-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenetic Architecture of Abdominal Pigmentation in
Body pigmentation contributes to the spectacular biodiversity present in nature and mediates mate choice, mimicry, and physiological functions such as thermoregulation and UV resistance. Thus, pigmentation is a significant contributor to fitness. In order to understand how complex traits such as pigmentation evolve, we must first identify the genetic variants underlying phenotypic variation. We used the Drosophila melanogaster Genetic Reference Panel, a wild derived population of fully sequenced inbred fly lines, to identify the contributions of both known and novel genetic variants to natural variation in abdominal pigmentation in female flies. Our results show that genetic variation within many biological pathways contributes to variation in D. melanogaster pigmentation.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005163
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005163Summary
Body pigmentation contributes to the spectacular biodiversity present in nature and mediates mate choice, mimicry, and physiological functions such as thermoregulation and UV resistance. Thus, pigmentation is a significant contributor to fitness. In order to understand how complex traits such as pigmentation evolve, we must first identify the genetic variants underlying phenotypic variation. We used the Drosophila melanogaster Genetic Reference Panel, a wild derived population of fully sequenced inbred fly lines, to identify the contributions of both known and novel genetic variants to natural variation in abdominal pigmentation in female flies. Our results show that genetic variation within many biological pathways contributes to variation in D. melanogaster pigmentation.
Introduction
Body pigmentation is a conspicuous trait that is variable within species, giving rise to natural variation, polyphenism and sexual dimorphism [1–4]. It also varies between species, contributing to species recognition, mate choice, thermoregulation, protection (warning signals), mimicry, and crypsis [5–7]. Changes in pigmentation are often adaptive and vital to the fitness of the organism [5,6].
Not only is body pigmentation ecologically relevant, in Drosophila it is a relatively simple and easily measured phenotype to study the genetic architecture of natural variation in complex traits [2,7–10]. Each tergite of female D. melanogaster generally has a stripe of dark coloration (melanin) on a lighter tan background (sclerotin). During pre - and post-ecdysis, the epidermal cells underlying the cuticle secrete tyrosine-derived catecholamines into the cuticle for sclerotinization and melanization [11,12]. The melanin/sclerotin biosynthetic pathway and its underlying genetic basis have been well studied. However, many of the genes known to affect D. melanogaster pigmentation do not form part of this pathway or any parallel pathway [5,13]. Furthermore, the genes that lead to natural variation in body pigmentation are not necessarily the same genes that are directly involved in the biosynthesis of melanin and sclerotin. By mapping the genetic basis of natural variation in body pigmentation, we may discover new genes affecting pigment biosynthesis as well as regulatory regions that determine when and where pigmentation will develop [3,13].
We used the D. melanogaster Genetic Reference Panel (DGRP) to perform a genome-wide association (GWA) study of natural variation in the proportion of melanization on female abdominal tergites 5 and 6. The DGRP consists of 205 sequenced inbred lines derived from a single North American population, facilitating GWA analyses for quantitative traits when all genetic variants are known. Local linkage disequilibrium (LD) in the DGRP is low and thus favorable for identifying candidate genes and even causal polymorphisms [14,15]. We identified single nucleotide polymorphisms (SNPs) affecting three genes previously known to contribute to variation in abdominal pigmentation, bric-à-brac 1 (bab1), tan (t), and ebony (e). However, we also identified novel candidate genes and showed that these contribute to abdominal pigmentation using mutations and RNAi knock-down constructs. Many of these novel genes affect other well-studied pathways and phenotypes, such as wing and bristle development, providing evidence for widespread pleiotropy. Four of the novel genes affecting pigmentation are computationally predicted genes with previously unknown functions. Based on their mutant or RNAi knockdown phenotypes, we have named them pinstripe (pns, CG7852), triforce (tfc, CG9134), plush (ph, CG1887), and farmer (frm, CG10625).
Results
Quantitative genetics of pigmentation
