-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSystemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
Mutations that activate a RAS oncogene are found in a large proportion of human cancers. In this study, we have used the roundworm Caenorhabditis elegans (C. elegans) as a model to investigate how the genetic composition of the animal affects the outcome of oncogenic RAS mutations that activate the MAPK pathway. By comparing the effects of activated RAS/MAPK signaling in two genetically different C. elegans strains, we have identified the monoamine oxidase A (MAOA) gene amx-2 as a negative regulator of RAS/MAPK signaling. MAOA enzymes are primarily known to catalyze the degradation of the neurotransmitters dopamine and serotonin. Here, we show that a specific serotonin degradation product that is produced by MAOA (5-HIAA) inhibits RAS signaling in different organs of C. elegans. Thus, by producing the inhibitory serotonin metabolite 5-HIAA the MAOA enzyme systemically controls the activation of the RAS/MAPK pathway.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005236
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005236Summary
Mutations that activate a RAS oncogene are found in a large proportion of human cancers. In this study, we have used the roundworm Caenorhabditis elegans (C. elegans) as a model to investigate how the genetic composition of the animal affects the outcome of oncogenic RAS mutations that activate the MAPK pathway. By comparing the effects of activated RAS/MAPK signaling in two genetically different C. elegans strains, we have identified the monoamine oxidase A (MAOA) gene amx-2 as a negative regulator of RAS/MAPK signaling. MAOA enzymes are primarily known to catalyze the degradation of the neurotransmitters dopamine and serotonin. Here, we show that a specific serotonin degradation product that is produced by MAOA (5-HIAA) inhibits RAS signaling in different organs of C. elegans. Thus, by producing the inhibitory serotonin metabolite 5-HIAA the MAOA enzyme systemically controls the activation of the RAS/MAPK pathway.
Introduction
Human cancer is a complex polygenic disease caused by somatic mutations in oncogenes and tumor suppressor genes together with inherited polymorphisms in cancer susceptibility genes. Many of the oncogenes and tumor suppressor genes that are mutated in different cancer types have been investigated in detail. However, relatively little is known about the effect of the genetic background on disease onset and progression. It thus remains a challenge to identify functional links between oncogenic traits and associated natural variants [1,2].
The components of the RAS/MAPK signaling pathway are mutated in a large fraction of human tumors. In particular, activating (“gain-of-function”) mutations in HRAS and KRAS are among the most prevalent tumor initiating mutations found in human cancer cells [3]. Thanks to the strong conservation of this pathway in metazoans, genetic studies in model organisms, such as the nematode Caenorhabditis elegans, have provided important insights into various factors modulating RAS/MAPK signaling [4]. Moreover, C. elegans has become a platform species for quantitative genetic analyses of various phenotypes and pathways in order to identify and characterize polymorphic genes [5,6]
In this study, we have used quantitative genetics to explore how the genetic background affects the phenotypes caused by the activating G13E (n1046) mutation in the C. elegans ras gene let-60 [7]. The n1046 mutation is homologous to the HRAS and KRAS mutations that are frequently found in human cancer cells [3]. For the purpose of this study, we compared RAS/MAPK signaling in two highly diverse genetic backgrounds, C. elegans varieties Bristol (N2) and Hawaii (CB4856) [8]. Compared to the reference strain N2, the Hawaiian CB4856 strain on average contains one polymorphism every 412 bp with around 75% of all genes carrying at least one coding polymorphism [9].
To measure the activity of the RAS/MAPK pathway in different genetic backgrounds, vulval induction can be used as a quantifiable and reproducible readout. During vulval development, the anchor cell in the somatic gonad secretes the EGF-like ligand that activates via an EGFR family receptor tyrosine kinase the RAS/MAPK signaling pathway in the adjacent vulval precursor cells (VPCs) [10]. In combination with a lateral NOTCH signal, RAS/MAPK signaling induces three of the six VPCs to adopt a 2°-1°-2° pattern of vulval cell fates (Fig 1A). Mutations that hyperactivate RAS/MAPK signaling, such as the n1046 allele, cause the differentiation of more than three and up to six VPCs and a Multivulva phenotype, while mutations that reduce RAS/MAPK signaling result in the induction of fewer than three VPCs and a Vulvaless phenotype. Hence, the average number of induced VPCs per animal, the vulval induction (VI), is a quantitative measure of RAS/MAPK signaling output in the VPCs [10,11]. Besides the vulva, RAS/MAPK signaling is activated in a variety of other tissues in C. elegans at different developmental stages, such as the meiotic germ cells in the hermaphrodite gonads, the excretory duct cell precursor in the embryo or the chemosensory neurons during olfaction in adults [4]. Using a quantitative genetics approach, we aimed at identifying globally acting as well tissue-specific modifiers of RAS/MAPK signaling. Here, we describe the identification of the polymorphic monoamine oxidase amx-2 gene as a global negative regulator of the RAS/MAPK pathway. amx-2 encodes a mitochondrial monoamine oxidase type A (MAOA) that catalyzes the oxidative deamination of biogenic amines such as dopamine (DA) and serotonin (5-HT) [12]. We further show that AMX-2 activity in intestinal cells controls the levels of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA), which acts as systemic inhibitor of MAPK phosphorylation.
