-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCell Cycle Control by the Master Regulator CtrA in
In order to propagate, all living cells must ensure that their genetic material is faithfully copied and properly partitioned into the daughter cells before division. These coordinated processes of DNA replication and cell division are termed the “cell cycle” and are controlled by a complex network of regulatory proteins in all organisms. In the class Alphaproteobacteria, the regulation of the cell cycle is closely linked to cellular differentiation processes that are vital for survival in the environment. In these bacteria, the cell cycle regulator CtrA is thought to serve as the primary link between the coordination of the cell cycle and cellular differentiation. The alphaproteobacterium, Sinorhizobium meliloti, an important model symbiont of alfalfa plants, undergoes a striking cellular differentiation that is vital to the formation of an efficient symbiosis dedicated to the conversion of atmospheric nitrogen to biologically available organic nitrogen. However, the link between cellular differentiation and cell cycle control in S. meliloti has not been made. In this study, we showed that S. meliloti cells without CtrA are similar to the symbiotic form. By the identification of the genes whose expression is directly and indirectly controlled by CtrA, we found that CtrA regulates vital cell cycle processes, including DNA replication and cell division, but through different genetic pathways than in other alphaproteobacteria. We importantly show that the levels of CtrA protein are governed by an essential cell cycle regulated proteolysis, which may also be an important mode of CtrA down-regulation during symbiosis to drive cellular differentiation.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005232
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005232Summary
In order to propagate, all living cells must ensure that their genetic material is faithfully copied and properly partitioned into the daughter cells before division. These coordinated processes of DNA replication and cell division are termed the “cell cycle” and are controlled by a complex network of regulatory proteins in all organisms. In the class Alphaproteobacteria, the regulation of the cell cycle is closely linked to cellular differentiation processes that are vital for survival in the environment. In these bacteria, the cell cycle regulator CtrA is thought to serve as the primary link between the coordination of the cell cycle and cellular differentiation. The alphaproteobacterium, Sinorhizobium meliloti, an important model symbiont of alfalfa plants, undergoes a striking cellular differentiation that is vital to the formation of an efficient symbiosis dedicated to the conversion of atmospheric nitrogen to biologically available organic nitrogen. However, the link between cellular differentiation and cell cycle control in S. meliloti has not been made. In this study, we showed that S. meliloti cells without CtrA are similar to the symbiotic form. By the identification of the genes whose expression is directly and indirectly controlled by CtrA, we found that CtrA regulates vital cell cycle processes, including DNA replication and cell division, but through different genetic pathways than in other alphaproteobacteria. We importantly show that the levels of CtrA protein are governed by an essential cell cycle regulated proteolysis, which may also be an important mode of CtrA down-regulation during symbiosis to drive cellular differentiation.
Introduction
The alphaproteobacterium Sinorhizobium meliloti can thrive in the soil as a free-living organism or as a nitrogen-fixing symbiotic partner with compatible legume hosts [1]. The S. meliloti-legume symbiosis involves multiple developmental stages, during which the bacteria coordinate their cell proliferation with the development of the host plant cells [2,3]. A key step in this symbiosis is the striking differentiation of S. meliloti cells into enlarged, polyploid (16–32 copies of the genome) nitrogen-fixing bacteroids within the specialized host cells that comprise the developing nodule [4]. Differentiation of bacteroids in S. meliloti-legume symbiosis is driven in part by nodule specific cysteine-rich peptides (NCRs) that are produced by the host legume [5,6]. These peptides, such as NCR247, can provoke in free-living cells many of the changes associated with bacteroid differentiation including the increase in cell size and endoreduplication of the genome [7]. The uncoupling of DNA replication from cell division in S. meliloti during symbiosis stands in stark contrast to the cell cycle of free-living S. meliloti, where DNA replication is tightly coupled to cell division [8].
The involvement of the cell cycle regulatory network in cellular differentiation programs, such as cyst formation in Rhodospirillum centenum and the asymmetric division of Caulobacter crescentus, is a common theme in Alphaproteobacteria [9,10]. In C. crescentus and presumably in other alphaproteobacteria, cellular differentiation is largely governed by the response regulator CtrA [10–14]. C. crescentus divides asymmetrically to produce two morphologically different cells, a motile swarmer cell and a sessile stalked cell [15]. The two cell types are also distinct in their replicative capacities. The stalked cell, which lacks active CtrA, can immediately initiate DNA replication and re-enter the cell cycle, while in the swarmer cell, the origin of replication is bound and inhibited by phosphorylated CtrA resulting in a G1 arrest [16,17]. As a transcription factor, phosphorylated CtrA binds ca. 200 promoter regions and controls the transcription of about 95 genes over the course of the cell cycle, thereby modulating diverse processes including polar morphogenesis and cell division [18]. The expression, activity and stability of C. crescentus CtrA are highly regulated during the cell cycle through transcriptional regulation, phosphorylation and regulated proteolysis [19–27].
An essential, functional homolog of C. crescentus CtrA is present in S. meliloti and has been implicated in the symbiotic cellular differentiation program [28]. The genetic circuit controlling CtrA in S. meliloti at the transcriptional and posttranslational levels has been predicted using bioinformatics and all the regulatory factors identified in C. crescentus are conserved on the sequence level in S. meliloti [11]. Genetic experiments on a few of these putative regulators of CtrA have revealed a striking link between symbiosis and cell cycle regulation [29–33]. In addition, gene expression profiling of S. meliloti at different stages of the symbiosis indicated that expression of ctrA is strongly down-regulated in bacteroids once differentiation begins [34], and Western blot analysis of purified bacteroids revealed that CtrA protein levels are very low in mature bacteroids [33]. More specifically, down regulation of CtrA during symbiosis may be caused by exposure to NCR peptides, as in vitro treatment of S. meliloti with a sub-lethal dose of the NCR peptide, NCR247, significantly attenuates ctrA expression [35]. Collectively, these observations suggest that NCR peptides and perhaps other plant factors modulate the cell cycle in part by affecting the level of CtrA activity. It is thus crucial to gain a deeper understanding of the factors governing the S. meliloti cell cycle, especially of the cell processes governed by CtrA and the regulatory mechanisms controlling CtrA activity.
In this study, we sought to understand the mechanisms regulating cell cycle regulation in S. meliloti by analyzing the effects of CtrA depletion in S. meliloti free-living cells. We aimed to define the direct and indirect transcriptional regulons of S. meliloti CtrA and probing regulatory mechanisms, such as regulated proteolysis that possibly govern CtrA levels during the S. meliloti cell cycle. As global analysis of the CtrA transcriptional network has not been performed in detail in an alphaproteobacterium other than C. crescentus, this work provides the first insight into how this highly conserved genetic network can evolve to fit the distinct lifestyles of this diverse group of bacteria. Furthermore, the model of CtrA cell cycle regulation in S. meliloti developed in this work will be pivotal in the future elucidation of how the bacterial cell cycle is modulated by plant factors during the symbiosis.
Results
Depletion of CtrA in S. meliloti causes “bacteroid-like” cell cycle changes
The current working model of cell cycle regulation in Alphaproteobacteria is largely based on the regulatory interactions identified in C. crescentus, thanks to their level of conservation in other species [11]. Cell cycle regulation in C. crescentus, especially the governance of replicative and morphological asymmetry, is centered on the master regulator CtrA, which can inhibit DNA replication initiation by directly binding the origin of replication and also acts as a transcriptional regulator regulating hundreds of genes [18]. Although CtrA is highly conserved in S. meliloti, the activity of CtrA as a transcription factor and the role of CtrA in regulating cell cycle functions have not been investigated. Therefore, in order to more clearly understand the role of CtrA in cellular differentiation during symbiosis, we focused our investigation on understanding the role of CtrA as a master regulator of the cell cycle in S. meliloti.
Previous work has demonstrated that ctrA (SMc00654) is an essential gene in S. meliloti [28]. To study the effects of loss of CtrA function in S. meliloti we constructed a conditional CtrA depletion strain utilizing the pSRK expression system based on IPTG induction [36]. We transduced a marked deletion of ctrA (ΔctrA) to cells harboring an IPTG-inducible copy of ctrA on the pSRK-Km vector to create the strain ΔctrA-PlacctrA (see methods) (S1 Table). In the presence of 1mM IPTG, ΔctrA-PlacctrA cells were viable, exhibited normal morphology (Fig 1A–1C), and produced CtrA (S1 Fig). However, upon removal of IPTG, ΔctrA-PlacctrA cells ceased growth, lost viability and developed aberrant morphologies (Fig 1A–1C). These phenotypic changes coincided with the rapid decrease of CtrA protein over time to undetectable levels (Fig 1D). Cells depleted of CtrA were elongated, swollen and a fraction of cells became Y-shaped (Fig 1C). The phenotypic effects caused by CtrA depletion are reminiscent of the morphology of differentiated bacteroids in which CtrA is also absent (Fig 1C) [33]. Interestingly, despite these aberrant morphologies, cells depleted of CtrA maintained their membrane integrity, as indicated by the lack of propidium iodide incorporation (S2 Fig).
