-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
Stalled translation during elongation is an aberrant phenomenon during protein synthesis. Thus, once detected, it is subjected to quality control in which mRNA and a nascent protein are rapidly degraded. Although the mechanism of degradation for stalled translation is reasonably well understood, the initial processes, including those for detecting stalled translation, have not been determined. The ubiquitin proteasome pathway has been determined to function in the degradation of a nascent protein during stalled translation. Because a ubiquitin signal is one of the most versatile of cellular signals, we investigated the roles of various ubiquitination mechanisms in the budding yeast Saccharomyces cerevisiae using ubiquitin mutants that inhibited the polymerization of specific ubiquitin chains. We identified a role of non-proteasomal K63 polyubiquitination in stalled translation surveillance. Moreover, a K63R ubiquitin mutant barely inhibited the quality control pathway for nonstop translation, indicating distinct mechanisms for these highly related quality control pathways. These findings provide insights into the fundamental mechanisms for the initial processes of stalled translation surveillance and further emphasize the versatility of ubiquitin signals in cellular systems.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005197
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005197Summary
Stalled translation during elongation is an aberrant phenomenon during protein synthesis. Thus, once detected, it is subjected to quality control in which mRNA and a nascent protein are rapidly degraded. Although the mechanism of degradation for stalled translation is reasonably well understood, the initial processes, including those for detecting stalled translation, have not been determined. The ubiquitin proteasome pathway has been determined to function in the degradation of a nascent protein during stalled translation. Because a ubiquitin signal is one of the most versatile of cellular signals, we investigated the roles of various ubiquitination mechanisms in the budding yeast Saccharomyces cerevisiae using ubiquitin mutants that inhibited the polymerization of specific ubiquitin chains. We identified a role of non-proteasomal K63 polyubiquitination in stalled translation surveillance. Moreover, a K63R ubiquitin mutant barely inhibited the quality control pathway for nonstop translation, indicating distinct mechanisms for these highly related quality control pathways. These findings provide insights into the fundamental mechanisms for the initial processes of stalled translation surveillance and further emphasize the versatility of ubiquitin signals in cellular systems.
Introduction
In addition to accurate protein synthesis in the ribosome, translational quality control pathways make significant contributions for appropriate gene expression [1]. Once aberrant mRNA templates, such as those with premature stop codons or those without stop codons, are detected during protein synthesis, mRNAs and nascent proteins are rapidly degraded by nucleases and the proteasome as quality controls pathways [2]. Stalled translation during elongation is also recognized as an aberrant translation that is subject to quality control [3]. A robust secondary RNA structure [4], consecutive polybasic amino acids [5], and rare codons [6] have been reported to induce strong translational stalling that is potentially susceptible to quality control surveillance.
The quality control for stalled translation surveillance involves a complex machinery, which includes a number of complexes and factors. mRNA degradation is initiated by an endonuclease, which remains to be identified, and proceeds via the actions of exonucleases. Kem1/Xrn1, a component of a processing body for mRNA turnover, is responsible for 5’ to 3’ mRNA degradation and a multiprotein complex, a so-called exosome, functions in 3’ to 5’ mRNA degradation in association with a Ski complex and Ski7 [3]. A complex of Dom34/Hbs1, which forms a structure that mimics tRNA/EF1α [7], functions in the disassembly of a stalled ribosome [3, 8]. A scaffold protein, Asc1, was also shown to be involved in the stalled translation surveillance by a gene knockout analysis, although its precise role is unknown [9, 10]. In addition, E3 ubiquitin ligases and their associated factors such as Rqc1 have been identified [10, 11]. Although numerous factors have been identified, the initial steps in stalled translation surveillance, such as detection of aberrance and triggering of subsequent degradation, have not been revealed.
Two E3 ligases, Hel2 and Ltn1, function in the quality control for stalled translation in apparently distinct manners [12]. Hel2 was reported to function in histone ubiquitination [13] and deleting the Hel2 gene resulted in enhanced expression of a full-length protein from mRNA with a stall signal in the midst of its reading frame [10]. Ltn1 was reported to be involved in polyubiquitination for proteasomal degradation [11]. A global analysis of cotranslational ubiquitination suggested that Hel2 and Ltn1 function in distinct manners [14]. However, the distinct roles of these E3 ligases remain unclear.
Ubiquitination is one of the most versatile cellular signals because polyubiquitin can be synthesized by linkage at a specific lysine or N-terminal methionine residues in various cellular processes [15]. In addition to polyubiquitination, monoubiquitination has been reported to function as a cellular signal [16, 17], which further establishes the versatility of ubiquitin signals. Some regulatory pathways are governed by multiple ubiquitin signals. For example, NF-κB activation is regulated by at least K11, K48, K63, and linear polyubiquitin chains [18].
In this report, we demonstrate a unique role of non-proteasomal K63 polyubiquitin in the quality control for stalled translation in the budding yeast Saccharomyces cerevisiae using a number of ubiquitin mutants that interfere with specific polyubiquitination at each lysine residue. Inhibiting K63 polyubiquitination resulted in the expression of a full-length product from mRNA with a stall signal and the K63 polyubiquitination is dependent on Hel2, which provided insights into the initial processes involved in stalled translation surveillance. This same ubiquitin mutant barely affected the quality control for nonstop translation, suggesting that initial steps are distinct between these two related quality control pathways.
Results
Distinct roles of Hel2 and Ltn1 E3 ubiquitin ligases in the quality control for stalled translation
A CGA (arginine) codon was reported to be an exceptional rare codon that caused a profound translational stall in the budding yeast S. cerevisiae [19]. By utilizing this property, we conducted genetic screenings to identify those factors that could function during the early stages of quality control for stalled translation at consecutive CGA (arginine) codons. For this purpose, we constructed a reporter system using 12 CGA codons inserted ahead of a reporter gene (hereafter, CGA reporter) and a reference reporter without this CGA repeat (blank reporter) (Fig 1A). Any functional defects in the quality control for stalled translation due to the genetic alterations of the responsible genes could be detected by facilitated growth due to the enhanced expression of the reporter gene.