Fig. 1. QTL mapping of let-60 ras modifiers. 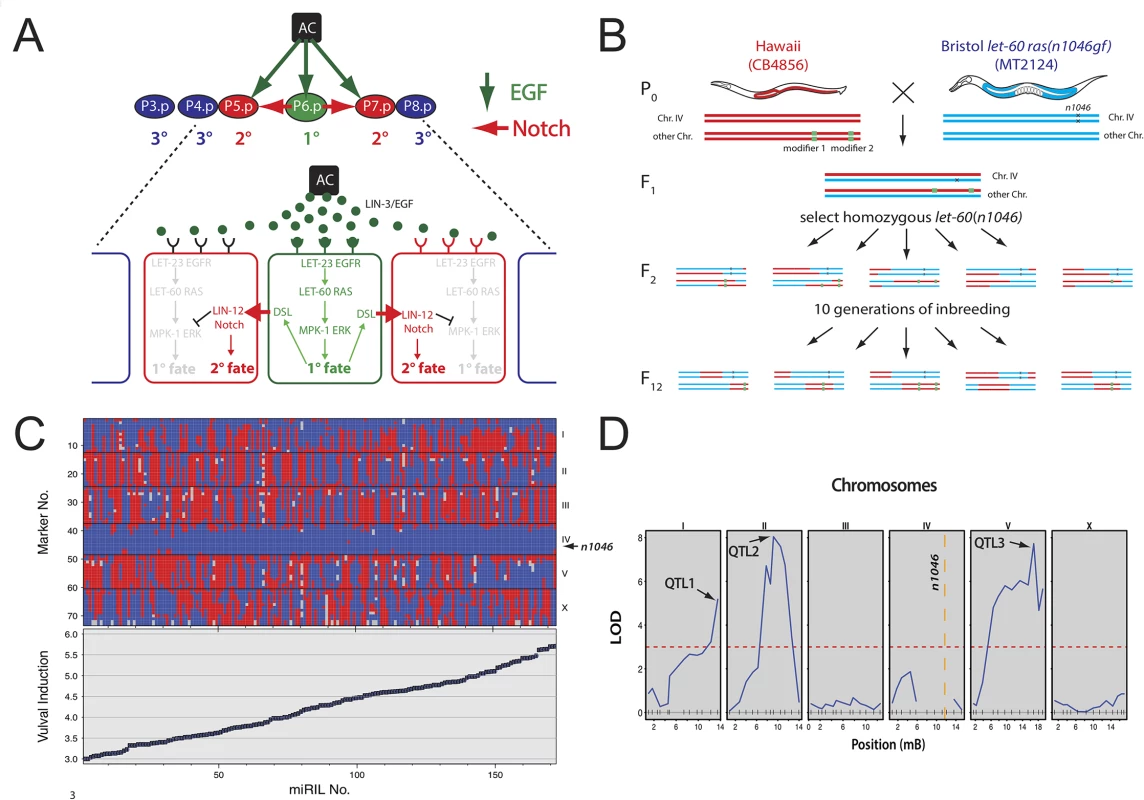
(A) RAS/MAPK signaling induces three VPCs. P6.p receives most of the inductive EGF signal from the anchor cell and activates the EGFR/RAS/MAPK pathway inducing the 1° cell fate (green arrows). Lateral signaling via the Notch pathway induces the 2° cell fate in the neighboring VPCs P5.p and P7.p (red arrows). The remaining VPCs (blue) adopt the non-vulval 3° cell fate. (B) Crossing scheme to generate the let-60(n1046gf) miRILs. Hawaii males (red) were crossed with Bristol let-60(n1046gf) mutants (blue). For each example animal, the two chromosomes IV carrying the n1046 mutation and another arbitrary chromosome pair are shown. Random segregation of the two parental genomes was allowed except for the let-60(gf) mutation that was kept homozygous from F2 generation onwards. After ten generations of self-fertilization to drive all regions to homozygosity, 228 independent miRILs were obtained. (C) Genotypes and phenotypes of the let-60(gf) miRILs sorted by increasing VI. Genotypes determined by FLP mapping [15] are plotted on the y-axis versus the miRIL numbers on the x-axis. Hawaii genotypes are indicated with red, Bristol genotypes with blue and missing genotypes with gray colors. The VIs for each miRIL are shown below the genotypes. Error bars indicate the standard error of the mean. (D) QTL mapping identified three regions (QTL1 through QTL3) above the threshold LOD score of 3 (dotted red line). In each of the panels showing chromosomes I through X, the locations of the FLP markers used for genotyping are indicated on the x-axis with vertical lines. For the exact locations of the FLPs used, see Materials and Methods and [15]. Results
Identification of RAS/MAPK modifiers by quantitative C. elegans genetics
To identify polymorphic modifiers of the RAS/MAPK pathway, we generated a set of 228 “mutation included recombinant inbred lines” (miRILs) between the Bristol strain MT2124 that carries the activating let-60 ras(n1046gf) mutation [7] and the Hawaiian CB4856 strain (Fig 1B). Since small genetic variations are efficiently buffered in a wild-type genome [13,14], the inclusion of the let-60(gf) allele created a sensitized genetic background, allowing us to identify genetic modifiers that increase or decrease RAS/MAPK signaling. After 10 generations of inbreeding and genotyping using fragment length polymorphisms (FLPs) [15], 173 of the miRILs homozygous for the n1046 allele were used for further analysis (Fig 1C, top) (see Materials and Methods for details on genotyping and the selection of informative miRILs). In addition, we quantified RAS/MAPK signaling output in each of these miRILs by measuring the VI of at least 20 animals. While the let-60(gf) allele in the Bristol background exhibits a VI of 3.7±0.06 (n = 100), the VIs of the miRILs varied between 3.0 and 5.7 (Fig 1C, bottom). Quantitative trait loci (QTL) mapping [14] identified at least three loci on chromosomes I (QTL1), II (QTL 2) and V (QTL 3) above the threshold LOD score of 3 that are associated with variation in the VI (Fig 1D). For QTL1, the Bristol genotype is associated with a decreased VI, while for QTL2 and QTL3 the Bristol genotype is associated with an increased VI (S1 Fig). To estimate the effect size of each QTL and explore how the QTLs affect the VI when combined, we used two mapping models, one where the QTLs have additive effects and another one where they show an interaction (S1 Table). This analysis did not detect any significant interactions between the QTLs. Since the let-60(gf) mutation maps to chromosome IV, our approach did not permit us to identify QTLs on this chromosome. Moreover, the genetic incompatibility between the Bristol and Hawaii genomes caused by the zeel-1 and peel-1 loci on the left arm of chromosome I may have prevented the detection of QTLs in this region [16]. To confirm and refine the mapping of the detected QTLs, introgression lines (ILs) carrying defined segments of the Hawaii genome in the QTL regions of interest were crossed to the let-60(gf) Bristol strain [17]. Lines homozygous for the introgressions and the let-60(gf) mutation were compared to sibling lines without introgressions to identify those introgressions that cause significant differences in the VI (see Materials and Methods). The results for the fine mapping of QTL1 are shown in Fig 2A and for all QTLs in S2 Fig IL mapping revealed that QTL1 is composed of two adjacent QTLs, termed 1a and 1b, and that QTL1b maps to an interval of 1.43 Mbp containing 142 polymorphic genes (Fig 2A). Through this approach, we have identified several regions in the C. elegans genome that contain modifiers of the RAS/MAPK pathway. Notably, for QTL1a and QTL1b the Bristol genotype caused reduced RAS/MAPK activity, while for QTL2 and QTL3 the Bristol background increased RAS/MAPK activity.
