-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCo-chaperone p23 Regulates . Lifespan in Response to Temperature
Temperature is a critical environmental factor that affects ageing in both cold-blooded and warm-blooded species. In invertebrate animals, lifespan varies inversely with temperature, with higher temperature resulting in faster development but shorter lifespan. This phenomenon has been usually attributed to passive changes in metabolic rate, but recent work suggests that this process is regulated. In this study, we identify the co-chaperone protein p23 in the nematode C. elegans as an important modulator of longevity in response to temperature. Co-chaperones bind to client proteins to assist in their folding or stabilize their shape, thereby regulating their activity. Remarkably, deletion of p23 results in animals that are long lived at high temperatures and short lived at low temperatures relative to normal wild type animals. Our experiments indicate that p23 regulates lifespan through the neurosensory apparatus. These in turn impinge on key longevity regulators that mediate the transcriptional outputs of insulin/IGF, heat shock response and steroidal signaling. These studies suggest that complexes formed by p23 play a central role in regulating longevity in response to temperature.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005023
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005023Summary
Temperature is a critical environmental factor that affects ageing in both cold-blooded and warm-blooded species. In invertebrate animals, lifespan varies inversely with temperature, with higher temperature resulting in faster development but shorter lifespan. This phenomenon has been usually attributed to passive changes in metabolic rate, but recent work suggests that this process is regulated. In this study, we identify the co-chaperone protein p23 in the nematode C. elegans as an important modulator of longevity in response to temperature. Co-chaperones bind to client proteins to assist in their folding or stabilize their shape, thereby regulating their activity. Remarkably, deletion of p23 results in animals that are long lived at high temperatures and short lived at low temperatures relative to normal wild type animals. Our experiments indicate that p23 regulates lifespan through the neurosensory apparatus. These in turn impinge on key longevity regulators that mediate the transcriptional outputs of insulin/IGF, heat shock response and steroidal signaling. These studies suggest that complexes formed by p23 play a central role in regulating longevity in response to temperature.
Introduction
Temperature dramatically impacts the lifespan of ectotherms, with lower temperatures typically extending and higher temperatures shortening life [1–3]. The conventional view is that temperature passively affects the rate of chemical reactions and metabolism, thereby influencing species longevity. An emerging body of evidence, however, indicates that changes in longevity in response to temperature also reflect a regulated process entailing important organismal adaptations [4–6].
Like other ectotherms, the nematode Caenorhabditis elegans shows clear temperature dependent influences on development and lifespan [2]. At the normal cultivation temperature (20°C), animals typically live three weeks. At low temperature (15°C) they live approximately ten days longer, and at high temperature (25°C) ten days shorter. A handful of identified loci have been recently shown to mediate changes in lifespan in response to temperature. At warm temperatures, signaling from thermotaxis neurons promotes normal lifespan, and activates steroid hormone signaling to maintain longevity [4]. Animals compromised in thermotaxis genes or steroid hormone production display shortened life. In addition, animals will mount a heat shock response when exposed to acute heat stress, which helps preserve organismal viability. Thermotaxis neurons regulate the organismal heat shock response through the heat shock transcription factor HSF-1 [7]. Overexpression of hsf-1 and downstream chaperones can extend lifespan [8,9]. In contrast, longevity at cool temperatures requires the cold sensitive TRPA-1 channel [5], which works through Ca2+ signaling, protein kinase-C PKC-2, and serum and glucocorticoid kinase SGK-1 to activate the forkhead transcription factor DAF-16/FOXO, a crucial regulator of longevity. Evidently the mechanisms governing longevity at low or high temperature appear somewhat different and few components affect both [4].
Temperature effects on organismal longevity are not limited to ectotherms. Notably, temperature sensing neurons involved in homeostatic control of core body temperature affect murine lifespan [6]. Higher temperatures in these neurons trigger a lowering of the core body temperature and correlate with extended lifespan. Several long-lived mouse models including the Ames Dwarf, Growth Hormone Receptor knockout, and FGF21 transgenic mice have associated a lower core body temperature reminiscent of nutrient induced torpor [10,11]. Furthermore cold exposure extends lifespan and suppresses tumorigenesis in rats [12]. These studies suggest an intimate but relatively unexplored relationship between nutrient and thermal sensing, metabolism and longevity.
At the cellular level, chaperones and co-chaperones facilitate protein folding and assembly, often in response to thermal stress [13,14]. One such co-chaperone is p23 [15]. p23 complexes with the HSP90 chaperone and inhibits its ATPase activity [16–18], thereby stabilizing association with client proteins such as steroid receptor transcription factors, heat shock factor, and others [19–25]. These interactions play an important role in regulating transcriptional events. p23 also displays HSP90 independent chaperone-like activity [26], and is implicated in various cellular functions [27]. Additionally the protein reportedly harbors prostaglandin E2 synthase (PGS) activity in vitro [28], although this is debated [29]. Interestingly, p23 upregulation has also been implicated in tumorigenesis presumably through its interactions with HSF1, steroid receptors or growth regulated kinases [30]. Indeed, several anti-cancer drugs being developed target chaperones and co-chaperones, highlighting the clinical importance of these pathways. p23 knockout mice reveal an early peri-natal lethal phenotype, with defects in lung and skin development, but its organismal roles remain largely unknown [29,31].
Here, we used C. elegans to explore the role of p23 function in metazoan biology. We found that daf-41, the C. elegans homolog of co-chaperone p23, has a novel role in regulation of lifespan at both high and low temperatures, as well as in the formation of the long lived dauer stage. Remarkably, daf-41 mutants provoked longevity at warm temperatures, but short lived phenotypes at cold temperatures, thus equalizing the temperature response. daf-41 interacted with insulin signaling, heat shock factor, and steroidal signaling to regulate lifespan by distinct mechanisms at different temperatures. Our findings implicate daf-41 as a central player in the thermal regulation of longevity.
Results
daf-41/p23 is a novel regulator of dauer formation
DAF-41/ZC395.10 is the sole C. elegans homolog of co-chaperone p23/cytosolic prostaglandin E synthase-3. DAF-41/p23 is broadly conserved in evolution, with approximately 45% peptide similarity to the human homolog (Fig. 1A). The protein contains an N-terminal HSP20-like co-chaperone domain, implicated in HSP90 binding, and a C-terminal region, implicated in intrinsic chaperone activity (Fig. 1B). daf-41(ok3052) is a deletion allele and presumptive null that removes co-chaperone and chaperone domains; daf-41(ok3015) harbors an in-frame deletion of the co-chaperone domain, leaving the chaperone domain intact (Fig. 1B).
Fig. 1. daf-41/ZC395.10 regulates dauer formation and stress resistance. 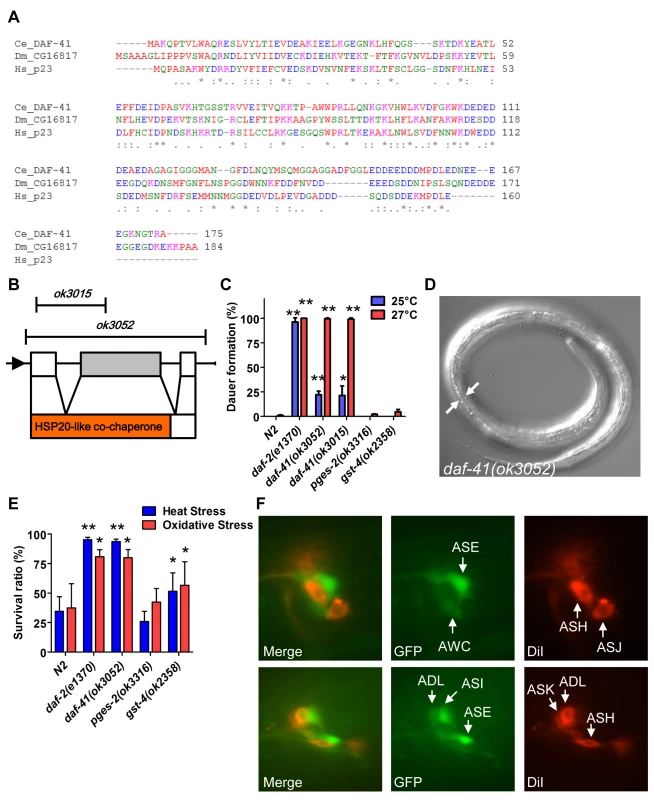
(A) An alignment of protein sequences between C. elegans DAF-41, D. melanogaster CG16817 and Homo sapiens p23/PTGES3. The similarity between DAF-41 and p23/PTGES3 is 44.6%. (B) Schematic illustration of the daf-41, and deletion alleles of ok3015 and ok3052. Black arrows indicate the direction of transcription. Red area indicates HSP20-like co-chaperone domain. (C) daf-41 mutants constitutively formed dauer larvae (Daf-c) weakly at 25°C and strongly at 27°C. (D) Dauer alae of daf-41(ok3052) animals grown at 27°C are indicated by the white arrows. (E) daf-41(ok3052) worms were resistant for oxidative stress (20mM of H2O2, 2.5 hrs) and heat stress (35°C, 8 hrs). gst-4(ok2358) worms were also slightly stress tolerant. (F) daf-41p::gfp (i.e. dpy-5(e907); sEx10796 [rCes daf-41::gfp + pCeh361]) worms were labeled with DiI and photos taken at the young adult stage. Patterns of gene expression of daf-41p::gfp (green), DiI (red), and merged figures are shown, with arrows indicating individual neurons. Under conditions of food scarcity, overcrowding and elevated temperature, C. elegans larvae enter the dauer diapause, a stage specialized for survival and dispersal. We found that both daf-41(ok3052) and daf-41(ok3015) alleles were constitutively prone to form dauer larvae (Daf-c phenotype) at elevated temperature, yielding approximately 25% dauer larvae at 25°C, and nearly 100% dauer larvae at 27°C (Fig. 1C-D). By contrast, mutants containing deletions of other putative prostaglandin synthase homologs, pges-2/mPGES2, gst-4/PGDS, had little observable dauer phenotype at these temperatures. By inference, the co-chaperone function of daf-41 may be more important for Daf-c phenotypes.