Fig. 1. Comparisons of the effects of deleting Hel2 and Ltn1. 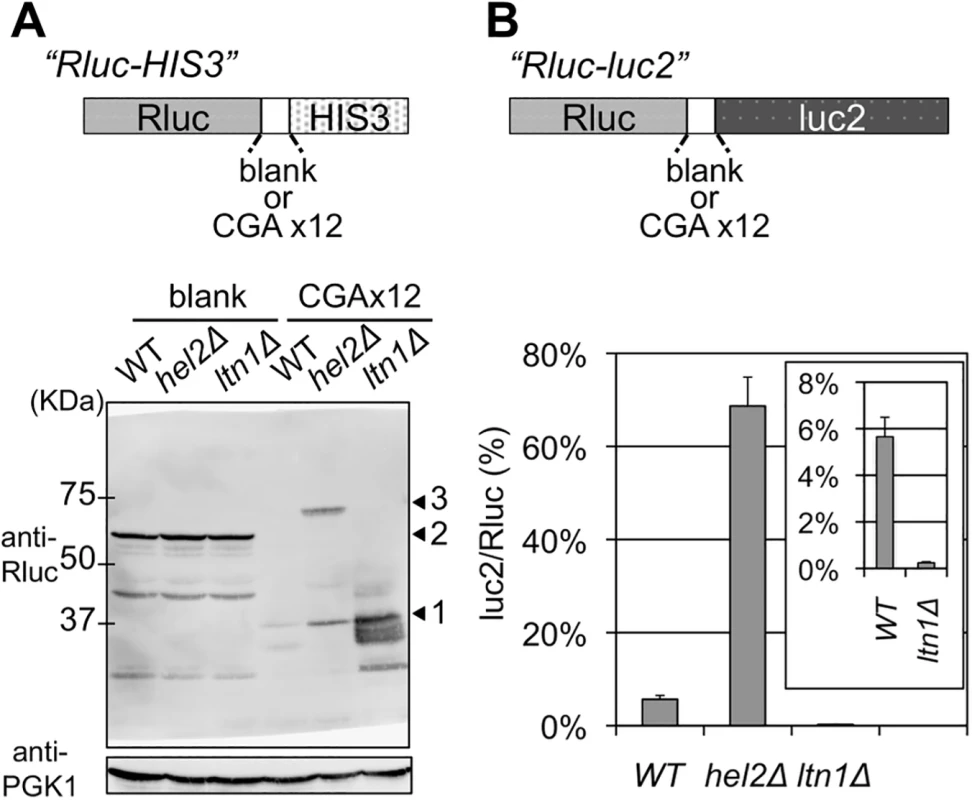
(A) Western blot analysis for Rluc-HIS3 reporters from the wild-type (WT), hel2∆, and ltn1∆ strains (BY4727, SKY61, S18-E01). A schematic image of reporter fusion genes, Rluc-HIS3, either with or without 12 CGA codon repeats, is illustrated above. The expression of fusion protein was detected using a Rluc antibody. PGK1 was used as a loading control. Arrowhead 1 indicates the Rluc protein alone, arrowhead 2 indicates the Rluc-blank-HIS3 protein, and arrowhead 3 indicates the Rluc-CGA x12-HIS3 protein. (B) Dual luciferase assay for Rluc-CGA x12-luc2 reporter in the wild-type (WT), hel2∆, and ltn1∆ strains (HRKW-2, SKY113, HRKW-6). A schematic image of reporter fusion genes, Rluc-luc2, either with or without 12 CGA codon repeats, is illustrated above. Bars indicate luc2/Rluc ratios. Percentages were standardized using the results of a dual luciferase assay for Rluc-blank-luc2 in the wild-type strain (HRKW-1). The inset indicates the results with the ltn1∆ strain with an appropriately adjusted range. Average luc2/Rluc ratios and standard deviations were determined from three independent measurements. In our genetic screening with this consecutive CGA reporter system, we identified an ubiquitin E3 ligase, Hel2, as a factor for the quality control of our CGA stalled translation system (S1 Fig). In a previous report, Hel2 was also reported to be involved in the quality control for stalled translation caused by a consecutive lysine [10]. Thus, this result suggested a common role of Hel2 in two different types of the quality control for stalled translation.
However, the genetic analysis with our CGA reporter system did not detect any effects by Ltn1 (S1 Fig), which is also an ubiquitin E3 ligase that has been reported to function in stalled translation [10]. These results clearly suggested distinct roles of the two E3 ubiquitin ligases, Hel2 and Ltn1, in the quality control system for stalled translation, as previously reported [10, 12].
Western blot analysis for the reporter construct in the ltn1∆ strain detected a highly elevated expression of stalled Rluc protein, which is situated upstream of the CGA stall signal (Fig 1A), due to the inhibition of rapid proteolysis of stalled products, as previously reported [10, 11]. In contrast, comparable amounts of full-length proteins and the stalled Rluc protein were detected in the hel2∆ strain (Fig 1A) consistently as previously reported [12].
Next, for quantitative analysis, an alternative assay construct was introduced, in which the reporter HIS3 was replaced with firefly luciferase (luc2), as previously reported [12, 20]. The expressions of both the full-length and stalled proteins could be monitored by a dual luciferase method (Fig 1B). These results were recorded as percentages of luc2/Rluc ratios. The luc2/Rluc ratios for the conditions of interest were standardized by the luc2/Rluc ratio derived from the blank reporter in a wild-type strain, which was set at 100% (S2A Fig). The results for the blank reporter were equivalent to 100% for the wild-type, ltn1∆ and hel2∆ strains (S2A Fig). For the wild-type strain, the average luc2/Rluc ratio due to the CGA reporter was 5.66% (Fig 1B and S1 Table). The average with the ltn1∆ strain was 0.24%, which was much lower than that with the wild-type strain and consistent with the specific enrichment of the stalled Rluc product detected by western blotting (Fig 1A). In comparison, the average with the hel2∆ strain was increased to 68.66% (Fig 1B). This indicated a marked increase in the full-length product Rluc-luc2 compared with the stalled product Rluc. This again provided evidence for distinguishable mechanisms in which these two E3 ligases participated, consistently as previously reported [10, 12]. These results prompted us to investigate the temporal and mechanistic roles of ubiquitination in the quality control for stalled translation.
Non-proteasomal K63 polyubiquitination involvement in the quality control for stalled translation
We constructed a series of ubiquitin mutants to investigate the involvement of specific type of polyubiquitination linkages in the Hel2 related quality control of stalled translation, particularly in the reduction of a full-length product. Each of these ubiquitin mutants had an arginine substitution at one of the lysine residues for a specific polyubiquitination linkage (Fig 2A). Thus, incorporation of these mutants in a polyubiquitin chain inhibits further elongation and results in substantial reduction of polyubiquitination effect (Fig 2B) [21–23]. These ubiquitin mutants were overexpressed in yeast strains with stalled translation reporters. The cellular expression levels of ubiquitin were shown to be relatively comparable to each other (Fig 2C).
Fig. 2. Effect of K63 polyubiquitination on the quality control for stalled translation. 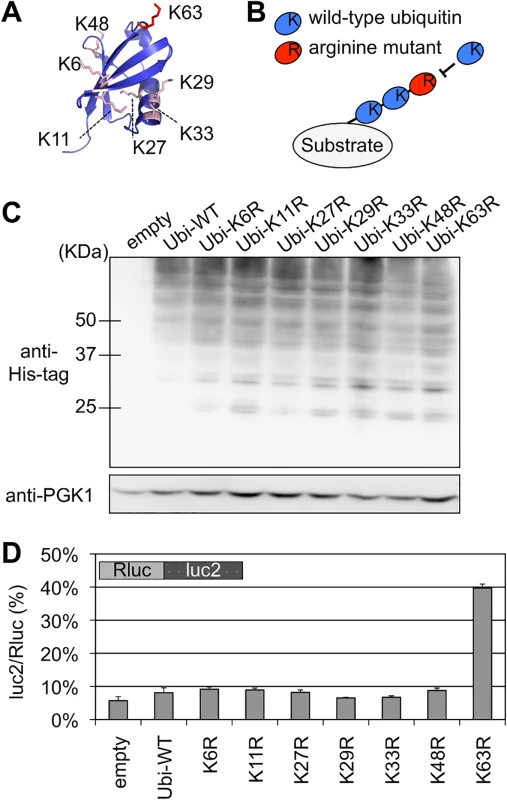
(A) Structure of Ubiquitin protein and location of the lysine residues for polyubiquitination [id: 1UBQ]. K63 (red) and other lysine residues (pink) are shown in sticks. The structural model was generated using MolFeat version 3.5 (Fiatlux, Tokyo, Japan). (B) Schematic illustration for the inhibition of polyubiquitination by arginine mutants. Arginine mutants were expressed from expression plasmids in yeast cells harboring endogenous ubiquitin genes on its genome. An incorporation of an arginine mutant into the (poly-) ubiquitin chain terminates further elongation. (C) Cellular ubiquitination levels of wild-type and ubiquitin mutants with a single arginine substitution (K6R, K11R, K27R, K29R, K33R, K48R, K63R). Ubiquitin genes were fused with His-tag and expressed from plasmids in the wild-type strain (BY4727). Cellular ubiquitination levels were detected by antibody for His-tag. PGK antibody was used as loading control. (D) Dual luciferase assay for the effects of arginine mutants of ubiquitin protein on the Rluc-CGA x12-luc2 reporter. Plasmids that included ubiquitin arginine mutants were introduced into a wild-type strain (HRKW-2). Ubi-WT indicates wild-type ubiquitin. Average luc2/Rluc ratios and standard deviations were determined from three independent measurements. Although these ubiquitin mutants barely affected the luc2/Rluc ratios of blank reporter (S2B Fig), expression of the K63R ubiquitin mutant markedly increased the luc2/Rluc ratio of the CGA reporter construct to 39.7% (Fig 2D and S1 Table). Consistently expression of K63R ubiquitin enhanced the colony growth on the assay plates (S3 Fig). Furthermore as shown by western blot analysis (Fig 3A), the expression of full-length reporter protein, which appeared at the same position of the full-length product in the hel2∆ strain, was increased by the expression of K63R ubiquitin (Fig 3A lane K63R). Contrastingly, expression of the full-length mRNA was less markedly affected by deletion of Hel2 and expression of K63R as compared with expression of the full-length protein (Fig 3B), suggesting that K63 polyubiquitination as well as Hel2 have more direct roles on protein turnover.