Fig. 2. AMX-2 negatively regulates RAS/MAPK signaling. 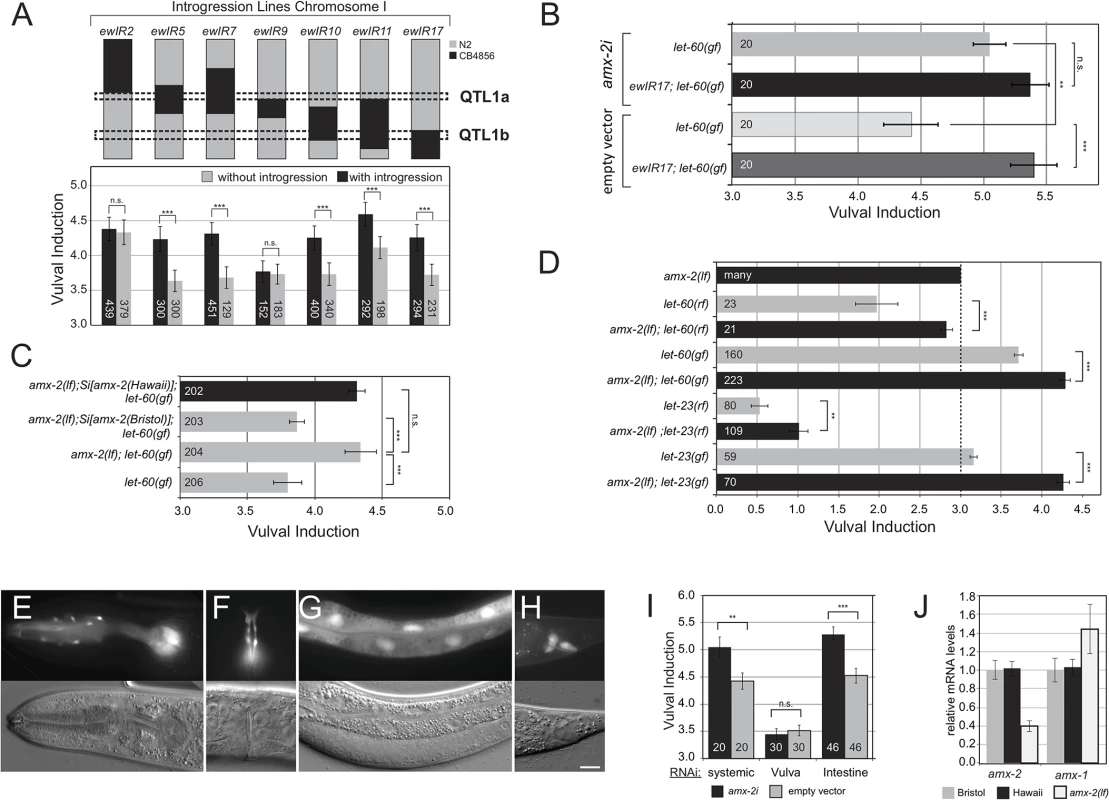
(A) Fine-mapping of QTL1 with ILs. For each IL, the regions containing the Hawaii (black) genome in the Bristol (grey) background are indicated, and the corresponding VIs are plotted below. Black columns indicate the average VI of three independent lines carrying an introgression and gray columns the average VI of three sibling lines without introgression. Dashed boxes indicate the QTL1a and QTL1b sub-regions. (B) Allele-specific effects of amx-2 RNAi compared to empty vector controls. (C) Two copies of Bristol but not Hawaii amx-2 rescue the increased VI of amx-2(ok1235); let-60(n1046gf) double mutants. (D) Epistasis analysis of amx-2(ok1235). The dashed line indicates the wild-type VI of 3. (E-H) Expression pattern of a transcriptional Pamx-2::gfp reporter in the pharynx and head neurons (E), the adult vulva (F), the intestine (G) and some rectal cells (H) of L4 larvae. The scale bar is 10μm. (I) Tissue-specific amx-2 RNAi. Knock-down in the intestine but not the vulval cells increases the VI of let-60(n1046gf) mutants (J) Quantitative PCR of amx-2 and amx-1. Expression levels were normalized to the N2 wild-type Bristol strain. Error bars in (A) to (I) indicate the standard error of the mean and in (J) the standard deviation measured in three independent experiments. The numbers of animals scored are shown inside the columns. *** indicates p<0.001, ** p<0.01, *<0.05 and n.s. p>0.1 in a Student’s t-test. The polymorphic amx-2 gene negatively regulates RAS/MAPK signaling
Since the QTL1b region does not contain any known regulators of RAS/MAPK signaling, we performed RNAi knockdown of 107 of the 142 genes in this region in let-60(gf) single mutants as well as in let-60(gf) mutants carrying the ewIR17 introgression, which spans QTL1b. We envisioned two possible scenarios that are not mutually exclusive: (1) The QTL1b region in the Bristol strain may contain a negative regulator of RAS/MAPK signaling that is inactive or weakly active in the Hawaii background. (2) The Hawaii background may contain a positive regulator of RAS/MAPK signaling that is inactive or weakly active in the Bristol background. We thus screened for candidates exhibiting allele-specific RNAi effects (S2 Table). Note that when grown on the E.coli strain HT115 that is commonly used in RNAi feeding experiments [18], the let-60(n1046) allele exhibits an increased VI compared to animals grown on standard OP50 bacteria [19]. Knockdown of five genes significantly increased the VI in the let-60(gf) but not in the ewIR17; let-60(gf) background, defining potential negative regulators of RAS/MAPK signaling that are active in the Bristol background (highlighted in green in S2 Table), whereas knockdown of ten genes reduced the VI in the ewIR17; let-60(gf) but not in the let-60(gf) background, defining potential positive regulators active in the Hawaii background (highlighted in blue in S2 Table). These data suggested that the QTL1b region is oligogenic, containing several polymorphic modifiers of RAS/MAPK signaling. Of particular interest was the amx-2 gene because it fulfilled the criteria of a polymorphic negative regulator of RAS/MAPK signaling acting in the Bristol strain, but being inactive in the Hawaii strain. amx-2 RNAi had no significant effect on the ewIR17; let-60(gf) background, but amx-2 RNAi caused a robust increase in the VI of let-60(gf) mutants (Fig 2B). Furthermore, the amx-2(ok1235) deletion mutant, which most likely represents a null allele (www.wormbase.org), increased the VI of let-60(gf) mutants in the Bristol background (Fig 2C). To individually assess the activities of the Bristol and Hawaii amx-2 variants, we generated single-copy insertions on chromosome II [20] of a 7.8 kb genomic fragment spanning the amx-2 locus that was isolated either from the Bristol or the Hawaii genome. These single-copy transgenes were then introduced (homozygously) into the amx-2(lf); let-60(gf) background. Insertion of the Bristol but not the Hawaii amx-2 variant reduced the VI of amx-2(lf); let-60(gf) double mutants to the value observed in let-60(gf) single mutants (Fig 2C). These results confirmed the different physiological activities of the two amx-2 variants. In addition, amx-2(lf) partially suppressed the Vulvaless phenotype caused by reduction-of-function mutations in let-60 ras [7] or the EGFR homolog let-23 [10] and enhanced the Multivulva phenotype of the let-23 gain-of-function mutation sa62 [21] (Fig 2D). We thus conclude that the Bristol variant of the amx-2 gene inhibits RAS/MAPK signaling in the VPCs.
amx-2 in intestinal cells inhibits RAS/MAPK signaling cell non-autonomously
To determine the site of amx-2 action, we generated transcriptional Pamx-2::gfp reporters. amx-2 was expressed in head neurons, the intestine and in a subset of cells of the rectum and in the adult vulva (Fig 2E–2H). However, we did not observe any amx-2 expression in the VPCs during vulval induction, though amx-2 reporter levels could be below the detection limit. Since neurons have a low sensitivity to RNAi [22], yet amx-2i efficiently phenocopied the amx-2(lf) phenotype, we suspected that amx-2 might act in intestinal cells, where we detected strongest expression. Intestine-specific amx-2 RNAi using an rde-1(lf); let-60(gf); Pelt-2::rde-1(+) strain [23] increased the VI to a similar degree as systemic RNAi, while vulva-specific RNAi using the Plin-31::rde-1(+) transgene [24] had no detectable effect, which is consistent with lack of detectable amx-2 reporter expression in the VPCs (Fig 2I, note that the overall lower VI in the vulva-specific RNAi strain is due to the genetic background [24]). Taken together, AMX-2 most likely acts in the intestinal cells to negatively regulate RAS/MAPK signaling in the VPCs.
