminate, but it was found in other brain-associated tissues as well. In addition, Pdf receptor mutant brains lacked this caspase activation that was observed at ZT7 (Figs 4B for ZT7 and S5B for ZT19). Moreover, most of the activated caspase was detected in tissue surrounding the PDF+ axons in the optic lobes (S5C Fig). Taken together with the generation of activated caspase in large areas of the brain in pdfGAL4>UAS-spagRNAi (or—dbtK/R) flies, the results demonstrate that signaling by PDF is required for this broad activation pattern, and in fact it is even needed in an autocrine manner for expression in the PDF+ cells that also express PDF receptor [30], because no activation is seen in these cells in the absence of the PDF receptor (Fig 4B). The CM1 antibody, a marker for Caspase-9 like Dronc activity, was also used to detect Dronc activity, and showed a similar pattern as the anti-Dronc antibody used (Fig 3C). In addition, RNAi to Dronc eliminated most of the signal, confirming that the caspase signal we detect is indeed from Dronc (Fig 3C). Since all Dronc signal is eliminated with expression of dronc RNAi with the timGAL4 driver, the results suggest that broader Dronc activation requires Dronc activation in TIM+ cells, which then signal a corresponding increase in the surrounding tissue via PDF signaling.
Tau is a substrate of Dronc when Dbt or Spag are knocked down
Since spag was initially found in a screen involving neurodegeneration and caspases have been implicated in the cleavage of key proteins associated with diseases such as AD, we examined whether Tau was a substrate for Dronc. First, we expressed HA-tagged Drosophila Tau (dTau) in S2 cells and either treated the cells with UV irradiation to induce widespread caspase activation or used RNAi to knock down either Spag or Dbt. When either Spag or Dbt was knocked down cleavage of dTau-HA was detected (Fig 5A). To confirm that Dronc was the main caspase involved in this cleavage we used RNAi and targeted either Dronc or Drice, the other main caspase involved in cell death. When Dronc was silenced along with Spag or Dbt, cleavage was inhibited, but when we targeted Drice the cleavage was still detected (Fig 5A).
Fig. 5. Spag or Dbt reduction leads to Dronc-dependent Tau cleavage and neurodegeneration in eyes overexpressing hTau. 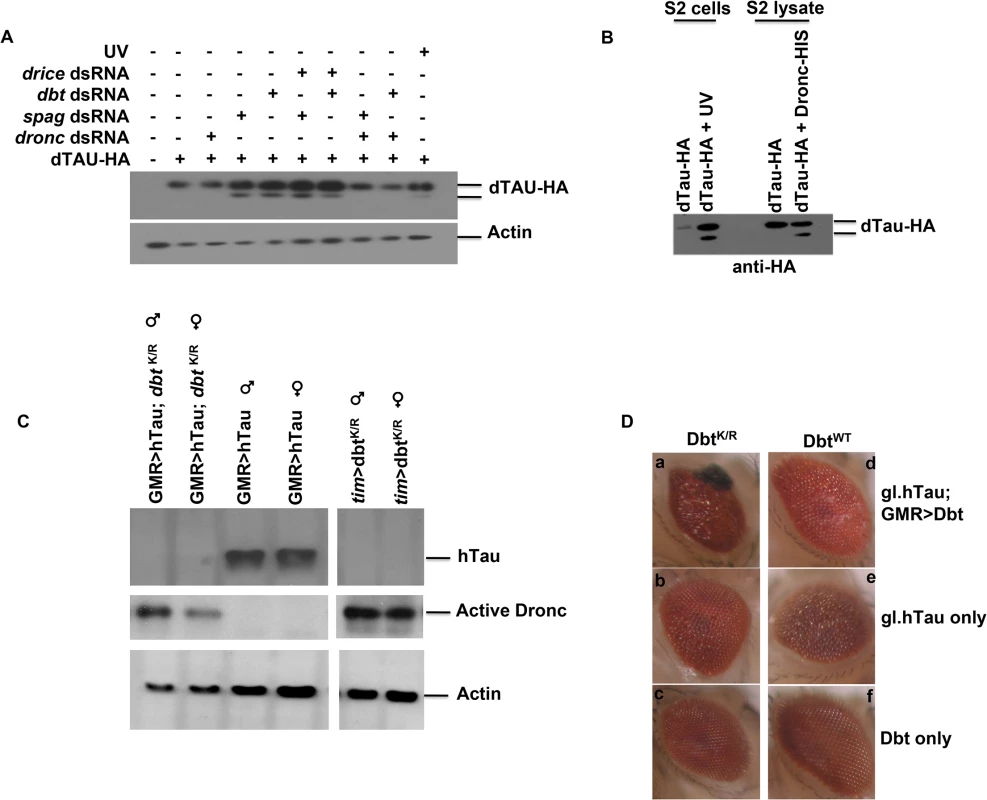
(A) S2 cells were transfected with Drosophila HA-tagged Tau +/- RNAi to spag, dbt alone or with either dronc or drice RNAi. Samples were then immunoblotted for dTau using anti-HA antibody. “UV” cells were treated with UV light to induce caspase activation, and this also produced cleavage of Tau. dronc RNAi inhibited Tau cleavage, while drice RNAi did not. (B) Drosophila HA-tagged tau was transfected in S2 cells and either UV treated to induce caspase activation or harvested and incubated with active recombinant Dronc. Samples were then immunoblotted for dTau using anti-HA. (C) Male and female flies were collected at ZT7 and immunoblotted for hTau, active Dronc and actin. Adult flies expressing DbtK/R in the eye with the indicated drivers in the presence or absence of hTau have activated Dronc, while expression of hTau alone did not produce activated Dronc. Flies expressing both hTau and DbtK/R show reduced Tau expression. (D) Adult flies expressing both DbtK/R and hTau produce an enhanced disrupted eye phenotype. Adult eyes of various genotypes were imaged and representative examples are shown. (a) gl.htau; GMRGAL4>UAS-dbtK/R (b) GMRGAL4 >/+; gl.htau (c) GMRGAL4>UAS-dbtK/R (d) gl.htau; GMRGAL4>UAS-dbtWT (e) GMRGAL4 >/+; gl.htau (f) GMRGAL4>UAS-dbtWT. See also S1 Table for quantification of eye size differences. To further confirm that Dronc was targeting dTau we incubated the dTau lysate with active recombinant Dronc. Samples from cells that were UV-irradiated showed a cleavage product for dTau. In addition, when active Dronc was used we also detected the same cleavage product (Fig 5B), which was not detected with lysates of S2 cells not treated with UV or active Dronc. This suggests that dTau is indeed a substrate for Dronc.