Fig. 3. Comparison of the effects of Hel2 deletion and K63R expression on protein and mRNA levels in stalled translation. 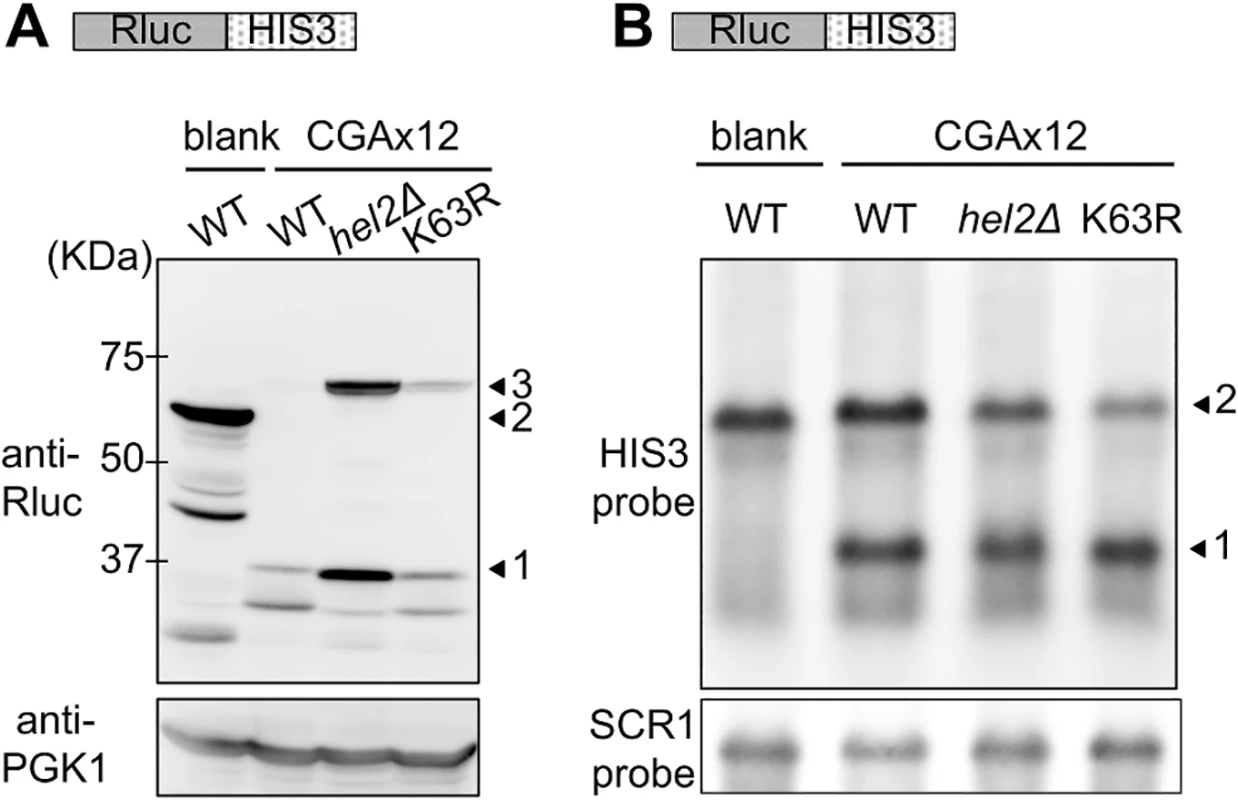
(A) Western blot analysis for the effects of K63R ubiquitin on Rluc-HIS3 reporter. blank indicates the result of the Rluc-blank-HIS3 reporter, and CGAx12 indicates the results of the Rluc-CAAx12-HIS3 reporter. WT indicates the wild-type strain (BY4727) without K63R ubiquitin, hel2∆ indicates the hel2∆ strain (SKY61), and K63R indicates the wid-type strain expressing K63R ubiquitin. Arrowhead 1 indicates the Rluc protein alone, arrowhead 2 indicates the Rluc-blank-HIS3 protein, and arrowhead 3 indicates the Rluc-CGA x12-HIS3 protein. PGK antibody was used as loading control. (B) Northern blot analysis for the effects of K63R ubiquitin on Rluc-HIS3 reporter. blank and CGAx12 as well as WT, hel2∆, and K63R are as in (A). The reporter mRNA was probed by HIS3 complementary DNA fragment. Arrowhead 1 indicates the cleaved HIS3 mRNA and arrowhead 2 indicates the full-length mRNA. SCR1 mRNA was detected as a loading control. To further confirm the role of K63 polyubiquitin, we tested two additional ubiquitin mutants, one is K0 ubiquitin, in which all of the lysine residues are substituted to arginine, and the other is K63only ubiquitin, in which all of the lysine residues except K63 are substituted to arginine, thus K63 serves as a solo lysine for polyubiquitination. The cellular ubiquitination levels of K0 ubiquitin and K63only ubiquitin were substantially lower than wild-type ubiquitin (Fig 4A), therefore both ubiquitin mutants were also expressed from multicopy plasmid. Although the effect was less marked than K63R ubiquitin presumably due to the cellular ubiquitination level, K0 ubiquitin expressed from the multicopy plasmid increased the luc2/Rluc ratio (Fig 4B and S1 Table). In the colony growth assay, K0 ubiquitin on both plasmids induced significant growth on a plate lacking histidine (Fig 4C). Contrastingly, K63only ubiquitin on neither of the plasmids affected at all (Fig 4B and 4C), suggesting that the K63 solely is sufficient to abolish the inhibitory effect of K0 ubiquitin. These results suggested that the K63 polyubiquitin signal is predominantly responsible for the reduction of a full-length product in the quality control.