Many Daf-c loci in the dauer signaling pathways, such as the daf-2/Insulin receptor (InsR) mutant, provoke resistance to various forms of stress [32,33]. Similarly daf-41 mutants displayed resistance to oxidative stress induced by H2O2 challenge comparable in strength to daf-2/InsR mutants (Fig. 1E). daf-41 mutants exposed to heat stress at 35°C were also significantly resistant (Fig. 1E). Other PGS mutants had little effect on oxidative and heat resistance. Altogether these results demonstrate that daf-41 is a novel Daf-c gene involved in stress tolerance.
To clarify daf-41 function, we examined its expression pattern. A promoter fusion to gfp, daf-41p::gfp, revealed expression most prominently in anterior and posterior neurons (Fig. 1F) including amphids (e.g. ASE, AWC, ASI, ADL) and phasmid sensory neurons, as well as peripheral neurons and ventral cord motorneurons. We also observed strong expression in body wall muscle and pharynx, as well as occasional expression in vulva, seam and intestine (S1A-B Fig).
daf-41 regulates dauer formation via insulin/IGF, and steroidal signaling
In the dauer signaling pathways, environmental cues are detected by the neurosensory apparatus, and integrated by cGMP, TGF-β and insulin/IGF signaling. Ultimately these pathways converge on steroidal signaling to mediate the choice between arrest at the dauer diapause or continuous development to reproductive adult [34]. To understand where daf-41 acts in the dauer signaling pathways, we performed genetic epistasis experiments for dauer formation at 27°C. We first combined daf-41 Daf-c mutations with dauer formation defective (Daf-d) mutations in TGF-β signaling (daf-5/Ski), insulin/IGF signaling (daf-16/FOXO), and steroidal signaling (daf-12/FXR) [35–39]. As expected, null mutation of the steroid receptor, daf-12—a master regulator of dauer formation—completely suppressed daf-41 Daf-c phenotypes. Mutation of daf-16 partially suppressed daf-41 Daf-c phenotypes, while daf-5 mutation had little or no effect (Fig. 2A). At 25°C, daf-41 Daf-c phenotypes were also suppressed by daf-12. These experiments reveal that daf-41 acts upstream of daf-16, and daf-12, and in parallel to daf-5, to prevent dauer formation, resembling loci acting early in the dauer signaling pathways (Fig. 2H) [40].
Fig. 2. Genetic interactions of daf-41 with dauer signaling pathways. 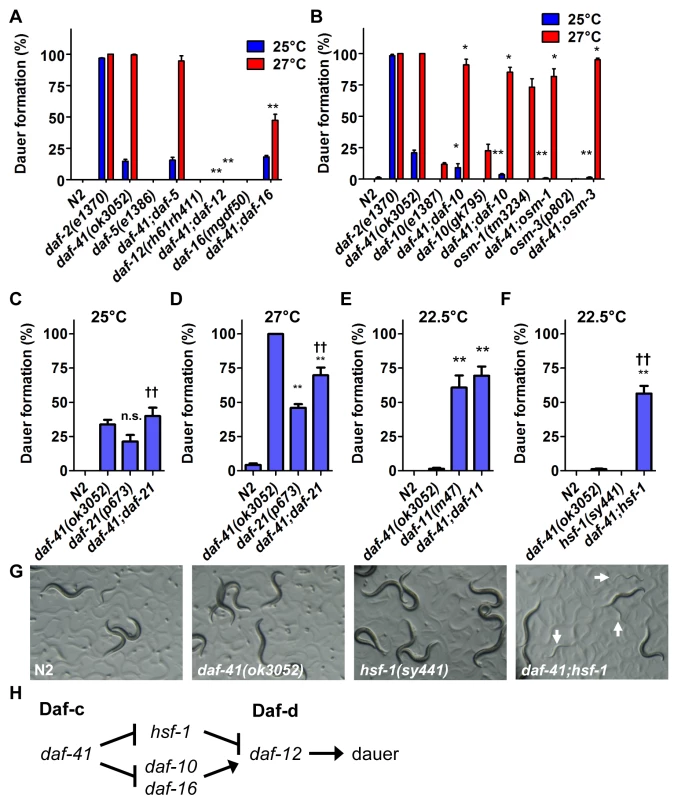
(A) daf-41(ok3052) Daf-c phenotypes were partially suppressed in af-16(mgDf50) and completely suppressed in daf-12(rh61rh411) backgrounds. (B) daf-41(ok3052) Daf-c phenotypes were suppressed in various chemotaxis mutant backgrounds. (C-D) daf-21(p673) had no additive effect on Daf-c phenotypes at 25°C, and modestly reduced dauer formation at 27°C in the daf-41(ok3052) background. (E) daf-11(m47) had no additive effect on dauer formation in the daf-41(ok3052) background at 22.5°C. (F) hsf-1(sy441) strongly enhanced dauer formation of daf-41(ok3052) at 22.5°C. (G) Cultures of daf-41(ok3052), hsf-1(sy441) and daf-41;hsf-1 are shown grown at 22.5°C. White arrows indicate dauer larvae. All error bars indicate S.D. *, p<0.05; **, p<0.01 versus daf-41(ok3052); ††, p<0.01 versus daf-21(p673)A or hsf-1(sy441) by t-test. (H) daf-41 regulates dauer formation via daf-10, daf-12 and daf-16 similar to daf-21. However, hsf-1 suppresses dauer formation in daf-21 but not daf-41. Note that the model reflects genetic interactions, not necessarily direct biochemical interactions. daf-41 affects dauer formation and chemotaxis through neurosensory signaling
We next analyzed interactions between daf-41 and neurosensory machineries of thermotaxis and chemotaxis, which variously affect dauer formation. We made double mutants with neurosensory transduction mutants (daf-10/IFT122, osm-1 and osm-3), which are Daf-d at 25°C and often Daf-c at 27°C [41,42]. We also examined thermotaxis mutants (pkc-1, ttx-3) that modulate dauer formation dependent on temperature and signaling pathway (e.g. ttx-3 suppresses daf-7/TGF-β Daf-c phenotype at 25°C, but enhances it at 15°C) [43,44].
We found that mutations in the chemotaxis genes, daf-10, osm-1 and osm-3, significantly suppressed Daf-c phenotypes of daf-41(ok3052) at 25°C and partially at 27°C (Fig. 2B). (Precedence for weaker Daf-c mutants suppressing stronger Daf-c mutants has been seen previously [45]). Consistent with a role proximal to the chemotaxis machinery, daf-41(ok3052) worms were chemotaxis defective for isoamylalcohol, benzaldehyde and 2,4,5-trimethylthiazoline (S2A Fig). By contrast, the thermotaxis loci did not appreciably affect daf-41(ok3052) Daf-c phenotypes at the examined temperatures (S3A-B Fig), although pkc-1 had a minor effect at 27°C. Thus daf-41 might work closely to chemotaxis loci and downstream or parallel to the thermotaxis loci.
Neurosensory cilia normally contact the environment through sensilla in head and tail, and typically fill with the lipophilic dye DiI. Mutants with defective neurosensory cilia structure, including daf-10 and osm-3 fail to fill with DiI because dendritic endings are not exposed [46]. We found that daf-41 null mutants had normal DiI filling similar to wild type worms (S2B Fig). Altogether these results show that daf-41 affects chemotaxis function, but not sensory cilia structure.
Interactions with HSP90 and cGMP signaling
In its capacity as co-chaperone, p23 is known to complex with HSP90 [16–18,47]. The C. elegans homolog of HSP90, daf-21, functions early in the dauer signaling pathways at the level of chemosensory processing, similar to daf-41. daf-21(p673) is a weak gain-of-function (gf) amino acid substitution that renders animals Daf-c [48]. Genetic epistasis studies show that Daf-c phenotypes are suppressed by daf-10 and other chemosensory mutants [40]. cGMP signaling represents another branch of the dauer signaling pathways that works at a similar level as daf-21. Mutations in the daf-11/transmembrane guanylyl cyclase provoke Daf-c phenotypes, which are similarly suppressed by chemosensory mutants [40,48]. Finally both daf-21 and daf-11 exhibit chemosensory deficits [49].