DbtK/R expressed with hTau leads to activation of Dronc, cleavage of Tau and an enhanced disrupted eye phenotype
Expression of human Tau (hTau) in fly eyes produces neurodegeneration and has been used as a fly model for tauopathies [16,17]. Therefore, we expressed DbtWT or DbtK/R along with hTau in the fly eye using the eye specific GMR driver to determine if Dbt might enhance eye neurodegeneration. When DbtK/R was expressed activated Dronc was detected, both with and without hTau expression, while expression of hTau alone was not sufficient to activate Dronc (Fig 5C). Expression of DBTK/R and hTau with GMR-GAL4 led to significant reductions in Tau levels at ZT7 (Fig 5C). Moreover, when DbtK/R was expressed along with hTau there was significantly increased disruption in the eye (Fig 5D and S1 Table). The enhanced disruption of the eye was manifested by the appearance of melanized patches in the eye (Fig 5D) and a decreased average surface area of the eye (S1 Table). Expression of DbtWT did not lead to enhancement of the eye phenotype (Fig 5D and S1 Table).
Cell death-independent expression of activated Dronc in young flies and circadian effects on the cell death pathway in older flies
To determine whether Dronc activation led to classical apoptosis we looked at another marker of apoptosis. Diap1 is an antiapoptotic protein that regulates cell death in flies by binding to and inhibiting the activity of Dronc. When cell death occurs Diap1 is targeted for degradation/cleavage, thereby freeing Dronc [31]. We examined Diap1 levels in fly heads and observed no difference between young wild type and timGAL4>UAS-spag RNAi or dbtK/R flies in which Dronc activation occurs (Fig 6A). This lack of effect on Diap1 levels was also observed in S2 cells treated with spag or dbt RNAi or expressing DbtK/R (Fig 6D). However, when flies aged a Diap1 cleavage product was detected in the spag RNAi and dbtK/R flies (Fig 6B) and activated caspase expression was also detected. Interestingly, aged spag RNAi flies showed activated caspase expression at all time points examined (Fig 6B). To determine if this pathway occurred naturally in aging flies we collected wild type Canton S (C.S.) fly heads at 60 days. Aged flies showed a mobility shift of Dbt at ZT 7 and 19 that was similar to what we observed in spag RNAi flies (Fig 6C). In addition, increased active caspase expression was detected at ZT7 and 19 for the aged flies (Fig 6C) and Diap1 cleavage was detected from ZT7 to 19 for the aged W.T. flies (Fig 6C). These results demonstrate that wild type flies exhibit the same circadian-dependent activation of apoptotic pathways that are produced in spag RNAi, dbtK/R and ClkJrk flies at younger ages, and suggest that reductions in activity of these circadian genes accelerate an age-dependent pathway that leads to activation of the apoptotic pathway.
Fig. 6. Apoptosis is not detected in young flies but is in older spag RNAi and wild type flies. 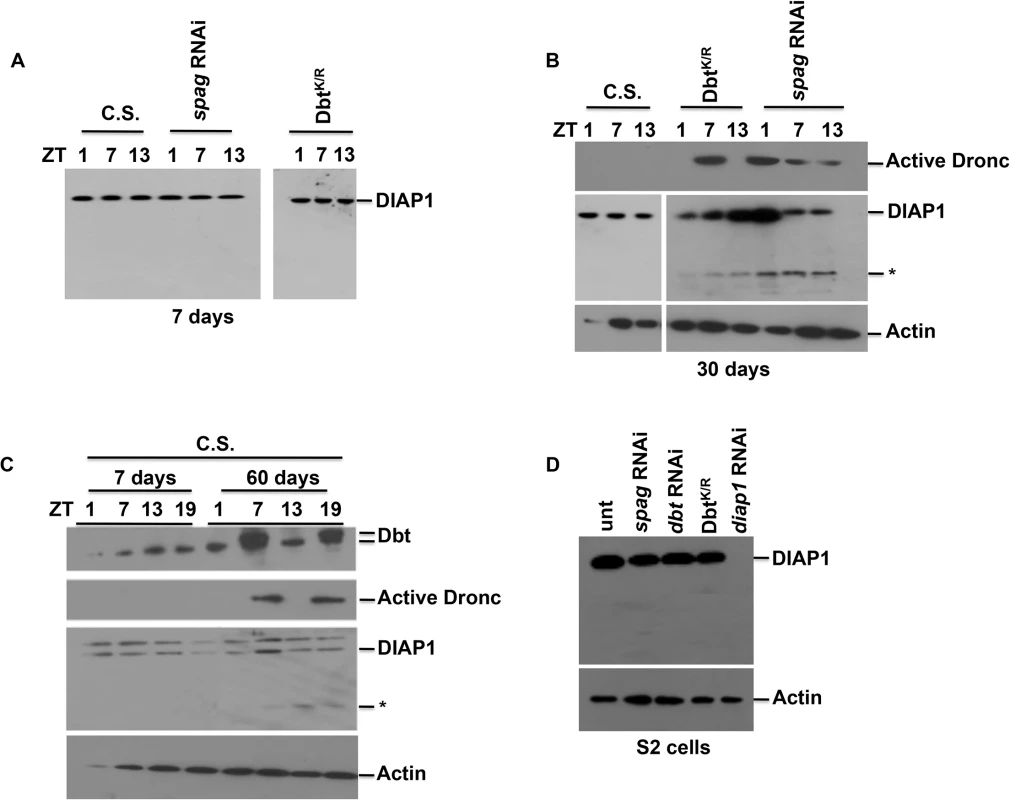
(A) Fly heads were harvested from 7-day old or (B) 30-day old wild type flies Canton S flies (C.S.) or flies expressing the indicated transgene with the timGAL4 driver at various times indicated and immunoblotted for Diap1, active Dronc or actin. Only 30-day old flies with reduced spag or dbt activity produced cleaved Diap1 (indicated by *), and spag RNAi flies produced active Dronc at all times at 30 days. (C) Fly heads from C.S. flies were collected at 7 and 60 days and immunoblotted for Dbt, active Dronc, Diap1 and actin. 60 day old C.S. flies showed Dbt mobility shift, active Dronc expression and Diap1 reduction at ZT7 and 19. Note that there were no spag RNAi flies still alive at this time. (D) S2 cells treated with spag, dbt, diap1 dsRNA or stably expressing DbtK/R were immunoblotted for Diap1, demonstrating reduced Diap1 only with dsRNAi for diap1. spag RNAi flies have reduced climbing and lifespan