Fig. 4. Effects of K0 and K63only ubiquitin mutants on stalled translation. 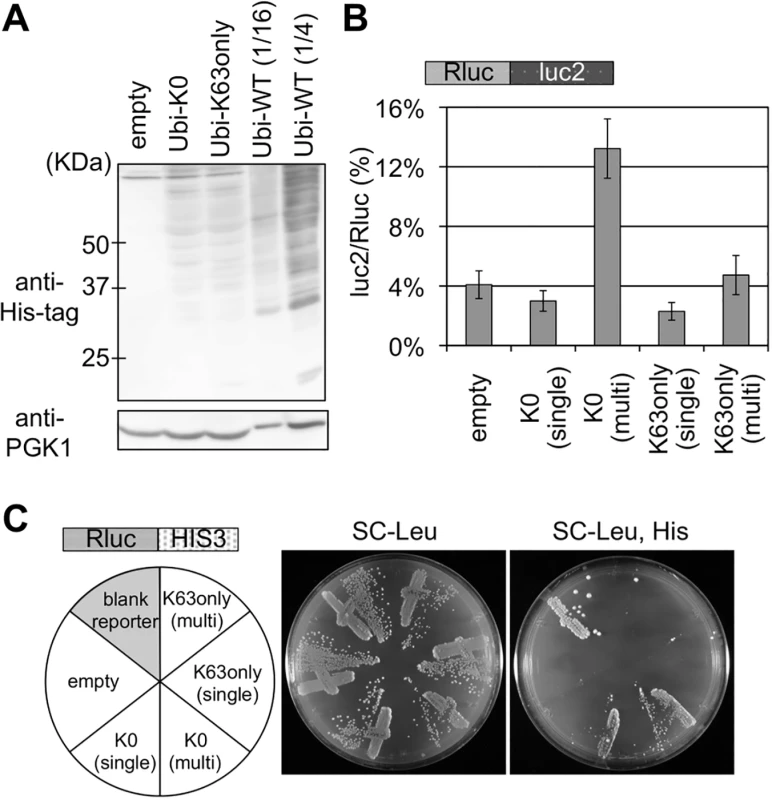
(A) Cellular ubiquitination levels of K0 and K63only ubiquitin mutants. Ubiquitinated proteins were detected as above. Amount of the protein sample from a cell expressing His-tagged wild-type ubiquitin are decreased to 1/16 and 1/4 dilution. (B) Dual luciferase assay for the of K0 and K63only ubiquitin mutants on the Rluc-luc2 reporter. Plasmids bearing K0 or K63only ubiquitin mutants were introduced into a wild-type strain harboring Rluc-luc2 reporter strain (HRKW-2). (single) indicates the transformant with ubiquitin expression from single copy plasmids, and (multi) indicates that from multiple copy plasmids. Average luc2/Rluc ratios and standard deviations were determined from three independent measurements. (C) Genetic colony growth test for the effect of K0 and K63only ubiquitin mutants on the Rluc-HIS3 reporter (SKY24). Types of ubiquitin vectors are indicated on the left. Transformant colonies were streaked on the SC-Leucine (Leu) (middle), and SC-Leucine, Histidine (Leu, His) (right) plates, and colony growth was monitored for 4 days at 30°C. In order to test the roles by K63 polyubiquitination in stalled translation on other stall signals, several additional stall signals such as poly CGA, poly lysine, poly arginine and poly GGN, as in preceding reports [5, 24], are inserted between the luciferase genes. As shown in S4 Fig (luc2/Rluc ratios of WT columns), stall effects of most of the additional signals were much weaker than that of the CGA signal, so as the effect of the genetic variants (S4 Fig and S2 Table), resulting in low significance scores. Nevertheless, importantly, the elongated consecutive polylysine signal, polylysine (x24), significantly increased the stall effect. For this polylysine (x24), luc2/Rluc ratios were markedly decreased by deletion of Ltn1 and the ratio increased by deletion of Hel2 as well as K63R expression, suggesting the involvement of Hel2 and K63 polyubiquitination for the quality control of this stall signal, consistently with the previous reports [10, 11].
One of the primary functions of polyubiquitination is to induce proteolysis by the proteasome [15, 25]. Thus, we tested the effects of the proteasome inhibitor, MG132. Addition of MG132 gave similar results to those by deleting Ltn1, as previously reported [11], i.e., enhanced expression of upstream Rluc protein (Fig 5A) and a decrease in the luc2/Rluc ratio (Fig 5B and S1 Table). These effects of MG132 indicated that the proteasome was irrelevant for the expression of a full-length protein from stalled translation. Thus, this suggests that the function of a K63 polyubiquitin chain for the quality control of a stalled ribosome is not mediated by the proteasome. Consistently, addition of MG132 and another proteasome inhibitor, PS341, at different concentrations did not markedly alter the fold differences in luc2/Rluc ratios between transformants of an empty vector (-K63R) and a K63R ubiquitin mutant (+K63R) (Fig 5C and S1 Table). Yeast strains that expressed only K63R ubiquitin were reported to be sensitive to anisomycin [26]. Consistent with this, overexpressing our K63R ubiquitin mutant increased the sensitivity to anisomycin (S5A Fig), indicating that our K63R ubiquitin mutant was valid. Taken together, these results indicated that K63 polyubiquitination functions as a non-proteasomal signal to inhibit the expression of a full-length protein from stalled translation.
Fig. 5. Effects of proteasome inhibitors, MG132 and PS341, on stalled translation and K63R expression. 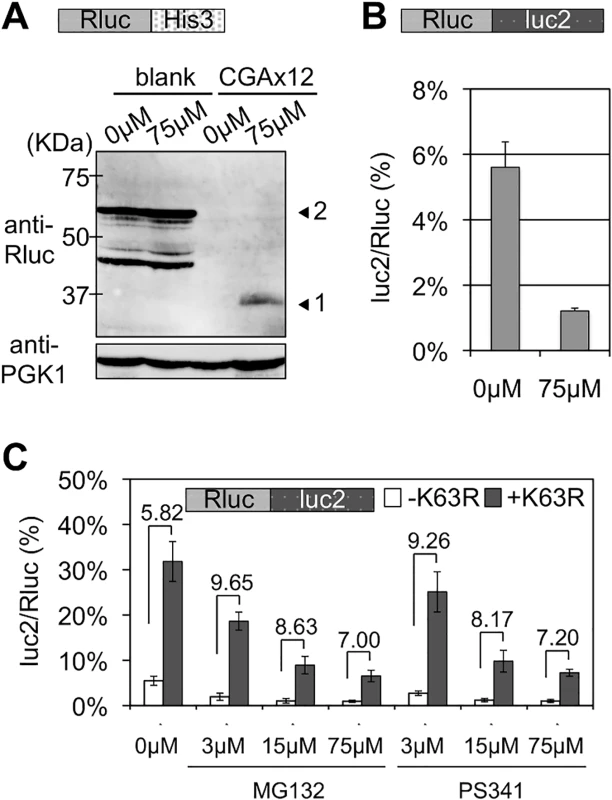
(A) Western blot analysis for effect of MG132. Vectors with Rluc-HIS3 reporters were introduced into a wild-type strain (BY4727). Yeast transformants were incubated for 5 h with either DMSO (e.g., 0 μM MG132) or 75 μM MG132. Arrowhead 1 indicates the Rluc protein only and arrowhead 2 indicates the Rluc-blank-HIS3 protein. PGK1 was used as a loading control. (B) Dual luciferase assay for the effect of MG132 on wild-type strain with Rluc-CGA x12-luc2 reporter (HRKW-2). MG132 was used as in (A). (C) The empty or K63R ubiquitin mutation plasmids were introduced into the pdr5∆ yeast strain harboring Rluc-luc2 reporter (SKY142). The transformants were incubated in the presence of MG132 or PS341 at indicated concentrations for 2 h. Fold differences between the percentages of luc2/Rluc ratios between the transformants with the absence (-K63R) or presence (+K63R) of K63R expression are indicated over the top of the bar graphs. Temporal order of K63 polyubiquitin signals during the quality control for stalled translation
The quality control for stalled translation involves factors other than Hel2 and Ltn1, such as Not4, Asc1, Dom34, Hbs1, a Ski complex, and a RQC complex. Deleting these factors did not markedly affect the ratio when using a blank reporter (S2C Fig). Then, as shown in Fig 6A, deleting these factors either decreased or increased the luc2/Rluc ratio with our CGA reporter (“-K63R” in Fig 6A and S1 Table), as previously reported [3, 9, 10]. Among them, the results of not4∆ strains seem somewhat inconsistent among previous reports [5, 11, 14, 27], presumably reflecting multiple roles of Not4 in gene expression and strain variability as previously discussed [14, 27]. The not4∆ strains in this study exhibited slower growth on YPD medium, as well as high sensitivity to azetidine-2-carboxylic acid (AZC) (S6 Fig), as previously reported [14, 27], supporting the validities of our strains.
