-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFrameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
Inherited diseases affecting only the nails in humans are rare; however, humans do not support themselves entirely on one appendage. Horses bear their entire weight on their third toe, resulting in a large amount of force on each hoof. An inherited disease characterized by a phenotype restricted to separation and breaking of the dorsal hoof wall was identified in a specific breed of pony, the Connemara. This disease has been termed hoof wall separation disease (HWSD). Parents of affected ponies appeared clinically normal, suggesting an autosomal recessive mode of inheritance. A genome-wide association analysis identified a region associated with HWSD which was further assessed through whole genome next-generation sequencing and genotyping of potential variants. Here, we present the discovery of a frameshift variant, leading to a premature stop codon in SERPINB11 of HWSD-affected ponies. Significantly decreased expression of the SERPINB11 transcript was identified in the hoof capsule of HWSD-affected ponies. This study describes the first genetic variant associated with a hoof wall specific phenotype and suggests a role of SERPINB11 in maintaining hoof wall structure.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005122
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005122Summary
Inherited diseases affecting only the nails in humans are rare; however, humans do not support themselves entirely on one appendage. Horses bear their entire weight on their third toe, resulting in a large amount of force on each hoof. An inherited disease characterized by a phenotype restricted to separation and breaking of the dorsal hoof wall was identified in a specific breed of pony, the Connemara. This disease has been termed hoof wall separation disease (HWSD). Parents of affected ponies appeared clinically normal, suggesting an autosomal recessive mode of inheritance. A genome-wide association analysis identified a region associated with HWSD which was further assessed through whole genome next-generation sequencing and genotyping of potential variants. Here, we present the discovery of a frameshift variant, leading to a premature stop codon in SERPINB11 of HWSD-affected ponies. Significantly decreased expression of the SERPINB11 transcript was identified in the hoof capsule of HWSD-affected ponies. This study describes the first genetic variant associated with a hoof wall specific phenotype and suggests a role of SERPINB11 in maintaining hoof wall structure.
Introduction
Many of the signaling molecules involved in patterning ectodermal derivatives, such as teeth and hair, are also involved in organizing mammalian distal limb appendages, including nails, claws and hooves [1]. Perissodactyla (odd-toed ungulates) are a diverse group of mammals that include the horse, zebra and donkey. These animals bear their weight almost entirely on the third toe. In the modern horse, the non-weight bearing digits have atrophied while the third digit has enlarged. The nail has also evolved to become fully interdigitated with the underlying soft tissue and to form a full weight bearing structure, the hoof. As horses are prey animals, the development of hooves illustrates a major evolutionary innovation based on the need for rapid acceleration and sustained speed. Since only the hooves touch the ground, the remaining portions of the foot have become parts of the limb, substantially increasing the length of stride. Additionally, raising the heel and digits off the ground increased the number of joints that move the limbs forward and thereby increased the rate of stride. Although these modifications substantially increase the potential speed and acceleration of these animals, extensive structural integrity of the hoof is required to support all of the body weight. On average, adult horses and ponies weigh 1000 and 880 lbs, respectively. At an evenly balanced stance when motionless, this places an average of 220–250 lbs of force on each limb. When in motion, ground reaction forces increase at various phases of the gait, resulting in additional force applied to each hoof [2].
Ectodermal dysplasias (EDs) are a heterogenous group of congenital disorders characterized by alterations in two or more ectodermal structures (hair, teeth, nails or sweat glands) [3]. Genetic conditions that exclusively involve the nails are rarer and have been classified as nonsyndromic congenital nail disorders (NDNC). Currently, there are ten characterized NDNCs in people, of which four have associated genetic alterations. Leukonychia (NDNC3), characterized by white discoloration of the nails, is caused by an alteration in PLCD1 [4]; Anonychia/hyponychia (NDNC4, absence of hypoplasia of nails) due to an alteration in RSPO4 [5]; toenail dystrophy (NDNC8), caused by an alteration in COL7A1 [6] and onychodystrophy (NDNC10), associated with an alteration in FZD6 [7]. There are currently six NDNCs for which a genetic alteration has not been identified, including isolated congenital onychodysplasia (NDNC7), a disease characterized by longitudinal streaks, thinning and splitting at the distal nail edge of all finger and toenails [8].
This study describes an inherited disorder, termed Hoof Wall Separation Disease (HWSD), characterized by separation and breaking of the dorsal hoof wall in the Connemara pony. Without the integrity of the hoof wall, ponies cannot support their weight effectively. The associated chronic inflammation often leads to laminitis, a debilitating condition characterized by separation of the third phalanx from the epidermal laminae that connect the bone to the dorsal hoof wall. Chronic laminitic episodes in horses are very painful and often warrant euthanasia. There are no known alterations that affect the hoof wall in horses and a comparative approach was not feasible due to the unique nature of the hoof. Our goal was to identify the molecular etiology of the disease in order to reduce its prevalence through genetic testing and to provide insight into this unique ectodermal structure. Genome-wide association analysis, coupled with whole genome next-generation sequencing, identified a frameshift variant in SERPINB11 associated with this novel, hoof specific phenotype in Connemara ponies. SERPINB11 remains an uncharacterized protein in humans [9,10] and further investigation of the potential role of SERPINB11 in NDNCs may be warranted.
Results
Phenotypic Description
Two clinically-affected Connemara females were examined at 5-months and 1-year of age. The onset of hoof pathology in these two index cases had become evident at 3 and 5-months of age, respectively. With the exception of the hooves, physical examinations revealed no other abnormalities; haircoat, underlying skin, mucous membranes and mucocutaneous junctions appearing normal. Abnormal sweating was not reported in either case. In both cases, all four hooves displayed a dorsal hoof wall separation at the sole with a normal coronary band appearance (Fig 1A). Proliferative horn was evident on the solar aspect of all four hooves (Fig 1B). The 5-month old pony, which had markedly proliferative solar horn, was lame on both front feet at the walk while the yearling, which had undergone a recent hoof trimming, appeared sound at the walk. Distal extremity radiographs of both front feet and the dental arcade revealed no abnormalities.