Because daf-41/p23 may regulate dauer entry through the chemosensory axis, we sought to dissect genetic interactions between daf-41, daf-21 and daf-11. Although both daf-41(ok3052) and daf-21(p673) yielded Daf-c phenotypes, daf-41;daf-21 worms did not show synthetic enhancement of dauer formation at 25°C (Fig. 2C). Furthermore, daf-41 phenotypes were weakly suppressed by daf-21 mutation at 27°C (conversely daf-21 phenotypes were weakly enhanced by daf-41) (Fig. 2D). Likewise the daf-41(ok3052) null allele had little effect on dauer formation of daf-11(m47) at 22.5°C, where daf-11’s Daf-c phenotypes were partially penetrant (Fig. 2E). Whereas mutants in independent pathways typically give strong synergy, the modest interactions observed above suggest that daf-41 could work in a proximal or overlapping pathway with daf-21 and daf-11.
In mammals, HSP90 complexes are known to negatively regulate the activity of the heat shock transcription factor HSF1 [23,24]. Recently, it was reported that the hsf-1(sy441) missense mutation suppresses the Daf-c phenotypes of daf-21 and daf-11 at 23°C [50], suggesting that dauer formation depends upon active hsf-1(+). We therefore analyzed genetic interactions between hsf-1 and daf-41 around this temperature. Surprisingly, instead of suppression, hsf-1(sy441) enhanced daf-41 Daf-c phenotypes at 22.5°C (Fig. 2F). Similarly the egg laying defect of hsf-1(sy441) was strikingly enhanced in the daf-41 mutant background (S4 Fig). These synthetic interactions suggest that daf-41 and hsf-1 could work closely together, or identify parallel pathways converging on the same process (Fig. 2H).
daf-41 regulates longevity in response to temperature
Because daf-41 mutants showed clear temperature dependent dauer and stress resistance phenotypes, we wondered whether daf-41 would influence ageing at various temperatures. Increasing temperature is well known to reduce longevity in ectotherms, including wild type C. elegans (Fig. 3A-C, Table 1). daf-41 mutants exhibited an altered temperature dependent longevity, revealing a leveling out of the lifespan curves to those typically seen at 20°C: animals were long lived at 25°C, normal lived at 20°C, and short lived at 15°C relative to wild type (Fig. 3A-E). Other PGS mutants showed normal temperature dependent lifespan phenotypes (Fig. 3A-C, Table 1).
Fig. 3. daf-41 mutants extend lifespan at elevated temperatures and shorten life span at lower temperatures. 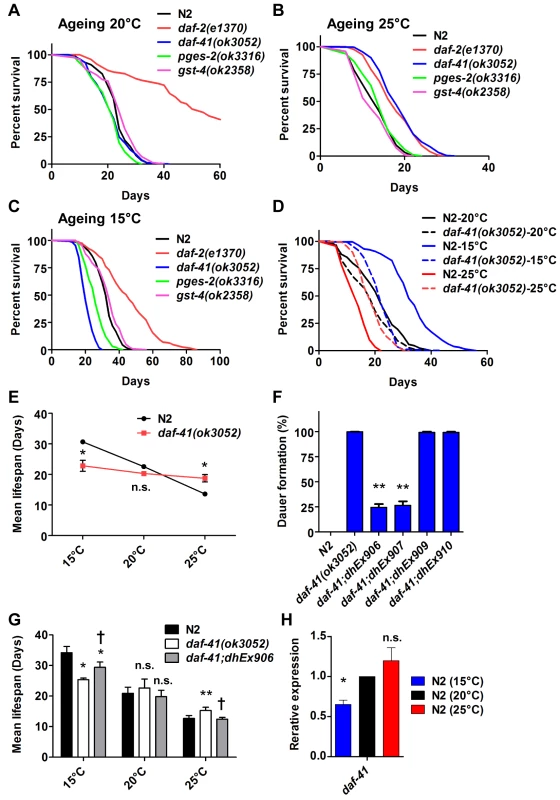
(A) daf-41(ok3052) mutant animals have a lifespan similar to wild type N2 worms at 20°C (B) daf-41(ok3052) animals showed extended lifespan at 25°C (C) daf-41(ok3052) animals showed reduced lifespan at 15°C. (D) Lifespan curves of N2 and daf-41(ok3052) at different temperatures were plotted onto the same graph as indicated. (E) Mean lifespan of 3 individual experiments were plotted. Error bars, S.D. *, p<0.05; n.s., no significant difference versus N2 by t-test. (F) daf-41(+) transgenes rescued Daf-c phenotypes of daf-41(ok3052) at 27°C. dhEx906 and dhEx907 are independent daf-41(+) transgenic lines. dhEx909 and dhEx910 are pges-2(+) transgenes under control of daf-41 5’ and 3’ regulatory elements. Error bars, S.D.; **, p<0.001 versus non-transgenic worms by t-test. (G) Mean lifespan from 3 individual experiments at 15°C, 20°C and 25°C were plotted. Error bars, S.D.; *, p<0.05; **, p<0.01 versus N2; †, p<0.05 versus daf-41(ok3052); n.s., no significant difference by t-test. (H) Gene expression of daf-41 was slightly reduced at 15°C, but no significant differences were measured between 20°C and 25°C. Error bars, S.D. *, p<0.05; n.s., no significance by t-test. Tab. 1. Ageing data of daf-41 mutants. 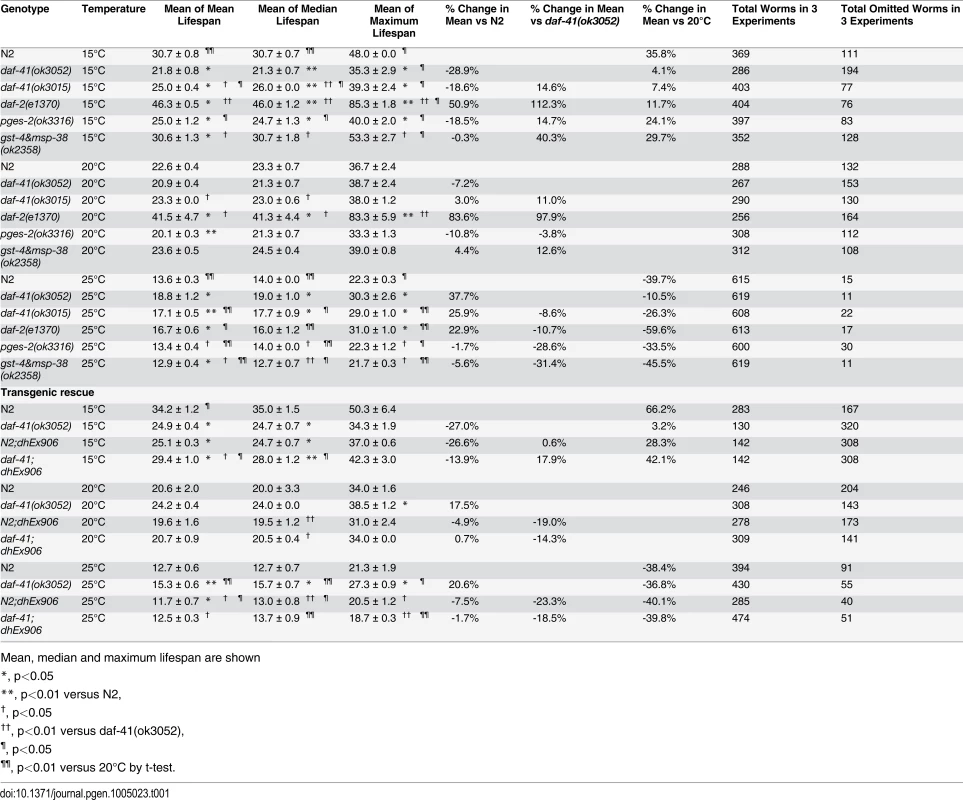
Mean, median and maximum lifespan are shown To determine if the observed longevity phenotypes were due to lesions in daf-41, we performed rescue experiments with the daf-41(+) transgene. As expected, daf-41(ok3052) mutant animals harboring daf-41(+) were readily rescued for Daf-c phenotypes, while the pges-2(+) control transgene expressed under daf-41 regulatory elements did not rescue (Fig. 3F). We next tested the influence of the transgenes on ageing. First, we found that overexpression of daf-41 and pges-2 had no effect on ageing in both N2 and daf-41(ok3052) at 20°C (Fig. 3G and S5A Fig). However, the daf-41 transgene clearly reversed the daf-41(ok3052) longevity phenotype at 25°C, and partially rescued the short-lived phenotype at 15°C (Fig. 3G and S5B-C Fig). In sum, these results reveal that daf-41(+) regulates longevity in response to temperature. Consistent with a role in thermal regulated processes, daf-41 mRNA increased modestly with temperature (Fig. 3H).