Expression of amx-1 is increased in amx-2(lf) mutants
To investigate a possible redundancy between the MAOA amx-2 and the MAOB gene amx-1, we measured the transcript levels of amx-2 and its paralog amx-1 by quantitative real-time PCR. The abundance of amx-2 and amx-1 transcripts was not significantly different between the Bristol and Hawaii backgrounds (Fig 2J). However, amx-2 transcript levels were around 60% decreased and amx-1 levels around 40% increased in amx-2(lf) mutants. Possibly, the elevated amx-1 expression can partially compensate for a loss of amx-2 expression.
The 5-HT metabolite 5-HIAA acts as systemic inhibitor of RAS/MAPK signaling
amx-2 encodes a member of the mitochondrial monoamine oxidase (MAO) family [25]. Sequence alignments of the catalytic domains of different MAOs indicated that AMX-2 is most closely related to the ancestor of the mammalian MAOA, MAOB and L-amino oxidases (S3 Fig). The Hawaii AMX-2 variant possesses two coding polymorphisms in the catalytic domain (V410I and N461S) and another four in the C-terminal region (R521G, T532S, N535S and L617P) (S4 Fig). MAOs are key enzymes in the degradation of the neurotransmitters 5-HT and DA (Fig 3A) [12]. The products of the DA and 5-HT deamination reactions, 3,4-dihydroxyphenylacetaldehyde and 5-hydroxyindole-acetaldehyde respectively, are further oxidized by aldehyde dehydrogenases into 3,4-dihydroxyphenylacetic acid and 5-hydroxyindoleacetic acid (5-HIAA), which in humans are secreted through the kidneys (Fig 3A) [26]. Consistent with the predicted function of AMX-2 in degrading 5-HT, total extracts of amx-2(lf) worms contained elevated levels of 5-HT when compared to wild-type extracts (Fig 3B).
Fig. 3. Systemic inhibition of RAS/MAPK signaling by Serotonin and its metabolites. 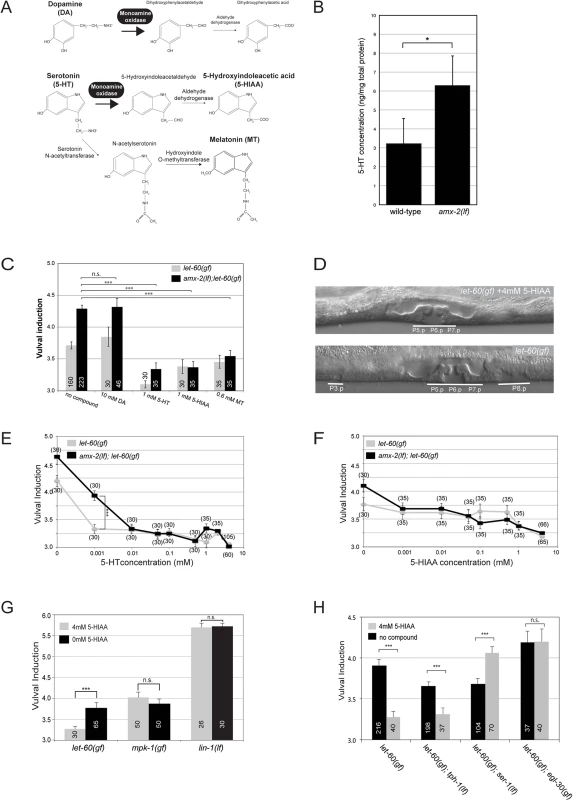
(A) Function of MAOA in DA and 5-HT degradation. (B) 5-HT levels in total extracts of wild-type and amx-2(ok1235) animals. (C) Effect of DA, 5-HT and its metabolites on the VI of let-60(n1046gf) single and amx-2(ok1235); let-60(n1046gf) double mutants. (D) Examples of (top) a 5-HIAA treated and (bottom) an untreated let-60(n1046gf) L4 larva. The normal vulva and the ectopically induced cells are underlined. (E) Dose-dependent reduction of the VI by 5-HT and (F) 5-HIAA treatments. Note in (E) the different sensitivities of the two strains to 1μM 5-HT. (G) Effect of 5-HIAA on mutations activating the EGFR/RAS/MAPK pathway at different levels. (H) Resistance of some 5-HT pathway mutants to 5-HIAA treatment. Error bars indicate the standard error of the mean. The numbers of animals scored are indicated in brackets or inside the columns. *** indicates p<0.001, ** p<0.01, and n.s. p>0.1 in a Student’s t-test. We thus investigated if AMX-2 inhibits RAS/MAPK signaling by controlling the levels of DA, 5-HT or their metabolites. The addition of 10mM DA to the growth medium had no significant effect on the VI of let-60(gf) single or amx-2(lf); let-60(gf) double mutants (Fig 3C). However, 1mM 5-HT as well as 1mM of the 5-HT metabolite 5-HIAA caused a strong reduction of the VI in both backgrounds and a suppression of the Multivulva phenotype (Fig 3C and 3D). Addition of 0.6mM melatonin (MT), another 5-HT metabolite (Fig 3A), had a slightly weaker yet significant effect on the VI (Fig 3C). We conclude that the 5-HT metabolites, in particular 5-HIAA, inhibit RAS/MAPK signaling. To test the sensitivity of the RAS/MAPK pathway to 5-HT and 5-HIAA, we performed dose-response experiments in the presence and absence of amx-2. For both compounds, the maximum reduction of the VI was observed at concentrations above 1mM (Fig 3E and 3F). However, let-60(gf) single mutants displayed a higher sensitivity to low concentrations (1μM) of 5-HT than amx-2(lf); let-60(gf) double mutants, while the effects of 5-HIAA were independent of the amx-2 genotype. Overall, 5-HT exerted a slightly stronger effect than 5-HIAA, suggesting that additional 5-HT metabolites besides 5-HIAA may inhibit RAS/MAPK signaling.
To determine at which step 5-HIAA regulates the RAS/MAPK pathway, we examined a strain expressing an activated form of the MAPK MPK-1 along with the MAPKK MEK-2 [27] (mpk-1(gf)). Application of 4mM 5-HIAA did not alter the VI of mpk-1(gf) mutants (Fig 3G). Also, 5-HIAA did not affect a lf mutation in lin-1, which encodes an ETS family transcription factor that represses vulval induction downstream of MPK-1 [28] (Fig 3G). Taken together, these results indicate that 5-HIAA inhibits RAS/MAPK signaling upstream of MPK-1.
5-HIAA acts via the 5-HT receptor SER-1 and the EGL-30 Gqα pathway
We further characterized the inhibitory effect of 5-HIAA on the RAS/MAPK pathway by testing mutants in the 5-HT pathway for their response to 5-HIAA treatment. A mutation in the tryptophan hydroxylase gene tph-1, which is essential for 5-HT biosynthesis [29], slightly reduced the VI in let-60(n1046gf) animals in the absence of 5-HIAA (Fig 3H). However, treatment of tph-1(lf); let-60(n1046gf) double mutants with 4mM 5-HIAA further reduced the VI, indicating that 5-HIAA acts in the absence of endogenous 5-HT and hence does not compete with 5-HT. By contrast, the VI of let-60(n1046gf) animals carrying a mutation in the 5-HT receptor gene ser-1 [30] was not reduced by 5-HIAA treatment. Surprisingly, the VI of let-60(gf); ser-1(lf) double mutants was even increased after 5-HIAA treatment. Moreover, a gain-of-function mutation in egl-30, which encodes a Gqα protein acting in the 5-HT pathway [31], rendered let-60(n1046gf) mutants resistant to 5-HIAA and caused a slight increase of the VI in untreated animals (Fig 3H). Since the SER-1/EGL-30 pathway plays an essential role in 5-HT stimulated egg laying [30], we tested the effects of 5-HIAA on the egg laying rate with and without 5-HT stimulation. While 5-HIAA treatment alone caused a slight reduction in the egg laying rate, 5-HIAA did not significantly compete with the 5-HT stimulated increase in egg laying (S5 Fig). We conclude that 5-HIAA acts via the SER-1 receptor and the downstream EGL-30 Gqα signaling pathway to repress RAS/MAPK activity. However, the inhibitory effect of 5-HIAA is independent of 5-HT activity.
