-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
Rothmund Thomson Syndrome (RTS), RAPADILINO Syndrome and Baller-Gerold Syndrome are very rare human syndromes associated with mutations in RECQL4. RECQL4 is important for controlling how cells divide and for preventing genome damage. Patients with RECQL4 mutations have problems with bone formation and a low bone mass, similar to osteoporosis. RTS patients have a highly increased risk of developing bone cancer (osteosarcoma). The role of RECQL4 in normal bone development and osteosarcoma formation is largely unknown. We have used mouse models to understand the specific role of Recql4 in bone development. Mice with Recql4 removed specifically from their bone cells have shortened bones and a reduced rate of bone formation. Therefore, RECQL4 is essential for normal bone development. Interestingly, the animals with no Recql4 in bone cells did not develop osteosarcoma. Using mouse models of osteosarcoma, we observed delayed cancer formation when Recql4 was also deleted. Further analysis demonstrated that bone cancer could not arise from Recql4 null cells even with concurrent p53 deletion. These studies clarify the role of RECQL4 in both normal and malignant bone biology and suggest that RECQL4 mutations that cause osteosarcoma most likely result in proteins with reduced, but not absent, function.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005160
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005160Summary
Rothmund Thomson Syndrome (RTS), RAPADILINO Syndrome and Baller-Gerold Syndrome are very rare human syndromes associated with mutations in RECQL4. RECQL4 is important for controlling how cells divide and for preventing genome damage. Patients with RECQL4 mutations have problems with bone formation and a low bone mass, similar to osteoporosis. RTS patients have a highly increased risk of developing bone cancer (osteosarcoma). The role of RECQL4 in normal bone development and osteosarcoma formation is largely unknown. We have used mouse models to understand the specific role of Recql4 in bone development. Mice with Recql4 removed specifically from their bone cells have shortened bones and a reduced rate of bone formation. Therefore, RECQL4 is essential for normal bone development. Interestingly, the animals with no Recql4 in bone cells did not develop osteosarcoma. Using mouse models of osteosarcoma, we observed delayed cancer formation when Recql4 was also deleted. Further analysis demonstrated that bone cancer could not arise from Recql4 null cells even with concurrent p53 deletion. These studies clarify the role of RECQL4 in both normal and malignant bone biology and suggest that RECQL4 mutations that cause osteosarcoma most likely result in proteins with reduced, but not absent, function.
Introduction
Rothmund-Thomson syndrome (RTS), RAPADILINO Syndrome and Baller-Gerold Syndrome are rare autosomal recessive disorders that are associated with mutations in the DNA helicase RECQL4. RECQL4 belongs to a family of RecQ helicases that are important in the regulation of DNA repair and replication, and constitutes five human members: BLM, WRN, RECQL4, RECQ1 and RECQ5 [1]. Along with RECQL4, WRN and BLM are also associated with human hereditary disorders involving both skeletal defects and cancer predisposition [1]. Mutations in WRN cause Werner Syndrome, in BLM Bloom Syndrome and when RECQL4 is mutated RTS and related syndromes arise [1,2]. Abnormalities in skeletal development are a defining feature of the human disorders associated with mutations in RecQ helicases, such as short stature, osteoporosis, low bone mass and polydactyly [3–6]. In addition to the developmental phenotypes shared across RecQ helicase mutation kindreds, RTS patients are predisposed to develop osteosarcoma (OS) [6,7]. However, neither the role of RECQL4 in osteoblast biology nor the influence of RECQL4 mutations in the initiation and maintenance of OS have been defined.
OS is the most common primary tumor of bone and it is currently treated with chemotherapy and surgical resection [8–10]. It presents bi-modally, primarily in children and teenagers with a second incidence after the age of 70 [11]. Recent data has begun to shed light on the complex genetics of conventional OS [12], and have revealed that OS is characterised by multiple somatic mutations and chromosomal aberrations [13]. Strikingly, with the exception of TP53, there are few highly recurrent somatic alterations [12]. These data highlight that OS is a disease most broadly characterised by a tolerance of genomic instability and mutation burden. Whilst these studies have begun to provide information about the landscape of the OS genome, the initiating events that enable OS formation are less clear. Conversely, human familial cancer syndromes have provided great insight into the genetic initiating events of OS.
Three familial syndromes are strongly associated with a predisposition to OS: Li-Fraumeni syndrome (TP53 mutations), hereditary retinoblastoma (RB1 mutations) and RTS. Indeed, mutations in the TP53 occur in >90% of conventional OS, and this observation is supported by murine OS models that demonstrate a p53 pathway mutation dependence [12,14–18]. In contrast to both TP53 and RB1 pathways, mutations in RECQL4 have not been observed in sporadic OS. Indeed, elevated levels of RECQL4 have been reported in sporadic OS, a result potentially confounded by the close genomic linkage of the RECQL4 locus with MYC, which is amplified in many OS [19,20]. Despite the apparently contradictory nature of the data, about 30% of RTS patients with deleterious RECQL4 mutations develop OS [7]. RTS associated mutations in RECLQ4 are notable in that they display an unusually high proportion of mutations that impact on splicing, possibly resulting from the presence of numerous short introns, and that mutations spare the N-termial region of protein [21–23]. A primary function of RECQL4 is thought to be mediated by its ATP-dependent helicase RecQ domain, and RTS associated mutations cluster to this region [21]. These clinical observations raise several important questions about the role of RECQL4 in osteoblast biology and tumorigenesis: firstly, what is the function of RECQL4 that leads to the benign skeletal defects observed in RTS patients? Secondly, what role does RECQL4 play in OS initiation and maintenance?
Previous attempts to generate Recql4 deficient mice have yielded three non-conditional alleles with divergent phenotypes. The first reported allele deleted exons 5–8, resulting in early embryonic lethality [24]. A second allele, generated by an in-frame deletion of exon 13, encoding part of the RecQ helicase domain, resulted in 95% of the mice dying within two weeks of birth [25]. The small proportions of viable homozygous mutants displayed severe growth retardation, but were not reported to develop OS. The third allele replaced part of exon 9 through to 13 with a PGK-Hprt mini gene cassette [26]. Although most homozygous mutant mice were viable, a small proportion displayed skeletal defects such as polydactyly and shorter limbs. These mice also developed cancers including OS, at low penetrance (5% incidence of cancer overall, 40% of cancers were OS (n = 2 animals)). The latter two alleles were both characterised by the presence of aberrant Recql4 transcripts within the cells, which potentially resulted in the generation of C-terminal truncated proteins. These limitations confound the understanding of the in vivo role of Recql4 in both normal development and tumor formation.
We recently described a conditional murine Recql4 allele generated by flanking exons 9 and 10 with loxP elements [27]. This region was selected to enable inactivation of the helicase domain and reflect the preponderance of mutations in this region in humans with RTS. In the mouse, exon 9 encodes the start of the ATP dependent RecQ helicase domain. Germ-line deletion of exons 9 and 10 resulted in embryonic lethality prior to E10.5, most similar to the phenotype reported with deletions of exons 5–8. Somatic deletion of Recql4 in adult mice resulted in the rapid development of a fully penetrant bone marrow failure-like syndrome and death of the Recql4 deficient animals [27]. This model precluded analysis of the role of Recql4 in skeletal development and OS. We have now used targeting deletion of this conditional allele within the osteoblast lineage to understand the requirement for Recql4 in normal skeletal homeostasis and OS development.
Results
Generation of osteoblast specific Recql4 deficient mice
As a result of the embryonic lethality of germ-line Recql4-/- animals and the lethal, non-skeletal phenotype upon somatic deletion of Recql4 in adult mice [27], we moved to a lineage restricted deletion model to examine the role of Recql4 in skeletal development and malignancy. To achieve osteoblast-restricted deletion of Recql4, we crossed the Recql4fl/fl mice with two Cre strains active at distinct stages of osteoblast development (Fig 1A). Firstly, we used the Osterix1-GFP::Cre (Osx-Cre) transgenic mouse where Cre is active in the proliferative osteoblast progenitor populations [28]. Secondly, to delete Recql4 in the committed mature osteoblast/osteocyte population we used the Dentin matrix protein 1-Cre (Dmp1-Cre) transgenic line [29]. In both cases, the Rosa26-eYFP strain that reports Cre activity through the expression of eYFP was included to allow for tracing and isolation of Cre exposed cells [30]. The distinct spatiotemporal expression of Osx and Dmp1 allows for in vivo assessment of the requirement for Recql4 in osteoblast lineage development.