Because metabolic rates increase with temperature and vary inversely with longevity, we wondered whether daf-41 mutants altered mitochondrial metabolism. When we measured O2 consumption of daf-41 mutants at different temperatures, however, we saw no significant differences from wild type (S5D Fig). We also examined reproductive potential of daf-41(ok3052) worms and found that mutants produced nearly the same number of progeny as wild type at 20°C (S5E Fig).
daf-41 regulates longevity via insulin signaling and the heat stress response
To better understand the nature of daf-41 longevity, we next performed genetic epistasis experiments with known longevity regulators, including daf-16/FOXO, hsf-1/heat shock factor, daf-12/FXR steroid receptor, and daf-10/IFT122 [8,51–54]. Intriguingly, daf-16 was epistatic at all temperatures. At 25°C daf-16(mgDf50) completely abolished the longevity of daf-41(ok3052) (Fig. 4B, Table 2). At 20°C daf-16 mutation reduced lifespan of daf-41(ok3052) similar to wild type N2 (Fig. 4A). At 15°C, daf-16 mutation did not further reduce the short lifespan of daf-41(ok3052) mutants (Fig. 4C). Moreover, daf-16 mutation had the effect of restoring temperature dependent regulation of lifespan to daf-41 mutants (Fig. 4D, Table 2). Although daf-16 mRNA levels were little affected by daf-41, several major target genes of daf-16, including sod-3, dod-3, and lipl-4 [55] were elevated in daf-41(ok3052) in a daf-16 dependent manner (Fig. 4E). No differences in DAF-16 nuclear localization were seen between WT and daf-41(ok3052), since both showed moderately elevated translocation at 25°C, (S6A-B Fig). These data support the notion that daf-41(+), either directly or indirectly inhibits the activity but not localization of DAF-16/FOXO at elevated temperatures. We therefore performed qRT-PCR analysis for insulin like peptides and found that expression of several ins genes were changed by temperature shift and in daf-41(ok3052) worms. In particular, ins-1, ins-5, ins-7, ins-10, ins-11, ins-12, ins-17, ins-18, ins-27 and ins-37 were up-regulated in daf-41(ok3052) worms at 25°C, and only ins-13 was down-regulated at 25°C (S7 Fig), consistent with modulation of IIS.
Fig. 4. daf-41 longevity is dependent on daf-16/FOXO. 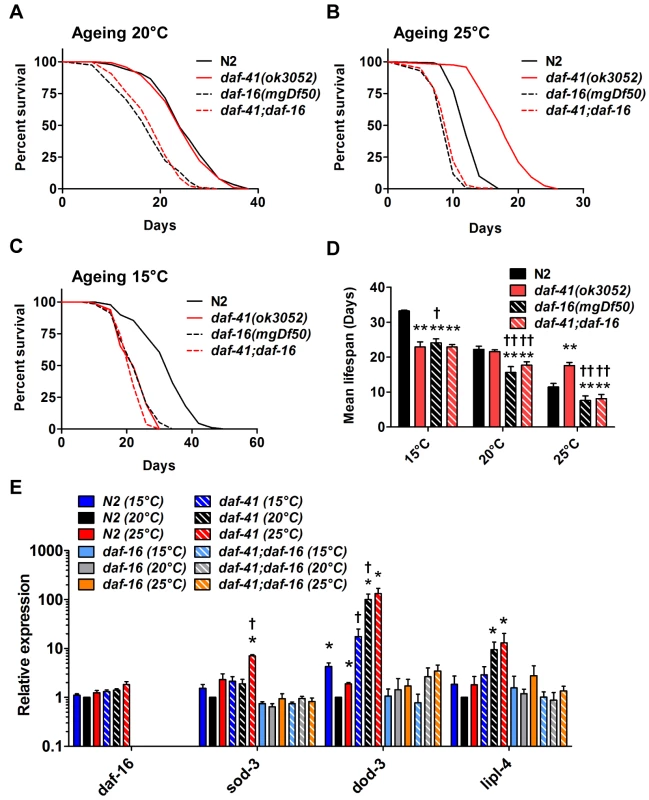
(A) daf-16(mgDf50) equally reduced the lifespan of daf-41(ok3052) and N2 worms at 20°C. (B) daf-16(mgDf50) abolished daf-41 longevity at 25°C. (C) daf-16(mgDf50) did not further reduce the short life span of daf-41(ok3052) worms at 15°C. (D) Mean lifespan from 3 individual experiments were plotted for the indicated genotypes. Error bars; S.D.; **,p<0.01 versus N2; ††, p<0.01 versus daf-41(ok3052) by t-test. (E) DAF-16 target genes, sod-3, dod-3 and lipl-4, were significantly upregulated in response to warm temperature in daf-41(ok3052) relative to N2. n = 4 biological replicates. Error bars, S.E.M; *, p<0.05 versus N2 of 20°C; †, p<0.05 versus daf-41(ok3052) of 20°C by t-test. Tab. 2. Ageing data of daf-41(ok3052) in various mutant backgrounds. 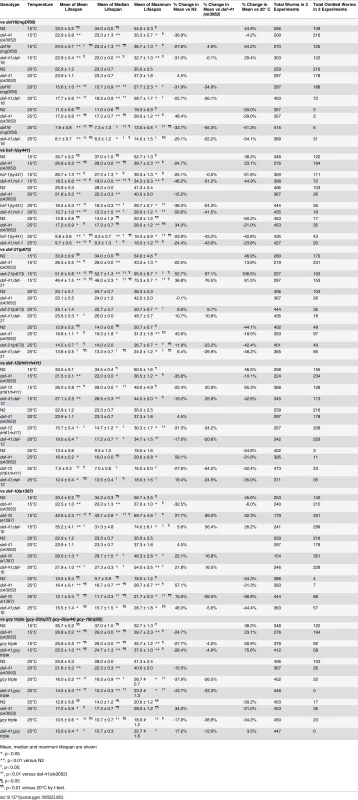
Mean, median and maximum lifespan are shown We next examined genetic interactions with the heat shock transcription factor hsf-1, which is required for normal lifespan at various temperatures [8,9]. Longevity of daf-41(ok3052) was completely abolished in the hsf-1(sy441) background at 25°C, suggesting the two genes work in a unified pathway (Fig. 5B, Table 2). By contrast, the daf-41;hsf-1 double mutant showed additive short-lived phenotypes at 15°C and 20°C (Fig. 5A, C and D, Table 2) presumably because hsf-1(sy441) is non-null. Although hsf-1 itself was not transcriptionally regulated, major target genes of hsf-1, including hsp-16.2, hsp-70, and hsp-4 [56], showed augmented expression in the daf-41 background compared to wild type at 25°C (Fig. 5E). These results indicate that daf-41(+) directly or indirectly inhibits the activity of HSF-1 at 25°C, to influence transcription and longevity. Recently, it has been reported that HSF-1 forms nuclear foci in response to heat shock but not by reduced IIS [57]. Similarly, we failed to detect foci formation of HSF-1 at 20°C and 25°C daf-41(ok3052) mutants (S8A-B Fig).