5-HIAA attenuates RAS/MAPK signaling in multiple organs of C. elegans
Besides the VPCs, RAS/MAPK signaling is required in several other organs of C. elegans [4]. Hence, let-60(gf) mutants exhibit multiple defects besides a Muv phenotype. For example, the temperature-sensitive let-60(ga89gf) allele causes accelerated exit of meiotic germ cells from the pachytene stage, resulting in the accumulation of many immature oocytes in the proximal gonad arm at the restrictive temperature [32,33] (Fig 4A). Moreover, let-60(n1046gf) mutants frequently contain two duct cells expressing the lin-48::gfp marker [34] (Fig 4B). Treatment with 4mM 5-HIAA partially suppressed the let-60(gf) phenotypes both in the germ line and the duct cell (Fig 4A and 4B). To measure the global effect of 5-HT and 5-HIAA treatment on MAPK activation, we quantified the levels of activated, phosphorylated MPK-1 in total extracts of L4 larvae [11]. Treatment with 5-HT and 5-HIAA caused a similar reduction in phospho-MPK-1 levels in let-60(gf) mutants. However, in the amx-2(lf); let-60(gf) background 5-HIAA exerted a stronger effect than 5-HT (Fig 4C). Thus, 5-HIAA supplemented into the culture medium exerts a systemic effect to inhibit RAS/MAPK signaling in different organs of C. elegans.
Fig. 4. 5-HIAA inhibits RAS/MAPK signaling and MPK-1 phosphorylation in multiple organs of C. elegans. 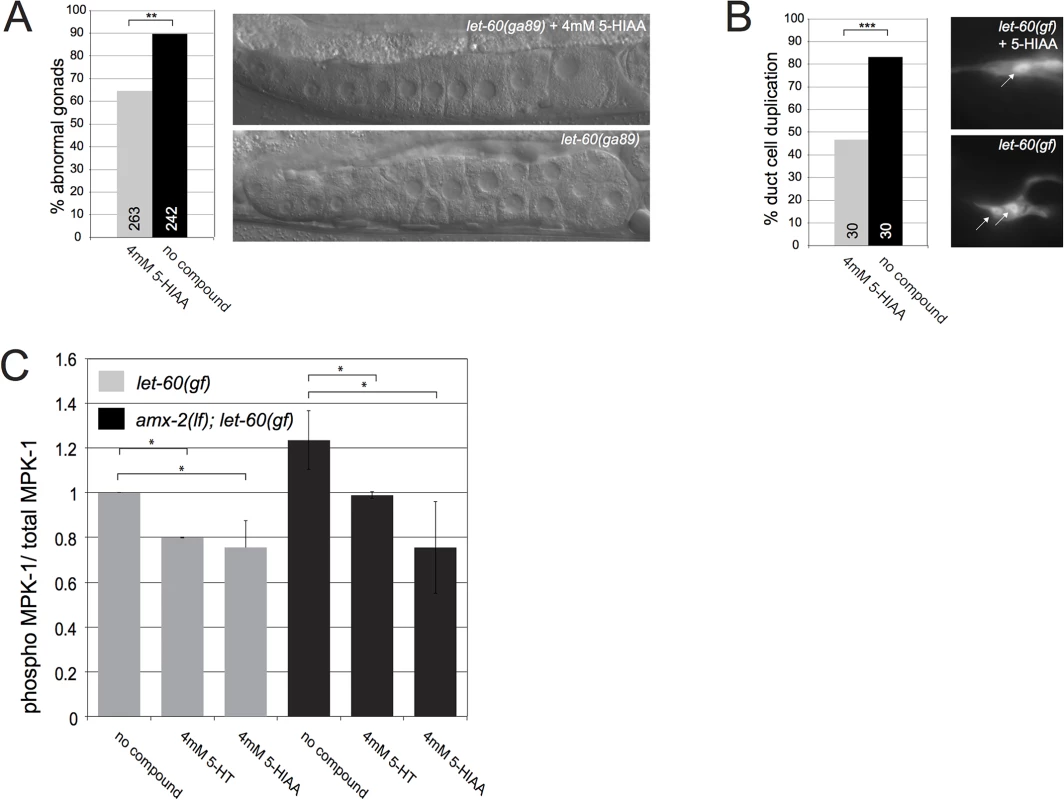
(A) Partial suppression of the germline defect in let-60(ga89ts) mutants treated with 5-HIAA and grown at 25°C. The images show the gonads of 5-HIAA treated (top) and untreated (bottom) young adults. Note the regularly stacked oocytes in 5-HIAA treated and the irregularly stacked and smaller oocytes in untreated animals. (B) Partial suppression of the duct cell duplication phenotype in let-60(n1046gf) mutants by 5-HIAA. The images show the single duct cell in a 5-HIAA treated let-60(n1046gf) L4 larva (top) and the two duct cells in an untreated larva (bottom). The arrows point at the nuclei of the duct cells expressing the lin-48::gfp marker. (C) MPK-1 phosphorylation in total extracts of let-60(n1046gf) single and amx-2(ok1235); let-60(n1046gf) double mutant larvae treated with 4mM 5-HT or 5-HIAA. The ratios of phosphoMPK-1 to total MPK-1 levels were determined in three independent experiments as described in [11] and Materials and Methods. Values were normalized to the ratios in untreated animals. The numbers of animals scored are indicated in brackets or inside the columns. *** indicates p<0.001 and ** p<0.01 in a Student’s t-test Discussion
We have identified several genetic modifiers of the oncogenic RAS/MAPK signaling pathway by comparing miRILs derived from the backgrounds of two highly diverged C. elegans isolates. The two parental strains used in this study display a level of sequence divergence that is comparable to the genetic variation observed in the human population [35]. The genetic modifiers of RAS/MAPK signaling we identified through this quantitative approach could not have been found in conventional forward genetic screens, as each locus alone only exerts a minor effect. Interestingly, both genetic backgrounds analyzed contain QTLs that enhance (i.e. QTLs 2 and 3 for Bristol) as well as QTLs that reduce (i.e. QTL 1 for Bristol) the relative strength of RAS/MAPK signaling. Thus, each isogenic background may represent a balanced state exhibiting intermediate RAS/MAPK pathway activity thanks to the opposing effects of the different modifiers. The interplay of these modifiers may be necessary to keep the activity of the RAS/MAPK pathway within a certain range and avoid the detrimental effects caused by increased or reduced RAS/MAPK signaling.