Fig. 1. CtrA plays an essential role in S. meliloti. 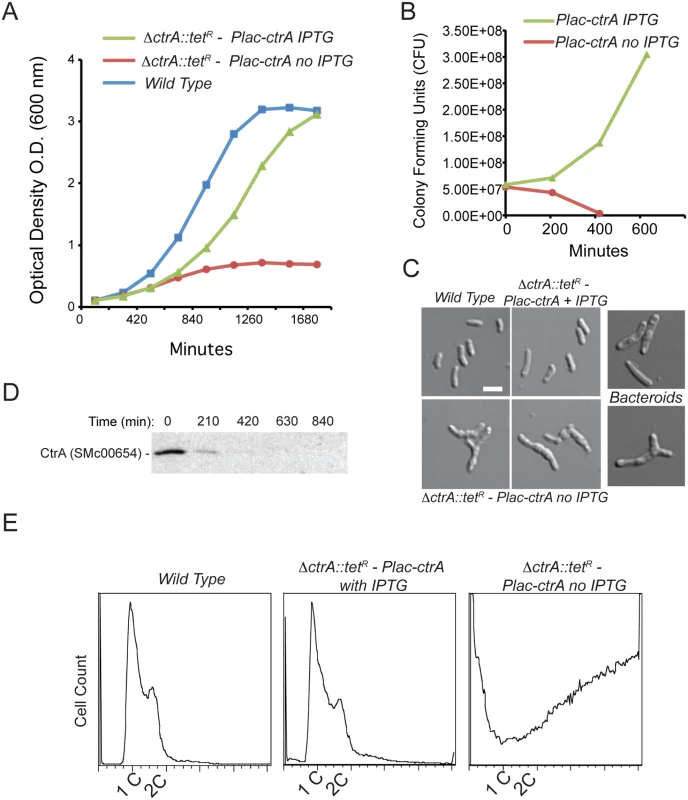
A. Optical density (OD600) of wild type S. meliloti and the CtrA depletion strain grown with and without IPTG, error bars represent standard errors. Mid-log phase cells depleted of CtrA show a stable OD level suggesting an impairment of normal growth. B. CFU of the experiments in (A) showing that cells without ctrA expression lost viability. C. Morphology of S. meliloti after 7 hours of CtrA depletion compared with wild type and bacteroid S. meliloti; cells appear elongated and enlarged (bar corresponds to 2 μm). D. Immunoblot analysis using anti-CtrA antibodies over a time course of CtrA depletion. E. FACS analysis of S. meliloti CtrA depletion strain after 8 hours +IPTG (control) and—IPTG (CtrA depleted) showing increased DNA content of up to 20 copies per cell in cells depleted of CtrA. To quantify the observed genome amplification we measured DNA content using flow cytometry and found a striking increase in DNA content in CtrA-depleted cells compared to wild type cells and ΔctrA-PlacctrA cells grown in the presence of IPTG (Fig 1E). CtrA-depleted cells contained ca. 20 times the amount of DNA per cell as ΔctrA-PlacctrA cells supplemented with IPTG as well as wild type, log phase S. meliloti cells. In CtrA-depleted cells the 1N and 2N peaks were lost indicating a complete de-coupling of DNA replication initiation and cell division, indicating that CtrA serves as an essential link between these two processes. Unlike the C. crescentus paradigm, the S. meliloti chromosomal origin of replication does not contain CtrA-binding motifs [11] and is not bound by CtrA protein (see S4 Table), so if CtrA governs DNA replication initiation it likely does so through an indirect, unknown mechanism. Finally, we used qPCR to determine the ratio of the three replicons that comprise the S. meliloti genome and found that the ratio between the replicons did not significantly change after 4 hours of CtrA depletion (S3 Fig). These observations indicate that CtrA function is required to equally repress the replication of all S. meliloti three replicons.
Collectively these data support the hypothesis down-regulation of CtrA activity during symbiosis could contribute to the elongation and genome amplification observed in differentiating bacteroids [28,33,35].
CtrA regulates the transcription of at least 126 genes in S. meliloti
To determine how the activity of CtrA as a transcription factor affects important cell cycle functions, we used microarray analysis to measure gene expression changes upon CtrA depletion in S. meliloti (S2 Table). For this experiment, exponential phase cultures of ΔctrA-Plac-ctrA S. meliloti grown in the presence of 1mM IPTG were washed and split into control (+IPTG) and ctrA depletion (–IPTG) sub-cultures. RNA was isolated from these cultures at different time points post-split and used for microarray-based gene expression analysis. We found that the expression of 126 genes changed significantly during CtrA depletion (Fig 2A, S3 Table). We validated the results by testing the expression of several key differentially expressed genes using qPCR and reporter lacZ fusions and found similar expression patterns as was detected by the microarray experiments (Fig 2B and S4A Fig). Hierarchical clustering of the expression data from each time point showed strong correlation between gene expression in control vs. CtrA depleted samples and a strong temporal effect of CtrA depletion on gene expression (Fig 2A).
Fig. 2. CtrA regulates the expression of at least 126 S. meliloti genes. 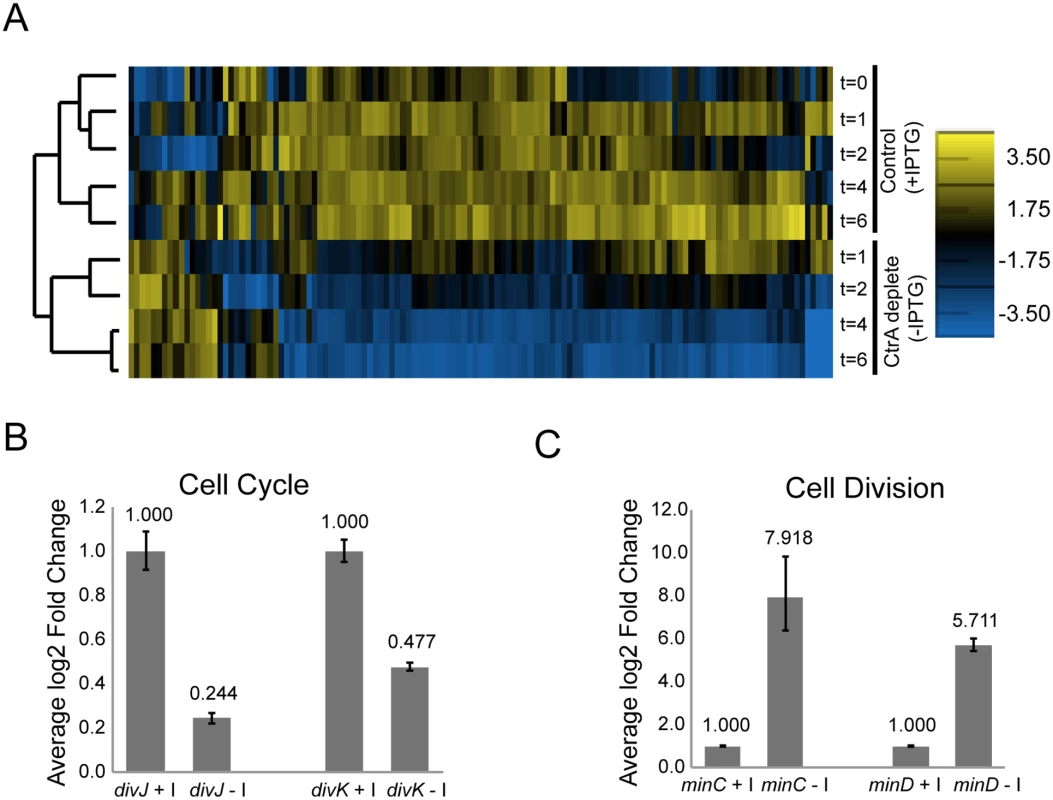
A. Hierarchical clustered expression profiles for 126 genes in cells expressing ctrA (control; +IPTG) and in cells depleted of ctrA (-IPTG) at several time points (t = 0, 1, 2, 4 and 6 hours) following the initiation of the—IPTG or +IPTG treatment. Normalized log2 expression levels are shown for each gene. The scale for expression level is located on the right. B. Fold change in divJ and divK expression in cells after depletion of CtrA (-I, IPTG) for two hours relative to control cells expressing CtrA (+I). Expression of divJ and divK in each sample was normalized to the expression of the control gene smc00128. Shown are data from a representative biological replicate. Error bars indicate standard error. C. Fold change in minC and minD expression in cells after depletion of CtrA (-I, IPTG) for four hours relative to control cells expressing CtrA (+I). Data normalization was performed as in B. Shown are data from a representative biological replicate. Error bars indicate standard error. Differentially expressed genes were found on each of the three S. meliloti replicons, with the majority (80%) located on the chromosome (S2 Table). Most of the genes (~86%) affected by depletion of CtrA were down-regulated, and the degree of down-regulation was greater the longer CtrA was depleted (Fig 2A). Thus, CtrA primarily functions as a positive regulator of transcription in S. meliloti. Among these positively regulated genes are many cell cycle regulators (i.e. ctrA, divJ, divK, sciP), motility genes (i.e. mcp, che, flg, flaAB, fli), cell envelope components and several hypothetical proteins. The transcription of the genes encoding cell cycle regulators DivJ and DivK was significantly down-regulated after 2 hours of CtrA depletion in both the microarray and qPCR experiments (Fig 2B), strongly indicating that CtrA serves as a transcriptional activator of these two genes. Conversely, qPCR analysis revealed that expression of the cell division regulators minC and minD was strongly up-regulated in the absence of CtrA, indicating that CtrA is a transcriptional repressor of this operon (Fig 2B and 2C). The effect of CtrA depletion on the expression of minCD was particularly interesting because MinCD is a strong inhibitor of FtsZ ring formation and overexpression of MinCD in S. meliloti inhibits cell division [37]. Therefore, the increased expression of minCD may contribute to the block in cell division in cells depleted of CtrA.
Analysis of CtrA binding sites by Chromatin Immunoprecipitation—Deep Sequencing (ChIP-Seq)
To discover the chromatin regions directly bound by CtrA in S. meliloti, we used ChIP-Seq analysis [38] (Materials and Methods). Starting from an exponential culture of S. meliloti cells, CtrA cross-linked DNA fragments of an average length of 300 base pairs were immunoprecipitated with antibodies raised against C. crescentus CtrA (CC3035), which can specifically bind S. meliloti CtrA [33]. Immunoprecipitated DNA was then deep-sequenced producing millions of ca. 50 nucleotide reads, which were mapped onto the S. meliloti genome to create a distribution of the number of reads per nucleotide (Fig 3A and S4 Table that shows the list of the 198 peaks across all three S. meliloti replicons). Correlating with our gene expression data, the chromosome contained most of the peaks (76%) while pSymB and pSymA contained only 15% and 10%, respectively. CtrA binding sites were mostly present in intergenic regions (79%), in line with a predicted regulatory role and with the microarray results [39] (see next section for more details).