Fig. 1. Deletion of Recql4 in osteoblast precursors results in dwarfism. 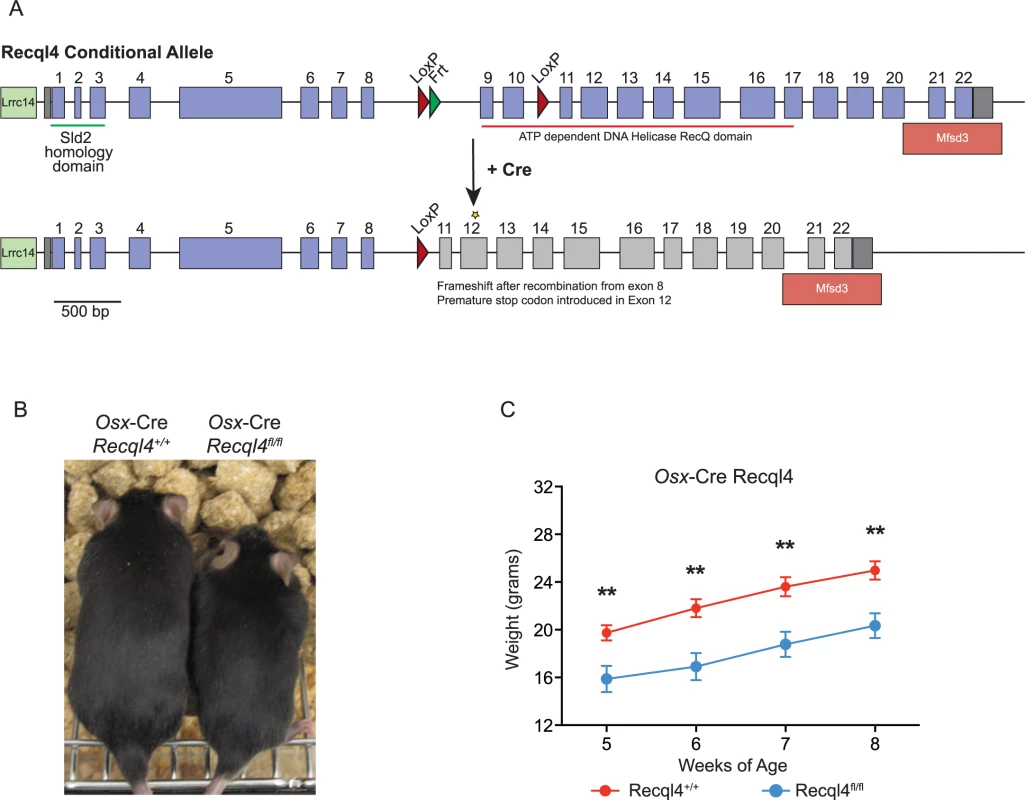
(A) Diagram of Cre-mediated Recql4 deletion. (B) Representative photograph of male Osx-Cre Recql4+/+ and Recql4fl/fl mice. (C) Gross body weights of male Osx-Cre Recql4 mice. n ≥ 7 for both genotypes. Data presented as mean±SEM. **P<0.01 compared with Recql4+/+, t-test. Reduced skeletal growth in Osx-Cre Recql4fl/fl mice
It had previously been reported that heterozygous germ-line Recql4 mutant mice displayed a skeletal phenotype, although their human counterparts (parents/siblings of RTS patients) do not present with any apparent skeletal changes [3,27,31]. We assessed skeletal parameters by micro-computed tomography (μCT) in male germ-line exon 9–10 deficient Recql4+/- mutants. We observed no significant changes in any bone parameter, nor did we observe any phenotype (malignant or otherwise) in the Recql4+/- mice upon extended aging.
We next investigated the effects of Recql4 deletion from the pre-osteoblast populations using Osx-Cre on normal skeletal development. Only male mice were assessed for all genotypes to exclude any influence of sex differences in bone structure, and all controls in these studies are Osx-Cre+ Recql4+/+ to control for the effects of the transgene alone on bone [32,33]. While Osx-Cre Recql4fl/+ had no phenotype and were no different to Osx-Cre Recql4+/+ controls, Osx-Cre Recql4fl/fl mice were smaller than Osx-Cre Recql4+/+ control littermates as evidenced by a significantly lower body weight (Fig 1B and 1C). Osx-Cre Recql4fl/fl mice had shorter tibia at 9 weeks of age (Fig 2A and 2B), while there was less mineralized bone within the long bones when assessed by von Kossa staining (Fig 2C). Although efficient genomic recombination of Recql4 in adult tissues was only observed in bone tissue (Fig 2D), we had previously observed a selection against stable deletion of Recql4 in hematopoietic cells [27]. To ascertain if stable deletion was occurring in osteoblast precursors, we isolated hematopoietic and vascular marker negative (CD45-Lin-CD31-) cells from the collagenase digested compact long bones. These were fractionated into eYFP positive and negative populations, distinguishing cells that have expressed Cre during development and would be expected to have Recql4 recombined (eYFP+ve), from Cre negative cells. Genomic DNA was isolated from each population from Osx-Cre Recql4fl/fl and Osx-Cre Recql4+/+ control littermates. Essentially complete deletion of exons 9 and 10 was observed in the eYFP+ve fraction from the Osx-Cre Recql4fl/fl (Fig 2E and 2F). This demonstrates that stable deletion was achieved with no recovery of non-deleted/heterozygous cells to confound the interpretation of the phenotype. Nonetheless, during these cell isolations a significantly lower proportion of eYFP positive phenotypic osteoblastic cells was noted in the Osx-Cre Recql4fl/fl mice compared to controls (Fig 2E and 2F).
Fig. 2. Osx-Cre Recql4fl/fl mice have reduced bone length. 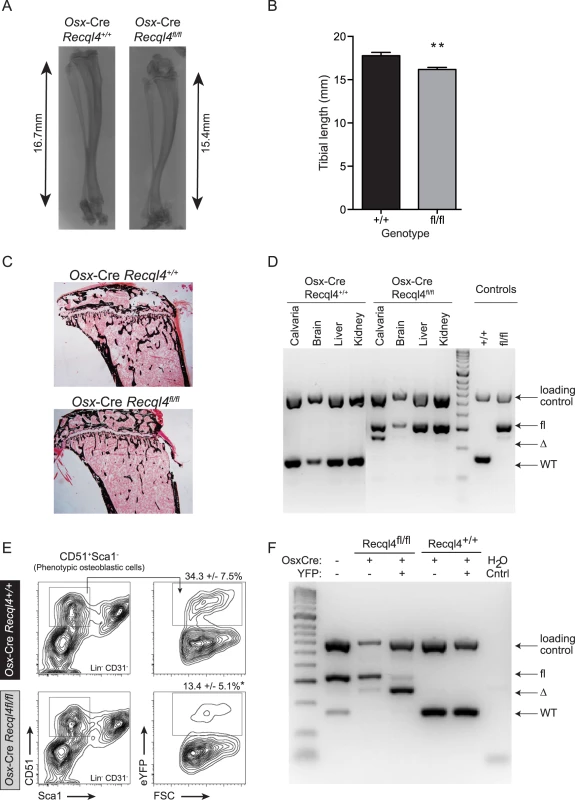
(A) X-ray of tibia from male Osx-Cre Recql4+/+ and Recql4fl/fl mice. (B) Measurement of tibial length from Osx-Cre Recql4+/+ and Recql4fl/fl mice. n≥ 9 for both genotypes. Data presented as mean±SEM. **P<0.01 compared with Recql4+/+, t-test. (C) Representative photo of Von Kossa staining of 9 week old Osx-Cre Recql4 tibiae. (D) Detection of Recql4 genomic excision in various tissues from Osx-Cre mice. (E) Percentage of FACS-sorted osteoblastic cells (Lin- CD31- CD51+ Sca1-) from Osx-Cre Recql4 mice. Data presented as mean±SEM. *P<0.05 compared with Recql4+/+, t-test. (F) Osteoblastic cells from Osx-Cre R26YFP Recql4 mice were sorted into eYFP+ve and eYFP-ve fractions prior to genomic DNA extraction and assessment of Recql4 genomic excision. The effect of Recql4 deletion from the early stages of osteoblast differentiation on skeletal architecture was assessed by μCT analysis of the trabecular bone within the secondary spongiosa and the diaphyseal cortical bone of the tibia. Qualitative assessment of pseudo-colored volume-rendered images of Osx-Cre Recql4fl/fl tibiae showed an apparently lower density with less trabecular bone (Fig 3A). This trabecular phenotype presented with a 17% reduction in bone volume as a proportion of total volume (BV/TV) (Fig 3B) compared to controls. Correspondingly, there was a 16% decrease in trabecular number (Tb.N) and a 17% increase in trabecular separation (Tb.Sp.), with no change in trabecular thickness (Tb.Th) (Fig 3B). We further characterized the bone structure and remodeling by histomorphometry within the proximal secondary spongiosa of the tibia. The low trabecular bone mass observed by μCT was associated with similar, but not statistically significant reductions in total bone volume and trabecular number, and increased trabecular separation in this second site (S1A Fig). There was no change in osteoid surface, thickness or volume, nor in the numbers of osteoblasts (N.Ob/B.Pm) or osteoclasts (N.Oc/B.Pm) per unit of bone perimeter (S1C Fig). Despite this, dynamic histomorphometry showed a significant 36% and 42% decrease in both mineral apposition rate (MAR) and bone formation rate (BFR/BS) respectively in Osx-Cre Recql4fl/fl mice, with no change in the mineralising surface per bone unit surface (MS/BS) compared to control mice (Fig 3C). Within the cortical bone, Osx-Cre Recql4fl/fl tibiae showed 10% and 14% reduction in cortical thickness (Ct.Th) and cortical bone area (Ct.Ar) respectively, with slight, but non-significant decrease in cortical cross-sectional bone area (Tt.Ar) and cortical bone area fraction (Ct.Ar/Tt.Ar) in the Recql4 deficient animals (Fig 3D and 3E). Collectively, these data demonstrate an essential requirement for Recql4 in osteoblast function.