We characterized natural variation in the proportion of melanization of tergites 5 (T5) and 6 (T6) in females for 175 DGRP lines (Figs 1 and 2 and S1 Table). Averaged across all lines, the mean pigmentation scores are 1.44 for T5 and 2.55 for T6 (Fig 2A). There is significant genetic variation in pigmentation among lines for both tergites (PT5 = 4.68 x 10–48 and PT6 = 6.65 x 10–96; S2 Table), with broad sense heritabilities (H2) of H2T5 = 0.66 and H2T6 = 0.88. The phenotypic (rP(T5,T6) = 0.63 ± 0.059) and genetic (rG(T5,T6) = 0.72 ± 0.053) correlations (± standard error) between the tergites for proportion of pigmentation are high but significantly different from unity, suggesting they have different genetic bases (Fig 2B). The high broad sense heritabilities for abdominal pigmentation traits provide a favorable scenario for GWA studies.
Fig. 1. Natural variation in T5 and T6 pigmentation in the DGRP. 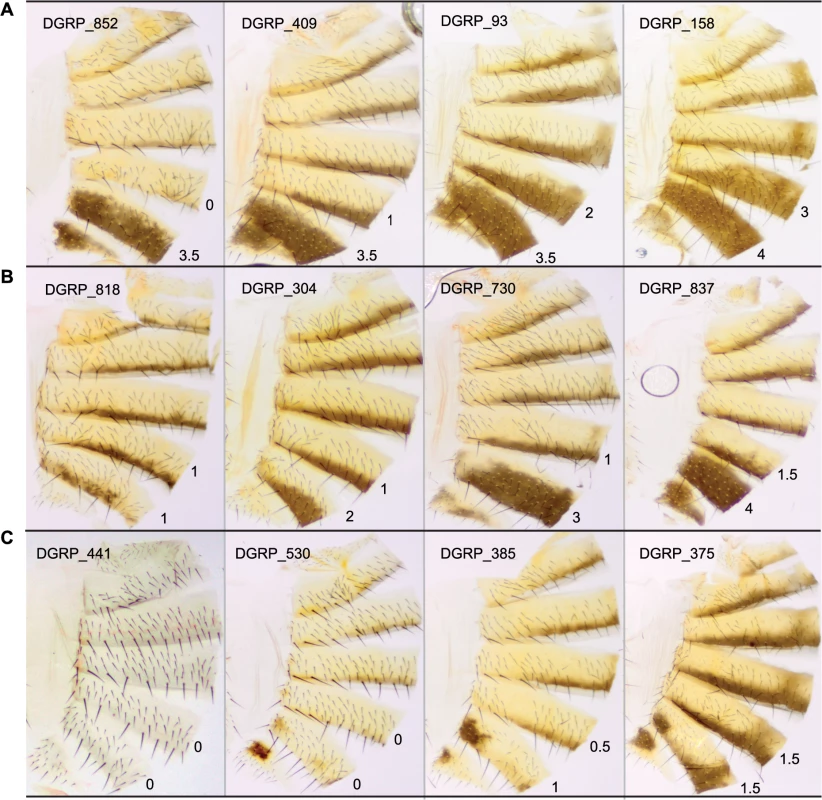
Images display one half of the fly abdominal cuticle, split along the dorsal midline. Pigmentation scores are given for T5 (upper) and T6 (lower). DGRP lines are denoted by DGRP_XXX in the upper left corner of each image. (A) Variation in T5. (B) Variation in T6. (C) Variation in spatial patterning of pigmentation. Fig. 2. Natural variation in female abdominal pigmentation. 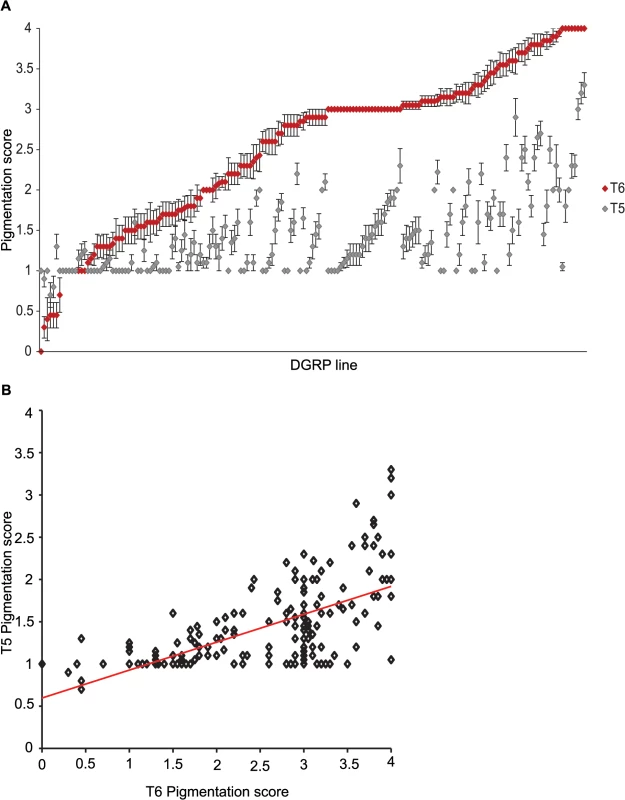
(A) T5 (gray) and T6 (red). DGRP lines are in order from least to most pigmentation on T6. (B) Scatter plot of T5 and T6 line means. Genome-wide association analyses
We performed genome-wide association analyses on the proportion of T5 and T6 melanization to identify genomic regions harboring variants contributing to natural variation in female abdominal pigmentation. The DGRP lines vary in Wolbachia infection status and karyotype for several common polymorphic inversions. We did not find significant associations of Wolbachia infection (PT5 = 0.58 and PT6 = 0.92) nor inversion karyotype on T5 or T6 pigmentation; however, the difference in pigmentation between T5 and T6 was significantly affected by ln(2L)t (P = 0.04) and In(2R)NS (P = 0.01) (S3 Table). For each GWA analysis, we used both a mixed model that accounted for any effects of Wolbachia, inversions, and cryptic relatedness and a regression model that corrected for all of the aforementioned effects except for cryptic relatedness [15]. Combining all of these models, we identified a total of 155 variants associated with pigmentation for any trait at a nominal reporting threshold of P < 10–5 (S4 Table). Of these, 84 were associated with T5 pigmentation, 34 with T6 pigmentation, 28 with the average of T5 and T6, and 35 with the difference in pigmentation between T5 and T6. A total of 84 candidate genes were implicated by these associated variants. Since variants associated with the average of the two posterior tergites were largely the same as those associated with either T5 or T6 alone, we focus our subsequent analyses on T5, T6 and the difference between them (Fig 3 and S4 and S5 Tables). Among the genes harboring SNPs associated with variation in abdominal pigmentation, we find genes with well documented effects on pigmentation (t, e, bab1); osa, a transcription factor recently shown to affect pigmentation; and a large group of novel candidate genes [2,9,16]. The identification of t, e, and bab1 as prominent contributors to variation in abdominal pigmentation instills confidence in the efficacy of our GWA analyses, as described below.
Fig. 3. Genome-wide association analyses. 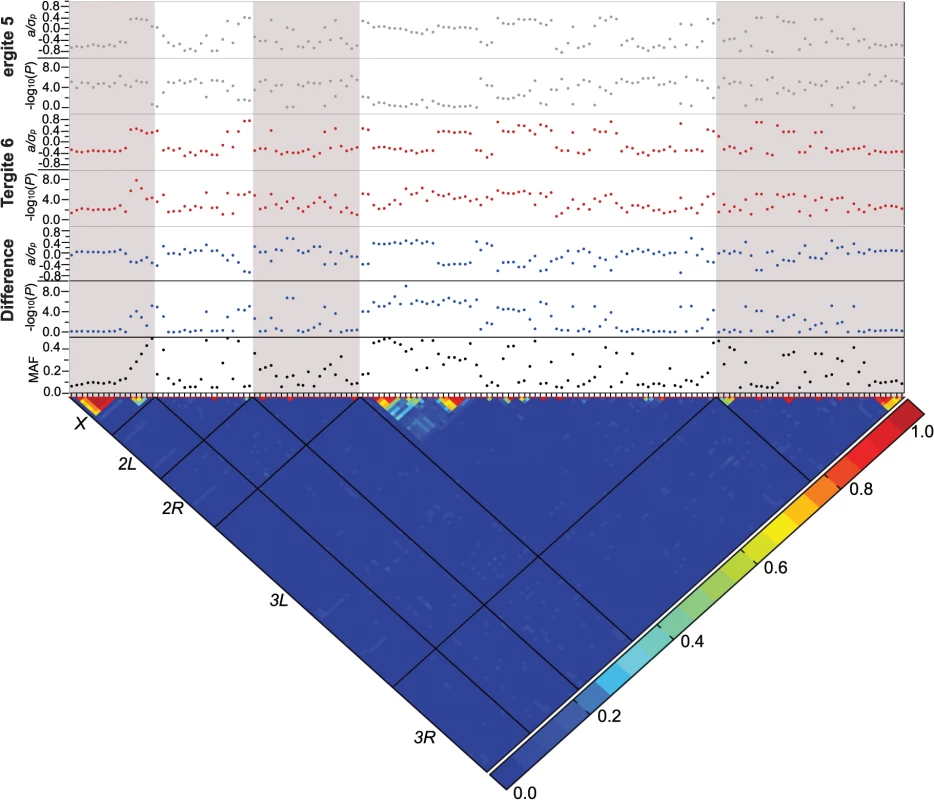
Results are depicted for T5, T6, and the T5-T6 difference. A nominal P ≤ 10–5 is indicated with a red line for each trait. The triangular heat map depicts the degree of LD, r2, between variants. The five major chromosome arms are delineated by the black lines. Red corresponds to complete LD and blue to absence of LD. The upper panels show the mixed model significance threshold (-log10(P)) and the effect size in phenotypic standard deviation units (a/σp) for each trait. The minor allele frequency (MAF) is shown on the bottom panel. Only a few variants exceeded a strict Bonferroni correction for multiple tests (P = 2.64 × 10–8): a SNP 41 bp upstream of Gr8a and 528 bp downstream of CG15370—the cis-regulatory region of t—in the T6 and average of T5 and T6 analyses (X_9121129_SNP); and two SNPs in the first intron of bab1 in the analysis of the difference between T5 and T6 (3L_1084990_SNP and 3L_1084199_SNP; S4 Table). The three SNPs that achieved Bonferroni significance levels were all at intermediate frequency and had moderately large effects. The minor allele of the polymorphism in the t cis-regulatory element (CRE) was associated with reduced pigmentation, while the minor alleles of the bab1 intronic polymorphisms were both associated with increased pigmentation in T6.
Although the other variants do not reach individual Bonferroni-corrected significance levels, quantile-quantile plots (S1 Fig) indicate a systematic departure from random expectation below P < 10–5, justifying our choice of this reporting threshold and suggesting that the top associations are enriched for true positives. Indeed, the SNP in the t CRE that reached Bonferroni significance in the T6 analysis was also significant in the T5 analysis at the more lenient reporting threshold, and two additional polymorphisms in the t CRE were significant at P < 10–5: X_9121177_SNP in the T5 and T6 analyses, and X_9121094_SNP in the T6 analysis.