Fig. 3. ChIP-Seq analysis reveals direct targets of CtrA. 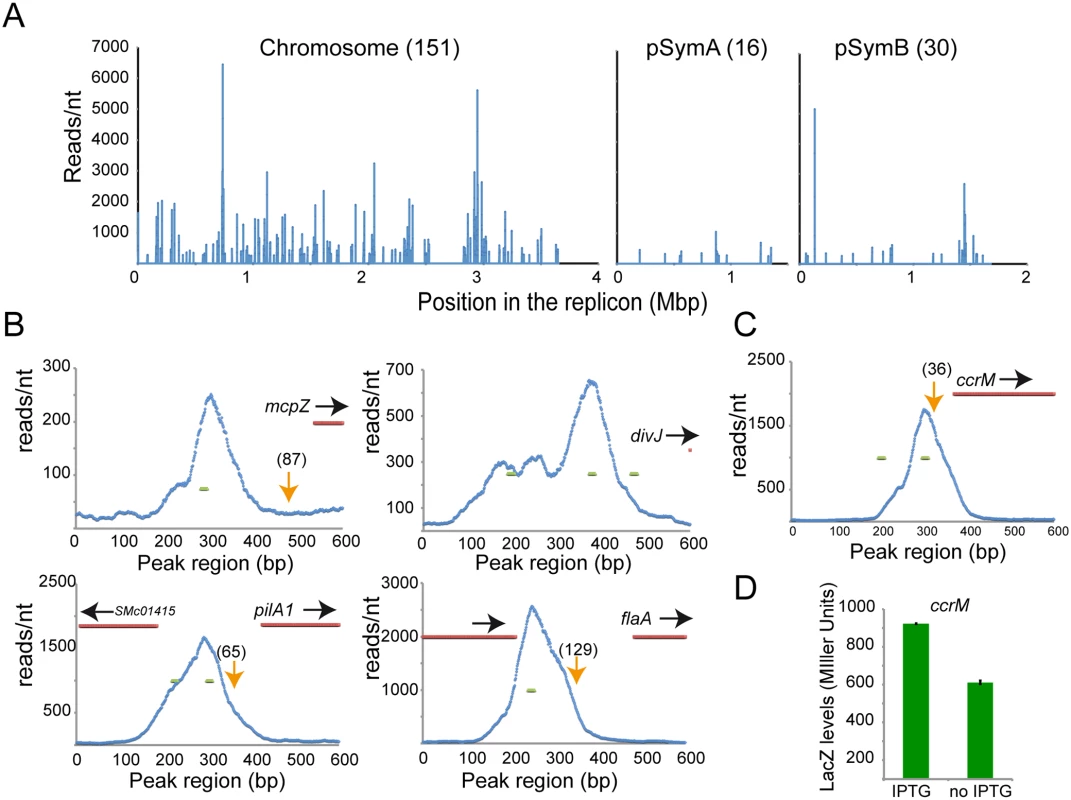
Genes directly regulated by CtrA. A. Representation of all CtrA binding sites in the three circular replicons of S. meliloti (here represented as linear starting from the origin of replication). B. Promoter region of several genes detected by microarrays. Transcriptional start sites, previously defined [39], are represented as orange arrows (numbers between brackets represent the distance form ATG). In blue the plot of reads per nucleotide measured by ChIP-Seq analysis in a 600bp long region including the beginning of the coding sequence (in red). Green lines represent predicted CtrA binding site [11]. C. ChIP-Seq of the ccrM promoter region. In blue the plot of reads per nucleotide measured by ChIP-Seq analysis. Green lines represent predicted CtrA binding site. D. Beta-galactosidase activity assay using a LacZ fusion of the ccrM promoter in cells (BM249) after depletion of CtrA (no IPTG) for two hours relative to control cells expressing CtrA (+IPTG). Error bars indicate standard error. We identified CtrA binding sites in the upstream regulatory regions of several key regulators of flagellum, pili, chemotaxis and cell cycle (Fig 3B) that were also identified by the microarray analysis. In particular, CtrA binding sites were detected in the promoter regions of mcpZ, pilA1, flaA and divJ and these binding sites overlapped with regions containing previously identified CtrA consensus sequences [11]. In order to confirm the direct transcriptional control of these genes by CtrA, their promoters were fused with lacZ and tested for their dependency on CtrA. Results showed that CtrA is a direct positive regulator of those genes (S4A Fig). Several genes were found to be bound by CtrA in the ChIP-Seq experiment (i.e. rcdA, cpdR1, ccrM and pleC), but not found to be differentially regulated in the CtrA depletion microarray. It is possible that these genes are direct transcriptional targets of CtrA, but the effect of CtrA depletion on their expression was missed due to the stringent cutoffs of the microarray data analysis (see Materials and Methods). These genes could also be subject to multiple levels of transcriptional regulation, which could compensate for the absence of CtrA in the system. Therefore, we used quantitative PCR and lacZ fusions to independently measure the effect of CtrA depletion on the expression of rcdA, cpdR1, ccrM and pleC. We found that ccrM, whose promoter was bound by CtrA in the ChIP-Seq analysis (Fig 3C), was significantly downregulated (Fig 3D) while pleC, cpdR and rcdA gave no significant changes consistent with the microarray analysis (S4 Fig).
Identification of the direct and indirect regulons of S. meliloti CtrA
Combining our gene expression data from the CtrA depletion microarray and the CtrA ChIP-Seq analysis we were able to identify both the direct and indirect regulons of CtrA in S. meliloti. A total of 54 genes were both differentially expressed upon CtrA depletion and bound by CtrA in the ChIP-Seq analysis. These genes represent the experimentally determined direct regulon of S. meliloti CtrA and include genes encoding components of the cell envelope, motility and chemotaxis regulators, and signaling proteins (Fig 4A). The remaining 72 genes, which were differentially regulated upon CtrA depletion but not bound by CtrA in the ChIP-Seq experiment, comprise the indirect CtrA regulon in S. meliloti (Fig 4B). The proteins encoded by these genes are involved in many different cellular functions, with the most represented functional groups being motility and chemotaxis genes, metabolism genes and hypothetical genes (Fig 4B).
Fig. 4. Expression profiles of direct and indirect targets of CtrA upon CtrA depletion. 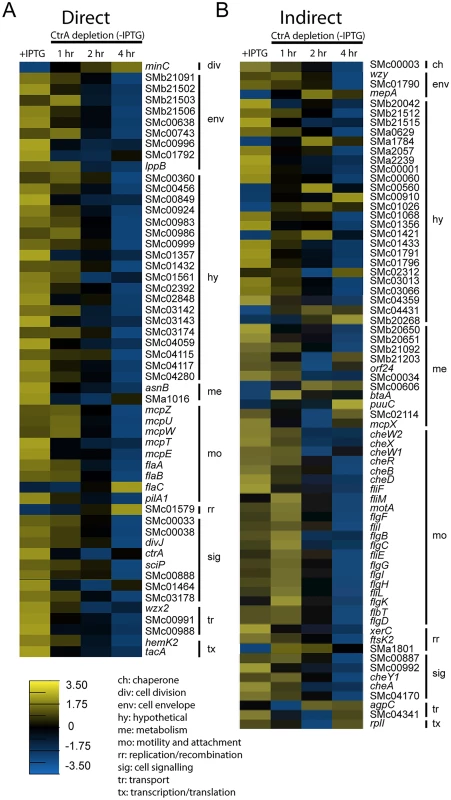
Expression profile of genes directly (A) and indirectly (B) controlled by CtrA. Shown are the average log2 expression levels for each gene in control cells (+IPTG) and the average log2 expression levels for each gene across each time point in cells depleted of CtrA (-IPTG). The scale for expression level is at the bottom of figure panel. Genes are grouped by functional classification explained in the legend on the bottom. Taking advantage of previous analysis of transcription start sites (TSSs) in S. meliloti [39] we mapped the ChIP-seq peaks of the 54 genes of the direct regulon of CtrA with respect of their TSSs (S8 Table). Out of 54, 36 genes had a TSS downstream the ChIP-seq peak; also we mapped the predicted CtrA binding sites, defined as full or half sites as previously described [18], identifying CtrA predicted motifs in 47/54 promoters. This analysis suggests that ChIP-seq data are consistent with CtrA binding promoters of genes, whose expression change was detected by microarrays.
The majority of motility and chemotaxis genes are indirect targets of CtrA with the exception of the methyl-accepting chemotaxis genes mcpT, mcpU, mcpW and mcpZ; the flagellin genes flaA, flaB and flaC; and the pili gene pilA1. The genes encoding the primary flagellar apparatus of S. meliloti (i.e. flgBCDH and fliEFIL) were indirectly regulated by CtrA (Fig 4B), which is different from the direct regulation of these genes observed in C. crescentus [18]. This observation reinforces the hypothesis of a transcriptional regulatory hierarchy of S. meliloti flagellar and chemotaxis genes [40] and suggests that CtrA regulates the transcription of most of these genes with the exception of fla and mcp genes indirectly through secondary regulators. Because the promoter of the two-component response regulator rem contained a CtrA-binding motif and Rem regulates flagellar and chemotaxis genes, it was postulated that Rem could be the intermediate regulator [41]. However, rem was not differentially expressed in our CtrA depletion microarray experiment, nor was it directly bound by CtrA in our ChIP-Seq analysis, suggesting that CtrA likely acts through a different secondary regulator to control the transcription of flagellar and chemotaxis genes during the cell cycle (S4B Fig) [40,41].
In C. crescentus, CtrA directly activates the expression of antagonists of CtrA function (e.g. cpdR, rcdA, clpP, sciP, and divK) [11,27]. Interestingly, we found that only the direct regulation of sciP and divJ by CtrA was conserved in S. meliloti. SciP is an important modulator CtrA activity in C. crescentus swarmer cells [42,43]. Previous work has shown that transcription of sciP is cell cycle regulated in S. meliloti and, like in C. crescentus, activated only in the later stages of the cell cycle [40]. It is interesting that although S. meliloti does not exhibit the same level of morphological asymmetry as C. crescentus, the cell cycle and CtrA mediated regulation of SciP is conserved between the two species. Future studies on the function of SciP will provide insights on how this protein regulates CtrA function and the cell cycle. In contrast to sciP, the direct regulation of rcdA and cpdR1, which encode homologs of two proteins involved in regulation of CtrA proteolysis in C. crescentus by CtrA, was not conserved in S. meliloti. Although our ChIP-Seq analysis detected CtrA binding sites within the promoter regions of rcdA and cpdR1, no transcriptional effect was observed under CtrA depletion conditions (S4C Fig). Also unlike the C. crescentus paradigm, our data indicate that CtrA indirectly regulates divK transcription. The architecture of transcriptional control by CtrA on the DivK module in S. meliloti (divJ and cbrA are directly - while divK is indirectly-controlled) is very different from C. crescentus control by CtrA, which is only on divK [27]. Thereby this complex architecture gives an additional degree of freedom in the primary negative feedback loop that regulates CtrA function in S. meliloti.