Since spag regulates a pathway that leads to the activation of caspases by reduction of Dbt, and this then leads to the cleavage of Tau by these activated caspases and ultimately to expression of apoptotic markers, we wanted to determine if there were behavioral and lifespan manifestations. The circadian locomotor assays initially suggested that there were effects as flies age. Locomotor behavior is preserved in young flies. However, as transgenic flies with reduction of spag by RNAi age, they tend to die during the locomotor assay (Table 1). The loss of the climbing response has been used to monitor age-related changes in Drosophila. Normal Drosophila show a strong negative geotactic response. When tapped to the bottom of a vial they rapidly climb to the top of the vial, and most flies remain there. Flies with knockdown of spag initially climb as well as control flies. However, over time they decline in performance more rapidly than controls (Fig 7A). The progressive, accelerated decline in climbing ability in spag RNAi flies demonstrates a functional deficit produced by knockdown of spag in clock cells. In addition, spag RNAi flies had a reduced lifespan compared to control flies (Fig 7B). By contrast, expression of DbtK/R in circadian cells did not produce accelerated death and loss of climbing ability in comparison with wild type Canton S flies, indicating that the effects of Dbt activity reduction are not as severe as those of spag reduction (See figure legend for discussion of statistics).
Fig. 7. spag RNAi flies have a shorter lifespan and reduced climbing ability. 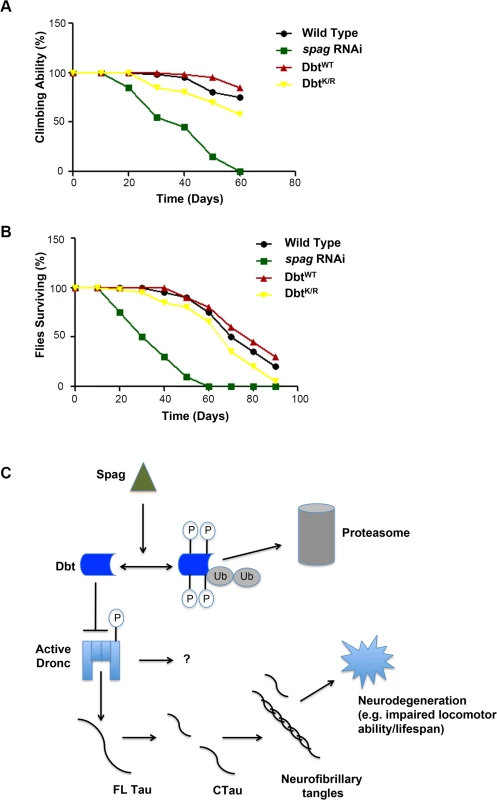
(A) Climbing ability score. Quantification of the climbing performance of spag RNAi,-DbtWT,-DbtK/R and control wild type flies as a function of age. Flies of each genotype were scored in three replicate groups of 10 for each genotype, and all showed the same trends. Genotypes: UAS-dcr; timGAL4>/UAS-spag RNAi (31253), timGAL4>dbtWT,-dbtK/R, Canton S wild type flies. A Kruskal-Wallis nonparametric ANOVA indicated a significant effect of genotype on the time at which flies lost their climbing ability (H[3, N = 120] = 61, p<0.0001); the multiple comparisons p values indicated that the spag RNAi genotype differed significantly from all the others (p<0.0001), while the other three did not differ significantly. (B) Survival curves for the same genotypes as in (A). Flies were maintained at 25°C, transferred every 10 days in three replicate batches for each genotype, and scored for survival. All three replicates showed the same trend. A Kruskal-Wallis nonparametric ANOVA indicated a significant effect of genotype on lifespan (H3[N = 400] = 197, p<0.001). The multiple comparisons p values indicated that the spag RNAi genotype differed significantly from the other three while the dbtK/R genotype differed only from the spag RNAi and the dbtWT (p<0.002); no other comparisons differed significantly. (C) Model of Spag and Dbt regulation of caspases. Dbt regulates caspases by phosphorylation, which inhibits caspase activity in normal conditions. When spag is removed Dbt is targeted for phosphorylation and degradation by the proteasome, freeing the caspase and allowing for cleavage of Tau and possibly other substrates (“?”) and production of tauopathy Discussion
We have identified a new player (spag) that links circadian signaling, cell death and tauopathies together. Orthologs of Spag in yeast and humans function as co-chaperones of Hsp90 to regulate its activity and recruit client proteins for Hsp90. They are part of multiprotein complexes that contribute to biogenesis of cellular machineries like RNA polymerase, ribonucleoproteins and Phosphatidyl-Inositol 3-kinase-related kinases [32,33]. Recently, Drosophila Spag has been shown to associate with Hsp90 and Hsp70 to likewise contribute to assembly of several of these factors [34]. The human ortholog of Spag (RPAP3) is a binding partner for a WD40 repeat protein that is involved in apoptosis. RPAP3 contains TPR domains and regulates apoptosis induced by several stimuli [35,36].