Fig. 5. daf-41(ok3052) longevity is hsf-1 dependent. 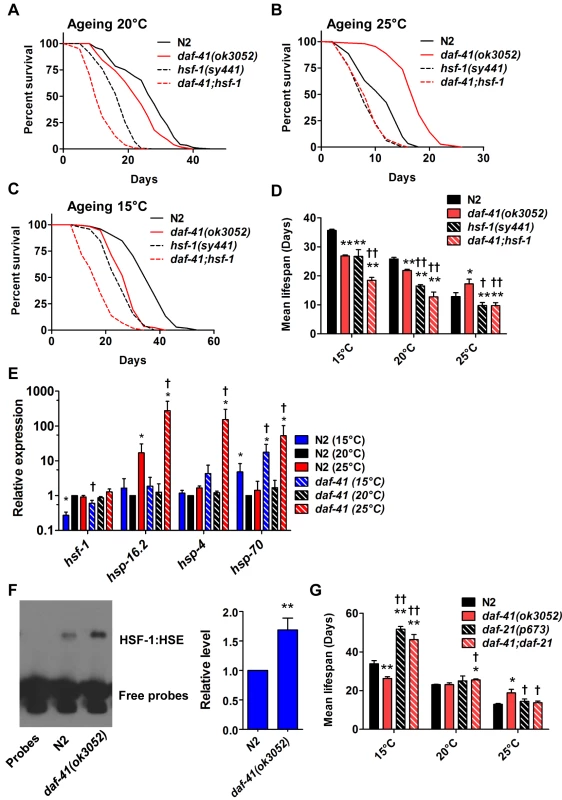
(A) hsf-1(sy441) shortened both N2 and daf-41(ok3052) life span at 20°C. (B) hsf-1(sy441) abolished daf-41(ok3052) longevity at 25°C. (C) hsf-1(sy441) further reduced daf-41(ok3052) short life span at 15°C. (D) Mean lifespan from of 3 individual experiments were plotted for indicated genotypes and conditions. Error bars, S.D.; *, p<0.05; **, p<0.01 versus N2; †, p<0.05; ††, p<0.01 versus daf-41(ok3052) by t-test. (E) daf-41(ok3052) enhanced the upregulation of HSF-1 target genes, hsp-16.2, hsp-4, and hsp-70, in response to warm temperature. n = 4 biological replicates. Error bars, S.E.M; *, p<0.05 versus N2 of 20°C; †, p<0.05 versus daf-41(ok3052) of 20°C by t-test. (F) HSF-1 binding activity to HSE was 1.5 fold increased in daf-41(ok3052) at 25°C. Error bars, S.E.M; **, p<0.01 versus N2. (G) At 15°C, daf-21(p673) mutation enhanced the longevity of N2 and daf-41(ok3052). At 20°C, daf-21 mutant animals lived slightly longer than N2. At 25°C, daf-21(p673) animals lived slightly longer than WT but the mutation reduced longevity in the daf-41(ok3052) background. Given the genetic interactions with hsf-1 for dauer formation, longevity, and gene transcription described above, we wondered whether daf-41 affects assembly of HSF-1 nuclear complexes. To measure this, we prepared nuclear extracts from wild type and daf-41 mutants and performed electrophoretic mobility shift assays on the heat shock factor response element as established previously [58]. First we found that daf-41 mutation had no effect on mRNA or protein levels of HSF-1 (S8C Fig). Second, we observed that daf-41 nuclear extracts showed a clear and reproducible 1.5–2 fold higher occupancy of the heat shock factor response element, compared to WT controls (Fig. 5F). These results suggest that DAF-41(+) normally affects the assembly or disassembly of HSF-1 transcriptional complexes.
Because p23 and HSP90 are known to work together, we asked whether they would have similar effects on lifespan. At 25°C, both daf-41(ok3052) and daf-21(p673)gf worms were longer lived than wild type; however, the daf-41;daf-21 double mutant had longevity phenotypes more similar to the daf-21 single mutant (Fig. 5G, S9A-B Fig, Table 2). The convergent behavior at 25°C suggests they could work together at this temperature, similar to dauer. By contrast at 15°C, daf-21(p673) worms were extremely long-lived; this longevity was additive to the short-lived phenotype of daf-41(ok3052) mutant animals. The distinct behavior of daf-41/p23 and daf-21/hsp90 at 15°C, suggests they may regulate lifespan at this temperature through different pathways (S9C Fig).
The steroid receptor DAF-12 also influenced the longevity phenotypes of daf-41(ok3052) (Fig. 6A-D, Table 2). At 25°C, daf-12 mutation partially reduced the longevity of daf-41 mutants in an additive manner, but was not epistatic, i.e. daf-12 daf-41 did not give the same life span as daf-12 itself. The expression levels of the DA biosynthetic gene, daf-36/Rieske, and two DAF-12 target genes, cdr-6 and fard-1, were also increased at higher temperatures. Therefore, daf-12(+) could work in parallel, or function as a minor branch of daf-41 signaling to promote longevity. More interestingly at 15°C, daf-12 mutation suppressed the short lived phenotypes of daf-41, restoring near normal life span. At this temperature, cdr-6 and fard-1 genes decreased in expression in daf-41 mutants relative to WT (Fig. 6E). These interactions suggest that daf-41(+) could prevent life shortening properties of DAF-12 steroidal signal transduction at lower temperatures.
Fig. 6. The short life span of daf-41(ok3052) at 15°C is daf-12 dependent. 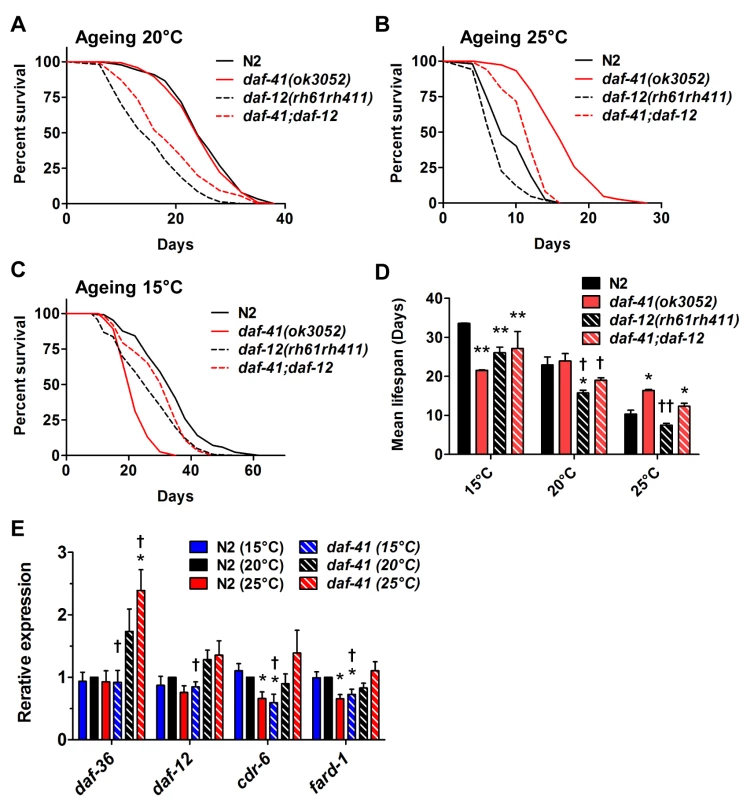
(A-B) daf-12(rh61rh411) reduced longevity of daf-41(ok3052) at 20°C and 25°C. (C) daf-12(rh61rh411) partly rescued the short life span of daf-41(ok3052) at 15°C. (D) Mean lifespan from 3 individual experiments are plotted with indicated genotypes and conditions. Error bars, S.D.; *, p<0.05; **, p<0.01 versus N2; †, p<0.05; ††, p<0.01 versus daf-41(ok3052) by t-test. (E) Transcriptional targets of DAF-12, cdr-6 and fard-1, were reduced with temperature in N2, but this tendency was reversed in the daf-41(ok3052) background. n = 4 biological replicates. Error bars, S.E.M; *, p<0.05 versus N2 of 20°C; †, p<0.05 versus daf-41(ok3052) of 20°C by t-test. We also examined interactions with daf-10/IFT122, which affects neuronal cilia, and is long lived [46,54]. At 15°C and 20°C, daf-10 mutation did not affect daf-41 and showed additive phenotypes, i.e. daf-10 increased lifespan of both N2 and daf-41(ok3052) independent of temperature (Fig. 7A, S10A-C Fig, Table 2). At 25°C, however, daf-41 and daf-41;daf-10 had similar degrees of lifespan extension, suggesting that the two activities might converge on a common process.