The molecular characterization of one particular region (QTL 1b) identified the monoamine oxidase gene amx-2 as a negative regulator of RAS/MAPK signaling in multiple organs of C. elegans. Though, the RNAi analysis of the QTL1b region indicated that this region contains possibly up to ten additional polymorphic modifiers of RAS/MAPK signaling besides amx-2. Single-copy gene insertion experiments [20] demonstrated that the Bristol variant can fully rescue an amx-2 deletion allele, while insertion of the Hawaii locus had no significant effect in this assay, indicating that amx-2 activity in the Hawaii background is severely reduced or even absent.
The identification of a monoamine oxidase as a negative regulator of RAS/MAPK signaling was initially a surprising result, since MAOA is primarily known for its role in degrading neurotransmitters in the nervous system [12]. However, we observed strong AMX-2 expression in non-neuronal tissues, especially in the intestinal cells. The 5-HT metabolites such as 5-HIAA that result from AMX-2 catalysis are likely to be released into the body cavity in order to modulate RAS/MAPK signaling in distant organs. Such a globally acting regulatory mechanism may be useful to rapidly adjust RAS/MAPK signaling in response to changing environmental conditions, after food intake and to adapt the speed of reproduction [36]. Epistasis analysis by applying exogenous 5-HIAA points at a step downstream of RAS and upstream of MAPK that is repressed by 5-HIAA. Hence, 5-HIAA may simultaneously repress the RAS/MAPK pathway activated by various receptor tyrosine kinases in different tissues [4]. The observation that 5-HT exerts an inhibitory effect even in amx-2(0) mutants may be explained by the presence of additional redundant MAOs, notably AMX-1, and by spontaneous oxidation of 5-HT. Our epistasis analysis further indicates that 5-HIAA acts via the SER-1 receptor, which activates the EGL-30 Gqα signaling pathway [31]. One possible scenario is that 5-HIAA and 5-HT exert opposing effects on SER-1, such that the balance between 5-HT and 5-HIAA levels determines the strength of EGL-30 activation, which in turn promotes RAS/MAPK signaling. In line with this model, Moghal et al. [37] have previously shown that egl-30 signaling in neuronal cells positively regulates vulval induction under different environmental conditions.
The role of 5-HT as a neurotransmitter in the mammalian nervous system is well documented [12]. However, over 90% of the 5-HT in the human body is found outside of the nervous system, especially in enterochromaffin cells of the intestine [38]. Remarkably, Rybaczyk et al. [39] reported that the expression of the human 5-HT degrading enzyme MAOA, the closest AMX-2 homolog, is consistently down-regulated across many human tumor types. The functional implications and mechanisms of reduced MAOA expression in cancer cells are unclear. Our findings that systemic application of the 5-HT metabolite 5-HIAA globally inhibits RAS/MAPK signaling may explain the physiological consequences of MAOA down-regulation. Tumors expressing low levels of MAOA may generate less oncostatic 5-HIAA and at the same time contain higher levels of 5-HT, which can promote tumor growth and survival via cross-talk to the RAS/MAPK pathway [40,41]. Thus, MAOA levels may set a global threshold for the activation of the RAS/MAPK cascade by different extracellular signals. To our knowledge, 5-HIAA is the first endogenous small molecule that acts as a systemic inhibitor of the RAS/MAPK pathway.
Materials and Methods
General methods and strains used
Strains were maintained on NGM agar seeded with OP50 bacteria at 20°C [42], unless otherwise stated. C. elegans Bristol refers to the wild-type N2 strain and Hawaii to CB4856 [8]. Transgenic lines were generated as described in [32,43].
Mutations used
LG I: amx-2(ok1235), egl-30(tg26) [31]; LG II: let-23(sa62)[21], let-23(sy1) [44], tph-1(n4622) [29]; LG IV: let-60(ga89) [32], let-60(n1046) [7], let-60(n2021) [7], lin-1(n304) [45]; LG V: rde-1(ne219) [46] LG X: ser-1(ok345) [47]. Transgenic strains: rde-1(ne219); duIs[Pelt-2::rde-1(+); pRF4] [23], let-60(n1046); rde-1(ne209); zhEx418[Plin-31::rde-1(+); myo-2::mCherry] [24], gaIS37[HS-mpk-1, dmek] [27], let-60(n1046); saIS14[lin-48p::gfp] [34], zhEx533[Pamx-2::gfp, Pmyo-2::mcherry], amx-2(ok1235); zhSi73[amx-2 Bristol]; let-60(n1046gf); zhSi74[amx-2 Hawaii]; let-60(n1046gf) (all this study).
Generation and genotyping of let-60(n1046) miRILs and Ils
miRILs were generated by crossing CB4856 males with MT2124(let-60(n1046)) hermaphrodites. In the F2 generation, lines homozygous for the n1046 allele were singled out and allowed to self-fertilize for 10 more generations to reach homozygosity by random cloning of individuals. At generation F12, lines were regarded as isogenic and frozen for long-term storage. All 228 miRIL lines were genotyped with the following 72 FLP markers as described in [15]: zh1-17; zh1-10a; zh1-07; zh1-18a; zh1-03; zh1-27; zh1-34; zh1-01;zh1-23; zh1-15; zh1-08; zh1-06; zh2-04a; zh2-16; zh2-07; zh2-13; zh2-19; zh2-02; zh2-20; zh2-25; zh2-27; zh2-09; zh2-10; zh2-12; zh3-17a; zh3-07; zh3-06; zh3-08; zh3-28; zh3-15; zh3-04; zh3-02; zh3-05a; zh3-35; zh3-10a; zh3-11; zh3-13; zh4-04a; zh4-5; zh4-06; zh4-16; zh4-08; zh4-17; zh4-18; zh4-19; zh4-20; zh4-21; zh4-12; zh5-13; zh5-03a; zh5-14; zh5-05; zh5-16; zh5-17; zh5-18; zh5-11; zh5-12; zh5-08; zh5-21/22; zh5-09zhX-17; zhX-08; zhX-13; zhX-15; zhX-10; zhX-24; zhX-07; zhX-12; zhX-11; zhX-21a; zhX-06; zhX-23. miRILs that contained a 100% Bristol genotype and miRILs lacking the n1046 allele were excluded from further analysis, and miRIls with identical genotypes were combined. These criteria reduced the 228 initial miRILs to 173 informative lines. To generate ILs in the n1046 background, the ewIR ILs from [14,17] were crossed with the MT2124(let-60(n1046)) mutant. For the exact breakpoints of the ILs used, see [17]. FLP mapping with 7 to 8 markers in the respective regions was used to identify and verify lines homozygous for the introgressions and exclude the presence of additional recombination events. Control siblings without an introgression were isolated in parallel, and the multivulva phenotype was used to identify homozygous let-60(n1046) lines. To quantify the VI, at least three independent introgression lines were compared to three sibling lines containing the let-60(n1046) allele but no introgression.
Phenotyping
To measure the VI, vulval induction was scored in L4 larvae using Nomarski optics as described [48], and the average number of induced VPCs per animal was calculated. The duct cell duplication phenotype was scored using the lin-48::gfp marker to visualize the duct cells using fluorescence microscopy [34]. The oocyte maturation phenotype was scored in 2 day old adults under Nomarski optics microscopy.