We also found that CtrA directly regulates the expression of 19 hypothetical proteins and indirectly regulates the expression of 24 hypothetical proteins in S. meliloti (Fig 4B). Ortholog analysis using Microbes Online [44] revealed that orthologs of most of these hypothetical genes are not present in C. crescentus, except for SMc00910, SMc02312 and SMc02848 (CC0705, CC2340 and CC3721, respectively in C. crescentus). However these genes are not regulated by CtrA in C. crescentus [18]. It will be especially interesting to determine the role of these genes in the cell cycle and physiology of S. meliloti in future work.
Our data also revealed that minC and minD are the only characterized cell division genes directly controlled by CtrA in S. meliloti (Fig 4A). The analysis revealed that CtrA is a repressor of minCD transcription, strongly suggesting that CtrA contributes to the expression pattern of minCD observed during the S. meliloti cell cycle [30]. In S. meliloti and many other bacteria, MinC and MinD repress cell division by inhibiting FtsZ polymerization and Z-ring formation [45]. In S. meliloti, CtrA may promote FtsZ polymerization and cell division by repressing minCD in predivisional cells. Placement of the Z-ring is not regulated by the Min system in C. crescentus and instead CtrA directly controls the transcription of ftsQA [18,46,47]. Thus CtrA regulation of cell division in S. meliloti may have specifically evolved to control FtsZ polymerization indirectly through the Min system.
In order to test this possibility we used M12 phage to transduce a min operon deletion cassette [37] into the ctrA depletion strain (BM249). Since the min operon is dispensable for proper growth of S. meliloti [37] this strain (EB1441) was viable when grown in 1mM IPTG. We hypothesized that deletion of the min cassette may partially rescue the arrested cell division phenotype of the ctrA depletion strain, however without supplemental IPTG EB1441 was unable to divide normally (no colonies were recovered after 7 days transducing the min::Spec without IPTG). However, when EB1441 was grown in 1mM IPTG and then transferred in to medium lacking IPTG (CtrA-loss of function) EB1441, similar cell division defects as the ctrA depletion strain were observed (S7 Fig). This result indicates that, although the overexpressed min system may contribute to the cell division defect of the CtrA depletion, it is not the only mechanism involved. For example, nucleoid occlusion may serve as a potent mechanism to block cell division, especially due to the severe amplification of the genome in CtrA depleted cells [48]. Moreover, CtrA may act on cell division through unknown genes of its regulon that may have an important role in S. meliloti cell division control.
Regulated proteolysis, but not transcriptional autoregulation is an important mechanism of CtrA regulation in S. meliloti
Bioinformatic prediction [11], our ChIP-Seq experiment and DNase I footprinting analysis [28] identified several CtrA binding sites in both the P1 and P2 promoter region of ctrA (Fig 5B). To determine if the ctrA auto-regulation observed in C. crescentus was conserved in S. meliloti, the lacZ fusions of ctrA P1 and P2 were tested in the ctrA depletion strain (Fig 5A). Upon ctrA depletion, both P1 and P2 showed mild changes of expression compared to non-depleted cells suggesting that, differently from C. crescentus, S. meliloti CtrA does not strongly activate its own promoters (Fig 5C). However, we observed a mild but significant decrease of CtrA expression by P1 in CtrA depleted cells compared to cells supplemented with IPTG, suggesting that CtrA may positively regulate its own transcription at P1, but CtrA depletion had no significant effect on transcription from P2 (Fig 6A). Thus, contrary to C. crescentus where P2 is strongly regulated by CtrA, S. meliloti CtrA only weakly activates its P1 promoter. Therefore, it is likely that other factors are involved in the transcriptional regulation of CtrA in S. meliloti, and future work will focus on identification of these regulators.
Fig. 5. Structure of the ctrA promoter in S. meliloti. 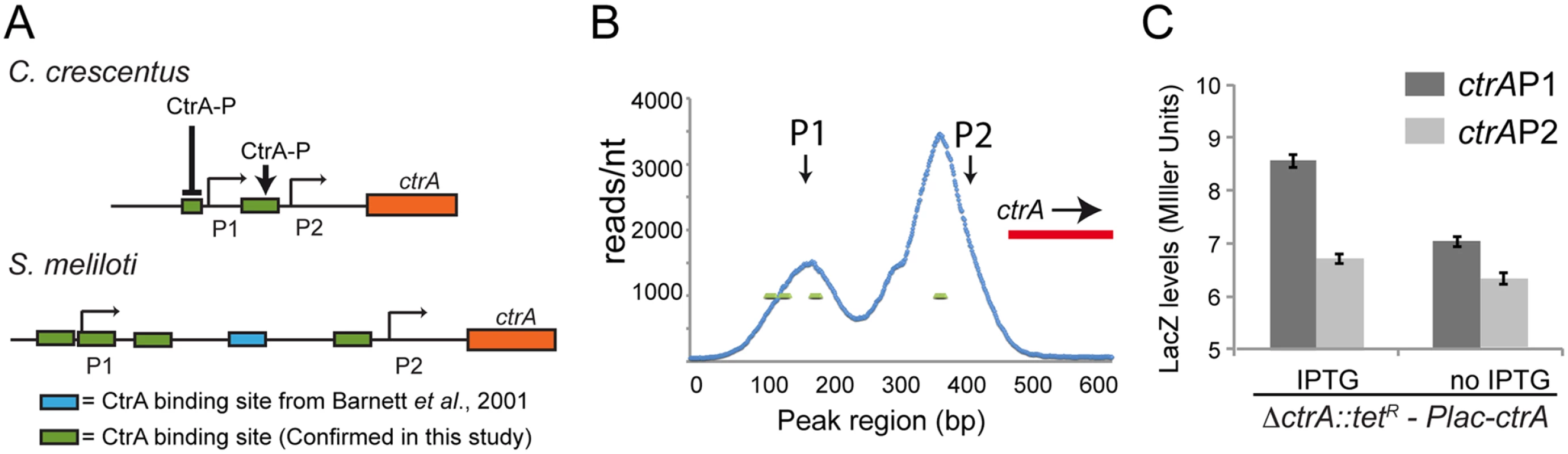
A. Promoters of ctrA in C. crescentus and S. meliloti. Both promoters have two transcriptional sites (P1 and P2). In C. crescentus P2 is activated by CtrA-P while P1 is activated by GcrA and repressed by CtrA-P [20,64,72]. In S. meliloti the P1 and P2 transcriptional start sites have been previously defined by primer extension [28]. ChIP-Seq results identified 4 binding sites of CtrA in S. meliloti upstream P1 and P2 while previously a fifth one was discovered by DNase I footprinting [28]. The presence of CtrA binding sites suggests a potential control of transcription by CtrA; B. Details of the ChIP-Seq using antibodies against CtrA (blue) of the ctrA promoter region. C. Promoters P1 and P2 were fused to lacZ measuring the beta—galactosidase activity depleting CtrA for 4 hours. Fig. 6. Proteolysis of CtrA is essential in S. meliloti and requires at least CpdR, RcdA and the last three amino acids of CtrA. 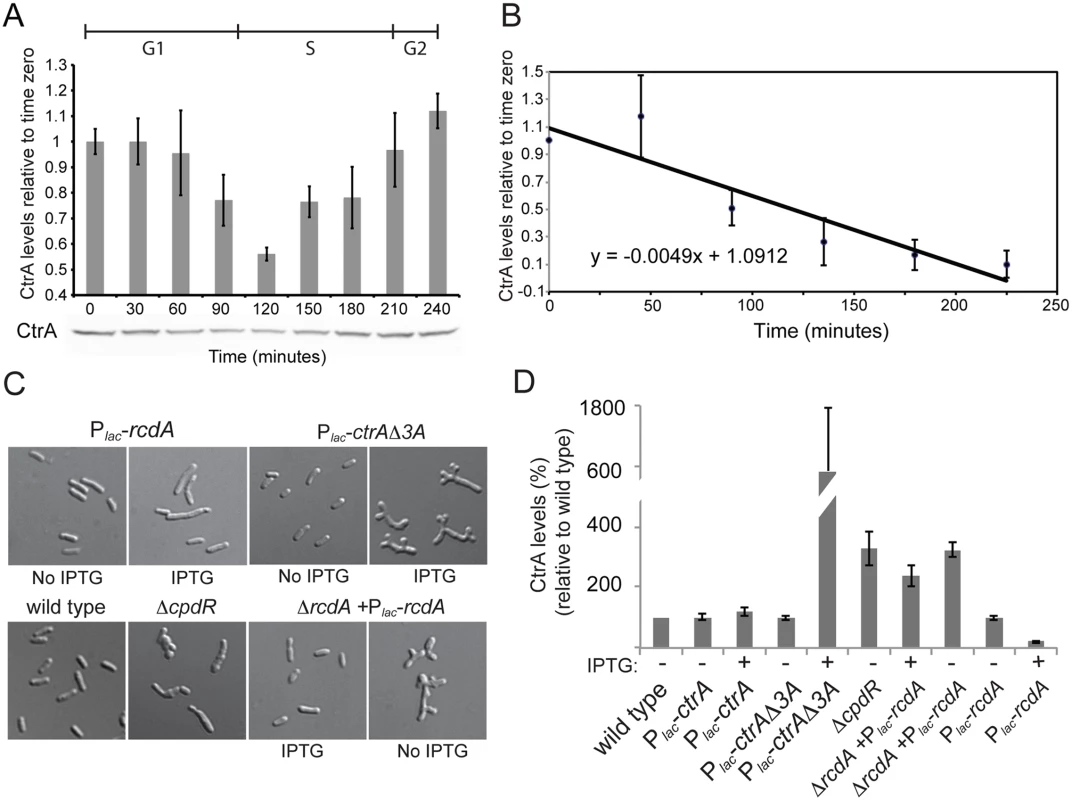
A. CtrA protein level changes during the cell cycle with a minimum around 120 min that corresponds to the G1-S transition [40]. Cells were synchronized and samples were collected every 30 minutes. CtrA antibodies were used to detect the protein level, protein levels were normalized for cell number and error bars represent standard error; B. Pulse-chase experiment of showing decrease over time of radiolabeled CtrA in S. meliloti cells. Values are averages from three separate experiments and the error bars represent standard deviation. C. Morphology of CtrA degradation defective mutants. CpdR- [29], although barely vital, shows compromised cell morphology. Cell depleted of RcdA for 7 hours also have altered morphology. Over-expression of rcdA for 7 hours causes cell elongation and division defects (Fig 1C). Overexpression of a stable version of CtrA (lacking the last three amino acids) for 7 hours causes altered cell morphology similar to that of the RcdA depletion strain. D. CtrA protein levels (% of CtrA in wild type cells) in the genetic backgrounds described in the panel C. Cell lysates were normalized for protein content, error bars represent standard error of three different replicates. Another important mode of CtrA regulation in C. crescentus is regulated proteolysis by the ClpXP protease [49]. It is unknown whether CtrA activity in S. meliloti is subject to a similar posttranslational regulatory network as it is in C. crescentus. The regulated proteolysis of CtrA is especially interesting in the context of S. meliloti symbiosis since regulated proteolysis represents an efficient mechanism by which CtrA could be eliminated from differentiating