Fig. 7. daf-41 partially interacts with the chemosensory and thermosensory apparatus to regulate longevity. 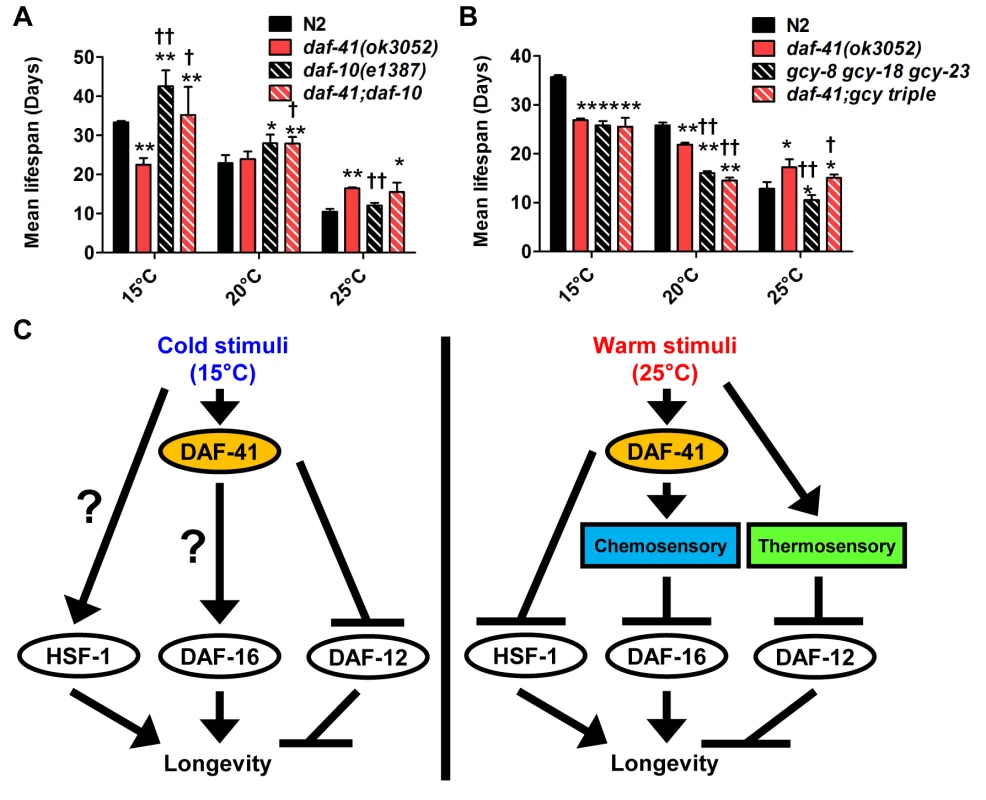
(A) Mean lifespan of 3 individual experiments were plotted. The triple mutant of gcy-23(nj37) gcy-8(oy44) gcy-18(nj38) (gcy triple) caused a parallel reduction of lifespan in N2 and daf-41(ok3052), respectively at 25°C. The gcy triple mutant did not further shorten the life span of daf-41(ok3052) at 15°C. (B) daf-10 mutation increased lifespan in parallel to daf-41 at 15°C and 20°C. daf-10 mutation did not further extend the life span of daf-41(ok3052) worms at 25°C. Error bars, S.D.; *, p<0.05; **, p<0.01 versus N2; †, p<0.05; ††, p<0.01 versus daf-41(ok3052) by t-test. (C) A schematic model describing the regulatory mechanism of longevity by daf-41 at different temperatures. At 25°C, daf-41 negatively regulates the transcriptional activities of DAF-16 and HSF-1 and their down-regulation results in normal life span. Thermotaxis and steroidal signaling may regulate longevity in parallel to daf-41. At 15°C, daf-41 (+) contributes to longevity possibly via daf-16/FOXO. daf-41(+) may also prevent life shortening activities of daf-12(+), while hsf-1 may promote longevity in parallel. These are working models that we interpret with caution, and may reflect direct or indirect interactions. Finally we examined the effect of genes involved in thermotaxis. These genes are implicated in the systemic heat shock response [7]. Moreover, recent work has shown that longevity arising from RNAi knockdown of the pat-4/integrin-linked kinase depends completely on genes involved in thermotaxis (ttx-3 and gcy-8), hsf-1, but not daf-16 [59]. For our studies, we used the soluble guanylyl cyclase (gcy) triple mutant, gcy-23(nj37) gcy-8(oy44) gcy-18(nj38), which is defective in thermotaxis due to signaling defects in the thermotaxis neurons [60]. As expected, we observed the gcy triple mutant to be short-lived at 25°C (Fig. 7B, S10F Fig, Table 2). Unexpectedly, the gcy triple mutant equally shortened the lifespan of daf-41 and wild type, suggesting parallel pathways. At 15°C, the gcy triple mutant did not further shorten daf-41 lifespan, possibly suggesting a convergent mechanism (S10D-E Fig, Table 2).
Discussion
How temperature influences animal lifespan is not well understood. In this work, we demonstrate that daf-41/p23 co-chaperone PTGES3 homolog modulates longevity in response to temperature, and regulates entry and exit from the long-lived dauer stage. On the one hand, daf-41(+) promotes normal adult longevity at cold temperature (15°C). On the other hand, daf-41(+) limits lifespan at warm temperature (25°C), but has little influence on longevity at 20°C. Thus the overall effect seen in daf-41 mutants is an equalization of the lifespan curve so that animals appear as if they are living at 20°C. Surprisingly, no difference in respiratory rates (O2 consumption) between wild type and daf-41 animals was detected at these temperatures, suggesting that daf-41’s effect on longevity is likely through mechanisms independent of mitochondrial metabolism. These intriguing observations reveal that daf-41 plays a unifying role in regulatory mechanisms that modulate lifespan in reaction to temperature, and imply that co-chaperone/chaperone complexes may mediate this response.
Although the detailed molecular mechanism by which daf-41 mediates these temperature dependent effects is not entirely understood, our genetic epistasis experiments suggest that daf-41 may directly or indirectly impinge on several transcriptional longevity regulatory mechanisms, including DAF-16/FOXO, HSF-1, and DAF-12 steroidal signaling, to regulate lifespan in response to temperature. Several lines of evidence argue that daf-16/FOXO is a critical mediator in these circuits. At warm temperature, lifespan extension of daf-41 mutants was abolished in the daf-16 mutant background, at low temperatures the daf-41;daf-16 double mutants did not live shorter than daf-41 alone, suggesting convergent mechanisms. Overall daf-16 mutation restored temperature dependent lifespan regulation to daf-41 mutants. As further support for a link to FOXO function, daf-16 partly suppressed daf-41 Daf-c phenotypes, placing daf-16 downstream for both dauer formation and longevity. Consistent with working in a signaling pathway, we found that daf-41(+) negatively regulated transcription of DAF-16 targets (sod-3, dod-3, lipl-4) in response to warm temperature although no clear effect was seen at low temperatures with these genes. Several insulin-like peptides were up or down regulated in daf-41 mutants in response to warm temperature, suggesting that daf-41 has the potential to affect IIS. However, we could not detect obvious differences in DAF-16 nuclear localization, as is often seen with mutations that only weakly affect signaling (Henderson et al. 2001). Conceivably daf-41 could regulate components of IIS or DAF-16 complexes themselves.
Heat shock factor, hsf-1, often works in tandem with daf-16/FOXO [8,9] and consistently, was also required for daf-41 induced longevity at elevated temperatures. Accordingly, several hsf-1 target genes (hsp-16.2, hsp-70, hsp-4) were expressed in daf-41 mutants under these conditions. Although HSF-1 mRNA or protein levels were unchanged in the daf-41 background, nuclear extracts from daf-41 mutants showed increased occupancy of the heat shock response element. Importantly, this result suggests that daf-41(+) influences either directly or indirectly the assembly/disassembly of the HSF-1 complex. This finding is consistent with observation the p23-HSP90 complex has been shown to inhibit the activity of the HSF-1 complex in other systems [23,25]. The observed short-lived phenotypes of daf-41 with hsf-1 at low temperatures were additive, possibly because the hsf-1 allele is temperature sensitive and non-null [56].
daf-41’s interactions with nuclear receptor daf-12 suggest an intriguing role for steroid signaling. At 15°C, daf-12 mutation suppressed daf-41 short-lived phenotypes, suggesting that daf-41 modulates life extending effects of daf-12. Steroid signaling has previously been implicated in lifespan regulation albeit at high temperatures: daf-9 hypomorphs as well as thermotaxis mutants are particularly short-lived at 25°C, in a manner dependent upon daf-12 [4]. At these temperatures, thermotaxis loci as well as hsf-1(+) promote expression of the hormone biosynthetic gene daf-9/CYP27A1 [4,50]. These observations suggest that normal lifespan at 25°C depends on adequate stimulation of steroidal signaling, thereby preventing life shortening activities of the unliganded DAF-12. At 25°C, daf-12 mutation shortened the life span of daf-41 to a similar extent as wild type, suggesting parallel pathways. Alternately daf-12 could contribute partially toward daf-41 longevity, since daf-12 target genes as well as daf-36 increase expression at this temperature. At this point, it is unclear whether the activating or repressing functions of the receptor are responsible for longevity.
In mammals, p23 together with HSP90, immunophilins and other chaperones, binds to unliganded steroid receptors in the cytosol, maintaining the receptor in a primed state competent for rapid ligand binding; upon binding hormone the steroid receptor enters the nucleus where interacts with transcriptional coregulators. Thereafter, p23 helps disassemble transient transcriptional complexes to facilitate hormone sampling and repeated rounds of transcription [15,20–22,26]. The p23-HSP90 complex also regulates type II nuclear receptors, such as the thyroid receptor [20], which constitutively reside in the nucleus, a situation more resembling that of DAF-12. Further studies on the role of steroidal signaling at low and high temperature may help clarify the whether similar mechanisms are at work in C. elegans.