QTL mapping and data storage
QTL mapping was performed using a single marker model on the per miRIL averages. Significance threshold was estimated using 1000 permutations [14]. All QTL data, phenotypes, QTL profiles and genotypes are stored in www.WormQTL.org [49].
RNA interference
Gene knock-down was carried out using RNAi feeding according to [18]. For intestine-specific RNAi, OLB11(rde-1(ne219); duIs[Pelt-2::rde-1(+); pRF4]) [23] was crossed with the MT2124(let-60(n1046)) strain. For vulva-specific RNAi, the strain AH2927(rde-1(ne219lf); let-60(n1046); zhEx418[Plin-31::rde-1; Pmyo-2::mcherry]) described in [24] was used.
Generation of single-copy insertion lines
A 7.8 kb genomic fragment spanning the entire amx-2 locus was amplified with the primers OTS123 (GATTTTGGAGAAGAAACGAGGG) and OTS124 (ACTTCACTATGTTCCTCTACCG) using either Bristol or Hawaii genomic DNA as template and subcloned into the XhoI restriction site of pCFJ151 [20]. Single-copy insertions of the amx-2 Bristol and amx-2 Hawaii containing plasmids into the ttTi5605 region on chromosome II were generated using the protocol by [20] to yield zhSi73 and zhSi74, respectively. The insertions were verified by PCR amplification using primers flanking the insertion site before crossing them into the amx-2(lf); let-60(gf) background. For each genotype, at least three independent lines were scored.
Transcriptional amx-2 reporters
Primers OTS219 (AAA AGG ATC CTT AGG TTT ATT GCT GGA AAA AT) and OTS220 (AAA AGG ATC CCC TTA ACC AAA TTT CAT ACC C) were used to amplify 4kb of upstream promoter region. The PCR fragment was further cloned into the BamHI restriction site of pPD95.67 to generate a the Pamx-2::gfp transcriptional reporter plasmid that was co-injected at 50ng/μl with 2.5ng/μl of the pharyngeal Pmyo-2::mcherry marker.
Measurement of 5-HT levels
Animals were grown in 100ml liquid cultures and harvested by flotation on 50% sucrose. Worm pellets were resuspended in 2ml PBS buffer and lysed using a swing-mill homogenizer followed by high-speed centrifugation to remove insoluble debris. Total protein concentrations were measured in each sample using the amidoblack staining assay [50]. 5-HT levels were determined with an ELISA kit according to the manufacturer’s instructions (BA E-5900, Labor Diagnostika Nord) and normalized to the total protein concentrations in the extracts. The average 5-HT concentrations for each genotype were determined with two separate measurements, each done in triplicate using extracts obtained from two independently grown cultures.
Treatment of C. elegans with 5-HT and its metabolites
Standard NGM plates were supplemented with the indicated concentrations of serotonin (5-HT) (H9523, Sigma), 5-Hydroxyindoleacetic acid (5-HIAA) (H8876, Sigma), dopamine (DA) (H8502, Sigma) or Melatonin (MT) (M5250 Sigma) and kept in dark at 4°C prior to use.
Quantification of ERK phosphorylation
Phospho MPK-1 levels in total extracts of C. elegans L4 larvae were determined by Western blotting as described in [11]. As loading controls, total MPK-1 levels were quantified on parallel blots loaded with the same amounts of protein (20μg) from the identical samples. Protein bands were quantified using the integrated density function in ImageJ. The ratios of phospho-MPK-1 to total MPK-1 levels were calculated for each extract and normalized to the ratios in untreated controls. Antibodies used: anti-MAP Kinase (Sigma-Aldrich, M5670), anti-phosphoMAP Kinase, Activated (Diphosphorylated ERK-1&2, Sigma-Aldrich, M8159).
Supporting Information
Zdroje
1. Eichler EE, Flint J, Gibson G, Kong A, Leal SM, et al. (2010) Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 11 : 446–450. doi: 10.1038/nrg2809 20479774
2. Stessman HA, Bernier R, Eichler EE (2014) A genotype-first approach to defining the subtypes of a complex disease. Cell 156 : 872–877. doi: 10.1016/j.cell.2014.02.002 24581488
3. Prior IA, Lewis PD, Mattos C (2012) A comprehensive survey of Ras mutations in cancer. Cancer Res 72 : 2457–2467. doi: 10.1158/0008-5472.CAN-11-2612 22589270
4. Sundaram MV (2006) RTK/Ras/MAPK signaling. WormBook: 1–19. doi: 10.1895/wormbook.1.80.1
5. Gaertner BE, Phillips PC (2010) Caenorhabditis elegans as a platform for molecular quantitative genetics and the systems biology of natural variation. Genetics research 92 : 331–348. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=21429266&retmode=ref&cmd=prlinks. doi: 10.1017/S0016672310000601 21429266
6. Kammenga JE, Phillips PC, De Bono M, Doroszuk A (2008) Beyond induced mutants: using worms to study natural variation in genetic pathways. Trends Genet 24 : 178–185. doi: 10.1016/j.tig.2008.01.001 18325626
7. Beitel GJ, Clark SG, Horvitz HR (1990) Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348 : 503–509. http://www.google.com/search?client=safari&rls=en-us&q=Caenorhabditis+elegans+ras+gene+let-60+acts+as+a+switch+in+the+pathway+of+vulval+induction&ie=UTF-8&oe=UTF-8. 2123303
8. Andersen EC, Gerke JP, Shapiro JA, Crissman JR, Ghosh R, et al. (2012) Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat Genet 44 : 285–290. doi: 10.1038/ng.1050 22286215
9. Thompson O, Edgley M, Strasbourger P, Flibotte S, Ewing B, et al. (2013) The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Research 23 : 1749–1762. doi: 10.1101/gr.157651.113 23800452
10. Sternberg PW (2005) Vulval development. WormBook: 1–28.
11. Nakdimon I, Walser M, Fröhli E, Hajnal A (2012) PTEN Negatively Regulates MAPK Signaling during Caenorhabditis elegans Vulval Development. PLoS Genet 8: e1002881. doi: 10.1371/journal.pgen.1002881 22916028
12. Tipton KF, Boyce S, O'Sullivan J, Davey GP, Healy J (2004) Monoamine Oxidases: Certainties and Uncertainties. Current Medicinal Chemistry 11 : 1965–1982. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15279561&retmode=ref&cmd=prlinks. 15279561
13. Milloz J, Duveau F, Nuez I, Félix M-A (2008) Intraspecific evolution of the intercellular signaling network underlying a robust developmental system. Genes Dev 22 : 3064–3075. doi: 10.1101/gad.495308 18981482
14. Snoek LB, Orbidans HE, Stastna JJ, Aartse A, Rodriguez M, et al. (2014) Widespread Genomic Incompatibilities in Caenorhabditis elegans. G3 (Bethesda).