bacteroids [33–35]. To shed light on the mechanism of CtrA regulation in S. meliloti and to understand how CtrA levels may be downregulated in the bacteroid during symbiosis, we first checked if CtrA protein levels dynamically changed over the cell cycle as in C. crescentus. S. meliloti cells were synchronized as previously described [30] and CtrA levels were measured over the course of the cell cycle by immunoblotting (Fig 6B). We observed a drop in CtrA levels around the G1-S transition in a similar fashion as in C. crescentus. Although S. meliloti CtrA contains a putative C-terminal ClpX targeting tag [22], it has not been demonstrated that S. meliloti CtrA is subject to the same cell cycle regulated proteolysis as observed in C. crescentus [21,22]. To test whether S. meliloti CtrA may be regulated by proteolysis during the cell cycle, we determined the half-life of CtrA using pulse/chase analysis. We pulsed mid-log phase cultures with 35S-labeled methionine and cysteine, chased with unlabeled methionine and cysteine, and pulled down the S. meliloti CtrA protein using a C. crescentus CtrA polyclonal antibody [50]. We directly determined the half-life of 35S labeled S. meliloti CtrA to be 141 minutes during a ~220 minute cell cycle (Fig 6B), as compared to the 53 minute half-life of C. crescentus CtrA in unsynchronized culture during a 160 minute cell cycle [19,20]. These results suggest that CtrA in S. meliloti is subjected to active proteolysis although it is difficult to directly compare half lives between the two species due to the likely differing ratios of G1-arrested and dividing cells in unsynchronized cultures.
We next wanted to test the effect of inhibiting CtrA proteolysis in S. meliloti to assess its importance in cell cycle regulation. In C. crescentus, both ctrADD, where the C-terminal alanine residues were converted to aspartate residues, and ctrAΔ3, where the last three amino acids were deleted, produce stabilized versions of CtrA [21,22]. We first attempted to introduce these two alleles of ctrA into S. meliloti to assess the effects of CtrA stabilization. Introducing both the ctrADD and ctrAΔ3 allele to S. meliloti via expression from a medium copy plasmid or by direct integration into the native ctrA locus by the sacB suicide method yielded no transconjugants, suggesting that the stable derivatives of CtrA are lethal in S. meliloti. To confirm the lethality of these putative non-degradable CtrA alleles in S. meliloti, ctrAΔ3 was cloned into an inducible pSRK-Km derivative plasmid under a Plac promoter and mated into S. meliloti. Upon induction of CtrAΔ3 expression loss of viability was observed (S5 Fig), with dramatic cell cycle morphological defects after 6 hours of induction (Fig 6C) that corresponded with increased CtrA protein levels (Fig 6D). Our results hence suggest that proper proteolytic regulation of CtrA levels are essential in S. meliloti.
If proteolysis of CtrA is essential in S. meliloti, mutations in genes coding for putative co-factors of proteolysis should lead to severe phenotypes and result in an abnormal increase of CtrA levels. CpdR and RcdA both play a crucial role in controlling CtrA proteolysis in C. crescentus [26,51]. CpdR was previously shown to have physiologically important roles in S. meliloti but its direct link to CtrA proteolysis was not examined [29]. Immunoblotting revealed that CtrA levels were ca. 3 times higher in a cpdR1 mutant relative to wild type cells (Fig 6D), consistent with a role for CpdR1 in promoting CtrA degradation in S. meliloti and suggesting that the striking phenotype of a cpdR1 mutant might be due, in part, to elevated CtrA levels (Fig 6C) [29]. However, when we attempted to construct a ΔrcdA derivative of S. meliloti, we could only do so in the presence of plasmid bearing the rcdA gene expressed under its native promoter or Plac (S5 Table). We were also unable to transduce the ΔrcdA into wild-type S. meliloti in the absence of ectopically expressed rcdA (S5 Table), further indicating that rcdA is an essential gene in S. meliloti.
To gain insights onto how RcdA affects cell viability and CtrA protein levels, we used the previously described pSRK system to create a rcdA depletion strain. Cells depleted of RcdA were elongated and branched with irregular and enlarged bodies, a phenotype reminiscent of cells lacking cpdR1 and cells expressing non-degradeable CtrAΔ3 protein [29] (Fig 6C). We next examined the effect of varying RcdA levels on CtrA levels by immunoblotting. CtrA levels were elevated in the rcdA depletion and were reduced under conditions of rcdA overexpression (Fig 6D). Collectively, these results strongly suggest that, as in C. crescentus, RcdA, CpdR and the last three amino acids of CtrA are all required for correct proteolysis of CtrA. Differently from Caulobacter, proteolysis plays an essential role in S. meliloti as any impairement of CtrA proteolysis causes a cell cycle arrest.
Discussion
In this work we present significant progress towards the understanding of S. meliloti cell cycle regulation and differentiation in bacteroids, specifically involving the conserved master cell cycle regulator CtrA (Fig 7A). We showed that CtrA-deprived cells are unable to divide, exhibit cell elongation, branching and a sharp increase in DNA content. These combined phenotypes result in a clear loss of viability. Through microarray-based gene expression analysis coupled with ChIP-Seq analysis we defined the direct and indirect regulons of CtrA and discovered that, in agreement with the loss of function phenotypes, CtrA acts as a transcriptional regulator controlling essential functions that are required for proper cell cycle progression, such as cell division, chromosome methylation, motility and cell envelope biogenesis. Similarly to C. crescentus, CtrA is subjected to several regulatory mechanisms. We showed that the concentration of CtrA drops at the moment of the G1 to S transition similarly to C. crescentus [22]. Taken together with the striking genome amplification observed during CtrA depletion, these data suggest that CtrA is a repressor of DNA replication initiation that must be inactivated at the G1-S transition and perhaps during bacteroid differentiation. Furthermore, perturbations of the last three C-terminal residues of CtrA results in a marked accumulation of CtrA protein, suggesting that like in C. crescentus, S. meliloti CtrA levels are regulated by proteolysis, likely by the ClpXP protease [49]. We also found that levels of CtrA are higher in mutants lacking functional CpdR and RcdA, which are key regulators of CtrA proteolysis in C. crescentus [26,51]. Surprisingly, however, unlike C. crescentus, the disruption of CtrA proteolysis is lethal in S. meliloti as both deletion of rcdA and expression of ctrAΔ3 are lethal.
