-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaWindpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
Effective tissue homeostasis requires a proper balance between the removal of dead cells and production of new cells. Due to environmental challenges, the Drosophila midgut epithelial cells are damaged from time to time and intestinal stem cells (ISC) can accelerate their proliferative rate to replace the lost midgut epithelium. The JAK/STAT pathway plays essential roles in these progresses. Upon damage, Upd ligands produced by dying enterocytes (ECs) activate JAK/STAT signaling in ISCs to promote their proliferation and differentiation. However, after damage how JAK/STAT signaling is switched from a highly active state to a homeostatic state is not yet fully understood. In this study, we identified the leucine rich repeats (LRR) protein Windpipe (Wdp) as a novel negative feedback regulator of JAK/STAT signaling during intestinal development. Wdp expression was induced by high levels of JAK/STAT signaling in intestines. And loss of Wdp leads to midgut homeostasis loss and increased ISC proliferation. Furthermore, we found Wdp in turn negatively regulates JAK/STAT signaling activity through promoting Domeless receptor endocytosis and lysosomal degradation. In this way, high levels of JAK/STAT signaling is switched off by Wdp, which ensure ISCs return to the homeostatic state after tissue damage.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005180
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005180Summary
Effective tissue homeostasis requires a proper balance between the removal of dead cells and production of new cells. Due to environmental challenges, the Drosophila midgut epithelial cells are damaged from time to time and intestinal stem cells (ISC) can accelerate their proliferative rate to replace the lost midgut epithelium. The JAK/STAT pathway plays essential roles in these progresses. Upon damage, Upd ligands produced by dying enterocytes (ECs) activate JAK/STAT signaling in ISCs to promote their proliferation and differentiation. However, after damage how JAK/STAT signaling is switched from a highly active state to a homeostatic state is not yet fully understood. In this study, we identified the leucine rich repeats (LRR) protein Windpipe (Wdp) as a novel negative feedback regulator of JAK/STAT signaling during intestinal development. Wdp expression was induced by high levels of JAK/STAT signaling in intestines. And loss of Wdp leads to midgut homeostasis loss and increased ISC proliferation. Furthermore, we found Wdp in turn negatively regulates JAK/STAT signaling activity through promoting Domeless receptor endocytosis and lysosomal degradation. In this way, high levels of JAK/STAT signaling is switched off by Wdp, which ensure ISCs return to the homeostatic state after tissue damage.
Introduction
The JAK/STAT pathway is evolutionarily conserved from Drosophila to mammals, and plays important roles in various developmental processes including cellular proliferation, innate immune response and stem cell development [1–4]. Dysregulation of the JAK/STAT pathway is associated with many human diseases, such as immune disorders and cancers [5–7]. Therefore, the JAK/STAT pathway is tightly controlled by various regulators and mechanisms to ensure proper signaling. While the core components of this pathway are well-documented, it is less understood how the duration of its signal activity is temporally regulated.
Drosophila is an excellent model to investigate the regulation of JAK/STAT signaling. Compared with various isoforms of the JAK/STAT pathway components in mammals [8–10], Drosophila has a relatively simple signal transduction cascade: a one-pass transmembrane receptor, Domeless (Dome) [11, 12]; a tyrosine JAK kinase, Hopscotch (Hop) [13]; a transcription factor, STAT92E [14, 15]; and three different ligands including Unpaired (Upd) [16], Upd2 [17], and Upd3 [18]. In the canonical pathway, binding of Dome receptor with its extracellular ligands induces Dome dimerization or oligomerization, which leads to juxtaposition of Hop. Hop molecules cross-phosphorylate each other and then phosphorylate Dome to generate docking sites for cytoplasmic STAT92E. Once bound to the Dome/Hop complex, STAT92E molecules are phosphorylated, form dimers, and then translocate into the nucleus, where they bind to defined STAT92E binding sites, and regulate the transcription of downstream target genes [1, 19]. This signaling transduction is under tight control at multiple steps to avoid improper signal activation [1, 9]. Several negative feedback regulators such as Socs36E and Ptp61F are identified to be involved in switching off JAK/STAT signaling during developmental processes [20–22].
JAK/STAT signaling pathway plays important roles in Drosophila adult midgut homeostasis and tissue regeneration. Due to dietary stress, tissue injury, or pathogen infection, intestinal epithelial cells turn over rapidly and midgut homeostasis is maintained by intestinal stem cells (ISC). A basally localized ISC divides asymmetrically to give rise to a renewed ISC and a non-dividing, undifferentiated enteroblast (EB). EBs then differentiate into either absorptive enterocytes (EC) or secretory enteroendocrine cells (ee) [23, 24]. Several signaling pathways including Notch, JAK/STAT, EGFR, Hippo, insulin, BMP and Wnt have been shown to regulate the maintenance, proliferation, and differentiation of ISCs [23–41]. Under physiological conditions, JAK/STAT signaling promotes ISC proliferation and is also required for the differentiation of ECs and ee cells [26–28, 42, 43]. In addition, the JAK/STAT pathway plays crucial roles during midgut regeneration. After bacterial infection or physical injury, the expression of Upd ligands such as Upd3 is induced. Secreted Upd ligands from ECs activate JAK/STAT signaling in ISCs, which rapidly increase their proliferation rate to replenish the damaged midgut epithelium [26, 44–46]. However, the mechanisms of how highly activated JAK/STAT signaling returns to normal levels after injury remain poorly understood.
Drosophila Windpipe (Wdp) is a single-pass transmembrane protein containing four leucine-rich repeats (LRR) in the extracellular domain, and is highly expressed in the developing trachea [47]. Currently, the biological function of this protein has not been defined. In this study, we have identified wdp as a novel component of the JAK/STAT pathway. We showed that Wdp is positively regulated by JAK/STAT signaling. Loss of wdp results in disruption of midgut homeostasis under physiological conditions, and potentiates tissue regeneration under damage conditions. Conversely, ectopically expressed Wdp negatively regulates JAK/STAT signaling. Importantly, we demonstrate that Wdp can promote Dome internalization and subsequent lysosomal degradation. Together, we propose that Wdp controls intestinal homeostasis by interfering with JAK/STAT signaling activity via a negative feedback mechanism.
Results
Wdp expression is positively regulated by JAK/STAT signaling in Drosophila midguts
Although the JAK/STAT pathway is required for regulating midgut homeostasis under physiological conditions and damage-induced tissue regeneration, how JAK/STAT signaling executes its functions during these processes remains largely unknown. So far, only a few STAT92E target genes including Socs36E, zfh1, and chinmo, have been identified [48–50]. To further explore the regulatory mechanism of the JAK/STAT pathway in midguts, we performed ChIP-Seq experiments aimed at identifying novel downstream targets of the JAK/STAT pathway. ChIP-Seq experiments were carried out in intestines ectopically expressing Upd and STAT92E in the progenitor cells (esgts>upd; STAT). In these experiments, we identified about 200 candidates with at least one peak (p<0.01) in the gene regulatory region. The previously well-characterized JAK/STAT downstream targets, including Domeless [51], Socs36E [20], and STAT92E [52, 53], were recovered in our experiments (S1 Table), indicating that our ChIP approach is workable to identify potential novel targets.
From the candidate genes, wdp, a gene previously shown to be highly expressed in the developing trachea [47], was identified. Wdp was ranked in the top 10% through our bioinformatics analysis of ChIP results. At least 3 significant peaks (p<0.01) containing conserved STAT92E binding sites (TTCN3/4GAA) [15] were found in the 5’ UTR and genomic region of wdp (Fig 1A). Moreover, wdp mRNA levels as determined by RT-qPCR were increased in response to ectopic JAK/STAT signaling in esgts>upd intestines (Fig 1B). To further analyze the transcriptional regulation of wdp by JAK/STAT signaling, we identified four potential STAT92E binding sites (BS1-, BS2-, BS3 - and BS4), and generated luciferase reporters that contain these potential binding sites. With the addition of Upd expressing S2 cells, the luciferase activity in cells transfected with BS2-, BS3 - or BS4 - luciferase constructs was obviously increased (Fig 1C). These results suggest that the expression of Wdp might be regulated by JAK/STAT signaling through BS2, BS3 and BS4 binding sites.