15. Zipperlen P, Nairz K, Rimann I, Basler K, Hafen E, et al. (2005) A universal method for automated gene mapping. Genome Biol 6: R19. 15693948
16. Seidel HS, Rockman MV, Kruglyak L (2008) Widespread genetic incompatibility in C. elegans maintained by balancing selection. Science 319 : 589–594. doi: 10.1126/science.1151107 18187622
17. Doroszuk A, Snoek LB, Fradin E, Riksen J, Kammenga J (2009) A genome-wide library of CB4856/N2 introgression lines of Caenorhabditis elegans. Nucleic Acids Res 37: e110. doi: 10.1093/nar/gkp528 19542186
18. Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, et al. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 : 231–237. http://www.nature.com/nature/journal/v421/n6920/full/nature01278.html. 12529635
19. Nakdimon I (2011) Regulation of the C. elegans RAS/MARK Pathway by the Tumor Suppressor PTEN DAF-18 and Nutritional Cues. Ph.D. thesis. The University of Zurich. http://opac.nebis.ch/ediss/20121450.pdf
20. Frøkjær-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, et al. (2008) Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40 : 1375–1383. doi: 10.1038/ng.248 18953339
21. Katz WS, Lesa GM, Yannoukakos D, Clandinin TR, Schlessinger J, et al. (1996) A point mutation in the extracellular domain activates LET-23, the Caenorhabditis elegans epidermal growth factor receptor homolog. Mol Cell Biol 16 : 529–537. 8552080
22. Timmons L, Court DL, Fire A (2001) Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 : 103–112. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11223248&retmode=ref&cmd=prlinks. 11223248
23. Pilipiuk J, Lefebvre C, Wiesenfahrt T, Legouis R, Bossinger O (2009) Increased IP3/Ca2+ signaling compensates depletion of LET-413/DLG-1 in C. elegans epithelial junction assembly. Dev Biol 327 : 34–47. http://www.sciencedirect.com/science/article/pii/S0012160608013754. doi: 10.1016/j.ydbio.2008.11.025 19109941
24. Haag A, Gutierrez P, Bühler A, Walser M, Yang Q, et al. (2014) An In Vivo EGF Receptor Localization Screen in C. elegans Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling. PLoS Genet 10: e1004341. doi: 10.1371/journal.pgen.1004341 24785082
25. Hobert O (2013) The neuronal genome of Caenorhabditis elegans. WormBook: 1–106.
26. Ambroziak W, Pietruszko R (1991) Human aldehyde dehydrogenase. Activity with aldehyde metabolites of monoamines, diamines, and polyamines. J Biol Chem 266 : 13011–13018. 2071588
27. Lackner M, Kim S (1998) Genetic analysis of the Caenorhabditis elegans MAP kinase gene mpk-1. Genetics 150 : 103–117. 9725833
28. Tan PB, Lackner MR, Kim SK (1998) MAP kinase signaling specificity mediated by the LIN-1 Ets/LIN-31 WH transcription factor complex during C. elegans vulval induction. Cell 93 : 569–580. 9604932
29. Sze JY, Victor M, Loer C, Shi Y, Ruvkun G (2000) Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403 : 560–564. 10676966
30. Carnell L, Illi J, Hong SW, McIntire SL (2005) The G-protein-coupled serotonin receptor SER-1 regulates egg laying and male mating behaviors in Caenorhabditis elegans. J Neurosci 25 : 10671–10681. 16291940
31. Bastiani CA, Gharib S, Simon MI, Sternberg PW (2003) Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics 165 : 1805–1822. 14704167
32. Eisenmann DM, Kim SK (1997) Mechanism of activation of the Caenorhabditis elegans ras homologue let-60 by a novel, temperature-sensitive, gain-of-function mutation. Genetics 146 : 553–565. 9178006
33. Stetak A, Gutierrez P, Hajnal A (2008) Tissue-specific functions of the Caenorhabditis elegans p120 Ras GTPase activating protein GAP-3. Dev Biol 323 : 166–176. doi: 10.1016/j.ydbio.2008.08.026 18805410
34. Berset TA, Hoier EF, Hajnal A (2005) The C. elegans homolog of the mammalian tumor suppressor Dep-1/Scc1 inhibits EGFR signaling to regulate binary cell fate decisions. Genes Dev 19 : 1328–1340. 15901674
35. Rockman MV, Skrovanek SS, Kruglyak L (2010) Selection at linked sites shapes heritable phenotypic variation in C. elegans. Science 330 : 372–376. doi: 10.1126/science.1194208 20947766
36. Lopez AL III, Chen J, Joo H-J, Drake M, Shidate M, et al. (2013) DAF-2 and ERK Couple Nutrient Availability to Meiotic Progressionduring Caenorhabditis elegans Oogenesis. Dev Cell 27 : 227–240. doi: 10.1016/j.devcel.2013.09.008 24120884
37. Moghal N (2003) Modulation of EGF receptor-mediated vulva development by the heterotrimeric G-protein G q and excitable cells in C. elegans. Development 130 : 4553–4566. 12925583
38. Buffa R, Capella C, Fontana P, Usellini L, Solcia E (1978) Types of endocrine cells in the human colon and rectum. Cell Tissue Res 192 : 227–240. 699014
39. Rybaczyk LA, Bashaw MJ, Pathak DR, Huang K (2008) An indicator of cancer: downregulation of Monoamine Oxidase-A in multiple organs and species. BMC Genomics 9 : 134. doi: 10.1186/1471-2164-9-134 18366702
40. Cowen DS, Sowers RS, Manning DR (1996) Activation of a mitogen-activated protein kinase (ERK2) by the 5-hydroxytryptamine1A receptor is sensitive not only to inhibitors of phosphatidylinositol 3-kinase, but to an inhibitor of phosphatidylcholine hydrolysis. J Biol Chem 271 : 22297–22300. 8798386
41. Rocca Della GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, et al. (1999) Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J Biol Chem 274 : 4749–4753. 9988712
42. Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94. Available: http://www.genetics.org/content/77/1/71.long. 4366476
43. Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10 : 3959–3970. 1935914
44. Aroian RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW (1990) The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature 348 : 693–699. 1979659
45. Beitel GJ, Tuck S, Greenwald I, Horvitz HR (1995) The Caenorhabditis elegans gene lin-1 encodes an ETS-domain protein and defines a branch of the vulval induction pathway. Genes Dev 9 : 3149–3162. 8543158
46. Qadota H, Inoue M, Hikita T, Köppen M, Hardin JD, et al. (2007) Establishment of a tissue-specific RNAi system in C. elegans. Gene 400 : 166–173. 17681718
47. Dernovici S, Starc T, Dent JA, Ribeiro P (2007) The serotonin receptor SER-1 (5HT2ce) contributes to the regulation of locomotion in Caenorhabditis elegans. Devel Neurobio 67 : 189–204.
48. Sternberg PW, Horvitz HR (1986) Pattern formation during vulval development in C. elegans. Cell 44 : 761–772. http://www.sciencedirect.com/science/article/pii/0092867486908421. 3753901
49. Snoek LB, Van der Velde KJ, Arends D, Li Y, Beyer A, et al. (2013) WormQTL—public archive and analysis web portal for natural variation data in Caenorhabditis spp. Nucleic Acids Res 41: D738–D743. doi: 10.1093/nar/gks1124 23180786
50. Schaffner W, Weissmann C (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem 56 : 502–514. 4128882
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