Fig. 1. Hoof from a Connemara pony foal affected with hoof wall separation disease (HWSD). 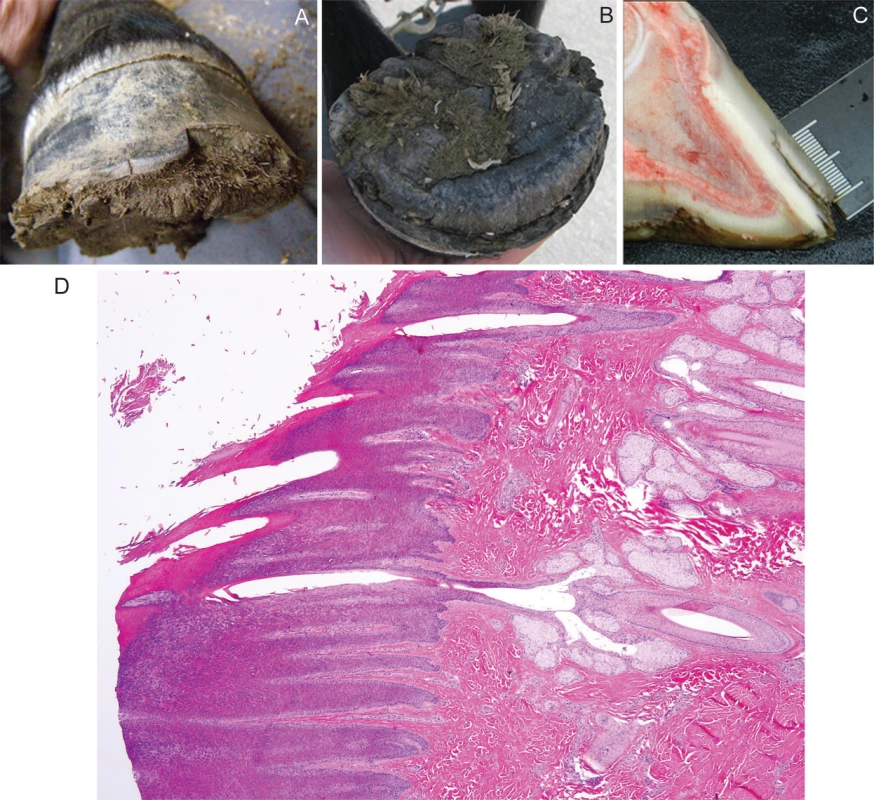
(A) Note the dorsal hoof wall separation at the sole and (1B) proliferative horn on the solar aspect of the hoof (1B). (1C) A sagittal section of a post-mortem HWSD-affected hoof demonstrates that the dorsal hoof wall fissure occurs outside of the white line. (1D) Coronary band, periople (hematoxylin & eosine stain): normal transition from haired skin into periople is evident. Hooves from three additional HWSD-affected female ponies (aged 1, 4 and 5 years) underwent complete gross examination. Age of onset in these three cases was less than 6-months of age. In the three cases, all four hooves showed variable degrees of splitting within the dorsal hoof wall, most prominent along the distal margin and variably spreading more proximally. The horn of the hoof wall was brittle and easily broken while the horn of the sole appeared stronger. The coronary band appeared normal. All four feet were bisected sagittally. Coffin bone rotation, an indication of laminitis where the toe of the distal phalanx (i.e. coffin bone) has dropped due to loss of lamellae support, was evident in 2/3 ponies (aged 4 and 5 years). The white line was intact and there was no hyperaemia or scarring in the corium or lamina. In the one HWSD pony (1-year of age) with no evidence of coffin bone rotation, the distance of the white line to the horn capsule measured 1 cm and was consistent proximal to distal. This horn to white line distance is within the normal radiographic reference range reported for adult ponies [11]. The dorsal hoof wall separation was outside of the white line (Fig 1C). Histologic examination of coronary band (Fig 1D), periople and proximal lamina, skeletal muscle and liver performed in one of three ponies (1-year of age) revealed no pathologic changes.
Genome Wide Association (GWA)
A case-control allelic GWA was performed on the 51,453 SNPs that passed quality control. Initial genomic inflation (λ = 1.48) was reduced (λ = 1.09) by eliminating seven unaffected outliers identified by multi-dimensional scaling (Fig 2A and 2B). Within this new sample set, there was a positive association between HWSD and a 1.7 Mb region on equine chromosome 8 (Fig 2C and 2D (S3 Table)); top SNVs at chr8 : 80,772,490 and chr8 : 80,648,576 praw = 1.37x10 = 10, pcorrected = 1.92x10-5). Ponies affected with HWSD had a distinct homozygous haplotype within the identified region; the same haplotype was not observed in control animals in the homozygous state. SNP genotypes of the associated region are provided in S4 Table.
Fig. 2. Genome-wide case-control allelic association study results of Connemara ponies with HWSD (15 cases, 24 controls). 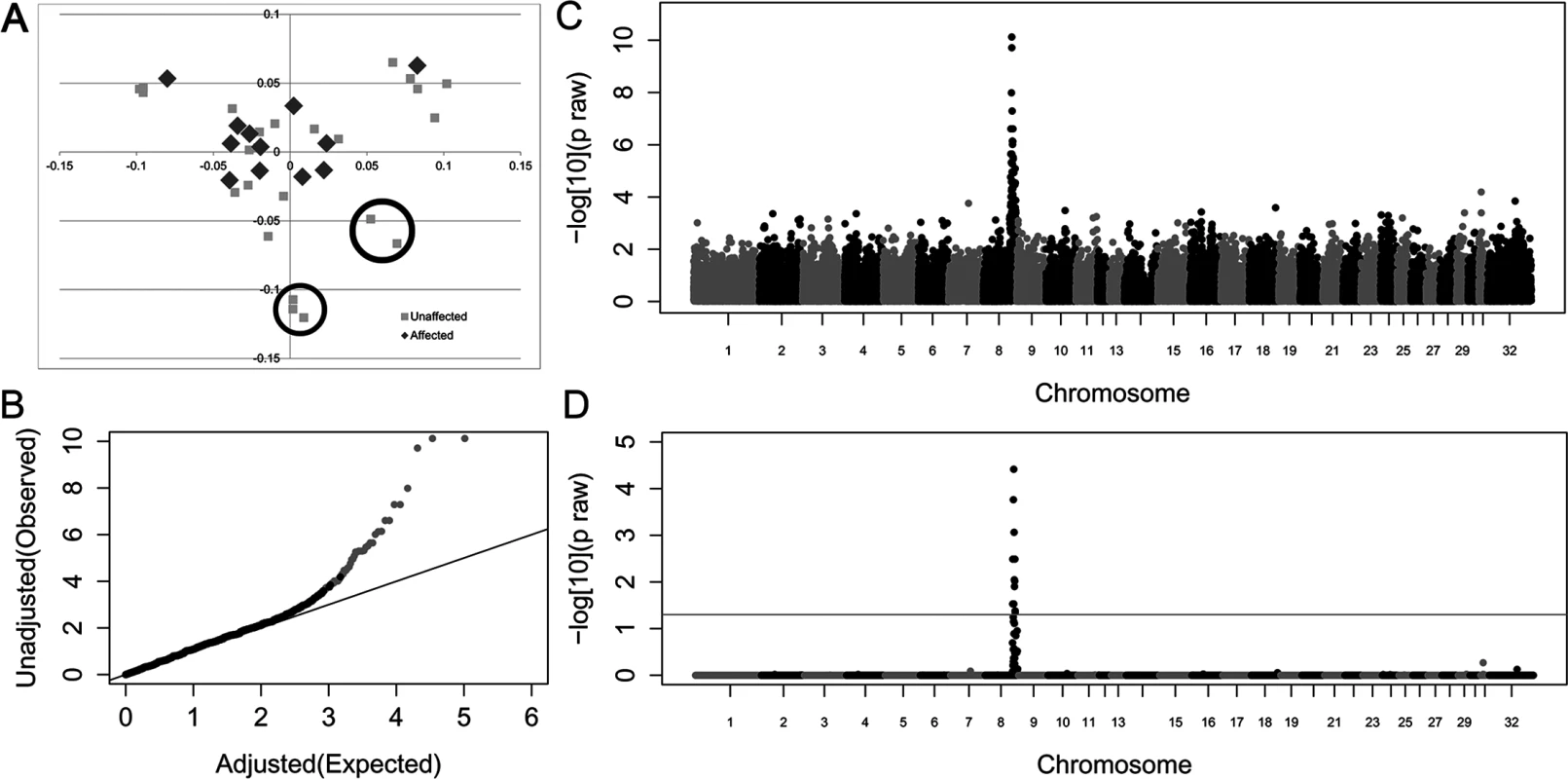
(2A) Multi-dimensional scaling plot. Controls circled contributed most to genomic inflation and were removed from analysis (2B). Quantile-Quantile plot of genome-wide association results after removing 7 control ponies. Grey dots represent the observed versus expected p-values of all SNPs. Black dots represent the observed versus expected p-values after removal of all SNPs on chromosome 8. The solid grey line represents the null hypothesis: observed p-values equal expected p-values. (2C) Manhattan plot of—log10 of raw p-values by chromosome. The lowest p-values are on chromosome 8. (2D) 52K Max (T) permutation results. The line that parallels the X axis denotes genome-wide significance (p<0.05;-log10>1.3). Variant Identification
The homozygous region identified in affected ponies (79,936,024–81,676,900 bp), contains a family of SERPINB genes. Based on the Equus caballus 2.0 genome assembly [12], horses have three additional copies of SERPINB3/B4 within this interval as compared to humans (Fig 3A). The rest of the genes, orientations and order are conserved with respect to human. Due to limited information regarding comparative diseases related to the SERPIN gene family, whole-genome next generation sequencing was performed instead of selectively sequencing predicted candidate genes. Whole-genome sequencing was performed on two Connemara HWSD cases and two unaffected Connemara ponies that were homozygous reference for the associated haplotype. A published Quarter horse whole-genome sequence was used as an additional control [13].