When we knock down spag in circadian cells using RNAi we observed reduction in Dbt levels and an increase in the activated caspase Dronc in fly heads. Interestingly, this mostly occurred during the day (ZT7) or after extended light treatment at night, increased as flies age and did not occur in constant darkness. Accumulation of activated Dronc was also observed with expression of the kinase dead form of Dbt (DbtK/R) in circadian cells. This suggests that some factor present or active during the light might regulate the Spag-Dbt pathway and confer a transient sensitivity to caspase activation after extended light treatment.
One feature that is common to various neurodegenerative diseases is the acceleration of the age-related disruption of the daily cycle of sleep and wake. Our work suggests that the most immediate way for the clock to influence neurodegeneration is by circadian gene-dependent control over the expression of pro-neurodegenerative factors. While these factors are not expressed in young flies with normal circadian clocks, the mutant clocks that we have produced in flies resemble those produced in aging wild type flies, in which Dbt modification, activated caspase expression and cleaved Diap1 are detected at ZT7 and ZT19. Prior work has shown the circadian function is blunted along with reduced healthspan in aging flies [37–39]. Circadian dysfunction would enhance their susceptibility to light-dependent neurodegeneration. In a previous report, the period gene was mutated in flies with sniffer gene mutations causing neurodegeneration. The flies with both mutations displayed faster neurodegeneration and had shorter lifespans compared to flies with single mutations. This suggests that disrupted circadian rhythms can accelerate the process of neurodegeneration [40].
What is the nature of this mechanism? Previous work linked Spag to Huntington’s disease and Hsp90 and a possible role in aggregation [25]. In addition, caspases have been shown to be involved in the cleavage of Tau, a protein associated with AD, and this caspase-mediated cleavage of Tau is associated with its aggregation. This led us to examine whether Tau was cleaved by the caspase Dronc in the Spag-Dbt pathway. HA-dTau was cleaved in S2 cells when we knocked down either Dbt or Spag, and this cleavage was prevented when we knocked down Dronc, but not the effector caspase Drice. This result is consistent with cleavage of Tau by Dronc in response to lowered Dbt and Spag activity.
We examined whether long-term reductions of Spag and Dbt activity in circadian cells have any detrimental effects on the fly. While younger flies with reduced Spag or Dbt activity did not exhibit expression of cell death marker Diap1, older flies with chronic reductions in Spag or Dbt activity exhibited elevated levels of Diap1 cleavage—a marker of the apoptotic pathway. These results suggest that chronic reductions in Spag or Dbt activity eventually produce deleterious effects.
Flies with reduced spag levels had more cleaved Diap1 compared to dbtK/R flies, higher levels of active Dronc and more rapid decline of climbing proficiency and lifespan. This puzzled us since both lines activate Dronc and lead to dTau cleavage, so we expected identical phenotypes. One possibility is that since Spag has been shown to interact with Hsp90, Spag might regulate Hsp90 and control the level of aggregation that occurs. In such a model, if Spag is eliminated and can no longer regulate Hsp90, Hsp90 might no longer interact with dTau and therefore no longer function to reduce dTau aggregates. The defect in Hsp90 function is not predicted for dbtK/R flies, which retain Spag. In addition, Hsp90 is a known regulator of cell death and has been shown to inhibit apoptosis [41]. Removal of Spag might lead to dysregulation of Hsp90, preventing it from regulating components of the cell death pathway and causing a higher level of activated caspase than observed with Dbt inhibition alone.
Is this pathway evolutionarily conserved? To address this we used the fly eye and expressed human Tau along with DbtWT or DbtK/R. When hTau and DbtK/R were coexpressed together an enhanced disrupted eye phenotype was produced together with Donc activation and Tau cleavage, and the phenotype is less severe when hTau is coexpressed with DbtWT. Since loss of Dbt kinase activity leads to Dronc activation, Dbt may be inhibiting Dronc by direct phosphorylation of Dronc, or alternatively there may be another intermediate target of Dbt. Prior work in mammals has demonstrated a link between reduced circadian clock function and neurodegeneration, as well as a link between CKIδ/ɛ and apoptosis [42–45]. Reduced clock function has been produced by alterations to circadian transcriptional regulators, with increased neurodegeneration produced in response to reactive oxygen species and induction of apoptosis. CKI regulation of cell death and cell cycle arrest has been linked to effects on the mitotic spindle, p53 and cell surface receptors involved in cell death. It is likely that circadian clocks and CKI affect apoptosis and neurodegeneration at multiple steps in addition to the ones outlined in this manuscript. However, the direct effects of the clock components on Dbt levels and the consequent expression of an activated initiator caspase suggest that these events may be upstream and global mediators of circadian effects on apoptosis and neurodegeneration.
It has been shown that these cell death components that are normally involved in destruction can also play critical roles in nonapoptotic events such as dendrite pruning, which occurs during development to create proper neural circuits. In Drosophila, caspase activity is detected locally in the degenerating dendrites and mutation of Dronc preserves most of the dendrite morphology [46]. In this instance caspases are not activated in the context of apoptosis, but in cell survival processes.