Fig. 1. Wdp expression is positively regulated by JAK/STAT signaling in Drosophila intestines. 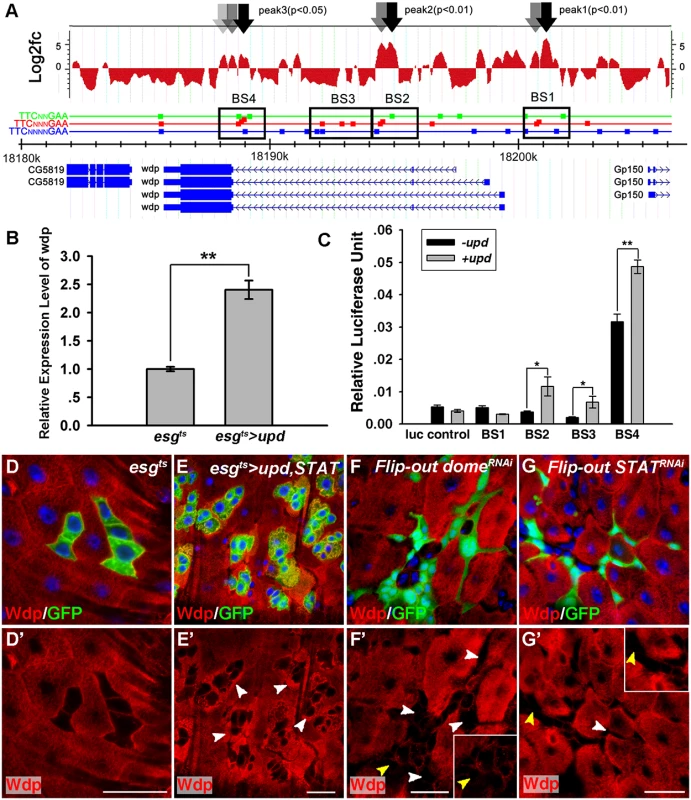
(A) ChIP analysis was performed to monitor the binding of STAT92E to wdp genomic regions with STAT92E antibody using adult intestines expressing Upd and STAT92E under the esgts driver for 10 days at 29°C. The localization of four putative STAT92E-binding sites (BS1-4) is indicated by a black square frame. The square boxes with green, red or blue colors represent putative STAT92E binding sites localized in wdp genomic region with 2, 3 or 4 spacers respectively. (B) wdp mRNA expression was obviously increased in esgts>upd intestines at 29°C for 7 days using RT-qPCR quantification. Mean ± SD are shown. **p<0.01. (C) The relative activity of the indicated luciferase vectors, which contain different putative STAT92E binding sites (BS1-4) from wdp genomic regions, upon addition of Upd expressing cells. Mean ± SD are shown. *p<0.1, **p<0.01. (D and D’) Wdp (red, by Wdp) is ubiquitously expressed in both small progenitor cells and large nuclei ECs in control midguts at 29°C for 7 days. (E and E’) Wdp expression (red, by Wdp) was significantly increased around the GFP+ clusters (arrowheads) in esgts >upd, STAT midguts at 29°C for 7 days. (F and F’) Wdp expression (red, by Wdp) was reduced in the Flip-out clones (arrowheads) knocking down Dome at 29°C for 7 days. Square box is the enlarged image of the position labeled by yellow arrowhead. (G and G’) Wdp expression (red, by Wdp) was reduced in the Flip-out clones (arrowheads) knocking down STAT at 29°C for 7 days. Square box is the enlarged image of the position labeled by yellow arrowhead. Blue indicates DAPI staining. Scale bars, 20μm. Next we wanted to determine whether the expression of Wdp is regulated by JAK/STAT signaling in vivo in Drosophila posterior midgut. First, the expression pattern of Wdp was examined by immunostaining with anti-Wdp antibody. The specificity of anti-Wdp antibody was verified (S1G–S1I Fig). Then we determined Wdp expression pattern in midguts and imaginal discs (S1 Fig). Wdp was ubiquitously expressed in wild-type intestines (Fig 1D, 1D’ and S1A–S1D’ Fig). When we used esgts to overexpress Upd and STAT in progenitor cells, we found Wdp protein levels were increased around the GFP+ clusters (Fig 1E and 1E’). Furthermore, we blocked JAK/STAT signaling activity by expressing dome RNAi or STAT RNAi, and found Wdp expression was reduced in dome RNAi/STAT RNAi expressing cells (Fig 1F–1G’ and S2A–S2D’ Fig). Besides, Wdp expression was also reduced in STAT92E06346 mutant clones (S2E–S2F’ Fig). To further confirm the regulation of Wdp by JAK/STAT signaling in ISCs, we first generated Notch264-39 mutant ISC clusters and found Wdp was mainly localized on the cell membrane in ISCs. However, Wdp expression levels were reduced in the ISC clusters deficient for stat92E with simultaneous Notch knockdown (S2G–S2H’ Fig), indicating that Wdp expression was reduced in JAK/STAT signaling deficient ISCs. Taken together, these data indicate that wdp, as a putative target of STAT92E, is positively regulated by JAK/STAT signaling in Drosophila intestines.
Loss of wdp disrupts midgut homeostasis under physiological conditions and potentiates tissue regeneration under damage conditions
Next, we examined the possible functions of wdp in midgut homeostasis. We generated 2 alleles of wdp mutants by imprecise excision of P{wHy}wdpDG23704 (S1E Fig) and selected wdp1 for further experiment. wdp1 is likely a functional null mutant, as half of the wdp coding sequence is removed. Consistently, wdp transcription in wdp1/1 homozygotes was abolished compared with WT (S1F Fig). Homozygous wdp1/1 flies are semi-lethal with a few escapers displaying no visual phenotypes.
To examine the function of Wdp in the posterior midgut, we used esg-lacZ, Dl-lacZ and Su(H)GBE-lacZ to mark progenitor cells, ISCs and EBs respectively. Compared with the controls, the number of esg-lacZ positive cells was significantly increased in wdp1/1 mutant intestines (Fig 2A, 2B and 2Q). Similar phenotype in wdp1/2 trans-heterozygotes was observed (S3E–S3G Fig), excluding the existence of possible background mutations. We also found an increased number of Dl-lacZ and Su(H)GBE-lacZ positive cells in wdp1/1 intestines (Fig 2C–2F and 2Q). Moreover, the number of 10×STAT GFP positive cells was obviously increased (Fig 2G and 2H). In addition, 10×STAT GFP seems to appear in the large putative EC cells (arrows in Fig 2H). These results suggest that midgut homeostasis is disrupted upon wdp loss under normal conditions.
Fig. 2. Loss of wdp disrupts midgut homeostasis under normal conditions and potentiates DSS-induced midgut regeneration. 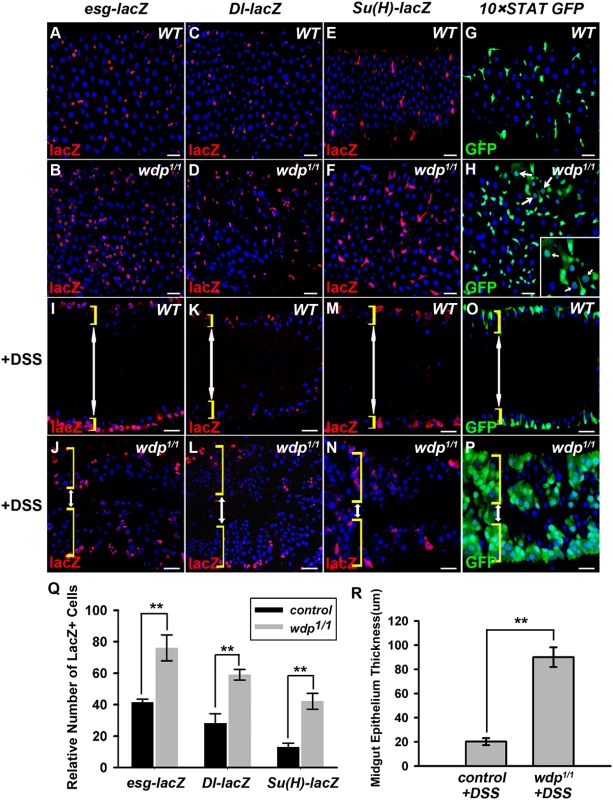
(A and B) The progenitor cells (red, by esg-lacZ) in control (A) or wdp1/1 adult midguts (B) at 25°C for 7 days. (C and D) ISCs (red, by Dl-lacZ) in control (C) or wdp1/1 adult midguts (D) at 25°C for 7 days. (E and F) EBs [red, by Su(H)GBE-lacZ] in control (E) or wdp1/1 adult midguts (F) at 25°C for 7 days. (G and H) 10×STAT GFP positive cells in control (G) or wdp1/1 adult midguts (H) at 25°C for 7 days. The appearance of 10×STAT GFP in putative ECs was observed in wdp1/1 mutants (arrows in H). Square box in H shows the enlarged image of the position labeled by white arrows. (I and J) The progenitor cells (red, by esg-lacZ) in the cross-section of midgut epithelium from control (I) or wdp1/1 adults (J) upon 3% DSS treatment at 29°C for 4 days. Both sides of the midgut epithelium are shown. The yellow brackets indicate the intestinal wall and the white double-headed arrows indicate the intestinal lumen. (K and L) ISCs (red, by Dl-lacZ) in the cross-section of midgut epithelium from control (K) or wdp1/1 adults (L) upon 3% DSS treatment at 29°C for 4 days. (M and N) EBs [red, by Su(H)GBE-lacZ] in the cross-section of midgut epithelium from control (M) or wdp1/1 adults (N) upon 3% DSS treatment at 29°C for 4 days. (O and P) 10×STAT GFP positive cells in the cross-section of midgut epithelium from control (O) or wdp1/1 adults (P) upon 3% DSS treatment at 29°C for 4 days. (Q) Quantification of the relative number of esg-lacZ, Dl-lacZ or Su (H)-lacZ positive cells in wdp1/1 adult midguts at 25°C for 7 days. Mean±SD are shown. n = 5–10 intestines. **p<0.01. (R) The thickness of midgut epithelium (μm) from control or wdp1/1 adults upon 3% DSS treatment at 29°C for 4 days. Mean±SD are shown. n = 8–10 intestines. **p<0.01. Blue indicates DAPI staining. Scale bars, 20μm. We further examined the roles of Wdp under damage conditions. Midgut regeneration of wdp1/1 adults was monitored in response to dextran sulfate sodium (DSS) feeding, which is used to investigate ISC proliferation and tissue regeneration upon damage [54]. Adult flies aged at 3 or 4 days were treated with 3% DSS for 4 days. Under DSS treatment, wdp1/1 adults showed dramatic hyperplasia and extensive multilayering of the midgut epithelium compared with controls (Fig 2I–2P and 2R). Moreover, the number of progenitor cells and 10×STAT GFP positive cells was also increased (Fig 2I–2P), indicating that tissue damage induced midgut regeneration was abnormally enhanced in the absence of wdp. Collectively, these data indicate that loss of wdp disrupts midgut homeostasis under normal conditions and potentiates tissue regeneration under damage conditions.
Wdp inhibits ISC proliferation and restricts ISC overproliferation induced by ectopic JAK/STAT signaling
We further examined whether Wdp is involved in regulating ISC activity. First, mosaic analysis with repressible cell marker (MARCM) approach was used to generate GFP positively marked clones for wdp1 mutants [55]. The control ISC clones contained an average of 7–8 cells per clone 6 days after clone induction (ACI) (Fig 3A, 3D and 3E). In contrast, the wdp1 mutant ISC clones contained up to 30 cells per clone 6d ACI (Fig 3B, 3D and 3F). Moreover, the number of Dl/Pros positive cells, which mark ISC/ee respectively, was increased in wdp1 mutant ISC clones compared with controls (Fig 3A and 3B). In addition, Brdu incorporation within wdp1 mutant clones was also enhanced (Fig 3E, 3F and 3H), suggesting that loss of Wdp led to the increased proliferation of ISCs.