Fig. 6. Temporal regulation of K63 polyubiquitination and Hel2 with other factors for stalled translation. 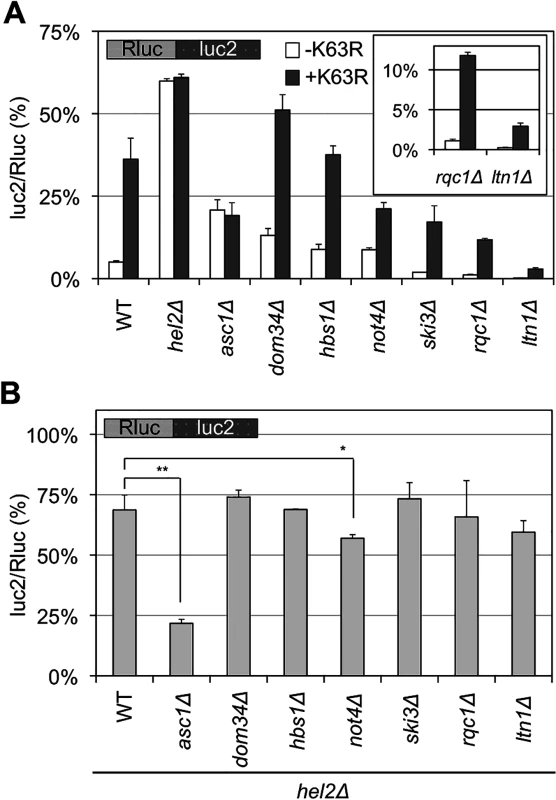
(A) The luc2/Rluc ratios for Rluc-luc2 reporter in the wild-type (WT) and single knockout strains (as indicated) in the presence (+K63R) or absence (-K63R) of K63R expression. The inset shows the results for the rqc1∆ and ltn1∆ strains. The average luc2/Rluc ratios and their standard deviations were obtained for three independent measurements. (B) The luc2/Rluc ratios for Rluc-luc2 reporter in the strains with double knockout of Hel2 and other factors involved in stalled translation. All strains are hel2∆. Thus, as examples, WT in this figure indicates a hel2∆ single knockout strain, and asc1∆ indicates the hel2∆asc1∆ strain. The average luc2/Rluc ratios and their standard deviations were obtained for three independent measurements. *p < 0.05, **p < 0.01. Next, the luc2/Rluc ratios in those genetic backgrounds were monitored in the presence of K63R ubiquitin to analyze the order of action of K63 polyubiquitination. In the asc1∆ and hel2∆ strains, K63R expression barely affected the luc2/Rluc ratios (Fig 6A), whereas in other strains, the luc2/Rluc ratios were increased to the expression of K63R (Fig 6A). The temporal relationships of Hel2 with respect to other factors were also analyzed by determining the luc2/Rluc ratios of double knockout strains with Hel2 (Fig 6B and S1 Table). The hel2∆asc1∆ strain exhibited a luc2/Rluc ratio of 21.75% (Fig 6B), which was close to the ratio with the asc1∆ single knockout strain (19.23%; S7 Fig), whereas other strains had rather comparable ratios (60%–74%) close to the hel2∆ single knockout strain (68.66%; Fig 6B) except for the not4∆hel2∆ strain (discussed later). For comparisons, the luc2/Rluc ratios of double knockout strains with Asc1 were examined, except for not4∆asc1∆ strain, which could not be constructed in our strain background. All strains with asc1∆ background exhibited comparable luc2/Rluc ratios (S7 Fig and S1 Table). Further, the hel2∆ and asc1∆ strains exhibited hyper-sensitive growth to anisomycin (S5B Fig). Anisomycin inhibits translation elongation [28], thus anisomycin sensitivities induced by expression of K63R ubiquitin and deletion of Hel2 and Asc1 supported the functional relevance of Hel2, Asc1, and K63 polyubiquitination in the quality control of stalled translation.
These results suggest that the effect of K63 polyubiquitination in this study depends on the functions of Hel2 and Asc1. And the K63 polyubiquitination act independently with other factors, such as Dom34/Hbs1 complex, Ski3, Ltn1, and Rqc1. It was also indicated that Asc1 functions prior to these other factors, including Hel2, for stalled translation surveillance (Fig 6B and S7 Fig). The early functions of Asc1 and Hel2 were consistently suggested by the expression of a full-length protein from a polylysine reporter in previous report [10].
Differential roles of putative K63 polyubiquitin signals in two related quality control pathways
Next, we investigated the effects of K63 polyubiquitination and Hel2 on the quality control for nonstop translation. mRNA without an in-frame stop codon is rapidly degraded by exosomes and a Ski complex in an mRNA surveillance system, called nonstop mRNA decay (NSD) [29]. A nascent protein derived from nonstop mRNA is rapidly degraded by the ubiquitin proteasome pathway in collaboration with the E3 ligase Ltn1 [11]. A Dom34/Hbs1 complex was also reported to function during nonstop translation [24]. Because many factors participate with similar roles in the quality control pathways for both nonstop and stalled translations, the distinction between these pathways is ambiguous.
A nonstop HIS3 reporter allele was employed for the evaluation of the role of K63 polyubiquitin in nonstop translation (Fig 7A) [29]. Nonstop HIS3 mRNA and its nascent product are substrates for nonstop translation surveillance, and thus, expression of HIS3 enzyme from the nonstop mRNA is not sufficient for cell viability in a medium without histidine. Upon disrupting the genes responsible for nonstop translation surveillance, the strains restore sufficient expression of HIS3 enzyme for normal growth.