Fig. 3. Osx-Cre Recql4fl/fl mice have a low bone mass. 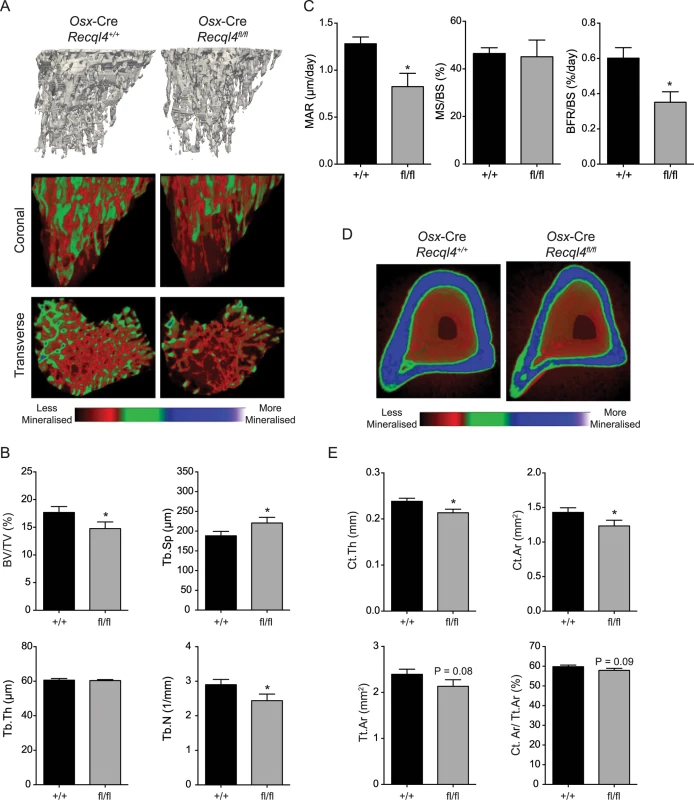
(A) Representative images of reconstructed trabecular region of the proximal tibial secondary spongiosa (top) and color-coded quantitative mineralization images (bottom) from male Osx-Cre Recql4+/+ and Recql4fl/fl mice. (B) Trabecular bone volume (BV/TV), trabecular separation (Tb.Sp), trabecular number (Tb.N), trabecular thickness (Tb.Th) of Osx-Cre Recql4 tibiae. n≥ 8 for both genotypes. (C) Mineralization apposition rate (MAR), mineralizing surface per unit bone surface (MS/BS) and bone formation rate per unit bone surface (BFR/BS) from the dynamic histomorphometric analysis of the proximal tibial secondary spongiosa. n = 5–6 for both genotypes. (D) Representative images of reconstructed cortical bone with colour-coded quantitative mineralisation from Osx-Cre Recql4+/+ and Recql4fl/fl mice. (E) Cortical thickness (Ct.Th), cortical bone area (Ct. Ar), total cortical cross-sectional bone area (Tt.Ar) and cortical bone area fraction (Ct.Ar/Tt.Ar) of Osx-Cre Recql4 cortical tissue. n≥ 8 for both genotypes. Data presented as mean±SEM. *P<0.05, compared with Recql4+/+, t-test. Given the known role of the bone marrow microenvironment in regulating hematopoietic homeostasis, we also assessed hematopoiesis in the Osx-Cre Recql4fl/fl and Osx-Cre Recql4+/+ littermates. We detected no differences in peripheral blood composition nor bone marrow hematopoiesis in the osteoblast restricted Recql4-deficient animals (S2A–S2C Fig). Recql4 deficient osteoblasts are able to support hematopoiesis normally.
The deletion of Recql4 in mature osteoblastic cells did not result in a bone phenotype
Given the striking effect of Recql4 deletion from pre-osteoblast stages on skeletal development, we sought to determine what impact deletion of Recql4 would have when restricted to mature osteoblasts and osteocytes. To this end, we used the Dmp1-Cre transgenic line [29,34] (Fig 4A). Efficient genomic deletion of Recql4 was seen in bone tissues, however unlike Osx-Cre, we also observed genomic recombination of Recql4 in muscle tissue, as previously reported for this Cre line (Fig 4B) [35]. Littermate male Dmp1-Cre Recql4+/+ and Dmp1-Cre Recql4fl/fl mice were comparable in weight and overall size (Fig 4C) and tibial lengths were the same between the cohorts (Fig 4D). Analysis by μCT of the trabecular and cortical bone regions of the tibia did not demonstrate any differences in those parameters assessed (Fig 4E and 4F). Collectively, the data indicate that although Recql4 is required in the proliferating and differentiating osteoblastic population, it is not required in mature osteoblast and osteocyte populations to maintain bone homeostasis.
Fig. 4. Dmp1-Cre Recql4fl/fl mice do not display a bone phenotype. 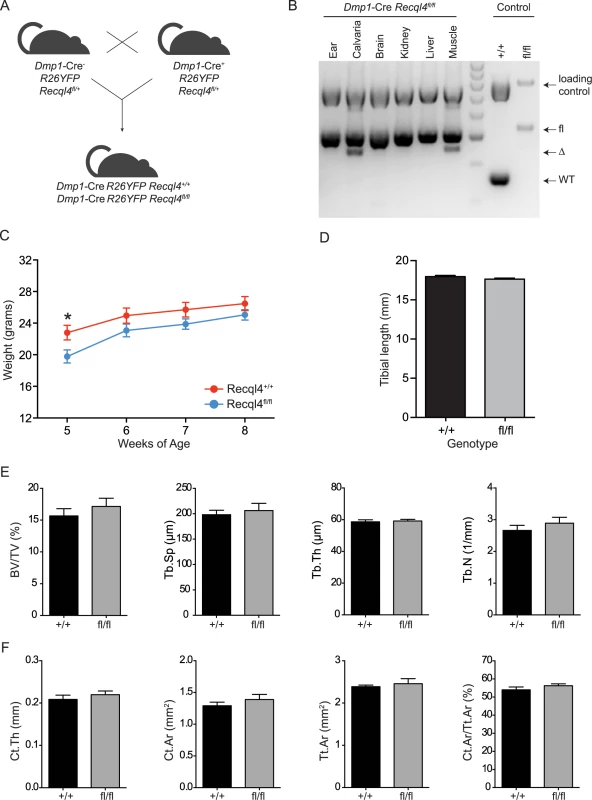
(A) Schematic of generating Dmp1-Cre Recql4+/+ and Recql4fl/fl mice. (B) The assessment of Recql4 genomic excision in various tissues from Dmp1-Cre mice. (C) Gross body weights of male Dmp1-Cre Recql4 mice. n = 4 for Recql4+/+, n = 6 for Recql4fl/fl. (D) Measurement of tibiae from Dmp1-Cre Recql4+/+ and Recql4fl/fl mice. n = 4 for Recql4+/+, n = 8 for Recql4fl/fl. (E) Trabecular bone volume (BV/TV), trabecular separation (Tb.Sp), trabecular number (Tb.N), trabecular thickness (Tb.Th) of Dmp1-Cre Recql4 tibiae. (F) Cortical thickness (Ct.Th), cortical bone area (Ct. Ar), total cortical cross-sectional bone area (Tt.Ar) and cortical bone area fraction (Ct.Ar/Tt.Ar) of Dmp1-Cre Recql4 cortical tissue. Data from 9 week old male mice; n = 4 for Recql4+/+, n = 8 for Recql4fl/fl. Data presented as mean±SEM. *P<0.05 compared with Recql4+/+, t-test. Depletion of Recql4 resulted in inhibition of cell proliferation and osteogenic differentiation
We initially planned to use FACS-purified, eYFP positive osteoblastic cells from the Osx-Cre Recql4+/+ and Osx-Cre Recql4fl/fl mice to delineate the role of Recql4 (Fig 2E). However, the low yields and very poor proliferation of the eYFP positive cells from Osx-Cre Recql4fl/fl cells rendered them unsuitable for further analysis [36,37]. We therefore utilized primary long bone osteoblastic cells from Rosa26-CreERT2 Recql4 mice [27]. Tamoxifen (4-OHT) was added to these cultures for up to 21 days to activate Cre mediated deletion of Recql4 and cell proliferation assays were performed during the first and third week.
Rosa26-CreERT2 Recql4fl/+ cells (control, referred to as Recql4Δ/+) proliferated comparably to non-tamoxifen treated cultures for the entire culture period (21 days). When assessed 7 days after the addition of tamoxifen, gene recombination was apparent in the Rosa26-CreERT2 Recql4fl/fl cells (homozygous deletion of Recql4, referred to as Recql4Δ/Δ), but only slightly more than 60% of the floxed allele had been deleted. Between days 2 and 7, there was no difference in proliferation rates between Recql4Δ/+ and Recql4Δ/Δ cells (Figs 5A, 5B and S3). At day 14 after tamoxifen addition, recombination of the Recql4 allele had increased to closer to 80% in both sets of genotypes (Figs 5B and S3). At this time, the tamoxifen-treated Recql4 Δ/Δ primary osteoblasts failed to proliferate (Fig 5A), while non-tamoxifen treated Recql4Δ/Δ cells proliferated similarly to controls (Recql4Δ/+ ± tamoxifen). The more complete the genomic deletion of Recql4, the more profound the proliferation arrest was apparent (Figs 5B and S3). These studies demonstrate a requirement for Recql4 in normal osteoblast expansion, and suggest that the in vivo phenotype may be attributed to the impaired proliferation of Recql4 deficient osteoblastic cells.