Fig. 3. Wdp inhibits ISC proliferation and restricts the ISC overproliferation caused by ectopic JAK/STAT signaling. 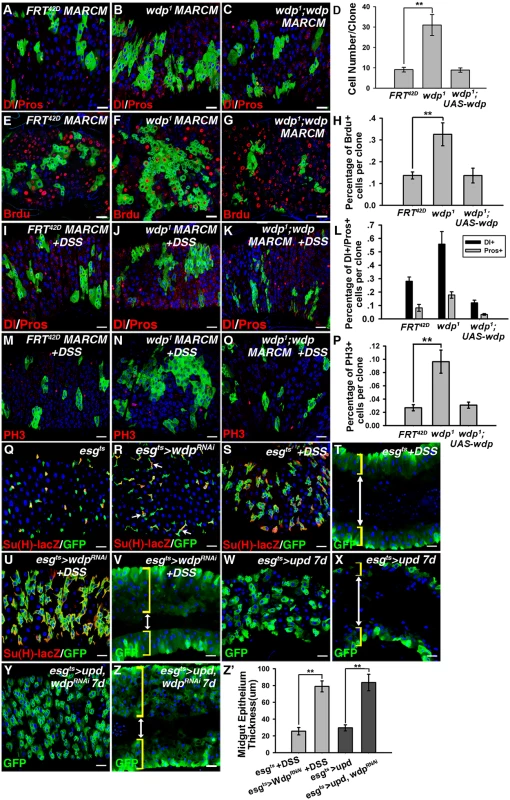
(A-C) Dl/Pros in MARCM control clones (A), MARCM clones of wdp1 cells (B) and MARCM clones of wdp1 cells with simultaneous Wdp expression (C). The overproliferation of ISC observed in wdp1 mutant MARCM clones (B) was rescued in the presence of transgenic wdp (C). (D) Quantification of clone size 6D ACI, including control, wdp1 mutant and wdp1 mutant while expressing transgenic wdp MARCM clones. Mean±SD are shown. n = 9 intestines. **p<0.01. (E-G) Brdu incorporation (Red, by Brdu) in MARCM control clones (E), wdp1 mutant clones (F) and wdp1 mutant clones with simultaneous Wdp expression (G). The increased Brdu incorporation in wdp1 mutant MARCM clones (F) was rescued with the simultaneous Wdp expression (G). (H) Quantification about the percentage of Brdu incorporation per clone with different genotypes. Mean±SD are shown. n = 6–9 intestines. **p<0.01. (I-P) Adult flies with control (I and M), wdp1 mutant (J and N) and wdp1 mutant while expressing transgenic Wdp (K and O) MARCM clones were treated with DSS at 29°C for 4 days. Under stress conditions, the number of Dl/Pros positive cells within wdp1 clones was also increased compared with controls (I and J). The overabundance of PH3 positive cells due to wdp depletion (M and N) was suppressed in the presence of transgenic wdp (O). (L) Quantification about the percentage of Dl+ and Pros+ cells per clone with indicated genotypes under DSS treatment. Mean±SD are shown. n = 8–10 intestines. (P) Quantification about the percentage of PH3+ cells per clone in intestines containing different MARCM clones under DSS treatment. Mean±SD are shown. n = 11–15 intestines. **p<0.01. (Q and R) The number of GFP+ cells was mildly increased upon knockdown of wdp using Su(H)GBE-lacZ; esgts driver (R) compared with controls (Q) at 29°C for 7 days. (S-V) Adult flies of esgts or esgts >wdp RNAi were treated with 3% DSS for 4 days at 29°C. Cross-section of midgut epithelium with the indicated genotypes was shown in T and V. Upon DSS treatment, the number of GFP+ clusters (S and U) as well as the thickness of midgut epithelium (T and V) from esgts >wdp RNAi midguts were significantly increased compared with controls. The intestinal lumen is indicated by white double-headed arrows and the intestinal wall by yellow brackets. (W-Z) The overproliferation of ISCs caused by upd expression (W and X) using the esgts driver was strikingly enhanced with simultaneous wdp knockdown (Y and Z). Cross-section of midgut epithelium with the indicated genotypes was shown in X and Z. (Z’) Quantification of midgut epithelium thickness (μm) with the indicated genotypes. Mean±SD are shown. n = 7–10 intestines. **p<0.01. Blue indicates DAPI staining. Scale bars, 20μm. We also examined the role of wdp in regulating ISC proliferation under damage conditions. Adult flies carrying MARCM clones of various genotypes were fed with DSS. Consistently, we found the size of wdp1 mutant clones was obviously enlarged, and the number of Dl/Pros positive cells was also increased within wdp1 clones compared with controls under damage conditions (Fig 3I, 3J and 3L). Similarly, the number of PH3 positive cells per gut was enhanced in the intestines containing wdp1 mutant clones (Fig 3M, 3N and 3P). It is important to mention that the increased ISC proliferation of wdp1 mutants under physiological conditions or damage conditions could be rescued by simultaneous wdp expression (Fig 3C, 3G, 3K and 3O), confirming that the observed defects were derived from loss of Wdp activity.
We also knocked down Wdp in progenitor cells by expressing wdp RNAi driven by esgts. A mild increase in GFP+ cells was observed when wdp was knocked down in the progenitor cells (Fig 3Q and 3R). Moreover, when the wdp knockdown flies were treated with DSS, the number of GFP+ cells was increased, and the midgut epithelium exhibited extensive multilayering compared with controls (Fig 3S–3V). This suggests that the tissue damage induced ISC proliferation was enhanced in the absence of wdp. Taken together, the above data derived from wdp mutant clones and RNAi experiments indicate that wdp restricts ISC proliferation under normal and regenerative conditions.
As wdp expression could be induced by ectopic JAK/STAT signaling in the intestines, we examined its role under high levels of JAK/STAT signaling. When upd was ectopically expressed using the esgts driver, ISC proliferation was obviously increased (Fig 3W and 3X). Surprisingly, simultaneous knockdown of wdp enhanced the excessive ISC proliferation induced by ectopic Upd expression, as determined by the increase in GFP+ cells and a thickened midgut epithelium (Fig 3Y–3Z’). These results indicate that Wdp restricts ISCs from excessive proliferation caused by ectopic JAK/STAT signaling.
Wdp downregulates JAK/STAT signaling activity
To gain insights into the mechanistic role of wdp in the JAK/STAT pathway, we examined its potential regulation of JAK/STAT signaling in other developmental processes. Eye imaginal disc is a good model to investigate JAK/STAT signaling [56, 57]. We detected the expression of Wdp in 3rd instar eye discs (S1J Fig). 10×STAT GFP is used as the signaling readout [58], which is detected throughout the posterior part of early 3rd instar eye discs (Fig 4A and 4A’). When wdp was ectopically expressed using mirror-Gal4 in the dorsal compartment, the levels of 10×STAT GFP were obviously reduced (Fig 4B and 4B’). Consistently, the activity of 10×STAT GFP was also decreased in the flip-out clones overexpressing Wdp compared with surrounding WT cells (Fig 4C–4D’). On the contrary, we detected enhanced expression region of 10×STAT GFP in the wdp1/1 homozygous eye discs (S4A–S4C Fig). Moreover, Wdp knockdown using mirror-gal4 also led to the enlarged 10×STAT GFP region in the dorsal compartment of 3rd instar eye discs (S4D–S4D‴ Fig). These data indicate that Wdp negatively regulates JAK/STAT signaling in eye discs. In contrast, Wingless (Wg), Hedgehog (Hh), and Decapentaplegic (Dpp) signaling pathways were not affected when wdp was ectopically expressed (S5 Fig), suggesting that Wdp mainly regulates JAK/STAT signaling in imaginal discs.
Fig. 4. Wdp negatively regulates JAK/STAT signaling. 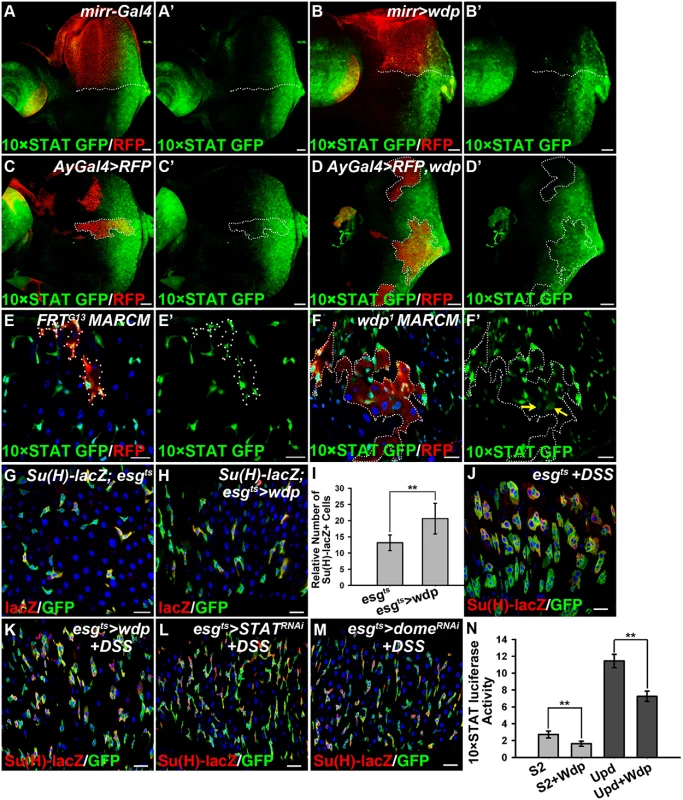
(A and A’) In WT, 10×STAT GFP is highly expressed throughout the posterior part of early 3rd instar eye discs. All the eye discs shown here are oriented anterior left, dorsal up. D/V boundary is shown by the dotted line. (B and B’) The levels of 10×STAT GFP (B’) were reduced in the dorsal compartment of early 3rd instar larva eye discs upon wdp expression using mirrorGal4. CD8-mRFP was used to mark the dorsal compartment. (C-C’) The activity of 10×STAT GFP in early 3rd instar larva eye discs bearing the control flip-out clones (Act>y+>Gal4, UAS-RFP) marked by the presence of RFP and dotted lines. (D-D’) The activity of 10×STAT GFP was decreased in Wdp overexpressing clones marked by RFP expression and dotted lines compared with surrounding WT cells in early 3rd instar larva eye discs. (E-F’) 10×STAT GFP was used to monitor the activity of JAK/STAT signaling in intestinal MARCM clones marked by the presence of RFP. The activity of 10×STAT GFP was increased in wdp1 MARCM clones compared with surrounding wild-type cells (F and F’). Yellow arrows in F’ indicate the appearance of 10×STAT GFP in putative ECs. Dotted lines denote the position of MARCM clone cells. (G-I) A slightly increased number of EBs was found upon overexpression of wdp (H) using esgts driver at 29°C for 10 days compared with controls (G). I shows quantification of Su(H)GBE-lacZ positive cells. Mean±SD are shown. n = 8 intestines. **p<0.01. (J-M) Adult flies of esgts (J), esgts>wdp (K), esgts>STAT RNAi (L) or esgts>Dome RNAi (M) were treated with DSS for 4 days at 29°C before dissection. Overexpression of wdp using the esgts driver (K) blocked the formation of large GFP+ clusters caused by DSS treatment. The phenotype was reminiscent of flies with STAT or Dome knockdown using esgts driver (L and M) when fed with DSS. (N) The activity of 10×STAT luciferase reporter in S2 cells transfected with UAS-wdp. Wdp expression was able to suppress the basal luciferase activity as well as Upd-induced upregulation of 10×STAT luciferase activity. Mean±SD are shown. **p<0.01. Blue indicates DAPI staining in E-M. Scale bars, 20μm. As mentioned above, loss of Wdp could potentiate Upd induced ISC proliferation (Fig 3W–3Z’), implying its regulation of JAK/STAT signaling in posterior midguts. To verify this hypothesis, we examined JAK/STAT signaling by detecting the activity of 10×STAT GFP in RFP positively marked intestinal MARCM clones. In wdp1 mutant clones, the levels of 10×STAT GFP were mildly increased when compared with surrounding wild-type cells (Fig 4F and 4F’). Moreover, in wdp1 mutant clones we detected 10×STAT GFP in the large cells (putative EC cells) as well as in small progenitors (arrows in Fig 4F’), implying JAK/STAT signaling maybe abnormally activated in ECs. However, in control clones there seems no obvious difference of 10×STAT GFP activity between RFP+ clones and RFP - cells. Besides, 10×STAT GFP was restricted in small progenitors (Fig 4E and 4E’). Furthermore, we examined the destabilized 10×STAT DGFP reporter [58] in wdp1/1 intestines and found the activity of 10×STAT DGFP was obviously increased compared with controls (S4E–S4F’ Fig). Consistently, 10×STAT DGFP also appeared in large ECs in wdp1/1 homozygotes (S4E–S4F’ Fig). These results suggest that JAK/STAT signaling was upregulated in the absence of wdp. Meanwhile, we used the esgts driver to overexpress wdp in the progenitor cells and found mild increase of EBs under normal conditions (Fig 4G–4I). However, when esgts>wdp flies were treated with DSS, the damage-induced tissue regeneration was suppressed. In contrast to the large GFP+ clusters containing both small diploid progenitors and large polyploid cells in the controls, there were no large GFP+ cells observed in the clusters of the esgts>wdp midguts (Fig 4J and 4K). This was reminiscent of intestines with deficient JAK/STAT signaling by STAT or Dome knockdown under DSS treatment (Fig 4L and 4M). Altogether, these data suggest that Wdp could interfere with JAK/STAT signaling in posterior midguts.