Fig. 3. Genes and segregating variants within the region of homozygosity shared by HWSD-affected ponies. 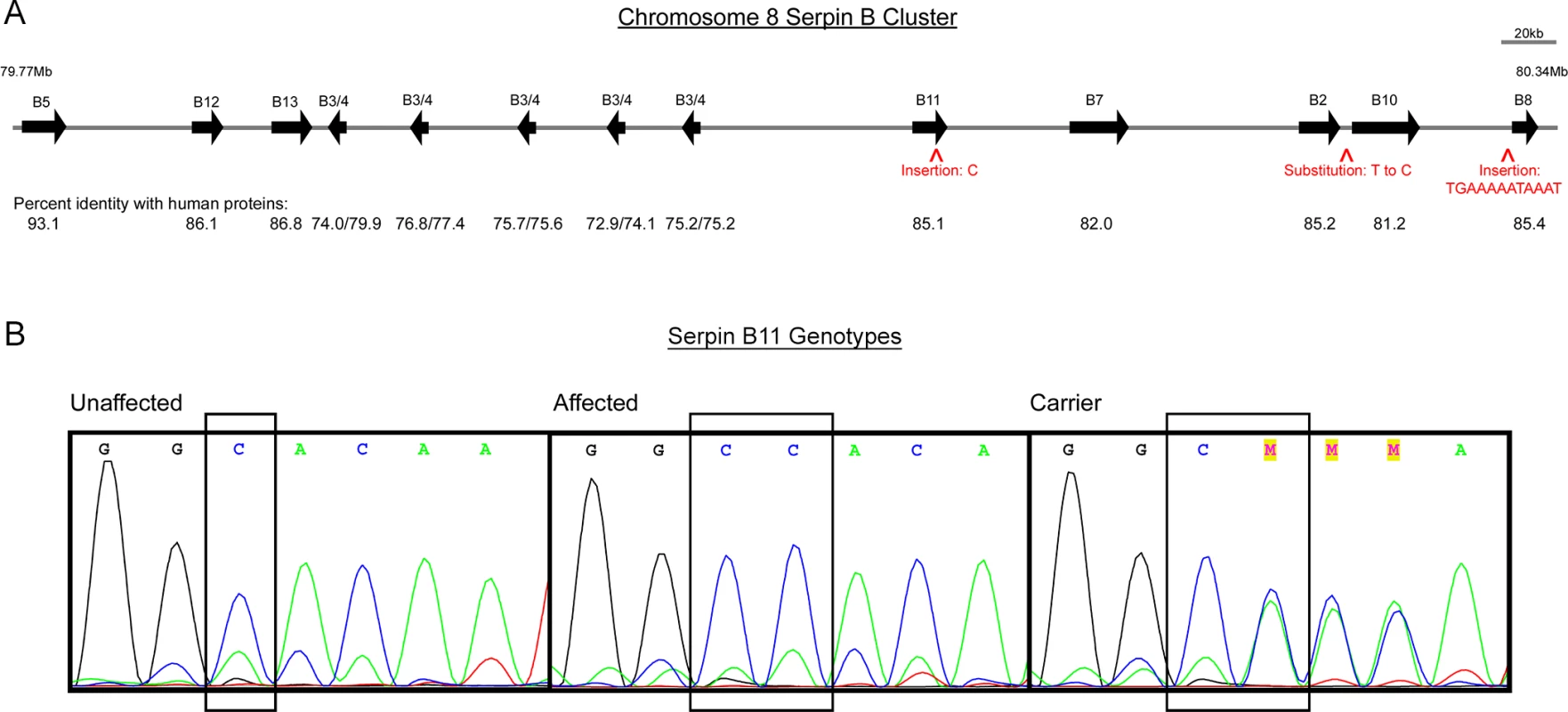
(3A) Scaled schematic representation of SERPINB gene family situated between 79.77Mb and 80.34Mb on equine chromosome 8. Arrows show the relative positions of the SERPINB genes in the cluster, and aligned numbers report the percent identity of the horse proteins compared to corresponding human proteins at the amino acid level. Five copies of equine SERPINB3/4 are variably closer to human B3 or B4. Carets depict locations of variants that segregate with HWSD: two insertions and one substitution. (3B) Chromatograms of SERPINB11 exon 5 sequences where a variant of C to C/C was found in affected Connemara ponies. From left to right: unaffected ponies have a genotype of “C/C”; affected Connemara ponies have a genotype of “CC/CC”; carrier Connemara ponies have a genotype of “C/CC”. Sequencing revealed a total of 9,758 single nucleotide variants (SNVs) within the interval, of which 363 segregated with HWSD in the two cases and three controls (S5 Table). Of these 363 SNVs, 16 were located within annotated genes or were fewer than 700 base pairs from the ATG. Although the promoters for the genes are not identified in horses, we selected 700 base pairs in order to cover key regulatory regions close to the start of translation. One additional SNV was chosen based on its location between SerpinB2 and SerpinB10. These gene-associated SNVs were selected for follow-up genotyping within a larger sample set. In addition, sequencing identified 1,230 small insertions and deletions (indels), of which 28 segregated with disease (S6 Table); 11 were within or close (<700 bp) to genes. Of these 11 indels, 6 were intronic, 1 was coding, and 4 were up/downstream. The coding and up/downstream indels were tagged for genotyping in a larger sample set. Of the 22 variants (17 SNVs and 5 indels) genotyped on the custom Sequenom panel, 21 passed quality control. Three variants were unique to the 23 affected Connemara ponies and heterozygous in 27 obligate carriers (Table 1). Although one indel failed Sequenom genotyping, we retained it in our analysis (Table 1). None of these variants were identified in the 169 non-Connemara equines also genotyped on the Sequenom array, however 82 unaffected Connemara ponies were heterozygous for the three variants and 244 were wild-type.