These data also highlight the importance of bab1 with respect to female abdominal pigmentation: we found a total of 21 polymorphisms (20 SNPs, one indel) in the first intron of this gene that are associated with natural variation in pigmentation in one or more analyses (Fig 4 and S4 Table). One bab1 SNP is unique to the T5 analysis, six bab1 SNPs are common between the T6 and T5—T6 difference analysis, and the remaining bab1 variants are unique to the difference in pigmentation between T5 and T6. Twelve of the bab1 variants are located within the minimal functional cis-regulatory regions as reported by REDfly or within other transcription factor binding sites (S4 and S6 Tables) [17]. Three SNPs (3L_1084990_SNP, 3L_1085137_SNP, and 3L_1085230_SNP) are located in the bab1 middle dimorphic element which contain binding sites for the transcription factors caudal (cad) and dl (dorsal) [3]. All of the polymorphisms segregating in bab1 associated with pigmentation have minor allele frequencies ranging from 0.22 to 0.49 and moderate effects. Interestingly, the direction of the effects is both positive (the minor allele is associated with reduced pigmentation) and negative (the minor allele is associated with increased pigmentation), such that variants in the dl and cad cis-regulatory modules have positive effects while those in the latter regions of the intron have largely negative effects (the exceptions are 3L_1093297_SNP in the latter region of the intron and 3L_1099962_SNP in the T5 GWAS, which have positive effects). The functionality of these bab1 CREs has been thoroughly investigated [3]. However, similar to the results of Bickel et al. [18], nine of the bab1 variants from this study are in regions outside of the known cis-regulatory regions. These variants may indicate the presence of a not-yet-described regulatory element, or the structure of the regulatory elements in this region may be more complex than previously thought (Fig 4).
Fig. 4. Pairwise linkage disequilibrium among the associated bab1 variants. 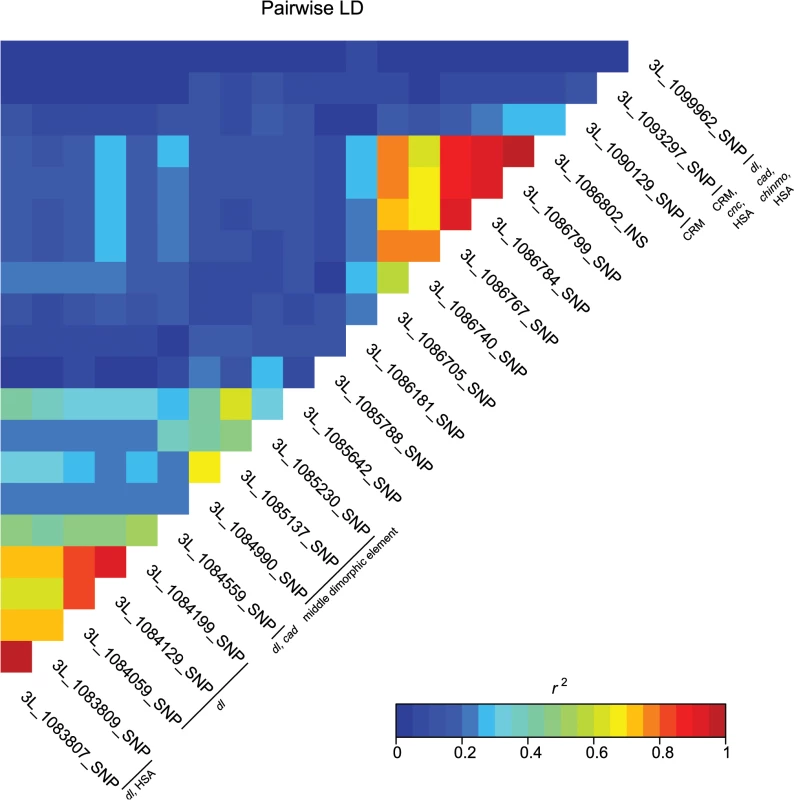
Heat map of LD (r2) of the 21 bab1 variants. Individual variants, cis-regulatory regions and transcription factor binding sites are labeled on the diagonal. A majority of the variants associated with variation in pigmentation are located within intronic or intergenic regions, suggesting they could affect gene regulation. In support of this hypothesis, we found many of these variants are located in annotated regulatory sites (S4 and S6 Tables). In total, variants associated with pigmentation were located in 24 different transcription factor binding sites (TFBS, each of which contain numerous variants), 17 cis-regulatory modules (CRM), 1 polycomb response element (PRE), and 31 hot spot analysis sites (HSA; where one or more 41 tested TFs bind to a given site) (S6 Table). TFBS for dl and cad are the most frequent of all TFBS, containing 28 and 22 associated variants, respectively. Two intergenic TFBS for bab1 (FBsf0000214860 and FBsf0000214320) were tagged by 3R_25139342_SNP, 3R_25139132_SNP, and 2R_16793853_SNP. A few variants are located in more than one regulatory site (S4 Table).
Variance in pigmentation explained by top variants
We asked what fraction of the total broad sense heritability was explained by variants in bab1, t and e using stepwise regression to select the top associations for pigmentation genes. The R2 from these models for each trait gives the heritability explained by the known genes. These loci explain 25.62%, 37.55%, 31.17% and 36.58% of the heritability for T5, T6, and the average and difference of T5 and T6, respectively; consistent with the intermediate allele frequencies and large effects of their top associated variants. Next, we used genomic best linear unbiased prediction (GBLUP) to estimate the total variance explained by all top variants. All variants explain 59.77%, 34.32%, 47.44% and 51.61% of the heritability for T5, T6, and the average and difference of T5 and T6, respectively. With the exception of T6, for which most of the variance is explained by the known pigmentation genes, substantial additional variance in proportion of pigmented cuticle is contributed by variants in novel genes. Finally, we estimated the faction of heritability explained for each variant as well as the fraction of heritability explained after accounting for the variance explained by the pigmentation genes. On average, the variants in novel candidate genes explained an additional 7.3% (T5), 4.5% (T6), 5.8% (average of T5 and T6) and 2.8% (difference between T5 and T6) of the heritability (S2 Fig and S4 Table)
Comparison with previous studies
e, t, and bab1 have been associated with variation in D. melanogaster female pigmentation in other populations [2,9,16]. We compared the top variants in these genes in our analyses with those from prior studies [9,16,19]. Three of the four SNPs identified by Bastide et al. [9] are identical to the three t CRE SNPs associated with our T5 and T6 analyses (X_9121094_SNP, X_9121129_SNP, and X_9121177_SNP). The bab1 SNP identified in the Bastide et al. [9] study did not overlap with our results nor those of Bickel et al. [18]. None of the top bab1 variants in this study were significant in the study of Bickel et al. [18], although three of our significant variants were also polymorphic in the Bickel data set (3L_1085788_SNP, 3L_1086799_SNP, and 3L_1086802_INS). Both the e CRE SNPs associated with pigmentation in the DGRP T6 analysis (3R_17063120_SNP) and the Bastide et al. study [9] (3R_17064232_SNP) are located within the CRE regulating e expression in the haltere (e_C [19]; S4 Table). The SNP at 3R_17064232 was also reported in the Pool and Aquadro study of light and dark African D. melanogaster [16]. The concordance among these datasets indicates that the haltere regulatory element may also control expression in the abdomen and warrants further investigation.