Interactions with chemosensory mutants suggest a role within the sensory apparatus. Indeed, daf-41 longevity at warm temperatures converged with daf-10/IFT122, and both mutants stimulate daf-16, suggesting they could work through a similar chemosensory mechanism [54]. Consistent with a proximal role in chemosensory signaling, daf-41 mutants exhibited chemosensory deficits and chemosensory mutants suppressed the daf-41 Daf-c phenotypes. Accordingly, daf-41 was expressed in several chemosensory neurons including ASE, AWC, ADL, and ASI but was not obviously expressed in the main thermosensory neurons AFD and AIY, although AWC and ASI also reportedly contribute to thermosensation [61]. Ultimately, tissue specific dissection of daf-41 activities may help illuminate what cells mediate these interactions. It is also noteworthy that daf-41 did not further shorten the lifespan of the gcy triple mutant at 15°C, suggesting it could work through thermotaxis circuits at low temperature.
p23 is known to interact directly with HSP90 to modulate various client proteins [15,16], and recently, C. elegans p23 and HSP90 have been shown to physically interact in vitro [47]. Consistent with the possibility of working together, both daf-41/p23 and daf-21/HSP90 act at the level of chemosensory processing with respect to dauer formation (this work; [49]), and modulated one anothers’ dauer and longevity phenotypes at 25°C. That daf-41 null and daf-21 gain of function have similar phenotypes could indicate that the wild type activities work in opposition for these processes. On the other hand, daf-41 and daf-21 had several divergent phenotypes: whereas daf-41 Daf-c phenotypes were enhanced by hsf-1 mutation (Fig. 2H, this work), daf-21 Daf-c phenotypes were suppressed [50]. Furthermore, whereas daf-41 mutants were short-lived at 15°C, daf-21 mutants were long-lived, and regulated life span differently. Thus some p23 phenotypes might arise from HSP90 dependent as well as independent processes, as has been noted previously [26,27]. We interpret these experiments with caution since daf-21 mutations are gain-of-function and non-null. Intriguingly, HSP90 in Candida albicans has been shown to regulate the switch to pseudohyphal growth in response to temperature [62], perhaps analogous to the thermal regulation of dauer formation or longevity.
Based on the observed genetic interactions we suggest the following model for longevity regulation. At elevated temperatures, p23 directly or indirectly inhibits the transcriptional activities of HSF-1 and perhaps DAF-16, thus limiting lifespan at these temperatures, while DAF-12/FXR and thermotaxis signaling work in parallel (Fig. 7C). Given the convergence with daf-10 longevity, we suggest that daf-41(+) might work through the chemosensory apparatus to impinge upon DAF-16. More speculatively, at lower temperatures, p23 stimulates DAF-16/FOXO and inhibits DAF-12/FXR, possibly through the thermotaxis apparatus, while HSF-1 works in parallel to promote lifespan extension.
Given the intriguing genetic interactions described here, it would be interesting to investigate whether DAF-41 and/or HSP90 binds and regulates the activities of HSF-1, DAF-16, or DAF-12 by protein-protein interaction. Alternately DAF-41 could interact with upstream components of these signaling pathways, including kinases, temperature sensitive channels, guanylyl cyclases, or cilia proteins. Conceivably, thermal regulation in these circuits could result from thermal influences on protein-protein interactions.
The results here and elsewhere reveal that regulation of longevity at different temperatures works by distinct mechanisms. This is perhaps not surprising, given that different stresses challenge the organism at low and high temperatures. Moreover, ectotherms must have evolved optima for growth and reproduction within a temperature range. With this in mind, we suggest that daf-41 could play distinct roles at low and high temperatures. Alternately daf-41 may be part of an adaptive response to temperature in which optima shifted towards higher temperatures have a consequent tradeoff at lower temperatures, and vice versa. Further elucidation of daf-41/p23 complexes and physiology in C. elegans should help illuminate the mechanism of thermal regulation of metazoan longevity.
Methods
Strains
Strains were obtained from the Caenorhabditis Genetics Center (CGC, USA) and National Bio Resource Project (NBRP, Japan). All strains were outcrossed at least 4 times to wild type N2 before further analysis. All strains in this work are itemized in S1 Table. Genotypes of mutants were confirmed by PCR, sequencing and phenotyping. Primer sets for genotyping are listed in S2 Table.
Ageing experiments
All lifespans were measured as previously described [52]. Strains for ageing experiments were maintained at the respective cultivation temperature of 15°C and 20°C for more than 3 generations before analysis, unless indicated otherwise. Progeny were collected by egg laying and bleaching. Ageing experiments were performed with and without 2′fluoro-5′deoxyuridine (FUdR, Sigma) to prevent contamination of next generation progeny and to reduce bagging phenotypes of Egl mutants. Strains were treated with FUdR as described previously [63]. For ageing experiments at warm temperature, worms were cultivated at 20°C until the young adult stage, then moved to 25°C to start the ageing analysis in order to bypass dauer formation and minimize internal hatching phenotypes. Each experiment started with more than 150 worms. Sterile, escaped, internally hatched, and exploded worms were censored on the day of loss. Experiments were performed at least 3 times and the mean lifespan calculated. Worms were transferred onto new OP plates every 2–3 days from the end of reproductive period, and scored for survival every 2–4 days. Statistical analyses were performed by Kaplan–Meier method with GraphPad Prism software (GraphPad Software, Inc.)
Generation of transgenic strains
The regions of the daf-41/ZC395.10 promoter, coding region and 3' UTR, as well as pges-2 coding region and its 3' UTR were amplified by PCR. The promoter region was inserted in front of gfp in the L3781 plasmid and coding regions inserted after gfp. daf-41p::gfp::daf-41::3'UTR and daf-41p::gfp::pges-2::3'UTR plasmids were confirmed by sequencing and co-injected into daf-41(ok3052) worms with coel::RFP plasmid as an injection marker. Both transgenic strains were outcrossed with N2 to generate the following strains: N2; daf-41p::gfp::daf-41::3'UTR, N2;daf-41p::gfp::pges-2::3'UTR, daf-41(ok3052); daf-41p::gfp::daf-41::3'UTR, and daf-41(ok3052);daf-41p:: gfp::pges-2::3'UTR.
Dauer formation assays
For dauer formation assays, all strains were maintained at 20°C and eggs collected by egg laying or bleaching. Greater than 50 eggs were transferred onto 3cm OP plates and cultured at 20°C, 22.5°C, 25°C and 27°C, respectively. Dauer formation fraction was typically scored at 60 hrs, and dauer exit ratio was scored at 84 hrs.
Stress resistance analysis
All strains were maintained at 20°C and Day 1 young adults were used for analysis. For heat stress analysis, 50–100 adult worms were transferred onto fresh 6 cm OP plates and shifted to 35°C. Fraction survival was scored 8 hrs after heat shock. For oxidative stress analysis, 50–100 adult worms were collected by washing off plates and transferred into 24 well plastic plates filled with 20mM of hydrogen peroxide (SIGMA) in M9 buffer. Experiments were performed with 5 biological replicates with 3 technical replicates for each mutant.
qRT-PCR analysis
Synchronized worms were prepared at different temperatures. Worms were collected in TRIzol (Invitrogen) at L4 stage and frozen in liquid nitrogen. Total RNA was extracted by RNeasy Mini kit (QIAGEN) and Superscript III First Strand Synthesis System (Invitrogen) was used for cDNA generation. qRT-PCR was performed with Power SYBR Green master mix (Applied Biosystems) on a 7900HT Fast Real-Time PCR System (Applied Biosystems). ama-1 was used as internal control for mRNA quontification. For each analysis, qRT-PCR was performed with at least three biological replicates. Primer sequences are listed in S2 Table.
Preparation of nuclear protein extracts
WT and daf-41(ok3052) worms were harvested and frozen down at day 1 of adulthood. Frozen worm pellets were first homogenized in an equal volume of 2X NPB buffer (20 mM HEPES, pH 7.6, 20 mM KCl, 3 mM MgCl2, 2mM EDTA, 0.5 M sucrose, 1 mM dithiothreitol, protease inhibitors, and phosphatase inhibitors) using a Kontes Pellet Pestle tissue grinder. The suspension was then centrifuged (4000 g, 5 min, 4°C) and the pellets were further homogenized by 20 strokes with pestle A of a Dounce homogenizer. The pellet was resuspended in NPB buffer with 0.25% NP-40 and 0.1% Triton-X100, centrifuged again, and washed three more times with the same buffer. The nuclear pellet was extracted with four volumes of HEG buffer (20 mM HEPES, pH 7.9, 0.5 mM EDTA, 10% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, and protease inhibitors) at 4°C for 45 min. Finally, the nuclear fraction was collected by centrifugation at 14,000 g for 15 min at 4°C. Protein concentrations were determined with a Bradford assay kit (Bio-Rad, Hercules, CA).
Electrophoretic mobility shift assays (EMSA)
EMSAs were carried out as previously described [58]. In brief, 1 μg of nuclear extract (described above) was mixed with 1 mg/mL of poly (dI-dC) and 1 nM of a biotin-labeled oligonucleotide containing the heat-shock element (HSE) sequence of hsp-16.1 [58], and incubated for 15 min at room temperature in binding buffer [20 mM HEPES, pH 7.6, 5 mM EDTA, 1 mM dithiothreitol, 150 mM KCl, 50 mM (NH4)2SO4, and 1% Tween 20 (v/v)]. After incubation, the samples were separated by native 3.5% PAGE and the HSF-1::HSE DNA complexes were visualized using a LightShift Chemiluminescent EMSA kit (Pierce, Rockford, IL).
Additional details for DiI staining, chemotaxis analysis, oxygen consumption measurement and microscopy analysis are described in S1 Text.