Fig. 7. Model of CtrA network in S. meliloti. 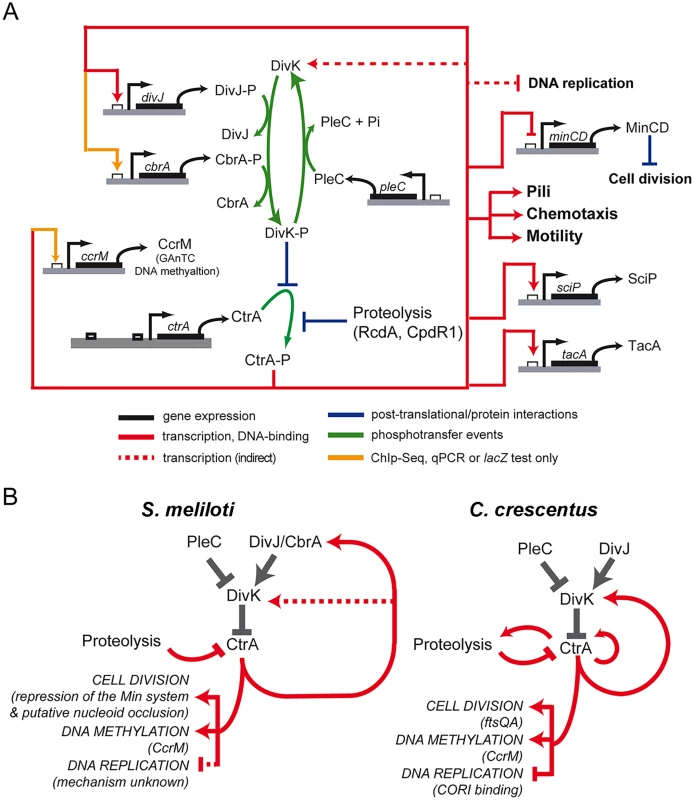
A. Scheme of genes regulated by CtrA. As reported in the legend two kinds of connections are reported: in red those confirmed by both ChIP-Seq and microarray and in yellow those not detected by microarrays but confirmed by other techniques. Phosphorylation of CtrA is essential [28] and the roles of DivJ, PLeC and CbrA have been previously described [33]. Despite the representation here, there is no indication of the preferred form of CtrA subjected to proteolysis. CtrA working on the promoters of genes is a simplification to represent of the direct effect of CtrA on transcription of the gene. B. Comparison between the circuit regulating cell cycle in S. meliloti and C. crescentus. Although the two organisms share the same logic of cell cycle regulatory circuit, differences in the factors connected and involved in the regulation of specific functions are present. The comparison between the S. meliloti CtrA circuit architecture with C. crescentus helped us to understand several general principles of the alphaproteobacterial cell cycle (Fig 7B). First, CtrA plays a crucial role in directly regulating genes involved in motility, chemotaxis and cell division. Motility was identified as an ancestral functional of this regulator in Magnetospirillum magnetotacticum and other alphaproteobacteria [13]. In most cases, however, the regulation of these similar processes is achieved through the control of different target genes. For example, CtrA controls cell division through ftsAQ in C. crescentus, while in S. meliloti this control is likely carried out by other means, such as CtrA repression of the Min system together with other unknown mechanisms, including for example nucleoid occlusion. This suggests that the CtrA regulon has changed significantly during the evolution of alphaproteobacteria and that different regulatory networks have evolved in the same phylogenetic group. A second general feature of the CtrA circuit in alphaproteobacteria concerns the link between CtrA and the DivK/DivJ module, which directly controls the phosphorylation status and indirectly controls the stability of CtrA. In both C. crescentus and S. meliloti, this module is controlled by CtrA via transcriptional regulation. In C. crescentus this important feedback loop is routed through the divK promoter, which is directly activated by CtrA [20]. In S. meliloti, divK transcription is only indirectly affected by CtrA depletion, and instead, CtrA feedback regulation of this module happens directly through the transcriptional activation of divJ by CtrA (Fig 7). The gene encoding the DivJ cognate kinase, cbrA, may also regulated directly by CtrA as its promoter was bound in the CtrA ChIP-Seq experiment, but significant differential expression of cbrA under conditions of CtrA depletion was only detected by lacZ-fusions and not by microarrays (S6 Fig). Previous work has found that altering the native promoter of divK in C. crescentus causes severe cell cycle defects [27], while in S. meliloti altering divJ transcription leads to an uncoordinated progression through the cell cycle, in particular, its overexpression causes a G2 block [33]. These and other interesting divergences in the wiring of the S. meliloti and C. crescentus cell cycle network are just the tip of the iceberg in uncovering how alphaproteobacteria have evolved species-specific wiring of these highly conserved cell cycle components to fit their unique lifestyles and diverse cellular differentiation programs.
Perhaps most importantly towards the understanding of symbiotic interactions with legume hosts, our findings provide insight into how S. meliloti cell cycle regulation may be altered during bacteroid differentiation to produce the bacteroid phenotypes of cell elongation and endoreduplication. Within the host nodule cells, S. meliloti is exposed to a microaerobic environment, which activates the FixJ/FixL two-component system. Activation of this system elicits a significant transcriptional response including expression of genes coding for the nitrogen fixation machinery [52–55]. Another important trigger of the bacteroid differentiation process is a large class of nodule-specific cysteine rich (NCR) peptides produced by the host legume [4,7,35]. The specific cellular targets of these peptides are unknown, although recent work has provided strong evidence that at least one of these peptides, NCR247, targets diverse cellular processes in S. meliloti [7,35,56], including the cell cycle. Furthermore, treatment of S. meliloti with NCR247 directly affects the transcription of CtrA and many other genes included in the direct and indirect CtrA regulons established in this work [35]. The observations that ctrA transcriptional downregulation coincides with bacteroid differentiation within the nodule [34], that CtrA is largely absent from mature bacteroids [33], and that an NCR peptide specifically perturbs the CtrA transcriptional regulon [35] all point to CtrA as an important regulatory node through which the legume host manipulates the bacterial cell cycle. Interestingly the dissection of gene expression changes in the differentiation region of the nodule revealed that the formation of bacteroids is associated increased concentration of rcdA transcript [34], which correlates with our observation that rcdA overexpression enhances CtrA degradation (Fig 6D). This work provides the first global overview of the CtrA cell cycle regulon in S. meliloti, which it will facilitate the exploration how NCR peptides and other plant factors affect the function of CtrA and other important cell cycle regulators to drive bacteroid differentiation in S. meliloti.
Materials and Methods
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in S6 Table. E. coli strains were grown in liquid or solid lysogeny broth (LB) (Sigma Aldrich) [57] at 37°C supplemented with appropriate antibiotics: kanamycin (50 μg ml-1 in broth and agar), tetracycline (10 μg ml-1 in broth and agar) and gentamycin (15 μg ml-1 in liquid broth, 20 μg ml-1 in agar). S. meliloti strains were grown in broth or agar TY [58] supplemented when necessary with kanamycin (200 μg ml-1 in broth and agar), streptomycin (500 μg ml-1 in broth and agar), tetracycline (1 μg ml-1 in liquid broth, 2 μg ml-1 in agar), spectinomycin (50 μg ml-1 in broth and agar) and gentamycin (20 μg ml-1 in broth and agar). For negative selection 1% sucrose was added to agar plates. Depletion conditions were tested growing cells to mid-log phase (OD600 = 0.6) in media containing IPTG (1mM for ctrA, and 80μM for rcdA), and then resuspended at OD600 = 0.1/0.2, after 2 washes, in media lacking IPTG. Synchronization experiments were performed as described previously [40].
Strain constructions and general techniques
Deletion mutants of ctrA and rcdA were constructed by two-step recombination of deletion cassettes, conducted as previously described using derivatives of the integrative plasmid pNPTS138 [33]. The first integration of the plasmid has been done by conjugation; 1 × 109 S. meliloti and 0.5 × 109 E. coli S17-1 cells [59] were used and incubated 24 h at 30°C. As simple deletion of the gene was not possible the procedure was performed in strain carrying a complementation plasmid. Deletions were verified by PCR using primers external to the area of recombination (see primers in S7 Table).
Complementation plasmids (pMR10 derivatives) [60], pSRK [36] derivatives (to study depletion and overexpression conditions) and pRKlac290 [61] derivatives (P-lacZ transcriptional reporter strains) were introduced by electroporation [62].
For transduction, M12 phage [63] and bacteria (in LB containing 2.5 mM CaCl2 and 2.5 mM MgSO4) were mixed to give a multiplicity of infection 1/2 (phage per cell). The mixture was incubated at 30°C for 30 min and subsequently plated on LB plates with the appropriate antibiotics [33].
For the efficiency-of-plating (EOP) assays showed in S5 Fig, cultures were grown to mid exponential phase (OD600 ≈ 0.55) in TY medium. Each sample was serially diluted up to 10-6 in TY, and spread onto TY agar with and without IPTG (1 mM). After 3 to 5 days of growth at 30°C, the number of colonies was determined. The average and standard deviation for each strain were derived from three independent cultures.
B-galactosidase assays and western blots were performed as previously described [64].
More details on cloning are in supporting S1 Text.
Pulse-chase analysis of CtrA in S. meliloti
Pulse-chase analysis was performed as described in (Chen, Sabio and Long 2008). S. meliloti was grown in SMM media to mid-log phase (OD600 ~0.6–0.7). Cultures were pulsed with 20uCi of 35[S]met and cys EasyTagEXPRESS protein labeling mix (Perkin Elmer) and chased with 100x chase solution (0.4% methionine, 0.3% cysteine). Time points were taken and pelleted at 10,000xg for 3 minutes every 45 minutes including 0 minutes after chase. Each time point was resuspended in 10x TEN (100mM Tris pH8, 10mM EDTA pH8, 0.25 NaN3), pelleted and resuspended in 1X TEN and put on ice. Once all time points were collected each sample was pelleted again and then resuspended in 50uL TES (10mM Tris pH8, 1mM EDTA pH8, 1% SDS). All samples were incubated at 100C for 10 minutes, 1mL of IP buffer (50mM Tris, pH7.5, 150mM NaCl, 1% Triton X-100) plus Sigma protease inhibitors (1 : 250) were added to each tube the pellet was resuspended and the samples were pelleted again. The supernatant was pre-cleared by incubation with 25mL of 50% slurry Protein A agarose (Pierce) at 4C for 20 minutes. 750uL of supernatant was transferred to a new tube and 1uL of CtrA antibody (C. crescentus CtrA antibody R308 gift of Michael Laub) and 25uL of 50% slurry Protein A agarose and rotated for 4 hours at 4C. Samples were washed 3x with IP buffer and 1x with IP buffer without Triton. Samples were resuspended in 12uL of 2x sample buffer and stored at -80C. Samples were run on 4–20% Tris-HCl gel and the gel was dried. Dried gels were exposed Amersham Biosciences Storage Phosphor Screen and developed using a Typhoon imager.
Chromatin Immunoprecipitation (ChIp)
Mid-log phase cells (80 ml, OD600 of 0.6) were cross-linked in 10 mM sodium phosphate (pH 7.6) and 1% formaldehyde at room temperature for 10 min and on ice for 30 min thereafter, washed thrice in phosphate buffered saline (PBS) and lysed with lysozyme 2.2 mg ml-1 in TES (Tris-HCl 10 mM pH 7.5, EDTA 1 mM, NaCl 100 mM). Lysates (Final volume 1ml) were sonicated (Branson Digital Sonicator 450, Branson Sonic Power. Co., www.bransonic.com/) on ice using 10 bursts of 20 sec (50% duty) at 30% amplitude to shear DNA fragments to an average length of 0.3–0.5 kbp and cleared by centrifugation at 14,000 rpm for 2 min at 4°C. Lysates were normalized by protein content by measuring the absorbance at 280 nm; ca. 7.5 mg of protein was diluted in 1 mL of ChIP buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl [pH 8.1], 167 mM NaCl plus protease inhibitors (Roche, www.roche.com/) and pre-cleared with 80 μL of protein-A agarose (Roche, www.roche.com/) and 100 μg BSA. Polyclonal antibodies to CtrA [33] were added to the remains of the supernatant (1 : 1,000 dilution), incubated overnight at 4°C with 80 μL of protein-A agarose beads pre-saturated with BSA, washed once with low salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.1), 150 mM NaCl), high salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.1), 500 mM NaCl) and LiCl buffer (0.25 M LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, 10 mM Tris-HCl (pH 8.1) and twice with TE buffer (10 mM Tris-HCl (pH 8.1) and 1 mM EDTA). The protein•DNA complexes were eluted in 500 μL freshly prepared elution buffer (1% SDS, 0.1 M NaHCO3), supplemented with NaCl to a final concentration of 300 mM and incubated overnight at 65°C to reverse the crosslinks. The samples were treated with 2 μg of Proteinase K for 2 h at 45°C in 40 mM EDTA and 40 mM Tris-HCl (pH 6.5). DNA was extracted using QIAgen minelute kit and resuspended in 30 μl of Elution Buffer. ChIp DNA sequencing was performed using Illumina MySeq and analyzed as previously described [64]. Raw fastq data are available upon request.