We further assessed the activity of 10×STAT luciferase reporter in S2 cells transfected with wdp. Consistently, we found Wdp expression was able to suppress the basal 10×STAT luciferase as well as Upd-induced upregulation of 10×STAT luciferase activity (Fig 4N). Taken together, the above data derived from different tissues indicate that Wdp is a negative regulator of the JAK/STAT signaling pathway.
Wdp acts downstream of Upd but upstream of Hop
To determine the level at which Wdp modulates JAK/STAT signaling, we examined the epistatic relationship between Wdp and the JAK/STAT pathway components. When hop was ectopically expressed using mirrorGal4 in eye discs, we observed increased JAK/STAT activity, as determined by an expanded expression region of 10×STAT GFP in the dorsal compartment which is marked by CD8-mRFP (Fig 5A and 5A’, arrow). Simultaneous expression of wdp failed to suppress the elevated JAK/STAT signaling caused by hop overexpression (Fig 5B, 5B’ and 5C), suggesting that Wdp acts upstream of Hop. Consistent with previous study [28], ectopic expression of Hop in midguts using the esgts driver led to weak expansion of esg positive cells (Fig 5D and 5D’). ISC over-proliferation caused by hop expression was not affected in the presence of wdp (Fig 5E, 5E’ and 5F). Thus, these epistatic experiments performed in both the midguts and eye discs placed Wdp upstream of Hop.
Fig. 5. Wdp functions downstream of Upd but upstream of Hop. 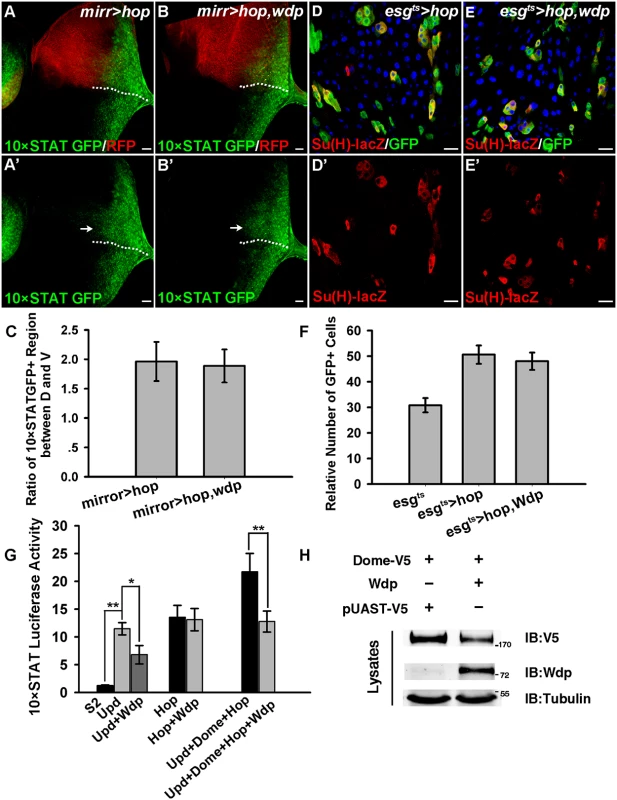
(A and A’) The activity and the expression regions of 10×STAT GFP were noticeably enhanced (arrow in A’) when hop3w was ectopically expressed using mirrorGal4 in the dorsal compartment marked by CD8-mRFP of early 3rd instar larva eye discs. D/V boundary is shown by the dotted line. All the eye discs shown here are oriented dorsal up, posterior right. (B and B’) Simultaneous expression of wdp was unable to suppress the increased 10×STAT GFP activity due to the hop3w overexpression in the dorsal compartment (arrow in B’). (C) Quantification about the ratio of 10×STAT GFP expression region (along the A-P axis) between dorsal and ventral part in early 3rd instar eye discs with indicated genotypes. Mean±SD are shown. n = 9–10 discs. (D-D’) Ectopic expression of hop3w using the esgts driver promotes ISC proliferation at 29°C for 7 days. (E-E’) Simultaneous expression of wdp cannot suppress overproliferation of ISC caused by ectopic hop3w expression using the esgts driver at 29°C for 7 days. (F) Quantification of the relative number of esgts>GFP cells with indicated genotypes. Mean±SD are shown. n = 12–15 intestines. (G) The increased activity of 10×STAT luciferase due to Upd expression can be suppressed by cotransfection of UAS-wdp. However, wdp overexpression can’t block the constitutive activation of JAK/STAT signaling caused by hop. The increased 10×STAT luciferase activity resulting from cotransfection of UAS-upd, dome and hop was obviously suppressed in the presence of wdp. Mean±SD are shown. *p<0.1, **p<0.01. (H) S2 cells transfected with UAS-dome-V5 alone or along with wdp were lysed and assessed for total Dome levels using V5 antibody. Blue indicates DAPI staining in D and E. Scale bars, 20μm. To further confirm the genetic epistasis between Wdp and Hop, we assessed the activity of 10×STAT luciferase reporter in S2 cells cotransfected with wdp and hop-V5 vectors. Consistently, the increased 10×STAT luciferase activity resulting from hop expression could not be blocked by cotransfection of wdp. However, ectopic expression of wdp was able to suppress the enhanced activity of 10×STAT luciferase caused by Upd expression, indicating that Wdp acts downstream of Upd. In addition, increased 10×STAT luciferase activity resulting from simultaneous transfection of UAS-upd, dome, and hop was significantly suppressed by cotransfection with wdp (Fig 5G). Moreover, we found Wdp expression could autonomously suppress the upregulation of JAK/STAT signaling caused by ectopic Upd expression in imaginal discs (S6 Fig), further confirming that Wdp functions downstream of Upd. Taken together, Wdp acts upstream of Hop but downstream of Upd to inhibit JAK/STAT signaling.
Wdp interacts with Domeless and promotes its endocytosis and lysosomal degradation
The JAK/STAT pathway is under tight control at various steps by different regulators and regulatory mechanisms. Since Wdp functions upstream of Hop and downstream of Upd, we examined the possible regulation of Wdp to the Dome receptor. Importantly, the total levels of Dome were reduced in S2 cells coexpressing Wdp (Fig 5H), implying that Wdp may affect the stability of Dome. Previous study showed that JAK/STAT signaling is negatively regulated by endocytic trafficking [59]. One possibility is that Wdp promotes Dome endocytosis for subsequent degradation. To test this, we performed the following experiments. First, when S2 cells were transfected with Dome-HA alone, Dome was mainly localized on the cell membrane, with a few punctates detected in the cytoplasm (Fig 6B, 6B’ and 6D). However, when co-expressed with Wdp, the majority of Dome was present in the cytoplasm as vesicle-like punctates (Fig 6C, 6C’ and 6D), implying the endocytosis of Dome is enhanced. To further determine whether Wdp could promote Dome endocytosis, we carried out time-lapse imaging experiments. After the live S2 cells expressing Dome-GFP were incubated with endocytic dye FM 4–64 at room temperature for 1h, we examined the dynamics of Dome-GFP and chased its co-localization with FM 4–64 at different time points. As shown in Fig 6E, in the absence of Wdp the majority of Dome-GFP was localized on the cell membrane and little co-localization with FM 4–64 was detected. When cotransfected with wdp, Dome-GFP was mainly observed as intracellular particles, which were partially co-localized with FM 4–64 (see arrowheads in Fig 6F). Furthermore, we observed newly formed Dome-GFP endocytic vesicles trafficking from the cell membrane (S1 Movie). All of these tissue culture data based on the overexpressed Wdp suggest that Wdp can promote Dome internalization.