Tab. 1. Variants segregating with HWSD phenotype. 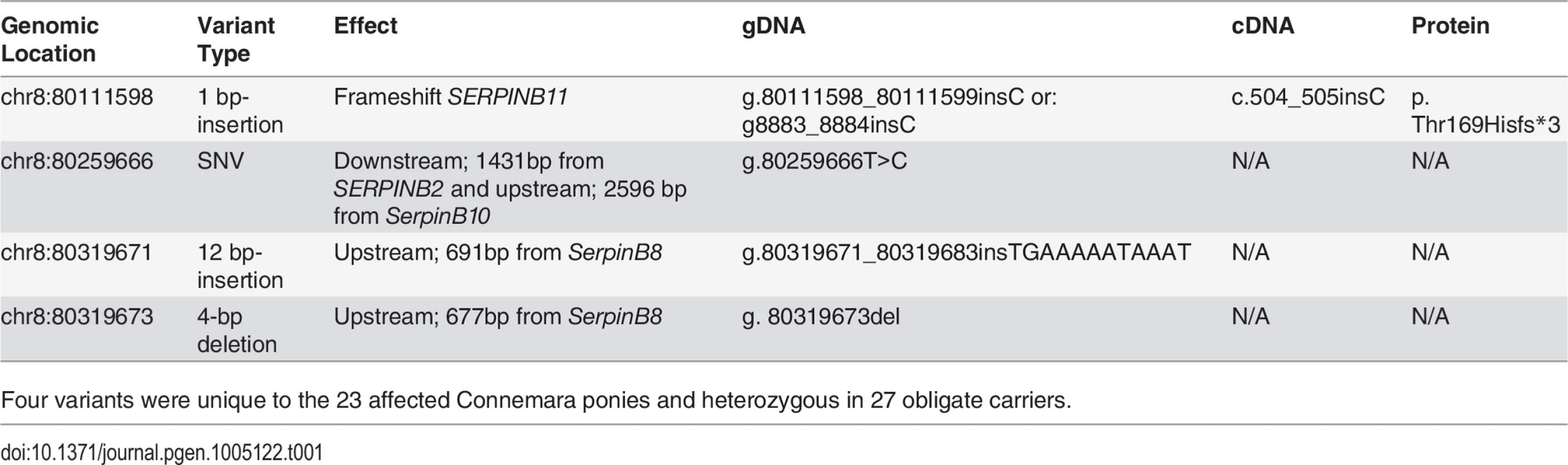
Four variants were unique to the 23 affected Connemara ponies and heterozygous in 27 obligate carriers. On chromosome 8, base pair 80,259,666 is located downstream of SERPINB2 and upstream of SERPINB10; 80,319,671 and the position of the four-base-pair deletion that failed quality control (80,319,673) are both within the rolling circle (RC) repeat element Helitron3Na_Mam located upstream of SERPINB8; 80,111,598 is in the fifth exon of SERPINB11 (Fig 3). The insertion within SERPINB11 introduces a frameshift that first alters the two amino acids following residue 168, and then introduces a premature STOP codon. 55% of the protein is predicted to be truncated, including a large portion of the serpin scaffold and the entire reactive site loop [9].
Gene Expression
As potential regulatory variants were not identified in our analysis and in order to prioritize the four remaining candidate variants, expression analysis of SERPINB2, SERPINB8, SERPINB10 and SERPINB11 was performed. These were the four genes closest to segregating SNVs and indels (Fig 3). Evaluation of mRNA levels in coronary band samples from four HWSD-affected ponies and four unaffected controls revealed that coronary band SERPINB11 expression was significantly reduced in affected ponies. Relative Expression Software Tool (REST) analysis indicated down-regulation (0.064; S.E. range 0.010–0.274) in the affected group by a mean factor of 16 and a probability that the difference between sample and control groups was due only to chance [P(H1)] of <0.001 (Fig 4A). By contrast, REST showed no difference in expression of SERPINB2, SERPINB8, or SERPINB10 between the affected and unaffected sample groups (Fig 4B).
Fig. 4. Expression analysis of SERPINB2, SERPINB8, SERPINB10 and SERPINB11. 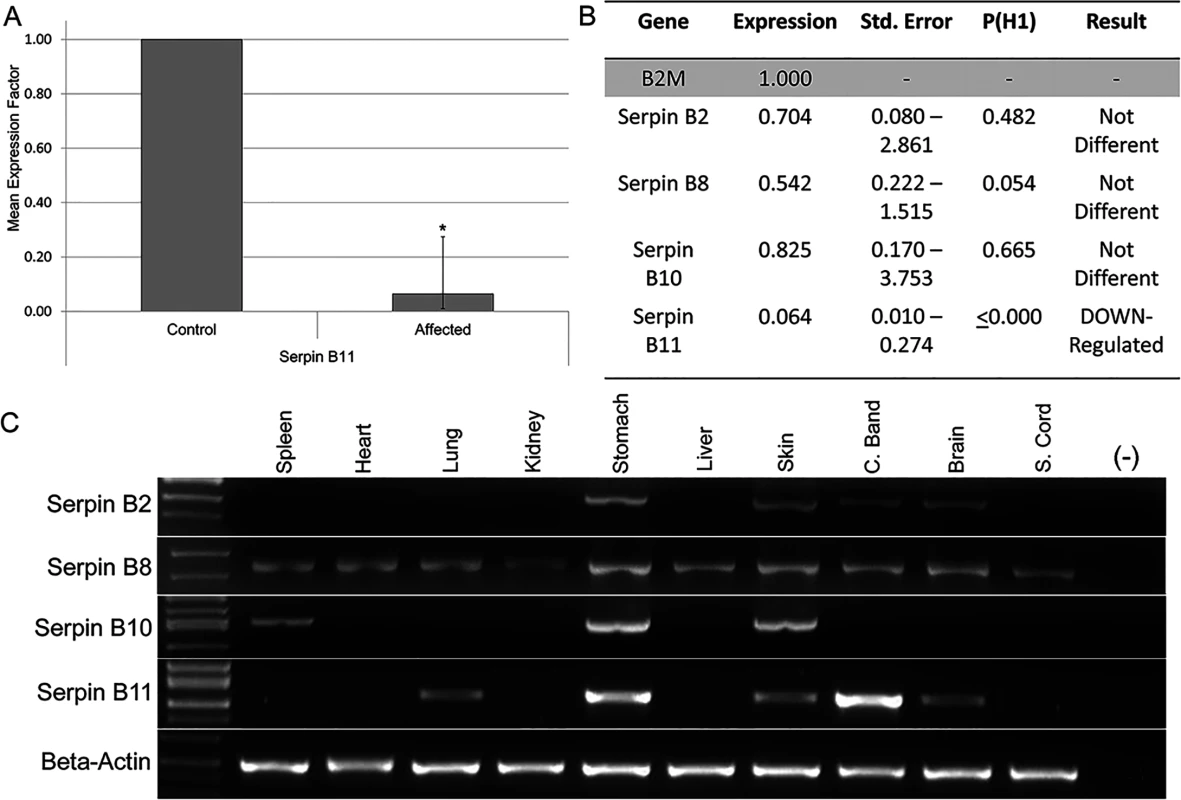
(4A) Relative SERPINB11 gene expression ratio of coronary band cDNA from four HWSD-affected Connemara ponies compared to four unaffected horses. Relative expression levels were normalized to the housekeeping gene B2M. Statistical significance is reported as p<0.001(*). B. Relative SERPINB2, B8, B10, and B11 gene expression levels in four affected Connemara ponies, compared to four unaffected horses and normalized to the housekeeping gene B2M. P(H1) = Probability of alternate hypothesis that difference between sample and control groups is due only to chance. P(H1) is significant (p<0.05) for only SERPINB11. C. Gel image of PCR amplification of SERPINB2, B8, B10, and B11 from spleen, heart, lung, kidney, stomach, liver, skin, coronary band, and spinal cord cDNA of an unaffected horse. Beta-actin was used to control for cDNA concentrations. SERPINB11 was also found to be a very abundant transcript in the coronary band of an unaffected horse, relative to its levels in other tissues. RT-PCR showed gene expression in lung, stomach, skin, coronary band, and brain. Levels were subjectively highest in the stomach and coronary band. By contrast, SERPINB2 appeared most highly expressed in stomach and skin; SERPINB8 was widely expressed and most abundant in stomach and skin; SERPINB10 was minimally expressed in coronary band and most prominent in stomach and skin (Fig 4C).
Penetrance, Allele Frequency, and Carrier Frequency
The SERPINB11 frameshift variant was found to be homozygous in a total of 31 affected ponies indicating complete penetrance. The severity of phenotype ranged from mild (cleft between dorsal hoof wall and white line apparent on solar aspect of hoof but the pony was able to be maintained with frequent hoof trimming and shoeing) to severe (Fig 1). Within the entire 423-Connemara-pony data set, allele frequency was 18.7% and a total of 96 ponies were heterozygous for the SERPINB11 insertion. The heterozygous animals are all phenotypically unaffected by HWSD. Within a 324-pony subset of individuals unrelated to the affected animals, carrier frequency for the variant is 14.8%.