Supporting Information
Zdroje
1. Miquel J, Lundgren PR, Bensch KG, Atlan H (1976) Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev 5 : 347–370. 823384
2. Klass MR (1977) Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev 6 : 413–429. 926867
3. Lee WS, Monaghan P, Metcalfe NB (2013) Experimental demonstration of the growth rate—lifespan trade-off. Proc Biol Sci 280 : 20122370. doi: 10.1098/rspb.2012.2370 23235704
4. Lee SJ, Kenyon C (2009) Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol 19 : 715–722. doi: 10.1016/j.cub.2009.03.041 19375320
5. Xiao R, Zhang B, Dong Y, Gong J, Xu T, et al. (2013) A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152 : 806–817. doi: 10.1016/j.cell.2013.01.020 23415228
6. Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, et al. (2006) Transgenic mice with a reduced core body temperature have an increased life span. Science 314 : 825–828. 17082459
7. Prahlad V, Cornelius T, Morimoto RI (2008) Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320 : 811–814. doi: 10.1126/science.1156093 18467592
8. Hsu AL, Murphy CT, Kenyon C (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300 : 1142–1145. 12750521
9. Morley JF, Morimoto RI (2004) Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell 15 : 657–664. 14668486
10. Brown-Borg HM, Bartke A (2012) GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci 67 : 652–660. doi: 10.1093/gerona/gls086 22466316
11. Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, et al. (2012) The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1: e00065. doi: 10.7554/eLife.00065 23066506
12. Holloszy JO, Smith EK, Vining M, Adams S (1985) Effect of voluntary exercise on longevity of rats. J Appl Physiol (1985) 59 : 826–831. 4055572
13. Craig EA, Gambill BD, Nelson RJ (1993) Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev 57 : 402–414. 8336673
14. Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381 : 571–579. 8637592
15. Johnson JL, Beito TG, Krco CJ, Toft DO (1994) Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol Cell Biol 14 : 1956–1963. 8114727
16. Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, et al. (2006) Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 440 : 1013–1017. 16625188
17. Richter K, Walter S, Buchner J (2004) The Co-chaperone Sba1 connects the ATPase reaction of Hsp90 to the progression of the chaperone cycle. J Mol Biol 342 : 1403–1413. 15364569
18. McLaughlin SH, Sobott F, Yao ZP, Zhang W, Nielsen PR, et al. (2006) The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J Mol Biol 356 : 746–758. 16403413
19. Johnson JL, Toft DO (1994) A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J Biol Chem 269 : 24989–24993. 7929183
20. Freeman BC, Felts SJ, Toft DO, Yamamoto KR (2000) The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev 14 : 422–434. 10691735
21. Freeman BC, Yamamoto KR (2002) Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296 : 2232–2235. 12077419
22. Wochnik GM, Young JC, Schmidt U, Holsboer F, Hartl FU, et al. (2004) Inhibition of GR-mediated transcription by p23 requires interaction with Hsp90. FEBS Lett 560 : 35–38. 14987994
23. Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94 : 471–480. 9727490
24. Bharadwaj S, Ali A, Ovsenek N (1999) Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 In vivo. Mol Cell Biol 19 : 8033–8041. 10567529
25. Zelin E, Zhang Y, Toogun OA, Zhong S, Freeman BC (2012) The p23 molecular chaperone and GCN5 acetylase jointly modulate protein-DNA dynamics and open chromatin status. Mol Cell 48 : 459–470. doi: 10.1016/j.molcel.2012.08.026 23022381
26. Freeman BC, Toft DO, Morimoto RI (1996) Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science 274 : 1718–1720. 8939864
27. Echtenkamp FJ, Zelin E, Oxelmark E, Woo JI, Andrews BJ, et al. (2011) Global functional map of the p23 molecular chaperone reveals an extensive cellular network. Mol Cell 43 : 229–241. doi: 10.1016/j.molcel.2011.05.029 21777812
28. Tanioka T, Nakatani Y, Kobayashi T, Tsujimoto M, Oh-ishi S, et al. (2003) Regulation of cytosolic prostaglandin E2 synthase by 90-kDa heat shock protein. Biochem Biophys Res Commun 303 : 1018–1023. 12684036
29. Lovgren AK, Kovarova M, Koller BH (2007) cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis. Mol Cell Biol 27 : 4416–4430. 17438133
30. Calderwood SK (2013) Molecular cochaperones: tumor growth and cancer treatment. Scientifica (Cairo) 2013 : 217513. doi: 10.1155/2013/217513 24278769
31. Grad I, McKee TA, Ludwig SM, Hoyle GW, Ruiz P, et al. (2006) The Hsp90 cochaperone p23 is essential for perinatal survival. Mol Cell Biol 26 : 8976–8983. 17000766
32. Honda Y, Honda S (1999) The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J 13 : 1385–1393. 10428762
33. Lithgow GJ, White TM, Melov S, Johnson TE (1995) Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A 92 : 7540–7544. 7638227
34. Fielenbach N, Antebi A (2008) C. elegans dauer formation and the molecular basis of plasticity. Genes Dev 22 : 2149–2165. doi: 10.1101/gad.1701508 18708575
35. Bell LR, Stone S, Yochem J, Shaw JE, Herman RK (2006) The molecular identities of the Caenorhabditis elegans intraflagellar transport genes dyf-6, daf-10 and osm-1. Genetics 173 : 1275–1286. 16648645
36. da Graca LS, Zimmerman KK, Mitchell MC, Kozhan-Gorodetska M, Sekiewicz K, et al. (2004) DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGF beta pathway to regulate C. elegans dauer development. Development 131 : 435–446. 14681186
37. Lin K, Dorman JB, Rodan A, Kenyon C (1997) daf-16: An HNF-3/forkhead family member that can function to double the life-span of C. elegans. Science 278 : 1319–1322. 9360933
38. Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, et al. (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389 : 994–999. 9353126
39. Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL (2000) daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev 14 : 1512–1527. 10859169
40. Thomas JH, Birnby DA, Vowels JJ (1993) Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics 134 : 1105–1117. 8375650
41. Riddle DL, Swanson MM, Albert PS (1981) Interacting genes in nematode dauer larva formation. Nature 290 : 668–671. 7219552
42. Ailion M, Thomas JH (2000) Dauer formation induced by high temperatures in C. elegans. Genetics 156 : 1047–1067. 11063684
43. Monje JM, Brokate-Llanos AM, Perez-Jimenez MM, Fidalgo MA, Munoz MJ (2011) pkc-1 regulates daf-2 insulin/IGF signalling-dependent control of dauer formation in Caenorhabditis elegans. Aging Cell 10 : 1021–1031. doi: 10.1111/j.1474-9726.2011.00747.x 21933341
44. Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, et al. (1997) Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron 19 : 345–357. 9292724
45. Coburn CM, Mori I, Ohshima Y, Bargmann CI (1998) A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult Caenorhabditis elegans: a distinct pathway for maintenance of sensory axon structure. Development 125 : 249–258. 9486798
46. Perkins LA, Hedgecock EM, Thomson JN, Culotti JG (1986) Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 117 : 456–487. 2428682
47. Eckl JM, Drazic A, Rutz DA, Richter K (2014) Nematode Sgt1-homologue D1054.3 binds open and closed conformations of Hsp90 via distinct binding sites. Biochemistry 53 : 2505–2514. doi: 10.1021/bi5000542 24660900
48. Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, et al. (2000) A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in caenorhabditis elegans. Genetics 155 : 85–104. 10790386
49. Vowels JJ, Thomas JH (1994) Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics 138 : 303–316. 7828815
50. Barna J, Princz A, Kosztelnik M, Hargitai B, Takacs-Vellai K, et al. (2012) Heat shock factor-1 intertwines insulin/IGF-1, TGF-beta and cGMP signaling to control development and aging. BMC Dev Biol 12 : 32. doi: 10.1186/1471-213X-12-32 23116063
51. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366 : 461–464. 8247153
52. Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A (2001) A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell 1 : 841–851. 11740945
53. Hsin H, Kenyon C (1999) Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399 : 362–366. 10360574
54. Apfeld J, Kenyon C (1999) Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402 : 804–809. 10617200
55. Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, et al. (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 : 277–283. 12845331
56. Hajdu-Cronin YM, Chen WJ, Sternberg PW (2004) The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics 168 : 1937–1949. 15611166
57. Morton EA, Lamitina T (2013) Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 12 : 112–120. doi: 10.1111/acel.12024 23107491
58. Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL (2012) HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell 148 : 322–334. doi: 10.1016/j.cell.2011.12.019 22265419
59. Kumsta C, Ching TT, Nishimura M, Davis AE, Gelino S, et al. (2013) Integrin-linked kinase modulates longevity and thermotolerance in C. elegans through neuronal control of HSF-1. Aging Cell.
60. Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, et al. (2006) Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 172 : 2239–2252. 16415369
61. Beverly M, Anbil S, Sengupta P (2011) Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J Neurosci 31 : 11718–11727. doi: 10.1523/JNEUROSCI.1098-11.2011 21832201
62. Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, et al. (2009) Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol 19 : 621–629. doi: 10.1016/j.cub.2009.03.017 19327993
63. Mitchell DH, Stiles JW, Santelli J, Sanadi DR (1979) Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol 34 : 28–36. 153363
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