Fig. 5. The depletion of Recql4 in vitro causes proliferation arrest. 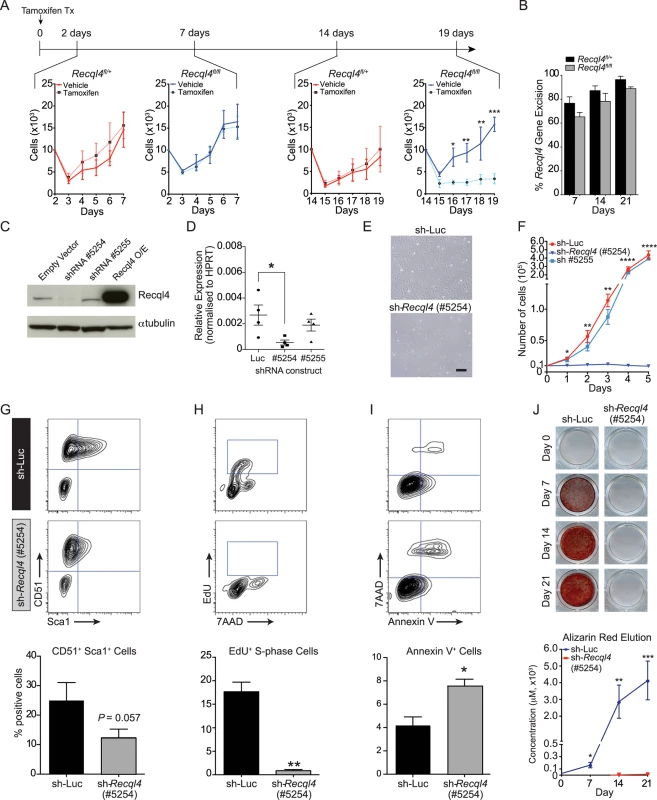
(A) Primary long bone osteoblastic cells were derived from Rosa26-CreERT2 Recql4 mice treated with tamoxifen and subjected to proliferation assays. n = 3 independent cultures per genotype. (B) The assessment of Recql4 genomic excision from tamoxifen-treated osteoblastic cells. n = 3 independent cultures per genotype. (C) Immuno-blot of Recql4 protein levels from Kusa4b10 cells, with α-tubulin as a loading control. (D) Quantitative real-time PCR (qPCR) analysis of Recql4 from Kusa4b10 cells. Relative gene expression levels were normalised to the expression of Hprt. n = 4 biological replicates. (E) Light microscopic images of control (shRNA-Luc) and sh-Recql4 (#5254) Kusa4b10 cells (200X magnification). (F) shRNA-Luc and sh-Recql4 (#5254) cells were seeded in 6-well plates and cell counts were performed at 24hr intervals. n = 4 independently infected cultures. (G-I) Control (shRNA-Luc) and sh-Recql4 (#5254) cells were seeded 24 hrs prior to flow cytometry analysis, and were stained with antibodies against CD51 and Sca1 (G), EdU and 7AAD (H), and Annexin V and 7AAD (I). Representative FACS plots shown on the top, percentages of stained cells summarised in bar graphs at bottom. n = 3–4 biological replicates. (J) shRNA-Luc and sh-Recql4 (#5254) cells were subjected to osteogenic differentiation and stained with Alizarin Red. Representative photos are shown at top, while the quantification of eluted Alizarin Red dye is shown at the bottom. n = 3 biological replicates; All graphical data presented as mean±SEM. *P<0.05, **P<0.01, ***P>0.0005, ****P>0.00001 compared with control, t-test or 2-way ANOVA. To allow for a more detailed analysis of osteoblast proliferation and differentiation, we moved to a short hairpin RNA (shRNA) approach. We utilized the Kusa4b10 murine cell line which has the capacity to differentiate into osteoblastic or adipogenic lineages [38]. We identified one shRNA (#5254), amongst numerous screened, which reduced the expression of Recql4 at both the transcript and protein levels in Kusa4b10 cells (Figs 5C, 5D, S4A, and S4B). Controls were either a second shRNA against Recql4 (#5255) that did not reduce transcript levels, or a luciferase-targeting shRNA.
The knockdown of Recql4 caused a striking phenotype in the Kusa4b10 cells. In particular, the cells displayed a ‘flattened’ morphology as compared to the controls, and proliferation was completely blocked (Fig 5E and 5F). The cell surface phenotype of the knockdown cells changed, with a reduction in the expression of Sca-1, a marker of proliferation, but no change in CD51 expression (Fig 5G) [39,40]. Depletion of Recql4 led to a 2-fold increase in apoptosis, as evidenced by increased Annexin V staining (Fig 5I). The most pronounced difference, however, became apparent when we assessed the cell cycle distribution of Recql4 knockdown cells. EdU pulse-labelling demonstrated a near complete absence of DNA replication or S-phase populations in the knock-down cells compared to controls (Fig 5H). In contrast to recent reports that assessed the acute depletion of RECQL4 with shRNA or siRNA [41], we did not observe an increase in senescence associated ß-galactosidase positive cells in Recql4-knockdown cultures compared to control infected cultures when assessed at similar time points post knockdown (S4C and S4D Fig). Taken together with the primary osteoblast analysis, these Recql4 depletion leads to a profound proliferation arrest and an elevated level of apoptosis without appreciable effects on senescence.
To determine if the loss of Recql4 affected osteoblast differentiation in addition to proliferation, control and knock-down Kusa4b10 cells were placed under osteoblastic differentiation conditions. To account for the reduced proliferative potential of the Recql4 depleted cells, the cells were seeded to be fully confluent at the start of differentiation. The knockdown of Recql4 led to a near complete failure in mineralization, as assessed by alizarin red staining, over a 21 day differentiation time-course (Fig 5J). All wells were visually confirmed to have a confluent sheet of cells, yet the Recql4 depleted cells were unable to mineralise under these assay conditions. Profiling of markers of osteoblastic maturation and differentiation by qPCR revealed that whilst Runx2 expression was not affected, all markers of osteoblast differentiation downstream of this were significantly reduced in Recql4 knockdown cells, consistent with the failure to mineralize (S4E Fig). These data suggest that Recql4 is not only required for normal proliferation of osteoblast precursors but also contributes to their maturation in vitro, a result consistent with the low bone formation rate in the presence of unchanged osteoblast numbers in the Osx-Cre Recql4fl/fl mice.
The concurrent loss of p53 does not rescue Recql4 deficient osteoblast proliferation
Recql4 has been proposed to interact with p53, which has been reported to inhibit osteoblast proliferation [15,42]. To explore the relationship between the two genes, we treated primary long bone osteoblastic cells from R26-CreERT2 Recql4fl/flp53fl/fl mice with tamoxifen and subjected them to proliferation assays as described previously (Fig 6A). After 14 days, the Recql4+/+p53Δ/Δ cells displayed increased proliferation compared to its non-tamoxifen treated isogenic counterpart. In contrast, concurrent and equally efficient, deletion of both Recql4 and p53, resulted in proliferation kinetics similar to the Recql4Δ/Δ single mutants (Figs 4A and 6B–6D). This demonstrated that the loss of p53 is insufficient to rescue the proliferative defect of Recql4Δ/Δ osteoblasts in vitro.
Fig. 6. Concurrent loss of p53 does not modify the Recql4 phenotype. 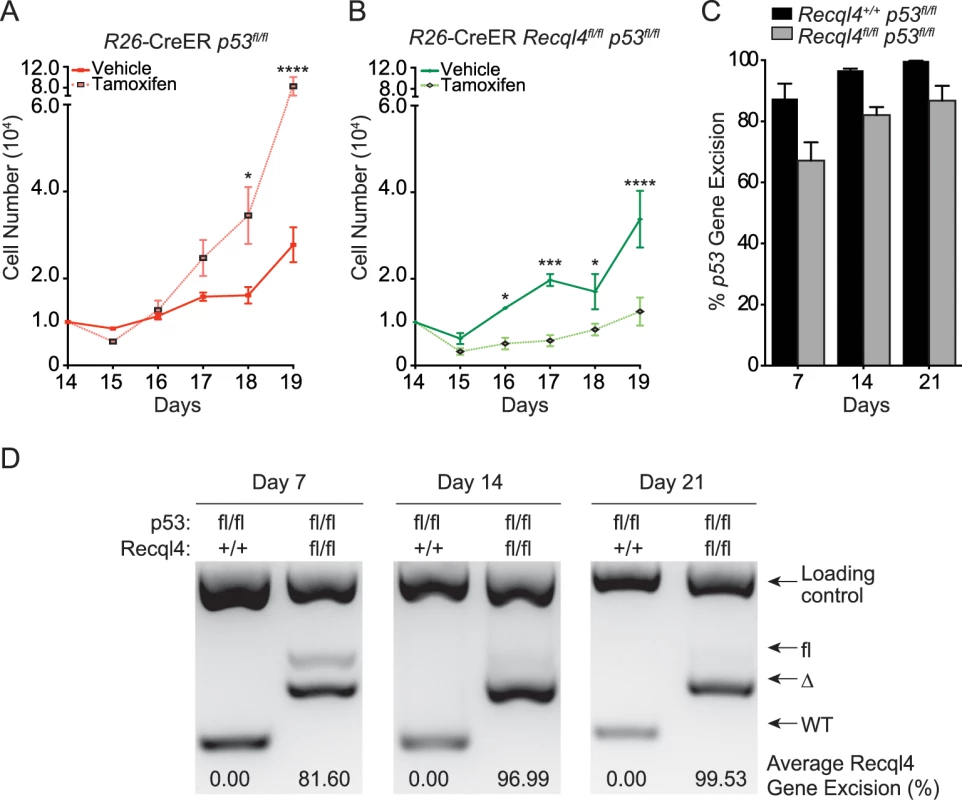
(A) and (B): Primary long bone osteoblastic cells were derived from R26-CreERT2 Recql4 p53 mice. They were then treated with tamoxifen and subjected to proliferation assays. n = 3–4 independent cultures per genotype; Data presented as mean±SEM. *P<0.05, **P<0.01, ***P>0.0005, ****P>0.00001 compared with vehicle, 2-way ANOVA. (C) The assessment of p53 genomic excision from tamoxifen-treated long bone osteoblastic cells from R26-CreERT2 Recql4 p53 mice. Data presented as mean±SEM. (D) The assessment of Recql4 genomic excision from tamoxifen-treated long bone osteoblastic cells from R26-CreERT2 Recql4 p53 mice. Osx-Cre Recql4fl/fl mice do not spontaneously develop osteosarcoma
Given the high incidence of OS in RTS patients, we established cohorts of Osx-Cre Recql4+/+, Osx-Cre Recql4fl/+ and Osx-Cre Recql4fl/fl mice that were allowed to age and were monitored for the development of cancer, particularly OS. These cohorts were followed over a 100 week period. No animals were found with OS, irrespective of the genotype (Fig 7A and S1 Table). Whilst we may not have a sufficiently large sample size to resolve a low incidence of OS development within these specific cohorts, no OS has ever been observed in the Osx-Cre Recql4fl/fl mice within our entire colony (>100 Osx-Cre Recql4fl/fl mice of various ages up to 2 years). This demonstrates that complete deletion of Recql4 in osteoblast precursors is not sufficient to initiate OS in the mouse at high frequency.