Validation of candidate genes
We selected 30 novel candidate genes based on the GWA results for functional validation using mutant alleles and RNAi knockdown (S7 Table). We phenotyped Exelixis insertion lines [20] and RNAi knockdown lines [21] with their appropriate controls for the proportion of melanization on T5 and T6 as done for the DGRP (S8 and S9 Tables). Wherever possible we tested both mutant and RNAi lines for the same gene as independent forms of validation. We used three GAL4 drivers for the UAS-RNAi lines. tubulin-GAL4/TM3, Sb (tub-GAL4) and ubiquitin-GAL4/CyO (ubi-GAL4) are ubiquitously expressed, while the pannier driver, y1 w1118; P{w[+mW.hs] = GawB}pnrMD237/TM3, P{w[+mC] = UAS-y.C}MC2, Ser1 (pnr-GAL4), has restricted expression in the midline [22]. The use of the pnr-GAL4 driver adds a spatial component to the validation experiments and allows for more precise testing of the candidate genes (S3 Fig). As positive controls, we also tested RNAi constructs for e and t (S3 Fig and S8 and S9 Tables).
We evaluated 15 Exelixis transposon insertions in candidate genes for effects on pigmentation (See Methods; Fig 5A and S7 Table). Six of these mutations affected the proportion of melanization on T5 (P < 0.0001 for all significant mutations): CG9134e00088, CG7852c04511, Exchange factor for arf6 (Efa6f03476), Fish-lips (Filif04573), and Glucose transporter 1 (Glut1d05758) showed increased melanization; and krotzkopf verkehrt (kkvc06225) showed decreased melanization (Figs 5A and 6). Twelve of the mutations affected the proportion of melanization on T6 (P < 0.0001 for all significant mutations). CG9134e0008, Efa6f03476, Filif04573, and Glut1d05758 showed increased melanization; and CG10625e01211, CG7852c04511, division abnormally delayed (dallyf01097), kayak (kayf02002), kkvc06225, klarsicht (klard05910), locomotion defects (locod09879), and Sucbe01940 showed decreased melanization (Figs 5A and 6). CG7852c04511 had increased pigmentation on T5 and decreased pigmentation on T6 (Fig 6C). CG33298d10678a, multiple wing hairs (mwhd01620), and Kinesin-like protein at 61F (Klp61Ff02870) were not significantly different from the control. Efa6f03476 also has a light and somewhat elongated thoracic trident; this thoracic pigmentation is completely absent in the control flies (S4 Fig).
Fig. 5. Validation of pigmentation candidate genes using Exelixis insertion mutants and RNAi knockdown. 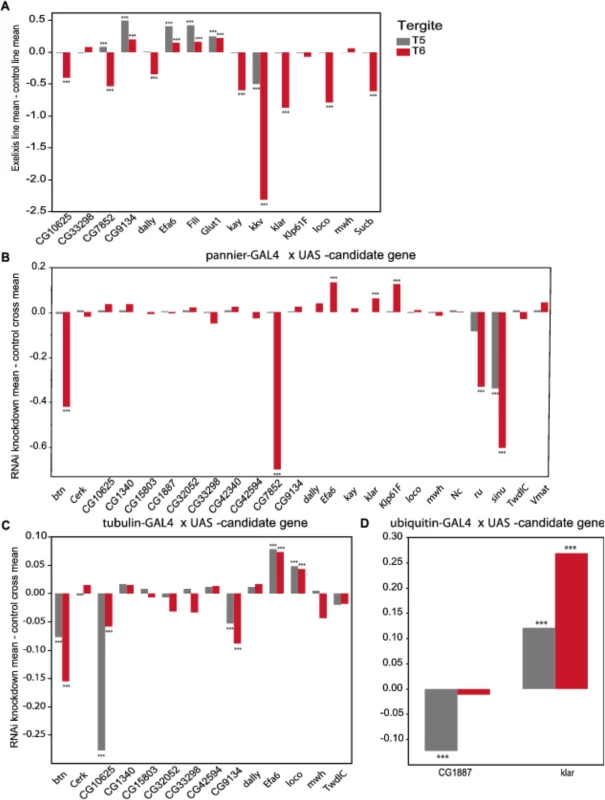
The y-axis in all panels is the deviation of the appropriate control line mean from the experimental line mean. Increases and decreases in the proportion of melanization are given above and below the x-axis, respectively. (A) Exelixis insertion mutants. (B) pnr-GAL4 x RNAi-UAS lines. (C) tub-GAL4 x RNAi-UAS lines. (D) ubi-GAL4 x RNAi-UAS-lines. ***: P<0.0001. Fig. 6. Female abdominal cuticles of Exelixis transposon insertion lines with significant effects on pigmentation. 
(A) Exelixis w1118 control. (B) CG10625e01211. (C) CG7852c04511. (D) CG9134e00088. (E) dallyf01097. (F) Efa6f03476. (G) Filif04573. (H) Glut1d05758. (I) kayf02002. (J) kkvc06225. (K) klard05910. (L) locod09879. (M) Sucbe01940. ns = not significant. ↑ and ↓ indicate significantly increased and decreased proportions of dark melanin, respectively. T5 = tergite 5 and T6 = tergite 6. Of the 28 candidate genes, 26 were available as RNAi knockdown constructs (S7 and S8 Tables). We crossed all of these constructs to the pnr-GAL4 driver, and obtained viable female progeny from all crosses except for kkv and Fili. We found that seven of these knockdown mutations affected the proportion of melanization of T5 and/or T6 (Figs 5B and 7A–7I, P < 0.0001 in all cases). Efa6, klar, and Klp61F knockdowns increased the proportion of dark melanin on T6; buttonless (btn) and CG7852 decreased it. Knockdown of roughoid (ru) and sinuous (sinu) showed decreases in pigmentation for both T5 and T6.
Fig. 7. Female abdominal cuticles of with significant effects on pigmentation in lines with targeted RNAi knockdown constructs. 
A-J: pnr-GAL4 x UAS-RNAi genotypes. (A) VDRC KK library control. (B) btn. (C) CG7852. (D) Klp61F. (E) ru. (F) VDRC GD library control. (G) Efa6. (H) klar. (I) sinu. J-Q: tub-GAL4 x UAS-RNAi genotypes. (J) VDRC KK library control. (K) btn, (L) CG10625. (M) CG9134. (N) VDRC GD library control. (O) Efa6. (P) loco. Q-T: ubi-GAL4 x RNAi-UAS genotypes. (Q) VDRC KK library control. (R) CG1887. (S) VDRC GD control. (T) klar. ns = not significant. ↑ and ↓ indicate significantly increased and decreased proportions of dark melanin, respectively. We also crossed the 26 UAS-RNAi constructs to an ubiquitously expressed tub-GAL4 driver, and found that 11 (42%) were lethal in both sexes, suggesting pleiotropic effects on vital functions: Vesicular monoamine transporter (Vmat), Klp61F, CG7852, CG1887, klar, ru, sinu, Nedd2-like caspase (Nc), kkv, kay, and CG42340 (Table 1). Of the 15 UAS-RNAi/tub-GAL4 knockdown alleles available for testing, six had significant (P < 0.0001) effects on pigmentation. Knockdowns of btn, CG10625, and CG9134 had decreased proportions of dark melanin on T5 and T6; Efa6 and loco knockdowns showed increases in pigmentation on both tergites (Figs 5C and 7).