Fig. 6. Wdp expression promotes Dome endocytosis and alters its subcellular localization in S2 cells. 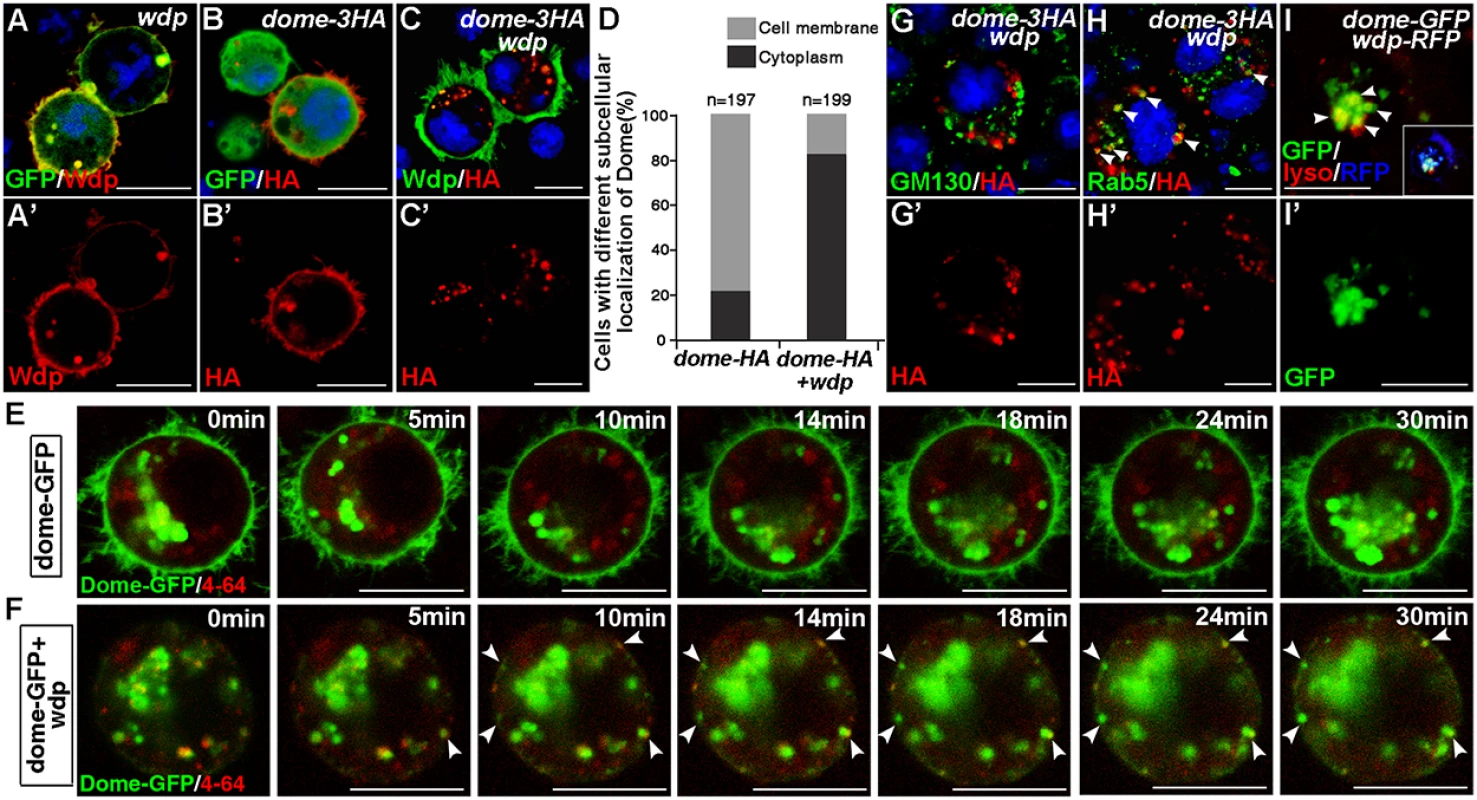
(A and A’) Wdp (red, by Wdp) was localized on the cell membrane in S2 cells cotransfected with UAS-wdp and GFP-GPI vectors. (B-C’) The subcellular localization of Dome in S2 cells transfected with the indicated vectors. In S2 cells expressing dome-HA alone, Dome-HA was mainly localized on the cell membrane and only few punctates were detected in the cytoplasm (B and B’). However, in S2 cells cotransfected with dome-HA and wdp, the majority of Dome-HA was detected as punctate particles in the cytoplasm instead of on the cell membrane (C and C’). (D) Ratio of S2 cells with Dome localized either on the cell membrane or in the cytoplasm when they were transfected with indicated vectors. (E and F) S2 cells transfected with dome-GFP alone (E) or in combination with wdp (F) were treated with 5μg/ml endocytic dye FM4-64 for 1h. And then time-lapse imaging was performed to detect dynamics of Dome-GFP and chase its co-localization with FM 4–64 at indicated time points. Arrowheads in F indicate the newly formed endocytic vesicles containing Dome-GFP on the cell membrane. (G-H’) S2 cells expressing Dome-HA and Wdp were immunostained with GM130 (G and G’) or Rab5 antibody (H and H’) to mark cis-Golgi or early endosome respectively. Dome-HA was partially co-localized with Rab5 (H, arrowheads). However, no obvious co-localization between Dome-HA and GM130 was observed (G). (I and I’) S2 cells cotransfected with dome-GFP and wdp-RFP were live stained with 50nM lysotracker solution for 2h. Dome-GFP was found to partially co-localize with the lysotracker as arrowheads shown in I. Square box is reduced image showing the overall expression of Dome-GFP (green), Wdp-RFP (blue) and lyso-tracker (red). Blue indicates DAPI staining in A-C and G-H. Scale bars, 10μm. We further examined the co-localization of Dome with various vesicular markers in S2 cells. Little co-localization was observed between intracellular Dome and the cis-Golgi apparatus as marked by GM130 (Fig 6G and 6G’), implying the presence of intracellular Dome-GFP was not due to defects in exocytosis. However, a large amount of Dome intracellular particles were co-localized with the early endosome marker Rab5 (Fig 6H and 6H’). In addition, high levels of Dome-GFP were present in the lysosomes as labeled by lyso-tracker in live cells (Fig 6I and 6I’). Moreover, the appearance of Dome intracellular punctates observed in Wdp-coexpressing cells was partially suppressed by Rab5 dsRNA treatment (S7 Fig), indicating that the accumulation of Dome intracellular punctates was a result of Rab5-mediated endocytosis. Interestingly, the subcellular localization of other membrane proteins such as CD8-mRFP or GFP-GPI was not affected when coexpressed with Wdp (S8 Fig), suggesting that Wdp specifically promotes Dome endocytosis.
We also examined whether Wdp functions similarly in vivo. We generated flip-out clones overexpressing Dome alone or along with Wdp in the wing and eye imaginal discs. Consistent with previous reports [59–61], Dome-V5, as a transmembrane receptor, was mainly localized on the cell membrane, and also formed some intracellular punctate structures which could correspond to endocytic vesicles (Fig 7A–7A‴). Importantly, coexpression with Wdp caused a significant change in the subcellular localization of Dome (Fig 7B–7B‴). In the presence of Wdp, Dome was totally disappeared from the cell membrane, but was found as intracellular punctates (Fig 7B–7B‴), where they partially colocalized with the early endosome marker Rab5 (Fig 7C–7C‴). In addition, we also observed the same phenomena in the eye discs (S9 Fig). Therefore, these data suggest that enhanced Wdp expression could promote Dome internalization in the wing and eye discs.
Fig. 7. Wdp interacts with Dome to promote its internalization for lysosomal degradation. 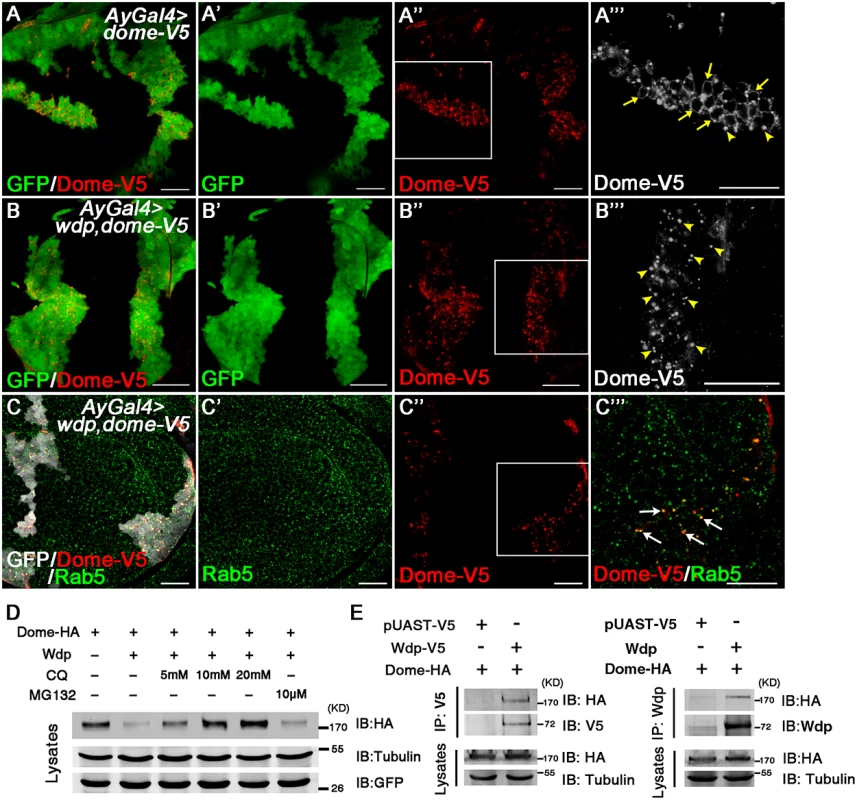
(A-A‴) In wing discs bearing GFP positively marked clones overexpressing Dome-V5 (Act>y+>Gal4, UAS-GFP, UAS-dome-V5), Dome-V5 was mainly localized on the cell membrane (yellow arrows) despite some intracellular punctates (yellow arrowheads). A‴ is the enlarged image of the position labeled by square box in A”. (B-B‴) Coexpression with Wdp alters the subcellular localization of Dome-V5. In wing discs bearing GFP positively marked clones expressing Dome-V5 together with Wdp (Act>y+>Gal4, UAS-GFP, UAS-dome-V5, UAS-wdp), Dome-V5 was depleted from cell membrane but detected as cytoplasmic punctate structures (yellow arrowheads). B‴ is the enlarged image of the position labeled by square box in B”. (C-C‴) The intracellular particles of Dome-V5 in the presence of Wdp were partially colocalized with early endosomes marked by Rab5 staining (white arrows). C‴ is the enlarged image of the position labeled by square box in C”. (D) To detect whether the internalized Dome was degraded, different concentrations of Chloroquine (5, 10 or 20mM) or 10 uM MG132 were used to treat S2 cells transfected with UAS-dome-HA and UAS-wdp. Then cell lysates were analyzed by western blotting with the indicated antibodies. (E) Wdp interacts with Dome in transfected S2 cells. HA-tagged dome, V5-tagged wdp (or no tagged wdp), or pUAST-V5 were transfected individually or together into S2 cells. Cell lysates were immunoprecipitated and analyzed by western blotting with the antibodies indicated. All the wing discs shown here are oriented anterior left, dorsal up. Scale bars, 20μm. To further determine whether Dome is degraded in the lysosomes after being internalized, S2 cells were treated with Chloroquine (CQ), a lysosomal inhibitor. Interestingly, we found the reduction of Dome levels caused by Wdp co-expression was restored upon Chloroquine treatment but not upon MG132 treatment, suggesting that the internalized Dome is undergoing lysosomal degradation rather than proteasome degradation (Fig 7D). This result is in agreement with the recently published paper showing that Dome undergoes lysosomal degradation [62]. Taken together, our data indicate that Wdp functions to promote Dome endocytosis through the endosomes, and subsequently to the lysosomes for degradation.