Discussion
Phenotype
Based on our clinical and histologic assessment, we have defined the phenotype of HWSD in the Connemara pony to include an early age of onset (within the first 6-months of life) and characteristic splitting and separation of the dorsal hoof wall (Fig 1A). Lesions are specific to the dorsal hoof wall and do not appear to involve any other ectodermal structures. Laminitis may be a sequelae to HWSD.
Splitting of the dorsal hoof wall may be observed in inflammatory or infection conditions of the equine hoof, including white line disease or as a complication of laminitis or solar abscessation. For this study, we characterized HWSD-affected ponies based upon the following criteria: (1) Connemara pony breed (2) age of onset within the first six months of life and (3) characteristic clinical signs, supported by digital photographs of all four feet. Other diseases, including white line disease and abscessation, are associated with infection; the sole or white line is visibly discolored and unhealthy on examination. With HWSD, the sole and white line appear healthy, with the exception of proliferative sole in the animal’s effort to support the limb on a structure other than the dorsal hoof wall. The Connemara pony originated in the County Galway of western Ireland and the breed standard is characterized by hard strong hooves [14]. These ponies are often unshod when used for performance activities (jumping, dressage, driving), evidence of a strong and healthy hoof within the breed. Based on the breed standard, this makes the phenotype of HWSD all the more striking in affected individuals. The age for the control ponies within this study was set at >2 years. We have not observed any reports of an older age of onset of HWSD; most ponies are affected within the first 6 months of life.
In the 23 ponies positive for the identified SERPINB11 variant, all demonstrated an abnormal dorsal hoof wall. However, variable expressivity was apparent in HWSD-affected cases. Mild HWSD cases demonstrated evidence of the dorsal hoof wall separation on the solar aspect of the hoof without severe splitting evident on a lateral view. In more severely affected cases (Fig 1), the dorsal hoof wall separation was readily apparent. We did not identify any potential genetic modifiers that could account for the variability in phenotype. Owners of HWSD-affected ponies often notice that the splitting of the distal hoof wall worsens when the environmental conditions change from dry to wet or vice versa. Management typically consists of frequent trimming of hooves and, in some cases, glue-on shoes as the dorsal hoof wall of most HWSD-affected ponies will split if nails are used.
The dorsal hoof wall is composed of keratins, which provide strength, hardness and insolubility due to disulfide bonds between and within the long chain fibrous molecules [15]. There are dozens of different keratin molecules, with molecular weights in the range of 40–70 kDa and varying degrees of hardness and sulfur concentration [16]. Terminally differentiated keratinocytes, originating from the coronary band, are arranged in specialized tubular and intertubular configurations in four distinct zones [17]. The gradient in tubule density mirrors the gradient in water content across the hoof wall, with the innermost layers of the hoof having the highest relative water content, which confers high crack resistance [18]. In healthy horses, by the time the shock of the impact with the ground reaches the first phalanx, about 90% of the energy has been dissipated, mainly at the innermost hoof wall layer (i.e. the lamellar interface) [19]. Distal hoof wall splitting does not, in it of itself, result in lameness. Rather, repeated stress on the innermost layer of the lamellar interface results in separation of the interdigitating lamellae from the distal phalanx (i.e. laminitis). As laminitis progresses, radiographs of the distal phalanx may reveal the separation of the dorsal hoof wall from the distal phalanx. Although radiographs were unremarkable in the examined 5-month old filly, the lameness was most likely due to lamellar inflammation that had not yet progressed to full separation.
Genome-Wide Association
The original population of ponies used in this study was stratified, which was markedly improved by removing seven control ponies visualized as outliers on the multidimensional scaling plot (Fig 2A). Alternatively, a mixed model approach could have been utilized to correct for the level of stratification; however, removal of the seven control ponies did not affect power to detect a significant association on ECA8. The associated region on ECA8 encompassed ~1.7 Mb, which contained 13 annotated SERPBINB genes. None of these genes have been previously associated with any of the human EDs nor was there any supporting evidence documenting expression of these genes in any ectodermal structures.
Whole Genome Sequencing Reveals a Frameshift Variant in SERPINB11
Targeted re-sequencing of the ~1.7 Mb candidate region was considered; however, it has become more cost effective to perform whole-genome sequencing, when an autosomal recessive mode of inheritance is suspected. With Mendelian disorders, putative functional variants may more readily be uncovered on smaller sample sets using whole genome sequencing whereas if a more complex mode of inheritance was likely, capture re-sequencing could allow for many individuals to be sequenced for the particular region of interest at a relatively comparable cost [20].
Sequencing revealed 363 SNVs and 28 indels that segregated with HWSD, with 17 and 11, respectively, located within or adjacent to annotated genes. After genotyping a larger population of HWSD-affected and unaffected equids, four segregating variants remained (Table 1), including a 4-bp deletion that had failed Sequenom genotyping. Of these four variants, only one was coding. The insertion within SERPINB11 introduced a frameshift, leading to a premature stop codon. Based upon the severity of the HWSD phenotype, priority was placed on coding variants as we presumed the variant would alter the amino acid structure of the protein involved. In addition, q-RT-PCR data demonstrated decreased expression of SERPINB11 in coronary band tissue, where keratinocytes of dorsal hoof wall originate. The decision to focus on the one coding variant for HWSD was validated in this study; however, we acknowledge that non-coding variants have been increasingly associated with disease [21]. The three other HWSD-segregating variants may be a part of the haplotype on which the frameshift variant originated.
SERPINB11 in Humans
Across species, serpins represent that largest and most functionally diverse family of serine protease inhibitors. Some serpins exhibit alternative functions, such as hormone transport and blood pressure regulation [22]. Serpins have been classified into clades according to their sequence similarity. Clades are classified as A-P, with clades A-I representing human serpins [22]. Serpins have a highly conserved secondary structure, with three β-sheets (A, B and C), nine α-helices and a reactive center loop (RCL), which serve as bait for the target proteases. The tertiary structure allows for a conformational change critical to protease inhibitor activity [22]. Serpins exist as monomeric proteins in their native state, which is defined by an exposed RCL that allows it to interact with the protease. Serpins can transition back and forth between latent and active forms [10].
The SERPINB clade is considered the ovalbumin or ov-serpin clade, based on their high sequence similarity to chicken ovalbumin, and exist intracellularly [23]. In humans, clusters of genes on HSA6 and 18 have evolved from a common ancestor by one or two interchromosomal duplications with several intrachromosomal duplications [24]. A similar clustering is evident in the horse, with the clusters of SERPINB genes located on ECA8 and 20 [12]. Of the clade B serpins, only human SERPINB11 and mouse Serpinb11 have yet to be characterized [9,10]. Current evidence suggests that, in mice, Serpinb11 can function as a trypsin inhibitor yet SERPINB11 has lost inhibitory activity in humans and may have evolved a non-inhibitory function [9]. The inhibitor function of SERPINB11 has not been assessed in the horse. In the chicken, there is no orthologue for human SERPINB11, suggesting that these genes were either lost in the chicken or arose after the merging of avian and mammalian lineages [25]. Based on these limited studies of SERPINB11, it may be that there exist different roles, including varying abilities to function as protease inhibitors, of SERPINB11 between species.