Tab. 1. Summary of candidate gene validation experiments. 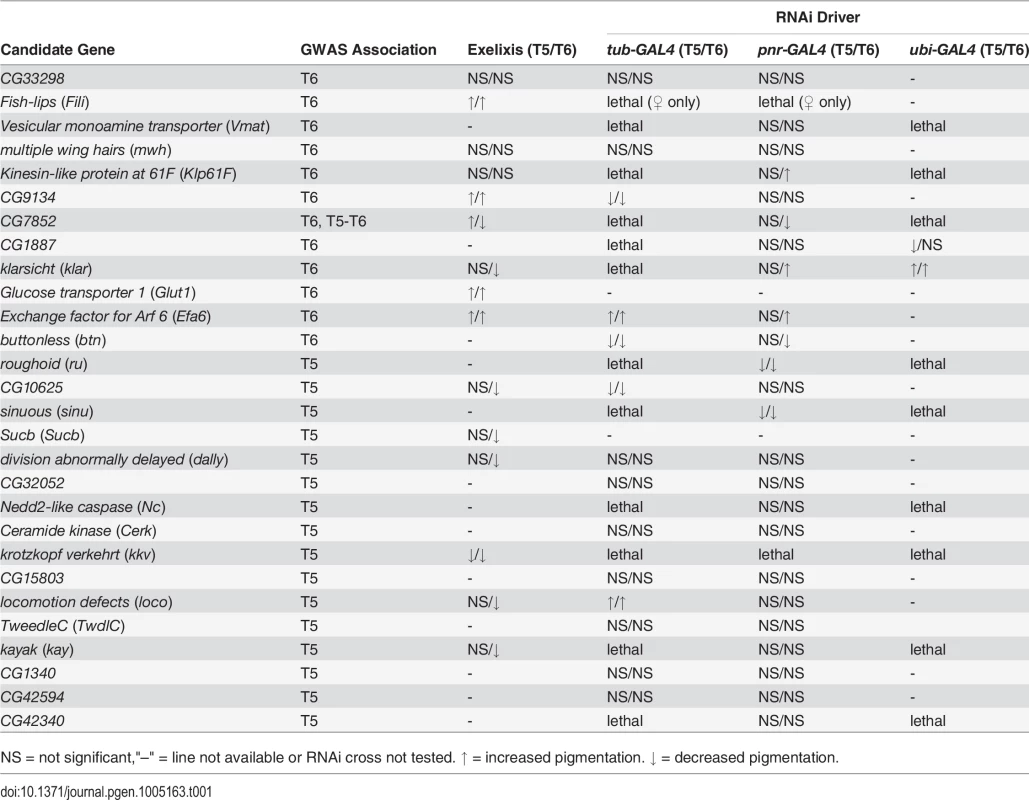
NS = not significant,"–" = line not available or RNAi cross not tested. ↑ = increased pigmentation. ↓ = decreased pigmentation. Next, we crossed the 11 UAS-RNAi constructs that were lethal when crossed to the tub-GAL4 driver to another ubiquitously expressed GAL4 driver, ubi-GAL4. Only two RNAi constructs were viable when driven by ubi-GAL4, CG1887 and klar, and both had significant (P < 0.0001) effects on abdominal pigmentation (Figs 5D and 7 and Table 1). The CG1887 knockdown showed a decrease in T5 melanization. Although T6 did not show a significant difference in the proportion of melanin in the CG1887 knockdown (P = 0.71), the dark melanin that is present is a light brown melanin that is only slightly darker than the adjacent sclerotin (S5 Fig). The CG1887 knockdown flies have obvious qualitative differences in overall body coloration from the control. The cuticle as a whole is semi-transparent and its strength is compromised as it ruptures easily when probed with forceps. The third thoracic leg of these progeny is also malformed and bristle number and patterning is highly disrupted (S5 Fig). All progeny of the cross die within 24 hours of eclosion; thus, pigmentation scoring was performed 8 hours after eclosion. The klar knockdown shows an increase of melanization on both tergites. Similar to the Efa6 mutant, this cross leads to a relative darkening of the thoracic trident compared to the surrounding cuticle (S4 Fig). In summary, we found that 17 of the 28 candidate genes tested affected female abdominal pigmentation and that for 12 of these genes, both tergites are affected (Table 1 and S8 and S9 Tables).
Discussion
The DGRP lines show substantial natural variation in female abdominal pigmentation, ranging from lines with no dark melanin to complete melanization on tergites 5 and 6. Despite being sampled from a single population, the lines span the range of pigmentation difference between the well-studied sister species D. yakuba and D. santomea. D. santomea is the lightest member of the D. melanogaster species subgroup; however, several of the DGRP lines are lighter than D. santomea (e.g., DGRP_441, Fig 1C). Utilizing the substantial genetic variation and a mapping population that is powerful to detect common variants associated with the variation, especially those with moderate to large effects (S6 Fig), we identified a total of 155 genetic variants associated with variation in female abdominal pigmentation using GWA analyses.
We identified variants in four genes previously shown to affect adult D. melanogaster pigmentation: bab1, t, e, and osa [2,16,23,24]. Most of the bab1 SNPs were associated only with the difference between T5 and T6 pigmentation, suggesting that variation in bab1 may underlie the genetic and phenotypic correlations between the traits. Most of the bab1 and t minor alleles are at moderate frequencies in the DGRP. These SNPs could be neutral or could be maintained segregating by a balance of unknown selective forces. We identified three SNPs in the CRE of t that were also found in the European populations studied by Bastide et al. [9], indicating that these SNPs are maintained in distant populations. Of note, our study is the first to associate natural variation in pigmentation with genetic variation in the transcription factor osa.
A majority of the variants identified in this study are in intronic or intergenic regions. Among the total regulatory elements identified, dl and cad binding sites were the most highly represented, suggesting a role for these two TFs on pigmentation patterning. Over half of the genetic variants located within bab1 were in known regulatory regions including some for dl and cad binding. We also identified a SNP upstream of e that is located within a cis-regulatory region consistent with the studies of African and European D. melanogaster [9,16,19]. These results implicate cis-regulatory evolution, which likely limits negative pleiotropic effects, as a major contributor to phenotypic variation within the DGRP population.
In addition to genes previously known to affect Drosophila pigmentation, we identified many novel candidate genes. We showed that 61% of the candidate genes affect the proportion of dark pigmentation on tergites 5 and 6 using mutant and RNAi knockdown experiments. These genes are known to be involved in processes such as sugar binding and transport, vesicle formation and transport, and cuticle formation. We summarize what is known from the literature about these candidate genes below and speculate about their roles in pigmentation and phenotypic evolution.
Prior to molting and eclosion, insects accumulate tyrosine-derivatives conjugated with hydrophilic molecules such as glucose, phosophate, and sulfate in the hemolymph. This keeps the reactive pigment precursors in an inert state until the organism is ready to molt or eclose [13,25–29]. We identified two genes, triforce (tfc, CG9134) and Glut1, which may facilitate the transport of glucose or glucose conjugates from the hemolymph to the epidermal cells. tfc is a C-type lectin with a carbohydrate binding domain and Glut1 is a membrane bound glucose transporter [30].
Several of our new pigmentation genes have roles in relatively well-described developmental pathways. These include kay, dally, Fili, and ru. kay is a transcription factor in the JNK signal transduction pathway [31]. It is required for decapentaplegic (dpp) expression, wing and leg development, and the elongation of the cells in the epidermis [32]. dpp expression in the tergite corresponds to the midline pigment stripe, and ectopic expression of dpp in pupae expands this stripe [32]. Furthermore, dpp signal transduction is potentiated by dally [33]. Additionally, dpp and Epidermal growth factor receptor (Egfr) signaling work synergistically to specify the lateral tergite cell fate [32. ru, also known as Rhomboid-3 (Rho-3), activates Egfr signaling and thus may help determine epidermal cell fate in the developing tergites; however, there are several Rho proteins capable of this activation [34]. Fili is a transmembrane protein that is involved with apoptosis in the wing imaginal disc and we speculate may facilitate proper tergite differentiation during metamorphosis [35].