We then investigated whether Wdp interacts with Dome to promote its endocytosis. We transfected HA-tagged Dome and wdp (or V5-tagged wdp) into S2 cells and found HA-tagged Dome could co-immunoprecipitate with both Wdp and V5-tagged Wdp (Fig 7E). These data indicate that Wdp interacts with Dome in transfected cells. Taken together, our data suggest that Wdp interacts with Dome and then promote its internalization from the cell membrane into the early endosomes, and finally to the lysosomes for degradation. In this way, Wdp attenuates JAK/STAT signaling to avoid uncontrolled signaling activation.
Discussion
In this study we have provided evidence that the LRR protein Wdp is a novel component of the JAK/STAT pathway that acts in a negative feedback manner to modulate JAK/STAT signaling activity and control intestinal homeostasis. Our in vivo and in vitro data indicate that wdp expression levels are positively regulated by JAK/STAT signaling. Loss of wdp disrupts midgut homeostasis under both physiological and damage conditions. Conversely, ectopic expression of Wdp leads to the reduction of JAK/STAT signaling activity. Mechanistically, we show that Wdp can interact with Dome, and promote Dome internalization and lysosomal degradation, thereby reducing JAK/STAT signaling activity.
Wdp controls intestinal homeostasis through interfering with JAK/STAT signaling activity
Midgut homeostasis is tightly controlled by different signaling pathways. During tissue damage, JAK/STAT, EGFR, JNK and Hippo signaling pathways are required for ISC proliferation and midgut regeneration [26, 30, 32, 33, 46, 63–65]. On the other hand, other signaling pathways, such as BMP signaling, may negatively regulate intestinal homeostasis after injury, although there exists some controversy about the function of BMP signaling during Drosophila intestinal development [36–39]. However, the mechanism of how ISC activity returns to quiescence after injury remains largely unknown. Here, we demonstrate that Wdp controls intestinal homeostasis through interfering with JAK/STAT signaling activity to avoid tissue hyperplasia.
Our data indicate that loss of Wdp disrupts midgut homeostasis under normal conditions and potentiates tissue regeneration under damage conditions (Figs 2 and 3). The proliferation rate of ISCs mutant for wdp is increased, while the differentiation of EC and ee cells is not inhibited (Fig 3 and S3A–S3D Fig). In addition, ectopic Wdp expression suppressed the damage induced tissue regeneration. Our data further demonstrate that Wdp controls intestinal homeostasis through interfering with JAK/STAT signaling activity (Fig 4). First, Wdp acts as a JAK/STAT downstream target and its expression levels are positively regulated by JAK/STAT signaling (Fig 1 and S2 Fig). Second, Wdp functions in a negative feedback loop to modulate JAK/STAT signaling activity (Fig 4 and S4 Fig). It is interesting to note that JAK/STAT signaling is mainly activated in ISCs and EBs [26]. However, we found that Wdp expression levels seem higher in ECs compared with progenitor cells (S1A–S1D’ Fig). One explanation is that low levels of Wdp in progenitors may guarantee high levels of JAK/STAT signaling, while high levels of Wdp in ECs may serve to reduce Dome levels thereby making ECs insensitive to Upd ligands. Consistent with this view, previous work showed that Dome is mainly expressed in the progenitors but not in their progeny [26]. Moreover, we found Wdp knock down using EC specific Myo1Ats also leads to the disruption of midgut homeostasis and the presence of 10×STAT GFP in putative EC cells (S4G–S4H’ Fig), suggesting that JAK/STAT signaling is activated upon wdp knockdown in ECs. On the other hand, we found Wdp expression was reduced but not totally eliminated in JAK/STAT signaling deficient cells (S2 Fig), suggesting that the basal level of Wdp in intestines (especially in ECs) may also be regulated by other regulatory mechanisms or signaling pathways. Further experiments are needed to clarify this issue.
It’s important to mention that Wdp expression could be induced under injury conditions, such as DSS or bleomycin treatment (S2I Fig). Consistent with our results, two recent studies also identified wdp as an upregulated gene upon Ecc15 and Pseudomonas entomophila (P.e) infection through their microarray data respectively [44, 66]. These stress conditions are also associated with the activation of JAK/STAT signaling [26, 46]. Therefore, their findings are consistent with our view that Wdp can be induced by the JAK/STAT pathway and then restrict its signaling activity in restoring intestinal homeostasis after tissue damage.
We further demonstrated the regulation of Wdp to JAK/STAT signaling in eye discs and S2 cells. 10×STAT GFP activity was decreased in eye discs overexpressing Wdp (Fig 4A–4D’) while increased in wdp mutant eye discs (S4A–S4D‴ Fig). Similarly, a reduction of 10×STAT luciferase activity was also observed in S2 cells transfected with Wdp (Fig 4N). Thus, we propose that Wdp is also likely to modulate JAK/STAT signaling activity for proper development of other tissues.
Taken together, we conclude that Wdp is involved in controlling intestinal homeostasis through interfering with JAK/STAT signaling in a negative feedback manner.
Wdp inhibits JAK/STAT signaling through promoting Dome endocytosis
Previously, several studies have addressed the roles of endocytosis in regulating JAK/STAT signal pathway. The Noselli lab found blocking internalization led to an inhibition of JAK/STAT signaling activity [61], while the Zeidler group reported the opposite results [59]. Moreover, several recent studies demonstrate that loss of ept/tsg101 or Rabex-5, two endocytic tumor suppressor genes, also induced JAK/STAT signaling activation and tissue overgrowth [67, 68]. Yet, the regulatory mechanism of how Dome receptors are internalized remains largely unknown. Here we demonstrate that Wdp promotes Dome endocytosis and subsequent lysosomal degradation. First, in S2 cells Wdp ectopic expression induces the formation of Dome endocytotic vesicles which were colocalized with the early endosome marker and lysosome marker (Fig 6). Second, we found Wdp expression can also promote Dome endocytosis in wing and eye imaginal discs. Furthermore, the decreased Dome levels caused by Wdp expression can be suppressed by CQ treatment (Fig 7). All of these data argue that Wdp acts to promote Dome endocytosis from the cell membrane, first into the early endosomes, and finally into the lysosomes for degradation. Previous work are mainly about Dome receptors undergo ligands induced endocytosis [59, 61], while in this work we show that Wdp is able to promote Dome internalization in a Upd independent manner. Our coimmnoprecipitation data indicate Wdp can interact with Dome (Fig 7E). Moreover, S1 Movie shows that Dome-GFP are aggregated on the cell membrane before they are internalized in the presence of Wdp. Therefore, one possible mechanism is that Wdp interacts with Dome, induces the aggregation of Dome on the cell membrane and then promotes Dome endocytosis. Further experiments are needed to define the detailed mechanism.
A model for the role of Wdp in regulating JAK/STAT pathway during tissue damage
On the basis of our findings, the following model is proposed (Fig 8A and 8B): Wdp regulates intestinal homeostasis through its modulation of JAK/STAT signaling. Under physical conditions, low levels of Wdp in progenitors are needed to maintain proper levels of JAK/STAT signaling activity, while high levels of Wdp in ECs reduce Dome levels to ensure these cells are insensitive to JAK/STAT signaling. When midgut epithelium is damaged by environmental challenges, high levels of JAK/STAT signaling activity are induced to replenish the damaged midgut. Then Wdp expression is highly induced in the intestines to reduce Dome levels, thereby switching off the overactivated JAK/STAT signaling. Through this way, ISC proliferative rate returns to normal levels to avoid tissue hyperplasia. While other mechanisms or regulators are likely to be involved in regulating intestinal homeostasis, our data suggest that Wdp is one of the key regulators in this process through interfering with JAK/STAT signaling activity.