Most research efforts into the characterization of major structural proteins of the equine hoof wall are targeted at the lamellae, as this is the site of biomechanical failure in equine laminitis. One study focused on characterizing proteins of the equine hoof wall that included the laminar epithelium (i.e. stratum internum), outer highly cornified hoof wall (stratrum medium) and coronary band epithelium [26]. From this investigation, it was evident that the keratin types within the coronary band epithelium were highly similar to those found in the stratum medium. Additionally, the high-sulfur and high-tyrosine protein components were rich in cysteine only in the stratum medium. Experimental studies from the same laboratory have determined that 35S-cysteine is preferentially taken up into the terminally differentiating hoof wall layers [26]. These cysteine-rich proteins are thought to contribute to stabilization of the interfibrillar matrix of the stratum medium through disulfide bonding. If SERPBINB11 retains cysteine peptidase inhibitory activity in the horse, as it does in the mouse [9], it may function to inhibit proteolytic cleavage of cysteine residues in the terminally differentiating hoof wall layer. A loss of SERPINB11 function could therefore result in a loss of peptidase inhibition and structural failure of the cysteine-rich hoof wall upon impact. Alternatively, SERPINB11 may play a specific role in relative keratinocyte proportions in the equine hoof wall, thereby weakening the overall structure and allowing it to fragment at the most distal end. Although keratinocytes may appear histologically intact at the level of the coronary band, the most distal aspect (i.e. most mature portion of the cell that is undergoing the majority of concussive impact) may be structurally abnormal. The distal dorsal hoof wall is difficult to evaluate histologically unless tissue is embedded in acrylic, which was not performed in this study. Further histologic investigation of the entire population of mature keratinocytes within the dorsal hoof wall of HWSD-affected ponies is warranted.
A bacterial artificial chromosome transgene expressing Cre under the control of Serpinb7 regulatory elements was recently developed [27]. Although the original aim of the study was to evaluation the expression in kidney mesangial cells, the authors discovered that the Serpinb7-Cre transgene mediated loxP-recombination in all epidermal layers of the skin, hair follicle cells and the epithelium of the mouse forestomach and esophagus. The transgene colocalized with Keratin10 and Keratin14 in the suprabasal and basal layer of the epidermis, respectively [27]. Similar to these expression patterns, the expression of SERPINB11 in our study was highest in the coronary band and stomach, with expression also detected in skin.
In humans, SERBPINB11 is located on chromosome 18q21.33. To date, there have been no reports of naturally occurring alterations of clade B serpins leading to a disease phenotype in humans. The only disease association of SERPINB11 is with endometroid ovary carcinoma [28]. Of the six characterized NDNCs that do not have an associated genetic alteration to date, none map to this region, including isolated congenital onychodysplasia (NDNC7), which phenotypically resembles HWSD with thinning and splitting at the distal nail edge of all finger and toenails [8]. Of interest, SERPINB11 was identified as a potential candidate gene for adaptive evolution in Yoruba [29].
Another mechanistic alternative is that SERPINB11 functions as member of a protein chaperoning complex, similar to the role of SERPINF1 and SERPINH1 in association with procollagen. A loss-of-function alteration in SERPINF1 has been demonstrated to cause osteogenesis imperfecta type VI in humans [30] while variants in SERPINH1 have been associated with ostogenesis imperfecta in both humans [31] and dogs [32]. In a similar manner, SERPINB11 may have a role as a chaperone protein for those proteins involved in hoof wall structure.
cDNA Sequencing and Tissue Expression of SERPINB11
In the horse, we identified one full-length transcript variant from hoof capsule. In humans, there have been seven major SERPINB11 transcripts identified; three correspond to a protein product with various splice variants and one contains an insertion, leading to a frameshift and premature stop codon at position 90 that results in a nonfunctional variant (NP_001278207) [9]. There are no deleterious effects of this truncated transcript reported in humans. However, based on the results from this study, further investigation into the potential role of the truncated transcript in nail health may be warranted. Tissue expression of SERPINB11 in humans demonstrates expression in the tonsil, lung, placenta and prostate while Serpinb11 demonstrates expanded expression in mice with transcripts identified in eye, lung, lymphocytes, thymus, stomach, uterus, heart, brain, liver, skeletal muscle and whole embryonic tissue at day 7, 15 and 17 [9]. Tissue expression of SERPINB11 in the horse was similar to mouse and included lung, stomach and brain (Fig 4A). However, expression of SERPINB11 in the horse was strongest in the coronary band, or most proximal part of the hoof capsule. The coronary band is the tissue from which the dorsal hoof wall arises and is analogous to a human cuticle. To the authors’ knowledge, expression of SERPINB11 in skin and nail tissue has not been examined in humans and mice.
Conclusions
The results of this study demonstrate a strong association of the SERPINB11 c.504_505insC variant with the HWSD phenotype. Additionally, HWSD is the first disease to be described that results in a hoof-specific phenotype, with no other ectodermal structures affected. Further studies are necessary to determine the mechanism by which SERPINB11 maintains structural integrity of the hoof wall of healthy ungulates and if SERPINB11 plays a similar role in nail and claw health of non-ungulate species.
Materials and Methods
Ethics Statement
Blood samples from index cases were collected at the University of California, Davis School of Veterinary Medicine William R. Pritchard Veterinary Medical Teaching Hospital. Additional samples were drawn by private veterinarians and mailed by individual Connemara owners. All animal samples were obtained following protocol number 17491 approved by the University of California Davis Institutional Animal care and Use Committee.
Phenotype
Two affected Connemara ponies were evaluated at the University of California, Davis (UCD) Veterinary Medical Teaching Hospital in 2011 by a board-certified equine internist (CF). Following humane euthanasia, hooves from three additional affected ponies were evaluated by a board-certified pathologist (VKA). Distal extremity radiographs were available from the five index cases. From these index cases and the histologic assessment of affected hooves, the phenotype of HWSD was established. For additional cases, inclusion criteria as an HWSD case consisted of (1) Connemara pony breed (2) age of onset within the first six months of life and (3) clinical signs consistent with a receding dorsal hoof wall and secondary solar proliferation, supported by digital photographs of all four feet. Inclusion criteria for unaffected animals in the genome wide association study consisted of (1) Connemara pony breed (2) >2 years of age (3) no apparent hoof pathology, supported by digital photographs of all four feet when available. Distal extremity radiographs of affected horses were evaluated, when available. Control animals used for genotyping of putative functional variants were reported by their owners to be >2 years of age and had no apparent hoof pathology.
Genomic DNA
DNA was collected and purified from all horses (Gentra Puregene blood kit, Quiagen, Valencia, CA).
RNA and cDNA
Hooves from four affected Connemara ponies [3 female (aged 2.5, 3, and 4.5 years) and 1 male (6 months)], including three of the index cases, were available for RNA purification. Four unaffected [2 female (1.5 years), 2 male (1 and 5 years)] horses were euthanized for reasons unrelated to this study and hooves collected as controls. All tissue samples were flash frozen in liquid nitrogen and stored at -80°C until RNA isolation. RNA was isolated (RNeasy Fibrous Tissue Mini Kit, Quiagen, Valencia, CA) and cDNA synthesized (QuantiTect Reverse Transcription Kit, QIAGEN, Valencia, CA). Negative reverse transcriptase controls were made simultaneously and the final products were assessed with the housekeeping gene, ACTB, as previously described [33].
Genome Wide Association Study
15 affected (4 male, 11 female) and 24 unaffected (7 male, 17 female) Connemara ponies were genotyped on the Illumina SNP70 Genotyping Beadchip (Illumina, San Diego, CA). Quality control was implemented using PLINK (Purcell et al 2007) and Single Nucleotide Polymorphisms (SNPs; based on EquCab2.0) were excluded with a minor allele frequency (MAF) <5% or genotyping call rate <90%. A case-control standard allelic genome-wide association (GWA) was performed using PLINK. Population stratification was determined using genomic inflation values in PLINK and visualized using multi-dimensional scaling (MDS) and quantile-quantile (Q-Q) plots. After removing seven control animals based upon the MDS plot, a repeat case-control standard allelic GWA analysis was performed with the remaining 15 affected and 17 unaffected (4 male, 13 female) animals. To correct for multiple testing, 52,000 permutations were performed. Manhattan plots and QQ plots were generated using the ggplot2 package [34] implemented in R v.3.1.1 [35].