Four validated candidate genes are involved with vesicle formation and transport: pinstripe (pns, CG7852, which describes the vertical stripe of pigment remaining on T6 in the RNAi knockdown), Efa6, Klp61F, and klar. pns is predicted to have Rab guanyl-nucleotide exchange factor activity, which facilitates vesicle transport by activating Rab proteins [36,37]. Rab5 works in conjunction with Megalin to remove the Yellow protein from the tanning D. melanogaster wing [38]. Efa6 is also involved with Rab signaling and vesicle mediated transport [36]. Klp61F is a kinesin motor, and klarsicht (klar) regulates microtubule-dependent vesicle transport along microtubules. Both could be involved in transporting vesicles of enzymes and/or dopamine-derivatives to and from the cuticle. Together these genes may represent components of the little-known transport mechanism for cuticle tanning in D. melanogaster.
farmer (frm, CG10625—the not quite fully developed stripes of pigment on tergites 5 and 6 are similar to the tan lines on the arms of a farmer after much time spent in the sun), kkv, and sinu, and loco may affect cuticle development and structure. frm is a cuticle structural protein [36]. kkv is one of two chitin synthase genes in D. melanogaster [39]. sinu is a claudin required for septate junction organization, cell-cell adhesion, and proper localization of proteins involved in chitin filament assembly in D. melanogaster [40]. loco regulates G protein signaling, and Gγ1 signaling is required for proper septate junction protein localization including sinu [41,42]. These proteins may help maintain the structural integrity of the adult cuticle and our study shows that when perturbed, they affect pigmentation.
Sucb and ph may affect the organism more broadly. Sucb is a succinate-CoA synthetase in the Krebs cycle [36]. It is plausible that variation in energy production due to genetic variation in key enzymes could indirectly affect variation in adult pigmentation by altering resources available for cuticle development. ph is a CD36 homologue, and dipteran CD36 family members may have roles in transport of cholesterol and steroids during ecdysone synthesis [43]. Since ecdysone is required for proper insect development and molting, disrupted transport of ecdysone precursors may explain the severe RNAi phenotype for this gene.
In summary, we provide evidence that genetic variation at a number of steps in regulatory, developmental, and transport may pathways contribute to natural variation in abdominal pigmentation. These findings exemplify the pleiotropic nature of these genes which may limit their potential as adaptive targets [44–46]. Several of the mutant and RNAi knockdown lines were lethal or had strong debilitating effects providing some support of this. It is known that the large-effect genes, t and e, are pleiotropic as well [23]. The difference may be that not all pigmentation genes have the necessary regulatory modules to alleviate pleiotropic effects. However, these candidate genes may contain tissue - or stage-specific gene regulatory architectures since most of the GWAS associated SNPs are in intronic and intergenic regions. Furthermore, a distinction should be made between pleiotropic genes and pleiotropic variants. A given gene may be pleiotropic, while particular variants within that gene may not be [47]. Additionally, in the DGRP lines allelic variants are homozygous. In nature, alleles with detrimental effects may be tempered in the heterozygous state or epistatic interactions may arise with differing combinations of polymorphic loci.
Consistent with other DGRP studies, we identified many more genetic variants associated with pigmentation than previous studies [48–51]. We suspect earlier studies may have only had the ability to identify the major effect loci and missed the more polygenic variation at these other loci. Most used only a small number of fly lines and thus interrogated a smaller sample of allelic variation, analyzed only known pigmentation genes, or the limited sample size of the mapping population gave reduced statistical power to detect variants with smaller effects. For example, the Winter's Lines, a panel of 144 recombinant inbred lines used to map the effect of bab1 on pigmentation in female D. melanogaster, were generated from only two gravid wild caught females [2]. The study of Bastide et al. [9] pooled individuals with extreme phenotypes for sequencing. This approach may filter out variants that lead to intermediate phenotypes and select for large effect variants. Pool and Aquadro focused only on e sequences among the 25 African lines eliminating any possibility of identifying additional loci [16]. The DGRP is more representative of natural populations and harbors many more polymorphic loci that may contribute to phenotypic variation and evolution [52]. Given both the population sample and the genome-wide coverage of polymorphisms, this study is perhaps the most comprehensive analysis of variation in Drosophila pigmentation to date.
It is important to acknowledge that gene expression knockdown and mutant analyses are only an approximate confirmation of causative SNPs. Genes implicated by the GWA analyses that do not confirm in these functional tests may be true positives and contribute to variation in pigmentation, but they do not change pigmentation when gene expression is reduced. Future studies should test effects of individual SNPs and further characterize the mechanisms though which the candidate genes affect variation in pigmentation; their potential interactions with variants in the candidate genes with major effects; and their allele frequency distributions in different populations. These studies will help elucidate the contribution of these novel variants to adaptive phenotypic evolution or whether they are population-specific deleterious variants that are maintained segregating by mutation-selection balance.
Our results open the door for new hypotheses to be tested on the transport of dopamine derivatives and conjugates from the hemolymph to the cuticle, the formation and movement of vesicles within epidermal cells, the mechanisms of regulatory and developmental pathways during tergite differentiation, the interactions of chitin filaments with cell adhesion and cuticle proteins, and how metabolic and hormonal regulation could lead to variation in pigmentation. Genetic variants that affect these processes could potentially serve as targets of adaptive evolution or sexual selection in natural populations. This study is a start. However, much more work is needed to draw mechanistic inferences about these novel candidate genes and their contributions to the evolution of pigmentation.
Materials and Methods
Drosophila stocks and phenotyping
The DGRP consists of 205 inbred lines with complete genome sequences. We scored female flies of 175 DGRP lines—aged 5 to 8 days—for the proportion of melanization on abdominal tergites 5 and 6. Two independent replicates for each DGRP line were reared and five individuals were scored from each replicate vial (N = 10 flies per line). The flies were reared in vials at a controlled adult density (CAD) of 10 males and 10 females on cornmeal-molasses-agar medium at 25°C, 75% relative humidity, and a 12-h light-dark cycle. The parental generation was allowed to lay eggs for 3 days. Each fly was visually assessed by a single observer for the percentage of brown/black melanin covering each tergite; the scores ranged from 0 for no dark pigmentation to 4 for 100% dark pigmentation in increments of 0.5.
Statistical and quantitative genetic analyses
We partitioned variation in pigmentation into genetic and environmental components using an ANOVA model of form Y = μ + L + T + L×T + R(L×T) + ε, where Y is phenotype, μ is the overall mean, L is the random effect of line, T is the fixed effect of tergite, R is the random effect of replicate vial, and ε is the residual. We also performed reduced ANOVAs separately for each tergite of form Y = μ + L + R (L) + ε. We estimated variance components for the random effects using REML. We computed the broad-sense heritability (H2) of pigmentation for each tergite separately as H2 = σ2L/ (σ2L + σ2ε), where σ2L is the among-line variance component and σ2ε is the error variance. We computed the genetic correlation between the tergites (rT5,T6) as CovT5,T6/ σLT5σLT6, where CovT5,T6 is the covariance in pigmentation score between tergites 5 and 6. All analyses were performed with version 9.3 of the SAS System for Windows (2013 SAS Institute Inc.).
Genome-wide association analysis
To identify genomic regions harboring variants contributing to natural variation in the proportion of tergite melanization, we conducted a GWA study for each tergite. The DGRP lines are also segregating for Wolbachia infection and for the following common inversions: In(2L)t, In(2R)NS, In(3R)P, In(3R)K, and In(3R)Mo. We performed GWA studies in two stages. In the first stage, we adjusted the line means for the effects of Wolbachia infection and major inversions. We then used the adjusted line means to fit a linear mixed model in the form of Y = Xb + Zu + e, where Y is the adjusted phenotypic values, X is the design matrix for the fixed SNP effect b, Z is the incidence matrix for the random polygenic effect u, and e is the residual. The vector of polygenic effects u has a covariance matrix in the form of Aσ2, where σ2 is the polygenic variance component. We fitted this linear mixed model using the FastLMM program (version 1.09) [53]. We performed these single marker analyses for the 1,897,337 biallelic variants (SNPs and indels) with minor allele frequencies ≥ 0.05 whose Phred scale quality scores were at least 500 and genotypes whose sequencing depths were at least one and genotype quality scores at least 20 [15]. All segregating sites within lines were treated as missing data. Additionally, we performed single marker tests for association on line means that were adjusted for the effects of Wolbachia infection and major inversions but not corrected for the relationship matrix. Significant variants were annotated using the 5.49 Release of the Flybase annotations.