Fig. 8. Model for the function of Wdp. 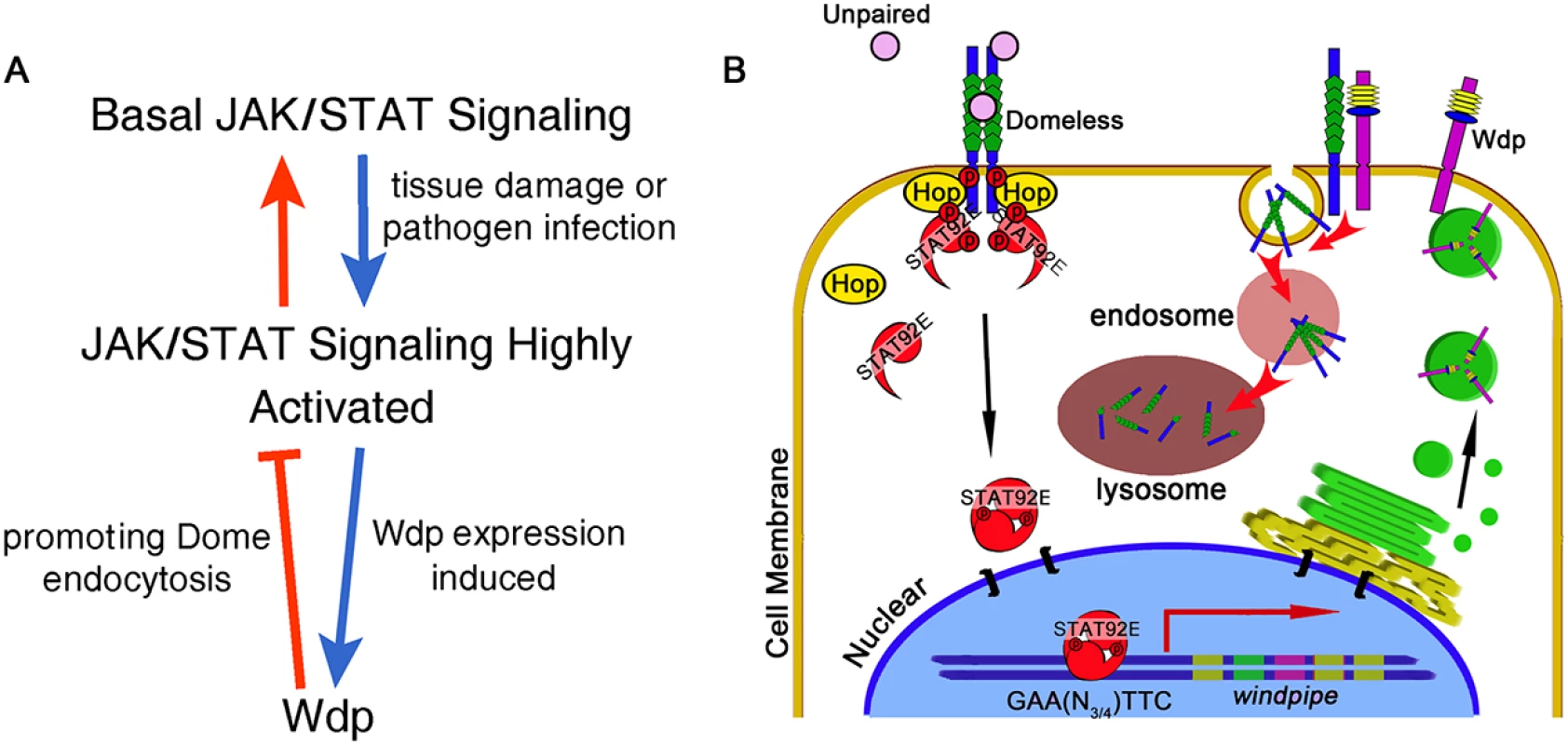
During tissue damage or pathogen infection induced midgut regeneration, the JAK/STAT pathway is highly activated (A). In response to extracellular signaling, STAT92E dimers translocate into the nucleus, bind to its consensus binding sites at the genomic region of wdp and then promote its transcription. Newly synthesized Wdp protein is transported to the cell membrane, where it interacts with Dome and promotes Dome internalization from the cell membrane finally into the lysosomes for subsequent degradation (B). Through this negative feedback manner, Wdp restricts the signal duration and ensures JAK/STAT signaling returns to the normal levels after injury in Drosophila intestines. Methods
Fly genetics
Information for alleles and transgenes used can be found either in FlyBase or as noted: P{wHy}wdpDG23704(BL20481), wdp1, wdp2, w1118, esg-lacZ, Dl-lacZ, Su(H)GBE-lacZ (gift from Sarah Bray), 10×STAT GFP(II), 10×STAT GFP(III), 10×STAT DGFP, FRT42D, FRTG13, FRT82B, wdp RNAi(75B), UAS-upd/cyo, UAS-STAT, esgGal4-gal80ts-UAS-GFP/cyo (gift from Norbert Perrimon), Su(H)GBE-LacZ; esgGal4-gal80ts-UAS-GFP, UAS-wdp(36B)/cyo, UAS-wdp(86F)/Tm6B, UAS-STAT RNAi (BL33637), UAS-domeless RNAi (BL34618), UAS-hop 3w (gift from Rongwen Xi), UAS-dome-V5, UAS-RFP, EnGal4, mirrorGal4, Myo1A Gal4;tub Gal80ts (gift from Steven. Hou), Notch264-39, STAT92E06346–FRT82B (gift from Rongwen Xi), UAS-Notch RNAi; STAT92E06346-FRT82B (gift from Rongwen Xi). The genotypes of all flies used in this paper can be found in S1 Text.
Constructs
pUAST-wdp, wdp-V5 and wdp-RFP were constructed by cloning the wdp cDNA into pUAST-attB, pUAST-attB-V5 and pUAST-RFP vectors respectively. Dome-V5 and hop-V5 were constructed by insertion of the coding region, from transgenic lines UAS-Dome (a gift from S. Hou) and UAS-Hop3w (a gift from Rongwen Xi), into pUAST-V5-attB vector. Dome was excised from pUAST-dome-V5 and inserted in pUAST-3HA or pUAST-GFP to generate pUAST-dome-3HA or pUAST-dome-GFP vectors respectively. pAC5.1-upd-V5 was made by cloning upd cDNA into pAC5.1-V5 vectors. UAS-wdp RNAi was made by cloning annealed oligos ctagcagtAGAGGAGAGCGATGTTAGACCtagttatattcaagcataGGTCTAACATCGCTCTCCTCTgcg and aattcgcAGAGGAGAGCGATGTTAGACCtatgcttgaatataactaGGTCTAACATCGCTCTCCTCTactg into EcoRI/ NheIsites of pWalium20 vector[69] and was confirmed to be functional (S1H and S1I Fig). 10×STAT luciferase vector was generated by subcloning firefly luciferase gene into 10×STAT Gal4 vector. To determine the binding sites of STAT92E in wdp genomic regions, we generated luciferase vectors containing putative binding regions based on the ChIP results. Primers used for constructing luciferase vectors can be found in S1 Text.
Wdp antibody generation
We generated polyclonal antibody specific for Drosophila Wdp protein by choosing the hydrophilic polypeptides 480-550aa and 591-661aa as the antigen. GST-tagged Wdp antigen was expressed in E. coli BL21 (DE3) and purified with GST affinity chromatography. Using this antigen, we generated and further separated mouse polyclonal antibody of Drosophila Wdp.
MARCM clone
MARCM clones in the adult midguts were induced by heat-shocking 3–4 day-old females for 75 min at 37°C. Adult guts were dissected and examined 6 days after clone induction.
Flip-out clone
For Flip-out clones in adult midguts, crosses were set up and cultured at 25°C. Flies were heat-shocked at 37°C for 75 minutes 3 days after eclosion and then dissected 6 days later. For Flip-out clones in wing or eye discs, crosses were kept at 25°C. Larvae were heat-shocked for 90 minutes at 37°C 48 hours after egg deposition and dissected at late 3rd instar larva stage.
Feeding experiments
Female adult flies at age 3 or 4 days were used to perform feeding experiments. Flies were cultured in an empty vial containing chromatography paper wet with 3% dextran sulfate sodium (MP Biomedicals) or 25μg/mL bleomycin (Sigma) dissolved in 5% glucose solution with heat inactivated yeast for 4 days at 29°C.
Antibodies used for immunostaining, immunoprecipitation, and western blotting
Fixation and antibody staining in imaginal discs were performed as described [70]. Fixation and antibody staining in cultured cells were performed as described [71]. Fixation and antibody staining in midguts were performed as described [37]. Primary antibodies used for the immunostaining were: mouse anti-Wdp (1 : 1000), chicken anti-lacZ (Abcam, 1 : 1000), mouse anti-Dl (DSHB, 1 : 50), mouse anti-Pros (DSHB, 1 : 200), rabbit anti-PH3 (Millipore, 1 : 2000), mouse anti Brdu (DSHB, 1 : 200), rabbit anti Pdm1(1 : 1000, gift from Xiaohang Yang), mouse anti-V5 (Invitrogen, 1 : 3000), mouse anti-HA (Abmart, 1 : 500), rabbit anti-GM130 (Abcam, 1 : 200), rabbit anti-Rab5 (Abcam, 1 : 200), Gp anti-Sens (1 : 200), rabbit anti-Sal (1 : 100), and Rat anti-Ci (DSHB, 1 : 5). The primary antibodies were detected by fluorescent-conjugated secondary antibodies from Jackson ImmunoResearch Laboratories, Inc. The primary antibodies used for IP and western blot were: rabbit anti-V5 (Sigma, 1 : 1000), rabbit anti-HA (Santa Cruz, 1 : 1000), mouse anti-Wdp (1 : 500), rabbit anti-GFP (Abmart, 1 : 1000) and mouse anti-tubulin (Abmart, 1 : 1000).
Brdu incorporation
Adult flies with MARCM clones were reared on standard corn meal food with 0.2mg/ml BrdU (Sigma) at 25°C for 4 days before dissection. Then midguts were treated with 3M HCl at 37°C for 30 min, and the reaction was stopped by washing with PBT twice.
RT-qPCR
RNA was extracted from 20 intestines of female adults using RNA pre pure kit (TIANGEN) and complementary DNA (cDNA) was synthesized with the M-MLV Reverse transcriptase (Promega). qPCR was performed using GoTaq qPCR Master Mix kit (Promega) on CFX96 Real-time PCR system(Bio-Rad). Experiments were performed in 3 biological independent replicates, each also contained 3 repeats. All the results are shown as Mean±SD of the biological replicates. Ribosomal gene RpL11 was used as normalization control. Primers used for qPCR are listed in S1 Text.
ChIP-Seq
The identification of STAT92E target genes in adult intestines was carried out through ChIP assay and ChIP-high throughput sequencing technique. JAK/STAT signaling was activated using esgts to overexpress Upd and STAT92E at 29°C for 10 days. Then about 400 adult intestines were dissected and cross-linked with 1% formaldehyde for 15 minutes. After washing process to remove the formaldehyde, intestinal tissue was lysed with RIPA buffer which contains 1% SDS on ice for 30 minutes. The sonication of chromatin was performed using the Covaris (AFA) system with 3% power output for 5 minutes each on 100μl lysate. STAT92E-bound chromatin fragments were enriched by immunoprecipitation with mouse raised STAT92E antibody. Most chromatin fragments resulting from sonication occurred between 200 and 400 bp. The process of dilution, antibody incubation, protein G pull down, beads washing, DNA complex elution, de-link, RNAse A / proteinase K digestion and DNA extraction are all performed according to standard protocols. The high throughput sequencing process was carried out using the Illumina solexa system.
Cell culture, transfection, coimmunoprecipitation and western blotting
Drosophila S2 cells were maintained at 25°C in HyQ SFX-insect cell culture medium. All transfection experiments were carried out using Effectene Transfection Reagent (QIAGEN).
For Wdp and Dome interaction experiments, S2 cells were transfected in 60mm dishes with 200ng Arm-Gal4, 200ng pUAST-dome-HA and 200ng pUAST-V5 control vector, or pUAST-wdp (with or without V5 tag). Then S2 cells were lysed in 200μl RIPA buffer without SDS on ice for 30 minutes. RIPA buffer includes 50 mM Tris-HCl (pH 7.8), 150 mM NaCl, 5 mM EDTA (pH 8.0), 0.5% Triton X-100, 0.5% NP-40, 0.5%DOC, complete protease inhibitor cocktail tablets (Roche), and phosphatase inhibitor cocktail tablets (Roche). After centrifugation, the suspension of lysates was added with antibody and incubated for 3h at 4°C, and then added with BSA blocked protein G beads and rotated overnight at 4°C. The immunocomplexes were collected by centrifugation and washed with 1 ml of RIPA buffer three times.