Microscopy
S. meliloti cells were grown to mid-log phase, fixed in 70% ethanol, washed and concentrated with GTE (50 mM glucose, 10 mM EDTA, 20 mM Tris,pH 7.5). Bacteroids were extracted as previously described [33]. Samples were stained with Hoechst 33324 (Cf 5μg/ml) and Propidium iodide (Cf 2μg/ml) for 30 minutes at RT. Samples were deposited on microscope slides coated with 0.1% poly-L-lysine. Images were processed with ImageJ [65].
Microarray hybridization and analysis
Exponential phase cells grown in the presence of 1mM IPTG were pelleted by centrifugation washed twice with 0.85% saline solution, and split into two cultures that contained either 1mM IPTG or no IPTG (CtrA depletion). RNA was isolated from triplicate samples of t = 0 S. meliloti cells as well as from +IPTG (control) and—IPTG (ctrA depleted) 1, 2, 4 and 6 hours after depletion were converted to cDNA and hybridized to custom Agilent microarrays containing 6046 S. melioti ORF (GPL18182). To determine which S. meliloti genes may be transcriptionally regulated by CtrA, the log2 fold change (logFC) of expression between average triplicate-IPTG and +IPTG samples at 1, 2, and 4 hours were calculated using the limma package in R. The 6-hours time point, although highly correlative with the earlier time points, was excluded from the analysis due to a lack of replicate samples. RNA isolation, cDNA synthesis and labeling, and microarray hybridization are as described [40]. Only one channel was used for hybridization.
Data were normalized as previously described [35]. Normalized microarray data of IPTG-treated and non-treated (CtrA-depletion) samples were directly compared by using the Limma package in R [66,67]. A linear model was fitted to the normalized log2 values for each gene at the 1-, 2, - and 4-hour time points and used to generate estimated coefficients and standard errors for the compared samples. Moderated t-statistics, moderated F-statistics and log-odds of differential expression using an empirical Bayes approach were applied to the parameter estimates and standard errors from the linear models for each probe. P-value adjustment for multiple testing was performed using the Benjamini-Hochberg false discovery rate procedure. Genes identified as differentially expressed had logFC values ≥ 1.0 or ≤ –1.0 and an adjusted p value of ≤ 0.05.
For heat map generation, the replicate-average log2 expression values for the 126 differentially expressed gene identified above were row normalized across the time points as described [35]. Normalized values were then clustered by using Gene Cluster 3.0 and the city block similarity metric with complete linkage clustering. For the heat map of direct and indirect targets of CtrA, the average log2 expression for each gene in +IPTG samples (1-, 2 - and 4-hour) and the replicate-average log2 expression values for a gene at each time point was row normalized and clustered as described above. The microarray data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus [68,69] and are accessible through GEO Series accession number GSE68218 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE68218).
FACS analysis
Flow cytometric analysis of DNA content in S. meliloti cells was performed as previously described [40].
Quantitative RT-PCR
The 2-ΔΔCT method was used to determine the expression level of indicated genes [70]. The fold change in gene expression in CtrA-depleted cells was plotted relative to gene expression in CtrA-replete cells. The expression level of the control gene smc00128 [71] was used to normalize expression data in cells replete with CtrA and cells lacking CtrA. Oligonucleotide primers are available upon request.
Supporting Information
Zdroje
1. Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol. 2007;5 : 619–633. doi: 10.1038/nrmicro1705 17632573
2. Oke V, Long SR. Bacteroid formation in the Rhizobium-legume symbiosis. Curr Opin Microbiol. 1999;2 : 641–646. 10607628
3. Foucher F, Kondorosi E. Cell cycle regulation in the course of nodule organogenesis in Medicago. Plant Mol Biol. 2000;43 : 773–786. 11089876
4. Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc Natl Acad Sci U S A. 2006;103 : 5230–5235. doi: 10.1073/pnas.0600912103 16547129
5. Mergaert P, Nikovics K, Kelemen Z, Maunoury N, Vaubert D, Kondorosi A, et al. A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol. 2003;132 : 161–173. doi: 10.1104/pp.102.018192 12746522
6. Alunni B, Kevei Z, Redondo-Nieto M, Kondorosi A, Mergaert P, Kondorosi E. Genomic organization and evolutionary insights on GRP and NCR genes, two large nodule-specific gene families in Medicago truncatula. Mol Plant-Microbe Interact MPMI. 2007;20 : 1138–1148. doi: 10.1094/MPMI-20-9-1138 17849716
7. Farkas A, Maróti G, Durgő H, Györgypál Z, Lima RM, Medzihradszky KF, et al. Medicago truncatula symbiotic peptide NCR247 contributes to bacteroid differentiation through multiple mechanisms. Proc Natl Acad Sci U S A. 2014;111 : 5183–5188. doi: 10.1073/pnas.1404169111 24706863
8. Gibson KE, Kobayashi H, Walker GC. Molecular determinants of a symbiotic chronic infection. Annu Rev Genet. 2008;42 : 413–441. doi: 10.1146/annurev.genet.42.110807.091427 18983260
9. Hallez R, Bellefontaine A-F, Letesson J-J, De Bolle X. Morphological and functional asymmetry in alpha-proteobacteria. Trends Microbiol. 2004;12 : 361–365. doi: 10.1016/j.tim.2004.06.002 15276611
10. Bird TH, MacKrell A. A CtrA homolog affects swarming motility and encystment in Rhodospirillum centenum. Arch Microbiol. 2011;193 : 451–459. doi: 10.1007/s00203-011-0676-y 21243338
11. Brilli M, Fondi M, Fani R, Mengoni A, Ferri L, Bazzicalupo M, et al. The diversity and evolution of cell cycle regulation in alpha-proteobacteria: a comparative genomic analysis. BMC Syst Biol. 2010;4 : 52. doi: 10.1186/1752-0509-4-52 20426835
12. Bellefontaine A-F, Pierreux CE, Mertens P, Vandenhaute J, Letesson J-J, De Bolle X. Plasticity of a transcriptional regulation network among alpha-proteobacteria is supported by the identification of CtrA targets in Brucella abortus. Mol Microbiol. 2002;43 : 945–960. 11929544
13. Greene SE, Brilli M, Biondi EG, Komeili A. Analysis of the CtrA pathway in Magnetospirillum reveals an ancestral role in motility in alphaproteobacteria. J Bacteriol. 2012;194 : 2973–2986. doi: 10.1128/JB.00170-12 22467786
14. Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84 : 83–93. 8548829
15. Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30 : 377–407. doi: 10.1146/annurev.mi.30.100176.002113 185940
16. Skerker JM, Laub MT. Cell-cycle progression and the generation of asymmetry in Caulobacter crescentus. Nat Rev Microbiol. 2004;2 : 325–337. doi: 10.1038/nrmicro864 15031731
17. Quon KC, Yang B, Domian IJ, Shapiro L, Marczynski GT. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci U S A. 1998;95 : 120–125. 9419339
18. Laub MT, Chen SL, Shapiro L, McAdams HH. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci U S A. 2002;99 : 4632–4637. doi: 10.1073/pnas.062065699 11930012
19. Reisenauer A, Shapiro L. DNA methylation affects the cell cycle transcription of the CtrA global regulator in Caulobacter. EMBO J. 2002;21 : 4969–4977. 12234936
20. Holtzendorff J, Hung D, Brende P, Reisenauer A, Viollier PH, McAdams HH, et al. Oscillating global regulators control the genetic circuit driving a bacterial cell cycle. Science. 2004;304 : 983–987. doi: 10.1126/science.1095191 15087506
21. Ryan KR, Judd EM, Shapiro L. The CtrA response regulator essential for Caulobacter crescentus cell-cycle progression requires a bipartite degradation signal for temporally controlled proteolysis. J Mol Biol. 2002;324 : 443–455. 12445780
22. Domian IJ, Quon KC, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90 : 415–424. 9267022
23. Jacobs C, Domian IJ, Maddock JR, Shapiro L. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell. 1999;97 : 111–120. 10199407
24. Chen YE, Tsokos CG, Biondi EG, Perchuk BS, Laub MT. Dynamics of two Phosphorelays controlling cell cycle progression in Caulobacter crescentus. J Bacteriol. 2009;191 : 7417–7429. doi: 10.1128/JB.00992-09 19783630
25. Jacobs C, Ausmees N, Cordwell SJ, Shapiro L, Laub MT. Functions of the CckA histidine kinase in Caulobacter cell cycle control. Mol Microbiol. 2003;47 : 1279–1290. 12603734
26. Iniesta AA, McGrath PT, Reisenauer A, McAdams HH, Shapiro L. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc Natl Acad Sci U S A. 2006;103 : 10935–10940. doi: 10.1073/pnas.0604554103 16829582
27. Biondi EG, Reisinger SJ, Skerker JM, Arif M, Perchuk BS, Ryan KR, et al. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444 : 899–904. doi: 10.1038/nature05321 17136100
28. Barnett MJ, Hung DY, Reisenauer A, Shapiro L, Long SR. A homolog of the CtrA cell cycle regulator is present and essential in Sinorhizobium meliloti. J Bacteriol. 2001;183 : 3204–3210. doi: 10.1128/JB.183.10.3204-3210.2001 11325950