Fig. 7. Effects of K63 polyubiquitination and Hel2 on quality control for non-stop translation. 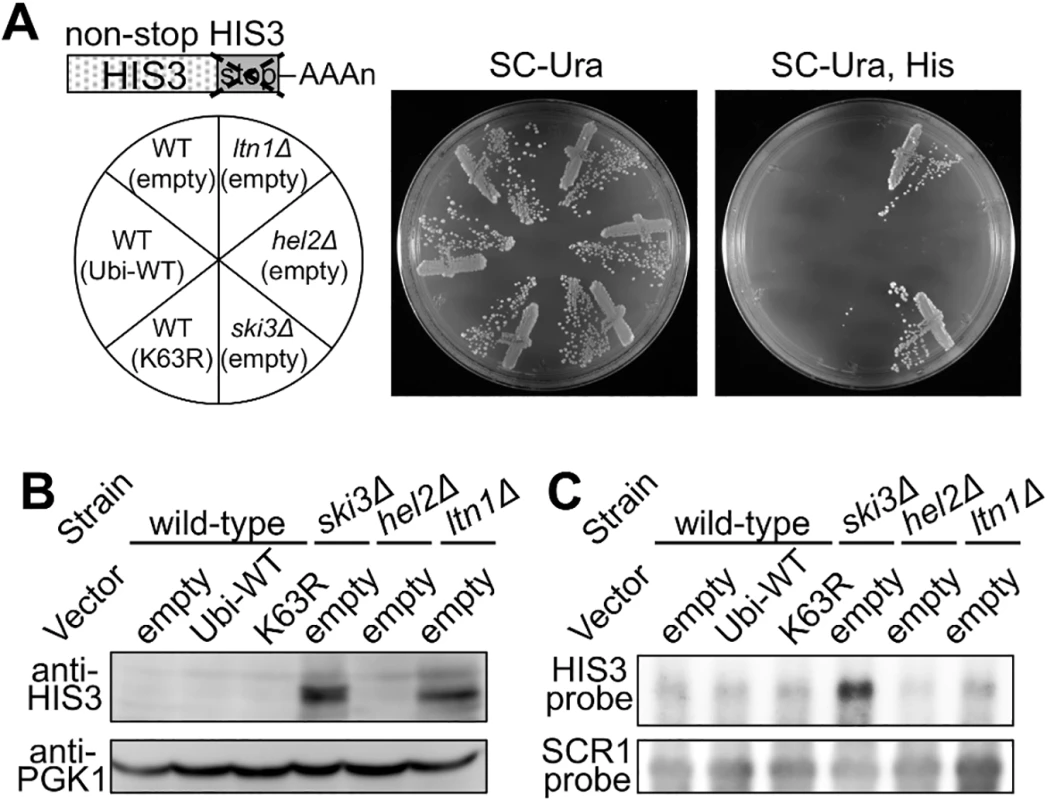
(A) Genetic colony growth test for the nonstop HIS3 reporter. A schematic image of a nonstop HIS3 allele is illustrated above. Empty vector (empty), wild-type ubiquitin (Ubi-WT), or K63R ubiquitin (K63R) plasmids were introduced into the wild-type, ski3∆, hel2∆, or ltn1∆ strains harboring nonstop HIS3 reporter (S17-A08, S17-A09, SKY123, SKY137). Types of knockout alleles and vectors are indicated on the left. Transformant colonies were streaked on the SC-Uracil (Ura) (middle), and SC-Uracil, Histidine (Ura, His) (right) plates, and colony growth was monitored for 3 days at 30°C. (B) Western blot analysis for the nonstop HIS3 reporter. Protein samples were extracted from the transformants of the nonstop HIS3 reporter strains as in (A). The expression of HIS3 protein was detected using a HIS3 polyclonal antibody. PGK1 was used as a loading control. (C) Northern blot analysis for the nonstop HIS3 reporter. mRNA samples were extracted from the transformants of the nonstop HIS3 reporter strains as in (A). The nonstop HIS3 reporter mRNA was probed by HIS3 complementary DNA fragment. SCR1 mRNA was detected as a loading control. The nonstop HIS3 allele was integrated into the genomes of a his3∆ strain as well as Ski3, Hel2, and Ltn1 deletion strains, and then their cellular expressions were assessed by histidine auxotrophic growth, western blotting, and northern blotting. The ski3∆ and ltn1∆ strains were viable on a plate that lacked histidine, whereas the K63R ubiquitin transformant and hel2∆ strain were not viable (Fig 7A). Western blot showed increased HIS3 protein expression in the ski3∆ and ltn1∆ strains but not in the K63R ubiquitin transformant or the hel2∆ strain (Fig 7B). Also, northern blot analysis showed that nonstop HIS3 mRNA was stabilized in the ski3∆ strain and the mRNA level did not increased in the ltn1∆ strain, as reported previously [30]. The deletion of Hel2 and expression of K63R ubiquitin hardly affected the stability of the reporter mRNA (Fig 7C). These results indicated that K63 polyubiquitination and Hel2 are not involved in the quality control for nonstop translation.
To confirm this speculation further, we tested the involvement of K63 polyubiquitination and Hel2 in another type of nonstop translation derived from self-cleavage of hammerhead ribozyme inserted in reporter gene [31, 32]. Except for Ski7 [31], it is reported that quality control of ribozyme-derived nonstop translation is accomplished by common factors with the nonstop mRNAs with polyA tail [24, 31, 33]. Hammerhead ribozyme sequence was inserted at the junction of a fusion gene of Rluc and HIS3 gene (S8A Fig) and expression of Rluc protein was monitored by western blot. Similarly to the results of nonstop allele of HIS3, upstream Rluc protein derived from self-cleaved mRNA was detected in the ski3∆ and ltn1∆ strains, but overexpression of K63R and deletion of Hel2 hardly enhanced the level of Rluc protein (S8B Fig).
Recently, Hel2 had been reported to weakly affect the quality control of nonstop translation [14] and this is inconsistent to our speculation mentioned above. Taking the high complexity of the quality control systems as well as the yet-unknown cryptic factors behind the genetic backgrounds into consideration, it is possible to speculate that the inconsistency arises from certain masking effects by other factors collaborating with K63 polyubiquitination and Hel2 in the quality control of nonstop mRNA.
Collectively with two type of reporter genes, the nonstop HIS3 allele and the hammerhead derived nonstop mRNA, our results suggested that K63 polyubiquitination as well as Hel2 have much more significant roles on the quality control of stalled translation that occurs during elongation.
Discussion
During the quality control for stalled translation, at least two E3 ubiquitin ligases, Ltn1 and Hel2, are distinctly involved (Fig 1) as reported previously [10, 12]. Ubiquitin is used for versatile cellular signals through polymerization via its lysine residues [15]. In this study, using ubiquitin mutants with Lys-to-Arg substitutions, which inhibit a specific type of polymerization linkage, we identified a unique role of K63 polyubiquitination in stalled translation surveillance in budding yeast S. cerevisiae (Figs 2, 3, 4 and S3). In stalled translation, the K63 polyubiquitination did not serve as a proteasomal signal (Fig 5), functioned in the presence of Hel2 and Asc1, and was independent of other known steps (Fig 6A). These findings suggest that multiple types of polyubiquitination signals and ubiquitin ligases are involved in the quality control for stalled translation, i.e., K48 polyubiquitination for proteasomal degradation and K63 polyubiquitination in this study. Our findings of K63 polyubiquitination provide important clues to elucidate the mechanisms of stalled translation surveillance and for novel roles of ubiquitin signals in cellular systems.
K63 polyubiquitination involvement in various pathways, such as those for cell signaling, permease trafficking, and DNA repair, have been extensively investigated [34–36]. In these pathways, a K63 polyubiquitin chain recruits factors for succeeding reactions through direct recognitions by K63 polyubiquitin specific receptors [37–41]. In this study, because K63 polyubiquitination was not involved in proteasomal degradation (Fig 5), it can be assumed that K63 polyubiquitination in stalled translation functions in recruiting related factors, and thereby serves as an intermediate between the recognition of stalled translation and degradation and/or dissociation of stalled machineries. In this scenario, a receptor of the K63 polyubiquitin chain for stalled translation must play an important role in transmitting a ubiquitin signal to subsequent reactions.