Variance in pigmentation explained by top variants
For each variant, we calculated two variance components. First, to calculate the variance explained by a variant without adjusting for variants in known pigmentation genes, we fitted a linear model for the adjusted line means for only the focal variant and used the R2 of the model to represent the variance explained by it. Second, to calculate additional variance explained by a focal variant after accounting for variants in known pigmentation genes, we first used stepwise selection to select the top associations for each pigmentation gene (tan, ebony, or bab1), requiring P-values to be smaller than 10–5 if more than one variant entered the model, and no P-value requirement if there was only one variant. The R2 of this baseline model (different for each of the four traits) is the variance explained by the pigmentation genes. We added each focal variant to the baseline model and calculated the difference between the R2 of the new model and the R2 of the baseline model, which represented the additional variance explained by the variant after accounting for the pigmentation genes. To calculate total variance explained by all significant variants, we used a mixed model approach because of the large number of variants. We computed the variance/covariance matrix based on the genotype matrix and estimated the variance components using the rrBLUP R package.
Validation of candidate genes
We tested 12 of the 13 genes implicated by the T6 pigmentation GWA analysis, none of which were previously known to affect variation in pigmentation in D. melanogaster: CG33298, Fili, Vmat, mwh, Klp61F, CG9134, CG7852, CG1887, klar, Glut1, Efa6, and btn. From the T5 pigmentation GWA analysis, we selected candidate genes that (1) had an Exelixis mutant [20] or VDRC RNAi [21] line available at the time of the study; (2) are involved in development, especially of the cuticle or epidermis, or pigmentation according to FlyBase and the available literature; and (3) show mRNA expression patterns similar to the regulatory genes, bab1 and Dsx, and genes in the pigmentation biosynthesis pathway (such as t and e), a peak of expression at 24 hr after puparium formation and 2–4 days after puparium formation, respectively, according to the modENCODE tissue and temporal expression data [27,52]. This resulted in 16 additional candidate genes: ru, CG10625, sinu, Sucb, dally, CG32052, Nc, Cerk, kkv, CG15803, loco, TwdlC, kay, CG1340, CG42594, and CG42340. For each candidate gene, we tested either an Exelixis transposon insertion line [20], a VDRC RNAi line [21], or when possible, both a mutation and RNAi construct. We assessed the proportion of melanization for both T5 and T6 for all candidate genes.
We evaluated 15 Exelixis transposon insertion lines: CG33298d10678a, Filif04573, mwhd01620, Klp61Ff02870, CG9134e00088, CG7852c04511, klard05910, Glut1d05758, Efa6f03476, CG10625e01211, Sucbe01940, dallyf01097, kkvc06225, locod09879, and kayf02002. The Exelixis progenitor w1118 line was used as a control. The KK and GD library progenitor lines were used to make control crosses for the RNAi knockdown experiments. Males from the GAL4 driver line were crossed to virgin females of the VDRC UAS line for all crosses. Three GAL4 driver lines were used for the RNAi crosses. All VDRC UAS lines were crossed with the full-body tubulin-GAL4/Sb driver and a pannier-GAL4 driver (y1 w1118; P{w+mW.hs = GawB}pnrMD237/TM3, P{w+mC = UAS-y.C}MC2, Ser1). In instances of lethality with the tubulin-GAL4/Sb driver, the lines were crossed to another full-body ubiquitin-GAL4/Cy driver. All GAL4-driver lines were obtained from the Bloomington, Indiana Drosophila Stock Center. We tested in total 26 RNAi knockdown constructs: CG33298, Fili, Vmat, mwh, Klp61F, CG9134, CG7852, CG1887, klar, Efa6, btn, ru, CG10625, sinu, dally, CG32052, Nc, Cerk, kkv, CG15803, loco, TwdlC, kay, CG1340, CG42594, and CG42340.
We reared three independent replicates for each Exelixis transposon insertion line, for each RNAi cross and for the appropriate controls under the same conditions as the DGRP lines, but in 8 oz. bottles with a controlled adult density of 20 males and 20 virgin females. We scored the proportion of melanization on T5 and T6 for 50 5–8 day old female progeny per replicate (N = 150 flies per genotype) from each Exelixis line or RNAi cross. In a few instances where viability was low fewer than 50 individuals per replicate were scored: pnr-GAL4 x sinu (N = 23), ubi-GAL4 x CG1887 (N = 90), and ubi-GAL4 x klar (N = 95). Means of test lines were compared to those of the appropriate controls with a Dunnett's test, which corrects for multiple testing, using JMP Pro 10.0.0 (2012 SAS Institute Inc.)
Dissection and photography
After mutant lines and RNAi knockdown progeny were scored for pigmentation, they were preserved in a 3 : 1 ethanol/glycerol solution and stored at 4°C until dissection for imaging. The fly cuticles were dissected from the abdomen and mounted to a glass slide using Permount and a glass cover slip. All photographs were taken with an Olympus DP25 microscope camera on an Olympus SZ61 stereo microscope.
Supporting Information
Zdroje
1. Lande R (1980) Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34 : 292–305.
2. Kopp A, Graze RM, Xu S, Carroll SB, Nuzhdin S V (2003) Dimorphic Traits in Drosophila melanogaster. Genetics 787 : 771–787.
3. Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, et al. (2008) The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134 : 610–623. doi: 10.1016/j.cell.2008.06.052 18724934
4. Simpson SJ, Sword GA, Lo N (2011) Polyphenism in insects. Curr Biol 21: R738–R749. doi: 10.1016/j.cub.2011.06.006 21959164
5. True JR (2003) Insect melanism: the molecules matter. Trends Ecol Evol 18 : 640–647. doi: 10.1016/j.tree.2003.09.006
6. Wittkopp PJ, Carroll SB, Kopp A (2003) Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet 19 : 495–504. 12957543
7. Llopart A, Elwyn S, Lachaise D, Coyne JA (2002) Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution 56 : 2262–2277. 12487356
8. Carbone MA, Llopart A, DeAngelis M, Coyne JA, Mackay TFC (2005) Quantitative trait loci affecting the difference in pigmentation between Drosophila yakuba and Drosophila santomea. Genetics 171 : 211–225. 15972457
9. Bastide H, Betancourt A, Nolte V, Tobler R, Stöbe P, et al. (2013) A genome-wide, fine-scale map of natural pigmentation variation in Drosophila melanogaster. PLoS Genet 9: e1003534. doi: 10.1371/journal.pgen.1003534 23754958
10. Cooley AM, Shefner L, McLaughlin WN, Stewart EE, Wittkopp PJ (2012) The ontogeny of color: developmental origins of divergent pigmentation in Drosophila americana and D. novamexicana. Evol Dev 14 : 317–325. doi: 10.1111/j.1525-142X.2012.00550.x 22765203
11. Andersen SO (2010) Insect cuticular sclerotization: a review. Insect Biochem Mol Biol 40 : 166–178. doi: 10.1016/j.ibmb.2009.10.007 19932179
12. Moussian B (2010) Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol 40 : 363–375. doi: 10.1016/j.ibmb.2010.03.003 20347980
13. Wright TR (1987) The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet 24 : 127–222. 3124532
14. Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, et al. (2012) The Drosophila melanogaster Genetic Reference Panel. Nature 482 : 173–178. doi: 10.1038/nature10811 22318601
15. Huang W, Massouras A, Inoue Y, Peiffer J, Rámia M, et al. (2014) Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res 24 : 1193–1208. doi: 10.1101/gr.171546.113 24714809
16. Pool JE, Aquadro CF (2007) The genetic basis of adaptive pigmentation variation in Drosophila melanogaster. Mol Ecol 16 : 2844–2851. 17614900
17. Gallo SM, Gerrard DT, Miner D, Simich M, Des Soye B, et al. (2011) REDfly v3.0: toward a comprehensive database of transcriptional regulatory elements in Drosophila. Nucleic Acids Res 39: D118–D123. doi: 10.1093/nar/gkq999 20965965
18. Bickel RD, Kopp A, Nuzhdin SV (2011) Composite effects of polymorphisms near multiple regulatory elements create a major-effect QTL. PLoS Genet 7: e1001275. doi: 10.1371/journal.pgen.1001275 21249179
19. Rebeiz M, Pool JE, Kassner VA, Aquadro CF, Carroll SB (2009) Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population. Science 326 : 1663–1667. doi: 10.1126/science.1178357 20019281
20. Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, et al. (2004) A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet 36 : 283–287. 14981521
21. Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 : 151–156. 17625558
22. Wittkopp PJ, True JR, Carroll SB (2002) Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 129 : 1849–1858. 11934851
23. True JR, Yeh S-D, Hovemann BT, Kemme T, Meinertzhagen I, et al. (2005) Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet 1: e63. 16299587
24. Rogers WA, Grover S, Stringer SJ, Parks J, Rebeiz M, et al. (2014) A survey of the trans-regulatory landscape for Drosophila melanogaster abdominal pigmentation. Dev Biol 385 : 417–432. doi: 10.1016/j.ydbio.2013.11.013 24269556
25. Kramer KJ, Hopkins TL (1987) Tyrosine metabolism for insect cuticle tanning. Arch Insect Biochem Physiol 6 : 279–301.
26. Hopkins TL, Morgan TD, Mueller DD, Tomer KB, Kramer KJ (1995) Identification of catecholamine β-glucosides in the hemolymph of the tobacco hornworm, Manduca sexta (L.), during development. Insect Biochem Mol Biol 25 : 29–37.
27. Hopkins TL, Morgan TD, Kramert KJ (1984) Catecholamines in haemolymph and cuticle during larval, pupal, and adult development of Manduca sexta (L.). Insect Biochem 14 : 533–540.
28. Fukami Y, Lipmann F (1982) Purification of a specific reversible tyrosine-O-phosphate phosphatase. Proc Natl Acad Sci U S A 79 : 4275–4279. 6181504
29. Chen PS, Mitchell HK, Neuweg M (1978) Tyrosine glucoside in Drosophila busckii. Insect Biochem 8 : 279–286.
30. modENCODE Consortium, Roy S, Ernst J, Kharchenko P V, Kheradpour P, et al. (2010) Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330 : 1787–1797. doi: 10.1126/science.1198374 21177974
31. Jurgens G, Wieschaus E, Nusslein-Volhard C, Kluding H (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux’s Arch Dev Biol 193 : 283–295.
32. Kopp A, Blackman RK, Duncan I (1999) Wingless, decapentaplegic and EGF receptor signaling pathways interact to specify dorso-ventral pattern in the adult abdomen of Drosophila. Development 126 : 3495–3507. 10409497
33. Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, et al. (2003) Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development 130 : 1515–1522. 12620978
34. Urban S, Lee JR, Freeman M (2002) A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J 21 : 4277–4286. 12169630
35. Adachi-Yamada T, Harumoto T, Sakurai K, Ueda R, Saigo K, et al. (2005) Wing-to-leg homeosis by spineless causes apoptosis regulated by fish-lips, a novel leucine-rich repeat transmembrane protein. Mol Cell Biol 25 : 3140–3150. 15798200
36. St Pierre SE, Ponting L, Stefancsik R, McQuilton P (2014) FlyBase 102—advanced approaches to interrogating FlyBase. Nucleic Acids Res 42: D780–D788. doi: 10.1093/nar/gkt1092 24234449
37. Vázquez-Martínez R, Malagón MM (2011) Rab proteins and the secretory pathway: the case of rab18 in neuroendocrine cells. Front Endocrinol 2 : 1.
38. Riedel F, Vorkel D, Eaton S (2011) Megalin-dependent yellow endocytosis restricts melanization in the Drosophila cuticle. Development 138 : 149–158. Available: http://dev.biologists.org/content/138/1/149.short. Accessed 7 May 2014. doi: 10.1242/dev.056309 21138977
39. Moussian B, Schwarz H, Bartoszewski S, Nusslein-Volhard C (2005) Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol 264 : 117–130. 15747378
40. Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ (2004) Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol 164 : 313–323. 14734539
41. Yu F, Wang H, Qian H, Kaushik R, Bownes M, et al. (2005) Locomotion defects, together with Pins, regulates heterotrimeric G-protein signaling during Drosophila neuroblast asymmetric divisions. Genes Dev 19 : 1341–1353. 15937221
42. Lin YR, Kim K, Yang Y, Ivessa A, Sadoshima J, et al. (2011) Regulation of longevity by regulator of G-protein signaling protein, Loco. Aging Cell 10 : 438–447. doi: 10.1111/j.1474-9726.2011.00678.x 21255223
43. Herboso L, Talamillo A, Pérez C, Barrio R (2011) Expression of the Scavenger Receptor Class B type I (SR-BI) family in Drosophila melanogaster. Int J Dev Biol 55 : 603–611. doi: 10.1387/ijdb.103254lh 21948708
44. Jeong S, Rebeiz M, Andolfatto P, Werner T, True J, et al. (2008) The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132 : 783–793. doi: 10.1016/j.cell.2008.01.014 18329365
45. Carroll SB (2008) Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134 : 25–36. doi: 10.1016/j.cell.2008.06.030 18614008
46. Stern DL (2000) Perspective: Evolutionary developmental biology and the problem of variation. Evolution (N Y) 54 : 1079–1091.
47. Carbone MA, Jordan KW, Lyman RF, Harbison ST, Leips J, et al. (2006) Phenotypic variation and natural selection at Catsup, a pleiotropic quantitative trait gene in Drosophila. Curr Biol 16 : 912–919. 16682353
48. Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, et al. (2012) The Drosophila melanogaster Genetic Reference Panel. Nature 482 : 173–178. doi: 10.1038/nature10811 22318601
49. Harbison ST, McCoy LJ, Mackay TFC (2013) Genome-wide association study of sleep in Drosophila melanogaster. BMC Genomics 14 : 281. doi: 10.1186/1471-2164-14-281 23617951
50. Weber AL, Khan GF, Magwire MM, Tabor CL, Mackay TFC, et al. (2012) Genome-wide association analysis of oxidative stress resistance in Drosophila melanogaster. PLoS One 7: e34745. doi: 10.1371/journal.pone.0034745 22496853
51. Swarup S, Huang W, Mackay TFC, Anholt RRH (2013) Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proc Natl Acad Sci U S A 110 : 1017–1022. doi: 10.1073/pnas.1220168110 23277560
52. Mackay TFC, Stone E A, Ayroles JF (2009) The genetics of quantitative traits: challenges and prospects. Nat Rev Genet 10 : 565–577. doi: 10.1038/nrg2612 19584810
53. Lippert C, Listgarten J, Liu Y, Kadie CM, Davidson RI, et al. (2011) FaST linear mixed models for genome-wide association studies. Nat Meth 8 : 833–835. doi: 10.1038/nmeth.1681 21892150
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