29. Kobayashi H, De Nisco NJ, Chien P, Simmons LA, Walker GC. Sinorhizobium meliloti CpdR1 is critical for co-ordinating cell cycle progression and the symbiotic chronic infection. Mol Microbiol. 2009;73 : 586–600. doi: 10.1111/j.1365-2958.2009.06794.x 19602145
30. Gibson KE, Barnett MJ, Toman CJ, Long SR, Walker GC. The symbiosis regulator CbrA modulates a complex regulatory network affecting the flagellar apparatus and cell envelope proteins. J Bacteriol. 2007;189 : 3591–3602. doi: 10.1128/JB.01834-06 17237174
31. Gibson KE, Campbell GR, Lloret J, Walker GC. CbrA is a stationary-phase regulator of cell surface physiology and legume symbiosis in Sinorhizobium meliloti. J Bacteriol. 2006;188 : 4508–4521. doi: 10.1128/JB.01923-05 16740957
32. Sadowski C, Wilson D, Schallies K, Walker G, Gibson KE. The Sinorhizobium meliloti sensor histidine kinase CbrA contributes to free-living cell cycle regulation. Microbiol Read Engl. 2013; doi: 10.1099/mic.0.067504-0
33. Pini F, Frage B, Ferri L, De Nisco NJ, Mohapatra SS, Taddei L, et al. The DivJ, CbrA and PleC system controls DivK phosphorylation and symbiosis in Sinorhizobium meliloti. Mol Microbiol. 2013;90 : 54–71. doi: 10.1111/mmi.12347 23909720
34. Roux B, Rodde N, Jardinaud M-F, Timmers T, Sauviac L, Cottret L, et al. An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser-capture microdissection coupled to RNA sequencing. Plant J Cell Mol Biol. 2014;77 : 817–837. doi: 10.1111/tpj.12442
35. Penterman J, Abo RP, De Nisco NJ, Arnold MFF, Longhi R, Zanda M, et al. Host plant peptides elicit a transcriptional response to control the Sinorhizobium meliloti cell cycle during symbiosis. Proc Natl Acad Sci U S A. 2014;111 : 3561–3566. doi: 10.1073/pnas.1400450111 24501120
36. Khan SR, Gaines J, Roop RM 2nd, Farrand SK. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl Environ Microbiol. 2008;74 : 5053–5062. doi: 10.1128/AEM.01098-08 18606801
37. Cheng J, Sibley CD, Zaheer R, Finan TM. A Sinorhizobium meliloti minE mutant has an altered morphology and exhibits defects in legume symbiosis. Microbiol Read Engl. 2007;153 : 375–387. doi: 10.1099/mic.0.2006/001362-0
38. Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316 : 1497–1502. doi: 10.1126/science.1141319 17540862
39. Schlüter J-P, Reinkensmeier J, Barnett MJ, Lang C, Krol E, Giegerich R, et al. Global mapping of transcription start sites and promoter motifs in the symbiotic α-proteobacterium Sinorhizobium meliloti 1021. BMC Genomics. 2013;14 : 156. doi: 10.1186/1471-2164-14-156 23497287
40. De Nisco NJ, Abo RP, Wu CM, Penterman J, Walker GC. Global analysis of cell cycle gene expression of the legume symbiont Sinorhizobium meliloti. Proc Natl Acad Sci U S A. 2014; doi: 10.1073/pnas.1400421111
41. Rotter C, Mühlbacher S, Salamon D, Schmitt R, Scharf B. Rem, a new transcriptional activator of motility and chemotaxis in Sinorhizobium meliloti. J Bacteriol. 2006;188 : 6932–6942. doi: 10.1128/JB.01902-05 16980496
42. Tan MH, Kozdon JB, Shen X, Shapiro L, McAdams HH. An essential transcription factor, SciP, enhances robustness of Caulobacter cell cycle regulation. Proc Natl Acad Sci U S A. 2010;107 : 18985–18990. doi: 10.1073/pnas.1014395107 20956288
43. Gora KG, Tsokos CG, Chen YE, Srinivasan BS, Perchuk BS, Laub MT. A cell-type-specific protein-protein interaction modulates transcriptional activity of a master regulator in Caulobacter crescentus. Mol Cell. 2010;39 : 455–467. doi: 10.1016/j.molcel.2010.06.024 20598601
44. Dehal PS, Joachimiak MP, Price MN, Bates JT, Baumohl JK, Chivian D, et al. MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res. 2009; gkp919. doi: 10.1093/nar/gkp919
45. Shih Y-L, Zheng M. Spatial control of the cell division site by the Min system in Escherichia coli. Environ Microbiol. 2013;15 : 3229–3239. doi: 10.1111/1462-2920.12119 23574354
46. Hung DY, Shapiro L. A signal transduction protein cues proteolytic events critical to Caulobacter cell cycle progression. Proc Natl Acad Sci U S A. 2002;99 : 13160–13165. doi: 10.1073/pnas.202495099 12237413
47. Wortinger M, Sackett MJ, Brun YV. CtrA mediates a DNA replication checkpoint that prevents cell division in Caulobacter crescentus. EMBO J. 2000;19 : 4503–4512. doi: 10.1093/emboj/19.17.4503 10970844
48. Rothfield L, Taghbalout A, Shih Y-L. Spatial control of bacterial division-site placement. Nat Rev Microbiol. 2005;3 : 959–968. doi: 10.1038/nrmicro1290 16322744
49. Jenal U, Fuchs T. An essential protease involved in bacterial cell-cycle control. EMBO J. 1998;17 : 5658–5669. doi: 10.1093/emboj/17.19.5658 9755166
50. Fields AT, Navarrete CS, Zare AZ, Huang Z, Mostafavi M, Lewis JC, et al. The conserved polarity factor podJ1 impacts multiple cell envelope-associated functions in Sinorhizobium meliloti. Mol Microbiol. 2012;84 : 892–920. doi: 10.1111/j.1365-2958.2012.08064.x 22553970
51. McGrath PT, Iniesta AA, Ryan KR, Shapiro L, McAdams HH. A dynamically localized protease complex and a polar specificity factor control a cell cycle master regulator. Cell. 2006;124 : 535–547. doi: 10.1016/j.cell.2005.12.033 16469700
52. David M, Daveran ML, Batut J, Dedieu A, Domergue O, Ghai J, et al. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988;54 : 671–683. 2842062
53. Galinier A, Garnerone AM, Reyrat JM, Kahn D, Batut J, Boistard P. Phosphorylation of the Rhizobium meliloti FixJ protein induces its binding to a compound regulatory region at the fixK promoter. J Biol Chem. 1994;269 : 23784–23789. 8089150
54. Monson EK, Ditta GS, Helinski DR. The oxygen sensor protein, FixL, of Rhizobium meliloti. Role of histidine residues in heme binding, phosphorylation, and signal transduction. J Biol Chem. 1995;270 : 5243–5250. 7890634
55. Gilles-Gonzalez MA, Ditta GS, Helinski DR. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991;350 : 170–172. doi: 10.1038/350170a0 1848683
56. Tiricz H, Szucs A, Farkas A, Pap B, Lima RM, Maróti G, et al. Antimicrobial nodule-specific cysteine-rich peptides induce membrane depolarization-associated changes in the transcriptome of Sinorhizobium meliloti. Appl Environ Microbiol. 2013;79 : 6737–6746. doi: 10.1128/AEM.01791-13 23995935
57. Sambrook JJ, Russell DDW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2001.
58. Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84 : 188–198. 4612098
59. Simon R, Priefer U, Pühler A. A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Nat Biotechnol. 1983;1 : 784–791. doi: 10.1038/nbt1183-784
60. Roberts RC, Toochinda C, Avedissian M, Baldini RL, Gomes SL, Shapiro L. Identification of a Caulobacter crescentus operon encoding hrcA, involved in negatively regulating heat-inducible transcription, and the chaperone gene grpE. J Bacteriol. 1996;178 : 1829–1841. 8606155
61. Alley MR, Gomes SL, Alexander W, Shapiro L. Genetic analysis of a temporally transcribed chemotaxis gene cluster in Caulobacter crescentus. Genetics. 1991;129 : 333–341. 1660425
62. Ferri L, Gori A, Biondi EG, Mengoni A, Bazzicalupo M. Plasmid electroporation of Sinorhizobium strains: The role of the restriction gene hsdR in type strain Rm1021. Plasmid. 2010;63 : 128–135. doi: 10.1016/j.plasmid.2010.01.001 20097223
63. Finan TM, Hartweig E, LeMieux K, Bergman K, Walker GC, Signer ER. General transduction in Rhizobium meliloti. J Bacteriol. 1984;159 : 120–124. 6330024
64. Fioravanti A, Fumeaux C, Mohapatra SS, Bompard C, Brilli M, Frandi A, et al. DNA Binding of the Cell Cycle Transcriptional Regulator GcrA Depends on N6-Adenosine Methylation in Caulobacter crescentus and Other Alphaproteobacteria. PLoS Genet. 2013;9: e1003541. doi: 10.1371/journal.pgen.1003541 23737758
65. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9 : 671–675. 22930834
66. Smyth GK. limma: Linear Models for Microarray Data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer New York; 2005. pp. 397–420. Available: http://link.springer.com/chapter/10.1007/0-387-29362-0_23
67. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3: Article3. doi: 10.2202/1544-6115.1027 16646809
68. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30 : 207–210. doi: 10.1093/nar/30.1.207 11752295
69. Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2013;41: D991–D995. doi: 10.1093/nar/gks1193 23193258
70. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25 : 402–408. doi: 10.1006/meth.2001.1262 11846609
71. Krol E, Becker A. Global transcriptional analysis of the phosphate starvation response in Sinorhizobium meliloti strains 1021 and 2011. Mol Genet Genomics MGG. 2004;272 : 1–17. doi: 10.1007/s00438-004-1030-8
72. Domian IJ, Reisenauer A, Shapiro L. Feedback control of a master bacterial cell-cycle regulator. Proc Natl Acad Sci U S A. 1999;96 : 6648–6653. 10359766
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