It has been reported that the rate of translation elongation is extensively regulated for a variety of reasons [42–44]. Therefore, a quality control surveillance system must be able to distinguish between aberrantly stalled states that are to be eliminated and rather slow translations that should be normally maintained. It was also reported that the length of a polyubiquitin chain was crucial for efficient recognition by its receptors [40–41]. In many cases, the length of a polyubiquitin chain is regulated by the balance between the actions of ubiquitin ligases and deubiquitinating enzymes [45]; therefore, putative deubiquitinating enzymes possibly participate in the dynamic regulation of stalled translation surveillance. Taken together, it is tempting to hypothesize that K63 ubiquitination and deubiquitinating activities are regulated in response to the elongation rate of ribosomes even in regular translation, and an aberrant stall that cannot be resumed results in substantial elongation of a K63 polyubiquitin chain beyond a threshold to induce recruitment of factors for degradation and dissociation of stalled translation, as illustrated (S9 Fig).
In our study, the Hel2 depletion and K63R ubiquitin expression exhibited certain similarities in yeast. Both resulted in increased expression patterns of full-length proteins (Fig 3), and their temporal orders of action were closely related (Fig 6). Furthermore, both of these barely affected the quality control for nonstop translation (Fig 7 and S8 Fig). However, despite our efforts, the target substrates for the Hel2 E3 ubiquitin ligase and the K63 ubiquitin enzyme responsible for this stalled translation system remain to be identified, although our results strongly suggest that there is a direct connection between them.
In this report, among the factors tested, Asc1 was the only factor that was clearly indicated to function prior to Hel2 and K63 polyubiquitination. But, it is noteworthy that the luc2/Rluc ratio of the not4∆hel2∆ double knockout strain was slightly, but significantly lower than that of the hel2∆ strain (Fig 6B). Previously, Not4 was proposed to preserve translation elongation, as deletion of Not4 reduced the full-length protein from mRNA with stall signals [27]. In our study, the luc2/Rluc ratio of the asc1∆ strain was lower than that of the hel2∆ strain and the K63R expressed strain (Fig 6B), suggesting the bidirectional role of Asc1 in protein synthesis; one is elimination of aberrant stalled translation by the quality control with Hel2 and K63 polyubiquitination, and the other is inhibition of translation through inefficient mRNA motifs such stall signals. Further, the fact that not4∆asc1∆ strain could not be constructed suggests the overlapping roles of Asc1 and Not4 for the preservation of translation elongation. And thus it is worth mentioning that the Asc1/Not4 related pathway(s) could be distinct in some parts from the pathway Hel2 and K63 polyubiquitination are involved.
Previously, a possibility of a common mechanism for stalled translation during elongation (no-go type translation) and nonstop translation could not be ruled out because many of the factors, particularly a Dom34/Hbs1 complex, were involved in the quality controls of both aberrances [2]. In this study, our results suggested that the initial processes of the aberrant translation surveillances are distinct because K63R polyubiquitin affect no-go type stall translation more significantly than nonstop translation (Figs 2, 3, 7 and S8). Thus, we assume that aberrant translations are detected by a specific mechanism and are degraded by common mechanisms. In addition, we assume that K63 polyubiquitin is not significantly involved for the quality control of nonstop translation because nonstop is rather obvious for a surveillance system than a cumulative type of aberrance as no-go type stall translation. However, still more efforts are required to reveal the functional mechanism of K63 polyubiquitination in quality control pathways.
It is currently thought that ribosome dissociation, endonucleolytic cleavage, and/or the proteasomal degradation of a nascent protein should occur after an aberrant stall is detected [2]. Because our K63R mutant expressed full-length protein from mRNA with a stalled signal (Figs 2 and 3A) but did not affected the mRNA level (Fig 3B), we assume that K63 polyubiquitination is involved in inducing the ribosome dissociation or proteasomal degradation. Thus, identifying factors essential for regulating K63 polyubiquitination, such as substrates, receptors, and potential deubiquitinating enzymes of K63 polyubiquitination, will be critical for elucidating the detection mechanism of an aberrant stall and the regulatory relationships among the pathways that are involved at the site of stalled translation.
Materials and Methods
Strains and plasmids
The S. cerevisiae strains used in study are listed in S2 Table and the plasmids used are listed in S3 Table.
Media
Yeast media were YPD or synthetic media, which were prepared with the appropriate dropout mix (ForMedium; Hunstanton, UK); 2% agar was added for plates. For analysis using MG132, yeast strains were grown in a synthetic medium, which included 0.17% yeast nitrogenous base without ammonium sulfate, 0.1% proline, 2% glucose, and 0.003% SDS with the appropriate dropout mix (ForMedium; Hunstanton, UK), as previously described [46]. Yeast cells were incubated for either 5 h for wild-type strain (BY4727) or 2 h for the pdr5∆ strain (SKY142) after adding MG132 (Merck Millipore, Darmstadt, Germany); the concentrations used are indicated in the figures.
Dual luciferase assay
Yeast cells were grown in appropriate media to log phase at 30°C and then collected by centrifugation. Whole cell lysates were prepared by vigorous shaking with glass beads on a FastPrep 24 (MP Biomedicals, Santa Ana, CA, USA). To determine luciferase activities, a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) and a GloMax 96 Microplate Luminometer (Promega, Madison, WI, USA) were used according to the manufacturer’s instructions.
Western blotting
Whole cell lysates were prepared by vigorous shaking with glass beads on a FastPrep 24 (MP Biomedicals, Santa Ana, CA, USA). Lysates were separated by SDS-PAGE and then transferred to PVDF membranes (Merck Millipore, Darmstadt, Germany). Transferred proteins were probed using anti-Rluc (Merck Millipore, Darmstadt, Germany), anti-HIS3 (custom polyclonal antibody from Takara Bio, Shiga, Japan), or anti-PGK (Invitrogen, Carlsbad, CA, USA) antibodies, as indicated in the figures. Antibodies used for probing were detected with ImmunoStar LD (Wako, Osaka, Japan). Images were acquired with LAS-3000 (Fujifilm, Tokyo, Japan).
Northern blotting
RNA was isolated by RNAiso Plus (Takara Bio, Shiga, Japan) with vigorous shaking with glass beads on a FastPrep 24 (MP Biomedicals, Santa Ana, CA, USA). Isolated RNA were separated by denaturing formaldehyde agarose gel and transferred to Hybond-N+ (GE Helthcare, Little Chalfont, UK). RNA was detected by DIG Northern Starter Kit (Roche Diagnostics, Basel, Switzerland). Images were acquired with LAS-3000 (Fujifilm, Tokyo, Japan). Customized oligo DNAs with 5' modification of digoxigenin were used as probes. Probes used in this report are: HIS3 probe [5'-CGACAACTGCGTACGGCCTGTTCGAAAGAT - 3'] and SCR1 probe [5'-ATCCCGGCCGCCTCCATCAC - 3'].