Fig. 7. The loss of Recql4 does not initiate osteosarcoma. 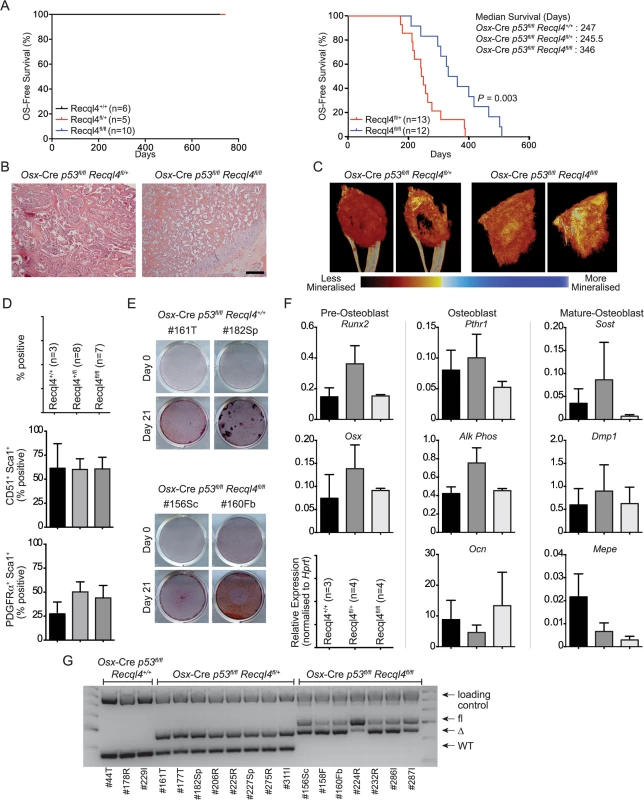
(A) Kaplan-Meier survival plots of Osx-Cre Recql4 (left) and Osx-Cre p53fl/fl Recql4 (right) mice. P value calculated by Log-Rank statistical test. (B) H&E stained sections of primary OS tumors from Osx-Cre p53fl/fl Recql4 animals of indicated genotype. (C) Representative reconstructed μCT images of primary OS tumors from Osx-Cre p53fl/fl Recql4fl/+ and Recql4fl/fl mice. (D) Flow cytometry percentages of tumor cells stained with CD51, Sca1 and PDGFRα. n = 3 for Recql4+/+, n = 8 for Recql4fl/+, n = 7 for Recql4fl/fl; Data presented as mean±SEM. (E) Representative photos of Alizarin Red-stained tumor cells that were subjected to osteogenic differentiation conditions. (F) qPCR profiling of Osx-Cre p53fl/fl Recql4+/+, Recql4fl/+ and Recql4fl/fl tumors for the indicated genes. n = 3–4; Data presented as mean±SEM. (G) Assessment of genomic excision of Recql4 in tumor-derived cell lines. 40ng of genomic DNA was used for PCR and subjected to gel electrophoresis. Increased OS-free survival in Osx-Cre p53fl/flRecql4fl/fl double knock-outs
The only mutation to occur in all human OS is the loss or mutation of TP53 [12]. Experimentally, loss of p53 from within the osteoblast lineage is sufficient to initiate OS with near complete penetrance [14,17]. To determine whether deletion of Recql4 in an Osx-Cre p53fl/fl background would accelerate OS initiation and shorten OS-free survival, we established cohorts of Osx-Cre p53fl/flRecql4+/+, Osx-Cre p53fl/flRecql4fl/+ and Osx-Cre p53fl/flRecql4fl/fl mice. OS was observed the in Osx-Cre p53fl/flRecql4+/+ mice as expected with a median onset of 247 days (Fig 7A and S1 Table; larger comparison cohort from our colonies previously reported: median survival time 226 days [43]). The loss of one allele of Recql4 did not significantly affect OS-free survival time compared to the Osx-Cre p53fl/flRecql4+/+ mice, consistent with the lack of phenotype in Recql4 heterozygous animals and humans (Fig 7A and S1 Table). However, and unexpectedly, Osx-Cre p53fl/flRecql4fl/fl mice had a significantly increased OS-free survival, with a median survival time of 346 days compared to Osx-Cre p53fl/flRecql4fl/+ controls (Fig 7A and S1 Table).
The Osx-Cre p53fl/flRecql4fl/fl mice more frequently presented with multiple primary tumors as compared to the Recql4 wildtype or heterozygous cohorts. The majority of the primary OS were distributed in axial locations (eg., the rib-cage and the vertebrae). We have not observed any non-OS tumors, such as hibernoma, in these cohorts independent of the genotype. Interestingly, while there was no difference in macroscopic metastatic incidence between Recql4+/+ and Recql4fl/fl tumour-bearing mice (including comparison to larger historic cohorts within the same facility), the Recql4fl/+ mice presented with an increased metastatic incidence at autopsy compared to either the Osx-Cre p53fl/flRecql4+/+ or Osx-Cre p53fl/flRecql4fl/fl mice. Irrespective of Recql4 status, metastatic tumors were most commonly located in the lung.
To understand if loss of Recql4 altered the biology of the OS that arose, we analysed a range of tumor characteristics. There were no grossly apparent differences between the three cohorts in the histology of the OS as assessed by either histology or μCT scans of primary tumors (Figs 7B, 7C and S5). Cell surface markers PDGFRa, CD51 and Sca-1 were screened on primary cell cultures derived from tumors of the individual genotypes and there was no difference based on Recql4 status (Fig 7D). The OS cell cultures behaved similarly when placed under differentiation inducing culture conditions (Fig 7E). There were no differences in the expression of a panel of osteoblast differentiation stage markers as assessed by qPCR (Fig 7F). Therefore, the only appreciable differences between OS arising in the Osx-Cre p53fl/flRecql4fl/+ and Osx-Cre p53fl/flRecql4fl/fl mice was the survival time and metastatic frequency. The explanation for the increased OS-free survival in the double knock-outs became apparent when we assessed the status of the Recql4 allele in the OS cells. In all cases, complete genomic recombination of the single floxed Recql4 allele was seen in the OS from Osx-Cre p53fl/flRecql4fl/+ mice. In striking contrast, in no case did we observe complete deletion of both floxed Recql4 alleles in the Osx-Cre p53fl/flRecql4fl/fl mice (Fig 7G). This demonstrates that mutations resulting in null alleles of Recql4 are not initiating events in OS and that loss of Recql4 does not co-operate with p53 mutation to initiate OS. The increased OS-free survival Osx-Cre p53fl/flRecql4fl/fl mice is most likely a result of selective pressure to retain an intact allele of Recql4 in the tumor that arises.
Discussion
In humans, mutations of RECQL4 are associated with a series of related syndromes RTS, RAPADILINO Syndrome and Baller-Gerold Syndrome. In common amongst these syndromes, and other human disorders associated with mutations in RecQ helicases, are defects in skeletal homeostasis leading to skeletal abnormalities including low bone mass, and in the case of RTS, OS [1]. Here we demonstrate that Recql4 is required for the expansion and normal differentiation of osteoblast precursor populations. In vivo we demonstrated that the deficiency of Recql4 in Osx-Cre expressing osteoblastic cells resulted in shorter bones and reduced bone mass (trabecular and cortical) that resulted from a reduced bone formation rate. The absence of changes in osteoclastogenesis as assessed by histomorphometry, and hematopoiesis more generally, indicate that the phenotype was osteoblast cell intrinsic. When deletion of Recql4 was restricted to more mature populations using Dmp1-Cre, the mice did not demonstrate a skeletal phenotype. Therefore, Recql4 is required for the normal proliferation and expansion of the pre-osteoblast populations, but it is dispensable for mature osteoblasts/osteocytes, including the functions of osteocytes that regulate mineralization. At the resolution of histomorphometry it is only possible to describe the nature of the cells (osteoblast lineage etc) not whether they are still proliferative or viable, as we have described in other mutants [36]. As Osx-Cre expressing cells turn over regularly [44] it is as expected that a static view of osteoblasts per bone surface may not yield a difference but the overall bone formation rate and amount of bone in the entire limb are reduced. This is consistent with a quantitative rather than a qualitative defect. In primary osteoblast cultures with induced deletion of Recql4 we saw normal proliferation for the first 14 days of culture and then suppressed proliferation in the Recql4Δ/Δ. This is very similar to the phenotype we previously reported in primary B - and T-cell cultures [27]. In keeping with a dilutional model of Recql4 function in DNA replication, as demonstrated in Xenopus [45], cells null for the Recql4 gene proliferate normally for 2 weeks in vitro and for multiple weeks in vivo continue to contribute to bone formation. However, once they reach critically low levels of Recql4 protein they most likely cease proliferation and, ultimately, undergo apoptosis. Concurrent deletion of p53 was not able to rescue the proliferative defect observed in Recql4-deficient cells. This is consistent with the failure of p53 deletion to rescue the lethal bone marrow failure that occurs upon widespread somatic deletion of Recql4 in adult mice [27]. Collectively, these studies lead us to conclude that the primary physiological function of Recql4 is in cell proliferation and DNA replication [46,47].
RECQL4 has well characterized roles in several critical cellular functions including DNA replication and genome stability [1,2,47,48]. Additional roles have been proposed based on studies in cell culture models including regulation of senescence, interactions with the mitochondria and p53 [42,49]. Our studies of osteoblast development and prior work on hematopoiesis are relevant to our understanding of the normal in vivo function of Recql4. The most prominent feature of Recql4 deficiency, as opposed to hypomorphic mutation, is a failure in cell proliferation. In the hematopoietic system, a rapid fully penetrant bone marrow failure results from the deletion of Recql4 [27]. The concurrent deletion of p53 did not alter the hematopoietic failure, consistent with a primary DNA replication/S-phase cell cycle defect. We show that in vivo deletion of Recql4 from proliferative pre-osteoblast populations, but not mature osteoblasts/osteocytes, caused a low bone mass phenotype. In primary osteoblast cultures, loss of Recql4 led to a failure to proliferate once gene deletion had efficiently occurred. As with hematopoiesis, p53 deletion did not rescue the proliferative failure associated with Recql4 deficiency in osteoblast cultures. We conclude that there is little evidence to support a direct genetic interaction between Recql4 and p53 pathways. This is supported by the recent data from Lu and colleagues who reported mild or no genetic rescue of a Recql4 deficient phenotype in the limb buds or growth plates by concurrent deletion of p53 [50]. The activation of p53 in Recql4 deficient cells most likely represents a secondary effect of impaired cell cycle progression and transcription, ultimately leading to apoptosis (S5C Fig). We did not find any increase in senescent cells as was recently reported in human fibroblasts infected with shRNA against RECQL4. It is possible that the mechanism that inhibits cell proliferation is different between the two cell types [41], but this seems less likely given the similarities observed in primary cell cultures of hematopoietic cells and osteoblasts.