A possible role for the Spag-Dbt-Dronc pathway in dendritic or axonal pruning/remodeling is intriguing in light of the existing literature on the role of the circadian clock and light in these processes[47–50]. There is circadian remodeling of lateral neuron (PDF+) axon branching patterns as well as the size and synapses of several noncircadian neurons in the optic lobes, which are extensively innervated by the PDF+ axons. These circadian changes require a functional circadian clock, are enhanced by light, and in the case of some optic lobe changes require signals from the lateral neurons. Intriguingly accumulation of activated Dronc in the optic lobe at ZT7 in timGAL4>UAS-spag RNAi (or UAS-dbtK/R) flies also required light and was produced by PDF signaling from the lateral neurons; therefore, this activation may in fact be due to hyperactivation of the same pathways that trigger normal circadian neuronal remodeling in the optic lobes. In wild type flies, some of the optic lobe neurons exhibit largest axon size in the morning and the evening, suggesting that caspases (presumably below the level of detection) might contribute to pruning during the middle of the day, at times when activated caspases are detected here. It is not certain whether expression of activated Dronc in the optic lobe cells not expressing the spag RNAi (or dbtK/R) also involves reductions in Dbt activity in those cells or is produced by a different pathway in response to PDF signaling, but it is likely that Dbt reductions in these cells also occur, as the reductions in Dbt detected in immunoblots of total head extracts can be quite complete. We would argue that PDF signaling is important for global DBT reductions, casapse activation and Tau cleavage, and that these are produced cell autonomously in S2 cells by spag RNAi or DBTK/R expression. Reductions in spag may also trigger the non-cell autonomous reductions in Dbt levels and caspase activation, or these may be triggered by a spag-independent mechanism in response to spag decreases in the PDF+ cells. Caspase involvement in synapse degeneration has previously been suggested to contribute to AD pathologies [51].
We have identified a new mechanism of Tau cleavage and AD. We propose a model in which Spag regulates Dbt levels by regulating Dbt ubiquitination and/or phosphorylation, and when removed leads to the targeted degradation of Dbt. This removal of Dbt removes the inhibition on the caspase Dronc, leading to accumulation of its activated form and targeted cleavage of Tau (Fig 7C). This is the first identification of a mechanism activating caspases in the context of AD and other tauopathies and sheds new light on the underlying mechanism that regulates the disease state and its connection to the circadian clock. Recently, we identified another TPR-containing protein (Bride of Dbt, or Bdbt) that interacts with Dbt to enhance Dbt activity and regulate its phosphorylation state [29]. It will be interesting to determine if this protein is part of any Spag-Dbt complexes that might regulate AD and apoptosis. Furthermore, it will be important to establish the genetic, environmental or aging processes that could interact with the mechanism to activate it during normal aging to produce AD or potentially other outcomes that negatively impact health and lifespan.
Materials and Methods
Fly stocks
Drosophila RNAi stocks for spaghetti (CG13570) were obtained from the Bloomington Drosophila Stock Center (stock number: 31253, targeting nucleotides 778–1200 of the transcript) and the Vienna Drosophila RNAi Center (VDRC stock numbers: 23896, targeting nucleotides 886–1233 of the transcript; 103353KK, targeting nucleotides 780–1200). In addition, the dronc RNAi line 23033 was obtained from the VDRC. The expression of hTau was under the control of the glass (gl) promoter provided by George Jackson [17] for Fig 5D and S1 Table, or alternatively a UAS-hTau fly line from Mel Feany [16] was used to express human Tau with the GMR-GAL4 driver on the X chromosome (Bloomington stock center). The UAS-dbt-myc lines and the timGAL4 and UAS-dcr; timGAL4 driver lines have been described previously [27,28]. The pdfGAL4 driver line (stock number 6899) and the Pdf receptor (CG13758) null mutant line (Pdfr5304; line 33068) were obtained from the Bloomington Drosophila Stock Center. ClkJrk [52], pero, perS and perL mutations [53] were used alone or together with the dbtK/R constructs as described in the text.
Locomotor activity analysis
UAS-spag RNAi lines were crossed with UAS-dcr2; timGal4 lines. Progeny were then continuously reared at 23°C in a 12 hr:12 hr LD cycle for one more week after collection of adults (or longer where indicated) to insure complete RNAi knock-down effects, and loaded onto monitors (Trikinetics, Waltham, MA) for behavioral monitoring in constant darkness (DD) for at least 5 days. Actogram activity records and periodogram analysis to determine periods were employed as previously described with ClockLab [27].
Immunoblot analysis
Fly heads were collected at ZT 1, 7, 13, and 19 or S2 cells at the times indicated in 1X SDS loading buffer, homogenized and stored at -80°C until analyzed. Samples were subjected to SDS-PAGE, transferred onto nitrocellulose and probed with appropriate antibodies: anti-DbtC (1 : 2000) [27], anti-activated Dronc (1 : 100) [26], anti-Actin (1 : 1000) (Developmental Studies Hybridoma Bank), anti-Diap1 (1 : 100) [54], anti-Tau (Developmental Studies Hybridoma Bank) and anti-HA (1 : 500) (Covance PRB-101P), and signals were detected with the appropriate secondary antibodies and the ECL detection procedure. Anti-activated Dronc was purified by protein affinity purification. Antisera were applied to a protein G bead column bound with full length inactive Dronc and the flow-through was collected. This was followed by incubation of the flow-through with protein G beads bound with active Dronc. The beads were eluted with 0.1M glycine, pH 2.7 and used.
Adult brain immunofluorescence
Brains were dissected from flies at ZT7 and ZT19, fixed, permeablized and incubated with anti-Dronc (1 : 100), anti-CM1 (Cell Signaling #9661), anti-PDF (1 : 5000) (PDF C7, Developmental Studies Hybridoma Bank) or anti-Myc (1 : 1000) (Santa Cruz sc-789-G) antibody overnight at 4°C. The following day samples were incubated with fluorescently-labeled secondary antibodies (anti-rabbit IgG Alexa fluor 488 or anti-mouse IgG Alexa fluor 568, 1 : 1000). The brains were examined using an Olympus Fluoview confocal, and Z stacks were obtained.