Supporting Information
Zdroje
1. Wolff S, Weissman J, Dillin A (2014) Differential Scales of Protein Quality Control. Cell 157 : 52–64. doi: 10.1016/j.cell.2014.03.007 24679526
2. Shoemaker C, Green R (2012) Translation drives mRNA quality control. Nat Struct Mol Biol 19 : 594–601. doi: 10.1038/nsmb.2301 22664987
3. Doma MK, Parker R (2006) Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440 : 561–564. 16554824
4. Hosoda N, Kobayashi T, Uchida N, Funakoshi Y, Kikuchi Y, et al. (2003) Translation termination factor eRF3 mediates mRNA decay through the regulation of deadenylation. J Biol Chem 278 : 38287–38291. 12923185
5. Dimitrova LN, Kuroha K, Tatematsu T, Inada T (2009) Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J Biol Chem 284 : 10343–10352. doi: 10.1074/jbc.M808840200 19204001
6. Letzring DP, Dean KM, Grayhack EJ (2010) Control of translation efficiency in yeast by codon-anticodon interactions. RNA 16 : 2516–2528. doi: 10.1261/rna.2411710 20971810
7. Kobayashi K, Kikuno I, Kuroha K, Saito K, Ito K, et al. (2010) Structural basis for mRNA surveillance by archaeal Pelota and GTP-bound EF1α complex. Proc Natl Acad Sci U S A 107 : 17575–17579. doi: 10.1073/pnas.1009598107 20876129
8. Shoemaker CJ, Eyler DE, Green R (2010) Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science 330 : 369–372. doi: 10.1126/science.1192430 20947765
9. Kuroha K, Akamatsu M, Dimitrova L, Ito T, Kato Y, et al. (2010) Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation arrest. EMBO Rep 11 : 956–961. doi: 10.1038/embor.2010.169 21072063
10. Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, et al. (2012) A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151 : 1042–1054. doi: 10.1016/j.cell.2012.10.044 23178123
11. Bengtson M, Joazeiro CA (2010) Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 467 : 470–473. doi: 10.1038/nature09371 20835226
12. Letzring D, Wolf AS, Brule CE, Grayhack EJ (2013) Translation of CGA codon repeats in yeast involves quality control components and ribosomal protein L1. RNA 19 : 1208–1217. doi: 10.1261/rna.039446.113 23825054
13. Singh RK, Gonzalez M, Kabbaj MH, Gunjan A (2012) Novel E3 ubiquitin ligases that regulate histone protein levels in the budding yeast Saccharomyces cerevisiae. PLoS One 7:e36295. doi: 10.1371/journal.pone.0036295 22570702
14. Duttler S, Pechmann S, Frydman J (2013) Principles of cotranslational ubiquitination and quality control at the ribosome. Mol Cell 50 : 379–393. doi: 10.1016/j.molcel.2013.03.010 23583075
15. Kulathu Y, Komander D (2012) Atypical ubiquitylation—the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat Rev Mol Cell Biol 13 : 508–523. doi: 10.1038/nrm3394 22820888
16. Hicke L (2001) Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol 2 : 195–201. 11265249
17. Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, et al. (2003) Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol 5 : 461–466. 12717448
18. Iwai K (2012) Diverse ubiquitin signaling in NF-κB activation. Trends Cell Biol 22 : 355–364. doi: 10.1016/j.tcb.2012.04.001 22543051
19. Chen L, Muhlrad D, Hauryliuk V, Cheng Z, Lim MK, et al. (2010) Structure of the Dom34-Hbs1 complex and implications for no-go decay. Nat Struct Mol Biol 17 : 1233–1240. doi: 10.1038/nsmb.1922 20890290
20. Grentzmann G, Ingram JA, Kelly PJ, Gesteland RF, Atkins JF (1998) A dual-luciferase reporter system for studying recoding signals. RNA 4 : 479–486. 9630253
21. Arnason T, Ellison MJ (1994) Stress resistance in Saccharomyces cerevisiae is strongly correlated with assembly of a novel type of multiubiquitin chain. Mol Cell Biol 14 : 7876–7883. 7969127
22. Flick K, Ouni I, Wohlschlegel JA, Capati C, McDonald WH, et al. (2004) Proteolysis-independent regulation of the transcription factor Met4 by a single Lys 48-linked ubiquitin chain. Nat Cell Biol 6 : 634–641. 15208638
23. Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A, et al. (2010) UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci U S A 107 : 1355–1360. doi: 10.1073/pnas.0912802107 20080579
24. Tsuboi T, Kuroha K, Kudo K, Makino S, Inoue E, et al. (2012) Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3' end of aberrant mRNA. Mol Cell 46 : 518–529. doi: 10.1016/j.molcel.2012.03.013 22503425
25. Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, et al. (1989) A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243 : 1576–1583. 2538923
26. Spence J, Gali RR, Dittmar G, Sherman F, Karin M, et al. (2000) Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell 102 : 67–76 10929714
27. Halter D, Collart MA, Panasenko OO (2014) The Not4 E3 ligase and CCR4 deadenylase play distinct roles in protein quality control. PLoS One 9:e86218. doi: 10.1371/journal.pone.0086218 24465968
28. Grollman AP (1967) Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J Biol Chem 242 : 3226–33. 6027796
29. van Hoof A, Frischmeyer PA, Dietz HC, Parker R (2002) Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295 : 2262–2264. 11910110
30. Defenouillère Q, Yao Y, Mouaikel J, Namane A, Galopier A, et al. (2013) Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products. Proc Natl Acad Sci U S A 110 : 5046–51. doi: 10.1073/pnas.1221724110 23479637
31. Meaux S, van Hoof A (2006) Yeast transcripts cleaved by an internal ribozyme provide new insight into the role of the cap and poly(A) tail in translation and mRNA decay. RNA 12 : 1323–37. 16714281
32. Khvorova A, Lescoute A, Westhof E, Jayasena SD (2003) Sequence elements outside the hammerhead ribozyme catalytic core enable intracellular activity Nat Struct Biol 10 : 708–12. 12881719
33. Matsuda R, Ikeuchi K, Nomura S, Inada T (2014) Protein quality control systems associated with no-go and nonstop mRNA surveillance in yeast. Genes Cells 19 : 1–12. doi: 10.1111/gtc.12106 24261871
34. Skaug B, Jiang X, Chen ZJ (2009) The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem 78 : 769–796. doi: 10.1146/annurev.biochem.78.070907.102750 19489733
35. Huen MS, Chen J. (2010) Assembly of checkpoint and repair machineries at DNA damage sites. Trends Biochem Sci 35 : 101–108. doi: 10.1016/j.tibs.2009.09.001 19875294
36. Lauwers E, Erpapazoglou Z, Haguenauer-Tsapis R, André B (2010) The ubiquitin code of yeast permease trafficking. Trends Cell Biol 20 : 196–204. doi: 10.1016/j.tcb.2010.01.004 20138522
37. Kanayama A, Seth RB, Sun L, Ea CK, Hong M, et al. (2004) TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell 15 : 535–548. 15327770
38. Cj Wu, Conze DB, Li T, Srinivasula SM, Ashwell JD (2006) Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation. Nat Cell Biol 8 : 398–406. 16547522
39. Kim H, Chen J, Yu X (2007) Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316 : 1202–1205. 17525342
40. Sobhian B, Shao G, Lilli DR, Culhane AC, Moreau LA, et al. (2007) RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316 : 1198–1202. 17525341
41. Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, et al. (2009) Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell 136 : 1098–1109. doi: 10.1016/j.cell.2009.03.007 19303852
42. Lu J, Deutsch C (2008) Electrostatics in the ribosomal tunnel modulate chain elongation rates. J Mol Biol 384 : 73–86. doi: 10.1016/j.jmb.2008.08.089 18822297
43. Li GW, Oh E, Weissman JS (2012) The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 484 : 538–541. doi: 10.1038/nature10965 22456704
44. Spencer PS, Siller E, Anderson JF, Barral JM (2012) Silent substitutions predictably alter translation elongation rates and protein folding efficiencies. J Mol Biol 422 : 328–335. doi: 10.1016/j.jmb.2012.06.010 22705285
45. Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, et al. (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123 : 773–786. 16325574
46. Liu C, Apodaca J, Davis LE, Rao H (2007) Proteasome inhibition in wild-type yeast Saccharomyces cerevisiae cells. Biotechniques 42 : 158, 160, 162. 17373478
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