Whole-Genome Next Generation Sequencing
Sequence data was generated using an Illumina HiSeq. Library preparation and sequencing was performed by the UC Davis Genome Center. Average library insert size was 300 bases. Four samples (2 affected [1 female, 1 male] and 2 unaffected [1 female, 1 male]) were bar-coded and pooled and 2 lanes of sequence of 100bp paired-end reads were obtained. An average of 79.2M reads were obtained per sample. Average read length after trimming was 98.25bp, resulting in 5.4-6X coverage for each of the four horses. Sequence data was processed on a 2U custom built rack server with 256GB DDR3 memory, 2 x Xeon(R) E5-2690 Eight-Core Processor (32 virtual cores), 22TB HD and Ubuntu 12.04.2 operating system. Read quality was assessed using qrqc (version 1.9.1) [36], while Scythe (version 0.990) [37] and Sickle (version 1.20) [38] were used for Illumina adapter & quality trimming. The Burrows-Wheeler Aligner (BWA version 0.6.2-r126) [39] was used to align reads to the horse genome (UCSC assembly ID: equCab2). Sequenced reads from an American Quarter Horse [13] were downloaded and used as an additional control. To call variants, the Genome Analysis Toolkit (GATK version 2.5-2-gf57256b) [40] best practices for variant calling was followed, including duplicate removal, indel realignment, base quality score recalibration, and SNP and INDEL discovery and genotyping across all samples using GATK recommended hard filtering parameters [41,42]. Variant effect prediction was assessed using SnpEff [21] (version 3.5f) [43]. Of the segregating variants identified, UCSC was used to identify the syntenic region on hg19 [12] and and the ECR browser (http://ecrbrowser.dcode.org/) used to identify potential highly conserved non-coding regions. FASTq files have been uploaded for the sequence data to the NCBI-SRA (submission # SRP052751).
SNP and Indel Genotyping
A custom Sequenom SNP panel (GeneSeek, Lincoln, NE) was designed to genotype the 17 SNPs and 5 indels that segregated with the affected phenotype on an additional 369 Connemara ponies, 18 non-Connemara ponies, 50 Arabians, 51 Quarter Horses, and 50 Thoroughbreds. An additional 54 Connemara ponies were genotyped only for SERPINB11, using the forward primer CAAGGGGATGAGGGAGTTCT and reverse primer CCTCACTTAGCCGAAAAGGA; the 296bp product was sequenced and assessed for the presence of a 1bp insertion.
Expression Analysis
RT-PCR was performed to evaluate relative expression of SERPINB2, SERPINB8, SERPINB10, SERPINB11 in the spleen, heart, lung, kidney, stomach, liver, skin, coronary band, brain, and spinal cord of one control horse. The predicted cDNA sequences for each SERPIN gene were pulled from the equCab2 assembly, as viewed on the UCSC Genome Browser [12] and PRIMER3 software [44] was used to design primers (S1 Table). Beta-Actin cDNA was also amplified to ensure equal loading of template, with previously published primers [45]. PCR was performed with the following cycling conditions: 12 minute melt at 95°C; 40 cycles of 30 seconds at 94°C, 30 seconds at 58°C, and 3 minutes at 72°C; final 20-minute extension at 72°C. PCR products were Sanger sequenced to ensure that the correct product was amplified. The SERPINB11 primer set was also used to sequence multiple affected ponies to ensure that the frameshift variant was detectable in transcribed cDNA.
Quantitative Expression Analysis
Primers for the four SERPIN genes (B2, B8, B10, B11) surrounding disease-associated variants were designed using the PRIMER3 software loaded with server settings for qPCR (Untergasser et al., 2012) (S2 Table). cDNA sequences were pulled from the equCab2 assembly [12]. Primers were selected only if pairs spanned at least one intron, did not bind to regions with an identified SNP, produced a single product with In-Silico PCR, and showed a single BLAT search result. Primers were used to PCR amplify from control coronary band cDNA, and products were sequenced to confirm specificity. Three reference genes were identified as potential internal controls: beta-actin (ACTB), beta-2-microglobulin (B2M), and ubiquitin B (UBB) [33]. All primers were synthesized by Eurofins MWG Operon, Huntsville, AL, USA (http://www.operon.com). Housekeeping gene stability was determined by calculating Ct mean, standard deviation, and coefficient of variation [46]. B2M demonstrated the most stable expression profile across our eight coronary band cDNA samples. qRT-PCR Reactions were performed in a 10uL reaction volume using the QIAGEN Rotor-Gene SYBR Green PCR Kit. Primers are listed in S2 Table. Each tube contained 20ng cDNA, and 0.5μM final primer concentration for each forward and reverse primer. PCR was performed on a Rotor-Gene Q 72-well thermocycler (QIAGEN, Valencia, CA) as follows: 5 minutes at 95°C; 40 cycles of 20 seconds at 95°C and 40 seconds at 60°C; melt curve ramping from 50°C to 99°C, rising by 1°C at each step. Each reaction was run in triplicate. Each run included a no-template control, negative-RT control, and standard concentration (40ng, 20ng, 10ng, 5ng, 2.5ng) cDNA for each SERPIN gene under investigation and B2M. Quantitative RT-PCR results were analyzed by group-wise comparison between quantification cycle values obtained from coronary band samples donated by four affected and four unaffected animals. The Relative Expression Software Tool (REST) platform was used to evaluate expression levels [47]. Experiments showing group-based differences were repeated and analyzed in triplicate.
Carrier Frequency
Pedigree information was collected for all genotyped ponies, using Certificates of Registry issued by regional Connemara societies and the Pedigree Online All Breed Database (www.allbreedpedigree.com). To assess carrier frequency in the general Connemara population, a sample set that excluded affected individuals and close relatives (siblings, half-siblings, sires, dams, grand-sires, and grand-dams) was created. Carrier frequency was calculated by dividing the total number of unrelated animals heterozygous for the frameshift variant by the total number of animals within the unrelated sample set.