The low trabecular and cortical bone volume of Osx-Cre Recql4fl/fl mice mirrors the low bone mass seen clinically in RTS patients [3–6]. However, unlike RTS and related patients, we did not see evidence of additional skeletal abnormalities such as polydactyly, radial ray defects or cleft palate as has been reported in Recql4 hypomorphic mice [26]. The low bone mass phenotype is conserved and the severity of the skeletal abnormalities is likely impacted by two important differences between patient and mouse model data. Firstly, in patients, the mutant RECQL4 proteins are present in all cells throughout the body and during all stages of skeletal development. Secondly, in the murine model, Osx-Cre does not delete Recql4 from all bone forming cells. This results in a chimeric setting where Recql4 wild-type cells are present and able to partially compensate for the deficiencies in bone formation that the Recql4 null cells have. There are less Recql4-deficient cells in the bone as evidenced by the lower proportions of eYFP positive osteoblastic cells (Fig 2E). A completely Recql4 deficient skeletal compartment would be expected to have a more profound phenotype. Consistent with this interpretation, it was recently reported that a more severe skeletal disturbance was achieved using Prx1-Cre to delete Recql4 exons 5–8 [50,51]. This study was restricted to early developmental time points due to the impaired survival of the Prx1-Cre Recql4fl/fl animals and so no analysis of adult animals was reported. Prx1-Cre deletes in the early developing limb bud mesenchymal cells and leads to near complete deletion in the appendicular skeleton. The Prx1-Cre Recql4fl/fl mice had growth retardation and more severe limb developmental defects than those we observed with Osx-Cre. It should be noted that the Osx-Cre model we have used was not restricted in activity to the postnatal skeleton and is expressed throughout skeletal development. We therefore can not exclude a contribution from developmental effects or deletion in other mesenchymal populations, such as chondrocytes, to the adult bone phenotype that we describe. Therefore, our data and that of Lu and colleagues [50] indicate that the primary function of RECQL4 in osteoblast precursors is to enable the appropriate expansion of cell populations required for normal skeletal formation. The low bone mass phenotype is consistent, albeit varying in severity, amongst different mesenchymal targeted Cre strains and Recql4 alleles. This analysis provides an explaination for the low bone mass that is a hallmark of RECQL4 mutations in humans and our modeling demonstrates that this intrinsic function of RECQL4 within the skeletal system.
RTS is notable as the third familial syndrome impacted by OS, in addition to Li-Fraumeni and hereditary retinoblastoma kindreds. However, unlike TP53 and RB1, mutations in RECQL4 are not a common feature of sporadic OS and appear restricted to RTS-related OS. A role of Recql4 mutations in OS has not been revealed by previous mouse models because the viable mutants have hypomorphic Recql4 alleles, leading to transcription of the the N-terminal regions [25,26]. One question that arises from these observations is whether OS in RTS patients represents the same disease as Li-Fraumeni and hereditary retinoblastoma kindred associated OS, and more importantly, sporadic OS in human. Our analyses demonstrate that the osteoblastic-restricted complete loss of function Recql4 alleles are not able to initiate OS in the mouse. Indeed, we have not seen tumor formation in Recql4 deficient animals or aged Recql4+/- animals of any type. The absence of OS formation in skeletally restricted Recql4 null mutants was also recently reported when Recql4 was deleted with Prx1-Cre [51]. Prx1-Cre deletes in the early limb bud mesenchymal cells and is active earlier than the Osx-Cre that we utilized. The results from the independent studies of an absence of OS initiation in Recql4 deficient skeletal models suggests that the failure to initiate OS does not reflect the choice of models, but rather, reflects the biology of Recql4 null alleles.
As RTS patients have a 30% incidence of OS this was somewhat unexpected, but not without precedence given our previous analysis of Osx-Cre pRbfl/fl animals which also do not develop OS despite the high rate of OS in hereditary retinoblastoma kindreds and the rate of RB1 mutation in conventional OS [12,17]. Unexpectedly, homozygous deletion of Recql4 delayed OS development in a fully penetrant Osx-Cre p53fl/fl model, directly contrasting with the acceleration of OS development when loss of Rb was combined with p53 deletion. This observation was explained by the incomplete deletion of Recql4 in the OS that did arise in the Osx-Cre Recql4fl/fl p53fl/fl mice. The increased OS-free survival is most likely because the Osx-Cre p53fl/fl Recql4fl/fl are unable to proliferate, a conclusion supported by our in vitro data, and the cell that ultimately gives rise to the tumor is being selected for incomplete deletion of the Recql4 locus. This is an inefficient process. This result demonstrates that OS most likely does not arise from the Recql4 null cells, most plausibly due to the profoundly impaired proliferation of these cells. It further suggests that the OS initiating cells require some level, albeit low based on the analysis of RTS patients, of RECQL4 function to enable OS initiation and maintenance [7]. The OS predisposition in RTS patients is most likely accounted for by the mutation spectrum in these patients resulting in the generation of C-terminal truncated RECQL4 proteins. These alleles are predicted to retain the essential role of RECQL4 in DNA replication, but disable RecQ helicase dependent functions. Thus, our working hypothesis is that aneuploidy and tumor predisposition can only arise in cells retaining the Sld2-homology regions of RECQL4 where basal cell proliferation is intact but genomic stability is compromised [46,47,52]. Therefore, together with the present data, we conclude that the RECQL4 is not an OS tumor suppressor when the mutation results in a null allele.
Our results demonstrate an essential, non-redundant role for Recql4 in the expansion and proliferation of osteoblast precursors that is not required once the cells become mature osteoblasts. Complete null alleles of Recql4 do not initiate OS and loss of Recql4 does not potentiate OS initiated by p53 deletion. That OS does not arise in Recql4 null cells reflects an essential function for Recql4 in the proliferation and expansion of the cells. These studies demonstrate that the low bone mass is a result of the loss of RECQL4 function, but tumorigenesis most likely requires mutant RECQL4 activity. The precise function of mutant RECQL4 in OS initiation remains to be defined. Collectively our study reveals that Recql4 is essential for normal skeletal homeostasis and that the greatly elevated rates of OS in RTS patients are not recapitulated by the complete absence of Recql4.
Materials and Methods
Ethics statement
All animal experiments were approved by the Animal Ethics Committee of St Vincent’s Hospital, Melbourne. AEC #044/10.
Mice
Recql4fl/fl mice (C57BL/6-Recql4tm2272Arte) were generated by TaconicArtemis GmbH (Cologne, Germany); full details of the allele have been previously described [27]. Osx-Cre, DMP1-Cre, Rosa26-eYFP and p53fl/fl animals have been previously described and were on a C57Bl/6 background [17,28,29,53]. 5-week old male mice were weighed weekly over a 4-week period. All histomorphometry and microCT was done on 9 week old male mice.
Histology and histomorphometry
Tumour tissue was obtained from Osx-Cre Recql4 p53fl/fl mice, and it was fixed in 2–4% paraformaldehyde overnight at 4°C. After embedding in paraffin, the tissue was sectioned and stained with hematoxlyin and eosin. Pathological assessment of the tumor sections was performed blinded to the sample genotype. For dynamic histomorphometry, in vivo labeling of bone formation was performed with intra-peritoneal injection of 8-week old male mice with alizarin (Sigma Aldrich) and calcein (Sigma Aldrich) 7 days and 2 days respectively before tissue collection [54]. Tibia were fixed in 4% paraformaldehyde and embedded in methylmethacrylate, 5μm sections were stained with von Kossa to identify mineralized tissue and toluidine blue for static histomorphometry [53,55]. Histomorphometric analysis was carried out in the secondary spongiosa of the proximal tibia metaphysis (Osteomeasure, Osteometrics, Atlanta, GA, USA) as previously described [56].
MicroCT analysis of bone parameters
Ex vivo microCT was performed on tibiae and tumor material using the SkyScan 1076 system (Bruker-microCT, Kontich, Belgium). Images were acquired using the following settings: 9μm pixel size, 0.5mm aluminum filter, 50kV voltage and 100μA current, 2400 ms exposure time, rotation 0.5°, frame averaging = 1. Images were reconstructed and analysed with SkyScan software programs NRecon (version 1.6.3.3), DataViewer (version 1.4.4), CT Analyser (CTan, version 1.12.0.0) and CTVox (version 2.2.0). The trabecular analysis region of interest (ROI) was determined by identifying the start of the mineralized zone of the proximal growth plate and calculating 16.5% of the total tibial length towards the tibial mid-shaft, where we then analysed an ROI of 13.5% of the total tibial length. Analysis of trabecular bone structure was completed using adaptive thresholding (mean of min and max values) in CTan with threshold set at 45–255 for trabecular bone. Cortical analyses were performed 35% of the total tibial length distal from the mineralized zone of the proximal growth plate, and extending for 12% of the total tibial length; the threshold values for cortical bone were set to 79–255 and global thresholding algorithm was used. The 3-dimensional visualization of trabecular and cortical bone was performed with CTVox, where volume-rendered images were pseudo-colored based on grey-scale (pixel) intensity that is reflective of bone mineralization.