For lysosome or proteasome inhibition assay, S2 cells were treated with 5, 10 or 20 mM Chloroquine (Sigma-Aldrich) or 10 uM MG132 (Sigma-Aldrich) respectively for 24 h before harvesting.
For western blotting, immunoprecipitated proteins were separated in SDS-PAGE and then blotted onto PVDF membranes. The membranes were stained with primary antibody overnight at 4°C, as anti-V5, anti-HA, anti-Wdp to detect interaction between Wdp and Dome. Antibody HA was used to examine the effects of Wdp on Dome levels. Followed by washing, PVDF membranes were incubated with secondary antibodies carrying infrared fluorophore, and then analyzed using Odyssey system (GENE).
Luciferase assay
S2 cells were seeded in 24-wells plate. Cells in each well were transfected with 5ng Renilla-luciferase, 30ng 10×STAT-luciferase reporter (or other reporters) and 30 ng other vectors as shown in figures. After 12h, cells were mixed with Upd transfected cells. After an additional 48h, S2 cells were washed with PBS and then lysed using Passive Lysis Buffer (Promega). Firefly-luciferase and Renilla-luciferase activity were detected using GLOMA Multi Detection System (Promega). All the results are from twice independent experiments each containing 3 repeats.
Live cell imaging
For labeling of endocytic vesicles, S2 cells were treated with 5μg/ml FM4-64 (Molecular Probes, Inc.) at 25°C for 1h. S2 cells were then washed twice with medium and then incubated at 25°C for another 1h. Then 200ul of cell suspension was applied to a microscope slide. Images were captured by a Zeiss LSM780 inverted confocal microscope and movies were made from time-lapse images using Corel Video Studio X4. For labeling of lysosomes, S2 cells were incubated with cell culture containing lyso-tracker (Invitrogen) at a final concentration of 50 nM at 25°C for 2h.
Supporting Information
Zdroje
1. Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006 Jul;133(14):2605–16. 16794031
2. Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004 Apr;198 : 72–82. 15199955
3. Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007 Apr 20;316(5823):402–4. 17446390
4. Hou SX, Zheng Z, Chen X, Perrimon N. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev Cell. 2002 Dec;3(6):765–78. 12479803
5. Schindler CW. Series introduction. JAK-STAT signaling in human disease. J Clin Invest. 2002 May;109(9):1133–7. 11994400
6. Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006 Nov;25(5):745–55. 17088085
7. Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003 Nov;197(2):157–68. 14502555
8. O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002 Apr;109 Suppl:S121–31. 11983158
9. Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004 Mar 15;117(Pt 8):1281–3. 15020666
10. Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003 Nov;3(11):900–11. 14668806
11. Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001 Oct 30;11(21):1700–5. 11696329
12. Chen HW, Chen X, Oh SW, Marinissen MJ, Gutkind JS, Hou SX. mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 2002 Feb 1;16(3):388–98. 11825879
13. Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994 Feb 1;8(3):300–12. 8314084
14. Hou XS, Melnick MB, Perrimon N. Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996 Feb 9;84(3):411–9. 8608595
15. Yan R, Small S, Desplan C, Dearolf CR, Darnell JE Jr. Identification of a Stat gene that functions in Drosophila development. Cell. 1996 Feb 9;84(3):421–30. 8608596
16. Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998 Oct 15;12(20):3252–63. 9784499
17. Hombria JC, Brown S, Hader S, Zeidler MP. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol. 2005 Dec 15;288(2):420–33. 16277982
18. Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003 Sep;5(3):441–50. 12967563
19. Hombria JC, Brown S. The fertile field of Drosophila Jak/STAT signalling. Curr Biol. 2002 Aug 20;12(16):R569–75. 12194841
20. Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene. 2002 Jul 18;21(31):4812–21. 12101419
21. Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 2005 Aug 15;19(16):1861–70. 16055650
22. Muller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, Boutros M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature. 2005 Aug 11;436(7052):871–5. 16094372
23. Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006 Jan 26;439(7075):475–9. 16340959
24. Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006 Jan 26;439(7075):470–4. 16340960
25. Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007 Feb 16;315(5814):988–92. 17303754
26. Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009 Jun 26;137(7):1343–55. doi: 10.1016/j.cell.2009.05.014 19563763
27. Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010 Feb 1;338(1):28–37. doi: 10.1016/j.ydbio.2009.10.045 19896937
28. Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2010 Feb;2(1):37–49. doi: 10.1093/jmcb/mjp028 19797317
29. Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009 Feb;136(3):483–93. doi: 10.1242/dev.026955 19141677
30. Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011 Jan 7;8(1):84–95. doi: 10.1016/j.stem.2010.11.026 21167805
31. Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011 Mar;138(6):1045–55. doi: 10.1242/dev.056671 21307097
32. Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21064–9. doi: 10.1073/pnas.1012759107 21078993
33. Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010 Sep 14;20(17):1580–7. doi: 10.1016/j.cub.2010.07.041 20727758
34. Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010 Dec;137(24):4135–45. doi: 10.1242/dev.060483 21098564
35. Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18702–7. doi: 10.1073/pnas.1109348108 22049341
36. Li H, Qi Y, Jasper H. Dpp signaling determines regional stem cell identity in the regenerating adult Drosophila gastrointestinal tract. Cell Rep. 2013 Jul 11;4(1):10–8. doi: 10.1016/j.celrep.2013.05.040 23810561
37. Li Z, Zhang Y, Han L, Shi L, Lin X. Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev Cell. 2013 Jan 28;24(2):133–43. doi: 10.1016/j.devcel.2012.12.010 23369712
38. Tian A, Jiang J. Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. Elife. 2014;3:e01857. doi: 10.7554/eLife.01857 24618900
39. Guo Z, Driver I, Ohlstein B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J Cell Biol. 2013 Jun 10;201(6):945–61. doi: 10.1083/jcb.201302049 23733344
40. Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008 Oct 23;455(7216):1119–23. doi: 10.1038/nature07329 18806781
41. Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009 Jul;136(13):2255–64. doi: 10.1242/dev.035196 19502486
42. Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010 Apr 1;109(5):992–9. doi: 10.1002/jcb.22482 20082318
43. Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011 Jun 1;354(1):31–43. doi: 10.1016/j.ydbio.2011.03.018 21440535
44. Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009 Feb 19;5(2):200–11. doi: 10.1016/j.chom.2009.01.003 19218090
45. Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009 Jul 17;325(5938):340–3. doi: 10.1126/science.1173164 19520911
46. Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009 Oct 1;23(19):2333–44. doi: 10.1101/gad.1827009 19797770
47. Huff JL, Kingsley KL, Miller JM, Hoshizaki DK. Drosophila windpipe codes for a leucine-rich repeat protein expressed in the developing trachea. Mech Dev. 2002 Feb;111(1–2):173–6. 11804793
48. Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010 Apr 20;18(4):556–68. doi: 10.1016/j.devcel.2010.02.006 20412771
49. Karsten P, Hader S, Zeidler MP. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mech Dev. 2002 Sep;117(1–2):343–6. 12204286
50. Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008 Jul 3;3(1):44–54. doi: 10.1016/j.stem.2008.05.001 18593558
51. Ghiglione C, Devergne O, Georgenthum E, Carballes F, Medioni C, Cerezo D, et al. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development. 2002 Dec;129(23):5437–47. 12403714
52. Ichiba M, Nakajima K, Yamanaka Y, Kiuchi N, Hirano T. Autoregulation of the Stat3 gene through cooperation with a cAMP-responsive element-binding protein. J Biol Chem. 1998 Mar 13;273(11):6132–8. 9497331
53. Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005 Jul 28;436(7050):563–7. 16049490
54. Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009 Jan 9;4(1):49–61. doi: 10.1016/j.stem.2008.10.016 19128792
55. Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001 May;24(5):251–4. 11311363
56. Wang YH, Huang ML. Organogenesis and tumorigenesis: insight from the JAK/STAT pathway in the Drosophila eye. Dev Dyn. 2010 Oct;239(10):2522–33. doi: 10.1002/dvdy.22394 20737505
57. Singh SR, Chen X, Hou SX. JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophila. Cell Res. 2005 Jan;15(1):1–5. 15686618
58. Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007 Jan;7(3):323–31. 17008134
59. Vidal OM, Stec W, Bausek N, Smythe E, Zeidler MP. Negative regulation of Drosophila JAK-STAT signalling by endocytic trafficking. J Cell Sci. 2010 Oct 15;123(Pt 20):3457–66. doi: 10.1242/jcs.066902 20841381
60. Sotillos S, Diaz-Meco MT, Moscat J, Castelli-Gair Hombria J. Polarized subcellular localization of Jak/STAT components is required for efficient signaling. Curr Biol. 2008 Apr 22;18(8):624–9. doi: 10.1016/j.cub.2008.03.055 18424141
61. Devergne O, Ghiglione C, Noselli S. The endocytic control of JAK/STAT signalling in Drosophila. J Cell Sci. 2007 Oct 1;120(Pt 19):3457–64. 17855388
62. Stec W, Vidal O, Zeidler MP. Drosophila SOCS36E negatively regulates JAK/STAT pathway signaling via two separable mechanisms. Mol Biol Cell. 2013 Sep;24(18):3000–9. doi: 10.1091/mbc.E13-05-0275 23885117
63. Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8 : 152. doi: 10.1186/1741-7007-8-152 21176204
64. Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008 Oct 9;3(4):442–55. doi: 10.1016/j.stem.2008.07.024 18940735
65. Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010 Dec;137(24):4147–58. doi: 10.1242/dev.052506 21068063
66. Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11414–9. 16061818
67. Gilbert MM, Beam CK, Robinson BS, Moberg KH. Genetic interactions between the Drosophila tumor suppressor gene ept and the stat92E transcription factor. PLoS One. 2009;4(9):e7083. doi: 10.1371/journal.pone.0007083 19787055
68. Thomas C, Strutt D. Rabaptin-5 and Rabex-5 are neoplastic tumour suppressor genes that interact to modulate Rab5 dynamics in Drosophila melanogaster. Dev Biol. 2014 Jan 1;385(1):107–21. doi: 10.1016/j.ydbio.2013.09.029 24104056
69. Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, Yang-Zhou D, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011 May;8(5):405–7. doi: 10.1038/nmeth.1592 21460824
70. Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008 Jan;14(1):120–31. 18160348
71. Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004 Apr;131(7):1563–75. 14998928
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