Drosophila Tau cleavage assay
Drosophila S2 cells were transiently transfected with dTau-HA and 24h later cells were treated with dsRNA corresponding to dronc, dbt, spag or drIce and harvested 48h later. For UV treatment, cells were harvested 24h later. Samples were analyzed by immunoblot using anti-HA antibody.
For treatment with active Dronc-His, S2 cells were harvested 24h after dTau-HA transfection, resuspended in Buffer A (25mM Tris·Cl pH 8.0, 50mM NaCl, 10mM DTT) and lysed by 4x freeze-thaw. After lysis, cells were spun down at 13000rpm and the supernatant was collected and incubated with active Dronc-His [54] for 4h at 30°C. The reaction was stopped by the addition of 5X SDS loading buffer and analyzed for dTau-HA by immunoblot analysis. As a positive control, extracts were analyzed from S2 cells expressing dTau-HA and treated with UV light.
Quantification of eye size in flies expressing hTau and/or Dbt-MYC
Fly eye size was measured from photomicrogaphs using the ImageJ program (open source program, NIH). A circumference was drawn around the eye and the area was obtained from the measure command. The values (number of pixels) were then tabulated and averaged and statistics were performed using the Statistica (Statsoft OK) software.
RT-PCR analysis
Fly heads (50–100) or S2 cells were collected and frozen in liquid nitrogen. Total RNA was isolated using the Trizol Reagent (Invitrogen), and cDNA synthesis was performed using the Taqman Reverse Transcription Kit (Life Technologies). PCR was performed using gene specific primers for spag and actin for 20 cycles, and the products were analyzed on an agarose gel.
Spag and Dbt rescue or overexpression experiments
S2 cells stably expressing Spag or Dbt were untreated or treated with dsRNA corresponding to spag or dbt. Forty eight hours after dsRNA addition cells were harvested and analyzed for active Dronc, Dbt or actin. Plasmids used for this experiment were the following: pMT-dbt-myc, pMT-dbtK/R-myc, pAC-spag-ha.
Primers used to generate dsRNA and the procedure for treatment of S2 cells with dsRNAi
dbt (nt 321–674):
forward 5’-TAATACGACTCACTATAGGGGCGCGTGGGTAACAAATATC-3’,
reverse 5’-TAATACGACTCACTATAGGGTGTATGTAATCGATGCGGGA-3’,
dronc (nt 939–1454):
forward 5’-TAATACGACTCACTATAGGGATGGTGGGGATAGTGCCATA-3’, reverse 5’-TAATACGACTCACTATAGGGTGTCAGGCCACTTCTCCTCT-3’,
drIce (nt 653–968):
forward 5’ - TAATACGACTCACTATAGGGACTGCCGCTACAAGGACATT-3’,
reverse 5’ - TAATACGACTCACTATAGGGGCGTGCACTGGAATCTTGTA-3’,
diap1(nt 491–1019):
forward 5’-TAATACGACTCACTATAGGGCCGGCATGTACTTCACACAC-3’, reverse 5’-TAATACGACTCACTATAGGGTTCTGTTTCAGGTTCCTCGG-3’,
spag set 1 (nt 735–1242):
forward 5’-TAATACGACTCACTATAGGGCAAAAGTGGGCCAAACTTTAC-3’
reverse 5’TAATACGACTCACTATAGGGTTCTGGGCTGCGTTCTAT-3’,
spag set 2 (nt22-227):
forward 5’-TAATACGACTCACTATAGGGGGAGCGCTAGCAACAGAAAT-3’
reverse 5’-TAATACGACTCACTATAGGGGCGACTTCTGGAGCTCTTTC-3’,
PCR fragments were produced from Drosophila genomic DNA with these primer sets. dsRNAi was produced and transfected into S2 cells by the procedures of the Perrimon lab (http://www.flyrnai.org/DRSC-HOME.html), using the T7 promoters encoded by the primers. The cells were harvested at the times indicated in the figure legends. In one experiment, the proteasome inhibitor MG132 was added to a concentration of 50–100 μM, and in another MYC-tagged Dbt was overexpressed.
Generation of spag expression clones
A cDNA clone for CG13570 (RE03224) was obtained from the Drosophila Genomics Resource Center (DGRC, Bloomington, IN). We used the DGRC Gateway collection in order to clone spag into vectors allowing expression in S2 cells. A full-length spag ORF was generated by PCR (Phusion, cat# F-553S, NEB) and cloned into pENTR/SD/D-TOPO (Cat# K240020, Life Technologies) by TOPO-mediated cloning. This clone was used to generate plasmids containing an HA tag. The clonase enzymatic mixture (Cat# 11791–019) was purchased from Life Technologies. spag was cloned into the pGateway vector pAHW (Cat#1095) to generate an N-terminal 3xHA-tag plasmid. The plasmid were driven by an Act5c promoter allowing for constitutive expression in S2 cells.
Generation of dTau-HA plasmid
For the expression of Drosophila Tau (dTau) in S2 cells, the pFLC1-TAU cDNA (Drosophila Genomics Resource Center, Clone # RE16764) was cloned into the pAc S2 cell expression vector. The N-terminal HA tag was added using the following primers:
Tau N-TER Forward, 5’-GCGCGAATTCGCGACCCTATGGCGTACCCGTACGACGTGCCGGACTACGCGATGGCGGATGTCCTGGAGAAAAGCTCACTG 3’,
Tau N-TER DRA Reverse, 5’—GGCGCATGTCCGACTTGTACC 3’
The DNA was digested with DraIII and EcoRI and swapped for the original fragment in the pAc-Tau.