Cell culture
The Kusa4b10, long-bone primary osteoblastic cells and mouse OS cell lines (no authentication performed) were cultured in αMEM (Lonza), 10% non heat inactivated FBS (SAFC Biosciences) and 1% Penicillin/Streptamycin/Glutamine (Life Technologies). OS cell cultures were derived by mincing tumor tissue with a scalpel. The resulting tumor homogenate was transferred to a 6 well plate and allowed to establish in standard culture conditions. Primary cultures were passaged at 60–80% confluence with media changes every 2–3 days. The primary osteoblastic cells were derived from long bone tissue, cleaned and crushed lightly with a mortar-pestle. The suspension was rinsed and diluted with PBS, and this process was repeated until the majority of hematopoietic cells had been removed (solution was clear). The bone fragments were placed in 15ml of collagenase I (3mg/ml) (Worthington), and incubated in a shaking 37°C water bath for 45 minutes. 35ml of PBS + 2% FBS was then added and the cell suspension was sieved. The resulting population of long-bone derived cells was centrifuged at 400g for 5 minutes, the cells were resuspended in culture media and plated onto a 6-well plate. On the next day, the 6-well plate was washed with PBS before adding fresh culture media to remove floating debris. By 48 hours post-derivation, the cells were ready for experiments. For experiments involving Rosa26-CreERT2 Recql4 cells, cultures were treated with 500nM Tamoxifen (4-OHT, Merck) to induce Cre activity.
Cell preparations and flow cytometry
The primary long-bone osteoblastic cells from Osx-Cre Recql4 mice were obtained as described previously [43,53]. Briefly, bone fragments were crushed and colllagenase-digested in a similar process to the derivation of these cells for in vitro experimentation. After pelleting the bone derived cells, they were stained with antibodies (Hematopoietic lineage markers, CD31, CD51, Sca1, CD45), and separated on the FACSAria cell sorter (BD Biosciences). Osteoblastic cells (Lineage-, CD31-, CD51+, Sca1+/-) were obtained and fractionated into YFP+ and YFP- populations.
Assessment of genomic excision
Tissues from various organs and FACS-sorted osteoblastic cells were subjected to genomic DNA extraction as directed by the manufacturer (Isolate II genomic DNA kit, Bioline). PCR was performed using 40ng of genomic DNA with the sequence-verified primers and conditions (S2 Table). Following that, the PCR products were subjected to electrophoresis on 2.5% agarose gels and visualized on the VersaDoc imaging system (Bio-Rad). The DNA bands were quantitated with the ImageJ software (NIH).
shRNA
The Recql4 shRNA were in the pLKO.1 vector and were purchased from Sigma-Aldrich (S4 Table). A Luciferase shRNA plasmid was used as a negative control (S4 Table). Ecotropic lentivirus was generated by transient transfection of 293T cells using calcium phosphate and the psPax2 (Addgene plasmid #12260) and pCMV-Eco Envelope plasmid (Cell Biolabs) using standard methods. Kusa4b10 cells were infected by spinoculation in 6-well plates. The cells were passaged onto fresh plates after 48 hours with media containing puromycin (Sigma). The cells were used after 2 days of antibiotic selection when non infected cells had died.
Apoptosis, cell cycle and senescence analysis
shRNA infected and selected Kusa4b10 cells were harvested and seeded at 100,000 cells per well in a 6 well plate. On the next day the cells were stained with AnnexinV-APC antibody (eBioscience) in 1X binding buffer (0.01M HEPES, pH7.4; 0.14M NaCl; 2.5mM CaCl2, eBioscience) for 15 mins at room temperature in the dark. The cells were then washed with 1X binding buffer and 5μg/ml of 7AAD was added to each sample prior to flow cytometry analysis. Both early apoptotic (AnnexinV+/7AAD-) and late apoptotic (AnnexinV+/7AAD+) cell populations were added together to determine the total number of apoptotic cells.
For cell cycle analysis the cells were treated with 10μM EdU (Life Technologies) for 1 hour at 37°C. Cells were then harvested, washed in PBS with 1% BSA, and fixed with 2% paraformaldehyde for 15 min at room temperature in the dark. The cells were washed with PBS with 1% BSA and stored as a pellet at 4°C. Cells were processed as described by the manufacturer (EdU labelling kit, Life Technologies) The cells were subjected to flow cytometry to assess the cellular incorporation of EdU.
For cellular senescence 4–5 days post infection/selection, SA-ß-gal staining was performed with Senescence ß-Galactosidase Staining Kit according to the manufacturer’s instructions (Cell Signalling). Briefly, the cells were washed with PBS, then fixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS for 15 min at room temperature. After rinsing the cells with PBS, the fixed cells were incubated with X-gal staining solution and visualised with an inverted microscope.
In vitro differentiation
Osteoblastic cells were seeded and cultured for 3–4 days. After the cells reached confluence, the media was replaced with differentiation media (αMEM, 15% FBS, 50μg/ml L-ascorbic acid, 10mM beta-glycerophosphate) and thereafter changed 2 times per week. Cells were collected on indicated days for analysis.
For mineralization the cells were washed 3 times (1x PBS), fixed with 70% ethanol for 30 mins and stored at 4°C in fixative. After washing the cells 3 times in water, they were stained with 0.5% Alizarin Red S (Sigma Aldrich) in water for 30 mins, then 3 washes in water and a 15 min wash in 1x PBS. 1 ml of 10% Hexadecylpyridinium chloride monohydrate (CTP, Sigma Aldrich) in DPBS was added and the plates incubated overnight at room temperature with shaking to elute and quantitate the Alizerin Red S. Absorbance was measured on a PolarStar Plate Reader at OD562nm.
Gene expression analysis
RNA was extracted from OS tissue as previously described [43]. RNA was also extracted from cell lines with the Isolate II RNA kit (Bioline). cDNA was synthesised with the Tetro kit (Bioline) with oligo-(dT) primers. Both SYBR-green and multiplex based probe qPCR were performed on the Stratagene Mx3000P machine (Agilent Technologies) with gene-specific primers (S3 Table). The relative gene expression was normalized to the HPRT housekeeping gene and calculated by the 2-ΔCT method.
Immunoblot
RIPA buffer extracted protein lysates from shRNA-transduced and selected Kusa4b10 cells were quantified with the Quick-start Bradford assay (Bio-Rad), and separated on a 4–12% Bis-Tris gel in MOPS buffer as directed by the manufacturer (Life Technologies). The gel was then transferred onto a PVDF membrane (Merck-Millipore). The membrane was blocked with 5% milk in TBST and incubated overnight at 4°C with the polyclonal rabbit anti-mouse Recql4 antibody (GL Biochem, 1 : 500) and the monoclonal anti-mouse α-tubulin antibody (Sigma, 1 : 5000) as previously described [27]. Subsequently, the blot was probed with horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies respectively (Thermo Scientific, 1 : 2000). The enhanced chemiluminescence kit (GE Amersham) was applied to the blot, and bands were visualized by exposing the WB to X-ray film.
Cell preparations and flow cytometry analysis
Peripheral blood was counted on a hematologic analyser (Sysmex KX-21N, Roche Diagnostics). Spleens were weighed and crushed in FACS Buffer (PBS + 2% FBS) to make single-cell suspensions. The bone marrow was recovered by flushing the femurs with FACS Buffer. The bone marrow cell suspensions were then stained with anti-mouse antibodies. Antibodies against murine CD2, CD3e, CD4, CD5, CD8a, CD11b, CD19, CD31, CD34, CD41, CD43, CD44, CD45.1, CD45.2, CD51, B220, CD71, PDGFRα (CD140a), F4/80, Gr1, IgM, Mac1, Sca1, and Ter119 were either biotinylated or conjugated with FITC, phycoerythrin, phycoerythrin-Cy5, peridinin chlorophyll protein-Cy5.5, phycoerythrin-Cy7, allophycocyanin, or allophycocyanin Alexa 750 and were all obtained from eBioscience, Biolegend, BD Biosciences or Life Technologies. Biotinylated antibodies were detected with streptavidin conjugated with Alexa-Fluor 488 (Life Technologies) or Brilliant Violet 605 (eBiosciences) [27,53]. FACS data was collected on an LSRIIFortessa (BD Biosciences) and analyzed with FlowJo software Version 9.0 (Treestar).
Kaplan-Meier survival analysis and statistical analysis
Mice were monitored daily until they reached humane endpoint criteria. Once the criteria were met, the mice were euthanised. The Kaplan-Meier survival plots, the Log-Rank statistical test and the unpaird 1-tailed Student t-tests with were prepared using Prism 6.0 (Graphpad Software). Census dates are the date of euthanasia of the tumor-bearing animal. All data is presented as mean ± S.E.M. A P value <0.05 was considered significant.