Supporting Information
Zdroje
1. Hamrick MW (2001) Development and evolution of the mammalian limb: adaptive diversification of nails, hooves, and claws. Evol Dev 3 : 355–363. 11710767
2. Hodson E, Clayton HM, Lanovaz JL (2000) The forelimb in walking horses: 1. Kinematics and ground reaction forces. Equine Vet J 32 : 287–294. 10952376
3. Freire-Maia N (1971) Ectodermal dysplasias. Hum Hered 21 : 309–312. 5139249
4. Kiuru M, Kurban M, Itoh M, Petukhova L, Shimomura Y, et al. (2011) Hereditary leukonychia, or porcelain nails, resulting from mutations in PLCD1. Am J Hum Genet 88 : 839–844. doi: 10.1016/j.ajhg.2011.05.014 21665001
5. Bergmann C, Senderek J, Anhuf D, Thiel CT, Ekici AB, et al. (2006) Mutations in the gene encoding the Wnt-signaling component R-spondin 4 (RSPO4) cause autosomal recessive anonychia. Am J Hum Genet 79 : 1105–1109. 17186469
6. Sato-Matsumura KC, Yasukawa K, Tomita Y, Shimizu H (2002) Toenail dystrophy with COL7A1 glycine substitution mutations segregates as an autosomal dominant trait in 2 families with dystrophic epidermolysis bullosa. Arch Dermatol 138 : 269–271. 11843659
7. Frojmark AS, Schuster J, Sobol M, Entesarian M, Kilander MB, et al. (2011) Mutations in Frizzled 6 cause isolated autosomal-recessive nail dysplasia. Am J Hum Genet 88 : 852–860. doi: 10.1016/j.ajhg.2011.05.013 21665003
8. Hamm H, Karl S, Brocker EB (2000) Isolated congenital nail dysplasia: a new autosomal dominant condition. Arch Dermatol 136 : 1239–1243. 11030770
9. Askew DJ, Cataltepe S, Kumar V, Edwards C, Pace SM, et al. (2007) SERPINB11 is a new noninhibitory intracellular serpin. Common single nucleotide polymorphisms in the scaffold impair conformational change. J Biol Chem 282 : 24948–24960. 17562709
10. Heit C, Jackson BC, McAndrews M, Wright MW, Thompson DC, et al. (2013) Update of the human and mouse SERPIN gene superfamily. Hum Genomics 7 : 22. doi: 10.1186/1479-7364-7-22 24172014
11. Cripps PJ, Eustace RA (1999) Radiological measurements from the feet of normal horses with relevance to laminitis. Equine Vet J 31 : 427–432. 10505960
12. Karolchik D, Barber GP, Casper J, Clawson H, Cline MS, et al. (2014) The UCSC Genome Browser database: 2014 update. Nucleic Acids Res 42: D764–770. doi: 10.1093/nar/gkt1168 24270787
13. Doan R, Cohen ND, Sawyer J, Ghaffari N, Johnson CD, et al. (2012) Whole-genome sequencing and genetic variant analysis of a Quarter Horse mare. BMC Genomics 13 : 78. doi: 10.1186/1471-2164-13-78 22340285
14. British Connemara Pony Society [Internet]. (2008) Breed Standard. Available from: http://www.britishconnemaras.co.uk/index.php?id=208.
15. Priestley GC. An introduction to the skin and its diseases. In: Priestley GC, editors. Molecular Aspects of Dermatology. Chichester: John Wiley & Sons; 1993. p.1–17.
16. Carter RA, Shekk V, de Laat MA, Pollitt CC, Galantino-Homer HL (2010) Novel keratins identified by quantitative proteomic analysis as the major cytoskeletal proteins of equine (Equus caballus) hoof lamellar tissue. J Anim Sci 88 : 3843–3855. doi: 10.2527/jas.2010-2964 20622188
17. Reilly JD, Cottress DF, Martin RJ, Cuddeford D (1996) Tubule density in equine hoof horn. Biomimetics 4 : 23–36. doi: 10.1002/(SICI)1097-0193(1996)4 : 1<23::AID-HBM2>3.0.CO;2-R 20408184
18. Thomason JJ, Biewener AA, Bertram JEA (1992) Surface strin on the equine hoof wall in vivo: implications for the material design and functional morphology of the wall. J Exper Biol 166 : 145–165.
19. Dyhre-Poulsen P, Smedegaard HH, Roed J, Korsgaard E (1994) Equine hoof function investigated by pressure transducers inside the hoof and accelerometers mounted on the first phalanx. Equine Vet J 26 : 362–366. 7988538
20. Shendure J (2011) Next-generation human genetics. Genome Biol 12 : 408. doi: 10.1186/gb-2011-12-9-408 21920048
21. Maher B (2012) ENCODE: The human encyclopaedia. Nature 489 : 46–48. 22962707
22. Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, et al. (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276 : 33293–33296. 11435447
23. Remold-O'Donnell E (1993) The ovalbumin family of serpin proteins. FEBS Lett 315 : 105–108. 8417965
24. Scott FL, Eyre HJ, Lioumi M, Ragoussis J, Irving JA, et al. (1999) Human ovalbumin serpin evolution: phylogenic analysis, gene organization, and identification of new PI8-related genes suggest that two interchromosomal and several intrachromosomal duplications generated the gene clusters at 18q21-q23 and 6p25. Genomics 62 : 490–499. 10644448
25. Benarafa C, Remold-O'Donnell E (2005) The ovalbumin serpins revisited: perspective from the chicken genome of clade B serpin evolution in vertebrates. Proc Natl Acad Sci U S A 102 : 11367–11372. 16055559
26. Grosenbaugh DA, Hood DM (1992) Keratin and associated proteins of the equine hoof wall. Am J Vet Res 53 : 1859–1863. 1280927
27. Wang Y, Guo Q, Casey A, Lin C, Chen F (2012) A new tool for conditional gene manipulation in a subset of keratin-expressing epithelia. Genesis 50 : 899–907. doi: 10.1002/dvg.22046 22764128
28. Pletscher-Frankild S, Palleja A, Tsafou K, Binder JX, Jensen LJ (2014) DISEASES: Text mining and data integration of disease-gene associations. Methods.
29. Voight BF, Kudaravalli S, Wen X, Pritchard JK (2006) A map of recent positive selection in the human genome. PLoS Biol 4: e72. 16494531
30. Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, et al. (2011) Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 88 : 362–371. doi: 10.1016/j.ajhg.2011.01.015 21353196
31. Christiansen HE, Schwarze U, Pyott SM, AlSwaid A, Al Balwi M, et al. (2010) Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet 86 : 389–398. doi: 10.1016/j.ajhg.2010.01.034 20188343
32. Drogemuller C, Becker D, Brunner A, Haase B, Kircher P, et al. (2009) A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet 5: e1000579. doi: 10.1371/journal.pgen.1000579 19629171
33. Bogaert L, Van Poucke M, De Baere C, Peelman L, Gasthuys F, et al. (2006) Selection of a set of reliable reference genes for quantitative real-time PCR in normal equine skin and in equine sarcoids. BMC Biotechnol 6 : 24. 16643647
34. Wickham H (2009) ggplot2: elegant graphics for data analysis. New York: Springer.
35. Team RDC (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
36. http://www.bioconductor.org/packages/release/bioc/html/qrqc.html.
37. https://github.com/ucdavis-bioinformatics/scythe.
38. Joshi NA FJ (2011) Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files (Version 1.20) [Software]. Available at https://githubcom/najoshi/sickle
39. Li H, &Durbin R. (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25 : 1754–1760. doi: 10.1093/bioinformatics/btp324 19451168
40. McKenna A, Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., & DePristo M. A. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20 : 1297–1303. doi: 10.1101/gr.107524.110 20644199
41. Auwera GA, Carneiro, M. O., Hartl, C., Poplin, R., del Angel, G., Levy‐Moonshine, A., et al. (2013) From FastQ Data to High‐Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Current Protocols in Bioinformatics: 11–10.
42. DePristo MA, Banks E., Poplin R., Garimella K. V., Maguire J. R., Hartl C., et al. (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics 43 : 491–498. doi: 10.1038/ng.806 21478889
43. Cingolani P PA, Wang le L, Coon M, Nguyen T, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6 : 80–92. doi: 10.4161/fly.19695 22728672
44. Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, et al. (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40: e115. 22730293
45. Fintl C, Pearson GT, Mayhew IG, Stewart Lowden C, Hopwood PA, et al. (2010) Comparative analysis of c-kit gene expression and c-Kit immunoreactivity in horses with and without obstructive intestinal disease. Vet J 186 : 64–69. doi: 10.1016/j.tvjl.2009.07.015 19716327
46. Zhang YW, Davis EG, Bai J (2009) Determination of internal control for gene expression studies in equine tissues and cell culture using quantitative RT-PCR. Vet Immunol Immunopathol 130 : 114–119. doi: 10.1016/j.vetimm.2009.01.012 19269038
47. Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30: e36. 11972351
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