Climbing and lifespan assays
Flies were maintained at 24°C on a 12 hr:12hr light/dark cycle. For aging studies, virgin male flies were isolated and maintained in 20 per vial and transferred to a fresh vial every 10 days. The number of dead flies was recorded.
For the climbing assay, flies were sorted into groups of ten per vial and tested. A climbing apparatus was prepared for each group, with two empty polystyrene vials vertically joined by tape facing each other. For the lower vial, we measured a vertical distance of 8 cm above the bottom surface and marked each vial by drawing a circle around the entire circumference of the vial. We transferred a group of ten flies into the lower vial and allowed the flies to acclimatize to the new setting for 1 minute before conducting the assay. Flies were gently tapped down to the bottom of the vial and the number of flies that climbed above the 8 cm mark by 10 seconds after the tap was measured[55]. This was repeated with the same group ten times, allowing for a 1 minute rest period between each trial. The average number of flies per group that passed the 8-cm mark as a percentage of total flies was recorded.
Supporting Information
Zdroje
1. Kim TW, Pettingell WH, Jung YK, Kovacs DM, Tanzi RE (1997) Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science 277 : 373–376. 9219695
2. Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, et al. (1999) Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell 97 : 395–406. 10319819
3. de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, et al. (2010) Caspase activation precedes and leads to tangles. Nature 464 : 1201–1204. doi: 10.1038/nature08890 20357768
4. Sanchez Mejia RO, Friedlander RM (2001) Caspases in Huntington's disease. Neuroscientist 7 : 480–489. 11765125
5. Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, et al. (2010) Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet 6: e1001026. doi: 10.1371/journal.pgen.1001026 20664788
6. Marx J (2001) Neuroscience. New leads on the 'how' of Alzheimer's. Science 293 : 2192–2194. 11567120
7. McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, et al. (2003) Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci U S A 100 : 715–720. 12522260
8. Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, et al. (2002) Caspase-9 activation and caspase cleavage of tau in the Alzheimer's disease brain. Neurobiol Dis 11 : 341–354. 12505426
9. Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, et al. (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A 100 : 10032–10037. 12888622
10. Iijima-Ando K, Iijima K (2010) Transgenic Drosophila models of Alzheimer's disease and tauopathies. Brain Struct Funct 214 : 245–262. doi: 10.1007/s00429-009-0234-4 19967412
11. Heidary G, Fortini ME (2001) Identification and characterization of the Drosophila tau homolog. Mech Dev 108 : 171–178. 11578871
12. Chen X, Li Y, Huang J, Cao D, Yang G, et al. (2007) Study of tauopathies by comparing Drosophila and human tau in Drosophila. Cell Tissue Res 329 : 169–178. 17406902
13. Chee FC, Mudher A, Cuttle MF, Newman TA, MacKay D, et al. (2005) Over-expression of tau results in defective synaptic transmission in Drosophila neuromuscular junctions. Neurobiol Dis 20 : 918–928. 16023860
14. Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, et al. (2004) GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol Psychiatry 9 : 522–530. 14993907
15. Mershin A, Pavlopoulos E, Fitch O, Braden BC, Nanopoulos DV, et al. (2004) Learning and memory deficits upon TAU accumulation in Drosophila mushroom body neurons. Learn Mem 11 : 277–287. 15169857
16. Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, et al. (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293 : 711–714. 11408621
17. Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, et al. (2002) Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34 : 509–519. 12062036
18. Hardin PE (2011) Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet 74 : 141–173. doi: 10.1016/B978-0-12-387690-4.00005-2 21924977
19. Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, et al. (1998) double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94 : 83–95. 9674430
20. Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, et al. (1998) The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94 : 97–107. 9674431
21. Guan J, Li H, Rogulja A, Axelrod JD, Cadigan KM (2007) The Drosophila casein kinase Iepsilon/delta Discs overgrown promotes cell survival via activation of DIAP1 expression. Dev Biol 303 : 16–28. 17134692
22. Weldemichael DA, Grossberg GT (2010) Circadian rhythm disturbances in patients with Alzheimer's disease: a review. Int J Alzheimers Dis 2010.