Supporting Information
Zdroje
1. Chu WK, Hickson ID (2009) RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer 9 : 644–654. doi: 10.1038/nrc2682 19657341
2. Brosh RM Jr. (2013) DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer 13 : 542–558. doi: 10.1038/nrc3560 23842644
3. Larizza L, Roversi G, Volpi L (2010) Rothmund-Thomson syndrome. Orphanet J Rare Dis 5 : 2. doi: 10.1186/1750-1172-5-2 20113479
4. Siitonen HA, Kopra O, Kaariainen H, Haravuori H, Winter RM, et al. (2003) Molecular defect of RAPADILINO syndrome expands the phenotype spectrum of RECQL diseases. Hum Mol Genet 12 : 2837–2844. 12952869
5. Simon T, Kohlhase J, Wilhelm C, Kochanek M, De Carolis B, et al. (2010) Multiple malignant diseases in a patient with Rothmund-Thomson syndrome with RECQL4 mutations: Case report and literature review. Am J Med Genet A 152A: 1575–1579. doi: 10.1002/ajmg.a.33427 20503338
6. Wang LL, Levy ML, Lewis RA, Chintagumpala MM, Lev D, et al. (2001) Clinical manifestations in a cohort of 41 Rothmund-Thomson syndrome patients. Am J Med Genet 102 : 11–17. 11471165
7. Wang LL, Gannavarapu A, Kozinetz CA, Levy ML, Lewis RA, et al. (2003) Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. J Natl Cancer Inst 95 : 669–674. 12734318
8. Chou AJ, Geller DS, Gorlick R (2008) Therapy for osteosarcoma: where do we go from here? Paediatr Drugs 10 : 315–327. 18754698
9. Jaffe N, Carrasco H, Raymond K, Ayala A, Eftekhari F (2002) Can cure in patients with osteosarcoma be achieved exclusively with chemotherapy and abrogation of surgery? Cancer 95 : 2202–2210. 12412175
10. Rajani R, Gibbs CP (2012) Treatment of Bone Tumors. Surg Pathol Clin 5 : 301–318. 22328909
11. Mirabello L, Troisi RJ, Savage SA (2009) International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 125 : 229–234. doi: 10.1002/ijc.24320 19330840
12. Chen X, Bahrami A, Pappo A, Easton J, Dalton J, et al. (2014) Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep 7 : 104–112. doi: 10.1016/j.celrep.2014.03.003 24703847
13. Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, et al. (2011) Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144 : 27–40. doi: 10.1016/j.cell.2010.11.055 21215367
14. Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, et al. (2008) Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci U S A 105 : 11851–11856. doi: 10.1073/pnas.0805462105 18697945
15. Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, et al. (2006) Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol 172 : 909–921. 16533949
16. Lin PP, Pandey MK, Jin F, Raymond AK, Akiyama H, et al. (2009) Targeted mutation of p53 and Rb in mesenchymal cells of the limb bud produces sarcomas in mice. Carcinogenesis 30 : 1789–1795. doi: 10.1093/carcin/bgp180 19635748
17. Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, et al. (2008) Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 22 : 1662–1676. doi: 10.1101/gad.1656808 18559481
18. Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, et al. (2014) Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci U S A 111: E5564–5573. doi: 10.1073/pnas.1419260111 25512523
19. Maire G, Yoshimoto M, Chilton-MacNeill S, Thorner PS, Zielenska M, et al. (2009) Recurrent RECQL4 imbalance and increased gene expression levels are associated with structural chromosomal instability in sporadic osteosarcoma. Neoplasia 11 : 260–268, 263p following 268. 19242607
20. Ueda T, Healey JH, Huvos AG, Ladanyi M (1997) Amplification of the MYC Gene in Osteosarcoma Secondary to Paget's Disease of Bone. Sarcoma 1 : 131–134. 18521214
21. Monnat RJ Jr. (2010) Human RECQ helicases: roles in DNA metabolism, mutagenesis and cancer biology. Semin Cancer Biol 20 : 329–339. doi: 10.1016/j.semcancer.2010.10.002 20934517
22. Wang LL, Worley K, Gannavarapu A, Chintagumpala MM, Levy ML, et al. (2002) Intron-size constraint as a mutational mechanism in Rothmund-Thomson syndrome. Am J Hum Genet 71 : 165–167. 12016592
23. Siitonen HA, Sotkasiira J, Biervliet M, Benmansour A, Capri Y, et al. (2009) The mutation spectrum in RECQL4 diseases. Eur J Hum Genet 17 : 151–158. doi: 10.1038/ejhg.2008.154 18716613
24. Ichikawa K, Noda T, Furuichi Y (2002) [Preparation of the gene targeted knockout mice for human premature aging diseases, Werner syndrome, and Rothmund-Thomson syndrome caused by the mutation of DNA helicases]. Nippon Yakurigaku Zasshi 119 : 219–226. 11979727
25. Hoki Y, Araki R, Fujimori A, Ohhata T, Koseki H, et al. (2003) Growth retardation and skin abnormalities of the Recql4-deficient mouse. Hum Mol Genet 12 : 2293–2299. 12915449
26. Mann MB, Hodges CA, Barnes E, Vogel H, Hassold TJ, et al. (2005) Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund-Thomson syndrome. Hum Mol Genet 14 : 813–825. 15703196
27. Smeets MF, DeLuca E, Wall M, Quach JM, Chalk AM, et al. (2014) The Rothmund-Thomson syndrome helicase RECQL4 is essential for hematopoiesis. J Clin Invest 124 : 3551–3565. doi: 10.1172/JCI75334 24960165
28. Rodda SJ, McMahon AP (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133 : 3231–3244. 16854976
29. Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, et al. (2007) DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res 86 : 320–325. 17384025
30. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, et al. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1 : 4. 11299042
31. Yang J, Murthy S, Winata T, Werner S, Abe M, et al. (2006) Recql4 haploinsufficiency in mice leads to defects in osteoblast progenitors: Implications for low bone mass phenotype. Biochem Biophys Res Commun 344 : 346–352. 16600186
32. Davey RA, Clarke MV, Sastra S, Skinner JP, Chiang C, et al. (2012) Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res 21 : 885–893. doi: 10.1007/s11248-011-9581-z 22160436
33. Kim BT, Mosekilde L, Duan Y, Zhang XZ, Tornvig L, et al. (2003) The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J Bone Miner Res 18 : 150–155. 12510817
34. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, et al. (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17 : 1235–1241. doi: 10.1038/nm.2448 21909103
35. Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, et al. (2011) Lrp5 functions in bone to regulate bone mass. Nat Med 17 : 684–691. doi: 10.1038/nm.2388 21602802
36. Tonna S, Takyar FM, Vrahnas C, Crimeen-Irwin B, Ho PW, et al. (2014) EphrinB2 signaling in osteoblasts promotes bone mineralization by preventing apoptosis. FASEB J 28 : 4482–4496. doi: 10.1096/fj.14-254300 24982128
37. Zhang C, Cho K, Huang Y, Lyons JP, Zhou X, et al. (2008) Inhibition of Wnt signaling by the osteoblast-specific transcription factor Osterix. Proc Natl Acad Sci U S A 105 : 6936–6941. doi: 10.1073/pnas.0710831105 18458345
38. Allan EH, Ho PW, Umezawa A, Hata J, Makishima F, et al. (2003) Differentiation potential of a mouse bone marrow stromal cell line. J Cell Biochem 90 : 158–169. 12938165
39. Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, et al. (2005) G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 106 : 3020–3027. 16037394
40. Van Vlasselaer P, Falla N, Snoeck H, Mathieu E (1994) Characterization and purification of osteogenic cells from murine bone marrow by two-color cell sorting using anti-Sca-1 monoclonal antibody and wheat germ agglutinin. Blood 84 : 753–763. 7519072
41. Lu H, Fang EF, Sykora P, Kulikowicz T, Zhang Y, et al. (2014) Senescence induced by RECQL4 dysfunction contributes to Rothmund-Thomson syndrome features in mice. Cell Death Dis 5: e1226. doi: 10.1038/cddis.2014.168 24832598
42. De S, Kumari J, Mudgal R, Modi P, Gupta S, et al. (2012) RECQL4 is essential for the transport of p53 to mitochondria in normal human cells in the absence of exogenous stress. J Cell Sci 125 : 2509–2522. doi: 10.1242/jcs.101501 22357944
43. Mutsaers AJ, Ng AJ, Baker EK, Russell MR, Chalk AM, et al. (2013) Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone 55 : 166–178. doi: 10.1016/j.bone.2013.02.016 23486187
44. Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, et al. (2012) Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10 : 259–272. doi: 10.1016/j.stem.2012.02.003 22385654
45. Collart C, Allen GE, Bradshaw CR, Smith JC, Zegerman P (2013) Titration of four replication factors is essential for the Xenopus laevis midblastula transition. Science 341 : 893–896. doi: 10.1126/science.1241530 23907533
46. Abe T, Yoshimura A, Hosono Y, Tada S, Seki M, et al. (2011) The N-terminal region of RECQL4 lacking the helicase domain is both essential and sufficient for the viability of vertebrate cells. Role of the N-terminal region of RECQL4 in cells. Biochim Biophys Acta 1813 : 473–479. doi: 10.1016/j.bbamcr.2011.01.001 21256165
47. Kohzaki M, Chiourea M, Versini G, Adachi N, Takeda S, et al. (2012) The helicase domain and C-terminus of human RecQL4 facilitate replication elongation on DNA templates damaged by ionizing radiation. Carcinogenesis 33 : 1203–1210. doi: 10.1093/carcin/bgs149 22508716
48. Singh DK, Karmakar P, Aamann M, Schurman SH, May A, et al. (2010) The involvement of human RECQL4 in DNA double-strand break repair. Aging Cell 9 : 358–371. doi: 10.1111/j.1474-9726.2010.00562.x 20222902
49. Gupta S, De S, Srivastava V, Hussain M, Kumari J, et al. (2014) RECQL4 and p53 potentiate the activity of polymerase gamma and maintain the integrity of the human mitochondrial genome. Carcinogenesis 35 : 34–45. doi: 10.1093/carcin/bgt315 24067899
50. Lu L, Harutyunyan K, Jin W, Wu J, Yang T, et al. (In Press) RECQL4 Regulates p53 Function in vivo During Skeletogenesis. Journal of Bone and Mineral Research.
51. Lu L, Jin W, Liu H, Wang LL (2014) RECQ DNA helicases and osteosarcoma. Adv Exp Med Biol 804 : 129–145. doi: 10.1007/978-3-319-04843-7_7 24924172
52. Kitao S, Lindor NM, Shiratori M, Furuichi Y, Shimamoto A (1999) Rothmund-thomson syndrome responsible gene, RECQL4: genomic structure and products. Genomics 61 : 268–276. 10552928
53. Singbrant S, Russell MR, Jovic T, Liddicoat B, Izon DJ, et al. (2011) Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood 117 : 5631–5642. doi: 10.1182/blood-2010-11-320564 21421837
54. Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, et al. (2009) Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res 24 : 1572–1585. doi: 10.1359/jbmr.090320 19338457
55. Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH (2007) Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129 : 1081–1095. 17574022
56. Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, et al. (2000) Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest 106 : 1095–1103. 11067862
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