23. Cermakian N, Lamont EW, Boudreau P, Boivin DB (2011) Circadian clock gene expression in brain regions of Alzheimer 's disease patients and control subjects. J Biol Rhythms 26 : 160–170. doi: 10.1177/0748730410395732 21454296
24. Sterniczuk R, Dyck RH, Laferla FM, Antle MC (2010) Characterization of the 3xTg-AD mouse model of Alzheimer's disease: part 1. Circadian changes. Brain Res 1348 : 139–148. doi: 10.1016/j.brainres.2010.05.013 20471965
25. Zhang S, Binari R, Zhou R, Perrimon N (2010) A genomewide RNA interference screen for modifiers of aggregates formation by mutant Huntingtin in Drosophila. Genetics 184 : 1165–1179. doi: 10.1534/genetics.109.112516 20100940
26. Yoo SJ, Huh JR, Muro I, Yu H, Wang L, et al. (2002) Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol 4 : 416–424. 12021767
27. Muskus MJ, Preuss F, Fan JY, Bjes ES, Price JL (2007) Drosophila DBT lacking protein kinase activity produces long-period and arrhythmic circadian behavioral and molecular rhythms. Mol Cell Biol 27 : 8049–8064. 17893330
28. Fan JY, Preuss F, Muskus MJ, Bjes ES, Price JL (2009) Drosophila and vertebrate casein kinase Idelta exhibits evolutionary conservation of circadian function. Genetics 181 : 139–152. doi: 10.1534/genetics.108.094805 18957703
29. Fan JY, Agyekum B, Venkatesan A, Hall DR, Keightley A, et al. (2013) Noncanonical FK506-binding protein BDBT binds DBT to enhance its circadian function and forms foci at night. Neuron 80 : 984–996. doi: 10.1016/j.neuron.2013.08.004 24210908
30. Im SH, Taghert PH (2010) PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol 518 : 1925–1945. doi: 10.1002/cne.22311 20394051
31. Muro I, Means JC, Clem RJ (2005) Cleavage of the apoptosis inhibitor DIAP1 by the apical caspase DRONC in both normal and apoptotic Drosophila cells. J Biol Chem 280 : 18683–18688. 15774476
32. Boulon S, Marmier-Gourrier N, Pradet-Balade B, Wurth L, Verheggen C, et al. (2008) The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. J Cell Biol 180 : 579–595. doi: 10.1083/jcb.200708110 18268104
33. Eckert K, Saliou JM, Monlezun L, Vigouroux A, Atmane N, et al. (2010) The Pih1-Tah1 cochaperone complex inhibits Hsp90 molecular chaperone ATPase activity. J Biol Chem 285 : 31304–31312. doi: 10.1074/jbc.M110.138263 20663878
34. Benbahouche NE, Iliopoulos I, Torok I, Marhold J, Henri J, et al. (2014) Drosophila Spag is the homolog of RNA Polymerase II Associated Protein 3 (RPAP3), and recruits the Heat Shock Proteins 70 and 90 (Hsp70 and Hsp90) during the assembly of cellular machineries. J Biol Chem 289 : 6236–6247. doi: 10.1074/jbc.M113.499608 24394412
35. Itsuki Y, Saeki M, Nakahara H, Egusa H, Irie Y, et al. (2008) Molecular cloning of novel Monad binding protein containing tetratricopeptide repeat domains. FEBS Lett 582 : 2365–2370. doi: 10.1016/j.febslet.2008.05.041 18538670
36. Yoshida M, Saeki M, Egusa H, Irie Y, Kamano Y, et al. (2013) RPAP3 splicing variant isoform 1 interacts with PIH1D1 to compose R2TP complex for cell survival. Biochem Biophys Res Commun 430 : 320–324. doi: 10.1016/j.bbrc.2012.11.017 23159623
37. Umezaki Y, Yoshii T, Kawaguchi T, Helfrich-Forster C, Tomioka K (2012) Pigment-dispersing factor is involved in age-dependent rhythm changes in Drosophila melanogaster. J Biol Rhythms 27 : 423–432. doi: 10.1177/0748730412462206 23223368
38. Rakshit K, Krishnan N, Guzik EM, Pyza E, Giebultowicz JM (2012) Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol Int 29 : 5–14. doi: 10.3109/07420528.2011.635237 22217096
39. Luo W, Chen WF, Yue Z, Chen D, Sowcik M, et al. (2012) Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging Cell 11 : 428–438. doi: 10.1111/j.1474-9726.2012.00800.x 22268765
40. Krishnan N, Rakshit K, Chow ES, Wentzell JS, Kretzschmar D, et al. (2012) Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol Dis 45 : 1129–1135. doi: 10.1016/j.nbd.2011.12.034 22227001
41. Arya R, Mallik M, Lakhotia SC (2007) Heat shock genes—integrating cell survival and death. J Biosci 32 : 595–610. 17536179
42. Tamaru T, Hattori M, Ninomiya Y, Kawamura G, Vares G, et al. (2013) ROS Stress Resets Circadian Clocks to Coordinate Pro-Survival Signals. PLoS One 8: e82006. doi: 10.1371/journal.pone.0082006 24312621
43. Beyaert R, Vanhaesebroeck B, Declercq W, Van Lint J, Vandenabele P, et al. (1995) Casein kinase-1 phosphorylates the p75 tumor necrosis factor receptor and negatively regulates tumor necrosis factor signaling for apoptosis. J Biol Chem 270 : 23293–23299. 7559483
44. Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, et al. (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123 : 5389–5400. doi: 10.1172/JCI70317 24270424
45. Li C, Macdonald JI, Hryciw T, Meakin SO (2010) Nerve growth factor activation of the TrkA receptor induces cell death, by macropinocytosis, in medulloblastoma Daoy cells. J Neurochem 112 : 882–899. doi: 10.1111/j.1471-4159.2009.06507.x 19943845
46. Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, et al. (2010) Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J Neurosci 30 : 6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010 20445064
47. Fernandez MP, Berni J, Ceriani MF (2008) Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol 6: e69. doi: 10.1371/journal.pbio.0060069 18366255
48. Mehnert KI, Cantera R (2011) Circadian rhythms in the morphology of neurons in Drosophila. Cell Tissue Res 344 : 381–389. doi: 10.1007/s00441-011-1174-x 21562943
49. Damulewicz M, Pyza E (2011) The clock input to the first optic neuropil of Drosophila melanogaster expressing neuronal circadian plasticity. PLoS One 6: e21258. doi: 10.1371/journal.pone.0021258 21760878
50. Sivachenko A, Li Y, Abruzzi KC, Rosbash M (2013) The transcription factor Mef2 links the Drosophila core clock to Fas2, neuronal morphology, and circadian behavior. Neuron 79 : 281–292. doi: 10.1016/j.neuron.2013.05.015 23889933
51. D'Amelio M, Cavallucci V, Cecconi F (2010) Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 17 : 1104–1114. doi: 10.1038/cdd.2009.180 19960023
52. Allada R, White NE, So WV, Hall JC, Rosbash M (1998) A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93 : 791–804. 9630223
53. Konopka RJ, Benzer S (1971) Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 68 : 2112–2116. 5002428
54. Means JC, Muro I, Clem RJ (2006) Lack of involvement of mitochondrial factors in caspase activation in a Drosophila cell-free system. Cell Death Differ 13 : 1222–1234. 16322754
55. Ali YO, Escala W, Ruan K, Zhai RG (2011) Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J Vis Exp. Mar 11;(49). pii: 2504.
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



